Abstract
Ketamine has emerged as a transformative and mechanistically novel pharmacotherapy for depression. Its rapid onset of action, efficacy for treatment-resistant symptoms, and protection against relapse distinguish it from prior antidepressants. Its discovery emerged from a reconceptualization of the neurobiology of depression and, in turn, insights from the elaboration of its mechanisms of action inform studies of the pathophysiology of depression and related disorders. It has been 25 y since we first presented our ketamine findings in depression. Thus, it is timely for this review to consider what we have learned from studies of ketamine and to suggest future directions for the optimization of rapid-acting antidepressant treatment.
Keywords: depression, stress, antidepressant, ketamine, neuroplasticity
The discovery of the rapid antidepressant effects of R,S-ketamine (ketamine) was hailed simultaneously as the most improbable and transformative advance in depression pharmacotherapy in many decades. In the 1990 s, ketamine was known as a dissociative anesthetic (1) with nociceptive efficacy (2, 3) and uses in veterinary medicine (4). Ketamine misuse was a public health concern (5–7). Nicknamed “Special K,” its profound effects on consciousness were called the “K hole” (8). The initiation of schizophrenia-related studies with ketamine in humans in the 1990s (9–11) stimulated controversy because of its psychoactive effects (12). Keatmine targeted glutamate receptors and produced response within hours of administration of a single dose. From the start, there were concerns as to whether its risks outweighed its benefits (13). The risks associated with ketamine treatment are real, and they have informed optimal clinical practice. However, when used appropriately, the remarkable efficacy of intermittent subanesthetic ketamine or the more potent of its two isomers at N-methyl-D-aspartate glutamate receptors (NMDAR), S-ketamine (Esketamine) can have a transformative positive impact on the lives of people suffering from depression, and potentially, the public health burden associated with treatment-resistant symptoms of depression. This review begins by characterizing advances in the conceptual framework for the biology of depression that set the stage for the discovery of the antidepressant effects of ketamine. It then describes the therapeutic impact of ketamine and considers mechanisms underlying its efficacy. Lastly, it addresses progress made in enhancing ketamine efficacy and safety.
The Neurobiology of Depression: Beyond the Monoamine Hypothesis
Historically, psychiatric psychopharmacology progressed more rapidly than pathophysiology. By 1957, pioneers identified the principal medication classes used currently to treat depression, the monoamine oxidase inhibitors, monoamine transporter antagonists, lithium, and the antipsychotics (14–17). These medications provided clues to the biology of depression, leading to monoamine-centric hypotheses (18, 19). Monoamine depletion studies led by Charney and colleagues at Yale (20) clearly implicated ongoing monoamine availability in the sustained efficacy of monoamine transporter antagonist antidepressants. However, the failure of monoamine depletion to produce depression in healthy individuals (21) challenged the notion that depression was simply a deficit in monoamine signaling.
At that point, Charney and Krystal broadened their focus to encompass the intrinsic signaling mechanisms of the cortex and limbic system. We now know that depression is associated with altered cortico-limbic structure (22), functional connectivity (23, 24), and functional regulation of circuits regulating mood (25, 26). These insights are driving circuit-based interventions for depression (27, 28). The molecular and cellular underpinnings of these alterations are emerging from postmortem studies characterizing the transcriptomic, epigenomic, and proteomic landscape of depression (29–33). A complete assessment of the biology of depression, however, is beyond the scope of this review. Here, we highlight three relevant recently characterized depression-related alterations in glutamate synaptic signaling [see for review: (34, 35)]:
The first characteristic is reduced glutamate synaptic efficacy as reflected in reduced amplitude of sensory evoked potentials (36) and in reduced cortical functional connectivity (23). Also, a recent study in PTSD with or without comorbid major depression (37) reported reduced synaptic strength as reflected by reduced “energy per cycle,” i.e., decreased metabolic activity (tricarboxylic acid cycle activity) per each molecule of glutamate released by neurons.
The second characteristic is reduced synaptic density. Preclinical studies described reductions in synaptic density and dendritic retraction in chronically stressed animals (38, 39). Human postmortem findings also report reduced synaptic density and reductions in genes coding for synaptic proteins (40, 41). Lower synaptic density in depression is also evident in vivo where it is associated with cortical circuit dysregulation (42).
A third characteristic is disrupted synaptic glutamate homeostasis. Preclinical research (43, 44) suggests that stress-related disruption of glial function, particularly glutamate transport, elevates extracellular glutamate levels, overstimulates extrasynaptic N-methyl-D-aspartate (NMDA) receptors (NMDAR), downregulates glutamate synaptic function, contributes to synaptic pruning, and produces depression-like behavior in animals. Analyses of postmortem tissue from depressed patients also reveal reductions in glial integrity (45) and downregulation of membrane glutamate transporters (46).
Ketamine: Clinical Efficacy Tied to Restoration of Synaptic Efficacy and Synaptic Density
Clinical Efficacy.
Ketamine and Esketamine efficacy contrasts with traditional antidepressant treatments. The first trial of subanesthetic ketamine (0.5 mg/kg, administered intravenously over 40 min) revealed antidepressant effects from a single dose that emerged over a few hours and became more pronounced over the following days (47). The first replication of this study mirrored these findings in patients with treatment-resistant depression symptoms (48). Subsequent clinical trials of ketamine and Esketamine replicated and extended these findings (49, 50). Ketamine and Esketamine produce response rates over 50% and remission rates between 30% and 50%, much higher than one would expect for a traditional antidepressant prescribed for treatment-resistant symptoms, i.e., response rates of <20% and remission rates of <15% (51) and comparable to electroconvulsive therapy (52). With ongoing treatment, the frequency of ketamine dosing can be reduced without loss of efficacy (53, 54). The durability of ketamine efficacy during long-term treatment is impressive. In a randomized Esketamine discontinuation study, only approximately 25% of patients relapsed during Esketamine treatment in the year following responding to Esketamine plus a new antidepressant. Responders who stopped Esketamine but continued their antidepressant had a relapse rate of over 57% (55). In other words, Esketamine shows signs of superior protection against depression relapse in comparison to a newly initiated antidepressant (55) than traditional antidepressants in comparison to placebo (56). Overall, there is no clear evidence that tolerance develops to its therapeutic effects during long-term treatment (57).
Safety concerns limit Esketamine to clinic settings and efforts to develop strategies for in-home ketamine treatment have raised clinical concerns. The principal medical side effects of ketamine and Esketamine are elevated blood pressure, nausea and vomiting, and dissociation (57). These effects are generally managed by pretreatment optimization of hypertension management and a serotonin-3 receptor antagonist for nausea. Preparing patients for the dissociative effects prior to treatment, supporting them during drug administration, and debriefing patients following treatment are usually sufficient to manage these symptoms. Rarely, patients benefit from additional supportive care or benzodiazepine administration. When ketamine is administered outside of clinic settings, there is an additional risk of misuse of the prescribed ketamine. This concern is amplified by evidence that rates of ketamine recreational use increased significantly in the United States since the Food and Drug Administration (FDA) approval of Esketamine (7).
Reversing Stress and Depression Effects on Glutamate Synaptic Signaling through Restoration of Synaptic Efficacy and Synaptic Density.
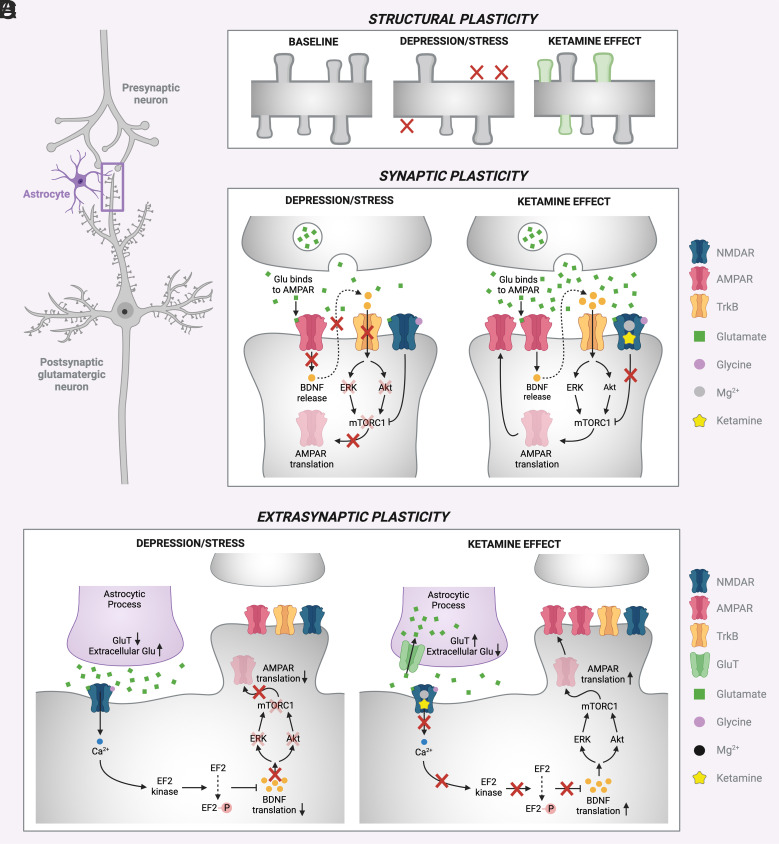
Preclinical studies provide foundational insights into mechanisms underlying the efficacy of ketamine in patients (Fig. 1). The antidepressant effects of ketamine share features with other forms of neuroplasticity whereby transient circuit activation produces long-lasting potentiation of synaptic signaling, sometimes referred to as Hebbian plasticity (58). The first step in the processes leading to antidepressant efficacy of ketamine is inhibition of interneuron activity (59), resulting in disinhibition of glutamate release. The importance of this step is supported by evidence that chemogenic inhibition of prefrontal cortex interneurons (60) or knockdown of GluN2B NMDAR subunits on somatostatin (SST) and parvalbumin (PV) interneurons but not glutamatergic neurons (61) prevents or occludes the antidepressant efficacy of ketamine. The importance of the resulting glutamate neuronal activation is supported by the convergent antidepressant effects of local infralimbic cortex ketamine administration and pharmacological (62) and optogenetic (63) activation of the same brain region. In stressed animals, activation of NMDAR (64) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPAR) (65) restore synaptic efficacy, elevate brain-derived neurotrophic factor (BDNF) levels, trigger local release of BDNF, and activate signaling cascades downstream from the receptor for BDNF, tropomyosin receptor kinase B (Trk B) receptors, including the mammalian target for rapamycin (mTOR). mTOR activation, in turn, drives restoration of dendritic spines pruned by stress-related processes (65, 66). The onset of antidepressant behavioral effects produced by ketamine precedes the emergence of regrown spines (66), suggesting that restoration of synaptic efficacy and other processes initiate antidepressant effects. However, antidepressant behavioral effects persist with the same timescale as the newly regrown spines (67) and interference with spine restoration shortens the duration of ketamine’s antidepressant effects (66). Thus, mTOR-dependent spine restoration appears to be related directly to the duration of ketamine efficacy.
Fig. 1.
Schematic of proposed mechanisms of ketamine’s antidepressant effect (A): (B) structural plasticity is induced leading to growth of dendritic spines in the prefrontal cortex, (C) enhanced synaptic efficacy, (D) extrasynaptic NMDA receptors’ role in homeostatic plasticity.
Translational research studies now provide evidence in healthy humans and depressed patients that support aspects of the models for ketamine efficacy outlined in the prior two paragraphs:
Support for a link between ketamine-related glutamate release and antidepressant response.
The ability of ketamine to stimulate human cortical glutamate release was demonstrated using a direct 13C-magnetic resonance spectroscopy (MRS) technique (68) and an indirect positron emission tomography (PET) method (69, 70). Using the latter approach, the magnitude of glutamate release correlated with the magnitude of depression improvement (71).
Support for an association between ketamine-related enhancement of synaptic efficacy and antidepressant response.
Preliminary studies suggest that antidepressant response is associated with an increase in the amplitude of sensory evoked potentials and stimulus-induced high-frequency cortical activity, as changes were observed in ketamine responders but not in ketamine nonresponders or healthy individuals (72, 73). Effective ketamine treatment also may ameliorate deficits in resting cortical functional connectivity (74).
Support for a role for synaptic regrowth in ketamine-related antidepressant response.
A pilot PET study of synaptic density provided evidence for the existence of at least two mechanisms contributing to ketamine efficacy (75). Ketamine did not affect synaptic density 24 h after a single dose in healthy individuals (n = 9) or depressed individuals without synaptic deficits (n = 6). While the depressed individuals without synaptic deficits improved after ketamine, their improvement was unrelated to changes in synaptic density and not associated with the degree of dissociative symptoms, i.e., a behavioral marker of the degree of NMDAR target engagement. However, in patients with depression with synaptic deficits (n = 6), ketamine increased synaptic density in a manner that was correlated with both clinical improvement and degree of dissociative symptoms.
Ketamine and Restoration of Homeostatic Plasticity within the Microcircuit
Ketamine effects on stress-related homeostatic plasticity also may contribute to its antidepressant effects (58, 76). By homeostatic plasticity, we refer to synaptic neuroadaptations to changes in neural activity that restore the balance between excitation and inhibition within microcircuits (77) and that complement input-specific synaptic plasticity (78). Depression produces synaptic downscaling as a response to impaired glutamate homeostasis as a consequence of astroglial dysfunction (43, 79) or enhanced tonic glutamate release (58). In both cases, NMDAR blockade by ketamine alleviates a homeostatic “break” on synaptic efficacy and neurotrophic signaling, in part, by reducing the phosphorylation of eukaryotic translation elongation factor-2 (eEF2) and activation of eEF2 kinase. These effects complement the ability of ketamine-induced glutamate release to drive restorative plasticity.
Homeostatic plasticity also may contribute to the ability to progressively reduce the frequency of ketamine dosing over time during long-term treatment. Treatments typically begin with twice-weekly ketamine infusions and then gradually decrease this frequency over time. It is not yet clear why the duration of ketamine’s therapeutic effects increases over time. One wonders if the increased duration of efficacy is related to increasing persistence of dendritic spines. If so, then steps that protect these spines might extend the duration of ketamine efficacy. Ketamine’s enhancement of AMPAR activation and raising of BDNF levels produces phosphorylation of methyl CpG binding protein 2 (MeCP2) Ser421, a protein implicated in ketamine’s effects on homeostatic plasticity (58) contributing to the prolongation but not the initiation of its antidepressant effects (80, 81). MeCP2, in turn, regulates mTOR signaling (82, 83) and, downstream, dysbindin (84). Dysbindin, in turn, is implicated in homeostatic regulation of glutamate release (85) as well as the outgrowth, maturation, and maintenance of dendritic spines (86, 87). Thus, the extended duration of ketamine efficacy with repeated dosing may engage specific proteins that might be targeted by novel treatments to extend the duration of ketamine efficacy.
Glutamatergic alterations in major depression are paralleled by disturbances in gamma-aminobutyric acid (GABA) signaling, reflected in lower cortical GABA levels in vivo (88, 89). These reductions are prominent in severely ill patients with psychosis or with prominent blunting of mood reactivity and vegetative signs of depression (90). Postmortem studies also describe compromised GABA neuronal integrity, particularly for SST GABA interneurons. In patients with treatment-resistant symptoms and GABA deficits, serotonin transporter antagonist (91), transcranial magnetic stimulation (92), and electroconvulsive therapy (93) treatments restored cortical GABA levels. One pilot study reported ketamine-related restoration of cortical GABA levels (94), a finding that was not replicated (95).
In animal studies, ketamine corrects stress-related reductions in GABA neuronal markers and GABA physiologic signals (inhibitory postsynaptic potentials) within cortico-limbic microcircuits (96, 97). Its induction of Hebbian and homeostatic synaptic plasticity is likely to occur in synapses between glutamate and GABA neurons (35), as well as between glutamate neurons. In animals, stress produces significant changes in GABA neurons, particularly in SST interneurons (35, 98). SST neurons modulate cortical functional connectivity by gating the efficacy of inputs to distal dendrites (99, 100). In stressed animals, SST neurons show reduced expression of molecular markers of functional integrity (101). GABA deficits in mice hemizygous for knockout of the γ2 subunit of the GABAA receptor exhibit homeostatic adaptations including downregulation of glutamate receptors and glutamate synapses (97). Thus, chronic GABA deficits associated with stress appear to produce allostatic adaptations that maladaptively restore excitation/inhibition balance but at a reduced setpoint for both GABA and glutamate synaptic connectivity. Ketamine also reverses the stress-related changes in glutamate and GABA signaling, maintaining excitation/inhibition balance but at normal levels (96, 97). α5-preferring GABAA receptor agonism may produce similar effects (102).
From Ketamine to Next-Generation Rapid-Acting Antidepressant Treatment
In the space below, we identify key questions related to ketamine efficacy that point to strategies optimizing NMDAR antagonist antidepressant treatments.
Protect the Integrity of the Restored Synaptic Connectivity Produced by Ketamine.
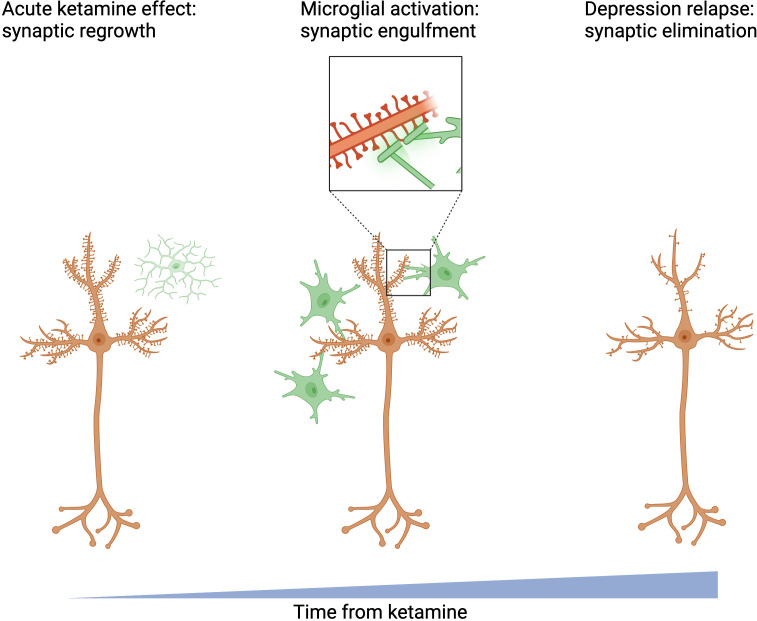
We (J.H.K., S.T.W., G.S., S.J., and A.P.K.) have treated patients who seem to have a complete therapeutic response to a single dose of ketamine only to see this improvement fade unless another dose of ketamine is administered. Clearly, restoration of an optimistic, positive psychological attitude in these cases is not sufficient on its own to prevent relapse. As the maintenance of ketamine’s antidepressant effects depends directly on restoration of lost spines (66), relapse may reflect the impermanence of regrown spines (67) or post-ketamine increases in spine elimination (Fig. 2). Thus, strategies that might prolong the persistence of the regrown spines might also extend ketamine efficacy. Two types of interventions are already shedding light on paths to extend ketamine efficacy: behavioral interventions and mTOR inhibition.
Fig. 2.
Potential mechanism limiting ketamine's duration of action.
Psychotherapy may augment and prolong the antidepressant efficacy of ketamine, as suggested by a pilot study of cognitive behavioral therapy delivered on the days following a ketamine infusion (103). Neural mechanisms underlying this effect are not well understood. Ketamine affects memory reconsolidation as well as the subsequent separation of memory engrams in the hippocampus. Experiences, such as fear extinction, can by themselves engender the kind of dendritic spine growth in the frontal cortex that is characteristic of ketamine’s effects (104). Also, there may be nonspecific immunological and neurotrophic effects of psychotherapy similar to another behavioral intervention, exercise. Exercise has robust antidepressant effects in humans (105) and animals (106). In animals, exercise raises brain BDNF levels, activates mTOR, promotes neurogenesis, and increases spinogenesis. It also protects newly created spines by reducing microglial inflammatory functions and promoting their neurotrophic functions (104, 107). Psychotherapy is a form of enrichment of the social environment. In animals, environmental enrichment raises BDNF levels, activates Akt/mTOR signaling, promotes synaptic growth, and protects dendritic integrity (108–113). Thus, it is possible that psychotherapies enhance and sustain ketamine efficacy through synergistic activity-dependent forms of neuroplasticity.
Another potential strategy for maintenance of ketamine-induced synapses is engagement of perineuronal nets (PNNs), which stabilize synapses and regulate synaptic plasticity. This extracellular matrix compartment coats PV interneurons (114), a potential initial target for ketamine’s antidepressant effects. The integrity of PNNs in the ventral hippocampus may be required for sustained antidepressant effects of ketamine (115), while repeated ketamine doses cause degradation of PNNs elsewhere (116–118).
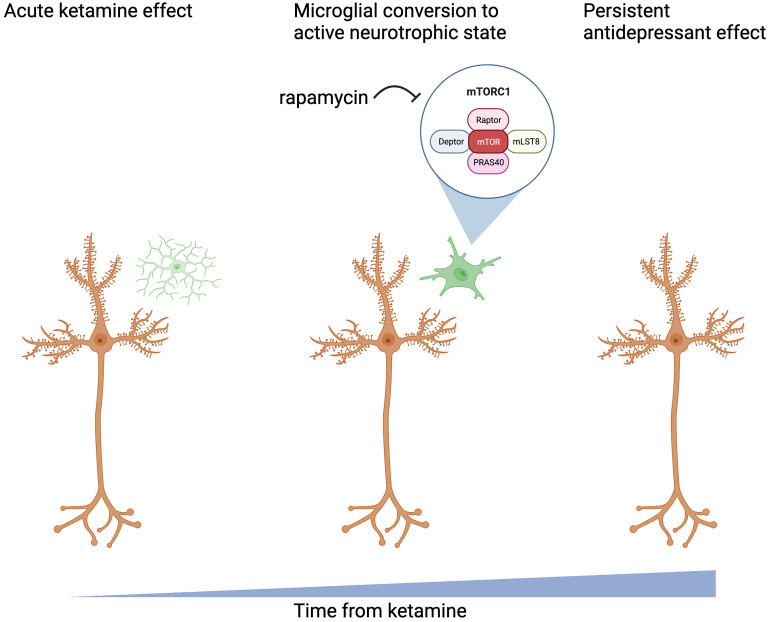
Low-dose mTOR inhibition may extend the duration of ketamine efficacy. In a within-subject study of 20 depressed patients, pretreatment with the mTOR inhibitor, rapamycin, increased ketamine response rates at 2 wk from 13 to 41% (119). This finding contrasted with the blockade of ketamine’s antidepressant effects by intracortical rapamycin, which produced much higher brain exposure to rapamycin (65). The extension of ketamine antidepressant effects by rapamycin might reflect the protection of regrown synapses from elimination by microglia (Fig. 3). Rapamycin may accomplish this by inhibiting mTOR, enhancing autophagy, and promoting the repolarization of microglia, i.e., inhibiting the inflammatory functions of microglia (120–122) and promoting their neurotrophic activity (123–126). Rapamycin also may paradoxically enhance ketamine activation of mTOR through negative feedback loops induced by mTORC1 inhibition, such as phosphatidylinositol-3 kinase (PI3K), AKT serine-threonine protein kinase (AKT), and extracellular signal-regulated kinase (ERK) activation (127, 128).
Fig. 3.
Potential avenue of extension of ketamine effect by mTOR inhibition.
Neuroinflammation appears to be an important contributor to treatment resistance of depression symptoms (129). The synergy of rapamycin, an immunosuppressant, and ketamine highlights the importance of antiinflammatory effects of ketamine to its clinical efficacy. The antiinflammatory effects of ketamine include reductions in proinflammatory cytokine release, inhibition of the proinflammatory kinase, glycogen synthase kinase 3β (GSK3β) (130), effects on the kynurenine pathway, interference with interferon signaling, reduction of microglial inflammatory polarization, and enhancement of microglial autophagic activity (129, 131–133).
Enhancing Ketamine Effects on Neuroplasticity via NMDAR Subtype Selectivity.
In randomized trials, ketamine is effective in a narrow dose range centered on 0.5 mg/kg administered intravenously over 40 min (47), but not at 0.2 mg/kg (134–136). At 1.0 mg/kg, ketamine has greater dissociative effects but not greater efficacy (135), while at anesthetic doses, ketamine is not antidepressant (137).
The narrow therapeutic dose range for ketamine limits its candidate primary brain targets to just a few including NMDARs and perhaps hyperpolarization-activated cyclic nucleotide-gated potassium channel 1 (HCN1) and TrkB receptors (138). The optimal ketamine dose for raising extracellular glutamate levels coincides with the typical antidepressant dose (139). Ketamine loses efficacy at anesthetic doses where it suppresses glutamate release (140). At these doses, ketamine blocks presynaptic NMDARs and HCN1 channels that promote glutamate release (141). Knockout of HCN1 channels prevents the emergence of ketamine’s antidepressant and neuroplastic effects (141). Thus, blockade of presynaptic NMDARs and HCN1 channels may limit ketamine efficacy.
Optimizing subunit selectivity might improve NMDAR antagonist tolerability or efficacy, although the path forward is not clear. Ketamine produces a higher affinity use-dependent blockade of the NMDAR cation channel and a lower affinity allosteric inhibition of channel opening, associated with anesthetic doses (142). Ketamine competes with magnesium for binding to the cation channel. This competition conveys greater ketamine potency at NMDARs with lower magnesium affinity, receptors bearing GluN2C and GluN2D subunits, relative to receptors bearing GluN2A or GluN2B subunits (143–145), although some studies challenge this view (146). Subunit selectivity may create a path to focus NMDAR antagonism on targets that promote efficacy and avoid those that may impede its effectiveness. Psychedelics, for example, enhance glutamate release and activate mTOR without blocking synaptic NMDARs or HCN1 channels (147–149) and single doses of psychedelic drugs appear to produce longer-duration antidepressant effects (150–152) than single doses of ketamine.
GluN2B-selective NMDAR antagonists appear to produce antidepressant effects in patients at doses that also evoke dissociative symptoms (153). In animals, GluN2B subunit-selective antagonists produce antidepressant effects via Hebbian plasticity (65, 154) and normalization of homeostatic adaptations (155–157). Knockdown of GluN2B subunits prevents or occludes the expression of ketamine’s antidepressant effects (80, 158). There may be drawbacks of blocking GluN2B-containing receptors. GluN2Bs figure prominently among postsynaptic NMDARs and blockade may reduce Hebbian plasticity (64). Also, GluN2B receptors contribute to presynaptic glutamate release (159) and blockade of these receptors reduces glutamate release that might underlie therapeutic neuroplasticity. One might infer that low doses of GluN2B-preferring NMDAR antagonists would produce restorative neuroplasticity without interfering with efficacy. However, low doses of the GluN2B-preferring NMDAR antagonist, memantine, were ineffective (160).
Selective blockers of the highest affinity targets for ketamine, GluN2C- and GluN2D-containing NMDARs, also might be considered. These NMDAR subtypes are well-represented in interneuron populations (161–165), although they are present in fewer excitatory synapses than GluN2B or GluN2A. GluN2D knockout mice do not exhibit glutamatergic neuronal activation in response to ketamine (166). The GluN2D-prefering NMDAR antagonist, S-methadone (146), showed efficacy in a preclinical study (167), where it activated mTOR and induced synaptic regrowth. Despite an encouraging preliminary report (168), press releases suggest that S-methadone failed in Phase III clinical trials. Questions related to the optimal dosing of GluN2D-selective antagonists remain. Selective antagonism of GluN2D-containing NMDARs has yet to be tested. Although GluN2C-containing NMDARs are the highest affinity target for ketamine (169), they appear to contribute to dissociative but not antidepressant effects. GluN2C knockout animals show attenuated behavioral abnormalities but not reduced antidepressant efficacy (170).
Optimize Ketamine Effects on Synaptic and Downstream Signaling.
Proteins in the signaling cascades activated by ketamine might be targeted as 1) alternative monotherapies for depression, 2) as adjunctive strategies to augment ketamine efficacy, or 3) as a strategy for creating a “nondissociative” ketamine via synergy, i.e., combination of a subdissociative dose of ketamine with an agent that conveys full efficacy. Drugs targeting proteins in ketamine-activated signaling cascades have shown promise in preclinical antidepressant studies (76). Because ketamine inhibits GABA neuronal activation and increases glutamate release, drugs that inhibit GABAA receptor signaling (171, 172) or block glutamate release-inhibiting metabotropic glutamate receptor-2 (173, 174) would be expected to reproduce or augment ketamine’s antidepressant effects. As activation of NMDA and AMPA glutamate receptors mediates a component of ketamine efficacy, drugs that facilitate activation of NMDARs (62, 175–177) or AMPARs (AMPAkines) (178–181) might also augment ketamine efficacy. In addition, drugs that raise BDNF levels, enhance TrkB receptor activation, or directly augment the activation of key steps in Akt/GSK-3/mTOR signaling (182–184) might similarly enhance ketamine’s efficacy. As has been suggested for GSK-3 inhibition, this strategy for combination treatment may enable a subdissociative dose of ketamine to achieve full efficacy (184).
Improve Ketamine Safety by Reducing the Dissociative Effects and Abuse Liability.
The primary strategy employed to date to develop a nondissociative NMDAR antagonist antidepressant has been to simply test subdissociative doses. To date, this strategy has not yielded a treatment with superior efficacy to ketamine. One might argue that the combination of buproprion and dextromethorphan is an effective antidepressant (185), but this medication has yet to show a ketamine-like clinical profile of rapid benefits and efficacy for treatment-resistant symptoms.
To date, tests of pharmacologic combination strategies to attenuate ketamine-induced dissociation have not yet yielded viable treatment approaches. Lamotrigine (186), lorazepam (187), and nimodipine (188) attenuate ketamine-induced dissociative symptoms in humans. Pretreatment with an mGluR2 agonist (189) also attenuated ketamine-related working memory impairment in healthy subjects. However, lamotrigine, lorazepam, and mGluR2 agonism reduce ketamine increases in cortical excitability (190–193) and thereby may interfere with ketamine efficacy. While nimodipine also reduces cortical excitability (194) and protects against NMDAR antagonist toxicity (195), it has yet to be tested in combination with ketamine during depression treatment. Clozapine (196), but not haloperidol (197, 198), attenuates ketamine-induced psychosis in people with schizophrenia or healthy subjects. Glycine transporter-1 (GlyT1) antagonists also reduced ketamine-induced psychosis in one study (199), but this finding was not replicated with another GlyT1 inhibitor (200).
Ketamine misuse may be the greatest risk associated with ketamine treatment. This risk is well-managed when ketamine or Esketamine are administered solely within clinics and patients do not have ketamine access at home. However, growing anecdotal reports of misuse of ketamine prescribed for home use are a concern (201, 202). We (J.H.K. and G.S.) have seen cases of individuals prescribed “take home” ketamine who subsequently developed compulsive ketamine use. Many NMDAR antagonists have abuse liability including ketamine (203), nitrous oxide (204, 205), dextromethorphan (206), phencyclidine (207), and ethanol (208). R-ketamine, the ketamine enantiomer of ketamine with reduced potency at NMDA and opioid receptors, also appears to have reduced abuse liability than S-ketamine in animals (209). Pharmacologic strategies for reducing the abuse liability of ketamine while preserving efficacy have yet to bear fruit. The euphoric effects of ketamine are not attenuated by pretreatment with the dopamine D2 receptor antagonist, haloperidol (198), or the opioid receptor antagonist, naltrexone (210). While we could not replicate the blockade of antidepressant effects of ketamine by naltrexone (211, 212), ketamine’s indirect facilitation of endogenous opioid signaling may contribute to its antidepressant effects (213). In contrast, in recovering ethanol-dependent patients, nimodipine reduced ketamine-induced euphoria (188). Thus, combining ketamine with a blockade of voltage-dependent calcium channels may reduce both dissociation risks and abuse liability.
Conclusions
Ketamine and Esketamine, with brexanolone, MDMA, and the psychedelics, have ushered in a first generation of rapid-acting antidepressants. Ketamine targets aspects of the neurobiology of depression that had not been linked so directly to prior treatments and it has led to the characterization of novel forms of antidepressant-related neuroplasticity. Twenty-five years have passed since we (J.H.K.) first presented the ketamine findings in depression. Thus, it is timely to work toward a next era of rapidly acting antidepressants that complement or even supersede ketamine and Esketamine in safety and efficacy. This review highlighted both general and specific strategies that might be pursued:
Protecting the integrity of regrown synapses through exercise, psychotherapy, and medications, like mTORC1 inhibitors, that promote repolarization of microglia, shifting them from proinflammatory to neurotrophic functional states.
Enhancing ketamine’s ability to engage synaptic plasticity through avoiding HCN1 antagonism and optimizing NMDAR subtype selectivity.
Targeting the downstream signaling mechanisms induced by ketamine as alternative monotherapies, combination treatments that augment ketamine efficacy, or that yield a nondissociative rapid-acting antidepressant, i.e., combination of a subdissociative dose of ketamine with another agent that augments its efficacy.
Development of combination treatments or new chemical entities that preserve efficacy but reduce the dissociative symptoms and abuse liability of ketamine.
These strategies also have implications for the optimization of psychedelic treatments for depression. Psychedelics increase glutamate release and activate mTOR (214), but they do not block NMDARs. It is not clear whether this difference contributes to the long-lasting antidepressant effects of psychedelics (151). However, psychedelics may not block maladaptive homeostatic plasticity as they do not block NMDARs, raising the possibility that ketamine and psychedelics have differing profiles of clinical efficacy. Nevertheless, the convergent effects of ketamine and psychedelics upon many downstream signaling mechanisms (147, 149, 215) may suggest that strategies outlined above for augmenting ketamine efficacy and safety might apply to psychedelics. In turn, efforts to create “nonhallucinogenic” psychedelics via biased 5-HT2A receptor agonism, partial 5-HT2A receptor agonism, combinations of 5-HT2A receptor agonists and antagonists, and other strategies (216–222) may suggest strategies for improving upon ketamine.
This is an opportune moment to press forward toward safer and more effective treatments for depression. Prior to the approval of Esketamine, psychiatry seemed to be backing away from its most effective treatments, perhaps as an expression of therapeutic nihilism. For example, the number of sites delivering electroconvulsive therapy has declined (223). Yet, the need is high. Depression is a leading contributor to the global burden of disease (224), in part due to ineffectively treated depression. The STAR*D study suggested that approximately one-third of patients do not achieve remission over 1 y despite multiple treatment attempts (225). An earlier study suggested that if patients do not attain clinical response over 1 y, only 20% of these patients will respond over the subsequent 4 y (226).
Psychiatry is increasingly interventional in addressing the treatment-resistant symptoms of depression, highlighted by the recent revisiting of deep brain stimulation as a depression treatment (28). As psychotherapists, psychiatrists engage in one of the most intense, invasive, and prolonged interventions in all of medicine. Ketamine, Esketamine, brexanolone, psychedelics, and MDMA are all intensive psychopharmacologic interventions that powerfully modulate consciousness, carry medical risks, but also offer paths to address the nihilism arising from the limited efficacy of standard treatment options. This is a very hopeful moment for psychiatric psychopharmacology and one that may profoundly impact the global burden of depression.
Acknowledgments
A.P.K. acknowledges the use of Biorender software for figure preparation. The work associated with this review was supported by the National Center for posttraumatic stress disorder (PTSD) of the US Department of Veterans Affairs (J.H.K., A.P.K., S.J., M.J.G., and I.E.), the Yale Center for Clinical Investigation (UL1TR001863; J.H.K., M.J.G., S.T.W., and I.E.), Nancy Taylor Foundation (I.E.), American Foundation for Suicide Prevention (J.H.K.), National Institute of Mental Health (S.T.W., G.S., and S.J.), Janssen Pharmaceuticals (G.S. and S.T.W.), Oui Therapeutics (S.T.W.), Sage Therapeutics (S.T.W.), and Freedom Biosciences (S.J. and A.P.K.). Illustration assistance was done by Jocelyne Rondeau. We acknowledge the following financial interests in the past three years: G.S. has received research contracts from Johnson & Johnson/Janssen, Merck, and the Usona Institute over the past 36 mo. A.P.K. received research funding from Freedom Biosciences, Transcend Therapeutics, State of Connecticut Department of Mental Health and Addiction Services (Ribicoff Laboratories) and Department of Defense.
Author contributions
J.H.K., S.J., M.J.G., S.T.W., G.S., and I.E. contributed to discussions that led to writing of the paper and critical review that reshaped the paper during preparation; A.P.K. contributed to discussions that led to writing of the paper and critical review that reshaped the paper during preparation and prepared figures included in the paper; and J.H.K., A.P.K., S.J., M.J.G., S.T.W., G.S., and I.E. wrote the paper.
Competing interests
S.T.W.: Janssen Research Foundation, Sage Therapeutics; J.H.K.: (consultations less than $5,000/y) Aptinyx, Inc., BioXcel, Biogen, Idec, MA, Bionomics, Limited (Australia), Boehringer Ingelheim International, Cerevel Pharmaceuticals, Epiodyne, Inc., Esai, Inc., Janssen Research & Development, Jazz Pharmaceuticals, Inc., Otsuka America Pharmaceutical, Inc., PsychoGenics, Inc., Sunovion Pharmaceuticals, Inc., Takeda Pharmaceuticals (Stock/Options) Biohaven Pharmaceuticals, Cartego Therapeutics, Damona Pharmaceuticals, Delix Therapeutics, Inc., EpiVario, Inc., Freedom Biosciences, Neumora Therapeutics, Inc., Response Pharmaceuticals, Rest Therapeutics, Spring Health, Inc., Tempero Bio, Inc., Terran Biosciences, Tetricus Inc. (nonmonetary support, i.e., provision of drug) Cerevel Pharmaceuticals, Novartis. G.S. has served as consultant to Ancora, Aptinyx, Atai, Axsome Therapeutics, Biogen, Biohaven Pharmaceuticals, Boehringer Ingelheim International GmbH, Bristol-Myers Squibb, Clexio, Cowen, Denovo Biopharma, ECR1, EMA Wellness, Engrail Therapeutics, Freedom Biosciences, Gilgamesh, Intra-Cellular Therapies, Janssen, KOA Health, Levo therapeutics, Lundbeck, Merck, MiCure, Navitor Pharmaceuticals, Neurocrine, Novartis, Noven Pharmaceuticals, Otsuka, Perception Neuroscience, Praxis Therapeutics, Relmada Therapeutics, Sage Pharmaceuticals, Seelos Pharmaceuticals, Taisho Pharmaceuticals, Valeant, Vistagen Therapeutics, and XW Labs. G.S. holds equity in Biohaven Pharmaceuticals, Freedom Biosciences, Gilead, Relmada, and Tetricus. J.H.K. stands to benefit from patents held by Yale University that were licensed to Janssen Pharmaceuticals (ketamine), Biohaven Pharmaceuticals (riluzole), Freedom Biosciences (NMDA-R antagonist plus MTORC inhibitor), and Spring Health (precision medicine strategy). G.S. is a co-inventor on a US patent (#8,778,979) held by Yale University and a co-inventor on US Provisional Patent Application No. 047162-7177P1 (00754) licensed to Janssen Pharmaceuticals.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
There are no data underlying this work.
References
- 1.Domino E. F., Chodoff P., Corssen G., Pharmacologic effects of CI-581, a new dissociative anesthetic, in man. Clin. Pharm. Ther. 6, 279–291 (1965). [DOI] [PubMed] [Google Scholar]
- 2.Noppers I., et al. , Ketamine for the treatment of chronic non-cancer pain. Expert Opin. Pharmacother. 11, 2417–2429 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Bell R. F., Eccleston C., Kalso E. A., Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst. Rev. 11, CD003351 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Wright M., Pharmacologic effects of ketamine and its use in veterinary medicine. J. Am. Vet. Med. Assoc. 180, 1462–1471 (1982). [PubMed] [Google Scholar]
- 5.Ng S. H., Tse M. L., Ng H. W., Lau F. L., Emergency department presentation of ketamine abusers in Hong Kong: A review of 233 cases. Hong Kong Med. J. 16, 6–11 (2010). [PubMed] [Google Scholar]
- 6.Tang W. K., et al. , Psychiatric morbidity in ketamine users attending counselling and youth outreach services. Subst. Abus 36, 67–74 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Palamar J. J., Rutherford C., Keyes K. M., Trends in ketamine use, exposures, and seizures in the United States. Am. J. Public Health 111, 2046–2049 (2021), 10.2105/ajph.2021.306486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muetzelfeldt L., et al. , Journey through the K-hole: Phenomenological aspects of ketamine use. Drug Alcohol. Depend. 95, 219–229 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Krystal J. H., et al. , Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen. Psychiatr y 51, 199–214 (1994). [DOI] [PubMed] [Google Scholar]
- 10.Lahti A. C., Holcomb H. H., Medoff D. R., Tamminga C. A., Ketamine activates psychosis and alters limbic blood flow in schizophrenia. Neuroreport 6, 869–872 (1995). [DOI] [PubMed] [Google Scholar]
- 11.Malhotra A. K., et al. , NMDA receptor function and human cognition: The effects of ketamine in healthy volunteers. Neuropsychopharmacology 14, 301–307 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Carpenter W. T. Jr., The schizophrenia ketamine challenge study debate. Biol. Psychiatry 46, 1081–1091 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Schatzberg A. F., A word to the wise about ketamine. Am. J. Psychiatry 171, 262–264 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Loomer H. P., Saunders J. C., Kline N. S., A clinical and pharmacodynamic evaluation of iproniazid as a psychic energizer. Psychiatric Res. Rep. 8, 129–141 (1957). [PubMed] [Google Scholar]
- 15.Kuhn R., Treatment of depressive states with an iminodibenzyl derivative (G 22355). Schweiz. Med. Wochenschr. 87, 1135–1140 (1957). [PubMed] [Google Scholar]
- 16.Cade J. F., Lithium salts in the treatment of psychotic excitement. Med. J. Aust. 2, 349–352 (1949). [DOI] [PubMed] [Google Scholar]
- 17.Delay J., Deniker P., Harl J. M., Therapeutic use in psychiatry of phenothiazine of central elective action (4560 RP). Ann. Med. Psychol. (Paris) 110, 112–117 (1952). [PubMed] [Google Scholar]
- 18.Schildkraut J. J., Kety S. S., Biogenic amines and emotion. Science 156, 21–37 (1967). [DOI] [PubMed] [Google Scholar]
- 19.Coppen A. J., Depressed states and indolealkylamines. Adv. Pharmacol. 1962, 283–291 (1968). [DOI] [PubMed] [Google Scholar]
- 20.Krystal J. H., Abdallah C. G., Sanacora G., Charney D. S., Duman R. S., Ketamine: A paradigm shift for depression research and treatment. Neuron 101, 774–778 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salomon R. M., Miller H. L., Krystal J. H., Heninger G. R., Charney D. S., Lack of behavioral effects of monoamine depletion in healthy subjects. Biol. Psychiatry 41, 58–64 (1997). [DOI] [PubMed] [Google Scholar]
- 22.Schmaal L., et al. , ENIGMA MDD: Seven years of global neuroimaging studies of major depression through worldwide data sharing. Trans. Psychiatry 10, 172 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murrough J. W., et al. , Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Hum. Brain Mapp. 37, 3214–3223 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser R. H., Andrews-Hanna J. R., Wager T. D., Pizzagalli D. A., Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72, 603–611 (2015), 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayberg H. S., Limbic-cortical dysregulation: A proposed model of depression. J. Neuropsychiatry Clin. Neurosci. 9, 471–481 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Pizzagalli D. A., Toward a better understanding of the mechanisms and pathophysiology of anhedonia: Are we ready for translation? Am. J. Psychiatry 179, 458–469 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ressler K. J., Mayberg H. S., Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat. Neurosci. 10, 1116–1124 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scangos K. W., et al. , Closed-loop neuromodulation in an individual with treatment-resistant depression. Nat. Med. 27, 1696–1700 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramaker R. C., et al. , Post-mortem molecular profiling of three psychiatric disorders. Genome Med. 9, 72 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labonté B., et al. , Sex-specific transcriptional signatures in human depression. Nat. Med. 23, 1102–1111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girgenti M. J., et al. , Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat. Neurosci. 24, 24–33 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Martins-de-Souza D., et al. , Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Trans. Psychiatry 2, e87 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H., et al. , Functional annotation of the human PTSD methylome identifies tissue-specific epigenetic variation across subcortical brain regions. medRxiv [Preprint] (2023). 10.1101/2023.04.18.23288704 (Accessed 25 April 2023). [DOI]
- 34.Krystal J. H., et al. , Glutamate and GABA systems as targets for novel antidepressant and mood stabilizing treatments. Mol. Psychiatry 7, S71–S80 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Duman R. S., Sanacora G., Krystal J. H., Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 102, 75–90 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kangas E. S., Vuoriainen E., Lindeman S., Astikainen P., Auditory event-related potentials in separating patients with depressive disorders and non-depressed controls: A narrative review. Int. J. Psychophysiol. 179, 119–142 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Averill L. A., et al. , Reduced energy per cycle, a marker of glutamatergic synaptic strength, in individuals diagnosed with PTSD and depression. medRxiv [Preprint] (2021). 10.1101/2023.04.18.23288704 (Accessed 25 April 2023). [DOI]
- 38.Magarinos A. M., McEwen B. S., Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69, 89–98 (1995). [DOI] [PubMed] [Google Scholar]
- 39.Radley J. J., et al. , Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 16, 313–320 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Duric V., et al. , Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int. J. Neuropsychopharmacol. 16, 69–82 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang H. J., et al. , Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 18, 1413–1417 (2012), 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes S. E., et al. , Lower synaptic density is associated with depression severity and network alterations. Nat. Commun. 10, 1529 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popoli M., Yan Z., McEwen B. S., Sanacora G., The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 13, 22–37 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hardingham G. E., Bading H., Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 11, 682–696 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajkowska G., Stockmeier C. A., Astrocyte pathology in major depressive disorder: Insights from human postmortem brain tissue. Curr. Drug Targets 14, 1225–1236 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medina A., et al. , Glutamate transporters: A key piece in the glutamate puzzle of major depressive disorder. J. Psychiatr. Res. 47, 1150–1156 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Berman R. M., et al. , Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Zarate C. A. Jr., et al. , A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Jawad M. Y., et al. , The efficacy and safety of adjunctive intranasal esketamine treatment in major depressive disorder: A systematic review and meta-analysis. Expert Opin. Drug Saf. 21, 841–852 (2022), 10.1080/14740338.2022.2058488. [DOI] [PubMed] [Google Scholar]
- 50.Bahji A., Zarate C. A., Vazquez G. H., Efficacy and safety of racemic ketamine and esketamine for depression: A systematic review and meta-analysis. Expert Opin. Drug Saf. 21, 863–866 (2022), 10.1080/14740338.2022.2047928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaynes B. N., et al. , Treating depression after initial treatment failure: Directly comparing switch and augmenting strategies in STAR*D. J. Clin. Psychopharmacol. 32, 114–119 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Anand A., et al. , Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N. Engl. J. Med. 388, 2315–2325 (2023). [DOI] [PubMed] [Google Scholar]
- 53.Alnefeesi Y., et al. , Real-world effectiveness of ketamine in treatment-resistant depression: A systematic review & meta-analysis. J. Psychiatr Res. 151, 693–709 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Jeon H. J., et al. , Long-term safety and efficacy of esketamine nasal spray plus an oral antidepressant in patients with treatment-resistant depression- an asian sub-group analysis from the SUSTAIN-2 study. Clin. Psychopharmacol. Neurosci. 20, 70–86 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daly E. J., et al. , Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 76, 893–903 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gueorguieva R., Chekroud A. M., Krystal J. H., Trajectories of relapse in randomised, placebo-controlled trials of treatment discontinuation in major depressive disorder: An individual patient-level data meta-analysis. The Lancet Psychiatry 4, 230–237 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaki N., et al. , Long-term safety and maintenance of response with esketamine nasal spray in participants with treatment-resistant depression: Interim results of the SUSTAIN-3 study. Neuropsychopharmacology 48, 1225–1233 (2023), 10.1038/s41386-023-01577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kavalali E. T., Monteggia L. M., Rapid homeostatic plasticity and neuropsychiatric therapeutics. Neuropsychopharmacology 48, 54–60 (2022), 10.1038/s41386-022-01411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ali F., et al. , Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nat. Commun. 11, 72–72 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fogaça M. V., et al. , Inhibition of GABA interneurons in the mPFC is sufficient and necessary for rapid antidepressant responses. Mol. Psychiatry 26, 3277–3291 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerhard D. M., et al. , GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J. Clin. Invest. 130, 1336–1349 (2019), 10.1172/JCI130808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pothula S., et al. , Cell-type specific modulation of NMDA receptors triggers antidepressant actions. Mol. Psychiatry 26, 5097–5111 (2021). [DOI] [PubMed] [Google Scholar]
- 63.Fuchikami M., et al. , Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc. Natl. Acad. Sci. U.S.A. 112, 8106–8111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zanos P., et al. , NMDA receptor activation-dependent antidepressant-relevant behavioral and synaptic actions of ketamine. J. Neurosci. 43, 1038–1050 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li N., et al. , mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329, 959–964 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moda-Sava R. N., et al. , Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science (New York, N.Y.) 364, eaat8078 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phoumthipphavong V., Barthas F., Hassett S., Kwan A. C., Longitudinal effects of ketamine on dendritic architecture in vivo in the mouse medial frontal cortex. eNeuro 3 (2016), 10.1523/ENEURO.0133-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdallah C. G., et al. , The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology 43, 2154–2160 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeLorenzo C., et al. , In vivo ketamine-induced changes in [(1)(1)C]ABP688 binding to metabotropic glutamate receptor subtype 5. Biol. Psychiatry 77, 266–275 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holmes S. E., et al. , Measuring the effects of ketamine on mGluR5 using [(18)F]FPEB and PET. J. Cereb Blood Flow Metab 40, 2254–2264 (2019), 10.1177/0271678x19886316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esterlis I., et al. , Ketamine-induced reduction in mGluR5 availability is associated with an antidepressant response: An [11C]ABP688 and PET imaging study in depression. Mol. Psychiatry 23, 824–832 (2017), 10.1038/mp.2017.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nugent A. C., et al. , Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol. Psychiatry 24, 1040–1052 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nugent A. C., Wills K. E., Gilbert J. R., Zarate C. A. Jr., Synaptic potentiation and rapid antidepressant response to ketamine in treatment-resistant major depression: A replication study. Psychiatry Res. Neuroimaging 283, 64–66 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abdallah C. G., et al. , Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology 42, 1210–1219 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holmes S. E., et al. , Imaging the effect of ketamine on synaptic density (SV2A) in the living brain. Mol. Psychiatry 27, 2273–2281 (2022), 10.1038/s41380-022-01465-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krystal J. H., Sanacora G., Duman R. S., Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol. Psychiatry 73, 1133–1141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turrigiano G., Abbott L. F., Marder E., Activity-dependent changes in the intrinsic properties of cultured neurons. Science 264, 974–977 (1994). [DOI] [PubMed] [Google Scholar]
- 78.Turrigiano G. G., The dialectic of Hebb and homeostasis. Philos. Trans. R. Soc. Lond B Biol. Sci . 372, 20160258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delgado J. Y., Fink A. E., Grant S. G. N., O’Dell T. J., Opazo P., Rapid homeostatic downregulation of LTP by extrasynaptic GluN2B receptors. J. Neurophysiol. 120, 2351–2357 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim J. W., Suzuki K., Kavalali E. T., Monteggia L. M., Bridging rapid and sustained antidepressant effects of ketamine. Trends Mol. Med. 29, 275–364 (2023), 10.1016/j.molmed.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim J. W., et al. , Sustained effects of rapidly acting antidepressants require BDNF-dependent MeCP2 phosphorylation. Nat. Neurosci. 24, 1100–1109 (2021), 10.1038/s41593-021-00868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buist M., et al. , Differential sensitivity of the protein translation initiation machinery and mTOR signaling to MECP2 gain- and loss-of-function involves MeCP2 isoform-specific homeostasis in the brain. Cells 11, 1442 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhai K., Liu B., Teng J., MicroRNA-212-3p regulates early neurogenesis through the AKT/mTOR pathway by targeting MeCP2. Neurochem. Int. 137, 104734 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Fei E., Chen P., Zhang Q., Zhong Y., Zhou T., Protein kinase B/Akt1 phosphorylates dysbindin-1A at Serine 10 to regulate neuronal development. Neuroscience 490, 66–78 (2022). [DOI] [PubMed] [Google Scholar]
- 85.Dickman D. K., Davis G. W., The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science 326, 1127–1130 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ito H., et al. , Dysbindin-1, WAVE2 and Abi-1 form a complex that regulates dendritic spine formation. Mol. Psychiatry 15, 976–986 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Jia J. M., Hu Z., Nordman J., Li Z., The schizophrenia susceptibility gene dysbindin regulates dendritic spine dynamics. J. Neurosci. 34, 13725–13736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanacora G., et al. , Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen. Psychiatry 56, 1043–1047 (1999). [DOI] [PubMed] [Google Scholar]
- 89.Abdallah C. G., et al. , Prefrontal cortical GABA abnormalities are associated with reduced hippocampal volume in major depressive disorder. Eur. Neuropsychopharmacol. 25, 1082–1090 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanacora G., et al. , Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch. Gen. Psychiatry 61, 705–713 (2004). [DOI] [PubMed] [Google Scholar]
- 91.Sanacora G., Mason G. F., Rothman D. L., Krystal J. H., Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatry 159, 663–665 (2002). [DOI] [PubMed] [Google Scholar]
- 92.Dubin M. J., et al. , Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J. Psychiatry Neurosci. 41, E37–E45 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanacora G., et al. , Increased cortical GABA concentrations in depressed patients receiving ECT. Am. J. Psychiatry 160, 577–579 (2003). [DOI] [PubMed] [Google Scholar]
- 94.Milak M. S., et al. , A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder. Mol. Psychiatry 21, 320–327 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valentine G. W., et al. , The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 191, 122–127 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ghosal S., et al. , Ketamine rapidly reverses stress-induced impairments in GABAergic transmission in the prefrontal cortex in male rodents. Neurobiol. Dis. 134, 104669–104669 (2019). [DOI] [PubMed] [Google Scholar]
- 97.Ren Z., et al. , Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol. Psychiatry 80, 457–468 (2016), 10.1016/j.biopsych.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fee C., Banasr M., Sibille E., Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: Cortical microcircuit and therapeutic perspectives. Biol. Psychiatry 82, 549–559 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chiu C. Q., et al. , Compartmentalization of GABAergic inhibition by dendritic spines. Science 340, 759–762 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Higley M. J., Localized GABAergic inhibition of dendritic Ca(2+) signalling. Nat. Rev. Neurosci. 15, 567–572 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Banasr M., et al. , Characterization of GABAergic marker expression in the chronic unpredictable stress model of depression. Chronic Stress (Thousand Oaks) 1, 2470547017720459 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fee C., et al. , Behavioral deficits induced by somatostatin-positive GABA neuron silencing are rescued by alpha 5 GABA-A receptor potentiation. Int. J. Neuropsychopharmacol. 24, 505–518 (2021), 10.1093/ijnp/pyab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wilkinson S. T., et al. , Cognitive behavioral therapy to sustain the antidepressant effects of ketamine in treatment-resistant depression: A randomized clinical trial. Psychother. Psychosom. 90, 318–327 (2021). [DOI] [PubMed] [Google Scholar]
- 104.Xu Z., et al. , Fear conditioning and extinction induce opposing changes in dendritic spine remodeling and somatic activity of layer 5 pyramidal neurons in the mouse motor cortex. Sci. Rep. 9, 4619 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chekroud S. R., et al. , Association between physical exercise and mental health in 1.2 million individuals in the USA between 2011 and 2015: A cross-sectional study. Lancet Psychiatry 5, 739–746 (2018). [DOI] [PubMed] [Google Scholar]
- 106.Yau S. Y., et al. , Sustained running in rats administered corticosterone prevents the development of depressive behaviors and enhances hippocampal neurogenesis and synaptic plasticity without increasing neurotrophic factor levels. Cell Trans. 23, 481–492 (2014). [DOI] [PubMed] [Google Scholar]
- 107.Salter M. W., Stevens B., Microglia emerge as central players in brain disease. Nat. Med. 23, 1018–1027 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Chen M., et al. , Enriched environment ameliorates learning and memory deficits in hepatic encephalopathy mice by restoration of the structure of dendrites and dendritic spines. Brain Res. 1804, 148264 (2023). [DOI] [PubMed] [Google Scholar]
- 109.Pamidi N., Yap C. G., Nayak N., Environmental enrichment preserves hippocampal neurons in diabetes and stressed rats. Histol. Histopathol. 37, 385–395 (2022). [DOI] [PubMed] [Google Scholar]
- 110.Ashokan A., Hegde A., Mitra R., Short-term environmental enrichment is sufficient to counter stress-induced anxiety and associated structural and molecular plasticity in basolateral amygdala. Psychoneuroendocrinology 69, 189–196 (2016). [DOI] [PubMed] [Google Scholar]
- 111.Hu X. L., Bergström S. A., Brink M., Rönnbäck A., Dahlqvist P., Enriched environment increases spinophilin mRNA expression and spinophilin immunoreactive dendritic spines in hippocampus and cortex. Neurosci. Lett. 476, 79–83 (2010). [DOI] [PubMed] [Google Scholar]
- 112.Landers M. S., Knott G. W., Lipp H. P., Poletaeva I., Welker E., Synapse formation in adult barrel cortex following naturalistic environmental enrichment. Neuroscience 199, 143–152 (2011). [DOI] [PubMed] [Google Scholar]
- 113.Ramírez-Rodríguez G., et al. , Environmental enrichment induces neuroplastic changes in middle age female Balb/c mice and increases the hippocampal levels of BDNF, p-Akt and p-MAPK1/2. Neuroscience 260, 158–170 (2014). [DOI] [PubMed] [Google Scholar]
- 114.Härtig W., Brauer K., Brückner G., Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport 3, 869–872 (1992). [DOI] [PubMed] [Google Scholar]
- 115.Donegan J. J., Lodge D. J., Hippocampal perineuronal nets are required for the sustained antidepressant effect of ketamine. Int. J. Neuropsychopharmacol. 20, 354–358 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matuszko G., Curreli S., Kaushik R., Becker A., Dityatev A., Extracellular matrix alterations in the ketamine model of schizophrenia. Neuroscience 350, 13–22 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Kaushik R., et al. , Fine structure analysis of perineuronal nets in the ketamine model of schizophrenia. Eur. J. Neurosci. 53, 3988–4004 (2021). [DOI] [PubMed] [Google Scholar]
- 118.Venturino A., et al. , Microglia enable mature perineuronal nets disassembly upon anesthetic ketamine exposure or 60-Hz light entrainment in the healthy brain. Cell Rep. 36, 109313 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abdallah C. G., et al. , Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology 45, 990–997 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xie L., et al. , mTOR signaling inhibition modulates macrophage/microglia-mediated neuroinflammation and secondary injury via regulatory T cells after focal ischemia. J. Immunol. 192, 6009–6019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goldshmit Y., et al. , Rapamycin increases neuronal survival, reduces inflammation and astrocyte proliferation after spinal cord injury. Mol. Cell Neurosci. 68, 82–91 (2015). [DOI] [PubMed] [Google Scholar]
- 122.Guden D. S., et al. , mTOR inhibition as a possible pharmacological target in the management of systemic inflammatory response and associated neuroinflammation by lipopolysaccharide challenge in rats. Can J. Physiol. Pharmacol. 99, 921–934 (2021). [DOI] [PubMed] [Google Scholar]
- 123.Bai J., et al. , Exercise facilitates the M1-to-M2 polarization of microglia by enhancing autophagy via the BDNF/AKT/mTOR pathway in neuropathic pain. Pain Phys. 25, E1137–E1151 (2022). [PubMed] [Google Scholar]
- 124.Chen L., et al. , Everolimus (RAD001) ameliorates vascular cognitive impairment by regulating microglial function via the mTORC1 signaling pathway. J. Neuroimmunol. 299, 164–171 (2016). [DOI] [PubMed] [Google Scholar]
- 125.He T., et al. , Sestrin2 regulates microglia polarization through mTOR-mediated autophagic flux to attenuate inflammation during experimental brain ischemia. J. Neuroinflammation 17, 329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li D., et al. , mTORC1 pathway disruption ameliorates brain inflammation following stroke via a shift in microglia phenotype from M1 type to M2 type. FASEB J. 30, 3388–3399 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Chen X. G., et al. , Rapamycin regulates Akt and ERK phosphorylation through mTORC1 and mTORC2 signaling pathways. Mol. Carcinog. 49, 603–610 (2010). [DOI] [PubMed] [Google Scholar]
- 128.Soares H. P., Ni Y., Kisfalvi K., Sinnett-Smith J., Rozengurt E., Different patterns of Akt and ERK feedback activation in response to rapamycin, active-site mTOR inhibitors and metformin in pancreatic cancer cells. PLoS One 8, e57289 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johnston J. N., et al. , Inflammation, stress and depression: An exploration of ketamine’s therapeutic profile. Drug Discov. Today 28, 103518 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kalkman H. O., Inhibition of microglial GSK3β activity is common to different kinds of antidepressants: A proposal for an in vitro screen to detect novel antidepressant principles. Biomedicines 11, 806 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu Y., Ding X., Wu X., Huang S., Ketamine inhibits LPS-mediated BV2 microglial inflammation via NMDA receptor blockage. Fundam. Clin. Pharmacol. 34, 229–237 (2019), 10.1111/fcp.12508. [DOI] [PubMed] [Google Scholar]
- 132.Ho M.-F., Zhang C., Zhang L., Li H., Weinshilboum R. M., Ketamine and active ketamine metabolites regulate STAT3 and the Type I interferon pathway in human microglia: Molecular mechanisms linked to the antidepressant effects of ketamine. Front Pharmacol. 10, 1302–1302 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu M., et al. , Ketamine regulates the autophagy flux and polarization of microglia through the HMGB1-RAGE axis and exerts antidepressant effects in mice. J. Neuropathol. Exp. Neurol. 81, 931–942 (2022). [DOI] [PubMed] [Google Scholar]
- 134.Su T. P., et al. , Dose-related effects of adjunctive ketamine in taiwanese patients with treatment-resistant depression. Neuropsychopharmacology 42, 2482–2492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fava M., et al. , Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol. Psychiatry 25, 1592–1603 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Abdallah C. G., et al. , Dose-related effects of ketamine for antidepressant-resistant symptoms of posttraumatic stress disorder in veterans and active duty military: A double-blind, randomized, placebo-controlled multi-center clinical trial. Neuropsychopharmacology 47, 1574–1581 (2022), 10.1038/s41386-022-01266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ainsworth N. J., Sepehry A. A., Vila-Rodriguez F., Effects of ketamine anesthesia on efficacy, tolerability, seizure response, and neurocognitive outcomes in electroconvulsive therapy: A comprehensive meta-analysis of double-blind randomized controlled trials. J. ECT 36, 94–105 (2020). [DOI] [PubMed] [Google Scholar]
- 138.Casarotto P. C., et al. , Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 184, 1299–1313.e1219 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moghaddam B., Adams B., Verma A., Daly D., Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 17, 2921–2927 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anis N. A., Berry S. C., Burton N. R., Lodge D., The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. British J. Pharmacol. 79, 565–575 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang K., et al. , Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast-acting antidepressant responses of ketamine. Sci. Signal 9, ra123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Orser B. A., Pennefather P. S., MacDonald J. F., Multiple mechanisms of ketamine blockade of N-methyl-D-aspartate receptors. Anesthesiology 86, 903–917 (1997). [DOI] [PubMed] [Google Scholar]
- 143.Clarke R. J., Glasgow N. G., Johnson J. W., Mechanistic and structural determinants of NMDA receptor voltage-dependent gating and slow Mg2+ unblock J. Neurosci. 33, 4140–4150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Glasgow N. G., Siegler Retchless B., Johnson J. W., Molecular bases of NMDA receptor subtype-dependent properties. J. Physiol. 593, 83–95 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Glasgow N. G., Wilcox M. R., Johnson J. W., Effects of Mg(2+) on recovery of NMDA receptors from inhibition by memantine and ketamine reveal properties of a second site. Neuropharmacology 137, 344–358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fava M., et al. , Esmethadone-HCl (REL-1017): A promising rapid antidepressant. Eur. Arch Psychiatry Clin. Neurosci. 273, 1463–1476 (2023), 10.1007/s00406-023-01571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.De Gregorio D., et al. , Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission. Proc. Natl. Acad. Sci. U.S.A. 118, e2020705118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aleksandrova L. R., Phillips A. G., Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol. Sci. 42, 929–942 (2021), 10.1016/j.tips.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 149.Kwan A. C., Olson D. E., Preller K. H., Roth B. L., The neural basis of psychedelic action. Nat. Neurosci. 25, 1407–1419 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carhart-Harris R., et al. , Trial of psilocybin versus escitalopram for depression. N. Engl. J. Med. 384, 1402–1411 (2021). [DOI] [PubMed] [Google Scholar]
- 151.Goodwin G. M., et al. , Single-dose psilocybin for a treatment-resistant episode of major depression. N. Engl. J. Med. 387, 1637–1648 (2022). [DOI] [PubMed] [Google Scholar]
- 152.Goodwin G. M., et al. , Single-dose psilocybin for a treatment-resistant episode of major depression: Impact on patient-reported depression severity, anxiety, function, and quality of life. J. Affect Disord 327, 120–127 (2023). [DOI] [PubMed] [Google Scholar]
- 153.Preskorn S. H., et al. , An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J. Clin. Psychopharmacol. 28, 631–637 (2008). [DOI] [PubMed] [Google Scholar]
- 154.Li N., et al. , Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry 69, 754–761 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Küpper M., et al. , GluN2B inhibition rescues impaired potentiation and epileptogenicity at associational-commissural CA3 synapses in a model of anti-NMDAR encephalitis. Neurosci. Lett. 795, 137031 (2023). [DOI] [PubMed] [Google Scholar]
- 156.Bridi M. C. D., et al. , Two distinct mechanisms for experience-dependent homeostasis. Nat. Neurosci. 21, 843–850 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miller O. H., Moran J. T., Hall B. J., Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology 100, 17–26 (2016). [DOI] [PubMed] [Google Scholar]
- 158.Miller O. H., et al. , GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. Elife 3, e03581 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abrahamsson T., et al. , Differential regulation of evoked and spontaneous release by presynaptic NMDA receptors. Neuron 96, 839–855.e835 (2017). [DOI] [PubMed] [Google Scholar]
- 160.Zarate C. A. Jr., et al. , A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am. J. Psychiatry 163, 153–155 (2006). [DOI] [PubMed] [Google Scholar]
- 161.Alsaad H. A., et al. , In the telencephalon, GluN2C NMDA receptor Subunit mRNA is predominately expressed in glial cells and GluN2D mRNA in interneurons. Neurochem. Res. 44, 61–77 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Garst-Orozco J., et al. , GluN2D-mediated excitatory drive onto medial prefrontal cortical PV+ fast-spiking inhibitory interneurons. PLoS One 15, e0233895 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gupta S. C., et al. , The NMDA receptor GluN2C subunit controls cortical excitatory-inhibitory balance, neuronal oscillations and cognitive function. Sci. Rep. 6, 38321 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hanson E., et al. , Tonic activation of GluN2C/GluN2D-containing NMDA receptors by ambient glutamate facilitates cortical interneuron maturation. J. Neurosci. 39, 3611–3626 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ravikrishnan A., et al. , Region-specific expression of NMDA receptor GluN2C Subunit in parvalbumin-positive neurons and astrocytes: Analysis of GluN2C expression using a novel reporter model. Neuroscience 380, 49–62 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Han D. H., et al. , Abolished ketamine effects on the spontaneous excitatory postsynaptic current of medial prefrontal cortex neurons in GluN2D knockout mice. Mol. Brain 14, 174 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fogaça M. V., et al. , N-Methyl-D-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology 44, 2230–2238 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fava M., et al. , REL-1017 (Esmethadone) as adjunctive treatment in patients with major depressive disorder: A phase 2a randomized double-blind trial. Am. J. Psychiatry 179, 122–131 (2022). [DOI] [PubMed] [Google Scholar]
- 169.Khlestova E., Johnson J. W., Krystal J. H., Lisman J., The role of GluN2C-containing NMDA receptors in ketamine’s psychotogenic action and in Schizophrenia models. J. Neurosci. 36, 11151–11157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tarrés-Gatius M., Miquel-Rio L., Campa L., Artigas F., Castañé A., Involvement of NMDA receptors containing the GluN2C subunit in the psychotomimetic and antidepressant-like effects of ketamine. Transl. Psychiatry 10, 427 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Trullas R., Ginter H., Skolnick P., A benzodiazepine receptor inverse agonist inhibits stress-induced ulcer formation. Pharmacol. Biochem. Behav. 27, 35–39 (1987). [DOI] [PubMed] [Google Scholar]
- 172.Braestrup C., Nielsen M., Honore T., Jensen L. H., Petersen E. N., Benzodiazepine receptor ligands with positive and negative efficacy. Neuropharmacology 22, 1451–1457 (1983). [DOI] [PubMed] [Google Scholar]
- 173.Campo B., et al. , Characterization of an mGluR2/3 negative allosteric modulator in rodent models of depression. J. Neurogenet. 25, 152–166 (2011). [DOI] [PubMed] [Google Scholar]
- 174.Yoshimizu T., Shimazaki T., Ito A., Chaki S., An mGluR2/3 antagonist, MGS0039, exerts antidepressant and anxiolytic effects in behavioral models in rats. Psychopharmacology (Berl) 186, 587–593 (2006). [DOI] [PubMed] [Google Scholar]
- 175.Malkesman O., et al. , Acute D-serine treatment produces antidepressant-like effects in rodents. Int. J. Neuropsychopharmacol. 15, 1135–1148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Heresco-Levy U., et al. , A randomized add-on trial of high-dose d-cycloserine for treatment-resistant depression. Int. J. Neuropsychopharmacol. 16, 501–506 (2013). [DOI] [PubMed] [Google Scholar]
- 177.Chen M.-H., et al. , Maintenance of antidepressant and antisuicidal effects by D-cycloserine among patients with treatment-resistant depression who responded to low-dose ketamine infusion: A double-blind randomized placebo-control study. Neuropsychopharmacology 44, 2112–2118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pilc A., Machaczka A., Kawalec P., Smith J. L., Witkin J. M., Where do we go next in antidepressant drug discovery? A new generation of antidepressants: A pivotal role of AMPA receptor potentiation and mGlu2/3 receptor antagonism. Expert Opin. Drug Discov. 17, 1131–1146 (2022). [DOI] [PubMed] [Google Scholar]
- 179.Kadriu B., et al. , Positive AMPA receptor modulation in the treatment of neuropsychiatric disorders: A long and winding road. Drug Discov. Today 26, 2816–2838 (2021), 10.1016/j.drudis.2021.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Davis-Reyes B. D., et al. , Subanesthetic ketamine with an AMPAkine attenuates motor impulsivity in rats. Behav. Pharmacol. 32, 335–344 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lauterborn J. C., Lynch G., Vanderklish P., Arai A., Gall C. M., Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J. Neurosci. 20, 8–21 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sengupta S., et al. , Discovery of NV-5138, the first selective Brain mTORC1 activator. Sci. Rep. 9, 4107 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kato T., et al. , Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation. J. Clin. Invest. 129, 2542–2554 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu R. J., et al. , GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology 38, 2268–2277 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.E. o. t. M. Letter, Dextromethorphan/bupropion (Auvelity) for depression. Med. Lett. Drugs Ther. 64, 201–203 (2022). [PubMed] [Google Scholar]
- 186.Anand A., et al. , Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: Support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch. Gen. Psychiatry 57, 270–276 (2000). [DOI] [PubMed] [Google Scholar]
- 187.Krystal J. H., et al. , Interactive effects of subanesthetic ketamine and subhypnotic lorazepam in humans. Psychopharmacology (Berl) 135, 213–229 (1998). [DOI] [PubMed] [Google Scholar]
- 188.Krupitsky E. M., et al. , Attenuation of ketamine effects by nimodipine pretreatment in recovering ethanol dependent men: Psychopharmacologic implications of the interaction of NMDA and L-type calcium channel antagonists. Neuropsychopharmacology 25, 936–947 (2001). [DOI] [PubMed] [Google Scholar]
- 189.Krystal J. H., et al. , Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 179, 303–309 (2005). [DOI] [PubMed] [Google Scholar]
- 190.Farber N. B., Jiang X. P., Heinkel C., Nemmers B., Antiepileptic drugs and agents that inhibit voltage-gated sodium channels prevent NMDA antagonist neurotoxicity. Mol. Psychiatry 7, 726–733 (2002). [DOI] [PubMed] [Google Scholar]
- 191.Brody S. A., Geyer M. A., Large C. H., Lamotrigine prevents ketamine but not amphetamine-induced deficits in prepulse inhibition in mice. Psychopharmacology (Berl) 169, 240–246 (2003). [DOI] [PubMed] [Google Scholar]
- 192.Nakao S., et al. , Halothane and diazepam inhibit ketamine-induced c-fos expression in the rat cingulate cortex. Anesthesiology 85, 874–882 (1996). [DOI] [PubMed] [Google Scholar]
- 193.Homayoun H., Jackson M. E., Moghaddam B., Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J. Neurophysiol. 93, 1989–2001 (2005). [DOI] [PubMed] [Google Scholar]
- 194.Lu Y. M., Zhang J. T., Zhao F. Q., Li F., Effects of nimodipine on l-glutamate-induced seizures and Ca2+ influx in hippocampus in freely moving rats. Zhongguo Yao Li Xue Bao 12, 297–300 (1991). [PubMed] [Google Scholar]
- 195.Sharp F. R., et al. , Phencyclidine induction of the hsp 70 stress gene in injured pyramidal neurons is mediated via multiple receptors and voltage gated calcium channels. Neuroscience 62, 1079–1092 (1994). [DOI] [PubMed] [Google Scholar]
- 196.Malhotra A. K., et al. , Clozapine blunts N-methyl-D-aspartate antagonist-induced psychosis: A study with ketamine. Biol. Psychiatry 42, 664–668 (1997). [DOI] [PubMed] [Google Scholar]
- 197.Lahti A. C., Koffel B., LaPorte D., Tamminga C. A., Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 13, 9–19 (1995). [DOI] [PubMed] [Google Scholar]
- 198.Krystal J. H., et al. , Interactive effects of subanesthetic ketamine and haloperidol in healthy humans. Psychopharmacology (Berl) 145, 193–204 (1999). [DOI] [PubMed] [Google Scholar]
- 199.D’Souza D. C., et al. , Glycine transporter inhibitor attenuates the psychotomimetic effects of ketamine in healthy males: Preliminary evidence. Neuropsychopharmacology 37, 1036–1046 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.D’Souza D. C., et al. , Dose-related target occupancy and effects on circuitry, behavior, and neuroplasticity of the glycine transporter-1 inhibitor PF-03463275 in healthy and Schizophrenia subjects. Biol. Psychiatry 84, 413–421 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Roxas N., Ahuja C., Isom J., Wilkinson S. T., Capurso N., A potential case of acute ketamine withdrawal: Clinical implications for the treatment of refractory depression. Am. J. Psychiatry 178, 588–591 (2021). [DOI] [PubMed] [Google Scholar]
- 202.Schak K. M., et al. , Potential risks of poorly monitored ketamine use in depression treatment. Am. J. Psychiatry 173, 215–218 (2016). [DOI] [PubMed] [Google Scholar]
- 203.Morgan C. J., Monaghan L., Curran H. V., Beyond the K-hole: A 3-year longitudinal investigation of the cognitive and subjective effects of ketamine in recreational users who have substantially reduced their use of the drug. Addiction (Abingdon, England) 99, 1450–1461 (2004). [DOI] [PubMed] [Google Scholar]
- 204.Yamakura T., Harris R. A., Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol. Anesthesiology 93, 1095–1101 (2000). [DOI] [PubMed] [Google Scholar]
- 205.van Amsterdam J. G., Nabben T., van den Brink W., Increasing recreational nitrous oxide use: Should we worry? A narrative review. J. Psychopharmacol. 36, 943–950 (2022). [DOI] [PubMed] [Google Scholar]
- 206.Murray S., Brewerton T., Abuse of over-the-counter dextromethorphan by teenagers. South Med. J. 86, 1151–1153 (1993). [DOI] [PubMed] [Google Scholar]
- 207.Davis B. L., The PCP epidemic: A critical review. Int. J. Addict 17, 1137–1155 (1982). [DOI] [PubMed] [Google Scholar]
- 208.Krystal J. H., Petrakis I. L., Mason G., Trevisan L., D’Souza D. C., N-methyl-D-aspartate glutamate receptors and alcoholism: Reward, dependence, treatment, and vulnerability. Pharmacol. Ther. 99, 79–94 (2003). [DOI] [PubMed] [Google Scholar]
- 209.Bonaventura J., et al. , Pharmacological and behavioral divergence of ketamine enantiomers: Implications for abuse liability. Mol. Psychiatry 26, 6704–6722 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Madonick S. M., et al. , Naltrexone blockade of ketamine intoxication in healthy humans. Alcoholism: Clin. Exp. Res. 23, 103A (1999). [Google Scholar]
- 211.Williams N. R., et al. , Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am. J. Psychiatry 175, 1205–1215 (2018), 10.1176/appi.ajp.2018.18020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yoon G., Petrakis I. L., Krystal J. H., Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry 76, 337–338 (2019), 10.1001/jamapsychiatry.2018.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Klein M. E., Chandra J., Sheriff S., Malinow R., Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc. Natl. Acad. Sci. U.S.A. 117, 2656–2662 (2020), 10.1073/pnas.1916570117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Marek G. J., Wright R. A., Schoepp D. D., Monn J. A., Aghajanian G. K., Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J. Pharmacol. Exp. Ther 292, 76–87 (2000). [PubMed] [Google Scholar]
- 215.Davoudian P. A., Shao L. X., Kwan A. C., Shared and distinct brain regions targeted for immediate early gene expression by ketamine and psilocybin. ACS Chem. Neurosci. 14, 468–480 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cameron L. P., et al. , A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 589, 474–479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gumpper R. H., Roth B. L., Psychedelics: Preclinical insights provide directions for future research. Neuropsychopharmacology (2023), 10.1038/s41386-023-01567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lewis V., et al. , A non-hallucinogenic LSD analog with therapeutic potential for mood disorders. Cell Rep. 42, 112203 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ginzel K. H., Mayer-Gross W., Prevention of psychological effects of d-lysergic acid diethylamide (LSD 25) by its 2-brom derivative (BOL 148). Nature 178, 210 (1956). [DOI] [PubMed] [Google Scholar]
- 220.Clark L. D., Bliss E. L., Psychopharmacological studies of lysergic acid diethylamide (LSD-25) intoxication; effects of premedication with BOL-128 (2-bromo-d-lysergic acid diethylamide), mescaline, atropine, amobarbital, and chlorpromazine. AMA Arch Neurol. Psychiatry 78, 653–655 (1957). [DOI] [PubMed] [Google Scholar]
- 221.Shao L. X., et al. , Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron 109, 2535–2544.e4 (2021), 10.1016/j.neuron.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rosenblat J. D., Leon-Carlyle M., Ali S., Husain M. I., McIntyre R. S., Antidepressant effects of psilocybin in the absence of psychedelic effects. Am. J. Psychiatry 1820, 395–396 (2023), 10.1176/appi.ajp.20220835. [DOI] [PubMed] [Google Scholar]
- 223.Case B. G., et al. , Declining use of electroconvulsive therapy in United States general hospitals. Biol. Psychiatry 73, 119–126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.G. B. o. D. Study, Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 9, 137–150 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gaynes B. N., et al. , What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 60, 1439–1445 (2009). [DOI] [PubMed] [Google Scholar]
- 226.Keller M. B., et al. , Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Arch. Gen. Psychiatry 49, 809–816 (1992). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data underlying this work.