Abstract
Over the past several decades, the incidence of early-onset colorectal cancer (EOCRC; in patients <50 years old) has increased at an alarming rate. Although robust and scientifically rigorous epidemiological studies have sifted out environmental elements linked to EOCRC, our knowledge of the causes and mechanisms of this disease is far from complete. Here, we highlight potential risk factors and putative mechanisms that drive EOCRC and suggest likely areas for fruitful research. In addition, we identify inconsistencies in the evidence implicating a strong effect of increased adiposity and suggest that certain behaviours (such as diet and stress) might place nonobese and otherwise healthy people at risk of this disease. Key risk factors are reviewed, including the global westernization of diets (usually involving a high intake of red and processed meats, high-fructose corn syrup and unhealthy cooking methods), stress, antibiotics, synthetic food dyes, monosodium glutamate, titanium dioxide, and physical inactivity and/or sedentary behaviour. The gut microbiota is probably at the crossroads of these risk factors and EOCRC. The time course of the disease and the fact that relevant exposures probably occur in childhood raise important methodological issues that are also discussed.
Early-onset colorectal cancer (EOCRC) is the second most common cancer and the third leading cause of cancer mortality in people <50 years of age in the USA1. The incidence of EOCRC has been on the rise over the past four decades1 and is expected to increase by >140% by 2030 (refs2,3). Incidence rates are inversely associated with age4, and the rise in incidence and mortality from EOCRC is global2,5,6.
Despite a lack of complete datasets and rigorous research, established cancer drivers have been linked to EOCRC (such as diet, sedentary lifestyle, smoking and alcohol)1,5,7–9. In addition, consensus exists that EOCRC is a pathologically, epidemiologically, anatomically, metabolically and biologically different disease to late-onset colorectal cancer (LOCRC; in patients >50 years old)10. Therefore, EOCRC must be investigated, evaluated and managed differently to LOCRC. We suggest that several known and unknown-but-suspected risk factors might explain this alarming trend in the younger population. Important to this discussion, bio-behaviours (that is, behaviours that affect biological process, such as diet, stress and exercise) have undergone a generational shift, including the westernization of diets (calorie-dense and nutrient-sparse) and an increase in physical inactivity, leading to poor (colonic) health. Several solutions to address these bio-behavioural risk factors are outlined in detail throughout this Review.
To fully appreciate the genesis of EOCRC (and the premise underlying this Review), it is essential to fully understand what is known about exposomal elements and the putative mechanisms by which the exposome11,12 (possibly at critical periods of development) drives this disease. The exposome encompasses the totality of human environmental (that is, nongenetic) exposures from conception onwards. The exposome consists of three overlapping domains: the general external environment (for example, socioeconomic factors, education, climate factors, social capital and stress); specific external environment (such as radiation, infections, tobacco, alcohol, prescription drugs and antibiotics, diet and physical activity); and internal environment (for example, metabolic factors, hormones, gut microbiota, inflammation and oxidative stress)11. We contend that the general external environment, such as perceived stress and low socioeconomic status associated with poor nutrition, probably contribute to the increased incidence of EOCRC. We also discuss the possibility that specific external environmental factors such as antibiotics, diet and physical activity contribute to EOCRC and explore putative mechanisms. Given that the microbiome and inflammation are key internal exposome players, and are widely recognized as being guardians of colorectal cancer (CRC)13, we focus on these players as mechanisms at the crossroads of the exposome and EOCRC.
Anatomy and pathology of EOCRC
The most consistent observation about EOCRC borne out by the epidemiology is presentation at an advanced stage — not only because of a more aggressive pathology but often as a result of a delay of up to 6 months from symptom onset to diagnosis14. EOCRCs are typically found in the rectum and distal colon (left side) with a high percentage of mucosal and signet cell pathology relative to LOCRC (although percentages remain small)15,16. The appearance of EOCRC on the left side gives clues as to the behaviour, causes and treatment of such cancers. For example, left-sided colon cancers are smaller, have lower recurrence rates, and longer disease-free survival than right-sided colon cancers17,18. Left-sided tumour size tends to correlate positively with lymph node involvement, and left-sided and right-sided CRCs respond differently to treatment17.
Cancers in the distal colon and rectum (important in the context of EOCRC) are associated with a high intake of red and processed meat, high lifetime alcohol intake, and low fish and poultry intake19–23. Risk is decreased on the left side by consumption of dark yellow vegetables and fruits, including apples24. Micronutrients such as calcium, dietary polyphenols, garlic, choline and vitamin D tend to be more closely associated with reduced risk of left-sided colon cancer25–31. Fibre intake and dairy consumption reduces CRC risk throughout the colon26, and zinc reduces rectal cancer risk in women32. Interestingly, cyclooxygenase 2 (COX2) inhibitors are chemopreventive in familial adenomatous polyposis (a disease of the distal colon and rectum)33,34 but not Lynch syndrome (a disease of the right and/or proximal colon)35. Aspirin (which targets both COX1 and COX2) seems to be a chemopreventive for the proximal colon, but not the distal colon or rectum36. Such findings are worth considering when deciding which putative exposomal elements to pursue as prime suspects, for delineating the mechanisms by which they behave, and for addressing primary and secondary chemopreventive measures.
Finally, although obesity does not seem to be anatomically selective for driving proximal colon versus distal colon versus rectal cancers37, it substantially increases the risk of CRC in patients with Lynch syndrome; this increased CRC risk is abrogated by aspirin38. Of particular importance to this discussion is that the rise in incidence of EOCRC is largely because of increased rates of rectal cancer39. Indeed, rectal cancer differs from distal colon cancer with regard to tissue histology, cancer pathology and aggressiveness35. Although molecular similarities exist between colon and rectal cancers, molecular differences exist at the somatic and proteomic levels40,41, and therefore the exposomal elements might be divergent. Delineation of exposomal elements affecting the rectum versus the colon is a critical step to understanding this disease for chemoprevention and treatment strategies.
Genetic and epigenetic elements in EOCRC
Hereditary syndromes and family history
Family history and hereditary conditions account for ~30% of EOCRCs1,42,43. The total prevalence of mutational burden is estimated at 16% in EOCRC, with half of these being Lynch syndrome mutations and the other half being other mutations (including adenomatous polyposis coli (APC), monoallelic and biallelic MutYH, and BRCA1/BRCA2 (REF.43)). Importantly, a negative family history does not exclude cancer hereditary syndromes44 (for example, owing to poor communication among families or other yet-to-be discovered inherited genes). Thus, more research is needed to fully elucidate the genetic profiles of patients with EOCRC.
Having a first-degree relative with a large or histologically advanced adenoma increases the lifetime risk of CRC by up to fourfold45,46. Therefore, guidelines recommend that such individuals initiate CRC screening at 70 years of age47. Unfortunately, adherence to this recommendation in the young is low48. Improving identification of — and screening in — this population is an immediate step to curb the rising rates of EOCRC. Barriers involved in such efforts need to be addressed, including patient and provider awareness of the risk on the basis of family history49. Additionally, educational efforts to promote CRC screening in average-risk individuals starting at 50 years of age might have unintentionally deterred age-appropriate screening in those at high risk. Physicians must recognize the risks and convey these risks to their patients as well as promote individual knowledge of family background. A concerted educational effort for both the general public and health-care providers to routinely initiate a risk assessment for CRC and develop a plan for age-appropriate CRC screening prior to 40 years of age would save lives.
Although we might discover new genes coming from Mendelian inheritance in certain families at high risk of EOCRC, these factors would be unlikely to exert a materially large effect on reversing the trend in EOCRC in entire populations. Certainly, the advancement of deep learning tied to whole-genome deep sequencing might shed more light on the genetics of EOCRC50. However, regardless of genetic background, the problem of recognition, awareness and education in this cohort remains. For example, many patients find out they have Lynch syndrome after a CRC diagnosis51. Even screening adherence rates in known mutation carriers are highly variable and often sub-par (as low as 53%)52,53. Ongoing efforts to recognize these high-risk families and improve screening adherence in mutation carriers can have a major effect on familial cancer risks, which should, in turn, have an effect on the overall rate of EOCRC. Just as these educational deficiencies are being addressed in innovative ways (such as social media campaigns and personalized web-based interfaces)54,55, accurate and appropriate screening techniques are also needed for these families. Guidelines for the genetic evaluation and management of hereditary CRC syndromes have been reviewed, assessed and updated on the basis of current knowledge and rigorous science56–59. To this end, deep learning algorithms that consider surrogate biomarkers and exposomal factors in combination with genetic profiling, as well as the integration of microbiome profiles, inflammatory load and other mechanisms that drive EOCRC, will advance our understanding of the disease. Indeed, such risk models have been developed for LOCRC cohorts60–63 and for hereditary cancer syndromes such as Lynch syndrome56,64,65. However, sensitivity and specificity are far from perfect even in these models.
EOCRC has a different signature to LOCRC
EOCRCs tend to be microsatellite stable (MSS) and neardiploid, and multiple alterations of chromosome number, chromosomal rearrangements, or gene amplification and/or deletion of oncogenes and/or tumour suppressors continue to be identified. Up to 63% of EOCRCs with MSS are euploid (chromosomal instability-negative)66. EOCRC is also associated with a higher percentage of synchronous (5.8% versus 1.2% for LOCRC) and metachronous (4.0% versus 1.6% for LOCRC) tumours67. Microsatellite and chromosome-stable tumours are common in EOCRC and are associated with a positive family history and rectal location (60% of microsatellite and chromosome-stable tumours are rectal)68. Another recognized feature of EOCRC is genome-wide hypomethylation in a subset of patients1,42,69, which seems to be correlated with chromosomal instability and poor prognosis66,69. Some of the key players involved in LOCRC, including KRAS codon 12 mutations, have been identified as drivers of EOCRC66,70. Indeed, it would also be wise to catalogue differences in molecular signatures of rectal versus distal colon cancer. To this end, subclassifications of EOCRC on the basis of genomic signatures have been proposed71. For more details on molecular changes associated with EOCRC the reader is guided to other reviews2,42,44,72. Interesting and consistent findings in young people with CRC include a relatively high rate of KRAS mutations, LINE-1 hypomethylation and TP53 mutations69,70,73. BRAFV600E mutations and/or APC mutations occur infrequently in EOCRC73–76.
Exposomal elements in EOCRC
Although genetic predisposition is extremely relevant in EOCRC, it does not account for the observed trends in diagnosis. Approximately 70% of EOCRCs might be driven by the exposome in the presence or absence of a previous somatic mutation(s), or rare gene variants (with variable degrees of penetrance). Exposome science suggests that certain windows of vulnerability (for disease risk) and opportunity (for health promotion) can be leveraged for prevention purposes. As for CRC in older individuals, epidemiological studies of EOCRC have identified diet77–79, alcohol80, smoking14,81 and lack of physical activity82 as risk factors. As some of these factors are becoming more predominant early in life and, therefore, becoming more prevalent in successive generations, questions arise as to whether exposomal elements — especially in the early years of life79 — could interact with underlying genetic background factors to trigger EOCRC. Indeed, for an algorithm that generates a lifestyle index (encompassing smoking, alcohol consumption, diet, waist–hip ratio and exercise participation), a high score is associated with a 27% reduction in risk of rectal cancer in Chinese men83. Given the increasing incidence of rectal cancer in the young8,39,84,85, similar studies are worth pursuing in other parts of the world.
To sift out the suspects affecting EOCRC, the following facts about the disease must be considered: one, EOCRC incidence and mortality have been increasing since the 1980s8,39,84,85; two, EOCRC is a global phenomenon2,6; three, CRC development is linked to chronic inflammation86 and dysbiosis87; four, EOCRC occurs mostly in the distal colon and rectum39; five, evidence suggests that CRC can develop as a result of insult years earlier88,89; six, specific early-life exposomal elements (some linked to EOCRC such as diet and obesity) effect the onset of disease later in life90,91; and seven, people across the BMI spectrum develop EOCRC (although there is a propensity towards patients with EOCRC being overweight)39,81,92,93.
With this knowledge, it makes sense to focus on the exposomal elements that meet the following metrics: first, the exposomal element must have a similar temporal trend to that of EOCRC; second, the trend should be global; third, the exposomal element must have inflammatory or microbiome-modifying properties or evidence of an effect on the distal colon or rectum; and fourth, the exposomal element should be present during development (conception to adulthood). With such benchmarks in mind, some unusual suspects might become prime suspects. Although alcohol and smoking seem to be associated with EOCRC, this link is demonstrated mostly in the older EOCRC subcohort94. Substantial direct exposure from alcohol and cigarettes that affects the pathology of the colon during childhood is unlikely. Epidemiological studies have, so far, failed to reach a conclusion regarding physical activity. Some studies suggest that physical activity does not distinguish between the right and left colon95, whereas other studies suggest that physical activity suppresses cancers of the right colon but neither those of the left colon nor rectum96–99. Independent of exercise and obesity, prolonged sedentary television viewing time (a surrogate for an inactive lifestyle) is associated with risk of EOCRC, particularly of the rectum9.
Against this backdrop, the exposomal elements that match all four metrics are shown in TABLE 1. Although additional information and many more experiments are necessary to imply causation100, these benchmarks provide an initial, logical framework for identifying putative exposomal factors driving EOCRC and a rational scientific premise for study. Importantly, new exposomal factors and new mechanisms will probably be discovered in experiments moving forward. Given the increasing rates of EOCRC, such discoveries within and outside the purview of the four metrics will be welcome news to those with EOCRC. Several examples that did not reach the metrics are outlined in BOX 1.
Table 1 |.
Exposomal elements driving EOCRC
| Exposomal element | Temporal trend | Global trend | Effect on inflammation/microbiome or known effect on distal colon or rectum | Exposure during development (conception to adulthood) |
|---|---|---|---|---|
| Westernized diets | Yes140 | Yes140 | Yes138,148 | Yes129,130 |
| Red and processed meat | Yes20,140,157,158 | Yes20,140,157,158 | Yes160,161,253,254 | Yes20,157,158 |
| Obesity | Yes101,103,140 | Yes101,103,140 | Yes108,109 | Yes105–107 |
| Stress | Yes118 | Yes117 | Yes255,256 | Yes118,119,257 |
| Antibiotics | Yes258 | Yes168 | Yes169–171 | Yes162 |
| Synthetic dyes | Yes186,200 | Yes186,200 | Yes192,193,259,260 | Yes200 |
| Monosodium glutamate | Yes261,262 | Yes261,262 | Yes201,202,263–265 | Yes261 |
| Titanium dioxide | Yes266 | Yes266 | Yes206,208,209,267 | Yes206,207,266 |
| High-fructose corn syrup | Yes210,215,268 | Yes210,215,268 | Yes216,269 | Yes217 |
Key exposomal suspects driving early-onset colorectal cancer (EOCRC) emerge when four metrics are fulfilled: first, a temporal relationship exists, similar to EOCRC; second, exposure is global, as is EOCRC; third, molecular evidence exists of inflammatory or microbiome-modifying properties or evidence of an effect on the distal colon or rectum; and four, exposure occurs at any time during development from conception until adulthood.
Box 1 |. potential exposomal elements affecting early-onset colorectal cancer.
Dietary emulsifiers
Trans-fatty acids
Acrylamide
Sodium nitrate/nitrite
A1 β-caseins
Obesity
Globally, 2.16 billion adults are predicted to be overweight, and 1.12 billion to be obese, by 2030 (REFS101,102). Food habits have deteriorated worldwide owing to cheap, readily available high-calorie sweeteners, advances in food processing, and the influence of technology on food and behaviour. There is no question that obesity is increasing globally101–103. Unsurprisingly, therefore, many studies have linked obesity to EOCRC39,81,92,93. A reasonable hypothesis (at least for a portion of EOCRC cases) is that the increased EOCRC incidence rates are a result of the generational shift towards a higher BMI104. Supporting this understanding (and key to EOCRC) is the fact that obesity and body fatness have been linked to CRC later in life105–107. Owing to the decade(s)-long process of carcinogenesis, a further hypothesis is that the diagnosis of cancer in the second to fourth decade of life might be a consequence of exposure decades earlier (that is, before adulthood). However, studies have yet to be published linking body fatness in infancy or maternal obesity to EOCRC; furthermore, datasets for such studies are difficult to find, and need to be identified or created.
The mechanisms linking obesity and EOCRC are poorly understood but might involve an interaction with the internal exposome (for example, microbiome and inflammation) and other specific exposomal elements (such as food additives and low-quality foods). Indeed, obesogenicity is associated with dysbiosis and inflammation in humans108,109. Moreover, body fatness during childhood and/or adolescence has been associated with unfavourable metabolic profiles that might exacerbate the development of CRC93,110. Thus, a reasonable hypothesis is that the detrimental role of body fatness and/or obesity on later CRC risk might have started earlier in life (such as through maternal obesity or obesity during infancy and childhood). Dysbiosis and/or inflammation might be at the mechanistic crossroads of obesity and EOCRC.
Notably, although obesity is associated with colon cancer37, evidence is weaker that it drives rectal cancer92,96,106,111. This finding is important because the observed increase in EOCRC is largely driven by an increased incidence of rectal cancers4,112,113. Furthermore, both nonobese and obese people develop EOCRC. These findings all support the scientific premise that exposomal elements outside of the worldwide obesity epidemic contribute to EOCRC. Complicating this picture, evidence exists that caloric restriction in childhood can increase CRC risk later in life110,114.
Perceived stress
Perceived stress (individual perception of psychosocial stress) is an external exposomal element that requires particular attention in the context of EOCRC. Not only does stress increase the risk of rectal cancer115, but stress during pregnancy can increase the risk of CRC in offspring116. The scientific premise for this hypothesis is strong given the following factors: first, global increases in perceived stress (including childhood and maternal perceived stress) parallel increases in EOCRC in the past four decades39,117–119; second, a reduced amount of sleep drives stress, obesity and CRC (and vice versa)116,120–122; third, obesity is linked to EOCRC and prenatal stress is associated with obesity in the offspring116; fourth, psychosocial stress increases the risk of diabetes and diabetes is linked to EOCRC116,123; fifth, stress is associated with reduced physical activity and deterioration in diet124; and sixth, the inflammatory milieu, innate immunity, function of immune cells and the microbiome are compromised under stress116, and a compromised immune system helps drive CRC125. Stress also causes genetic, epigenetic and microbial changes not only in the stressed individual but in the offspring of that stressed individual116. Such generational transfer, including aberrant DNA methylation, has been linked to the genesis of CRC126. Because psychosocial stress modulates microbiota signatures in the gastrointestinal tract127, and gut microbiota have a key role CRC development128, stress-induced dysbiosis and inflammatory load might also have a mechanistic role in EOCRC116.
Diet
A large and consistent body of literature shows that the adoption of a western diet, which is rich in red meat, high in saturated fat and low in fibre, exerts a negative effect on the colon and that healthier regimens, such as a Mediterranean diet, promote a healthy colon129. Interestingly, a western dietary pattern increases risk specifically in the distal colon and rectum129,130 (EOCRC tends to affect the distal colon or rectum), whereas a Mediterranean diet seems to protect the entire colon and rectum from CRC. A western dietary pattern also has been shown to be associated with tumours that are KRAS wild-type, BRAF wild-type, have no or a low CpG island methylator phenotype (CIMP) and are MSS130. Given that a large subset of patients with EOCRC tend to have tumours that are KRAS+/+ (refs73,131), BRAF+/+ (refs66,73,76,132–134), CIMPlo (refs74,75,135–137), and MSS42,75, linking diet to molecular features of EOCRC (and subsets of EOCRC) would advance our knowledge.
A western diet also drives gut dysbiosis138 and inflammation139, and an increasing number of children (worldwide) are eating diets high in refined carbohydrates, added sugars, fats and animal sources140. Arguing against linking a western diet to EOCRC is the understanding from an epidemiological standpoint that EOCRC is increasing both in areas with heavy consumption of a western diet (such as the USA and Canada)8,85,141 and of a Mediterranean diet (for example, Egypt)142. However, global food supplies are increasingly homogeneous143, and countries with people traditionally consuming a Mediterranean diet have been adopting an increasingly westernized diet144,145. Likewise, we have observed this trend in other parts of Africa, Asia and Latin America145,146.
Augmenting the unhealthy nature of a westernized diet is the cooking style typically used. For example, frying (especially deep-frying) can generate pro-inflammatory and pro-carcinogenic advanced glycation end-products (AGEs)147. These molecules are highly oxidant compounds formed through the nonenzymatic reaction between reducing sugars and free amino acids. Animal-derived foods that are high in fat and protein are generally AGE-rich and prone to new AGE formation during cooking. By contrast, nutrient-rich foods such as vegetables, fruits, whole grains and milk contain relatively few AGEs, even after cooking147. Cooking time, cooking style, cooking temperature and the presence of moisture also dictate the level of AGEs. AGEs contribute to metabolic syndrome147, drive gut dysbiosis148 and might have a role in type 2 diabetes mellitus, cardiovascular disease and even Alzheimer disease149. Additionally, AGEs are transferred through maternal blood, prematurely raising levels of AGEs in children to adult norms, preconditioning them to abnormally high oxidative stress and inflammation and thus possibly to early onset of disease, such as diabetes147 and possibly EOCRC.
The Dietary Inflammatory Index (DII) was developed to characterize the inflammatory potential of diet. Just as a Mediterranean diet has low AGE levels150, the same diet has a particularly low DII151. Diet-associated inflammation, as measured by the DII, is strongly and consistently related to CRC incidence and mortality across a wide variety of racial and ethnic groups152. The DII has also been used to quantify the relationship between food and inflammation and other risk factors including weight gain and obesity153–155. Given the evidence linking diet, inflammation and CRC, a higher DII score might contribute to EOCRC, as we have seen in numerous studies among older individuals with CRC156. However, this hypothesis has not been tested in a direct and rigorous manner.
Red and processed meat
A role for red and processed meat in CRC development has been proposed, largely on the basis of evidence from epidemiological studies, especially in those populations consuming a westernized diet20,157,158. Red and processed meat reaches the four metrics for study in that consumption and production have increased globally and in children since the 1960s159. In addition, red and processed meats have pro-inflammatory and dysbiosis-promoting properties160,161. We predict that inferring causation of EOCRC by red or processed meat will be supported by future mechanistic studies.
Antibiotics
Antibiotic over-use is a serious public health concern. More than 1 million doses of antibiotics are prescribed unnecessarily in the USA every year, and 50% of infants are exposed directly to antibiotics for >5 days162. Furthermore, indirect antibiotic exposure through pregnancy is high and can have persistent effects on the infant microbiota after birth163. Antibiotic overexposure at an early age has been correlated with multiple health disorders, including obesity164,165. Epidemiological studies support an association between antibiotic exposure and CRC166–168.
Adding to the scientific premise that antibiotics influence colon health and CRC genesis, repeated short-term or long-term exposure (possibly at windows of vulnerability) contributes to antibiotic resistance and alters the gut microbiota with pro-inflammatory and pro-carcinogenic consequences169–171. The suggestion of developmental windows of vulnerability to antibiotics is supported by studies consistently showing that antibiotic use in infancy increases the risk of childhood obesity172,173 (which is linked to EOCRC). Although animal models support the notion that heavy antibiotic use can drive gastrointestinal cancers174, studies are not always consistent175–177. Some studies have shown that antibiotics can protect against CRC, probably owing to the fact that specific microorganisms (for example, Fusobacterium) can drive CRC178 . Thus, inconsistencies across studies are not surprising and highlight the need for carefully controlled, scientifically rigorous studies that consider and delineate ‘bad’ versus ‘good’ bacteria, developmental timing and exposure, and type and dose of antibiotic. Addressing this knowledge gap is critical to counter the effects of repeated exposure or long-term antibiotic use. Notably, other drugs targeting the gastrointestinal tract, such as proton-pump inhibitors, have also been associated with gut dysbiosis179, and thus might also affect the risk of EOCRC.
Dietary additives
Changes in agricultural practices over the past four decades have resulted in a considerable shift in food quality and consumption both globally and regionally (reviewed in detail elsewhere180). The health consequences resulting from these changes are only beginning to be understood; however, the consequences generally fit with the models proposed here in that the result is an increase in consumption of energy-dense foods (leading to obesity) and a decline in nutrient content (which affects everyone, regardless of weight). Furthermore, some of the fillers and additives are themselves carcinogenic181.
Ingredients that have found their way into our food supply range from thoroughly tested chemicals that, so far, have been found to be inert, to known carcinogens or pre-carcinogens such as nitrates and nitrites in processed meats. Indeed, nitrate exposure through drinking water has been shown to be associated with CRC182, and intake of nitrite-containing processed meat is associated with increased CRC risk183. Mechanistically, nitrite consumption can lead to the formation of N-nitroso compounds, some of which are carcinogenic. The addition and subtraction of food ingredients is too vast to cover in this Review, and the historical nature of changes in food content over the past 40 years has been covered elsewhere184. Indeed, many of the new exposomal elements found in contemporary diets meet our four metrics as summarized in TABLE 1 and outlined below.
Synthetic food colouring.
Toxicity and carcinogenicity studies on synthetic food colouring have been reviewed elsewhere185–188. Synthetic dyes are added to our food and consumed throughout the world. Three dyes (Allura Red, tartrazine and Sunset Yellow) account for 90% of all dyes used in food in the USA189. They are used to attract consumers and are especially attractive to children. Importantly, dye consumption per person has increased fivefold since 1955 (REF185). Thus, in the context of EOCRC, these synthetic products are highly suspect and require scientific scrutiny. Synthetic food dyes are in breakfast cereals, candy, snacks, beverages, vitamins, and other products aimed at children. In 2010, the European Union placed warning labels on foods that contain synthetic food dyes. Although the implications of such measures are yet to emerge (for EOCRC), it is concerning that measures have not been taken in the USA, nor in most other countries outside of the European Union. This fact is alarming because of the scientific premise supporting a role for synthetic dyes in carcinogenesis.
Allura Red is used as an example because it is a highly common synthetic dye189 and meets all metrics outlined in TABLE 1. Allura Red (like tartrazine, Sunset Yellow and other synthetic food colourings) is a sulfonated mono azo dye and, as such, is metabolized by intestinal bacteria190,191 through azo-reduction and has pro-inflammatory properties185–187,192,193. The Acceptable Daily Intake (ADI) for Allura Red is currently set at 7 mg/kg daily on the basis of antiquated data194. Although this ADI was confirmed by a joint Food and Agriculture Organization–WHO Expert Committee on Food Additives in 2016 (REF.187), the lack of scientifically rigorous original studies regarding the impact of Allura Red on health is clear; the committee could draw from only seven original studies since 2010. Strikingly (and consistent with our findings from searching the biomedical literature), original data examining the effect of Allura Red on carcinogenesis is lacking. Of the four studies regarding the effect of Allura Red on the colon191,195–198, three of these studies (albeit conducted by one group) found colonic DNA damage in rats following consumption of 10 mg/kg daily of Allura Red191,197,198. The other study found negative results, although the authors were affiliated with the International Association of Color Manufacturers and The Coca-Cola Company195. Regarding human exposure, 10 mg/kg daily in rats is the equivalent of 72 mg daily for a 30-kg human child199. Although average human exposure to Allura Red is below the ADI187, one serving of some popular beverages that children consume contains >50 mg Allura Red187,200. Considering these facts, we suggest that Allura Red is a key prime suspect that needs scientific attention and has been understudied in the context of carcinogenesis and EOCRC.
Monosodium glutamate.
Monosodium glutamate (MSG) is produced through the fermentation of starch, sugar beets, sugar cane or molasses and was introduced as a food flavouring in the early 1900s. It is a common food additive used to intensify and enhance the flavour of savoury dishes. It is found in a variety of processed foods such as frozen dinners, salty snacks and canned soups, and is also often added to restaurant foods. MSG is worth considering as an ingredient stimulating EOCRC as it meets the metrics for hypothesis testing (TABLE 1). In particular, global consumption of MSG has increased in the past 50 years195, and it has pro-inflammatory properties196. Additionally, MSG is used to induce obesity and diabetes (both of which are linked to EOCRC)39,93,123 in animal models201. Interestingly, the MSG diabetes model renders mice more susceptible to azoxymethane-induced CRC202.
Titanium dioxide.
Titanium dioxide (TiO2) is a naturally occurring metal oxide and is an engineered nanomaterial commonly used in daily consumer products, including food. The food additive TiO2 (also known as E171) is commonly used as a whitening and brightening agent in confectionery, white sauces and icing (all foods typically targeted towards, and consumed by, children). In the USA, the FDA approved the use of food-grade TiO2 in 1966 with the stipulation that levels must not exceed 1% of the food weight203. However, the increasingly common use of TiO2 leads to substantial levels of daily dietary intake. Human exposure analyses on foods consumed among American and British populations report that children <10 years of age have higher exposure to TiO2 than adults204,205. Although the reader is guided to other reviews on the subject of TiO2 in food and health204,205, insufficient research is being carried out regarding the impact of TiO2 on colon carcinogenesis. Importantly in the context of EOCRC, TiO2 as a food additive has been demonstrated to facilitate growth of colitis-associated colorectal tumours in animals206,207. In addition, food-grade TiO2 changes the expression of colonic genes involved in immune responses, oxidative stress, DNA repair, xenobiotic metabolism, cancer pathway signalling and, interestingly, genes involved in olfactory and serotonin signalling207–209. As with the other suspects discussed, TiO2 reaches the metrics already outlined (TABLE 1) to support the scientific premise of studying the effect of TiO2 on EOCRC.
High-fructose corn syrup.
High-fructose corn syrup (HFCS) has been used in beverages for decades. The technology to produce it was developed in the 1960s and it was introduced to the food and beverage industry as a liquid sweetener alternative to sucrose (sugar) in the 1970s. Made from abundant corn, by the mid-1980s HFCS had fully replaced sucrose in most beverages in the USA210. Recognizing that EOCRC is linked to obesity39,81,92,93 and that obesity is associated with high consumption of HFCS211, examining the effect of HFCS on EOCRC makes sense. The literature provides a compelling scientific premise for study. Consumption of fructose-rich beverages leads to increased gain in body weight212, and intermediate biomarkers associated with obesity can be reversed if HFCS is replaced by glucose213. The harmful effects of fructose also can be found from the first months of life. Children of mothers who consume fructose have increased body weight, food intake and circulating levels of leptin, and decreased insulin sensitivity214. Importantly, HFCS meets the four metrics for investigation (TABLE 1). In particular, consumption has increased in the USA and globally since the early 1970s215. HFCS also has pro-inflammatory and dysbiotic properties216 and children are generally exposed to higher doses than adults217. Only in the past few years have mechanistic animal experiments started to reveal the effect of HFCS on the gut. HFCS-treated mice show a substantial increase in gut tumour size and tumour grade in Apcmin/+ mice in the absence of obesity and metabolic syndrome218,219. The effect of HFCS on the distal colon and rectum is unknown.
Microbiome link to EOCRC
The scientific premise supporting a mechanistic link between gut microbial dysbiosis and CRC is strong87,220,221. Approximately 1,000 different species of microorganisms, comprised of trillions of cells, reside in the gut221. Although the overall picture remains blurry, the microbiota provides many targets for the exposome. Indeed, specific microorganisms (such as Fusobacterium nucleatum, Escherichia coli, Bacteroides fragilis and Salmonella enterica) have been identified as having a key role in colon carcinogenesis178,222. Infection with pathogens could contribute to neoplastic development through different mechanisms, including intestinal dysbiosis, inflammation, evasion of tumoural immune response and activation of protumoural signalling pathways, such as β-catenin222.
Gut microbiota and their host share a symbiotic and intricate relationship that benefits both the microbiome and the host. Microorganisms maintain gastrointestinal homeostasis and (under healthy circumstances) protect the gut against inflammation and cancer. However, certain elements of the exposome (that is, any general external exposomal element (such as stress), specific exposomal elements (such as antibiotics and synthetic food dyes), or internal exposomal elements (for example, inflammation))11,12 can affect the gut microbiome leading to dysbiosis (FIG. 1). In turn, dysbiosis can have a direct effect on the mechanisms that lead to CRC. For example, certain microbiota can mediate the effects of diet on colon cancer risk by their generation of butyrate, folate and biotin (molecules known to have a key role in the regulation of epithelial proliferation). Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures223. High-fat diets can cause intestinal dysbiosis, leading to the accumulation of harmful bacterial products such as lipopolysaccharides that can enter the intestinal circulation and cause inflammation224. As another example, dietary emulsifiers (used to aid texture and extend the shelf-life of processed foods) modulate the gut microbiota and drive colitis and metabolic syndrome225. Given that both colitis and obesity are associated with EOCRC93,105–107,226,227, a reasonable hypothesis is that dietary emulsifiers drive EOCRC as well. Initial studies have shown that these agents cause dysbiosis and increase the incidence of CRC in animal models228.
Fig. 1 |. The effect of the exposome and early-life environmental exposures on microbiome health.
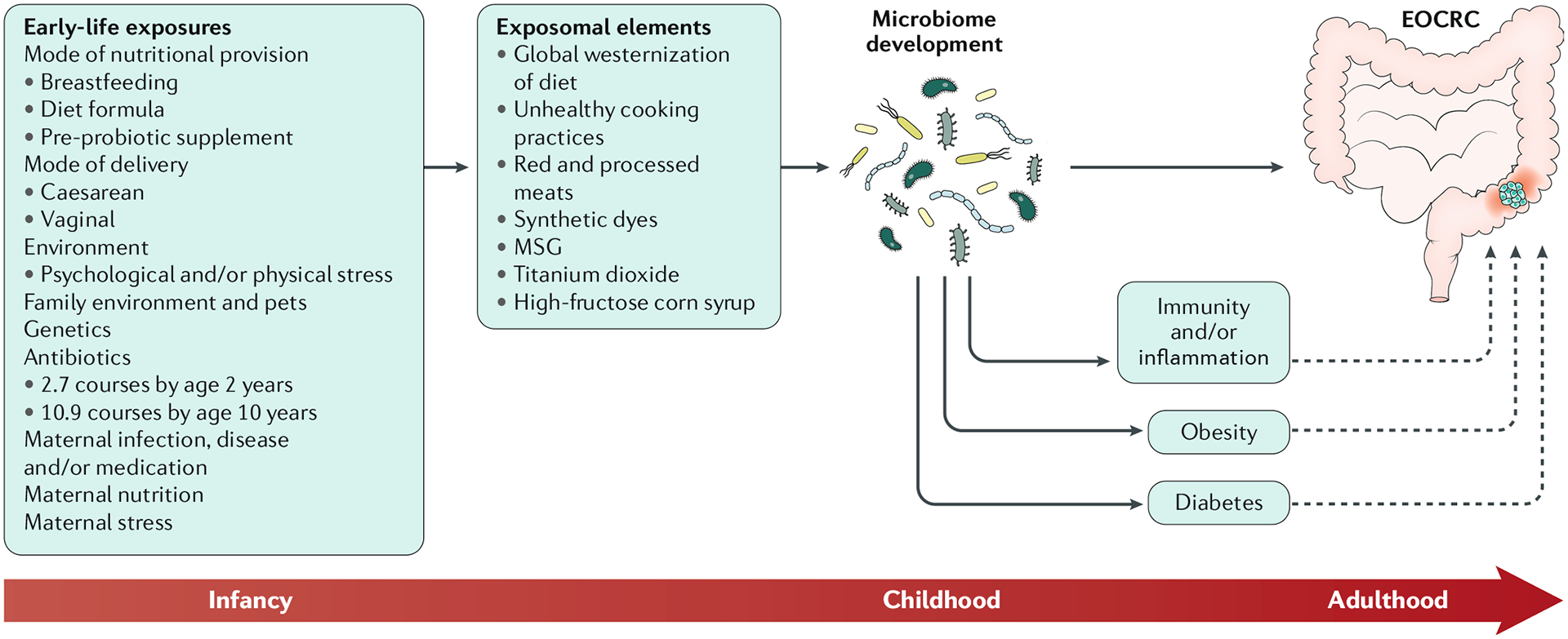
Exposomal elements that modulate the gut microbiome include not only those elements meeting the four metrics discussed in this Review (such as stress, antibiotics and dietary factors; TABLE 1) but also elements previously thought to be disconnected from colon health, such as birth mode, breastfeeding behaviours and maternal stress and nutrition. In turn, given the role of the microbiome in disease genesis (and the role of the microbiome in maintaining gut health) it probably has a key role in guiding colonic health and development of colorectal cancer. This role might or might not be mediated by obesogenic pathologies. EOCRC, early-onset colorectal cancer; MSG, monosodium glutamate.
Exposomal elements that modulate the gut microbiome include not only those elements meeting the above metrics (such as stress, antibiotics and dietary factors) but also elements previously thought to be disconnected from colon health, such as birth mode, breastfeeding behaviours and maternal stress and nutrition116,229,230. Exposure to antibiotics, stress and harmful dietary components can lead to microbial dysbiosis, and these exposures can occur during development. Furthermore, the degree to which the microbiome is at the crossroads of the exposome and EOCRC might be dictated by the timing of exposure. However, testing the hypothesis that dysbiosis in early human development causes molecular changes and dangerous lesions that render the colon at increased risk of transformation in early adulthood is a particular challenge. For example, samples would need to be collected (stool and preferably colonic tissue, and preferably at multiple times during development) during a specific (yet unknown) time frame, and then linked to CRC development decades later. As yet we are unaware of the existence of such a valuable resource. The integration of other confounding exposomal elements during development (probably involving diet) adds to the complexity of solving the EOCRC problem in the context of microbial dysbiosis. The advancement of machine learning and artificial intelligence in biomedical research and personalized medicine might help to address these issues.
Conclusions
Regrettably, the alarming rise in EOCRC described by epidemiological studies has yet to be followed up by well-designed observational and intervention studies in humans or mechanistic animal experiments. A working group, Fight Colorectal Cancer, has been convened to determine priorities for research of EOCRC231. Consistent with this Review, recommendations were made for prioritizing targeted, large, epidemiological studies and the need to tease out the causative factors and the genes involved in a scientifically rigorous fashion. Here, we have complemented these recommendations by rationally identifying prime suspects worth further investigation. To address the rise in EOCRC, some solutions can be deployed now (for example, awareness through educating physicians and patients), some can be deployed with additional work to overcome barriers (such as novel or modified screening techniques and surrogate end points, and improved protocols and guidelines); and some solutions can be deployed with money, time, ingenuity and scientific rigour (for example, to arrive at a better understanding of the mechanisms and gene–environment interactions) (FIG. 2).
Fig. 2 |. Solutions for EOCRC.
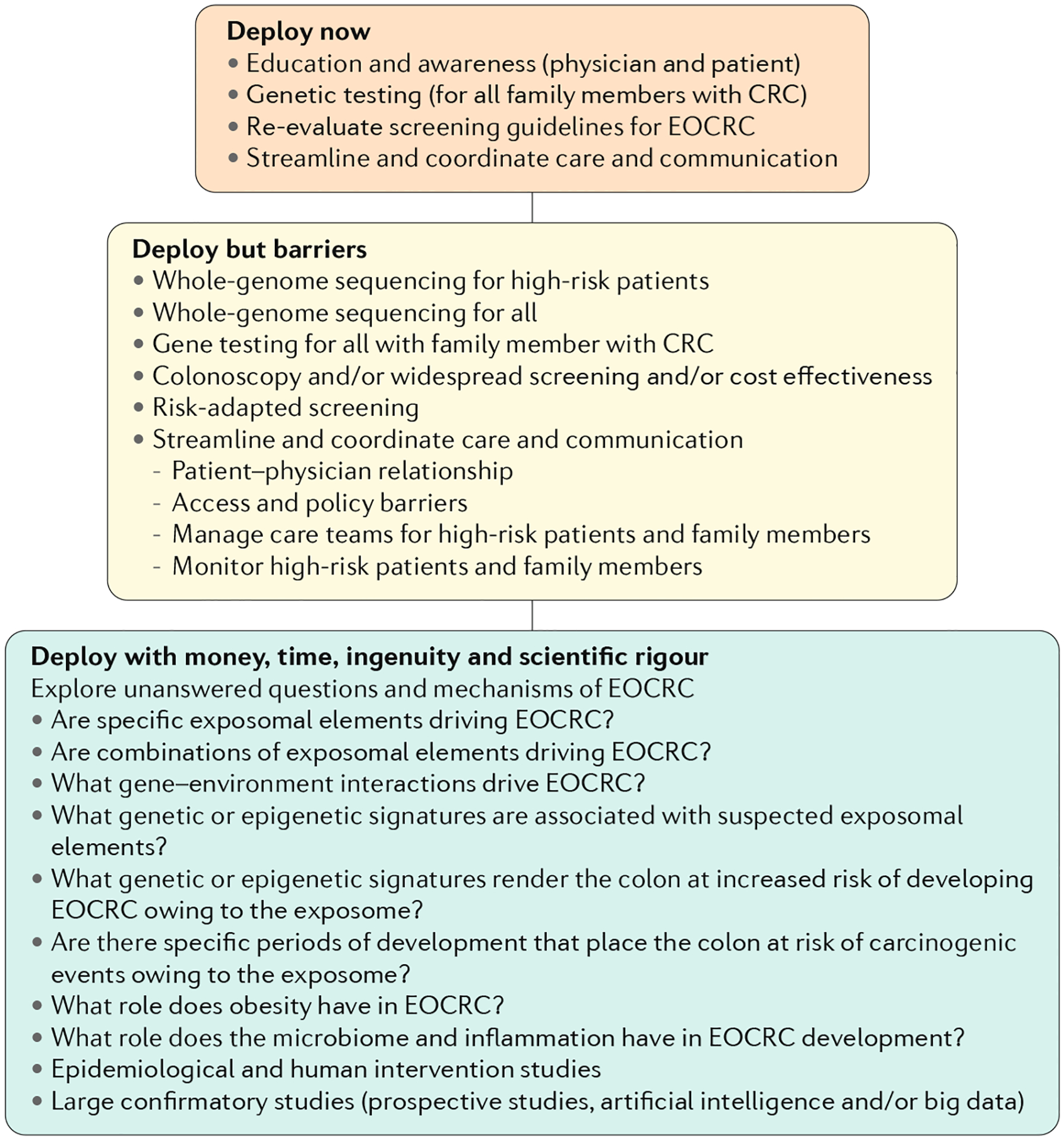
To address the rise in early-onset colorectal cancer (EOCRC), solutions can be deployed now, deployed with additional work to overcome barriers and deployed with money, time, ingenuity and scientific rigour. CRC, colorectal cancer.
Our understanding of how ingredients that have become common in foods over the past four decades might individually increase or combine to increase the risk of EOCRC is woeful. This factor is highlighted by the finding that, despite a wide swathe of the (global) population (particularly children) being exposed200, only four articles relevant to the effect of Allura Red on colon carcinogenesis could be identified191,195–198. Importantly, food constituents rarely exert their effects individually and so these agents should be considered as part of larger (usually unhealthy) dietary patterns (which is why the DII was developed).
How this global nutrition transition affects the colon remains confusing. Future efforts should explore the effect of timing and dose of suspected elements, and the mechanisms by which they might drive EOCRC. Does one or more of the exposomal elements highlighted in Table 1 drive CRC at a young age? Do these elements interact with the genetic background of the individual? What genetic factor(s) increase the risk for sporadic EOCRC? Is age at exposure critical to risk? We hope that such questions will be answered, and that this Review sparks additional questions and hypothesis testing. On the basis of the evidence and logical clues outlined above, the globalization of western diets, fast-food cooking styles, the infiltration of our food by poorly understood artificial ingredients and processing techniques might help to explain the increasing incidence of EOCRC. Until mechanistic studies are carried out, however, we will not know for sure. In addition, high levels of stress and the increasing use of antibiotics place the colon at increased risk of cancer development. The microbiome and/or the inflammasome are likely to be at the crossroads of the link between these exposomal elements and EOCRC.
We posit that if other elements of the exposome are uncovered as prime suspects through attaining all four EOCRC metrics (TABLE 1), then they should be seriously investigated. With access to big data, other exposomal suspects might become clear moving forward. Only after the hypotheses are tested and the clues are investigated can we tackle this challenging disease in a specific and deliberate manner. In the interim, aiming for a healthy lifestyle index (restricting a western-style diet and encouraging a Mediterranean or other mainly plant-based diet), reducing consumption of low-nutrient additives (such as artificially coloured foods and synthetic food colourings), reducing stress, maintaining a healthy weight, and reducing gastrointestinal-targeting drug consumption (especially antibiotics) will probably reduce EOCRC risk. An attainable goal is to use machine and deep learning (that is, artificial intelligence) algorithms in connecting exposomics to taxonomics to generate a weighted-risk signature for targeted chemoprevention of EOCRC.
Key points.
The alarming rise in early-onset colorectal cancer (EOCRC) over the past four decades described by epidemiological studies and cancer registry data requires coordination and follow-up with mechanistic in vitro testing, animal experimentation and human intervention studies.
EOCRC occurs in both people who are obese and those who are nonobese, and the rising incidence is global.
Some solutions to EOCRC can be deployed now (for example, awareness campaigns); some can be deployed with additional work to overcome barriers (such as identifying surrogate end points); and some can deployed with money, time, ingenuity and scientific rigour (for example, uncovering mechanisms and gene–environment interactions).
Key elements driving EOCRC are exposed when four metrics are fulfilled: one, a temporal relationship exists that follows that of EOCRC; two, exposure is global, as with EOCRC; three, evidence exists of inflammatory or microbiome-modifying properties or evidence of an effect on the distal colon or rectum; and four, exposure occurs during development from conception to adulthood.
The following elements reach all four of the above metrics: a westernized diet including red and processed meats; consumption of monosodium glutamate, titanium dioxide, high-fructose corn syrup and synthetic dyes; obesity; stress; and widespread use of antibiotics.
Delineation of exposomal elements attacking the rectum versus colon and their interactions with genetics is a critical step to understanding this disease for purposes of chemoprevention and treatment.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Patel SG & Ahnen DJ Colorectal cancer in the young. Curr. Gastroenterol. Rep 20, 15 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Mauri G et al. Early-onset colorectal cancer in young individuals. Mol. Oncol 13, 109–131 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey CE et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA Surg. 150, 17–22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy CC, Singal AG, Baron JA & Sandler RS Decrease in incidence of young-onset colorectal cancer before recent increase. Gastroenterology 155, 1716–1719 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut 68, 2179–2185 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Vuik FE et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 68, 1820–1826 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel P & De P Trends in colorectal cancer incidence and related lifestyle risk factors in 15–49-year-olds in Canada, 1969–2010. Cancer Epidemiol. 42, 90–100 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD & Jemal A Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970–2014. JAMA 318, 572–574 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen LH et al. Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer. JNCI Cancer Spectr. 2, pky073 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoffel EM & Murphy CC Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology 158, 341–353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wild CP, Scalbert A & Herceg Z Measuring the exposome: a powerful basis for evaluating environmental exposures and cancer risk. Env. Mol. Mutagen 54, 480–499 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Wild CP Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomarkers Prev 14, 1847–1850 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Lucas C, Barnich N & Nguyen HTT Microbiota, inflammation and colorectal cancer. Int. J. Mol. Sci 18, 1310 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connell LC, Mota JM, Braghiroli MI & Hoff PM The rising incidence of younger patients with colorectal cancer: questions about screening, biology, and treatment. Curr. Treat. Options Oncol 18, 23 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Yeo H et al. Early-onset colorectal cancer is distinct from traditional colorectal cancer. Clin. Colorectal Cancer 16, 293–299.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Yeo SA, Chew MH, Koh PK & Tang CL Young colorectal carcinoma patients do not have a poorer prognosis: a comparative review of 2,426 cases. Tech. Coloproctol 17, 653–661 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Modest DP et al. Exploring the effect of primary tumor sidedness on therapeutic efficacy across treatment lines in patients with metastatic colorectal cancer: analysis of FIRE-3 (AIOKRK0306). Oncotarget 8, 105749–105760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim DR, Kuk JK, Kim T & Shin EJ Comparison of oncological outcomes of right-sided colon cancer versus left-sided colon cancer after curative resection: which side is better outcome? Medicine 96, e8241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norat T et al. Meat, fish, and colorectal cancer risk: the European prospective investigation into cancer and nutrition. J. Natl Cancer Inst 97, 906–916 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstein AM et al. Processed and unprocessed red meat and risk of colorectal cancer: analysis by tumor location and modification by time. PLoS One 10, e0135959 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nimptsch K et al. Dietary intakes of red meat, poultry, and fish during high school and risk of colorectal adenomas in women. Am. J. Epidemiol 178, 172–183 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akhter M et al. Alcohol consumption is associated with an increased risk of distal colon and rectal cancer in Japanese men: the Miyagi cohort study. Eur. J. Cancer 43, 383–390 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Bongaerts BW, van den Brandt PA, Goldbohm RA, de Goeij AF & Weijenberg MP Alcohol consumption, type of alcoholic beverage and risk of colorectal cancer at specific subsites. Int. J. Cancer 123, 2411–2417 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Annema N, Heyworth JS, McNaughton SA, Iacopetta B & Fritschi L Fruit and vegetable consumption and the risk of proximal colon, distal colon, and rectal cancers in a case-control study in Western Australia. J. Am. Dietetic Assoc 111, 1479–1490 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Zhang X et al. Calcium intake and colorectal cancer risk: results from the nurses’ health study and health professionals follow-up study. Int. J. Cancer 139, 2232–2242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hjartaker A et al. Subsite-specific dietary risk factors for colorectal cancer: a review of cohort studies. J. Oncol 2013, 703854 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu K, Willett WC, Fuchs CS, Colditz GA & Giovannucci EL Calcium intake and risk of colon cancer in women and men. J. Natl Cancer Inst 94, 437–446 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Oh K, Willett WC, Wu K, Fuchs CS & Giovannucci EL Calcium and vitamin D intakes in relation to risk of distal colorectal adenoma in women. Am. J. Epidemiol 165, 1178–1186 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Larsson SC, Bergkvist L, Rutegard J, Giovannucci E & Wolk A Calcium and dairy food intakes are inversely associated with colorectal cancer risk in the cohort of Swedish men. Am. J. Clin. Nutr 83, 667–673 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Cho E et al. Dietary choline and betaine and the risk of distal colorectal adenoma in women. J. Natl Cancer Inst 99, 1224–1231 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang ZJ et al. Dietary polyphenols and colorectal cancer risk: the Fukuoka colorectal cancer study. World J. Gastroenterol 19, 2683–2690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X et al. A prospective study of intakes of zinc and heme iron and colorectal cancer risk in men and women. Cancer Causes Control. 22, 1627–1637 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higuchi T et al. A randomized, double-blind, placebocontrolled trial of the effects of rofecoxib, a selective cyclooxygenase-2 inhibitor, on rectal polyps in familial adenomatous polyposis patients. Clin. Cancer Res 9, 4756–4760 (2003). [PubMed] [Google Scholar]
- 34.Steinbach G et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med 342, 1946–1952 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Paschke S et al. Are colon and rectal cancer two different tumor entities? A proposal to abandon the term colorectal cancer. Int. J. Mol. Sci 19, 2577 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothwell PM et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 376, 1741–1750 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Ma Y et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 8, e53916 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Movahedi M et al. Obesity, aspirin, and risk of colorectal cancer in carriers of hereditary colorectal cancer: a prospective investigation in the CAPP2 study. J. Clin. Oncol 33, 3591–3597 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Siegel RL et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J. Natl Cancer Inst 108, 1–6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imperial R et al. Comparative proteogenomic analysis of right-sided colon cancer, left-sided colon cancer and rectal cancer reveals distinct mutational profiles. Mol. Cancer 17, 177 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strum WB & Boland CR Clinical and genetic characteristics of colorectal cancer in persons under 50 years of age: a review. Dig. Dis. Sci 64, 3059–3065 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Pearlman R et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 3, 464–471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stigliano V, Sanchez-Mete L, Martayan A & Anti M Early-onset colorectal cancer: a sporadic or inherited disease? World J. Gastroenterol 20, 12420–12430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch KL, Ahnen DJ, Byers T, Weiss DG & Lieberman DA First-degree relatives of patients with advanced colorectal adenomas have an increased prevalence of colorectal cancer. Clin. Gastroenterol. Hepatol 1, 96–102 (2003). [DOI] [PubMed] [Google Scholar]
- 46.Lieberman DA, Prindiville S, Weiss DG & Willett W Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA 290, 2959–2967 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Rex DK et al. Colorectal cancer screening: recommendations for physicians and patients from the US multi-society task force on colorectal cancer. Am. J. Gastroenterol 112, 1016–1030 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Tsai MH, Xirasagar S, Li YJ & de Groen PC Colonoscopy screening among US adults aged 40 or older with a family history of colorectal cancer. Prev. Chronic Dis 12, E80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hogan NM et al. Awareness and uptake of family screening in patients diagnosed with colorectal cancer at a young age. Gastroenterol. Res. Pract 2015, 194931 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang K et al. Dissecting cancer heterogeneity based on dimension reduction of transcriptomic profiles using extreme learning machines. PLoS One 13, e0203824 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pi S, Nap-Hill E, Telford J & Enns R Recognition of Lynch syndrome amongst newly diagnosed colorectal cancers at St. Paul’s hospital. Can. J. Gastroenterol. Hepatol 2017, 9625638 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Claes E et al. Predictive testing for hereditary nonpolyposis colorectal cancer: subjective perception regarding colorectal and endometrial cancer, distress, and health-related behavior at one year post-test. Genet. Test 9, 54–65 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Halbert CH et al. Colon cancer screening practices following genetic testing for hereditary nonpolyposis colon cancer (HNPCC) mutations. Arch. Intern. Med 164, 1881–1887 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Wu HC et al. Developing screening services for colorectal cancer on Android smartphones. Telemed. J. E. Health 20, 687–695 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schultz M, Seo SB, Holt A & Regenbrecht H Family history assessment for colorectal cancer (CRC) risk analysis — comparison of diagram- and questionnaire-based web interfaces. BMC Med. Inform. Decis. Mak 15, 95 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giardiello FM et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US multi-society task force on colorectal cancer. Am. J. Gastroenterol 109, 1159–1179 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Syngal S et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am. J. Gastroenterol 110, 223–2623 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta S et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 3.2017. J. Natl Compr. Canc Netw 15, 1465–1475 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Stoffel EM, Mangu PB & Limburg PJ Hereditary colorectal cancer syndromes: American Society of Clinical Oncology clinical practice guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology clinical practice guidelines. J. Oncol. Pract 11, e437–e441 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Hippisley-Cox J & Coupland C Identifying patients with suspected colorectal cancer in primary care: derivation and validation of an algorithm. Br. J. Gen. Pract 62, e29–e37 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tao S, Hoffmeister M & Brenner H Development and validation of a scoring system to identify individuals at high risk for advanced colorectal neoplasms who should undergo colonoscopy screening. Clin. Gastroenterol. Hepatol 12, 478–485 (2014). [DOI] [PubMed] [Google Scholar]
- 62.Chiu HM et al. A risk-scoring system combined with a fecal immunochemical test is effective in screening high-risk subjects for early colonoscopy to detect advanced colorectal neoplasms. Gastroenterology 150, 617–625.e13 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Koning NR et al. Identification of patients at risk for colorectal cancer in primary care: an explorative study with routine healthcare data. Eur. J. Gastroenterol. Hepatol 27, 1443–1448 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Schneider R et al. Colorectal carcinoma with suspected Lynch syndrome: a multidisciplinary algorithm. Zentralbl. Chir 140, 591–599 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Choi YH, Briollais L, Green J, Parfrey P & Kopciuk K Estimating successive cancer risks in Lynch syndrome families using a progressive three-state model. Stat. Med 33, 618–638 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Pilozzi E et al. Left-sided early-onset vs late-onset colorectal carcinoma: histologic, clinical, and molecular differences. Am. J. Clin. Pathol 143, 374–384 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Liang JT et al. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br. J. Surg 90, 205–214 (2003). [DOI] [PubMed] [Google Scholar]
- 68.Boardman LA et al. A search for germline APC mutations in early onset colorectal cancer or familial colorectal cancer with normal DNA mismatch repair. Genes. Chromosomes Cancer 30, 181–186 (2001). [PubMed] [Google Scholar]
- 69.Antelo M et al. A high degree of LINE-1 hypomethylation is a unique feature of early-onset colorectal cancer. PLoS One 7, e45357 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watson R, Liu TC & Ruzinova MB High frequency of KRAS mutation in early onset colorectal adenocarcinoma: implications for pathogenesis. Hum. Pathol 56, 163–170 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Arriba M et al. Unsupervised analysis of array comparative genomic hybridization data from early-onset colorectal cancer reveals equivalence with molecular classification and phenotypes. Neoplasia 19, 28–34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boland PM, Yurgelun MB & Boland CR Recent progress in Lynch syndrome and other familial colorectal cancer syndromes. CA Cancer J. Clin 68, 217–231 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lieu CH et al. Comprehensive genomic landscapes in early and later onset colorectal cancer. Clin. Cancer Res 25, 5852–5858 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liang J, Kalady MF & Church J Young age of onset colorectal cancers. Int. J. Colorectal Dis 30, 1653–1657 (2015). [DOI] [PubMed] [Google Scholar]
- 75.Ballester V, Rashtak S & Boardman L Clinical and molecular features of young-onset colorectal cancer. World J. Gastroenterol 22, 1736–1744 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Willauer AN et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 125, 2002–2010 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeh CC, Hsieh LL, Tang R, Chang-Chieh CR & Sung FC MS-920: DNA repair gene polymorphisms, diet and colorectal cancer risk in Taiwan. Cancer Lett. 224, 279–288 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Khan NA et al. Dietary practices, addictive behavior and bowel habits and risk of early onset colorectal cancer: a case control study. Asian Pac. J. Cancer Prev 16, 7967–7973 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Nimptsch K & Wu K Is timing important? The role of diet and lifestyle during early life on colorectal neoplasia. Curr. Colorectal Cancer Rep 14, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rosato V et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control. 24, 335–341 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Kim JY et al. Different risk factors for advanced colorectal neoplasm in young adults. World J. Gastroenterol 22, 3611–3620 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boyle T et al. Timing and intensity of recreational physical activity and the risk of subsite-specific colorectal cancer. Cancer Causes Control. 22, 1647–1658 (2011). [DOI] [PubMed] [Google Scholar]
- 83.Zhang QL et al. The joint effects of major lifestyle factors on colorectal cancer risk among Chinese men: a prospective cohort study. Int. J. Cancer 142, 1093–1101 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siegel RL & Jemal A Percentage of colorectal cancer diagnosed in adults aged younger than 50 years. Cancer 122, 1462–1463 (2016). [DOI] [PubMed] [Google Scholar]
- 85.Siegel RL et al. Colorectal cancer statistics, 2017. CA Cancer J. Clin 67, 177–193 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Dulai PS, Sandborn WJ & Gupta S Colorectal cancer and dysplasia in inflammatory bowel disease: a review of disease epidemiology, pathophysiology, and management. Cancer Prev. Res 9, 887–894 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao Z, Guo B, Gao R, Zhu Q & Qin H Microbiota disbiosis is associated with colorectal cancer. Front. Microbiol 6, 20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keinan-Boker L, Vin-Raviv N, Liphshitz I, Linn S & Barchana M Cancer incidence in Israeli Jewish survivors of World War II. J. Natl Cancer Inst 101, 1489–1500 (2009). [DOI] [PubMed] [Google Scholar]
- 89.Hughes LA et al. Childhood and adolescent energy restriction and subsequent colorectal cancer risk: results from the Netherlands cohort study. Int. J. Epidemiol 39, 1333–1344 (2010). [DOI] [PubMed] [Google Scholar]
- 90.Liu PH et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 5, 37–44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruder EH et al. Adolescent and mid-life diet: risk of colorectal cancer in the NIH-AARP diet and health study. Am. J. Clin. Nutr 94, 1607–1619 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jensen BW et al. Childhood body mass index and height in relation to site-specific risks of colorectal cancers in adult life. Eur. J. Epidemiol 32, 1097–1106 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Hidayat K, Yang CM & Shi BM Body fatness at an early age and risk of colorectal cancer. Int. J. Cancer 142, 729–740 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Koo JE et al. Prevalence and risk factors of advanced colorectal neoplasms in asymptomatic Korean people between 40 and 49 years of age. J. Gastroenterol. Hepatol 32, 98–105 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Boyle T, Keegel T, Bull F, Heyworth J & Fritschi L Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J. Natl Cancer Inst 104, 1548–1561 (2012). [DOI] [PubMed] [Google Scholar]
- 96.Nunez C, Nair-Shalliker V, Egger S, Sitas F & Bauman A Physical activity, obesity and sedentary behaviour and the risks of colon and rectal cancers in the 45 and up study. BMC Public Health 18, 325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Longnecker MP, Gerhardsson le Verdier M, Frumkin H & Carpenter C A case-control study of physical activity in relation to risk of cancer of the right colon and rectum in men. Int. J. Epidemiol 24, 42–50 (1995). [DOI] [PubMed] [Google Scholar]
- 98.Halle M & Schoenberg MH Physical activity in the prevention and treatment of colorectal carcinoma. Dtsch. Arztebl. Int 106, 722–727 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moradi T et al. Occupational physical activity and risk for cancer of the colon and rectum in Sweden among men and women by anatomic subsite. Eur. J. Cancer Prev 17, 201–208 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hill AB Observation and experiment. N. Engl. J. Med 248, 995–1001 (1953). [DOI] [PubMed] [Google Scholar]
- 101.Malik VS, Willett WC & Hu FB Global obesity: trends, risk factors and policy implications. Nat. Rev. Endocrinol 9, 13–27 (2013). [DOI] [PubMed] [Google Scholar]
- 102.Sung H et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J. Clin 69, 88–112 (2018). [DOI] [PubMed] [Google Scholar]
- 103.Stokes A, Ni Y & Preston SH Prevalence and trends in lifetime obesity in the US, 1988–2014. Am. J. Prev. Med 53, 567–575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alati R et al. Generational increase in obesity among young women: a prospective analysis of mother–daughter dyads. Int. J. Obes 40, 176–180 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Kantor ED et al. Adolescent body mass index and erythrocyte sedimentation rate in relation to colorectal cancer risk. Gut 65, 1289–1295 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Renehan AG et al. Body mass index at different adult ages, weight change, and colorectal cancer risk in the National Institutes of Health-AARP cohort. Am. J. Epidemiol 176, 1130–1140 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Levi Z et al. Adolescent body mass index and risk of colon and rectal cancer in a cohort of 1.79 million Israeli men and women: a population-based study. Cancer 123, 4022–4030 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Ziccardi P et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation 105, 804–809 (2002). [DOI] [PubMed] [Google Scholar]
- 109.Kasai C et al. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and nextgeneration sequencing. BMC Gastroenterol. 15, 100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang W et al. Lipidomic profiling reveals soluble epoxide hydrolase as a therapeutic target of obesity-induced colonic inflammation. Proc. Natl Acad. Sci. USA 115, 5283–5288 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Larsson SC & Wolk A Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am. J. Clin. Nutr 86, 556–565 (2007). [DOI] [PubMed] [Google Scholar]
- 112.Segev L, Kalady MF & Church JM Left-sided dominance of early-onset colorectal cancers: a rationale for screening flexible sigmoidoscopy in the young. Dis. Colon Rectum 61, 897–902 (2018). [DOI] [PubMed] [Google Scholar]
- 113.Sjoblom T et al. The consensus coding sequences of human breast and colorectal cancers. Science 314, 268–274 (2006). [DOI] [PubMed] [Google Scholar]
- 114.Brand MP, Peeters PH, van Gils CH & Elias SG Pre-adult famine exposure and subsequent colorectal cancer risk in women. Int. J. Epidemiol 46, 612–621 (2017). [DOI] [PubMed] [Google Scholar]
- 115.Kikuchi N et al. Perceived stress and colorectal cancer incidence: the Japan collaborative cohort study. Sci. Rep 7, 40363 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Q et al. Maternal stress and early-onset colorectal cancer. Med. Hypotheses 121, 152–159 (2018). [DOI] [PubMed] [Google Scholar]
- 117.Cohen S, Janicki-Deverts D & Miller GE Psychological stress and disease. JAMA 298, 1685–1687 (2007). [DOI] [PubMed] [Google Scholar]
- 118.Twenge JM et al. Birth cohort increases in psychopathology among young Americans, 1938–2007: a cross-temporal meta-analysis of the MMPI. Clin. Pyschol. Rev 30, 145–154 (2010). [DOI] [PubMed] [Google Scholar]
- 119.Xin Z, Niu J & Chi L Birth cohort changes in Chinese adolescents’ mental health. Int. J. Psychol 47, 287–295 (2012). [DOI] [PubMed] [Google Scholar]
- 120.Sluggett L, Wagner SL & Harris RL Sleep duration and obesity in children and adolescents. Can J. Diabetes 43, 146–152 (2019). [DOI] [PubMed] [Google Scholar]
- 121.Jiao L et al. Sleep duration and incidence of colorectal cancer in postmenopausal women. Br. J. Cancer 108, 213–221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thompson CL et al. Short duration of sleep increases risk of colorectal adenoma. Cancer 117, 841–847 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vu HT et al. Diabetes mellitus increases risk for colorectal adenomas in younger patients. World J. Gastroenterol 20, 6946–6952 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hebert JR et al. Considering the role of stress in populations of high-risk, underserved community networks program centers. Prog. Community Health Partnersh 9, 71–82 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tilg H, Adolph TE, Gerner RR & Moschen AR The intestinal microbiota in colorectal cancer. Cancer Cell 33, 954–964 (2018). [DOI] [PubMed] [Google Scholar]
- 126.Tse JWT, Jenkins LJ, Chionh F & Mariadason JM Aberrant DNA methylation in colorectal cancer: what should we target? Trends Cancer 3, 698–712 (2017). [DOI] [PubMed] [Google Scholar]
- 127.Carson TL et al. Associations between race, perceived psychological stress, and the gut microbiota in a sample of generally healthy black and white women: a pilot study on the role of race and perceived psychological stress. Psychosom. Med 80, 640–648 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Louis P, Hold GL & Flint HJ The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol 12, 661–672 (2014). [DOI] [PubMed] [Google Scholar]
- 129.Castello A et al. Low adherence to the western and high adherence to the Mediterranean dietary patterns could prevent colorectal cancer. Eur. J. Nutr 58, 1495–1505 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Mehta RS et al. Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology 152, 1944–1953.e1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chang DT et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod. Pathol 25, 1128–1139 (2012). [DOI] [PubMed] [Google Scholar]
- 132.Kirzin S et al. Sporadic early-onset colorectal cancer is a specific sub-type of cancer: a morphological, molecular and genetics study. PLoS One 9, e103159 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khan SA et al. Colorectal cancer in the very young: a comparative study of tumor markers, pathology and survival in early onset and adult onset patients. J. Pediatr. Surg 51, 1812–1817 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Roon EH et al. Early onset MSI-H colon cancer with MLH1 promoter methylation, is there a genetic predisposition? BMC Cancer 10, 180 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tapial S et al. Cimp-positive status is more representative in multiple colorectal cancers than in unique primary colorectal cancers. Sci. Rep 9, 10516 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Perea J et al. Classifying early-onset colorectal cancer according to tumor location: new potential subcategories to explore. Am. J. Cancer Res 5, 2308–2313 (2015). [PMC free article] [PubMed] [Google Scholar]
- 137.Kim HC et al. Aberrant CpG island methylation in early-onset sporadic gastric carcinoma. J. Cancer Res. Clin. Oncol 131, 733–740 (2005). [DOI] [PubMed] [Google Scholar]
- 138.Statovci D, Aguilera M, MacSharry J & Melgar S The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front. Immunol 8, 838 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Keefe SJ Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol 13, 691–706 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Popkin BM, Adair LS & Ng SW Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev 70, 3–21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brenner DR et al. Increasing colorectal cancer incidence trends among younger adults in Canada. Prev. Med 105, 345–349 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Veruttipong D et al. Age distribution, polyps and rectal cancer in the Egyptian population-based cancer registry. World J. Gastroenterol 18, 3997–4003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Khoury CK et al. Increasing homogeneity in global food supplies and the implications for food security. Proc. Natl Acad. Sci. USA 111, 4001–4006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Musaiger AO Overweight and obesity in the eastern Mediterranean region: can we control it? East. Mediterr. Health J 10, 789–793 (2004). [PubMed] [Google Scholar]
- 145.Odegaard AO, Koh WP, Yuan JM, Gross MD & Pereira MA Western-style fast food intake and cardiometabolic risk in an Eastern country. Circulation 126, 182–188 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Danaei G et al. The global cardiovascular risk transition: associations of four metabolic risk factors with national income, urbanization, and western diet in 1980 and 2008. Circulation 127, 1493–1502, 1502e1–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Uribarri J et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Dietetic Assoc 110, 911–916.e12 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yacoub R et al. Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS One 12, e0184789 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gupta A & Uribarri J Dietary advanced glycation end products and their potential role in cardiometabolic disease in children. Horm. Res. Paediatr 85, 291–300 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rodriguez JM et al. Reduction of serum advanced glycation end-products with a low calorie Mediterranean diet. Nutr. Hops 31, 2511–2517 (2015). [DOI] [PubMed] [Google Scholar]
- 151.Mayr HL et al. Randomization to 6-month Mediterranean diet compared with a low-fat diet leads to improvement in dietary inflammatory index scores in patients with coronary heart disease: the AUSMED heart trial. Nutr. Red 55, 94–107 (2018). [DOI] [PubMed] [Google Scholar]
- 152.Harmon BE et al. The dietary inflammatory index is associated with colorectal cancer risk in the multiethnic cohort. J. Nutr 147, 430–438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ramallal R et al. Inflammatory potential of diet, weight gain, and incidence of overweight/obesity: the SUN cohort. Obesity 25, 997–1005 (2017). [DOI] [PubMed] [Google Scholar]
- 154.Alipoor E et al. Dietary inflammatory index and parameters of diet quality in normal weight and obese patients undergoing hemodialysis. Nutr. 61, 32–37 (2019). [DOI] [PubMed] [Google Scholar]
- 155.Park YM et al. Dietary inflammatory potential and risk of mortality in metabolically healthy and unhealthy phenotypes among overweight and obese adults. Clin. Nutr 38, 682–688 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shivappa N et al. Dietary inflammatory index and colorectal cancer risk — a meta-analysis. Nutrients 9, 1043 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Martin CA, Milinsk MC, Visentainer JV, Matsushita M & de-Souza NE Trans fatty acidforming processes in foods: a review. An. Acad. Bras. Cienc 79, 343–350 (2007). [DOI] [PubMed] [Google Scholar]
- 158.Zhao Z et al. Red and processed meat consumption and colorectal cancer risk: a systematic review and meta-analysis. Oncotarget 8, 83306–83314 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ritchie H & Roser M Meat and dairy production. Our World in Data https://ourworldindata.org/meat-production (2019). [Google Scholar]
- 160.Chai W et al. Dietary red and processed meat intake and markers of adiposity and inflammation: the multiethnic cohort study. J. Am. Coll. Nutr 36, 378–385 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Thogersen R et al. Ingestion of an inulin-enriched pork sausage product positively modulates the gut microbiome and metabolome of healthy rats. Mol. Nutr. Food Res 62, e1800608 (2018). [DOI] [PubMed] [Google Scholar]
- 162.Esaiassen E et al. Antibiotic exposure in neonates and early adverse outcomes: a systematic review and meta-analysis. J. Antimicrob. Chemother 72, 1858–1870 (2017). [DOI] [PubMed] [Google Scholar]
- 163.Azad MB et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG 123, 983–993 (2016). [DOI] [PubMed] [Google Scholar]
- 164.Wang JL et al. Infection, antibiotic therapy and risk of colorectal cancer: a nationwide nested case-control study in patients with type 2 diabetes mellitus. Int. J. Cancer 135, 956–967 (2014). [DOI] [PubMed] [Google Scholar]
- 165.Scott FI et al. Administration of antibiotics to children before age 2 years increases risk for childhood obesity. Gastroenterology 151, 120–129.e5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dik VK, van Oijen MG, Smeets HM & Siersema PD Frequent use of antibiotics is associated with colorectal cancer risk: results of a nested case–control study. Dig. Dis. Sci 61, 255–264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Boursi B, Haynes K, Mamtani R & Yang YX Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiol. Drug Saf 24, 534–542 (2015). [DOI] [PubMed] [Google Scholar]
- 168.Klein EY et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl Acad. Sci. USA 115, E3463–E3470 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ananthakrishnan AN et al. Environmental triggers in IBD: a review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol 15, 39–49 (2018). [DOI] [PubMed] [Google Scholar]
- 170.Knoop KA, McDonald KG, Kulkarni DH & Newberry RD Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 65, 1100–1109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rigoni R et al. Intestinal microbiota sustains inflammation and autoimmunity induced by hypomorphic RAG defects. J. Exp. Med 213, 355–375 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bailey LC et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatrics 168, 1063–1069 (2014). [DOI] [PubMed] [Google Scholar]
- 173.Rasmussen SH et al. Antibiotic exposure in early life and childhood overweight and obesity: a systematic review and meta-analysis. Diabetes Obes. Metab 20, 1508–1514 (2018). [DOI] [PubMed] [Google Scholar]
- 174.Kaur K et al. Antibiotic-mediated bacteriome depletion in Apcmin/+ mice is associated with reduction in mucus-producing goblet cells and increased colorectal cancer progression. Cancer Med. 7, 2003–2012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang C et al. Salinomycin inhibits the growth of colorectal carcinoma by targeting tumor stem cells. Oncol. Rep 34, 2469–2476 (2015). [DOI] [PubMed] [Google Scholar]
- 176.Hattori N et al. Antibiotics suppress colon tumorigenesis through inhibition of aberrant DNA methylation in an azoxymethane and dextran sulfate sodium colitis model. Cancer Sci. 110, 147–156 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hardiman KM, Liu J, Feng Y, Greenson JK & Fearon ER Rapamycin inhibition of polyposis and progression to dysplasia in a mouse model. PLoS One 9, e96023 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bullman S et al. Analysis of fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Imhann F et al. Proton pump inhibitors affect the gut microbiome. Gut 65, 740–748 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kearney J Food consumption trends and drivers. Phil. Trans. R. Soc. Lond. B Biol. Sci 365, 2793–2807 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ferguson LR Natural and man-made mutagens and carcinogens in the human diet. Mutat. Res 443, 1–10 (1999). [PubMed] [Google Scholar]
- 182.Espejo-Herrera N et al. Colorectal cancer risk and nitrate exposure through drinking water and diet. Int. J. Cancer 139, 334–346 (2016). [DOI] [PubMed] [Google Scholar]
- 183.Crowe W, Elliott CT & Green BD A review of the in vivo evidence investigating the role of nitrite exposure from processed meat consumption in the development of colorectal cancer. Nutrients 11, 2673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nicole W Secret ingredients: who knows what’s in your food? Environ. Health Perspect 121, A126–A133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kobylewski S & Jacobson MF Toxicology of food dyes. Int. J. Occup. Environ. Health 18, 220–246 (2012). [DOI] [PubMed] [Google Scholar]
- 186.Oplatowska-Stachowiak M & Elliott CT Food colors: existing and emerging food safety concerns. Crit. Rev. Food Sci. Nutr 57, 524–548 (2017). [DOI] [PubMed] [Google Scholar]
- 187.FAO/WHO. Evaluation of certain food additives: eightysecond report of the joint FAO/WHO expert committee on food additives world health organ. Tech. Rep. Ser 82, 1–162 (2016). [Google Scholar]
- 188.Ijssennagger N, van der Meer R & van Mil SWC Sulfide as a mucus barrier-breaker in inflammatory bowel disease? Trends Mol. Med 22, 190–199 (2016). [DOI] [PubMed] [Google Scholar]
- 189.Potera C The artificial food dye blues. Environ. Health Perspect 118, A428 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Feng J, Cerniglia CE & Chen H Toxicological significance of azo dye metabolism by human intestinal microbiota. Front. Biosci 4, 568–586 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shimada C, Kano K, Sasaki YF, Sato I & Tsudua S Differential colon DNA damage induced by azo food additives between rats and mice. J. Toxicol. Sci 35, 547–554 (2010). [DOI] [PubMed] [Google Scholar]
- 192.Khayyat LI, Essawy AE, Sorour JM & Soffar A Sunset yellow and allura red modulate Bcl2 and COX2 expression levels and confer oxidative stress-mediated renal and hepatic toxicity in male rats. PeerJ 6, e5689 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Meyer SK et al. Hepatic effects of tartrazine (E102) after systemic exposure are independent of oestrogen receptor interactions in the mouse. Toxicol. Lett 273, 55–68 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.World Health Organization. Evaluation of certain food additives (twenty-third report of the joint FAO/WHO expert committee on food additives). Tech. Rep. Ser 648, 13 (1980). [PubMed] [Google Scholar]
- 195.Bastaki M, Farrell T, Bhusari S, Pant K & Kulkarni R Lack of genotoxicity in vivo for food color additive allura red AC. Food Chem. Toxicol 105, 308–314 (2017). [DOI] [PubMed] [Google Scholar]
- 196.Bastaki M, Farrell T, Bhusari S, Pant K & Kulkarni R Lack of genotoxicity in vivo for food color additive tartrazine. Food Chem. Toxicol 105, 278–284 (2017). [DOI] [PubMed] [Google Scholar]
- 197.Tsuda S et al. DNA damage induced by red food dyes orally administered to pregnant and male mice. Toxicol. Sci 61, 92–99 (2001). [DOI] [PubMed] [Google Scholar]
- 198.Sasaki YF et al. The comet assay with 8 mouse organs: results with 39 currently used food additives. Mutat. Res 519, 103–119 (2002). [DOI] [PubMed] [Google Scholar]
- 199.Nair A, Morsy MA & Jacob S Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug. Dev. Res 79, 373–382 (2018). [DOI] [PubMed] [Google Scholar]
- 200.Stevens LJ, Burgess JR, Stochelski MA & Kuczek T Amounts of artificial food colors in commonly consumed beverages and potential behavioral implications for consumption in children: revisited. Clin. Pediatr 54, 1228–1230 (2015). [DOI] [PubMed] [Google Scholar]
- 201.Sasaki Y et al. Dose dependent development of diabetes mellitus and non-alcoholic steatohepatitis in monosodium glutamate-induced obese mice. Life Sci. 85, 490–498 (2009). [DOI] [PubMed] [Google Scholar]
- 202.Hata K et al. Monosodium glutamate-induced diabetic mice are susceptible to azoxymethane-induced colon tumorigenesis. Carcinogenesis 33, 702–707 (2012). [DOI] [PubMed] [Google Scholar]
- 203.Talbot P et al. Food-grade TiO2 is trapped by intestinal mucus in vitro but does not impair mucin O-glycosylation and short-chain fatty acid synthesis in vivo: implications for gut barrier protection. J. Nanobiotechnol 16, 53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Winkler HC, Notter T, Meyer U & Naegeli H Critical review of the safety assessment of titanium dioxide additives in food. J. Nanobiotechnol 16, 51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Baranowska-Wojcik E, Szwajgier D, Oleszczuk P & Winiarska-Mieczan A Effects of titanium dioxide nanoparticles exposure on human health-a review. Biol. Trace Elem. Res 193, 118–129 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Urrutia-Ortega IM et al. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food Chem. Toxicol 93, 20–31 (2016). [DOI] [PubMed] [Google Scholar]
- 207.Bettini S et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep 7, 40373 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Proquin H et al. Gene expression profiling in colon of mice exposed to food additive titanium dioxide (E171). Food Chem. Toxicol 111, 153–165 (2018). [DOI] [PubMed] [Google Scholar]
- 209.Proquin H et al. Transcriptomics analysis reveals new insights in E171-induced molecular alterations in a mouse model of colon cancer. Sci. Rep 8, 9738 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.White JS in Beverage Impacts on Health and Nutrition 2nd edn (eds Wilson T & Temple NJ) 285–301 (Humana Press, 2016). [Google Scholar]
- 211.Tappy L Fructose-containing caloric sweeteners as a cause of obesity and metabolic disorders. J. Exp. Biol 221, jeb164202 (2018). [DOI] [PubMed] [Google Scholar]
- 212.Alexander Bentley R, Ruck DJ & Fouts HN U.S. obesity as delayed effect of excess sugar. Econ. Hum. Biol 36, 100818 (2019). [DOI] [PubMed] [Google Scholar]
- 213.Jin R et al. Dietary fructose reduction improves markers of cardiovascular disease risk in Hispanic-American adolescents with NAFLD. Nutrients 6, 3187–3201 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zheng J, Feng Q, Zhang Q, Wang T & Xiao X Early life fructose exposure and its implications for long-term cardiometabolic health in offspring. Nutrients 8, 685 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bray GA, Nielsen SJ & Popkin BM Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr 79, 537–543 (2004). [DOI] [PubMed] [Google Scholar]
- 216.Panasevich MR et al. High-fat, high-fructose, highcholesterol feeding causes severe NASH and cecal microbiota dysbiosis in juvenile Ossabaw swine. Am. J. Phys. Endocrinol. Metab 314, E78–E92 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Morgan RE Does consumption of high-fructose corn syrup beverages cause obesity in children? Pediatr. Obes 8, 249–254 (2013). [DOI] [PubMed] [Google Scholar]
- 218.Goncalves MD et al. High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363, 1345–1349 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Toop CR & Gentili S Fructose beverage consumption induces a metabolic syndrome phenotype in the rat: a systematic review and meta-analysis. Nutrients 8, 577 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chassaing B, Vijay-Kumar M & Gewirtz AT How diet can impact gut microbiota to promote or endanger health. Curr. Opin. Gastroenterol 33, 417–421 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Guinane CM & Cotter PD Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Ther. Adv. Gastroenterol 6, 295–308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hernandez-Luna MA, Lopez-Briones S & Luria-Perez R The four horsemen in colon cancer. J. Oncol 2019, 5636272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sobhani I et al. Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures. Proc. Natl Acad. Sci. USA 116, 24285–24295 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Huang P & Liu Y A reasonable diet promotes balance of intestinal microbiota: prevention of precolorectal cancer. Biomed Res. Int 2019, 3405278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chassaing B et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519, 92–96 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Levi Z et al. Measured body mass index in adolescence and the incidence of colorectal cancer in a cohort of 1.1 million males. Cancer Epidemiol. Biomarkers Prev 20, 2524–2531 (2011). [DOI] [PubMed] [Google Scholar]
- 227.Stidham RW & Higgins PDR Colorectal cancer in inflammatory bowel disease. Clin. Colon. Rectal Surg 31, 168–178 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Viennois E, Merlin D, Gewirtz AT & Chassaing B Dietary emulsifier-induced low-grade inflammation promotes colon carcinogenesis. Cancer Res. 77, 27–40 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Akagawa S et al. Effect of delivery mode and nutrition on gut microbiota in neonates. Ann. Nutr. Metab 74, 132–139 (2019). [DOI] [PubMed] [Google Scholar]
- 230.Tamburini S, Shen N, Wu HC & Clemente JC The microbiome in early life: implications for health outcomes. Nat. Med 22, 713 (2016). [DOI] [PubMed] [Google Scholar]
- 231.Dwyer AJ et al. A summary of the fight colorectal cancer working meeting: exploring risk factors and etiology of sporadic early-age onset colorectal cancer. Gastroenterology 157, 280–288 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chassaing B, Van de Wiele T, De Bodt J, Marzorati M & Gewirtz AT Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 66, 1414–1427 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Roberts CL et al. Translocation of Crohn’s disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut 59, 1331–1339 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Roberts CL, Rushworth SL, Richman E & Rhodes JM Hypothesis: increased consumption of emulsifiers as an explanation for the rising incidence of Crohn’s disease. J. Crohns Colitis 7, 338–341 (2013). [DOI] [PubMed] [Google Scholar]
- 235.Lewis JD & Abreu MT Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 152, 398–414.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 236.Aguayo-Patron SV & Calderon de la Barca AM Old fashioned vs. ultra-processed-based current diets: possible implication in the increased susceptibility to type 1 diabetes and celiac disease in childhood. Foods 6, 100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bel S et al. Dietary exposure of the Belgian population to emulsifiers E481 (sodium stearoyl-2-lactylate) and E482 (calcium stearoyl-2-lactylate). Food Addit. Contam 35, 828–837 (2018). [DOI] [PubMed] [Google Scholar]
- 238.Mozaffarian D et al. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr 79, 606–612 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Dhaka V, Gulia N, Ahlawat KS & Khatkar BS Trans fats-sources, health risks and alternative approach — a review. J. Food Sci. Technol 48, 534–541 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.McKelvey W, Greenland S & Sandler RS A second look at the relation between colorectal adenomas and consumption of foods containing partially hydrogenated oils. Epidemiology 11, 469–473 (2000). [DOI] [PubMed] [Google Scholar]
- 241.Slattery ML, Benson J, Ma KN, Schaffer D & Potter JD Trans-fatty acids and colon cancer. Nutr. Cancer 39, 170–175 (2001). [DOI] [PubMed] [Google Scholar]
- 242.Vinikoor LC et al. Consumption of trans-fatty acid and its association with colorectal adenomas. Am. J. Epidemiol 168, 289–297 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hsu HT et al. Kinetics for the distribution of acrylamide in French fries, fried oil and vapour during frying of potatoes. Food Chem. 211, 669–678 (2016). [DOI] [PubMed] [Google Scholar]
- 244.Kumar J, Das S & Teoh SL Dietary acrylamide and the risks of developing cancer: facts to ponder. Front. Nutr 5, 14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Raju J et al. Negligible colon cancer risk from foodborne acrylamide exposure in male F344 rats and nude (nu/nu) mice-bearing human colon tumor xenografts. PLoS One 8, e73916 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hogervorst JG et al. Dietary acrylamide intake and the risk of colorectal cancer with specific mutations in KRAS and APC. Carcinogenesis 35, 1032–1038 (2014). [DOI] [PubMed] [Google Scholar]
- 247.Esposito F, Nardone A, Fasano E, Triassi M & Cirillo T Determination of acrylamide levels in potato crisps and other snacks and exposure risk assessment through a margin of exposure approach. Food Chem. Toxicol 108, 249–256 (2017). [DOI] [PubMed] [Google Scholar]
- 248.Kadawathagedara M et al. Dietary acrylamide intake during pregnancy and anthropometry at birth in the French EDEN mother–child cohort study. Environ. Res 149, 189–196 (2016). [DOI] [PubMed] [Google Scholar]
- 249.Luopajarvi K et al. Enhanced levels of cow’s milk antibodies in infancy in children who develop type 1 diabetes later in childhood. Pediatr. Diabetes 9, 434–441 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Llewellyn SR et al. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology 154, 1037–1046.e2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bajka BH, Clarke JM, Cobiac L & Topping DL Butyrylated starch protects colonocyte DNA against dietary protein-induced damage in rats. Carcinogenesis 29, 2169–2174 (2008). [DOI] [PubMed] [Google Scholar]
- 252.Toden S, Bird AR, Topping DL & Conlon MA Resistant starch attenuates colonic DNA damage induced by higher dietary protein in rats. Nutr. Cancer 51, 45–51 (2005). [DOI] [PubMed] [Google Scholar]
- 253.Ley SH et al. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am. J. Clin. Nutr 99, 352–360 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shen Q, Chen YA & Tuohy KM A comparative in vitro investigation into the effects of cooked meats on the human faecal microbiota. Anaerobe 16, 572–577 (2010). [DOI] [PubMed] [Google Scholar]
- 255.Merlot E, Couret D & Otten W Prenatal stress, fetal imprinting and immunity. Brain Behav. Immun 22, 42–51 (2008). [DOI] [PubMed] [Google Scholar]
- 256.Foster JA, Rinaman L & Cryan JF Stress & the gut-brain axis: regulation by the microbiome. Neurobiol. Stress 7, 124–136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jacob T, Itzchak EB & Raz O Stress among healthcare students — a cross disciplinary perspective. Physiother. Theory Pract 29, 401–412 (2013). [DOI] [PubMed] [Google Scholar]
- 258.Sharland M et al. Antibiotic prescribing in general practice and hospital admissions for peritonsillar abscess, mastoiditis, and rheumatic fever in children: time trend analysis. Br. Med. J 331, 328–329 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Raposa B et al. Food additives: sodium benzoate, potassium sorbate, azorubine, and tartrazine modify the expression of NFkappaB, GADD45alpha, and MAPK8 genes. Physiol. Int 103, 334–343 (2016). [DOI] [PubMed] [Google Scholar]
- 260.Brown JP Reduction of polymeric azo and nitro dyes by intestinal bacteria. Appl. Environ. Microbiol 41, 1283–1286 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sano C History of glutamate production. Am. J. Clin. Nutr 90, 728s–732s (2009). [DOI] [PubMed] [Google Scholar]
- 262.Tennant DR Review of glutamate intake from both food additive and non-additive sources in the European Union. Ann. Nutr. Metab 73, 21–28 (2018). [DOI] [PubMed] [Google Scholar]
- 263.Zanfirescu A, Cristea AN, Nitulescu GM, Velescu BS & Gradinaru D Chronic monosodium glutamate administration induced hyperalgesia in mice. Nutrients 10, E1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Feng Z et al. Monosodium L-glutamate and dietary fat exert opposite effects on the proximal and distal intestinal health in growing pigs. Appl. Physiol. Nutr. Metab 40, 353–363 (2015). [DOI] [PubMed] [Google Scholar]
- 265.Nagata M et al. Type 2 diabetes mellitus in obese mouse model induced by monosodium glutamate. Exp. Anim 55, 109–115 (2006). [DOI] [PubMed] [Google Scholar]
- 266.Weir A, Westerhoff P, Fabricius L, Hristovski K & von Goetz N Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol 46, 2242–2250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gholinejad Z, Khadem Ansari MH & Rasmi Y Titanium dioxide nanoparticles induce endothelial cell apoptosis via cell membrane oxidative damage and p38, PI3K/Akt, NF-κB signaling pathways modulation. J. Trace Elem. Med. Biol 54, 27–35 (2019). [DOI] [PubMed] [Google Scholar]
- 268.Klurfeld DM, Foreyt J, Angelopoulos TJ & Rippe JM Lack of evidence for high fructose corn syrup as the cause of the obesity epidemic. Int. J. Obes 37, 771–773 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Charrez B, Qiao L & Hebbard L The role of fructose in metabolism and cancer. Horm. Mol. Biol. Clin. Invest 22, 79–89 (2015). [DOI] [PubMed] [Google Scholar]


