Abstract
Lung cancer remains the leading cause of cancer-related mortality worldwide. The main issue is the absence of a screening test available in clinical practice; the identification of noninvasive biomarkers is thus an urgent clinical necessity. Currently, low-dose computed tomography (LD-CT) demonstrates a 20% reduction in lung cancer mortality. However, it is not particularly suitable for clinical practice because of its costs, radiation, and false-positive rate. Several studies have therefore focused on research into biomarkers in body fluids. Despite the power of certain molecules to distinguish lung cancer patients from healthy subjects, no biomarker has yet been shown to significantly and reliably influence clinical decisions or to be translated from the laboratory to clinical practice. In this paper, we provide an overview of the peer-reviewed biomedical literature published in the last 10 years on the research regarding biomarkers for the early diagnosis of lung cancer via a comprehensive analysis of the reviews published this past year. Our main objective is to highlight the limitations and strengths of studies on predictive lung cancer biomarkers to stimulate further investigation for early diagnosis. Finally, we discuss future perspectives on managing clinical trials for biomarker research and their integration into clinical practice.
Keywords: early diagnosis, lung cancer, biomarkers, body fluids, research management
1. Introduction
Lung cancer is an aggressive neoplasm and is the leading cause of cancer-related deaths worldwide, with an estimated 1.8 million deaths [1]. The five-year survival rate is associated with the stage of the disease—67% for stage I and 23% for stage III—and the mortality is also strongly associated with late diagnosis [2]. This scenario is aggravated by the absence of a noninvasive screening test, for example, mammography and the fecal occult blood test currently in use for other aggressive neoplasms such as breast cancer and colorectal cancer (survival rate 60–80% respectively). Although low-dose computed tomography (LDCT) has shown a 20% reduction in mortality [3], its application remains limited to the high-risk population (heavy smokers aged 50–80 years), excluding the growing number of young individuals (<50 years) diagnosed with advanced-stage lung cancer [4,5]. Furthermore, the prevalence of false positives leading to unnecessary invasive diagnostic procedures, coupled with the high costs of the methodology, renders it unsuitable for integration into screening initiatives in low-income developing countries [6]. Concerning clinical practice, there is a pressing need for an alternative solution to address the key questions such as noninvasiveness and test reliability while favoring easily obtainable biological samples that can be analyzed with cost-effective tools and reagents, thus making it feasible for adoption even in less industrialized countries. According to the National Institute of Health (NIH), a biomarker is defined as “a characteristic used to measure and evaluate objectively normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention” [7]. In this regard, during the last decade, a considerable number of research studies have focused on the investigation of new technologies for the identification of biomarkers that should be suitable for mass screening, tackling the complexity of the biological and histological heterogeneity of lung cancer. Several biological molecules such as proteins, microRNAs (miRNAs), circulants tumor cells (CTCs), tumor DNA (ctDNA), and volatile organic compounds (VOCs) have been investigated to understand their predictive value. Another key point of early detection is the issue of sample choice. Body fluids such as blood (serum and plasma), urine, stools, exhaled breath, sputum, and saliva meet clinical needs because of their simplicity of collection and noninvasiveness [8,9].
Finally, this review aims to contribute to the literature concerning biomarkers for the early diagnosis of lung cancer. We focused on the state of the art as well as promising biomarkers while discussing the challenges and tips for the discovery of biomarkers and their transition into clinical practice.
Phases for the Discovery of Lung Cancer Biomarkers
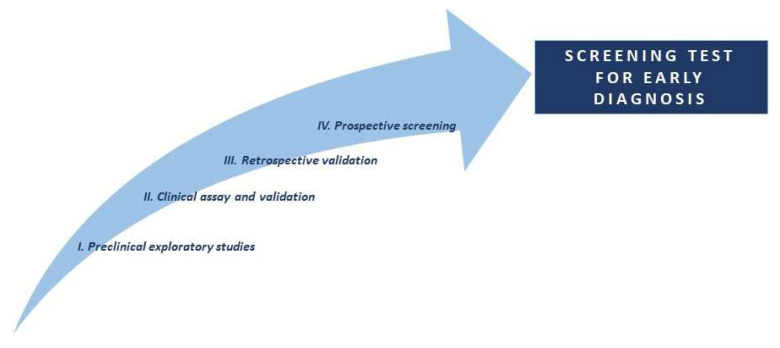
The investigation of biomarkers and their translation into clinical practice are the main issues in the development of a screening test. In 2001, Pepe et al. proposed four consecutive phases to reach the final validation of the biomarkers (Figure 1).
Figure 1.
Diagram of the discovery biomarkers stages: I comparison of tumor tissue with nontumor tissue to identify characteristics unique to the neoplasm, to identify possible biomarkers (e.g., proteins, mRNA, genes). II. Designing a case–control study to assess whether the biomarker is uniquely expressed in both tumor tissue and other noninvasive biological samples (e.g., blood) with the aim of distinguishing cancer patients from healthy subjects. III. Collection of noninvasive samples from cancer patients who have not yet received a diagnosis and from subjects who have not developed cancer. This aspect could confirm the biomarker’s ability to detect cancer in a preclinical stage, making it suitable as a screening test. IV. Individuals who tested positive with biomarkers are referred for further diagnostic evaluation. This phase also helps to identify the number of false-positive cases undergoing further assessment.
The first phase is the identification of the specific biomarkers of the neoplasia by comparing the analysis of tumor and healthy tissue. A crucial factor in the first phase is the stratification of the study cohort, particularly the control group, to ensure that other factors such as age, gender, race, and possibly lifestyle-related characteristics (smoking habits) do not influence the biomarker’s expression, which would result in an overlap between cases and controls. In the second phase, the biomarkers detected in the first phase are searched in samples that do not require invasive procedures for collection (e.g., blood, urine, and respiratory exhalation). In phase III, a comparison is made between individuals who have cancer (not diagnosed at the time of biomarker analysis) and individuals who have not developed the disease. In phase IV, patients who test positive in the screening (using only the biomarker) are referred for further diagnostic evaluation. This phase also helps to identify the number of false-positive cases undergoing further assessment.
Although the research path for a biomarker may seem simple, in practice, it is a highly complex and expensive process. The validation phases require a large number of samples to ensure the appropriate statistical validity of the data, as well as samples that reflect the biological variability of the population [10].
2. Materials and Methods
Selection of Articles
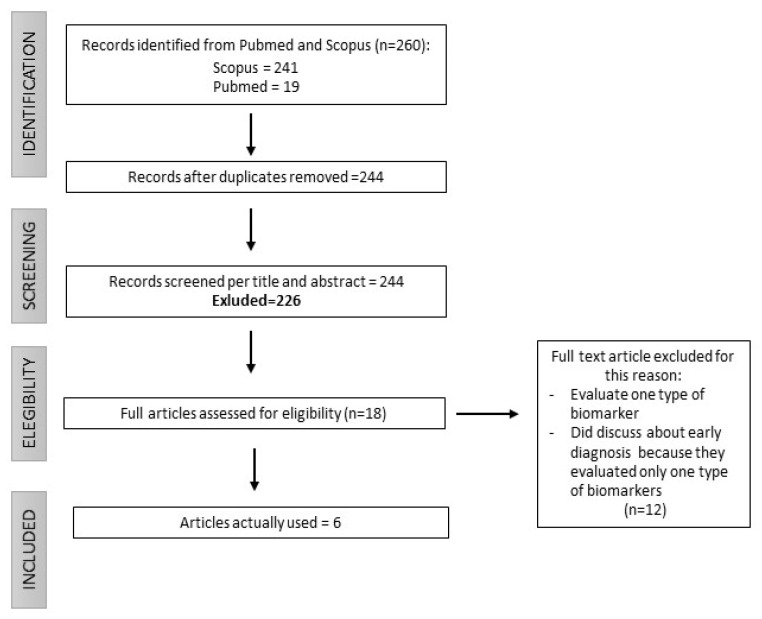
We studied the review publications related to biomarkers for the early diagnosis of lung cancer that were indexed on the PubMed and Scopus scientific literature databases, using the keywords “lung cancer” AND “early diagnosis” AND “biomarkers”. Specifically, we focused on reviews published between 2022 and 2023 that encompassed the literature from the past 10 years. The search produced 260 results; after the removal of duplicates, the review articles were screened by examining the titles and abstracts. After a further screening based on the reading of the entire article, six reviews were selected for the final text (Figure 2).
Figure 2.
Flowchart.
Through the comparative analysis of the reviews, we assessed the biomarkers investigated to date, the types of samples used, and the analytical techniques employed. The ultimate goal was to identify which studies are currently in the validation phase and which biomarkers hold future potential as predictive elements for lung cancer. Additionally, we aimed to shed light on the limitations and key factors that lead to a biomarker reaching the validation phase.
3. Results: Current and Promising Lung Cancer Biomarkers
3.1. Circulating Blood Proteins and Autoantibodies
Circulating proteins can stem from various sources, including the overexpression of cancer cells, increased secretion from diseased tissue, or inflammation linked to malignancy. The proteome has been widely studied in the oncological field to identify serum proteins as potential biomarkers for the early diagnosis of lung cancer. Among the most interesting studies conducted in the last 10 years, CancerSEEK reported a panel of eight proteins (CA-125, CEA, HGF, Myeloperoxidase, OPN, Prolactin, and TIMP-1) effective in distinguishing lung cancer patients from healthy controls [11]. Moreover, the combination with cfDNA increases the sensitivity of this protein panel [11,12]. In another study, a panel of three proteins and one autoantibody (NY-ESO-1) were assessed, and a sensitivity of 71% and specificity of 88% were observed [13]. Mazzone et al. performed a separate clinical trial with the same test (PAULA), demonstrating a sensitivity and specificity of 49% and 96%, respectively [14]. A prospective proteomic study based on two proteins (LG3BP and C163A) integrated with clinical and imaging features showed a sensitivity of 97% and a specificity of 44% [15]. A more recent project involves the development of a 36-protein multiplex assay for the risk assessment of lung cancer. However, more studies should be conducted to demonstrate that these approaches are suitable to implement in clinical practice [9]. Cancer cells stimulate the immune system through the release of protein inducing the production of circulating autoantibodies against tumor-associated antigens (TAAs). EarlyCDT, a panel of seven autoantibodies (p53, NY-ESO-1, CAGE, GBU4-5, HuD, MAGEA4, and SOX2), is commercially available as a blood test to assess the risk of malignancy in people with solid pulmonary nodules [16]. A clinical trial with EarlyCDT on a symptomatic lung cancer patient showed a sensitivity of 41% and a specificity of 91%, and a follow-up study on a high-risk cohort revealed a sensitivity and a specificity of 37% and 91%, respectively [17]. Moreover, Qiang Du et al. tested p53, PGP9.5, SOX2, GAGE7, GBU4-5, MAGEA1, and CAGE and found no statistically significant difference between stages I/II and III/IV, concluding that the test is capable of detecting both early and advanced stages. This phenomenon could be related to the amplification of the immune system. Further studies will be needed to understand the potential prognostic power of proteins and TAAs [18,19]. Their stability in the serum allows them to be detected via immunoenzymatic assays (ELISAs) and makes TAAs possible biomarkers for the early diagnosis of lung cancer [20].
3.2. microRNA (miRNAs)
MiRNAs are small noncoding RNAs that are involved as regulators of gene expression at the post-transcriptional level. They can be aberrantly expressed in many pathological processes as well as in cancer. MiRNAs can be detected in different body fluids such as urine, sputum, and blood (serum and plasma) [21]. In 2002, Calin et al. reported the involvement of microRNAs in lung cancer pathogenesis [22]. They preserve their stability from initial development to metastasis formation, making them appealing biomarkers for the diagnosis and prognosis of lung cancer [8,9,21]. An early study conducted on lung tissue detected 12 miRNAs expressed differently between lung cancer tissue and benign lung tissue [23]. In addition, studies on miRNAs in sputum have shown that the combination of multiple miRNAs can differentiate lung cancer patients from healthy individuals with a sensitivity of 73% to 80% and a specificity of 91% to 96% [24]. Two further studies have compared different miRNA panels in lung cancer patients before and after lung cancer resection and in healthy controls. Le HB et al. showed an increased expression in the serum of miR-21, miR-205, miR-30d, and miR-24 before lung cancer surgery. The same miRNA was upregulated in the serum of early-stage lung cancer patients in comparison to healthy subjects, suggesting their role as a screening biomarker as well as for postoperative disease relapse [25]. Moreover, an 18-month postsurgery follow-up conducted by Leidinger et al. demonstrated a significant reduction in the expression levels of miRNA over time after the surgery [26]. Currently, the miR-Test [27] and MSC (microRNA signature classifier) [28] are undergoing validation. The serum signature of miRNA identified in high-risk subjects enrolling in a screening program with LDCT showed a sensitivity and specificity of 77.8% and 74.8%, respectively [27]. Sozzi et.al. based on 24 miRNA expression ratios stratified the population into low, inter-mediate, or high risk of lung cancer [29]. Their study revealed 87% sensitivity and 81% specificity. Both studies exhibited a reduction in the LDCT false-positive rate [27,28,29].
3.3. Circulating Tumor Cells (CTCs) and Circulating Tumor DNA (ctDNA)
CTCs are derived from the primary tumor mass. During this process, the cells detached from the tumor mass enter the circulatory stream. CTCs were evaluated in a group of 168 patients with chronic obstructive pulmonary disease (COPD) followed with annual CT scans for 4 years. It was found that COPD patients who tested positive for CTCs in the annual CT screening developed lung nodules 1–4 years later. These studies suggest that CTCs could be used for early diagnosis [30,31]. Another study showed that the sensitivity and specificity of CTCs for diagnosing lung cancer were 73.2% and 84.1%, respectively [32], while Wang et al. obtained a sensitivity of 77.7% and a specificity of 89.5%. The comparison between the sensitivity of stage I and stage II revealed that the two values almost overlapped (69.8% and 72.2%) [33]. A study of a larger lung cancer patient cohort demonstrated sensitivity and specificity values similar to other studies, but with the combination of CEA and additional biomolecules, these values could be increased to 84.21% and 88.78%, respectively [32]. Emerging research with negative enrichment fluorescence in situ hybridization methods or the FISH approach demonstrated that the sensitivity and specificity were increased (89–100%) [31,33,34].
ctDNA is a part of cell-free DNA derived from tumor cells. The concentration of ctDNA in plasma varies from 0.01% to 90% [35]. Newman et al. observed a 100% rate of ctDNA in patients with stage II-IV lung cancer, while a 50% rate was observed in early-stage patients [36]. The combination with protein showed a specificity of 99% and a sensitivity of 59% [11,12]. Using deep sequencing (CAPP-seq), Chabon et al. investigated cancer profiling to analyze the ctDNA. This approach demonstrated that ctDNA levels were low in early-stage lung cancer. The same research group developed and validated a machine learning method (Lung-CLiP) using the findings described above in conjunction with other molecular features, and a specificity of 96% was achieved [37]. Phomaryova demonstrated that in lung cancer patients, the concentration of ctDNA is eight times higher than that in healthy individuals [38]. Furthermore, studies report that high concentrations of circulating ctDNA are correlated with a worse clinical outcome [34]. However, ctDNA has demonstrated poor sensitivity, and most patients have levels of less than 0.1%, which is challenging to detect in the blood [9].
3.4. Future Directions and Challenges: Volatile Organic Compounds (VOCs)
Since the 1970s, volatile organic compounds have been used in the field of medicine [39]. Lung cancer studies emphasize the presence of VOCs in exhaled breath [40]. The most widely used approach for the analysis of respiratory VOCs is gas chromatography combined with mass spectrometry (GC/MS) [41]. This method has shown a discriminatory power to detect the specific volatile compounds of lung cancer patients. In one study, GC/MS combined with artificial neural networks showed a sensitivity of 80% and specificity of 91% [42]. In a prospective pilot study, Peled et al. demonstrated the potential of breath analysis to distinguish malignant nodules from benign nodules in high-risk subjects [43]. Another promising measurement device in the field of early diagnosis is the electronic nose (e-nose). This emergent technology is based on the binding of VOCs to different sensors or sensor arrays within handheld devices. The investigators analyzed 214 breath samples using an e-nose with 11 gas sensors. The experimental results revealed an accuracy of 95.75%, a sensitivity of 94.78%, and a specificity of 96.96% [44]. Shlomi D et al. compared patients with benign lung nodules and patients with lung cancer. Moreover, the lung cancer group was divided into two subgroups: patients who harbored the EGFR mutation and lung cancer patients with wild-type EGFR. This study showed the discriminatory power to distinguish the early LC from benign nodules and had 87% accuracy [45]. Two other studies used an e-nose to detect a specific lung cancer signature (in lung cancer patients vs. high-risk healthy controls) with a sensitivity of 81% and specificity of 91% [46]. Moreover, Gasparri et al. demonstrated that an e-nose with 12 sensors has a greater sensitivity to lung cancer at stage I with respect to stage II/III/IV (92% and 58%, respectively) [47]. Additionally, a recent multicentric case–control study yielded a sensitivity of 95% and a specificity of 49% [48].
So far, more than 100 volatile urinary biomarkers have been suggested as being related to cancer. Urinary VOC patterns in cancer patients are often different from those found in the urine samples of control subjects, and these differences also depend on cancer type and stage [49]. In 2023, investigators isolated for the first time five specific VOCs of early-stage lung cancer (I/II) with a specificity and sensitivity of 85% and 90%, respectively [50]. Results with greater robustness are warranted before these may be fully integrated into workflows or incorporated into clinical guidelines.
All suitable biomarkers are shown in Table 1.
Table 1.
Selected lung cancer biomarkers.
| Study | Population | Method | Biomarkers | Main Results |
|---|---|---|---|---|
| Xu BJ [11] | 40 LC 8 HR |
MALDI-MS | Proteins | 75% accuracy |
| Doseeva V [13] | 75 LC 75 HR |
IMMUNOASSAY xMAP | Proteins and autoantibody | 77% sensitivity 80% specificity |
| Mazzone PJ [14] | 155 LC 245 HR |
IMMUNOASSAY MAGPIX | Proteins and autoantibody | 74% sensitivity 80% specificity |
| Silvestri GA [15] | 29 LC 149 HR |
MS | Proteins | 97% sensitivity 44% specificity |
| Chapman CJ [17] | 235 LC 266 HR |
ELISA | Autoantibodies | 92 % accuracy |
| Du Q [18] | 305 LC 74 HR |
ELISA | Autoantibodies | 56.53% sensitivity 91.60% specificity |
| Yu L [24] | 64 LC 58 HR |
qRT-PCR | miRNA | 80.6% sensitivity 91.7% specificity |
| Montani F [27] | 74 LC 115 HR |
NA | miRNA | 77.8% sensitivity 74.8% specificity |
| Sozzi G [29] | 69 LC 870 HR |
PCR | miRNA | 87% sensitivity 81% specificity |
| Yu Y [32] | 153 LC 93 H |
RT-PCR + FISH | CTCs | 67.2% sensitivity for stage I 84.1% specificity |
| Katz RL [33] | 107 LC 100 H |
FISH | CTCs | 89% sensitivity 100% specificity |
| Newman AM [36] | 13LC 13 H |
CAPP-Seq | ctDNA | 96% specificity |
| Ponomaryova AA [38] | 60 LC 32 H |
TaqMan PCR (MSP) | cirDNA | 87% sensitivity 75% specificity |
| Rudnicka J [42] | 86 LC 41 H |
GC/MS | VOCs | 80% sensitivity 91.23% specificity |
| Shlomi D [45] | 89 LC 30 H |
eNOSE | VOCS |
83% accuracy 79% sensitivity 85% specificity |
| McWilliams A [46] | 25 LC 166 H |
eNOSE | VOCs | 80% accuracy |
| Gasparri R [47] | 70 LC 76 H |
eNOSE | VOCs | 81% sensitivity 91% specificity |
| Hanai Y [49] | 20 LC 20 H |
GC/MS | VOCs | 95% sensitivity 70–100% specificity |
| Gasparri R [50] | 46 LC 81 H |
GC/MS | VOCs | 85% sensitivity 90% specificity |
LC = lung cancer patients; H = healthy subjects.
4. Discussion
The early diagnosis of lung cancer ranks among the most crucial health issues. The five-year survival is strongly correlated with stage (90% stage I vs. 10% stage IV) [51]. Considerable advances have been made in metastatic lung cancer diagnosis and treatment by finding numerous disease subtypes defined by specific oncogenic driver mutations (EGFR, ALK, ROS1, BRAF, HER2, MET, RET or KRASG12C, and PD-L1). This has led to the development of a range of molecularly targeted therapies, which have exerted a significant impact on patient survival rates [52]. By contrast, although numerous studies have been conducted to search for useful biomarkers for early diagnosis, none of the investigated molecules have been incorporated into clinical practice. Currently, in a clinical setting, serum tumor markers are increasingly being used as a supplement to radiological examinations (CT and PET) for therapy monitoring and disease recurrence. Studies on lung cancer patients have demonstrated that proteins such as CYFRA 21-1, CEA, and NSE can be used to determine the lung cancer subtype or are correlated with the stage and prognosis of the disease. Additionally, the integration of ctDNA into the protein panel has shown potential for therapy monitoring [11,13,14,15]. However, the serum concentration of these biomarkers also increases in the presence of other malignancies, rendering them nonspecific. Thus, despite the emerging evidence of their potential in early diagnosis and treatment monitoring, they have not yet been incorporated into clinical guidelines. Further studies will be necessary to determine whether proteomic analyses could be cost-effective for lung cancer screening, even in low-income countries [9]. On the other hand, TAAs can be detected in the blood by means of the ELISA assay, one of the most specific and straightforward tests for detecting circulating biomolecules, widely used in research settings and clinics worldwide [53]. Furthermore, one study reported that lung cancer patients have TAA levels that are 30% higher than those in healthy individuals [54]. Du Q et al. revealed that TAA levels do not differ among different stages of the disease, and they can be used to detect both early and advanced stages of lung cancer, suggesting that this phenomenon may be related to immune amplification. This characteristic could be crucial for early diagnosis [18]. However, studies described in the peer-reviewed biomedical literature have shown a low test sensitivity (30–40%), limiting their use in detecting high-risk subjects [16,17,18,19]. Therefore, additional studies will be needed to understand the combination of antibodies with greater sensitivity and specificity for early-stage lung cancer.
Concerning microRNAs, a significant number of research studies have explored their potential role as a biomarker in the early diagnosis of lung cancer. MicroRNA has been studied alone or in combination with other biomolecules, showing high sensitivity and specificity in distinguishing lung cancer subjects from healthy controls [24,26,29]. As described in the Results section, the miR-Test and MSC are the only studies currently ongoing and are pending validation. Moreover, the miR-Test has presented accuracy, sensitivity, and specificity of less than 80%, as well as detection sensitivity between stage I (69%) and stages II/III (71%), which is not particularly significant [27]. At the same time, the MSC has shown a greater effect only in combination with CT [29]. All these miRNA studies conducted so far suffer some degree of limitation such as a small sample size, the selection and inclusion of patients in advanced stages, epidemiological diversity, and poor homogeneity in experimental protocols [8,19].
Other interesting biomolecules are the CTCs. These are already used in thoracic oncology for the molecular characterization of lung cancer, suggesting their potential role in the early diagnosis of lung cancer. Indeed, recent data have shown that lung cancer patients have CTC levels ten times higher than patients with other types of cancer, and their presence in the circulation is associated with a worse prognosis [35]. However, CTC isolation and detection remain complex procedures that require the development of new technologies. Furthermore, high specificity (96–100%) and low sensitivity (26–68%) have been reported in the literature [32,33]. ctDNA, like CTCs, has a circulating concentration ranging from 0.01% to 90% depending on the lung cancer burden and its progression [35]. As demonstrated in the literature, its concentration is much higher in advanced stages than in early stages (100% vs. 50%), resulting in levels eight times higher than in healthy individuals [11,12,38]. While these characteristics make ctDNA a promising biomarker for early diagnosis, its low plasma quantity and half-life have challenged its clinical applicability. Further research on biological samples other than plasma could be useful in assessing the future applicability of ctDNA. However, future studies will be necessary to explore the diagnostic power of CTCs and ctDNA for early lung cancer diagnosis and their utility in clinical practice.
During the last decade, the volatolomic profile has garnered much interest in the field of the early diagnosis of lung cancer. Various types of VOCs can be detected in exhaled breath because they have low solubility in the blood and are thus excreted in the breath within minutes of their formation. Several analytical methods for VOC detection have been recently developed. The most widely used analytical method is gas chromatography–mass spectrometry (GC/MS). However, MS-based analysis is expensive and requires skilled staff. Other innovative and cost-effective tools are available for potential future applications in clinical practice, such as artificial sensors (electronic nose). Studies have demonstrated the ability of the electronic nose (e-nose) to distinguish between lung cancer patients and healthy subjects, particularly with greater sensitivity in diagnosing stage I lung cancer compared to stages II, III, or IV. Furthermore, the combined use of GC/MS and e-nose could enhance the method’s specificity, sensitivity, and accuracy. Recent research has unveiled the potential of urinary volatile compounds for early nonsmall cell lung cancer (NSCLC) diagnosis, indicating the ability of GC/MS to detect not only specific volatile compounds related to the lung cancer group but also to discriminate specific compounds associated with the early stages. Future research will be necessary to implement the results obtained thus far.
5. Study Limitations
Literature studies underline the potential of several biomarkers such as circulating blood proteins and autoantibodies, miRNA, CTCs, ctDNA, and VOCs to differentiate lung cancer patients from healthy subjects, through noninvasive body fluid analysis. However, some limitations such as a small sample size, limited stratification of the population including few early stages of lung cancer patients [31], the lack of a long follow-up, and the lack of a standard approach limit scientific robustness and their translation in clinical practice.
6. Future Perspectives
The near future should be focused on establishing a multicenter clinical trial involving a large number of participants, with the aim of improving the population stratification involved in the study. The group of lung cancer patients should be stratified based on the radiological characteristics of the nodule (e.g., ground-glass opacity, solid nodule, and spiculated nodule) alongside histological factors (TNM staging), with a particular emphasis on early stages. Simultaneously, the group of high-risk healthy subjects should be stratified based on their family cancer history, smoking history, and existing lung conditions. Furthermore, studies should include both prospective and retrospective analyses, along with the planning of a follow-up to monitor the behavior of biomarkers in high-risk healthy subjects, in order to identify the potential presence of a malignant lung nodule in individuals who have not yet received a diagnosis.
The results should be analyzed by establishing a universal database that is accessible to a global network of research centers dedicated to early lung cancer diagnosis. Data sharing is paramount for resource synergy and optimization, reducing costs in biomarker research. Ideally, a consortium would be established, whereby biomarkers for mass population screening are discussed and evaluated. Each participant should use a variety of approaches and data collection methods and make them transparently available in the public domain. Furthermore, great technological strides have witnessed the development and deployment of artificial intelligence and machine learning tools that can be used to process, overlay, and integrate molecular biomarkers with clinical and epidemiological data.
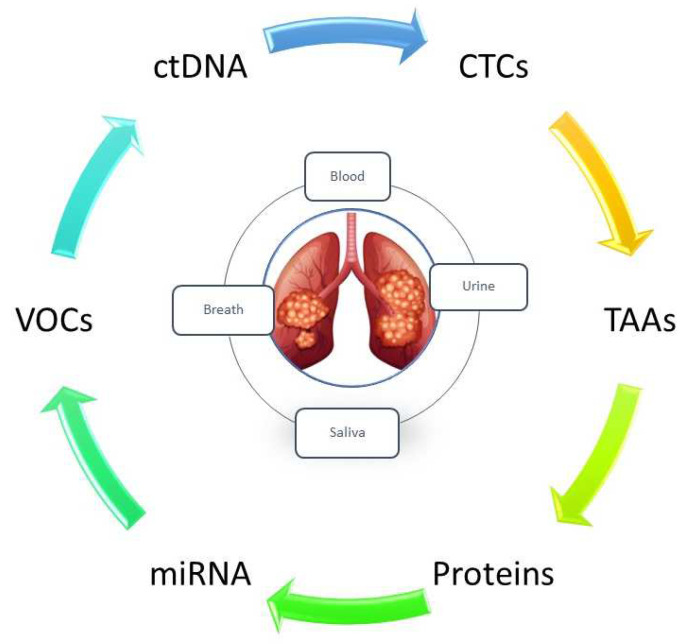
The milestone we aspire to reach is a noninvasive and relatively cost-effective diagnostic tool that can significantly impact clinical decision making. This tool should be robust and readily implementable in the clinical practice of all healthcare facilities, even those in resource-constrained countries (Figure 3).
Figure 3.
Overview of liquid biopsy and promising biomarkers for the early diagnosis of lung cancer.
7. Conclusions
In conclusion, despite the potential role demonstrated by the described biomarkers in distinguishing patients with lung cancer from healthy individuals, to date, there are no biomarkers available in clinical practice for the early diagnosis of lung cancer. Future research should focus more on population stratification and the use of standardizable and reproducible methodologies, as well as on the organization of long-term follow-ups, particularly for the population at high risk. Collaboration among multiple researchers could play a fundamental role.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Leiter A., Veluswamy R.R., Wisnivesky J.P. The global burden of lung cancer: Current status and future trends. Nat. Rev. Clin. Oncol. 2023;20:624–639. doi: 10.1038/s41571-023-00798-3. [DOI] [PubMed] [Google Scholar]
- 3.Wood D.E., Kazerooni E.A., Aberle D., Berman A., Brown L.M., Eapen G.A., Ettinger D.S., Ferguson J.S., Hou L., Kadaria D., et al. NCCN Guidelines® Insights: Lung Cancer Screening, Version 1.2022. J. Natl. Compr. Cancer Netw. 2022;20:754–764. doi: 10.6004/jnccn.2022.0036. [DOI] [PubMed] [Google Scholar]
- 4.Dingillo G., Bassiri A., Badrinathan A., Alvarado C.E., Sinopoli J., Tapias L., Linden P., Towe C.W. Lung Cancer in Young Patients is Associated With More Advanced Disease but Better Overall Survival. J. Surg. Res. 2023;292:307–316. doi: 10.1016/j.jss.2023.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Salcedo P., Wilson D.O., De-Torres J.P., Weissfeld J.L., Berto J., Campo A., Alcaide A.B., Pueyo J., Bastarrika G., Seijo L.M., et al. Improving selection criteria for lung cancer screening. The potential role of emphysema. Am. J. Respir. Crit. Care Med. 2015;191:924–931. doi: 10.1164/rccm.201410-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang H.-T., Wang P.-H., Chen W.-F., Lin C.-J. Risk Assessment of Early Lung Cancer with LDCT and Health Examinations. Int. J. Environ. Res. Public Health. 2022;19:4633. doi: 10.3390/ijerph19084633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biomarkers Definitions Working Group. Atkinson A.J., Jr., Colburn W.A., DeGruttola V.G., DeMets D.L., Downing G.J., Hoth D.F., Oates J.A., Peck C.C., Spilker B.A., et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 8.Saman H., Raza A., Patil K., Uddin S., Crnogorac-Jurcevic T. Non-Invasive Biomarkers for Early Lung Cancer Detection. Cancers. 2022;14:5782. doi: 10.3390/cancers14235782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casagrande G.M.S., Silva M.d.O., Reis R.M., Leal L.F. Liquid Biopsy for Lung Cancer: Up-to-Date and Perspectives for Screening Programs. Int. J. Mol. Sci. 2023;24:2505. doi: 10.3390/ijms24032505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepe M.S., Etzioni R., Feng Z., Potter J.D., Thompson M.L., Thornquist M., Winget M., Yasui Y. Phases of biomarker development for early detection of cancer. JNCI J. Natl. Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 11.Xu B.J., Gonzalez A.L., Kikuchi T., Yanagisawa K., Massion P.P., Wu H., Mason S.E., Olson S.J., Shyr Y., Carbone D.P., et al. MALDI-MS derived prognostic protein markers for resected non-small cell lung cancer. Proteom. Clin. Appl. 2008;2:1508–1517. doi: 10.1002/prca.200800094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen J.D., Li L., Wang Y., Thoburn C., Afsari B., Danilova L., Douville C., Javed A.A., Wong F., Mattox A., et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359:926–930. doi: 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doseeva V., Colpitts T., Gao G., Woodcock J., Knezevic V. Performance of a multiplexed dual analyte immunoassay for the early detection of non-small cell lung cancer. J. Transl. Med. 2015;13:55. doi: 10.1186/s12967-015-0419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzone P.J., Wang X.-F., Han X., Choi H., Seeley M., Scherer R., Doseeva V. Evaluation of a Serum Lung Cancer Biomarker Panel. Biomark. Insights. 2018;13:1177271917751608. doi: 10.1177/1177271917751608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvestri G.A., Tanner N.T., Kearney P., Vachani A., Massion P.P., Porter A., Springmeyer S.C., Fang K.C., Midthun D., Mazzone P.J., et al. Assessment of Plasma Proteomics Biomarker’s Ability to Distinguish Benign From Malignant Lung Nodules: Results of the PANOPTIC (Pulmonary Nodule Plasma Proteomic Classifier) Trial. Chest. 2018;154:491–500. doi: 10.1016/j.chest.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jett J.R., Dyer D., Kern J., Rollins D., Phillips M. Screening for lung cancer with the EarlyCDT-Lung and computed tomography. J. Thorac. Oncol. 2015;10:S306. [Google Scholar]
- 17.Chapman C.J., Healey G.F., Murray A., Boyle P., Robertson C., Peek L.J., Allen J., Thorpe A.J., Hamilton-Fairley G., Parsy-Kowalska C.B., et al. EarlyCDT®-Lung test: Improved clinical utility through additional autoantibody assays. Tumor Biol. 2012;33:1319–1326. doi: 10.1007/s13277-012-0379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Q., Yu R., Wang H., Yan D., Yuan Q., Ma Y., Slamon D., Hou D., Wang H., Wang Q. Significance of tumor-associated autoantibodies in the early diagnosis of lung cancer. Clin. Respir. J. 2018;12:2020–2028. doi: 10.1111/crj.12769. [DOI] [PubMed] [Google Scholar]
- 19.Paez R., Kammer M.N., Tanner N.T., Shojaee S., Heideman B.E., Peikert T., Balbach M.L., Iams W.T., Ning B., Lenburg M.E., et al. Update on Biomarkers for the Stratification of Indeterminate Pulmonary Nodules. Chest. 2023;164:1028–1041. doi: 10.1016/j.chest.2023.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solassol J., Maudelonde T., Mange A., Pujol J.-L. Clinical relevance of autoantibody detection in lung cancer. J. Thorac. Oncol. 2011;6:955–962. doi: 10.1097/JTO.0b013e318215a0a4. [DOI] [PubMed] [Google Scholar]
- 21.Marmor H.N., Zorn J.T., Deppen S.A., Massion P.P., Grogan E.L. Biomarkers in Lung Cancer Screening: A Narrative Review. Curr. Chall. Thorac. Surg. 2023;5:5. doi: 10.21037/ccts-20-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calin G.A., Dumitru C.D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K., et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanaihara N., Caplen N.J., Bowman E., Seike M., Kumamoto K., Yi M., Stephens R.M., Okamoto A., Yokota J., Tanaka T., et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Yu L., Todd N.W., Xing L., Xie Y., Zhang H., Liu Z., Fang H., Zhang J., Katz R.L., Jiang F. Early detection of lung adenocarcinoma in sputum by a panel of microRNA markers. Int. J. Cancer. 2010;127:2870–2878. doi: 10.1002/ijc.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le H.-B., Zhu W.-Y., Chen D.-D., He J.-Y., Huang Y.-Y., Liu X.-G., Zhang Y.-K. Evaluation of dynamic change of serum miR-21 and miR-24 in pre- and post-operative lung carcinoma patients. Med. Oncol. 2012;29:3190–3197. doi: 10.1007/s12032-012-0303-z. [DOI] [PubMed] [Google Scholar]
- 26.Leidinger P., Keller A., Backes C., Huwer H., Meese E. MicroRNA expression changes after lung cancer resection: A follow-up study. RNA Biol. 2012;9:900–910. doi: 10.4161/rna.20107. [DOI] [PubMed] [Google Scholar]
- 27.Montani F., Marzi M.J., Dezi F., Dama E., Carletti R.M., Bonizzi G., Bertolotti R., Bellomi M., Rampinelli C., Maisonneuve P., et al. miR-Test: A blood test for lung cancer early detection. JNCI J. Natl. Cancer Inst. 2015;107:djv063. doi: 10.1093/jnci/djv063. [DOI] [PubMed] [Google Scholar]
- 28.Pastorino U., Boeri M., Sestini S., Sabia F., Milanese G., Silva M., Suatoni P., Verri C., Cantarutti A., Sverzellati N., et al. Baseline computed tomography screening and blood microRNA predict lung cancer risk and define adequate intervals in the BioMILD trial. Ann. Oncol. 2022;33:395–405. doi: 10.1016/j.annonc.2022.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Sozzi G., Boeri M., Rossi M., Verri C., Suatoni P., Bravi F., Roz L., Conte D., Grassi M., Sverzellati N., et al. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: A correlative MILD trial study. J. Clin. Oncol. 2014;32:768–773. doi: 10.1200/JCO.2013.50.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilie M., Hofman V., Long E., Selva E., Vignaud J.-M., Padovani B., Mouroux J., Marquette C.H., Hofman P. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS ONE. 2014;9:e111597. doi: 10.1371/journal.pone.0111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang L., Li J., Zhang R. Liquid biopsy for early diagnosis of non-small cell lung carcinoma: Recent research and detection technologies. Biochim. Biophys. Acta Rev. Cancer. 2022;1877:188729. doi: 10.1016/j.bbcan.2022.188729. [DOI] [PubMed] [Google Scholar]
- 32.Yu Y., Chen Z., Dong J., Wei P., Hu R., Zhou C., Sun N., Luo M., Yang W., Yao R., et al. Folate receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl. Oncol. 2013;6:697–702. doi: 10.1593/tlo.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz R.L., Zaidi T.M., Pujara D., Shanbhag N.D., Truong D., Patil S., Mehran R.J., El-Zein R.A., Shete S.S., Kuban J.D. Identification of circulating tumor cells using 4-color fluorescence in situ hybridization: Validation of a noninvasive aid for ruling out lung cancer in patients with low-dose computed tomography–detected lung nodules. Cancer Cytopathol. 2020;128:553–562. doi: 10.1002/cncy.22278. [DOI] [PubMed] [Google Scholar]
- 34.Lei Y., Sun N., Zhang G., Liu C., Lu Z., Huang J., Zhang C., Zang R., Che Y., Mao S., et al. Combined detection of aneuploid circulating tumor-derived endothelial cells and circulating tumor cells may improve diagnosis of early stage non-small-cell lung cancer. Clin. Transl. Med. 2020;10:e128. doi: 10.1002/ctm2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herath S., Rad H.S., Radfar P., Ladwa R., Warkiani M., O’byrne K., Kulasinghe A. The Role of Circulating Biomarkers in Lung Cancer. Front. Oncol. 2022;11:801269. doi: 10.3389/fonc.2021.801269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newman A.M., Bratman S.V., To J., Wynne J.F., Eclov N.C.W., Modlin L.A., Liu C.L., Neal J.W., Wakelee H.A., Merritt R.E., et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chabon J.J., Hamilton E.G., Kurtz D.M., Esfahani M.S., Moding E.J., Stehr H., Schroers-Martin J., Nabet B.Y., Chen B., Chaudhuri A.A., et al. Integrating genomic features for non-invasive early lung cancer detection. Nature. 2020;580:245–251. doi: 10.1038/s41586-020-2140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponomaryova A.A., Rykova E.Y., Cherdyntseva N.V., Skvortsova T.E., Dobrodeev A.Y., Zav’yalov A.A., Bryzgalov L.O., Tuzikov S.A., Vlassov V.V., Laktionov P.P. Potentialities of aberrantly methylated circulating DNA for diagnostics and post-treatment follow-up of lung cancer patients. Lung Cancer. 2013;81:397–403. doi: 10.1016/j.lungcan.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Sinues P.M.-L., Zenobi R., Kohler M. Analysis of the exhalome: A diagnostic tool of the future. Chest. 2013;144:746–749. doi: 10.1378/chest.13-1106. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt F., Kohlbrenner D., Malesevic S., Huang A., Klein S.D., Puhan M.A., Kohler M. Mapping the landscape of lung cancer breath analysis: A scoping review (ELCABA) Lung Cancer. 2022;175:131–140. doi: 10.1016/j.lungcan.2022.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Chen X., Muhammad K.G., Madeeha C., Fu W., Xu L., Hu Y., Liu J., Ying K., Chen L., Yurievna G.O. Calculated indices of volatile organic compounds (VOCs) in exhalation for lung cancer screening and early detection. Lung Cancer. 2021;154:197–205. doi: 10.1016/j.lungcan.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Rudnicka J., Kowalkowski T., Buszewski B. Searching for selected VOCs in human breath samples as potential markers of lung cancer. Lung Cancer. 2019;135:123–129. doi: 10.1016/j.lungcan.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Peled N., Hakim M., Bunn P.A., Miller Y.E., Kennedy T.C., Mattei J., Mitchell J.D., Hirsch F.R., Haick H. Non-invasive breath analysis of pulmonary nodules. J. Thorac. Oncol. 2012;7:1528–1533. doi: 10.1097/JTO.0b013e3182637d5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L., Li W., He Z., Chen W., Liu H., Chen K., Pi X. Detection of lung cancer with electronic nose using a novel ensemble learning framework. J. Breath. Res. 2021;15:026014. doi: 10.1088/1752-7163/abe5c9. [DOI] [PubMed] [Google Scholar]
- 45.Shlomi D., Abud M., Liran O., Bar J., Gai-Mor N., Ilouze M., Onn A., Ben-Nun A., Haick H., Peled N. Detection of Lung Cancer and EGFR Mutation by Electronic Nose System. J. Thorac. Oncol. 2017;12:1544–1551. doi: 10.1016/j.jtho.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 46.McWilliams A., Beigi P., Srinidhi A., Lam S., MacAulay C.E. Sex and Smoking Status Effects on the Early Detection of Early Lung Cancer in High-Risk Smokers Using an Electronic Nose. IEEE Trans. Biomed. Eng. 2015;62:2044–2054. doi: 10.1109/TBME.2015.2409092. [DOI] [PubMed] [Google Scholar]
- 47.Gasparri R., Santonico M., Valentini C., Sedda G., Borri A., Petrella F., Maisonneuve P., Pennazza G., D’amico A., Di Natale C., et al. Volatile signature for the early diagnosis of lung cancer. J. Breath. Res. 2016;10:016007. doi: 10.1088/1752-7155/10/1/016007. [DOI] [PubMed] [Google Scholar]
- 48.Kort S., Brusse-Keizer M., Schouwink H., Citgez E., de Jongh F.H., van Putten J.W., Borne B.v.D., Kastelijn E.A., Stolz D., Schuurbiers M., et al. Diagnosing Non-Small Cell Lung Cancer by Exhaled Breath Profiling Using an Electronic Nose. Chest. 2022;163:697–706. doi: 10.1016/j.chest.2022.09.042. [DOI] [PubMed] [Google Scholar]
- 49.Hanai Y., Shimono K., Matsumura K., Vachani A., Albelda S., Yamazaki K., Beauchamp G.K., Oka H. Urinary volatile compounds as biomarkers for lung cancer. Biosci. Biotechnol. Biochem. 2012;76:679–684. doi: 10.1271/bbb.110760. [DOI] [PubMed] [Google Scholar]
- 50.Gasparri R., Capuano R., Guaglio A., Caminiti V., Canini F., Catini A., Sedda G., Paolesse R., Di Natale C., Spaggiari L. Volatolomic urinary profile analysis for diagnosis of the early stage of lung cancer. J. Breath. Res. 2022;16:046008. doi: 10.1088/1752-7163/ac88ec. [DOI] [PubMed] [Google Scholar]
- 51.Ning J., Ge T., Jiang M., Jia K., Wang L., Li W., Chen B., Liu Y., Wang H., Zhao S., et al. Early diagnosis of lung cancer: Which is the optimal choice? Aging. 2021;13:6214–6227. doi: 10.18632/aging.202504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otano I., Ucero A.C., Zugazagoitia J., Paz-Ares L. At the crossroads of immunotherapy for oncogene-addicted subsets of NSCLC. Nat. Rev. Clin. Oncol. 2023;20:143–159. doi: 10.1038/s41571-022-00718-x. [DOI] [PubMed] [Google Scholar]
- 53.Tabatabaei M.S., Ahmed M. Enzyme-Linked Immunosorbent Assay (ELISA) Methods Mol. Biol. 2022;2508:115–134. doi: 10.1007/978-1-0716-2376-3_10. [DOI] [PubMed] [Google Scholar]
- 54.Lubin R., Zalcman G., Bouchet L., Trédaniel J., Legros Y., Cazals D., Hirsch A., Soussi T. Serum p53 antibodies as early markers of lung cancer. Nat. Med. 1995;1:701–702. doi: 10.1038/nm0795-701. [DOI] [PubMed] [Google Scholar]