Abstract
Histoplasma capsulatum is a pathogenic fungus that exists in two distinct forms. The saprophytic mycelial phase inhabits moist soil environments; once inhaled, hyphae and conidia convert to a unicellular yeast phase that is capable of parasitizing macrophage phagolysosomes. Yeasts cultures, but not mycelial cultures, release large quantities of a calcium-binding protein (CBP) which may be important in calcium acquisition during intracellular parasitism. In this study, we show that the gene encoding CBP (CBP1) is transcriptionally regulated. To identify promoter sequences that are important for yeast phase-specific activity, we created a series of fusions between successively truncated CBP1 5′ untranslated regulatory sequences and the Eschericha coli lacZ gene. The fusions were constructed on a telomeric shuttle plasmid that can replicate autonomously in the fungus. By assaying for β-galactosidase activity from H. capsulatum transformants, we identified a 102-bp region that mediates promoter activation and yeast phase promoter activity. Base pair substitution analysis suggests that the sequences between 839 and 877 bp upstream of the start codon are the most important for this positive regulation.
The dimorphic fungus Histoplasma capsulatum causes a pulmonary infection that can develop into potentially life-threatening disseminated disease, especially in an immunocompromised individual. Infection occurs when the host inhales aerosolized conidia and hyphal fragments from contaminated soil. In the 37°C environment of the lung, the fungus converts to a unicellular yeast phase. This form of the organism is able to parasitize alveolar macrophages, which can act as a vehicle for dissemination throughout the host (6). The transition from the saprophytic mycelial phase to the parasitic yeast phase is thought to be an essential prerequisite for pathogenicity.
H. capsulatum yeasts reside within macrophage phagolysosomes (7), where the environment is unsuitable for survival of most microorganisms. Microbes that live in a phagolysosome must develop mechanisms to deal with this hostile environment. Little is known about the mechanisms by which H. capsulatum survives and proliferates within this particular niche, but it is likely that some of these mechanisms are yeast phase specific. The first such candidate to be identified is an abundant, extracellular protein that is produced by yeast cultures but not by mycelial cultures (3). This protein is called calcium-binding protein (CBP) because of its ability to bind 45CaCl2 in vitro, and we have hypothesized that this protein is important for Ca2+ acquisition by yeasts inside phagolysosomes. In support of this hypothesis, we found that yeasts, unlike mycelia, grow well in a low-Ca2+ environment (3) and that purified CBP facilitates the uptake of 45CaCl2 by yeasts (2). Calcium levels in phagolysosomes have never been measured directly, but studies with Salmonella typhimurium suggest that calcium concentrations in this compartment are low (8). S. typhimurium has a two-component regulatory system that represses gene expression in response to high concentrations of magnesium or calcium. These repressed genes are normally expressed when S. typhimurium inhabits macrophage phagolysosomes, implying that this environment is low in both magnesium and calcium.
A limited number of Histoplasma genes which are transcribed only in the yeast phase have been identified (5, 9, 12, 15), but little is known about the mechanisms of their regulation. Until recently, the molecular tools necessary for the characterization of promoter regulatory sequences have not been available. Our laboratory has developed an Escherichia coli-H. capsulatum shuttle plasmid which exists as a circular replicon in E. coli and as a linear, telomeric plasmid in H. capsulatum (17). The plasmid is maintained in H. capsulatum by uracil prototrophy selection. A UV-mutagenized, uracil-auxotrophic strain, which has a defect that can be complemented by the Podospora anserina URA5 gene (PaURA5), serves as the host strain (21). Plasmids are linearized to expose the telomeric sequences at the ends of the DNA and then are introduced into the host strain by an efficient electroporation procedure. Nearly all transformants retain the DNA as linear, extrachromosomal plasmids that replicate at a high copy number and are unmodified except for the addition of extra telomeric sequences at the ends (17). Using this plasmid as a vector, our laboratory has also developed lacZ as a reporter gene in H. capsulatum (18). The original construct showed that an in-frame insertion of the E. coli lacZ gene into the H. capsulatum URA5 gene (HcURA5) (18a) resulted in the production of β-galactosidase.
In this study, we examine the genetic basis of phase-specific production of CBP by H. capsulatum. We show by Northern blot analysis that the CBP1 gene is transcriptionally regulated. To identify the 5′ untranslated region (UTR) sequences that promote yeast phase-specific activity, we constructed a shuttle plasmid with a promoterless lacZ gene. Various versions of the CBP1 5′ UTR sequence were cloned upstream of lacZ so that their ability to promote lacZ expression could be evaluated in H. capsulatum. We show that a 102-bp region mediates promoter activation and that promoter sequences up to and including this region are sufficient for yeast phase-specific promoter activity. Further base substitution analysis suggests that the sequences between -839 and -877 are the most important for activation. This study provides the first functional evidence for positive regulation of a phase-specific gene in a dimorphic fungal pathogen.
MATERIALS AND METHODS
H. capsulatum strains and culture conditions.
A UV-mutagenized (20) isolate of ATCC strain G217B was used for all experiments described here. This strain designated G217Bura5-23 is a uracil auxotroph that has the same growth rate as its parent and parasitizes macrophages in vitro as effectively as its parent (15a). Growth of this strain requires supplementation with 100 μg of uracil per ml of medium.
H. capsulatum yeasts were grown at 37°C in HMM broth or on HMM plates (19). Liquid cultures were grown in an orbital shaker in an atmosphere of 95% air–5% CO2 and maintained by a 1:25 dilution into fresh HMM every 3 days. Mycelial cultures were grown in the same media but at 25°C. For the Northern blot experiments, 30-ml broth cultures were inoculated heavily with mycelia and incubated for 8 days on an orbital shaker in ambient air. The cultures used for the β-galactosidase assays were inoculated with a small mycelial patch and incubated in the same manner for 4 weeks.
Preparation of total RNA from H. capsulatum.
Yeast RNA was extracted by the method of Lodge et al. (14). Briefly, a 3-day 25-ml HMM broth culture was harvested by centrifugation and washed in an equal volume of ice-cold RNA buffer (200 mM Tris-HCl, 50 mM NaCl, 10 mM EDTA [pH 7.4]). After resuspension in 8 ml of RNA buffer, we added 15 ml of a phenol-chloroform-isoamyl alcohol solution (24:24:1) along with 5 ml of acid-washed glass beads. This mixture was vortexed for 2 min with periodic chilling on ice. After centrifugation at 10,000 × g for 10 min, the aqueous phase was recovered and then extracted two more times with 15 ml of the phenol-chloroform-isoamyl alcohol solution. Total RNA from the supernatant was precipitated by addition of an equal volume of 100% ethanol and centrifugation at 12,000 × g for 10 min. The precipitated RNA was washed two times with 75% ethanol, dried, resuspended in diethyl pyrocarbonate-treated deionized water, and stored at −20°C.
Mycelial RNA extracts were prepared as described by Harris et al. (11). Organisms were grown in HMM broth, pelleted at 500 × g, washed, and resuspended in UNET buffer (8 M urea, 0.15 M NaCl, 1 mM EDTA, 0.1 M Tris [pH 7.5]). Phenol was added to a final concentration of 20%, and the organisms were Dounce homogenized with periodic chilling on ice. Sodium dodecyl sulfate was added to a final concentration of 2%, and the mixture was extracted repeatedly with phenol. RNA was precipitated, dried, and resuspended as described above.
Northern analysis.
Yeast and mycelial RNAs (18 μg) were separated by 0.9% agarose gel electrophoresis. RNA was transferred to a Nytran membrane (Schleicher and Schuell, Inc., Keene, N.H.), using a vacuum blotter (Bio-Rad Laboratories, Hercules, Calif.). The filter was probed with either H. capsulatum CBP1 coding sequence or ribosomal DNA sequences (17a) which were labeled by random priming (Rediprime kit; Amersham Life Science, Inc., Arlington Heights, Ill.). After overnight hybridization at 68°C and subsequent washing of the filters, radioactivity was detected with a Bio-Rad phosphorimager.
Primer extension.
Primer extension reactions were done with the Promega (Madison, Wis.) Avian myeloblastosis virus reverse transcriptase primer extension system. Approximately 18 μg of total RNA, prepared from a G217Bura5-23 transformant which carried either pJBP40 or pJBP41, was used in the reaction. Primer LACZPE was used to identify the 5′ end of the CBP1-lacZ fusion transcripts, and primer CBPPE was used to map the 5′ end of the endogenous CBP1 transcript (Table 1). Sequencing reactions were performed with the T7 Sequenase version 2.0 DNA sequencing kit (Amersham Life Science), using primer CBPPE and plasmid pJB1 (2) as the template. Radioactivity was detected using a Bio-Rad phosphorimager.
TABLE 1.
Primers used
| Name | Sequence (5′-3′) |
|---|---|
| CBPPE | GGAGCGATAACCTTGGAGAAAAG |
| LACZPE | CGACGGGATCTTCGACTCTAGA |
| PUC19F | ATACCGCATCAGGCGCC |
| UCURAT | CCGAATTGTGCAGGTCGACTCTAGAGG |
| URATERMF | ACCTGCACAATTCGGCGTCCCAACTGC |
| SPH1URA | ACATGCATGCGGATGTGCTGTATCGCATCG |
| BAMURAP | CGGGATCCAAGCTTTATATAAACTTGAGAG |
| XBAURAP | GCTCTAGACATTGTAGCAATTCCCGCT |
| CBP1 | CGGGATCCGAACGAACCAATCAGAACAC |
| CBP5 | GCTCTAGACATTTTGAATGACGAAGTGG |
| CBP8 | CGGGATCCGCGTAATGAAAAACGAAGTCG |
| CBP9 | CGGGATCCCCTATTAAATATCGTACAGTA |
| CBP10 | CGGGATCCTACCCCGCCATAGCGAATTG |
| CBP12 | CGGGATCCTTATACTGATGTCTGAACAAT |
| CBPAR | GACATGCACGTAGTCTGGCGGGGTATGACTTCTG |
| CBPAF | GACTACGTGCATGTCTTTCATGTTGCAAGGGCTAA |
| CBPBR | GACATGCACGTAGTCAACCATGAAAGAAGCCAATTC |
| CBPBF | GACTACGTGCATGTCTAATGTTCAACACAAAAGTTC |
| CBPCR | GACATGCACGTAGTCTTGAACATTAAAGTTTAGCCC |
| CBPCF | GACTACGTGCATGTCTAACCAATGGTCACTAACTT |
| CBPDR | GACATGCACGTAGTCCCATTGGTTAACTTGAACTT |
| CBPDF | GACTACGTGCATGTCTCCTATTAAATATCGTACAGT |
| BglII linker | GAAGATCTTC |
Sequencing.
We obtained additional 5′ CBP1 sequence either by manual sequencing with the T7 Sequenase version 2.0 DNA sequencing kit or by a commercial automated sequencing service provided by Joan Strange at the University of Montana. Both strands of the DNA were sequenced. The CBP1 5′ UTRs of plasmids pJBP39 and pJBP40 were sequenced to ensure that no misincorporations occurred during amplification. These plasmids were sequenced by a commercial automated sequencing service provided by Retrogen (San Diego, Calif.).
Plasmid construction.
Plasmid pJBP33 was constructed in three stages. The first stage began by moving HcURA5 3′ termination sequences into the polylinker of pUC19. The polylinker provided additional restriction sites that would ultimately be used for cloning the lacZ gene and 5′ UTR sequences upstream of the lacZ gene. To facilitate the insertion of HcURA5 sequences into the polylinker, a fusion was made by a multistep PCR procedure (4). First the pUC19 polylinker was amplified with primers PUC19F and UCURAT, and the HcURA5 region was amplified from pWU75 (18a) with primers URATERMF and SPH1URA (Table 1). Then these PCR products were used as the template for a third amplification with primers PUC19F and SPH1URA. The final PCR product was digested with EcoRI and SphI and cloned into a EcoRI/SphI-digested pUC19. A BglII site was created upstream of the HcURA5 sequences by cloning a BglII linker (Table 1) in the HincII site of the modified polylinker. This site would eventually be used for cloning of the lacZ gene. The second stage was to move a portion of the polylinker that contained HcURA5 sequences and additional upstream restriction sites into the telomeric plasmid, pWU55 (18). A 360-bp BamHI/SphI fragment of the modified polylinker was cloned into the BamHI and SphI sites of pWU55. Briefly, pWU55 was created by cloning into the HpaI site of pWU1 (16) a cassette containing the Tn5 kanamycin resistance gene flanked on both ends with telomeric repeat sequences. The final stage was to clone the 3.1-kb BamHI lacZ gene from pWU77 (18) into the BglII site of the pWU55 derivative to create pJBP33.
Insertion of the 5′ UTR sequence from CBP1 or HcURA5 into the BamHI/XbaI sites of pJBP33 required (i) amplification of the sequence with a forward primer that has a BamHI site at the 5′ end and a reverse primer that has a XbaI site at the 5′ end, (ii) digestion of the amplified fragment with BamHI and XbaI, and (iii) ligation of the fragment with BamHI/XbaI-digested pJBP33. Primers BAMURAP and XBAURAP (Table 1) were used to amplify a 395-bp fragment of the HcURA5 5′ UTR from pWU75. This fragment was cloned into pJBP33 to create pJBP20. The template for amplification of the CBP1 5′ UTR was pJB1 (2), and the reverse primer was always CBP5. The forward primers, sizes of the amplified products, and resulting plasmids are as follows: CBP1, 564 bp, pJBP35; CBP8, 685 bp, pJBP38; CBP9, 782 bp, pJBP39; CBP10, 883 bp, JBP40; CBP12, 1,102 bp, pJBP41 (Table 1). The DNA polymerase used in all of the amplification reactions was Pfu, the high-fidelity enzyme from Stratagene.
To create the four 15-bp substitutions in the CBP1 5′ UTR, a multistep PCR protocol was used (4). First, the region 5′ to the substitution was amplified with forward primer CBP12 and a reverse primer that hybridized just upstream of the substituted region. This primer also had a nonhybridizing 5′ tail of the sequence 5′-GACTACGTGCATGTC-3′ , which we had chosen to replace the wild-type sequence. Next, the region 3′ to the substituted bases was amplified with the reverse primer CBP5 and a forward primer that hybridized just downstream of the substituted region with the same nonhybridizing 5′ tail. These two PCR products were used as the template in a third amplification with CBP12 as the forward primer and CBP5 as the reverse primer. The final product was digested with BamHI and XbaI and cloned into pJBP33 as described above. The new sequence created a BsaAI site in the CBP1 5′ UTR, and so a digest of the new plasmids with BsaAI confirmed the presence and location of this sequence. The reverse primer for the first amplification and the forward primer for the second amplification for each plasmid are as follows: CBPAR and CBPAF for pJBP44; CBPBR and CBPBF for pJBP45; CBPCR and CBPCF for pJBP46; and CBPDR and CBPDF for pJBP47 (Table 1).
Electrotransformation of DNA into H. capsulatum.
Prior to its introduction into yeasts, each plasmid (approximately 3 μg) was linearized by digestion with PacI. The linear plasmid was purified away from the fragment carrying the kanamycin resistance gene by agarose gel electrophoresis, and then the plasmid was ethanol precipitated to concentrate it. Plasmid DNA was resuspended in 2 μl of sterile deionized water. Introduction of DNA into yeasts was achieved by an electroporation procedure (18). Briefly, 5 ml of a 2-day culture was centrifuged at 300 × g for 5 min. The supernatant was discarded, and the yeasts were resuspended in 5 ml of warm (37°C) 10% (wt/vol) mannitol. Again the yeasts were recovered by centrifugation as described above and resuspended in 200 μl of 10% mannitol. The yeasts were transferred to 0.2-cm cuvettes and mixed with the resuspended plasmid at room temperature. The electroporations were done at a capacitance of 25 μF, a resistance setting of 600 Ω, and a voltage of 0.75 kV. Time constants were between 8 and 12 ms. The electroporated cells were directly plated onto HMM without uracil and incubated at 37°C for about 2 weeks.
Cytoplasmic extracts and assays for β-galactosidase and total protein.
H. capsulatum yeasts from a 5-ml 3-day culture were pelleted by centrifugation at 800 × g for 3 min. Yeasts were washed once in 5 ml of 0.1 M sodium phosphate buffer (pH 7.5), pelleted, and resuspended in 1 ml of 0.1 M sodium phosphate buffer with 0.75 ml 0.5-mm zirconia/silica beads (Biospecs, Bartlesville, Okla.). Prior to disruption in a Mini-8 Beadbeater (Biospecs), the resuspended yeasts were placed on ice for 4 min. Disruption was achieved in two 1-min beatings separated by a 4-min chilling on ice. Extracts were then centrifuged at 4°C for 30 min at 16,000 × g. The supernatants of each extract were recovered and stored on ice.
H. capsulatum mycelial extracts were prepared by pouring a culture through a Millipore filtering manifold with a 2.5-cm-pore-size glass fiber filter. The harvested mycelia were washed once with 0.1 M sodium phosphate buffer and then transferred into a microcentrifuge tube for disruption as described above for yeasts.
To determine β-galactosidase activity, 10 μl of each extract was diluted 1:10 in ice-chilled microtiter wells containing 1 mM MgCl2, 4 5 mM β-mercaptoethanol, 0.4 mg of o-nitrophenyl-β-D-thiogalactopyranoside per ml, and 10 mM sodium phosphate buffer (pH 7.5) (18). The enzyme kinetics of each reaction was measured by a Molecular Devices Precision Microplate Reader. Reaction mixtures were maintained at 37°C while the optical density (OD) at 405 nm was determined every 30 s for 15 min. β-Galactosidase standards, prepared by diluting β-galactosidase (Sigma grade VIII) in 0.1 M sodium phosphate buffer to concentrations of 0 to 3,000 mU/ml, were assayed to generate a standard curve for the conversion of Vmax (mOD/minute) to milliunits of β-galactosidase activity per ml. The Bio-Rad protein assay was used to standardize total protein in 5-μl aliquots of each extract.
Nucleotide sequence accession number.
The sequence data shown in Fig. 3 have been submitted to the DDBJ/EMBL/GenBank databases under accession no. AF006209.
FIG. 3.
The 5′ UTR sequence of CBP1. The sequence is numbered so that the translation start site is +1. The two ATG codons are underlined and in boldface. The first amino acids of CBP are indicated below the sequence. Arrows mark the two transcription start sites, and a potential TATA sequence is boxed. Plasmids carrying 15-bp sequence substitutions are indicated below the sequences (underlined) that were altered.
RESULTS
The CBP1 gene is transcriptionally regulated.
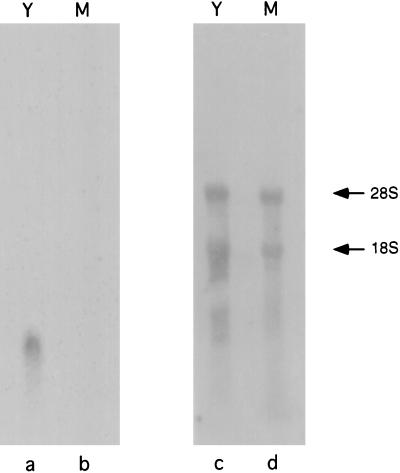
Previously, Batanghari and Goldman (3) showed that CBP is produced by yeast cultures, but not mycelial cultures, of H. capsulatum. To determine whether this phase-specific production is regulated at the level of CBP1 transcription, a Northern blot analysis was performed with total RNA that had been isolated from either yeast or mycelial H. capsulatum (Fig. 1). A ribosomal DNA probe indicated that equal amounts of yeast and mycelial RNA were present in all lanes. However, the CBP1 probe hybridized only with yeast phase RNA, and no signal was apparent with mycelial extracts. These results clearly indicate that the CBP1 promoter is much more active in the yeast phase than in the mycelial phase.
FIG. 1.
Northern analysis of yeast (Y) and mycelial (M) total RNAs. Lanes a and b were probed with CBP1 coding sequence, and lanes c and d were probed with rRNA sequences. The arrows indicate the 28S and 18S rRNA bands.
Sequence analysis and mapping of the transcription start site.
Since the CBP1 promoter is developmentally regulated, a thorough analysis of the 5′ UTR could reveal sequences important in phase-specific gene activation and/or repression. The first step was to obtain additional 5′ sequence and to map the transcription and translation start sites. Primer extension analysis revealed two transcription start sites of equal intensity (Fig. 2). A potential TATA box is located 47 bp upstream from the first start site of transcription, but there is no apparent CAAT box (Fig. 3). Previously, we were unable to identify the translation start site for CBP1 because there are two potential ATG codons and the mature CBP protein is a processed form that lacks the amino terminus. However, our primer extension results indicate that the second ATG codon must be the start site of translation. Moreover, the sequences surrounding this codon (TCAA ATG CT) show good homology to a consensus Kozak sequence for filamentous fungi (TCA[C/A][A/C]ATG[G/T]C) (1).
FIG. 2.
Primer extension analysis of the CBP1 transcript. Lanes a to d are chain termination sequencing reactions with the termination base indicated at the top; lane e is the primer extension reaction. The bases located at the 5′ ends of the CBP1 transcript are indicated by asterisks.
Telomeric reporter plasmids to study CBP1 regulation.
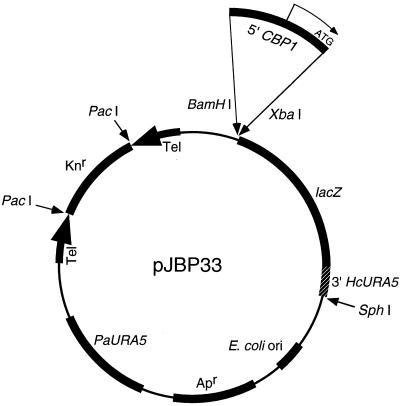
With more than 1 kb of confirmed sequence information, we constructed a series of plasmids to identify the essential regulatory sequences in the CBP1 5′ UTR. For this purpose, we created an E. coli-H. capsulatum shuttle vector that carried a promoterless lacZ gene (Fig. 4). To express the lacZ gene efficiently in H. capsulatum, we fused it to the 3′ region of the HcURA5 gene, which supplied termination sequences. Plasmid pJBP33 and its derivatives were linearized with PacI prior to electroporation into H. capsulatum. The presence of linear, extrachromosomal plasmids in Histoplasma transformants was confirmed by Southern blot analysis (data not shown).
FIG. 4.
Expression plasmid pJBP33. Locations of relevant coding and regulatory sequences are indicated. Apr, ampicillin resistance gene; Knr, kanamycin resistance gene; Tel, telomeric sequences. Large arrows indicate orientations of the telomeric sequences. Amplified 5′ UTR sequences were cloned into the BamHI and XbaI sites as depicted (see Materials and Methods).
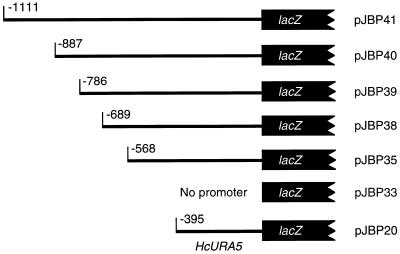
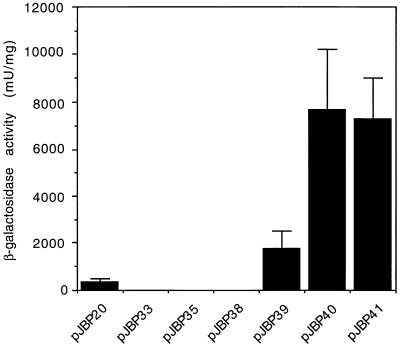
To identify the minimum region of the CBP1 5′ UTR that is necessary for yeast phase activity, we prepared successively truncated versions of the promoter, up to and including the start codon, and cloned them upstream of the lacZ gene at the BamHI and XbaI sites of pJBP33 (see Materials and Methods) (Fig. 5). Cell extracts from yeast transformants that carried these plasmids were assayed for β-galactosidase activity and total protein so that enzyme activity could be normalized to protein concentration. For a positive control, HcURA5 5′ UTR sequences were also cloned into pJBP33, and Histoplasma transformants with this plasmid were assayed. The observation that pJBP33 transformants had no detectable β-galactosidase activity confirmed that there was no fortuitous promoter activity originating in the parent plasmid to drive lacZ expression (Fig. 6). When we analyzed the series of truncated CBP1-lacZ fusions, we found that 786 bp of CBP1 5′ UTR (pJBP39) had some promoter activity, but an additional 102 bp of upstream (pJBP40) increased promoter activity about 4.5-fold. However, a construct with an additional 223 bp (pJBP41) had approximately the same promoter activity as the pJBP40 construct.
FIG. 5.
Promoter-lacZ fusions. The thin lines represent 5′ UTR sequences from either CBP1 (pJBP35, -38, -39, -40, and -41) or HcURA5 (pJBP20) which were fused to the lacZ gene by being cloned into pJBP33. The numbers indicate how many base pairs upstream of the start codon were included.
FIG. 6.
Yeast phase promoter activity in successively truncated CBP1 5′ UTR sequences. Each bar represents the relative β-galactosidase activity from at least three H. capsulatum transformants that carried the plasmid indicated below the bar. The absence of a bar corresponds to samples with β-galactosidase levels of <50 mU/ml.
We performed primer extension analysis on RNA from the pJBP40 and pJBP41 yeast transformants to determine whether transcription of the CBP1-lacZ fusions was initiating at the same location as in the endogenous CBP1 gene. We found that transcription was originating at the same two bases in the lacZ fusions, but the intensity of the CBP1-lacZ fusion signal was weaker than that of the endogenous CBP1 gene (data not shown).
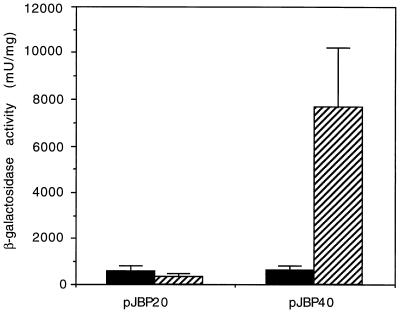
To determine whether 887 bp of CBP1 5′ UTR was sufficient for phase-specific promoter activity, we compared β-galactosidase activity from pJBP40 transformants in the yeast phase to that of the same transformants in the mycelial phase. The HcURA5-lacZ fusion (pJBP20) was used as a constitutive promoter control. As expected, the promoter activities from HcURA5 sequences were similar in the two phases. In contrast, the β-galactosidase activity from the minimum CBP1 promoter sequence (pJBP40) was about 12-fold greater in the yeast phase than in the mycelial phase (Fig. 7). Therefore, this portion of the CBP1 5′ UTR also corresponds to the region responsible for yeast phase-specific promoter activity.
FIG. 7.
Phase-specific regulation with the minimum CBP1 promoter sequence. Each bar represents the relative β-galactosidase activity from at least three H. capsulatum transformants that carried the plasmid indicated below the bar. Mycelial phase β-galactosidase activity is represented by solid bars, and yeast phase activity is represented by hatched bars.
Mutational analysis of the CBP1 5′ UTR.
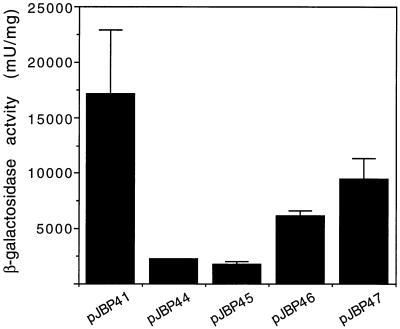
In an effort to confirm and further define the sequences that are important for CBP1 promoter activity, we substituted 15 bp of sequence in four evenly spaced regions between -887 and -785 of the CBP1 5′ UTR. These substitutions were made in the largest length of CBP1 5′ UTR (1,110 bp) and cloned into pJBP33 so that the promoter activity of these mutated sequences could be assessed. We chose to substitute sequences rather than to delete them so that we would not alter the spacing between other promoter sequences. The stretches of 15 bp wild-type sequences were substituted with an arbitrary sequence, 5′-GACTACGTGCATGTC-3′ . The locations of these substitutions and the plasmids in which these versions of the promoter were cloned are shown in Fig. 3. Promoter activities of these mutant sequences and the wild-type sequence were assayed in the yeast phase of H. capsulatum. Although none of the substituted sequences had wild-type activity, the two constructs with the most 5′-proximal substitutions were much less active, with about 8-fold (pJBP44) and 10-fold (pJBP45) decreases in activity (compared to the wild-type level). The other two substituted sequences had approximately threefold (pJBP46) and twofold (pJBP47) decreases in activity. These results confirm that the sequences between -887 and -786 are important for promoter activity and suggest that the sequences between -877 and -839 are the most important.
DISCUSSION
Most of the organisms responsible for systemic mycoses are the dimorphic ascomycetes H. capsulatum, Blastomyces dermatitidis, Paracoccidiodes brasiliensis, and Coccidioides immitis. Each of these organisms exists in a saprophytic mycelial form outside the host and switches to a parasitic yeast form within the host. This transition undoubtedly requires the expression of a new subset of genes. Because these organisms have traditionally posed severe biological and technical challenges, few molecular genetic tools have been developed for them. Studies to examine regulation of dimorphism have largely been limited to the identification of phase-specific genes or proteins (5, 9, 10, 12, 13, 15). Ours is the first attempt to pinpoint phase-specific promoter elements, with the aid of a reporter gene, in any of these dimorphic fungal pathogens.
The first step toward understanding phase-specific gene regulation is to identify a phase-specific gene. The CBP1 gene of H. capsulatum is an attractive candidate because it encodes an abundant yeast phase-specific product which is hypothesized to play a role in pathogenesis (2, 3). Therefore, we predicted that CBP1 would have a strong promoter and that it would be transcriptionally regulated, since this is the most common type of regulation. Northern blot analysis confirmed that CBP1 is transcriptionally regulated, with mycelial phase expression below the limits of detection by this technique. The temperature restrictions on yeast growth (37°C) versus mycelial growth (25°C) do not allow us to distinguish whether CBP1 expression is merely temperature sensitive or whether regulation is more complex; we therefore refer to CBP1 transcriptional regulation simply as phase specific.
We then assessed the promoter activity of 5′ UTR sequences by shuttling CBP1-lacZ fusions into H. capsulatum and assaying for β-galactosidase activity. These promoter-lacZ fusions were introduced into H. capsulatum on a telomeric plasmid because this is the most reliable method available for introducing and maintaining foreign DNA in this organism. The most common fate of transforming nontelomeric DNA into H. capsulatum is random integration into the chromosome (21). This event often results in multiple integrations, tandem duplications, and/or rearrangement of the transformed DNA and adjacent sequences. In contrast, electroporation with linearized telomeric plasmids yield transformants that maintain the plasmid extrachromosomally, unmodified except for the possible addition of more telomeric sequences repeats (17). These plasmids are multicopy but the exact copy number is not known, and there is probably some variation in copy number between transformants. This may be the cause for variation in β-galactosidase activity between isolates with the same plasmids. However, based on results of assays for β-galactosidase activity from multiple transformants, we believe that the effects of variation in copy number are minimized.
By assaying for promoter activity of successively truncated CBP1 5′ UTR fragments in the yeast phase, we identified a 102-bp region, between bp -786 and -887 upstream of the start codon, which significantly increases promoter activity. We also showed that promoter sequences up to and including this region (887 bp) are sufficient for yeast phase-specific activity. These results suggest that the CBP1 promoter is positively regulated in the yeast phase and that the sequences between -786 and -887 mediate promoter activation. Substitution analysis at four regions of the promoter helped to localize the important sequences more precisely. It is not surprising that all of the promoter constructs containing substituted sequences were less active than the wild-type 1,110-bp promoter, since a 15-bp stretch of substituted bases anywhere in this promoter would likely affect activity to some degree. However, the two most 5′ substitutions (pJBP44 and pJBP45) have a dramatic effect on promoter activity (8- and 10-fold less activity, respectively), suggesting that these sequences are necessary for phase-specific promoter activity.
Although these results suggest that the promoter is positively regulated in the yeast phase, we have not ruled out the possibility that the promoter is also regulated by repression in the mycelial phase. Promoter activity from our series of truncated constructs assayed in the mycelial phase (data not shown) shows no evidence of repression. However, repression may be mediated by sequences close to the transcriptional start site, and our constructs would probably be too large to address this question.
The CBP1-lacZ fusions have the same start site of transcription as the endogenous CBP1 gene, which suggests that the same sequences which promote transcription of the endogenous gene also promote transcription in the fusions. However, there was significantly less transcript from the fusions than from the endogenous gene. It is possible that all of the elements required for wild-type promoter activity are not present even in the largest fusion construct (pJBP41). For most genes in filamentous fungi, 400 bp of promoter sequence would be sufficient (1), but regulated promoters could require enhancer elements which may be several kilobases away. It is also possible that the fusion transcript is not as stable as the transcript from the endogenous gene. For instance, the HcURA5 3′ termination sequences which are fused to lacZ may not be optimal and thus could interfere with stability of the transcript.
We have demonstrated that certain CBP1 promoter sequences are necessary for activation, but the precise mechanism remains undefined. The simplest hypothesis is that a yeast phase trans-acting protein binds to the promoter in this region and activates transcription. A more complicated hypothesis is that these sequences are important for the structure of the promoter. If this promoter is regulated by the same mechanism as other yeast phase-specific genes, then we might expect to find similar sequences in the promoters of these genes. Only four genes that are preferentially transcribed in the yeast phase have been reported: yps-3 (12), cdc2 (5), Ole1 (9), and hsp82 (15). However, there are no reported functional analyses of the promoters for these genes, and a comparison of the available sequences does not reveal any striking similarities (data not shown). Future progress in understanding phase-specific transcription will require the identification of additional regulated genes, a functional analysis of their promoter sequences, and the identification of trans-acting regulators.
FIG. 8.
Yeast phase promoter activity in CBP1 5′ UTR with substituted sequences (Fig. 3). Each bar represents the relative β-galactosidase activity from at least three H. capsulatum transformants that carried the plasmid indicated below the bar. β-Galactosidase activity from the pJBP44 construct is the result of two transformants, and so no error bar is shown.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants A125584 (to W.E.G.) and A107172 (to Washington University School of Medicine) from the National Institutes of Health. W.E.G. is a recipient of the Burroughs Wellcome Fund Scholar Award in Molecular Pathogenic Mycology. J.B.P. was supported by a Lucille P. Markey Pathway postdoctoral fellowship.
REFERENCES
- 1.Ballance D J. Sequences important for gene expression in filamentous fungi. Yeast. 1986;2:229–236. doi: 10.1002/yea.320020404. [DOI] [PubMed] [Google Scholar]
- 2.Batanghari J W, Deepe G S, Jr, Di Cera E, Goldman W E. Histoplasma acquisition of calcium and expression of CBP1 during intracellular parasitism. Mol Microbiol. 1998;27:531–540. doi: 10.1046/j.1365-2958.1998.00697.x. [DOI] [PubMed] [Google Scholar]
- 3.Batanghari J W, Goldman W E. Calcium dependence and binding in cultures of Histoplasma capsulatum. Infect Immun. 1997;65:5257–5261. doi: 10.1128/iai.65.12.5257-5261.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cormack B. Mutagenesis of cloned DNA. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1997. [Google Scholar]
- 5.Di Lallo G, Gargano S, Maresca B. The Histoplasma capsulatum cdc2 gene is transcriptionally regulated during the morphologic transition. Gene. 1994;140:51–57. doi: 10.1016/0378-1119(94)90729-3. [DOI] [PubMed] [Google Scholar]
- 6.Eissenberg L G, Goldman W E. Histoplasma variation and adaptive strategies for parasitism: new perspectives on histoplasmosis. Clin Microbiol Rev. 1991;4:411–421. doi: 10.1128/cmr.4.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eissenberg L G, Schlesinger P H, Goldman W E. Phagosome-lysosome fusion in P388D1 macrophages infected with Histoplasma capsulatum. J Leukocyte Biol. 1988;43:483–491. doi: 10.1002/jlb.43.6.483. [DOI] [PubMed] [Google Scholar]
- 8.García Véscovi E, Soncini F C, Groisman E A. Magnesium as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 9.Gargano S, Di Lallo G, Kobayachi G S, Maresca B. A temperature-sensitive strain of Histoplasma capsulatum has an altered Δ9-fatty acid desaturase gene. Lipids. 1995;30:899–906. doi: 10.1007/BF02537480. [DOI] [PubMed] [Google Scholar]
- 10.Goldani L Z, Picard M, Sugar A M. Sythesis of heat-shock proteins in mycelia and yeast forms of Paracoccidioides brasiliensis. J Med Microbiol. 1994;40:124–128. doi: 10.1099/00222615-40-2-124. [DOI] [PubMed] [Google Scholar]
- 11.Harris G S, Keath E J, Medoff J. Expression of α- and β-tubulin genes during dimorphic-phase transitions of Histoplasma capsulatum. Mol Cell Biol. 1989;9:2042–2049. doi: 10.1128/mcb.9.5.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keath E J, Abidi F E. Molecular cloning and sequence analysis of yps-3, a yeast-phase-specific gene in the dimorphic fungal pathogen Histoplasma capsulatum. Microbiology. 1994;140:759–767. doi: 10.1099/00221287-140-4-759. [DOI] [PubMed] [Google Scholar]
- 13.Keath E J, Painter A A, Kobayashi G S, Medoff G. Variable expression of a yeast-phase-specific gene in Histoplasma capsulatum strains differing in thermotolerance and virulence. Infect Immun. 1989;57:1384–1390. doi: 10.1128/iai.57.5.1384-1390.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodge J K, Johnson R L, Weinberg R A, Gordon J I. Comparison of myristoyl-CoA: protein N-myristoyltransferases from three pathogenic fungi: Cryptococcus neoformans, Histoplasma capsulatum, and Candida albicans. J Biol Chem. 1994;269:2996–3009. [PubMed] [Google Scholar]
- 15.Minciottei G, Gargano S, Maresca B. Molecular cloning and expression of hsp82 gene of the dimorphic pathogenic fungus Histoplasma capsulatum. Biochim Biophys Acta. 1992;1131:103–107. doi: 10.1016/0167-4781(92)90106-a. [DOI] [PubMed] [Google Scholar]
- 15a.Woods, J. P. Unpublished data.
- 16.Woods J P, Goldman W E. In vivo generation of linear plasmids with addition of telomeric sequences by Histoplasma capsulatum. Mol Microbiol. 1992;6:3603–3610. doi: 10.1111/j.1365-2958.1992.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 17.Woods J P, Goldman W E. Autonomous replication of foreign DNA in Histoplasma capsulatum: role of native telomeric sequences. J Bacteriol. 1993;175:636–641. doi: 10.1128/jb.175.3.636-641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Woods, J. P., and W. E. Goldman. Unpublished data.
- 18.Woods, J. P., E. L. Heinecke, and W. E. Goldman. Electrotransformation and expression of bacterial genes encoding hygromycin phosphotransferase and β-galactosidase in the pathogenic fungus Histoplasma capsulatum. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 18a.Woods, J. P., D. M. Retallack, E. L. Heinecke, and W. E. Goldman. Unpublished data.
- 19.Worsham P L, Goldman W E. Quantitative plating of Histoplasma capsulatum without addition of conditioned medium or siderophores. J Med Vet Mycol. 1988;26:137–143. [PubMed] [Google Scholar]
- 20.Worsham P L, Goldman W E. Selection and characterization of ura5 mutants of Histoplasma capsulatum. Mol Gen Genet. 1988;214:348–352. doi: 10.1007/BF00337734. [DOI] [PubMed] [Google Scholar]
- 21.Worsham P L, Goldman W E. Development of a genetic transformation system for Histoplasma capsulatum: complementation of uracil auxotrophy. Mol Gen Genet. 1990;221:358–362. doi: 10.1007/BF00259400. [DOI] [PubMed] [Google Scholar]