Abstract
Sepsis is a dysregulated host response to an infection, characterized by organ failure. The pathophysiology is complex and incompletely understood, but mitochondria appear to play a key role in the cascade of events that culminate in multiple organ failure and potentially death. In shaping immune responses, mitochondria fulfil dual roles: they not only supply energy and metabolic intermediates crucial for immune cell activation and function but also influence inflammatory and cell death pathways. Importantly, mitochondrial dysfunction has a dual impact, compromising both immune system efficiency and the metabolic stability of end organs. Dysfunctional mitochondria contribute to the development of a hyperinflammatory state and loss of cellular homeostasis, resulting in poor clinical outcomes. Already in early sepsis, signs of mitochondrial dysfunction are apparent and consequently, strategies to optimize mitochondrial function in sepsis should not only prevent the occurrence of mitochondrial dysfunction, but also cover the repair of the sustained mitochondrial damage. Here, we discuss mitochondrial quality control (mtQC) in the pathogenesis of sepsis and exemplify how mtQC could serve as therapeutic target to overcome mitochondrial dysfunction. Hence, replacing or repairing dysfunctional mitochondria may contribute to the recovery of organ function in sepsis. Mitochondrial biogenesis is a process that results in the formation of new mitochondria and is critical for maintaining a pool of healthy mitochondria. However, exacerbated biogenesis during early sepsis can result in accumulation of structurally aberrant mitochondria that fail to restore bioenergetics, produce excess reactive oxygen species (ROS) and exacerbate the disease course. Conversely, enhancing mitophagy can protect against organ damage by limiting the release of mitochondrial-derived damage-associated molecules (DAMPs). Furthermore, promoting mitophagy may facilitate the growth of healthy mitochondria by blocking the replication of damaged mitochondria and allow for post sepsis organ recovery through enabling mitophagy-coupled biogenesis. The remaining healthy mitochondria may provide an undamaged scaffold to reproduce functional mitochondria. However, the kinetics of mtQC in sepsis, specifically mitophagy, and the optimal timing for intervention remain poorly understood. This review emphasizes the importance of integrating mitophagy induction with mtQC mechanisms to prevent undesired effects associated with solely the induction of mitochondrial biogenesis.
Keywords: Sepsis, Mitochondrial quality control, Mitochondrial biogenesis, Mitochondrial dynamics, Mitophagy
Graphical abstract
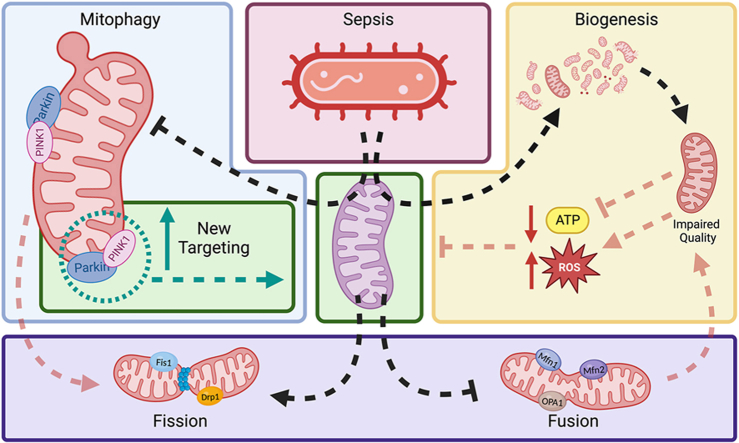
Under healthy conditions (depicted in purple), mitochondria maintain a delicate balance of mtQC mechanisms to ensure optimal mitochondrial mass and function. However, in sepsis (highlighted in the red block), multiple factors disrupt this equilibrium. As sepsis progresses, mitochondrial dysfunction becomes evident, characterized by reduced ATP production and increased ROS [1]. This dysfunctional state triggers a series of responses aimed at mitigating damage and restoring mitochondrial health. Excessive mitochondrial proliferation (depicted in the yellow block) occurs as a compensatory mechanism, attempting to counteract the deteriorating mitochondrial function [2]. Simultaneously, mitophagy is inhibited (depicted in the blue block), further contributing to mitochondrial dysfunction [3]. Additionally, increased mitochondrial fission (highlighted in the purple block) is observed as a response to sepsis-induced stress [4]. To restore cellular homeostasis, it is crucial to promote patent mitophagy and shift the balance towards fusion, allowing for the removal of faulty mitochondria. Only after successful induction of mitophagy can upregulation of mitochondrial biogenesis be beneficial for restoring mitochondrial mass and bioenergetics. This graphical abstract highlights the intricate interplay between mtQC mechanisms in sepsis, emphasizing the importance of restoring mitochondrial health to maintain overall cellular homeostasis.
Highlights
-
•
Mitochondrial dysfunction drives inflammation and organ damage.
-
•
Mitophagy and biogenesis can be induced pharmacologically, and hold promise for future therapeutic avenues.
-
•
The key lies in the timing of the interventions promoting mitochondrial quality control in sepsis.
-
•
Induction of mitophagy and mitochondrial fusion during sepsis improves outcomes in pre-clinical models.
-
•
Induction of mitochondrial biogenesis after sepsis improves cognition in rats.
1. Introduction
Sepsis is a systemic host response to an infection that is characterized by organ failure and is among the leading causes of death worldwide [5,6]. The World Health Organization (WHO) declared sepsis a global health priority in 2017 and adopted a resolution to improve the prevention, diagnosis, and management of sepsis. Despite the extensive research being conducted, the pathophysiology of sepsis remains only partially understood [6]. Sepsis survival has increased over the last decades, but mortality remains around 20% [7,8]. Currently, the most important outcome predictor remains time-to-antibiotics, in case sepsis patients present with hypotension [9] as therapeutic options directed at the molecular cause of sepsis induced organ dysfunction are lacking. The mainstay of treatment therefore remains to be antimicrobial therapy and supportive care [6,10]. There is a long-established recognition that the host response, rather than the pathogen itself, is responsible for much of the tissue damage observed in sepsis [6,8,11]. In particular, the hyperinflammatory response that can occur in sepsis can lead to widespread tissue damage and organ failure [6,8,12], exposing sepsis survivors increased all-cause mortality risk as well as functional and cognitive impairments [13,14]. Additionally, mitochondrial dysfunction is considered to play a key role in the induction of organ damage and failure in sepsis [15].
Mitochondrial quality control (mtQC) is the process by which mitochondria maintain their ability to respirate and regulate their multiple effector functions. Mitochondrial DNA (mtDNA) is replicated and transcribed in the mitochondrial matrix. Damage to mtDNA can lead to mitochondrial dysfunction and disease [[16], [17], [18]]. Besides energy production, mitochondria are involved in cellular processes such as calcium homeostasis, the generation of reactive oxygen species (ROS), and the initiation of cell death [19]. In sepsis, mitochondria play a crucial role in maintenance of endothelial cell homeostasis [20,21], where dysfunction can lead to both micro- and macrovascular complications. Mitochondria further play a critical role in the immune system in sepsis, as they are central to both the energy metabolism of immune cells and the regulation of inflammation through ROS generation and inflammasome assembly [22].
Sepsis leads to mitochondrial electron transport chain (ETC) complex dysfunction, particularly of complexes I, III and IV [1]. Dysfunctional mitochondria can contribute to the development of a (hyper)inflammatory state [23,24] by increasing ROS production during infection in response to pathogen-associated molecular patterns (PAMPs) [25], damage-associated molecular patterns (DAMPs) [26], and pro-inflammatory cytokines released by leukocytes as part of the host immune response [27]. In response to pro-inflammatory cytokines during sepsis, skeletal muscle cells produce and release nitric oxide (NO). In septic patients, excessive NO production links to an increased requirement for vasopressor drugs to maintain blood pressure, and findings show a positive correlation between tissue nitrite/nitrate concentrations and disease severity, as well as between complex I activity and both reduced ATP and glutathione concentrations. Conversely, vasopressor requirement is inversely correlated with both complex I activity and ATP concentrations, and between nitrite/nitrate levels and both complex I activity and reduced glutathione concentrations. These findings underpin organ failure resulting from bioenergetic malfunction [28]. Mitochondrial dysfunction in sepsis leads to increased oxidative stress, which is responsible for most of the cell damage observed [[29], [30], [31]]. The oxidative stress can lead to damage to the mitochondrial membrane, which results in increased mitochondrial permeability and leakage of mitochondrial DNA into the circulation, where it may act as a DAMP, thereby perpetuating the systemic inflammatory response [32]. Finally, in mice and human fibroblasts mitochondrial dysfunction induces cellular senescence and accelerates aging [33], which may very well account for the increased morbidity and mortality observed in sepsis patients [5,9,14]. This observation further supports the correlation between mitochondrial dysfunction, inflammation, and clinical outcomes in sepsis.
To summarise, in addition to promoting immune system activation, mitochondrial dysfunction also contributes to the organ failure in sepsis and poor clinical outcome. Based on the current knowledge, mitochondria have a dual role during infections: while they play a crucial role in generating cellular energy and regulating the innate immune response, damage to mitochondria leads to dysfunctional mitochondria with impaired ATP production and excessive ROS formation contributing to inflammation and organ damage. Yet, how mitochondrial quality control mechanisms influence the course of sepsis is currently unclear. Thus, this review discusses the effects of modulating mitochondrial quality control mechanisms and their association with sepsis outcomes.
2. Relevance of patent mitochondria in sepsis
2.1. Mitochondrial dysfunction and immune cell activation
Having established mitochondria as central organelles in the context of sepsis and its outcomes, we now delve deeper into the necessity of mitigating mitochondrial damage and the ramifications of dysfunctional mitochondria for disease progression in sepsis. Our focus will be directed towards understanding mitochondria as catalysts for inflammation, contributors to vascular complications, and mediators of cell death and end-organ dysfunction in sepsis (Fig. 1).
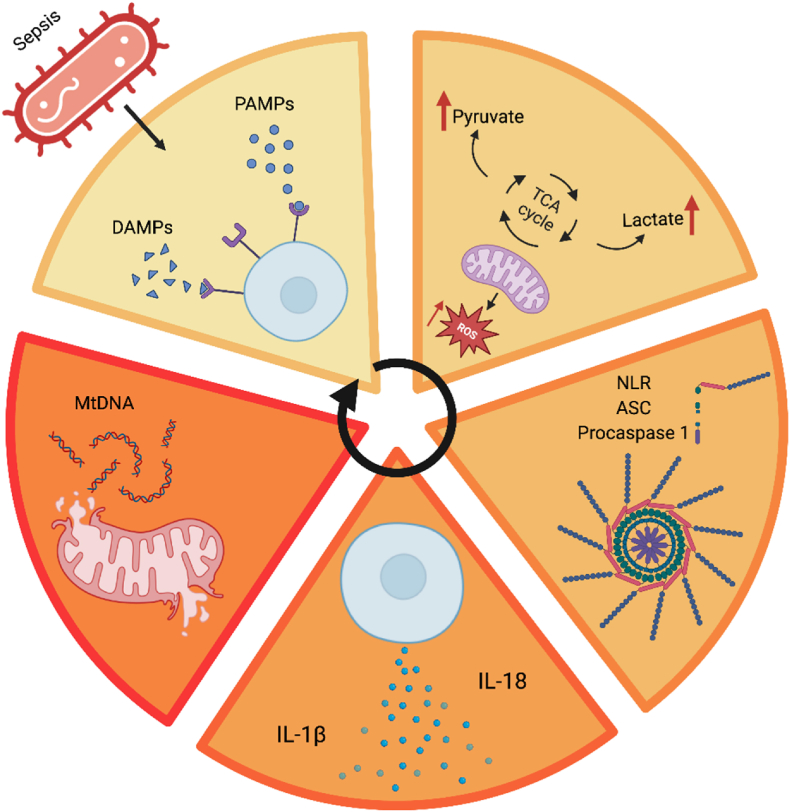
Fig. 1.
Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs) interact with cell surface receptors, including Toll-like receptors (TLRs) and other pattern recognition receptors (PRRs), initiating the cascade of mitochondrial dysfunction and inflammation in sepsis [25,26]. Mitochondria within the cell emit reactive oxygen species (ROS) molecules, symbolizing oxidative stress which in turn triggers the formation of the inflammasome, key components such as NOD-like receptors (NLRs), apoptosis-associated speck-like protein (ASC), and procaspase 1 come together, marking the initiation of the inflammatory response [34,35]. Cytokines, such as IL-1β and IL-18, are secreted from the cell and result in mitochondrial damage [36,37]. Fragments of mitochondrial DNA released from the damaged mitochondria act as DAMPs. A feedback loop is formed perpetuating the cycle of inflammation and cellular response. Further mitochondrial damage results in the upregulation of the glycolytic pathway (Warburg effect) [38,39].
Mitochondria play an important role in the innate immune system. One way that mitochondria act as immune defence effectors is by activating the inflammasome [40]. Mitochondria from both immune and non-immune cells increase ROS production during infection in response to both pathogen-associated molecular patterns (PAMPs) [25] and DAMPs [26]. ROS are important signalling molecules that contribute to pathogen (particularly bacterial) clearance, while excessive ROS generation can lead to tissue damage [41]. PAMPs from the invading pathogens, as well as DAMPs from injured tissues, can stimulate the NLRP3 inflammasome [34,35]. Mitochondrial DNA (mtDNA) is a central DAMP in the propagation of the immune response and associated damage [17,42]. When mtDNA is released from damaged or stressed cells, it can enter neighbouring cells and activate the inflammasome by binding to the protein NOD-like receptor family, pyrin domain-containing 3 (NLRP3) [43,44]. NLRP3 is responsible for initiating the production of pro-inflammatory cytokines such as interleukin-1 beta (IL-1β) and IL-18 [36,37]. In turn, pro-inflammatory cytokines released as part of the host response contribute to the systemic inflammatory response seen in sepsis, causing tissue damage and organ dysfunction [27,45].
In sepsis, mitochondria play a dual role in both aiding pathogen clearance and causing tissue damage. Mitochondrial components, like DNA and ROS, can activate the inflammasome, in turn increasing levels of pro-inflammatory cytokines. This activation, exacerbated by signals from both damaged cells and bacterial elements, intensifies inflammation, resulting in further tissue harm and organ dysfunction. Grasping the mitochondrial role in the immune response offers insights into sepsis pathophysiology.
2.2. Metabolic reprogramming contributes to (micro)vascular and organ dysfunction in sepsis
Metabolic reprogramming refers to the adaptive shifts cells undergo in their energy production and consumption pathways, optimizing their function in response to specific environmental cues or challenges [46]. Metabolic reprogramming, which is essential for mounting an adequate immune response, particularly influences neutrophils and macrophages by leveraging mitochondria for citrate production, fatty acid synthesis, and ATP generation, thereby facilitating rapid proliferation and effector function. This metabolic shift towards aerobic glycolysis is known as the Warburg effect [38,39]. Neutrophils and macrophages require relatively large amounts of ATP to carry out their effector functions and are among the first cells to undergo metabolic reprogramming in sepsis [47]. Although aerobic glycolysis is less efficient than oxidative phosphorylation for energy production, the Warburg effect allows rapid generation of metabolic intermediates and ATP required to allow fast proliferation of leukocytes [38,48]. Next to generating ATP, glycolysis also leads to the production of lactate and pyruvate, of which the latter normally enters the Krebs cycle for oxidative phosphorylation. However, both lactate and pyruvate can act as radical scavengers during infection, thereby precluding the toxic effects of free radicals, but also leading to a depletion of lactate and pyruvate to fuel the Krebs cycle [49]. Eventually, almost all bodily cells undergo metabolic reprogramming to cope with the energy depletion and with the potential shortage of oxygen in case of shock associated with sepsis [48].
Mitochondrial dysfunction in endothelial cells contribute to the pathophysiology of sepsis. Under healthy conditions, these mitochondria play a crucial role in cell signalling, primarily attributed to their ability to generate reactive oxygen species (ROS) and maintain calcium homeostasis, which is essential for proper endothelial cell function [50]. Impaired vascular contractility, increased vascular permeability, and reduced nitric oxide (NO) bioavailability due to the activation of endothelial cells by inflammatory cytokines collectively culminate in what is generally referred to as endothelial dysfunction [50]. Furthermore, dysfunctional mitochondria within endothelial cells contribute to oxidative stress and damage to crucial cellular components, including lipids, proteins, and DNA, further compromising endothelial cell function [51].
In the context of sepsis, microvascular dysfunction is characterized by impaired regulation of blood flow, heightened vascular permeability, and disrupted cell-cell interactions [52]. Mitochondrial dysfunction in endothelial cells plays a significant role in the development of these abnormalities by disrupting normal cellular signalling pathways, promoting inflammation, and inducing oxidative stress [52]. Impaired endothelial cell function and compromised vascular integrity stand as critical contributors to the pathophysiology of sepsis, driving its progression and associated complications.
The kidney is among the most vulnerable organs in the sepsis population and sepsis induced acute kidney injury (sepsis-AKI) is strongly associated with poor outcomes [53,54]. Accordingly, prevention of sepsis-AKI and recovery of function are on the forefront of sepsis research. Mitochondrial metabolic reprogramming significantly affects the kidney, with mitochondrial dysfunction and altered metabolism being recognized as key driving forces in the aetiology of sepsis-AKI [55]. Upon mitochondrial failure, (an)aerobic glycolysis takes over ATP production as evidenced in kidney tubular epithelial cells (i.e. Warburg effect), but this process also leads to the production of lactate [56]. However, lactate can lead to (intracellular) acidification, swelling of cells and thereby compromise microvascular function and perfusion of the organ [57]. Inhibiting glycolysis partially restores mitochondrial membrane potential and decreases ROS production, resulting in reduced mitochondrial and kidney injury [56]. Preservation of mitochondrial function with the chromanol-based compound SUL-138 in lipopolysaccharide (LPS) challenged endothelial cells and sepsis in mice induced by caecal ligation and puncture (CLP) improved mitochondrial complex I and complex IV activity and limited mitochondrial ROS generation, resulting in lower systemic inflammation and dampening of acute kidney injury [58].
To sum up, (an)aerobic glycolysis increases ROS production, while changes in metabolism during the acute inflammatory response modify mitochondrial bioenergetics and lead to mitochondrial injury, which in turn result in organ dysfunction. Particularly, endothelial ROS production impairs NO signalling, which impairs endothelial (organ)function, particularly vasomotor control and barrier function of the endothelium, which can be restored through supporting mitochondrial complexes I and IV function.
2.3. Mitochondrially-mediated mechanisms of cell death in sepsis
Mitochondria play a significant role in cell death regulation, where cell damage and cell death occur even in non-severe cases of sepsis. Changes in mitochondrial membrane potential and assembly of the mitochondrial permeability transition pore (MPT) can drive necrosis [59] and apoptosis [60]. Sepsis associated apoptosis, triggered by the release of cytochrome c from stressed or damaged mitochondria, is observed in lymphocytes and epithelial cells [61]. In severe sepsis, lymphocyte apoptotic cell death plays a crucial role in the development of shock and multi-organ dysfunction syndrome (MODS), contributing to the impaired immune response frequently encountered in the immune-paralysis phase of sepsis [62]. Likewise, diaphragm mitochondria of septic mice of exhibit increased proton leak and downregulation of superoxide dismutase 2 (SOD2), potentially resulting in cell death from apoptosis and ferroptosis mediated by ROS [63].
Iron-dependent cell injury is of particular relevance among the pathways leading to cell injury because it plays a critical role in promoting a cascade of events that exacerbate tissue damage in sepsis. Sepsis increases iron-dependent cell injury and death through lipid peroxidation, specifically ferroptosis. Intracellular iron levels increase due to upregulated expression of transferrin receptor 1 (TFR1) and divalent metal transporter 1 (DMT1) activity [64], while macrophages release iron from damaged erythrocytes after phagocytosis [65]. The accumulation of ROS and expansion of the iron-pool promote ferroptosis, a necrotic form of cell death. Ferroptosis releases DAMPs from cells [66], perpetuating inflammation. Inflammasome formation and caspase activation, induced by inflammation, cause gasdermin cleavage [67]. Cleaved gasdermin forms membrane pores that promote cytolysis leading to pyroptosis, another necrotic form of cell death [68]. Yes-associated protein 1 (YAP1) acts as a ferroptosis suppressor by regulating the intracellular labile iron pool. YAP1 knockout sepsis mice contract more severe acute lung injury and show lowered ferroptosis defence as compared to wildtype animals, as demonstrated by the downregulation of GPx4, TH1, and SCL7A11, and concurrent increases in promotors of lipid peroxidation, including SFXN1 and NCOA4. In LPS-stimulated lung epithelial cells, YAP1 deficiency was associated with increased labile iron pool and mitochondrial dysfunction, leading to ferroptosis. Accordingly, YAP1 overexpression prevented the degradation of ferric iron to ferrous iron and conferred protection from mitochondrial dysfunction [69]. In summary, iron-dependent cell injury represents a key pathway that amplifies tissue damage and inflammation.
In sepsis, mitochondria act as central regulators of cell fate, driving both cell survival and death through mechanisms intimately linked with their function and structure. Disturbances in mitochondrial function, such as the release of cytochrome c and changes in membrane potential, not only initiate cell death but can perpetuate further cellular demise and activate other forms of regulated cell death.
3. Mitochondrial quality control
Assessing the severity of sepsis and predicting its clinical course is challenging in clinical practice. Nonetheless, numerous biomarkers have emerged as potential indicators to assess disease severity. Notably, specific biomarkers associated with the immunosuppressive phase of sepsis, such as anti-inflammatory cytokines and changes in the cell surface markers of monocytes and lymphocytes, have been investigated. Monocytes in sepsis play essential roles in phagocytosis and antigen presentation but can also trigger dangerous inflammation through excessive cytokine production [70]. Monocyte distribution width (MDW) is a valuable marker for early sepsis detection, especially in the emergency department. In regard to lymphocytes, sepsis can result in severe lymphopenia resulting from increased apoptosis, reduced T-cell proliferation, and cytokine production [[71], [72], [73]]. Specifically, lymphocyte subsets, especially CD4 T lymphocytes, assessed upon hospital admission in patients subsequently developing sepsis, have emerged as promising biomarkers for identifying those at a heightened risk of an unfavourable outcome [74]. In sepsis, neutrophils are stimulated but struggle to migrate effectively to infection sites, contributing to the condition and even organ failure [75]. Neutrophil CD64 has been identified as a promising biomarker for outcome prediction among patients with an infection. High nCD64 expression during early sepsis might be associated with a better prognosis [76,77].
It is important to know there is no one biomarker that can accurately predict the outcome of sepsis, but by employing a multi-marker panel comprising both pro- and anti-inflammatory biomarkers, it may be possible to identify patients who will progress towards severe sepsis at an early stage, before significant organ dysfunction ensues [78]. Various mitochondrial parameters, including mitochondrial membrane potential and oxidative stress, have been suggested as potential biomarkers for assessing clinical recovery and the response to oxygen therapy in sepsis [15]. Improvement in mitochondrial parameters during treatment may indicate a positive outcome in sepsis patients. In patients with sepsis-associated acute kidney injury (sepsis-AKI), disturbances in mitochondrial quality control are evident. These patients often exhibit a reduction in mitochondrial mass, increased oxidative stress, and decreased expression of mitochondrial biogenesis markers, all of which suggest a decrease in mitochondrial quality control [91]. As a result of these mechanisms, septic patients have shown changes in mitochondrial morphology, such as fragmentation and swelling, prompting the development of drugs targeting mitochondria to enhance sepsis survival [79]. Given the role of mitochondria as drivers of inflammation and organ failure, we propose that measuring mitochondrial function and markers of mitochondrial quality control could serve as future guides for assessing both the severity, progression and treatment effect of sepsis.
Mitochondria form highly dynamic intracellular networks that execute quality control mechanisms to regulate their population numbers and network structure through processes including mitochondrial biogenesis (mitogenesis), fission/fusion (mitochondrial dynamics), and autophagic degradation (mitophagy). Mitochondrial homeostasis is ensured through the delicate balance of these quality control processes [80,81]. Mitochondria respond to stress by changing their mass, shape and interconnectivity. Changes in mitochondrial mass reflect alterations between mitochondrial biogenesis and mitophagy, whereas changes in fission/fusion dynamics are reflected by the interconnectivity of the mitochondrial reticulum [30]. Additionally, fission and mitophagy play a crucial role in pruning dysfunctional parts of the mitochondrial network.
The relationship between sepsis and mitochondrial dysfunction has been the subject of extensive research, which has identified mitochondrial damage as a major contributor to sepsis morbidity and mortality [1,30,82] (see also references below). A prominent example is that mitochondrial membrane depolarization in thrombocytes correlates with clinical disease severity in patients with sepsis during the progression of the disease [83]. Since septic patients may already present with mitochondrial dysfunction, it will not be possible to fully prevent it in sepsis. Thus, efforts should be expanded to promote the recovery of mitochondrial function. As such, we propose that the first phase of mitochondrial sepsis management comprises the limitation of further mitochondrial damaged by preventing ROS generating, while the second phase should aim to induce mitochondrial quality control (mtQC) mechanisms to recover mitochondria integrity and function. Based on the above, it seems to be biologically plausible that such an approach would combine promoting mitophagy for removal of damaged and dysfunctional mitochondria and stimulating biogenesis to increase the pool of functional mitochondria for bioenergetics.
3.1. Mitogenesis and biogenesis
Though sometimes used interchangeably, mitochondrial biogenesis and mitogenesis denote distinct but interrelated processes. Within the overarching process of mitochondrial biogenesis, mitogenesis refers to the initiation of mitochondrial division or fission, facilitated by the dynamin-related protein 1 (DRP1). It entails the replication of mitochondrial DNA and the proliferation of existing mitochondria, a process comparable to mitosis. In contrast, mitochondrial biogenesis delineates a broader concept, emphasizing the enhancement – rather than mere preservation – of mitochondrial mass by producing new mitochondria [84]. This comprehensive process is underpinned by the synthesis of mitochondrial DNA and the assembly of mitochondrial structures. Central to this is the TFAM-PCG1a-NRF1/2 pathway (Fig. 2). For uniformity throughout this review, we employ the term "mitochondrial biogenesis" when referring to this specific pathway. This approach underscores the emphasis on proteins localized at mitochondria yet are encoded in the nuclear genome regulated by transcription factors including the PGC1 family, Nrf1 and Nrf2, and on nuclear-encoded mitochondrial transcription factors such as TFAM, TFB1M, and TFB2M [85].
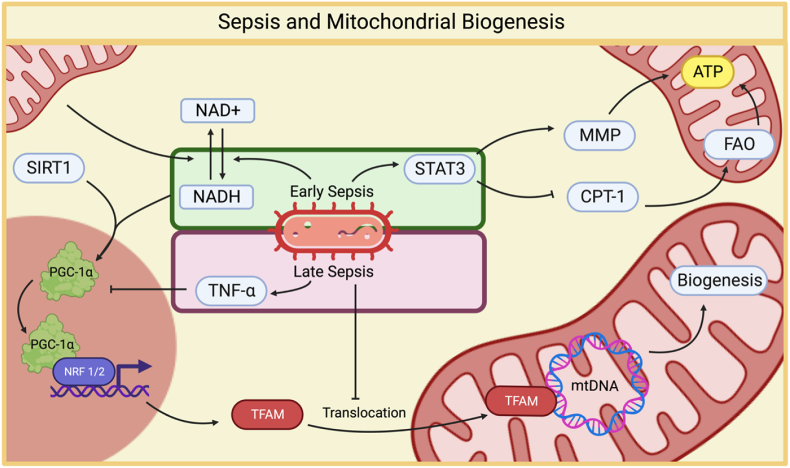
Fig. 2.
The relationship between sepsis and mitochondrial biogenesis. During the initial stages of sepsis, there is a notable downregulation of mitochondrial biogenesis. This reduction is primarily driven by elevated levels of the cytokine TNFα, which leads to decreased levels of PGC-1α [86]. As sepsis progresses to its later stages, mitochondrial dysfunction becomes increasingly evident. In response to this dysfunction, a defensive cellular response is initiated to counteract oxidative stress and prevent cell death. STAT3 enhances the mitochondrial membrane potential, thus improving ATP production efficiency, which is crucial for cellular energy supply. In an effort to maintain energy production, cells increase fatty acid oxidation. This is achieved, in part, by inhibiting the degradation of CPT1, a key enzyme involved in fatty acid transport into mitochondria [87] Additionally, the increased expression of Nrf1 and Nrf2, along with TFAM, suggests an upregulated response to cope with dysfunctional mitochondria [2]. However, a notable challenge emerges in the form of impaired TFAM translocation into the mitochondria [88]. This impairment potentially explains the contradictory results observed regarding mitochondrial biogenesis in sepsis.
3.2. TFAM-PGC1α-NRF1/2-pathway controls both biogenesis and mitogenesis
The core transcription initiation complex to reproduce mtDNA is a specialized assembly of proteins, involving mitochondrial RNA polymerase (POLRMT), transcription factor A (TFAM), and transcription factor B2M (TFB2M), that binds to specific DNA sequences, facilitating the commencement of transcription by positioning and guiding the RNA polymerase to initiate RNA synthesis from the DNA template [89].
Central to the process of mitochondrial biogenesis is the TFAM-PGC1α-NRF1/2-pathway, a pivotal regulator of the mitochondrial life cycle and its response to stress. TFAM, in particular, is vital in the generation of new mitochondria. TFAM achieves this by binding to distinct mitochondrial DNA sequences, promoting their transcription and replication [80]. It oversees mitochondrial DNA replication and transcription, while the mitochondrial transcription specificity factor (TFBM) assists in bringing RNA polymerase to the mitochondrial DNA promoter, thereby forming the core transcription initiation complex for human mitochondria [90]. Together, their upregulation fosters increased mitochondrial gene expression and protein production. Interestingly, even though overall TFAM expression is heightened in peripheral blood mononuclear cells (PBMCs) of sepsis patients compared to controls, a marked decrease in its level inside mitochondria suggests an impediment in mitochondrial TFAM uptake. The reduced intramitochondrial TFAM, observed in sepsis patients, leads to a corresponding decrease in mitochondrial DNA copy numbers, mtND1 expression, and overall cellular ATP content [88]. This was further evidenced by a similar behaviour of LPS-stimulated PBMCs from healthy individuals [88]. Moreover, the interplay between TFAM and TFB2M within the core transcription initiation complex appears compromised in septic patients. The skewed ratio of intra-over extramitochondrial TFAM, brought about by increased extramitochondrial levels of TFAM - but reduced intramitochondrial levels of TFAM - might explain the sepsis paradox of increased TFAM expression, yet absence of bioenergetic recovery due to the diminished mitochondrial TFAM presence. Post-mortem sepsis studies have shown a downregulation in genes tied to mtQC, including those related to TFAM (biogenesis), which likely contributes to the aforementioned discrepancy [91].
PGC-1 proteins, particularly PGC‐1α, are pivotal in mtDNA replication, with emerging research positioning PGC‐1α as the central regulator of mitochondrial biogenesis [81]. Furthermore, PGC-1α is recognized as the cornerstone in overseeing the mitochondrial lifecycle and response to oxidative stress [92]. Notably, there is noticeable mitochondrial swelling and dysfunction within tubular epithelial cells during sepsis- AKI. This is further compounded by a selective suppression in the expression of oxidative phosphorylation genes and a corresponding decline in mitochondrial biogenesis, mainly attributed to diminished PGC-1α levels. Interestingly, elevated levels of the pro-inflammatory cytokine TNF-α correlate with decreased PGC-1α abundance and reduced oxygen consumption — a sign of mitochondrial failure. However, augmenting PGC-1α levels can counter this trend. The essential role of PGC-1α in preserving renal function is further underscored by experiments in tubule-specific PGC-1α knockout mice, that compared to wildtype mice show pronounced increases AKI severity upon LPS injection, as evidenced by heightened serum creatine and urea nitrogen levels [86]. Additionally, in mice PGC-1α is a critical player in restoring renal recovery by enhancing nicotinamide adenine biosynthesis and thereby correcting ischemia-induced energy shortages and restoring renal function [93]. Hence, PGC-1α emerges as a vital mechanism in safeguarding kidney function during sepsis, primarily by averting mitochondrial failure through induction of mitochondrial biogenesis.
Nuclear factor erythroid 2-related factor 2 (Nrf2) stands as a central transcription factor, governing the expression of various antioxidant and cytoprotective genes to counter oxidative stress, inflammation, and toxins. In mice, activation of Nrf2 through Heme oxygenase-1 (HO-1) sparks mitochondrial biogenesis, effectively safeguarding them from fatal Staphylococcus aureus (S. aureus) sepsis [94]. Protection can be further enhanced by carbon monoxide (CO) inhalation that leads to upregulation of Nrf2-dependent genes, amplifying mitochondrial biogenesis, curtailing inflammation, and thus enhancing sepsis survival. The protective effect of CO, however, diminishes when both Nrf2 and Akt1 are knocked down, emphasizing their combined role in mitochondrial biogenesis [94]. Mirroring this, sub-lethal S. aureus sepsis in mice instigates renal mitochondrial injury but also ignites renal mtDNA repair and mitochondrial biogenesis [95]. During sepsis-induced renal inflammation, there is a notable increase in mtDNA repair and mitochondrial biogenesis, as evidenced by heightened 8-oxoguanine DNA glycosylase (OGG1) activity. This upsurge in OGG1, along with augmented expression of Nrf1, Nrf2, and TFAM, suggests a defensive response aimed at counteracting oxidative stress and cell death. Moreover, the rise in renal mitochondrial mass and the central role of Nrf2 in driving mitochondrial biogenesis imply an inherent healing mechanism following systemic bacterial invasion [2]. The toxic effects of LPS on myocardium can be effectively countered by the induction of the Nrf2 and Nrf1 pathways. Songorine, an active C20-diterpene alkaloid, leverages this protective mechanism by bolstering cardiac function through Nrf2 induction [2]. Upon exposure to LPS, cardiomyocytes typically experience mitochondrial ROS generation and a calcium surge, but these effects are mitigated by songorine. Moreover, songorine enhances antioxidant defences, boosts mitochondrial gene expression associated with fatty acid β-oxidation and electron transport, and invigorates the Nrf1 and TFAM pathways in an Nrf2-dependent fashion. However, in Nrf2-deficient mice exposed to LPS, the cardioprotective efficacy of songorine diminishes, underscoring its modulatory action through the Nrf2 and Nrf1 pathways. In conclusion, ensuring efficient transport of these transcription factors into mitochondria is paramount, as their impaired uptake might exacerbate conditions, highlighting the importance of maintaining these protein levels within mitochondria.
LPS treatment elevates mitochondrial biogenesis in rats, with PGC‐1α and TFAM being upregulated, leading to a 20% surge in mitochondrial mass within 24 h [96]. This contradicts human findings as one of the characteristics of sepsis-induced mitochondrial dysfunction is impaired translocation of TFAM [97] despite cytosolic upregulation [88]. Additionally, a recent review concluded that sepsis is associated with decreased intramitochondrial TFAM levels. Phosphorylation of human TFAM impairs DNA binding and consequently to initiate transcription [98], however we are not aware of literature directly demonstrating increased TFAM phosphorylation in human sepsis. Intriguingly, the above introduced augmented biogenesis in rats is not reflected in improved mitochondrial function [96]. Mitochondrial respiration showed vulnerabilities: complex I activity was curtailed by 30% at 6 and 24 h post-LPS treatment, and complex II function similarly dipped at 24 h after LPS challenge. Alongside this functional reduction, mitochondrial ultrastructure manifested various anomalies, including internal vesicles, disrupted cristae, and swelling. Building on this observation, similar findings have been noted in diaphragms of septic CLP mice. Despite the beneficial upregulation of mitochondrial biogenesis markers in other contexts, the septic diaphragms tell a more disheartening story. A significant decline in the quantity and quality of mitochondria is evident, which might be attributed to the suppressed expression of PGC-1α [63]. This septic state also witnesses a downregulation of respiratory chain complexes, most notably complex III and IV, leading to subdued oxygen consumption tied to ADP phosphorylation. The broader picture suggests that mitochondria show a compounded impairment during sepsis: downregulated mitochondrial biogenesis and oxidative-stress-induced damage, culminating in diminished mitochondrial mass.
Early mitochondrial biogenesis appears paramount to survive sepsis induced critical illness. Patients who survived showed a higher muscle ATP levels and reduced phosphocreatine to ATP ratio and exhibited (early) induction of mitochondrial biogenesis and antioxidant defence [99]. The induction of mitochondrial biogenesis and antioxidant defence seems to counterbalance mitochondrial protein depletion, thus preserving mitochondrial functionality and energy balance. However, there was still depletion of respiratory protein subunits and their transcripts (PPARGC1A, NRF1, and TFAM). Notably, only survivors manifested increased levels of the mitochondrial oxidative stress protein manganese superoxide dismutase (MnSOD) and the mitochondrial biogenesis associated transcript PPARGC1A. The contrast is stark with non-survivors, whose reduced mtQC response might increase their vulnerability to mitochondrial damage, possibly hampering cellular energy functionality and thwarting recovery to a normal state [100].
3.3. Sirtuins and STAT3: integrated modulators of mitochondrial biogenesis and cellular energy metabolism
Sirtuins and STAT3 are key modulators of mitochondrial dynamics and cellular bioenergetics, whose modulation could improve outcomes for sepsis patients, given the protective role of early mitochondrial biogenesis in critical illness. Silent information regulator 2-related histone deacetylases, i.e. sirtuins (SIRTs), are a 7 member protein family serving as critical nexuses in cellular activities, encompassing aging, inflammation, and bioenergetics, especially in the realm of mitochondrial biogenesis. Two if its members have been implicated in sepsis, specifically SIRT1 and SIRT3. SIRT1 mandates the presence of nicotinamide adenine dinucleotide (NAD) for the deacetylation of its target substrates [101,102]. The cellular redox balance of NAD+ and NADH, intrinsically connected to catabolic metabolism, casts SIRT1 as a metabolic sensor that coherently aligns metabolic imbalances with transcriptional responses. This understanding is strengthened by SIRT1's interaction and NAD + -dependent deacetylation of PGC-1α [103]. SIRT1, primarily recognized as a nuclear/cytosolic protein [[104], [105], [106]], exerts a pivotal role in sepsis by guiding inflammation and mitochondrial dynamics through two main avenues. First, it activates PGC-1α via deacetylation, enabling PGC-1α to ally with the mitochondrial transcription factor A (TFAM). This partnership is fundamental for directing mitochondrial DNA replication and transcription [104,105]. Secondly, SIRT1 enhances the expression of the strictly mitochondrial SIRT3 via the RELB pathway, an NF-κB transcription factor family member [107]. As sepsis takes hold, there is a profound alteration in monocyte behaviour and a shift in their mitochondrial fuel preference, both driven by the SIRT1-RELB-SIRT3 axis [108]. Therefore, current research underscores the dominant influence of SIRTs on mitochondrial biogenesis via PGC-1α [109].
Signal transducer and activator of transcription 3 (STAT3) is a multifaceted protein that is implicated across a spectrum of cellular activities, from proliferation and survival to energy metabolism, all while regulating mitochondrial calcium levels. Upon exposure to LPS, STAT3 augments the mitochondrial membrane potential, thus enhancing ATP production efficiency. Moreover, STAT3 bolsters mitochondrial biogenesis and heightens fatty acid oxidation by inhibiting the degradation of carnitine palmitoyl transferase 1a (CPT1a), an enzyme responsible for facilitating the transport of long-chain fatty acids into the mitochondria, through its interaction with ubiquitin-specific peptidase 50 (USP50). However, in absence of USP50, STAT3 levels rise and CPT1a remains active and abundant within in the cell, leading to improved fatty acid oxidation, and as a result reduced lipid damage. The overexpression of STAT3 in mitochondria, however, augments the severity of LPS-induced sepsis as reflected in enhanced NF-κB activity [87]. Thus, STAT3 function appears to be dualistic, acting as both a protective agent by enhancing ATP production and a damaging agent by the escalation of sepsis when overexpressed in mitochondria.
In summary, mitochondrial biogenesis is central to sepsis progression. Despite the valuable perspectives gained from clinical observations of the involvement of mtQC in sepsis, there is a notable gap in experimental evidence from human-based interventions. Biomarkers reflecting mitochondrial biogenesis and function may adequately and timely assess sepsis severity and predict the clinical outcome. It is crucial, however, to acknowledge the existing gaps and limitations in the current body of research that support this hypothesis. Although some studies have indicated that recovery from sepsis likely depends on early activation of mitochondrial biogenesis and a balanced biogenesis response, as excessive biogenesis can be counterproductive in causing structural anomalies and reduced mitochondrial functionality, it requires further validation through extensive research. However, harnessing the potential of the interconnected pathways driving mitochondrial biogenesis might offer novel therapeutic avenues for sepsis.
3.4. Pharmacological compounds for enhancing biogenesis in sepsis recovery
Pharmacological activation of mitochondrial biogenesis can be achieved with a large range of compounds (Fig. 3). While the effects of many of these compounds in the context of sepsis have yet to be thoroughly investigated, the fundamental pathways responsible for stimulating biogenesis are well-established in the literature. The proteasome inhibitor and anticancer drug bortezomib can prevent degradation of unbound (DNA-free) TFAM [98]. In a murine macrophage sepsis model bortezomib decreased pro-inflammatory cytokine levels and increased survival after LPS challenge, in keeping bortezomib treatment in rats prior to CLP increased seven day survival and decreased lung inflammation [110]. Given TFAM's central role in biogenesis, prevention of its degradation through bortezomib may hold therapeutic potential. The drug bezafibrate activates a group of receptors known as peroxisome proliferator-activated receptors (PPARs) and is commonly used to treat dyslipidaemia [111]. In mice, bezafibrate stimulates the expression of the key regulator PGC-1α in the heart and skeletal muscles [112]. It also increases respiratory capacity and restores oxidative phosphorylation function in fibroblasts with complex III or complex deficiencies from patients with inherited genetic mitochondrial disorders [113]. In a murine model of Huntington's disease, bezafibrate improved mitochondrial numbers and function in brain and muscle tissue by bringing PGC-1α levels back to normal. Thereby attenuating neuronal atrophy, i.e. neurodegeneration, and preventing the conversion of oxidative to glycolytic muscle fibres. Finally, bezafibrate protected against oxidative damage substantiated amongst other by reduced DNA-damage and lipid peroxidation [114].
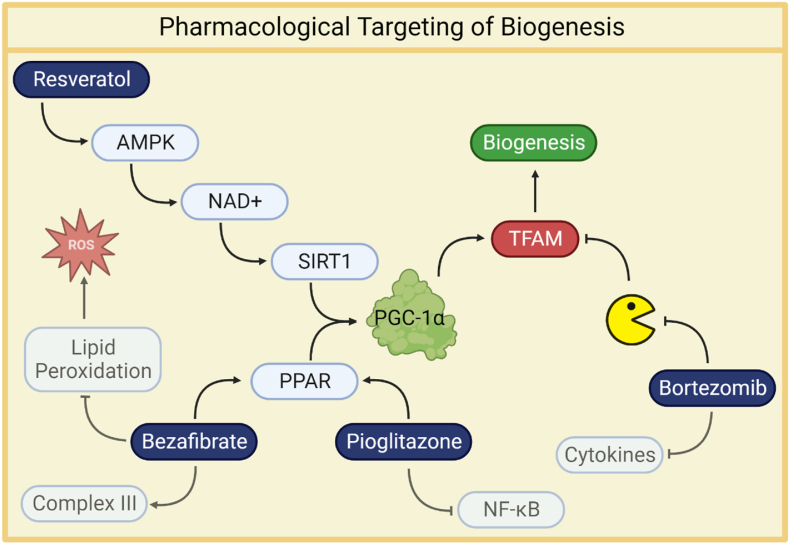
Fig. 3.
Resveratrol activates AMP-activated protein kinase (AMPK), leading to an increase in cellular NAD + levels, which subsequently activates SIRT1 [116]. This dual activation of AMPK and SIRT1 together promotes mitochondrial biogenesis through the activation of PGC-1α [118]. Bezafibrate, on the other hand, activates peroxisome proliferator-activated receptors (PPARs) and enhances respiratory capacity while protecting against oxidative damage [112]. Pioglitazone, a PPARγ agonist, stimulates PPAR and TFAM expression [122], mitigating the inflammatory response by partially inhibiting NF-κB activation [123]. Additionally, bortezomib prevents the degradation of unbound (DNA-free) TFAM [98], which is central to mitochondrial biogenesis, suggesting therapeutic potential in preserving TFAM integrity.
Resveratrol is known for its ability to activate sirtuins, particularly SIRT1, in mammals [115]. Resveratrol activates AMP-activated protein kinase (AMPK), leading to increased cellular NAD + levels, which in turn activates SIRT1 [116]. AMPK activation is considered a crucial factor in the mechanism of action of resveratrol on SIRT1 and mitochondrial biogenesis. AMPK is activated through phosphorylation at Thr172. This activation is influenced by AMP and ADP binding, reflecting cellular energy status as indicated by AMP/ATP and ADP/ATP ratios [117]. AMPK acts as a metabolic sensor that responds to changes in cellular energy levels. The combined action of activated AMPK and SIRT1 promotes mitochondrial biogenesis via PGC-1α activation [118]. Overall, resveratrol has demonstrated therapeutic potential in a wide range of diseases, often attributed to its antioxidant or anti-inflammatory, however, its impact has not yet been extensively studied in a sepsis model.
PPARγ agonists, such as pioglitazone [119], have garnered attention for their potential to stimulate biogenesis in several neurodegenerative diseases [120,121]. This research presents promising avenues for addressing conditions characterised by inflammatory brain symptoms. Notably, in patients with type 2 diabetes, pioglitazone treatment has demonstrated a significant increase in both mitochondrial copy number and the expression of genes related to mitochondrial biogenesis [122] including peroxisome proliferator–activated receptor, PGC-1α and TFAM. Furthermore, in the context of sepsis, pioglitazone mitigates the inflammatory response, by partially inhibiting NF-κB activation [123]. This effect was demonstrated in a CLP mouse model treated with pioglitazone. It is worth noting, however, the study did not explore its effects on mitochondrial function.
4. Mitophagy
On the one hand, mitochondria are instrumental in orchestrating an immune response during sepsis, but on the other hand, they can inadvertently exacerbate harm. While mitochondrial dysfunction predominantly leads to an increase in ROS production at the expense of ATP generation, inducing mitogenesis and mitochondrial biogenesis can provide new therapeutic targets to reinstate cellular bioenergetic homeostasis. However, with an understanding of the associated pitfalls, our attention now pivots to the critical process of clearing damaged mitochondria, called mitochondrial autophagy, or in short: mitophagy. Autophagy and mitophagy are intertwined processes that degrade and recycle cellular components in response to stress; while mitophagy targets dysfunctional mitochondria, autophagy addresses broader cellular elements, reflecting their shared mechanisms and roles in cellular health [124]. As such, mitophagy is a selective form of autophagy, which removes mitochondria as a response to various stimuli, such as nutrient starvation, oxidative stress or programmed mitochondrial removal [125]. As one of the mechanisms of mitochondrial quality control, mitophagy balances mitochondrial ROS production by removing dysfunctional mitochondria responsible for the overproduction of ROS. Sepsis induces early autophagy, but leads to impaired autophagy in later stages. Induction of autophagy by rapamycin reduced the severity of tubular epithelial injury and preserved renal function after CLP in mice [126].
There are different pathways that regulate mitophagy (Fig. 4), however the best-studied is the pathway mediated by phosphatase and tensin homolog (PTEN)-induced kinase PINK1 and E3-ubiquitin ligase Parkin [127]. PINK1 accumulates at the OMM (outer mitochondrial membrane) in response to mitochondrial injury, increased ROS, depolarization, or accumulation of misfolded proteins. The accumulated PINK1 is then auto-phosphorylated, which in turn phosphorylates ubiquitin to recruit Parkin from the cytosol to the mitochondrial membrane [128]. Parkin, when recruited and activated, drives the ubiquitination of various mitochondrial inner proteins, which recruit and activate more Parkin (through positive feedback). Phosphorylated Parkin delivers ubiquitinated mitochondria to autophagosomes, resulting in removal of damaged mitochondria by mitophagy [129].
Fig. 4.
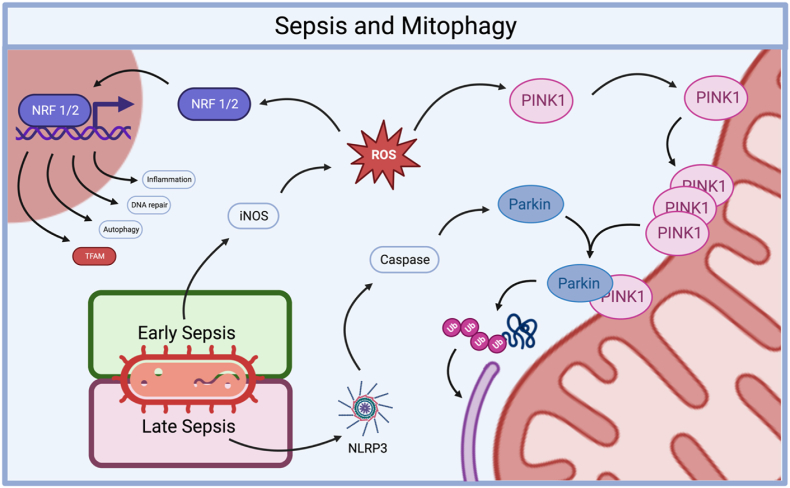
The relationship between sepsis and PINK1-Parkin mediated mitophagy. In the early stages of sepsis, the induction of iNOS leads to the production of ROS which subsequently initiates two critical processes: The activation of mitophagy, mediated by Nrf2, which orchestrates the induction of the PINK1-Parkin pathway. And the stimulation of mitochondrial biogenesis [130]. In the later phases of sepsis, mitophagy becomes impaired. This impairment may be attributed to the cleavage of Parkin by caspases activated by NLRP3 [131]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4.1. PINK1-parkin mediated mitophagy
Phosphatase and tensin homolog (PTEN)-induced kinase PINK1 and E3-ubiquitin ligase Parkin [127] are important regulators of mitophagy, with relevance to regulation of immune function and preserving organ function in sepsis. PINK1 accumulates at the outer mitochondrial membrane (OMM) in response to mitochondrial injury, increased ROS levels, mitochondrial membrane depolarization, or accumulation of misfolded proteins. The accumulated PINK1 is then auto-phosphorylated, which in turn phosphorylates ubiquitin to recruit Parkin from the cytosol to the mitochondrial membrane [128]. Parkin, when recruited and activated, drives the ubiquitin-mediated autophagy and mitophagy which recruits and activates more Parkin through a positive feedback loop. Phosphorylated Parkin delivers ubiquitinated mitochondria to autophagosomes, resulting in removal of damaged mitochondria by mitophagy [129]. By this process, a reduction in PINK1 inhibits mitophagy, inadvertently elevating mitochondrial fragmentation through dynamin-related protein 1 (Drp1)-related mitochondrial fission, which, however, can be counteracted by parkin overexpression. Hence, PINK1 primarily ensures protection by bolstering mitophagy and curbing mitochondrial fragmentation [132].
Mitophagy is essential to maintain cellular homeostasis by preserving mitochondrial function. Knock-out of PINK1 and parkin exacerbates AKI in septic mice, induced by either LPS or CLP. Additionally, knockdown of PINK1 and Parkin in renal proximal tubular cells (RPTC) treated with LPS lowers the accumulation of the autophagy adaptor optineurin, indicative of reduced autophagy in the cells. In line with this, genetic silencing of optineurin reduces LPS-induced mitophagy. These findings demonstrate the importance of the PINK1-Parkin-optineurin pathway in conferring protection from sepsis-AKI by induction of mitophagy [133]. In murine hepatocytes, LPS induces expression of inducible nitric oxide synthase (iNOS) and subsequent production of ROS. Subsequently, the iNOS-ROS signalling pathway triggers activation of mitophagy and mitochondrial biogenesis in hepatocytes via Nrf2, which mediates the induction of PINK1 and Parkin, in response to iNOS-ROS signalling [130].
In early murine sepsis-AKI mitophagy is elevated, but impaired in the later phase, potentially due to the cleavage of the mitophagy promotor Parkin via caspases activated by NLRP3: a damage response protein that is part of the inflammasome. Impaired mitophagy is associated with the accumulation of damaged mitochondria, oxidative stress, and cell death. Indeed, loss of mitophagy increased mitochondrial-associated apoptosis increased over time during sepsis-AKI in mice. Therefore, promoting mitophagy during sepsis-AKI may be a promising therapeutic strategy to prevent apoptosis and attenuate AKI [131]. Sepsis-AKI can also be attenuated via promoting Nrf2 nuclear translocation. The activation of Nrf2 is an important adaptive response to cellular stress, as it allows cells to upregulate the defence against oxidative stress and increases the expression of genes involved in inflammation, DNA repair, and autophagy. The renal protective effects of mitophagy are further supported by Gao et al. (2020) [3] who demonstrated that polydatin treatment protects from sepsis-AKI through induction of Parkin-mediated mitophagy. Treatment with polydatin prevented mitochondrial dysfunction by reducing mitochondrial mass via upregulation of mitophagy by activating SIRT1 and inhibiting the formation of the NLRP3-inflammasome, and partially precluded AKI in septic mice. Additionally, the PINK1-Parkin-pathway seems to play an important role in modulation sepsis-induced muscle atrophy. Accumulation of dysfunctional, damaged mitochondria are considered crucial catalysts for muscle catabolism. Parkin counteracts catabolism and its overexpression prevented a decrease in the content of mitochondrial subunits of NADH dehydrogenase and cytochrome C oxidase, and attenuated sepsis-induced muscle wasting in CLP-sepsis mice [134].
4.2. Non-PINK1--parkin-mediated mitophagy: Beclin-1 and MKK3
While the PINK1-Parkin pathway is the most well-known pathway for mitophagy, there are several other pathways that can mediate this process, each with their own unique regulatory mechanisms. Beclin-1, also known as autophagy-related protein 6 (ATG6), is a protein that plays a key regulatory role in autophagy and mitophagy [135]. Administration of the Beclin-1 peptide protects mitochondria associated membranes (MAMs), specialized regions that govern interaction between endoplasmic reticulum (ER) and mitochondria, in LPS-stimulated human cardiomyocytes, while loss of Beclin-1 has the opposite effect [136]. Consistent with this work, upregulation of Beclin-1 promotes autophagy and mitophagy and reduces the release mitochondrial DAMPs, resulting in reduced inflammation in LPS-stimulated mice [137]. Mitophagy can also be regulated by the mitogen-activated protein kinase 3 (MKK3), a protein kinase that is involved in the regulation of cellular responses to stress and inflammation involving modulation of mitochondrial biogenesis and mitophagy. Activation of MKK3 is increased in peripheral blood mononuclear cells (PBMCs) from septic patients compared with non-septic controls. Wildtype CLP mice sustained higher inflammatory cell infiltration in the lungs, increased levels of oxidative stress, whereas MKK3 knockout mice have lower levels of mitochondrial injury, with increased mitochondrial biogenesis and mitophagy, reduced lung injury and improved survival in sepsis [138]. The findings suggest a critical role for MKK3-driven mitophagy in maintaining pool of healthy mitochondria in sepsis with relevance to preservation of organ function and survival.
In summary, mitophagy plays a crucial role in promoting beneficial outcomes as demonstrated in murine sepsis models by reducing oxidative stress, maintaining mitochondrial function, and decreasing inflammation, fibrosis, and organ injury. The PINK1-parkin pathway is a critical regulator of mitophagy, and overexpression of Parkin attenuates sepsis-induced muscle wasting. Furthermore, treatment with rapamycin, polydatin, and Beclin-1 peptide can enhance (mitochondrial) autophagy, leading to improved outcomes in sepsis. Targeting (mitochondrial) autophagy seems a promising therapeutic strategy to lower morbidity and mortality from sepsis. Further research is needed to better understand the molecular mechanisms underlying mitophagy regulation in sepsis and to develop targeted interventions that can enhance the beneficial effects.
4.3. Pharmacological options of mitophagy induction
Rapamycin (Sirolimus) is an immunosuppressant used to prevent organ rejection in transplant recipients [139] and to treat certain autoimmune diseases [140]. It suppresses T-lymphocyte activation and proliferation through mammalian target of rapamycin (mTOR) inhibition. In experimental models of murine spinal cord [141] and rat cerebral ischemia [142], rapamycin showed promise in promoting rapamycin significantly boosted mitophagy by facilitating the translocation of p62 and Parkin to the mitochondria. Another investigation revealed that rapamycin upregulated the expression of mitophagy-promoting genes, such as PINK1 and PARKIN [143]. Additionally, a third study [144] highlighted how rapamycin effectively rescued mitochondrial myopathy through the synchronized activation of autophagy and the enhancement of lysosomal biogenesis. Furthermore, in a sepsis model induced by CLP in mice [145], pretreatment with rapamycin has been associated with decreased inflammation, pyroptosis suppression, and reduced organ damage, ultimately contributing to improved survival rates (Fig. 5).
Fig. 5.
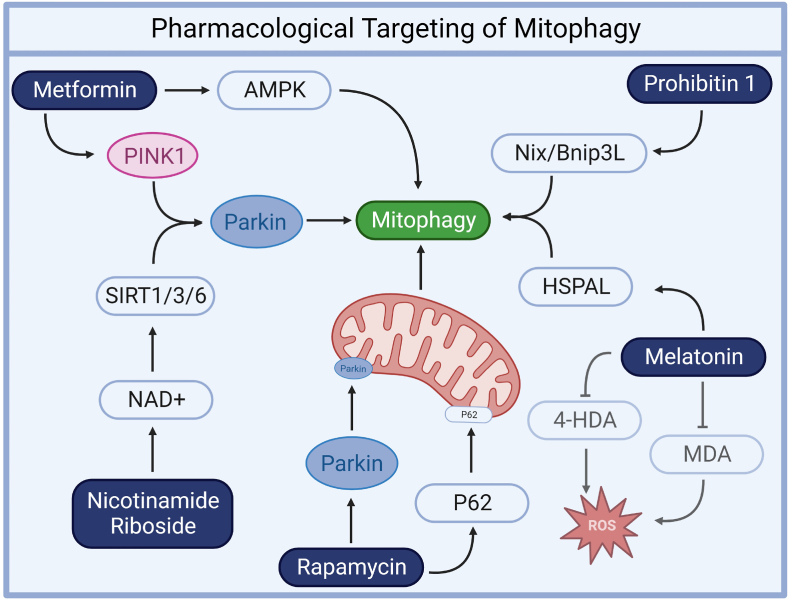
Metformin enhances AMPK activation and mitochondrial function, reducing pro-inflammatory cytokines and mitigating mitochondrial oxidative stress [148]. It also upregulates mitophagy markers like PINK1 and Parkin, preserving mitochondrial integrity [147]. NR regulates NAD + -dependent deacetylases, including SIRT1, SIRT3, and SIRT6, which facilitate mitophagy by deacetylating key proteins like PINK1 and Parkin [[150], [151], [152]]. Rapamycin significantly boosts mitophagy by promoting the translocation of p62 and Parkin to mitochondria and increasing the expression of mitophagy-promoting genes, such as PINK1 and PARKIN [143]. Melatonin enhances mitophagy by elevating the expression of heat shock 70 kDa protein 1L (HSPLA) [155] and reduces lipid peroxidation products like malondialdehyde (MDA) and 4-hydroxylalkenals (4-HDA) [157]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Metformin, a widely prescribed drug for type 2 diabetes, is known for its primary antidiabetic effects through the inhibition of mitochondrial complex I activity, resulting in improved glycaemic control [146]. Recent research has unveiled its broader applicability in enhancing mitochondrial function and promoting mitophagy. Studies involving type 2 diabetes patients have demonstrated metformin's ability to reduce HbA1c levels, alleviate mitochondrial oxidative stress, and upregulate mitophagy markers like PINK1 and Parkin as well as preserving mitochondrial integrity [147]. Additionally, metformin enhances AMPK activation and mitochondrial function, reversing pro-inflammatory cytokine increases observed in diabetic patients [148]. In the context of sepsis management, metformin's antioxidant and anti-inflammatory properties are gaining attention, as it reduces reactive oxygen species production, suppresses pro-inflammatory cytokines, modulates inflammation-related transcription factors, and maintains mitochondrial membrane potential, all without evidence of lactic acidosis at therapeutic doses in human diabetic PBMCs [148]. These findings highlight the potential repurposing of metformin for broader clinical applications beyond diabetes management, particularly in sepsis and associated organ failure, due to its multifaceted effects encompassing mitophagy stimulation, improved mitochondrial function, and inflammation attenuation.
NAD + precursors, such as nicotinamide riboside (NR), hold promise as a therapeutic avenue. In human fibroblasts NR activates sirtuins and promotes mitophagy, impacting cellular health and mitochondrial function positively [149]. NAD + plays a crucial role in regulating NAD + -dependent deacetylases [[150], [151], [152]], including SIRT1, SIRT3, and SIRT6, all of which have been demonstrated to facilitate mitophagy by deacetylating essential proteins in the process. These deacetylation events involve key players like PINK1 and Parkin, and further underscore the significance of NAD+ in governing mitophagy-related mechanisms across different SIRT proteins. NR treatment can decrease mitochondrial content while maintaining quality, and elevated NAD+/NADH ratios, along with SIRT1 activation, yield similar effects, including decreased mitochondrial content, increased autophagy, and mitochondrial fragmentation. In sepsis mouse models, NR administration prior to sepsis onset elevated NAD + levels, reducing oxidative stress, inflammation, and caspase-3 activity in lung and heart tissues, improving pulmonary microvascular permeability and myocardial function, and reducing mortality [153]. NR also suppressed plasma high mobility group box-1 (HMGB1) levels, prevented ROS production and apoptosis, and enhanced NAD + content, while inhibiting SIRT1 offset these benefits, emphasizing the importance of the NAD+/SIRT1 signalling pathway in NR-mediated protection. As NR is already a health supplement, its potential to prevent organ injury in sepsis represents a promising therapeutic strategy, highlighting its role in enhancing cellular health and mitigating mitochondrial dysfunction through sirtuin activation and mitophagy promotion.
Melatonin, both endogenously produced by the pineal gland and available as a supplement, has emerged as a promising therapeutic agent with the dual capacity to promote mitophagy and provide protection against mitochondrial dysfunction and oxidative stress [154]. The relationship between melatonin and mitophagy is complex as some studies suggest that melatonin enhances mitophagy, while others suggest that it represses it. One study found that melatonin enhances mitophagy in human mesenchymal stem cells under oxidative stress by upregulating the expression of heat shock 70 kDa protein 1L (HSPLA) [155]. However, another study suggests that melatonin may provide beneficial effects on mitochondrial function by repressing mitophagy [156]. In a study involving septic newborns, melatonin's antioxidant properties were evaluated, revealing that melatonin treatment significantly reduced elevated levels of lipid peroxidation products, specifically malondialdehyde (MDA) and 4-hydroxylalkenals (4-HDA), commonly seen in septic infants compared to healthy controls [157]. This reduction in MDA and 4-HDA levels substantiates a mitigation of oxidative stress. This underscores melatonin's potential as an antioxidant and protective agent against oxidative stress in sepsis, with implications for improving clinical outcomes and increasing survival rates among septic patients, making it a promising therapeutic candidate for conditions marked by mitochondrial dysfunction, oxidative damage, and inflammation, such as sepsis. Overall, the relationship between melatonin and mitophagy is an area of active research, and more studies are needed to fully understand the mechanisms involved.
Prohibitin 1 (PHB1) and prohibitin 2 (PHB2), collectively known as prohibitins, are ubiquitously expressed proteins. PHB1 is an inner mitochondrial membrane (IMM) protein that forms a heterodimeric complex with PHB2. This complex stabilizes the structure of the mitochondrial membrane and regulates various mitochondrial functions, including mitophagy [158,159]. PHB1 has been shown to induce mitophagy in response to increased mitochondrial reactive oxygen species (ROS) through binding to the mitophagy receptor Nix/Bnip3L, independently of Parkin [159]. In the context of sepsis, the role of PHB1 becomes even more significant. An analysis of samples from 348 sepsis patients revealed an inverse correlation between PHB1 expression and the severity of sepsis [160]. It plays a vital role in regulating NLRP3 inflammasomes, reducing PHB1 levels increases cytoplasmic mtDNA levels and intensifies the activation of the NLRP3 inflammasome. When a mitophagy inhibitor is applied, it reverses the inflammasome activation induced by PHB1 knockdown. The cardioprotective impact of PHB1 in sepsis is evident by its ability to promote survival and protect the circulatory system [161]. Enhancing PHB1 levels, whether through overexpression or recombinant human PHB1 treatment, boosts the antioxidant and anti-inflammatory response in HL-1 cardiomyocytes. This protection extends to shielding the cells from mitochondrial dysfunction and cytokine-induced toxicity. Notably, when recombinant human PHB1 was administered, it mitigated inflammation, revived cardiac contractility, and restored ATP production in mice subjected to a lipopolysaccharide challenge. In conclusion, the multifaceted role of prohibitin 1 (PHB1) in maintaining mitochondrial integrity, regulating inflammasome activity, and its cardioprotective effects during sepsis underscores its potential as a valuable target for therapeutic interventions in severe inflammatory disorders (Table 1).
Table 1.
Summary of pharmacological agents affecting mitochondrial quality control mechanisms.
| Compound | Model | Effect | Reference |
|---|---|---|---|
| Biogenesis | |||
| Bortezomib | HeLa cells | Prevention of TFAM degradation. Reduction of inflammatory cytokines and increased survival | Lu et al. (2013) Han et al. (2015) |
| Bezafibrate | Mice | Upregulation of PGC-1α expression in skeletal and heart muscles | Hondares et al. (2007) |
| Fibroblasts from patients with genetic mitochondrial disorders | Restoration of PGC-1α levels, improved mitochondrial copy numbers. Restoration of brain function and protection from oxidative damage by lipid peroxidation | Sirvastava et al. (2009) | |
| Murine Huntington | Johri et al. (2012) | ||
| Resveratrol | Saccharomyces ervisiae | Activation of AMPK resulting in increased cellular NAD + availability allowing for SIRT1 activation | Howitz et al. (2003) |
| HepG2 cells/LDL receptor deficient mice | Zang et al. (2006) | ||
| Pioglitazone | DM II patients | Upregulation of biogenesis gene expression (PGC-1α, TFAM) and elevated mitochondrial copy numbers | Bogacka et al. (2005) |
| CLP mice | Inhibition of inflammatory response via NF-κB activation | Kaplan et al. (2014) | |
| Rosiglitazone | CLP rats | Activation of biogenesis. Improved brain ATP availability, oxygen consumption. Ameliorated long term cognitive impairment | Manfredini et al. (2019) |
| Mitophagy | |||
| Rapamycin | Transient middle cerebral artery occlusion rats | Promotion of mitophagy via p62 and Parkin mitochondrial translocation | Li et al. (2014) |
| U87MG cells | Upregulation of mitophagy gene expression (PINK1, Parkin) | Lenzi et al. (2021) | |
| Mice | Rescue of mitochondrial myopathy via autophagy and lysosomal biogenesis | Civiletto et al. (2018) | |
| CLP mice | Reduction of inflammation and suppression of pyroptosis limiting organ damage | Wang et al. (2019) | |
| Rescue of cognitive impairment via enhanced autophagy | Liu et al. (2017) | ||
| Rapamycin/Rilmenidine | CLP rats | Activation of autophagy. Improved brain ATP availability, oxygen consumption. Ameliorated long term cognitive impairment | Manfredini et al. (2019) |
| Metformin | DM II patients | Reduction of HbA1c levels, mitochondrial oxidative stress and upregulation of PINK1, Parkin | Bhansali et al. (2020) |
| Enhanced AMPK activation and reversed pro inflammatory cytokine signalling | Marañón et al. (2022) | ||
| Nicotinamide riboside (NR) | Human fibroblasts | Promotion of mitophagy and sirtuin activation | Jang et al. (2012) |
| LPS mice | Elevation of NAD+, reduced oxidative stress, inflammation and caspase-3. | Hong et al. (2018) | |
| Melatonin | Human mesenchymal stem cells | Enhanced mitophagy via expression of HSPLA | Yoon et al. (2019) |
| Mouse granulose cells | Repression of mitophagy | Jiang et al. (2021) | |
| Septic newborns | Reduction of lipid peroxidation products (MDA, 4-HAD) | Gitto et al. (2001) | |
| Prohibitin 1 (PHB1) | PHB1 deficient mice/Crohn's disease patients/Mode-K cells | Induction of mitophagy via Nix/Bnip3L (Parkin independent) | Alula et al. (2023) |
| Sepsis patient material | Regulation of the NLRP3 inflammasome and management of cytoplasmic mtDNA levels. | Chen et al. (2023) | |
| LPS treated HL-1 cardiomyocytes | Enhanced antioxidant and anti-inflammatory responses | Mattox et al. (2021) | |
| LPS mice | Mitigation of inflammation. Restoration of ATP production and cardiac contractility. | Mattox et al. (2021) | |
| Fission/Fusion | |||
| Procynidin | LPS mice | Increased Nrf2 nuclear translocation, reduction in ROS, increased fusion-to-fission ratio. | Liu et al. (2020) |
| LPS treated lung tissue and lung epithelial cells | Limitation of inflammatory response, oxidative stress and apoptosis | Ning et al. (2022) | |
| Streptozotocin mice | Promotion of SIRT3 dependent SOD2 deacetylation. | Liu et al. (2017) | |
4.4. Mitophagy vs biogenesis
While biogenesis and mitophagy have opposing functions, they are counterparts of the same coin and are closely interlinked with one another. Despite their opposing actions, both play essential roles in protecting against organ damage and death during sepsis.
Mitophagy and mitochondrial biogenesis collaboratively balance cellular energy production and oxidative stress control. For instance, in THP-1 cells following LPS exposure [162], mitophagy curtails oxidative stress by clearing dysfunctional mitochondria, while concurrent upregulation of mitochondrial biogenesis ensures sustained energy provision. Together, they orchestrate a cellular defence against the adverse effects of unregulated inflammation during sepsis [162]. Interestingly, while being opposite processes, both mitophagy and mitochondrial biogenesis are increased in survival and recovery of sepsis. HO-1 is an inducible enzyme that can be upregulated in response to various stressors, including inflammation, oxidative stress, and hypoxia [163]. Its upregulation is a protective response aimed at reducing oxidative stress, inflammation, and cellular damage. Induction of HO-1 by hemin improved mitochondrial function by decreasing lipid peroxidation, increasing mitochondrial biogenesis and mitophagy, and decreasing fission-related protein in CLP mice. Further, hemin treatment decreased proinflammatory cytokine levels in liver, organ damage and death of septic mice. The beneficial action of hemin in sepsis has been attributed to the downregulation of TLR4 expression as, similar to hemin, treatment with a TLR4 antagonist attenuated sepsis-induced mortality, inflammatory response, and mitochondrial dysfunction [163]. In line with mitochondria-protective effects mediated by HO-1 induction, Shi et al. (2019) [164] demonstrated that septic lung injury in rats can be attenuated by the induction of HO-1 through the PI3K/Akt pathway resulting in increased mitochondrial quality control mechanisms, evidenced by enhanced mitochondrial fission/fusion, mitochondrial biogenesis, mitophagy and a lower rate of apoptosis. It was accompanied by less lung injury and a higher survival rate. LY294002, a PI3K inhibitor, or knockdown of PI3K suppressed Akt phosphorylation and attenuated induction of HO-1, thereby reversing the beneficial effects evoked by hemin pre-treatment. Together, these results suggest that HO-1 activation, through the PI3K/Akt pathway plays a critical role in protecting the lung from oxidative injury in sepsis by regulating mitochondrial quality control and may be a therapeutic target for preventing sepsis-related lung injury.
Mitochondrial quality control mechanisms play a vital role in maintaining mitochondrial function. Amidst the ongoing scientific discourse surrounding the importance of these mechanisms, we offer an alternative perspective: the key lies in the timing of the interventions promoting mtQC in sepsis seem key and should be the focus of further research is this area. Manfredini et al. (2019) [165] demonstrated mitochondrial dysfunction is associated with long-term cognitive impairment in rat sepsis. Sepsis was induced both acutely (24 h) and in the long term (10 days. in adult Wistar rats using CLP. A week later, they were treated with activators for biogenesis and autophagy or saline as a control. Cognitive impacts were assessed through behavioural tests, while brain mitochondrial functions were evaluated at various time intervals post-sepsis. Treatment with rosiglitazone, which activates mitochondrial biogenesis, or rapamycin and rilmenidine, which activate autophagy, proved to be effective interventions. These treatments improved brain ATP levels, enhanced oxygen consumption, and importantly, ameliorated the long-term cognitive impairment observed in sepsis survivors. Thus, mitophagy and mitochondrial biogenesis are two functionally opposed mechanisms that are together essential to maintain a healthy pool of mitochondria. This study underscores the crucial role of mitochondrial dysfunction in long-term cognitive impairment following sepsis. Moreover, it highlights the efficacy of treatments that regulate mitochondrial function, especially during the later stages of sepsis, as potential therapeutic strategies to mitigate cognitive impairment in sepsis survivors. In line with these findings, a study conducted in a CLP mouse model demonstrated that early administration of Rapamycin effectively rescued cognitive impairment by boosting autophagy [166]. Taken together, it would be reasonable to conclude that early administration of mitophagy-enhancing drugs during sepsis may prove beneficial by eliminating damaged and malfunctioning mitochondria. Conversely, the administration of biogenesis-enhancing drugs at a later stage could aid in replenishing the necessary mitochondrial population, thus promoting healthy mitochondrial function.
5. Mitochondrial dynamics
Mitochondrial dynamics, specifically fission and fusion, involve structural changes in existing mitochondria and plays a critical role in regulating cellular bioenergetics. Mitochondria are dynamic organelles which change morphology by undergoing coordinated cycles of fission or fusion and movement within the cell [167]. Mitochondrial fission is defined as the division of one mitochondrion into two (smaller) daughter mitochondria, while conversely mitochondrial fusion refers to the opposite process, the union of two mitochondria that results in one larger mitochondrion [168]. Both fission and fusion processes are regulated by guanosine triphosphatases (GTPases), which are members of the dynamin family, that divide and fuse the two lipid bilayers that surround the mitochondria [169], the IMM and the OMM. The IMM forms invaginations called cristae that surround the mitochondrial matrix and contain most of the fully assembled complexes of the ETC and ATP synthase, as well as substantial amounts of the soluble electron transport protein cytochrome C [170], while the OMM surrounds the IMM and the intermembrane space. The balance between mitochondrial fusion and fission plays a vital role in maintaining mitochondrial function, structure, and turnover [169]. Specifically, fission and fusion are intricately linked to the regulation of mitophagy, as fission usually targets dysfunctional mitochondria for mitophagy [171]. Conversely, the fusion process is essential for the exchange of mitochondrial contents and enabling repair of damaged mitochondria [172,173], a process sometimes referred to as cross-complementation, which ultimately influences mitochondrial biogenesis [174]. Hereby, fission and fusion can allow selective removal of damaged mitochondrial components through redistribution followed by mitophagy [161]. Sepsis leads to a shift in the fission/fusion balance towards fission [175], which could aid to clear damage mitochondrial components via redistribution and mitophagy, but can also contribute to increased oxidative stress and cell death in sepsis (Fig. 6).
Fig. 6.
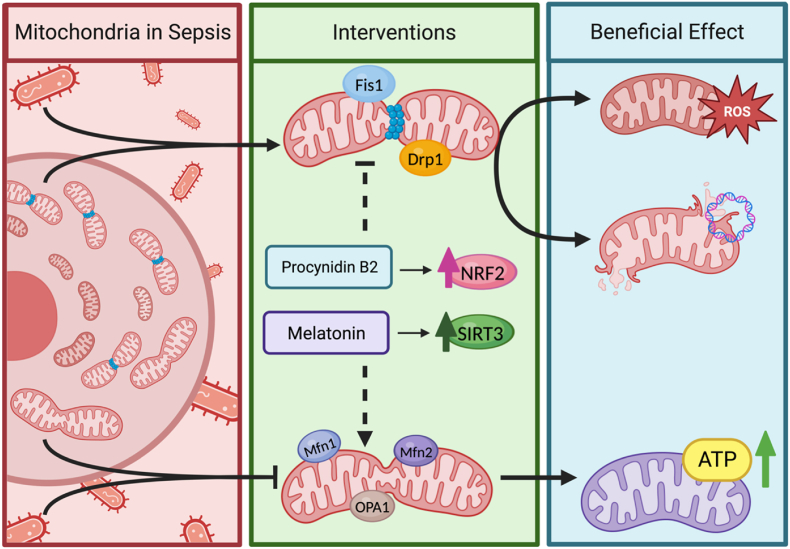
Dysregulated mitochondrial dynamics in sepsis. In prolonged sepsis, the fission-to-fusion ratio favours fission. This imbalance causes the accumulation of damaged mitochondria, oxidative stress, and cell death. Treatment with procyanidin B2 (PCB2) promotes Nrf2 translocation into the nucleus, which indirectly upregulates mitochondrial fusion while inhibiting fission [176]. Melatonin increases the activity and expression of SIRT3, which preserves mitochondrial quality control mechanisms and alleviates sepsis-induced injury [177]. This highlights the potential therapeutic benefits of targeting mitochondrial dynamics and quality control in sepsis.
The adverse effects of mitochondrial fission on organ dysfunction in ICU-admitted sepsis patients are emphasized by the findings of Huang et al. (2021) [4]. MtQC-related biomarkers PGC-1α (promotor of mitochondrial biogenesis), fission protein 1 (Fis1)(promotor of mitochondrial fission), mitofusin2 (Mfn2)(promotor of mitochondrial fusion), and Parkin (promotor of mitophagy) differ between healthy individuals and through the stages of sepsis severity. In this population, the levels of PGC-1α, Mfn2, and Parkin were lowest in healthy individuals, while the levels of Fis1 were highest in the septic shock group – thus supporting a shift from fusion to fission in patients with sepsis as well. Lowered levels of biomarkers for mitochondrial biogenesis and increased levels of fission biomarkers correlated with disease severity and progression, as measured by the Sequential Organ Failure Assessment (SOFA) score. This suggests that monitoring these biomarkers could help predict outcome and guide treatment in septic patients. Induction of sepsis by CLP in mice, leads to sepsis-AKI and disturbs fission and fusion as manifested by a relative increase in fission over fusion [176]. Treatment with procyanidin B2 (PBC2) shifted this balance back towards fusion, reinforcing mitochondrial integrity. This not only curbed mitochondrial-mediated apoptosis but also rectified impaired mitophagy. The underlying mechanisms behind PBC2's efficacy were multifaceted. One notable pathway involved the increased nuclear translocation of the transcription factor Nrf2, which plays a role in both the reduction of ROS accumulation and a decrease in mitochondrial damage. Furthermore, Nrf2's translocation to the nucleus enhanced mitochondrial biogenesis, increasing the fusion-to-fission ratio. Moreover, PBC2's promotion of mitophagy effectively diminished the build-up of damaged mitochondria, which subsequently decreased associated apoptosis and cellular damage [176]. Organ dysfunction in ICU-admitted sepsis patients are emphasized by the findings of Huang et al. (2021) [4]. MtQC-related biomarkers including PGC-1α Fis1, Mfn2, and Parkin differ between healthy individuals and through the stages of sepsis severity. In this population, the levels of PGC-1α, Mfn2, and Parkin were lowest in healthy individuals, while the levels of Fis1 were highest in the septic shock group – highlighting the importance of preventing mitochondrial scattering through fission. Additionally, both reduced biogenesis and increased fission markers correlated with disease severity and progression (SOFA score), suggesting that monitoring these biomarkers could help predict outcome and guide treatment in septic patients. Promoting fusion by melatonin confers protection against acute lung injury [177] by reducing the inflammatory response, oxidative stress, and apoptosis in LPS-treated lung tissues and LPS-treated lung epithelial cells. Melatonin also inhibited mitophagy and restored fatty acid oxidation via the activation of the SIRT3/SOD2 signalling pathway. in LPS-treated lung epithelial cells in vitro and in vivo. The interaction of melatonin with the melatonin receptor 1B (MTNR1B) that plays a pivotal role in regulating glucose homeostasis and insulin secretion, may explain the enhanced glucose uptake and improved fatty acid oxidation [177]. The protective effects of melatonin seem to be, at least in part, mediated through SIRT3. Inhibition of SIRT3 negated melatonin's defensive capabilities against acute lung injury. Melatonin not only boosted the activity and expression of SIRT3 but also promoted the SIRT3-dependent deacetylation of superoxide dismutase 2 (SOD2), thereby increasing mitochondrial antioxidative capacity [178].
In essence, the alteration in mitochondrial dynamics, characterized by increased fission and decreased fusion events in sepsis, either leads to or reflects the accumulation of damaged mitochondria, oxidative stress, and subsequent cell death. This aberrant fission-to-fusion ratio can be further exacerbated by impaired mitophagy, which allows the accumulation of (fissioned, fragmented) damaged mitochondria. Promoting clearance of damaged mitochondria by mitophagy and inducing mitochondrial fusion could reveal to be a potential therapeutic strategy to attenuate organ dysfunction and improve outcomes in sepsis.
5.1. Future perspectives
Whether or not mitochondrial biogenesis, mitophagy, fission/fusion or all three mechanisms are key to improve sepsis outcomes, therapies should ascertain not to increase the amount of circulating mtDNA as elevated mtDNA in the circulation acts as a DAMP and induces remote inflammation. Higher levels of circulating mtDNA are associated with systemic inflammation, organ dysfunction [12] and long-term mortality [179]. On the other hand, intact mitochondrial biogenesis seems crucial as lowered circulatory mtDNA levels are associated with frailty and all-cause mortality in the general population [180]. Age is inversely associated with mtDNA copy number, with a higher mtDNA copy number in women relative to men. Potentially, this observation may in part account for better outcomes of female sepsis patients as observed by Nasir et al., 2015 [181].
On a final note, future research should also assess the effect of currently used medication on mitochondrial quality control, as some drugs may have agonistic or antagonistic functions. For example, propofol, a widely used sedative drug in patients admitted to ICUs, induces mitochondrial dysfunction characterized by bioenergetic dysfunction manifested by reduced ATP production and a reduced mitochondrial mass in isolated human skeletal muscle cells [182]. Noradrenaline, a commonly used vasopressor, increases mitochondrial network size and turnover. Noradrenaline also reversed or prevented the occurrence propofol-induced mitochondrial dysfunction and restored fatty acid oxidation capacity. Although both propofol and noradrenaline reduced mitochondrial membrane potential and amplified ROS production, their combined effects were not additive at typical sub-maximal dosages used in patients. Potentially, noradrenaline can prevent propofol-induced impairment of mitochondrial function in human skeletal muscle cells [182]. More importantly, the mainstay of current treatment – antibiotics – also may or may not confer immunomodulatory and bioenergetic effects [183,184]. Given the hypothesis that mitochondria share a bacterial ancestry, it is reasonable to consider the potential impact of bacterial antibiotics on mitochondrial function. Research has demonstrated that certain bacterial antibiotics can induce oxidative stress and harm in mammalian cells [185]. This leads to issues like mitochondrial dysfunction and excessive ROS (reactive oxygen species) production. It's worth noting that antibiotics have the potential to affect both the immune system and the body's energy processes [183]. This could potentially impede the host's ability to combat infections and maintain organ function. Some of these adverse effects may stem from their impact on mitochondria, which are already compromised due to the underlying septic condition. However, it's important to acknowledge that the findings from cell culture and animal models may not perfectly align with results from clinical settings. For example, one study found no discernible impact of antibiotics on the mitochondrial bioenergetics of lymphocytes of septic patients [184]. In summary, are and will most likely remain the main source of disease control in sepsis, and discontinuing their use is not a viable option, however antibiotics appear to have an effect on mitochondrial function in vitro, and further research is needed to corroborate these finding in clinical practice.
6. Conclusion
Sepsis-induced mitochondrial dysfunction is a complex process that is primarily triggered by increased production of NO and ROS resulting in oxidative stress, mitochondrial membrane damage, and ultimately, cell death. Concomitantly, sepsis triggers release of mtDNA into the circulation, propagating systemic inflammation. Hereby, persisting mitochondrial dysfunction and oxidative stress are major contributors to the observed cell damage and organ dysfunction in sepsis.
Mitochondrial quality control (mtQC) is essential to mitigate organ damage due to persisting mitochondrial dysfunction, diligently facilitating both repair mechanisms and the upkeep of mitochondrial integrity. Mitophagy, the process of selective removal of damaged mitochondria, is an essential mechanism for the maintenance of a healthy pool of mitochondria. Inducing mitophagy reduces oxidative stress, inflammation, fibrosis, and organ injury in sepsis and attenuates sepsis-induced muscle wasting. Sepsis leads to an imbalance between fission-fusion, favouring fission over fusion and leading to the accumulation of damaged mitochondria given the reduced rate of mitophagy. Additionally, these processes combined with dysregulated mitochondrial biogenesis underlies the reduction in mitochondrial mass and persistence of mitochondrial dysfunction, leading to impaired bioenergetics and loss of cellular homeostasis.
Overall, understanding the interconnectedness of mitochondrial dysfunction and mitochondrial quality control over the course of sepsis will provide the insights to develop targeted mitochondria-directed therapies to prevent and recover from organ damage. Based on current knowledge, preventing excessive mitochondrial fission, while promoting fusion and mitophagy during sepsis and stimulating biogenesis timely after sepsis, may help attenuate organ dysfunction and improve outcomes in sepsis.
Figures were created with BioRender.com.
Authors' contributions
F.M. Lira Chavez and L.P. Gartzke performed the literature search, analysed the articles, wrote the manuscript, and designed the figures. F.E. van Beuningen participated in the extended literature search and the information extraction. S.E. Wink participated in revising the manuscript and designing the figures. G. Krenning, H.R. Bouma and R.H. Henning participated interpreting the results and revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Availability of data and materials
All data/literature analysed during this study are included in this published article.
Financial Support and sponsorship
Funding bodies were not involved in the design of the study and collection, analysis, and interpretation of data and in authoring the article.
Ethical Approval and consent to participate
Ethical Approval is not applicable for this article.
Consent for publication
There are no human subjects in this article and informed consent is not applicable.
Copyright
Authors retain copyright of their works.
Declaration of competing interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Funding bodies were not involved in the design of the study and collection, analysis, and interpretation of data and in authoring the article.
Acknowledgements
This work was supported by GUIDE and University Medical Centre Groningen (PhD grant F.M.L.C and MD/PhD grant to L.P.G.) as well as Consejo Nacional de Humanidades, Ciencias y Tecnologias (CONAHCYT) (PhD grant F.M.L.C). Many thanks to Nora Spraakman for her valuable insights and contributions regarding readability and input.
Data availability
No data was used for the research described in the article.
References
- 1.Garrabou G., et al. The effects of sepsis on mitochondria. J. Infect. Dis. Feb. 2012;205(3):392–400. doi: 10.1093/infdis/jir764. [DOI] [PubMed] [Google Scholar]
- 2.Li Y., et al. Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis. Redox Biol. Jan. 2021;38 doi: 10.1016/j.redox.2020.101771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao Y., et al. Role of Parkin-mediated mitophagy in the protective effect of polydatin in sepsis-induced acute kidney injury. J. Transl. Med. 2020;18(1):114. doi: 10.1186/s12967-020-02283-2. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang W., Wang X., Zhang H., Wang G., Xie F., Liu D. Serum mitochondrial quality control related biomarker levels are associated with organ dysfunction in septic patients. Shock. Sep. 2021;56(3):412–418. doi: 10.1097/SHK.0000000000001737. [DOI] [PubMed] [Google Scholar]
- 5.Rudd K.E., et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. Jan. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angus D.C., van der Poll T. Severe sepsis and septic shock. N. Engl. J. Med. 2013;369(9):840–851. doi: 10.1056/NEJMra1208623. Aug. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson E.K., Rubenstein A.R., Radin G.T., Wiener R.S., Walkey A.J. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit. Care Med. Mar. 2014;42(3):625–631. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotchkiss R.S., Moldawer L.L., Opal S.M., Reinhart K., Turnbull I.R., Vincent J.-L. Sepsis and septic shock. Nat. Rev. Dis. Prim. 2016;2 doi: 10.1038/nrdp.2016.45. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seymour C.W., et al. Time to treatment and mortality during mandated emergency care for sepsis. N. Engl. J. Med. Jun. 2017;376(23):2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans L., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. Nov. 2021;47(11):1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott B.N.V., Sarkar T., Kratofil R.M., Kubes P., Thanabalasuriar A. Unraveling the host's immune response to infection: seeing is believing. J. Leukoc. Biol. 2019;106(2):323–335. doi: 10.1002/JLB.4RI1218-503R. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmons J.D., et al. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann. Surg. 2013;258(4):591–596. doi: 10.1097/SLA.0b013e3182a4ea46. ; discussion 596, Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Slikke E.C., Beumeler L.F.E., Holmqvist M., Linder A., Mankowski R.T., Bouma H.R. Understanding post-sepsis syndrome: how can clinicians help? Infect. Drug Resist. Sep. 2023;16:6493–6511. doi: 10.2147/IDR.S390947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jentzer J.C., Lawler P.R., Van Houten H.K., Yao X., Kashani K.B., Dunlay S.M. Cardiovascular events among survivors of sepsis hospitalization: a retrospective cohort analysis. J. Am. Heart Assoc. Feb. 2023;12(3) doi: 10.1161/JAHA.122.027813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nedel W., Deutschendorf C., Portela L.V.C. Sepsis-induced mitochondrial dysfunction: a narrative review. World J. Crit. Care Med. Jun. 2023;12(3):139–152. doi: 10.5492/wjccm.v12.i3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P., Sampath H. Mitochondrial DNA integrity: role in health and disease. Cells. 2019;8(2) doi: 10.3390/cells8020100. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadalutti C.A., Ayala-Peña S., Santos J.H. Mitochondrial DNA damage as driver of cellular outcomes. Am. J. Physiol. Cell Physiol. Feb. 2022;322(2):C136–C150. doi: 10.1152/ajpcell.00389.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tresse E., et al. Mitochondrial DNA damage triggers spread of Parkinson's disease-like pathology. Mol. Psychiatry, Oct. 2023 doi: 10.1038/s41380-023-02251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kowaltowski A.J., Vercesi A.E. Mitochondrial damage induced by conditions of oxidative stress. Free Radic. Biol. Med. Feb. 1999;26(3–4):463–471. doi: 10.1016/s0891-5849(98)00216-0. [DOI] [PubMed] [Google Scholar]
- 20.Dolmatova E.V., Wang K., Mandavilli R., Griendling K.K. The effects of sepsis on endothelium and clinical implications. Cardiovasc. Res. Jan. 2021;117(1):60–73. doi: 10.1093/cvr/cvaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J., et al. Mitochondria in sepsis-induced AKI. J. Am. Soc. Nephrol. Jul. 2019;30(7):1151–1161. doi: 10.1681/ASN.2018111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrieux P., Chevillard C., Cunha-Neto E., Nunes J.P.S. Mitochondria as a cellular hub in infection and inflammation. Int. J. Mol. Sci. 2021;22:21. doi: 10.3390/ijms222111338. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jassim A.H., Inman D.M., Mitchell C.H. Crosstalk between dysfunctional mitochondria and inflammation in glaucomatous neurodegeneration. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.699623. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Horssen J., van Schaik P., Witte M. Inflammation and mitochondrial dysfunction: a vicious circle in neurodegenerative disorders? Neurosci. Lett. Sep. 2019;710 doi: 10.1016/j.neulet.2017.06.050. [DOI] [PubMed] [Google Scholar]
- 25.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. May 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 26.Grazioli S., Pugin J. Mitochondrial damage-associated molecular patterns: from inflammatory signaling to human diseases. Front. Immunol. May 2018;9:832. doi: 10.3389/fimmu.2018.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchi S., Guilbaud E., Tait S.W.G., Yamazaki T., Galluzzi L. Mitochondrial control of inflammation. Nat. Rev. Immunol. Mar. 2023;23(3):159–173. doi: 10.1038/s41577-022-00760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brealey D., et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–223. doi: 10.1016/S0140-6736(02)09459-X. Jul. [DOI] [PubMed] [Google Scholar]
- 29.Galley H.F. Oxidative stress and mitochondrial dysfunction in sepsis. Br. J. Anaesth. 2011;107(1):57–64. doi: 10.1093/bja/aer093. Jul. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H., Feng Y.-W., Yao Y.-M. Potential therapy strategy: targeting mitochondrial dysfunction in sepsis. Mil. Med. Res. 2018;5(1):41. doi: 10.1186/s40779-018-0187-0. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagar H., Piao S., Kim C.-S. Role of mitochondrial oxidative stress in sepsis. Acute Crit. Care. May 2018;33(2):65–72. doi: 10.4266/acc.2018.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lubkin D.T., Bishawi M., Barbas A.S., Brennan T.V., Kirk A.D. Extracellular mitochondrial DNA and N-formyl peptides in trauma and critical illness: a systematic review. Crit. Care Med. Dec. 2018;46(12) doi: 10.1097/CCM.0000000000003381. 2018–2028. [DOI] [PubMed] [Google Scholar]
- 33.Wiley C.D., et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metabol. Feb. 2016;23(2):303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin L., Batra S., Jeyaseelan S. Deletion of Nlrp3 augments survival during polymicrobial sepsis by decreasing autophagy and enhancing phagocytosis. J. Immunol. Feb. 2017;198(3):1253–1262. doi: 10.4049/jimmunol.1601745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S., et al. NLRP3 inflammasome deficiency protects against microbial sepsis via increased lipoxin B4 synthesis. Am. J. Respir. Crit. Care Med. Sep. 2017;196(6):713–726. doi: 10.1164/rccm.201604-0892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu Y., Huang Y., Chen M., Yang Y., Li X., Zhang W. Mitochondrial DNA in NLRP3 inflammasome activation. Int. Immunopharm. Jul. 2022;108 doi: 10.1016/j.intimp.2022.108719. [DOI] [PubMed] [Google Scholar]
- 37.Danielski L.G., Giustina A.D., Bonfante S., Barichello T., Petronilho F. The NLRP3 inflammasome and its role in sepsis development. Inflammation. 2020;43(1):24–31. doi: 10.1007/s10753-019-01124-9. Feb. [DOI] [PubMed] [Google Scholar]
- 38.Escoll P., Buchrieser C. Metabolic reprogramming of host cells upon bacterial infection: why shift to a Warburg-like metabolism? FEBS J. Jun. 2018;285(12):2146–2160. doi: 10.1111/febs.14446. [DOI] [PubMed] [Google Scholar]
- 39.Van Wyngene L., Vandewalle J., Libert C. Reprogramming of basic metabolic pathways in microbial sepsis: therapeutic targets at last? EMBO Mol. Med. 2018;10(8) doi: 10.15252/emmm.201708712. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Moltke J., Ayres J.S., Kofoed E.M., Chavarría-Smith J., Vance R.E. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 2013;31:73–106. doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen G.T., Green E.R., Mecsas J. Neutrophils to the roscue: mechanisms of NADPH oxidase activation and bacterial resistance. Front. Cell. Infect. Microbiol. 2017;7:373. doi: 10.3389/fcimb.2017.00373. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuck J.L., et al. Mitochondrial DNA damage-associated molecular patterns mediate a feed-forward cycle of bacteria-induced vascular injury in perfused rat lungs. Am. J. Physiol. Lung Cell Mol. Physiol. May 2015;308(10):L1078–L1085. doi: 10.1152/ajplung.00015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019;20(13) doi: 10.3390/ijms20133328. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tschopp J., Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. Mar. 2010;10(3):210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 45.Berghe T.V., et al. Simultaneous targeting of interleukin-1 and interleukin-18 is required for protection against inflammatory and septic shock. Crit. Care. 2014;18(S2) doi: 10.1186/cc14023. Apr. [DOI] [PubMed] [Google Scholar]
- 46.Smith R.L., Soeters M.R., Wüst R.C.I., Houtkooper R.H. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr. Rev. 2018;39(4):489–517. doi: 10.1210/er.2017-00211. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinberg S.E., Sena L.A., Chandel N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity. Mar. 2015;42(3):406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J., Zhou G., Wang X., Liu D. Metabolic reprogramming consequences of sepsis: adaptations and contradictions. Cell. Mol. Life Sci. 2022;79(8):456. doi: 10.1007/s00018-022-04490-0. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maus M., Marin P., Israël M., Glowinski J., Prémont J. Pyruvate and lactate protect striatal neurons against N-methyl-D-aspartate-induced neurotoxicity. Eur. J. Neurosci. Sep. 1999;11(9):3215–3224. doi: 10.1046/j.1460-9568.1999.00745.x. [DOI] [PubMed] [Google Scholar]
- 50.Kirkman D.L., Robinson A.T., Rossman M.J., Seals D.R., Edwards D.G. Mitochondrial contributions to vascular endothelial dysfunction, arterial stiffness, and cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. May 2021;320(5):H2080–H2100. doi: 10.1152/ajpheart.00917.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theofilis P., et al. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines. 2021;9(7) doi: 10.3390/biomedicines9070781. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lush C.W., Kvietys P.R. Microvascular dysfunction in sepsis. Microcirculation. 2000;7(2):83–101. doi: 10.1038/sj.mn.7300096. Apr. [DOI] [PubMed] [Google Scholar]
- 53.Zarbock A., et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat. Rev. Nephrol. Jun. 2023;19(6):401–417. doi: 10.1038/s41581-023-00683-3. [DOI] [PubMed] [Google Scholar]
- 54.James M.T., Bhatt M., Pannu N., Tonelli M. Long-term outcomes of acute kidney injury and strategies for improved care. Nat. Rev. Nephrol. Apr. 2020;16(4):193–205. doi: 10.1038/s41581-019-0247-z. [DOI] [PubMed] [Google Scholar]
- 55.Tang C., Cai J., Yin X.-M., Weinberg J.M., Venkatachalam M.A., Dong Z. Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. May 2021;17(5):299–318. doi: 10.1038/s41581-020-00369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji R., et al. The Warburg effect promotes mitochondrial injury regulated by uncoupling protein-2 in septic acute kidney injury. Shock. May 2021;55(5):640–648. doi: 10.1097/SHK.0000000000001576. [DOI] [PubMed] [Google Scholar]
- 57.Li X., et al. Lactate metabolism in human health and disease. Signal Transduct. Targeted Ther. 2022;7(1):305. doi: 10.1038/s41392-022-01151-3. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Star B.S., van der Slikke E.C., van Buiten A., Henning R.H., Bouma H.R. The novel compound SUL-138 counteracts endothelial cell and kidney dysfunction in sepsis by preserving mitochondrial function. Int. J. Mol. Sci. 2023;24(7) doi: 10.3390/ijms24076330. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonora M., et al. Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene. 2015;34(12):1475–1486. doi: 10.1038/onc.2014.96. Mar. [DOI] [PubMed] [Google Scholar]
- 60.Zaib S., Hayyat A., Ali N., Gul A., Naveed M., Khan I. Role of mitochondrial membrane potential and lactate dehydrogenase A in apoptosis. Anti Cancer Agents Med. Chem. 2022;22(11):2048–2062. doi: 10.2174/1871520621666211126090906. [DOI] [PubMed] [Google Scholar]
- 61.Zhang M., Zheng J., Nussinov R., Ma B. Release of cytochrome C from bax pores at the mitochondrial membrane. Sci. Rep. 2017;7(1):2635. doi: 10.1038/s41598-017-02825-7. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hotchkiss R.S., et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. Jul. 1999;27(7):1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Oliveira T.S., et al. Sepsis disrupts mitochondrial function and diaphragm morphology. Front. Physiol. Sep. 2021;12 doi: 10.3389/fphys.2021.704044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pieracci F.M., Barie P.S. Iron and the risk of infection. Surg. Infect. 2005;6(Suppl 1):S41–S46. doi: 10.1089/sur.2005.6.s1-41. [DOI] [PubMed] [Google Scholar]
- 65.Recalcati S., Cairo G. Macrophages and iron: a special relationship. Biomedicines. 2021;9:11. doi: 10.3390/biomedicines9111585. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu Y., et al. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Dis. 2021;7(1):193. doi: 10.1038/s41420-021-00579-w. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng D., Liwinski T., Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020;6:36. doi: 10.1038/s41421-020-0167-x. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsuchiya K. Inflammasome-associated cell death: pyroptosis, apoptosis, and physiological implications. Microbiol. Immunol. 2020;64(4):252–269. doi: 10.1111/1348-0421.12771. Apr. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J., et al. YAP1 alleviates sepsis-induced acute lung injury via inhibiting ferritinophagy-mediated ferroptosis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.884362. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu J., Li L., Luo J. Diagnostic and prognostic value of monocyte distribution width in sepsis. J. Inflamm. Res. Jul. 2022;15:4107–4117. doi: 10.2147/JIR.S372666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monneret G., Venet F. A rapidly progressing lymphocyte exhaustion after severe sepsis. Crit. Care. 2012;16(4):140. doi: 10.1186/cc11416. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hotchkiss R.S., Osmon S.B., Chang K.C., Wagner T.H., Coopersmith C.M., Karl I.E. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J. Immunol. Apr. 2005;174(8):5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 73.Sheikh Motahar Vahedi H., Bagheri A., Jahanshir A., Seyedhosseini J., Vahidi E. Association of lymphopenia with short term outcomes of sepsis patients; a brief report. Arch. Acad. Emerg. Med. Jan. 2019;7(1):e14. [PMC free article] [PubMed] [Google Scholar]
- 74.Polilli E., et al. Circulating lymphocyte subsets as promising biomarkers to identify septic patients at higher risk of unfavorable outcome. BMC Infect. Dis. 2021;21(1):780. doi: 10.1186/s12879-021-06481-1. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shen X.-F., Cao K., Jiang J.-P., Guan W.-X., Du J.-F. Neutrophil dysregulation during sepsis: an overview and update. J. Cell Mol. Med. 2017;21(9):1687–1697. doi: 10.1111/jcmm.13112. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin W.-P., Li J.-B., Zheng X.-F., An L., Shao H., Li C.-S. Effect of neutrophil CD64 for diagnosing sepsis in emergency department. World J. Emerg. Med. 2020;11(2):79–86. doi: 10.5847/wjem.j.1920-8642.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeh C.-F., Wu C.-C., Liu S.-H., Chen K.-F. Comparison of the accuracy of neutrophil CD64, procalcitonin, and C-reactive protein for sepsis identification: a systematic review and meta-analysis. Ann. Intensive Care. 2019;9(1):5. doi: 10.1186/s13613-018-0479-2. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faix J.D. Biomarkers of sepsis. Crit. Rev. Clin. Lab Sci. 2013;50(1):23–36. doi: 10.3109/10408363.2013.764490. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shu Q., She H., Chen X., Zhong L., Zhu J., Fang L. Identification and experimental validation of mitochondria-related genes biomarkers associated with immune infiltration for sepsis. Front. Immunol. May 2023;14 doi: 10.3389/fimmu.2023.1184126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Picca A., Lezza A.M.S. Regulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions: useful insights from aging and calorie restriction studies. Mitochondrion. 2015;25:67–75. doi: 10.1016/j.mito.2015.10.001. Nov. [DOI] [PubMed] [Google Scholar]
- 81.Popov L.-D. Mitochondrial biogenesis: an update. J. Cell Mol. Med. May 2020;24(9):4892–4899. doi: 10.1111/jcmm.15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. Jan. 2014;5(1):66–72. doi: 10.4161/viru.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gründler K., et al. Platelet mitochondrial membrane depolarization reflects disease severity in patients with sepsis and correlates with clinical outcome. Crit. Care. 2014;18(1):R31. doi: 10.1186/cc13724. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rackham O., Filipovska A. Organization and expression of the mammalian mitochondrial genome. Nat. Rev. Genet. 2022;23(10):606–623. doi: 10.1038/s41576-022-00480-x. Oct. [DOI] [PubMed] [Google Scholar]
- 85.Gureev A.P., Shaforostova E.A., Popov V.N. Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1α signaling pathways. Front. Genet. May 2019;10:435. doi: 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tran M., et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Invest. Oct. 2011;121(10):4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li R., et al. Mitochondrial STAT3 exacerbates LPS-induced sepsis by driving CPT1a-mediated fatty acid oxidation. Theranostics. Jan. 2022;12(2):976–998. doi: 10.7150/thno.63751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rahmel T., et al. Mitochondrial dysfunction in sepsis is associated with diminished intramitochondrial TFAM despite its increased cellular expression. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-78195-4. 21029, Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shutt T.E., Bestwick M., Shadel G.S. The core human mitochondrial transcription initiation complex: it only takes two to tango. Transcription. 2011;2(2):55–59. doi: 10.4161/trns.2.2.14296. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gleyzer N., Vercauteren K., Scarpulla R.C. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell Biol. Feb. 2005;25(4):1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van der Slikke E.C., Star B.S., van Meurs M., Henning R.H., Moser J., Bouma H.R. Sepsis is associated with mitochondrial DNA damage and a reduced mitochondrial mass in the kidney of patients with sepsis-AKI. Crit. Care. 2021;25(1):36. doi: 10.1186/s13054-020-03424-1. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abu Shelbayeh O., Arroum T., Morris S., Busch K.B. PGC-1α is a master regulator of mitochondrial lifecycle and ROS stress response. Antioxidants. May 2023;12(5) doi: 10.3390/antiox12051075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tran M.T., et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531(7595):528–532. doi: 10.1038/nature17184. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.MacGarvey N.C., et al. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am. J. Respir. Crit. Care Med. Apr. 2012;185(8):851–861. doi: 10.1164/rccm.201106-1152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bartz R.R., et al. Staphylococcus aureus sepsis induces early renal mitochondrial DNA repair and mitochondrial biogenesis in mice. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0100912. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vanasco V., et al. Cardiac mitochondrial biogenesis in endotoxemia is not accompanied by mitochondrial function recovery. Free Radic. Biol. Med. Dec. 2014;77:1–9. doi: 10.1016/j.freeradbiomed.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 97.Huang M., Cai S., Su J. The pathogenesis of sepsis and potential therapeutic targets. Int. J. Mol. Sci. 2019;20:21. doi: 10.3390/ijms20215376. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu B., et al. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease. Mol. Cell. Jan. 2013;49(1):121–132. doi: 10.1016/j.molcel.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carré J.E., et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am. J. Respir. Crit. Care Med. Sep. 2010;182(6):745–751. doi: 10.1164/rccm.201003-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Puthucheary Z.A., et al. Metabolic phenotype of skeletal muscle in early critical illness. Thorax. 2018;73(10):926–935. doi: 10.1136/thoraxjnl-2017-211073. Oct. [DOI] [PubMed] [Google Scholar]
- 101.Cantó C., Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. Apr. 2009;20(2):98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buck S.W., Gallo C.M., Smith J.S. Diversity in the Sir2 family of protein deacetylases. J. Leukoc. Biol. Jun. 2004;75(6):939–950. doi: 10.1189/jlb.0903424. [DOI] [PubMed] [Google Scholar]
- 103.Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. Mar. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 104.Aquilano K., Vigilanza P., Baldelli S., Pagliei B., Rotilio G., Ciriolo M.R. Peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC-1alpha) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. J. Biol. Chem. Jul. 2010;285(28):21590–21599. doi: 10.1074/jbc.M109.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aquilano K., Baldelli S., Pagliei B., Ciriolo M.R. Extranuclear localization of SIRT1 and PGC-1α: an insight into possible roles in diseases associated with mitochondrial dysfunction. Curr. Mol. Med. Jan. 2013;13(1):140–154. doi: 10.2174/1566524011307010140. [DOI] [PubMed] [Google Scholar]
- 106.Michishita E., Park J.Y., Burneskis J.M., Barrett J.C., Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell. Oct. 2005;16(10):4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Potthoff M.J., et al. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J. Clin. Invest. Sep. 2007;117(9):2459–2467. doi: 10.1172/JCI31960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu T.F., Vachharajani V., Millet P., Bharadwaj M.S., Molina A.J., McCall C.E. Sequential actions of SIRT1-RELB-SIRT3 coordinate nuclear-mitochondrial communication during immunometabolic adaptation to acute inflammation and sepsis. J. Biol. Chem. Jan. 2015;290(1):396–408. doi: 10.1074/jbc.M114.566349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kong S., Cai B., Nie Q. PGC-1α affects skeletal muscle and adipose tissue development by regulating mitochondrial biogenesis. Mol. Genet. Genom. May 2022;297(3):621–633. doi: 10.1007/s00438-022-01878-2. [DOI] [PubMed] [Google Scholar]
- 110.Han S.H., et al. The effect of bortezomib on expression of inflammatory cytokines and survival in a murine sepsis model induced by cecal ligation and puncture. Yonsei Med. J. Jan. 2015;56(1):112–123. doi: 10.3349/ymj.2015.56.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tenenbaum A., Motro M., Fisman E.Z. Dual and pan-peroxisome proliferator-activated receptors (PPAR) co-agonism: the bezafibrate lessons. Cardiovasc. Diabetol. 2005;4:14. doi: 10.1186/1475-2840-4-14. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hondares E., Pineda-Torra I., Iglesias R., Staels B., Villarroya F., Giralt M. PPARdelta, but not PPARalpha, activates PGC-1alpha gene transcription in muscle. Biochem. Biophys. Res. Commun. Mar. 2007;354(4):1021–1027. doi: 10.1016/j.bbrc.2007.01.092. [DOI] [PubMed] [Google Scholar]
- 113.Srivastava S., Diaz F., Iommarini L., Aure K., Lombes A., Moraes C.T. PGC-1alpha/beta induced expression partially compensates for respiratory chain defects in cells from patients with mitochondrial disorders. Hum. Mol. Genet. May 2009;18(10):1805–1812. doi: 10.1093/hmg/ddp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Johri A., et al. Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington's disease. Hum. Mol. Genet. 2012;21(5):1124–1137. doi: 10.1093/hmg/ddr541. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Howitz K.T., et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. Sep. [DOI] [PubMed] [Google Scholar]
- 116.Zang M., et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. Aug. 2006;55(8):2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 117.Hardie D.G., Carling D., Gamblin S.J. AMP-activated protein kinase: also regulated by ADP? Trends Biochem. Sci. Sep. 2011;36(9):470–477. doi: 10.1016/j.tibs.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 118.Cantó C., et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060. doi: 10.1038/nature07813. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Omae T., Nagaoka T., Tanano I., Yoshida A. Pioglitazone, a peroxisome proliferator-activated receptor-γ agonist, induces dilation of isolated porcine retinal arterioles: role of nitric oxide and potassium channels. Invest. Ophthalmol. Vis. Sci. Aug. 2011;52(9):6749–6756. doi: 10.1167/iovs.10-6826. [DOI] [PubMed] [Google Scholar]
- 120.Jia L., Wang J., Cao H., Zhang X., Rong W., Xu Z. Activation of PGC-1α and mitochondrial biogenesis protects against prenatal hypoxic-ischemic brain injury. Neuroscience. Apr. 2020;432:63–72. doi: 10.1016/j.neuroscience.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 121.Vázquez-González D., Corona J.C. Pioglitazone enhances brain mitochondrial biogenesis and phase II detoxification capacity in neonatal rats with 6-OHDA-induced unilateral striatal lesions. Front. Neurosci. 2023;17 doi: 10.3389/fnins.2023.1186520. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bogacka I., Xie H., Bray G.A., Smith S.R. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. May 2005;54(5):1392–1399. doi: 10.2337/diabetes.54.5.1392. [DOI] [PubMed] [Google Scholar]
- 123.Kaplan J., Nowell M., Chima R., Zingarelli B. Pioglitazone reduces inflammation through inhibition of NF-κB in polymicrobial sepsis. Innate Immun. 2014;20(5):519–528. doi: 10.1177/1753425913501565. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang J. Autophagy and mitophagy in cellular damage control. Redox Biol. 2013;1(1):19–23. doi: 10.1016/j.redox.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pickles S., Vigié P., Youle R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. Feb. 2018;28(4):R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sunahara S., et al. Influence of autophagy on acute kidney injury in a murine cecal ligation and puncture sepsis model. Sci. Rep. 2018;8(1):1050. doi: 10.1038/s41598-018-19350-w. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bakula D., Scheibye-Knudsen M. Mitophaging: mitophagy in aging and disease. Front. Cell Dev. Biol. 2020;8:239. doi: 10.3389/fcell.2020.00239. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Doblado L., et al. Mitophagy in human diseases. Int. J. Mol. Sci. 2021;22(8) doi: 10.3390/ijms22083903. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin Q., et al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. Sep. 2019;26 doi: 10.1016/j.redox.2019.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cyr A., et al. Endotoxin engages mitochondrial quality control via an iNOS-reactive oxygen species signaling pathway in hepatocytes. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/4745067. Oct. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu J.-X., et al. Disturbance of mitochondrial dynamics and mitophagy in sepsis-induced acute kidney injury. Life Sci. 2019;235 doi: 10.1016/j.lfs.2019.116828. Oct. [DOI] [PubMed] [Google Scholar]
- 132.Wu Y., Chen L., Qiu Z., Zhang X., Zhao G., Lu Z. PINK1 protects against dendritic cell dysfunction during sepsis through the regulation of mitochondrial quality control. Mol. Med. 2023;29(1):25. doi: 10.1186/s10020-023-00618-5. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Y., et al. The PINK1/PARK2/optineurin pathway of mitophagy is activated for protection in septic acute kidney injury. Redox Biol. Jan. 2021;38 doi: 10.1016/j.redox.2020.101767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leduc-Gaudet J.-P., et al. Parkin overexpression attenuates sepsis-induced muscle wasting. Cells. 2020;9(6) doi: 10.3390/cells9061454. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cao Y., Klionsky D.J. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. Oct. 2007;17(10):839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- 136.Sun Y., Cai Y., Qian S., Chiou H., Zang Q.S. Beclin-1 improves mitochondria-associated membranes in the heart during endotoxemia. FASEB Bioadv. Mar. 2021;3(3):123–135. doi: 10.1096/fba.2020-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun Y., et al. Beclin-1-Dependent autophagy protects the heart during sepsis. Circulation. 2018;138(20):2247–2262. doi: 10.1161/CIRCULATIONAHA.117.032821. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mannam P., et al. MKK3 regulates mitochondrial biogenesis and mitophagy in sepsis-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. Apr. 2014;306(7):L604–L619. doi: 10.1152/ajplung.00272.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Augustine J.J., Bodziak K.A., Hricik D.E. Use of sirolimus in solid organ transplantation. Drugs. 2007;67(3):369–391. doi: 10.2165/00003495-200767030-00004. [DOI] [PubMed] [Google Scholar]
- 140.Mayer D.F., Kushwaha S.S. Transplant immunosuppressant agents and their role in autoimmune rheumatic diseases. Curr. Opin. Rheumatol. May 2003;15(3):219–225. doi: 10.1097/00002281-200305000-00008. [DOI] [PubMed] [Google Scholar]
- 141.Li Q., et al. Rapamycin enhances mitophagy and attenuates apoptosis after spinal ischemia-reperfusion injury. Front. Neurosci. 2018;12:865. doi: 10.3389/fnins.2018.00865. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li Q., et al. Rapamycin attenuates mitochondrial dysfunction via activation of mitophagy in experimental ischemic stroke. Biochem. Biophys. Res. Commun. Feb. 2014;444(2):182–188. doi: 10.1016/j.bbrc.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 143.Lenzi P., et al. Rapamycin ameliorates defects in mitochondrial fission and mitophagy in glioblastoma cells. Int. J. Mol. Sci. May 2021;22(10) doi: 10.3390/ijms22105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Civiletto G., et al. Rapamycin rescues mitochondrial myopathy via coordinated activation of autophagy and lysosomal biogenesis. EMBO Mol. Med. 2018;10(11) doi: 10.15252/emmm.201708799. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang Z., et al. Protective effects of rapamycin induced autophagy on CLP septic mice. Comp. Immunol. Microbiol. Infect. Dis. Jun. 2019;64:47–52. doi: 10.1016/j.cimid.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 146.Nasri H., Rafieian-Kopaei M. Metformin: current knowledge. J. Res. Med. Sci. Jul. 2014;19(7):658–664. [PMC free article] [PubMed] [Google Scholar]
- 147.Bhansali S., Bhansali A., Dutta P., Walia R., Dhawan V. Metformin upregulates mitophagy in patients with T2DM: a randomized placebo-controlled study. J. Cell Mol. Med. Mar. 2020;24(5):2832–2846. doi: 10.1111/jcmm.14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.de Marañón A.M., et al. Metformin modulates mitochondrial function and mitophagy in peripheral blood mononuclear cells from type 2 diabetic patients. Redox Biol. 2022;53 doi: 10.1016/j.redox.2022.102342. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jang S., Kang H.T., Hwang E.S. Nicotinamide-induced mitophagy: event mediated by high NAD+/NADH ratio and SIRT1 protein activation. J. Biol. Chem. 2012;287(23) doi: 10.1074/jbc.M112.363747. 19314, Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Amjad S., et al. Role of NAD+ in regulating cellular and metabolic signaling pathways. Mol. Metabol. 2021;49 doi: 10.1016/j.molmet.2021.101195. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xie N., et al. NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Targeted Ther. 2020;5(1):227. doi: 10.1038/s41392-020-00311-7. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aman Y., et al. The NAD+-mitophagy axis in healthy longevity and in artificial intelligence-based clinical applications. Mech. Ageing Dev. Jan. 2020;185 doi: 10.1016/j.mad.2019.111194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hong G., et al. Administration of nicotinamide riboside prevents oxidative stress and organ injury in sepsis. Free Radic. Biol. Med. Aug. 2018;123:125–137. doi: 10.1016/j.freeradbiomed.2018.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zarezadeh M., et al. Melatonin effectiveness in amelioration of oxidative stress and strengthening of antioxidant defense system: findings from a systematic review and dose-response meta-analysis of controlled clinical trials. Clin. Nutr. ESPEN. Apr. 2022;48:109–120. doi: 10.1016/j.clnesp.2022.01.038. [DOI] [PubMed] [Google Scholar]
- 155.Yoon Y.M., Kim H.J., Lee J.H., Lee S.H. Melatonin enhances mitophagy by upregulating expression of heat shock 70 kDa protein 1L in human mesenchymal stem cells under oxidative stress. Int. J. Mol. Sci. 2019;20(18) doi: 10.3390/ijms20184545. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiang Y., Shen M., Chen Y., Wei Y., Tao J., Liu H. Melatonin represses mitophagy to protect mouse granulosa cells from oxidative damage. Biomolecules. 2021;11(7) doi: 10.3390/biom11070968. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gitto E., et al. Effects of melatonin treatment in septic newborns. Pediatr. Res. Dec. 2001;50(6):756–760. doi: 10.1203/00006450-200112000-00021. [DOI] [PubMed] [Google Scholar]
- 158.Alula K.M., et al. Inner mitochondrial membrane protein Prohibitin 1 mediates Nix-induced, Parkin-independent mitophagy. Sci. Rep. 2023;13(1):18. doi: 10.1038/s41598-022-26775-x. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oyang L., et al. The function of prohibitins in mitochondria and the clinical potentials. Cancer Cell Int. 2022;22(1):343. doi: 10.1186/s12935-022-02765-x. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen S., Ma J., Yin P., Liang F. The landscape of mitophagy in sepsis reveals PHB1 as an NLRP3 inflammasome inhibitor. Front. Immunol. Jun. 2023;14 doi: 10.3389/fimmu.2023.1188482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mattox T.A., et al. Prohibitin-1 is a dynamically regulated blood protein with cardioprotective effects in sepsis. J. Am. Heart Assoc. Jul. 2021;10(14) doi: 10.1161/JAHA.120.019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Widdrington J.D., et al. Exposure of monocytic cells to lipopolysaccharide induces coordinated endotoxin tolerance, mitochondrial biogenesis, mitophagy, and antioxidant defenses. Front. Immunol. 2018;9:2217. doi: 10.3389/fimmu.2018.02217. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Park J.-S., Choi H.-S., Yim S.-Y., Lee S.-M. Heme oxygenase-1 protects the liver from septic injury by modulating TLR4-mediated mitochondrial quality control in mice. Shock. Aug. 2018;50(2):209–218. doi: 10.1097/SHK.0000000000001020. [DOI] [PubMed] [Google Scholar]
- 164.Shi J., et al. PI3K/Akt pathway-mediated HO-1 induction regulates mitochondrial quality control and attenuates endotoxin-induced acute lung injury. Lab. Invest. Dec. 2019;99(12):1795–1809. doi: 10.1038/s41374-019-0286-x. [DOI] [PubMed] [Google Scholar]
- 165.Manfredini A., et al. Mitochondrial dysfunction is associated with long-term cognitive impairment in an animal sepsis model. Clin. Sci. Sep. 2019;133(18):1993–2004. doi: 10.1042/CS20190351. [DOI] [PubMed] [Google Scholar]
- 166.Liu W., Guo J., Mu J., Tian L., Zhou D. Rapamycin protects sepsis-induced cognitive impairment in mouse Hippocampus by enhancing autophagy. Cell. Mol. Neurobiol. Oct. 2017;37(7):1195–1205. doi: 10.1007/s10571-016-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ishihara N., Otera H., Oka T., Mihara K. Regulation and physiologic functions of GTPases in mitochondrial fusion and fission in mammals. Antioxidants Redox Signal. 2013;19(4):389–399. doi: 10.1089/ars.2012.4830. Aug. [DOI] [PubMed] [Google Scholar]
- 168.Tilokani L., Nagashima S., Paupe V., Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 2018;62(3):341–360. doi: 10.1042/EBC20170104. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Youle R.J., van der Bliek A.M. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–1065. doi: 10.1126/science.1219855. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kühlbrandt W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015;13:89. doi: 10.1186/s12915-015-0201-x. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Twig G., Shirihai O.S. The interplay between mitochondrial dynamics and mitophagy. Antioxidants Redox Signal. May 2011;14(10):1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Twig G., et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. Jan. 2008;27(2):433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Scott I., Youle R.J. Mitochondrial fission and fusion. Essays Biochem. 2010;47:85–98. doi: 10.1042/bse0470085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Picca A., et al. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Rev. Cardiol. Sep. 2018;15(9):543–554. doi: 10.1038/s41569-018-0059-z. Nat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Arulkumaran N., et al. Mitochondrial function in sepsis. Shock. 2016;45(3):271–281. doi: 10.1097/SHK.0000000000000463. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu J.-X., et al. Protection of procyanidin B2 on mitochondrial dynamics in sepsis associated acute kidney injury via promoting Nrf2 nuclear translocation. Aging (Albany NY) Aug. 2020;12(15):15638–15655. doi: 10.18632/aging.103726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ning L., et al. A novel mechanism for the protection against acute lung injury by melatonin: mitochondrial quality control of lung epithelial cells is preserved through SIRT3-dependent deacetylation of SOD2. Cell. Mol. Life Sci. Nov. 2022;79(12):610. doi: 10.1007/s00018-022-04628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu X., et al. Sirt3-dependent deacetylation of SOD2 plays a protective role against oxidative stress in oocytes from diabetic mice. Cell Cycle. 2017;16(13):1302–1308. doi: 10.1080/15384101.2017.1320004. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.van der Slikke E.C., et al. Association between oxidized nucleobases and mitochondrial DNA damage with long-term mortality in patients with sepsis. Free Radic. Biol. Med. Feb. 2022;179:156–163. doi: 10.1016/j.freeradbiomed.2021.12.305. [DOI] [PubMed] [Google Scholar]
- 180.Ashar F.N., et al. Association of mitochondrial DNA levels with frailty and all-cause mortality. J. Mol. Med. Feb. 2015;93(2):177–186. doi: 10.1007/s00109-014-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nasir N., Jamil B., Siddiqui S., Talat N., Khan F.A., Hussain R. Mortality in sepsis and its relationship with gender. Pakistan J. Med. Sci. Q. Oct. 2015;31(5):1201–1206. doi: 10.12669/pjms.315.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Krajčová A., et al. Effect of noradrenaline on propofol-induced mitochondrial dysfunction in human skeletal muscle cells. ICMx. 2022;10(1):47. doi: 10.1186/s40635-022-00474-3. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Miller M., Singer M. Do antibiotics cause mitochondrial and immune cell dysfunction? A literature review. J. Antimicrob. Chemother. Apr. 2022;77(5):1218–1227. doi: 10.1093/jac/dkac025. [DOI] [PubMed] [Google Scholar]
- 184.Nedel W.L., et al. Antibiotic therapy does not alter mitochondrial bioenergetics in lymphocytes of patients with septic shock - a prospective cohort study. Mitochondrion. Sep. 2022;66:7–12. doi: 10.1016/j.mito.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 185.Kalghatgi S., et al. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells. Sci. Transl. Med. 2013;5(192):192ra85. doi: 10.1126/scitranslmed.3006055. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data/literature analysed during this study are included in this published article.
No data was used for the research described in the article.