Abstract
The identification of exported proteins by fusion studies, while well developed for gram-negative bacteria, is limited for gram-positive bacteria, in part due to drawbacks of available export reporters. In this work, we demonstrate the export specificity and use of the Staphylococcus aureus secreted nuclease (Nuc) as a reporter for gram-positive bacteria. Nuc devoid of its export signal (called ΔSPNuc) was used to create two fusions whose locations could be differentiated. Nuclease activity was shown to require an extracellular location in Lactococcus lactis, thus demonstrating the suitability of ΔSPNuc to report protein export. The shuttle vector pFUN was designed to construct ΔSPNuc translational fusions whose expression signals are provided by inserted DNA. The capacity of ΔSPNuc to reveal and identify exported proteins was tested by generating an L. lactis genomic library in pFUN and by screening for Nuc activity directly in L. lactis. All ΔSPNuc fusions displaying a strong Nuc+ phenotype contained a classical or a lipoprotein-type signal peptide or single or multiple transmembrane stretches. The function of some of the predicted signals was confirmed by cell fractionation studies. The fusions analyzed included long (up to 455-amino-acid) segments of the exported proteins, all previously unknown in L. lactis. Homology searches indicate that several of them may be implicated in different cell surface functions, such as nutrient uptake, peptidoglycan assembly, environmental sensing, and protein folding. Our results with L. lactis show that ΔSPNuc is well suited to report both protein export and membrane protein topology.
Most exported proteins are targeted for transport by a primary export signal comprising a hydrophobic domain. The signal can be present at the protein N terminus and cleaved during transport (i.e., signal peptide), but it can also remain embedded in the membrane (i.e., transmembrane segment) (63). Exported proteins are estimated to represent about 20% of total cellular proteins in gram-negative bacteria (39, 44), and contribute to various essential processes like nutrient uptake, macromolecular transport and assembly, envelope biogenesis and integrity, motility, cell division, energy generation, scavenging and detoxification, signal transduction, stress resistance, cell communication, and virulence in the case of pathogens.
Several years ago, the elegant strategy of translational fusion to an export-specific reporter protein was designed to specifically isolate genes encoding exported proteins. This kind of reporter is translocation competent but unable to direct its own export (it corresponds to a signal peptideless form of an exported protein), and its activity requires an extracytoplasmic location. Among a library of proteins N-terminally fused to such a reporter, only fusions having the proper signal are exported and active. This strategy was first described for Escherichia coli using alkaline phosphatase (PhoA) as a reporter (16, 36); since then it has been applied to many gram-negative bacteria, particularly pathogens (for reviews, see references 24 and 35 and references therein).
Export-specific reporters have a potentially important use in gram-positive bacteria, not only for protein identification and structural analyses, but also for technological applications. Most studies directly adopted the gram-negative reporters available, PhoA and the E. coli TEM β-lactamase (BlaM) (5). The Bacillus licheniformis α-amylase, AmyL, has also been used (17). Surprisingly, relatively few fusion studies allowed identification and characterization of the exported proteins (32, 42). In many cases, only the export signal was characterized (17, 18, 43, 51, 54, 55), possibly because only very short polypeptides (60 amino acids) were fused to the reporter.
The rather limited results obtained by using reporter fusions may reveal that the reporters used are not fully adapted for use in gram-positive bacteria. (i) Fusions to gram-negative reporters PhoA and BlaM seem to display little activity and/or to be less stable in gram-positive bacteria, probably because of improper folding (42, 54). Both PhoA (active as a dimer) and BlaM folding require disulfide bond formation, which is catalyzed by DsbA in various gram-negative bacteria (3, 22); it is not yet clear whether such a process exists in gram-positive bacteria (19). Furthermore, altered codon usage and GC content may decrease expression of reporter genes. (ii) Selection of BlaM fusions has been routinely performed in E. coli, possibly due to difficulties of direct ampicillin resistance selection in gram-positive bacteria (43, 51, 54). Such preselection may create a bias due to species specificity of export signals, which, for signal peptides, are significantly longer in gram-positive bacteria (65). (iii) AmyL, a reporter of gram-positive origin, may be the best suited for use in gram-positive bacteria. However, the plate detection test results in loss of cell viability (18a), and thus its use requires replica plating (17, 18).
The above-mentioned considerations led us to design a protein export reporter which would be suitable for use in a broad host range of gram-positive bacteria. The reporter we chose is based on the Staphylococcus aureus secreted nuclease (Nuc), a small, stable, monomeric, extensively studied enzyme (EC 3.1.31.1 [9]), having a mature form devoid of cysteine residues (50). Nuc is efficiently secreted by various gram-positive bacteria as an active 168-amino-acid polypeptide which may undergo subsequent proteolytic cleavage of the N-terminal 19- to 21-amino-acid propeptide to give rise to another active form, called NucA (27, 30, 31, 38, 58). The enzymatic activity test for Nuc is sensitive and nontoxic to colonies (28, 29, 50). Several features of Nuc thus make it a potentially optimal candidate for reporting protein export in gram-positive bacteria.
In this study, we show that a truncated form of Nuc lacking its export signal (called ΔSPNuc) is an export-specific reporter. A shuttle vector, pFUN (for fusion to nuclease), was designed to specifically identify genes encoding exported proteins as translational fusions to ΔSPNuc. pFUN was developed and used to study protein export in Lactococcus lactis, a gram-positive microaerophilic industrial microorganism used in dairy fermentations (37). Despite the technological importance of surface and extracellular proteins in this organism, export of relatively few proteins (excluding plasmid- or transposon-encoded proteins) has been reported to date (4, 6, 12, 13, 15, 26, 40, 60–62). In this work, we characterize 16 previously unknown exported L. lactis proteins. Our results confirm that ΔSPNuc is a sensitive and specific export reporter for L. lactis and potentially for other gram-positive bacteria.
MATERIALS AND METHODS
Bacterial strains and media.
L. lactis subsp. cremoris MG1363 (11) strains were grown on M17 medium (59) supplemented with 1% glucose at 30°C without shaking. E. coli DH5α was grown on Luria-Bertani medium at 37°C with shaking. Plasmids were maintained by the addition of erythromycin (5 μg/ml in L. lactis) or ampicillin (100 μg/ml in E. coli).
Plasmids.
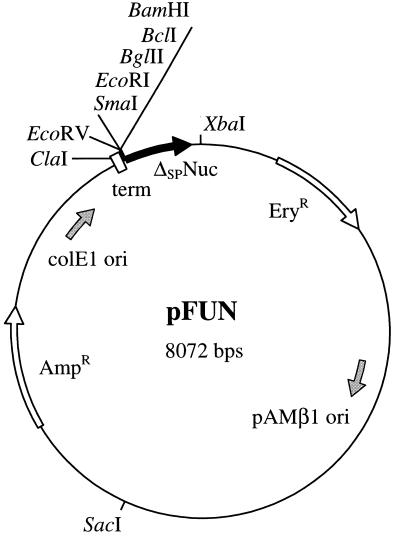
The shuttle vector pFUN is designed to identify genes encoding exported proteins as translational fusions to a reporter (see Fig. 3). It was constructed by cloning different modules in pBluescript plasmid pBS-KS+ to generate the complete cloning cassette. In the last step, the resultant plasmid was joined to the broad-host-range gram-positive vector, pIL252 (52). Constructions were carried out as follows. (i) An oligonucleotide linker, composed of 5′-CGATAGCCCGCCTAATGAGCGGGCTTTTTTTTGAT-3′ and 5′-ATCAAAAAAAAGCCCGCTCATTAGGCGGGCTAT-3′, encodes the trpA transcription terminator (in boldface type) (7); its extremities are compatible with ClaI and EcoRV. The linker was inserted into ClaI-EcoRV-cut pBS-KS+, thus generating pVE8001 (pBS::trpA). (ii) A second oligonucleotide linker, composed of 5′-AATTACCCGGGAATTCAGATCTTTGATCAAG-3′ and 5′-GATCCTTGATCAAAGATCTGAATTCCCGGGT-3′, introduces a multicloning site (SmaI, EcoRI, BglII, and BclI) with EcoRI and BamHI compatible extremities; it was cloned into the EcoRI and BamHI sites of pBS-KS+ (destroying the EcoRI cloning site but reconstituting the BamHI cloning site), thus generating pVE8002 (pBS::mcs). (iii) The reporter open reading frame (ORF) corresponds to Δnuc, the 518-bp Sau3A fragment of the S. aureus nuc gene (50), and to ΔSPNuc, the C-terminal polypeptide of 155 amino acids, lacking the signal peptide of the Nuc precursor and the first 13 amino acids of the mature form (Fig. 1) (27). This fragment was isolated from the cloned nuc gene in pBS::nuc (nuc PCR fragment cloned into pBS-KS+) (29) and cloned into the BamHI site of pBS-KS+, to generate pVE8003 (pBS::Δnuc); Δnuc has the same transcriptional orientation as the ampicillin resistance gene. (iv) The large ScaI-EcoRV fragment (1.8 kb) of pVE8001 was ligated to the small ScaI-EcoRV fragment (1.2 kb) of pVE8002 to create pVE8004 (pBS::trpAmcs) (in these plasmids, ScaI is a unique site within the ampicillin resistance gene). (v) The large ScaI-BamHI fragment (1.9 kb) of pVE8004 was ligated to the small ScaI-BamHI fragment (1.7 kb) of pVE8003 to create pVE8005 (pBS::trpAmcsΔnuc). (vi) To obtain the final plasmid, pFUN, pVE8005 was digested by SstII and treated with T4 DNA polymerase and pIL252 was digested by EcoRI and treated with Klenow enzyme; after heat inactivation, both plasmid DNAs were digested by XbaI. The large XbaI-blunted fragments of both pIL252 (4.6 kb) and pVE8005 (3.5 kb) were purified and joined to generate pFUN (8.1 kb). At each cloning step, constructions were verified by restriction enzyme digestion and DNA sequencing.
FIG. 3.
pFUN, a new probe vector for identification of genes encoding exported proteins. pFUN is an 8,072-bp shuttle vector that contains pAMβ1 (active in various lactic acid bacteria) and ColE1 (active in E. coli) replicons. A multicloning site (BamHI, BclI, BglII, EcoRI, and SmaI) allows the creation of translational fusions between genomic DNA fragments and the ORF (Δnuc) for ΔSPNuc (black arrow). If the inserted DNA supplies transcriptional and translational signals, a fusion with ΔSPNuc could be produced, and if the polypeptide fused to ΔSPNuc supplies an export signal, the fusion would display Nuc activity. Note that BamHI, BclI, and BglII sites were designed to allow fusions in the three ORFs and that a unique EcoRV site upstream of the multicloning site is also available. The trpA ρ-independent terminator (term) ensures that transcription of the translational fusion initiates from signals present on the inserted DNA. Unique restriction sites, genes conferring antibiotic resistance (open arrows on the plasmid), and origins of replication (grey arrows inside the plasmid) are shown. Further details of construction are given in Materials and Methods. AmpR, ampicillin resistance marker; EryR, erythromycin resistance marker.
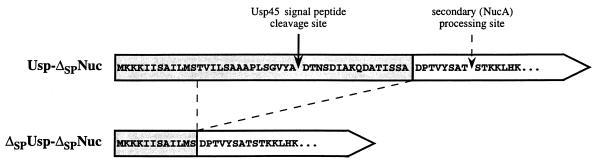
FIG. 1.
N-terminal sequences of Usp-ΔSPNuc and ΔSPUsp-ΔSPNuc fusion proteins. Sequences derived from Usp45 or from ΔSPNuc (i.e., C-terminal 155 amino acids of Nuc) are shown in shaded or white boxes, respectively. The solid arrow indicates the Usp45 signal peptide cleavage site (61). The dashed arrow indicates the secondary site of proteolysis, which results in the NucA form (i.e., C-terminal 147 amino acids of Nuc) (30). The dashed lines joining Usp-ΔSPNuc and ΔSPUsp-ΔSPNuc indicate the region of the former fusion which is absent in the latter.
pVE8009 and pVE8010 are pFUN derivatives containing translational fusions between usp45 (61) and Δnuc. For both, fusion expression is driven by the usp45 promoter and the translational start site, but one fusion protein contains an intact Usp45 signal peptide (Usp-ΔSPNuc, encoded by pVE8009), while the other (ΔSPUsp-ΔSPNuc, encoded by pVE8010) does not (Fig. 1). To generate pVE8009, plasmid pVE3588 (a pBS-SK+ derivative into which is cloned a PCR-amplified fragment containing the usp45 gene [kindly provided by Y. Le Loir and P. Langella]) was digested by BssHII followed by filling in with Klenow enzyme. After heat inactivation, DNA was digested by EcoRI and the 345-bp fragment was purified and introduced into pFUN after BamHI digestion, Klenow treatment followed by heat inactivation, and EcoRI digestion. To generate pVE8010, pVE3588 was digested by AccI and a 280-bp fragment was purified, treated with Klenow enzyme followed by heat inactivation, and further digested by EcoRI. A fragment of 245 bp was purified and introduced into pFUN after BamHI digestion, Klenow treatment followed by heat inactivation, and EcoRI digestion, thus generating pVE8010. Both constructions were verified by restriction enzyme digestion and DNA sequencing.
DNA manipulations.
Plasmid DNA was extracted from E. coli by the alkaline lysis method and from L. lactis by either the cesium chloride method or a modification of the alkaline lysis method as described previously (48). General procedures for DNA manipulations were performed as described previously (48), and enzymes were used as recommended by suppliers. Electroporation of L. lactis was performed as described previously (29). PCRs were performed essentially according to the protocol supplied by Perkin-Elmer on a DNA Thermal Cycler 9600, using Taq DNA polymerase (Boehringer Mannheim) as recommended. Oligonucleotides were synthesized with a DNA synthesizer (Oligo 1000; Beckman). All constructions were confirmed by sequencing using the dye termination method on a DNA Thermal Cycler 9600 (Perkin-Elmer), essentially according to the protocol supplied by Applied Biosystems.
Genomic libraries.
L. lactis MG1363 genomic libraries were constructed by cloning Sau3A fragments ranging in size from 0.5 to 1.3 kb into the BamHI site of pFUN. For experiments using E. coli DH5α as the intermediate recipient strain, recombinant E. coli clones were screened for Nuc activity and plasmids from Nuc+ clones were prepared either individually or mixed and used to transform L. lactis MG1363. Lactococcal transformants which were Nuc+ were further analyzed. In libraries established directly in L. lactis, the linearized vector was dephosphorylated with calf intestinal alkaline phosphatase (0.25 or 0.1 U per pmol of DNA; New England Biolabs) prior to ligation. The insertion rate was about 72% in the lactococcal libraries. From 2,500 clones screened, about 0.9% were strongly positive (Nuc+) and 0.4% gave a weak and delayed phenotype (Nuc+/−). Phenotypes were confirmed by additional Nuc activity tests.
Nuc activity plate test.
Nuc production by colonies of L. lactis MG1363 derivatives grown on brain heart infusion (Difco) agar plates was detected by a toluidine blue-DNA-agar overlay (see references 28 and 29 for composition and use), which does not affect viability. Note that toluidine blue-agar should be at 57°C for pouring (28, 29). The presence of pink halos reflects nuclease activity. About 150 to 300 colonies can be screened by this overlay on a standard petri plate and can be readily subcultured (29). Note that colonies of L. lactis should be screened for Nuc activity within 2 h of the overlay; clones producing a halo within 2 h are referred to as Nuc+, whereas those producing a weak halo after 2 h are referred to as Nuc+/− clones. We subsequently determined that prolonged incubation times favor the isolation of false positives, including the Nuc+/− clones.
Characterization of ΔSPNuc translational fusions.
Most fusion inserts in pFUN were characterized by PCR amplification from plasmid DNA preparations. One of the primers for PCR is complementary to the 5′ end of Δnuc (5′-AGTCGCAGGTTCTTTATG-3′), and the other corresponds to a sequence within the trpA ρ-independent terminator (5′-CTAATGAGCGGGCTTTTT-3′). Sequencing was performed either directly on plasmid DNA or on the PCR product obtained by using the primers described above (for each clone, three independent PCR products were combined to perform the sequencing). Primers were synthesized as needed to complete sequencing. For the most part, regions encoding the fusions were sequenced several times. On large DNA inserts, sequences distal from the translational fusion were not always determined, or were determined on one strand. The xbap and xnip programs (10) were used for fragment assembly and consensus sequence analyses. ORF start sites should be considered tentative assignments. The start sites chosen were, for the majority, ATG, and in a few cases GTG or TTG was chosen. In one case (tmp4-Δnuc), a rare start codon, ATA (45), appeared to be the most suitable start site. Classical signal peptides and their cleavage sites were predicted by using the Signal Peptide Prediction program for proteins from gram-positive bacteria (39). Transmembrane domains were predicted by using the Helical Transmembrane Regions program (46). The BLAST algorithm (on the National Center for Biotechnology Information server) was used for homology searches. Optimal alignment between two sequences was performed with the BestFit program (56).
Location studies, SDS-polyacrylamide gel electrophoresis (PAGE), Western blot, and zymograms.
We examined the distribution of all ΔSPNuc fusion proteins between the cell and culture medium of mid-exponential-phase L. lactis cells (optical density at 600 nm, ∼0.5). Both medium and cell fractions of a given strain were prepared from a starting volume equivalent to that of 1 ml of a culture at an optical density at 600 nm of 1. Culture medium was obtained by filtering cultures on 0.2-μm-pore-size filters (Sartorius). The filtrate was then precipitated with trichloroacetic acid (16% final concentration), and the resulting pellet was resuspended in 100 μl of 50 mM NaOH or washed in 80% acetone (comparable results were obtained by both procedures). Cells were harvested by 10 min of centrifugation at 4°C and a relative centrifugal force of 3,900 (model no. IK15; rotor 12024; Sigma). The cell pellet was washed once in TE (50 mM Tris-HCl, 50 mM EDTA) or TES buffer (50 mM Tris-HCl, 50 mM EDTA, and 20% sucrose) and precipitated with trichloroacetic acid (16% final concentration) followed by a wash in 80% acetone. Cells were then resuspended in TE or TES buffer containing lysozyme (final concentration, 5 to 10 mg/ml), incubated for 10 min at 37°C, and lysed with sodium dodecyl sulfate (SDS) (2 to 4% final concentration) in a final volume of 100 μl. Equal volumes of loading buffer (Tris-HCl, SDS, glycerol, bromophenol blue, and dithiothreitol at final concentrations of 60 mM, 2%, 10%, 0.01%, and 200 mM, respectively) were added to both medium and cell samples, and a volume of 20 μl was loaded onto a gel.
SDS-PAGE, electroblotting onto polyvinylidene difluoride membranes (Millipore), and immunoblotting were performed as described previously (48) or according to manufacturer recommendations. Anti-Nuc rabbit antibodies were kindly provided by J. R. Miller. Immunodetection was performed with protein G-horseradish peroxidase conjugate (Bio-Rad) and an enhanced chemiluminescence kit (Dupont-NEN) as recommended by the suppliers. Nuc enzyme activity was evaluated on zymograms of SDS-PAGE after removal of SDS, as described previously (31). Briefly, after Coomassie brilliant blue staining, the gel was washed with shaking for 1 h in 40 mM Tris-HCl at pH 7 and 25% isopropanol and then washed four times for 15 min in 40 mM Tris-HCl at pH 7. Proteins displaying Nuc activity were detected by a toluidine blue-agar overlay (with the same composition as that for the test plate).
Nucleotide sequence accession numbers.
The sequences of the pFUN inserts encoding the polypeptides fused to ΔSPNuc are new and have been assigned the following GenBank accession numbers: Exp1, U95828; Exp2, U95831; Exp3, U95827; Exp4, U95836; Exp5, U95835; Nlp1, U95829; Nlp2, U95830; Nlp3, U95834; Nlp4, U95838; Tmp1, U95832; Tmp2, U95833; Tmp3, U95837; Tmp4, U95839; Tmp5, U95840; Tmp6, U95841; Tmp7, U95842; Cyp1, AF015752; Cyp2, AF015749; Cyp3, AF015750; and Cyp4, AF015751. The pFUN accession number is AF038666.
RESULTS
ΔSPNuc is an export-specific reporter.
We first determined whether Nuc activity is contingent upon its export. The Nuc C-terminal 155-amino-acid polypeptide lacking its export signal and the first 13 amino acids of the mature form, called ΔSPNuc (Fig. 1), was tested for this purpose. This form retains activity when fused to the C terminus of various polypeptides (27). Two hybrid proteins were constructed with the L. lactis-secreted protein of unknown function, Usp45 (61). ΔSPNuc was fused C-terminally to N-terminal portions of the Usp45 precursor (Fig. 1). The hybrid Usp-ΔSPNuc has an intact signal peptide, while ΔSPUsp-ΔSPNuc does not. Low-copy-number plasmids encoding Usp-ΔSPNuc (pVE8009) or ΔSPUsp-ΔSPNuc (pVE8010) were introduced into L. lactis MG1363. A plate test revealed that only L. lactis colonies producing the Usp-ΔSPNuc fusion display a Nuc+ phenotype (Fig. 2, top). This shows that the plate test for Nuc activity can be used to discriminate between L. lactis cells producing ΔSPNuc fusions which contain an intact signal peptide and those which do not.
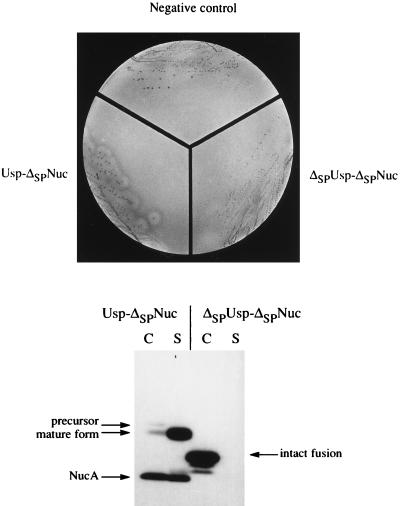
FIG. 2.
Specific detection of ΔSPNuc fusions which have an export signal in L. lactis. (Top) Nuc activity of Usp-ΔSPNuc but not of ΔSPUsp-ΔSPNuc is detected by plate tests. MG1363 strains containing plasmid pFUN (negative control), pVE8009 (Usp-ΔSPNuc), or pVE8010 (ΔSPUsp-ΔSPNuc) were streaked onto solid medium (brain heart infusion agar) and grown overnight. Colonies were overlaid with indicator medium containing toluidine blue, denatured DNA, and agar. Nuclease activity is detected by pink halos around colonies (Nuc+ phenotype) only in the case of MG1363(pVE8009), which produces Usp-ΔSPNuc. (Bottom) Location of Usp-ΔSPNuc and ΔSPUsp-ΔSPNuc fusion proteins in L. lactis. Cell (C) and supernatant (S) fractions of mid-exponential-phase cultures of L. lactis MG1363 derivative strains producing either Usp-ΔSPNuc or ΔSPUsp-ΔSPNuc fusion protein (from plasmids pVE8009 and pVE8010, respectively) were analyzed by SDS-PAGE and Western blotting using polyclonal Nuc antibodies. Putative forms of each fusion are indicated. Commercial NucA, used as a size reference (not presented), comigrates with the smallest degradation product of Usp-ΔSPNuc. Note that aberrant relative migration of Usp-ΔSPNuc forms (precursor, mature, and NucA) has previously been observed (30, 31) and that NucA in the cell fraction may be cytoplasmic or externally cell associated (31).
The locations of both hybrid proteins, Usp-ΔSPNuc and ΔSPUsp-ΔSPNuc, were determined. Supernatant and cell fractions of cultures of L. lactis MG1363(pVE8009) and MG1363(pVE8010) were subjected to SDS-PAGE and Western blotting using anti-Nuc polyclonal antibodies (Fig. 2, bottom). As expected, the majority of Usp-ΔSPNuc was in the supernatant fraction as two polypeptides, corresponding in size to the mature form and NucA (Fig. 1), while the cell fraction contained a higher-molecular-weight form, probably corresponding to the Usp-ΔSPNuc precursor. In contrast, ΔSPUsp-ΔSPNuc, as well as some degradation products, were present exclusively in the cellular fraction, thus confirming that export did not occur. Comparable amounts of Usp-ΔSPNuc and ΔSPUsp-ΔSPNuc were detected (Fig. 2, bottom). These results confirm that the majority of Usp-ΔSPNuc, but not ΔSPUsp-ΔSPNuc, is secreted. The plate test for Nuc activity is thus a faithful reporter of ΔSPNuc fusion export, and ΔSPNuc may be used to identify L. lactis exported proteins.
In vitro activity of ΔSPNuc fusions.
Nuc activity results in DNA and RNA degradation and would be lethal if expressed intracellularly, as observed for an intracellular signal peptideless form of Serratia marcescens secreted nuclease (1). However, we observed that L. lactis cells containing ΔSPUsp-ΔSPNuc grew normally, suggesting that intracellular ΔSPNuc fusions are inactive in vivo. We therefore tested the activity of Usp-ΔSPNuc and ΔSPUsp-ΔSPNuc in vitro by zymograms performed on cell extracts after SDS-PAGE (data not shown). Upon extraction, only the Usp-ΔSPNuc precursor (like the native Nuc precursor [30]) was inactive, possibly because of signal peptide antifolding activity (33). In contrast, both intracellular ΔSPUsp-ΔSPNuc forms and all processed Usp-ΔSPNuc forms gave strong Nuc+ signals. These results suggest that intracellular conditions are suboptimal for nuclease activity or alternatively that cytoplasmic forms of nuclease are improperly folded or aggregated and hence inactive. We found that nondenatured extracts of an L. lactis strain producing ΔSPUsp-ΔSPNuc did have Nuc activity (not shown). It is thus likely that the intrinsic activity of intracellular ΔSPNuc fusions is inhibited in the cell. Indeed, cytoplasmic pH (between 5.7 and 7.6 in lactococci [23]) and Ca2+ concentration (estimated to be 0.1 μM in E. coli [41]) are far from the optimal conditions for Nuc activity (100 μM Ca2+ at pH 9 or >100 μM Ca2+ at lower pH [9]).
Design of vector pFUN for identification of exported proteins in gram-positive bacteria.
Cloning vector pFUN (Fig. 3) was constructed to generate ΔSPNuc translational fusion libraries. pFUN contains a staphylococcal nuclease ORF (Δnuc) which lacks the necessary transcription and translational signals to produce ΔSPNuc. Multicloning sites (SmaI, EcoRI, BclI, BglII, and BamHI) are present upstream of a ΔSPNuc cassette. The last three of these sites are designed to create fusions in the three ORFs. Run-through transcription from the vector is prevented by a ρ-independent terminator placed adjacent to the multicloning site. Use of a plasmid like pFUN rather than a transposon insertion system should allow the identification of essential genes. pFUN contains a pBluescript moiety which confers high copy number to the vector in E. coli to facilitate DNA manipulation. It also replicates (via the pIL252 component) in various gram-positive bacteria, including L. lactis, and is maintained at a low copy number, which minimizes possible toxicity due to high-level production of fusion proteins.
Identification of L. lactis exported proteins with an intermediate cloning step in E. coli.
Previous searches for L. lactis export signals (with AmyL or BlaM reporters) employed an intermediate cloning step in E. coli (43, 51). To compare the efficiency of the ΔSPNuc reporter, we generated an L. lactis genomic library in pFUN with a prescreening step in E. coli (note that periplasmic Nuc displays a Nuc+ phenotype by the plate test, probably because of leakage across the outer membrane [29, 50]). An L. lactis MG1363 genomic library (Sau3A fragments) was cloned in pFUN (in the BamHI site), established in E. coli and screened for Nuc activity. Plasmid DNA prepared from E. coli Nuc+ clones, grown either individually or combined, was used to transform L. lactis MG1363 in which a new screening test was performed.
The majority (86%) of plasmids prepared from individual E. coli Nuc+ clones gave rise to Nuc− clones in L. lactis. It is likely that the high copy number of the vector in E. coli could result in toxic levels of fusion proteins, whether they are exported or not. Similar results were reported in previous studies on lactic acid bacterial export signals (18, 43, 51).
This prescreening procedure resulted in four recombinant plasmids which conferred Nuc+ phenotypes in both E. coli and L. lactis. All four polypeptides fused to ΔSPNuc were found to have an export signal (analyzed below). We noted that polypeptides varied in length from 91 to 320 amino acids and were markedly longer than those identified in similar L. lactis studies (24 to 84 amino acids [43, 51]).
Identification of L. lactis exported proteins directly in L. lactis.
To improve the efficiency of the fusion strategy, the pFUN lactococcal genomic library was established directly in L. lactis. Libraries of L. lactis MG1363 genomic DNA were constructed by cloning Sau3A fragments into the dephosphorylated BamHI site of pFUN (Fig. 3). Ligation mixtures were introduced directly in L. lactis MG1363 and screened for Nuc activity. A total of about 2,500 colonies were screened for the Nuc phenotype. A strong Nuc+ phenotype was present in 0.9% of clones; additional clones (0.4%) which gave a weak and delayed (Nuc+/−) phenotype were also characterized to better define the screening procedure. All of these clones, plus those isolated with an intermediate step in E. coli, were subjected to DNA sequence analysis.
Twenty nonredundant fusions were identified among the sequenced clones. Of the 16 fusions which displayed a strong Nuc+ phenotype, all contained a consensus export signal (putative classical- or lipoprotein-type signal peptides or putative transmembrane domains [see below]), suggesting that they are truly exported proteins. The four fusions which showed a Nuc+/− phenotype did not contain any recognizable export signal and probably correspond to cytoplasmic proteins (see below).
Isolation of putative cytoplasmic proteins and limits of the screening test.
The four polypeptides isolated as Nuc+/− fusions lack any classical export signals and were named Cyp (for putative cytoplasmic protein). One of these, Cyp4, is homologous to various cytoplasmic mannose-6-phosphate isomerases, while the other polypeptides show no significant homologies with known proteins. Why do these fusions have Nuc (albeit weak) activity? In one case, Cyp1, a stretch of eight contiguous hydrophobic amino acids (GAMVWLGG) could allow at least partial translocation of the fusion to the medium; it was previously shown that a short hydrophobic domain can act as a weak export signal (21, 67) (see below). For the three others, Cyp2, Cyp3, and Cyp4, no significant hydrophobic domain could be identified, and we attribute the Nuc+/− phenotype to partial cell lysis or leakage.
These results define the optimal use of our fusion strategy in L. lactis: screening for positive clones should be performed within a 2-h incubation period to avoid false-positive isolation and to reliably identify exported proteins.
Exp proteins.
Five L. lactis MG1363 polypeptides isolated as active ΔSPNuc fusions, named Exp (for putative extracellular protein), contain a potential N-terminal classical signal peptide (Table 1) composed of an N-terminal positively charged region, a central hydrophobic core, and a C-terminal cleavage region (44, 53, 63). Cleavage sites were predicted by using the Signal Peptide Prediction program with gram-positive data (39).
TABLE 1.
Putative export signals of ΔSPNuc fusions in L. lactis
| ΔSPNuc fusion | Sizea | Putative export signal sequencesb |
|---|---|---|
| Exp polypeptides | ||
| Exp1c | 320 | MKNLIPKKIKQVGILVGALLMLLSVLPVNLLGVMKVDA↓DSSQTEV-275 |
| Exp2c | 284 | MKKIAIIFCTLLMSLSVLSSFAVSA↓DTTTTNN-252 |
| Exp3 | 92 | VEKVKHEKGIIAFLTVLTILLTGAVKVSA↓DSTQAEI-56 |
| Exp4 | 144 | MKKINLALLTLATLMGVSSTAVVFA↓DDATSTG-112 |
| Exp5 | 455 | VRYSKISTKSKKNKQNKRAKRGSAKSKWWTAVKLFLIVFFSLIILGLA↓AGGAVFV-400 |
| Nlp polypeptides | ||
| Nlp1c | 140 | MKKKIFIALMASVSLFTLAA↓CGSGNKQ-113 |
| Nlp2c | 91 | MKSWKKVALGGASVLALATLAA↓CGSSASS-62 |
| Nlp3 | 110 | MKKILMLFAIPAVLLLAG↓CQKTADK-85 |
| Nlp4 | 94 | LKFKKLGLVMATVFAGAALVTLSG↓CSSSDSA-63 |
| Tmp polypeptides | ||
| Tmp1 | 40 | MKLKHIMLMTVLYIIATLSLVILSNQNILENDT-7 |
| Tmp2 | 111 | 48-KFKKFSGVEKVFYLTMVVAALVLAVGLLYVKTKTLEVQGN-23 |
| Tmp3 | 196 | 28-LSITFKVLRGLSVVFGIVFVLVGVFGAATGVGYFARLVEQTKIPP-123 |
| Tmp4 | 51 | IKHKKIIIITMILVLVLSLILSYGSIKYKSEQKMAQ-15 |
| Tmp5 | 273 | MKFIKKNKWALLASFFIPLILMVIVLAMTGIYWGSSRSILAGDAYHQYVAIHS- |
| -LYRNILHSGGSQGFLYTFTSGLGLNLYAFSAYYMGSFLMPFTFFFDV- | ||
| -KSMPDALYLFTIIKFGLIGLSSFVSFKN- | ||
| -MYQKLSNLTVLSISTAFALMSFLT- | ||
| -SQLEITMWLDVFILLPLIIWGLHRLMDE- | ||
| -RKRWLYFVSLLILFIQNYYFGFMVAIFLVLYFLARMTYEKW- | ||
| -SWTKVLDFVVSSTLAGIASLIMLLPMYLDLKSNNSD-16 | ||
| Tmp6 | 118 | 83-LPKHFFDIFKIACYIVIGYVLFFTIPYMVSPSSKL- |
| Tmp7 | 234 | MMLKKEWQAILKHKFFIIVIIALALVPAIYNYIFLGSMWDPYGKLNDLP-185 |
Number of amino acids fused to ΔSPNuc.
For Exp, Nlp, and Tmp polypeptides, classical signal peptides, lipoprotein signal peptides, and transmembrane domains, respectively, are presented. For the export signal sequences shown, charged amino acids are shown in italics, stretches of hydrophobic amino acids predicted to form a transmembrane domain are underlined, signal peptide cleavage regions (amino acids −3 to +1) are in boldface type, and predicted cleavage sites are indicated by arrows. Numbers to the left and/or right of the export signal indicate the length of polypeptide that is joined at that end in the fusion (the lack of a number signifies 0 amino acids). For Tmp5, the seven predicted transmembrane domains are presented. The start sites (the first amino acids [Met, Val, and Lev]) correspond to the most-N-terminal methionine, valine, or leucine and/or the first amino acid following a consensus ribosome-binding site. It should be noted that start sites are not confirmed, and alternative start sites are also possible.
Isolated in experiments with a prescreening step in E. coli. Exp2 and Nlp1 were also isolated directly in L. lactis.
Four Exp proteins have homology with previously identified proteins of various origins (Table 2). Exp1 is homologous to Usp45, the major MG1363 secreted protein, whose function is unknown (61). Exp2 appears to be a homolog of various penicillin-binding proteins, in particular, B. subtilis DacA. Exp3 shows homology with an L. lactis phage putative protein (ORF258). Although MG1363 was initially isolated as a phage- and plasmid-cured strain, it would not be surprising to find cryptic phage or phage remnants in such strains. Exp5 appears to be a complex polypeptide, composed of two distinct polypeptides fused in frame, apparently obtained by cocloning of two independent DNA fragments in pFUN. The N-terminal end (175 amino acids) shows similarity with exported penicillin-binding protein PBP1A of Streptococcus pneumoniae. It is fused in frame with a polypeptide segment (280 amino acids) which gives a high similarity score with the cytoplasmic protein UvrB (the B subunit of exonuclease ABC). These results indicate that a proportion of tripartite fusion Exp5-ΔSPNuc molecules is completely translocated, as attested by Nuc activity conferred by the C-terminal end of the fusion.
TABLE 2.
Homologs of polypeptides isolated as active ΔSPNuc fusions in L. lactis
| ΔSPNuc fusion | Protein homolog(s)a | Identity (%) | Aligned segmentb |
|---|---|---|---|
| Exp1 | Unidentified secreted protein Usp45 (L. lactis) | 29.7 | |
| P54 protein (Enterococcus faecium) | 24.8 | ||
| Exp2 | d,d-Carboxypeptidase DacA (S. pneumoniae) | 51.7 | |
| DacA (B. subtilis) | 42.0 | ||
| Exp3 | ORF258 (L. lactis phage BK5T) | 51.1 | |
| Exp5c | PBP1A (S. pneumoniae) | 53.5 | 175 (N-ter) |
| PBP1A (B. subtilis) | 36.8 | 175 (N-ter) | |
| Uvr402 (S. pneumoniae) | 88.1 | 280 (C-ter) | |
| UvrB, B subunit of exonuclease ABC (E. coli) | 66.7 | 280 (C-ter) | |
| Nlp1 | ss80 signal peptide (L. lactis; 30 aa identified) | 100.0 | 30 (N-ter) |
| ORF108, probable ABC transporter binding protein (B. subtilis) | 49.3 | ||
| Phosphate binding protein PstS (Mycobacterium intracellulare) | 28.9 | ||
| Nlp3 | Adhesin PsaA (S. pneumoniae) | 41.1 | |
| Manganese binding protein MntC (Synechocystis sp.) | 27.8 | ||
| Nlp4 | Protease maturation protein PrtM (Lactobacillus paracasei) | 36.4 | |
| PrtM (L. lactis) | 36.4 | ||
| Tmp2d | FtsL (S. pneumoniae; 76 aa) | 26.3 | 76 (C-ter) |
| Tmp3 | PBP1A (E. coli) | 27.8 | 156 (C-ter) |
| Tmp7 | PIP (L. lactis) | 25.2 |
The protein homologs presented have the highest identity scores and/or are characterized. Polypeptide lengths are given for those homologs for which only partial sequences are available. aa, amino acids.
Number of amino acids aligned to a database polypeptide. The lack of a number indicates that alignment was performed on the majority of the polypeptide. N-ter and C-ter indicate that homology is found at the N or C terminus, respectively, of the polypeptide fused to ΔSPNuc. With the exception of Tmp2 and Exp5, homologies correspond to the full length of polypeptides fused to ΔSPNuc.
Exp5 is a complex fusion, apparently obtained by cocloning and joining in frame a PBP1A homolog to a UvrB homolog. The significance of the homology between the Exp5 N terminus and PBP1A is reinforced by the homology between the ORFs upstream of exp5-Δnuc and pbp1A (S. pneumoniae). Note that Tmp3 and Exp5 N termini are both PBP1A homologs but differ from one another (only 25% identity).
Tmp2 appears to be a true FtsL homolog, as suggested by homology of the ORF found upstream of tmp2-Δnuc to S. pneumoniae ORFD and to B. subtilis ORFB, which are present upstream of ftsL in the pbpX and pbpB regions, respectively.
Lipoproteins.
Four L. lactis MG1363 polypeptides isolated as active ΔSPNuc fusions, named Nlp (for putative new lipoprotein), have a potential N-terminal signal peptide with the consensus cleavage region for lipoproteins (cleavage occurs in front of a cysteine residue which is acylated [44, 53, 63]) (Table 1).
Among these, three are homologous to proteins in databases (Table 2). Nlp1 is similar to a putative ABC transporter binding protein (Bacillus subtilis ORF108), and only its signal peptide was previously identified (51). Recent results from our laboratory suggest that Nlp1 is involved in stress response in L. lactis (34). Nlp3 is related to streptococcal adhesins and is also likely to be part of an ABC transporter (20). Nlp4 shows significant similarities to PrtM (a plasmid-encoded chaperone of PrtP protease of L. lactis), which is proposed to belong to a family of exported peptidyl prolyl isomerases (47).
Transmembrane proteins.
Seven L. lactis MG1363 polypeptides showing at least one potential transmembrane domain were identified as active ΔSPNuc fusions and were correspondingly named Tmp (for putative trans-membrane protein) (63) (Table 1). Transmembrane domains were predicted by using the Helical Transmembrane Regions program (46). Six Tmp’s (Table 1) contain only one putative transmembrane domain (i.e., monotopic proteins), while Tmp5 appears to be targeted to the membrane by seven putative transmembrane domains (i.e., polytopic protein). We can assign a C-out topology to all Tmp’s, as Nuc activity of a fusion indicates that the ΔSPNuc domain is exported. The Helical Transmembrane Regions program predicted the observed C-out topology for all Tmp’s except Tmp2. We noted that the putative hydrophobic transmembrane domain of Tmp2 is surrounded by numerous charged amino acids and might therefore be incorrectly analyzed with respect to the positive-inside rule (64). By considering a longer length (15 amino acids) on either side of the hydrophobic domain of Tmp2, the N terminus is more positively charged than its C terminus, which would predict a C-out orientation of ΔSPNuc, as observed in the present study.
Three Tmp’s show homology with previously identified proteins (Table 2). Tmp2 is homologous to FtsL, a putative essential cell division protein (14). Tmp3 is homologous to various penicillin-binding proteins from diverse bacteria. Tmp7 is homologous to PIP, the phage infection protein of L. lactis C2, which is a transmembrane protein required for infection by various phages (12). Although no significant homology was found with Tmp1, it is likely to be a sensor protein, as the ORF upstream of tmp1 is homologous to various regulator genes of two-component (sensor-regulator) systems (e.g., Synechococcus sphR and B. subtilis phoP). Furthermore, both tmp1 and the upstream gene were recently identified in a systematic search for two-component systems (38a).
Location of fusion proteins.
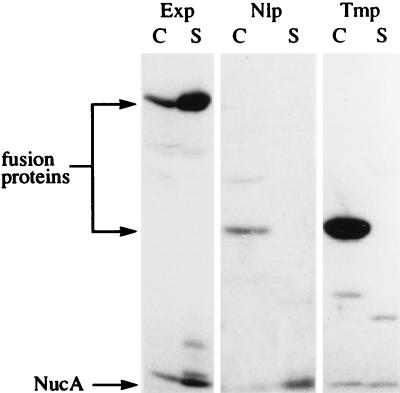
The locations of all exported ΔSPNuc fusions were examined; results with Exp1-ΔSPNuc, Nlp1-ΔSPNuc, and Tmp2-ΔSPNuc are presented in Fig. 4. Supernatant and cell fractions of cultures of L. lactis strains producing the fusions were subjected to SDS-PAGE and Western blotting using anti-Nuc polyclonal antibodies. Exp1-ΔSPNuc was found mainly in the medium, thus confirming that it is secreted. Full-size Nlp1-ΔSPNuc and full-size Tmp2-ΔSPNuc were almost exclusively associated with the cell fraction. The other exported fusion proteins examined (not shown) were each found in the expected locations, and in all cases, degradation products were detected. It is notable for lipoprotein and transmembrane fusions (e.g., see Fig. 4) that significant amounts of NucA were found in the medium; proteolytic release of the NucA domain is consistent with its surface location in those fusions.
FIG. 4.
Location of Exp1-ΔSPNuc, Nlp1-ΔSPNuc, and Tmp2-ΔSPNuc fusion proteins in L. lactis. Three L. lactis MG1363 derivative strains, each producing a representative member of each class of putative exported ΔSPNuc fusions (shown in parentheses), were examined: Exp (Exp1-ΔSPNuc), Nlp (Nlp1-ΔSPNuc), and Tmp (Tmp2-ΔSPNuc). Cell (C) and supernatant (S) fractions from mid-exponential-phase cultures of the above-mentioned strains were analyzed by SDS-PAGE and Western blotting using polyclonal Nuc antibodies. Putative forms of each fusion are indicated. Note that a band corresponding to a high-molecular-weight protein, visible in the cell fraction of the strain producing Nlp1-ΔSPNuc, is nonspecific and is observed in other samples.
Location of the tripartite fusion protein Exp5-ΔSPNuc was also examined (not shown). Western blotting revealed that Exp5-ΔSPNuc is highly unstable. Both cell and supernatant fractions contained degradation products but gave different gel profiles; NucA is the major band in the supernatant. These results indicate that at least a proportion of Exp5-ΔSPNuc is truly exported, thus proving that a cytoplasmic polypeptide can be translocated in L. lactis. Export of a cytoplasmic polypeptide as part of a tripartite fusion has previously been reported by using PhoA as a reporter (57); however PhoA activity was very low (57). In contrast, the rather strong Nuc activity observed in Exp5-ΔSPNuc suggests that the NucA domain at the C terminus of ΔSPNuc fusions serves as a sensitive probe for translocation.
The locations of all of the Cyp-ΔSPNuc fusions were also examined (not shown). Gel profiles of samples prepared from mid-log-phase liquid cultures showed that Cyp2-ΔSPNuc, Cyp3-ΔSPNuc, and Cyp4-ΔSPNuc fusions were exclusively cell associated; we consider it likely that cell lysis within colonies on plates accounts for their observed Nuc+/− phenotype. Interestingly, for Cyp1-ΔSPNuc, in addition to detecting the cell-associated forms, we also detected some degradation products and, in particular, NucA in the medium (not shown). This behavior is in agreement with the role of the short hydrophobic domain of Cyp1-ΔSPNuc as a weak export signal (see above).
Activity of fusion proteins.
Zymograms were performed on SDS-PAGE gels on concentrated nondenatured supernatant samples of L. lactis MG1363 strains producing Exp1-ΔSPNuc or Exp4-ΔSPNuc (not shown). For both fusion proteins, the entire-length mature forms (corresponding to 282 and 119 amino acids, respectively, fused to the reporter), as well as degradation products and NucA, were active, thus demonstrating that long N-terminal fusions to ΔSPNuc are enzymatically active.
Taken together, the above-mentioned results lead us to affirm the feasibility of pFUN as an export reporter and membrane protein topology probe of gram-positive bacteria.
DISCUSSION
ΔSPNuc is a reliable reporter for export studies.
In this study, we have developed and characterized an export reporter, ΔSPNuc, adapted for use in gram-positive bacteria. Analyses of fusion proteins obtained in L. lactis indicate that ΔSPNuc is well suited for such studies and has advantages over previously used reporters derived from E. coli. First, fusion protein lengths appear to be systematically longer than those reported in similar studies. Polypeptides joined to ΔSPNuc were up to 455 amino acids long, with an average of 146 amino acids. In contrast, polypeptides fused to reporters in other published systems were often all short or restricted in size (with average lengths between 36 and 83 amino acids [17, 18, 43, 51, 55]). The ability to isolate long polypeptide fusions with ΔSPNuc is consistent with the observation that Nuc activity is detected even in the presence of long N-terminal fusions. Second, in constructing a partial library in pFUN, we used a restricted size range of Sau3A DNA fragments, fused in only one of the three possible ORFs. The identification of 16 exported fusions under these specific conditions leads us to predict that the ΔSPNuc fusion library can be expanded to identify the majority of exported proteins. Third, Nuc appears to be highly active and stable in L. lactis. As little as 10 ng of Nuc can be detected in plate tests (data not shown). Assuming that a colony is about 109 cells, this corresponds to a detection limit of about 400 molecules per cell. We observed that, even if the fusion protein is degraded (as seen for Exp5-ΔSPNuc), the active NucA moiety is stable and can truly report translocation events that would otherwise go undetected because of subsequent improper protein folding and degradation.
The strategy we used, in which a fusion library is for the first time established directly in L. lactis, resulted in efficient detection of exported proteins. Sixteen ΔSPNuc fusions showed strong Nuc activity and contained identifiable export signals. Four fusions with presumably cytoplasmic polypeptides gave weak and delayed Nuc activity. Among them, one was exported, probably via a short hydrophobic stretch which could serve as a weak translocator (21, 67). Despite their confirmed cytoplasmic location, the Nuc+/− phenotype of the three others could be due to leakage or partial cell lysis inside the colonies on plates. Isolation of false-positive clones having weak enzymatic activity has previously been reported with fusions to PhoA or to other reporters in both prokaryotic (5, 8, 18, 36, 42, 66) and eukaryotic systems (25), and export-specific efficiency was comparable to what we observed with ΔSPNuc (25).
Taking together the above-mentioned considerations, the ΔSPNuc reporter appears to have a more stable export-specific activity and may be more versatile than other reporters presently used in gram-positive bacteria.
Location of exported L. lactis proteins.
The putative exported polypeptides identified in the present study each possess a primary export signal corresponding to either a classical signal peptide, a lipoprotein-type signal peptide, or a transmembrane domain (Table 1) (44, 53, 63). The signals were for the most part canonical in length and structure for gram-positive bacteria (53, 65), and many were confirmed to be functional (see, for example, Exp1-ΔSPNuc in Fig. 4). In one case, for Nlp1-ΔSPNuc, we demonstrated the surface location of the fusion by protease treatment: proteinase K treatment on intact cells degrades Nlp1-ΔSPNuc but has no effect on ΔSPUsp-ΔSPNuc (not shown).
Five secreted proteins (Exp1 to Exp4 and the N-terminal part of Exp5) were tentatively identified in this study. This is surprising if we consider that only one protein, Usp45, is detected in quantity in the culture medium of L. lactis strains (61). This may indicate that some secreted proteins are produced at very low levels. Alternatively, as proteins fused to ΔSPNuc are truncated at their C termini, cell wall targeting or sorting signals, described for numerous gram-positive bacterial surface proteins (2, 49), may have been removed. Thus, proteins predicted to be extracellular by fusion studies might actually be envelope associated. Further characterization will be needed to confirm their locations.
Seven polypeptides fused to ΔSPNuc are putative transmembrane proteins. In one case, the fused region was polytopic. We expect that the ΔSPNuc reporter will be a reliable probe of membrane protein topology which could be used to complement and confirm predictive modelling in L. lactis and other gram-positive bacteria.
Functions of exported L. lactis proteins.
Several of the identified exported proteins are of particular interest. Nlp4, which is a chromosomally encoded lipoprotein, is homologous to plasmid-encoded PrtM, an exported peptidyl prolyl isomerase specific for the folding of PrtP (also plasmid-encoded) proteinase (see reference 47 and references therein). The family of exported peptidyl prolyl isomerases is implicated in the correct folding of exported proteins as well as in long-term cell survival (47). Along these lines, Nlp4 may have technological interest for heterologous protein secretion and survival in L. lactis. Further studies will determine the specific role of Nlp4. Also of interest are Nlp1 and Nlp3, putative ABC transporter binding proteins; recent results from our laboratory suggest that Nlp1 is involved in stress response (34). Other identified proteins of potential interest include Tmp7, a homolog of L. lactis PIP (phage infection is a major economic concern in industrial processes), and Tmp1, which is likely to be a sensor protein. Finally, Exp2 and Tmp3 are putatively the first penicillin-binding proteins described for L. lactis.
Several exported proteins identified in the present study display no homology, or the homology gives no information about their functions. Nevertheless, we suspect that some of the exported proteins are involved in adaptation to environmental changes, and our fusion strategy provides us with a powerful tool to study the expression of the genes in response to environmental signals. Studies will be facilitated by the presence of the native expression signals in fusion genes as well as quantifiable Nuc activity. For example, Exp1 is homologous to Usp45, which is highly conserved in lactic acid bacteria but has no known function (61); by using ΔSPNuc fusions, expression of usp45 and exp1 genes can be examined as a function of growth conditions. Studying exported proteins involved in adaptation to various environments will be relevant for understanding and controlling culture irreproducibility, a major problem in fermentation processes with lactic acid bacteria.
Conclusions.
We have shown in this study that the ΔSPNuc reporter is efficient for the analysis of exported proteins of L. lactis. The broad host range of Nuc and the pFUN probe vector suggests that their use can be extended to other gram-positive bacteria. Using pFUN directly in L. lactis, we selectively identified chromosomal genes encoding putative exported proteins. All of the characterized genes were previously unknown in L. lactis. Homology studies suggest that some of them may be implicated in different cell surface functions, such as nutrient uptake, peptidoglycan assembly, environmental sensing, and protein folding. The strategy that we describe provides the groundwork for a systematic study of exported proteins in a food-grade microorganism and could be invaluable in selecting and characterizing genes which are selectively expressed under certain growth conditions. This type of approach will have important uses in systematic functional genome analyses, the follow-up of sequencing projects. It can also be used for technological applications to identify highly expressed surface proteins which cannot be readily deduced from sequence analysis.
ACKNOWLEDGMENTS
We are very grateful to Julie Debry and Erwan Seznec for technical assistance, to Sophie Sourice for DNA sequencing, and to Bertrand Nicolas for photography. Nuc antibodies were a generous gift of James Miller. Willem de Vos kindly provided usp45 DNA. We are indebted to Yves Le Loir and Philippe Langella for providing plasmids pBS::nuc and pVE3588 and for discussing their results prior to publication. We thank our colleagues M. van de Guchte, P. Langella, Y. Le Loir, E. Maguin, J.-C. Piard, and P. Serror for frequent discussion during the course of this work. We are thankful to M. van de Guchte and E. Maguin for helpful suggestions and critical reading of the manuscript.
REFERENCES
- 1.Ahrenholtz I, Lorenz M G, Wackernagel W. A conditional suicide system in Escherichia coli based on the intracellular degradation of DNA. Appl Environ Microbiol. 1994;60:3746–3751. doi: 10.1128/aem.60.10.3746-3751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba T, Schneewind O. Target cell specificity of a bacteriocin molecule: a C-terminal signal directs lysostaphin to the cell wall of Staphylococcus aureus. EMBO J. 1996;15:4789–4797. [PMC free article] [PubMed] [Google Scholar]
- 3.Bardwell J C, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 4.Bolhuis H, Poelarends G, van Veen H W, Poolman B, Driessen A J, Konings W N. The lactococcal lmrP gene encodes a proton motive force-dependent drug transporter. J Biol Chem. 1995;270:26092–26098. doi: 10.1074/jbc.270.44.26092. [DOI] [PubMed] [Google Scholar]
- 5.Broome-Smith J K, Tadayyon M, Zhang Y. β-Lactamase as a probe of membrane protein assembly and protein export. Mol Microbiol. 1990;4:1637–1644. doi: 10.1111/j.1365-2958.1990.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 6.Buist G, Kok J, Leenhouts K J, Dabrowska M, Venema G, Haandrikman A J. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J Bacteriol. 1995;177:1554–1563. doi: 10.1128/jb.177.6.1554-1563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christie G E, Farnham P J, Platt T. Synthetic sites for transcription termination and a functional comparison with tryptophan operon termination sites in vitro. Proc Natl Acad Sci USA. 1981;78:4180–4184. doi: 10.1073/pnas.78.7.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleavinger C M, Kim M F, Im J H, Wise K S. Identification of mycoplasma membrane proteins by systematic TnphoA mutagenesis of a recombinant library. Mol Microbiol. 1995;18:283–293. doi: 10.1111/j.1365-2958.1995.mmi_18020283.x. [DOI] [PubMed] [Google Scholar]
- 9.Cuatrecasas P, Fuchs S, Anfinsen C B. Catalytic properties and specificity of the extracellular nuclease of Staphylococcus aureus. J Biol Chem. 1967;242:1541–1547. [PubMed] [Google Scholar]
- 10.Dear S, Staden R. A sequence assembly and editing program for efficient management of large projects. Nucleic Acids Res. 1991;19:3907–3911. doi: 10.1093/nar/19.14.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geller B L, Ivey R G, Trempy J E, Hettinger-Smith B. Cloning of a chromosomal gene required for phage infection of Lactococcus lactis subsp. lactis C2. J Bacteriol. 1993;175:5510–5519. doi: 10.1128/jb.175.17.5510-5519.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godon J J, Jury K, Shearman C A, Gasson M J. The Lactococcus lactis sex-factor aggregation gene cluA. Mol Microbiol. 1994;12:655–663. doi: 10.1111/j.1365-2958.1994.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 14.Guzman L M, Barondess J J, Beckwith J. FtsL, an essential cytoplasmic membrane protein involved in cell division in Escherichia coli. J Bacteriol. 1992;174:7716–7728. [PMC free article] [PubMed] [Google Scholar]
- 15.Hagting A, Kunji E R, Leenhouts K J, Poolman B, Konings W N. The di- and tripeptide transport protein of Lactococcus lactis. A new type of bacterial peptide transporter. J Biol Chem. 1994;269:11391–11399. [PubMed] [Google Scholar]
- 16.Hoffman C S, Wright A. Fusions of secreted proteins to alkaline phosphatase: an approach for studying protein secretion. Proc Natl Acad Sci USA. 1985;82:5107–5111. doi: 10.1073/pnas.82.15.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hols P, Baulard A, Garmyn D, Delplace B, Hogan S, Delcour J. Isolation and characterization of genetic expression and secretion signals from Enterococcus faecalis through the use of broad-host-range α-amylase probe vectors. Gene. 1992;118:21–30. doi: 10.1016/0378-1119(92)90244-j. [DOI] [PubMed] [Google Scholar]
- 18.Hols P, Ferain T, Garmyn D, Bernard N, Delcour J. Use of homologous expression-secretion signals and vector-free stable chromosomal integration in engineering of Lactobacillus plantarum for α-amylase and levanase expression. Appl Environ Microbiol. 1994;60:1401–1413. doi: 10.1128/aem.60.5.1401-1413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Hols, P. Personal communication.
- 19.Ishihara T, Tomita H, Hasegawa Y, Tsukagoshi N, Yamagata H, Udaka S. Cloning and characterization of the gene for a protein thiol-disulfide oxidoreductase in Bacillus brevis. J Bacteriol. 1995;177:745–749. doi: 10.1128/jb.177.3.745-749.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkinson H F. Cell surface protein receptors in oral streptococci. FEMS Microbiol Lett. 1994;121:133–140. doi: 10.1111/j.1574-6968.1994.tb07089.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser C A, Preuss D, Grisafi P, Botstein D. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science. 1987;235:312–317. doi: 10.1126/science.3541205. [DOI] [PubMed] [Google Scholar]
- 22.Kamitani S, Akiyama Y, Ito K. Identification and characterization of an Escherichia coli gene required for the formation of correctly folded alkaline phosphatase, a periplasmic enzyme. EMBO J. 1992;11:57–62. doi: 10.1002/j.1460-2075.1992.tb05027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashket E R. Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol Rev. 1987;46:233–244. [Google Scholar]
- 24.Kaufman M R, Taylor R K. Identification of bacterial cell-surface virulence determinants with TnphoA. Methods Enzymol. 1994;235:426–448. doi: 10.1016/0076-6879(94)35159-7. [DOI] [PubMed] [Google Scholar]
- 25.Klein R D, Gu Q, Goddard A, Rosenthal A. Selection for genes encoding secreted proteins and receptors. Proc Natl Acad Sci USA. 1996;93:7108–7113. doi: 10.1073/pnas.93.14.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koivula T, Palva I, Hemila H. Nucleotide sequence of the secY gene from Lactococcus lactis and identification of conserved regions by comparison of four SecY proteins. FEBS Lett. 1991;288:114–118. doi: 10.1016/0014-5793(91)81015-z. [DOI] [PubMed] [Google Scholar]
- 27.Kovacevic S, Veal L E, Hsiung H M, Miller J R. Secretion of staphylococcal nuclease by Bacillus subtilis. J Bacteriol. 1985;162:521–528. doi: 10.1128/jb.162.2.521-528.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachica R V F, Genigeorgis C, Hoeprich P D. Metachromatic agar-diffusion methods for detecting staphylococcal nuclease activity. Appl Microbiol. 1971;21:585–587. doi: 10.1128/am.21.4.585-587.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Loir Y, Gruss A, Ehrlich S D, Langella P. Direct screening of recombinants in gram-positive bacteria using the secreted staphylococcal nuclease as a reporter. J Bacteriol. 1994;176:5135–5139. doi: 10.1128/jb.176.16.5135-5139.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Loir Y, Gruss A, Ehrlich S D, Langella P. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J Bacteriol. 1998;180:1895–1903. doi: 10.1128/jb.180.7.1895-1903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liebl W, Sinskey A J, Schleifer K H. Expression, secretion, and processing of staphylococcal nuclease by Corynebacterium glutamicum. J Bacteriol. 1992;174:1854–1861. doi: 10.1128/jb.174.6.1854-1861.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim E M, Rauzier J, Timm J, Torrea G, Murray A, Gicquel B, Portnoi D. Identification of Mycobacterium tuberculosis DNA sequences encoding exported proteins by using phoA gene fusions. J Bacteriol. 1995;177:59–65. doi: 10.1128/jb.177.1.59-65.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G, Topping T B, Randall L L. Physiological role during export for the retardation of folding by the leader peptide of maltose-binding protein. Proc Natl Acad Sci USA. 1989;86:9213–9217. doi: 10.1073/pnas.86.23.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maguin, E., and P. Duwat. Personal communication.
- 35.Manoil C, Mekalanos J J, Beckwith J. Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol. 1990;172:515–518. doi: 10.1128/jb.172.2.515-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manoil C, Beckwith J. TnphoA: a transposon probe for protein export signals. Proc Natl Acad Sci USA. 1985;82:8129–8133. doi: 10.1073/pnas.82.23.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKay L L, Baldwin K A. Applications for biotechnology: present and future improvements in lactic acid bacteria. FEMS Microbiol Rev. 1990;7:3–14. doi: 10.1111/j.1574-6968.1990.tb04876.x. [DOI] [PubMed] [Google Scholar]
- 38.Miller J R, Kovacevic S, Veal L E. Secretion and processing of staphylococcal nuclease by Bacillus subtilis. J Bacteriol. 1987;169:3508–3514. doi: 10.1128/jb.169.8.3508-3514.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Morel, P., and D. van Sinderen. Personal communication.
- 39.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of procaryotic and eucaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson D, Lauridsen A A, Tomoyasu T, Ogura T. A Lactococcus lactis gene encodes a membrane protein with putative ATPase activity that is homologous to the essential Escherichia coli ftsH gene product. Microbiology. 1994;140:2601–2610. doi: 10.1099/00221287-140-10-2601. [DOI] [PubMed] [Google Scholar]
- 41.Norris V, Chen M, Goldberg M, Voskuil J, McGurk G, Holland I B. Calcium in bacteria: a solution to which problem? Mol Microbiol. 1991;5:775–778. doi: 10.1111/j.1365-2958.1991.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 42.Pearce B J, Yin Y B, Masure H R. Genetic identification of exported proteins in Streptococcus pneumoniae. Mol Microbiol. 1993;9:1037–1050. doi: 10.1111/j.1365-2958.1993.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Martinez G, Kok J, Venema G, van Dijl J M, Smith H, Bron S. Protein export elements from Lactococcus lactis. Mol Gen Genet. 1992;234:401–411. doi: 10.1007/BF00538699. [DOI] [PubMed] [Google Scholar]
- 44.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero A, Garcia P. Initiation of translation at AUC, AUA and AUU codons in Escherichia coli. FEMS Microbiol Lett. 1991;68:325–330. doi: 10.1016/0378-1097(91)90377-m. [DOI] [PubMed] [Google Scholar]
- 46.Rost B, Casadio R, Fariselli P, Sander C. Transmembrane helices predicted at 95% accuracy. Protein Sci. 1995;4:521–533. doi: 10.1002/pro.5560040318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudd K E, Sofia H J, Koonin E V, Plunkett G R, Lazar S, Rouviere P E. A new family of peptidyl-prolyl isomerases. Trends Biochem Sci. 1995;20:12–14. doi: 10.1016/s0968-0004(00)88940-9. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 49.Schneewind O, Mihaylova Petkov D, Model P. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J. 1993;12:4803–4811. doi: 10.1002/j.1460-2075.1993.tb06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shortle D. A genetic system for analysis of staphylococcal nuclease. Gene. 1983;22:181–189. doi: 10.1016/0378-1119(83)90102-6. [DOI] [PubMed] [Google Scholar]
- 51.Sibakov M, Koivula T, von Wright A, Palva I. Secretion of TEM β-lactamase with signal sequences isolated from the chromosome of Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1991;57:341–348. doi: 10.1128/aem.57.2.341-348.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simon D, Chopin A. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie. 1988;70:559–566. doi: 10.1016/0300-9084(88)90093-4. [DOI] [PubMed] [Google Scholar]
- 53.Simonen M, Palva I. Protein secretion in Bacillus species. Microbiol Rev. 1993;57:109–137. doi: 10.1128/mr.57.1.109-137.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith H, Bron S, Van Ee J, Venema G. Construction and use of signal sequence selection vectors in Escherichia coli and Bacillus subtilis. J Bacteriol. 1987;169:3321–3328. doi: 10.1128/jb.169.7.3321-3328.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith H, de Jong A, Bron S, Venema G. Characterization of signal-sequence-coding regions selected from the Bacillus subtilis chromosome. Gene. 1988;70:351–361. doi: 10.1016/0378-1119(88)90207-7. [DOI] [PubMed] [Google Scholar]
- 56.Smith T F, Waterman M S. Comparison of bio-sequences. Adv Appl Math. 1981;2:482–489. [Google Scholar]
- 57.Snyder W B, Silhavy T J. β-Galactosidase is inactivated by intermolecular disulfide bonds and is toxic when secreted to the periplasm of Escherichia coli. J Bacteriol. 1995;177:953–963. doi: 10.1128/jb.177.4.953-963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suciu D, Inouye M. The 19-residue pro-peptide of staphylococcal nuclease has a profound secretion-enhancing ability in Escherichia coli. Mol Microbiol. 1996;21:181–195. doi: 10.1046/j.1365-2958.1996.6211341.x. [DOI] [PubMed] [Google Scholar]
- 59.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Environ Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tynkkynen S, Buist G, Kunji E, Kok J, Poolman B, Venema G, Haandrikman A. Genetic and biochemical characterization of the oligopeptide transport system of Lactococcus lactis. J Bacteriol. 1993;175:7523–7532. doi: 10.1128/jb.175.23.7523-7532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Asseldonk M, Rutten G, Oteman M, Siezen R J, de Vos W M, Simons G. Cloning of usp45, a gene encoding a secreted protein from Lactococcus lactis subsp. lactis MG1363. Gene. 1990;95:155–160. doi: 10.1016/0378-1119(90)90428-t. [DOI] [PubMed] [Google Scholar]
- 62.van Veen H W, Venema K, Bolhuis H, Oussenko I, Kok J, Poolman B, Driessen A J M, Konings W N. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc Natl Acad Sci USA. 1996;93:10668–10672. doi: 10.1073/pnas.93.20.10668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988;947:307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]
- 64.von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature. 1989;341:456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]
- 65.von Heijne G, Abrahmsen L. Species-specific variation in signal peptide design. Implications for protein secretion in foreign hosts. FEBS Lett. 1989;244:439–446. doi: 10.1016/0014-5793(89)80579-4. [DOI] [PubMed] [Google Scholar]
- 66.Wang R C, Seror S J, Blight M, Pratt J M, Broome-Smith J K, Holland I B. Analysis of the membrane organization of an Escherichia coli protein translocator, HlyB, a member of a large family of procaryote and eucaryote surface transport protein. J Mol Biol. 1991;217:441–454. doi: 10.1016/0022-2836(91)90748-u. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Broome-Smith J K. Identification of amino acid sequences that can function as translocators of β-lactamase in Escherichia coli. Mol Microbiol. 1989;3:1361–1369. doi: 10.1111/j.1365-2958.1989.tb00117.x. [DOI] [PubMed] [Google Scholar]