Abstract
Cannabis legalization continues to progress in many US states and other countries. Δ9-tetrahydrocannabinol (Δ9-THC) is the major psychoactive constituent in cannabis underlying both its abuse potential and the majority of therapeutic applications. However, the neural mechanisms underlying cannabis action are not fully understood. In this chapter, we first review recent progress in cannabinoid receptor research, and then examine the acute CNS effects of Δ9-THC or other cannabinoids (WIN55212-2) with a focus on their receptor mechanisms. In experimental animals, Δ9-THC or WIN55212-2 produces classical pharmacological effects (analgesia, catalepsy, hypothermia, hypolocomotion), biphasic changes in affect (reward vs. aversion, anxiety vs. anxiety relief), and cognitive deficits (spatial learning and memory, short-term memory). Accumulating evidence indicates that activation of CB1Rs underlies the majority of Δ9-THC or WIN55121-2’s pharmacological and behavioral effects. Unexpectedly, glutamatergic CB1Rs preferentially underlie cannabis action relative to GABAergic CB1Rs. Functional roles for CB1Rs expressed on astrocytes and mitochondria have also been uncovered. In addition, Δ9-THC or WIN55212-2 is an agonist at CB2R, GPR55 and PPARγ receptors and recent studies implicate these receptors in a number of their CNS effects. Other receptors (such as serotonin, opioid, and adenosine receptors) also modulate Δ9-THC’s actions and their contributions are detailed. This chapter describes the neural mechanisms underlying cannabis action, which may lead to new discoveries in cannabis-based medication development for the treatment of cannabis use disorder and other human diseases.
1. Introduction
Cannabis is the most commonly abused drug worldwide and accounts for half of all drug seizures by law enforcement (WHO, 2021). Since the 2000s, the general public has reported less perceived risk from cannabis, while diagnoses of cannabis use disorder (CUD) climb (Carliner, Brown, Sarvet, & Hasin, 2017). An estimated 147 million people in the world are cannabis users WHO, 2021. Recreational use remains the most prevalent (53.4%), but a growing community of individuals report purely medical use (10.5%) or a combination of medical and recreational use (36.1%; Schauer, King, Bunnell, Promoff, & McAfee, 2016). Therapeutic use of cannabis has a long history and an accumulating body of work has supported cannabinoids in the treatment of chronic spasticity and pain (Whiting et al., 2015). In this chapter, we explore the acute effects of cannabis from a neurobiological viewpoint. The majority of work in this vein has focused on Δ9-tetrahydrocannabinol (Δ9-THC), the primary psychoactive phytocannabinoid in cannabis that underlies its rewarding effects, but also the majority of therapeutic uses. Δ9-THC was first isolated from hashish by Rafael Mechoulam in 1964 (Gaoni & Mechoulam, 1964). This compound produces a number of physiological and behavioral changes in preclinical animal models including the classic tetrad effects (analgesia, catalepsy, hypothermia, hypolocomotion), a change in affective state either positive (reward, anxiety relief) or negative (aversion, anxiogenesis), and deleterious effects on cognition. Systemic reviews on the endocannabinoid system, pharmacology of cannabinoids, and their involvement and implications in various human diseases have previously been conducted and are beyond the scope of this chapter (Alexander, 2016; Leung, 2011; Mechoulam & Parker, 2013; Pertwee, 2005, 2006). Here we focus on research progress investigating the neural mechanisms underlying the behavioral effects of Δ9-THC and other cannabinoids in experimental animals. We first describe the receptor systems where cannabinoids bind followed by detailed region- and cell type-specific receptor mechanisms underlying Δ9-THC’s CNS effects.
2. Cannabinoid receptors
There are at least two types of cannabinoid receptors (CB1R and CB2R) identified. Δ9-THC, synthetic cannabinoids (WIN55,212-2, CP55940, HU-210), and the endocannabinoids (anandamide, AEA; 2-arachidonoyl glycerol, 2-AG) have high binding affinities at both the GPCR-coupled receptors. Cannabinoids also bind and activate other putative cannabinoid receptors, including G protein-coupled receptor 55 (GPR55), transient receptor potential vanilloid 1 (TRPV1) channel, and peroxisome proliferator-activated nuclear receptors (PPARs) (Fig. 1). In addition, cannabinoids may also indirectly act on other receptor systems such as opioid, adenosine, and serotonin receptors by diverse ways.
Fig. 1.
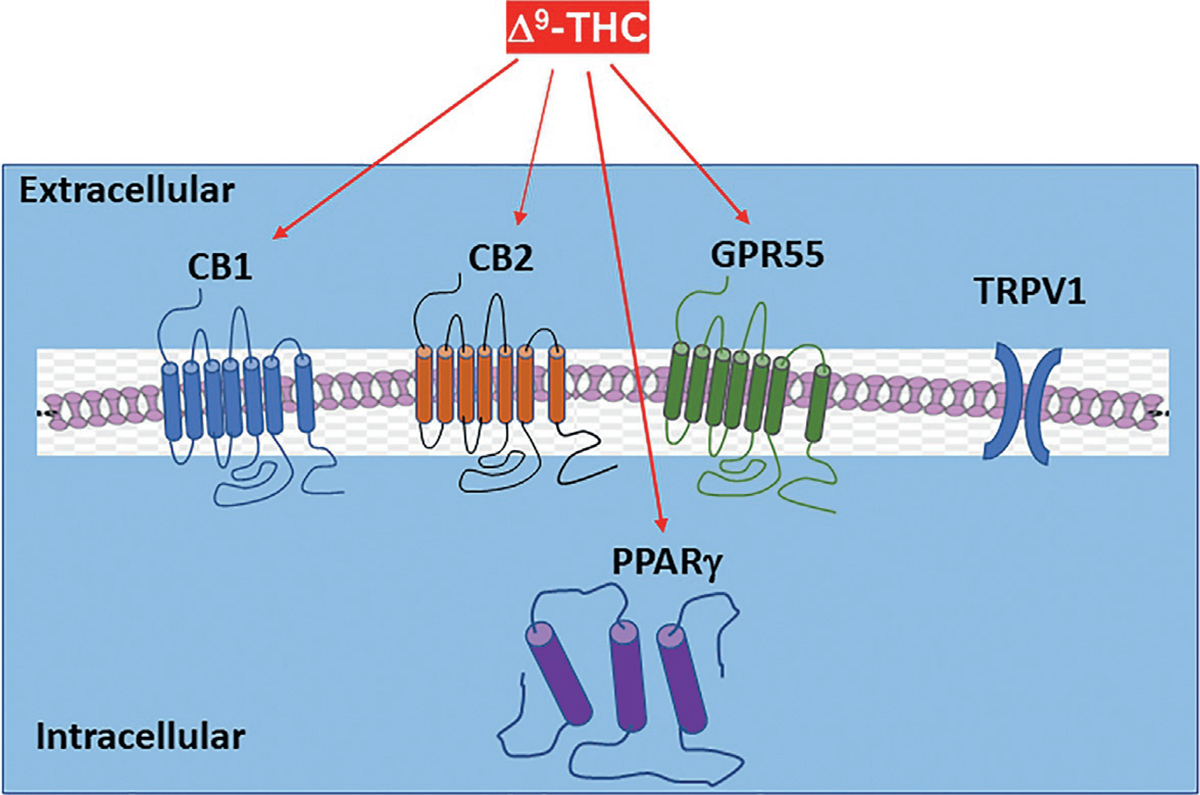
Major targets of Δ9-THC based on their receptor binding and functional assays as shown in Table 1.
Table 1 shows the receptor binding profiles of those commonly used cannabinoids to both CB1 and CB2 receptors and other putative receptors as shown in Fig. 1. In brief, these compounds are classified into four categories based on their chemical structures: classical, nonclassical, eicosanoid, and aminoalkylindole (Pertwee, 2008a, 2008b; Pertwee et al., 2010). The classical category includes dibenzopyran derivatives such as Δ9-THC and HU-210. Δ9-THC is a weak partial agonist at both the CB1R and CB2R with greater CB1R affinity and activity at GPR55 and PPARγ (Pertwee, 2008a, 2008b). HU-210 is a synthetic analog of Δ8-THC with 100–800-fold greater potency than Δ9-THC at the CB1R and CB2R, a prolonged duration of action, and activity at GPR55 and TRPV1 (Devane et al., 1992; Pertwee et al., 2010). CP55940 fits within the nonclassical nomenclature, containing compounds that are Δ9-THC derivatives and lack a pyran ring (Howlett et al., 2002). CP55940 has marginally lower affinity than HU-210 at the CB1R and CB2Rs and binds to GPR55, TRPV1 and PPARγ (Pertwee et al., 2010). Within the eicosanoid classification are the two main endocannabinoids: 2-AG and AEA. AEA is a partial agonist at both the cannabinoid receptors with even lower CB2R affinity than Δ9-THC (Pertwee, 2005). 2-AG has high affinity at the CB1R and somewhat less at the CB2R with greater efficacy observed at CB1Rs than CP55940. Both AEA and 2-AG bind to GPR55, TRVP1, and PPARγ (Pertwee et al., 2010). The fourth and final category, the aminoalkylindoles, have the most distinct chemical structures relative to the other subtypes (Ferraro et al., 2001). The aminoalkylindole WIN 55,212-2 is a full agonist at both the CB1Rand CB2Rwith greater affinity than Δ9-THCattheCB1R andactivity at TRVP1, PPARα and PPARγ (Howlett et al., 2002).
Table 1.
Receptor binding profiles of several major cannabinoids on CB1, CB2 and other putative cannabinoid receptors.
| Drug | CB1 (Ki, nM) | CB2 (Ki, nM) | GPR55 (EC50, nM) | TRPV1 (EC50, μM) | PPARγ (EC50, μM) |
|---|---|---|---|---|---|
| Anandamide (AEA) | 61–543 | 279–1940 | 18 | 0.16–1.15 | 8–10 |
| 2-Arachidonoylglycerol (2-AG) | 58–472 | 145, 1400 | 3 | 8.4–26 | 10–30 |
| Δ9-Tetrahydrocannabinol (THC) | 5.05–80.3 | 3.13–75.3 | 8 | >100 | 0.3 |
| WIN55,212-2 (WIN) | 1.89–123 | 0.28–16.2 | N.D. | >100 | 10 |
| CP55940 | 0.5–5.0 | 0.69–2.8 | 5 | >100 | 10 |
| HU-210 | 0.06–0.73 | 0.17–0.52 | 26 | 1.2 | N.D. |
Based on Pertwee (2008a) and Pertwee et al. (2010).
2.1. CB1 receptor
As stated above, Δ9-THC acts as a partial agonist at G-protein coupled CB1R (Iwamura, Suzuki, Ueda, Kaya, & Inaba, 2001). This receptor recruits Gi/o proteins and inhibits adenylate cyclase while increasing mitogen-activated protein kinase (Howlett, 2005; Pertwee, 2008a, 2008b). CB1R can also inhibit N-type and P/Q-type calcium currents, stimulate A-type outward potassium channels, and use Gs proteins to signal (Howlett et al., 2002; Jarrahian, Watts, & Barker, 2004).
Regional distribution of CB1R:
The first CB1R distribution studies used autoradiography with [3H]-CP55,940, a tritiated CB1R agonist (Herkenham et al., 1991, 1990) and found extraordinarily high levels of CB1Rs in the substantia nigra, globus pallidus, hippocampus, cerebellum, and cortex (Fig. 2). Autoradiographic studies using [3H]-WIN55,212-2 further confirmed this pattern in rat (Jansen, Haycock, Ward, & Seybold, 1992) and human brains (Glass, Faull, & Dragunow, 1997; Mato, Del Olmo, & Pazos, 2003). In situ hybridization (ISH) and immunohistochemistry (IHC) assays corroborated the autoradiographic reports and revealed that CB1Rs are highly expressed in a restricted set of forebrain neurons, particularly in the cortex, amygdala, and hippocampus (see reviews by Galaj & Xi, 2019; Hu & Mackie, 2015). These neurons project widely throughout the CNS, resulting in a dense network of CB1-positive axons (Bodor et al., 2005; Mackie, 2008). Double-label immunostaining and ISH experiments revealed that the cells expressing CB1Rs in the forebrain are primarily GABAergic and CCK-positive interneurons (Katona et al., 1999; Tsou, Mackie, Sañudo-Peña, & Walker, 1999).
Fig. 2.
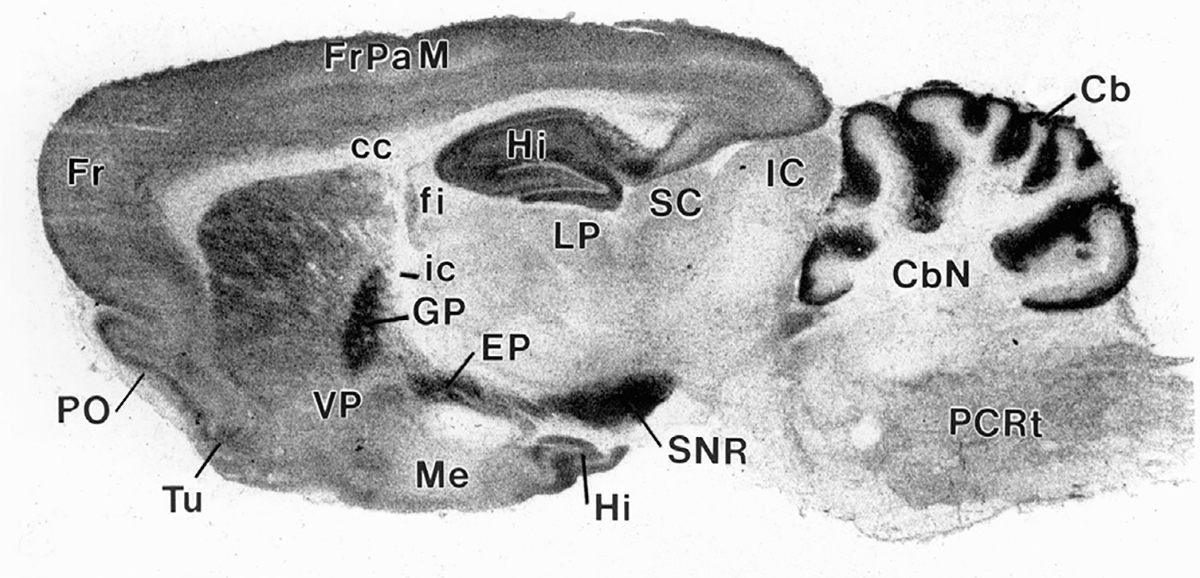
[3H]CP55,940 autoradiography demonstrating CB1R distribution in the rat brain. A high density of CB1Rs is expressed in the SNR, GP, Hi, and cerebellum. Fr, Frontal cortex; FrPaM, frontal primary motor cortex; PO, pre-olfactory bulb; Tu, olfactory tubercle; Hi, hippocampus; VP, ventral pallidum; Me, Median eminence; fi, fimbria of the hippocampus; ic, internal capsule; LP, lateral post thalamus nuclei; SC, superior colliculus; IC, inferior colliculus; Cb, cerebellum; CbN, cerebellar nuclei; CC, corpus cal losum; GP, globus pallidus; EP, entopeduncular nucleus (homolog of GPi); SNR, substantia nigra pars reticulata; PCRt, parvicellular reticular nuclei. (This image was provided by Dr. Miles Herkenham at NIMH, USA)
Neuronal CB1R:
RNAscope ISH is a highly sensitive and selective assay that we and others have used to characterize the cellular distributions of CB1Rs in the brain. High densities of CB1 mRNA have been detected in the cell bodies of both GABA and glutamate neurons in multiple brain regions including the cortex, thalamus, midbrain and cerebellum (Fig. 3; Han et al., 2017; Humburg et al., 2021; Vickstrom et al., 2021). This CB1 mRNA signal is highly specific as selective deletion of CB1Rs from either GABAergic neurons or glutamatergic neurons abolished CB1 mRNA staining in the corresponding cell types. Functional studies provide further information regarding cellular localization of CB1R. For example, electrophysiological assays demonstrate that CB1R activation inhibits GABA release in the midbrain, which may lead to postsynaptic (dopamine) neuron disinhibition (or activation; Lupica & Riegel, 2005; Szabo, Siemes, & Wallmichrath, 2002). This suggests that activation of GABAergic CB1Rs has functional consequences. Electrophysiological assays also demonstrate functional CB1R expression in glutamatergic neurons or their terminals in the midbrain and many other brain regions (Melis, Gessa, & Diana, 2000; Melis et al., 2004). We have examined CB1R expression in midbrain dopamine (DA) neurons. Under high magnification, CB1-immunostaining was found mainly in cell membranes and nerve fibers, but not in neuronal cell bodies (Han et al., 2017). Since nerve fibers from different neuronal types are always intertwined, IHC assays alone are not sufficient to identify whether midbrain DA neurons express CB1Rs. However, RNAscope ISH assays indicate that a subpopulation of midbrain DA neurons expresses CB1 mRNA (Fig. 3).
Fig. 3.
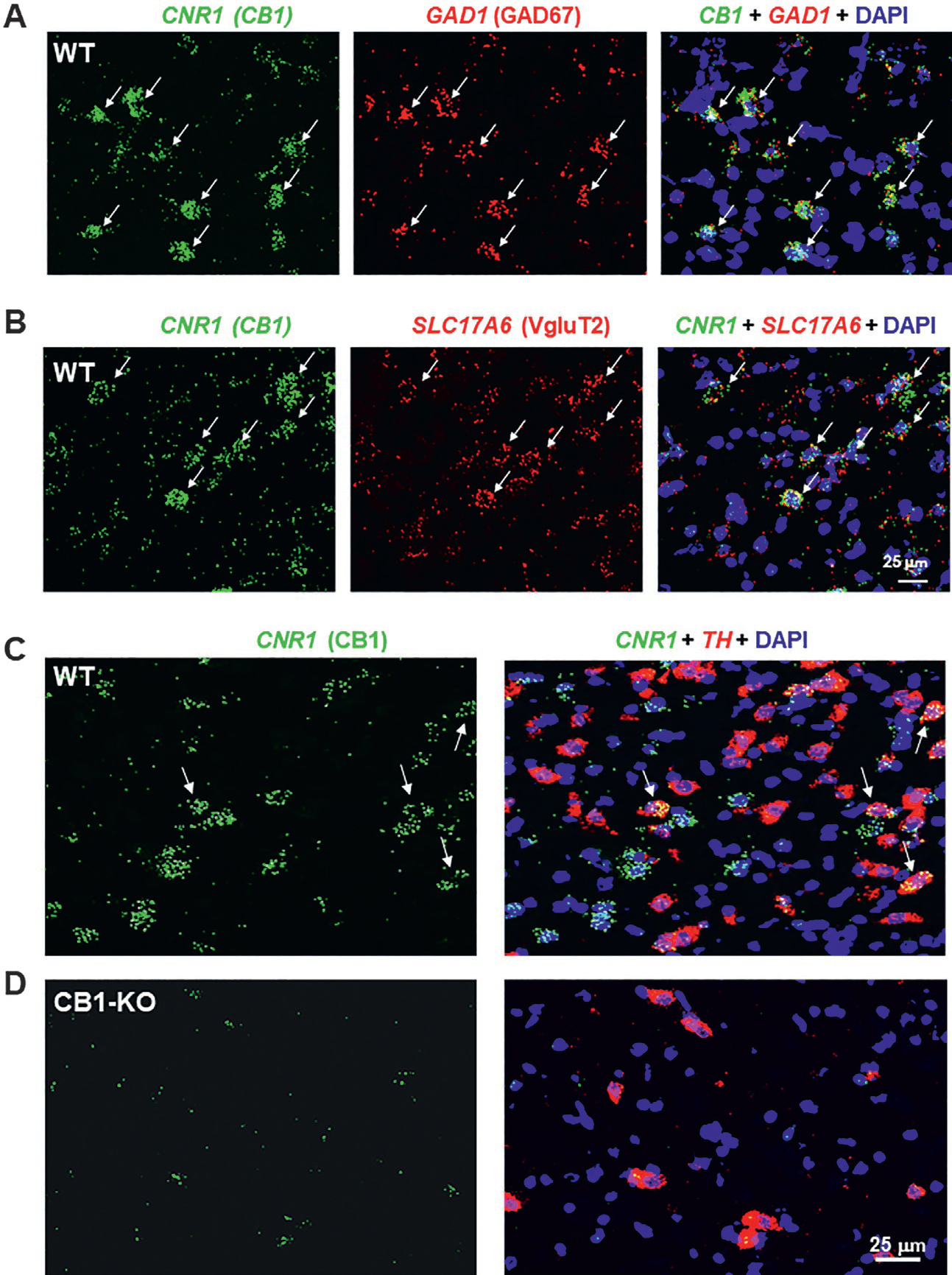
RNAscope ISH results, illustrating the cellular distributions of CB1Rs in the midbrain ventral tegmental area. CB1 mRNA was detected in GAD1-labeled GABAergic neurons (A), VgluT2-labeled glutamate neurons (B) and a small population of TH-labeled DA neurons (C) in the midbrain of WT, but not CB1-KO mice (D).
Endocannabinoids regulate physiological functions in the brain mainly through activation of CB1Rs that inhibit presynaptic GABA or glutamate release via a retrograde endocannabinoid-CB1R mechanism (Castillo, Younts, Chávez, & Hashimotodani, 2012; Piomelli, 2003). Specifically, presynaptic neuronal excitation increases glutamate release at excitatory synapses by activation of voltage-dependent Ca++ channels, which subsequently activates postsynaptic AMPA and NMDA receptors and depolarizes post-synaptic neurons. Meanwhile, glutamate may also activate postsynaptic mGluR1 or mGluR5, causing an increase in 2-AG synthesis in postsynaptic neurons. Postsynaptic neuronal depolarization may also elevate intracellular Ca++ and elicit 2-AG production. After being released from postsynaptic neurons, 2-AG retrogradely travels across the synapse to activate presynaptic CB1Rs. Presynaptic CB1Rs are Gi/o protein-coupled receptors (Howlett, 2005; Pertwee, 2008a, 2008b). Their activation leads to inhibition of presynaptic glutamate or GABA release (Hoffman, Laaris, Kawamura, Masino, & Lupica, 2010; Howlett et al., 2002; Howlett, Blume, & Dalton, 2010; Jarrahian et al., 2004; Laaris, Good, & Lupica, 2010). This neuronal CB1R-mediated inhibition leads to several types of short-term or long-term synaptic plasticity, such as depolarization-induced suppression of excitation at excitatory synapses, depolarization-induced suppression of inhibition at inhibitory synapses, or long-term depression, which are associated with endocannabinoid involvement in various brain functions (Galaj & Xi, 2019).
Glial CB1R:
CB1R has also been detected on non-neuronal cells such as astrocytes (Djeungoue-Petga & Hebert-Chatelain, 2017; Han et al., 2012; Oliveira da Cruz, Robin, Drago, Marsicano, & Metna-Laurent, 2016; Stella, 2010). Astrocytes were traditionally thought to provide nutrients to neurons and to maintain a functional homeostasis for neuronal functions. However, recent studies have indicated that astrocytes can regulate synaptic transmission and brain functions. For example, electrical stimulation of adjacent neurons can increase intracellular Ca++ levels in hippocampal astrocytes that express CB1R (Navarrete & Araque, 2008). This effect is mediated by a Gαq protein-phospholipase C signal pathway, rather than the Gαi/o protein-cAMP signal pathway observed in neurons. The increase in astrocyte Ca++ induces gliotransmitter release (Metna-Laurent & Marsicano, 2015; Mothet et al., 2000) and results in hetero-synaptic potentiation at excitatory or inhibitory synapses. Thus, glial CB1R-mediated neuronal excitation differs significantly from neuronal CB1R-mediated homosynaptic inhibition.
Less is known regarding CB1R present on microglia and mitochondria. Microglia are the immune cells of the CNS. They act as macrophages and can change phenotype based on their microenvironment. Microglia in an activated state dispense a substantial amount of nitric oxide (NO). Interestingly, administration of a CB1R agonist blocks this effect (Waksman, Olson, Carlisle, & Cabral, 1999) and microglia contain an anandamide binding site coupled to NO release (Stefano, Liu, & Goligorsky, 1996). As such, changes in NO production may mediate the effects of cannabinoids via microglial CB1Rs. On the other hand, mitochondria are organelles responsible for a cell’s energy production. They support basic brain functioning primarily via the process of mitochondrial respiration i.e., the conversion of oxygen and nutrients into ATP. CB1R on mitochondria (mtCB1Rs) modulates mitochondrial respiration (Bénard et al., 2012) and are neuroprotective (Ma et al., 2015). Further, mtCB1R mediates depolarization-induced suppression of inhibition, a form of short term synaptic plasticity in which glutamatergic neurons in the hippocampus are depolarized leading to the release of endocannabinoids and subsequent CB1R activation and decreased GABAergic activity (Bénard et al., 2012).
2.2. CB2 receptor
The cannabinoid CB2R was cloned in 1993 from human leukemia cells (Munro, Thomas, & Abu-Shaar, 1993). CB2R has 44% sequence homology with CB1Rs (Pertwee, 1997). They are G-protein coupled (Gi/o) and inhibit adenylate cyclase, leading to a decrease in cAMP signaling and neuronal inhibition (Patel, Davison, Pittman, & Sharkey, 2010). CB2R activation can also stimulate p42/p44 MAP kinase and elevate intracellular calcium (Cabral & Griffin-Thomas, 2008). Δ9-THC is a partial agonist at CB2R with relatively high affinity (Table 1; Iwamura et al., 2001).
Regional distribution of CB2R:
CB2R was initially referred to as “peripheral cannabinoid receptors” due to their predominant expression in peripheral tissues including immune cells, spleen, tonsils, lymph nodes, liver, and the gastrointestinal tract (Galiègue et al., 1995; Onaivi et al., 1999) and the failure to detect CB2R in the CNS. However, more advanced techniques such as RNAscope ISH and fluorescence-activated cell sorting (FACS) followed by RT-PCR assays have unearthed CB2R expression in the CNS including the spinal cord (Nent, Nozaki, Schmöle, Otte, & Zimmer, 2019), brain stem (Van Sickle et al., 2005), hippocampus (Li & Kim, 2015), ventral tegmental area (Zhang et al., 2017), and cerebellum (Gong et al., 2006).
Cellular distribution of CB2R:
The specific cell types within the CNS that express CB2R are somewhat controversial. The majority of work assumes that CB2Rs are expressed on microglia although more direct anatomical evidence is still needed. Recent studies have revealed neuronal CB2R expression on DA neurons in the midbrain (Zhang et al., 2015; Zhang et al., 2017), glutamate neurons in the red nucleus and hippocampus (Li & Kim, 2015; Stempel et al., 2016; Zhang et al., 2021) and GABA neurons in the striatum and cerebellum (Fig. 4; Li & Kim, 2015; Zhang, De Biase, et al., 2021) (Fig. 4). CB2Rs in the brain are located on the postsynaptic cells (Brusco, Tagliaferro, Saez, & Onaivi, 2008a, 2008b) and are inducible, showing upregulation under neuroinflammatory conditions (Atwood & Mackie, 2010; Maresz, Carrier, Ponomarev, Hillard, & Dittel, 2005).
Fig. 4.
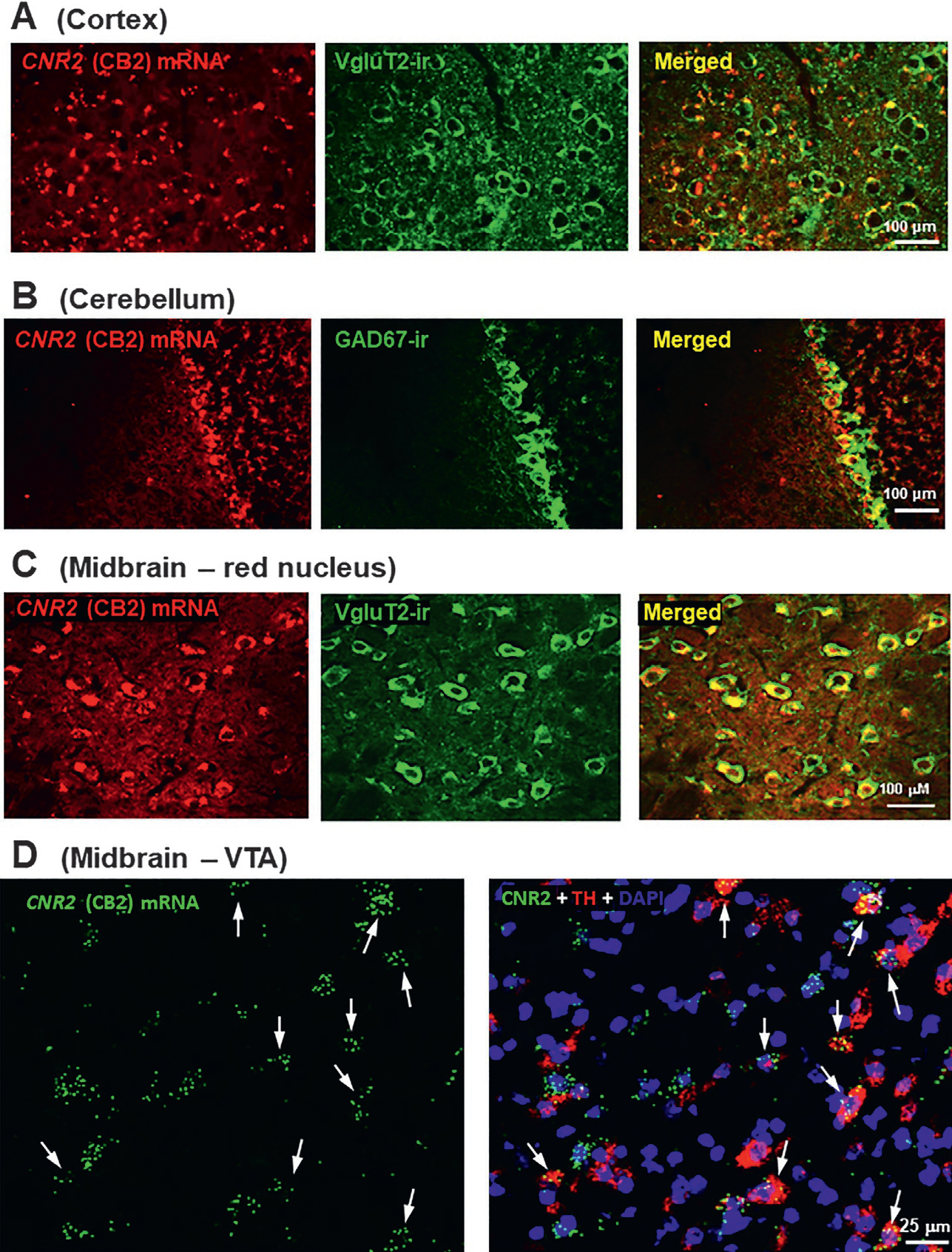
Conventional (A, B, C) and RNAscope (D) ISH results, illustrating the cellular distributions of CB2Rs in mouse brain. CB2 mRNA was detected in cortical VgluT2-labeled glutamatergic neurons (A), cerebellar GAD1-labeled GABAergic neurons (B), red nucleus VgluT2-labeled glutamatergic neurons (C), and VTA TH-labeled dopaminergic neurons (D).
CB2R transcripts:
An important finding in recent research is the unique distribution patterns of CB2 transcript (mRNA) isoforms (CB2A, CB2B, CB2C, CB2D) across species and tissue types (Liu et al., 2009; Zhang et al., 2015), which may, in part, explain why early assessments failed to detect CB2 mRNA in the brain (Galiègue et al., 1995; Munro et al., 1993; Schatz, Lee, Condie, Pulaski, & Kaminski, 1997). In humans and mice the CB2A isoform was found primarily in the testis and brain, whereas CB2B was expressed in the spleen and leukocytes (Liu et al., 2009). CB2C and CB2D isoforms were only detected in rats (Zhang et al., 2015). On the whole, CB2A is the predominant subtype (20–30-fold higher than CB2B; Zhang et al., 2014). However, in the mouse spleen CB2A is only about 3-fold higher than CB2B (Zhang et al., 2014). A direct comparison of brain and spleen CB2A mRNA levels revealed considerably greater expression in the spleen (50–100-fold). These findings suggest that brain CB2 mRNA is more likely to be detected with a probe that targets CB2A rather than CB2B transcript. However, brain CB2 expression is still detectable using riboprobes that recognize the encoding sequences on both CB2A and CB2B isoforms, by which CB2 mRNA was discovered in the cortex, hippocampus, and globus pallidus of non-human primates (Lanciego et al., 2011; Sierra et al., 2015). These findings indicate that expression of the CB2 gene is dependent on the isoform subtype and varies by species and region.
2.3. GPR55
Δ9-THC is an agonist at the orphan receptor GPR55 (Table 1). This receptor has been put forward as a putative “CB3 cannabinoid receptor,” given that both endocannabinoids (AEA, 2-AG) and synthetic cannabinoids (HU-210, CP55,940) are also able to bind (Table 1). However, GPR55 does not contain a quintessential cannabinoid binding pocket (Baker, Pryce, Davies, & Hiley, 2006) and has minimal receptor homology with CB1R (13.5%) or CB2R (14.4%; Elbegdorj, Westkaemper, & Zhang, 2013). GPR55 couples to G12 and G13 proteins and activates RhoA and Ca++ (Henstidge et al., 2009; Ryberg et al., 2007). GPR55 is distributed throughout the nervous system. In the periphery, it was been uncovered in the GI tract (Li et al., 2013), liver (Romero-Zerbo et al., 2011), pancreas (McKillop, Moran, Abdel-Wahab, & Flatt, 2013), and adipose tissue (Imbernon et al., 2014). QT-PCR assays indicate GPR55 mRNA expression in the striatum, substantia nigra, frontal cortex, hippocampus and cerebellum (Celorrio et al., 2017; Ryberg et al., 2007; Wu et al., 2013). In cell cultures, GPR55 and microglia colocalize (Pietr et al., 2009). In striatal or substantia nigra (SN) brain tissues, GPR55 mRNA was detected in neurons (colocalized with a neuronal marker), but not in microglia or astrocytes (Celorrio et al., 2017).
2.4. Peroxisome proliferator-activated receptors (PPARs)
PPARs are nuclear receptors with 3 isoforms (α, β, γ) that regulate gene expression (O’Sullivan, 2016). Activated PPARs dimerize retinoid X receptors and bind to DNA sequences termed PPAR response elements (Bishop-Bailey, 2000). Δ9-THC is an agonist at PPARγ (EC50~0.3μM; O’Sullivan, Tarling, Bennett, Kendall, & Randall, 2005) (Table 1), but does not bind to PPARα (Sun et al., 2007). However, one report demonstrated that Δ9-THC administration increased PPARα transcriptional activity (Takeda et al., 2014). PPARs are also activated by endocannabinoids (AEA, 2-AG) and fatty acids (oleic acid, arachidonic acid) and may function as lipid sensors, monitoring metabolic activity in vivo (Pertwee et al., 2010). PPARγ expression predominates in adipose tissue, but is also observed in the liver, large intestine, and spleen (Lehrke & Lazar, 2005; Vidal-Puig et al., 1996; Villapol, 2018). Within the CNS, PPARγ expression has been detected in the piriform cortex, ventral pallidum, caudate putamen (Moreno, Farioli-Vecchioli, & Cerù, 2004), and to a lesser extent the prefrontal cortex (PFC), nucleus accumbens (NAc), and amygdala (Warden et al., 2016). Immunohistochemical images showed colocalization of PPARγ in neurons, some staining in astrocytes, but not in microglia (Warden et al., 2016).
2.5. Transient receptor potential vanilloid 1 (TRPV1) channel
Six families of transient receptor potential channels (TRP) have been identified: canonical, vanilloid (TRPV), melastatin (TRPM), polycystin, mucolipin and ankyrin (TRPA). TRPs are ion channels with a nonselective cation pore and six transmembrane domains that are involved in sensory transduction. Δ9-THC has no effect on TRPV1 functional activity at 100 μM, while AEA is a potent TRPV1 agonist with a EC50 value of 0.16–1.15 μM (Table 1). Δ9-THC is a mild agonist at TRPV2 (EC50: ~0.65μM) and has intermediate effects at TRPA1 (EC50: ~0.23μM) and TRPM8 (IC50: ~0.16μM; De Petrocellis et al., 2011). TRPV2 is activated by elevations in temperature and inflammation (De Petrocellis, Nabissi, Santoni, & Ligresti, 2017) and distributed in the paraventricular nucleus, arcuate nucleus, nucleus of the solitary tract, locus coeruleus as well as a number of other regions in the rat forebrain and hindbrain (Nedungadi, Dutta, Bathina, Caterina, & Cunningham, 2012). TRPV2 is colocalized with neurons and to a lesser extent, astrocytes (Nedungadi et al., 2012; Shibasaki, Ishizaki, & Mandadi, 2013).
2.6. Other targets
Beyond the five main receptor systems described above, cannabinoids may also interact with other targets possibly by forming heterodimers or functioning as opioid receptor allosteric modulators. For the purposes of this book chapter, we will only discuss the few receptors implicated in cannabinoid action in later Sections 3–6.
Opioid receptors:
A significant amount of work indicates cross-talk between the endocannabinoid and endogenous opioid system. Opioid receptors are inhibitory GPCRs. There are four receptor subtypes: μ, δ, κ, and nociception, with endorphins, enkephalins, dynorphins, and nociceptin as the endogenous ligands, respectively. CB1R was reported to form heterodimers with μ, δ, and κ opioid receptors and signaling at μ opioid receptors (MORs) is reduced by CB1R agonism (Rios, Gomes, & Devi, 2006). Colocalization of CB1Rs and MORs has also been detected in striatal medium-spiny neurons and the dorsal horn of the spinal cord (Rodriguez, Mackie, & Pickel, 2001; Salio et al., 2001). In addition, Δ9-THC was reported to increase the rate of dissociation of MOR and δ opioid receptor (DOR) ligands from their orthosteric binding sites designating THC as an allosteric modulator at these receptors (Kathmann, Flau, Redmer, Tränkle, & Schlicker, 2006).
Adenosine receptors:
Cannabinoids also have activity at the adenosine receptors, which are divided into four subtypes: A1, A2A, A2B and A3. Prior work has demonstrated that CB1R antagonism prevents A1R activation (Savinainen, Saario, Niemi, Järvinen, & Laitinen, 2003). Heteromeric complexes between CB1Rs and A2ARs have been detected in the striatum (Ferre et al., 2011; Ferreira et al., 2015) and hippocampus (Aso et al., 2019). The endocannabinoids (2-AG, AEA), but not synthetic cannabinoids (WIN55,212-2, CP55940), were reported to function as negative allosteric modulators at the A3 receptor (Lane, Beukers, Mulder-Krieger, & Ijzerman, 2010).
Serotonin receptors:
Additionally, a subset of serotonergic receptors is targeted by cannabinoids. There are seven families of 5-HT receptors (5-HT1–7) and further subcategories within these classes. The majority of 5-HT receptors are GPCRs, not including the 5HT3R. CB1-5HT2A heterodimers have been identified in the hippocampus, caudate putamen, and somatosensory cortex (Viñals et al., 2015). The Δ9-THC metabolites, 11-hydroxy-Δ8-THC and 11-oxo-Δ8-THC, attenuated serotonin binding to 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT2C receptors (Kimura, Ohta, Watanabe, Yoshimura, & Yamamoto, 1998; Kimura, Yamamoto, Ohta, Yoshida, & Watanabe, 1996). Similarly, AEA weakened radioligand binding of 5-HT to 5-HT2A, 5-HT2B, and 5-HT2C (Kimura et al., 1998). On the other hand, HU210 increased 5-HT binding to 5-HT2Rs on rat cortical membranes (Cheer, Cadogan, Marsden, Fone, & Kendall, 1999). In addition, CB1R may also form heterodimers with dopamine D2Rs in the striatum (Marcellino et al., 2008)
3. Cannabinoid tetrad effects
High doses of cannabinoids such as Δ9-THC or WIN55,212-2 (a potent CB1R and CB2R agonist) produce classical tetrad effects—analgesia, hypothermia, catalepsy, and hypolocomotion, which are often used to determine whether a novel compound is cannabimimetic in nature. The receptor mechanisms mediating cannabinoid effects in the tetrad are not fully understood. However, transgenic mice with conditional knockouts of different cannabinoid receptors have been widely used to identify the neuronal populations that mediate Δ9-THC or other cannabinoid effects. Significant progress has been made. Here, we discuss each assay within the tetrad and the current knowledge regarding the neural underpinnings of Δ9-THC- or WIN55,212-2-induced changes.
3.1. Analgesia
In pre-clinical work, Δ9-THC induces a strong antinociceptive effect across multiple behavioral tests (hot plate, tail flick test, formalin test) and models of chronic pain (inflammatory pain, neuropathic pain; Casey, Atwal, & Vaughan, 2017; Craft, Haas, Wiley, Yu, & Clowers, 2017; Finn et al., 2004; Wang et al., 2020).
CB1R mechanisms:
Early work demonstrated that pharmacological blockade or genetic deletion of CB1Rs in CB1-KO mice blocked Δ9-THC- or WIN55,212-2-induced analgesia (Compton, Aceto, Lowe, & Martin, 1996; Ledent et al., 1999; Rinaldi-Carmona et al., 1994; Varvel et al., 2005; Wiley & Martin, 2003; Zimmer, Zimmer, Hohmann, Herkenham, & Bonner, 1999). To address the anatomical locus and the cell type-specific receptor mechanisms underlying cannabinoid modulation of pain, multiple cell-type specific CB1-KO mice have been developed with CB1R deleted from a restricted neuronal population. As stated above, CB1Rs are highly expressed on GABA and glutamatergic neurons. However, selective deletion of CB1Rs on cortical glutamatergic neurons (Glu-CB1-KO, generated by crossing CB1-floxed mice with NEX-Cre mice), forebrain GABAergic interneurons (GABA-CB1-KO, generated by crossing CB1-floxed mice with Dlx5/6-Cre mice), or dopamine D1 receptor-expressing neurons (Drd1-CB1-KO, generated by crossing CB1-floxed mice with D1-Cre mice) failed to alter Δ9-THC antinociception (Table 2; Monory et al., 2007). These findings indicate that CB1Rs on cortical GABAergic or glutamatergic neurons as well as D1-expressing neurons do not mediate cannabinoid antinociception.
Table 2.
Summary of cannabinoid (THC, WIN55,212-2, JWH133)-induced tetrad effects in transgenic animals.
| Transgenic mouse line | Analgesia | Hypothermia | Catalepsy | Hypoactivity | References |
|---|---|---|---|---|---|
| Wildtype | Normal THC or WIN effect | Normal effect | Normal effect | Normal effect | Monory et al. (2007) and Wang et al. (2020) |
| Global CB1-KO | No effect (to THC or WIN) | No effect | No effect | No effect | Ledent et al. (1999), Monory et al. (2007), Wang et al. (2020), and Zimmer, Zimmer, Hohmann, Herkenham, and Bonner (1999) |
| CaMK-CB1-KO ( xCaMK-Cre) | ↓ THC effect | No effect | No effect | No effect | Monory et al. (2007) |
| Glu-CB1-KO ( xNEX-Cre) | Normal THC effect | ↓ THC effect | Normal effect | ↓ THC effect | Monory et al. (2007) |
| GABA-CB1-KO ( xDlx5/6-Cre) | Normal THC effect | Normal effect | Normal effect | Normal effect | Monory et al. (2007) |
| D1-CB1-KO ( xDrd1-Cre) | Normal THC effect | ↓ THC effect | ↓ THC effect | Normal effect | Monory et al. (2007) |
| SNS-CB1-KO ( xNav1.8-Cre) | ↓ WIN effect | N/D | Normal effect | N/D | Agarwal et al. (2007) |
| Global CB2-KO | ↓ THC or WIN effect | Normal effect | ↓ THC or WIN effect | Normal effect (rotarod) ↓ THC effect (open-field) | Wang et al. (2020) and Li et al. (2021) |
| DA-CB2-KO ( xDAT-Cre) | Normal effect (to WIN) | Normal effect | ↓ WIN effect | Normal effect | Liu et al. (2017)) |
| CX3CR1-CB2-KO ( xCX3CR1-Cre) | Normal effect (to WIN, ACEA) | Normal effect | Normal effect | Normal effect | Liu, Canseco-Alba, Liang, Ishiguro, and Onaivi (2020) |
| Immune-CB2-KO (CB2flox xLysM-Cre) | Normal JWH133 effect | N/D | N/D | N/D | Cabañero et al. (2020) |
| Syn-CB2-KO ( xSyn-Cre) | ↓ JWH133 effect | N/D | N/D | N/D | Cabañero et al. (2020) |
| SNS-CB2-KO ( xNav1.8-Cre) | ↓ JWH133 effect in thermal pain | N/D | N/D | N/D | Cabañero et al. (2020) |
| GPR55-KO | Normal THC effect ↑ WIN effect | ↑ THC effect | ↑ THC effect | ↑ THC effect | Wang et al. (2020) |
Abbreviations: CaMK—Ca++/calmodulin-dependent protein kinase II; CB1flox—CB1-floxed mice; NEX—a gene encodes neuronal helix-loop-helix protein (a transcription factor), expressed in pyramidal neurons of the dorsal telencephalon during embryonic development; Dlx5/6—distal-less homeobox genes 5/6, expressed in forebrain GABAergic neurons during embryotic development; SNS—sensory neurons expressing Nav1.8 channel; DA—dopamine; LysM—lymphocytes/monocytes; Syn—Synapsin (a neuronal marker).
In a more recent report, De Giacomo and colleagues (2020) used a conditional rescue model in which CB1Rs are restored only in distinct neuronal subpopulations in full CB1-KO mice and compared to full CB1R-KO mice to determine whether CB1Rs in a given region can reproduce Δ9-THC antinociception. In line with the findings from conditional CB1-KO mice, the rescue of CB1R expression in dorsal telencephalic glutamate neurons (Glu-CB1-RS) or forebrain GABA neurons (GABA-CB1-RS) did not re-establish Δ9-THC-induced analgesia (De Giacomo et al., 2020), suggesting that activation of CB1Rs in forebrain GABA or glutamate neurons is insufficient to produce analgesic effects. In contrast, mice lacking CB1Rs on CaMKIIα-positive neurons (CaMK-CB1-KO, generated by CB1-floxed mice with CaMKIIα-Cre mice) demonstrated attenuated (but not abolished) Δ9-THC-induced analgesia (Monory et al., 2007), suggesting that CB1Rs in CaMKIIα-expressing neurons partially mediate Δ9-THC-induced analgesia. In addition, CB1Rs may still be expressed by GABAergic neurons in other brain regions in the forebrain of GABA-CB1-KO mice since the Dlx5/6 (distal-less homeobox 5 and 6) genes are expressed in progenitors of GABAergic interneurons only in developing forebrain and their expression strongly diminishes after birth (Dimidschstein et al., 2016). Moreover, in the striatum these genes are not GABA specific. Similarly, CB1Rs may still be expressed in glutamatergic neurons in other brain regions in the forebrain Glu-CB1-KO mice since the NEX gene is mainly expressed in pyramidal neurons of the dorsal telencephalon during embryonic development (Schwab et al., 2000). Thus, more studies are required to determine the role of CB1Rs in GABA or glutamate neurons of other non-forebrain regions in cannabinoid antinociception.
The major site of pain perception is the sensory nervous system, to be more precise, the sensory neurons in the dorsal root ganglia (DRG) as well as the dorsal horn neurons in the spinal cord, which also contain high densities of CB1Rs (Ahluwalia, Urban, Capogna, Bevan, & Nagy, 2000; Farquhar-Smith et al., 2000). To determine the role of CB1Rs in primary sensory neurons, a peripheral CB1-KO mouse line was developed, in which CB1Rs in DRG nociceptive (Nav1.8-expressing) sensory neurons were deleted (SNS-CB1-KO, generated by crossing CB1-floxed mice with SNS-Cre mice; Agarwal et al., 2007). The nociceptor-specific loss of CB1Rs substantially reduced analgesia produced by local and systemic, but not intrathecal, delivery of WIN55,212-2 (Table 2; Agarwal et al., 2007). This suggests that CB1Rs expressed on the peripheral terminals of nociceptors (DRG sensory neurons) are critical in cannabinoid-induced analgesia (Fig. 5). These findings are consistent with work demonstrating that systemic administration of a novel peripherally acting CB1R agonist, AZ11713908, produced robust analgesia (Yu et al., 2010). Interestingly, CaMKIIα is also highly expressed in DRG neurons (Carlton & Hargett, 2002), suggesting that a peripheral CB1R mechanism could also contribute to the reduction of Δ9-THC-induced analgesia observed in CaMK-CB1-KO mice. Thus, CB1R mechanisms in peripheral sensory neurons appear to be the primary mechanism underlying cannabinoid analgesic effects (Fig. 5).
Fig. 5.
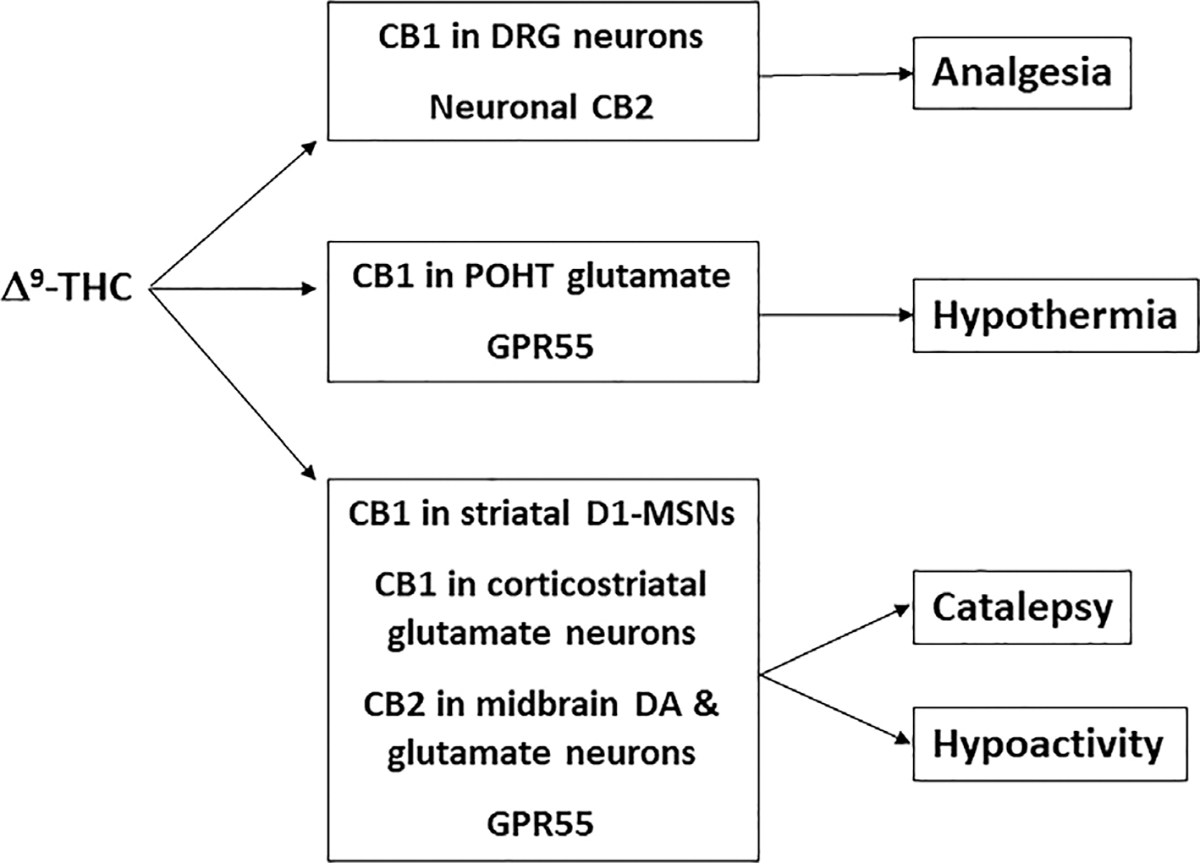
A summary of the major neural mechanisms underlying THC-induced tetrad effects.
CB2R mechanisms:
Although CB1R activation appears to be the primary mechanism underlying Δ9-THC’s analgesic effects, other targets have been discovered. For instance, CB2R agonists have been demonstrated to produce potent analgesic effects in animal models of chronic inflammatory and neuropathic pain (Maldonado, Baños, & Cabañero, 2016; Shang & Tang, 2017). Further, we have recently reported that deletion of CB2Rs in CB2-KO mice significantly reduces Δ9-THC- or WIN55,212-2-induced analgesia, implicating CB2Rs in cannabinoid antinociception (Wang et al., 2020). However, the anatomical substrates underlying CB2R-mediated analgesia are still unclear. Recently, it was reported that mice can learn to self-administer the CB2R agonist JWH133 to inhibit neuropathic pain (Cabañero et al., 2020). This behavior was blocked by global CB2-KO mice, suggesting a CB2R-mediated effect. Interestingly, selective deletion of CB2R from neurons in neuronal CB2-KO mice (CB2-floxed X Syn-Cre) caused an increase in JWH133 self-administration, while selective deletion of CB2R from immune cells in monocyte-specific CB2-KO mice (CB2-floxed X LysM-Cre) did not alter JWH133 self-administration, suggesting that increased spontaneous pain occurs in neuronal CB2-KO mice and high doses of JWH133 are required to relieve neuropathic pain (Cabañero et al., 2020) (Table 2). These findings provide clear evidence supporting a neuronal CB2R mechanism underlying CB2R-induced analgesia. However, selective deletion of CB2R from DGR neurons in peripheral neuronal CB2-KO mice (CB2-loxed X Nav1.8-Cre) did not significantly alter JWH133-produced analgesic effects, suggesting that a neuronal CB2R mechanism in the brain play a dominant role in JWH133- or other cannabinoid-induced analgesia (Fig. 5).
Other mechanisms:
Additionally, the TRPA1 channel is a receptor critically involved in thermal pain perception. Evidence has shown that TRPA1 is implicated in cannabinoid antinociception. Specifically, Akopian et al. (2008) found that WIN55,212-2 induces analgesia in a peripheral capsaicin pain model, which is absent in TRPA1-KO mice. JWH133 produced a significant reduction in either mechanical or thermal hypernociception in a neuropathic pain model (Cabañero et al., 2020). Genetic deletion of TRPA1 blocked JWH133-induced reduction in thermal, but not, mechanical, pain, suggesting possible involvement of TRPA1 in cannabinoid analgesia. In addition, the opioid system was reported to be involved in cannabinoid analgesia. Specifically, κ-opioid receptors (KORs) are involved in Δ9-THC-induced analgesia as the κ agonist dynorphin was increased by Δ9-THC administration and mice with a genetic deletion of dynorphin showed attenuated Δ9-THC-induced analgesia (Houser, Eads, Embrey, & Welch, 2000; Zimmer et al., 2001). However, KOR-KO mice displayed no change in Δ9-THC antinociception (Ghozland et al., 2002). Given that Δ9-THC does not bind to KORs, dynorphin may indirectly alter Δ9-THC’s action against pain. Unexpectedly, genetic deletion of GPR55 produced an opposite enhancement in WIN55,212-2-induced analgesia (Wang et al., 2020), suggesting involvement of GPR55 mechanism in cannabinoid analgesia.
3.2. Hypothermia
Intermediate to high doses of Δ9-THC produce a drop in body temperature across different routes of administration and species (Hayakawa et al., 2007; McMahon, Amin, & France, 2005; Taffe, Kevin, Creehan, & Vandewater, 2015; Taffe, Creehan, Vandewater, Kerr, & Cole, 2021; Varvel et al., 2006).
CB1R mechanism:
CB1Rs mediate the hypothermic effects of Δ9-THC as pharmacological antagonism or genetic deletion of CB1Rs blocks Δ9-THC-induced decreases in temperature (Hayakawa et al., 2007; Ledent et al., 1999; McMahon et al., 2005; Varvel et al., 2005; Zimmer et al., 1999). In contrast to the analgesic effects, hypothermic responses to Δ9-THC were significantly attenuated in CaMK-CB1-KO and forebrain Glu-CB1-KO mice, but not forebrain GABA-CB1-KO mice, implicating cortical glutamatergic neurons in Δ9-THC-induced hypothermia (Monory et al., 2007). The hypothermic effects of Δ9-THC are likely mediated mainly by CB1Rs expressed in the preoptic anterior hypothalamus (POAH), a major thermoregulatory brain area (Fitton & Pertwee, 1982; Rawls, Cabassa, Geller, & Adler, 2002). In forebrain Glu-CB1-RS and GABA-CB1-RS mice, CB1R expression is rescued in the hypothalamus relative to full CB1-KO mice (Gutierrez-Rodríguez et al., 2017; Remmers et al., 2017; Ruehle et al., 2013). Consistent with the data from conditional knockout mice, Glu-CB1-RS, but not GABA-CB1-RS, mice showed a partial rescue of Δ9-THC hypothermia. As such, glutamatergic CB1Rs may play a dominant role in Δ9-THC-mediated hypothermia. Glutamate tonically increases body temperature by binding to NMDA receptors in the preoptic hypothalamus (Sengupta, Jaryal, & Mallick, 2016). One report found that the hypothermic response to WIN55,212-2 is synergistically enhanced by NMDA receptor antagonism (Rawls, Cowan, Tallarida, Geller, & Adler, 2002). Microinjections of WIN55,212-2 into the POAH induced hypothermia (Rawls, Cabassa, et al., 2002). These findings suggest that cannabinoids may act on a glutamatergic pathway in the POAH to produce hypothermia (Fig. 5).
Non-CB1R mechanisms:
Additional non-CB1R receptor mechanisms have been implicated in Δ9-THC-induced temperature shifts. We found that blockade or deletion of CB2Rs in global CB2-KO mice or selective deletion of CB2Rs in midbrain DA neurons failed to alter Δ9-THC- or WIN55,121-2-induced hypothermia (Liu et al., 2017; Wang et al., 2020). In contrast, selective antagonism or genetic deletion of GPR55 receptors augmented hypothermia in response to Δ9-THC or WIN55,212-2 (Wang et al., 2020), suggesting that activation of GPR55 has a suppressive effect on Δ9-THC-induced hypothermia. No prior work has established a role for GPR55 in temperature control. However, knowledge of this receptor is limited, and future work should investigate this possibility. In addition, serotonergic 5-HT1A receptors and dopamine D2 receptors also regulate Δ9-THC-induced hypothermia in an opposing manner such that D2 receptor antagonists attenuate and 5-HT1A receptor antagonists potentiate Δ9-THC’s hypothermic effects and vice versa with their respective agonists (Malone & Taylor, 2001; Nava, Carta, & Gessa, 2000). Although Δ9-THC has no direct binding affinity at 5-HT1A and D2 receptors, these effects could be mediated indirectly via Δ9-THC metabolite activity at the 5-HT1AR and CB1-D2 heterodimer interactions.
3.3. Catalepsy
In rodents, Δ9-THC induces catalepsy at high doses (10mg/kg and above; Long et al., 2010; Metna-Laurent, Mondésir, Grel, Vallée, & Piazza, 2017). The most common behavioral assay of catalepsy is the bar test in which an animals forepaws are placed on a horizontal bar and the amount of time it takes them to move out of this unusual conformation and put both paws on the ground is recorded (Sanberg, Bunsey, Giordano, & Norman, 1988).
CB1R mechanism:
As with Δ9-THC induced hypothermia, blockage or deletion of CB1Rs effectively abolishes Δ9-THC’s cataleptic effects (Ledent et al., 1999; Lichtman & Martin, 1997; Tseng & Craft, 2004; Varvel et al., 2005; Zimmer, Zimmer, Hohmann, Herkenham, & Bonner, 1999). An early study found that deletion of CB1Rs on forebrain GABA or glutamate neurons failed to alter the cataleptic effects of Δ9-THC (Monory et al., 2007), demonstrating that CB1Rs on both neuronal cell types in the cortex do not mediate Δ9-THC-induced catalepsy. This is consistent with findings from conditional rescue mice in which neither dorsal telencephalic glutamatergic CB1Rs nor forebrain GABAergic CB1Rs were sufficient to rescue the cataleptic effect of Δ9-THC (De Giacomo et al., 2020).
Interestingly, deletion of CB1Rs from CaMKIIα (CaMK-CB1-KO) or D1-expressing neurons (Drd1-CB1-KO) abolished Δ9-THC-induced catalepsy (Monory et al., 2007), suggesting that CB1Rs on both types of neurons play a critically important role in catalepsy produced by cannabinoids. CaMKIIα is expressed in numerous neuronal cell types that project to a myriad of brain regions. Therefore, it is unknown exactly how CB1Rs in CaMKIIα-expressing neurons underlie cannabinoid-induced catalepsy. In contrast, D1Rs are mainly distributed in one population of GABAergic medium-spiny neurons (D1-MSNs) in the striatum and glutamatergic neurons in the cortex. It is well known that D1-MSNs regulate voluntary motor movements (van der Stelt & Di Marzo, 2003). Activation of CB1Rs on D1-MSNs likely inhibits GABAergic MSNs in the striatum, producing motor impairment. This is supported by the finding that microinjections of Δ9-THC into the nucleus accumbens (NAc) produced catalepsy (Sano et al., 2008) that was inhibited by both serotoninergic agonists and NMDA receptor antagonists (Nobuaki Egashira et al., 2006; Kinoshita et al., 1994). These findings suggest that CB1R expression in the striatum may be a primary brain region underlying Δ9-THC-induced catalepsy (Fig. 5).
CB2R mechanism:
In addition to CB1R, CB2R also plays a role in the cataleptic effects of Δ9-THC or WIN55212-2. Indeed, deletion and pharmacological antagonism of CB2Rs attenuated cataleptic behavior following Δ9-THC or WIN55,212-2 administration (Wang et al., 2020). Furthermore, selective deletion of CB2Rs from midbrain DA neurons attenuated WIN55,212-2-induced catalepsy (Liu et al., 2017), while deletion of CB2R from microglia (CB2-floxed X CX3CR1-Cre) had no effect on WIN55,212-2-induced catalepsy (Liu, Canseco-Alba, Liang, Ishiguro, & Onaivi, 2020), suggesting that a neuronal, not microglial, CB2R mechanism underlies cannabinoid-induced catalepsy. In addition, it was recently reported that CB2Rs are highly expressed in glutamate neurons in the red nucleus of the midbrain and modulate locomotor activity (Zhang, Shen, et al., 2021). These findings together suggest that activation of CB2Rs in the mesolimbic DA neurons and the motor circuit glutamate neurons at least in part underlies cannabinoid-induced catalepsy (Fig. 5).
GPR55 mechanism:
On the other hand, it was recently reported that GPR55 receptors are densely distributed in the striatum and administration of a GPR55 agonist (abnormal-cannabidiol) has been shown to block catalepsy produced by haloperidol (Marichal-Cancino, Fajardo-Valdez, E. Ruiz-Contreras, Mendez-Díaz, & Prospero-García, 2017; Celorrio et al., 2017), while pharmacological blockade of GPR55 potentiate Δ9-THC-induced catalepsy (Wang et al., 2020). Similarly, mice lacking GPR55 demonstrated enhanced Δ9-THC- or WIN55,212-2-induced catalepsy (Wang et al., 2020). These findings suggest that GPR55 activation may produce an anti-cataleptic effect. As such, the final behavioral expression of cannabinoid-induced catalepsy may depend on the respective contributions of CB1R, CB2R, and GPR55.
3.4. Hypolocomotion
Δ9-THC suppresses locomotor activity at doses of 3mg/kg and above. The open field locomotion test is most often utilized to measure changes in movement. Mice are placed in a large, empty container and the distance they travel is monitored. The rotarod test is another measure of locomotor performance, particularly motor coordination, in which mice are placed on an elevated revolving rod and the time it takes them to fall is recorded.
CB1R mechanism:
Like the other assays within the tetrad, Δ9-THC or WIN55,212-2 alters locomotor activity via activation of CB1Rs as deletion of CB1Rs abolished Δ9-THC and other cannabinoids-induced locomotor impairment (Ledent et al., 1999; Nguyen et al., 2016; Taffe, Creehan, & Vandewater, 2015; Zimmer et al., 1999). Findings from three different conditional CB1-KO mice strains (Glu-CB1-KO, CaMK-CB1-KO and VgluT2-CB1-KO) implicate glutamatergic neurons in Δ9-THC’s effects on locomotion (Monory et al., 2007; Han et al., 2017). These findings parallel work with conditional rescue mice in which restoration of CB1R expression in dorsal telencephalic glutamatergic neurons (Glu-CB1-RS mice) reestablished Δ9-THC-induced locomotor suppression (De Giacomo et al., 2020). In contrast, deletion of CB1Rs in forebrain GABA neurons failed to alter Δ9-THC-induced locomotor impairment (Monory et al., 2007). Similarly, GABA-CB1-RS mice with CB1R expression rescued in forebrain GABAergic neurons showed no evidence of Δ9-THC locomotor inhibition (De Giacomo et al., 2020). Previous work has demonstrated that cannabinoids attenuate excitatory glutamatergic input in the striatum (Brown, Brotchie, & Fitzjohn, 2003). Thus, CB1Rs on corticostriatal glutamatergic projection neurons likely mediate hypolocomotion produced by Δ9-THC (Monory et al., 2007) (Fig. 5).
The basal ganglia contains two major GABAergic neuronal populations—D1-MSNs and D2-MSNs. Both populations of neurons express CB1Rs (Hermann, Marsicano, & Lutz, 2002) and control basal ganglia motoric output (Graybiel, 2000). Activation of D1-MSNs enhances, while activation of D2-MSNs inhibits locomotion (Calabresi, Picconi, Tozzi, Ghiglieri, & Di Filippo, 2014; Kravitz et al., 2010). The D1-expressing MSNs have become a locus of interest since Δ9-THC treatment may directly inhibit this locomotor-enhancing population of neurons. However, CB1R deletion from D1-MSNs had no effect on Δ9-THC-induced hypolocomotion (Monory et al., 2007). Interestingly, the effects of Δ9-THC on overall locomotor activity (open field test) vs. motor coordination (rotarod) may have distinct neural underpinnings. Indeed, Blazquez and colleagues (2020) found that Glu-CB1-KO and WT mice had comparable deficits in motor coordination following Δ9-THC in direct contrast to the findings described above using the open-field. Further, the motor dyscoordinating effects of Δ9-THC were absent in Drd1-CB1-KO mice, indicating that D1-MSNs are critical for Δ9-THC-induced deficits in motor coordination (Blázquez et al., 2020) (Fig. 5).
CB2R mechanism:
In addition to CB1R mechanisms, dopaminergic CB2Rs may also underlie Δ9-THC-induced locomotor depression. We have previously reported that CB1 and CB2 receptors modulate locomotor activity in opposite directions (Li et al., 2021; Li et al., 2009; Wang et al., 2020; Xi et al., 2011). Specifically, genetic deletion of CB1Rs decreased basal locomotor activity, while genetic deletion of CB2Rs produced a moderate increase, indicating that activation of CB2Rs inhibits locomotor behavior (Li et al., 2021; Wang et al., 2020). Systemic or intra-NAc administration of JWH133, a selective CB2R agonist, inhibits basal level locomotion and decreases cocaine’s locomotor activating effects in a dose-dependent manner (Xi et al., 2011). Further, genetic deletion of CB2Rs blocked the Δ9-THC-induced reduction in open-field locomotion (Li et al., 2021; Wang et al., 2020), implicating CB2Rs in Δ9-THC’s locomotor suppressant effects. When CB2Rs are selectively deleted from midbrain DA neurons, mice show an increase in basal locomotor activity (Canseco-Alba et al., 2019). These findings suggest that dopaminergic CB2Rs contribute to Δ9-THC-induced hypolocomotion (Galaj & Xi, 2019; Jordan & Xi, 2019) (Fig. 5).
GPR55 mechanism:
Finally, GPR55-KO mice showed heightened Δ9-THC or WIN55,212-2-induced deficits in motor coordination on the rotarod (Wang et al., 2020). These findings complement prior work in which administration of a GPR55 agonist improved performance on the rotarod following selective lesions of striatal DA neurons (Fatemi, Abdollahi, Shamsizadeh, Allahtavakoli, & Roohbakhsh, 2021). This work demonstrates that GPR55 agonism is involved in motor coordination and has an obverse effect on Δ9-THC hypomotility in the tetrad, although the cell types and brain regions responsible are unknown.
In summary, Δ9-THC and other cannabinoids produce classical tetrad effects through multiple receptor mechanisms, including CB1R, CB2R and GPR55 with CB1R predominant (Table 2). Technical advances in detecting low level gene expression and the development of conditional transgenic animals have begun to uncover the region and cell type-specific subpopulations that underlie Δ9-THC’s effects in the tetrad. In brief, CB1R in peripheral primary sensory neurons of the DRG and CB2R in super-spinal neurons appear to be the major targets underlying THC-induced analgesia, while glutamatergic CB1Rs in the preoptic anterior hypothalamus are not only necessary, but also sufficient for Δ9-THC-induced hypothermia. The cataleptic and locomotor suppressant effects of Δ9-THC are likely mediated mainly by activation of CB1Rs on corticostriatal glutamatergic projection neurons and CB2Rs on midbrain DA neurons and red nucleus glutamate neurons (Fig. 5).
4. Cannabinoid subjective effects
The subjective experience of cannabis varies on the affective spectrum from person to person. The majority of human users report enjoyment, relaxation and laughter, while others describe paranoia, anxiety and depression (Green, Kavanagh, & Young, 2003). In preclinical work, negative or aversive effects of Δ9-THC are most commonly observed, particularly at high doses, whereas reward is rarer, difficult to replicate and only observed with low doses. A number of preclinical models of drug reward are utilized in addiction research. The gold standard is intravenous self-administration where animals are implanted with a jugular catheter and trained to make operant responses for drug infusions. Another commonly used model is place conditioning in which the amount of time spent in a context formerly associated with drug exposure is used as a measure of reward. Aversion can also be assessed in this model if time in the drug paired context drops considerably after conditioning. Lastly, intracranial self-stimulation (ICSS) is a behavioral test that assesses how drugs of abuse alter operant responding for electrical stimulation of the median forebrain bundle or optical stimulation of a specific phenotype of neurons such as DA neurons or glutamate neurons. A decrease or increase in brain-stimulation reward (BSR) thresholds denotes a rewarding or aversive drug effect, respectively. The following section will walk through studies investigating the neural mechanisms underlying Δ9-THC reward versus aversion using these behavioral models.
4.1. Cannabinoid reward
Self-administration of Δ9-THC has been demonstrated in squirrel monkeys at low doses (4μg/kg/infusion), but not in rhesus monkeys, an effect that can be blocked by rimonabant, a selective CB1R blocker (John et al., 2018; Justinova, Tanda, Redhi, & Goldberg, 2003; Mansbach, Nicholson, Martin, & Balster, 1994; Tanda, Munzar, & Goldberg, 2000). However, in rodents (rats and mice), Δ9-THC or WIN55,212-2 alone cannot maintain reliable self-administration possibly due to the limited reinforcing efficacy and anxiogenic effects of cannabinoids (Lefever, Marusich, Antonazzo, & Wiley, 2014; Takahashi & Singer, 1979). Interestingly, it was recently reported that passive Δ9-THC pre-exposure or co-administration of Δ9-THC with cannabidiol (CBD), a phytocannabinoid devoid of psychotomimetic effects, improved cannabinoid self-administration in rats (Spencer et al., 2018). A small number of studies have demonstrated Δ9-THC-induced place preferences and decreases in BSR thresholds at the low end of the dose range (0.075–1mg/kg), which are absent in the presence of a CB1R antagonist or in CB1-KO mice (Braida, Iosue, Pegorini, & Sala, 2004; Foll, Wiggins, & Goldberg, 2006; Gardner et al., 1988; Ghozland et al., 2002; Katsidoni, Kastellakis, & Panagis, 2013; Lepore, Liu, Savage, Matalon, & Gardner, 1996; Lepore, Vorel, Lowinson, & Gardner, 1995; Li et al., 2021; Soria et al., 2004; Valjent & Maldonado, 2000). Microinjections of Δ9-THC directly into the NAc shell and posterior ventral tegmental area (VTA) also support place preferences in rats, implicating these brain regions in Δ9-THC-induced reward (Zangen, Solinas, Ikemoto, Goldberg, & Wise, 2006).
GABAergic CB1R mechanism:
Δ9-THC administration produces a rise in DA concentration in the NAc (Tanda, Pontieri, & Chiara, 1997) and increases the firing rate of dopaminergic neurons in the VTA (French, Dillon, & Wu, 1997). Thus, it was hypothesized that CB1Rs on GABA neurons mediate the rewarding effects of Δ9-THC via disinhibition of VTA DA neurons (Fig. 6). This hypothesis is supported by electrophysiological data demonstrating decreased GABA activity in midbrain slices in the presence of Δ9-THC and WIN55,212-2 (Friend et al., 2017; Szabo et al., 2002). Additionally, transgenic FAAHC/A knock-in mice, which recapitulate the FAAH (fatty acid amide hydrolase) polymorphism and display decreased FAAH expression and elevated circulating AEA, produced an enhanced place preference in adolescent female FAAHC/A mice relative to controls (Burgdorf et al., 2020). Importantly, this increase in cannabinoid reward was accompanied by greater expression of GABAergic CB1Rs and lower expression of glutamatergic CB1Rs in the VTA (Burgdorf et al., 2020).
Fig. 6.
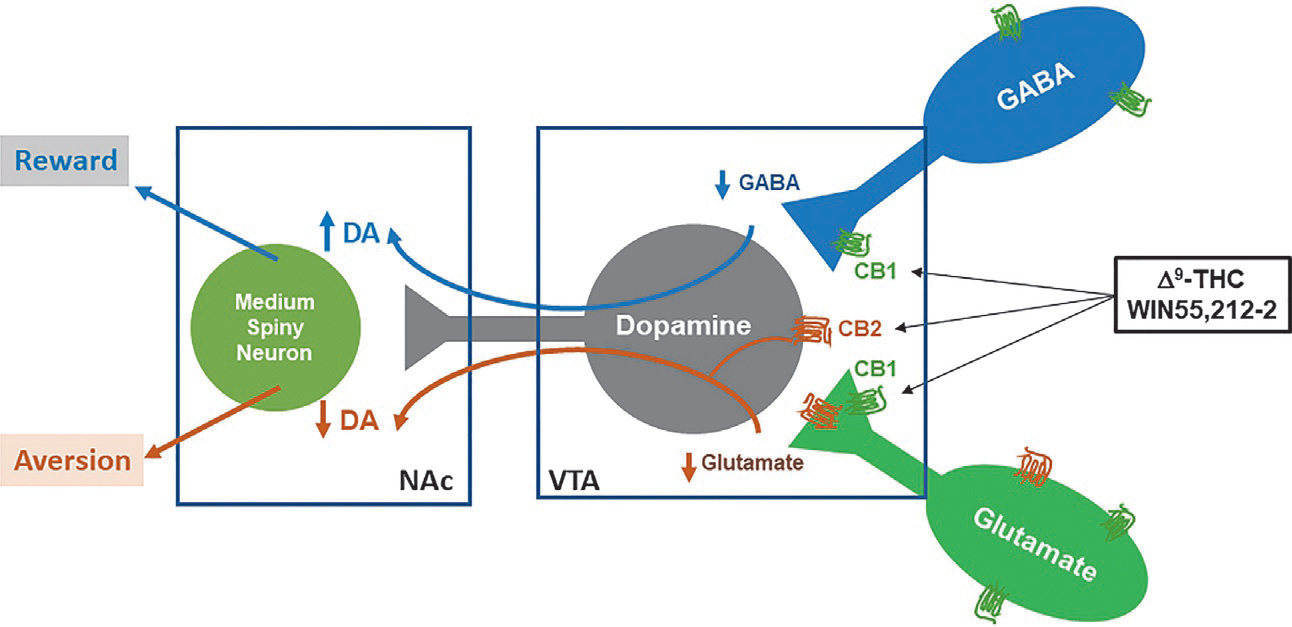
Neural mechanisms underlying cannabinoid reward vs. aversion. CB1Rs are expressed in VTA GABAergic neurons and glutamatergic neurons as well as their afferents projected from other brain regions to VTA DA neurons (data shown). CB2Rs are found in VTA DA neurons. Cannabinoids modulate the mesolimbic DA system via activation of brain CB1Rs and CB2Rs. Cannabinoids such as Δ9-THC or WIN55,212-2 produce rewarding effects by binding to CB1Rs on VTA GABAergic interneurons and/or their afferents, thereby reducing GABA-mediated disinhibition of VTA DA neurons and cannabinoid reward. Conversely, Δ9-THC or WIN55,212-2 may also produce aversive effects by activating CB1Rs on glutamatergic neurons in the VTA or glutamatergic afferents, and CB2Rs on midbrain DA neurons, thereby inhibiting VTA DA release to the NAc. The subjective effects of cannabinoids may thus depend on the balance of opposing CB1R and CB2R effects and individual differences in expression of cannabinoid receptors. DA, dopamine; GABA, γ-aminobutyric acid; NAc, nucleus accumbens; VTA, ventral tegmental area.
Non-CB1R mechanisms:
Other work has evaluated whether non-cannabinoid receptor systems are involved in the rewarding effects of Δ9-THC. In one report, the MOR antagonist, naltrexone, decreased Δ9-THC self-administration (Justinova, Tanda, Munzar, & Goldberg, 2004) and Δ9-THC place preferences were absent in MOR-KO mice (Ghozland et al., 2002). Δ9-THC increases β-endorphin release in the VTA, which could explain the lack of rewarding effects when MORs are antagonized or deleted (Solinas, Zangen, Thiriet, & Goldberg, 2004). Another series of studies implicated A2A adenosine receptors (A2ARs) in the rewarding effects of Δ9-THC. CB1Rs form heterodimers with A2ARs on presynaptic cells and activation of A2ARs counteracts the inhibitory effects of CB1Rs on glutamate release in corticostriatal terminals (Ferreira et al., 2015; Köfalvi et al., 2020). Antagonism of presynaptic A2ARs was shown to reduce Δ9-THC self-administration in squirrel monkeys, indicating that a decrease in cortical striatal glutamate attenuates Δ9-THC reward (Justinová et al., 2011). Similarly, inhibition of postsynaptic A2ARs potentiated Δ9-THC self-administration (Justinová, Redhi, Goldberg, & Ferre, 2014). These findings suggest that excess glutamate in corticostriatal brain regions, perhaps the NAc, may also contribute to the rewarding effects of Δ9-THC likely by stimulating dopamine release in the striatum. Finally, α7 nicotinic acetylcholine receptors (α7nAChRs) also play a role in Δ9-THC reward. Kynurenic acid (KYNA) acts as a negative allosteric modulator of α7nAChRs and increased levels of KYNA inhibit Δ9-THC-induced increases in NAc dopamine and Δ9-THC self-administration in squirrel monkeys (Justinova et al., 2013). α7nAChRs are located on glutamatergic neurons that project to the NAc shell (Dani & Bertrand, 2007; Secci et al., 2019). As such, suppression of glutamatergic activity by KYNA and subsequent decreases in NAc DA likely underlie the decrease in Δ9-THC reward. This work by Justinova and colleagues provides further evidence in support of a glutamatergic accumbal mechanism meditating Δ9-THC’s rewarding properties perhaps in conjunction with disinhibition of GABAergic tone in the VTA.
4.2. Cannabinoid aversion
The aversive effects of Δ9-THC are well documented in preclinical work. Subjects develop robust place aversions and increases in BSR thresholds in ICSS particularly at intermediate to high doses (3–20mg/kg; Braida et al., 2004; Hempel, Clasen, Nelson, Woloshchuk, & Riley, 2018; Katsidoni et al., 2013; Li et al., 2021; Mallet & Beninger, 1998a, b; Schramm-Sapyta et al., 2007; Spiller et al., 2019; Valjent & Maldonado, 2000; Vann et al., 2008; Wiebelhaus et al., 2015). We have recently tested cannabinoids in a new assay, optogenetic ICSS (oICSS), to further evaluate the rewarding (or reward-enhancing) versus aversive (or reward-attenuating) effects of a given drug (Han et al., 2017; Jordan et al., 2019; Newman et al., 2019). In this procedure, an adeno-associated virus (AAV) carrying a Cre-dependent channelrhodopsin 2 (ChR2) gene is microinjected into the VTA to express light-sensitive ChR2 in DA neurons of transgenic DA transporter (DAT)-Cre mice or glutamatergic neurons in VgluT2-Cre mice. Using this assay, we found that systemic administration of Δ9-THC or WIN55,212-2 dose-dependently inhibited oICSS maintained by optical stimulation of VTA DA neurons and shifted the stimulation-response curve rightward or downward (Humburg et al., 2021), suggesting that cannabinoids are aversive in mice.
Glutamatergic CB1R mechanism:
The receptor mechanisms underlying Δ9-THC’s aversive effects are not fully understood. In prior work, cannabinoid-induced reductions in BSR thresholds were prevented by CB1R antagonism (SR141716A or AM251; Katsidoni, Kastellakis, & Panagis, 2013; Spiller et al., 2019), pointing to a CB1R mechanism in cannabinoid-induced aversion. To determine the specific cell types involved, we have recently used optogenetics to stimulate VTA glutamate neurons in VgluT2-Cre mice (Han et al., 2017). Unexpectedly, Δ9-THC significantly inhibited oICSS maintained by optical stimulation of VTA glutamatergic neurons, but this effect was absent in VgluT2-CB1-KO mice. Similarly, deletion of CB1Rs on glutamate neurons prevented the expression of Δ9-THC place aversions (Han et al., 2017). These findings suggest that CB1Rs on VTA glutamate neurons are involved in Δ9-THC-induced aversion (Fig. 6). This fits with the model of Δ9-THC’s affective properties described above wherein increased expression of CB1Rs on GABA neurons and lower expression of CB1Rs on glutamate neurons shifts the affective properties of Δ9-THC towards reward (Burgdorf et al., 2020).
Dopaminergic CB2R mechanism:
Beyond CB1Rs, Δ9-THC’s aversive effects were also blocked by CB2R antagonism, as assessed by an increase in BSR thresholds in rats (Spiller et al., 2019). Additionally, genetic deletion of CB2Rs shifted Δ9-THC place conditioning from a place aversion in wildtype mice to a place preference in CB2-KO mice (Li et al., 2021), indicating that CB2Rs play a role in the initial aversive effects of Δ9-THC. CB2Rs are expressed on VTA dopaminergic neurons (Zhang et al., 2014; Zhang et al., 2017; Zhang et al., 2019) and can decrease the firing rate of these cells as well as diminish DA release in the NAc (Ma et al., 2019; Zhang et al., 2014; Zhang et al., 2017). These findings implicate CB2Rs on mesolimbic DA neurons in THC-induced aversion (Fig. 6).
PPAR mechanisms:
Little is known regarding the role of PPARs in cannabinoid action. However, we have recently explored the function of PPARs in Δ9-THC induced aversion using oICSS (Hempel, Bi, Klein, & Xi, 2021). Administration of Δ9-THC decreased responding for optical stimulation of VTA DA neurons in DAT-cre mice and this effect was attenuated by administration of a PPARα or PPARγ antagonist. As previously stated, Δ9-THC is a potent PPARγ agonist (Table 1), which delineates how PPARγ antagonism reduced Δ9-THC action on oICSS. PPARα receptors have been detected in the VTA and NAc at low levels (Warden et al., 2016). However, the mechanism through which PPARα modulates Δ9-THC’s aversive effects is still unclear. While Δ9-THC administration does produce changes in PPARα gene transcription, it does not bind to the α isoform.
Kappa opioid receptor mechanism:
Finally, κ-opioid receptors (KORs) have been investigated as a potential receptor target mediating Δ9-THC aversion. Mice with elevated expression of the opioid encoding gene prodynorphin, a precursor of the KOR agonist dynorphin, demonstrated enhanced Δ9-THC place aversions relative to controls (Cheng, Laviolette, van der Kooy, & Penninger, 2004). In the same vein, dynorphin-deficient and KOR-KO mice developed attenuated Δ9-THC place aversions as did mice administered a KOR antagonist prior to assessments of Δ9-THC aversion (Clasen et al., 2017; Ghozland et al., 2002; Zimmer et al., 2001). Microinjections of Δ9-THC into the posterior NAc shell produced a significant place aversion that was attenuated by KOR antagonism, implicating NAc KORs in the expression of Δ9-THC aversion (Norris, Szkudlarek, Pereira, Rushlow, & Laviolette, 2019).
5. Cannabinoid effects on anxiety
One of the most commonly cited reasons for cannabis use in humans is the relaxing effects of the drug (Ewusi Boisvert et al., 2020; Green, Kavanagh, & Young, 2003); however, some individuals experience anxiety under the influence (Spindle et al., 2018). In preclinical models, Δ9-THC has a biphasic effect on anxiety: anxiolytic at low doses and anxiogenic at high doses. The elevated plus maze (EPM) is a frequently used animal model in which rodents are placed on a raised apparatus containing two crossed arms – one of which is enclosed by walls and the other is open. Greater time spent in the open arms is a measure of decreased anxiety. The light dark test is another assay of anxiety that takes advantage of rodents’ preference for dark, enclosed spaces. Subjects have access to two compartments separated by a door – one compartment is open and well-lit and the other is dark and enclosed. Anxiolytic drugs increase the proportion of time spent in the light compartment. The following subsections will update the current understanding of the receptor mechanisms underlying the anxiolytic and anxiogenic effects of Δ9-THC.
5.1. Anxiolytic
As with the majority of cannabinoid effects on the central nervous system, administration of a CB1 antagonist blocks Δ9-THC-induced anxiolytic properties (Berrendero & Maldonado, 2002; Rubino et al., 2007). CB1Rs in the amygdala and prefrontal cortex (PFC) underlie this effect (Tiziana Rubino et al., 2007). CP55,940 also produces an anxiolytic-like response at a low dose (1μg/kg), which is absent in Glu-CB1-KO, but not GABA-CB1-KO mice (Rey, Purrio, Viveros, & Lutz, 2012), suggesting that glutamatergic CB1Rs in the PFC and amygdala mediate the initial anxiolytic effects of Δ9-THC. However, further work is needed to confirm this.
CB1R and CB2R mechanisms:
One study found that brief exposure to a predator odor produced an anxiety-like state in rats that was blocked by the selective monoacylglycerol lipase (MGL) inhibitors, KML29 and JZL184 (Ivy et al., 2020). MGL inhibitors prevent 2-AG degradation and elevate brain 2-AG concentrations, indicating that increased levels of 2-AG were anxiolytic in this model. Unexpectedly, the behavioral response to JZL184 was abolished by a CB2R, but not CB1R, antagonist. Specifically, the selective CB2R agonist, JWH133, produced anxiolytic-like effects in rats exposed to a predator odor stressor and this response was blocked by the CB2R antagonist AM630 (Ivy et al., 2020). However, early studies demonstrated that JZL184 produced marked anti-anxiety effects that were prevented by administration of the CB1R antagonist SR141716A (Kinsey, O’Neal, Long, Cravatt, & Lichtman, 2011; Sciolino, Zhou, & Hohmann, 2011). Thus, 2-AG relieves anxiety potentially through activation of both CB1Rs and CB2Rs.
Other receptor mechanisms:
Outside of the cannabinoid receptor family, other systems also modulate Δ9-THC’s anti-anxiety effect (Table 3). For instance, antagonism of the MOR and DOR suppressed the anxiolytic response to Δ9-THC, although no additional work has investigated this neurobiological mechanism (Berrendero & Maldonado, 2002). Additionally, serotonergic receptors are known to regulate anxiety and have been investigated in this context. Administration of a 5-HT1A receptor antagonist abolished the anxiolytic effect of Δ9-THC (Braida, Limonta, Malabarba, Zani, & Sala, 2007). Similarly, genetic deletion of 5-HT2A receptors (5-HT2ARs) blocked the anxiolytic response to Δ9-THC (Viñals et al., 2015). Viñals et al. (2015) further demonstrated that CB1Rs form heterodimeric complexes with 5-HT2ARs and perturbation of this relationship via transmembrane helix interference peptides suppressed the anti-anxiety effect of Δ9-THC. These heterodimers were found in the cortex, hippocampus and striatum, although it’s unknown exactly which regions mediate the anxiolytic effects of Δ9-THC.
Table 3.
Receptor mechanisms underlying Δ9-THC’s affective properties.
| Assay | Target | Antagonist | Knockout mouse | Results | References |
|---|---|---|---|---|---|
| Reward | |||||
| IVSA | CB1Rs | AM251 SR141716A |
N/A | Blocked | Freels et al. (2020, Justinova, Tanda, Redhi, and Goldberg (2003), and Spencer et al. (2018) |
| CPP | CB1Rs | SR141716A | CB1-KO | Blocked | Braida, Iosue, Pegorini, and Sala (2004), Li et al. (2021) |
| ICSS | CB1Rs | SR141716A | N/A | Blocked | Katsidoni, Kastellakis, and Panagis (2013) |
| IVSA | MORs | Naltrexone | N/A | Attenuated | Justinova, Tanda, Munzar, & Goldberg, 2004 |
| CPP | MORs | N/A | MOR-KO | Blocked | Ghozland et al. (2002) |
| IVSA | A2ARs | MSX-3 | N/A | Attenuated | Justinová et al. (2011) |
| IVSA | Presynaptic A2ARs Postsynaptic A2ARs |
SCH-442416 KW-6002 |
N/A | Attenuated Enhanced |
Justinová et al. (2011) and Justinová, Redhi, Goldberg, and Ferré (2014) |
| IVSA | α7nAChRs | Ro 61-8048 | N/A | Attenuated | Justinova et al. (2013) |
| Aversion | |||||
| ICSS | CB1Rs | SR141716A | N/A | Blocked | Katsidoni, Kastellakis, and Panagis (2013) |
| ICSS | CB2Rs | AM630 | N/A | Blocked | Spiller et al. (2019) |
| oICSS | PPARα/PPARγ | GW6471 GW9662 |
N/A | Attenuated | Hempel et al. (2021) |
| oICSS CPA | Subcortical glutamate-CB1Rs | N/A | VgluT2-CB1-KO | Attenuated Blocked |
Han et al. (2017) |
| CPA | CB2Rs | N/A | CB2-KO | Blocked | Li et al. (2021) |
| CPA | Prodynorphin gene | N/A | Dream-KO | Enhanced | Cheng, Laviolette, van der Kooy, and Penninger (2004) |
| CPA | Prodynorphin gene | N/A | Pdyn-KO | Attenuated | Zimmer et al. (2001)) |
| CPA | KORs | Nor-BNI | KOR-KO | Blocked | Clasen et al. (2017), Ghozland et al. (2002) and Norris, Szkudlarek, Pereira, Rushlow, and Laviolette (2019) |
| Anxiolytic effect | |||||
| LDB EPM | CB1Rs | SR141716A | N/A | Blocked | Berrendero and Maldonado (2002) and Rubino et al. (2007) |
| LDB | MORs DORs | β-funaltrexamine Naltrindole |
N/A | Blocked | Berrendero and Maldonado (2002) |
| EPM | 5-HT1ARs | WAY100635 | N/A | Blocked | Braida, Limonta, Malabarba, Zani, and Sala (2007) |
| EPM | 5-HT2ARs | N/A | 5-HT2AR-KO | Blocked | Viñals et al. (2015) |
| EPM | CB2Rs | AM630 | N/A | Blocked | Ivy et al. (2020) |
| Anxiogenic effect | |||||
| EPM | CB1Rs | AM251 | N/A | Blocked | Rubino et al. (2008) |
| EPM | PPARα | GW6471 | N/A | Attenuated | Hempel et al. (2021) |
| EPM Open field | Unknown | CBD | N/A | Attenuated | Liu et al. (2017), Murphy et al. (2017), and Todd and Arnold (2016) |
Abbreviations: IVSA—intravenous self-administration, CPP—conditioned place preference, CPA—conditioned place aversion, ICSS—intracranial self-stimulation, oICSS—optical ICSS, LDB—Light dark box, EPM—elevated plus maze, DREAM—downstream regulatory element antagonistic modulator, Pdyn, prodynorphin.
5.2. Anxiogenic
CB1Rs in the basolateral amygdala mediate the anxiogenic effects of Δ9-THC, as prior work has demonstrated that microinjections of Δ9-THC into this brain region produced anxiety that was attenuated by pretreatment with the CB1R antagonist AM251 (Rubino et al., 2008). Interestingly, mice lacking CB1Rs on GABAergic, but not glutamatergic neurons, failed to demonstrate anxiety in response to CP55,940 (50μg/kg; Rey, Purrio, Viveros, & Lutz, 2012). A GABAB receptor-related mechanism may also contribute to the anxiogenic effect as positive allosteric modulation of GABAB receptors blocked CP55,940-induced anxiety. Whether these findings extend to Δ9-THC’s anxiogenic response is unknown. In addition, we have recently discovered that inhibition of PPARα can attenuate the anxiety produced by 5mg/kg Δ9-THC (Hempel et al., 2021). PPARα is expressed brain regions that regulate anxiety including the amygdala and hippocampus (Kainu, Wikström, Gustafsson, & Pelto-Huikko, 1994; Warden et al., 2016). However, the function of PPARα in the CNS is still being explored and very little is currently known.
A number of studies have demonstrated that co-administration of CBD can inhibit the anxiogenic effects of Δ9-THC (Liu, Scott, & Burnham, 2021; Murphy et al., 2017; Szkudlarek et al., 2019; Todd & Arnold, 2016; Zuardi, Shirakawa, Finkelfarb, & Karniol, 1982). CBD targets a number of receptors, but could suppress the effects of Δ9-THC on anxiety via negative allosteric modulation of the CB1R or 5-HT1AR activation (Campos & Guimarães, 2008; Galaj & Xi, 2021; Laprairie, Bagher, Kelly, & Denovan-Wright, 2015; Russo, Burnett, Hall, & Parker, 2005). Prior work has demonstrated that CBD can block anxiety caused by restraint stress via activity at the 5-HT1AR (Resstel et al., 2009). Along these lines, Szkudlarek et al. (2019) found that intra-PFC injections of CBD suppressed Δ9-THC-induced anxiety in the EPM and 5-HT1ARs are highly expressed in PFC neurons. Further work is needed to ascertain if direct 5-HT1AR activation mediates CBD’s inhibitory effect on Δ9-THC in preclinical models of anxiety (for a summary see Table 3).
6. Cannabinoid cognitive effects
Acute exposure to cannabis impairs executive functions in human users across a number of domains such as attention, inhibitory control, psychomotor control, short term episodic memory, working memory and spatial memory (Crane, Schuster, Fusar-Poli, & Gonzalez, 2013; Crean, Crane, & Mason, 2011; Ranganathan & D’Souza, 2006). In animals, deficits in learning and memory are observed in spatial learning and memory, short term memory, repeated acquisition, habit formation, and fear conditioning (Goodman & Packard, 2015; Kangas et al., 2016; Prini et al., 2020; Resstel, Moreira, & Guimarães, 2009). A comprehensive review of the literature regarding the neurobiology of cannabinoids and cognition is beyond the scope of the present work. Here, we will focus on the neural mechanisms underlying acute effects of the most consistent Δ9-THC-induced neurocognitive impairments, namely spatial learning and memory and short-term memory. A number of different animal models have been used in this context. Two commonly employed tests are the Morris water maze (MWM) and the novel object recognition task (NOR). The MWM assesses spatial learning and memory and in this task, animals are placed in a tank filled with cloudy liquid and must find a hidden platform to escape. In the NOR, subjects are initially exposed to two objects and in a subsequent session they are presented with one familiar and one novel object. Time spent exploring the new object is a measure of short-term memory impairment (for a summary see Table 4).
Table 4.
Receptor mechanisms underlying Δ9-THC-induced learning and memory impairments.
| Assay | Target | Antagonist | Knockout mouse | Results | References |
|---|---|---|---|---|---|
| Spatial learning and memory | |||||
| RAM | CB1R | SR141716A | N/A | Blocked | Lichtman and Martin (1996) |
| MWM | CB1R | SR141716A | CB1-KO | Blocked | Varvel and Lichtman (2002) |
| MWM | Cell-type specific CB1Rs | N/A | GABA-CB1-KO Glu-CB1-KO GFAP-CB1-KO |
No effect No effect Blocked |
Han et al. (2012) |
| MWM | NDMARs | AP-5 | N/A | Blocked | Han et al. (2012) |
| MWM | COX-2 | NS-398 | COX-2-KO− | Blocked | Chen et al. (2013) |
| MWM | A1R | Caffeine | N/A | Enhanced | Sousa et al. (2011) |
| RAM | 5-HT | Clomipramine 5-MeODMT |
N/A | Attenuated Attenuated |
Egashira et al. (2002) |
| RAM | 5-HT2 | DOI | N/A | Attenuated | Egashira et al. (2002) |
| RAM | 5-HT1A | 8-OHDPAT | N/A | Reversed | Inui et al. (2004) |
| RAM | AchE | Physostigmine Tetrahydroaminoacridine |
N/A | Attenuated Attenuated |
Mishima, Egashira, Matsumoto, Iwasaki, and Fujiwara (2002) |
| Short-term memory | |||||
| DNMS | CB1R | SR141617A | N/A | Blocked | Hampson and Deadwyler (2000) |
| DNMP | CB1R | SR141617A | N/A | Attenuated | Hampson and Deadwyler (2000) and Mallet and Beninger (1998a, b) |
| NOR | Mitochondria-specific CB1R | N/A | DN22-CB1 | Blocked | Hebert-Chatelain et al. (2016) |
| NOR | PKC | NPC CHE | N/A | Blocked Blocked |
Busquets-Garcia et al. (2018) |
| DNMP | A1R | Caffeine CPT | N/A | Enhanced Enhanced |
Panlilio et al. (2012) |
| NOR | A2AR | SCH442416 KW-6002 | N/A | Attenuated No effect |
Aso et al. (2019) |
| NOR | 5-HT2AR | MDL 100907 | N/A | Reversed | Viñals et al. (2015) |
| NOR | 5-HT2AR | N/A | 5-HT2AR-K0 | Attenuated | Viñals et al. (2015) |
| DNMS | ACHe | Rivastigmine | N/A | Blocked | Goonawardena, Robinson, Hampson, and Riedel (2010) |
Abbreviations: RAM—radial arm maze, MWM—Morris water maze, DNMS—delayed nonmatch to sample, DNMP—delayed nonmatch to position, NOR—novel object recognition.
6.1. Spatial memory
CB1Rs mediate the impairment in spatial memory after acute administration of Δ9-THC as demonstrated by work in which CB1Rs are antagonized or genetically deleted (Lichtman & Martin, 1996; Varvel & Lichtman, 2002). Intracerebral microinjection studies have established that CB1Rs in the hippocampus and medial PFC (mPFC) underlie this effect (Egashira, Mishima, Iwasaki, & Fujiwara, 2002). Moreover, the synthetic cannabinoid, HU210, produced deficits in spatial memory that were present in the forebrain GABA-CB1-KO and Glu-CB1-KO mice, but absent in mice with a conditional deletion of CB1Rs in astrocytes (Han et al., 2012). In this report, antagonism of glutamatergic NMDA receptors (NMDARs) blocked the effects of HU210 on spatial memory and long-term synaptic depression in the hippocampus (Han et al., 2012). These findings indicate that cannabinoid-induced changes in spatial memory rely on stimulation of CB1Rs on astrocytes and subsequent release of glutamate and changes in NMDAR signaling that induce LTD. It’s unknown whether a similar pathway underlies the effects of Δ9-THC in the MWM. However, antagonism and genetic ablation of cyclooxygenase-2 (COX-2), an enzyme responsible for the conversion of arachidonic acid to prostanoids, blocked Δ9-THC impairments in spatial memory (Chen et al., 2013). These findings tie in with the astrocytic CB1R mechanism as COX-2 enhances prostaglandin E2 (PGE2) release from astrocytes (Chen et al., 2013) and PGE2 signaling can facilitate glutamatergic gliotransmission (Cali, Lopatar, Petrelli, Pucci, & Bezzi, 2014). Overall, astrocytic glutamate release appears to play a pivotal role in the expression of cannabinoid memory impairment.
A number of other pathways are involved in the effects of Δ9-THC on spatial memory. For instance, pretreatment with an adenosine A1 receptor (A1R) agonist (caffeine) significantly elevated the Δ9-THC-induced deficit in spatial memory (Sousa et al., 2011). A1Rs were detected on glutamatergic terminals in the hippocampus and stimulation of A1Rs disinhibited CB1R suppression of hippocampal glutamatergic signaling (Sousa et al., 2011). Activation of the serotonergic system had the opposite effect on Δ9-THC spatial memory impairments, i.e. the deficit was reversed by 5-HT1A and 5HT2 receptor agonism (Egashira et al., 2002; Inui et al., 2004). Δ9-THC also attenuated acetylcholine release in the dorsal hippocampus, which was blocked by administration of a 5-HT1AR agonist in agreement with prior work in which facilitation of acetylcholine release rescued Δ9-THC-induced memory deficits (Inui et al., 2004; Mishima, Egashira, Matsumoto, Iwasaki, & Fujiwara, 2002). These systems may work in concert with astrocyte mediated glutamate release to mediate Δ9-THC’s effects on spatial memory.
6.2. Short-term memory
Unsurprisingly, activation of CB1Rs mediates the Δ9-THC-induced deficits in short-term memory as confirmed by CB1R antagonism studies (Hampson & Deadwyler, 2000; Mallet & Beninger, 1998a, b). The hippocampus appears to be a locus of interest in this respect as in vivo recording from groups of hippocampal neurons during performance of a short-term memory task revealed attenuated firing strength following Δ9-THC administration (Hampson & Deadwyler, 2000). One group has demonstrated that mice lacking CB1Rs in hippocampal mitochondria fail to demonstrate a disruption in short-term memory following WIN55,212-2 administration (Hebert-Chatelain et al., 2016). CB1Rs on mitochondria inhibit soluble-adenylyl cyclase, which decreases protein kinase A phosphorylation and has downstream impacts on mitochondrial energetic activity (Hebert-Chatelain et al., 2016). However, recent work has shown that intra-hippocampal injections of a protein kinase C inhibitor blocked Δ9-THC’s effects on short-term memory (Busquets-Garcia et al., 2018). It’s not clear whether the differential activity at these two protein kinase families works in conjunction to produce cannabinoid memory deficits or if the cannabinoid agent selected drives activity at one or the other group of enzymes.
The adenosine system has also been implicated in Δ9-THC’s inhibitory effect on short-term memory. Administration of caffeine or a selective A1R antagonist potentiated Δ9-THC-induced short-term memory impairments (Panlilio et al., 2012). On the other hand, A2AR antagonism mediated the therapeutic effect of CBD on Δ9-THC short-term memory deficits (Aso et al., 2019). A1 and A2AR antagonists have recently been shown to differentially regulate synaptic plasticity in the hippocampus, which could underlie their opposing actions on cannabinoid memory changes (Reis et al., 2019). As described earlier, CB1Rs form heterodimers with 5HT2ARs and evidence of these structures has been detected in the hippocampus (Viñals et al., 2015). Both genetic deletion and pharmacological antagonism of 5HT2ARs reversed Δ9-THC-induced changes in short-term memory (Viñals et al., 2015). Finally, cholinergic hypofunction has been hypothesized to play a role in the cognitive deficits produced by cannabinoids. Goonawardena, Robinson, Hampson, and Riedel (2010) found that an acetylcholinesterase inhibitor blocked WIN55,212-2-induced memory impairing properties and restored the normal firing patterns of hippocampal neurons. Whether these findings can be applied to Δ9-THC is unknown.
7. Conclusion
The majority of Δ9-THC’s pharmacological and behavioral effects are mediated by activation of CB1Rs (Tables 2–4). However, the cell types and brain regions expressing these receptors varies depending on the behavior assessed. Despite the widespread expression of CB1Rs on GABAergic neurons, glutamatergic CB1Rs preferentially underlie many of Δ9-THC’s effects including hypothermia, hypolocomotion, aversion, and anxiety relief. New research has discovered functional roles for CB1Rs expressed on astrocytes and mitochondria in cannabinoid-induced cognitive impairments. This represents an exciting and promising area for future work, as non-neuronal CB1R expression has been mostly ignored. Similarly, Δ9-THC binds to a number of other targets such as CB2R, GPR55, PPARs. The data from our lab has demonstrated that activation of these non-CB1 receptors also modulate Δ9-THC-induced behavior (Hempel et al., 2021; Li et al., 2021; Spiller et al., 2019; Wang et al., 2020). A host of additional non-cannabinoid receptor systems have been implicated in Δ9-THC’s behavioral effects primarily serotonergic (5-HT1ARs & 5-HT2ARs), opioidergic (MORs & KORs), and adenosinergic (A1Rs & A2ARs) signaling. Activity at these receptors may stem from downstream activity following CB1R or CB2R stimulation. Given the increasing use of cannabis for both recreational and medicinal purposes, understanding the neurobiology of Δ9-THC’s CNS effects is of vital concern. Moreover, this research provides a basis for the design of pharmacotherapeutics for substance use disorders including cannabis use disorder as well as non-psychoactive alternatives for medical marijuana users.
Acknowledgments
This work was supported by the Intramural Research Program (IRP) at the National Institute on Drug Abuse (NIDA; DA000620-02), National Institutes of Health, U.S. Public Health Service, USA. We thank Dr. Mikes Herkenham at Section on Functional Neuroanatomy, Laboratory of Cellular and Molecular Regulation, National Institute on Mental Health (NIMH), Bethesda, MD, USA, for providing the original high quality image in Fig. 2.
Abbreviations
- Δ9-THC
Δ9-tetrahydrocannabinol
- AEA
Anandamide
- 2-AG
2-arachidonoyl glycerol
- ChR2
channelrhodopsin 2
- GABA
gamma-aminobutyric acid
- GPR55
G protein-coupled receptor 55
- GPCR
G protein-coupled receptor
- ISH
in situ hybridization
- NAc
nucleus accumbens
- oICSS
optical intracranial self-stimulation
- PPARα
peroxisome proliferator-activated nuclear receptor alpha
- PPARγ
peroxisome proliferator-activated nuclear receptor gamma
- TRPV1
transient receptor potential vanilloid 1 channel
- VTA
ventral tegmental area
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, et al. (2007). Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nature Neuroscience, 10(7), 870–879. 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Capogna M, Bevan S, & Nagy I (2000). Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience, 100(4), 685–688. 10.1016/S0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Patwardhan A, & Hargreaves KM (2008). Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. The Journal of Neuroscience, 28(5), 1064–1075. 10.1523/jneurosci.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH (2016). Therapeutic potential of cannabis-related drugs. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 64, 157–166. 10.1016/j.pnpbp.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Aso E, Fernández-Dueñas V, López-Cano M, Taura J, Watanabe M, Ferrer I, et al. (2019). Adenosine A2A-cannabinoid CB1 receptor heteromers in the hippocampus: Cannabidiol blunts Δ9-tetrahydrocannabinol-induced cognitive impairment. Molecular Neurobiology, 56(8), 5382–5391. 10.1007/s12035-018-1456-3. [DOI] [PubMed] [Google Scholar]
- Atwood BK, & Mackie K (2010). CB2: A cannabinoid receptor with an identity crisis. British Journal of Pharmacology, 160(3), 467–479. 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Pryce G, Davies WL, & Hiley CR (2006). In silico patent searching reveals a new cannabinoid receptor. Trends in Pharmacological Sciences, 27(1), 1–4. 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Bénard G, Massa F, Puente N, Lourenço J., Bellocchio L., Soria-Gómez E., et al. (2012). Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nature Neuroscience, 15(4), 558–564. 10.1038/nn.3053. [DOI] [PubMed] [Google Scholar]
- Berrendero F, & Maldonado R (2002). Involvement of the opioid system in the anxiolytic-like effects induced by Δ9-tetrahydrocannabinol. Psychopharmacology, 163(1), 111–117. 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- Bishop-Bailey D (2000). Peroxisome proliferator-activated receptors in the cardiovascular system. British Journal of Pharmacology, 129(5), 823–834. 10.1038/sj.bjp.0703149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez C, Ruiz-Calvo A, Bajo-Grañeras R, Baufreton JM, Resel E, Varilh M, et al. (2020). Inhibition of striatonigral autophagy as a link between cannabinoid intoxication and impairment of motor coordination. eLife, 9, e56811. 10.7554/eLife.56811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyíri G, Mackie K, Ledent C, Hájos N, et al. (2005). Endocannabinoid signaling in rat somatosensory cortex: Laminar differences and involvement of specific interneuron types. The Journal of Neuroscience, 25(29), 6845–6856. 10.1523/jneurosci.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Iosue S, Pegorini S, & Sala M (2004). Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. European Journal of Pharmacology, 506(1), 63–69. 10.1016/j.ejphar.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Malabarba L, Zani A, & Sala M (2007). 5-HT1A receptors are involved in the anxiolytic effect of Δ9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague–Dawley rats. European Journal of Pharmacology, 555(2), 156–163. 10.1016/j.ejphar.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Brown TM, Brotchie JM, & Fitzjohn SM (2003). Cannabinoids decrease corticostriatal synaptic transmission via an effect on glutamate uptake. The Journal of Neuroscience, 23(35), 11073–11077. 10.1523/jneurosci.23-35-11073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro P, Saez T, & Onaivi ES (2008a). Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse, 62(12), 944–949. 10.1002/syn.20569. [DOI] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro P, Saez T, & Onaivi ES (2008b). Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Annals of the New York Academy of Sciences, 1139(1), 450–457. [DOI] [PubMed] [Google Scholar]
- Burgdorf CE, Jing D, Yang R, Huang C, Hill MN, Mackie K, et al. (2020). Endocannabinoid genetic variation enhances vulnerability to THC reward in adolescent female mice. Science Advances, 6(7), eaay1502. 10.1126/sciadv.aay1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-Garcia A, Gomis-González M, Salgado-Mendialdúa V, Galera-López L, Puighermanal E, Martín-García E, et al. (2018). Hippocampal protein kinase C signaling mediates the short-term memory impairment induced by Delta9-tetrahydrocannabinol. Neuropsychopharmacology, 43(5), 1021–1031. 10.1038/npp.2017.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabañero D, Ramírez-López A, Drews E, Schmöle A, Otte DM, Wawrzczak-Bargiela A, et al. (2020). Protective role of neuronal and lymphoid canna-binoid CB2 receptors in neuropathic pain. eLife, 9, e55582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA, & Griffin-Thomas L (2008). Cannabinoids as therapeutic agents for ablating neuroinflammatory disease. Endocrine, Metabolic & Immune Disorders Drug Targets, 8(3), 159–172. 10.2174/187153008785700118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Ghiglieri V, & Di Filippo M (2014). Direct and indirect pathways of basal ganglia: a critical reappraisal. Nature Neuroscience, 17(8), 1022–1030. 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- Cali C, Lopatar J, Petrelli F, Pucci L, & Bezzi P (2014). G-protein coupled receptor-evoked glutamate exocytosis from astrocytes: Role of prostaglandins. Neural Plasticity, 2014, 254574. 10.1155/2014/254574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, & Guimarães FS (2008). Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology, 199(2), 223. 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- Canseco-Alba A, Schanz N, Sanabria B, Zhao J, Lin Z, Liu Q-R, et al. (2019). Behavioral effects of psychostimulants in mutant mice with cell-type specific deletion of CB2 cannabinoid receptors in dopamine neurons. Behavioural Brain Research, 360, 286–297. 10.1016/j.bbr.2018.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carliner H, Brown QL, Sarvet AL, & Hasin DS (2017). Cannabis use, attitudes, and legal status in the U.S.: A review. Preventive Medicine, 104, 13–23. 10.1016/j.ypmed.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton SM, & Hargett GL (2002). Stereological analysis of Ca2+/calmodulin-dependent protein kinase IIα-containing dorsal root ganglion neurons in the rat: Colocalization with isolectin Griffonia simplicifolia, calcitonin gene-related peptide, or vanilloid receptor 1. Journal of Comparative Neurology, 448(1), 102–110. 10.1002/cne.10250. [DOI] [PubMed] [Google Scholar]
- Casey SL, Atwal N, & Vaughan CW (2017). Cannabis constituent synergy in a mouse neuropathic pain model. Pain, 158(12), 2452–2460. 10.1097/j.pain.0000000000001051. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chávez AE, & Hashimotodani Y (2012). Endocannabinoid signaling and synaptic function. Neuron, 76(1), 70–81. 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celorrio M, Rojo-Bustamante E, Fernández-Suárez D, Sáez E, Estella-Hermoso de Mendoza A, Müller CE, et al. (2017). GPR55: A therapeutic target for Parkinson’s disease? Neuropharmacology, 125, 319–332. 10.1016/j.neuropharm.2017.08.017. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Cadogan AK, Marsden CA, Fone KCF, & Kendall DA (1999). Modification of 5-HT2 receptor mediated behaviour in the rat by oleamide and the role of cannabinoid receptors. Neuropharmacology, 38(4), 533–541. 10.1016/S0028-3908(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Zhang J, Fan N, Teng Z-Q, Wu Y, Yang H, et al. (2013). Δ9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell, 155(5), 1154–1165. 10.1016/j.cell.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H-YM, Laviolette SR, van der Kooy D, & Penninger JM (2004). DREAM ablation selectively alters THC place aversion and analgesia but leaves intact the motivational and analgesic effects of morphine. The European Journal of Neuroscience, 19(11), 3033–3041. 10.1111/j.0953-816X.2004.03435.x. [DOI] [PubMed] [Google Scholar]
- Clasen MM, Flax SM, Hempel BJ, Cheng K, Rice KC, & Riley AL (2017). Antagonism of the kappa opioid receptor attenuates THC-induced place aversions in adult male Sprague-Dawley rats. Pharmacology Biochemistry and Behavior, 163, 30–35. 10.1016/j.pbb.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DR, Aceto MD, Lowe J, & Martin BR (1996). In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): Inhibition of delta 9-tetrahydrocannabinol-induced responses and apparent agonist activity. The Journal of Pharmacology and Experimental Therapeutics, 277(2), 586–594. [PubMed] [Google Scholar]
- Craft RM, Haas AE, Wiley JL, Yu Z, & Clowers BH (2017). Gonadal hormone modulation of △9-tetrahydrocannabinol-induced antinociception and metabolism in female versus male rats. Pharmacology Biochemistry and Behavior, 152, 36–43. 10.1016/j.pbb.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, & Gonzalez R (2013). Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychology Review, 23(2), 117–137. 10.1007/s11065-012-9222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Crane NA, & Mason BJ (2011). An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. Journal of Addiction Medicine, 5(1), 1–8. 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, & Bertrand D (2007). Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annual Review of Pharmacology and Toxicology, 47(1), 699–729. 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- De Giacomo V, Ruehle S, Lutz B, Häring M, & Remmers F (2020). Differential glutamatergic and GABAergic contributions to the tetrad effects of Δ(9)-tetrahydrocannabinol revealed by cell-type-specific reconstitution of the CB1 receptor. Neuropharmacology, 179, 108287. 10.1016/j.neuropharm.2020.108287. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, et al. (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. British Journal of Pharmacology, 163(7), 1479–1494. 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Nabissi M, Santoni G, & Ligresti A (2017). Chapter Eight—Actions and regulation of ionotropic cannabinoid receptors. In Kendall D, & Alexander SPH (Eds.), Vol. 80. Advances in pharmacology (pp. 249–289). Academic Press. [DOI] [PubMed] [Google Scholar]
- Devane WA, Breuer A, Sheskin T, Jaerbe TU, Eisen MS, & Mechoulam R (1992). A novel probe for the cannabinoid receptor. Journal of Medicinal Chemistry, 35(11), 2065–2069. [DOI] [PubMed] [Google Scholar]
- Dimidschstein J, Chen Q, Tremblay R, Rogers SL, Saldi GA, Guo L, et al. (2016). A viral strategy for targeting and manipulating interneurons across vertebrate species. Nature Neuroscience, 19(12), 1743–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeungoue-Petga M-A, & Hebert-Chatelain E (2017). Linking mitochondria and synaptic transmission: The CB1 receptor. BioEssays, 39(12), 1700126. 10.1002/bies.201700126. [DOI] [PubMed] [Google Scholar]
- Egashira N, Matsuda T, Koushi E, Mishima K, Iwasaki K, Shoyama Y, et al. (2006). Involvement of 5-hydroxytryptamine1A receptors in Δ9-tetrahydrocannabinol-induced catalepsy-like immobilization in mice. European Journal of Pharmacology, 550(1), 117–122. 10.1016/j.ejphar.2006.08.051. [DOI] [PubMed] [Google Scholar]
- Egashira N, Mishima K, Iwasaki K, & Fujiwara M (2002). Intracerebral microinjections of delta 9-tetrahydrocannabinol: search for the impairment of spatial memory in the eight-arm radial maze in rats. Brain Research, 952(2), 239–245. 10.1016/s0006-8993(02)03247-x. [DOI] [PubMed] [Google Scholar]
- Egashira N, Mishima K, Katsurabayashi S, Yoshitake T, Matsumoto Y, Ishida J, et al. (2002). Involvement of 5-hydroxytryptamine neuronal system in Δ9-tetrahydrocannabinol-induced impairment of spatial memory. European Journal of Pharmacology, 445(3), 221–229. 10.1016/S0014-2999(02)01755-7. [DOI] [PubMed] [Google Scholar]
- Elbegdorj O, Westkaemper RB, & Zhang Y (2013). A homology modeling study toward the understanding of three-dimensional structure and putative pharmacological profile of the G-protein coupled receptor GPR55. Journal of Molecular Graphics and Modelling, 39, 50–60. 10.1016/j.jmgm.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewusi Boisvert E, Bae D, Pang RD, Davis JP, Kelley-Quon LI, Barrington-Trimis JL, et al. (2020). Subjective effects of combustible, vaporized, and edible cannabis: Results from a survey of adolescent cannabis users. Drug and Alcohol Dependence, 206, 107716. 10.1016/j.drugalcdep.2019.107716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Egertová M, Bradbury EJ, McMahon SB, Rice ASC, & Elphick MR (2000). Cannabinoid CB1 receptor expression in rat spinal cord. Molecular and Cellular Neuroscience, 15(6), 510–521. 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- Fatemi I, Abdollahi A, Shamsizadeh A, Allahtavakoli M, & Roohbakhsh A (2021). The effect of intra-striatal administration of GPR55 agonist (LPI) and antagonist (ML193) on sensorimotor and motor functions in a Parkinson’s disease rat model. Acta Neuropsychiatrica, 33(1), 15–21. 10.1017/neu.2020.30. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Tomasini MC, Gessa GL, Bebe BW, Tanganelli S, & Antonelli T (2001). The cannabinoid receptor agonist WIN 55,212-2 regulates glutamate transmission in rat cerebral cortex: An in vivo and in vitro study. Cerebral Cortex, 11(8), 728–733. 10.1093/cercor/11.8.728. [DOI] [PubMed] [Google Scholar]
- Ferre S, Quiroz C, Orru M, Guitart X, Navarro G, Cortes A, et al. (2011). Adenosine A2A receptors and A2A receptor heteromers as key players in striatal function. Frontiers in Neuroanatomy, 5(36). 10.3389/fnana.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SG, Gonçalves FQ., Marques JM., Tomé ÂR., Rodrigues RJ., Nunes-Correia I., et al. (2015). Presynaptic adenosine A2A receptors dampen cannabinoid CB1 receptor-mediated inhibition of corticostriatal glutamatergic transmission. British Journal of Pharmacology, 172(4), 1074–1086. 10.1111/bph.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Roe CH, Madjd A, Fone KC, Kendall DA, et al. (2004). Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. The European Journal of Neuroscience, 19(3), 678–686. 10.1111/j.0953-816x.2004.03177.x. [DOI] [PubMed] [Google Scholar]
- Fitton AG, & Pertwee RG (1982). Changes in body temperature and oxygen consumption rate of conscious mice produced by intrahypothalamic and intracerebroventricular injections of Δ9-tetrahydrocannabinol. British Journal of Pharmacology, 75(2), 409–414. 10.1111/j.1476-5381.1982.tb08802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foll BL, Wiggins M, & Goldberg SR (2006). Nicotine pre-exposure does not potentiate the locomotor or rewarding effects of Δ-9-tetrahydrocannabinol in rats. Behavioural Pharmacology, 17(2). Retrieved from http://journals.lww.com/behaviouralpharm/Fulltext/2006/03000/Nicotine_pre_exposure_does_not_potentiate_the.10.aspx. [DOI] [PubMed] [Google Scholar]
- Freels TG, Baxter-Potter LN, Lugo JM, Glodosky NC, Wright HR, Baglot SL, et al. (2020). Vaporized cannabis extracts have reinforcing properties and support conditioned drug-seeking behavior in rats. The Journal of Neuroscience, 40(9), 1897–1908. 10.1523/jneurosci.2416-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French ED, Dillon K, & Wu X (1997). Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport, 8(3). Retrieved from http://journals.lww.com/neuroreport/Fulltext/1997/02100/Cannabinoids_excite_dopamine_neurons_in_the.14.aspx. [DOI] [PubMed] [Google Scholar]
- Friend L, Weed J, Sandoval P, Nufer T, Ostlund I, & Edwards JG (2017). CB1-dependent long-term depression in ventral tegmental area GABA neurons: A novel target for marijuana. The Journal of Neuroscience, 37(45), 10943–10954. 10.1523/jneurosci.0190-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, & Xi Z-X (2019). Potential of cannabinoid receptor ligands as treatment for substance use disorders. CNS Drugs, 33(10), 1001–1030. 10.1007/s40263-019-00664-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, & Xi Z-X (2021). Possible receptor mechanisms underlying cannabidiol effects on addictive-like behaviors in experimental animals. International Journal of Molecular Sciences, 22(1), 134. Retrieved from https://www.mdpi.com/1422-0067/22/1/134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, et al. (1995). Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. European Journal of Biochemistry, 232(1), 54–61. 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, & Mechoulam R (1964). Isolation, structure, and partial synthesis of an active constituent of hashish. Journal of the American Chemical Society, 86(8), 1646–1647. [Google Scholar]
- Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, et al. (1988). Facilitation of brain stimulation reward by delta 9-tetrahydrocannabinol. Psychopharmacology, 96(1), 142–144. [DOI] [PubMed] [Google Scholar]
- Ghozland S, Matthes HWD, Simonin F, Filliol D, Kieffer BL, & Maldonado R (2002). Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22(3), 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Faull RLM, & Dragunow M (1997). Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience, 77(2), 299–318. 10.1016/S0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gong J-P, Onaivi ES, Ishiguro H, Liu Q-R, Tagliaferro PA, Brusco A, et al. (2006). Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Research, 1071(1), 10–23. 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Goodman J, & Packard MG (2015). The influence of cannabinoids on learning and memory processes of the dorsal striatum. Neurobiology of Learning and Memory, 125, 1–14. 10.1016/j.nlm.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Goonawardena AV, Robinson L, Hampson RE, & Riedel G (2010). Cannabinoid and cholinergic systems interact during performance of a short-term memory task in the rat. Learning & Memory, 17(10), 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM (2000). The basal ganglia. Current Biology, 10(14), R509–R511. [DOI] [PubMed] [Google Scholar]
- Green B, Kavanagh D, & Young R (2003). Being stoned: A review of self-reported cannabis effects. Drug and Alcohol Review, 22(4), 453–460. 10.1080/09595230310001613976. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Rodríguez A, Puente N, Elezgarai I, Ruehle S, Lutz B, Reguero L, et al. (2017). Anatomical characterization of the cannabinoid CB1 receptor in cell-type–specific mutant mouse rescue models. Journal of Comparative Neurology, 525(2), 302–318. 10.1002/cne.24066. [DOI] [PubMed] [Google Scholar]
- Hampson RE, & Deadwyler SA (2000). Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. The Journal of Neuroscience, 20(23), 8932–8942. 10.1523/jneurosci.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, He Y, Bi G-H, Zhang H-Y, Song R, Liu Q-R, et al. (2017). CB1 receptor activation on VgluT2-expressing glutamatergic neurons underlies Δ 9-tetrahydrocannabinol (Δ 9-THC)-induced aversive effects in mice. Scientific Reports, 7(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, et al. (2012). Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell, 148(5), 1039–1050. 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Hazekawa M, Ogata A, Fujioka M, et al. (2007). Δ9-tetrahydrocannabinol (Δ9-THC) prevents cerebral infarction via hypothalamic-independent hypothermia. Life Sciences, 80(16), 1466–1471. 10.1016/j.lfs.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Hebert-Chatelain E, Desprez T, Serrat R, Bellocchio L, Soria-Gomez E, Busquets-Garcia A, et al. (2016). A cannabinoid link between mitochondria and memory. Nature, 539(7630), 555–559. 10.1038/nature20127. [DOI] [PubMed] [Google Scholar]
- Hempel B, Bi GH, Klein B, & Xi ZX (2021). Peroxisome proliferator-activated receptors modulate optical brain-stimulation reward in DAT-Cre mice. Under review. [Google Scholar]
- Hempel BJ, Clasen MM, Nelson KH, Woloshchuk CJ, & Riley AL (2018). An assessment of concurrent cannabidiol and Δ9-tetrahydrocannabinol administration in place aversion and taste avoidance conditioning. Experimental and Clinical Psychopharmacology, 26(2), 205–213. 10.1037/pha0000188. [DOI] [PubMed] [Google Scholar]
- Henstidge CM, Balenga NAB, Ford LA, Ross RA, Waldhoer M, & Irving AJ (2009). The GPR55 ligand L-α-lysophosphatidylinositol promotes RhoA-dependent Ca2+ signaling and NFAT activation. The FASEB Journal, 23(1), 183–193. 10.1096/fj.08-108670. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn A, Johnson M, Melvin L, de Costa B, & Rice K (1991). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. The Journal of Neuroscience, 11(2), 563–583. 10.1523/jneurosci.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. (1990). Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences, 87(5), 1932–1936. Retrieved from https://www.pnas.org/content/pnas/87/5/1932.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann H, Marsicano G, & Lutz B (2002). Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience, 109(3), 451–460. 10.1016/S0306-4522(01)00509-7. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Laaris N, Kawamura M, Masino SA, & Lupica CR (2010). Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. The Journal of Neuroscience, 30(2), 545–555. 10.1523/jneurosci.4920-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser SJ, Eads M, Embrey JP, & Welch SP (2000). Dynorphin B and spinal analgesia: induction of antinociception by the cannabinoids CP55,940, Delta(9)-THC and anandamide. Brain Research, 857(1–2), 337–342. 10.1016/s0006-8993(00)01981-8. [DOI] [PubMed] [Google Scholar]
- Howlett AC (2005). Cannabinoid receptor signaling. In Pertwee RG (Ed.), Cannabinoids (pp. 53–79). Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. (2002). International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacological Reviews, 54(2), 161–202. 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Blume LC, & Dalton GD (2010). CB1 cannabinoid receptors and their associated proteins. Current Medicinal Chemistry, 17(14), 1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SS-J, & Mackie K (2015). Distribution of the endocannabinoid system in the central nervous system. In Pertwee RG (Ed.), Endocannabinoids (pp. 59–93). Cham: Springer International Publishing. [DOI] [PubMed] [Google Scholar]
- Humburg BA, Jordan CJ, Zhang H-Y, Shen H, Han X, Bi G-H, et al. (2021). Optogenetic brain-stimulation reward: A new procedure to re-evaluate the rewarding versus aversive effects of cannabinoids in dopamine transporter-Cre mice. Addiction Biology, 26(4), e13005. 10.1111/adb.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbernon M, Whyte L, Diaz-Arteaga A, Russell WR, Moreno NR, Vazquez MJ, et al. (2014). Regulation of GPR55 in rat white adipose tissue and serum LPI by nutritional status, gestation, gender and pituitary factors. Molecular and Cellular Endocrinology, 383(1), 159–169. 10.1016/j.mce.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Inui K, Egashira N, Mishima K, Yano A, Matsumoto Y, Hasebe N, et al. (2004). The serotonin1A receptor agonist 8-OHDPAT reverses delta 9-tetrahydrocannabinol-induced impairment of spatial memory and reduction of acetylcholine release in the dorsal hippocampus in rats. Neurotoxicity Research, 6(2), 153–158. 10.1007/bf03033218. [DOI] [PubMed] [Google Scholar]
- Ivy D, Palese F, Vozella V, Fotio Y, Yalcin A, Ramirez G, et al. (2020). Cannabinoid CB2 receptors mediate the anxiolytic-like effects of monoacylglycerol lipase inhibition in a rat model of predator-induced fear. Neuropsychopharmacology, 45(8), 1330–1338. 10.1038/s41386-020-0696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura H, Suzuki H, Ueda Y, Kaya T, & Inaba T (2001). In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. Journal of Pharmacology and Experimental Therapeutics, 296(2), 420–425. Retrieved from https://jpet.aspetjournals.org/content/jpet/296/2/420.full.pdf. [PubMed] [Google Scholar]
- Jansen EM, Haycock DA, Ward SJ, & Seybold VS (1992). Distribution of cannabinoid receptors in rat brain determined with aminoalkylindoles. Brain Research, 575(1), 93–102. 10.1016/0006-8993(92)90428-C. [DOI] [PubMed] [Google Scholar]
- Jarrahian A, Watts VJ, & Barker EL (2004). D2 dopamine receptors modulate Galpha-subunit coupling of the CB1 cannabinoid receptor. The Journal of Pharmacology and Experimental Therapeutics, 308(3), 880–886. 10.1124/jpet.103.057620. [DOI] [PubMed] [Google Scholar]
- John WS, Martin TJ, Solingapuram Sai KK, Nader SH, Gage HD, Mintz A, et al. (2018). Chronic Δ9-THC in rhesus monkeys: Effects on cognitive performance and dopamine D2/D3 receptor availability. Journal of Pharmacology and Experimental Therapeutics, 364(2), 300–310. 10.1124/jpet.117.244194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Humburg B, Rice M, Bi G-H, You Z-B, Shaik AB, et al. (2019). The highly selective dopamine D3R antagonist, R-VK4-40 attenuates oxycodone reward and augments analgesia in rodents. Neuropharmacology, 158, 107597. 10.1016/j.neuropharm.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, & Xi ZX (2019). Progress in brain cannabinoid CB(2) receptor research: From genes to behavior. Neuroscience and Biobehavioral Reviews, 98, 208–220. 10.1016/j.neubiorev.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinová Z, Ferre S, Redhi GH, Mascia P, Stroik J, Quarta D, et al. (2011). Reinforcing and neurochemical effects of cannabinoid CB1 receptor agonists, but not cocaine, are altered by an adenosine A2A receptor antagonist. Addiction Biology, 16(3), 405–415. 10.1111/j.1369-1600.2010.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Mascia P, Wu H-Q, Secci ME, Redhi GH, Panlilio LV, et al. (2013). Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nature Neuroscience, 16(11), 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinová Z, Redhi GH, Goldberg SR, & Ferre S (2014). Differential effects of presynaptic versus postsynaptic adenosine A2A receptor blockade on Δ9-tetrahydrocannabinol (THC) self-administration in squirrel monkeys. The Journal of Neuroscience, 34(19), 6480–6484. 10.1523/jneurosci.5073-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Munzar P, & Goldberg SR (2004). The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology, 173(1–2), 186–194. 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, & Goldberg SR (2003). Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology, 169(2), 135–140. 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Kainu T, Wikström AC, Gustafsson JA, & Pelto-Huikko M (1994). Localization of the peroxisome proliferator-activated receptor in the brain. Neuroreport, 5(18), 2481–2485. 10.1097/00001756-199412000-00019. [DOI] [PubMed] [Google Scholar]
- Kangas BD, Leonard MZ, Shukla VG, Alapafuja SO, Nikas SP, Makriyannis A, et al. (2016). Comparisons of Δ9-tetrahydrocannabinol and anandamide on a battery of cognition-related behavior in nonhuman primates. Journal of Pharmacology and Experimental Therapeutics, 357(1), 125–133. 10.1124/jpet.115.228189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Tränkle C, & Schlicker E (2006). Cannabidiol is an allosteric modulator at mu-and delta-opioid receptors. Naunyn-Schmiedeberg’s Archives of Pharmacology, 372(5), 354–361. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, et al. (1999). Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. The Journal of Neuroscience, 19(11), 4544–4558. 10.1523/jneurosci.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsidoni V, Kastellakis A, & Panagis G (2013). Biphasic effects of Delta9-tetrahydrocannabinol on brain stimulation reward and motor activity. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP), 16(10), 2273–2284. 10.1017/S1461145713000709. [DOI] [PubMed] [Google Scholar]
- Kimura T, Ohta T, Watanabe K, Yoshimura H, & Yamamoto I (1998). Anandamide, an endogenous cannabinoid receptor ligand, also interacts with 5-hydroxytryptamine (5-HT) receptor. Biological and Pharmaceutical Bulletin, 21(3), 224–226. [DOI] [PubMed] [Google Scholar]
- Kimura T, Yamamoto I, Ohta T, Yoshida H, & Watanabe K (1996). Changes in 5-HT receptor binding induced by tetrahydrocannabinol metabolites in bovine cerebral cortex. Research Communications in Alcohol and Substances of Abuse, 17(1–2), 57–69. [Google Scholar]
- Kinoshita H, Hasegawa T, Katsumata Y, Kameyama T, Yamamoto I, & Nabeshima T (1994). Effect of dizocilpine (MK-801) on the catalepsy induced by Δ9-tetrahydrocannabinol in mice. Journal of Neural Transmission/General Section JNT, 95(2), 137–143. 10.1007/BF01276432. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, O’Neal ST, Long JZ, Cravatt BF, & Lichtman AH (2011). Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacology Biochemistry and Behavior, 98(1), 21–27. 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köfalvi A, Moreno E, Cordomí A., Cai N-S., Fernández-Dueñas V., Ferreira SG., et al. (2020). Control of glutamate release by complexes of adenosine and cannabinoid receptors. BMC Biology, 18(1), 9. 10.1186/s12915-020-0739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, et al. (2010). Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature, 466(7306), 622–626. 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaris N, Good CH, & Lupica CR (2010). Δ9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus. Neuropharmacology, 59(1), 121–127. 10.1016/j.neuropharm.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Barroso-Chinea P, Rico AJ, Conte-Perales L, Callén L, Roda E, et al. (2011). Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. Journal of Psychopharmacology, 25(1), 97–104. 10.1177/0269881110367732. [DOI] [PubMed] [Google Scholar]
- Lane JR, Beukers MW, Mulder-Krieger T, & Ijzerman AP (2010). The endocannabinoid 2-arachidonylglycerol is a negative allosteric modulator of the human A3 adenosine receptor. Biochemical Pharmacology, 79(1), 48–56. 10.1016/j.bcp.2009.07.024. [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly MEM, & Denovan-Wright EM (2015). Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. British Journal of Pharmacology, 172(20), 4790–4805. 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. (1999). Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science, 283(5400), 401–404. 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lefever TW, Marusich JA, Antonazzo KR, & Wiley JL (2014). Evaluation of WIN 55,212-2 self-administration in rats as a potential cannabinoid abuse liability model. Pharmacology, Biochemistry, and Behavior, 118, 30–35. 10.1016/j.pbb.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, & Lazar MA (2005). The many faces of PPARγ. Cell, 123(6), 993–999. 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Lepore M, Liu X, Savage V, Matalon D, & Gardner EL (1996). Genetic differences in Δ9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sciences, 58(25), PL365–PL372. 10.1016/0024-3205(96)00237-8. [DOI] [PubMed] [Google Scholar]
- Lepore M, Vorel SR, Lowinson J, & Gardner EL (1995). Conditioned place preference induced by !D–9-tetrahydrocannabinol: Comparison with cocaine, morphine, and food reward. Life Sciences, 56(23–24), 2073–2080. 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- Leung L (2011). Cannabis and its derivatives: Review of medical use. The Journal of the American Board of Family Medicine, 24(4), 452–462. 10.3122/jabfm.2011.04.100280. [DOI] [PubMed] [Google Scholar]
- Li K, Fichna J, Schicho R, Saur D, Bashashati M, Mackie K, et al. (2013). A role for O-1602 and G protein-coupled receptor GPR55 in the control of colonic motility in mice. Neuropharmacology, 71, 255–263. 10.1016/j.neuropharm.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hempel BJ, Yang H-J, Han X, Bi G-H, Gardner EL, et al. (2021). Dissecting the role of CB1 and CB2 receptors in cannabinoid reward versus aversion using transgenic CB1- and CB2-knockout mice. European Neuropsychopharmacology, 43, 38–51. 10.1016/j.euroneuro.2020.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hoffman AF, Peng XQ, Lupica CR, Gardner EL, & Xi ZX (2009). Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice. Psychopharmacology, 204(1), 1–11. 10.1007/s00213-008-1432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, & Kim J (2015). Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience, 311, 253–267. 10.1016/j.neuroscience.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, & Martin BR (1996). Delta 9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology, 126(2), 125–131. 10.1007/bf02246347. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, & Martin BR (1997). The selective cannabinoid antagonist SR 141716A blocks cannabinoid-induced antinociception in rats. Pharmacology Biochemistry and Behavior, 57(1), 7–12. 10.1016/S0091-3057(96)00121-9. [DOI] [PubMed] [Google Scholar]
- Liu QR, Canseco-Alba A, Liang Y, Ishiguro H, & Onaivi ES. (2020). Low basal CB2R in dopamine neurons and microglia influences cannabinoid tetrad effects. International Journal of Molecular Sciences, 21(24), 9763. 10.3390/ijms21249763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Canseco-Alba A, Liang Y, Ishiguro H, & Onaivi ES (2020). Low Basal CB2R in Dopamine Neurons and Microglia Influences Cannabinoid Tetrad Effects. International Journal of Molecular Sciences, 21(24). 10.3390/ijms21249763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q-R, Canseco-Alba A, Zhang H-Y, Tagliaferro P, Chung M, Dennis E, et al. (2017). Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Scientific Reports, 7(1), 17410. 10.1038/s41598-017-17796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q-R, Pan C-H, Hishimoto A, Li C-Y, Xi Z-X, Llorente-Berzal A, et al. (2009). Species differences in cannabinoid receptor 2 (CNR2 gene): Identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genes, Brain and Behavior, 8(5), 519–530. 10.1111/j.1601-183X.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Scott BW, & Burnham WM (2021). Effects of cannabidiol and Δ9-tetrahydrocannabinol in the elevated plus maze in mice. Behavioural Pharmacology. 10.1097/fbp.0000000000000636. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Long LE, Chesworth R, Huang X-F, McGregor IS, Arnold JC, & Karl T (2010). A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP), 13(7), 861–876. 10.1017/S1461145709990605. [DOI] [PubMed] [Google Scholar]
- Lupica CR, & Riegel AC (2005). Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology, 48(8), 1105–1116. 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Ma Z, Gao F, Larsen B, Gao M, Luo Z, Chen D, et al. (2019). Mechanisms of cannabinoid CB(2) receptor-mediated reduction of dopamine neuronal excitability in mouse ventral tegmental area. eBioMedicine, 42, 225–237. 10.1016/j.ebiom.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Jia J, Niu W, Jiang T, Zhai Q, Yang L, et al. (2015). Mitochondrial CB1 receptor is involved in ACEA-induced protective effects on neurons and mitochondrial functions. Scientific Reports, 5(1), 12440. 10.1038/srep12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K (2008). Cannabinoid receptors: where they are and what they do. Journal of Neuroendocrinology, 20(Suppl 1), 10–14. 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Baños JE, & Cabañero D (2016). The endocannabinoid system and neuropathic pain. Pain, 157, S23–S32. 10.1097/j.pain.0000000000000428. [DOI] [PubMed] [Google Scholar]
- Mallet PE, & Beninger RJ (1998a). The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by Δ9-tetrahydrocannabinol or anandamide. Psychopharmacology, 140(1), 11–19. 10.1007/s002130050733. [DOI] [PubMed] [Google Scholar]
- Mallet PE, & Beninger RJ (1998b). Δ9-Tetrahydrocannabinol, but not the endogenous cannabinoid receptor ligand anandamide, produces conditioned place avoidance. Life Sciences, 62(26), 2431–2439. 10.1016/S0024-3205(98)00226-4. [DOI] [PubMed] [Google Scholar]
- Malone DT, & Taylor DA (2001). Involvement of somatodendritic 5-HT1A receptors in Δ9-tetrahydrocannabinol-induced hypothermia in the rat. Pharmacology Biochemistry and Behavior, 69(3), 595–601. 10.1016/S0091-3057(01)00567-6. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Nicholson KL, Martin BR, & Balster RL (1994). Failure of Delta(9)-tetrahydrocannabinol and CP 55,940 to maintain intravenous self-administration under a fixed-interval schedule in rhesus monkeys. Behavioural Pharmacology, 5(2), 219–225. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Carriba P, Filip M, Borgkvist A, Frankowska M, Bellido I, et al. (2008). Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology, 54(5), 815–823. 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, & Dittel BN (2005). Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. Journal of Neurochemistry, 95(2), 437–445. 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Marichal-Cancino BA, Fajardo-Valdez A, Ruiz-Contreras AE, Mendez-Díaz M, & Prospero-García O (2017). Advances in the physiology of GPR55 in the central nervous system. Current Neuropharmacology, 15(5), 771–778. 10.2174/1570159X14666160729155441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato S, Del Olmo E, & Pazos A (2003). Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. European Journal of Neuroscience, 17(9), 1747–1754. 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- McKillop AM, Moran BM, Abdel-Wahab YHA, & Flatt PR (2013). Evaluation of the insulin releasing and antihyperglycaemic activities of GPR55 lipid agonists using clonal beta-cells, isolated pancreatic islets and mice. British Journal of Pharmacology, 170(5), 978–990. 10.1111/bph.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Amin MR, & France CP (2005). SR 141716A differentially attenuates the behavioral effects of Δ9-THC in rhesus monkeys. Behavioural Pharmacology, 16(5–6), 363–372. Retrieved from https://journals.lww.com/behaviouralpharm/Fulltext/2005/09000/SR_141716A_differentially_attenuates_the.8.aspx. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, & Parker LA (2013). The endocannabinoid system and the brain. Annual Review of Psychology, 64(1), 21–47. 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- Melis M, Gessa GL, & Diana M (2000). Different mechanisms for dopaminergic excitation induced by opiates and cannabinoids in the rat midbrain. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 24(6), 993–1006. 10.1016/S0278-5846(00)00119-6. [DOI] [PubMed] [Google Scholar]
- Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, et al. (2004). Prefrontal Cortex Stimulation Induces 2-Arachidonoyl-Glycerol-Mediated Suppression of Excitation in Dopamine Neurons. The Journal of Neuroscience, 24(47), 10707–10715. 10.1523/jneurosci.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metna-Laurent M, & Marsicano G (2015). Rising stars: Modulation of brain functions by astroglial type-1 cannabinoid receptors. Glia, 63(3), 353–364. 10.1002/glia.22773. [DOI] [PubMed] [Google Scholar]
- Metna-Laurent M, Mondésir M, Grel A, Vallée M, & Piazza P-V (2017). Cannabinoid-Induced Tetrad in Mice. Current Protocols in Neuroscience, 80(1), 9.59.51–59.59.10. 10.1002/cpns.31. [DOI] [PubMed] [Google Scholar]
- Mishima K, Egashira N, Matsumoto Y, Iwasaki K, & Fujiwara M (2002). Involvement of reduced acetylcholine release in Δ9-tetrahydrocannabinol-induced impairment of spatial memory in the 8-arm radial maze. Life Sciences, 72(4), 397–407. 10.1016/S0024-3205(02)02274-9. [DOI] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schütz G, et al. (2007). Genetic Dissection of Behavioural and Autonomic Effects of Δ9-Tetrahydrocannabinol in Mice. PLoS Biology, 5(10), e269. 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, & Cerù MP (2004). Immunolocalization of peroxisome proliferator-activated receptors and retinoid x receptors in the adult rat CNS. Neuroscience, 123(1), 131–145. 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Mothet J-P, Parent AT, Wolosker H, Brady RO, Linden DJ, Ferris CD, et al. (2000). d-Serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proceedings of the National Academy of Sciences, 97(9), 4926–4931. 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, & Abu-Shaar M (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature, 365(6441), 61–65. 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Murphy M, Mills S, Winstone J, Leishman E, Wager-Miller J, Bradshaw H, et al. (2017). Chronic adolescent Δ9-tetrahydrocannabinol treatment of male mice leads to long-term cognitive and behavioral dysfunction, which are prevented by concurrent cannabidiol treatment. Cannabis and Cannabinoid Research, 2(1), 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava F, Carta G, & Gessa GL (2000). Permissive Role of Dopamine D2 Receptors in the Hypothermia Induced by Δ9-Tetrahydrocannabinol in Rats. Pharmacology Biochemistry and Behavior, 66(1), 183–187. 10.1016/S0091-3057(00)00231-8. [DOI] [PubMed] [Google Scholar]
- Navarrete M, & Araque A (2008). Endocannabinoids mediate neuron-astrocyte communication. Neuron, 57(6), 883–893. 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Nedungadi TP, Dutta M, Bathina CS, Caterina MJ, & Cunningham JT (2012). Expression and distribution of TRPV2 in rat brain. Experimental Neurology, 237(1), 223–237. 10.1016/j.expneurol.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nent E, Nozaki C, Schmöle A-C, Otte D, & Zimmer A (2019). CB2 receptor deletion on myeloid cells enhanced mechanical allodynia in a mouse model of neuropathic pain. Scientific Reports, 9(1), 7468. 10.1038/s41598-019-4-3858-4.31097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Cao J, Keighron JD, Jordan CJ, Bi G-H, Liang Y, et al. (2019). Translating the atypical dopamine uptake inhibitor hypothesis toward therapeutics for treatment of psychostimulant use disorders. Neuropsychopharmacology, 44(8), 1435–1444. 10.1038/s41386-019-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, et al. (2016). Inhaled delivery of Δ9-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology, 109, 112–120. 10.1016/j.neuropharm.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris C, Szkudlarek HJ, Pereira B, Rushlow W, & Laviolette SR (2019). The bivalent rewarding and aversive properties of Δ9-tetrahydrocannabinol are mediated through dissociable opioid receptor substrates and neuronal modulation mechanisms in distinct striatal sub-regions. Scientific Reports, 9(1), 9760. 10.1038/s41598-019-46215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SE, Tarling EJ, Bennett AJ, Kendall DA, & Randall MD (2005). Novel time-dependent vascular actions of Δ9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor gamma. Biochemical and Biophysical Research Communications, 337(3), 824–831. 10.1016/j.bbrc.2005.09.121. [DOI] [PubMed] [Google Scholar]
- Oliveira da Cruz JF, Robin LM, Drago F, Marsicano G, & Metna-Laurent M (2016). Astroglial type-1 cannabinoid receptor (CB1): A new player in the tripartite synapse. Neuroscience, 323, 35–42. 10.1016/j.neuroscience.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Chaudhuri G, Abaci AS, Parker M, Manier DH, Martin PR, et al. (1999). Expression of cannabinoid receptors and their gene transcripts in human blood cells. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 23(6), 1063–1077. 10.1016/S0278-5846(99)00052-4. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SE (2016). An update on PPAR activation by cannabinoids. British Journal of Pharmacology, 173(12), 1899–1910. 10.1111/bph.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Ferre S, Yasar S, Thorndike EB, Schindler CW, & Goldberg SR (2012). Combined effects of THC and caffeine on working memory in rats. British Journal of Pharmacology, 165(8), 2529–2538. 10.1111/j.1476-5381.2011.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KD, Davison JS, Pittman QJ, & Sharkey KA (2010). Cannabinoid CB2 Receptors in Health and Disease. Current Medicinal Chemistry, 17(14), 1394–1410. 10.2174/092986710790980041. [DOI] [PubMed] [Google Scholar]
- Pertwee RG (1997). Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacology & Therapeutics, 74(2), 129–180. [DOI] [PubMed] [Google Scholar]
- Pertwee R (2005). Pharmacological actions of cannabinoids. In Pertwee RG (Ed.), Cannabinoids (pp. 1–51). Berlin, Heidelberg: Springer Berlin Heidelberg. [DOI] [PubMed] [Google Scholar]
- Pertwee R (2006). Cannabinoid pharmacology: The first 66 years. British Journal of Pharmacology, 147(Suppl. 1), S163–S171. 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG (2008a). The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. British Journal of Pharmacology, 153(2), 199–215. 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG (2008b). Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addiction Biology, 13(2), 147–159. 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Di Marzo V, Elphick MR, et al. (2010). International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacological Reviews, 62(4), 588–631. 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietr M, Kozela E, Levy R, Rimmerman N, Lin YH, Stella N, et al. (2009). Differential changes in GPR55 during microglial cell activation. FEBS Letters, 583(12), 2071–2076. 10.1016/j.febslet.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Piomelli D (2003). The molecular logic of endocannabinoid signalling. Nature Reviews Neuroscience, 4(11), 873–884. 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Prini P, Zamberletti E, Manenti C, Gabaglio M, Parolaro D, & Rubino T (2020). Neurobiological mechanisms underlying cannabis-induced memory impairment. European Neuropsychopharmacology, 36, 181–190. 10.1016/j.euroneuro.2020.02.002. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, & D’Souza DC (2006). The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology, 188(4), 425–444. 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Cabassa J, Geller EB, & Adler MW (2002). CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2 [(4,5-dihydro-2-methyl-4 (4-morpholinylmethyl))-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1ij]quinolin-6-one]-induced hypothermia. Journal of Pharmacology and Experimental Therapeutics, 301(3), 963–968. 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Cowan A, Tallarida RJ, Geller EB, & Adler MW (2002). N-methyl-d-aspartate antagonists and WIN 55212-2 [4,5-dihydro-2-methyl-4(4-morpholinyl-methyl)-1-(1-naphthalenyl-carbonyl)-6H-pyrrolo[3,2,1-ij]quinolin-6-one], a cannabinoid agonist, interact to produce synergistic hypothermia. Journal of Pharmacology and Experimental Therapeutics, 303(1), 395–402. 10.1124/jpet.102.037473. [DOI] [PubMed] [Google Scholar]
- Reis SL, Silva HB, Almeida M, Cunha RA, Simões AP, & Canas PM (2019). Adenosine A1 and A2A receptors differently control synaptic plasticity in the mouse dorsal and ventral hippocampus. Journal of Neurochemistry, 151(2), 227–237. 10.1111/jnc.14816. [DOI] [PubMed] [Google Scholar]
- Remmers F, Lange MD, Hamann M, Ruehle S, Pape H-C, & Lutz B (2017). Addressing sufficiency of the CB1 receptor for endocannabinoid-mediated functions through conditional genetic rescue in forebrain GABAergic neurons. Brain Structure and Function, 222(8), 3431–3452. 10.1007/s00429-017-1411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LBM, Moreira FA, & Guimarães FS (2009). Chapter 16 Endocannabinoid System and Fear Conditioning. In Vol. 81. Vitamins & Hormones (pp. 421–440). Academic Press. [DOI] [PubMed] [Google Scholar]
- Resstel LBM, Tavares RF, Lisboa SFS, Joca SRL, Corrêa FMA, & Guimarães FS (2009). 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. British Journal of Pharmacology, 156(1), 181–188. 10.1111/j.1476-5381.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros M-P, & Lutz B (2012). Biphasic Effects of Cannabinoids in Anxiety Responses: CB1 and GABAB Receptors in the Balance of GABAergic and Glutamatergic Neurotransmission. Neuropsychopharmacology, 37(12), 2624–2634. 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. (1994). SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Letters, 350(2–3), 240–244. 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rios C, Gomes I, & Devi LA (2006). μ opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. British Journal of Pharmacology, 148(4), 387–395. 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, & Pickel VM (2001). Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21(3), 823–833. 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Zerbo S, Rafacho A, Díaz-Arteaga A, Suárez J, Quesada I, Imbernón M, et al. (2011). A role for the putative cannabinoid receptor GPR55 in the islets of Langerhans. The Journal of Endocrinology, 211(2), 177–185. [DOI] [PubMed] [Google Scholar]
- Rubino T, Guidali C, Vigano D, Realini N, Valenti M, Massi P, et al. (2008). CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology, 54(1), 151–160. 10.1016/j.neuropharm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Rubino T, Sala M, Viganò D, Braida D, Castiglioni C, Limonta V, et al. (2007). Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral [Delta]9-tetrahydrocannabinol in rats. Neuropsychopharmacology, 32(9), 2036–2045. 10.1038/sj.npp.1301330. [DOI] [PubMed] [Google Scholar]
- Ruehle S, Remmers F, Romo-Parra H, Massa F, Wickert M, Wörtge S, et al. (2013). Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: Distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. The Journal of Neuroscience, 33(25), 10264–10277. 10.1523/jneurosci.4171-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, & Parker KK (2005). Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochemical Research, 30(8), 1037–1043. 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, et al. (2007). The orphan receptor GPR55 is a novel cannabinoid receptor. British Journal of Pharmacology, 152(7), 1092–1101. 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio C, Fischer J, Franzoni MF, Mackie K, Kaneko T, & Conrath M (2001). CB1-cannabinoid and μ-opioid receptor co-localization on postsynaptic target in the rat dorsal horn. Neuroreport, 12(17), 3689–3692. Retrieved from https://journals.lww.com/neuroreport/Fulltext/2001/12040/CB1_cannabinoid_and___opioid_receptor.17.aspx. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Bunsey MD, Giordano M, & Norman AB (1988). The catalepsy test: its ups and downs. Behavioral Neuroscience, 102(5), 748–759. 10.1037//0735-7044.102.5.748. [DOI] [PubMed] [Google Scholar]
- Sano K, Mishima K, Koushi E, Orito K, Egashira N, Irie K, et al. (2008). Δ9-Tetrahydrocannabinol-induced catalepsy-like immobilization is mediated by decreased 5-HT neurotransmission in the nucleus accumbens due to the action of glutamate-containing neurons. Neuroscience, 151(2), 320–328. 10.1016/j.neuroscience.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Savinainen JR, Saario SM, Niemi R, Järvinen T, & Laitinen JT (2003). An optimized approach to study endocannabinoid signaling: Evidence against constitutive activity of rat brain adenosine A1 and cannabinoid CB1 receptors. British Journal of Pharmacology, 140(8), 1451–1459. 10.1038/sj.bjp.0705577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz AR, Lee M, Condie RB, Pulaski JT, & Kaminski NE (1997). Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicology and Applied Pharmacology, 142(2), 278–287. 10.1006/taap.1996.8034. [DOI] [PubMed] [Google Scholar]
- Schauer GL, King BA, Bunnell RE, Promoff G, & McAfee TA (2016). Toking, vaping, and eating for health or fun: marijuana use patterns in adults, U.S., 2014. American Journal of Preventive Medicine, 50(1), 1–8. 10.1016/j.amepre.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta N, Cha Y, Chaudhry S, Wilson W, Swartzwelder HS, & Kuhn C (2007). Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology, 191(4), 867–877. 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- Schwab MH, Bartholomae A, Heimrich B, Feldmeyer D, Druffel-Augustin S, Goebbels S, et al. (2000). Neuronal basic helix-loop-helix proteins (NEX and BETA2/Neuro D) regulate terminal granule cell differentiation in the hippocampus. Journal of Neuroscience, 20(10), 3714–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, & Hohmann AG (2011). Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacological Research, 64(3), 226–234. 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secci ME, Mascia P, Sagheddu C, Beggiato S, Melis M, Borelli AC, et al. (2019). Astrocytic mechanisms involving kynurenic acid control Δ9-tetrahydrocannabinol-induced increases in glutamate release in brain reward-processing areas. Molecular Neurobiology, 56(5), 3563–3575. 10.1007/s12035-018-1319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta T, Jaryal AK, & Mallick HN (2016). Effects of NMDA and non-NMDA ionotropic glutamate receptors in the medial preoptic area on body temperature in awake rats. Journal of Thermal Biology, 61, 1–7. 10.1016/j.jtherbio.2016.07.020. [DOI] [PubMed] [Google Scholar]
- Shang Y, & Tang Y (2017). The central cannabinoid receptor type-2 (CB2) and chronic pain. International Journal of Neuroscience, 127(9), 812–823. 10.1080/00207454.2016.1257992. [DOI] [PubMed] [Google Scholar]
- Shibasaki K, Ishizaki Y, & Mandadi S (2013). Astrocytes express functional TRPV2 ion channels. Biochemical and Biophysical Research Communications, 441(2), 327–332. 10.1016/j.bbrc.2013.10.046. [DOI] [PubMed] [Google Scholar]
- Sierra S, Luquin N, Rico AJ, Gómez-Bautista V, Roda E, Dopeso-Reyes IG, et al. (2015). Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism. Brain Structure and Function, 220(5), 2721–2738. 10.1007/s00429-014-0823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Zangen A, Thiriet N, & Goldberg SR (2004). β-Endorphin elevations in the ventral tegmental area regulate the discriminative effects of Δ-9-tetrahydrocannabinol. European Journal of Neuroscience, 19(12), 3183–3192. 10.1111/j.0953-816X.2004.03420.x. [DOI] [PubMed] [Google Scholar]
- Soria G, Castañe A, Berrendero F, Ledent C, Parmentier M, Maldonado R, et al. (2004). Adenosine A2A receptors are involved in physical dependence and place conditioning induced by THC. European Journal of Neuroscience, 20(8), 2203–2213. 10.1111/j.1460-9568.2004.03682.x. [DOI] [PubMed] [Google Scholar]
- Sousa VC, Assaife-Lopes N, Ribeiro JA, Pratt JA, Brett RR, & Sebastião AM (2011). Regulation of hippocampal cannabinoid CB1 receptor actions by adenosine A1 receptors and chronic caffeine administration: Implications for the effects of Δ9-tetrahydrocannabinol on spatial memory. Neuropsychopharmacology, 36(2), 472–487. 10.1038/npp.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S, Neuhofer D, Chioma VC, Garcia-Keller C, Schwartz DJ, Allen N, et al. (2018). A model of Δ9-tetrahydrocannabinol self-administration and reinstatement that alters synaptic plasticity in nucleus accumbens. Biological Psychiatry, 84(8), 601–610. 10.1016/j.biopsych.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller KJ, Bi GH, He Y, Galaj E, Gardner EL, & Xi ZX (2019). Cannabinoid CB(1) and CB(2) receptor mechanisms underlie cannabis reward and aversion in rats. British Journal of Pharmacology, 176(9), 1268–1281. 10.1111/bph.14625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, et al. (2018). Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: A crossover trial. JAMA Network Open, 1(7), e184841. 10.1001/jamanetworkopen.2018.4841.30646391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano GB, Liu Y, & Goligorsky MS (1996). Cannabinoid receptors are coupled to nitric oxide release in invertebrate immunocytes, microglia, and human monocytes*. Journal of Biological Chemistry, 271(32), 19238–19242. 10.1074/jbc.271.32.19238. [DOI] [PubMed] [Google Scholar]
- Stella N (2010). Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia, 58(9), 1017–1030. 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempel AV, Stumpf A, Zhang H-Y, Özdoğan T, Pannasch U, Theis A-K, et al. (2016). Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron, 90(4), 795–809. 10.1016/j.neuron.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Alexander SPH, Garle MJ, Gibson CL, Hewitt K, Murphy SP, et al. (2007). Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. British Journal of Pharmacology, 152(5), 734–743. 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Siemes S, & Wallmichrath I (2002). Short communication: Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. European Journal of Neuroscience, 15(12), 2057–2061. 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- Szkudlarek HJ, Desai SJ, Renard J, Pereira B, Norris C, Jobson CEL, et al. (2019). Δ-9-Tetrahydrocannabinol and Cannabidiol produce dissociable effects on prefrontal cortical executive function and regulation of affective behaviors. Neuropsychopharmacology, 44(4), 817–825. 10.1038/s41386-018-0282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Creehan KM, & Vandewater SA (2015). Cannabidiol fails to reverse hypothermia or locomotor suppression induced by Δ(9)-tetrahydrocannabinol in Sprague-Dawley rats. British Journal of Pharmacology, 172(7), 1783–1791. 10.1111/bph.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Creehan KM, Vandewater SA, Kerr TM, & Cole M (2021). Effects of Δ9-tetrahydrocannabinol (THC) vapor inhalation in Sprague-Dawley and Wistar rats. Experimental and Clinical Psychopharmacology, 29(1), 1–13. 10.1037/pha0000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RN, & Singer G (1979). Self-administration of Δ9-tetrahydrocannabinol by rats. Pharmacology Biochemistry and Behavior, 11(6), 737–740. 10.1016/0091-3057(79)90274-0. [DOI] [PubMed] [Google Scholar]
- Takeda S, Ikeda E, Su S, Harada M, Okazaki H, Yoshioka Y, et al. (2014). Δ9-THC modulation of fatty acid 2-hydroxylase (FA2H) gene expression: Possible involvement of induced levels of PPARα in MDA-MB-231 breast cancer cells. Toxicology, 326, 18–24. 10.1016/j.tox.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Munzar P, & Goldberg SR (2000). Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nature Neuroscience, 3(11), 1073–1074. 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, & Chiara GD (1997). Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common μ1 opioid receptor mechanism. Science, 276(5321), 2048. 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Todd SM, & Arnold JC (2016). Neural correlates of interactions between cannabidiol and Delta(9)-tetrahydrocannabinol in mice: implications for medical cannabis. British Journal of Pharmacology, 173(1), 53–65. 10.1111/bph.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AH, & Craft RM (2004). CB1 receptor mediation of cannabinoid behavioral effects in male and female rats. Psychopharmacology, 172(1), 25–30. 10.1007/s00213-003-1620-x. [DOI] [PubMed] [Google Scholar]
- Tsou K, Mackie K, Sañudo-Peña MC, & Walker JM (1999). Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience, 93(3), 969–975. 10.1016/S0306-4522(99)00086-X. [DOI] [PubMed] [Google Scholar]
- Valjent E, & Maldonado R (2000). A behavioural model to reveal place preference to Δ9-tetrahydrocannabinol in mice. Psychopharmacology, 147(4), 436–438. 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, & Di Marzo V (2003). The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. European Journal of Pharmacology, 480(1), 133–150. 10.1016/j.ejphar.2003.08.101. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, et al. (2005). Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science, 310(5746), 329–332. 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, et al. (2008). Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Δ9-tetrahydrocannabinol. Drug and Alcohol Dependence, 94(1–3), 191–198. 10.1016/j.drugalcdep.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Bridgen DT, Tao Q, Thomas BF, Martin BR, & Lichtman AH (2005). Δ9Tetrahydrocannbinol accounts for the antinociceptive, hypothermic, and cataleptic effects of marijuana in mice. Journal of Pharmacology and Experimental Therapeutics, 314(1), 329–337. 10.1124/jpet.104.080739. [DOI] [PubMed] [Google Scholar]
- Varvel SA, & Lichtman AH (2002). Evaluation of CB1 receptor knockout mice in the morris water maze. Journal of Pharmacology and Experimental Therapeutics, 301(3), 915–924. 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wiley JL, Yang R, Bridgen DT, Long K, Lichtman AH, et al. (2006). Interactions between THC and cannabidiol in mouse models of cannabinoid activity. Psychopharmacology, 186(2), 226–234. 10.1007/s00213-006-0356-9. [DOI] [PubMed] [Google Scholar]
- Vickstrom CR, Liu X, Liu S, Hu M-M, Mu L, Hu Y, et al. (2021). Role of endocannabinoid signaling in a septohabenular pathway in the regulation of anxiety- and depressive-like behavior. Molecular Psychiatry, 26(7), 3178–3191. 10.1038/s41380-020-00905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Puig A, Jimenez-Liñan M, Lowell BB, Hamann A, Hu E, Spiegelman B, et al. (1996). Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. The Journal of Clinical Investigation, 97(11), 2553–2561. 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S (2018). Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cellular and Molecular Neurobiology, 38(1), 121–132. 10.1007/s10571-017-0554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñals X, Moreno E, Lanfumey L, Cordomí A, Pastor A, de La Torre R, et al. (2015). cognitive impairment induced by Delta9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS Biology, 13(7), e1002194. 10.1371/journal.pbio.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman Y, Olson JM, Carlisle SJ, & Cabral GA (1999). The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. Journal of Pharmacology and Experimental Therapeutics, 288(3), 1357–1366. Retrieved from https://jpet.aspetjournals.org/content/jpet/288/3/1357.full.pdf. [PubMed] [Google Scholar]
- Wang XF, Galaj E, Bi GH, Zhang C, He Y, Zhan J, et al. (2020). Different receptor mechanisms underlying phytocannabinoid-versus synthetic cannabinoid-induced tetrad effects: Opposite roles of CB(1)/CB(2) versus GPR55 receptors. British Journal of Pharmacology, 177(8), 1865–1880. 10.1111/bph.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden A, Truitt J, Merriman M, Ponomareva O, Jameson K, Ferguson LB, et al. (2016). Localization of PPAR isotypes in the adult mouse and human brain. Scientific Reports, 6(1), 27618. 10.1038/srep27618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. (2015). Cannabinoids for medical use: A systematic review and meta-analysis. JAMA, 313(24), 2456–2473. 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Grim TW, Owens RA, Lazenka MF, Sim-Selley LJ, Abdullah RA, et al. (2015). Δ9-Tetrahydrocannabinol and endocannabinoid degradative enzyme inhibitors attenuate intracranial self-stimulation in mice. Journal of Pharmacology and Experimental Therapeutics, 352(2), 195–207. 10.1124/jpet.114.218677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, & Martin BR (2003). Cannabinoid pharmacological properties common to other centrally acting drugs. European Journal of Pharmacology, 471(3), 185–193. 10.1016/S0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2021). Cannabis. Alcohol, Drugs and Addictive Behaviors Unit. [Google Scholar]
- Wu C-S, Chen H, Sun H, Zhu J, Jew CP, Wager-Miller J, et al. (2013). GPR55, a G-protein coupled receptor for lysophosphatidylinositol, plays a role in motor coordination. PLoS One, 8(4), e60314. 10.1371/journal.pone.0060314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, et al. (2011). Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nature Neuroscience, 14(9), 1160–1166. 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XH, Cao CQ, Martino G, Puma C, Morinville A, St-Onge S, et al. (2010). A peripherally restricted cannabinoid receptor agonist produces robust anti-nociceptive effects in rodent models of inflammatory and neuropathic pain. Pain, 151(2), 337–344. 10.1016/j.pain.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Zangen A, Solinas M, Ikemoto S, Goldberg SR, & Wise RA (2006). Two brain sites for cannabinoid reward. The Journal of Neuroscience, 26(18), 4901–4907. 10.1523/jneurosci.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-Y, Bi GH, Li X, Li J, Qu H, Zhang SJ, et al. (2015). Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology, 40(4), 1037–1051. 10.1038/npp.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, De Biase L, Chandra R, Shen H, Liu QR, Gardner E, et al. (2021). Repeated cocaine administration upregulates CB2 receptor expression in striatal medium-spiny neurons that express dopamine D1 receptors in mice. Acta Pharmacologica Sinica. 10.1038/s41401-021-00712-6.34316031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-Y, Gao M, Liu Q-R, Bi G-H, Li X, Yang H-J, et al. (2014). Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proceedings of the National Academy of Sciences, 111(46), E5007–E5015. 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-Y, Gao M, Shen H, Bi G-H, Yang H-J, Liu Q-R, et al. (2017). Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addiction Biology, 22(3), 752–765. 10.1111/adb.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-Y, Shen H, Gao M, Ma Z, Hempel BJ, Bi G-H, et al. (2021). Cannabinoid CB2 receptors are expressed in glutamate neurons in the red nucleus and functionally modulate motor behavior in mice. Neuropharmacology, 189, 108538. 10.1016/j.neuropharm.2021.108538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Shen H, Jordan CJ, Liu QR, Gardner EL, Bonci A, et al. (2019). CB(2) receptor antibody signal specificity: correlations with the use of partial CB(2)-knockout mice and anti-rat CB(2) receptor antibodies. Acta Pharmacologica Sinica, 40(3), 398–409. 10.1038/s41401-018-0037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Valjent E, König M, Zimmer AM, Robledo P, Hahn H, et al. (2001). Absence of Δ-9-tetrahydrocannabinol dysphoric effects in dynorphin-deficient mice. The Journal of Neuroscience, 21(23), 9499–9505. Retrieved from http://www.jneurosci.org/content/21/23/9499.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, & Bonner TI (1999). Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proceedings of the National Academy of Sciences, 96(10), 5780–5785. 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Shirakawa I, Finkelfarb E, & Karniol IG (1982). Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology, 76(3), 245–250. [DOI] [PubMed] [Google Scholar]


