Abstract
More than a decade ago IL-1 blockade was suggested as an add-on therapy for the treatment of cancer. This proposal was based on the overall safety record of anti-IL-1 biologics and the anti-tumor properties of IL-1 blockade in animal models of cancer. Today, a new frontier in IL-1 activity regulation has developed with several orally active NLRP3 inhibitors currently in clinical trials including cancer. Despite an increasing body of evidence suggesting a role of NLRP3 and IL-1-mediated inflammation driving cancer initiation, immunosuppression, growth, and metastasis, NLRP3 activation in cancer remains controversial. In this review, we discuss the recent advances in the understanding of NLRP3 activation in cancer. Further, we discuss the current opportunities for NLRP3 inhibition in cancer intervention with novel small molecules.
Introduction
In 2010 we published a provocative review entitled “Why not treat human cancer with interleukin-1 blockade?” and suggested that clinical trials using IL-1 blockade should be considered since the safety and the clear benefits observed blocking IL-1 activity in several animal models of tumorigenesis and metastasis (Dinarello, 2010). More than 10 years later, this proposal based on preclinical data is now taking place in human studies. For example, the importance of IL-1 in tumor development has been recently confirmed in a randomized, placebo controlled clinical trial of over 10,000 subjects with atherosclerosis treated with a monoclonal antibody against IL-1β (Ridker, Everett, et al., 2017). A sub-analysis of patients demonstrated a lower incidence of lung cancer and a reduced cumulative incidence of fatal cancers in this population (Ridker, MacFadyen, et al., 2017). These and other data reveal the role of inflammatory cytokines such as IL-1β in cancer progression.
The past two decades also identified an important step forward in the understanding of IL-1 and IL-1 Family members biology with the characterization of the Nucleotide-binding domain and Leucine-rich Repeat (NLR) family, a sub-group of the Pattern Recognition Receptors (PRRs) family (Inohara, Chamaillard, McDonald, & Nunez, 2005). Several members of the NLR family assemble in multimolecular complexes termed inflammasomes. Inflammasomes are a major regulator of innate immune responses to both endogenous and exogenous stimuli mediating a potent inflammatory response. Specifically, NLRs convert the biologically inactive pro-IL-1β and pro-IL-18 into their active forms by the enzymatic activity of caspase-1 (Franchi, Eigenbrod, Munoz-Planillo, & Nunez, 2009). Although inflammasome function is critical for the host defense against infections, dysregulated NLRs activity leads to amplification of the inflammatory response and ultimately to tissue damage. In the context of tumorigenesis, several NLRs contribute to tumor progression by their ability to modulate the inflammatory response, creating a tumor permissive environment. Of the NLRs members, there is a particular interest on NLRP3. NLRP3 expression and activation is increased in several malignancies compared to the healthy matching tissue (Figure 1). This review summarizes the current knowledge on NLRP3 activation in cancer and the role of this NLR in cancer pathogenesis and progression.
Figure 1. NLRP3 expression profile in various cancers paired to normal tissues.
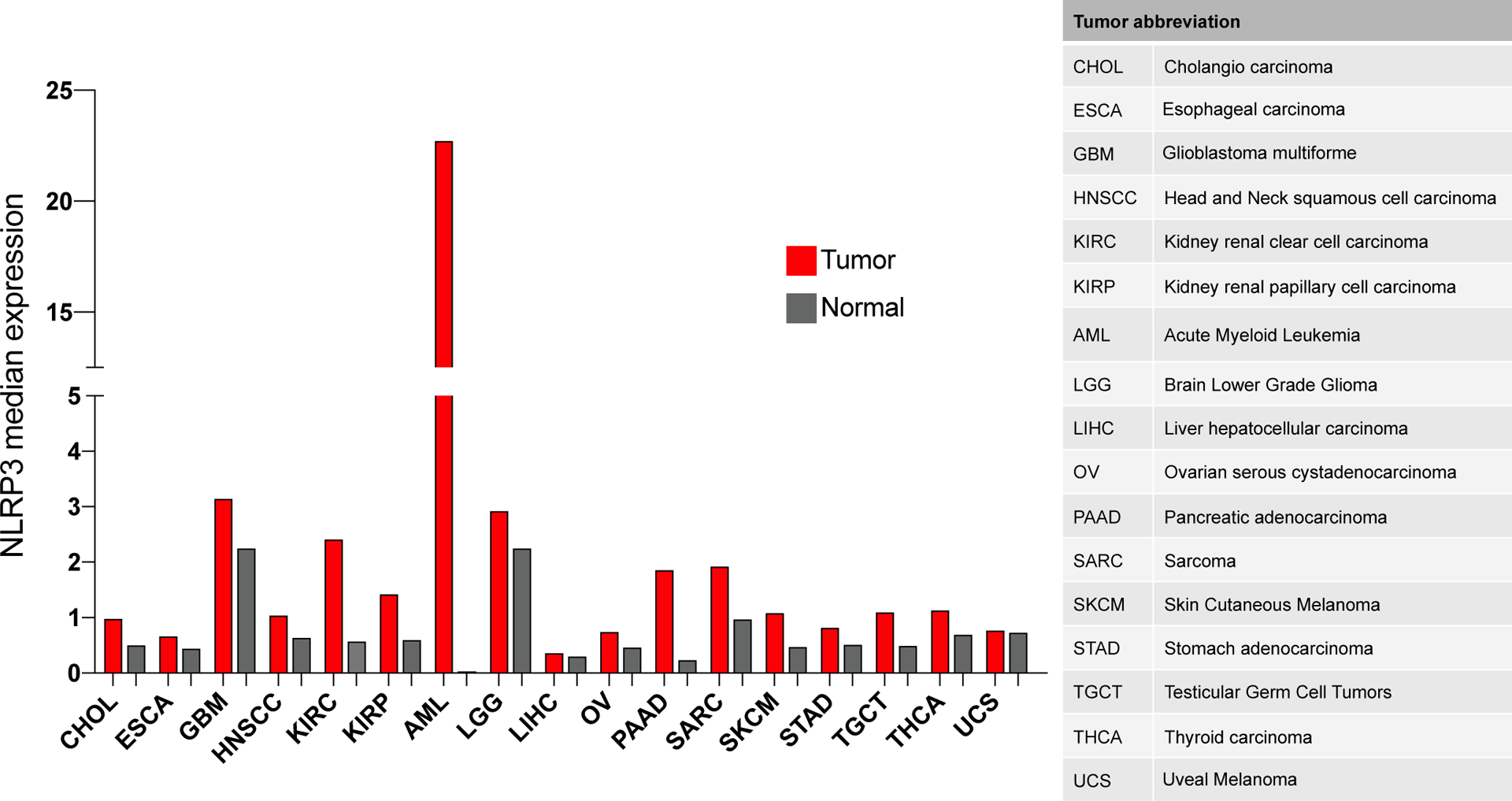
Data extrapolated from GEPIA (gene expression profiling interactive analysis) (Tang, et al., 2017).
NLRP3 activation and inflammasome formation
Unlike membrane bound Toll-like receptors (TLRs), NLRP3 and the other NLRs recognize Damage-Associated Molecular Patterns (DAMPs) and Pathogen-Associated Molecular Pattern molecules (PAMPs) in the host cytosol. Activation of NLRP3 results in the hydrolysis of ATP by the ATPase activity in the NACHT domain of NLRP3, which then triggers self-oligomerization of the protein. Following oligomerization, NLRP3 interacts with ASC (Apoptosis-associated Speck-like protein containing a CARD), resulting in the recruitment of procaspase-1 through CARD–CARD binding. The interaction between full length caspase-1 and NLRP3/ASC complexes induces the self-cleavage and activation of caspase-1 (p10 and p20), which mediates the conversion of the precursors of IL-1β and IL-18 into their biological active forms (Latz, Xiao, & Stutz, 2013). In addition to the processing of IL-1β and IL-18 by caspase-1, gasdermin D (GSDMD) is also processed by caspase-1. The resulting release of the N-terminal domain of GSDMD determines the formation of membrane pores that results in the release of IL-1β by a lytic form of cell death known as pyroptosis (He, et al., 2015). Figure 2 shows a schematic representation of NLRP3 activation and the subsequent NLRP3 inflammasome formation.
Figure 2. Schematic representation of the NLRP3 activation and the subsequent NLRP3 inflammasome formation.
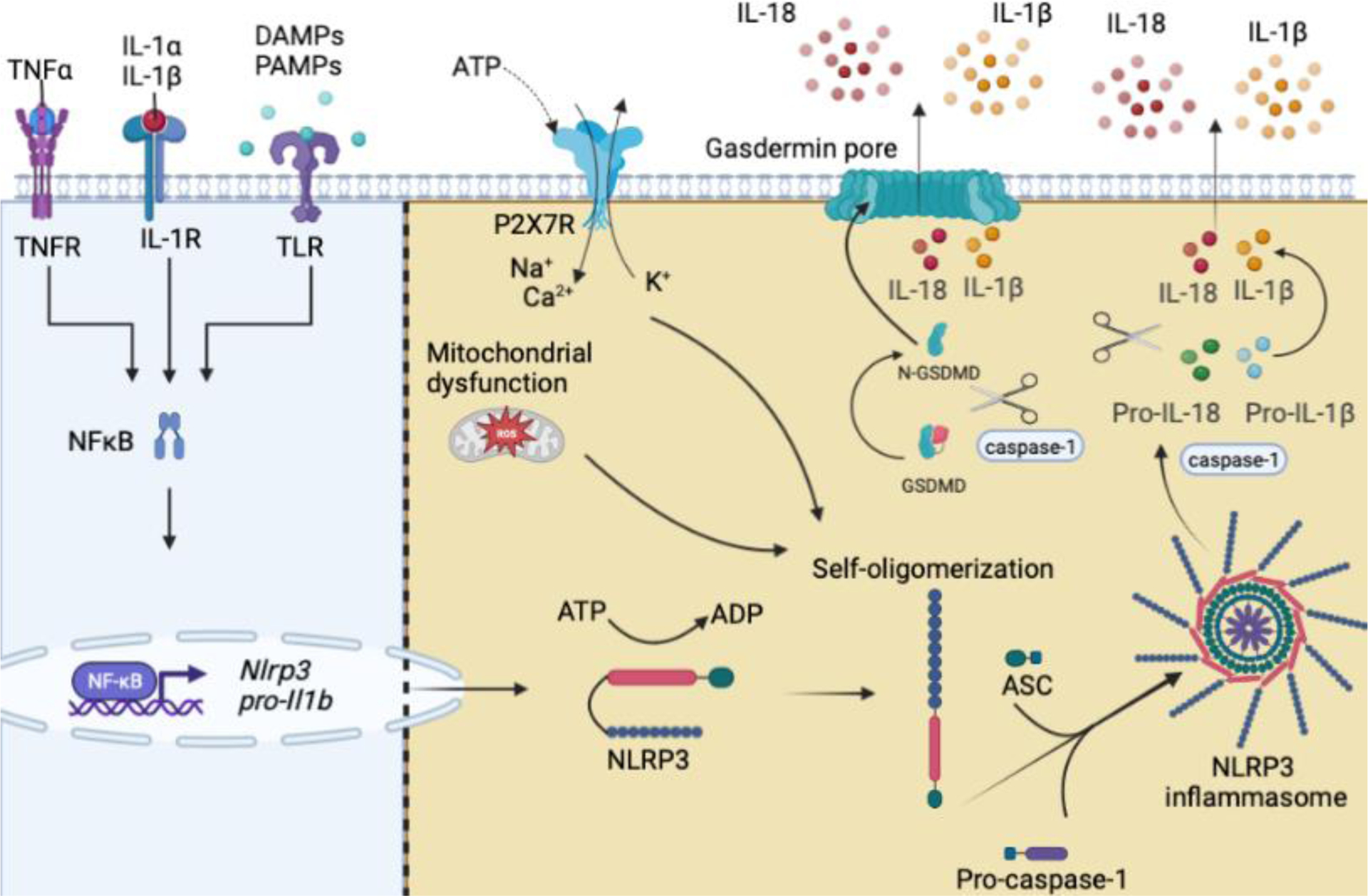
(Left) Engagement of TLRs by PAMPs and DAMPs or cytokine receptors by their respective cytokines (IL-1α, IL-1β, or TNFα) lead to upregulation of NFκB-mediated gene expression of inflammasome components, termed priming. (Right) Activation occurs through PAMPs and DAMPs that activate upstream signals resulting in NLRP3 self-oligomerization (in text). NLRP3 then interacts with ASC, pro-caspase-1 is recruited resulting in caspase-1 activation, activated caspase-1 then cleaves pro-IL-1β and pro-IL-18. Biologically active IL-1β and IL-18 are then secreted into the extracellular space. Abbreviations: interleukin-1 alpha (IL-1α), interleukin-1 beta (IL-1β), interleukin 1 receptor 1 (IL-1R1), tumor necrosis factor α (TNFα), tumor necrosis factor receptor (TNFR), toll-like receptor (TLR), damage-associated molecular patterns (DAMPs), pathogen-associated molecular patterns (PAMPS), ATP (adenosine triphosphate), ADP (adenosine diphosphate), GSDMD (gasdermin D), purinergic receptor P2X7 (P2X7R), nuclear factor-κB (NFκB), reactive oxygen species (ROS), apoptosis-associated speck-like protein containing a CARD (ASC).
NLRP3 is activated by a wide array of signals ranging from virus and bacterial infections, extracellular ATP, mitochondria damage/dysfunction, metabolic alarmin, misfolded protein, amyloid deposition, response to therapy and more; suggesting that NLRP3 is a key sensor for cellular stress and a possible guardian of the cellular homoeostasis. The resting cellular level of NLRP3 is generally low, and to reach the critical threshold to induce caspase-1 activation NLRP3 relies on the so called “priming” in order to upregulate NLRP3 expression. Notably, NLRP3 levels are elevated in several tumors and there are examples of tumor cells that are able to specifically activate NLRP3 activation in non-tumor cells. Some of these mechanisms are described in more details in this review but overall suggest that NLRP3 in the tumor microenvironment (TME) does not only function as an intracellular surveillant for danger signals but it may be exploited by tumor cells to provide a growth advantage and/or immune escape mechanism by regulating the inflammatory response.
NLRP3 activation in tumor progression
Inflammation has long been understood as the natural response of the immune system against tumors, a process designed to limit tumor progression. However, growing evidence in the last decades substantiates that inflammation is involved in mechanisms associated with tumorigenesis and poor clinical prognosis. IL-1β and its regulation via NLRP3 activation represents a classic example. In pancreatic ductal adenocarcinoma (PDAC), which represents over 90% of all pancreatic cancers, NLRP3 expression is elevated in cancer tissue compared to the adjacent normal tissue and the NLRP3 inflammasome signature was associated with poor overall survival in patients with PDAC (L. Zheng & Liu, 2022). In mouse models of PDAC, genetic deletion and pharmacological inhibition of NLRP3 with the small molecules OLT1177® and MNS, resulted in reduced tumor growth and increased survival (Amo-Aparicio, Daly, Hojen, & Dinarello, 2023; Daley, et al., 2017; H. Liu, Xu, Liang, & Liu, 2020). Mechanistically, it was shown that lack of or inhibition of NLRP3 enhances a Th1 immune response resulting in increased activation of cytotoxic T cells (Amo-Aparicio, et al., 2023; Daley, et al., 2017; H. Liu, et al., 2020). Recent observations also demonstrated NLRP3 activation in tumor-associated macrophages (TAMs), enhancing lung metastasis in pancreatic cancer models (Gu, et al., 2022). Constitutive expression of NLRP3 and spontaneous IL-1β release has been reported in human melanoma cells lines (Okamoto, et al., 2010; Tengesdal, Menon, et al., 2021). Genetic and pharmaceutical inhibition of NLRP3 reduced melanoma IL-1β release. The relevance of tumor-derived NLRP3 activation in melanoma was confirmed in vivo using B16F10 cells. NLRP3 deficient B16F10 cells exhibited reduced tumor progression and immunosuppression in an immunocompetent mouse model compared to the growth of native B16F10 cells (Tengesdal, Menon, et al., 2021). The role of NLRP3 in melanoma progression was further validated using two different NLRP3 inhibitors, OLT1177® and MCC950, in which both inhibitors reduced tumor growth and increased survival compared to the control mice (Tengesdal, Menon, et al., 2021; Theivanthiran, et al., 2020). Furthermore, in a recent study, it was demonstrated that melanoma NLRP3-dependent IL-1β production increases the immunosuppressive properties of myeloid-derived suppressor cells (MDSCs) via IL-6/STAT3 activation in MDSCs, which ultimately lead to T cell suppression and tumor growth (Tengesdal, Dinarello, et al., 2021).
In humans with breast cancer, IL-1β production in primary breast tumor biopsies correlated with disease severity (T. C. Wu, et al., 2018). In NLRP3 deficient mice, growth of orthotopically implanted E0771 breast cancer was significantly reduced (Tengesdal, et al., 2022). Consistently, mice depleted of NLRP3 or caspase-1 suppressed induction of IL-1β production in tumors (Guo, Fu, Zhang, Liu, & Li, 2016).
Increased expression of NLRP3 and IL-1β was also found in head and neck squamous cell carcinoma (HNSCC). Inhibition of NLRP3 with MCC950 reduced tumor growth and increases effector T cell infiltration in a mouse model of HNSCC (L. Chen, et al., 2018).
Increased NLRP3 and IL-1β production have been reported also in hematologic cancers. Patients with lymphoma and acute myeloid leukemia (AML) have significantly increased expression of NLRP3 (Hamarsheh, et al., 2020; Jia, et al., 2017; Zhao, et al., 2017). Notably, based on the public database GEPIA, AML displays the highest NLRP3 expression compared to matching non-tumor tissue (757.3 fold increase; Figure 1), suggesting a role for NLRP3 in this hematologic malignancy. Consistently, Zhong et al observed that NLRP3 activation in AML cells promotes proliferation while suppressing cell death mechanisms. In mice, the authors showed that NLRP3 upregulation increased leukemia burden in bone marrow, spleen and liver leading to reduced survival. Following genetic deletion of NLRP3, leukemia progression was inhibited (Zhong, et al., 2021).
Overall, these findings suggest a pathological role for NLRP3 in several cancers (Figure 3, Table 1). This is mediated by tumor intrinsic NLRP3 activation as observed in melanoma or by activation of the inflammasome in neighboring or infiltrating cells as demonstrated in breast and pancreatic cancer. Although the exact mechanisms of NLRP3 in tumor-promotion are not yet fully defined, it appears that, given its central role in modulating the inflammatory response, NLRP3 activation is able to suppress anti-tumor immunity generating a tumor permissive environment that promotes tumor progression and metastasis.
Figure 3. Dual role of NLRP3 in cancer.
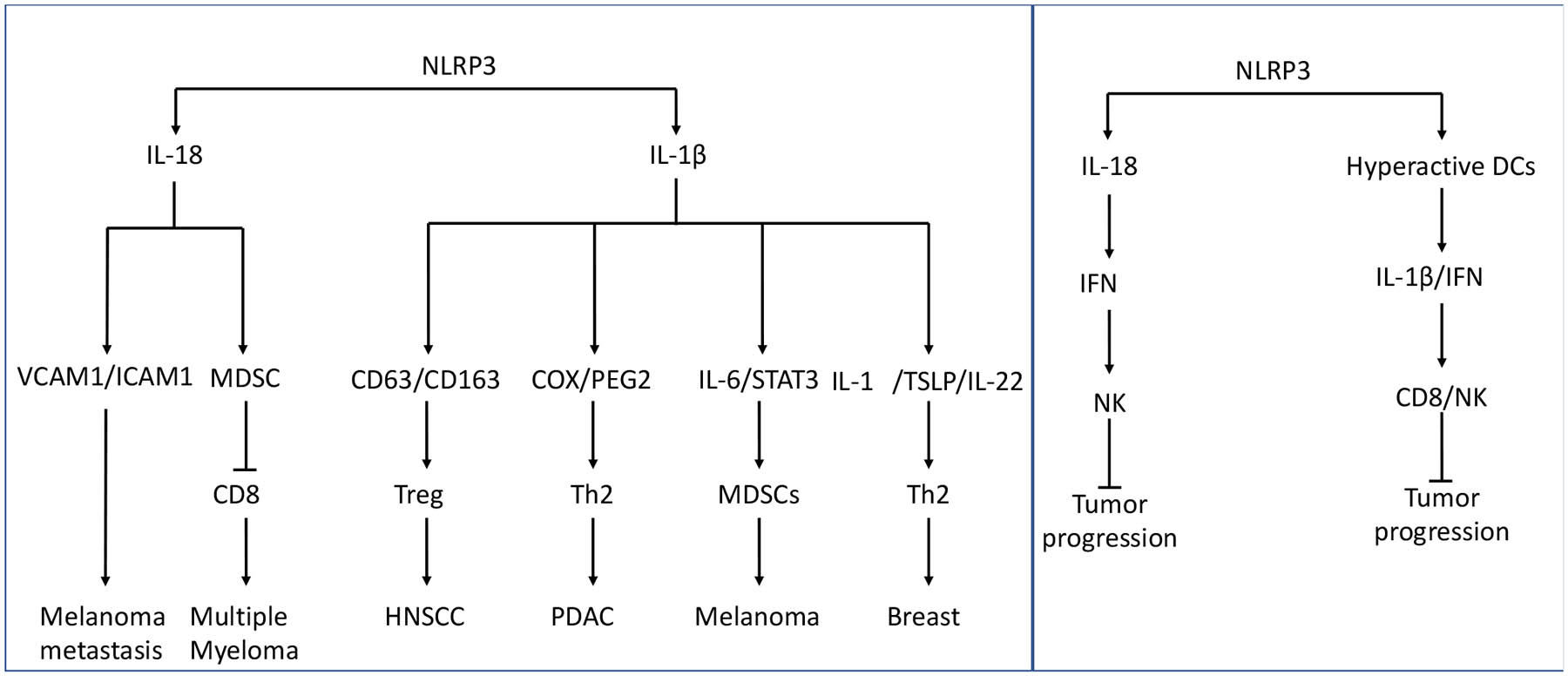
Schematization of pro-tumor (left) and anti-tumor (right) functions of NLRP3 in tumor.
Table 1.
NLRP3 in tumor progression and tumor suppression.
| Cancer | Expression of inflammasome components / activation | Immune response effect / outcome | Ref. | |
|---|---|---|---|---|
| PDAC | ↑ NLRP3* in tumor | Inhibition of NLRP3 ↑ cytotoxic T cell response | Amo-Aparicio 2023 et al., Zheng and Liu, 2022, Daley et al., 2017; Liu et al. | |
| ↑ NLRP3* in TAMs | ↑ Lung metastasis | Gu et al., 2022 | ||
| Melanoma | Constitutive ↑ NLRP3* and IL-1β release in melanoma cells | ↑ MDSC and ↓ CD8+ T and NK cell activity | Okamoto et al., 2010; Tengesdal, 2021 | |
| Pro-tumor | Breast | ↑ NLRP3* and IL-1β in tumor infiltrating myeloid cells | ↑ MDSC and ↓ CD8+ T cell activity | Wu et al., 2018a, Tengesdal et al., 2022 |
| HNSCC | ↑ NLRP3* and IL-1β in tumor | ↓ Effector T cell tumor infiltration | Chen et al., 2018 | |
| AML | ↑ NLRP3 and IL-1β | ↑ Myeloproliferation and chemotherapy resistance ↓Th1 cells |
Hamarsheh et al., 2020; Jia et al., 2017; Zhong et al., 2021 | |
| Lymphoma | ↑ IL-18 | ↑ Proliferation | Zhao et al., 2017 | |
| CRC | NLRP3 SNP (gain-of-function) | Associated with poor prognosis | Cambui, et al., 2020 | |
| HCC | ND | Inhibition of NLRP3 reduces tumor volume and metastasis | Chen et al., 2020a; Fan et al., 2014 | |
| CRC | ↑ NLRP3* | Suppresses metastasis | Dupaul-Chicoine et al., 2015 | |
| Anti-tumor | CLL | ↑ NLRP3* in vitro | Inhibition of cell proliferation and induces apoptosis | Salaro et al., 2016 |
| HCC | ↓ NLRP3* with advanced stages | NLRP3 agonist induced pyroptosis in HCC cells | Wei et al., 2015; Wei et al., 2019b | |
| Lung adenocarcinoma | ↑ NLRP3 | Reduced tumor growth, increased T cell activation and survival | Barsoumian et al., 2023 |
Arrows indicate ↑ (increase) and ↓ (decrease)
Indicates NLRP3 activation was demonstrated
ND Indicates mechanism not described
NLRP3 activation in tumor suppression
In colorectal cancer (CRC), NLRP3 activation suppressed metastatic growth functioning as a negative regulator of tumorigenesis by eliciting immune system activation (Dupaul-Chicoine, et al., 2015). Allen et al, observed that mice deficient in NLRP3 and treated with dextran sulfate sodium (DSS) and azoxymethane resulted in increased ulceration of the colon with fibrinogen deposition when compared to the control group. At 9 weeks, NLRP3-deficient mice showed significantly greater numbers and increased size of polyps compared to the wild type mice (Allen, et al., 2010). In the same study, NLRP3-deficient mice exhibited reduced inflammation as well as slower disease progression compared to ASC- or caspase-1 deficient mice. Thus, it is possible that other NLRs have a role in the initiation and progression of CRC. In contrast with this study, Hu et al showed, in a similar chemical CRC-induced model, that lack of NLRP3 was not associated with increased tumorigenesis when compared to the wild type mice (B. Hu, et al., 2010). Further, Bauer et al showed that deficiency in NLRP3 resulted in a less severe colitis following DSS in mice (Bauer, et al., 2010). Additionally, in a study with 215 CRC patients, the NLRP3 gain-of-function variant Gln705Lys (rs35829419) resulted in increased inflammation, which was associated with poor prognosis, suggesting a detrimental role for NLRP3 in CRC (Cambui, et al., 2020).
In blood lymphocytes from patients with chronic lymphocytic leukemia (CLL), NLRP3 expression is significantly reduced compared to blood lymphocytes from healthy donors (Salaro, et al., 2016). In vitro, NLRP3 overexpression inhibited cell proliferation and stimulated apoptosis, suggesting NLRP3 functions as a negative regulator for CLL progression (Salaro, et al., 2016).
In hepatocellular carcinoma (HCC), low NLRP3 expression is associated with advanced stages and poor pathological differentiation (Wei, et al., 2014). Further, in cultured HCC cells, induced expression of NLRP3 and inflammasome components as well as pharmacological NLRP3 activation, reduced HCC proliferation, induced pyroptosis and reduced the migratory ability of HCC cells (Wei, et al., 2015; Wei, Zhu, Zhu, Zhao, & Li, 2019). Although this study suggests a protective function for NLRP3 in HCC, other studies report that pharmacological inhibition of NLRP3 reduces tumor volume and metastatic potential in vitro and in vivo (W. Chen, Wei, Chen, Yang, & Wu, 2020; Fan, et al., 2014). Interestingly, it was recently reported that NLRP3 activation with a specific agonist (name and structure of the molecule not available), in combination with radiotherapy and immunotherapy (anti-PD-1), increased survival and reduced tumor progression in murine models of lung adenocarcinoma (Barsoumian, et al., 2023). Specifically, the author showed that intratumoral NLRP3 activation not only controlled growth of primary tumors but also showed abscopal responses in secondary and non-irradiated tumors. The authors concluded that NLRP3 activation in these models was able to increase antigen presentation, innate function, and T cell priming (Barsoumian, et al., 2023).
The seemingly contradictory function of NLRP3 in tumors suggests to be context (human vs mouse) and tissue-specific. This may be the result of an unexpected influence of NLRP3 activation on immune regulator markers like PD-1 and PD-L1 in the tumor or immune cells or an IL-18-mediated antitumor activity. The tissues specificity underlying the cancers where NLRP3 expression and/or activation is associated with positive outcomes represents an area in need of further research. Such studies should utilize singe-cell and spatial analysis to determine the cellular source of NLRP3 and the effect of its activation on the tumor microenvironment to shed light on what remains a conflicting picture. Figure 3 and Table 1 highlight some of the mechanisms associated with anti-and pro-tumor activity of NLRP3.
Mechanism of NLRP3 activation in cancer
Within the TME, endogenous and exogenous factors such as hypoxia, cellular stress, cellular damage, tumor and stroma cytokine secretion and infections determine activation of NLRP3 and influence tumor initiation and progression. In this section we review the main events that contribute to NLRP3 activation in cancer (Table 2).
Table 2:
Mechanisms for NLRP3 activation in tumor microenvironment.
| NLRP3 Trigger | Cancer(s) | Proposed mechanism(s) | Expression of inflammaso me components / activation | Immune response effect / outcome | Ref. |
|---|---|---|---|---|---|
| HIF1α | Lung, prostate, bladder, TNBC | HIF1α activates RIPK3 mediated phosphorylation of MLKL. NLRP3* and IL-1β release in macrophages. | ↑ NLRP3* and IL-1β in cancer cells. | Reduced tumor cell invasiveness, suppressed Warburg metabolism | Chen et al., 2020b; Huang et al., 2019; Panchanath an et al., 2016; Yang et al., 2023 |
| Mitochondrial dysfunction | AML | KrasG12D induced ROS | ↑ NLRP3* | ↑ Myeloproliferation | Hamarsheh et al., 2020 |
| GC | H. pylori induced ROS | ↑ NLRP3* and IL-1β | ↑ Lymph node metastasis and poor survival | Zhang et al., 2022; Wang et al., 2022). | |
| TNBC | ROS-mediated NLRP3 priming/activation | ↑ NLRP3*, IL-1β and caspase-1 | Promotes EMT | Si et al., 2020 | |
| ATP | Breast, HNSCC | ATP mediated K+ efflux through P2X7R in fibroblasts and cancer cells | ↑ NLRP3* and IL-1β | Tumor progression | Ershaid et al., 2019; Bae et al., 2017 |
| Microbiome | CRC, GC, PDAC | Priming of inflammasome components through NF-κB. Increased PD- L1 expression. | ↑ NLRP3 and IL-1β | ↑ Metastasis and immunosuppression | Wu et al., 2021; Li et al., 2018; Yin et al., 2021 |
| NLRP3 SNPs | PDAC, CRC, GC, bladder, melanoma | Gain-of-function NLRP3 variants | ↑ NLRP3*, IL-1β, IL-18 and caspase-1 | ↑ Risk of tumor development and disease severity | Miskiewicz et al., 2015; Cambui et al., 2020; Castano-Rodriguez et al., 2014; Xu et al., 2021; Verma et al., 2012a |
| Virus | PTLD, CC, OSCC | Hyperglycemia activates NLRP3, activating dormant EBV. HPV-mediated chronic inflammation | ↑ NLRP3*, IL-1β and caspase-1 | ↑ Chronic inflammation and tumor development | Burton et al., 2020; Fernandes et al., 2020; Scuderi et al., 2021 |
| Environmental | Mesothelioma, melanoma | Silica-induced lysosomal damage. UV-mediated DNA damage. | ↑ NLRP3* and IL-1β | ↑ Risk of tumor development | Hornung et al., 2008; Shukla et al., 2012; Hasegawa et al., 2016 |
| CHIP | Lung, prostate, breast, MDS | Somatic mutation-driven aberrant NLRP3 activation | ↑ NLRP3 and IL-1β, IL-18 and caspase-1 | ↑ Risk of tumor development and disease severity | Kessler et al., 2022; Tian et al., 2023; McLemore et al., 2022 |
Arrows indicate ↑ (increase) and ↓ (decrease)
Indicates NLRP3 activation was demonstrated
Hypoxia
As tumors grow, insufficient blood supply leads to hypoxia, thereby activating hypoxia-inducible factors (HIFs). HIFs are master transcription regulators and are implicated in mechanisms of cancer progression such as; angiogenesis, metabolic alterations, metastasis, and immunosuppression (reviewed in (Wicks & Semenza, 2022)). Over the last ten years NLRP3 has proven to be a causal factor in these same protumor mechanisms (reviewed in (Sharma & Kanneganti, 2021)). While the interplay of HIFs and NLRP3 in the setting of cancer is not fully understood, existing studies largely support the role of HIF1α as an activator of NLRP3. In macrophages, inflammasome activation due to ischemia is attributed to activation of receptor-interacting protein kinase 3 (RIPK3), which in turn phosphorylates mixed lineage kinase domain like protein (MLKL). Phosphorylated MLKL leads to disruption of the plasma membrane, resulting in NLRP3 activation and IL-1β secretion (Conos, et al., 2017; Gutierrez, et al., 2017). Additionally, Ouyang et al demonstrated cellular uptake of adenosine via the A2A receptor in macrophages results in downstream activation of NLRP3 in a HIF1α-dependent manner (Ouyang, et al., 2013). This is particularly relevant in line with reports of elevated adenosine in tumors compared to normal tissues (Blay, White, & Hoskin, 1997; Ohta, et al., 2006). In cancer cell specific studies, hypoxia-mediated HIF1α increases NLRP3 expression and activation in lung adenocarcinoma cells (A549), prostate cancer cells (PC-3) and bladder cancer cells (T24) (Y.-R. Chen, et al., 2020; J. J. Huang, Xia, Huang, & Li, 2019; Panchanathan, Liu, & Choubey, 2016). Additionally, suppression of HIF1α in triple-negative breast cancer cells inhibited NLRP3 activation, reduced invasiveness and suppressed Warburg metabolism (H. L. Yang, et al., 2023). Consistently, expression of HIF1α correlates with IL-1β in triple-negative breast cancer patients (Lappano, et al., 2020). Taken together these studies suggest that hypoxia induced stabilization of HIF1α contributes to NLRP3 inflammasome activation and subsequent IL-1β release from both host and tumor cells in the TME.
Mitochondria dysfunction
The classic tumor promoting characteristics of the TME (i.e. hypoxia, oncogene expression, loss of tumor suppressors and others) each contribute to mitochondrial dysfunction and generation of reactive oxygen species (ROS) in cancer cells (reviewed in (Reczek & Chandel, 2017)). Mitochondrial ROS is one of the earliest described ‘signal 2’ activators of the NLRP3 inflammasome (Sorbara & Girardin, 2011; R. Zhou, Yazdi, Menu, & Tschopp, 2011). Moreover, in both a murine model and patient samples Hamarsheh et al showed downstream ROS production after KrasG12D-RAC1 activation in myeloid leukemia activated NLRP3. The authors also reported that treatment with the NLRP3 inhibitor, MCC950, both myeloproliferation and cytopenia were reversed (Hamarsheh, et al., 2020).
Chronic Helicobacter pylori (H. pylori) infection is highly associated with development of gastric cancer (GC), offering additional evidence ROS-mediated activation of NLRP3 facilitates tumorigenesis in GC (F. Wang, Meng, Wang, & Qiao, 2014). For example, the H. pylori protein CagA increases ROS production, resulting in the activation of NLRP3 (Zhang, et al., 2022). Furthermore, NLRP3 expression correlates with lymph node metastasis and poor survival in GC (F. Wang, et al., 2014). In human MDA-MB-231 triple-negative breast cancer cells, ROS increases expression of NLRP3, caspase-1, and IL-1β (Si, et al., 2020). In the same study, inhibition of ROS decreased expression of inflammasome proteins, epithelial-mesenchymal transition (EMT) markers as well as cell migration, indicating ROS-mediated NLRP3 activation drives breast cancer cell invasiveness (Si, et al., 2020). Thus, both environmental and cancer cell-intrinsic factors result in mitochondrial dysfunction, accumulation of ROS and subsequent activation of NLRP3.
ATP
The TME is characterized by elevated cellular death due to high cell proliferation and the lack of blood carrying oxygen and nutrients for the cells. As a consequence, dying cells release ATP containing intracellular content into the TME. In non-malignant tissues, extracellular ATP (eATP) concentrations are quite low whereas intracellular ATP is high; however, in the TME eATP levels are high and activate P2X7 receptorsP2X7R (Allard, Longhi, Robson, & Stagg, 2017; Di Virgilio & Adinolfi, 2017). eATP is a potent DAMP and acts as a canonical ‘signal 2’ in NLRP3 activation through P2X7R-mediated potassium (K+) efflux (D. Ferrari, et al., 2006; Idzko, Ferrari, & Eltzschig, 2014). Ershaid et al reported that mammary gland fibroblasts stimulated with ATP activated NLRP3, thereby amplifying IL-1β signaling in the TME promoting breast cancer progression (Ershaid, et al., 2019). In a pivotal cancer study, Gilbert et al showed that prolonged ATP exposure leads to increased expression of non-pore functional P2X7R which is widely expressed in human cancers (Gilbert, et al., 2019). This effect was also reported in head and neck cancers, Bae et al demonstrated P2X7R as a regulator of NLRP3 activation in the head and neck cancer cell line A253 (Bae, et al., 2017). Therefore, increases in eATP offer continuous P2X7R-mediated NLRP3 activation.
Microbiome
Through its action on TLR4, the Gram-negative bacterial cell wall lipopolysaccharide (LPS) has long been established as the canonical priming mechanism for the NLRP3 inflammasome by promoting NF-κB signaling. In the intestinal mucosa, for example, LPS is present in higher concentrations in colorectal cancer lesions due to leaky barrier compared to healthy colonic tissue. Furthermore, LPS in the tumor activates NLRP3 and promote metastasis in colorectal cancer (X. Wu, et al., 2021). Chronic infection with the Gram-negative bacterium H. pylori is positively correlated with development of GC and induces PD-L1 expression in GC cells (Li, et al., 2018). In PDAC, Yin et al demonstrated gut-derived LPS induced PD-L1 expression on the human PDAC cell line, PANC-1. In the same study, the NLRP3 inhibitor BAY11-7802 reduced PD-L1 expression, a finding consistent with other reports of NLRP3-mediated PD-L1 expression (Yin, et al., 2021). Further evidence of chronic infection with LPS containing bacteria driving tumorigenesis takes place in lung cancer. Lung cancer risk is elevated in patients with chronic obstructive pulmonary disorder, a disease associated with chronic infection of the airways (Desai, et al., 2014). Liu et al, showed that chronic exposure to Pseudomonas aeruginosa-derived LPS promotes immunosuppressive genes in the TME, thereby facilitating tumor progression and resistance to immunotherapy (C. H. Liu, et al., 2021). Although, inflammasome activation was not measured directly in this study, cytokines directly induced by IL-1β were all upregulated (IL-17/KC(IL-8)/G-CSF) (Broudy, Kaushansky, Harlan, & Adamson, 1987; Kaplanski, et al., 1994; Sutton, Brereton, Keogh, Mills, & Lavelle, 2006). Thus, a picture emerges of chronic exposure to LPS creating a tumor-receptive environment through NLRP3 mediated mechanisms.
Cytokines
Cytokines regulate growth, cell trafficking, signaling, and differentiation of both stromal as well as tumor cells. Produced by cancer cells, some cytokines create optimal growth conditions within the TME, whereas cytokines secreted by stromal cells may influence the behavior of malignant cells. Depending on the TME, cytokines can modulate anti-tumoral responses, but during chronic inflammation they can also create a permissive environment for tumor progression and tissue invasion. In this section, we focus on cytokines that directly activate NLRP3 or act downstream of NLRP3 activation to affect tumor progression.
IL-1α
In the Interleukin Family of cytokines, IL-1α is unique. The primary transcript of the IL-1α precursor is constitutive in healthy as well malignant cells and the protein requires no processing into an active form to initiate an inflammatory response (B. Kim, et al., 2013; Suwara, et al., 2014). In an inflammatory milieu, IL-1α-containing cells, particularly epithelial cells in tumors, undergo a necrotic cell death, mostly due to rapid tumor growth without sufficient vascular support, releasing IL-1α into the extracellular space.
Regardless of signaling by either the IL-1α precursor or mature form, the release of IL-1α in the TME allows this cytokine to bind to IL-1R1 expressed on several immune and tumor cells and the activation of NF-κB. In addition to IL-1α mediated NF-κB gene expression, IL-1α triggers NLRP3 expression and activation in TAMs. The priming effect of IL-1α on NLRP3 was confirmed by a recent study where following stimulation with IL-1α, the human metastatic melanoma line 1205Lu secreted 2-fold more IL-1β in a NLRP3-dependent fashion compared to unstimulated 1205Lu cells (Tengesdal, Dinarello, et al., 2021). A recent study demonstrated that the IL-1α precursor binds to mitochondrial cardiolipin, which increases NLRP3, suggesting that IL-1α can induce NLRP3 activation by several mechanisms (Dagvadorj, et al., 2021).
In a placebo-controlled clinical study, in metastatic colorectal cancer, anti-IL-1α therapy was administered over four weeks (Hickish, et al., 2017). The findings included improved survival and reduction in markers of systemic inflammation including IL-6 and platelets (Hickish, et al., 2017). Although NLRP3 expression was not assessed in this study, as mentioned above, IL-1α primes NLRP3. Therefore, it cannot be excluded that some of the beneficial effects observed with anti-IL-1α treatment may include NLRP3 inhibition.
IL-1β
IL-1β is the prominent downstream pro-inflammatory cytokine following activation of NLRP3 in myeloid cells. In mice, IL-1β signaling leads to immunosuppression, angiogenesis and metastasis; moreover, tumors in mice treated with neutralizing antibodies to IL-1β fail to grow. More than 10,000 publications have investigated the role of this cytokine in nearly all known cancer models. Based on mouse cancer models, blocking IL-1 as a treatment for human cancer was proposed in 2010 (Dinarello, 2010). Considering the role of NLRP3 in the regulation of IL-1β activity, inhibition of NLRP3 in cancer model gained a significant interest in the past years 10 years. The important evidence for the role of NLRP3/IL-1β in cancer came from CANTOS study, a placebo-controlled and randomized world-wide study in 10,067 patients with atherosclerosis in order to prevent a second cardiovascular event (Ridker, Everett, et al., 2017). Patients with no known cancer were enrolled to receive anti-IL-1β (canakinumab) or placebo. Over the course of 4 years, a 67% reduction in the incidence of lung cancer was observed in patients treated with anti-IL-1β compared with those in the placebo cohort (p<0.0001) (Ridker, MacFadyen, et al., 2017). Furthermore, in the group treated with high dose anti-IL-1β, total fatal cancer mortality was significantly reduced compared with the placebo-treated group (p<0.0007). The reduction in lung cancer deaths was 77% (Ridker, MacFadyen, et al., 2017). Based on these data above, two follow-up trials in patients with local or metastatic lung cancer were treated with canakinumab in addition to radiation and/or chemotherapy. The first two trials (CANOPY-1 and 2) have failed to meet their primary efficacy endpoints (Lythgoe & Prasad, 2022).
Looking back, the benefit of canakinumab in preventing lung cancer was due to early treatment, suggesting the efficacy of blocking IL-1β in early stages of carcinogenesis.
IL-1 Receptor antagonist.
The IL-1 Receptor antagonist (IL-1Ra) is a naturally occurring cytokine in the IL-1 Family; anakinra is the recombinant form of IL-1Ra. IL-1Ra binds to IL-1R1 but unlike IL-1α or IL-1β, the binding of IL-1Ra to IL-1R1 does not result in signaling. Anakinra is used to arrest inflammation due to IL-1β or IL-1α. Although anakinra is approved for rheumatoid arthritis, systemic onset juvenile arthritis and recently for COVID-19, off-label anakinra is used broadly to treat IL-1-mediated diseases.
Anakinra has been used in several mouse models of cancer. In tumor models of B16 melanoma, IL-1Ra showed to be effective in reducing tumor growth (Lavi, Voronov, Dinarello, Apte, & Cohen, 2007; McKenzie, Oran, Dinarello, & Sauder, 1996; Vidal-Vanaclocha, et al., 1996; Vidal-Vanaclocha, Amezaga, Asumendi, Kaplanski, & Dinarello, 1994). In PDAC, IL-1Ra extended survival by reducing the IL-6-STAT3 axis (Dosch, et al., 2021). IL-1Ra also reduces NF-κB activation in PDAC-derived cells in vitro; tumor growth in mice treated with IL-1Ra alone or in combination with gemcitabine also reduced tumor growth (Zhuang, et al., 2016). In models of mouse breast cancer, anakinra significantly reduced the number of bone metastases compared to vehicle treated mice (Holen, et al., 2016). In addition, angiogenesis was also reduced by anakinra (Holen, et al., 2016). In humans, anakinra administered to women with HER2-negative metastatic breast cancer reduced the number of metastases. Inflammation in a humanized mouse model of breast cancer reveals a role for tumor-infiltrating CD11c+ myeloid cells; anakinra treatment prevented progression (T. C. Wu, et al., 2018). In the same study, analysis of peripheral blood leukocytes of patients with HER2-negative breast cancer revealed an IL-1β transcriptional signature, which was decreased with anakinra treatment (T. C. Wu, et al., 2018). A commentary of that study concluded that IL-1β-mediated inflammation in breast cancer is best treated with anakinra since blocking IL-1R1 prevents the inflammatory signal of either IL-1α or IL-1β (Dinarello, 2018). Relevant to NLRP3, it has been shown that IL-1Ra decreases NLRP3 expression, limiting the inflammasome-dependent inflammation (Iannitti, et al., 2016; Pariano, et al., 2021).
IL-18 and IL-18 Binding Protein
IL-18 is closely related to IL-1β in that the IL-18 precursor requires caspase-1 cleavage for conversion to active IL-18. The pro-inflammatory properties of IL-18 have been reviewed (Kaplanski, 2018). Historically, IL-18 was termed IFNγ-inducing factor (S. Nakamura, et al., 2000). Since IL-18 is a major inducer of IFNγ and IFNγ is a well-known activator of natural killer cells (NK cells), studies have focused on IL-18-induced NK tumor-killing mechanisms. Similar to IL-1, IL-18 also has a natural occurring inhibitor, the IL-18 Binding Protein (IL-18BP). The affinity of IL-18BP ranges from 0.5 nM to 0.04 nM (Novick, et al., 1999). Because of the unusual high binding of IL-18 to IL-18BP, most circulating IL-18 is bound to IL-18BP (Novick, et al., 2001).
Processed active IL-18 has both pro-tumor as well as an anti-tumor properties. As a pro-tumor cytokine, IL-18 induces endothelial adhesion molecules, for example VCAM and ICAM, which increase tumor spread and metastases (Vidal-Vanaclocha, et al., 1996). Blocking IL-18 in tumor models is accomplished using antibodies to IL-18, antibodies to the IL-18 receptor ligand bind chain or IL-18BP. Of these therapeutic strategies, IL-18BP would be the most effective because of its high affinity for IL-18 (Novick, et al., 1999). There are several mouse tumor models blocking IL-18. In metastatic B16 melanoma, adherence to liver endothelium was prevented by inhibition of caspase-1, anti-IL-18 or IL-18BP (Vidal-Vanaclocha, et al., 2000). Deficiency of IL-18 in a mouse model of multiple myeloma, protected mice from disease progression reducing MDSCs expansion (K. Nakamura, et al., 2018). In pancreatic cancer models, it was shown that activation of the IL-18 receptor in T cells associated with reduced migration, cell exhaustion and reduced production of effector T cell cytokines (Lutz, et al., 2023; Nasiri, et al., 2023; Stromnes, 2023). Interestingly, it was also showed that lack of NLRP3 resulted in similar T cells exhaustion suggesting a direct correlation between NLRP3 activation, IL-18 production, IL-18 receptor engagement and CD8 T cell function (Lutz, et al., 2023).
IL-18 also acts as an anti-tumor cytokine. Using replication-competent adenovirus expressing IL-18, vascular endothelial growth factor expression and lung metastasis were significantly reduced (C. Yang, et al., 2016). Since IL-18 induces IFNγ, IL-18 augmented IFNγ secretion promotes proliferation of cytotoxic T cells with increased anti-tumor activities (Figure 3). Human IL-18-expressing CD19 CAR T cells exhibited enhanced proliferation and antitumor activity using a melanoma xenograft model (B. Hu, et al., 2017). In line with the anti-tumor activity of IL-18, Douguet et al recently demonstrated that stimulation of P2X7R on dendritic cells induced an NLRP3-dependent IL-18-mediated cytotoxic T cell response, enhancing immune checkpoint inhibitor efficacy (Douguet, et al., 2021). As previously mentioned, the activity of IL-18 is naturally regulated by endogenous IL-18BP. The ‘decoy-resistant’ IL-18 (DR-18) showed to be unreceptive to IL-18BP activity while maintaining normal IL-18 agonist functions, which was shown to potentiate the anti-tumor efficacy of immunotherapy in preclinical models of colorectal carcinoma and melanoma (Cirella, et al., 2023; T. Zhou, et al., 2020). Therefore, DR-18 represents a promising new approach to exploit the anti-tumor properties of IL-18. Most of the anti-tumor activity of IL-18 have been described in NK and CD8 T cells. Notably, recent work showed a cytotoxic enhancing effect of IL-18 on γδT cells. Teo et al, showed that preactivation of γδT cells with IL-12/18/21 promoted antitumor activity of these cells and overcome immunotherapy resistance in a melanoma and HCC preclinical models (Teo, et al., 2023).
Clinically, only limited data are available on IL-18 as a tumor therapeutic. In a Phase2 randomized study, recombinant IL-18 was given as single agent to patients with untreated metastatic melanoma (Tarhini, et al., 2009). The study concluded that IL-18 was overall tolerated but the study was terminated for lack of efficacy (Tarhini, et al., 2009). We wonder if different outcomes would have been recorded if IL-18 was used in combination with immunomodulatory agents like checkpoints inhibitors. In that scenario, a lower dose of IL-18 may need to be considered.
In summary, IL-18 remains a dual function cytokine in cancer with pro- and anti-tumor activities (Figure 3).
IL-6
IL-6 is a growth factor for non-malignant cells, for example, IL-6-mediates growth of B- and plasma cells. In the rheumatoid joint, IL-6 promotes the growth of the fibroblast-like cells of the pannus, which contributes to the destructive nature of the rheumatoid joint. In general, anti-IL-6 antibodies target the membrane form of the IL-6 Receptor and prevent IL-6 signaling. Tocilizumab is a monoclonal antibody that targets the IL-6 Receptor and is often administered to suppress inflammation. In cancer, as tumors enlarge and inflammation increases, elevated circulating IL-6 levels indicate tumor-related inflammation. Novel approaches in antibody production have rendered a TME specific IL-6R antibody that is only functional in the presence of eATP, which is abundant in the TME, thereby increasing on-target efficacy (Mimoto, et al., 2020). Therefore, IL-6 Receptor antibodies are used in combination with standard therapies in cancer, i.e., excision, radiation, immunotherapy and chemotherapy.
Although often elevated in cancer, examples of IL-6 itself inducing malignant transformation are rarely demonstrated. However, children with radiation resistant craniopharyngioma are treated with anti-IL-6 Receptor monoclonal antibody. At the time of this review, there are two trials recruiting patients for anti-IL 6 Receptor treatment in craniopharyngioma (NCT03970226 and NCT05233397).
Several studies that administer IL-6 Receptor antibodies to inhibit cancer growth are in multiple myeloma. The pathogenesis of multiple myeloma is driven by the growth properties of IL-6 for B cells, also called plasma cells. Antibodies targeting IL-6 delay the transition of early multiple myeloma, often called smoldering multiple myeloma, to progressive multiple myeloma (Brighton, et al., 2019). However, since IL-6 production is often induced by IL-1, inhibition of IL-1 is effective in suppressing the growth properties of IL-6 (Lust & Donovan, 1999). In a phase II clinical trial, inhibition of IL-1 signaling with anakinra reduced IL-6 production resulting increased disease stability (Lust, et al., 2016). Notably, after 10 years of daily anakinra in 47 patients with a diagnosis of smoldering myeloma, the median overall survival was 9.5 years (Lust, et al., 2016). These data provide the rationale for intervention in early treatment high risk multiple myeloma patients to decrease IL-1 driven IL-6 production.
Idiopathic Castleman is a rare lymphoproliferative disorder with high circulating IL-6 levels and organ failure. Siltuximab is a chimeric antibody that targets IL-6 and prevents IL-6 binding to the IL-6 Receptor. A double blind, randomized, placebo-controlled trial revealed that siltuximab significantly reduces tumor and symptomatic responses compared to the placebo treated patients (van Rhee, et al., 2014).
Pyroptosis
As stated in the first paragraph, following activation of NLRP3, active caspase-1 processes the IL-1β and IL-18 precursors; in addition, caspase-1 also cleaves the inactive GSDMD into the pore forming GSDMN. The membrane pores allow the release of cell contents resulting in cell death, often termed “pyroptosis”. However, in the presence of specific inflammasome activators, i.e. OxPLs or DAMPs), human and murine mononuclear phagocytes and neutrophils release IL-1β in absence of pyroptosis resulting in a “hyperactive” state (Figure 4) (Carty, et al., 2019; K. W. Chen, et al., 2014; Di Gioia, et al., 2020; Evavold, et al., 2018; Gaidt, et al., 2016; Hatscher, et al., 2021; Zanoni, et al., 2016; Zanoni, Tan, Di Gioia, Springstead, & Kagan, 2017; Zhivaki, et al., 2020). Given the stimulatory activity of IL-1β on immune competent cells including T cells, a “hyperactive” state has been associated with prolonged immunostimulatory responses (Ben-Sasson, Hogg, et al., 2013; Ben-Sasson, et al., 2009; Ben-Sasson, Wang, Cohen, & Paul, 2013; Zanoni, et al., 2016; Zhivaki, et al., 2020; Zhivaki & Kagan, 2021). For example, Zhivaki et al showed that prolonged NLRP3 activation in dendritic cells (DCs) induced an effective and durable CD8+ response, associated with suppression of tumor progression in mice. Conversely, using the NLRP3 agonist alum, the authors demonstrated increased pyroptosis in DCs, thereby reducing the ability to maintain prolonged and effective antitumor immunity as shown by lower production of IFNγ and increased tumor progression (Zhivaki, et al., 2020). Thus, the method of NLRP3 activation may determine cell fate. Although the progression of tumors following NLRP3 activation is still largely unexplored, the data presented here support the relevance of NLRP3 and IL-1 in antitumor immunity. This is particularly relevant in the context of therapy-induced sterile inflammation where release of DAMPs from dying tumor cells, following therapies like chemotherapy and radiotherapy, has the potential to activate NLRP3 and an IL-1-mediated T cell response, as discussed in the following sections. However, cautions should be applied in an NLRP3-activation strategy considering the pleiotropic and pro-tumor activities associated with IL-1β and IL-18.
Figure 4. Pyroptotic-mediated fate in phagocytes.
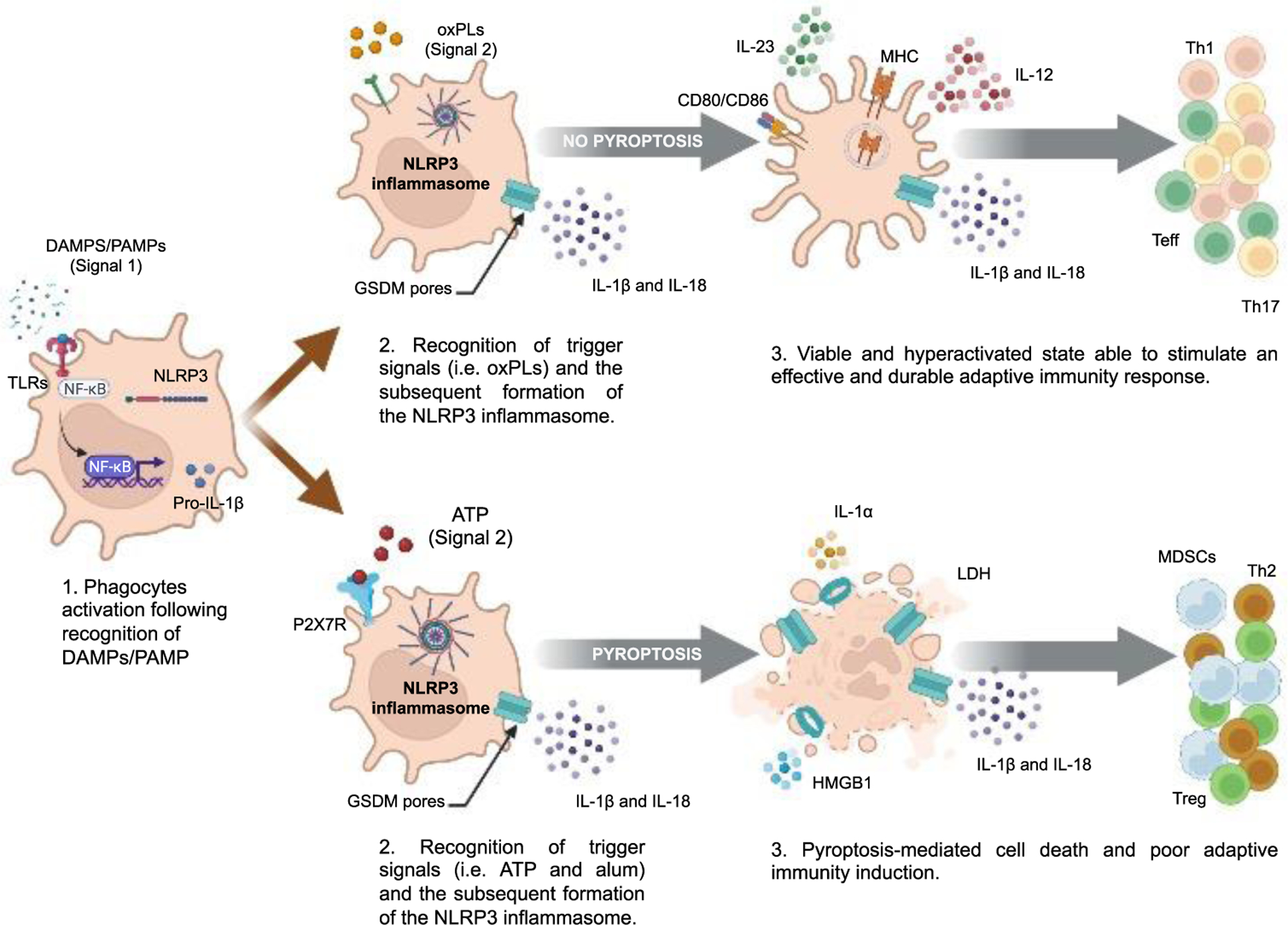
Following recognition of DAMPs or PAMPs, NF-κB translocases in the nuclei of phagocytes like dendritic cells (DCs) mediating the transcription of NLRP3 (inactive) and pro-IL-1β (1.). Next, inflammasome triggers (signal 2) will induce NLRP3 inflammasome formation (2.) Based on the nature of “signal 2”, a different fate for the cell can occur. For example, triggers like oxPLs (top) result in NLRP3 inflammasome formation and hyperactive DCs (3.). Hyperactive DCs remain viable and are able to induce a durable and effective adaptive anti-tumor response while releasing IL-1β and other cytokines like IL-12 and IL-23. On the other hand, triggers like ATP (bottom) result in NLRP3 inflammasome formation and a GSDM-mediated cell death failing in the induction of an effective anti-tumor immunity (3.). Abbreviations: LDH, lactate dehydrogenase enzyme; TLRs, Toll-like receptors; MDSCs, myeloid suppressor cells; HGMB1, High mobility group box 1.
Genetic alteration in NLRP3
A study of 337 patients with colorectal cancer (CRC) revealed that the single nucleotide polymorphism (SNP) Q705K in NLRP3 is predictive of disease progression and poor survival (Ungerback, et al., 2012). In immortalized monocytes, a separate study reported increased IL-1β and IL-18 production for the same SNP in NLRP3, suggesting that Q705K SNP is a gain-of-function alteration leading to an overactive NLRP3 inflammasome (Verma, Sarndahl, et al., 2012). Additionally, the prevalence of Q705K SNP in NLRP3 is higher in patients with pancreatic cancer compared to healthy controls (Miskiewicz, et al., 2015). Consistent with these findings, Cambui et al observed polymorphisms associated with increased NLRP3 activation are associated with poor prognosis in CRC (Cambui, et al., 2020). Furthermore, in both gastric and bladder cancer, SNPs in NLRP3 (rs10754558) are also associated with disease severity (Castano-Rodriguez, Kaakoush, Goh, Fock, & Mitchell, 2014; Xu, et al., 2021). In a male Swedish population, the NLRP3 variant rs35829419 increases the is overall risk of developing sporadic malignant melanoma (Verma, Bivik, et al., 2012). These studies reveal that genetic alterations increasing NLRP3 activity, are highly associated with disease progression in several cancers. Despite the relevance of these findings, further research is warranted to determine which cell types carry these alterations (tumor vs host), and the consequences of the increased NLRP3 activation in the TME composition, immune system response and whether they influence tumor cell evasion mechanisms and metastasis.
Viral induced tumors
Chronic viral infections, such as Epstein-Barr virus (EBV), have been linked to post-transplant lymphoproliferative disorder (PTLD), Burkitt’s Lymphoma and nasopharyngeal carcinoma (Nijland, Kersten, Pals, Bemelman, & Ten Berge, 2016; Rochford & Moormann, 2015; Tsao, Tsang, & Lo, 2017). EBV achieves persistent infection by immune evasion during periods of dormancy however, the replicative and lytic phases are likely responsible for EBV’s tumorigenic properties (Hong, et al., 2005; van Esser, et al., 2001). Burton et al reported how under hyperglycemic settings, NLRP3 interaction with the arrestin protein TXNIP activates caspase-1, which turns on the viral replication switch nudging EBV out of dormancy (Burton, Goldbach-Mansky, & Bhaduri-McIntosh, 2020). These findings are in line with NLRP3 activation driving insulin resistance and offer a novel explanation for the association of diabetes being the greatest risk factor for developing EBV PTLD as well as other EBV cancers (Francis, Johnson, Teixeira-Pinto, Craig, & Wong, 2018; Grant & Dixit, 2013; C. Hu, et al., 2015).
Human papilloma virus (HPV) is a known oncovirus in which long-term infection is associated with chronic inflammation and an increased risk of cervical cancer (CC) (J. V. Fernandes, et al., 2015). Consistently, increased cellular infiltration is present in cervical neoplasia compared to surrounding healthy tissue and CC patients present with elevated serum IL-1β levels compared to healthy controls (Donders & Vieira-Baptista, 2017; Qian, et al., 2010). Co-culture of CC cell lines and monocytes induced IL-1β in an NLRP3-dependent manner and PBMCs isolated from CC patients revealed elevated IL-1β after LPS stimulation (F. P. Fernandes, Leal, & Pontillo, 2020) suggesting a possible link between NLRP3 activation and HPV- mediated inflammation in CC. HPV infections have also been correlated with oropharyngeal squamous cell carcinoma (OSCC) (Ramqvist & Dalianis, 2010). Saliva from patients with OSCC have elevated levels of IL-1β, IL-6, IL-8 and TNFα (E. Ferrari, et al., 2021). Two-independent studies have found that inhibition of NLRP3 resulted in decreased OSCC tumor progression in mouse models, suggesting a tumor promoting role of NLRP3 in HPV positive OSCC (Scuderi, et al., 2021; H. Wang, et al., 2018).
Environmental agonist of NLRP3
Exposure to several environmental chemicals and air pollutants increases the risk of cancer (Hill, et al., 2023; Ochieng, et al., 2015). For example, inhalation of asbestos fiber-like crystalline silica is associated not only with the development of respiratory diseases like asbestosis but also lung cancer and malignant mesothelioma (Nishida & Yatera, 2022). Silica crystals induce the formation of the NLRP3 inflammasome by the recognition of lysosome damage via cathepsin B activation (Hornung, et al., 2008). Accordingly, Shukla et al suggested that NLRP3 activation following exposure to asbestos promotes the development of malignant mesothelioma (Shukla, et al., 2012). Chow et al reported that while NLRP3 was required for the IL-1β production by asbestos, NLRP3-deficient mice displayed a similar incidence of malignant mesothelioma and survival times as wild-type mice (Chow, Tschopp, Moller, & Smyth, 2012). These studies suggest that NLRP3 is fundamental for the inflammatory response following asbestos exposure. Arsenic is a group I carcinogen associated with cancers of the skin, lung, liver and kidney renal cell carcinoma (RCC) (Martinez, Vucic, Becker-Santos, Gil, & Lam, 2011). The relationship between arsenic and NLRP3 is controversial (Ahn, et al., 2018; Qiu, et al., 2018). Chung et al identified a significant correlation between high total urinary arsenic levels, the NLRP3 SNP rs1539019 and an increased risk of RCC (Chung, et al., 2020).
Ultraviolet (UV) radiation is the primary environmental risk factor for skin cancer (Kanavy & Gerstenblith, 2011). Hasegawa et al showed that UV radiation resulted in NLRP3 activation following DNA damage in keratinocytes (Hasegawa, Nakashima, & Suzuki, 2016). Melanoma, the leading cause of death among skin cancers, is characterized by elevated NLRP3/IL-1β levels and NLRP3 inhibition has been shown to significantly reduce melanoma tumor growth (Tengesdal, Dinarello, et al., 2021).
Clonal hematopoiesis of indeterminate potential (CHIP)
Clonal hematopoiesis of indeterminate potential (CHIP) is characterized by the clonal expansion of hematopoietic stem cells (HSCs) possessing somatic mutations commonly associated with cancer, but with no detectable hematological cancer (reviewed in (Calvillo-Arguelles, et al., 2019; Mitchell, Gopakumar, & Jaiswal, 2021)). CHIP is typified by the presence of one or more driver-mutations, the most common being loss-of-function in Dnmt3a and Tet2, genes involved in DNA methylation. The first studies on CHIP found significant associations with development of hematological malignancies and cardiovascular disease, CHIP has since been associated with numerous age-related inflammatory diseases (Jaiswal, et al., 2014; Jaiswal, et al., 2017). Importantly, IL-1β secretion is increased in an NLRP3-dependent manner in Tet2 deficient macrophages (Fuster, et al., 2017). Therefore, it has been proposed that the expansion of mutant HSCs in CHIP results in hyperinflammatory myeloid cells, potentiating inflammatory diseases (Belizaire, Wong, Robinette, & Ebert, 2023).
Research on the role CHIP plays in solid malignancies is emerging. Bolton et al initially found expansion of CHIP clones following chemotherapy (Bolton, et al., 2020). More recently, CHIP has been associated with an increased risk of lung cancer and prostate as well as a non-significant increase of breast cancer in individuals with high frequency of CHIP mutations, suggesting a causal role (Kessler, et al., 2022; Tian, et al., 2023).
How certain mutations associated with CHIP result in hyperinflammatory myeloid cells is highly relevant to cancer. McLemore et al recently demonstrated that Tet2−/−, Srsf2−/− myeloid cells and primary MDSCs bone marrow samples exhibit increased cytosolic R-loops accumulation, inducing cGAS/STING-mediated NLRP3 activation (McLemore, et al., 2022). NLRP3 activation resulted in induction of IFN-stimulated gene leading to pyroptosis and myeloid lineage skewing. Therefore, CHIP represents a condition that can characterize tumor-associated inflammation where NLRP3/IL-1β appear to play a central role.
NLRP3 activation in response to therapy
For advanced cancer, curative surgery is often not an option. In this section we summarize the current knowledge of NLRP3 activation in response to the main anti-tumor therapies (Table 3).
Table 3:
NLRP3 activation in response to therapy.
| Therapy class | Drug | Cancer | Proposed mechanism(s) | Expression of inflammasome components / activation | Immune response effect / outcome | Ref. |
|---|---|---|---|---|---|---|
| Chemotherapy | Doxorubicin & daunorubicin | N/A (in vitro BMDMs) | ND | ↑ NLRP3* and IL-1β | ↑ drug-related adverse events | Sauter et al., 2011 |
| Doxorubicin | ALL | ND | ↑ NLRP3*, IL-18 and caspase-1 | ↑ Therapy resistance | Zhongbo Hu et al. | |
| 5-FU | OSCC | ND | ↑ NLRP3* | ↑ Toxicity and poor clinical outcomes | Feng et al., 2017 | |
| 5-FU & gemcitabine | Lymphoma | Activation of MDSCs | ↑ NLRP3* and IL-1β | ↑ Therapy resistance and immunosuppression | Bruchard et al., 2013 | |
| Gemcitabine | TNBC | ND | ↑ NLRP3 and IL-1β | ↑ Therapy resistance | Zheng et al., 2020 | |
| Radiotherapy | Radiation | GBM and N/A (in vitro BMDMs) | ND | ↑ NLRP3*, IL-1β and caspase-1 | ↓ Survival ↑ tumor volume ↓ survival |
Liu et al., 2017 |
| Checkpoint inhibitors | Anti-PD-1 | Melanoma, breast and N/A (in vivo/in vitro myeloid cells) | Constitutive NLRP3 activation in melanoma cells mediate recruitment of MDSCs and expression of PD-L1 | ↑ NLRP3* and IL-1β | ↑ Tumor volume and immunosuppression ↓ survival |
Tengesdal et al., 2021b; Theivanthi ran et al., 2020; Tengesdal et al., 2021a |
| Anti-PD-1 | DLBCL | NLRP3 activation increased PD-L1 in DLBCL cells | ↑ NLRP3* | ↑ Tumor progression and immunosuppres sion | Lu et al., 2021; Li et al., 2015 | |
| TKIs | Vemurafenib and trametinib | Melanoma cells | ND | ↑ NLRP3 | ↑ Therapy resistance | Zhai et al., 2017 |
| Imatinib | CML | ND | ↑ IL-1β | ↑ Therapy resistance | Lee et al., 2016 | |
| Imatinib and mastinib | N/A (in vitro myeloid cells) | Therapy-induced lysosomal damage | ↑ NLRP3* and IL-1β | ↑ Therapy resistance and toxicity | Neuwirt et al., 2023; Huang et al., 2022 | |
| Cancer vaccine | NA | Lung, colorectal cancer, melanoma | Adjuvant-induced IL-1β production needed for adaptive immune response | ↑ NLRP3* and IL-1β | ↑ antigen presentation and acquired immunity activation | Ben-Sasson, Hogg, et al., 2013, Marty-Roix et al., 2016; Tahtinen et al., 2022; Tritto et al., 2009 |
Arrows indicate ↑ (increase) and ↓ (decrease)
Indicates NLRP3 activation was demonstrated
ND Indicates mechanism not described
NA Not applicable
Chemotherapy
Chemotherapy targets highly proliferative cancer cells by interfering with DNA and RNA synthesis, however, chemotherapy also affects immune cell activation, which ultimately changes the composition of the TME. Several chemotherapy agents increase NLRP3 activation which, in turn, initiate inflammation that influences the host immune response. Sauter et al, showed that doxorubicin and daunorubicin induce processing and release of IL-1β through activation of the NLRP3 inflammasome in bone marrow derived macrophages (Sauter, Wood, Wong, Iordanov, & Magun, 2011). In patients with acute lymphoblastic leukemia (ALL) serum IL-18 is elevated after chemotherapy. In vitro, ALL cells treated with doxorubicin increased NLRP3, caspase-1 and IL-18 expression (Zhongbo Hu, et al.). Additional evidence of chemotherapy-induced activation of NLRP3 has been reported in OSCC. OSCC patients treated with 5-FU (5-fluorouracil) exhibited increased NLRP3 expression and activation, which was associated with poorer clinical outcomes compared to patients who did not receive 5-FU. The authors then show that in both Nlrp3−/− and Caspase1−/− mice the toxicity of 5-FU was reduced in the OSCC mouse model (Feng, et al., 2017).
Unexpectedly, chemotherapy associated NLRP3 activation induces resistance to chemotherapeutic agents. In a study assessing the effects of gemcitabine and 5-FU on immune cell populations, Bruchard et al showed that both gemcitabine and 5-FU induced NLRP3 activation and IL-1β secretion in MDSCs, which limited efficacy of both chemotherapeutic agents in the murine lymphoblastic lymphoma model, EL4 (Bruchard, et al., 2013). Human triple-negative breast cancer (TNBC) cell lines exposed to prolonged gemcitabine expressed significantly higher NLRP3 expression and produced higher levels of IL-1β compared to untreated TNBC cell lines. In the same cells, gemcitabine-induced upregulation of NLRP3 expression reduced sensitivity to gemcitabine, which was reversed using the NLRP3 inhibitor CY-09 (Q. Zheng, Yao, Cai, & Zhou, 2020). These findings suggest a potential benefit in targeting NLRP3 in combination with chemotherapy in order to reduce immunosuppression and the development of chemotherapy resistance. Another benefit associated with the inhibition of NLRP3 in combination with chemotherapy is the reduction of the NLRP3-mediated tissue damage. This is particularly relevant in the context of cancer therapy-associated cardiotoxicity where chemo agents induce cardiac dysfunction by the activation of NLRP3 (Marchetti, et al., 2015; Mauro, et al., 2023). These studies suggest a potential benefit in targeting NLRP3 in combination with chemotherapy in order to reduce immunosuppression, tissue damage and the development of chemotherapy resistance.
The use of chemotherapy also stimulates an inflammasome-dependent antitumor immunity response. In mice, oxaliplatin, doxorubicin and mitomycin C increased ability of DCs to activate cytotoxic CD8 T cells against colon carcinoma (CT26) and melanoma (B16F10) (Casares, et al., 2005; Ghiringhelli, et al., 2009). In the aforementioned studies, mice depleted of NLRP3 and caspase-1 exhibit reduced responses to chemotherapy and an impaired CD8 T cell response.
Radiotherapy
Radiotherapy induces an intense inflammatory response that damages healthy tissue, limiting the application of curative radiation doses (J. H. Kim, Jenrow, & Brown, 2014). Several studies reveal NLRP3 mediates radiation-induced tissue damage (Mauro, et al., 2023; Sohn, et al., 2015; J. Wei, et al., 2019). In BMDMs, NLRP3 deficiency significantly reduced IL-1β secretion and cell death following radiation (Y. G. Liu, et al., 2017). In vivo, increased caspase-1-mediated inflammation and reduced survival was observed following radiation, and both were reversed in mice depleted of NLRP3 (Y. G. Liu, et al., 2017). In a xenograft model using human glioblastoma cell line U87, Liu et al observed that ionizing radiation induced NLRP3 activation and caspase-1 cleavage. Furthermore, inhibition of NLRP3 increased survival and reduced tumor volume compared to radiation treatment alone. Despite these observations suggesting a detrimental function of NLRP3 activation following radiotherapy, only limited data are available.
Immune checkpoint inhibitors
Immune checkpoint inhibitors represent a class of antibodies targeting cell surface receptors, which upon binding to their respective ligands results in inhibition of a particular immune response (Pardoll, 2012). In the case of antibodies targeting the programmed death ligand interaction (PD-1/PD-L1), long-term remission has been achieved in some patients, whereas other patients respond briefly or not at all (Wolchok, et al., 2017). Immune checkpoint molecules in a non-cancer setting promote self-tolerance, therefore, it is not unexpected that NLRP3 activation plays a role in the regulation of PD-1/PD-L1 axis.
In metastatic melanoma cells, NLRP3 is constitutively active, as shown initially in human melanoma cell lines and in human biopsies (Okamoto, et al., 2010; Tengesdal, Menon, et al., 2021; Theivanthiran, et al., 2020). IL-1β release from tumor cells is chronic and low-grade, which drives expansion and recruitment of immunoregulatory cells such as MDSCs into the TME. MDSCs possess several immunosuppressive properties, one of which is high PD-L1 expression, thereby dampening the efficacy of checkpoint inhibitors targeting the PD-1/PD-L1 axis. In two separate murine metastatic melanoma models, inhibition of NLRP3 reduced recruitment of MDSCs and improved efficacy of anti-PD-1 therapy (Tengesdal, Menon, et al., 2021; Theivanthiran, et al., 2020). Notably, NLRP3 inhibition with OLT1177® reduced PD-L1 expression in MDSCs isolated from melanoma bearing mice, supporting a direct role for NLRP3 activation in the regulation of immune checkpoint marker and response to therapy (Tengesdal, Dinarello, et al., 2021). The findings from these studies are the basis for a clinical trial designed to evaluate the safety and preliminary efficacy of dapansutrile (OLT1177®) in combination with pembrolizumab for the treatment of PD-1 refractory advanced/unresectable melanoma (NCT04971499).
In diffuse large B cell lymphoma cells, NLRP3 inflammasome activation increased PD-L1 expression which resulted in a reduction of cytotoxic T cells (Lu, et al., 2021). In vivo, the authors showed that NLRP3 blockade limited lymphoma growth reducing PD-L1 expression in the TME and PD-1/TIM-3- expressing T cells, MDSCs, TAMs, and regulatory T cells.
Using a murine model of metastatic breast cancer, NLRP3 inhibition markedly stunted tumor growth through reduced MDSCs infiltration into the TME and resulting in increased CD8+ T cells (Tengesdal, et al., 2022). Additionally, it was demonstrated that breast cancer cells induce PD-L1 expression in monocytes in a NLRP3-dependent manner. Consistently, Nlrp3−/− mice show markedly reduced tumor growth and upon treatment with anti-PD-1, tumors were completely ablated (Tengesdal, et al., 2022).
In a pan-cancer analysis, the association between NLRP3 and the immune checkpoints LAG3, PD-1, PD-L2, PD-L1, CTLA-4, TIGIT and ICOS exist for almost all the cancer types analyzed (Ding, et al., 2022). In conclusion, these data demonstrate the ability of NLRP3 to induce immune checkpoints expression in tumor and/or immune cells. Therapeutically, increased expression of NLRP3 limits the efficacy of checkpoint inhibitors. Therefore, NLRP3 inhibition as add-on therapy to checkpoint inhibitors could be beneficial especially in those patients that show low or no response to checkpoint inhibitors.
Targeted therapy
Tyrosine kinase inhibitors (TKIs) are widely used immunoregulatory drugs that target the signaling proteins regulating cell growth and motility. TKIs are approved for a growing number of cancers including, but not limited to non-small cell lung cancer (NSCLC), BRAF mutated melanoma, HER2+BC, chronic and acute myeloid leukemia (CML and AML respectively) (Pottier, et al., 2020). Prolonged use of tyrosine kinase inhibitors can result in treatment resistance, a major hurdle to achieving complete remission. Drug resistance with tyrosine kinase inhibitors involves both tumor-intrinsic mechanisms of resistance as well as off-target effects altering normal host cell function (Y. Yang, Li, Wang, Zhao, & Li, 2022). NLRP3 has been implicated in tumor-intrinsic mechanisms of drug resistance. For example, Zhai et al observed increased NLRP3 expression in melanoma cells that are resistant to BRAF and mitogen-activated protein kinase inhibitors when compared to parental cells (Zhai, Vaddi, Samson, Takegami, & Fujita, 2020). Additionally, CML cells resistant to the BCR-ABL inhibitor, imatinib, show increased IL-1β production (Lee, et al., 2016). Although the exact role of NLRP3 is yet to be determined in BCR-ABL inhibitor resistant CML patients, it is possible that NLRP3/IL-1β/IL-1R1 axis drives CML expansion.
The efficacy of TKIs may also be limited by off-target effects on host immune cell populations and adverse events. A new study by Neuwirt et al demonstrates how the TKIs imatinib and mastinib induce NLRP3-dependent IL-1β secretion from myeloid cells through lysosomal membrane destabilization (Neuwirt, et al., 2023). In line with imatinib inducing off-target effects, Huang et al reported that hepatotoxicity caused by imatinib was NLRP3-mediated, and that inhibition of NLRP3 may improve tyrosine kinase inhibitors efficacy one of the most common adverse events associated with imatinib (Han, Yee, Cho, & Gwak, 2020; F. R. Huang, et al., 2022). Thus, the addition of an NLRP3 inhibitor to TKIs may improve efficacy and limits adverse events.
Cancer vaccines
Similarly to vaccines designed for infection, therapeutic cancer vaccines are based on the administration of tumor specific, or tumor associated antigens to activate an innate and humoral specific anti-tumor immunity. The major limitation for tumor vaccines is the endogenous and low immunogenic antigens. In mice, immunization with heat-killed Listeria monocytogenes with IL-1β as an adjuvant reduces liver colony forming unites following live L. monocytogenes infection when compared to unvaccinated mice. The same study also showed that IL-1 immunized mice showed a significant reduction in HPV-transformed lung epithelial TC1 tumor growth when compared to the control groups (Ben-Sasson, Hogg, et al., 2013). The RNA vaccines induce the production of IL-1β and IL-1α as observed by Tahtinen et al. The host response to these vaccines is the up-regulation of IL-1Ra, which is required to mitigate the vaccine-induced toxicity (Tahtinen, et al., 2022). In vitro, primary human monocytes stimulated with RNA vaccine in presence of the NLRP3 inhibitor MCC950 significantly reduced IL-1β production (Tahtinen, et al., 2022).
Several vaccine adjuvants like alum, QS-1, MF-59 or the cationic liposomes in the RNA vaccines are known NLRP3 activators (Marty-Roix, et al., 2016; Tahtinen, et al., 2022; Tritto, Mosca, & De Gregorio, 2009).
Manna et al, generated a nanovaccine that induces the NLRP3 inflammasome formation (Manna, et al., 2023). In combination with checkpoint inhibition, the nanovaccine enhanced vaccine immunogenicity, induced an anti-tumor response and increased survival against several established tumors (Manna, et al., 2023). Despite these studies suggest that the NLRP3-induced IL-1β and IL-18 production function as effective vaccine adjuvants, we warrant caution. Considering the pleiotropic role of IL-1, the use of this cytokine or NLRP3 agonists during the design of cancer vaccines may determine different outcomes. Using dendritic cells vaccination against the poorly immunogenic melanoma cell line B16F10, van Deventer et al showed that mice lacking in NLRP3 led to a significant improvement in survival compared to control animals. The improved survival was associated with reduced accumulation of MDSCs in the tumor, and increased T cell-mediated antitumor immunity (van Deventer, et al., 2010). The role of NLRP3 in MDSCs activation and migration in melanoma shown in this study is in line with other reports displaying similar results (Tengesdal, Menon, et al., 2021).
Therapeutic considerations
The large body of evidence from pre-clinical and clinical trials suggest an overall tumor-promoting function of the pro-inflammatory cytokines of the IL-1 Family. Of these, the best characterized is IL-1β. Although agents against IL-1β activity are available and proven to be quite effective (anakinra, canakinumab and rilonacept), targeting this cytokine is less selective as it is activated by various inflammasomes depending on the type of PAMPs or DAMPs. Further, IL-1β is a pivotal cytokine of several immune-related pathways necessary for the host’s response to infections. IL-1 inhibitor agents can therefore increase the risk of opportunistic infections, limiting their application (Galloway, et al., 2011; Ridker, Everett, et al., 2017). Furthermore, the IL-1β antagonists anakinra, canakinumab, and rilonacept are peptides, hindering their ability to be administered orally or to penetrate the blood-brain barrier. An alternative approach in targeting IL-1, is the inhibition of NLRP3. In the past years, progress has been made in the development of NLRP3 inhibitors (Marchetti, 2019). Compared to IL-1 biologics, NLRP3 inhibitors have improved safety, specificity (no inhibition of other inflammasomes), a shorter half-life and the ability of crossing the brain blood barrier. In addition, NLRP3 inhibitors are orally active. These properties make NLRP3 inhibitors an attractive therapeutic strategy for the treatment of many inflammatory diseases but also for the inflammatory component in cancer. Relevant to this review and cancer treatment, it is important to consider that, contrary to biologics, to date, no data are available for chronic administration of NLRP3 inhibitors therefore, further studies are necessary to establish long-term effect and dosage of these small molecules.
To the best of our knowledge, only two specific NLRP3 inhibitors have been investigated in cancer. Dapansutrile in combination with pembrolizumab (anti-PD-1) is being administered in patients with advanced melanoma refractory to anti-PD-1 (NCT04971499). The NLRP3 inhibitor DFV890 is also currently being given patients with very low, low or intermediate risk of myelodysplastic syndromes as well as lower risk chronic myelomonocytic leukemia in order to assess safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy (NCT05552469).
Conclusions
In this review we describe NLRP3 activation in cancer. Some of the activation mechanisms are inherent to the TME, whereas others are dependent on environmental stimuli or therapeutic interventions (Figure 5). Regardless of the mechanism of NLRP3 activation, a picture emerges where unchecked inflammasome formation is often associated with tumor progression. Some preclinical studies focus on intrinsically activated NLRP3 in tumor cells as is the case of metastatic melanoma cell whereas other preclinical models of cancers focus on a paracrine action of tumor cells as in the case of breast and pancreatic cancer (tumor vs host). Independent of the origin, tumor cells exploit NLRP3 activation to induce an inflammatory response facilitating immunosuppression, angiogenesis, and immune evasion which ultimately generates a tumor permissive environment. Nevertheless, the pro0tumor effect of NLRP3 in cancer comes with exceptions. For example, in colorectal cancer NLRP3 activation appear to increase the immune system and to limit tumor progression. Another exception is represented by “hyperactivated” dendritic cells where NLRP3 activation results in IL-1β production independent of pyroptosis.
Figure 5. Mechanisms of NLRP3 activation in tumor.
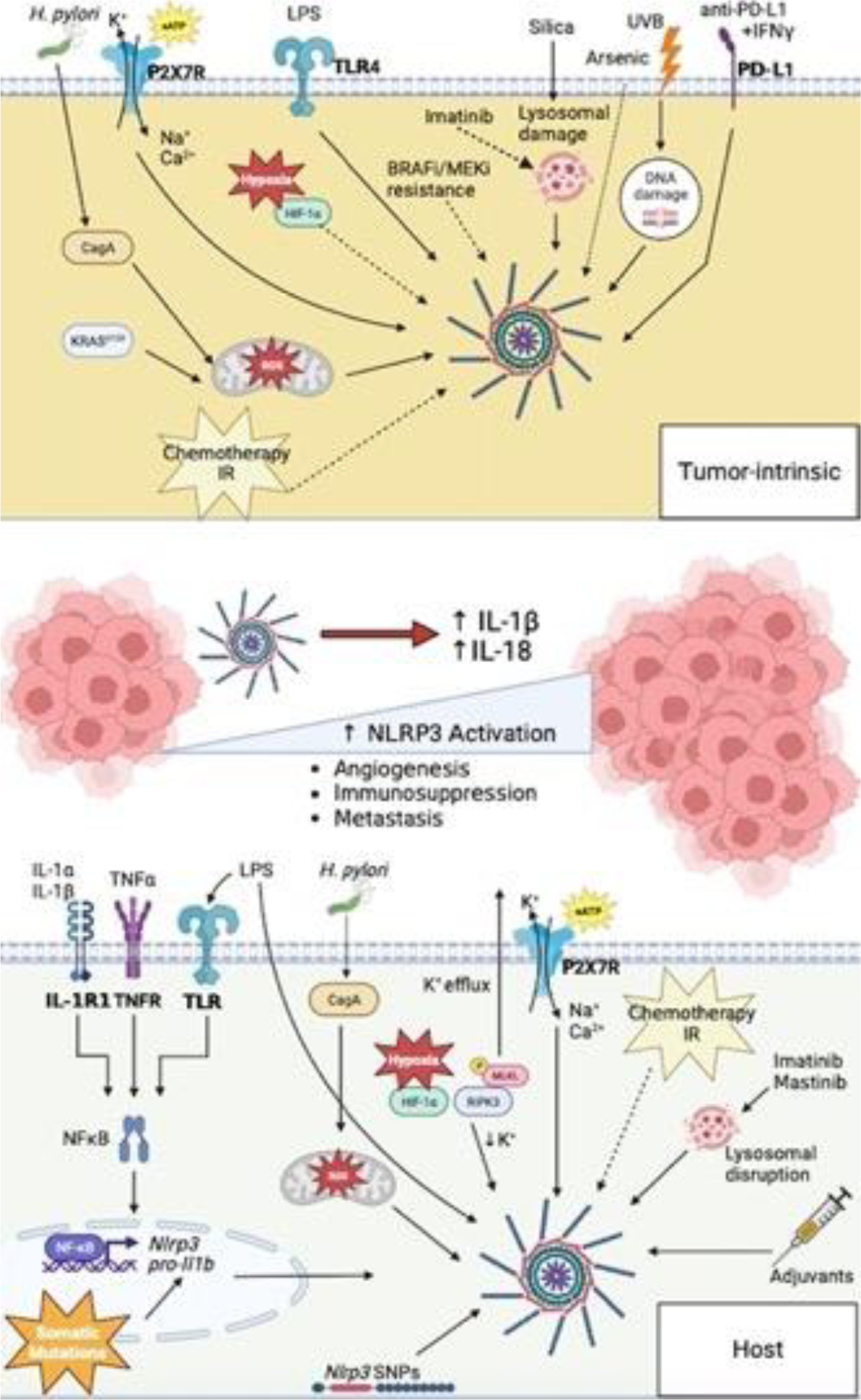
(Top) Tumor cell-intrinsic mechanisms of NLRP3 activation in the TME. (Bottom) Host cell-intrinsic mechanisms of NLRP3 activation in the TME. Aberrant activation of NLRP3 within the TME results in increased IL-1β and IL-18 secretion. Dysregulation of these cytokines result tumor promoting mechanisms, such as; angiogenesis, immunosuppression and metastasis. NLRP3 activation therefore represents a key immune checkpoint within the TME, acting as a master switch for inflammation-mediated tumor progression.
In summary, the role of NLRP3 in cancer appears to be tissue and cancer specific, which requires elucidation for targeting NLRP3. However, the ongoing clinical trials using NLRP3 inhibitor in cancer (NCT04971499 and NCT05552469) hopefully will provide further elucidation of the role of this protein in cancer.
Acknowledgments
This work was supported by the following grants: Wings of Hope for Pancreatic Cancer Research (C.M.), ACS IRG #22-154-59 from the American Cancer Society (C.M.), Cancer League of Colorado, Inc #−22051 (C.M.), IK2BX006147-01A2 (C.M.), NIH grant AI-15614 (C.A.D.) and the Interleukin Foundation. Some figures were created using BioRender (https://biorender.com/).
Abbreviations:
- 5-FU
5-fluorouracil
- ADP
adenosine diphosphate
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- ASC
apoptosis-associated speck-like protein containing a CARD
- ATP
adenosine triphosphate
- BMDM
bone marrow-derived macrophages
- CAR
chimeric antigen receptor
- CC
cervical cancer
- cGAS
cyclic GMP-AMP synthase
- CHIP
clonal hematopoiesis of indeterminate potential
- CHOL
cholangio carcinoma
- CLL
chronic lymphocytic leukemia
- CML
chronic myeloid leukemia
- COX-2
cyclooxygenase 2
- CRC
colorectal cancer
- DAMPs
damage-associated molecular patterns
- DCs
dendritic cells
- DLBCL
diffuse large B cell lymphoma
- DSS
dextran sulfate sodium
- eATP
extracellular adenosine triphosphate
- EBV
Epstein-Barr virus
- EMT
epithelial-mesenchymal transition
- ESCA
esophageal carcinoma
- GBM
glioblastoma multiforme
- GC
gastric cancer
- GSDMD
gasdermin D
- GSDMN
gasdermin N
- HCC
hepatocellular carcinoma
- HGMB1
high mobility group box 1
- HIF1α
hypoxia inducible factor 1 alpha
- HNSCC
head and neck squamous cell carcinoma
- HPV
human papilloma virus
- HSC
hematopoietic stem cells
- ICAM1
intercellular adhesion molecule 1
- ICD
immunogenic cell death
- IFNγ
interferon gamma
- IL-18
interleukin 18
- IL-18BP
interleukin-18 binding protein
- IL-1R1
interleukin-1 receptor 1
- IL-1Ra
interleukin-1 receptor antagonist
- IL-1α
interleukin-1 alpha
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- K+
potassium
- KRC
kidney renal clear cell carcinoma
- KRP
kidney renal papillary cell carcinoma
- LDH
lactate dehydrogenase
- LGG
brain lower grade glioma
- LIHC
liver hepatocellular carcinoma
- LPS
lipopolysaccharide
- MDSCs
myeloid-derived suppressor cells
- MLKL
mixed lineage kinase domain like protein
- NFκB
nuclear factor-κB
- NK
natural killer cell
- NLR
nucleotide-binding domain and leucine-rich repeat family
- NLRP3
NOD-, LRR- and pyrin domain-containing protein 3
- NSCLC
non-small cell lung cancer
- OSCC
oropharyngeal squamous cell carcinoma
- OV
ovarian serous cystadenocarcinoma
- OxPLs
oxidized phospholipids
- P2X7R
purinergic receptor P2X7
- PAAD
pancreatic adenocarcinoma
- PAMPs
pathogen-associated molecular patterns
- PD-1
programmed death protein 1
- PD-L1
programmed cell death ligand 1
- PDAC
pancreatic ductal adenocarcinoma
- PGE2
prostaglandin E2
- PRRs
pattern recognition receptors
- PTLD
post-transplant lymphoproliferative disorder
- RCC
renal cell carcinoma
- RIPK3
receptor-interacting protein kinase 3
- ROS
reactive oxygen species
- SARC
sarcoma
- SKCM
skin cutaneous melanoma
- SNP
single nucleotide polymorphism
- STAD
stomach adenocarcinoma
- STING
stimulator of interferon genes
- TAMs
tumor-associated macrophages
- TGCT
testicular germ cell tumors
- Th1
T helper type 1
- Th2
T helper type 2
- THCA
thyroid carcinoma
- TKIs
tyrosine kinase inhibitors
- TLRs
toll-like receptors
- TME
tumor microenvironment
- TNBC
triple-negative breast cancer
- TNFR
tumor necrosis factor receptor
- TNFα
tumor necrosis factor alpha
- Treg
regulatory T cells
- TSLP
thymic stromal lymphopoietin
- UCS
uveal melanoma
- UV
ultraviolet
- VCAM1
vascular cell adhesion molecule 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
I.W.T. serves as consultant for Olatec and receives compensation. C.A.D. serves as Chairman of Olatec’s Scientific Advisory Board, is co-Chief Scientific Officer, receives compensation, and has equity in Olatec. C.M. serves as Director for Olatec’s Innovative Science Program and has equity in Olatec.
Data availability
Data will be made available on request.
References
- Ahn E, Araki K, Hashimoto M, Li WY, Riley JL, Cheung J, Sharpe AH, Freeman GJ, Irving BA, & Ahmed R (2018). Role of PD-1 during effector CD8 T cell differentiation. Proceedings of the National Academy of Sciences of the United States of America, 115, 4749–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard B, Longhi MS, Robson SC, & Stagg J (2017). The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev, 276, 121–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, Herfarth HH, Jobin C, & Ting JP (2010). The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med, 207, 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amo-Aparicio J, Dominiguez A, Atif SM, Dinarello A, Azam T, Alula MK, Piper M, Lieu CH, Lentz RW, Leal AD, Badby SM, Messersmith WA, Karam SD, Dinarello CA, Pitts TM, & Marchetti C (2023). Pancreatic Ductal Adenocarcinoma Cells Regulate NLRP3 Activation to Generate a Tolerogenic Microenvironment. Cancer Research Commun, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JY, Lee SW, Shin YH, Lee JH, Jahng JW, & Park K (2017). P2X7 receptor and NLRP3 inflammasome activation in head and neck cancer. Oncotarget, 8, 48972–48982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoumian HB, He K, Hsu E, Bertolet G, Sezen D, Hu Y, Riad TS, Cortez MA, & Welsh JW (2023). NLRP3 agonist enhances radiation-induced immune priming and promotes abscopal responses in anti-PD1 resistant model. Cancer Immunol Immunother, 72, 3003–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, & Schnurr M (2010). Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut, 59, 1192–1199. [DOI] [PubMed] [Google Scholar]
- Belizaire R, Wong WJ, Robinette ML, & Ebert BL (2023). Clonal haematopoiesis and dysregulation of the immune system. Nat Rev Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M, Caucheteux S, Ratner-Hurevich M, Berzofsky JA, Nir-Paz R, & Paul WE (2013). IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med, 210, 491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, & Paul WE (2009). IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A, 106, 7119–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson SZ, Wang K, Cohen J, & Paul WE (2013). IL-1beta strikingly enhances antigen-driven CD4 and CD8 T-cell responses. Cold Spring Harb Symp Quant Biol, 78, 117–124. [DOI] [PubMed] [Google Scholar]
- Blay J, White TD, & Hoskin DW (1997). The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res, 57, 2602–2605. [PubMed] [Google Scholar]
- Bolton KL, Ptashkin RN, Gao T, Braunstein L, Devlin SM, Kelly D, Patel M, Berthon A, Syed A, Yabe M, Coombs CC, Caltabellotta NM, Walsh M, Offit K, Stadler Z, Mandelker D, Schulman J, Patel A, Philip J, Bernard E, Gundem G, Ossa JEA, Levine M, Martinez JSM, Farnoud N, Glodzik D, Li S, Robson ME, Lee C, Pharoah PDP, Stopsack KH, Spitzer B, Mantha S, Fagin J, Boucai L, Gibson CJ, Ebert BL, Young AL, Druley T, Takahashi K, Gillis N, Ball M, Padron E, Hyman DM, Baselga J, Norton L, Gardos S, Klimek VM, Scher H, Bajorin D, Paraiso E, Benayed R, Arcila ME, Ladanyi M, Solit DB, Berger MF, Tallman M, Garcia-Closas M, Chatterjee N, Diaz LA Jr., Levine RL, Morton LM, Zehir A, & Papaemmanuil E (2020). Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet, 52, 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighton TA, Khot A, Harrison SJ, Ghez D, Weiss BM, Kirsch A, Magen H, Gironella M, Oriol A, Streetly M, Kranenburg B, Qin X, Bandekar R, Hu P, Guilfoyle M, Qi M, Nemat S, & Goldschmidt H (2019). Randomized, Double-Blind, Placebo-Controlled, Multicenter Study of Siltuximab in High-Risk Smoldering Multiple Myeloma. Clin Cancer Res, 25, 3772–3775. [DOI] [PubMed] [Google Scholar]
- Broudy VC, Kaushansky K, Harlan JM, & Adamson JW (1987). Interleukin 1 stimulates human endothelial cells to produce granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor. J Immunol, 139, 464–468. [PubMed] [Google Scholar]
- Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F, Boireau W, Simon B, Ryffel B, Connat JL, Kanellopoulos J, Martin F, Rebe C, Apetoh L, & Ghiringhelli F (2013). Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med, 19, 57–64. [DOI] [PubMed] [Google Scholar]
- Burton EM, Goldbach-Mansky R, & Bhaduri-McIntosh S (2020). A promiscuous inflammasome sparks replication of a common tumor virus. Proc Natl Acad Sci U S A, 117, 1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvillo-Arguelles O, Jaiswal S, Shlush LI, Moslehi JJ, Schimmer A, Barac A, & Thavendiranathan P (2019). Connections Between Clonal Hematopoiesis, Cardiovascular Disease, and Cancer: A Review. JAMA Cardiol, 4, 380–387. [DOI] [PubMed] [Google Scholar]
- Cambui RAG, do Espirito Santo GF, Fernandes FP, Leal VNC, Galera BB, Favaro EGP, Rizzo LA, Elias RM, & Pontillo A (2020). Double-edged sword of inflammasome genetics in colorectal cancer prognosis. Clin Immunol, 213, 108373. [DOI] [PubMed] [Google Scholar]
- Carty M, Kearney J, Shanahan KA, Hams E, Sugisawa R, Connolly D, Doran CG, Munoz-Wolf N, Gurtler C, Fitzgerald KA, Lavelle EC, Fallon PG, & Bowie AG (2019). Cell Survival and Cytokine Release after Inflammasome Activation Is Regulated by the Toll-IL-1R Protein SARM. Immunity, 50, 1412–1424 e1416. [DOI] [PubMed] [Google Scholar]
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Metivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, & Kroemer G (2005). Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med, 202, 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano-Rodriguez N, Kaakoush NO, Goh KL, Fock KM, & Mitchell HM (2014). The NOD-like receptor signalling pathway in Helicobacter pylori infection and related gastric cancer: a case-control study and gene expression analyses. PLoS One, 9, e98899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Gross CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, & Schroder K (2014). The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell Rep, 8, 570–582. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang CF, Li YC, Deng WW, Mao L, Wu L, Zhang WF, Zhang L, & Sun ZJ (2018). Blockage of the NLRP3 inflammasome by MCC950 improves anti-tumor immune responses in head and neck squamous cell carcinoma. Cell Mol Life Sci, 75, 2045–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Wei T, Chen Y, Yang L, & Wu X (2020). Downregulation of IRAK1 Prevents the Malignant Behavior of Hepatocellular Carcinoma Cells by Blocking Activation of the MAPKs/NLRP3/IL-1beta Pathway. Onco Targets Ther, 13, 12787–12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-R, Yeh H-C, Chiu F-Y, Wu H-E, Fang H-C, & Li C-Y (2020). The role of HIF-1α in regulating NLRP3 inflammasome activation in bladder cancer. Journal of Clinical Oncology, 38, e17028–e17028. [Google Scholar]
- Chow MT, Tschopp J, Moller A, & Smyth MJ (2012). NLRP3 promotes inflammation-induced skin cancer but is dispensable for asbestos-induced mesothelioma. Immunol Cell Biol, 90, 983–986. [DOI] [PubMed] [Google Scholar]
- Chung CJ, Bao BY, Lin YC, Huang YL, Shiue HS, Ao PL, Pu YS, Huang CY, & Hsueh YM (2020). Polymorphism of nucleotide binding domain-like receptor protein 3 (NLRP3) increases susceptibility of total urinary arsenic to renal cell carcinoma. Sci Rep, 10, 6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirella A, Bolanos E, Di Trani CA, de Andrea CE, Sanchez-Gregorio S, Etxeberria I, Gonzalez-Gomariz J, Olivera I, Brocco D, Glez-Vaz J, Luri-Rey C, Azpilikueta A, Rodriguez I, Fernandez-Sendin M, Egea J, Eguren I, Sanmamed MF, Palencia B, Teijeira A, Berraondo P, & Melero I (2023). Intratumoral Gene Transfer of mRNAs Encoding IL12 in Combination with Decoy-Resistant IL18 Improves Local and Systemic Antitumor Immunity. Cancer Immunol Res, 11, 184–198. [DOI] [PubMed] [Google Scholar]
- Conos SA, Chen KW, De Nardo D, Hara H, Whitehead L, Nunez G, Masters SL, Murphy JM, Schroder K, Vaux DL, Lawlor KE, Lindqvist LM, & Vince JE (2017). Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci U S A, 114, E961–E969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagvadorj J, Mikulska-Ruminska K, Tumurkhuu G, Ratsimandresy RA, Carriere J, Andres AM, Marek-Iannucci S, Song Y, Chen S, Lane M, Dorfleutner A, Gottlieb RA, Stehlik C, Cassel S, Sutterwala FS, Bahar I, Crother TR, & Arditi M (2021). Recruitment of pro-IL-1alpha to mitochondrial cardiolipin, via shared LC3 binding domain, inhibits mitophagy and drives maximal NLRP3 activation. Proc Natl Acad Sci U S A, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley D, Mani VR, Mohan N, Akkad N, Pandian G, Savadkar S, Lee KB, Torres-Hernandez A, Aykut B, Diskin B, Wang W, Farooq MS, Mahmud AI, Werba G, Morales EJ, Lall S, Wadowski BJ, Rubin AG, Berman ME, Narayanan R, Hundeyin M, & Miller G (2017). NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J Exp Med, 214, 1711–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai H, Eschberger K, Wrona C, Grove L, Agrawal A, Grant B, Yin J, Parameswaran GI, Murphy T, & Sethi S (2014). Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc, 11, 303–309. [DOI] [PubMed] [Google Scholar]
- Di Gioia M, Spreafico R, Springstead JR, Mendelson MM, Joehanes R, Levy D, & Zanoni I (2020). Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat Immunol, 21, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, & Adinolfi E (2017). Extracellular purines, purinergic receptors and tumor growth. Oncogene, 36, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA (2010). Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev, 29, 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA (2018). An Interleukin-1 Signature in Breast Cancer Treated with Interleukin-1 Receptor Blockade: Implications for Treating Cytokine Release Syndrome of Checkpoint Inhibitors. Cancer Res, 78, 5200–5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Yan Y, Dong Y, Xu J, Su W, Shi W, Zou Q, & Yang X (2022). NLRP3 promotes immune escape by regulating immune checkpoints: A pan-cancer analysis. Int Immunopharmacol, 104, 108512. [DOI] [PubMed] [Google Scholar]
- Donders GG, & Vieira-Baptista P (2017). Bacterial vaginosis and inflammatory response showed association with severity of cervical neoplasia in HPV-positive women. Diagn Cytopathol, 45, 472–473. [DOI] [PubMed] [Google Scholar]
- Dosch AR, Singh S, Dai X, Mehra S, Silva IC, Bianchi A, Srinivasan S, Gao Z, Ban Y, Chen X, Banerjee S, Nagathihalli NS, Datta J, & Merchant NB (2021). Targeting Tumor-Stromal IL6/STAT3 Signaling through IL1 Receptor Inhibition in Pancreatic Cancer. Mol Cancer Ther, 20, 2280–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douguet L, Janho Dit Hreich S, Benzaquen J, Seguin L, Juhel T, Dezitter X, Duranton C, Ryffel B, Kanellopoulos J, Delarasse C, Renault N, Furman C, Homerin G, Feral C, Cherfils-Vicini J, Millet R, Adriouch S, Ghinet A, Hofman P, & Vouret-Craviari V (2021). A small-molecule P2RX7 activator promotes anti-tumor immune responses and sensitizes lung tumor to immunotherapy. Nat Commun, 12, 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, Rodrigue-Gervais IG, Breton V, Colpitts SL, Beauchemin N, & Saleh M (2015). The Nlrp3 Inflammasome Suppresses Colorectal Cancer Metastatic Growth in the Liver by Promoting Natural Killer Cell Tumoricidal Activity. Immunity, 43, 751–763. [DOI] [PubMed] [Google Scholar]
- Ershaid N, Sharon Y, Doron H, Raz Y, Shani O, Cohen N, Monteran L, Leider-Trejo L, Ben-Shmuel A, Yassin M, Gerlic M, Ben-Baruch A, Pasmanik-Chor M, Apte R, & Erez N (2019). NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat Commun, 10, 4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold CL, Ruan J, Tan Y, Xia S, Wu H, & Kagan JC (2018). The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity, 48, 35–44 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan SH, Wang YY, Lu J, Zheng YL, Wu DM, Li MQ, Hu B, Zhang ZF, Cheng W, & Shan Q (2014). Luteoloside suppresses proliferation and metastasis of hepatocellular carcinoma cells by inhibition of NLRP3 inflammasome. PLoS One, 9, e89961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Luo Q, Zhang H, Wang H, Chen W, Meng G, & Chen F (2017). The role of NLRP3 inflammasome in 5-fluorouracil resistance of oral squamous cell carcinoma. J Exp Clin Cancer Res, 36, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes FP, Leal VNC, & Pontillo A (2020). Cervical Cancer induces NLRP3 inflammasome activation in human peripheral blood monocytes. The Journal of Immunology, 204, 145.131–145.131. [Google Scholar]
- Fernandes JV, TA DEMF, JC DEA, Cobucci RN, MG DEC, Andrade VS, & JM DEA (2015). Link between chronic inflammation and human papillomavirus-induced carcinogenesis (Review). Oncol Lett, 9, 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, & Di Virgilio F (2006). The P2X7 receptor: a key player in IL-1 processing and release. J Immunol, 176, 3877–3883. [DOI] [PubMed] [Google Scholar]
- Ferrari E, Pezzi ME, Cassi D, Pertinhez TA, Spisni A, & Meleti M (2021). Salivary Cytokines as Biomarkers for Oral Squamous Cell Carcinoma: A Systematic Review. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, & Nunez G (2009). The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol, 10, 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis A, Johnson DW, Teixeira-Pinto A, Craig JC, & Wong G (2018). Incidence and predictors of post-transplant lymphoproliferative disease after kidney transplantation during adulthood and childhood: a registry study. Nephrol Dial Transplant, 33, 881–889. [DOI] [PubMed] [Google Scholar]
- Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, & Walsh K (2017). Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science, 355, 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, Robertson AA, Cooper MA, Graf T, & Hornung V (2016). Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity, 44, 833–846. [DOI] [PubMed] [Google Scholar]
- Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Watson KD, Lunt M, Consortium BCC, Symmons DP, & British Society for Rheumatology Biologics, R. (2011). The risk of serious infections in patients receiving anakinra for rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford), 50, 1341–1342. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, & Zitvogel L (2009). Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med, 15, 1170–1178. [DOI] [PubMed] [Google Scholar]
- Gilbert SM, Oliphant CJ, Hassan S, Peille AL, Bronsert P, Falzoni S, Di Virgilio F, McNulty S, & Lara R (2019). ATP in the tumour microenvironment drives expression of nfP2X7, a key mediator of cancer cell survival. Oncogene, 38, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RW, & Dixit VD (2013). Mechanisms of disease: inflammasome activation and the development of type 2 diabetes. Front Immunol, 4, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Deng W, Zhang Y, Chang Y, Shelat VG, Tsuchida K, Lino-Silva LS, & Wang Z (2022). NLRP3 activation in tumor-associated macrophages enhances lung metastasis of pancreatic ductal adenocarcinoma. Transl Lung Cancer Res, 11, 858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Fu S, Zhang J, Liu B, & Li Z (2016). Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci Rep, 6, 36107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez KD, Davis MA, Daniels BP, Olsen TM, Ralli-Jain P, Tait SW, Gale M Jr., & Oberst A (2017). MLKL Activation Triggers NLRP3-Mediated Processing and Release of IL-1beta Independently of Gasdermin-D. J Immunol, 198, 2156–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamarsheh S, Osswald L, Saller BS, Unger S, De Feo D, Vinnakota JM, Konantz M, Uhl FM, Becker H, Lubbert M, Shoumariyeh K, Schurch C, Andrieux G, Venhoff N, Schmitt-Graeff A, Duquesne S, Pfeifer D, Cooper MA, Lengerke C, Boerries M, Duyster J, Niemeyer CM, Erlacher M, Blazar BR, Becher B, Gross O, Brummer T, & Zeiser R (2020). Oncogenic Kras(G12D) causes myeloproliferation via NLRP3 inflammasome activation. Nat Commun, 11, 1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JM, Yee J, Cho YS, & Gwak HS (2020). Factors Influencing Imatinib-Induced Hepatotoxicity. Cancer Res Treat, 52, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Nakashima M, & Suzuki Y (2016). Nuclear DNA damage-triggered NLRP3 inflammasome activation promotes UVB-induced inflammatory responses in human keratinocytes. Biochem Biophys Res Commun, 477, 329–335. [DOI] [PubMed] [Google Scholar]
- Hatscher L, Lehmann CHK, Purbojo A, Onderka C, Liang C, Hartmann A, Cesnjevar R, Bruns H, Gross O, Nimmerjahn F, Ivanovic-Burmazovic I, Kunz M, Heger L, & Dudziak D (2021). Select hyperactivating NLRP3 ligands enhance the T(H)1- and T(H)17-inducing potential of human type 2 conventional dendritic cells. Sci Signal, 14. [DOI] [PubMed] [Google Scholar]
- He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, & Han J (2015). Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res, 25, 1285–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickish T, Andre T, Wyrwicz L, Saunders M, Sarosiek T, Kocsis J, Nemecek R, Rogowski W, Lesniewski-Kmak K, Petruzelka L, Apte RN, Mohanty P, Stecher M, Simard J, & de Gramont A (2017). MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol, 18, 192–201. [DOI] [PubMed] [Google Scholar]
- Hill W, Lim EL, Weeden CE, Lee C, Augustine M, Chen K, Kuan FC, Marongiu F, Evans EJ Jr., Moore DA, Rodrigues FS, Pich O, Bakker B, Cha H, Myers R, van Maldegem F, Boumelha J, Veeriah S, Rowan A, Naceur-Lombardelli C, Karasaki T, Sivakumar M, De S, Caswell DR, Nagano A, Black JRM, Martinez-Ruiz C, Ryu MH, Huff RD, Li S, Fave MJ, Magness A, Suarez-Bonnet A, Priestnall SL, Luchtenborg M, Lavelle K, Pethick J, Hardy S, McRonald FE, Lin MH, Troccoli CI, Ghosh M, Miller YE, Merrick DT, Keith RL, Al Bakir M, Bailey C, Hill MS, Saal LH, Chen Y, George AM, Abbosh C, Kanu N, Lee SH, McGranahan N, Berg CD, Sasieni P, Houlston R, Turnbull C, Lam S, Awadalla P, Gronroos E, Downward J, Jacks T, Carlsten C, Malanchi I, Hackshaw A, Litchfield K, Consortium TR, DeGregori J, Jamal-Hanjani M, & Swanton C (2023). Lung adenocarcinoma promotion by air pollutants. Nature, 616, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holen I, Lefley DV, Francis SE, Rennicks S, Bradbury S, Coleman RE, & Ottewell P (2016). IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget, 7, 75571–75584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong GK, Gulley ML, Feng WH, Delecluse HJ, Holley-Guthrie E, & Kenney SC (2005). Epstein-Barr virus lytic infection contributes to lymphoproliferative disease in a SCID mouse model. J Virol, 79, 13993–14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, & Latz E (2008). Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol, 9, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Elinav E, Huber S, Booth CJ, Strowig T, Jin C, Eisenbarth SC, & Flavell RA (2010). Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A, 107, 21635–21640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Ren J, Luo Y, Keith B, Young RM, Scholler J, Zhao Y, & June CH (2017). Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep, 20, 3025–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Ding H, Li Y, Pearson JA, Zhang X, Flavell RA, Wong FS, & Wen L (2015). NLRP3 deficiency protects from type 1 diabetes through the regulation of chemotaxis into the pancreatic islets. Proc Natl Acad Sci U S A, 112, 11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FR, Fang WT, Cheng ZP, Shen Y, Wang DJ, Wang YQ, & Sun LN (2022). Imatinib-induced hepatotoxicity via oxidative stress and activation of NLRP3 inflammasome: an in vitro and in vivo study. Arch Toxicol, 96, 1075–1087. [DOI] [PubMed] [Google Scholar]
- Huang JJ, Xia J, Huang LL, & Li YC (2019). HIF1alpha promotes NLRP3 inflammasome activation in bleomycininduced acute lung injury. Mol Med Rep, 20, 3424–3432. [DOI] [PubMed] [Google Scholar]
- Iannitti RG, Napolioni V, Oikonomou V, De Luca A, Galosi C, Pariano M, Massi-Benedetti C, Borghi M, Puccetti M, Lucidi V, Colombo C, Fiscarelli E, Lass-Florl C, Majo F, Cariani L, Russo M, Porcaro L, Ricciotti G, Ellemunter H, Ratclif L, De Benedictis FM, Talesa VN, Dinarello CA, van de Veerdonk FL, & Romani L (2016). IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat Commun, 7, 10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Ferrari D, & Eltzschig HK (2014). Nucleotide signalling during inflammation. Nature, 509, 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara, Chamaillard, McDonald C, & Nunez G (2005). NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem, 74, 355–383. [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, & Ebert BL (2014). Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med, 371, 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, & Ebert BL (2017). Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med, 377, 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Zhang C, Hua M, Wang M, Chen P, & Ma D (2017). Aberrant NLRP3 inflammasome associated with aryl hydrocarbon receptor potentially contributes to the imbalance of T-helper cells in patients with acute myeloid leukemia. Oncol Lett, 14, 7031–7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanavy HE, & Gerstenblith MR (2011). Ultraviolet radiation and melanoma. Semin Cutan Med Surg, 30, 222–228. [DOI] [PubMed] [Google Scholar]
- Kaplanski G (2018). Interleukin-18: Biological properties and role in disease pathogenesis. Immunol Rev, 281, 138–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski G, Farnarier C, Kaplanski S, Porat R, Shapiro L, Bongrand P, & Dinarello CA (1994). Interleukin-1 induces interleukin-8 secretion from endothelial cells by a juxtacrine mechanism. Blood, 84, 4242–4248. [PubMed] [Google Scholar]
- Kessler MD, Damask A, O’Keeffe S, Banerjee N, Li D, Watanabe K, Marketta A, Van Meter M, Semrau S, Horowitz J, Tang J, Kosmicki JA, Rajagopal VM, Zou Y, Houvras Y, Ghosh A, Gillies C, Mbatchou J, White RR, Verweij N, Bovijn J, Parikshak NN, LeBlanc MG, Jones M, Regeneron Genetics C, Collaboration G-RD, Glass DJ, Lotta LA, Cantor MN, Atwal GS, Locke AE, Ferreira MAR, Deering R, Paulding C, Shuldiner AR, Thurston G, Ferrando AA, Salerno W, Reid JG, Overton JD, Marchini J, Kang HM, Baras A, Abecasis GR, & Jorgenson E (2022). Common and rare variant associations with clonal haematopoiesis phenotypes. Nature, 612, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Lee Y, Kim E, Kwak A, Ryoo S, Bae SH, Azam T, Kim S, & Dinarello CA (2013). The Interleukin-1alpha Precursor is Biologically Active and is Likely a Key Alarmin in the IL-1 Family of Cytokines. Front Immunol, 4, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Jenrow KA, & Brown SL (2014). Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat Oncol J, 32, 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappano R, Talia M, Cirillo F, Rigiracciolo DC, Scordamaglia D, Guzzi R, Miglietta AM, De Francesco EM, Belfiore A, Sims AH, & Maggiolini M (2020). The IL1beta-IL1R signaling is involved in the stimulatory effects triggered by hypoxia in breast cancer cells and cancer-associated fibroblasts (CAFs). J Exp Clin Cancer Res, 39, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Xiao TS, & Stutz A (2013). Activation and regulation of the inflammasomes. Nat Rev Immunol, 13, 397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi G, Voronov E, Dinarello CA, Apte RN, & Cohen S (2007). Sustained delivery of IL-1 Ra from biodegradable microspheres reduces the number of murine B16 melanoma lung metastases. J Control Release, 123, 123–130. [DOI] [PubMed] [Google Scholar]
- Lee CR, Kang JA, Kim HE, Choi Y, Yang T, & Park SG (2016). Secretion of IL-1beta from imatinib-resistant chronic myeloid leukemia cells contributes to BCR-ABL mutation-independent imatinib resistance. FEBS Lett, 590, 358–368. [DOI] [PubMed] [Google Scholar]
- Li H, Xia JQ, Zhu FS, Xi ZH, Pan CY, Gu LM, & Tian YZ (2018). LPS promotes the expression of PD-L1 in gastric cancer cells through NF-kappaB activation. J Cell Biochem, 119, 9997–10004. [DOI] [PubMed] [Google Scholar]
- Liu CH, Chen Z, Chen K, Liao FT, Chung CE, Liu X, Lin YC, Keohavong P, Leikauf GD, & Di YP (2021). Lipopolysaccharide-Mediated Chronic Inflammation Promotes Tobacco Carcinogen-Induced Lung Cancer and Determines the Efficacy of Immunotherapy. Cancer Res, 81, 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Xu Y, Liang K, & Liu R (2020). Immune Cells Combined With NLRP3 Inflammasome Inhibitor Exert Better Antitumor Effect on Pancreatic Ductal Adenocarcinoma. Front Oncol, 10, 1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YG, Chen JK, Zhang ZT, Ma XJ, Chen YC, Du XM, Liu H, Zong Y, & Lu GC (2017). NLRP3 inflammasome activation mediates radiation-induced pyroptosis in bone marrow-derived macrophages. Cell Death Dis, 8, e2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Zhao Y, Pang Y, Ji M, Sun Y, Wang H, Zou J, Wang Y, Li G, Sun T, Li J, Ma D, Ye J, & Ji C (2021). NLRP3 inflammasome upregulates PD-L1 expression and contributes to immune suppression in lymphoma. Cancer Lett, 497, 178–189. [DOI] [PubMed] [Google Scholar]
- Lust JA, & Donovan KA (1999). The role of interleukin-1 beta in the pathogenesis of multiple myeloma. Hematol Oncol Clin North Am, 13, 1117–1125. [DOI] [PubMed] [Google Scholar]
- Lust JA, Lacy MQ, Zeldenrust SR, Witzig TE, Moon-Tasson LL, Dinarello CA, & Donovan KA (2016). Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol, 91, 571–574. [DOI] [PubMed] [Google Scholar]
- Lutz V, Hellmund VM, Picard FSR, Raifer H, Ruckenbrod T, Klein M, Bopp T, Savai R, Duewell P, Keber CU, Weigert A, Chung HR, Buchholz M, Menke A, Gress TM, Huber M, & Bauer C (2023). IL18 Receptor Signaling Regulates Tumor-Reactive CD8+ T-cell Exhaustion via Activation of the IL2/STAT5/mTOR Pathway in a Pancreatic Cancer Model. Cancer Immunol Res, 11, 421–434. [DOI] [PubMed] [Google Scholar]
- Lythgoe MP, & Prasad V (2022). Repositioning canakinumab for non-small cell lung cancer-important lessons for drug repurposing in oncology. Br J Cancer, 127, 785–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna S, Maiti S, Shen J, Weiss A, Mulder E, Du W, & Esser-Kahn AP (2023). Nanovaccine that activates the NLRP3 inflammasome enhances tumor specific activation of anti-cancer immunity. Biomaterials, 296, 122062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C (2019). The NLRP3 Inflammasome as a Pharmacological Target. J Cardiovasc Pharmacol, 74, 285–296. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Toldo S, Chojnacki J, Mezzaroma E, Liu K, Salloum FN, Nordio A, Carbone S, Mauro AG, Das A, Zalavadia AA, Halquist MS, Federici M, Van Tassell BW, Zhang S, & Abbate A (2015). Pharmacologic Inhibition of the NLRP3 Inflammasome Preserves Cardiac Function After Ischemic and Nonischemic Injury in the Mouse. J Cardiovasc Pharmacol, 66, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez VD, Vucic EA, Becker-Santos DD, Gil L, & Lam WL (2011). Arsenic exposure and the induction of human cancers. J Toxicol, 2011, 431287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty-Roix R, Vladimer GI, Pouliot K, Weng D, Buglione-Corbett R, West K, MacMicking JD, Chee JD, Wang S, Lu S, & Lien E (2016). Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants. J Biol Chem, 291, 1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro AG, Mezzaroma E, Toldo S, Melendez GC, Franco RL, Lesnefsky EJ, Abbate A, Hundley WG, & Salloum FN (2023). NLRP3-mediated inflammation in cardio-oncology: sterile yet harmful. Transl Res, 252, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie RC, Oran A, Dinarello CA, & Sauder DN (1996). Interleukin-1 receptor antagonist inhibits subcutaneous B16 melanoma growth in vivo. Anticancer Res, 16, 437–441. [PubMed] [Google Scholar]
- McLemore AF, Hou HA, Meyer BS, Lam NB, Ward GA, Aldrich AL, Rodrigues MA, Vedder A, Zhang L, Padron E, Vincelette ND, Sallman DA, Abdel-Wahab O, List AF, & McGraw KL (2022). Somatic gene mutations expose cytoplasmic DNA to co-opt the cGAS/STING/NLRP3 axis in myelodysplastic syndromes. JCI Insight, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimoto F, Tatsumi K, Shimizu S, Kadono S, Haraya K, Nagayasu M, Suzuki Y, Fujii E, Kamimura M, Hayasaka A, Kawauchi H, Ohara K, Matsushita M, Baba T, Susumu H, Sakashita T, Muraoka T, Aso K, Katada H, Tanaka E, Nakagawa K, Hasegawa M, Ayabe M, Yamamoto T, Tanba S, Ishiguro T, Kamikawa T, Nambu T, Kibayashi T, Azuma Y, Tomii Y, Kato A, Ozeki K, Murao N, Endo M, Kikuta J, Kamata-Sakurai M, Ishii M, Hattori K, & Igawa T (2020). Exploitation of Elevated Extracellular ATP to Specifically Direct Antibody to Tumor Microenvironment. Cell Rep, 33, 108542. [DOI] [PubMed] [Google Scholar]
- Miskiewicz A, Szparecki G, Durlik M, Rydzewska G, Ziobrowski I, & Gorska R (2015). The Q705K and F359L Single-Nucleotide Polymorphisms of NOD-Like Receptor Signaling Pathway: Association with Chronic Pancreatitis, Pancreatic Cancer, and Periodontitis. Arch Immunol Ther Exp (Warsz), 63, 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SR, Gopakumar J, & Jaiswal S (2021). Insights into clonal hematopoiesis and its relation to cancer risk. Curr Opin Genet Dev, 66, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kassem S, Cleynen A, Chretien ML, Guillerey C, Putz EM, Bald T, Forster I, Vuckovic S, Hill GR, Masters SL, Chesi M, Bergsagel PL, Avet-Loiseau H, Martinet L, & Smyth MJ (2018). Dysregulated IL-18 Is a Key Driver of Immunosuppression and a Possible Therapeutic Target in the Multiple Myeloma Microenvironment. Cancer Cell, 33, 634–648 e635. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Otani T, Ijiri Y, Motoda R, Kurimoto M, & Orita K (2000). IFN-gamma-dependent and -independent mechanisms in adverse effects caused by concomitant administration of IL-18 and IL-12. J Immunol, 164, 3330–3336. [DOI] [PubMed] [Google Scholar]
- Nasiri E, Student M, Roth K, Siti Utami N, Huber M, Buchholz M, Gress TM, & Bauer C (2023). IL18 Receptor Signaling Inhibits Intratumoral CD8(+) T-Cell Migration in a Murine Pancreatic Cancer Model. Cells, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwirt E, Magnani G, Cikovic T, Wohrle S, Fischer L, Kostina A, Flemming S, Fischenich NJ, Saller BS, Gorka O, Renner S, Agarinis C, Parker CN, Boettcher A, Farady CJ, Kesselring R, Berlin C, Backofen R, Rodriguez-Franco M, Kreutz C, Prinz M, Tholen M, Reinheckel T, Ott T, Gross CJ, Jost PJ, & Gross O (2023). Tyrosine kinase inhibitors can activate the NLRP3 inflammasome in myeloid cells through lysosomal damage and cell lysis. Sci Signal, 16, eabh1083. [DOI] [PubMed] [Google Scholar]
- Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, & Ten Berge IJ (2016). Epstein-Barr Virus-Positive Posttransplant Lymphoproliferative Disease After Solid Organ Transplantation: Pathogenesis, Clinical Manifestations, Diagnosis, and Management. Transplant Direct, 2, e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida C, & Yatera K (2022). The Impact of Ambient Environmental and Occupational Pollution on Respiratory Diseases. Int J Environ Res Public Health, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, & Rubinstein M (1999). Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity, 10, 127–136. [DOI] [PubMed] [Google Scholar]
- Novick D, Schwartsburd B, Pinkus R, Suissa D, Belzer I, Sthoeger Z, Keane WF, Chvatchko Y, Kim SH, Fantuzzi G, Dinarello CA, & Rubinstein M (2001). A novel IL-18BP ELISA shows elevated serum IL-18BP in sepsis and extensive decrease of free IL-18. Cytokine, 14, 334–342. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Nangami GN, Ogunkua O, Miousse IR, Koturbash I, Odero-Marah V, McCawley LJ, Nangia-Makker P, Ahmed N, Luqmani Y, Chen Z, Papagerakis S, Wolf GT, Dong C, Zhou BP, Brown DG, Colacci AM, Hamid RA, Mondello C, Raju J, Ryan EP, Woodrick J, Scovassi AI, Singh N, Vaccari M, Roy R, Forte S, Memeo L, Salem HK, Amedei A, Al-Temaimi R, Al-Mulla F, Bisson WH, & Eltom SE (2015). The impact of low-dose carcinogens and environmental disruptors on tissue invasion and metastasis. Carcinogenesis, 36 Suppl 1, S128–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, & Sitkovsky M (2006). A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A, 103, 13132–13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Liu W, Luo Y, Tanaka A, Cai X, Norris DA, Dinarello CA, & Fujita M (2010). Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J Biol Chem, 285, 6477–6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X, Ghani A, Malik A, Wilder T, Colegio OR, Flavell RA, Cronstein BN, & Mehal WZ (2013). Adenosine is required for sustained inflammasome activation via the A(2)A receptor and the HIF-1alpha pathway. Nat Commun, 4, 2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan R, Liu H, & Choubey D (2016). Hypoxia primes human normal prostate epithelial cells and cancer cell lines for the NLRP3 and AIM2 inflammasome activation. Oncotarget, 7, 28183–28194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM (2012). The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer, 12, 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariano M, Pieroni S, De Luca A, Iannitti RG, Borghi M, Puccetti M, Giovagnoli S, Renga G, D’Onofrio F, Bellet MM, Stincardini C, Della-Fazia MA, Servillo G, van de Veerdonk FL, Costantini C, & Romani L (2021). Anakinra Activates Superoxide Dismutase 2 to Mitigate Inflammasome Activity. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier C, Fresnais M, Gilon M, Jerusalem G, Longuespee R, & Sounni NE (2020). Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers (Basel), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian N, Chen X, Han S, Qiang F, Jin G, Zhou X, Dong J, Wang X, Shen H, & Hu Z (2010). Circulating IL-1beta levels, polymorphisms of IL-1B, and risk of cervical cancer in Chinese women. J Cancer Res Clin Oncol, 136, 709–716. [DOI] [PubMed] [Google Scholar]
- Qiu T, Pei P, Yao X, Jiang L, Wei S, Wang Z, Bai J, Yang G, Gao N, Yang L, Qi S, Yan R, Liu X, & Sun X (2018). Taurine attenuates arsenic-induced pyroptosis and nonalcoholic steatohepatitis by inhibiting the autophagic-inflammasomal pathway. Cell Death Dis, 9, 946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramqvist T, & Dalianis T (2010). Oropharyngeal cancer epidemic and human papillomavirus. Emerg Infect Dis, 16, 1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek CR, & Chandel NS (2017). The Two Faces of Reactive Oxygen Species in Cancer. Annual Review of Cancer Biology, 1, 79–98. [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, & Group CT (2017). Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med, 377, 1119–1131. [DOI] [PubMed] [Google Scholar]
- Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, & Group CT (2017). Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet, 390, 1833–1842. [DOI] [PubMed] [Google Scholar]
- Rochford R, & Moormann AM (2015). Burkitt’s Lymphoma. Curr Top Microbiol Immunol, 390, 267–285. [DOI] [PubMed] [Google Scholar]
- Salaro E, Rambaldi A, Falzoni S, Amoroso FS, Franceschini A, Sarti AC, Bonora M, Cavazzini F, Rigolin GM, Ciccone M, Audrito V, Deaglio S, Pelegrin P, Pinton P, Cuneo A, & Di Virgilio F (2016). Involvement of the P2X7-NLRP3 axis in leukemic cell proliferation and death. Sci Rep, 6, 26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter KA, Wood LJ, Wong J, Iordanov M, & Magun BE (2011). Doxorubicin and daunorubicin induce processing and release of interleukin-1beta through activation of the NLRP3 inflammasome. Cancer Biol Ther, 11, 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi SA, Casili G, Basilotta R, Lanza M, Filippone A, Raciti G, Puliafito I, Colarossi L, Esposito E, & Paterniti I (2021). NLRP3 Inflammasome Inhibitor BAY-117082 Reduces Oral Squamous Cell Carcinoma Progression. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma BR, & Kanneganti TD (2021). NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol, 22, 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Miller JM, Hillegass JM, MacPherson MB, Beuschel SL, Pass HI, & Mossman BT (2012). Abstract 5461: Role of the NLRP3 inflammasome in the development and drug resistance of malignant mesothelioma. Cancer Research, 72, 5461–5461. [Google Scholar]
- Si L, Fu J, Liu W, Hayashi T, Nie Y, Mizuno K, Hattori S, Fujisaki H, Onodera S, & Ikejima T (2020). Silibinin inhibits migration and invasion of breast cancer MDA-MB-231 cells through induction of mitochondrial fusion. Mol Cell Biochem, 463, 189–201. [DOI] [PubMed] [Google Scholar]
- Sohn SH, Lee JM, Park S, Yoo H, Kang JW, Shin D, Jung KH, Lee YS, Cho J, & Bae H (2015). The inflammasome accelerates radiation-induced lung inflammation and fibrosis in mice. Environ Toxicol Pharmacol, 39, 917–926. [DOI] [PubMed] [Google Scholar]
- Sorbara MT, & Girardin SE (2011). Mitochondrial ROS fuel the inflammasome. Cell Res, 21, 558–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes IM (2023). IL18 at the Crossroads between Chronic Inflammation and T-cell Exhaustion in Pancreatic Cancer. Cancer Immunol Res, 11, 400. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, & Lavelle EC (2006). A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med, 203, 1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwara MI, Green NJ, Borthwick LA, Mann J, Mayer-Barber KD, Barron L, Corris PA, Farrow SN, Wynn TA, Fisher AJ, & Mann DA (2014). IL-1alpha released from damaged epithelial cells is sufficient and essential to trigger inflammatory responses in human lung fibroblasts. Mucosal Immunol, 7, 684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahtinen S, Tong AJ, Himmels P, Oh J, Paler-Martinez A, Kim L, Wichner S, Oei Y, McCarron MJ, Freund EC, Amir ZA, de la Cruz CC, Haley B, Blanchette C, Schartner JM, Ye W, Yadav M, Sahin U, Delamarre L, & Mellman I (2022). IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol, 23, 532–542. [DOI] [PubMed] [Google Scholar]
- Tang Z, Li C, Kang B, Gao G, Li C, & Zhang Z (2017). GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res, 45, W98–W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarhini AA, Millward M, Mainwaring P, Kefford R, Logan T, Pavlick A, Kathman SJ, Laubscher KH, Dar MM, & Kirkwood JM (2009). A phase 2, randomized study of SB-485232, rhIL-18, in patients with previously untreated metastatic melanoma. Cancer, 115, 859–868. [DOI] [PubMed] [Google Scholar]
- Tengesdal IW, Dinarello A, Powers NE, Burchill MA, Joosten LAB, Marchetti C, & Dinarello CA (2021). Tumor NLRP3-derived IL-1beta drives the IL-6/STAT3 axis resulting in sustained MDSC-mediated immunosuppression. Front Immunol, 12, 661323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengesdal IW, Li S, Powers NE, May M, Neff CP, Joosten LAB, Marchetti C, & Dinarello CA (2022). Activation of Host-NLRP3 Inflammasome in Myeloid Cells Dictates Response to Anti-PD-1 Therapy in Metastatic Breast Cancers. Pharmaceuticals (Basel), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengesdal IW, Menon DR, Osborne DG, Neff CP, Powers NE, Gamboni F, Mauro AG, D’Alessandro A, Stefanoni D, Henen MA, Mills TS, De Graaf DM, Azam T, Vogeli B, Palmer BE, Pietras EM, DeGregori J, Tan AC, Joosten LAB, Fujita M, Dinarello CA, & Marchetti C (2021). Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. Proc Natl Acad Sci U S A, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo HY, Song Y, Yong KSM, Liu Y, Mei Y, Hanafi ZB, Zhu Y, Chua YL, Gascoigne NRJ, Chen Q, & Liu H (2023). IL12/18/21 Preactivation Enhances the Antitumor Efficacy of Expanded gammadeltaT Cells and Overcomes Resistance to Anti-PD-L1 Treatment. Cancer Immunol Res, 11, 978–999. [DOI] [PubMed] [Google Scholar]
- Theivanthiran B, Evans KS, DeVito NC, Plebanek M, Sturdivant M, Wachsmuth LP, Salama AK, Kang Y, Hsu D, Balko JM, Johnson DB, Starr M, Nixon A, Holtzhausen A, & Hanks BA (2020). A tumor-intrinsic PD-L1/NLRP3 inflammasome signaling pathway drives resistance to anti-PD-1 immunotherapy. J Clin Invest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R, Wiley B, Liu J, Zong X, Truong B, Zhao S, Uddin MM, Niroula A, Miller CA, Mukherjee S, Heiden BT, Luo J, Puri V, Kozower BD, Walter MJ, Ding L, Link DC, Amos CI, Ebert BL, Govindan R, Natarajan P, Bolton KL, & Cao Y (2023). Clonal Hematopoiesis and Risk of Incident Lung Cancer. J Clin Oncol, 41, 1423–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritto E, Mosca F, & De Gregorio E (2009). Mechanism of action of licensed vaccine adjuvants. Vaccine, 27, 3331–3334. [DOI] [PubMed] [Google Scholar]
- Tsao SW, Tsang CM, & Lo KW (2017). Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerback J, Belenki D, Jawad ul-Hassan A, Fredrikson M, Fransen K, Elander N, Verma D, & Soderkvist P (2012). Genetic variation and alterations of genes involved in NFkappaB/TNFAIP3- and NLRP3-inflammasome signaling affect susceptibility and outcome of colorectal cancer. Carcinogenesis, 33, 2126–2134. [DOI] [PubMed] [Google Scholar]
- van Deventer HW, Burgents JE, Wu QP, Woodford RM, Brickey WJ, Allen IC, McElvania-Tekippe E, Serody JS, & Ting JP (2010). The inflammasome component NLRP3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res, 70, 10161–10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Esser JW, van der Holt B, Meijer E, Niesters HG, Trenschel R, Thijsen SF, van Loon AM, Frassoni F, Bacigalupo A, Schaefer UW, Osterhaus AD, Gratama JW, Löwenberg B, Verdonck LF, & Cornelissen JJ (2001). Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and quantitatively predicts EBV-lymphoproliferative disease following T-cell--depleted SCT. Blood, 98, 972–978. [DOI] [PubMed] [Google Scholar]
- van Rhee F, Wong RS, Munshi N, Rossi JF, Ke XY, Fossa A, Simpson D, Capra M, Liu T, Hsieh RK, Goh YT, Zhu J, Cho SG, Ren H, Cavet J, Bandekar R, Rothman M, Puchalski TA, Reddy M, van de Velde H, Vermeulen J, & Casper C (2014). Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol, 15, 966–974. [DOI] [PubMed] [Google Scholar]
- Verma D, Bivik C, Farahani E, Synnerstad I, Fredrikson M, Enerback C, Rosdahl I, & Soderkvist P (2012). Inflammasome polymorphisms confer susceptibility to sporadic malignant melanoma. Pigment Cell Melanoma Res, 25, 506–513. [DOI] [PubMed] [Google Scholar]
- Verma D, Sarndahl E, Andersson H, Eriksson P, Fredrikson M, Jonsson JI, Lerm M, & Soderkvist P (2012). The Q705K polymorphism in NLRP3 is a gain-of-function alteration leading to excessive interleukin-1beta and IL-18 production. PLoS One, 7, e34977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Vanaclocha F, Alvarez A, Asumendi A, Urcelay B, Tonino P, & Dinarello CA (1996). Interleukin 1 (IL-1)-dependent melanoma hepatic metastasis in vivo; increased endothelial adherence by IL-1-induced mannose receptors and growth factor production in vitro. J Natl Cancer Inst, 88, 198–205. [DOI] [PubMed] [Google Scholar]
- Vidal-Vanaclocha F, Amezaga C, Asumendi A, Kaplanski G, & Dinarello CA (1994). Interleukin-1 receptor blockade reduces the number and size of murine B16 melanoma hepatic metastases. Cancer Res, 54, 2667–2672. [PubMed] [Google Scholar]
- Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, Fuentes AM, Anasagasti MJ, Martin J, Carrascal T, Walsh P, Reznikov LL, Kim SH, Novick D, Rubinstein M, & Dinarello CA (2000). IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci U S A, 97, 734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Meng W, Wang B, & Qiao L (2014). Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett, 345, 196–202. [DOI] [PubMed] [Google Scholar]
- Wang H, Luo Q, Feng X, Zhang R, Li J, & Chen F (2018). NLRP3 promotes tumor growth and metastasis in human oral squamous cell carcinoma. Bmc Cancer, 18, 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Wang H, Wang H, Wang B, Meng L, Xin Y, & Jiang X (2019). The role of NLRP3 inflammasome activation in radiation damage. Biomed Pharmacother, 118, 109217. [DOI] [PubMed] [Google Scholar]
- Wei Q, Guo P, Mu K, Zhang Y, Zhao W, Huai W, Qiu Y, Li T, Ma X, Liu Y, Chen X, & Han L (2015). Estrogen suppresses hepatocellular carcinoma cells through ERbeta-mediated upregulation of the NLRP3 inflammasome. Lab Invest, 95, 804–816. [DOI] [PubMed] [Google Scholar]
- Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X, Zhao W, Huai W, Guo P, & Han L (2014). Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab Invest, 94, 52–62. [DOI] [PubMed] [Google Scholar]
- Wei Q, Zhu R, Zhu J, Zhao R, & Li M (2019). E2-Induced Activation of the NLRP3 Inflammasome Triggers Pyroptosis and Inhibits Autophagy in HCC Cells. Oncol Res, 27, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks EE, & Semenza GL (2022). Hypoxia-inducible factors: cancer progression and clinical translation. J Clin Invest, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino MS, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbe C, Ascierto PA, Long GV, Cebon J, Sosman J, Postow MA, Callahan MK, Walker D, Rollin L, Bhore R, Hodi FS, & Larkin J (2017). Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med, 377, 1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC, Xu K, Martinek J, Young RR, Banchereau R, George J, Turner J, Kim KI, Zurawski S, Wang X, Blankenship D, Brookes HM, Marches F, Obermoser G, Lavecchio E, Levin MK, Bae S, Chung CH, Smith JL, Cepika AM, Oxley KL, Snipes GJ, Banchereau J, Pascual V, O’Shaughnessy J, & Palucka AK (2018). IL1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Res, 78, 5243–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Qian S, Zhang J, Feng J, Luo K, Sun L, Zhao L, Ran Y, Sun L, Wang J, & Xu F (2021). Lipopolysaccharide promotes metastasis via acceleration of glycolysis by the nuclear factor-kappaB/snail/hexokinase3 signaling axis in colorectal cancer. Cancer Metab, 9, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Huang R, Xia W, Jiang B, Xiao G, & Li Y (2021). Associations between inflammasome-related gene NLRP3 Polymorphisms (rs10754558 and rs35829419) and risk of bladder cancer in a Chinese population. J Clin Lab Anal, 35, e23973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Cao H, Liu N, Xu K, Ding M, & Mao LJ (2016). Oncolytic adenovirus expressing interleukin-18 improves antitumor activity of dacarbazine for malignant melanoma. Drug Des Devel Ther, 10, 3755–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HL, Lin PY, Vadivalagan C, Lin YA, Lin KY, & Hseu YC (2023). Coenzyme Q(0) defeats NLRP3-mediated inflammation, EMT/metastasis, and Warburg effects by inhibiting HIF-1alpha expression in human triple-negative breast cancer cells. Arch Toxicol. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li S, Wang Y, Zhao Y, & Li Q (2022). Protein tyrosine kinase inhibitor resistance in malignant tumors: molecular mechanisms and future perspective. Signal Transduct Target Ther, 7, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Pu N, Chen Q, Zhang J, Zhao G, Xu X, Wang D, Kuang T, Jin D, Lou W, & Wu W (2021). Gut-derived lipopolysaccharide remodels tumoral microenvironment and synergizes with PD-L1 checkpoint blockade via TLR4/MyD88/AKT/NF-kappaB pathway in pancreatic cancer. Cell Death Dis, 12, 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, Donado CA, Shao F, Wu H, Springstead JR, & Kagan JC (2016). An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science, 352, 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Di Gioia M, Springstead JR, & Kagan JC (2017). By Capturing Inflammatory Lipids Released from Dying Cells, the Receptor CD14 Induces Inflammasome-Dependent Phagocyte Hyperactivation. Immunity, 47, 697–709 e693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z, Vaddi PK, Samson JM, Takegami T, & Fujita M (2020). NLRP1 Functions Downstream of the MAPK/ERK Signaling via ATF4 and Contributes to Acquired Targeted Therapy Resistance in Human Metastatic Melanoma. Pharmaceuticals (Basel), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li C, Chen D, He X, Zhao Y, Bao L, Wang Q, Zhou J, & Xie Y (2022). H. pylori CagA activates the NLRP3 inflammasome to promote gastric cancer cell migration and invasion. Inflamm Res, 71, 141–155. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang C, Hua M, Wang R, Zhong C, Yu J, Han F, He N, Zhao Y, Liu G, Zheng N, Ji C, & Ma D (2017). NLRP3 inflammasome activation plays a carcinogenic role through effector cytokine IL-18 in lymphoma. Oncotarget, 8, 108571–108583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, & Liu H (2022). Prognostic association between NLRP3 inflammasome expression level and operable pancreatic adenocarcinoma. Int J Biol Markers, 37, 314–321. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Yao D, Cai Y, & Zhou T (2020). NLRP3 augmented resistance to gemcitabine in triple-negative breast cancer cells via EMT/IL-1beta/Wnt/beta-catenin signaling pathway. Biosci Rep, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivaki D, Borriello F, Chow OA, Doran B, Fleming I, Theisen DJ, Pallis P, Shalek AK, Sokol CL, Zanoni I, & Kagan JC (2020). Inflammasomes within Hyperactive Murine Dendritic Cells Stimulate Long-Lived T Cell-Mediated Anti-tumor Immunity. Cell Rep, 33, 108381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhivaki D, & Kagan JC (2021). NLRP3 inflammasomes that induce antitumor immunity. Trends Immunol, 42, 575–589. [DOI] [PubMed] [Google Scholar]
- Zhong C, Wang R, Hua M, Zhang C, Han F, Xu M, Yang X, Li G, Hu X, Sun T, Ji C, & Ma D (2021). NLRP3 Inflammasome Promotes the Progression of Acute Myeloid Leukemia via IL-1beta Pathway. Front Immunol, 12, 661939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhongbo Hu, M. S, Letterio John,, Targeting NLRP3 Inflammasome-Induced Therapy Resistance in ALL, Blood, Volume 136, S., 2020, Page 46, ISSN 0006–4971, https://doi.org/10.1182/, blood-2020–137520., (https://www.sciencedirect.com/science/article/pii/S0006497118719885), rates, A. B. P. a. l. l. A. i. t. m. c. c. c. a. t. m. f. c. o. d. f. c. b. y. o. a. S., Disclosures, & declare., N. r. c. o. i. t. [Google Scholar]
- Zhou R, Yazdi AS, Menu P, & Tschopp J (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature, 469, 221–225. [DOI] [PubMed] [Google Scholar]
- Zhou T, Damsky W, Weizman OE, McGeary MK, Hartmann KP, Rosen CE, Fischer S, Jackson R, Flavell RA, Wang J, Sanmamed MF, Bosenberg MW, & Ring AM (2020). IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature, 583, 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Ju HQ, Aguilar M, Gocho T, Li H, Iida T, Lee H, Fan X, Zhou H, Ling J, Li Z, Fu J, Wu M, Li M, Melisi D, Iwakura Y, Xu K, Fleming JB, & Chiao PJ (2016). IL1 Receptor Antagonist Inhibits Pancreatic Cancer Growth by Abrogating NF-kappaB Activation. Clin Cancer Res, 22, 1432–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


