Abstract
Anoxygenic photosynthetic growth of Rhodobacter sphaeroides 2.4.1 requires a functional fnrL gene, which encodes the anaerobic regulator, FnrL. Using transcriptional fusions to the puc operon in which the upstream FNR consensus-like sequence is either present or absent, we obtained results that suggest that FnrL has both a direct and an indirect role in puc operon expression. In addition to FnrL, several other factors, including the two-component Prr regulatory system and the transcriptional repressor PpsR, are known to mediate oxygen control of photosynthesis gene expression in this organism. Therefore, we examined the relationship between FnrL and these other regulatory elements. Our results indicate that while mutations of prr or ppsR can lead to an increase in expression of some photosynthesis genes under aerobic and anaerobic conditions, regardless of the presence or absence of FnrL, there remains an absolute requirement for a functional fnrL gene for photosynthetic growth. We examined the potential role(s) of FnrL in photosynthetic growth by considering several target genes which may be required for this growth mode.
In recent years, a number of factors that mediate oxygen control of photosynthesis (PS) gene expression in Rhodobacter sphaeroides 2.4.1, including the two-component Prr regulatory system (6, 7, 17), the PpsR repressor (11, 22, 23) and AppA antirepressor (10, 12), the tspO gene product (27), and the product of the fnrL gene (30), have been identified. The Prr regulatory system consists of a sensor histidine kinase encoded by prrB that responds to decreased oxygen tension and functions through the response regulator encoded by prrA (6, 7), although a target DNA sequence for PrrA has not been identified. The ppsR gene product functions as a transcriptional repressor under both aerobic and photosynthetic conditions, and an intact palindrome with the sequence motif TGTN12ACA is required for PpsR activity (11, 22, 23). The activity of PpsR, in turn, appears to be modulated by the appA gene product, which may be directly responsive to redox changes brought about by changes in oxygen tension and light intensity (12). The tspO gene encodes an outer membrane-localized polypeptide that appears to dampen the course of derepression of a number of PS genes in response to a decline in external oxygen. FnrL is the R. sphaeroides homolog of the Escherichia coli anaerobic regulatory protein, Fnr. The similarity of the helix-turn-helix motif of FnrL to the corresponding region in other members of the Fnr family of proteins (30) predicts the DNA recognition sequence TTGATN4ATCAA. This motif has been identified within the upstream sequences of several operons (29, 30), and we have determined for the hemA gene, whose upstream sequences contain an FNR consensus sequence, that FnrL increases transcription when oxygen tension is reduced (30).
Other factors that appear to be either signal generators or sensors have been identified. Analysis of the phenotype(s) associated with mutations in the ccoNOQP and/or rdxBHIS operons suggest that these operons encode proteins which form membrane-localized complexes that can generate or respond to a redox signal or intermediate indicative of a change from aerobiosis to anaerobiosis (21, 31). This signal or its absence ultimately results in increased transcription of PS genes under highly aerobic conditions in strains mutant for the membrane-responsive redox carriers. We have proposed that this altered transcriptional response is mediated through both FnrL and the Prr two-component activation system (31). Additionally, studies of the tspO gene product (27), which is the R. sphaeroides homolog of the eukaryotic peripheral benzodiazopene receptor (28), indicates it may play a role in oxygen control mediated through PpsR/AppA, since the spectrum of downstream activities of TspO and PpsR are similar.
Thus, do all of these regulatory genes belong to single or multiple regulatory circuit(s)? The TspO, PpsR, and AppA proteins appear to be involved exclusively in the regulation of PS gene expression. In contrast, the Prr system, in addition to being involved in PS gene expression (6, 7), is involved in the regulation of genes involved in both CO2 and N2 fixation (14, 24). Because photosynthesis is an obligately anaerobic process in R. sphaeroides, the precise role of the fnrL gene product is more difficult to ascertain, since the absence of FnrL results in a phenotype whereby the mutant is unable to grow both photosynthetically and by anaerobic respiration. However, recent studies involving a comparison of FnrL null strains of both R. sphaeroides and R. capsulatus clearly reveals that anoxygenic photosynthesis can be separated from general anaerobiosis when the role of FnrL is considered (29).
The pucBA genes of R. sphaeroides 2.4.1 encode the structural polypeptides of one of the two antenna complexes of the photosynthetic apparatus. These genes have previously been identified as a potential target for regulation by FnrL, since the upstream sequence of this operon contains sequences with similarity to the FNR consensus sequence (16). To more clearly define the role of FnrL in mediating oxygen control of PS gene expression, we determined whether fnrL plays a role in regulating the final levels of puc operon expression, and if so, at what point(s) such control is exercised.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The R. sphaeroides and E. coli strains and plasmids used in this study are described in Table 1. Additional details pertaining to some of the plasmids are described in Results.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | (φ80dlacZΔM15) ΔlacU169 recA1 endA1 hisdR17 supE44 thi-1 gyrA96 relA1 | 13 |
| DH5αphe | DH5α phe::Tn10dCm | 7 |
| HB101 | lacY1 galK2 supE44 ara14 proA2 rpsL20 recA13 xyl-5 mtl-1 hisdS20 mcrB mrr | 8 |
| R. sphaeroides | ||
| 2.4.1 | Wild type | W. Sistrom |
| JZ1678 | ΔfnrL::ΩKmr | 30 |
| JZ1692 | ΔfnrL::ΩSpr/Str | 30 |
| JZ1844 | ppsR+ ppsR::ΩKmr ΔfnrL::ΩSpr/Str | This study |
| JZ1846 | ppsR::ΩKmr ΔfnrL::ΩSpr/Str | This study |
| JZ1847 | ppsR::ΩKmr ΔfnrL::ΩSpr/Str | This study |
| Plasmids | ||
| pCF200 | RSF1010 derivative carrying a puc::lacZ transcriptional fusion (Fig. 1) | 16 |
| pCF250 | RSF1010 derivative carrying a puc::lacZ transcriptional fusion (Fig. 1) | 16 |
| pRK415 | Mob+ Tcr IncP | 15 |
| pRK2013 | Kmr IncP1 ColE1 Mob | 8 |
| pLK-1 | RSF1010 derivative carrying a puc::aph transcriptional fusion | 6 |
| pUI1649 | pRK415 derivative, prrB+ | 6 |
| pUI1650 | pRK415 derivative, prrB78 | 6 |
| pUI1650* | pRK415 derivative, prrB78* | This study |
| pUI1970 | pRK415 derivative, fnrL+ | 32 |
| p714SmH::Kmr::mob | ppsR::ΩKmr | 12 |
Growth conditions.
E. coli (19) and R. sphaeroides (4, 5) were cultured according to previously described protocols unless otherwise indicated. Growth of R. sphaeroides was monitored with a Klett-Summerson colorimeter with a no. 66 filter (1 Klett unit = 107 cells per ml). Alternatively, cell optical densities at 660 nm (OD660) were determined with a UV-1601PC spectrophotometer (Shimadzu, Columbia, Md.). When appropriate, either to provide selection for plasmids or to select for various recombinant strains, media were supplemented with antibiotics. Final concentrations for R. sphaeroides were 0.8 μg of tetracycline per ml and 50 μg of kanamycin, spectinomycin, and streptomycin (St) per ml; for E. coli, the final concentrations were 15 μg of tetracycline per ml, 50 μg of kanamycin, spectinomycin, and streptomycin per ml, and 100 μg of ampicillin per ml.
DNA manipulations and analyses.
Plasmid isolation was carried out according to standard protocols or manufacturers’ instructions, with plasmid Wizard (Promega Corp., Madison, Wis.) or Prep-a-Gene (Bio-Rad) kits. Restriction endonuclease treatment and other enzymatic treatment of DNA fragments and plasmids were performed according to standard procedures or following manufacturers’ instructions, with enzymes purchased from New England BioLabs, Inc. (Beverly, Mass.), Boehringer Mannheim Biochemicals (Indianapolis, Ind.), Bethesda Research Laboratories Life Technologies, Inc. (Gaithersburg, Md.), and Promega. DNA analysis was performed by using standard electrophoretic techniques. Southern hybridization of R. sphaeroides genomic DNA was performed as previously described (30). Labeling of DNA probes and detection of hybridizing sequences by chemiluminescence were performed with a NEBlot Phototope kit purchased from New England BioLabs.
Conjugation.
Plasmid mobilization into R. sphaeroides was performed as previously described (4). To introduce plasmids into strains of R. sphaeroides, triparental matings with E. coli HB101(pRK2013) (8) as a helper strain were used.
Construction of PpsR-FnrL null mutant strains.
The plasmid used for introducing the ppsR::ΩKmr mutation into FnrL− mutant strain JZ1692 (Table 1), p714SmH::Kmr::mob, is described in Table 1. Exconjugants in which all or portions of plasmid p714SmH::Kmr::mob had been integrated into the chromosome by homologous recombination were selected for by using the kanamycin resistance (Kmr) marker of the Ω cartridge within the ppsR coding sequences. Candidates of even-numbered crossovers were then scored for by using the outside drug marker, tetracycline. The presence of appropriate DNA sequences in recombinant strains JZ1844, JZ1846, and JZ1847 (Table 1) was confirmed by Southern hybridization analysis (results not shown).
β-Galactosidase assays.
Assays of β-galactosidase activity were performed on crude cell extracts as described previously (26), using reagent-grade o-nitrophenyl-β-d-galactopyranoside, purchased from Sigma Chemical Co. (St. Louis, Mo.) as the substrate. β-Galactosidase activity in R. sphaeroides is expressed in units, where 1 U corresponds to 1 μmol of o-nitrophenyl-β-d-galactopyranoside hydrolyzed per min per mg of protein.
Spectral analysis of cell extracts.
Crude cell-free lysates were prepared either by sonication in ICM buffer (10 mM KPO4, 1 mM EDTA [pH 7.2]) as previously described (25) or by passage through a French pressure cell (26). Spectra were recorded with a Shimadzu UV-1601PC spectrophotometer. The levels of B875 and B800-850 complexes were determined by the method of Meinhardt et al. (20).
Pigment extraction and analysis.
Pigments were extracted from R. sphaeroides cells and quantitated as described by Cohen-Bazire et al. (2).
Protein determinations.
Protein concentrations of cell extracts were determined with the Pierce (Rockford, Ill.) bicinchoninic acid protein assay reagent. As a reference, bovine serum albumin was used.
RESULTS
Analysis of puc operon expression in wild-type and FnrL− mutant strains.
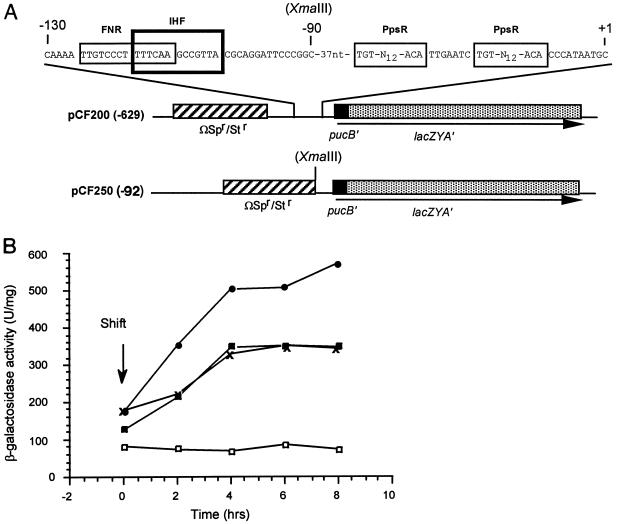
Two regulatory domains of the puc operon have been defined through the studies of Lee and Kaplan (16): the upstream regulatory sequences, spanning nucleotides (nt) −629 to −150 relative to the +1 site of transcription, and the downstream regulatory sequences, spanning nt −150 to the +1 site of transcription. An extensive analysis of these domains with respect to puc expression indicates the presence of as many as seven unique regulatory elements (16). Sequences similar to the FNR consensus sequence are located at nt −148 to −135, which partially overlap an integration host factor (IHF) binding site (Fig. 1A). To determine whether FnrL is involved in the regulation of puc expression, we used two different puc::lacZ transcriptional fusion plasmids: pCF200, in which upstream sequences from nt −629 to +175 relative to the start site of transcription of the puc operon are positioned in front of a promoterless lacZ gene, and pCF250, which contains nt −92 to +175 relative to the start site of transcription of the puc operon similarly positioned in front of the lacZ gene (Table 1 and Fig. 1A). Because FnrL− mutant strains are unable to grow either photosynthetically or anaerobically in the dark with dimethyl sulfoxide (DMSO) (29, 30), we have analyzed puc expression in response to diminishing oxygen tension. This analysis consisted of growing cells to low cell density while sparging with a mixture of 30% O2–69% N2–1% CO2 mixture. At time zero, the gas mixture was changed to 2% O2–97% N2–1% CO2; at various intervals, cell samples were removed and β-galactosidase activity present in crude cell extracts was determined. The kinetics of β-galactosidase production for wild-type 2.4.1 and FnrL− mutant strains bearing the two different puc::lacZ transcriptional fusion plasmids, following the shift to low oxygen, are shown in Fig. 1B. For pCF200, which contains the FNR consensus-like sequence, the level of LacZ induction in the FnrL− mutant after 4 h is approximately 65% of the wild-type level, which is virtually identical to the level of β-galactosidase activity present in wild-type cells containing pCF250, which lacks the FNR consensus-like sequence. On the other hand, expression from pCF250 in the FnrL− mutant showed no induction of β-galactosidase activity following the shift from high to low oxygen. This experiment was performed three additional times with similar results, as summarized in Table 2. These results suggest that FnrL normally activates puc operon expression when oxygen tensions are reduced to below threshhold levels. However, our analysis also suggests that there are at least two components which contribute to FnrL-mediated puc expression. These contributions are distinguishable when the results obtained for pCF200 are compared to those obtained for pCF250.
FIG. 1.
Analysis of puc operon expression in wild-type and FnrL− strains using puc::lacZ transcription fusion plasmids. (A) Schematic diagram of the relevant features of the puc::lacZ transcription fusion plasmids, pCF200 and pCF250. (B) β-Galactosidase activity versus time, following a shift from 30 to 2% oxygen, of cultures of R. sphaeroides 2.4.1(pCF200) (•), JZ1678(pCF200) (X), 2.4.1(pCF250) (▪), and JZ1678(pCF250) (□).
TABLE 2.
β-Galactosidase activities in extracts of R. sphaeroides following oxygen shift
| Strain | β-Galactosidase activitya (avg ± SD [n = 3])
|
|
|---|---|---|
| 0 h after shift | 4–6 h after shift | |
| 2.4.1(pCF200) | 110 ± 38 | 515 ± 52 |
| JZ1678(pCF200) | 145 ± 43 | 350 ± 34 |
| 2.4.1(pCF250) | 110 ± 24 | 386 ± 40 |
| JZ1678(pCF250) | 56 ± 19 | 108 ± 32 |
Expressed in units, defined as micromoles of o-nitrophenyl-β-d-galactopyranoside hydrolyzed per minute per milligram of protein extract.
The levels of spectral complexes present in cells at the conclusion of the analysis shown in Fig. 1B, i.e., after 8 h of incubation at 2% oxygen, were determined, and are shown in Table 3. These data indicate that while the growth conditions following the shift to lower oxygen levels induced the production of both B800-850 and B875 spectral complexes in the wild-type strains, virtually no complexes were detected in the FnrL− mutant strains. These results strongly support the conclusion that FnrL is involved, either directly or indirectly, in generalized PS gene expression leading to spectral complex formation. Such a conclusion is further supported by analysis of wild-type strains carrying multiple copies of the wild-type fnrL gene. A spectral analysis of membrane extracts from wild-type cells bearing the plasmid vector pRK415 alone, grown in 30% oxygen, revealed that the levels of B800-850 and B875 complexes were 0.16 (±0.05) and 0.25 (±0.19) nmol per mg of crude membrane protein, respectively. In contrast, the level of B800-850 and B875 complexes present under identical conditions in wild-type cells bearing pUI1970, which carries the wild-type fnrL gene, were 0.44 (±0.22) and 1.30 (±0.08) nmol per mg of crude membrane protein, respectively. Thus, while we have obtained evidence to suggest that FnrL directly activates puc expression when oxygen tension is reduced, these results further indicate that FnrL, in addition, has a broader role in the regulation of PS gene expression.
TABLE 3.
Levels of spectral complexes present in strains of R. sphaeroides 2.4.1 following oxygen shift
| Strain | Level (nmol/mg of crude membrane protein)
|
|
|---|---|---|
| B800-850 | B875 | |
| 2.4.1(pCF200) | 7.48 | 7.14 |
| JZ1678(pCF200) | NDa | 0.38 |
| 2.4.1(pCF250) | 5.62 | 6.92 |
| JZ1678(pCF250) | ND | 0.54 |
ND, none detected.
Phenotypic analysis of PpsR− FnrL− mutants.
While the evidence described above directly indicates that PS genes are regulated by FnrL, it is well established that there are other regulators which mediate oxygen control of PS gene expression in R. sphaeroides. One of these regulators is the transcriptional repressor encoded by the ppsR gene (11, 22, 23). It has been shown previously that disabling the ppsR gene in an otherwise wild-type background leads to a derepression of photopigment genes and puc operon expression under both aerobic and photosynthetic conditions (23). Thus, we addressed the question of whether relieving PpsR repression is able to overcome the FnrL− block to photosynthetic growth. We examined the consequences for growth characteristics of FnrL− mutant strains when a disruption (with an omega cassette) of the ppsR gene was also introduced.
Among the three plating conditions used to isolate the exconjugants (aerobic, anaerobic-dark/DMSO, and photosynthetic), colonies were only obtained on plates incubated under aerobic conditions. Since PpsR− mutants are stable and can grow under anaerobic and photosynthetic conditions, this result indicates that it is the absence of functional FnrL that prevents colony formation under anaerobic-dark/DMSO or photosynthetic conditions. Photopigment analysis of two structurally confirmed PpsR− FnrL− double mutant strains obtained under aerobic conditions, JZ1846 and JZ1847 (see Materials and Methods and Table 1), revealed that carotenoid levels were approximately 13-fold higher than in the PpsR+ FnrL− mutant, JZ1844 (Table 4). Bacteriochlorophyll (Bchl) levels were also elevated in the PpsR− FnrL− mutant strains. However, these levels were not consistent between the two independent double-mutant isolates, JZ1846 and JZ1847 (Table 4). This inconsistency in Bchl levels is not unexpected since, as has been observed previously, strains producing photosynthetic membranes under aerobic conditions are highly unstable and accumulate mutations in PS genes which include Bchl biosynthetic genes (6, 17). When tested individually, none of these strains were able to grow photosynthetically. Therefore, while disabling ppsR leads to a derepression of certain PS genes even in the absence of FnrL, this derepression alone is insufficient to bypass the FnrL requirement for photosynthetic growth.
TABLE 4.
Pigment levels present under aerobic conditions
| Strain | Relevant characteristics | Level (μg of pigment/OD660 of cell culture)
|
|
|---|---|---|---|
| Crt | Bchl | ||
| JZ1844 | PpsR+ FnrL− | 3 | 2 |
| JZ1846 | PpsR− FnrL− | 39 | 28 |
| JZ1847 | PpsR− FnrL− | 38 | 5 |
Phenotypic consequences of introducing the prrB78 allele in trans into an FnrL− mutant.
In addition to PpsR, which negatively regulates the expression of certain PS genes under aerobic conditions, the Prr regulatory system mediates the activation of PS gene expression in response to lowering oxygen tension. Thus, while both of these effectors mediate oxygen control of PS gene expression, they respond differently to changes in oxygen tension.
The prrB78 mutation, which is dominant in multicopy to the wild-type prrB allele (6), has been shown to confer oxygen insensitivity to the Prr regulatory system and results in the formation of photosynthetic complexes in the presence of high oxygen tensions (6). As is the case for derepression of PS gene expression when the ppsR gene is disabled (23), the prrB78 oxygen-insensitive phenotype is also observed in the presence of an intact fnrL gene (6). Therefore, the role of the fnrL gene in photosynthetic growth was further investigated in the presence of the prrB78 allele. We introduced plasmid pUI1650, bearing the prrB78 allele (6), into the FnrL− mutant strain, JZ1678.
FnrL− exconjugants containing plasmid pUI1650 were obtained only under aerobic conditions (Table 3), and colonies failed to appear on plates incubated under anaerobic (dark/DMSO) or photosynthetic conditions. When the prrB78 allele was introduced in trans into wild-type cells under anaerobic conditions, the cells were quite stable. However, under aerobic conditions, an array of differentially pigmented colonies arose (6). True wild-type exconjugants bearing the prrB78 allele can be visually identified by a deep red pigmentation associated with the synthesis of photopigments under aerobic conditions. Instability in the presence of oxygen was also associated with the introduction of pUI1650 into JZ1678, indicating that the prrB78 allele in trans was affecting photopigment gene expression in the FnrL− mutant as well. Nevertheless, these cells are incapable of photosynthetic growth, which suggests that FnrL is indispensable even in the presence of the prrB78 allele.
Presence of photosynthetic membranes in FnrL− mutants bearing the prrB78* allele in trans under aerobic conditions.
To quantitatively compare the effects on PS gene expression of prrB78 in trans in the presence and absence of FnrL, we needed to obtain a stable, pure culture of both wild-type 2.4.1 and JZ1678 containing pUI1650. However, as noted here and elsewhere (6), the presence of the prrB78 allele results in genetic instability, due to the formation of spectral complexes containing Bchl in the presence of oxygen. Restreaking wild-type exconjugants for stable single-colony isolates continued to give rise to a multiplicity of either differently pigmented or variegated colonies. However, one JZ1678 exconjugant colony type, which was more pigmented than JZ1678 exconjugants containing the vector alone, could be successfully purified, suggesting that either the FnrL− mutant background tolerated the prrB78 allele better than wild-type 2.4.1 or the prrB78 allele was modified. To distinguish between these two possible explanations, plasmid DNAs isolated from two independent stable JZ1678 exconjugants were reintroduced into both JZ1678 and wild-type 2.4.1. Unlike the parent plasmid pUI1650, bearing the prrB78 allele, the plasmid DNAs which had been passaged through JZ1678 produced virtually stable exconjugants with only rare pigmentation variants when introduced into either wild-type 2.4.1 or JZ1678. While the wild-type 2.4.1 exconjugants were visually more highly pigmented than cells without plasmid or containing only vector DNA, they were less pigmented than those exconjugants that could be identified among the exconjugants derived from unpassaged plasmid pUI1650.
To determine the basis for the difference between the passaged and unpassaged plasmid DNAs, we performed DNA sequence analysis of the entire prrB gene carried on plasmid DNA isolated from JZ1678. While our analysis confirmed the presence of the original prrB78 mutation, we also found that an 11-bp deletion had occurred at the 3′ end of the prrB gene, resulting in a frameshift such that 24 new amino acids were included at the site of the deletion, terminating with an ochre nonsense codon. These sequences are compared to the wild-type prrB DNA sequence in Fig. 2. We call this mutant allele prrB78* and refer to the plasmid bearing this allele as pUI1650*, to distinguish it from pUI1650, which bears the original prrB78 allele (6). It should be noted that the FnrL− exconjugants of prrB78* were again obtained on plates only under aerobic conditions.
FIG. 2.
Comparison of the relevant DNA sequences of mutant allele prrB78* and wild-type prrB, together with their predicted protein sequences.
The availability of these stable strains allowed us to carry out a comparative analysis. We prepared membrane extracts from JZ1678(pUI1650*) and wild-type 2.4.1(pUI1650*) cells grown in 30% oxygen. Spectral analysis of the membrane extracts revealed the presence of photosynthetic membranes in both strains (Table 5). Thus, it appears that the prrB78* mutation is dominant in both the wild-type and FnrL− mutant strains with respect to PS gene expression, in that photosynthetic complexes are present even under highly aerobic conditions. However, given the inability of this strain to produce colonies under photosynthetic conditions, it appears that the presence of the photosynthetic membranes alone cannot induce photosynthetic growth when fnrL is disabled. Thus, we believe that there are additional points of regulatory control that require, either directly or indirectly, the intervention of FnrL for their expression and which are essential for photosynthetic growth. The presence of the wild-type prrB allele present in multicopy in either wild-type 2.4.1 or the FnrL− mutant JZ1678 also led to the development of photosynthetic membranes under these same conditions, but the pigment-protein complexes were present at significantly lower concentrations than in the presence of multiple copies of the prrB78* allele (Table 5).
TABLE 5.
Levels of spectral complexes present under aerobic conditions in strains of R. sphaeroides 2.4.1
| Strain | Relevant characteristics | Level (nmol/mg of crude membrane protein; avg ± SD [n ≥ 3])
|
|
|---|---|---|---|
| B800-850 | B875 | ||
| 2.4.1(pRK415) | Wild type (plasmid vector) | NDa | 0.75 ± 0.14 |
| JZ1678(pRK415) | ΔfnrL::ΩKmr (plasmid vector) | ND | 0.11 ± 0.01 |
| 2.4.1(pUI1650*) | Wild type (prrB78* allele) | 0.55 ± 0.15 | 3.24 ± 0.64 |
| JZ1678(pUI1650*) | ΔfnrL::ΩKmr (prrB78* allele) | 0.37 ± 0.13 | 3.38 ± 0.49 |
| 2.4.1(pUI1649) | Wild type (wild type prrB) | 0.16 ± 0.11 | 1.82 ± 0.25 |
| JZ1678(pUI1649) | ΔfnrL::ΩKmr (wild type prrB) | 0.06 ± 0.04 | 1.06 ± 0.24 |
ND, none detected.
Growth of wild-type 2.4.1 and FnrL− mutant strains following a shift from aerobic to photosynthetic conditions.
As shown in Fig. 3A, we monitored the growth of wild-type cells bearing either the vector plasmid or the vector carrying one of three different alleles of prrB, following a shift from highly aerobic conditions to anaerobic photosynthetic conditions. For the analysis of wild-type cells bearing pUI1650, as previously demonstrated, plasmid pLK-1 was also present, to provide positive selection for the presence of the prrB78 allele (6). Cells containing pLK-1 and pUI1650 were grown in the presence of kanamycin at 300 μg/ml, the maximum level of resistance observed for these cells. This is somewhat less than the maximum resistance observed for PRRB78(pLK-1), bearing the prrB78 mutation on the chromosome, which under aerobic conditions can grow in the presence of kanamycin at 450 μg/ml (6). By selecting for growth in the presence of high levels of kanamycin, we can enrich for a population of cells containing the prrB78 allele, which, in addition to producing photosynthetic membranes under aerobic conditions, elevates expression of the Kmr gene, due to the fact that Kmr gene expression is under PS gene control.
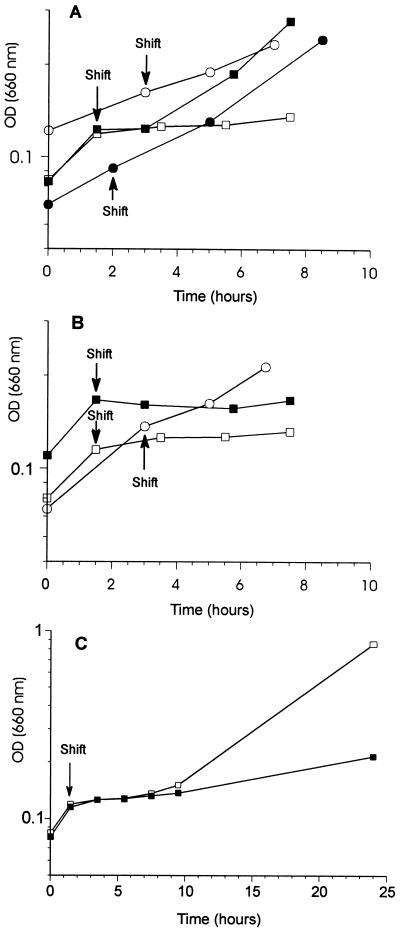
FIG. 3.
Schematic diagram of the growth of wild-type and FnrL− mutant strains of R. sphaeroides 2.4.1 bearing various plasmids following a shift from aerobic (30% oxygen) to photosynthetic (anaerobic, 10 W/m2) conditions. For each strain, all of at least three samples analyzed showed growth patterns similar to the examples presented here. (A) Growth of wild-type 2.4.1 cells bearing plasmids pRK415 (□), pUI1649 (▪), pUI1650 (○), and pUI1650* (•). (B) Growth of FnrL− mutant strain JZ1678 bearing plasmids pRK415 (□), pUI1649 (▪), and pUI1650* (○). (C) Extended growth of wild-type 2.4.1(pRK415) (□) and JZ1678(pRK415) (▪).
Following the shift to stringent anaerobic-photosynthetic conditions, growth of wild-type cells bearing the vector plasmid ceases for approximately 8 to 9 h before resuming, which is what is routinely observed for wild-type cells (1). In contrast, wild-type 2.4.1 cells bearing a plasmid with wild-type prrB sequences (pUI1649) were able to transit from aerobic growth to photosynthetic growth in 3 to 4 h. Most dramatic were the growth characteristics of wild-type cells containing pUI1650 (with pLK-1), which carries the prrB78 allele, or containing pUI1650*, which carries the prrB78* allele. For these cells, growth was virtually unaffected following the transition from aerobic to photosynthetic conditions, indicating that these cells contain a functional photosynthetic apparatus prior to the shift to anaerobiosis that is ready to function following the shift.
A similar analysis was performed for FnrL− mutant strains bearing either the vector plasmid or the wild-type prrB (pUI1649) or prrB78* (pUI1650*) allele. As shown in Fig. 3B, the results for the FnrL− mutant strain bearing the vector alone are similar to those for wild-type cells except that the former do not recover from the lag period and therefore do not grow photosynthetically. In the case of JZ1678(pUI1649), unlike the wild-type cells, there is a pronounced lag in growth of up to 7 h following the shift to photosynthetic conditions before growth resumes. In contrast, when prrB78* is present in multicopy in JZ1678, there is no lag observed following the shift to anaerobiosis in liquid cultures. Thus, it appears that prrB78* is sufficient to poise the cells under aerobic conditions for photosynthetic growth, regardless of the presence or absence of FnrL.
As shown in Fig. 3C, after extended incubation under photosynthetic conditions, the wild-type cells bearing the vector alone had recovered from the lag in growth and had undergone several culture doublings. However, during the same period, the culture of the FnrL− mutant strain bearing the vector had not yet doubled in optical density. For each of the strains used, at least three independent isolates were analyzed. All showed growth patterns similar to the examples presented in Fig. 3.
Since FnrL− mutants bearing pUI1650* failed to form colonies under photosynthetic conditions, the basis for the growth of the liquid culture of FnrL− mutant strain JZ1678 bearing pUI1650* under photosynthetic conditions was investigated. Plasmid-free segregants were isolated by culturing the FnrL− mutant strain bearing pUI1650* in the absence of antibiotic and under aerobic conditions. Eight segregants, which scored as tetracycline sensitive, were then analyzed individually for the ability to form colonies under aerobic and photosynthetic conditions. Among these, three isolates showed similar growth under both conditions. We believe that these results indicate that the increase in optical density observed for the liquid culture of JZ1678 (pUI1650*) was attributable to an extragenic suppressor mutation(s) which arose during the course of incubation of the nonrevertible FnrL− mutant strain under photosynthetic conditions. While photosynthetically competent pseudorevertants eventually arose from liquid cultures of FnrL− mutant strains bearing the vector alone (for example, Fig. 3C) or the wild-type prrB gene, or in the absence of any plasmid (results not shown), it appears that the presence of photosynthetic membranes under aerobic conditions in the FnrL− mutant strain bearing the prrB78* allele allowed the FnrL− mutant strain to bypass the normal lag phase while transiting from aerobic to photosynthetic growth. Thus, suppressor mutations accumulated more rapidly in this population than in those populations requiring the normal 7- to 8-h lag prior to the resumption of growth.
DISCUSSION
Our results indicate that FnrL has a dual role in the regulation of PS gene expression: (i) Transcriptional fusions between DNA sequences containing FNR consensus sequences and the lacZ gene indicate that FnrL probably acts at the FNR consensus sequence to regulate transcription of the puc operon in response to changes in oxygen levels. Within the upstream sequences of the puc operon, the putative target sequence is thought to be TTGTCN4TTCAA (16), which overlaps the binding site of IHF (16 and 18). Previous work has established by both in vivo and in vitro analyses that the IHF binding site plays a critical role in the enhanced control of puc operon transcription by oxygen and light (18). Because this transcriptional response involves a direct interaction between the functionally separate upstream and downstream regulatory sequences of the puc operon (16, 17), IHF binding might induce bending of the DNA molecule enabling interaction between FnrL and RNA polymerase to occur, leading to activation of transcription, in a manner resembling transcriptional activation of ς54 promoters. (ii) FnrL also appears to have an additional role in the regulation of puc operon expression, as well as in photosynthetic growth in general. We show here that a transcriptional fusion between puc upstream sequences entirely devoid of the FNR consensus sequence remains responsive to the presence of FnrL under conditions of low oxygen, but in the absence of FnrL, puc operon transcription is greatly diminished.
We have also shown here that the FnrL requirement for photosynthetic growth cannot be bypassed through either the absence of the PS repressor gene product, PpsR, or the presence of the trans-dominant prrB78* allele. In the FnrL+ background, either one of these mutations is capable of causing the formation of functional photosynthetic membranes under aerobic conditions, as indicated by the absence of any growth lag when such cells are subjected to stringent photosynthetic growth conditions following a shift from aerobic growth. It is also readily apparent that the presence of photosynthetic membranes in the presence of oxygen gives rise to strains which are genetically unstable. These same strains, however, are quite stable under anaerobiosis and show no growth impairment under those conditions.
What then is the critical role of FnrL in photosynthetic growth? Our results indicate that for R. sphaeroides 2.4.1, there must be PS genes which absolutely require FnrL-mediated regulation under the photosynthetic growth mode. Genes and/or operons which we might consider in this regard are those having upstream FNR consensus sequences. These include the ccoNQOP operon and the ctaD and ctaCBGE operons (29), which encode the cbb3 and aa3 cytochrome c terminal oxidases, respectively. It has previously been demonstrated that Cco− mutant strains are altered in PS gene expression; i.e., expression of both the puc and puf operons is elevated under aerobic conditions, and spectral complexes are present (21, 31). We proposed that the mechanism of Cco action may involve the production of an active redox component, which signals the Prr regulon and thereby leads to altered regulation of PS gene expression (21, 31). However, we have shown here that although prrB78* is dominant to FnrL− in the development of photosynthetic membranes, the presence of photosynthetic membranes alone cannot restore photosynthetic growth in the absence of a functional fnrL. It should be noted that unlike the Prr system, PpsR derepression of PS genes does not appear to involve FnrL activity. Rather, another effector, namely, AppA, may be involved in communicating anaerobiosis to this regulator (12). However, even here, the absence of a functional PpsR repressor is insufficient to overcome the fnrL mutation with respect to photosynthetic growth. Therefore, the mutation in fnrL is dominant to mutations which are known to result in constitutive formation of photosynthetic membranes.
Other genes that may require increased expression under photosynthetic conditions, and which have upstream FNR consensus sequences (29, 30), include the tetrapyrrole and Bchl biosynthetic genes hemA, hemZ, hemN, and bchE. Thus, the absence of FnrL may not permit the accelerated formation of tetrapyrrole intermediates or Bchl, essential for photosynthetic membranes, at levels that are adequate for anaerobic photosynthetic growth. We have already demonstrated a role for FnrL in the anaerobic regulation of hemA expression (30). Other workers (3, 9) have shown that inactivation of the hemN gene (previously referred to as hemF) of R. sphaeroides, which encodes one of two isozymes of the oxygen-independent coproporphyrinogen III oxidase (the other isozyme being encoded by hemZ), leads to an inability to grow photosynthetically. Interestingly, while the upstream sequences of the bchE genes of Rhodobacter capsulatus (GenBank accession no. Z11165) and R. sphaeroides both contain the target motif for PpsR (whose homolog in R. capsulatus is CrtJ), neither the bchE nor the hemA gene of R. capsulatus contains upstream FNR consensus sequences, nor has a second hemN/hemZ gene been found. The fact that FnrL− mutant strains of R. capsulatus are not altered in the ability to grow under photosynthetic conditions (29) potentially implicates these three genes. Whether it is FnrL regulation of the expression of one or another of these genes singly or in combination that is required for photosynthetic growth of R. sphaeroides is not known. However, these observations establish a clear focus for future studies.
It is possible to isolate suppressor strains that bypass the FnrL requirement for photosynthetic growth, as was shown here and elsewhere (30). Identification of these loci, together with analysis of those genes described above which are likely to be regulated by FnrL, will be critical to developing a full understanding of the regulatory components required for photosynthetic growth in this organism.
ACKNOWLEDGMENTS
We thank J. Eraso for kindly providing plasmids pUI1650, pUI1649, and pLK-1. We also thank M. Gomelsky for providing plasmid p714SmH::Kmr::mob, and we thank M. Gomelsky and N. Mouncey for helpful discussions.
This work was supported by Public Health Service grant GM15590 from the National Institutes of Health.
REFERENCES
- 1.Chory J, Donohue T J, Varga A R, Staehelin A, Kaplan S. Induction of the photosynthetic membranes of Rhodopseudomonas sphaeroides: biochemical and morphological studies. J Bacteriol. 1984;159:540–554. doi: 10.1128/jb.159.2.540-554.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen-Bazire G, Sistrom W R, Stanier R Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Comp Physiol. 1957;49:25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- 3.Coomber S A, Jones R M, Jordan P M, Hunter C N. A putative anaerobic coproporphyrinogen III oxidase in Rhodobacter sphaerroides. I. Molecular cloning, transposon mutagenesis and sequence analysis of the gene. Mol Microbiol. 1992;6:3159–3169. doi: 10.1111/j.1365-2958.1992.tb01772.x. [DOI] [PubMed] [Google Scholar]
- 4.Davis J, Donohue T J, Kaplan S. Construction, characterization, and complementation of a puf mutant of Rhodobacter sphaeroides. J Bacteriol. 1988;170:320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donohue T J, McEwan A G, Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides cytochrome c2 gene. J Bacteriol. 1986;168:962–972. doi: 10.1128/jb.168.2.962-972.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eraso J M, Kaplan S. Oxygen-insensitive synthesis of the photosynthesis membranes of Rhodobacter sphaeroides: a mutant histidine kinase. J Bacteriol. 1995;177:2695–2706. doi: 10.1128/jb.177.10.2695-2706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eraso J M, Kaplan S. prrA, a putative response regulator involved in oxygen regulation in photosynthesis gene expression in Rhodobacter sphaeroides. J Bacteriol. 1994;176:32–43. doi: 10.1128/jb.176.1.32-43.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figurski D H, Helinski D R. Replication of an origin containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson L C D, McGlynn P, Chaudhri M, Hunter C N. A putative anaerobic coproporphyrinogen III oxidase in Rhodobacter sphaeroides. II. Analysis of a region of the genome encoding hemF and the puc operon. Mol Microbiol. 1992;6:3171–3186. doi: 10.1111/j.1365-2958.1992.tb01773.x. [DOI] [PubMed] [Google Scholar]
- 10.Gomelsky M, Kaplan S. appA, a novel gene encoding a trans-acting factor involved in the regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1995;177:4609–4618. doi: 10.1128/jb.177.16.4609-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomelsky M, Kaplan S. Genetic evidence that PpsR from Rhodobacter sphaeroides 2.4.1 functions as a repressor of puc and bchF expression. J Bacteriol. 1995;177:1634–1637. doi: 10.1128/jb.177.6.1634-1637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomelsky M, Kaplan S. Molecular genetic analysis suggesting interactions between AppA and PpsR in regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1997;179:128–134. doi: 10.1128/jb.179.1.128-134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jessee J. New subcloning efficiency competent cells: >1 × 106 transformants/μg. Focus. 1986;8:9. [Google Scholar]
- 14.Joshi H M, Tabita F R. A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc Natl Acad Sci USA. 1996;93:14515–14520. doi: 10.1073/pnas.93.25.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 16.Lee J K, Kaplan S. cis-acting regulatory elements involved in oxygen and light control of puc operon transcription in Rhodobacter sphaeroides. J Bacteriol. 1992;174:1146–1157. doi: 10.1128/jb.174.4.1146-1157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J K, Kaplan S. Isolation and characterization of trans-acting mutations involved in oxygen regulation of puc operon transcription in Rhodobacter sphaeroides. J Bacteriol. 1992;174:1158–1171. doi: 10.1128/jb.174.4.1158-1171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J K, Wang S, Eraso J M, Gardner J, Kaplan S. Transcriptional regulation of puc operon expression in Rhodobacter sphaeroides. Involvement of an integration host factor-binding sequence. J Biol Chem. 1993;268:24491–24497. [PubMed] [Google Scholar]
- 19.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 20.Meinhardt S W, Kiley P J, Kaplan S, Crofts A R, Harayama S. Characterization of light-harvesting mutants of Rhodopseudomonas sphaeroides. I. Measurement of the efficiency of light energy transfer from light-harvesting complexes to the reaction center. Arch Biochem Biophys. 1985;236:130–139. doi: 10.1016/0003-9861(85)90612-5. [DOI] [PubMed] [Google Scholar]
- 21.O’Gara J P, Kaplan S. Evidence for the role of redox carriers in photosynthesis gene expression and carotenoid biosynthesis in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1997;179:1951–1961. doi: 10.1128/jb.179.6.1951-1961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penfold R J, Pemberton J M. A gene from the photosynthetic gene cluster of Rhodobacter sphaeroides induces in trans suppression of bacteriochlorophyll and carotenoid levels in R. sphaeroides and in R. capsulatus. Curr Microbiol. 1991;23:259–263. [Google Scholar]
- 23.Penfold R J, Pemberton J M. Sequencing, chromosomal inactivation, and functional expression of ppsR, a gene which represses carotenoid and bacteriochlorophyll synthesis in Rhodobacter sphaeroides. J Bacteriol. 1994;176:2869–2876. doi: 10.1128/jb.176.10.2869-2876.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian Y, Tabita F R. A global signal transduction system regulates aerobic and anaerobic CO2 fixation in Rhodobacter sphaeroides. J Bacteriol. 1996;178:12–18. doi: 10.1128/jb.178.1.12-18.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabaty M, Kaplan mgpS, a complex regulatory locus involved in the transcriptional control of the puc and puf operons in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 1996;178:35–45. doi: 10.1128/jb.178.1.35-45.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai T-N, Havelka W A, Kaplan S. A broad-host-range vector system for cloning and translational lacZ fusion analysis. Plasmid. 1988;19:175–188. doi: 10.1016/0147-619x(88)90037-6. [DOI] [PubMed] [Google Scholar]
- 27.Yeliseev A A, Kaplan S. A sensory transducer homologous to the mammalian peripheral-type benzodiazepine receptor regulates photosynthetic membrane complex formation in Rhodobacter sphaeroides 2.4.1. J Biol Chem. 1995;270:21167–21175. doi: 10.1074/jbc.270.36.21167. [DOI] [PubMed] [Google Scholar]
- 28.Yeliseev A A, Krueger K E, Kaplan S. A mammalian mitochondrial drug receptor functions as a bacterial “oxygen” sensor. Proc Natl Acad Sci USA. 1997;94:5101–5106. doi: 10.1073/pnas.94.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeilstra-Ryalls J H, Gabbert K, Kranz R, Kaplan S. Analysis of the fnrL gene and its function in Rhodobacter capsulatus. J Bacteriol. 1997;179:7264–7273. doi: 10.1128/jb.179.23.7264-7273.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeilstra-Ryalls J H, Kaplan S. Aerobic and anaerobic regulation in Rhodobacter sphaeroides 2.4.1: the role of the fnrL gene. J Bacteriol. 1995;177:6422–6431. doi: 10.1128/jb.177.22.6422-6431.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeilstra-Ryalls J H, Kaplan S. Control of hemA expression in Rhodobacter sphaeroides 2.4.1: regulation through alterations in cellular redox state. J Bacteriol. 1996;178:985–993. doi: 10.1128/jb.178.4.985-993.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeilstra-Ryalls, J. H., and S. Kaplan. 1996. Unpublished observations.