Abstract
Candida albicans is an opportunistic yeast and the primary etiological factor in oral candidiasis and denture stomatitis. The pathogenesis of C. albicans could be triggered by several variables, including environmental, nutritional, and biomaterial surface cues. Specifically, biomaterial interactions are driven by different surface properties, including wettability, stiffness, and roughness. Dental biomaterials experience repetitive (cyclic) stresses from chewing and biomechanical movements. Pathogenic biofilms are formed over these biomaterial surfaces under cyclic strain. This study investigated the effect of the cyclic strain (deformation) of biomaterial surfaces on the virulence of Candida albicans. Candida biofilms were grown over Poly (methyl methacrylate) (PMMA) surfaces subjected to static (no strain) and cyclic strain with different levels (and 0.2%). To evaluate the biomaterial-biofilm interactions, the biofilm characteristics, yeast-to-hyphae transition, and the expression of virulent genes were measured. Results showed the biofilm biomass and metabolic activity to be significantly higher when Candida adhered to surfaces subjected to cyclic strain compared to static surfaces. Examination of the yeast-to-hyphae transition showed pseudo-hyphae cells (pathogenic) in cyclically strained biomaterial surfaces, whereas static surfaces showed spherical yeast cells (commensal). RNA sequencing was used to determine and compare the transcriptome profiles of cyclically strained and static surfaces. Genes and transcription factors associated with cell adhesion (CSH1, PGA10, and RBT5), biofilm formation (EFG1), and secretion of extracellular matrix (ECM) (CRH1, ADH5, GCA1, and GCA2) were significantly upregulated in the cyclically strained biomaterial surfaces compared to static ones. Genes and transcription factors associated with virulence (UME6 and HGC1) and the secretion of extracellular enzymes (LIP, PLB, and SAP families) were also significantly upregulated in the cyclically strained biomaterial surfaces compared to static. For the first time, this study reveals a biomaterial surface factor triggering the pathogenesis of Candida albicans, which is essential for understanding, controlling, and preventing oral infections.
Keywords: biomaterial-microbial interactions, oral biofilms, candidiasis, denture stomatitis, C. albicans, Candida pathogenesis, PMMA, cyclic deformation
Graphical abstract
1. Introduction
Candida albicans (C. albicans) is generally a harmless commensal organism in the microflora of the oral cavity [1]. However, this fungus can become pathogenic under certain circumstances, causing a range of infections, such as invasive candidiasis [2, 3]. C. albicans can form pathogenic biofilms over biomaterials and medical devices such as dentures, catheters, prostheses, and endotracheal tubes, producing infections [4, 5]. For instance, more than 70% of patients with complete dentures suffer from denture stomatitis [6, 7]. Medical device-associated fungal infections are highly resistant to drugs and to the host immune system, leading to life-threatening complications [6–9]. This condition is worse in immunocompromised and medically compromised individuals [10, 11]. If untreated, invasive candidiasis can lead to persistent candidemia, a serious condition with a high risk of complications that have long-term health effects [12]. Therefore, understanding and controlling the formation of pathogenic fungal biofilms over medical devices and implanted biomaterials is of utmost importance for preventing local infections and systemic diseases [13].
The dangerous transformation of C. albicans from commensal to pathogenic is expressed in different ways, including a morphological change from yeast to hyphae, the expression of adhesins and invasins, the formation of biofilms, phenotypic switching, thigmotropism, and the secretion of aspartic proteinases, phospholipases, and lipases [1, 14–19]. For example, the hyphal shape allows the fungus to penetrate deep-seated tissues to produce local infections and cause disease [17]. The pathogenicity of Candida is triggered by multiple factors, including environmental and nutritional conditions, interactions with biomaterials, and the host [1, 20, 21]. From the environmental standpoint, different levels of temperature, pH, CO2, and oxygen, and nutrient availability may trigger pathogenicity [20, 22–28]. For example, C. albicans has yeast morphology at low extracellular pH levels (< 6) but transitions to a hyphal shape at high pH levels (> 7) [27]. Sensing the extracellular pH level is conducted via the RIM101 signal transduction pathway [29]. The dynamism exhibited by C. albicans in response to environmental cues impacts not only its shape, structure, and pathogenicity but also its interaction with immune cells and the progression of infections [30].
The formation of fungal pathogenic biofilms is a sequential process involving the adherence of yeast cells over a biomaterial surface, their proliferation, and the induction of hyphal formation and expression of virulence genes [31, 32]. Specifically, the Candida-biomaterial interactions are controlled by different biomaterial surface properties, including wettability [33], chemistry [34, 35], roughness/topography [36, 37], electrical charge density [38], stiffness [39], and potentially the combination thereof [21, 40–43]. For instance, increased biofilm quantity and pathogenicity are positively correlated with an increase in the biomaterial surface roughness and hydrophobicity [44]. These changes may affect the microbial community composition and the meta-transcriptional landscape, increasing the production of virulence factors and the progression of infection [20]. As a result, the formed biofilm becomes difficult to eradicate with increased potential to spread disease [21]. Candida biofilm-associated infections have limited treatment options [45], and therefore, understanding and controlling the biomaterial interactions is essential.
During clinical service, medical devices (or implanted biomaterials) experience repetitive (cyclic) stresses from biomechanical movements. For example, dentures must bear the repetitive forces from mastication (~500,000 times per year), and urinary catheters the repetitive forces from the muscular bladder complex [46, 47]. Repeated stresses are relevant to studying the fatigue failure of biomaterials and medical devices. However, this biomaterial factor has never been considered as a potential contributor to fungal-biomaterial interactions. In a clinical setting, Candida cells may adhere to these “cyclically stretched” biomaterial surfaces caused by the biomechanical movements. This repetitive surface strain (or deformation) may be affecting the microbe adhesion, biofilm formation, and pathogenesis, which in turn may be fueling the progression of infectious diseases such as denture stomatitis. In fact, our recent work indicated that when C .albicans adhered to a biomaterial surface subjected to cyclic strain, the biofilm biomass, metabolic activity, and number of viable cells were significantly higher than those obtained on similar surfaces under static conditions [48]. C. albicans switched its morphology from yeast to hyphae and expressed virulent genes. A mechanistic study is needed to unveil the pathways activated by this novel factor driving the pathogenesis of Candida. Denture stomatitis is a major oral infectious disease. Its etiology has been understood from the environmental conditions, host, cell strains, and some biomaterial surface factors. To the best of our knowledge, the etiology of this disease has never been investigated from this novel biomaterial cyclic strain/ deformation perspective. For the first time, this study investigates the effect of different cyclic biomaterial surface strain parameters on the pathogenicity of C. albicans for oral applications.
2. Materials and Methods
2.1. Fabrication and Characterization of PMMA Samples
Poly (methyl methacrylate) (PMMA) samples were fabricated by mixing liquid monomer of methylmethacrylate and PMMA powder (Original Truliner, Bosworth) by hand in a 1:1 weight ratio at room temperature. This material was chosen because it is common in denture use and different biomedical applications. The mixture was then poured into a stainless-steel mold with the desired dimensions until complete polymerization (~10 min). A mylar film was placed over the material’s surface to guarantee homogenous roughness along the sample surface. For the biofilm model, rectangular beams (25×5×1.6 mm3) were fabricated. Beams were stored in distilled water for 24 h at 37°C to release unreacted monomers that could influence the fungus-biomaterial interactions [49]. Physical and mechanical properties of the PMMA samples were evaluated, including degree of conversion (DC) (see Support Information (SI) SI-1), average roughness of the surfaces (SI-2), wettability (SI-3), flexural strength, and moduli (SI-4).
2.2. Validation of the Cyclic Strain of PMMA Surfaces
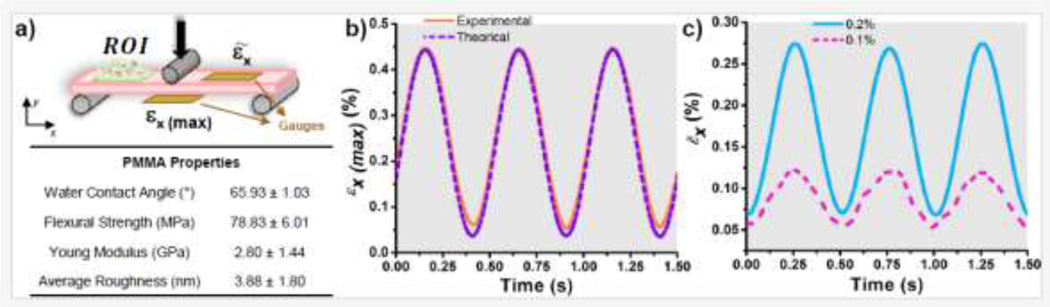
In the biofilm model, beams were subjected to cyclic (repetitive) loading to stretch biomaterial surfaces to a specific strain and frequency. The magnitude of the cyclic strain applied over the biomaterial surface was measured using strain gauges (6 mm grid, BF120–3AA, Zemic Europe). For strain measurements, beams were subjected to a 3-point bending configuration. A gauge was bonded at the center of the tension side of the beam to measure the maximum surface strain (Fig. 1a). A second gauge was bonded on the compression side of the beam exactly in between the two pins (Fig. 1a) to measure the average strain at this region of interest (ROI). The strain gauges were connected using a Wheatstone bridge excited with 5 volts. The gauges output voltage was measured with a data acquisition system, and the mechanical strain was calculated [50]. Beams were subjected to sinusoidal cyclic loadings (to N at 2 Hz) using a fatigue testing machine (ElectroForce 5500, TA Instruments, 200 N loadcell). The load range was chosen to resemble the mechanical stresses found during average mastication in denture users (~22 MPa) [51, 52]. To validate the experimental strain experienced by the biomaterial surfaces at the two locations, the Euler–Bernoulli beam theory for small deformations was employed with where is the applied load, the beam span, the beam width, the beam height, and the Young’s modulus. A comparison between the theoretical and experimental evaluation was conducted.
Figure 1.
a) Scheme of the cyclic loading configuration applied to the beam and used for the biofilm model showing the selected region of interest (ROI) where biofilms were evaluated and the position of the strain gauges for deformation validation. Summary table showing the physical and mechanical properties of the biomaterial (PMMA). b) Sinusoidal cyclic strain (deformation) applied to the biomaterial surface at the tension side and its comparison to the theoretical strain values. c) Average strains measured and calculated at the ROI corresponding to 0.1% and 0.2%.
2.3. Biofilm Model
2.3.1. Salivary Pellicle Formation
The biomaterial surfaces were coated with a saliva pellicle to provide necessary proteins [53–55]. Unstimulated saliva (5 to 10 mL per donor) was collected from healthy young (<35 years old) donors (N=40). Donors were screened for systemic diseases and the absence of active carious lesions and periodontal disease. Volunteers were asked to abstain from eating or drinking two hours before donation. After collection, individual saliva samples were combined and centrifuged at 5,000 rpm for 15 min. The supernatant was treated with dithiothreitol (2.5 mM final concentration) (PRV3151, Promega) for 10 min and mixed with phosphate-buffered saline (PBS) in a 1:1 ratio. The saliva/PBS mix was then sterilized using a 0.2 μm membrane filter. PMMA beams were sterilized by soaking in a 70% ethanol solution for 15 minutes, followed by air drying inside a biological safety cabinet under UV light (SI-5). To coat the surfaces with sterile saliva, beams were submerged in the saliva solution (1 mL) at 37°C for 16 h before fungal inoculation.
2.3.2. Fungal Culture
The C. albicans strain (SC5314) was grown on a yeast extract peptone dextrose (YPD) (10 g/L yeast extract, 20 g/L peptone, and 20 g/L dextrose) (DF0428, Thermo Fisher) agar plate at 30°C for 24 h under aerobic conditions. A single colony was harvested, suspended in 10 mL of fresh YPD media, and grown at 30°C with continuous shaking at 200 rpm for 20 h. Then, the cells were centrifuged at 6,000 rpm for 5 min, washed twice with PBS, and diluted to OD600=1.0 in PBS (equivalent to ~1×108 CFU/mL) to obtain a liquid culture (SI-6) [56]. Next, saliva pellicle-coated beams were submerged in 3 mL of the liquid culture and incubated for 3 h for cell adhesion. Then, beams were gently washed with PBS (3 times) to remove non-adherent cells and placed on the bending fixture (span: 20 mm) with 3 mL of fresh yeast nitrogen base (YNB) (239210, Difco) supplemented with 2% glucose (G7021, Sigma-Aldrich). This media was chosen since it deters hyphae formation caused by nitrogen or carbon starvation [57]. Next, the beams were subjected to cyclic mechanical loading under different surface strain magnitudes (= 0.1% and 0.2%) at 2 Hz throughout the incubation period for 24 h (Fig. 2a and SI-7). These cyclic strain conditions were selected to recreate dentures experiencing 10 and 20 MPa, respectively [51, 58]. Two temperatures were selected for incubation including 30°C to deter filamentous growth (transition to hyphae) and virulence [59], and at 37°C to simulate clinical conditions. After incubation, beams (N=6 per group per evaluation) were gently rinsed with PBS (3 times) to detach loose cells, and the tension side and borders of the beams were gently cleaned using a cell scraper to only study the biofilm formed at the ROI (Fig. 1a).
Figure 2.
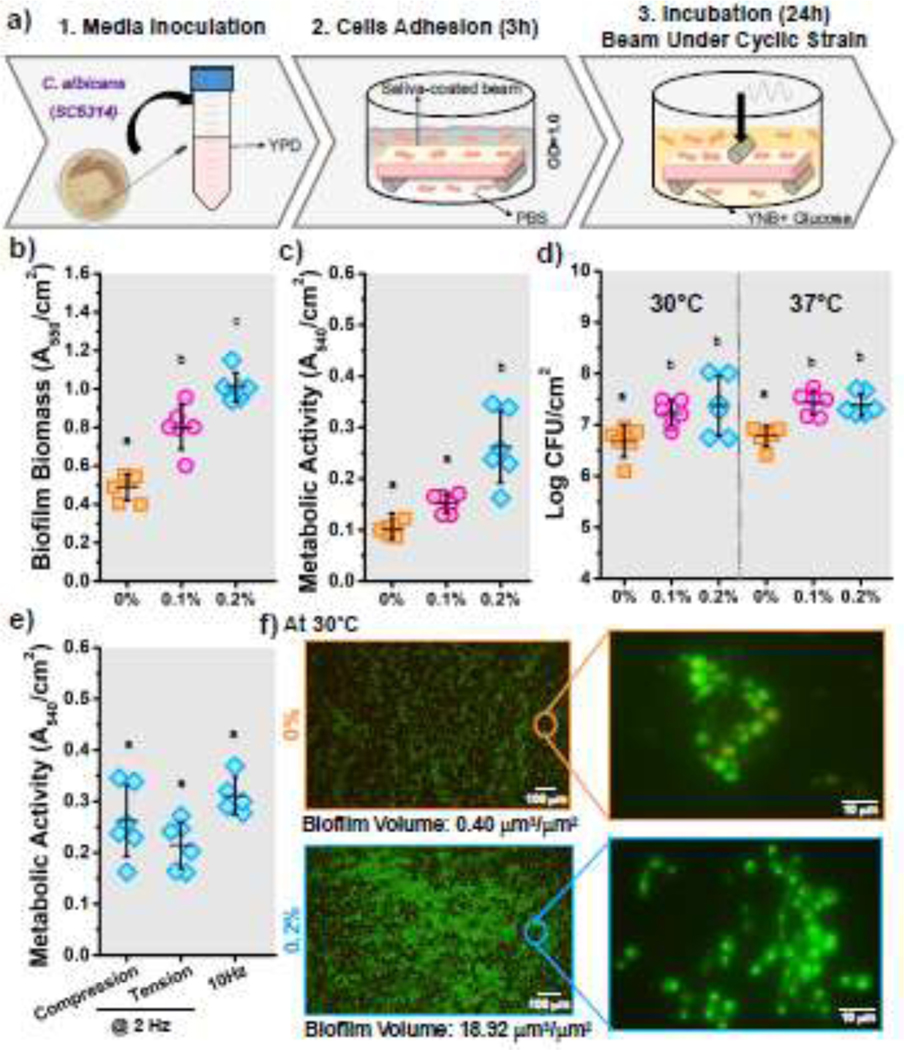
C. albicans biofilm–biomaterial interactions. (a) Schematic of the biofilm model used to cultivate C. albicans biofilms on PMMA samples under cyclic mechanical strain. b) Biofilm biomass, c) metabolic activity, and d) number of viable cells of biofilms cultivated under different cyclic strain conditions (0%, 0.1%, and 0.2%). e) Metabolic activity of C. albicans biofilms grown on surfaces subjected to different types of strain (tension or compression) and with different frequencies (2 Hz and 10 Hz). f) Representative fluorescence microscopy images of C. albicans biofilms on PMMA surfaces under different repetitive strains. Samples were stained with SYTO 9 (green) and propidium iodide (red) to indicate live and dead fungi, respectively. N=6 samples for each evaluation. Means with different letters are significantly different from each other (p ≤ 0.05).
2.4. Evaluation of the Fungal Biofilm-Biomaterial Interactions
2.4.1. Microbiological Evaluations: Biofilm Biomass, Metabolic Activity and Cell Viability
To evaluate the fungal biofilm-biomaterial interactions, the biofilm biomass and metabolic activity were evaluated using crystal violet (CV) and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assays respectively following previous protocols [60, 61]. For the biomass measurements, the beams were submerged in 1 mL solution of 0.1% CV (Sigma V5265) at room temperature for 20 min to stain the biofilms. As a de-staining solution, acetic acid 30% (v/v) (Ricca Chemical 1383032) was added to the samples and shaken by hand at room temperature until the biofilm was dissolved. Aliquots of the solution were transferred to a 96-well plate, and the absorbance at 550 nm (A550) was measured (Multiskan Sky, Thermo Scientific). For MTT measurements, beams were submerged in a 0.5 mg/mL MTT solution (Thermo Fisher Scientific V13154) and incubated at 37°C for 3 h. The MTT solution was replaced with an equal amount of dimethyl sulfoxide (DMSO) (Sigma BP231100) and shaken by hand in the dark until all formazan crystals were completely dissolved. The absorbance at 540 nm (A540) was measured to quantify metabolic activity. All absorbance values were normalized to the ROI area where the biofilms were studied (Fig. 1a). Cell viability was assessed by counting the number of colony-forming units (CFU). After incubation, beams were sonicated for 1 min at 40 kHz to detach the biofilm from the sample and then vortexed for 30 s in 1 mL of PBS [62]. Five 10-fold serial dilutions were prepared and plated on YPD agar plates for 24 h at 30°C under aerobic conditions. The colonies were counted manually and normalized with the beam surface ROI area.
2.4.2. Imaging of Biofilms
To visualize and quantify live and dead cells over the beam surface, biofilms were stained using the LIVE/DEAD BacLight viability kit (Thermo Fisher L7007) according to the manufacturer’s instructions. Briefly, a solution of fluorescent stain was prepared by mixing 3 μL of SYTO9 and PI in 1 mL of ultrapure water (final concentration of 6 μM for SYTO 9 and 30 μM for propidium iodide (PI)). Then, 200 μL of the staining solution was applied to the beam surface and incubated in a dark room for 20 min. Beams were gently rinsed with filter-sterilized water to remove the excess dye. Stained biofilms were visualized using a fluorescence microscope (EVOS M5000). Images were collected in a 50 z-stack traversing the biofilms along their thickness. The color images were converted to grayscale (unit 8, bitmap) to quantify the proportion of live (green image) and dead (red image) cells. A threshold was applied to obtain black-and-white pictures. The white pixels quantified above the threshold were counted as cells. The number of white pixels was computed for live and dead images separately. To estimate the biofilm volume, we manually identified the first (lower boundary) and last (upper boundary) images within the z-stack. The thickness of the biofilm was calculated by multiplying the number of images within the identified z-stack range. Image processing and calculations were performed using ImageJ. The yeast-to-hyphae transition was monitored under a light microscope (EVOS M5000) after 4 h of incubation at 30°C and 37°C. The hyphae formation (%) was calculated as the ratio of filamentous cells (i.e., hyphae and pseudo-hyphae) and the number of total cells at five random locations of the ROI.
2.4.3. Molecular Evaluations: RNA Sequencing
After incubation, the total RNA was extracted from biofilms using the RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions. RNA yield and quality were assessed using a NanoDrop (Thermo Fisher Scientific). Novogene Bioinformatics Technology Co. (Beijing, China) performed RNA sample quality testing (SI-8) and transcriptome analysis. RNA was sequenced from three biological replicates for each group. Sequencing was performed using an Illumina NovaSeq PE150. The reads obtained from each sample were filtered to remove adapters and low-quality reads. Clean sequencing reads were mapped to the reference genome using Hisat2 v2.0.5. Gene expression levels were normalized using the fragments per kilobase of transcript per million mapped reads method (FPKM) [63]. Differentially expressed genes (DEGs) were identified by comparing the expression levels of C. albicans cells grown in YPD medium overnight. Differential expression analysis was performed using DESeq2 v1.20.0 [64]. DESeq2 provides statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution. The resulting q-values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate [65]. Genes with |log2 (fold change (FC))|≥1 and statistical significance padj≤0.05 were considered differentially expressed. The log2 measurements of the DEGs were transformed to FC. To establish differences between static and cyclically strained biomaterial surfaces, the FC of the deformed materials was normalized to FC in the static surfaces (i.e., normalized FC= FCstrained/FCstatic). To validate the RNA-seq data, we performed real-time polymerase chain reaction (RT-qPCR) to evaluate changes in gene expression of selected genes between cyclically strained and static samples (SI-9).
3. Statistical Analysis
For all evaluations (biomass, MTT, CFU, yeast-to-hyphae transformation), data was expressed as the mean ± standard deviations. The assumption of normal distribution and homoscedasticity was assessed by Shapiro–Wilk’s and Levene’s tests. Statistical differences in the results were evaluated using one-way ANOVA with a significance of 0.05. The Tukey post hoc test was used for multiple comparisons with a 95% confidence level. All statistical analyses were completed using STATGRAPHICS Centurion XVII.
4. Results
4.1. Biomaterials’ Surface Properties
Biomaterial surface properties relevant to the microbe-biomaterial interactions were evaluated to guarantee that cyclic strain of the biomaterial surface was the only variable influencing fungal adhesion and biofilm formation. Physical and mechanical measurements included surface roughness (Ra: 3.88 nm), wettability (contact angle: 65.93°), and flexural stiffness (E=2.80 GPa) (see Support Information and Fig. 1a). All samples consistently rendered the same properties. The cyclic strain applied over the biomaterial surface was measured and validated (Fig. 1b). The stresses experienced by the beam surfaces were adjusted to represent cases experienced in clinical conditions. Experimental strain measurements showed an excellent agreement with theoretical results with deviations (<2%) (Fig. 1b). At the ROI, the application of the cyclic stresses was adjusted to render average strains of 0.1% and 0.2% for use in the biofilm model for biofilm-biomaterial evaluations (Fig. 1c).
4.2. Microbiological Biofilm-Biomaterial Interaction Evaluations
The formed biofilms were studied by measuring the biomass, metabolic activity, and cell viability and by visualizing the biofilms using fluorescence microscopy. First, the effect of the cyclic mechanical strain of the biomaterial surface on C. albicans biofilms was evaluated. Three different strain magnitudes (0%, 0.1%, and 0.2%) were used. Overall, cyclic strain of the biomaterial surface caused a significant increase in the biofilm biomass (Fig. 2b) and the metabolic activity (Fig. 2c) compared to static (=0%) surfaces. This increase, for both evaluations, was proportional to the strain magnitude, with higher cyclic strain rendering higher metabolic activity and biomass. Cell viability (CFU) was evaluated after incubating the biofilm at two different temperatures (30°C and 37°C). Overall, results showed an increase in the number of viable cells with increased magnitude of cyclic strain (Fig. 2d). An increase in cell viability was observed for both incubation temperatures.
Moreover, we evaluated the effect of the strain direction (tension or compression) and loading frequency (10 Hz) on the biofilms’ metabolic activity. No significant differences were observed when biofilms were formed on surfaces subjected to tensile or compression stresses and at a higher frequency rate (Fig. 2e). Live/dead imaging was used to visualize the formed biofilm and to confirm the changes in the biofilm biomass (Fig. 2f). For the static surfaces (=0%), dispersed agglomerations of spherical yeasts were observed. A compact biofilm was observed for cyclically strained biomaterial surfaces (=0.2%), comprised of elongated pseudo-hyphal structures and fewer round yeast cells. The biofilm volumes were quantified with higher quantities for the cyclically strained surfaces (18.9 μm3/μm2) compared to static ones (0.4 μm3/μm2) (Fig. 2f and SI-10).
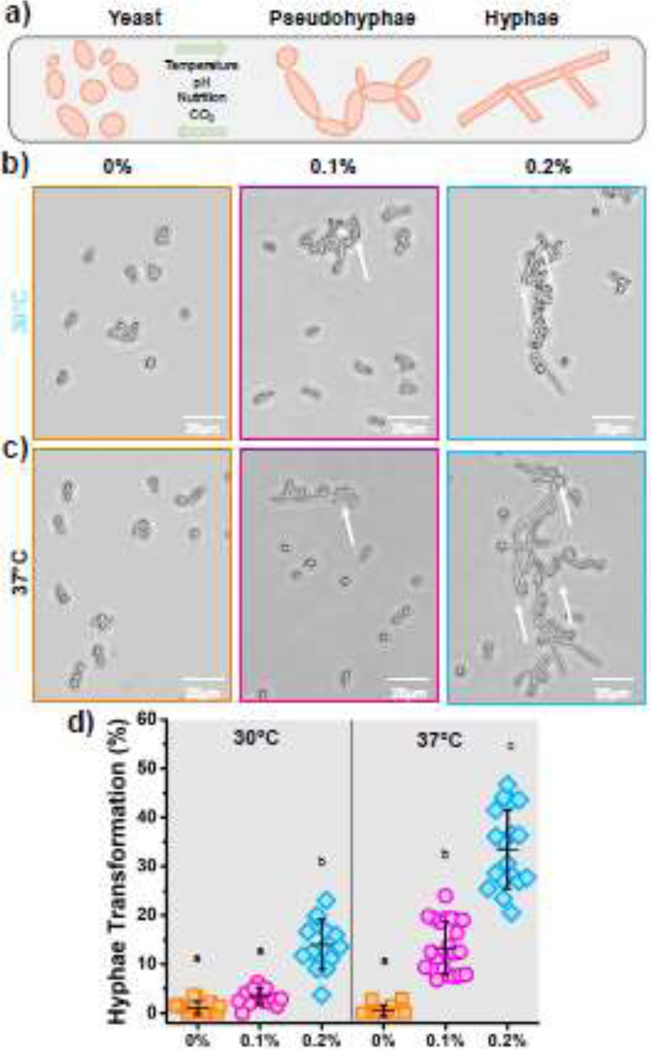
The yeast-to-hyphae transition was evaluated by microscopically examining the morphology of Candida cells (SI-10). Mainly spherical shaped cells were observed in static surfaces after incubation at both 30°C and 37°C (Fig. 3b and 3c). This morphology indicates the cell in yeast condition. No hyphae or pseudo-hyphae morphologies were observed under this biomaterial surface condition. For cyclically strained biomaterial surfaces (=0.2%), a combination of elongated pseudo-hyphae and oval-shaped cells were observed (Fig. 3b and 3c). The cyclic strain of the surface triggered a morphogenetic switching of the yeast cell. Candida filamentous forms such as hyphae or pseudohyphae are considered a virulence trait due to their contribution to host tissue invasion [66, 67]. To confirm these observations, we counted the number of filamentous (i.e., hyphae and pseudo-hyphae) cells. A significant increase in the percentage of yeast-to-hyphae transition was observed for both incubation temperatures in the cyclic strained group (=0.2%) (Fig. 3d). A higher transformation was measured for the samples incubated at 37°C (~35%) compared to samples incubated at 30°C and under the same cyclic strain (~15%).
Figure 3.
Yeast-to-hyphae transition evaluations. a) Scheme of the yeast-to-hyphae morphological transition of C. albicans. b) Photomicrographs showing the morphology of C. albicans cells growing over PMMA surfaces under cyclic strain (0%, 0.1%, and 0.2%) incubated at 30°C and c) 37°C. d) Percentage of hyphae formation after 4 h of incubation. The error bars were obtained from N = 6 measurements. Means with different letters are significantly different from each other (p ≤ 0.05).
4.3. Molecular Evaluations
To better understand these changes, we analyzed transcriptomic data of C. albicans cultivated on static (=0%) and cyclically strained surfaces (=0.2%) incubated at both 30°C and 37°C. Untreated Candida cells in planktonic state were also sequenced. Sequencing data exhibited an acceptable correlation among biological replicates (R2>0.8 for all groups) (SI-11). A total of 5,825 genes were found in the sequencing libraries. Examination of the RNA-seq data using principal component analysis (PCA) showed significant differences between biofilms formed under static and cyclically strained biomaterial surfaces at both incubation temperatures (Fig. 4a). Roughly, 5,630 genes were expressed in the treatment groups (static/cyclically strained) (Fig. 4b). Looking at the total of expressed genes of the cyclically strained biomaterial surface, roughly half of the genes were significantly up-or downregulated when compared to the static surfaces. To elucidate the effect of cyclic strained of biomaterial surfaces on gene expression, we ranked the top DEGs that were up- or down-regulated equally at both incubation temperatures (Fig. 4c and SI-12). This analysis enabled us to analyze DEG changes independently of the temperature. The highest upregulations were observed for RBR1 (a glycosylphosphatidylinositol (GPI)-anchored cell wall protein, 30-FC), CDC19 (a pyruvate kinase at yeast cell surface, 15-FC), and ARE2 (a Acyl CoA:sterol acyltransferase, 11-FC). The highest downregulation included ZCF2 (transcription factor required for adaptation to reactive sulfur species, 0.26-FC), PRS5 (a putative 5-phospho-ribosyl-1(alpha)-pyrophosphate synthetase, 0.34-FC), and PRS1 (phosphoribosylpyrophosphate synthetase, 0.34-FC).
Figure 4.
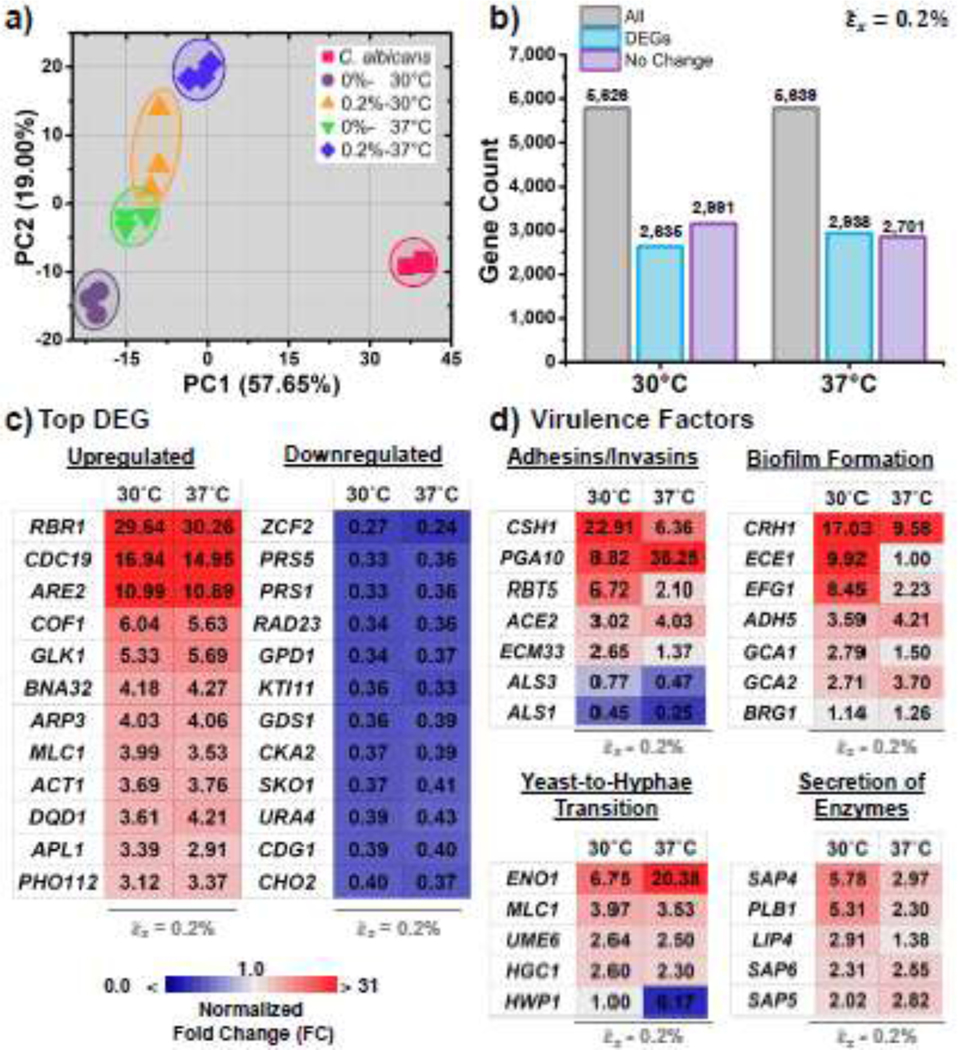
RNA- sequencing of Candida albicans biofilms under static (0%) and cyclically strained surfaces (0.2%). Two incubation temperatures were used including 30°C and 37°C. a) Principal component analysis (PCA) of the gene expression for planktonic cells (pink squares) and biofilms grown under both static and cyclically strained surfaces. b) Number of differentially expressed genes (DEGs) in cyclically strained samples incubated at 30°C and 37°C. c) Heatmap displaying fold changes (FC) in the top differentially expressed genes (DEGs). d) Heat map of the fold changes of genes related to virulence, including adhesion/invasion, biofilm formation/maturation, yeast-to-hyphae transition, and secretion of extracellular enzymes.
Selected genes related to the virulence factors of C. albicans [68–71] were analyzed and presented Fig. 4d and SI-13. At lower incubation temperatures (30°C), the cyclically strained biomaterial surfaces resulted in Candida with a significant upregulation of relevant genes. When analyzing adhesins and invasins, the highest upregulation was observed for CSH1 (23-FC), PGA10 (9-FC), and RBT5 (7-FC). CSH1 and PGA10 are genes associated with coding cell wall proteins [32]. RBT5 is a gene encoding a GPI-anchored protein required for cell wall integrity and host adhesion [72]. The highest downregulation was found in the ALS family genes encoding agglutinin-like proteins (ALS1 and ALS3, 0.45 and 0.77-FC, respectively) [73]. In genes involved in biofilm formation and maturation, significant upregulation was observed for CRH1 (17-FC), ECE1 (10-FC), and EFG1 (8.5-FC). CRH1 is a regulator of matrix production [74], ECE1 is involved in adhesion, biofilm formation, and filamentation [75], and EFG1 is a transcription factor associated with cell morphology and biofilm formation [76]. Looking at the expression of yeast-to-hyphae transformation genes, significant upregulation was found for ENO1 (involved in glycolysis and gluconeogenesis, 6.8-FC) [77], MLC1 (a Spitzenkorper and cytokinetic ring in hyphae, 4-FC) [78], and UME6 (a regulator of filamentous growth, 2.6-FC) [79]. The highest downregulation was measured for HWP1 (0.45-FC). Regarding the expression of genes associated with the secretion of enzymes, significant upregulation was found for SAP4 (6-FC), PLB1 (5.3-FC), and LIP4 (3-FC). SAP4 is a secretory aspartyl protease involved in hyphae formation and virulence [80], PLB1 is a phospholipase B [81], and LIP4 is a secreted lipase involved in the hydrolysis of ester bonds of triacylglycerols [82]. Taken together, the biomaterial surface under cyclic strain is triggering the pathogenesis of Candida by upregulating critical genes and transcription factors associated with virulence.
An analysis of the same selected genes/transcription factors at higher temperatures (37°C) was conducted. In some cases, the higher temperatures assist with increased upregulation. For example, the adhesion/invasion gene PGA10 increased from 9 to 36 FC with higher temperature (Fig. 4d). An opposite response was observed for CSH1 which decreased from 23 to 6 FC at higher temperature. Overall, incubation at 37°C decreased the upregulation of genes associated with biofilm formation (i.e., CRH1, ECE1, and EFG1) and secretion of enzymes genes (i.e., SAP4, PLB1, and LIP4). Expression of hyphal-specific genes UME6 (2.5-FC) and MLC1 (3.53-FC) remained similar at both temperatures. However, the ENO1 expression was increased at 37°C. To validate the RNA-seq results, RT-qPCR was performed in RNA extracted from static and cyclically strained surfaces incubated at 37°C. Genes involved with a protein cell surface, adhesion, biofilm formation, and virulence were selected (CDC19, ALS1, ALS3, and HWP1). Significant changes were observed for all the genes between the static and cyclically strained surfaces (SI-9). RT-qPCR results were consistent with those obtained from RNA-seq.
5. Discussion
Candida biofilm–biomaterial interactions are controlled by different biomaterial surface properties, including chemistry, charge, wettability, roughness, topography, and stiffness [21, 83, 84]. For example, biomaterial surfaces with higher roughness (Ra> 0.2 μm) promote cell adhesion [85, 86], while hydrophilic surfaces (contact angle <90°) [87] and negative electric charges (i.e., negative zeta potentials) reduce adhesion and biofilm formation [88]. These biomaterial surface properties are sensed by the cells, which respond by triggering virulence traits. In this study, for the first time, we revealed the effect of the biomaterial cyclic surface strain on the pathogenesis of C. albicans. The regulation of virulence by this biomaterial variable has not been explored for fungi or bacteria. In mammalian cells, the detection of cyclically strained biomaterial surfaces is an established driver of development and behavior [89–92]. Our findings evidenced an increase in the biofilm biomass, metabolic activity, number of viable cells, and number of hyphae-shaped cells, in addition to the significant expression of virulence-associated genes when Candida cells adhered and formed biofilms on these cyclically stretched substrate surfaces. This study suggests that Candida uses mechanical sensing of contact to a cyclically deformed substrate to induce pathogenesis and potentialize virulence traits. Understanding the mechanisms involved in this virulence activation is critical for the prevention of infections, given the multiple biomechanical forces to which biomaterials/medical devices are subjected in the body during clinical use.
C. albicans’ pathogenesis is multifactorial [1, 15]. Environmental cues triggering the pathogenesis include nutrients, temperature, pH, and oxygen, which in turn regulate the cells to adjust the morphology, increase metabolism and express virulence [93]. Additionally, Candida can sense abiotic biomaterial surfaces by detecting chemical or mechanical features present in the surface [1, 94]. Our study used environmental conditions and nutrients that deter and limit pathogenesis. Specifically, cells were cultured and incubated at 30°C in limiting YNB media (high nitrogen and carbon concentration, no amino acids, pH=5.4) and an atmosphere with low CO2 levels [57, 59, 95, 96]. Additionally, to study the possible contribution of media stirring on the virulence of Candida, we monitored the yeast-to-hyphae transformation after stirring the culture media with two conditions including shaking and actuator movement. Results showed no morphological changes (no transition from yeast-to-hyphae) under these conditions suggesting no influence on the studied phenomena (SI-14). Our results showed that when Candida adheres over static biomaterial surfaces under these limiting environmental conditions that deter pathogenesis, there is a limited virulence, represented by weak formation of biofilms (Fig. 2) and no hyphal formation (Fig. 3). However, when there is a cyclic strain in the biomaterial surface, the pathogenesis is triggered without relying upon chemical recognition of any specific host factor or environmental cues. Specifically, additional biofilm was formed, more yeast cells were present, and the yeast cells transformed into filamentous cells. In other words, when Candida adhered to repetitive strained (deformed) biomaterial surfaces, the biofilm was more robust and virulent than when growing on static biomaterial surfaces. Increased magnitude of biomaterial strain rendered increased traits of virulence (Figs. 3 and 4). Transition to hyphae also depended on the strain magnitude (0% transformation for =0% versus 14% for =0.2%). These findings in morphological transitions play a role in its virulence by increased adherence [97], biofilm formation [67], invasion of epithelial cell layers [98], and stress resistance [99]. To represent clinical cases and to determine the potential synergy for virulence, biofilm-biomaterial evaluations were conducted using an incubation temperature of 37°C and hyphal-inducing media (SI-15). At a microbiological level, the increase in incubation temperature potentialized the transformation from yeast to hyphae (Fig. 3d) without significantly increasing the yeast viability (Fig. 2d). These evaluations at body temperature are relevant since dentures, medical devices, and catheters are implanted and prone to fungal infections. Additionally, since C. albicans infects a broad range of host types, cyclic surface contact could serve as a nonspecific cue for host infection of materials such as tongue, dentures, and catheters.
To understand which genes mediate the ability of Candida to sense cyclic biomaterial surfaces and trigger virulence, we conducted RNA-seq (Fig. 4). First, we looked at the most significantly up-or down-regulated DEGs from static to cyclically strained surfaces independently of temperature (Fig. 4c). RBR1 ranked first in upregulation. This extracellular cell wall protein encodes GPI proteins required for filamentous growth at acidic pH and low temperature [100]. GPI proteins are involved in the adhesion to surfaces and virulence of C. albicans. The upregulation of this gene was only evidenced at the cyclically strained biomaterial surfaces and not expressed in static surfaces. RBR1 is known to be co-regulated by RIM101 and NRG1 [100], which are transcription factors associated with the response to extracellular pH and the regulation of chlamydospore formation and hyphal gene induction, respectively [101]. In Candida albicans, the CDC19 gene encodes for a pyruvate kinase, an enzyme involved in the glycolysis pathway [102]. Glycolysis is a central metabolic pathway particularly important for microorganisms like C. albicans that rely on fermentation for energy production. Deletion of CDC19 leads to a significant decrease in pyruvate kinase activity, which in turn impairs glycolysis, ATP production, and virulence [103–105]. In our study, CDC19 was significantly upregulated only in cyclically strained surfaces but not over static ones. Overall, CDC19 plays an essential role in the metabolism and viability of C. albicans, which can be explained by the increased metabolism (MTT) in the microbiological evaluations (Fig. 2).
Looking into adhesins/invasins genes, cyclic strain of the PMMA surfaces resulted in increased expression of CSH1 compared to static surfaces at both incubation temperatures. CSH1 is a cell surface protein contributing to the hydrophobicity of the cell [106]. It modulates important virulence attributes like adhesion and biofilm formation [107]. Hydrophobic cells can easily interact and adhere to solid surfaces (e.g., PMMA surfaces) [108]. In fact, the expression of CSH1 has been associated with increased biomaterial surface adhesion of C. albicans [108]. Also, this gene has been associated with the full adherence of biofilm [109]. The presence of hydrophobic proteins may influence the initial distribution of yeast cells on the biomaterial surface and determine a site for colonization [110]. Moreover, we analyzed some CFEM (common in several fungal extracellular membrane proteins) [111] such as RBT5 and PGA10 [112] (Fig. 4c). These two genes were significantly upregulated in cyclically strained surfaces compared to static ones. RBT5 is a protein required for the maintenance of the cell wall integrity, host adhesion, and hemoglobin utilization [113]. In addition, these genes have been associated with switching from the yeast form to filamentous growth (hyphal-specific functions) [114], an indication of virulence. Downregulation was observed for the ALS family genes in all biomaterial surfaces (static and cyclic) incubated at both temperatures, suggesting that surfaces under cyclic deformation are not related to changes in the agglutinin-like proteins.
Regarding biofilm formation and ECM secretion, we analyzed selected genes and transcription factors associated with each stage of biofilm formation, including adhesion, maturation, and dispersion (Fig. 4d) [115]. Our results showed that cyclic strain of the biomaterial surface upregulated biofilm formation genes such as CRH1 (which affects cell wall organization and virulence) [74]. In addition, we found the upregulation of genes involved in cell wall organization, remodeling, and production of ECM, such as ADH5 [115]. ADH5 is an alcohol dehydrogenase gene that acts positively in the production of ECM, while GCA2 is an extracellular glucoamylase [116]. Increased ECM enables biofilms to protect microbes against environmental damage and the host immune response [117]. The effect of mechanical stimulation on the production of ECM and tissue remodeling has been reported for other tissues [118]. Cardiovascular cells under cyclic strains respond by remodeling their ECM (i.e., increased synthesis of elastin, proteoglycans, glycosaminoglycans, matrix metalloproteinases (MMPs)) [119, 120]. Our results suggest that cyclically strained biomaterial surfaces potentialize Candida’s adherence and biofilm formation. It appears that cells are intending to adhere to the moving biomaterial surface to form robust and stickier biofilms with enhanced ECM.
Looking into the yeast-to-hyphae morphological transition, we analyzed different genes and transcription factors, including EFG1, UME6, HGC1, and HWP1 [57]. The hyphal morphology has the ability to penetrate the cell membrane of epithelial cells actively to accelerate the progression of infection [59]. Two of these transcription factors (EFG1 and UME6) were significantly upregulated with the cyclic biomaterial strain compared to static surfaces. EFG1 is a central transcriptional regulator of morphogenesis and biofilm formation [76]. It is involved in the cAMP-protein kinase A (PKA) pathway and controls multiple genes involved in adhesion. In our study, EFG1 is positively regulated by TPK1/TPK2 (upregulated, not shown) to promote filamentation by regulating the expression of UME6 [121], which is a key filament-specific transcriptional regulator required to maintain filamentation [122]. Changes in the regulation of these genes showed that cyclically strained biomaterials surfaces could promote biofilm formation and virulence by an EFG1-dependent pathway. The upregulation of HGC1 is essential for hyphal morphogenesis and for infection by facilitating the invasion of host tissues and escape from phagocytosis [123]. Candida transition from yeast to hyphae by activating the EFG1→ UME6 →HGC1 pathway has been related to the downregulation of HWP1 and ALS3 (as found in our results), suggesting that these genes are dispensable for Candida filamentation [79]. Additionally, deletion of BCR1 is related to the downregulation of adhesin factors, including ALS1, ALS3 and HWP1, causing a defective biofilm despite being able to form hyphal structures [124]. Moreover, the secretion of aspartic proteinases (SAP family) [80, 125] and phospholipases (PLB family) were also upregulated with the cyclic strained of biomaterial surfaces. Increased secretion of aspartic proteinases is involved in the hydrolysis of structural and functional proteins that defend the host tissue [126]. Additionally, phospholipases are related to cell penetration, adhesion to epithelial cells, and persistence of Candida infections [127]. All these results indicate that the cyclic strained surface triggers the transition to hyphal form facilitating the potential of cells to penetrate tissue, secrete toxic enzymes and cause disease. Further mechanistic studies are required to reveal the specific molecular pathways involved in the pathogenesis of Candida cells triggered by cyclic deformation.
Despite the potential impact of the present findings, this study has limitations. First, the biomaterial surfaces had a salivary coating. A protein layer on the surface of the biomaterial, such as saliva or blood plasma, can influence cell adhesion, morphology, and expression of virulence factors [35, 128]. For example, mucin coatings favor cell adhesion and biofilm formation, whereas lactoferrin coatings hinders [128, 129]. Clinically, the improper cleaning of dentures results in a layer of food debris and microorganisms that increase bacterial adhesion and biofilm formation that, combined with surface deformation, could alter the expression of virulence factors. In addition, the cyclic strain of the biomaterial surfaces may modulate the oral microbiome, which warrant further studies. Second, the experiments were performed on PMMA substrates since it is the prosthetic material of choice and is related to the development of oral candidiasis. However, futures studies should include the use of other materials involved in yeast infections (i.e., latex, silicone, polyvinyl chloride, among others) to not only verify the effect reported in this study but also unravel possible new interaction between chemical composition, cyclic strain of surfaces, and the Candida biofilms. Third, the use of different biomaterials in different body-type environments and in contact with different microbial species raises questions if the mechanism elucidated here for C. albicans cells may also be operative for other yeast species and bacterial strains. Finally, the oral cavity is a diverse and complex environment that houses numerous microorganisms, including bacteria, viruses, and fungi. For example, the surface of dentures and oral mucosa are frequently colonized by Candida species, Streptococcus mutans, and Staphylococcus aureus [130]. Future studies should evaluate how polymicrobial interactions between C. albicans, other microbial species, and the effect of cyclic deformation may further modulate the pathogenesis and virulence of Candida biofilms [131, 132].
6. Conclusions
For the first time, our study showed the effect of the cyclic strain of a biomaterial surface on the pathogenesis of C. albicans. Overall, the interactions of Candida with these repetitive stretched surfaces rendered increased formation, yeast-to-hyphae transition, and expression of virulence factors. In this study, PMMA surfaces were cyclically strained to 0.1% and 0.2% and incubated at 30°C and 37°C. The increase in biofilm formation was proportional to the biomaterial strain. Elevated temperatures (37°C) potentialized the transition from yeast to filamentous cells. RNA-seq revealed that Candida cells in the presence of cyclic strain perform mechanosensing by activating transcription factors encoding genes involved in cell wall (RBR1), metabolism (CDC19), yeast adhesion (CSH1, PGA10, RBT5), biofilm formation (CRH1, EFG1, ADH5), yeast-to-hyphal transition (UME6, HGC1) and secretion of aspartyl proteases (SAP family genes). Results from this work could be used to explore a novel mechanism in microbes triggering pathogenicity and the development of novel antimicrobial drugs.
Supplementary Material
Statement of Significance.
Fungal infections produced by Candida albicans are a significant contributor to various health conditions. Candida becomes pathogenic when certain environmental conditions change, including temperature, pH, nutrients, and CO2 levels. In addition, surface properties, including wettability, stiffness, and roughness, drive the interactions between Candida and biomaterials. Clinically, Candida adheres to biomaterials that are under repetitive deformation due to body movements. In this work, we revealed that when Candida adhered to biomaterial surfaces subjected to repetitive deformation, the microorganism becomes pathogenic by increasing the formation of biofilms and the expression of virulent factors related to hyphae formation and secretion of enzymes. Findings from this work could aid the development of new strategies for treating fungal infections in medical devices or implanted biomaterials.
7. Acknowledgements
This work was partly supported by the National Institute of Dental and Craniofacial Research (NIDCR) Award R21-DE030564. This work was also supported in part by the Temple University Maurice Kornberg School of Dentistry start-up fund and by the Office of Provost at Temple University strategic fund. In addition, we would like to acknowledge support from Dr. Dmitriy A. Dikin of the College of Engineering Nano Instrumentation Center (CoE NIC), Dr. Aaron P. Mitchell from the University of Georgia, Dr. MaryAnne Rizk from the University of Maryland and personnel of the Oral Microbiome Laboratory at Temple University for the valuable discussions during the progress of this work.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- [1].Mayer FL, Wilson D, Hube B, Candida albicans pathogenicity mechanisms, Virulence 4(2) (2013) 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pfaller MA, Diekema DJ, Epidemiology of Invasive Candidiasis: a Persistent Public Health Problem, Clinical Microbiology Reviews 20(1) (2007) 133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ, Invasive candidiasis, Nature Reviews Disease Primers 4(1) (2018) 18026. [DOI] [PubMed] [Google Scholar]
- [4].Gulati M, Nobile CJ, Candida albicans biofilms: development, regulation, and molecular mechanisms, Microbes and Infection 18(5) (2016) 310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ramage G, Borghi E, Rodrigues CF, Kean R, Williams C, Lopez-Ribot J, Our current clinical understanding of Candida biofilms: where are we two decades on?, APMIS n/a(n/a) (2023). [DOI] [PubMed] [Google Scholar]
- [6].Karajacob AS, Al-Maleki AR, Tay ST, Revisiting oral thrush in South-East Asian patients: A review of published studies (2000–2020), Journal of Oral Pathology & Medicine 51(1) (2022) 98–105. [DOI] [PubMed] [Google Scholar]
- [7].Vila T, Sultan AS, Montelongo-Jauregui D, Jabra-Rizk MA, Oral Candidiasis: A Disease of Opportunity, Journal of fungi (Basel, Switzerland) 6(1) (2020) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kojic Erna M, Darouiche Rabih O, Candida Infections of Medical Devices, Clinical Microbiology Reviews 17(2) (2004) 255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee J-H, Kim Y-G, Khadke SK, Lee J, Antibiofilm and antifungal activities of mediumchain fatty acids against Candida albicans via mimicking of the quorum-sensing molecule farnesol, Microbial biotechnology 14(4) (2021) 1353–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr., Calandra TF, Edwards JE Jr., Filler SG, Fisher JF, Kullberg B-J, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious A. Diseases Society of, Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 48(5) (2009) 503–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC, Hidden Killers: Human Fungal Infections, Science Translational Medicine 4(165) (2012) 165rv13–165rv13. [DOI] [PubMed] [Google Scholar]
- [12].Nucci M, Persistent Candidemia: Causes and Investigations, Current Fungal Infection Reports 5(1) (2011) 3–11. [Google Scholar]
- [13].Desai JV, Mitchell AP, Andes DR, Fungal biofilms, drug resistance, and recurrent infection, Cold Spring Harbor perspectives in medicine 4(10) (2014) a019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Staniszewska M, Virulence Factors in Candida species, Curr Protein Pept Sci 21(3) (2020) 313–323. [DOI] [PubMed] [Google Scholar]
- [15].Talapko J, Juzbašić M, Matijević T, Pustijanac E, Bekić S, Kotris I, Škrlec I, Candida albicans—The Virulence Factors and Clinical Manifestations of Infection, Journal of Fungi 7(2) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Calderone RA, Fonzi WA, Virulence factors of Candida albicans, Trends in Microbiology 9(7) (2001) 327–335. [DOI] [PubMed] [Google Scholar]
- [17].Lopes JP, Lionakis MS, Pathogenesis and virulence of Candida albicans, Virulence 13(1) (2022) 89–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rodríguez-Cerdeira C, Martínez-Herrera E, Carnero-Gregorio M, López-Barcenas A, Fabbrocini G, Fida M, El-Samahy M, González-Cespón JL, Pathogenesis and Clinical Relevance of Candida Biofilms in Vulvovaginal Candidiasis, Frontiers in microbiology 11 (2020) 544480–544480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cho E, Park Y, Kim KY, Han D, Kim HS, Kwon JS, Ahn HJ, Clinical Characteristics and Relevance of Oral Candida Biofilm in Tongue Smears, J Fungi (Basel) 7(2) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lamont RJ, Koo H, Hajishengallis G, The oral microbiota: dynamic communities and host interactions, Nature reviews Microbiology 16(12) (2018) 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zheng S, Bawazir M, Dhall A, Kim H-E, He L, Heo J, Hwang G, Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion, Frontiers in Bioengineering and Biotechnology 9(82) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Krsmanovic M, Biswas D, Ali H, Kumar A, Ghosh R, Dickerson AK, Hydrodynamics and surface properties influence biofilm proliferation, Advances in Colloid and Interface Science 288 (2021) 102336. [DOI] [PubMed] [Google Scholar]
- [23].Stoodley P, Sauer K, Davies DG, Costerton JW, Biofilms as Complex Differentiated Communities, Annual Review of Microbiology 56(1) (2002) 187–209. [DOI] [PubMed] [Google Scholar]
- [24].O’Toole G, Kaplan HB, Kolter R, Biofilm Formation as Microbial Development, Annual Review of Microbiology 54(1) (2000) 49–79. [DOI] [PubMed] [Google Scholar]
- [25].Bowen WH, Burne RA, Wu H, Koo H, Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments, Trends in Microbiology 26(3) (2018) 229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Garnett JA, Matthews S, Interactions in bacterial biofilm development: a structural perspective, Current protein & peptide science 13(8) (2012) 739–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Davis DA, How human pathogenic fungi sense and adapt to pH: the link to virulence, Current Opinion in Microbiology 12(4) (2009) 365–370. [DOI] [PubMed] [Google Scholar]
- [28].Vylkova S, Environmental pH modulation by pathogenic fungi as a strategy to conquer the host, PLOS Pathogens 13(2) (2017) e1006149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yuan X, Mitchell BM, Hua X, Davis DA, Wilhelmus KR, The RIM101 signal transduction pathway regulates Candida albicans virulence during experimental keratomycosis, Investigative ophthalmology & visual science 51(9) (2010) 4668–4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cottier F, Hall RA, Face/Off: The Interchangeable Side of Candida Albicans, Frontiers in cellular and infection microbiology 9 (2020) 471–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fanning S, Mitchell AP, Fungal biofilms, PLoS pathogens 8(4) (2012) e1002585-e1002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Finkel JS, Mitchell AP, Genetic control of Candida albicans biofilm development, Nature reviews. Microbiology 9(2) (2011) 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].De-la-Pinta I, Cobos M, Ibarretxe J, Montoya E, Eraso E, Guraya T, Quindós G, Effect of biomaterials hydrophobicity and roughness on biofilm development, Journal of Materials Science: Materials in Medicine 30(7) (2019) 77. [DOI] [PubMed] [Google Scholar]
- [34].Eguia A, Arakistain A, De-la-Pinta I, López-Vicente J, Sevillano E, Quindós G, Eraso E, Candida albicans biofilms on different materials for manufacturing implant abutments and prostheses, Medicina Oral, Patología Oral y Cirugía Bucal 25(1) (2020) e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bürgers R, Hahnel S, Reichert TE, Rosentritt M, Behr M, Gerlach T, Handel G, Gosau M, Adhesion of Candida albicans to various dental implant surfaces and the influence of salivary pellicle proteins, Acta Biomaterialia 6(6) (2010) 2307–2313. [DOI] [PubMed] [Google Scholar]
- [36].Lagree K, Mon HH, Mitchell AP, Ducker WA, Impact of surface topography on biofilm formation by Candida albicans, PloS one 13(6) (2018) e0197925-e0197925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Le PH, Nguyen DHK, Aburto-Medina A, Linklater DP, Crawford RJ, MacLaughlin S, Ivanova EP, Nanoscale Surface Roughness Influences Candida albicans Biofilm Formation, ACS Applied Bio Materials 3(12) (2020) 8581–8591. [DOI] [PubMed] [Google Scholar]
- [38].Cheong YA-O, Arce MA-O, Benito A, Chen D, Luengo Crisóstomo N, Kerai LA-O, Rodríguez GA-O, Valverde JA-O, Vadalia M, Cerpa-Naranjo AA-OX, Ren GA-O, Synergistic Antifungal Study of PEGylated Graphene Oxides and Copper Nanoparticles against Candida albicans. LID - 10.3390/nano10050819 [doi] LID - 819, (2079–4991 (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Puerner C, Kukhaleishvili N, Thomson D, Schaub S, Noblin X, Seminara A, Bassilana M, Arkowitz RA, Mechanical force-induced morphology changes in a human fungal pathogen, BMC Biology 18(1) (2020) 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Song F, Koo H, Ren D, Effects of material properties on bacterial adhesion and biofilm formation, Journal of dental research 94(8) (2015) 1027–1034. [DOI] [PubMed] [Google Scholar]
- [41].Schmalz G, Cieplik F, Biofilms on restorative materials, Oral Biofilms 29 (2021) 155–194. [DOI] [PubMed] [Google Scholar]
- [42].Busscher H, Rinastiti M, Siswomihardjo W, Van der Mei H, Biofilm formation on dental restorative and implant materials, Journal of dental research 89(7) (2010) 657–665. [DOI] [PubMed] [Google Scholar]
- [43].Holban AM, Farcasiu C, Andrei OC, Grumezescu AA-OX, Farcasiu AA-OX, Surface Modification to Modulate Microbial Biofilms-Applications in Dental Medicine. LID - 10.3390/ma14226994 [doi] LID - 6994, (1996–1944 (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nett J, Andes D, Candida albicans biofilm development, modeling a host–pathogen interaction, Current Opinion in Microbiology 9(4) (2006) 340–345. [DOI] [PubMed] [Google Scholar]
- [45].Vera-González N, Shukla A, Advances in Biomaterials for the Prevention and Disruption of Candida Biofilms, Frontiers in Microbiology 11(2251) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lohbauer U, Dental glass ionomer cements as permanent filling materials?—Properties, limitations future trends, Materials 3(1) (2009) 76–96. [Google Scholar]
- [47].Buschang P, Hayasaki H, Throckmorton G, Quantification of human chewing-cycle kinematics, Archives of Oral Biology 45(6) (2000) 461–474. [DOI] [PubMed] [Google Scholar]
- [48].Montoya C, Kurylec J, Baraniya D, Tripathi A, Puri S, Orrego S, Antifungal Effect of Piezoelectric Charges on PMMA Dentures, ACS Biomaterials Science & Engineering 7(10) (2021) 4838–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wekwejt M, Chen S, Kaczmarek-Szczepańska B, Nadolska M, Łukowicz K, Pałubicka A, Michno A, Osyczka AM, Michálek M, Zieliński A, Nanosilver-loaded PMMA bone cement doped with different bioactive glasses – evaluation of cytocompatibility, antibacterial activity, and mechanical properties, Biomaterials Science 9(8) (2021) 3112–3126. [DOI] [PubMed] [Google Scholar]
- [50].Hoffmann K, Applying the wheatstone bridge circuit, HBM Darmstadt, Germany: 1974. [Google Scholar]
- [51].Cheng YY, Cheung WL, Chow TW, Strain analysis of maxillary complete denture with three-dimensional finite element method, J Prosthet Dent, United States, 2010, pp. 309–18. [DOI] [PubMed] [Google Scholar]
- [52].Ravi N, Krishna DP, Manoj S, Chethan H, A Functional Stress Analysis in the Maxillary Complete Denture Influenced by the Position of Artificial Teeth and Load Levels: an In-vitro Study, Journal of Indian Prosthodontic Society 10(4) (2010) 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Al-Ahmad A, Wollensak K, Rau S, Guevara Solarte DL, Paschke S, Lienkamp K, Staszewski O, How Do Polymer Coatings Affect the Growth and Bacterial Population of a Biofilm Formed by Total Human Salivary Bacteria?-A Study by 16S-RNA Sequencing, Microorganisms 9(7) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bamford CV, Nobbs AH, Barbour ME, Lamont RJ, Jenkinson HF, Functional regions of Candida albicans hyphal cell wall protein Als3 that determine interaction with the oral bacterium Streptococcus gordonii, Microbiology (Reading) 161(Pt 1) (2015) 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fischer NG, Aparicio C, The salivary pellicle on dental biomaterials, Colloids and Surfaces B: Biointerfaces 200 (2021) 111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rodríguez-Tudela JL, Cuenca-Estrella M, Díaz-Guerra TM, Mellado E, Standardization of antifungal susceptibility variables for a semiautomated methodology, J Clin Microbiol 39(7) (2001) 2513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chow EWL, Pang LM, Wang Y, From Jekyll to Hyde: The Yeast-Hyphal Transition of Candida albicans, Pathogens (Basel, Switzerland) 10(7) (2021) 859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cheng YY, Li JY, Fok SL, Cheung WL, Chow TW, 3D FEA of high-performance polyethylene fiber reinforced maxillary dentures, Dent Mater, 2010. Academy of Dental Materials. Published by Elsevier Ltd, England, 2010, pp. e211–9. [DOI] [PubMed] [Google Scholar]
- [59].Kornitzer D, Regulation of Candida albicans Hyphal Morphogenesis by Endogenous Signals, Journal of fungi (Basel, Switzerland) 5(1) (2019) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Phumat P, Khongkhunthian S, Wanachantararak P, Okonogi S, Comparative inhibitory effects of 4-allylpyrocatechol isolated from Piper betle on Streptococcus intermedius, Streptococcus mutans, and Candida albicans, Archives of Oral Biology 113 (2020) 104690. [DOI] [PubMed] [Google Scholar]
- [61].Teodoro GR, Gontijo AVL, Salvador MJ, Tanaka MH, Brighenti FL, Delbem ACB, Delbem ÁCB, Koga-Ito CY, Effects of Acetone Fraction From Buchenavia tomentosa Aqueous Extract and Gallic Acid on Candida albicans Biofilms and Virulence Factors, Frontiers in Microbiology 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Barbosa JO, Rossoni RD, Vilela SFG, De Alvarenga JA, dos Santos Velloso M, de Azevedo Prata MC, Jorge AOC, Junqueira JC, Streptococcus mutans can modulate biofilm formation and attenuate the virulence of Candida albicans, PLoS One 11(3) (2016) e0150457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B, Mapping and quantifying mammalian transcriptomes by RNA-Seq, Nature Methods 5(7) (2008) 621–628. [DOI] [PubMed] [Google Scholar]
- [64].Anders S, Huber W, Differential expression analysis for sequence count data, Genome Biology 11(10) (2010) R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Li J, Witten DM, Johnstone IM, Tibshirani R, Normalization, testing, and false discovery rate estimation for RNA-sequencing data, Biostatistics (Oxford, England) 13(3) (2012) 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mukaremera L, Lee KK, Mora-Montes HM, Gow NAR, Candida albicans Yeast, Pseudohyphal, and Hyphal Morphogenesis Differentially Affects Immune Recognition, Front Immunol 8 (2017) 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Thompson DS, Carlisle PL, Kadosh D, Coevolution of morphology and virulence in Candida species, Eukaryotic Cell 10(9) (2011) 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chong PP, Chin VK, Wong WF, Madhavan P, Yong VC, Looi CY, Transcriptomic and Genomic Approaches for Unravelling Candida albicans Biofilm Formation and Drug Resistance-An Update, Genes 9(11) (2018) 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Alim D, Sircaik S, Panwar SL, The Significance of Lipids to Biofilm Formation in Candida albicans: An Emerging Perspective, Journal of fungi (Basel, Switzerland) 4(4) (2018) 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mba IE, Nweze EI, Mechanism of Candida pathogenesis: revisiting the vital drivers, European Journal of Clinical Microbiology & Infectious Diseases 39(10) (2020) 1797–1819. [DOI] [PubMed] [Google Scholar]
- [71].Cavalheiro M, Teixeira MC, Candida Biofilms: Threats, Challenges, and Promising Strategies, Front Med (Lausanne) 5 (2018) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mishra PK, Baum M, Carbon J, DNA methylation regulates phenotype-dependent transcriptional activity in Candida albicans, Proceedings of the National Academy of Sciences 108(29) (2011) 11965–11970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hoyer LL, The ALS gene family of Candida albicans, Trends in Microbiology 9(4) (2001) 176–180. [DOI] [PubMed] [Google Scholar]
- [74].Pardini G, De Groot PW, Coste AT, Karababa M, Klis FM, de Koster CG, Sanglard D, The CRH family coding for cell wall glycosylphosphatidylinositol proteins with a predicted transglycosidase domain affects cell wall organization and virulence of Candida albicans, J Biol Chem 281(52) (2006) 40399–411. [DOI] [PubMed] [Google Scholar]
- [75].Engku Nasrullah Satiman EAF, Ahmad H, Ramzi AB, Abdul Wahab R, Kaderi MA, Wan Harun WHA, Dashper S, McCullough M, Arzmi MH, The role of Candida albicans candidalysin ECE1 gene in oral carcinogenesis, Journal of Oral Pathology & Medicine 49(9) (2020) 835–841. [DOI] [PubMed] [Google Scholar]
- [76].Glazier VE, EFG1, Everyone’s Favorite Gene in Candida albicans: A Comprehensive Literature Review, Frontiers in cellular and infection microbiology 12 (2022) 855229–855229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Reyna-Beltrán E, Iranzo M, Calderón-González KG, Mondragón-Flores R, Labra-Barrios ML, Mormeneo S, Luna-Arias JP, The Candida albicans ENO1 gene encodes a transglutaminase involved in growth, cell division, morphogenesis, and osmotic protection, J Biol Chem 293(12) (2018) 4304–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Puerner C, Serrano A, Wakade RS, Bassilana M, Arkowitz RA, A Myosin Light Chain Is Critical for Fungal Growth Robustness in Candida albicans, mBio 12(5) (2021) e0252821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Banerjee M, Uppuluri P, Zhao XR, Carlisle PL, Vipulanandan G, Villar CC, López-Ribot JL, Kadosh D, Expression of UME6, a key regulator of Candida albicans hyphal development, enhances biofilm formation via Hgc1- and Sun41-dependent mechanisms, Eukaryotic cell 12(2) (2013) 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Staniszewska M, Bondaryk M, Malewski T, Schaller M, The expression of the Candida albicans gene SAP4 during hyphal formation in human serum and in adhesion to monolayer cell culture of colorectal carcinoma Caco-2 (ATCC), Open Life Sciences 9(8) (2014) 796–810. [Google Scholar]
- [81].Mukherjee PK, Chandra DM. Fau - Kuhn J, Kuhn MA. Fau - Ghannoum Dm, Ghannoum MA, Differential expression of Candida albicans phospholipase B (PLB1) under various environmental and physiological conditions, (1350–0872 (Print)). [DOI] [PubMed] [Google Scholar]
- [82].Hube B, Stehr F, Bossenz M, Mazur A, Kretschmar M, Schäfer W, Secreted lipases of Candida albicans: cloning, characterisation and expression analysis of a new gene family with at least ten members, Archives of Microbiology 174(5) (2000) 362–374. [DOI] [PubMed] [Google Scholar]
- [83].Kumamoto CA, Molecular mechanisms of mechanosensing and their roles in fungal contact sensing, Nat Rev Microbiol 6(9) (2008) 667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Le PH, Nguyen DHK, Medina AA, Linklater DP, Loebbe C, Crawford RJ, MacLaughlin S, Ivanova EP, Surface Architecture Influences the Rigidity of Candida albicans Cells, Nanomaterials 12(3) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Bürgers R, Schneider-Brachert W, Rosentritt M, Handel G, Hahnel S, Candida albicans adhesion to composite resin materials, Clinical Oral Investigations 13(3) (2009) 293–299. [DOI] [PubMed] [Google Scholar]
- [86].Nevzatoğlu EU, Özcan M, Kulak-Ozkan Y, Kadir T, Adherence of Candida albicans to denture base acrylics and silicone-based resilient liner materials with different surface finishes, Clinical Oral Investigations 11(3) (2007) 231–236. [DOI] [PubMed] [Google Scholar]
- [87].Yoshijima Y, Murakami K, Kayama S, Liu D, Hirota K, Ichikawa T, Miyake Y, Effectof substrate surface hydrophobicity on the adherence of yeast and hyphal Candida, Mycoses 53(3) (2010) 221–226. [DOI] [PubMed] [Google Scholar]
- [88].Klotz SA, Drutz DJ, Zajic JE, Factors governing adherence of Candida species to plastic surfaces, Infection and immunity 50(1) (1985) 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wang H, Ip W, Boissy R, Grood ES, Cell orientation response to cyclically deformed substrates: Experimental validation of a cell model, Journal of Biomechanics 28(12) (1995) 1543–1552. [DOI] [PubMed] [Google Scholar]
- [90].Wang JHC, Grood ES, The Strain Magnitude and Contact Guidance Determine Orientation-Response of Fibroblasts to Cyclic Substrate Strains, Connective Tissue Research 41(1) (2000) 29–36. [DOI] [PubMed] [Google Scholar]
- [91].Wang JHC, Grood ES, Florer J, Wenstrup R, Alignment and proliferation of MC3T3-E1 osteoblasts in microgrooved silicone substrata subjected to cyclic stretching, Journal of Biomechanics 33(6) (2000) 729–735. [DOI] [PubMed] [Google Scholar]
- [92].Faust U, Hampe N, Rubner W, Kirchgeßner N, Safran S, Hoffmann B, Merkel R, Cyclic Stress at mHz Frequencies Aligns Fibroblasts in Direction of Zero Strain, PLOS ONE 6(12) (2011) e28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nadeem SG, Shafiq A, Hakim ST, Anjum Y, Kazm SU, Effect of growth media, pH and temperature on yeast to hyphal transition in Candida albicans, Open Journal of Medical Microbiology 3 (2013). [Google Scholar]
- [94].Böttcher B, Hoffmann B, Garbe E, Weise T, Cseresnyés Z, Brandt P, Dietrich S, Driesch D, Figge MT, Vylkova S, The Transcription Factor Stp2 Is Important for Candida albicans Biofilm Establishment and Sustainability, Frontiers in Microbiology 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Heintz-Buschart A, Eickhoff H, Hohn E, Bilitewski U, Identification of inhibitors of yeast-to-hyphae transition in Candida albicans by a reporter screening assay, Journal of Biotechnology 164(1) (2013) 137–142. [DOI] [PubMed] [Google Scholar]
- [96].Chen H, Zhou X, Ren B, Cheng L, The regulation of hyphae growth in Candida albicans, Virulence 11(1) (2020) 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wilson D, Naglik JR, Hube B, The Missing Link between Candida albicans Hyphal Morphogenesis and Host Cell Damage, PLoS pathogens 12(10) (2016) e1005867-e1005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Desai JV, Candida albicans Hyphae: From Growth Initiation to Invasion, Journal of fungi (Basel, Switzerland) 4(1) (2018) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sudbery PE, Growth of Candida albicans hyphae, Nature Reviews Microbiology 9(10) (2011) 737–748. [DOI] [PubMed] [Google Scholar]
- [100].Lotz H, Sohn K, Brunner H, Mühlschlegel Fritz A, Rupp S, RBR1, a Novel pH-Regulated Cell Wall Gene of Candida albicans, Is Repressed by RIM101 and Activated by NRG1, Eukaryotic Cell 3(3) (2004) 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Murad AM, Leng P, Straffon M, Wishart J, Macaskill S, MacCallum D, Schnell N, Talibi D, Marechal D, Tekaia F, d’Enfert C, Gaillardin C, Odds FC, Brown AJ, NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans, Embo j 20(17) (2001) 4742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Xu YF, Zhao X, Glass DS, Absalan F, Perlman DH, Broach JR, Rabinowitz JD, Regulation of yeast pyruvate kinase by ultrasensitive allostery independent of phosphorylation, Mol Cell 48(1) (2012) 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Barelle CJ, Priest DM. Fau - Maccallum Cl, Maccallum NAR. Fau - Gow Dm, Gow FC. Fau - Odds Na, Odds AJP. Fau - Brown Fc, Brown AJ, Niche-specific regulation of central metabolic pathways in a fungal pathogen, (1462–5814 (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Askew C, Sellam A, Epp E, Hogues H, Mullick A, Nantel A, Whiteway M, Transcriptional regulation of carbohydrate metabolism in the human pathogen Candida albicans, PLoS Pathog 5(10) (2009) e1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Arita GS, Faria DR, Capoci IRG, Kioshima ES, Bonfim-Mendonça PS, Svidzinski TIE, Cell wall associated proteins involved in filamentation with impact on the virulence of Candida albicans, Microbiological Research 258 (2022) 126996. [DOI] [PubMed] [Google Scholar]
- [106].Danchik C, Casadevall A, Role of Cell Surface Hydrophobicity in the Pathogenesis of Medically-Significant Fungi, Frontiers in Cellular and Infection Microbiology 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Goswami RR, Pohare SD, Raut JS, Karuppayil SM, Cell surface hydrophobicity as a virulence factor in Candida albicans, Biosciences Biotechnology Research Asia 14(4) (2017) 1503. [Google Scholar]
- [108].Klotz SA, Surface-active properties of Candida albicans, Appl Environ Microbiol 55(9) (1989) 2119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sahni N, Yi S, Daniels KJ, Srikantha T, Pujol C, Soll DR, Genes Selectively Up-Regulated by Pheromone in White Cells Are Involved in Biofilm Formation in Candida albicans, PLOS Pathogens 5(10) (2009) e1000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Rodrigues AG, Mårdh PA, Pina-Vaz C, Martinez-de-Oliveira J, Fonseca AF, Germ tube formation changes surface hydrophobicity of Candida cells, Infect Dis Obstet Gynecol 7(5) (1999) 222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chaffin WL, Candida albicans Cell Wall Proteins, Microbiology and Molecular Biology Reviews 72(3) (2008) 495–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Pérez A, Pedrós B, Murgui A, Casanova M, López-Ribot JL, Martínez JP, Biofilm formation by Candida albicans mutants for genes coding fungal proteins exhibiting the eight-cysteine-containing CFEM domain, FEMS Yeast Research 6(7) (2006) 1074–1084. [DOI] [PubMed] [Google Scholar]
- [113].Weissman Z, Shemer R, Conibear E, Kornitzer D, An endocytic mechanism for haemoglobin-iron acquisition in Candida albicans, Mol Microbiol 69(1) (2008) 201–17. [DOI] [PubMed] [Google Scholar]
- [114].Braun BR, Head MX. Fau - Wang Ws, Wang AD. Fau - Johnson Mx, Johnson AD, Identification and characterization of TUP1-regulated genes in Candida albicans, (0016–6731 (Print)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Araújo D, Henriques M, Silva S, Portrait of Candida Species Biofilm Regulatory Network Genes, Trends in Microbiology 25(1) (2017) 62–75. [DOI] [PubMed] [Google Scholar]
- [116].Morici P, Fais R, Rizzato C, Tavanti A, Lupetti AA-O, Inhibition of Candida albicans Biofilm Formation by the Synthetic Lactoferricin Derived Peptide hLF1–11, (1932–6203 (Electronic)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Fazeli-Nasab B, Sayyed RZ, Mojahed LS, Rahmani AF, Ghafari M, Antonius S, Sukamto, Biofilm production: A strategic mechanism for survival of microbes under stress conditions, Biocatalysis and Agricultural Biotechnology 42 (2022) 102337. [Google Scholar]
- [118].Seliktar D, Nerem RM, Galis ZS, The Role of Matrix Metalloproteinase-2 in the Remodeling of Cell-Seeded Vascular Constructs Subjected to Cyclic Strain, Annals of Biomedical Engineering 29(11) (2001) 923–934. [DOI] [PubMed] [Google Scholar]
- [119].Gupta V, Grande-Allen KJ, Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells, Cardiovascular Research 72(3) (2006) 375–383. [DOI] [PubMed] [Google Scholar]
- [120].Butt RP, Laurent GJ, Bishop JE, Mechanical Load and Polypeptide Growth Factors Stimulate Cardiac Fibroblast Activity, Annals of the New York Academy of Sciences 752(1) (1995) 387–393. [DOI] [PubMed] [Google Scholar]
- [121].Lu Y, Su C, Liu H, Candida albicans hyphal initiation and elongation, Trends in microbiology 22(12) (2014) 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lu Y, Su C, Liu H, A GATA Transcription Factor Recruits Hda1 in Response to Reduced Tor1 Signaling to Establish a Hyphal Chromatin State in Candida albicans, PLOS Pathogens 8(4) (2012) e1002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Zheng X, Wang Y, Wang Y, Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis, The EMBO journal 23(8) (2004) 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, Phan Q-T, Edwards JE, Filler SG, Mitchell AP, Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo, PLoS pathogens 2(7) (2006) e63–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Naglik J, Albrecht A, Bader O, Hube B, Candida albicans proteinases and host/pathogen interactions, Cellular Microbiology 6(10) (2004) 915–926. [DOI] [PubMed] [Google Scholar]
- [126].de Bernardis F, Sullivan PA, Cassone A, Aspartyl proteinases of Candida albicans and their role in pathogenicity, Medical Mycology 39(4) (2001) 303–313. [DOI] [PubMed] [Google Scholar]
- [127].Schaller M, Borelli C, Korting HC, Hube B, Hydrolytic enzymes as virulence factors of Candida albicans, Mycoses 48(6) (2005) 365–377. [DOI] [PubMed] [Google Scholar]
- [128].Souza JGS, Costa RC, Sampaio AA, Abdo VL, Nagay BE, Castro N, Retamal-Valdes B, Shibli JA, Feres M, Barão VAR, Bertolini M, Cross-kingdom microbial interactions in dental implant-related infections: is Candida albicans a new villain?, iScience 25(4) (2022) 103994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kavanaugh Nicole L, Zhang Angela Q, Nobile Clarissa J, Johnson Alexander D, Ribbeck K, Berman J, Mucins Suppress Virulence Traits of Candida albicans, mBio 5(6) (2014) e01911–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Sampaio C, Pessan JP, Nunes GP, Magno MB, Maia LC, Exterkate R, Deng D, Monteiro DR, Are the counts of Streptococcus mutans and Staphylococcus aureus changed in complete denture wearers carrying denture stomatitis? A systematic review with meta-analyses, The Journal of Prosthetic Dentistry (2023). [DOI] [PubMed] [Google Scholar]
- [131].Du Q, Ren B, Zhou X, Zhang L, Xu X, Cross-kingdom interaction between Candida albicans and oral bacteria, Frontiers in Microbiology 13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Satala D, Gonzalez-Gonzalez M, Smolarz M, Surowiec M, Kulig K, Wronowska E, Zawrotniak M, Kozik A, Rapala-Kozik M, Karkowska-Kuleta J, The Role of Candida albicans Virulence Factors in the Formation of Multispecies Biofilms With Bacterial Periodontal Pathogens, Front Cell Infect Microbiol 11 (2021) 765942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.