Abstract
Norovirus is attributed to nearly 1 out of every 5 episodes of diarrheal disease globally and is estimated to cause approximately 200,000 deaths annually worldwide, with 70,000 or more among children in developing countries. Noroviruses remain a leading cause of sporadic disease and outbreaks of acute gastroenteritis even in industrialized settings, highlighting that improved hygiene and sanitation alone may not be fully effective in controlling norovirus. Strengths in global progress towards a Norovirus vaccine include a diverse though not deep pipeline which includes multiple approaches, including some with proven technology platforms (e.g., VLP-based HPV vaccines). However, several gaps in knowledge persist, including a fulsome mechanistic understanding of how the virus attaches to human host cells, internalizes, and induces disease.
Keywords: Vaccines, Policy, Global Health, Vaccine value
1. The global public health need for a vaccine
Norovirus is attributed to nearly 1 out of every 5 episodes of diarrheal disease globally and is estimated to cause approximately 200,000 deaths annually worldwide, with 70,000 or more among children in developing countries [1]. While norovirus is ubiquitous among all populations in high-, middle-, and low- income settings, incidence is highest in young children and at an earlier age among children in low-and-middle-income countries (LMICs). In regions where rotavirus vaccine has been introduced, norovirus-associated illness are often the leading cause of medically-attended visits for acute gastroenteritis in pediatric populations, likely via replacement [2–5] An individual will experience an average of three to eight norovirus illness episodes in their lifetime, of which at least one will occur by age of 5 years. Noroviruses are transmitted by multiple routes, but person-to-person spread predominates. Noroviruses remain a leading cause of sporadic disease and outbreaks of AGE in industrialized settings [1], highlighting that improved hygiene and sanitation alone may not be fully effective in controlling norovirus. Current infectious disease priorities are largely based on the burden associated with medically attended health events. However, based on current models, the overwhelming cost of norovirus is due to productivity losses resulting from acute illness. Productivity losses tend to go unrecognized but make up 94 % of the global economic burden of norovirus [6]. From a full public health value approach, focusing only on medically attended outcomes substantially underestimates the total economic impact of infections, particularly norovirus. In summary, due to the ubiquitous nature of norovirus and the substantial health and economic impacts across high-, middle-, and low-income countries, norovirus gastroenteritis should be considered a global health problem which cannot be addressed by hygiene and infrastructure alone. Table 1 and Table 2 provide a summary of the epidemiology, potential indirect public health impact, key populations and associated delivery strategies relevant to a norovirus vaccine.
Table 1.
Summary of epidemiology and potential indirect public health impact.
| Feature | Summary and evidence |
|---|---|
| Epidemiology | |
| Reservoir |
|
| At-risk populations |
|
| Mortality |
|
| Morbidity |
|
| Geographical and seasonal distribution |
|
| Gender distribution |
|
| Socio-economic status vulnerability(ies) (equity/wealth quintile) |
|
| Natural immunity |
|
| Pathogenic types, strains, and serotypes |
|
| Potential indirect impacts | |
| Anti-microbial resistance (AMR) threat |
|
| Epidemic and outbreak potential |
|
| Transmission route/potential |
|
| Acquired/herd immunity |
|
| Co-associated mortality |
|
| Economic burden | |
| Health facility costs/out of pocket costs/ productivity costs |
|
Table 2.
Overview of potential target and key population(s) and associated delivery strategy (ies).
| Target and key population(s) | Delivery strategy(ies) |
|---|---|
| LIC - Adult | Very difficult; adult VPD burden and surveillance systems to support vaccine programs lacking; no comprehensive WHO recommendation for adult immunization; vaccine delivery systems are not optimized.[49] |
| LIC - Pediatric | Key population of interest and established vaccine programs. Vaccine series would be ideally introduced prior to six months of age given epidemiology [31]. |
| MIC – adult | Very difficult; high risk groups of HIV-infected populations and access to vaccine care is more robust. Growing elderly population and traveler markets may exist. Norovirus may be an important vaccine to introduce into these populations and thus could be a pathway [49]. |
| MIC - Pediatric | Vaccination systems are in place for introduction in pediatric populations. Likely alignment with schedule of rotavirus vaccination. |
| HIC - Adult | Vaccination systems are in place for adult immunizations and high-risk target populations (elderly, hospital workers, education systems, travelers, etc). |
| HIC - Pediatric | Vaccination systems in place for introduction in pediatric populations. |
1.1. Current methods of surveillance, diagnosis, prevention, and treatment
Surveillance –
Many HICs such as Japan, the United States, Germany, and parts of China have implemented active and passive systems for monitoring norovirus infections and outbreaks [31,40–42]. However, most LMICs and regions of the world do not include assessing for norovirus at present. Although the Middle East and North Africa (MENA) region employ active and passive surveillance to monitor a number of disease pathogens, they currently do not include assessment for norovirus [43]. Similarly, in Latin America, there is no active surveillance of norovirus, and all data on disease distribution and determinants are collected through epidemiologic field studies [44,45].
Diagnosis –
Methods vary by country and geographic region based on available resources. In HICs, diagnosis is generally presumptive due to the appearance of one or more norovirus related symptoms such as loose stool or vomiting and occurrence during the winter months. When routine clinical laboratory diagnosis is sought, it is usually tested via realtime (RT)-PCR platforms. However, due to most cases not presenting to medical services and the lack of RT-PCR availability and testing generally, norovirus cases often go undiagnosed. This is an important problem, since most physicians and pediatricians in LMIC are unaware of norovirus as an important agent, and therefore, they do not see the need for a norovirus vaccine, which might limit its use once it becomes available (Riddle et al, manuscript in preparation).
Prevention –
Individual protection against norovirus (and other enteric viruses) occurs through routine practice of hygiene measures such as proper hand hygiene, safe food handling and preparation, surface cleaning and disinfection, and thorough laundry washing. From a population health perspective, identification and mitigation during outbreaks are important to mitigate spread with in communities.
Treatment –
Current standard of care for norovirus infection remains supportive, with focus on hydration and electrolyte replenishment. Antiemetics and antimotility agents may play a role in some patients [46].
1.2. Summary of knowledge and research gaps in epidemiology, potential indirect public health impact and economic burden
Epidemiology
Data underlying the illness burden estimates in adults and children over 5 years of age are sparse and subject to important under-reporting bias, especially in LMIC. Current estimates are significantly challenged by the fact that norovirus infections and detection of outbreaks largely go unrecorded as most people do not contact health care services because of the nature of the infection.
There is a need to genotype norovirus strains associated with more severe AGE in different parts of the world, specifically in pediatric populations, to select the best combination of antigens for an effective norovirus vaccine that could be used globally.
Data are lacking on the potential chronic health effects due to norovirus (e.g., post-infectious irritable bowel syndrome, constipation, dyspepsia, and gastroesophageal reflux disease).
There are significant gaps in global norovirus surveillance that impose barriers for accurate estimation of global norovirus disease burden.
Current observations on outbreak activity reflects the bias of information attainment from settings in congregate settings, and therefore may underestimate other important outbreak activity due to norovirus.
Indirect public health impact
Societal costs include direct and indirect (i.e., productivity losses due to absenteeism from work or school and mortality) costs.
Modelling suggests that productivity losses due to absenteeism is a large driver of total disease burden.
There is an important need to measure indirect effects of norovirus infections in LMIC in nutrition (growth velocity) and cognitive development, as seen with AGE in general.
For HIC (and LMIC as well), the impacts on work-force absences in healthcare and education settings, hospital infection control and isolation costs, as well as impacts on immunocompromised individuals also need evaluation.
Economic burden
Current estimates of the economic burden include studies that exclude vomiting-only norovirus episodes, which may represent 13 %–27 % of cases in the community [16,47], and thus likely underestimate the true cost burden of all norovirus illnesses.
There are limited data on treatment administered outside the formal health system (e.g., local pharmacy over the counter, traditional healers, oral rehydration) but would likely be substantial in the societal and individual costs of disease.
More reliable data on health care seeking behavior, hospitalizetion rates for norovirus, and missed productivity, especially for LMICs and older children and adults would be particularly useful.
2. Potential target populations and delivery strategies
2.1. High-income countries
While no formal target product profiles exist, from an epidemiological perspective, likely population segments for a norovirus vaccine could include all age-groups given the epidemiology of infection and disease burden associated with infection. Priority groups in HICs would likely include children, the elderly, and travelers as well as certain industries such as food-handling, health care and education systems where outbreaks are frequently known to occur and cause substantial disruptions. Of interest, Bartsch and co-workers employing modelling techniques, have suggested that the greatest potential economic and health benefits of a norovirus vaccination programme will be young children (under 5 years old) and the elderly (over 65 years old) [48]. Within HICs a norovirus vaccine could be integrated into the routine childhood vaccine schedule as well as routine adult immunization schedules. Traveler populations could be identified and immunized as part of routine pre-travel counselling.
2.2. Middle-income countries
As with HIC, norovirus is likely to impact all segments of the population in middle-income countries. Immunization systems are robust, and a pediatric vaccine would likely be able to be integrated into EPI schedules. However, many MICs have not introduced rotavirus vaccines with which a new norovirus vaccine may compete. From an adult vaccine preventable disease perspective, a recent review has captured current challenges broadly for adult vaccine introduction in middle- (and low-) income countries [49]. In the next decades, the number of adults over 65 years of age will grow to be more than the under-5 population, heavily concentrated in low- and middle-income countries. Current adult vaccine programs targeted at pneumococcal disease, influenza, and herpes zoster provide prescient examples of gaps and considerations for adult immunization programs in these countries. Lack of burden of disease in adults limits the potential public and governmental assessment of adult immunization program value. The few countries reporting adult immunization programs generally focus on high-risk groups, and a norovirus vaccine may not compete well with the other aforementioned vaccines. There is also a general lack of appropriate delivery platforms. Thus, a robust system for norovirus vaccination in adults may not be easily integrated into many MICs that could benefit from a norovirus vaccine, except for high-risk adult populations that some MICs target.
2.3. Low-income countries
Approximately 70 % of norovirus cases worldwide are known to occur in children between the ages of 6 and 23 months with the median age of infection in LMICs, where the burden of norovirus infection is highest, being between 6 and 16 months of age [31]. A vaccine should also provide protection before the peak of infection. Pediatric immunization could be implemented within the existing EPI or immunization schedule. Since the burden of it is greatest during the first year of life, immunizing early will have the greatest impact. It is estimated that a norovirus vaccine schedule completed by 6 or 12 months of age could prevent up to 85 % or 50 % of pediatric cases, respectively. The introduction of any new vaccine into routine vaccination programs in most LIC require a complex set of activities including mobilization and leverage of political will and country leadership; advocacy and communications; advanced planning for all aspects of vaccine introduction including processes, standard operating procedures, preparation of the cold chain, logistics, training, and monitoring and evaluation. Most countries in the LMIC, where the burden of disease is quite high, currently deliver vaccines against 13 vaccine preventable diseases (VPDs) at regular schedules within their immunization programme. The current EPI schedule is contact at birth, 6 weeks, 10 weeks, 14 weeks, 6 months, 9 months, 12 months and 18 months. Immunization could be implemented within this schedule. It should be noted that the success of a pediatric vaccine will depend very much on how it can be integrated into the current immunization programmes of countries. As stated above, issues with adult immunization programs in MIC would be the same for LIC.
While the above considerations have been broken out by socioeconomic strata, these likely have applicability across all settings. Other target population and use-case considerations across all socioeconomic strata are important to consider including situations when a novel pandemic strain might arise, utilization of the vaccine as part of an outbreak response in congregate, health care and educational settings, as well as the potential value of a norovirus vaccine in maternal vaccination programs.
3. Norovirus and its consideration as a public health priority by global, regional or country stakeholders
Norovirus vaccine stakeholders are thought to include global health organizations, low-income countries where mortality and morbidity are considerably high, and high-income countries in which burden of disease has been substantially defined and vaccine markets may support early introduction, as well as non-governmental philanthropic organizations (Table 3). For countries who have introduced rotavirus vaccines into their national immunization programs, norovirus would likely be a pathogen (along with shigella) for a vaccine target of interest. However, such an assessment is largely hypothetical as there is little documented evidence that defines the prioritized interest in global health intuitions and stakeholders. At the country-level there is better evidenced interest by the United States as well as China, though formal estimates of demand and vaccine uptake are lacking. Within the United States, health economic analyses have been conducted for US general population as well as the US Department of Defense.
Table 3.
Overview of non-commercial stakeholders engaged, their interest and potential demand.
| Stakeholders engaged | Summary of position/interest | Potential demand and uptake |
|---|---|---|
| WHO | No stated position: however, the present effort demonstrates that the WHO has interest in defining the value of a potential vaccine for global use. | No WHO-derived global demand or uptake forecasts. |
| China | No stated position on norovirus vaccine, though research advances in candidate vaccines and establishment of national surveillance systems suggest that this is a priority pathogen. Scientists from the Chinese Centers for Disease Control state that these efforts are essential to provide information about the evolving strain distribution and epidemiologic characteristics of norovirus outbreaks which contributes to the development of effective vaccines. | No formal demand or uptake estimates are available. Unclear the target populations that might be of interest. Given size, demand forecasts likely to be large [50]. |
| Centers for Disease Control and Prevention (CDC)/US | Not a stated position; however, interest is clear given activity and leadership in norovirus surveillance and epidemiology in the US (and globally). | No-CDC based demand forecasts, though economic analysis and burden of disease description efforts point towards important populations of interest including pediatrics, adult and the elderly [10]. |
| Bill & Melinda Gates Foundation | Norovirus is on a learning agenda but is not in the BMGF’s active enteric vaccine portfolio. | No demand forecast/uptake assessments described. |
| Department of Defense/US | No specific requirement for a norovirus vaccine articulated, however DoD has provided industry support (Ligocyte/Takeda) for norovirus vaccine development and testing. | No formal DoD vaccine demand/uptake forecast though economic analysis has described potential vaccine dose requirements and cost-effectiveness of a vaccine strategy (relative to other leading enteric pathogens) [51,52]. |
A norovirus vaccine should have a dual market potential with pediatric populations in LMIC and HIC, as well as adult populations in HIC and MICs. Private markets in MICs may be sizeable given the burden of disease of this pathogen in all segments and potential demand for such a vaccine. However, formal market analyses are clearly needed.
4. Existing guidance on preferences/preferred product attributes for vaccines against norovirus
Neither the WHO nor any other global priority setting body has developed a preferred product characteristic or target product profile for a norovirus vaccine.
5. Vaccine development
5.1. Probability of technical and regulatory success (PTRS):
There are a number of features currently known about a norovirus vaccine that are favorable towards development of successful norovirus vaccine. These include a diverse though not deep pipeline which includes multiple approaches, and some with technology platforms that have proven successful for other vaccines (e.g., VLP-based HPV vaccines).(Tan 2021) In addition, there is evidence for acquisition of immunity from natural exposure [53,54]. A fairly clear development pathway exists for pediatric populations in LMIC (e.g., rotavirus vaccine development), and there is a human challenge model that could provide opportunity for de-risking, though this model has not been extensively used [55–58].
However, development of a norovirus vaccine is not without challenges. Mechanistic understanding of how the virus attaches to human host cells, internalizes and induces disease is not fully elucidated, and which genotypes to include may rely on future emerging new strains as well as the level of cross-protection against different genotypes that are not included in the vaccine formulation [59,60]. Hence, the frequency of possible reformulation of a norovirus vaccine is currently unknown. There are no good animal disease models that recapitulate human disease, though the recently developed human intestinal enteroid system may provide newer tools to better understand biology and vaccine design optimization.[61].
Table 4 outlines the current expert assessment of the PTRS for a norovirus vaccine with a standardized rating provided according to Appendix A. Table 5 provides an overview of parameters that inform scientific feasibility of developing an effective vaccine for LMIC public market use.
Table 4.
Summary of indicators supporting an assessment of the probability of technical and regulatory success for a norovirus vaccine candidate.
| Probability of success theme | Indicator | Summary | Rating |
|---|---|---|---|
| Biological Feasibility | Most advanced vaccine candidate(s) |
|
Low-Moderate |
| Existence of immunity from natural exposure |
|
Moderate | |
| Understanding mechanisms of immunity |
|
Low | |
| Likelihood of vaccine protection against the majority of pathogenic strains |
|
Low | |
| Product Development Feasibility | Existence of animal models to facilitate vaccine development |
|
Low-Moderate |
| Existence of in vitro assays to facilitate vaccine development |
|
Moderate | |
| Ease of Clinical Development |
|
High | |
| Availability or need for human challenge models |
|
Moderate-High |
Table 5.
Overview of parameters that inform scientific feasibility of developing an effective vaccine for LMIC public market use.
| Parameter | Issues and evidence |
|---|---|
| Diagnosis/case ascertainment | Most studies related to norovirus impact have focused on case definitions (acute diarrhea characterized by increase/unformed stools, with or without vomiting or fever) together with stool testing for norovirus using mostly real-time RT-PCR. This methodology is robust with the caveat that the specific etiologic role of norovirus can be questioned due to high coinfection rates in some studies and to high asymptomatic infection in others. In young children, studies evaluating multiple pathogens frequently detect norovirus together with other viral (mainly rotavirus) or bacterial (mainly diarrheagenic E. coli) pathogens, which makes an etiologic assignment difficult. Conversely, detection of norovirus in fully asymptomatic individuals (although at a lower percent when compared to diarrhea individuals), also suggests that the sole detection of the pathogen does not imply a pathogenic role [27,62–65]. |
| Biomarkers/Correlates of risk and/or protection | While a number of correlates of protection have been explored, they have not allowed to consistently identify a correlate suitable for vaccine trials. HBGA-blocking antibodies have showed promising results, and together with studies in Human Intestinal Enteroids represent the most relevant advances in our understanding of virus diversity and their relationship with cell adhesion and infection [66,67]. |
| Sero-epidemiological data | Population-based studies suggest that seroepidemiology may be used as a tool to measure force of infection within a given population, and that there is genotype-specific homologous protective effect after natural exposure, with some evidence of short-term cross-genotype functional anti-body responses. Number of infections need to induce broadly protective natural antibody responses are not known, nor is the full duration of homologous and heterologous protection after natural exposure [53,54]. |
| Clinical endpoints | Clinical endpoints for vaccine studies are just recently being proposed. Following the “rotavirus model”, moderate to severe infection based on hospitalization and/or fluid replacement requirement, and/or Vesikari/modified Vesikari scores are being considered. Detection method will likely be realtime RT-PCR and protection against the clinical outcome caused by vaccine types will likely be the primary outcome. |
| Controlled Human infection model (CHIM) | Volunteer studies using a controlled human infection model have been developed and are used for early vaccine evaluations. Studies aiming to identify suitable challenge strains are in progress [55,56,68,69]. |
| Opportunity for innovative clinical trial designs | Current vaccine trials for the most advanced candidates are being evaluated in adult and child populations (see below). Trial designs are conventional and will include populations followed for up to 2 years for the primary outcome as well as reinfections and potential herd protection for family members. Human challenge studies have also been considered and could be introduced into LMIC settings given the risk of infections in adults globally. |
| Regulatory approach(es), including potential accelerated approval strategies |
|
| Potential for combination with other vaccines | Given the route of administration, schedule, number of doses and delivery strategy the combination of a norovirus vaccine with another respiratory and/or enteric pathogen, for example rotavirus would be attractive [1,9]. |
| Feasibility of meeting presentation and stability requirements | Likely, yes as vaccines in development utilize similar constructs and formulation to existing vaccines in the LMICs. |
| Vaccine platform | Depending on the vaccine construct, a norovirus VLP vaccine would be similar to other vaccines that are manufactured by developing world manufactures. |
| Large scale Manufacturer capacity/interest | Uncertain. Current vaccines in the pipeline are not yet developed by large manufactures. |
5.2. Overview of the vaccine candidates in the clinical pipeline:
There are currently-three VLP-based platform vaccine candidates and one adenovirus vector-based platform which have undergone clinical studies (Fig. 1 and Table 6). The most advanced candidate has produced several publications, from its early stage (Ligocyte) and later stages (Takeda) including phase I and II trials as well as formulation and dosing adjustments and active search of immune correlates of protection. This vaccine, currently under development by Hillevax, is the only candidate reporting clinical efficacy to date, and is moving forward to phase III trials in adults and children from 5 months of age. The other two VLP candidates, from Chinese manufacturers are in earlier stages, and peer-review results of the phase I trials have not yet been reported. The adenovirus vectored GI.1 vaccine using oral tablets was reported to be safe and immunogenic (using several immunologic parameters).
Fig. 1.
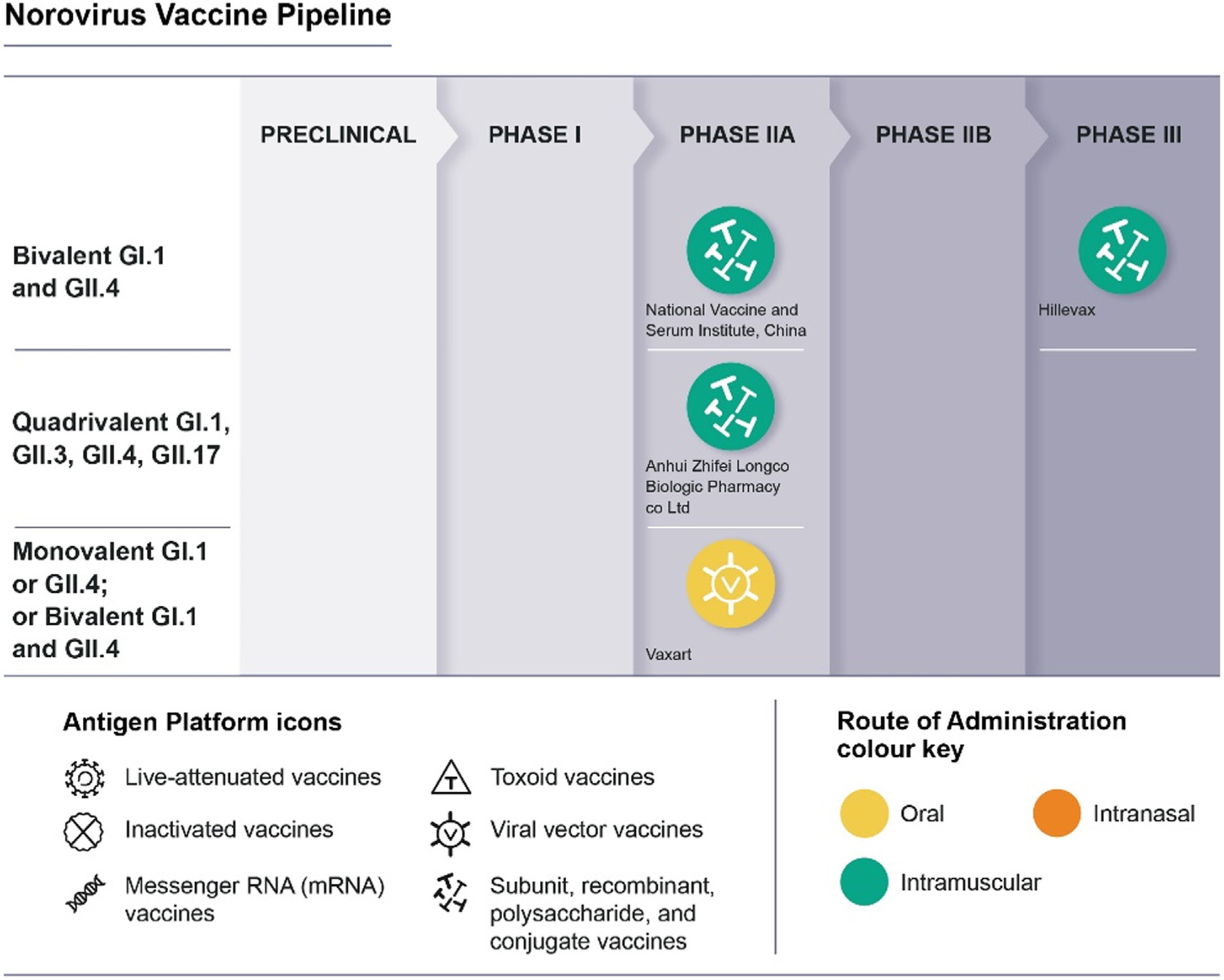
30th, 2022 (Credit: WHO).
Table 6.
Overview of vaccine candidates in the clinical pipeline.
| Candidate | Antigen platform | Developer/manufacturer | Phase of development, population, and location | Route of administration, no. of doses, schedule |
|---|---|---|---|---|
| Bivalent GI.1 and GII.4 | Virus-like particles Baculovirus expression system | Ligocyte followed by Takeda, now Hillevax | Clinical: Phase IIb and norovirus challenge completed in US healthy adults; advancing to phase 2b trials in children under 5 in the US, Colombia, Dominican Republic, Mexico, Panama and Peru | First delivered by intranasal route, and currently developed for intramuscular administration in two doses separated by 28–57 days [51,57,58,70]. |
| Bivalent GI.1 and GII.4 | Virus-like particles Hansenula polymorpha expression system | National Vaccine and Serum Institute, China Collaborators: Lanzhou Institute of Biological Products Co., ltd Beijing Zhong Sheng Heng Yi Pharmaceutical Technology Co., ltd. Zhengzhou University | Clinical: Phase I completed in Healthy People Aged 6 Months to 59 Years; Phase II trials in progress among Healthy People Aged 6 Months to 59 Years. China. | Developed for intramuscular administration in 2–3 doses separated by 28 days [71,72]. |
| Quadrivalent GI.1, GII.3, GII.4, GII.17 | Virus-like particles Pichia pastoris system expression system | Anhui Zhifei Longcom Biologic Pharmacy Co., ltd. | Clinical: Phase I/IIa ongoing in healthy people aged 6 weeks to 59 years (age-descending). China | Developed for intramuscular administration; doses under evaluation [73]. |
| Monovalent GI.1 or GII.4; or Bivalent GI.1 and GII.4 | Adenovirus vector-based vaccine platform including the VP1 gene; Co-expression with a doublestranded RNA adjuvant | Vaxart | Clinical: Phase I in adults completed (G1.1 vaccine); advancing to Phase II trials in the US. | Oral administration; currently evaluating number of doses [74]. |
6. Health, social and economic impact of a norovirus vaccine on burden of disease and transmission
A norovirus vaccine would have a number of different target populations and thus a variety of potential health, social and economic impacts (Table 7). These populations include young children in LMICs as well as those in HICs. In addition, there are healthy adults, as well as older adults where there is substantial burden. Finally, there are also identified specific at-risk populations such as healthcare workers or food-handlers. As such, there are many potential and important impacts that a vaccine may have. The tables below provided substantial detail on what has been considered thus far. From an age-based perspective, the youngest will have the highest rates of overall healthcare utilization, as well as are considered primary drivers of transmission in communities. In HIC, more severe disease is also found in the older age groups, with a majority of deaths occurring in elderly individuals aged 65 years and older. Travelers from HIC to LMIC are at increased risk of itinerary disrupting illness which not only has individual health impacts but could also impact the local economies through reduction of purchases/expenditures during travel. Military personnel in garrison, on ships and deployed on the ground in overseas locations also have a high incidence of norovirus illness which could impact readiness and operational effectiveness. Other high-risk groups include health-care workers and food-handlers, the latter of which would be important given that norovirus accounts for a considerable amount of domestically-acquired foodborne illness in HIC.
Table 7.
Overview of studies that address the potential value of a norovirus vaccine on health, social and economic impacts on disease burden and transmission.
| Policy question | Assessment method/measure | Additional information specific to models | Assumptions | Outcomes/interpretation |
|---|---|---|---|---|
| Understand the transmission dynamics of norovirus and to predict the likely impact of vaccination. | Dynamic age-specific mathematical model of norovirus transmission and vaccination. | Model fitted to age-stratified time series case notification data available from Germany. Includes the use of a self-reporting Markov model to account for variation by age and over time in the statutory reporting of norovirus in Germany. The model uses a sequential Monte Carlo particle filter. The estimated model was then extended and applied to investigate the potential impact of a range of immunization strategies. Sensitivity analyses were performed on the mode of vaccine action and other vaccine-related parameters. |
|
|
| Which age groups should be vaccinated to maximize population impact? | Deterministic, age-structured compartmental model of norovirus transmission and immunity in the U.S. population. | Model was fit to age-specific monthly norovirus-associated U.S. hospitalizations between 1996 and 2007 Simulated mass immunization ofbothpediatric and elderly populations |
|
|
| Is pediatric norovirus vaccination cost- effective in daycare settings? | Transmission-model-based cost-effectiveness analysis | A dynamic SEIR-like transmission model of norovirus outbreaks in daycare settings was calibrated to NORS outbreak data and adapted to include vaccination. The model incorporated detailed dynamics of pediatric transmission within daycare settings (including infection via human-to-human and fomite-to-human contacts). The economic analysis utilized an incremental cost-effectiveness ratio to compare costs and QALYS of vaccination and no vaccination (observed standard of care only, in which symptomatic children are excluded from daycare). The model did not include secondary transmission outside of daycare centers. |
|
|
| Clinical impact and cost effectiveness thresholds for vaccinating children or older adults in community settings | Age-structured compartmental transmission model and cost effectiveness analysis. |
|
|
|
| Cost effectiveness of norovirus vaccine compared to other enteric vaccines for military use | Modified version of an economic model developed to evaluate the cost-effectiveness of a vaccine acquisition strategy within the Department of Defense adapted to norovirus. |
|
|
|
| Cost effectiveness of norovirus vaccination in LMIC military population | Adapted economic model developed by the United States Department of Defense to evaluate cost-effectiveness of vaccine acquisition strategies utilizing a static decision tree model to compare cost-effectiveness of vaccine implementation. |
|
|
|
| Cost-effectiveness of norovirus vaccination in children in LMICs (Peru) | Markov decision model to evaluate cost- effectiveness of a two-dose norovirus vaccine in Peru’s routine childhood immunization schedule based on two recent estimates of NV incidence (one for peri-urban region, one for jungle region). |
|
|
|
| Potential economic value of human norovirus vaccine for the United States | Markov simulation model of vaccine cost- effectiveness from societal perspective (including direct medical costs and indirect costs) |
|
|
|
6.1. Summary of knowledge and research gaps in modelling health, social and economic impact on disease burden and transmission
Gaps in modelling literature include:
Impact of norovirus vaccination in settings where vulnerable population are at risk such as long-term care facilities.
Impact of norovirus vaccination across the whole population considering the role of and targeting vaccination among high transmission populations (e.g., healthcare workers, food workers and handlers).
Comparison of vaccination schedules in young children, considering maternal antibody interference and norovirus infection rates.
Impact of vaccination in LMICs. There are some models from middle income countries but few and none in LICs.
Models that consider the multi-strain dynamics and vaccine valency.
Models which consider the impact of COVID-19 and changes in underlying disease dynamics of norovirus, including the proportion of susceptible individuals in different age groups, and possible disruptions to inter-epidemic cycles.
Influential model inputs that need further definition to inform modelling studies include:
Care seeking behaviors and health care utilization for norovirus across diverse age groups and populations (especially in LMIC settings).
Protection and duration of protection of norovirus immunity following vaccination and/or viral infection.
- Impact of maternal antibodies on vaccine effectiveness in young children.
- Cost of norovirus vaccine and vaccine program implementation.
- In children.
- In older adults.
- In the military.
- Other targeted groups like HCWs.
- Vaccine uptake willingness of norovirus vaccine among priority populations.
- Children, older adults, the military.
Established cost-effectiveness cut off value across settings (not norovirus specific).
7. Policy considerations and Financing
The evidence required to support a global policy recommendation and financing from Gavi are quite similar to other acute enteric vaccines, most notably rotavirus vaccines for which there are a number of oral rotavirus vaccines already pre-qualified and under the Gavi investment. In addition, new parenteral rotavirus vaccines are currently under development also provide an example pathway for development, licensure and lessons learned from policy development and financing perspectives.
In general, the pathway for a norovirus vaccine would be quite similar to a rotavirus vaccine, although given the epidemiology of norovirus the evaluation of the full public health impact of disease extends beyond childhood given that norovirus incidence and burden of diseases is present and quantifiable across older children, adolescents, adults and the elderly. Given that the severity of illness and under 5 mortality attributable to norovirus appears less in children compared to rotavirus, the full public health burden for those age greater than 5 needs to be considered and enumerated.
Experience also indicates that country level preferences need to be considered early in the process of development. For example, recent evaluation on the hypothetical introduction of injectable or oral next generation rotavirus vaccines (NGRVs) identifies that vaccine delivery considerations were the most important preference drivers for national stakeholders, followed by improved efficacy and cost [80]. Interestingly, while national vaccine program stakeholders preferred a higher efficacy stand-alone injectable NGRV or existing oral live-attenuated rotavirus vaccines, health care providers strongly opposed an injectable rotavirus vaccine to a vaccine schedule. From both immunization program and provider stakeholder perspectives, combination vaccine approaches are much preferred compared to stand-alone vaccines and thus early considerations of combination norovirus vaccines with existing vaccines or those in advanced development (Phase III) could be considered.
Finally, considerations of Gavi financing for new vaccines is critical to garner interest by industry partners, though the potential dual market of a norovirus vaccine may provide non-Gavi financing from high and middle-income countries based on the potential value of preventable disease burden by vaccines.
From a non-Gavi eligible country perspective (e.g., MICs and HICs), vaccine policy and financing considerations would be largely driven by identification of substantial disease burden, demonstration of efficacy in a country or region, approval by a national regulatory authority and consideration and endorsement by a national immunization policy making body. Financing of the vaccine would likely be a blend of private and public market procurement.
Table 8 outlines a number of policy and financing considerations from an LIC perspective that apply in large part to all vaccines but have been made specific to a hypothetical norovirus vaccine as appropriate. Many considerations cannot be known at the present due to the early development process of current norovirus vaccines.
Table 8.
Overview of expectations of evidence that are likely to be required to support a global/regional/national policy recommendation, or financing for LICs.
| Parameter for policy/financing consideration | Assumptions | Guidance/reports available |
|---|---|---|
| Consensus building to define priority public health goals for the vaccine candidate |
|
WHO Technical document. From Vaccine Development to Policy: A Brief Review of WHO Vaccine-Related Activities and Advisory Processes (2017) [81]. WHO | WHO Preferred Product Characteristics (PPCs) [82]. |
| Facilitation and acceleration of product development to achieve WHO public health goals in accordance with WHO recommend norms and standards |
|
WHO Technical Document. From Vaccine Development to Policy: A Brief Review of WHO Vaccine-Related Activities and Advisory Processes (2017) [81]. |
| Registration of product by a functional National Regulatory Authority |
|
|
| Review of key evidence inputs by SAGE working group (WG) to inform optimal use of vaccine from public health perspective, including safety, operational issues and implementation research, and programmatic suitability, as well as the quality of the evidence, values and preferences, equity, feasibility, etc. |
|
WHO Guidance for the development of evidence- based vaccination-related recommendations [83]. |
| SAGE recommendations (from SAGE WG) to WHO are adapted into global policy published as Vaccine Position Paper |
|
WHO Supplement to WHO Vaccine Position Papers. Guideline Development Group [83]. |
| Concurrent with or following SAGE recommendation for widespread use, companies can submit their dossier for WHO prequalification |
|
FDA. WHO Vaccine Pre-qualification Program. 2018 [84,85]. WHO Guidance Documents. WHO - Prequalification of Medical Products (IVDs, Medicines, Vaccines and Immunization Devices, Vector Control) [86]. |
| Develop a package of information to meet Gavi’s approach to prioritizing adoption of support of new vaccines |
|
Gavi Vaccine Investment Strategy [80]. |
8. Access and implementation feasibility
Vaccines only work if they are actually used. And getting vaccine manufacturers to pursue development and licensure of a vaccine requires a strong market and one that is sustainable long term. Thus, in identification of market strength and sustainability, issues of vaccine access and implementation are of critical importance to consider in prioritizing, developing and establishing policy and financing of novel vaccines such as norovirus. Pertinent to a novel norovirus vaccine, the rotavirus vaccine experience is instructive and provides perspective on what might be relevant to consider. While rotavirus vaccines have consistently been shown to save lives in many LMICs, and to be highly cost-effective or even cost-saving in many, 110 countries have introduced rotavirus vaccines into their national immunization programs, 53 have accessed support from Gavi, and 65 countries have not expressed plans to introduce rotavirus vaccines, including 8 that are eligible for Gavi support.[87]
Despite the clear evidence of mortality reduction by rotavirus vaccines, the high emphasis on infant mortality reduction by global vaccine financers and regional immunization program authorities often presents a challenge for diseases where there is ‘relatively’ more morbidity than mortality. Such is the case with rotavirus and would appear to be even more of the case with norovirus. To offset the lesser case-fatality rate of norovirus and relevant to the situation of non-rotavirus adoption among many LMICs, a framework was used to consider the favorability of access and implementation and ultimately market strength and sustainability (Appendix B). While limited vaccine candidates are currently in the pipeline (and early stage at that), we considered both a parenterally delivered VLP-based vaccine construct (Hillevax, Boston, MA USA) and an oral adenovirus vectored- VLP-based construct (Vaxart, South San Francisco, CA USA) as prototype vaccines in the access and implementation framework. For simplicity we refer to these as oral novel norovirus vaccines and injectable novel norovirus vaccines.
Possibility of implementation within existing delivery systems
Both oral and injectable vaccines are common vaccine constructs on the market therefore theoretically implementing with existing delivery systems is possible. However, the unique product profiles and formulation characteristics of a vaccine would have important impacts on whether such a new vaccine could be integrated into existing systems. Cold storage requirements, shelf life, and package volumes would be important, but final formulations/-packaging for both constructs are not known at this time. Vaccines which do not require significant cold storage demands, have a long shelf life and are contained in environmentally friendly and space efficient packaging are optimum. Unique to a currently developed adenovirus-vectored norovirus vaccine by Vaxart, this vaccine is formulated as oral tablets (or liquid formulation for patients unable to swallow tablets) which are room temperature-stable. [88] While there are no existing systems which deliver tablet-based vaccines globally, such a formulation could be conceivably integrated and would be an alternative to the current crowded landscape of injectable vaccines.
Vaccine schedule is also an important feature to consider in this area of implementation feasibility. Current trials of available constructs are considering both single dose and two dose regimens separated by 1 or 2 months with boosters at 4 months (see vaccine section). Single dose regimens could be better accommodated in schedules, though multi-dose regimens may be required particularly in younger populations who are not already primed by natural history exposure.
Finally, potential for combinability with other licensed or near-to license vaccines is a consideration. An attractive combination would be a norovirus combined with rotavirus vaccine given the overlap in clinical condition, as well as the global epidemiology of norovirus. Oral and next generation parenteral rotavirus vaccines are currently being used or may be introduced into the near future. An alternative opportunity might also be to make a combined influenza-norovirus vaccine given the similar geographic distributions (global), strong overlap in seasonality, and the potential need to update the norovirus vaccine with prevailing genotypes as new genotypes emerge.
Thus, the possibility of implementation within existing delivery systems is seen as HIGH to VERY HIGH.
Commercial attractiveness
In support of favorability for this factor, based on our assessment there are large target populations in HIC and LMIC, both private and public markets. Though formal market assessments are needed, it would be likely that military and traveler markets would be quick to adopt such a vaccine, as well as food handlers and those working in congregate settings where norovirus outbreaks are known to frequently occur. In addition, given that norovirus is a recurrent illness among all age-groups and the potential that the vaccine might need to be readministered as genotype epidemiology changes, demand requirements may be higher than traditional pediatric vaccines. This may be seen as a downside as well if manufacturers are required to reformulate the vaccine at some frequency with safety and efficacy studies if correlates of protection are not established.
The potential downside for the attractiveness of a norovirus vaccine is whether the vaccine would meet considerations and be prioritized under Gavi’s Vaccine Investment Strategy. If nonmortality burden of disease is considered and augmented with broader population-based burden of disease estimates, the global public health value of a norovirus vaccine may be considerable and of high priority. If the vaccine has a large global market in HIC and middle-income countries who are willing to pay, this may also increase interest given the opportunity for cost-sharing of a high-volume vaccine with tiered pricing.
Given these reasons, the commercial attractiveness for a norovirus vaccine at present is assessed as MODERATE to HIGH.
Clarity of licensure and policy decision pathway
Rotavirus vaccines (and other acute diarrheal vaccines under development) have paved the way for clinical development, licensure and policy decision pathways. Given the attractiveness of a norovirus vaccine to HIC populations, it is highly likely that a recognized NRA would license the vaccine for their populations including pediatric age-groups. The WHO SAGE in working on rotavirus vaccines has an existing framework to consider a norovirus vaccine, though would need to be expanded to consider the full public health value of a vaccine that extends beyond children under 5 years.
Thus, we assess the clarity of licensure and policy decision pathway to be VERY HIGH for a norovirus vaccine.
Expected financing mechanism
A norovirus vaccine would likely have fairly expansive global interest in high income countries given the documented impact of disease burden and epidemiology. Less is known about the potential interest in LMICs with competing demands for existing licensed vaccines and other novel vaccines entering the market. Given the potential dual use market for a norovirus vaccine, large volume and tiered pricing strategies could provide cost of vaccine offsets for LMICs with advanced market commitment incentives.
Therefore, the expected financing mechanism is considered LOW to MODERATE.
Ease of uptake
A pediatric vaccine would likely be able to be integrated into an EPI schedule. However, depending on the timing of introduction there may be concerns about another separate injection for norovirus vaccine. Combination of the vaccine with penta- or hexavalent vaccines, or a combined diarrhea vaccine (with inactivated rotavirus or shigella) may be attractive but would require other clinical and technical development. Alternatively, an oral formulation may also increase uptake given it would not require additional injection. Adult and pediatric dose formulations could present a challenge as well. Use of norovirus in the HIC should not present a major barrier.
Thus, it is assessed that the ease of uptake is considered HIGH.
9. Conclusion
The consideration of norovirus as a target for vaccine development as a priority pathogen is complex. On one hand, its incidence and impact are noted in all regions of the world as well as in both younger and older age-groups, thus making the potential value of preventing disease vast. This would also appear to be a driving factor to encourage multiple stakeholders who might want to see such a vaccine developed. However, in contrast to a number of other priority pathogens for which vaccines are being developed, the economic and health burden attributed to norovirus infections are generally due to milder disease and there is often a premium placed on vaccines that prevent infections that commonly lead to death. However, if one were to look at this infection from a holistic perspective, there is a considerable global disease burden that should place this vaccine as a priority pathogen. Furthermore, norovirus is one of a number of enteric pathogens for which there are current vaccines available and underutilized (e.g., rotavirus) as well as vaccines under development that provided competition for another enteric vaccine to develop (e.g., shigella, non-Typhoid salmonella and ETEC). Given these alternative interventions that are underutilized and under development, norovirus may not present as a global public health priority pathogen. However, to solve the current problem of enteric burden of disease as well as the important long-term health manifestations that accompanies populations with high disease burden caused by multiple infections, a ‘this AND that’ is needed perspective can be taken.
There are many positive considerations regarding the state of science, and vaccine development pipeline that encourage a positive outlook on the probability of success that a safe and effective norovirus vaccine could be developed. Burden of disease data (and ongoing public health surveillance) are broadly available to provide strong data that guides vaccine need, though measures of impact on work-force productivity and the accounting of impacts of mild-to-moderate disease need focus. There are currently-four different norovirus vaccines under development, relying on proven technology (VLPs) and currently in phase 2 field efficacy trials. Furthermore, there is a controlled human infection model that could be utilized to support or accelerate (e.g., in HIC adults) vaccine licensure. For LMIC pediatric indication, there is an existing ‘playbook’ on how to develop, test and approve enteric vaccines, particularly given the robust pathways that rotavirus vaccines have been advanced through.
More work is needed to understand how a norovirus vaccine might fit in the current payer models of Gavi, as well as middle-income and higher-income public and private markets.
Data availability
No data was used for the research described in the article.
Supplementary Material
Acknowledgements
The team would like to sincerely thank the Office of Medical Research staff Meghana Veeramachaneni and Amber Emerson for their invaluable editing and project management assistance. This work in part was also supported by the Bill & Melinda Gates Foundation Seattle, WA [INV029307].
Declaration of Competing Interest
BAL reports personal fees from Epidemiological Research and Methods, LLC. MO acknowledges financial support from ANID PIA/ PUENTE AFB220003 and reports receiving grants and research support from Janssen Vaccines & Prevention B.V., Bharat Biotech International Limited, and HilleVax Inc. CFL declares that he receives research grant funding to his Institute from Takeda Vaccines Inc. and HilleVax Inc. for norovirus epidemiology and a norovirus vaccine phase II trial, respectively. All other authors declare no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This supplement was sponsored by the World Health Organization’s Immunization, Vaccines, and Biologicals unit. The authors alone are responsible for the views expressed in each article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Footnotes
The development of this Vaccine Value Profile has been commissioned by WHO’s Immunization, Vaccines and Biologicals (IVB) department on the recommendation of IVB’s Product Development for Vaccines Advisory Committee, to the Bill & Melinda Gates Foundation. All authors are independent subject matter experts and the authors alone are responsible for the views expressed in this manuscript.
Diclaimers
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, the World Health Organization or the authors’ affiliated institutions.
The current knowledge described in this document is largely based on studies from the pre-COVID-19-pandemic era. The impact of large-scale non-pharmaceutical interventions against SARSCoV-2 on the underlying epi of norovirus, including potential changes in its disease dynamics, the proportion of susceptible individuals in different age groups, and possible disruptions to the cycles between epidemics is unknown.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.03.034.
References
- [1].Lopman B Global burden of norovirus and prospects for vaccine development. CDC Foundation; 2015. Aug. 46 p. Available from: https://www.cdc.gov/norovirus/downloads/global-burden-report.pdf. [Google Scholar]
- [2].Burke RM, Mattison CP, Marsh Z, Shioda K, Donald J, Salas SB, et al. Norovirus and other viral causes of medically attended acute gastroenteritis across the age spectrum: results from the medically attended acute gastroenteritis study in the United States. Clin Infect Dis [Internet]. 2021;73:e913–20. Available from: Doi: 10.1093/cid/ciab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ballard S-B, Requena D, Mayta H, Sanchez GJ, Oyola-Lozada MG, Colquechagua Aliaga FD, et al. Enteropathogen changes after rotavirus vaccine scale-up. pediatrics [Internet]. 2022;149. Available from: Doi: 10.1542/peds.2020-049884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tarr GAM, Pang X-L, Zhuo R, Lee BE, Chui L, Ali S, et al. Attribution of pediatric acute gastroenteritis episodes and emergency department visits to norovirus genogroups I and II. J Infect Dis [Internet]. 2021;223:452–61. Available from: Doi: 10.1093/infdis/jiaa391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Payne DC, Vinjé J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med [Internet]. 2013;368:1121–30. Available from: Doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bartsch SM, Lopman BA, Ozawa S, Hall AJ, Lee BY. Global economic burden of norovirus gastroenteritis. PLoS ONE [Internet]. 2016;11:e0151219. Available from: Doi: 10.1371/journal.pone.0151219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wikswo ME, Kambhampati A, Shioda K, Walsh KA, Bowen A, Hall AJ, et al. Outbreaks of acute gastroenteritis transmitted by person-to-person contact, environmental contamination, and unknown modes of transmission–United States, 2009–2013. MMWR Surveill Summ [Internet]. 2015;64:1–16. Available from: Doi: 10.15585/mmwr.mm6412a1. [DOI] [PubMed] [Google Scholar]
- [8].Villabruna N, Koopmans MPG, de Graaf M. Animals as reservoir for human norovirus. Viruses [Internet]. 2019;11. Available from: Doi: 10.3390/v11050478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lopman BA, Steele D, Kirkwood CD, Parashar UD. The vast and varied global burden of norovirus: prospects for prevention and control. PLoS Med [Internet]. 2016;13:e1001999. Available from: Doi: 10.1371/journal.pmed.1001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE, et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS ONE [Internet]. 2013;8:e72788. Available from: Doi:10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pires SM, Fischer-Walker CL, Lanata CF, Devleesschauwer B, Hall AJ, Kirk MD, et al. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PLoS ONE [Internet]. 2015;10:e0142927. Available from: Doi: 10.1371/journal.pone.0142927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, et al. World health organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data synthesis. PLoS Med [Internet]. 2015;12:e1001921. Available from: Doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Asare EO, Hergott D, Seiler J, Morgan B, Archer H, Wiyeh AB, et al. Case fatality risk of diarrhoeal pathogens: a systematic review and meta-analysis. Int J Epidemiol [Internet]. 2022. Available from: Doi: 10.1093/ije/dyac098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pogreba-Brown K, Austhof E, Armstrong A, Schaefer K, Villa Zapata L, McClelland DJ, et al. Chronic gastrointestinal and joint-related sequelae associated with common foodborne illnesses: a scoping review. Foodborne Pathog Dis [Internet]. 2020;17:67–86. Available from: Doi: 10.1089/fpd.2019.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Porter CK, Faix DJ, Shiau D, Espiritu J, Espinosa BJ, Riddle MS. Postinfectious gastrointestinal disorders following norovirus outbreaks. Clin Infect Dis [Internet]. 2012;55:915–22. Available from: Doi: 10.1093/cid/cis576. [DOI] [PubMed] [Google Scholar]
- [16].Marshall JA, Hellard ME, Sinclair MI, Fairley CK, Cox BJ, Catton MG, et al. Incidence and characteristics of endemic norwalk-like virus-associated gastroenteritis. J Med Virol [Internet]. 2003;69:568–78. Available from: Doi:10.1002/jmv.10346. [DOI] [PubMed] [Google Scholar]
- [17].Ahmed SM, Lopman BA, Levy K. A systematic review and meta-analysis of the global seasonality of norovirus. PLoS ONE [Internet]. 2013;8:e75922. Available from: Doi: 10.1371/journal.pone.0075922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walter F, Ott JJ, Claus H, Krause G. Sex- and age patterns in incidence of infectious diseases in Germany: analyses of surveillance records over a 13-year period (2001–2013). Epidemiol Infect [Internet]. 2018;146:372–8. Available from: Doi: 10.1017/S0950268817002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cannon JL, Lopman BA, Payne DC, Vinjé J. Birth cohort studies assessing norovirus infection and immunity in young children: a review. Clin Infect Dis [Internet]. 2019;69:357–65. Available from: Doi: 10.1093/cid/ciy985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gruber JF, Bowman NM, Becker-Dreps S, Reyes Y, Belson C, Michaels KC, et al. Risk factors for norovirus gastroenteritis among Nicaraguan children. Am J Trop Med Hyg [Internet]. 2017;97:937–43. Available from: Doi: 10.4269/ajtmh.16-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Violato M, Taylor-Robinson D, Hungerford D, Gray A, O’Brien S, Iturriza-Gomara M. Family income and exposure to norovirus in childhood: findings from the UK Millennium Cohort Study. SSM Popul Health [Internet]. 2019;8:100445. Available from: Doi: 10.1016/j.ssmph.2019.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kreidieh K, Charide R, Dbaibo G, Melhem NM. The epidemiology of norovirus in the Middle East and North Africa (MENA) region: a systematic review. Virol J [Internet]. 2017;14:220. Available from: Doi: 10.1186/s12985-017-0877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Simmons K, Gambhir M, Leon J, Lopman B. Duration of immunity to norovirus gastroenteritis. Emerging Infect Dis [Internet]. 2013;19:1260–7. Available from: Doi: 10.3201/eid1908.130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rouhani S, Peñataro Yori P, Paredes Olortegui M, Siguas Salas M, Rengifo Trigoso D, Mondal D, et al. Norovirus infection and acquired immunity in 8 countries: results from the MAL-ED Study. Clin Infect Dis [Internet]. 2016;62:1210–7. Available from: Doi: 10.1093/cid/ciw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Saito M, Goel-Apaza S, Espetia S, Velasquez D, Cabrera L, Loli S, et al. Multiple norovirus infections in a birth cohort in a Peruvian Periurban community. Clin Infect Dis [Internet]. 2014;58:483–91. Available from: Doi: 10.1093/cid/cit763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jones TF, Yackley J. Foodborne disease outbreaks in the United States: a historical overview. Foodborne Pathog Dis [Internet]. 2018;15:11–5. Available from: Doi: 10.1089/fpd.2017.2388. [DOI] [PubMed] [Google Scholar]
- [27].Ashbaugh HR, Early JM, Johnson ME, Simons MP, Graf PCF, Riddle MS, et al. A multisite network assessment of the epidemiology and etiology of acquired diarrhea among U.S. military and western travelers (global travelers’ diarrhea study): a principal role of norovirus among travelers with gastrointestinal illness. Am J Trop Med Hyg [Internet]. 2020;103:1855–63. Available from: Doi:10.4269/ajtmh.20-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hungerford D, Jere KC, Bar-Zeev N, Harris JP, Cunliffe NA, Iturriza-Gómara M. Epidemiology and genotype diversity of norovirus infections among children aged <5 years following rotavirus vaccine introduction in Blantyre, Malawi. J Clin Virol [Internet]. 2020;123:104248. Available from: Doi: 10.1016/j.jcv.2019.104248. [DOI] [PubMed] [Google Scholar]
- [29].Hofmann FM, Olawumi E, Michaelis M, Stößel U, Hofmann F. Significance of norovirus in occupational health: a review of published norovirus outbreaks in Central and Northern Europe. Int Arch Occup Environ Health [Internet]. 2020;93:911–23. Available from: Doi: 10.1007/s00420-020-01543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Manuel CS, Moore MD, Jaykus L-A. Predicting human norovirus infectivity -recent advances and continued challenges. Food Microbiol [Internet]. 2018;76:337–45. Available from: Doi: 10.1016/j.fm.2018.06.015. [DOI] [PubMed] [Google Scholar]
- [31].Bucardo F, Reyes Y, Svensson L, Nordgren J. Predominance of norovirus and sapovirus in Nicaragua after implementation of universal rotavirus vaccination. PLoS ONE [Internet]. 2014;9:e98201. Available from: Doi:10.1371/journal.pone.0098201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Biswas JS, Lentaigne J, Hill NE, Harrison JJ, Mackenzie H, Akorli E, et al. Epidemiology and etiology of diarrhea in UK military personnel serving on the United Nations Mission in South Sudan in 2017: a prospective cohort study. Travel Med Infect Dis [Internet] 2019;28:34–40. Available from: Doi: 10.1016/j.tmaid.2018.12.004. [DOI] [PubMed] [Google Scholar]
- [33].Bennett SD, Sodha SV, Ayers TL, Lynch MF, Gould LH, Tauxe RV. Produce-associated foodborne disease outbreaks, USA, 1998–2013. Epidemiol Infect [Internet]. 2018;146:1397–406. Available from: Doi: 10.1017/S0950268818001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Neo FJX, Loh JJP, Ting P, Yeo WX, Gao CQH, Lee VJM, et al. Outbreak of caliciviruses in the Singapore military, 2015. BMC Infect Dis [Internet] 2017;17:719. 10.1186/s12879-017-2821-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].de Graaf M, Villabruna N, Koopmans MP. Capturing norovirus transmission. Curr Opin Virol [Internet] 2017;22:64–70. 10.1016/j.coviro.2016.11.008. [DOI] [PubMed] [Google Scholar]
- [36].Johnson PC, Mathewson JJ, DuPont HL, Greenberg HB. Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis [Internet] 1990;161:18–21. 10.1093/infdis/161.1.18. [DOI] [PubMed] [Google Scholar]
- [37].Rogawski McQuade ET, Liu J, Kang G, Kosek MN, Lima AAM, Bessong PO, et al. Protection from natural immunity against enteric infections and etiologyspecific diarrhea in a longitudinal birth cohort. J Infect Dis [Internet] 2020;222:1858–68. Available from: Doi: 10.1093/infdis/jiaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wyatt RG, Dolin R, Blacklow NR, DuPont HL, Buscho RF, Thornhill TS, et al. Comparison of three agents of acute infectious nonbacterial gastroenteritis by cross-challenge in volunteers. J Infect Dis [Internet] 1974;129:709–14. 10.1093/infdis/129.6.709. [DOI] [PubMed] [Google Scholar]
- [39].Mirelman AJ, Ballard SB, Saito M, Kosek MN, Gilman RH. Cost-effectiveness of norovirus vaccination in children in Peru. Vaccine [Internet] 2015;33:3084–91. 10.1016/j.vaccine.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gao Z, Liu B, Yan H, Li W, Jia L, Tian Y, et al. Norovirus outbreaks in Beijing, China, from 2014 to 2017. J Infect [Internet]. 2019;79:159–66. Available from: Doi: 10.1016/j.jinf.2019.05.019. [DOI] [PubMed] [Google Scholar]
- [41].Sakon N, Yamazaki K, Nakata K, Kanbayashi D, Yoda T, Mantani M, et al. Impact of genotype-specific herd immunity on the circulatory dynamism of norovirus: a 10-year longitudinal study of viral acute gastroenteritis. J Infect Dis [Internet] 2015;211:879–88. 10.1093/infdis/jiu496. [DOI] [PubMed] [Google Scholar]
- [42].Arowolo KO, Ayolabi CI, Lapinski B, Santos JS, Raboni SM. Epidemiology of enteric viruses in children with gastroenteritis in Ogun State, Nigeria. J Med Virol [Internet] 2019;91:1022–9. 10.1002/jmv.25399. [DOI] [PubMed] [Google Scholar]
- [43].Guarines KM, Mendes RPG, de Magalhães JJF, Pena L. Norovirus-associated gastroenteritis, Pernambuco, Northeast Brazil, 2014–2017. J Med Virol [Internet]. 2020;92:1093–101. Available from: Doi: 10.1002/jmv.25631. [DOI] [PubMed] [Google Scholar]
- [44].Degiuseppe JI, Barclay L, Gomes KA, Costantini V, Vinjé J, Stupka JA. Molecular epidemiology of norovirus outbreaks in Argentina, 2013–2018. J Med Virol [Internet] 2020;92:1330–3. 10.1002/jmv.25684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stewart S, Robertson C, Pan J, Kennedy S, Dancer S, Haahr L, et al. Epidemiology of healthcare-associated infection reported from a hospital-wide incidence study: considerations for infection prevention and control planning. J Hosp Infect [Internet] 2021;114:10–22. Available from: Doi: 10.1016/j.jhin.2021.03.031. [DOI] [PubMed] [Google Scholar]
- [46].Burch T Validation of quantitative microbial risk assessment using epidemiological data from outbreaks of waterborne gastrointestinal disease. Risk Anal [Internet] 2019;39:599–615. 10.1111/risa.13189. [DOI] [PubMed] [Google Scholar]
- [47].Rockx B, De Wit M, Vennema H, Vinjé J, De Bruin E, Van Duynhoven Y, et al. Natural history of human calicivirus infection: a prospective cohort study. Clin Infect Dis [Internet] 2002;35:246–53. 10.1086/341408. [DOI] [PubMed] [Google Scholar]
- [48].Bartsch SM, Lopman BA, Hall AJ, Parashar UD, Lee BY. The potential economic value of a human norovirus vaccine for the United States. Vaccine [Internet] 2012;30:7097–104. 10.1016/j.vaccine.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sauer M, Vasudevan P, Meghani A, Luthra K, Garcia C, Knoll MD, et al. Situational assessment of adult vaccine preventable disease and the potential for immunization advocacy and policy in low- and middle-income countries. Vaccine [Internet] 2021;39:1556–64. Available from: Doi: 10.1016/j.vaccine.2021.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jin M, Wu S, Kong X, Xie H, Fu J, He Y, et al. Norovirus outbreak surveillance, China, 2016–2018. Emerging Infect Dis [Internet]. 2020;26:437–45. Available from: Doi: 10.3201/eid2603.191183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sherwood J, Mendelman PM, Lloyd E, Liu M, Boslego J, Borkowski A, et al. Efficacy of an intramuscular bivalent norovirus GI.1/GII.4 virus-like particle vaccine candidate in healthy US adults. Vaccine [Internet] 2020;38:6442–9. 10.1016/j.vaccine.2020.07.069. [DOI] [PubMed] [Google Scholar]
- [52].Tallant A, Porter CK, Putnam SD, Tribble DR, Hooper TI, Riddle MS. Relative cost-effectiveness of a norovirus vaccine in the deployed military setting compared to a vaccine against campylobacter sp., ETEC, and shigella sp. Vaccine [Internet] 2014;32:5156–62. Available from: Doi: 10.1016/j.vaccine.2014.07.070. [DOI] [PubMed] [Google Scholar]
- [53].Arnold BF, Martin DL, Juma J, Mkocha H, Ochieng JB, Cooley GM, et al. Enteropathogen antibody dynamics and force of infection among children in low-resource settings. ELife [Internet] 2019:8. Available from: 10.7554/eLife.45594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kulkarni R, Lole K, Chitambar SD. Seroprevalence of antibodies against GII.4 norovirus among children in Pune, India. J Med Virol [Internet] 2016;88:1636–40. 10.1002/jmv.24495. [DOI] [PubMed] [Google Scholar]
- [55].Evaluation of infectivity and illness of Norwalk GI.1 virus Lot 001–09NV in the human challenge model. 2018 Oct 26 [last updated 2020 Jan 22; cited 2022 May 11] In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. 2018- . Available from: https://clinicaltrials.gov/ct2/show/NCT03721549. [Google Scholar]
- [56].Phase I norovirus challenge model. 2019 Nov 22 [last updated 2021 Apr 9; cited 2022 May 11]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. 2019- . Available from: https://clinicaltrials.gov/ct2/show/NCT04174560. [Google Scholar]
- [57].Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, et al. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med [Internet] 2011;365:2178–87. 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bernstein DI, Atmar RL, Lyon GM, Treanor JJ, Chen WH, Jiang X, et al. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis [Internet] 2015;211:870–8. Available from: Doi:10.1093/infdis/jiu497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tan M Norovirus vaccines: current clinical development and challenges. Pathogens [Internet] 2021:10. Available from: 10.3390/pathogens10121641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cates JE, Vinjé J, Parashar U, Hall AJ. Recent advances in human norovirus research and implications for candidate vaccines. Expert Rev Vaccines [Internet] 2020;19:539–48. 10.1080/14760584.2020.1777860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ettayebi K, Tenge VR, Cortes-Penfield NW, Crawford SE, Neill FH, Zeng X-L, et al. New insights and enhanced human norovirus cultivation in human intestinal enteroids. MSphere [Internet] 2021:6. Available from: Doi: 10.1128/mSphere.01136-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gaensbauer JT, Lamb M, Calvimontes DM, Asturias EJ, Kamidani S, Contreras-Roldan IL, et al. Identification of enteropathogens by multiplex pcr among rural and urban Guatemalan children with acute diarrhea. Am J Trop Med Hyg [Internet] 2019;101:534–40. Available from: Doi: 10.4269/ajtmh.18-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mathew S, Smatti MK, Al Ansari K, Nasrallah GK, Al Thani AA, Yassine HM. Mixed viral-bacterial infections and their effects on gut microbiota and clinical illnesses in children. Sci Rep [Internet] 2019;9:865. 10.1038/s41598-018-37162-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pouletty M, De Pontual L, Lopez M, Morin L, Poilane I, Pham LL, et al. Multiplex pcr reveals a high prevalence of multiple pathogens in traveller’s diarrhoea in children. Arch Dis Child [Internet] 2019;104:141–6. 10.1136/archdischild-2017-314327. [DOI] [PubMed] [Google Scholar]
- [65].Andersson M, Kabayiza J-C, Elfving K, Nilsson S, Msellem MI, Mårtensson A, et al. Coinfection with enteric pathogens in East African children with acute gastroenteritis-associations and interpretations. Am J Trop Med Hyg [Internet] 2018;98:1566–70. Available from: Doi: 10.4269/ajtmh.17-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ramani S, Estes MK, Atmar RL. Correlates of protection against norovirus infection and disease-where are we now, where do we go?. PLoS Pathog [Internet] 2016;12:e1005334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tenge VR, Hu L, Prasad BVV, Larson G, Atmar RL, Estes MK, et al. Glycan recognition in human norovirus infections. Viruses [Internet] 2021:13. Available from: 10.3390/v13102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Optimal human dose for GII.2 norovirus (snow mountain) challenge studies. 2015 Jun 16 [last updated 2018 Dec 7; cited 2021 Dec 20]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. 2015- . Available from: https://clinicaltrials.gov/ct2/show/NCT02473224. [Google Scholar]
- [69].New challenge pool of Norwalk virus inoculum. 2005 Aug 30 [last updated 2014 May 9; cited 2022 May 11]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. 2005- . Available from: https://clinicaltrials.gov/ct2/show/NCT00138476. [Google Scholar]
- [70].Efficacy and safety of two doses of HIL-214 in children. 2022 Mar 16 [last updated 2022 Jun 10; cited 2022 May 11]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. 2022- . Available from: https://clinicaltrials.gov/ct2/show/NCT05281094. [Google Scholar]
- [71].A clinical trial to evaluate the safety and immunogenicity of norovirus bivalent vaccine. 2019 Dec 6 [last updated 2021 Apr 22; cited 2022 May 3]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. 2019- . Available from: https://clinicaltrials.gov/ct2/show/NCT04188691. [Google Scholar]
- [72].Clinical trial to evaluate the recombinant norovirus bivalent (GI.1/GII.4) vaccine (hansenula polymorpha). 2021 Jun 28 [last updated 2022 May 6; cited 2022 Apr 17]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. 2021- . Available from: https://clinicaltrials.gov/ct2/show/NCT04941261. [Google Scholar]
- [73].Clinical trial of quadrivalent recombinant norovirus vaccine (pichia pastoris). 2020 Sep 24 [last updated 2020 Sep 25; cited 2022 Apr 18]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. 2020- . Available from: https://clinicaltrials.gov/ct2/show/NCT04563533. [Google Scholar]
- [74].Kim L, Liebowitz D, Lin K, Kasparek K, Pasetti MF, Garg SJ, et al. Safety and immunogenicity of an oral tablet norovirus vaccine, a phase I randomized, placebo-controlled trial. JCI Insight [Internet] 2018:3. Available from: 10.1172/jci.insight.121077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gaythorpe KAM, Trotter CL, Conlan AJK. Modelling norovirus transmission and vaccination. Vaccine [Internet] 2018;36:5565–71. 10.1016/j.vaccine.2018.07.053. [DOI] [PubMed] [Google Scholar]
- [76].Steele MK, Remais JV, Gambhir M, Glasser JW, Handel A, Parashar UD, et al. Targeting pediatric versus elderly populations for norovirus vaccines: a model-based analysis of mass vaccination options. Epidemics [Internet] 2016;17:42–9. 10.1016/j.epidem.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Steimle LN, Havumaki J, Eisenberg MC, Eisenberg JNS, Prosser LA, Pike J, et al. Cost-effectiveness of pediatric norovirus vaccination in daycare settings. Vaccine [Internet] 2021;39:2133–45. 10.1016/j.vaccine.2021.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bartsch SM, O’Shea KJ, Wedlock PT, Ferguson MC, Siegmund SS, Lee BY. Potential clinical and economic value of norovirus vaccination in the community setting. Am J Prev Med [Internet] 2021;60:360–8. 10.1016/j.amepre.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ballard S-B, Tallant A, Guerra RG, Quigley D, Stiegmann R, Mirelman AJ, et al. Application of a cost-effectiveness analysis of pathogen-specific vaccines against gastroenteritis to a military population in a developing country setting. Vaccine [Internet] 2020;38:2292–7. 10.1016/j.vaccine.2020.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].PATH. Injectable rotavirus vaccines – what value could they bring? Vaccines Work [Internet]. 2021. Oct 28 [cited 2022 May 12]; [about 6 screens]. Available from: https://www.gavi.org/vaccineswork/injectable-rotavirus-vaccines-what-value-could-they-bring. [Google Scholar]
- [81].World Health Organization. From vaccine development to policy: a brief review of WHO vaccine-related activities and advisory processes (2017) 2017:11 p. [Google Scholar]
- [82].Neuzil KM, Bresee JS, de la Hoz F, Johansen K, Karron RA, Krishnan A, et al. WHO Preferred Produc Characteristics for Next-Generation Influenza Vaccines Advisory Group. Data and product needs for influenza immunization programs in low- and middle-income countries: rationale and main conclusions of the WHO preferred product characteristics for next-generation influenza vaccines. Vaccine [Internet] 2017;35. Available from: 10.1016/j.vaccine.2017.08.088. [DOI] [PubMed] [Google Scholar]
- [83].World Health Organization. Guidance for the development of evidence-based vaccination-related recommendations. Version 8 2017:55 p. [Google Scholar]
- [84].Dellepiane N, Wood D. Twenty-five years of the WHO vaccines prequalification programme (1987–2012): lessons learned and future perspectives. Vaccine [Internet] 2015;33:52–61. 10.1016/j.vaccine.2013.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].WHO Vaccine Prequalification Program [Internet]. Silver Spring (MD): U.S. Food & Drug Administration; [updated 2018 Mar 23; cited 2022 May 12]. Available from: https://www.fda.gov/vaccines-blood-biologics/who-engagements/who-vaccine-prequalification-program. [Google Scholar]
- [86].World Health Organization. WHO - Prequalification of Medical Products (IVDs, Medicines, Vaccines and Immunization Devices, Vector Control). [cited 2022 May 12]. Available from: https://extranet.who.int/pqweb/.
- [87].Buttery JP, Kirkwood C. Rotavirus vaccine implementation: evidence to fill the gap? Lancet Glob Health [Internet]. 2021;9:e885–6. Available from: Doi:10.1016/S2214-109X(21)00265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Vaxart. Oral. [Internet]. Vaxart Inc 2021. San Francisco (CA): Vaxart; [cited 2022 May 10]. Available from: https://vaxart.com/portfolio-item/oral/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.


