Abstract
Purpose:
To determine the incidence of central serous chorioretinopathy (CSC) stratified by age, sex, and diagnosis with obstructive sleep apnea (OSA), and to determine whether some patients with newly diagnosed CSC may be candidates for OSA evaluation.
Design:
Retrospective cohort study.
Methods:
We used the IBM Marketscan database to select 59,016,145 commercially-insured patients in the United States between 2007 and 2016. We identified patients’ first diagnosis with CSC, and defined patients as having OSA if they had a diagnosis following a sleep study. We specified Cox proportional hazard models with interactions between age, sex, and OSA status to determine patients’ risk of developing CSC. We estimated the positive predictive value (PPV) that a new diagnosis of CSC would have in predicting a subsequent diagnosis of OSA.
Results:
Risk of CSC increased with age in years (HR=1.030, p<.001) and OSA diagnosis (HR=1.081, p=.033), and was lower in women (HR=0.284, p<.001). We estimated the annual incidence of CSC was 9.6 and 23.4 per 100,000 women and men, respectively. Incidence was higher in women and men with OSA (17.2 and 40.8 per 100,000). The PPV of CSC diagnosis as a predictor of OSA was highest in the fifth decade of life.
Conclusion:
The incidence of CSC in our patient sample is higher than previously reported. Risk of CSC is higher in men than in women, and OSA increases risk of CSC in both men and women. Some patients, particularly older males, may be good candidates for OSA evaluation following a CSC diagnosis.
INTRODUCTION
Central serous chorioretinopathy (CSC) is a disorder characterized by the formation of a localized neurosensory retinal detachment caused by leakage of fluid at the level of the retinal pigment epithelium (RPE). It is the fourth most common nonsurgical retinopathy after age-related macular degeneration, diabetic retinopathy, and branch retinal vein occlusion.1 It is more common in males than in females2–4 and primarily affects patients between the ages of 20 and 50.5,6The true incidence of CSC is unknown. The only population-based study in the United States reported an annual incidence of 9.9 cases per 100,000 men and 1.7 per 100,000 women in Olmstead County, Minnesota; a population-based study in Taiwan estimated an annual incidence of 27 cases per 100,000 men and 15 cases per 100,000 women.2,7
The pathophysiology of CSC remains poorly understood despite advances in imaging techniques and numerous studies of the disease.8 Many risk factors have been described in the literature, including psychologic stress,9,10 type A personality,11 corticosteroid medication use,12 elevation of endogenous steroids due to endocrine disorders such as Cushing’s syndrome13 or steroid-producing tumors,14 pregnancy,15 hypertension, and psychopharmacologic medication use.3
Obstructive sleep apnea (OSA) is characterized by recurrent episodes of partial or complete upper airway obstruction during sleep, leading to repetitive oxygen desaturation and/or cortical arousals with consequent autonomic nervous system activation.16OSA has been identified as a possible risk factor for the development of CSC,17,18 but the extent of this association is not fully understood. The intermittent episodes of asphyxia are believed to trigger increased sympathetic activity and the episodes of sudden arousal are physical stressors that may lead to elevated catecholamine levels.19,20This increased sympathetic drive is also present in CSC and can cause vascular endothelial dysfunction.21,22 It has also been hypothesized that the elevated cortisol levels may increase the likelihood of CSC development.22 Additionally, in both diseases, there is increased oxidative stress, platelet aggregation and hypercoagulability.23–25 Previous studies have noted co-occurrence of OSA in patients withCSC, but the magnitude of the association has been variable,17,18,26,27 and the extent to which the association is driven by other comorbidities, such as obesity, is unclear.28,29
We used administrative claims data to estimate the incidence of CSC in a national population of patients with employer-provided health insurance, the absolute risk of CSC conferred by an OSA diagnosis, and the feasibility of using a CSC diagnosis in an ophthalmologist’s office to identify undiagnosed cases ofOSA. This is the first estimate of the incidence of CSC in the United States drawn from a national sample, stratified by sex, age, and OSA diagnosis.
METHODS
Data source & sample selection
We used administrative insurance claims data from the IBM MarketScan database,one of the largest healthcare databases available for patients with employer-provided health insurance, including data from over 245 million unique patients since 1995, and data on patients in all 50 states. Our version of the database extends from 2007 to 2016.This database has been used in prior ophthalmic studies and in prior estimates of national disease incidence.30–36 MarketScan data are longitudinal, extending from the time patients first receive insurance coverage until they discontinue insurance, and include demographic information, diagnosis, procedural, and billing codes for patient encounters with health care providers. The institutional review board at Stanford University determined this secondary analysis of deidentified administrative data to be exempt from review.
We restricted this sample to include all patients in MarketScan with continuous insurance coverage, withat least 365 days of enrollment to avoid including patients with unobserved care. In sensitivity analyses, we limited the sample to patients with longer enrollment periods. In order to ensure that the diagnoses observed in our analysis were new diagnoses, rather than old diagnoses that were re-coded after patients changed insurers, we used a “lookback” period of 90 days, excluding patients whose first diagnosis of CSC or OSA occurred within their first 90 days of enrollment in the database.
Variable definitions
We categorized patients as having OSA if they received a diagnosis of OSA following a sleep study. OSA diagnosis was defined using International Classification of Diseases, 9th and 10th editions (ICD-9 and ICD-10) diagnosis codes (Supplemental Table 1). As a sensitivity analysis, we also created a more restrictive (but less inclusive) definition of sleep apnea, in which we defined patients as having OSA only if they received a diagnosis following a sleep study and then also had a record of receiving treatment with a continuous positive airway pressure (CPAP) device. Receipt of sleep studies and CPAP devices was identified using Current Procedural Terminology (CPT) codes (Supplemental Table 1). Diagnosis with CSC was identified as the first date in a patient’s record where an ICD-9 or ICD-10 code for CSC was listed (Supplemental Table 1).
Statistical analysis: Estimating overall incidence
To estimate the overall incidence of CSC in this sample, without adjusting for patient age, sex, or OSA, we constructed basic life tables by calculating the number of new diagnoses of CSC in each year, and dividing that by the total ‘time at risk’ for all patients in that year of the dataset. We calculated ‘time at risk’ in each year of the dataset according to the number of ‘person-years’ in that year. ‘Time at risk’ excludes patients who have already been diagnosed with CSC as they are no longer at risk for the disease. For example, if a patient began their insurance enrollment halfway through 2008, was diagnosed with CSC at the end of 2009, and then discontinued enrollment at the end of 2010, they would contribute 0.5 person-years to total time-at-risk in 2008, 1.0 person-years in 2009, and 0 person-years in 2010. These life tables allowed us to calculate overall incidence of CSC in the entire sample and compare those results with other studies that have estimated absolute risk of CSC in select populations.
Statistical analysis: Estimating risk of CSC
To estimate the absolute risk of CSC for patients depending on their age, sex, and OSA diagnosis, we conducted a time-to-event analysis beginning from a patient’s initial enrollment in the database, and extending until the date of their first diagnosis with CSC or, if they were never diagnosed with CSC, extending until they discontinued enrollment. We specified a Cox proportional hazards model, with patients’ age at enrollment, sex, and OSA diagnosis as covariates. As we were interested in estimating the effect of OSA on subsequent risk of developing CSC, patients were only coded as having OSA if their first diagnosis with OSA preceded their first diagnosis with CSC. To allow for a non-linear association between age and risk of CSC, we included a quadratic term for age. Additionally, in order to account for the possibility that age, sex, and OSA diagnosis may each affect risk of CSC differently depending on the values of the other covariates, we included interaction terms for all variables; for example, it is possible that age has a different effect on CSC risk for men and for women, and that OSA affects risk of CSC in young men differently than it affects risk for older women. We used the estimates from this model to calculate the annual risk of CSC for each patient.
We performed several sensitivity analyses to determine whether the risks estimated in this model changed substantially when OSA was coded differently, or when minimum follow-up time for patients in the model was adjusted. First, we re-estimated the model using the more restrictive definition of OSA, in which patients were only coded as having OSA if they completed a sleep study and also received a CPAP device. Second, we re-estimated the model restricting the sample to patients with several different minimum follow-up times, beginning with patients who had at least 1 year of enrollment in the database, and then increasing that requirement in 1-year increments, finishing with a model that only includes patients who had10 years of enrollment in the database. Increasing the minimum enrollment for the analysis reduced the sample size in terms of the number of patients the model used to estimate risk, but ensured that our calculations of risk were drawn from a sample of patients that were observed for a longer period, giving us more confidence that diagnoses of CSC really did represent patients’ first diagnosis, rather than a secondary diagnosis that was preceded by an unobserved index diagnosis occurring under a different insurance plan. Varying the follow-up time also allowed us to determine whether our estimated incidence of CSC was associated with how long patients were observed in the dataset. For example, patients with shorter periods of enrollment in the dataset may represent patients with poor insurance continuity, who may not receive appropriate preventive care, and may therefore have a higher burden of disease.37 Alternatively, it is possible that these patients may be less likely to be diagnosed with a disease even if it is present, due to less stable access to health services.38
Statistical analysis: Estimating the value of CSC as a screen for OSA
After estimating the overall incidence of CSC in our cohort and estimating the risk of CSC for individual patients depending on their age, sex, and OSA diagnosis, we reconsidered the potential relationship between CSC and OSA from the perspective of an ophthalmologist seeing a patient in clinic with newly diagnosed CSC. If OSA increases patients’ risk of developing CSC, then it is possible that a patient who presents to an ophthalmology clinic with new-onset CSC may have undiagnosed OSA. We analyzed whether CSC diagnosis might be used to screen for OSA in ophthalmology clinics, and whether ophthalmologists should consider referring patients with CSC for evaluation of suspected OSA. To assess whether ophthalmologists should consider referral in this population, we calculated the positive predictive value (PPV) of a CSC diagnosis in predicting a subsequent diagnosis of OSA. Our models of CSC risk relied on OSA diagnoses that precede CSC diagnosis, but in this analysis we considered the possibility that patients with CSC might have latent or undiagnosed OSA, and determined how many patients with newly diagnosed CSC would go on to develop their first diagnosis of OSA following their first CSC diagnosis. We stratified our estimates of PPV by patient age and sex, to determine whether CSC might be more useful for screening some populations than others.
RESULTS
We studied a population of 59,016,145 commercially-insured patients in the United States between 2007 and 2016. Women comprised 54% of the sample (N=32,018,719), and the mean age at the time of enrollment in the database was 33.1 years (SD 18.6). 39,254 patients were diagnosed with CSC. 1,539,006 patients were diagnosed with OSA, using the more inclusive definition of OSA (an ICD-9/10 diagnosis following a CPT code for a sleep study), and 1,000,130 patients were diagnosed with OSA under the most restrictive definition (ICD-9/10 diagnosis following a sleep study and a CPT code for a CPAP device).
Incidence of CSC
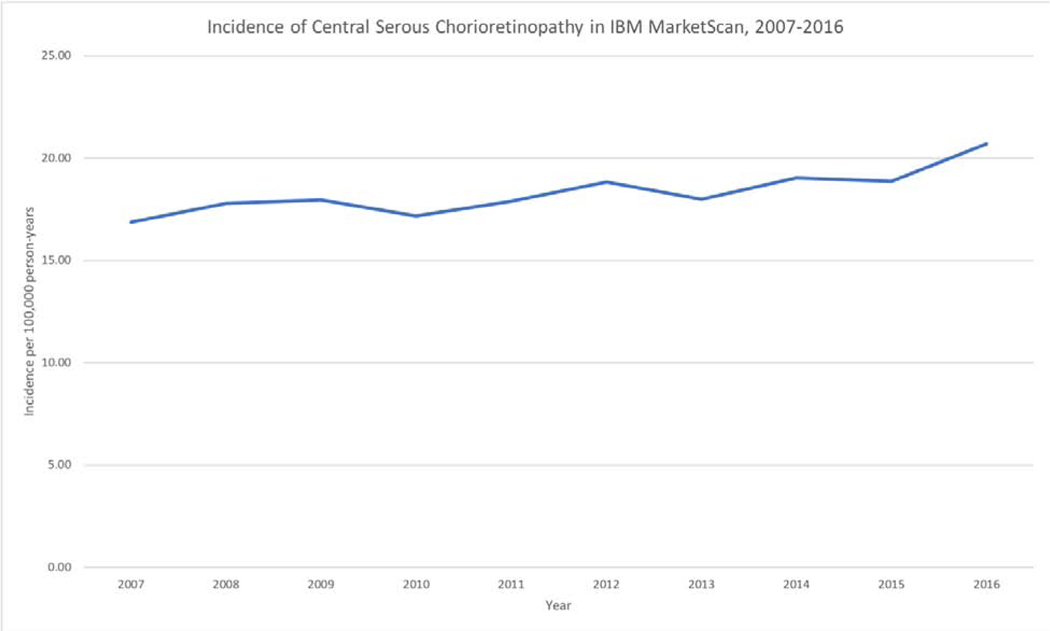
The overall incidence of CSC in this analysis was 18.3 per 100,000 person-years. The estimated incidence was quite stable between 2007 and 2016, ranging from a minimum of 16.9/100,000 person-years in 2007 to a maximum of 20.69/100,000 person-years in 2016. (Table 1, Figure 1).
Table 1:
Life tables illustrating incidence of central serous chorioretinopathy in the MarketScan database
| Year | Patients | Mean days at risk | Person-years at risk | New diagnoses of CSC | Rate of CSC per 100,000 Person-years |
|---|---|---|---|---|---|
|
| |||||
| 2007 | 16,763,128 | 340.1 | 15,619,882.7 | 2,636 | 16.88 |
| 2008 | 24,293,723 | 333.6 | 22,201,367.9 | 3,952 | 17.80 |
| 2009 | 26,537,499 | 333.2 | 24,221,873.2 | 4,352 | 17.97 |
| 2010 | 27,054,825 | 332.0 | 24,611,729.8 | 4,224 | 17.16 |
| 2011 | 28,829,932 | 329.9 | 26,053,593.8 | 4,656 | 17.87 |
| 2012 | 29,139,349 | 332.5 | 26,545,515.8 | 5,001 | 18.84 |
| 2013 | 24,736,057 | 332.4 | 22,524,883.9 | 4,054 | 18.00 |
| 2014 | 23,153,082 | 332.5 | 21,090,542.0 | 4,017 | 19.05 |
| 2015 | 18,325,421 | 332.4 | 16,687,665.6 | 3,150 | 18.88 |
| 2016 | 16,404,166 | 345.4 | 15,524,336.4 | 3,212 | 20.69 |
Figure 1:
Incidence of central serous chorioretinopathy in MarketScan over time
Risk of CSC by patient age, sex, and OSA diagnosis
Time-to-event analyses showed strong associations between age, sex, and OSA diagnosis and risk of developing CSC. An analysis of 15,250,959 patients with at least 5 years of continuous enrollment in the database found increased risk of CSC witheach year of age(HR= 1.030, p<.001)and OSA diagnosis (HR= 1.081, p=.033), and found decreased risk of CSC associated with female sex (HR=0.284, p<.001). These findings were robust in sensitivity analyses that varied the minimum follow-up time of patients in the sample; an analysis of 53,122,216 patients with at least 365 days of follow-up in the database also showed significant effects for age (HR=1.042 per year of life, p<.001), female sex (HR=0.292, p<.001), and OSA diagnosis (HR=1.117, p<.001).An analysis of 2,461,656 patients with 10 years of follow-upfound significant effects for age and sex, but not OSA when tested as an individual variable (age in years HR=1.013, p<.001,female HR=0.297, p<.001, OSA HR=1.116, p=.107), although OSA was still a significant contributor in to the overall model in joint F-tests of all interaction variables that included OSA (p<.001) .(Supplemental Table 2). Interaction terms between age, sex, and OSA diagnosis were jointly significant in all time-to-event analyses, regardless of minimum follow-up time (Supplemental Table 2). In a sensitivity analysis that used a more restrictive definition of OSA, OSA diagnosis was still associated with increased risk of CSC (HR=1.087, p=.044). (Supplemental Table 3).
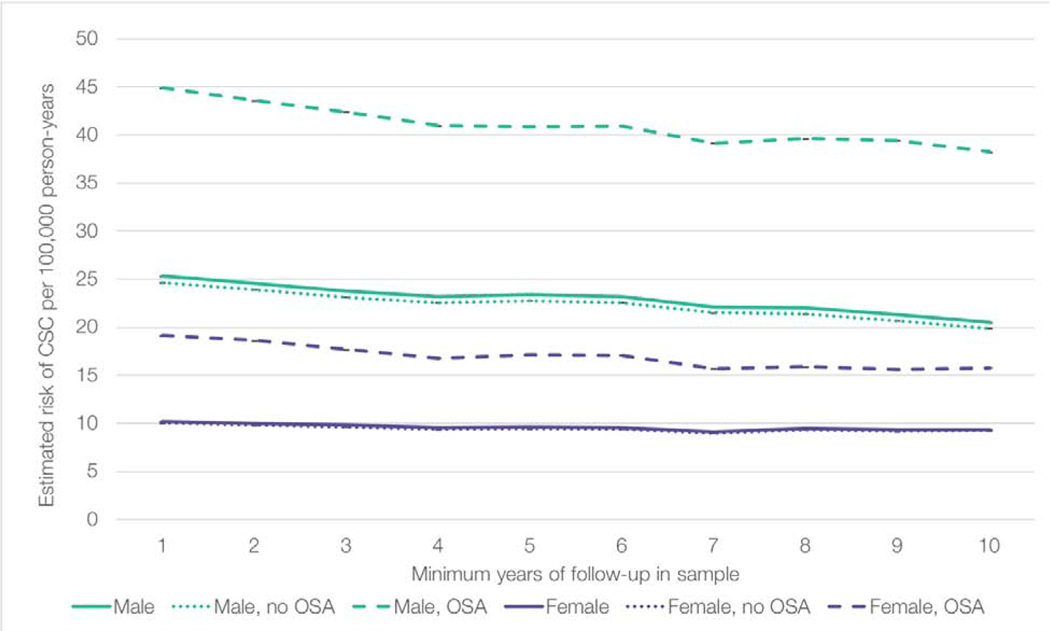
The results of each model in Supplemental Table 2, with minimum follow-up of 1 year up to 10 years, were used to estimate the annual risk of developing CSC among men and women with and without OSA (Figure 2). For all sub-groups, the estimated incidence of CSC decreased as the sample size decreased and minimum follow-up became longer. For example, the estimated incidence of men with OSA was approximately 44.9/100,000 patient-years in the model with one year of follow-up, and 38.2/100,000 patient-years in the model with 10 years of follow-up (p<.001). The relationship between age, sex, and OSA diagnosis appeared to be consistent in all models; OSA increased risk of CSC for both men and women, but the effect of sex was even larger than the effect of OSA – women with OSA were still at lower risk of CSC than men without OSA. Using the sample of patients with at least 5 years of follow-up within the database, we estimated that the annual CSC risk per 100,000 patients was as follows (maximum range of all models displayed in parentheses): 23.4 (20.5–25.3) for men overall, 22.8 (19.9–24.6) for men without OSA, 40.8 (38.2–44.9) for men with OSA, 9.6 (9.1–10.2) for women overall, 9.4 (9.2–10.0) for women without OSA, and 17.2 (15.8–19.1) for women with OSA. (Figure 2).
Figure 2:
Risk of central serous chorioretinopathy stratified by sex and obstructive sleep apnea, in models with different minimum follow-up time
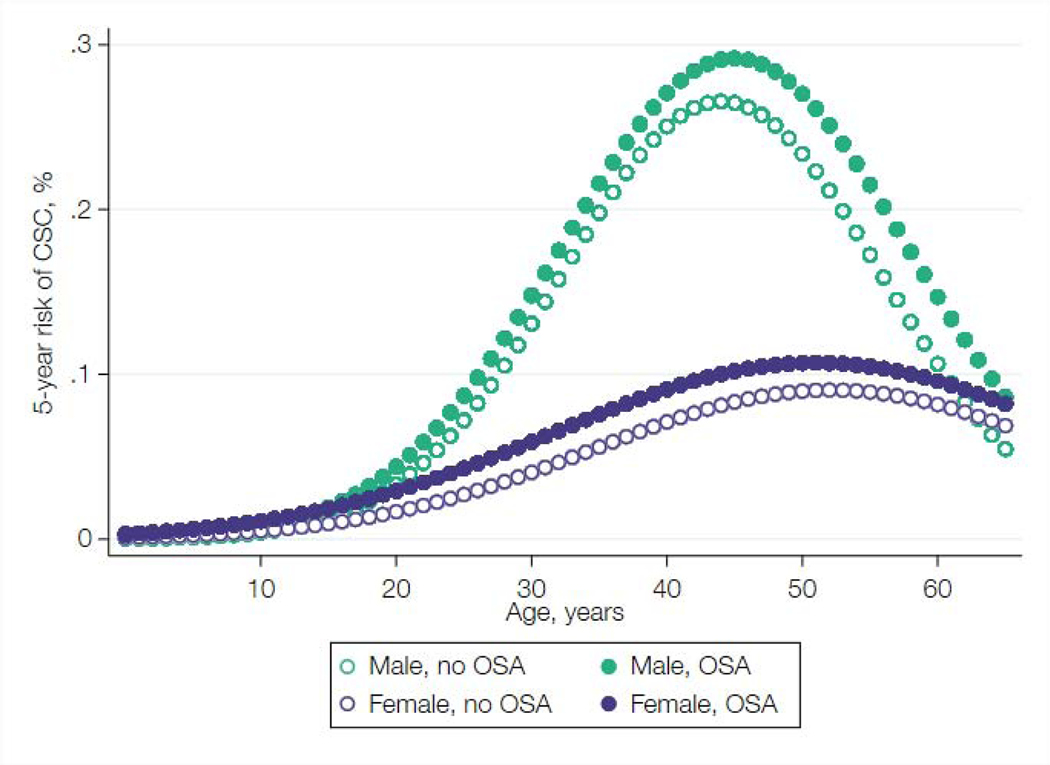
The results of the 5-year follow-up model (Supplemental Table 2, Column 5) were used to predict 5-year risk of developing CSC, stratified by age, sex, and OSA diagnosis(Figure 3). The results illustrative how risk of CSC is modified by each of these variables. In this sample, risk for men rose dramatically during their 20s and 30s, peaking after age 40, and then declined to match the risk for women around age 60. In contrast, risk for women peaked slightly later, after age 50, and in general was less dramatically affected by age. (Figure 3).
Figure 3:
Estimated 5-year risk of developing central serous chorioretinopathy stratified by age, sex, and obstructive sleep apnea.
Feasibility of CSC as a screening test for OSA
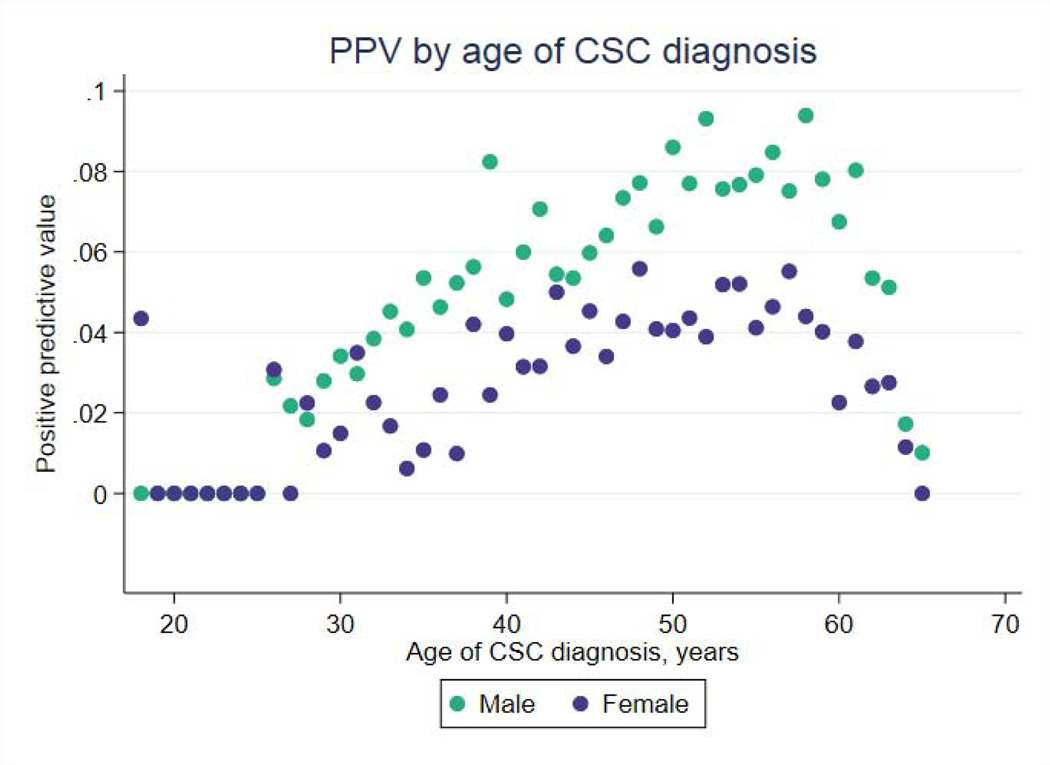
We investigated the feasibility of using CSC to predict OSA by estimating the positive predictive value of CSC diagnosis in predicting OSA diagnosis within one year. PPV ranged between 3% and 10% for men, and between 0% and 6% for women, peaking for both groups in the 5th and 6th decades of life, during which PPV was approximately 8–10% for men and 4–6% for women (Figure 4).
Figure 4:
Positive predictive value of central serous chorioretinopathy in predicting a subsequent diagnosis of obstructive sleep apnea within one year, by sex and age
DISCUSSION
We present the first model of CSC risk to use a national sample of patients in the United States. Under different minimum follow-up periods ranging from 1 year to 10 years, we estimate the annual incidence of CSC is 9.6 (min. 9.1, max. 10.2) per 100,000 women and 23.4 (min. 20.5, max. 25.3) per 100,000 men. This is appreciably higher than the previously reported incidence out of Olmsted County, which was limited to one geographic area and a smaller cohort.2 Incidence was higher among people with OSA, ranging from 17.2 (min. 15.8, max. 19.1) per 100,000 women, to 40.8 (min. 38.2, max. 44.9) per 100,000 men (Figure 2). Accurate estimates of disease incidence are critical for understanding how relative risk factors translate to absolute risk of disease for individual patients. Accurate estimates are also important for public health priorities, such as crafting appropriate health policy and predicting the future burden of disease in the U.S. population.
Our results confirm a strong association between OSA and CSC and we are the first study to estimate an absolute risk of OSA on CSC that is stratified by age and sex. Prior studies report the odds of OSA to be 1.6 and 3.7 times higher in CSC patients compared to the general population.18,39 These studies were limited by cohort size and by the lack of definitive diagnosis of OSA by overnight polysomnogram testing.39In contrast, we queried over 59 million patients and identified OSA patients only if they received a diagnosis following a sleep study, and performed a sensitivity analysis further restricting our definition of OSA to patients who had a sleep study as well as a record of receiving a CPAPdevice. Patients with a diagnosis of OSA were significantly more likely to receive a subsequent diagnosis of CSC. Our findings support a strong male predilection; men without OSA still had a higher estimated risk of CSC than women with OSA.
To make our findings applicable to clinical ophthalmologists, we explored the feasibility of using CSC as a screening test for undiagnosed OSA. Undiagnosed or untreated OSA is associated with excessive daytime sleepiness, impaired neurocognitive performance, an increased risk of motor vehicle and occupational accidents, a reduced quality of life, and cardiovascular and cerebrovascular morbidity and mortality.40,41A large 10-year prospective study found that untreated severe OSA independently increased the odds of fatal and nonfatal cardiovascular events by 2.87 and 3.17, respectively.42 Additionally, after adjusting for age, sex, body-mass-index (BMI), and prevalent coronary heart disease, the Sleep Heart Health Study found that individuals with OSA had four times the risk of atrial fibrillation compared with those without OSA.43 It has also been reported that 93% of women and 82% of men with moderate to severe OSA have not been clinically diagnosed, emphasizing the importance of recognizing associated medical comorbid conditions that may implicate OSA as an underlying diagnosis.44
If OSA increases patients’ risk of developing CSC, then it is possible that a patient who presents to an ophthalmology clinic with new-onset CSC may have undiagnosed OSA. We calculated the PPV of a new CSC diagnosis in predicting a subsequent diagnosis of OSA within one year. We used this estimate as a proxy for the PPV of CSC as a screening test if ophthalmologists referred all new CSC patients for OSA evaluation. We found a rise in PPV with age, that ultimately peaked in the 5th and 6th decades of life. This rise is likely driven by rising prevalence of obesity with age, as the PPV of any screening test rises with the prevalence of the diagnosis for which it is being screened.45,46In our sample, the estimated utility of CSC in screening for OSA is low in absolute terms – even individuals in their 5th and 6th decades of life had less than a 10% chance of receiving a new OSA diagnosis within one year of being diagnosed with CSC. If CSC were used as a screening test for OSA in the absence of other clinical information, it would result in a large number of unnecessary, and potentially expensive, evaluations for sleep apnea. However, our estimates of the PPV of CSC diagnosis for OSA may be an underestimate of the real-world utility of CSC in screening for OSA, as our models do not account for BMI and other risk factors for OSA that clinicians are likely to have access to when deciding whether to refer patients for OSA evaluation.47–49 This association should be further explored in the clinical setting, incorporating additional patient characteristics, clinical history, and findings on ocular exam and imaging.
Our findings suggestthat there are subgroups of patients, particularly males in their 5th and 6thdecade, who may reasonably be referred for OSA evaluation in the setting of new onset CSC. Our findings do not imply a causal link between OSA and CSC; further studies are needed to elucidate the relationship between these diseases. However, the efficacy of sleep study referral in the setting of a new CSC diagnosis does not necessarily depend on the direction of a causal pathway linking the diseases – the purpose of referral is to identify undiagnosed OSA, and the efficacy of referral depends on correlation between OSA and CSC.
Strengths and Limitations
Overall, our estimates of incidence are very stable throughout different years of the sample and over different follow-up periods, but we report the full range of estimates from all models in order to be transparent about variation in our estimates, since this variation is not captured in the p-value or 95% confidence interval for any individual model.
As with all studies of administrative claims data, our findings are limited by diagnostic coding errors and bya lack ofgranular clinical information. We do not have access to informative ocular imaging that could confirm our CSC diagnosis and further identify acute, recurrent, and chronic CSC as it relates to OSA diagnosis and treatment. We also recognize the possibility of coding error for entities that may present with CSC-like findings clinically. Additionally, the database does not include information that could be useful in predicting OSA diagnosis, such as patient’s BMI data.28 Our cohort is drawn from the commercially-insured population in the United States, but our results may not be applicable for estimating risk in the Medicare population or among patients who are under-insured or on Medicaid.
Conclusions
We estimate that the incidence of CSC in the United States is higher than previously reported. Men are at higher risk of CSC than women, and that OSA increases risk of CSC for both men and women. Some patients, particularly males in their 5th and 6th decade, with a new CSC diagnosis may be good candidates for OSA evaluation.
Supplementary Material
Supplemental Table 1: Diagnosis and procedural codes
Supplemental Table 2: Results of Cox proportional hazards models for all follow-up periods
Supplemental Table 3: Results of a Cox proportional hazards model with 5-year follow-up and with a more restrictive definition of obstructive sleep apnea
ACKNOWLEDGEMENTS:
Funding:
This research was supported by an unrestricted grant from the Research to Prevent Blindness, and the National Eye Institute P30-EY026877 awarded to the Department of Ophthalmology. The sponsor or funding organization had no role in the design or conduct of this research. This work was also supported by a grant from the Stanford School of Medicine MedScholars Fund awarded to Daniel Vail. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Meeting Presentation:
The American Academy of Ophthalmology Annual Meeting, 2019
Conflict of interest: no conflicting relationship exists for any author
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang M, Munch IC, Hasler PW, Prunte C, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126–145. doi: 10.1111/j.1600-0420.2007.00889.x [DOI] [PubMed] [Google Scholar]
- 2.Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology. 2008;115(1):169–173. doi: 10.1016/j.ophtha.2007.02.032 [DOI] [PubMed] [Google Scholar]
- 3.Tittl MK, Spaide RF, Wong D, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999;128(1):63–68. doi: 10.1016/S0002-9394(99)00075-6 [DOI] [PubMed] [Google Scholar]
- 4.Spitznas M, Huke J. Number, shape, and topography of leakage points in acute type I central serous retinopathy. Graefes Arch Clin Exp Ophthalmol. 1987;225(6):437–440. doi: 10.1007/BF02334172 [DOI] [PubMed] [Google Scholar]
- 5.Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996;103(12):2070–2079; discussion 2079–2080. doi: 10.1016/S0161-6420(96)30386-2 [DOI] [PubMed] [Google Scholar]
- 6.Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111(2):244–249. doi: 10.1016/j.ophtha.2003.09.024 [DOI] [PubMed] [Google Scholar]
- 7.Tsai DC, Chen SJ, Huang CC, et al. Epidemiology of idiopathic central serous chorioretinopathy in Taiwan, 2001–2006: a population-based study. PLoS One.2013;8(6):e66858. doi: 10.1371/journal.pone.0066858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013;58(2):103–126. doi: 10.1016/j.survophthal.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63(3):Suppl:1–139. [PubMed] [Google Scholar]
- 10.Gelber GS, Schatz H. Loss of vision due to central serous chorioretinopathy following psychological stress. Am J Psychiatry. 1987;144(1):46–50. doi: 10.1176/ajp.144.1.46 [DOI] [PubMed] [Google Scholar]
- 11.Yannuzzi LA. Type-A behavior and central serous chorioretinopathy. Retina. 1987;7(2):111–131. doi: 10.1097/00006982-198700720-00009 [DOI] [PubMed] [Google Scholar]
- 12.Wakakura M, Ishikawa S. Central serous chorioretinopathy complicating systemic corticosteroid treatment. Br J Ophthalmol. 1984;68(5):329–331. doi: 10.1136/bjo.68.5.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouzas EA, Scott MH, Mastorakos G, Chrousos GP, Kaiser-Kupfer MI. Central serous chorioretinopathy in endogenous hypercortisolism. Arch Ophthalmol. 1993;111(9):1229–1233. doi: 10.1001/archopht.1993.01090090081024 [DOI] [PubMed] [Google Scholar]
- 14.Thoelen AM, Bernasconi PP, Schmid C, Messmer EP. Central serous chorioretinopathy associated with a carcinoma of the adrenal cortex. Retina. 2000;20(1):98–99. doi: 10.1097/00006982-200001000-00020 [DOI] [PubMed] [Google Scholar]
- 15.Chumbley LC, Frank RN. Central serous retinopathy and pregnancy. Am J Ophthalmol. 1974;77(2):158–160. doi: 10.1016/0002-9394(74)90667-9 [DOI] [PubMed] [Google Scholar]
- 16.Amatoury J, Jordan AS, Toson B, Nguyen C, Wellman A, Eckert DJ. New insights into the timing and potential mechanisms of respiratory-induced cortical arousals in obstructive sleep apnea. Sleep. 2018;41(11). doi: 10.1093/sleep/zsy160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloos P, Laube I, Thoelen A. Obstructive sleep apnea in patients with central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246(9):1225–1228. doi: 10.1007/s00417-008-0837-0 [DOI] [PubMed] [Google Scholar]
- 18.Leveque TK, Yu L, Musch DC, Chervin RD, Zacks DN. Central serous chorioretinopathy and risk for obstructive sleep apnea. Sleep Breath. 2007;11(4):253–257. doi: 10.1007/s11325007-0112-3 [DOI] [PubMed] [Google Scholar]
- 19.Dimsdale JE, Coy T, Ziegler MG, Ancoli-Israel S, Clausen J. The effect of sleep apnea on plasma and urinary catecholamines. Sleep. 1995;18(5):377–381. [PubMed] [Google Scholar]
- 20.Grunstein RR, Stewart DA, Lloyd H, Akinci M, Cheng N, Sullivan CE. Acute withdrawal of nasal CPAP in obstructive sleep apnea does not cause a rise in stress hormones. Sleep. 1996;19(10):774–782. doi: 10.1093/sleep/19.10.774 [DOI] [PubMed] [Google Scholar]
- 21.Michael JC, Pak J, Pulido J, de Venecia G. Central serous chorioretinopathy associated with administration of sympathomimetic agents. Am J Ophthalmol. 2003;136(1):182–185. doi: 10.1016/S0002-9394(03)00076-X [DOI] [PubMed] [Google Scholar]
- 22.Kasasbeh E, Chi DS, Krishnaswamy G. Inflammatory aspects of sleep apnea and their cardiovascular consequences. South Med J. 2006;99(1):58–67; quiz 68–59, 81. [DOI] [PubMed] [Google Scholar]
- 23.Caccavale A, Romanazzi F, Imparato M, Negri A, Morano A, Ferentini F. Low-dose aspirin as treatment for central serous chorioretinopathy. Clin Ophthalmol. 2010;4:899–903. doi: 10.2147/OPTH.S12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iijima H, Iida T, Murayama K, Imai M, Gohdo T. Plasminogen activator inhibitor 1 in central serous chorioretinopathy. Am J Ophthalmol. 1999;127(4):477–478. doi: 10.1016/S00029394(98)00378-X [DOI] [PubMed] [Google Scholar]
- 25.Toraldo DM, Peverini F, De Benedetto M, De Nuccio F. Obstructive sleep apnea syndrome: blood viscosity, blood coagulation abnormalities, and early atherosclerosis. Lung. 2013;191(1):1–7. doi: 10.1007/s00408-012-9427-3 [DOI] [PubMed] [Google Scholar]
- 26.Yavas GF, Kusbeci T, Kasikci M, et al. Obstructive sleep apnea in patients with central serous chorioretinopathy. Curr Eye Res. 2014;39(1):88–92. doi: 10.3109/02713683.2013.824986 [DOI] [PubMed] [Google Scholar]
- 27.Chatziralli I, Kabanarou SA, Parikakis E, Chatzirallis A, Xirou T, Mitropoulos P. Risk Factors for Central Serous Chorioretinopathy: Multivariate Approach in a Case-Control Study. Curr Eye Res. 2017;42(7):1069–1073. doi: 10.1080/02713683.2016.1276196 [DOI] [PubMed] [Google Scholar]
- 28.Brodie FL, Charlson ES, Aleman TS, et al. Obstructive sleep apnea and central serous chorioretinopathy. Retina. 2015;35(2):238–243. doi: 10.1097/IAE.0000000000000326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji Y, Li M, Zhang X, Peng Y, Wen F. Poor Sleep Quality Is the Risk Factor for Central Serous Chorioretinopathy. J Ophthalmol. 2018;2018:9450297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheer D, Schwartz SW, Parr M, Zgibor J, Sanchez-Anguiano A, Rajaram L. Prevalence and incidence of narcolepsy in a US health care claims database, 2008–2010. Sleep. 2019;42(7). doi: 10.1093/sleep/zsz091 [DOI] [PubMed] [Google Scholar]
- 31.Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int. 2017;37(9):1551–1557. doi: 10.1007/s00296-017-3726-1 [DOI] [PubMed] [Google Scholar]
- 32.Broder MS, Chang E, Cherepanov D, Neary MP, Ludlam WH. INCIDENCE AND PREVALENCE OF ACROMEGALY IN THE UNITED STATES: A CLAIMS-BASED ANALYSIS. Endocr Pract. 2016;22(11):1327–1335. doi: 10.4158/EP161397.OR [DOI] [PubMed] [Google Scholar]
- 33.Rassen JA, Bartels DB, Schneeweiss S, Patrick AR, Murk W. Measuring prevalence and incidence of chronic conditions in claims and electronic health record databases. Clin Epidemiol. 2019;11:1–15. doi: 10.2147/CLEP.S181242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brilliant MH, Vaziri K, Connor TB Jr., et al. Mining Retrospective Data for Virtual Prospective Drug Repurposing: L-DOPA and Age-related Macular Degeneration. Am J Med. 2016;129(3):292–298. doi: 10.1016/j.amjmed.2015.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lykins J, Wang K, Wheeler K, et al. Understanding Toxoplasmosis in the United States Through “Large Data” Analyses. Clin Infect Dis. 2016;63(4):468–475. doi: 10.1093/cid/ciw356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang SV, Li N, Rice DS, et al. Using Healthcare Databases to Refine Understanding of Exploratory Associations Between Drugs and Progression of Open-Angle Glaucoma. Clin Pharmacol Ther. 2019;106(4):874–883. doi: 10.1002/cpt.1490 [DOI] [PubMed] [Google Scholar]
- 37.Gold R, DeVoe J, Shah A, Chauvie S. Insurance continuity and receipt of diabetes preventive care in a network of federally qualified health centers. Med Care. 2009;47(4):431–439. doi: 10.1097/MLR.0b013e318190ccac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavarreda SA, Gatchell M, Ponce N, Brown ER, Chia YJ. Switching health insurance and its effects on access to physician services. Med Care. 2008;46(10):1055–1063. doi: 10.1097/MLR.0b013e318187d8db [DOI] [PubMed] [Google Scholar]
- 39.Wu CY, Riangwiwat T, Rattanawong P, Nesmith BLW, Deobhakta A. ASSOCIATION OF OBSTRUCTIVE SLEEP APNEA WITH CENTRAL SEROUS CHORIORETINOPATHY AND CHOROIDAL THICKNESS: A Systematic Review and Meta-Analysis. Retina. 2018;38(9):1642–1651. doi: 10.1097/IAE.0000000000002117 [DOI] [PubMed] [Google Scholar]
- 40.Vanderveken OM, Boudewyns A, Ni Q, et al. Cardiovascular implications in the treatment of obstructive sleep apnea. J Cardiovasc Transl Res. 2011;4(1):53–60. doi: 10.1007/s12265-010-9238-y [DOI] [PubMed] [Google Scholar]
- 41.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 42.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7 [DOI] [PubMed] [Google Scholar]
- 43.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. doi: 10.1093/sleep/20.9.705 [DOI] [PubMed] [Google Scholar]
- 45.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea:rationale, design, and major findings of the Wisconsin Sleep Cohort study. Wmj. 2009;108(5):246–249. [PMC free article] [PubMed] [Google Scholar]
- 46.Jennum P, Riha RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J. 2009;33(4):907–914. doi: 10.1183/09031936.00180108 [DOI] [PubMed] [Google Scholar]
- 47.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderateweight change and sleep-disordered breathing. Jama. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015 [DOI] [PubMed] [Google Scholar]
- 48.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. Jama. 2004;291(16):2013–2016. doi: 10.1001/jama.291.16.2013 [DOI] [PubMed] [Google Scholar]
- 49.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence ofsleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Diagnosis and procedural codes
Supplemental Table 2: Results of Cox proportional hazards models for all follow-up periods
Supplemental Table 3: Results of a Cox proportional hazards model with 5-year follow-up and with a more restrictive definition of obstructive sleep apnea