Abstract
A Rhodobacter capsulatus reporter strain, carrying a constitutively expressed nifA gene and a nifH-lacZ gene fusion, was used for random transposon Tn5 mutagenesis to search for genes required for the NtrC-independent ammonium repression of NifA activity. A mutation in hvrA, which is known to be involved in low-light activation of the photosynthetic apparatus, released both ammonium and oxygen control of nifH expression in this reporter strain, demonstrating a regulatory link of nitrogen fixation and photosynthesis via HvrA. In addition, a significant increase in bacteriochlorophyll a (BChla) content was found in cells under nitrogen-fixing conditions. HvrA was not involved in this up-regulation of BChla. Instead, the presence of active nitrogenase seemed to be sufficient for this process, since no increase in BChla content was observed in different nif mutants.
Under anaerobic conditions in the light, the phototrophic purple bacterium Rhodobacter capsulatus is able to fix atmospheric dinitrogen. A large number of genes involved in synthesis and regulation of the molybdenum nitrogenase and the alternative, heterometal-free nitrogenase have been identified and characterized in detail (for a review, see reference 21). Both nitrogenase systems are only expressed under conditions of ammonium depletion, and a regulatory cascade resembling the general nitrogen regulation (ntr) system of enteric bacteria has been proposed for R. capsulatus (8, 13, 19, 20). Homologs of NtrB (NifR2), NtrC (NifR1), and GlnB (NifR5) are responsible for sensing and responding to the intracellular concentration of fixed nitrogen. Under nitrogen limitation the phosphorylated form of NtrC activates the transcription of a number of genes, including nifA1/nifA2 and anfA, which encode the transcriptional activators of the molybdenum and the alternative nitrogenase, respectively. However, in contrast to all known members of the NtrC family, R. capsulatus NtrC does not activate its target genes in concert with the RNA polymerase containing ς54 (9–11, 13, 19). The R. capsulatus rpoN (nifR4) gene encoding ς54 is part of the nitrogen fixation regulon and forms an autoregulatory circuit activated by NifA and AnfA, respectively (5, 21). These transcriptional activators in turn stimulate the expression of all other nitrogen fixation genes in a ς54-dependent manner. However, nitrogen fixation in R. capsulatus is not only regulated at the transcriptional level but also at the posttranslational level. In response to ammonium and darkness, the draTG gene products control the activity of dinitrogenase reductase of both nitrogenase systems by reversible, covalent ADP-ribosylation (23).
In addition, the NifA-dependent transcriptional activation is negatively regulated at the posttranslational level by ammonium in an NtrC-independent manner (12). In Klebsiella pneumoniae and Azotobacter vinelandii, an additional regulatory protein, NifL, is responsible for modulation of NifA activity in response to oxygen and fixed nitrogen. However, there is no evidence for a nifL-like gene in R. capsulatus (for a review, see reference 21). In addition, the domain structure of R. capsulatus NifA differs from NifA of K. pneumoniae and A. vinelandii (22) and corresponds to the rhizobial type of oxygen-sensitive NifA proteins (for a review, see reference 7), which in general seem to be independent of regulation by NifL homologs.
In phototrophic bacteria the high energy demand of the nitrogen fixation process can be fulfilled by photosynthesis. Genes encoding the reaction center, the light-harvesting complex and proteins involved in synthesis of the corresponding pigments, are only expressed under anaerobic conditions. In addition, synthesis of the photosynthetic apparatus is regulated by light intensity (for a review, see reference 1). Regulatory genes involved in this dual control are clustered in one region of the chromosome and include a two-component regulatory system (regA-regB) responsible for oxygen regulation (15, 26–28) and two genes (hvrA and hvrB) necessary for low-light activation (2, 3). Recently, the regA-regB systems in Rhodospirillum rubrum and Rhodobacter sphaeroides were shown not only to regulate photosystem biosynthesis and transcription of genes required for CO2 fixation but also to control the ability of these bacteria to grow under nitrogen-fixing conditions (15).
In this paper we present evidence that HvrA, which was previously shown to be responsible for light regulation of the photosynthetic apparatus, is also involved in the NtrC-independent ammonium control of nif gene expression. The observed increase of the bacteriochlorophyll a (BChla) content under conditions of nitrogen fixation further corroborates the hypothesis of a regulatory link of photosynthesis and nitrogen fixation.
Identification of an R. capsulatus gene (hvrA) involved in NtrC-independent ammonium regulation of nif gene expression.
Contrary to earlier models of nif gene regulation, constitutive expression of nifA1 did not result in ammonium-independent expression of a nifH-lacZ reporter gene fusion in R. capsulatus (12). This repression by high ammonium concentrations was not affected by mutations in ntrC or glnB (12). However, constitutively expressed nifA genes from K. pneumoniae and Rhizobium meliloti complemented an R. capsulatus nifA1 nifA2 double mutant, but neither of the heterologous nifA gene products was affected by ammonium, indicating that the ammonium sensitivity is a specific property of R. capsulatus NifA (21).
To identify genes involved in ammonium repression of NifA activity, we performed a random transposon Tn5 mutagenesis (18) of R. capsulatus W49I/R372I (a derivative of R. capsulatus B10S [18]) carrying a constitutively expressed nifA1 gene [nifA1(Con)] and a chromosomally integrated nifH-lacZYA reporter gene fusion (12). Tn5-induced mutants (kanamycin resistant) were screened for the ability to form deep blue colonies in the presence of ammonium on solid RCV medium supplemented with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) under anaerobic phototrophic growth conditions. Expression of the nifH-lacZ fusion in mutant strains identified by this procedure was quantified by measuring β-galactosidase activity of cells grown in liquid RCV medium containing either ammonium or serine as nitrogen source. Among 70,000 kanamycin-resistant mutants, three strains (M3-4, M7-1, and M8-20) were identified, which showed significant expression of the nifH-lacZ fusion in the presence of ammonium (Table 1).
TABLE 1.
R. capsulatus Tn5 mutants showing an increased expression of nifH-lacZ in the presence of ammonium
| Strain | Localization of Tn5a | Size of cloned EcoRI fragmentb | β-Galactosidase activityc
|
|
|---|---|---|---|---|
| NH4+ | Serine | |||
| Parental straind | 30 | 1200 | ||
| M3-4 | hvrA (nt. 326/327) | 2.8 kb | 680 | 1980 |
| M7-1 | PnifH (nt. 1237/1238) | 9.8 kb | 1150 | 900 |
| M8-20 | PnifH (nt. 1234/1235) | 9.8 kb | 790 | 640 |
Numbers in parentheses correspond to the sites of Tn5 insertions relative to the previously published sequences of hvrA (3) and nifH (29), respectively.
The given numbers do not include the size of Tn5.
Cells were cultivated in RCV medium with either 7.5 mM NH4+ or 9.5 mM serine as nitrogen source. β-Galactosidase activities expressed in mU/mg of protein (nmoles of o-nitrophenol × min−1 × mg of protein−1) were determined as described elsewhere (6, 24). Data are means of at least three independent experiments and standard error was less than 20% in each case.
The parental strain was R. capsulatus W49I/R372I [nifA1(Con) nifH- lacZYA].
Tn5-containing EcoRI fragments from these mutant strains were cloned and analyzed by DNA sequencing. Tn5 insertions in mutant strains M7-1 and M8-20 mapped within the nifH promoter region at distances of 52 and 55 bp upstream of the nifH start codon, respectively. It seemed reasonable that insertion of Tn5 at these positions created promoters constitutively expressing the nifH-lacZ fusion. In general, Tn5 insertions result in polar mutations, but nonpolar Tn5 insertions have also been described for the nifR3-R2-R1 (nifR3-ntrBC) gene region (14). Since promoter sequences are not yet well defined in R. capsulatus, these artificial promoters created by fusion of Tn5 and target sequences in the nifH promoter region were not obvious from the DNA sequence.
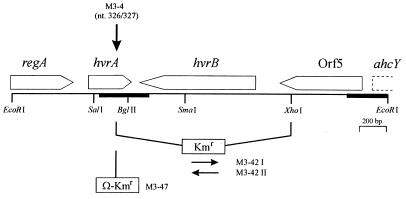
As shown in Fig. 1, the Tn5 insertion in M3-4 was localized within the hvrA gene, which is part of a regulatory gene cluster involved in the oxygen and light regulation of the photosynthetic apparatus (3). To ensure that the phenotype observed in M3-4 did not result from a secondary mutation, we reconstructed the hvrA mutation in the background of the parental strain W49I/R372I. For this purpose, mutant strains M3-47, M3-42I, and M3-42II were constructed (Fig. 1). For the construction of M3-47, the Tn5-containing EcoRI fragment from mutant M3-4 was first cloned into the mobilizable plasmid pWKR102A (4) prior to replacement of the Tn5-internal 3.4-kb HindIII fragment by a 2-kb HindIII fragment carrying a Ω-Km cassette. The resulting hybrid plasmid was introduced into the parental strain W49I/R372I, and marker rescue resulted in M3-47, carrying the Ω-Km cassette at the original site of Tn5 insertion. Mutant strains M3-42I and M3-42II, carrying a deletion encompassing the 3′ end of hvrA, the hvrB gene, and part of Orf5, were constructed in a similar manner. Mutants M3-42I and M3-42II are distinguished by the orientation of the Km resistance gene. Correct homogenotization of interposons was verified in each case by Southern hybridization (data not shown). As found for the original Tn5 mutant, the reconstructed interposon mutant strains M3-47, M3-42I, and M3-42II formed deep blue colonies on X-Gal plates in the presence of ammonium (data not shown), corroborating the conclusion that the mutation of hvrA indeed was responsible for the observed phenotype.
FIG. 1.
Organization of regulatory photosynthesis genes in R. capsulatus and localization of mutations affecting NifA-dependent expression of nif genes. The physical and genetic organization of the photosynthesis gene cluster has been described previously (2, 3). Heavy lines indicate the DNA fragments sequenced in this study. The position of Tn5 insertion in mutant M3-4 [nifA(Con) nifH-lacZ hvrA::Tn5] is marked by a vertical arrow. Numbers in parentheses correspond to the hvrA sequence determined by Buggy et al. (3). Locations of a hvrA interposon insertion (M3-47) and two deletions encompassing hvrA, hvrB, and Orf5 (M3-42I and M3-42II) are indicated. The sizes of the interposon cassettes are not drawn to scale.
Disruption of hvrA leads to nifH expression in the presence of ammonium and oxygen.
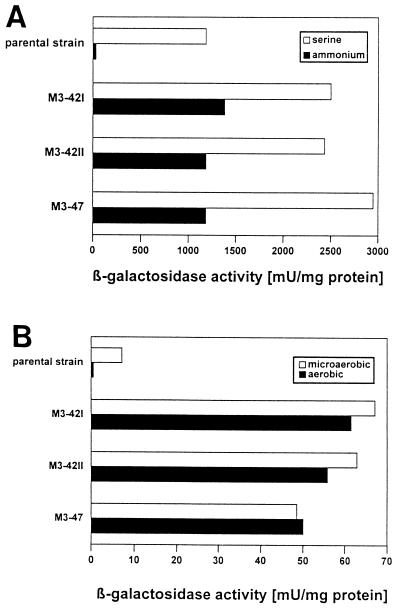
For a more detailed study of the ammonium regulation of nifH-lacZ expression in mutant strains M3-47, M3-42I, and M3-42II, in comparison to the parental strain W49I/R372I [nifA(Con) nifH-lacZYA], all strains were grown phototrophically in the presence of either ammonium or serine as nitrogen source under anaerobic conditions. In contrast to the parental strain, which showed little nifH expression in the presence of ammonium, the mutants strongly expressed the nifH-lacZ fusion under these conditions (Fig. 2A), and the levels of β-galactosidase activities were in the range of the maximum activity obtained with the parental strain under nitrogenase-derepressing conditions (serine as nitrogen source [22]). Moreover, in the presence of serine, the mutant strains showed about two times greater β-galactosidase activities than the parental strain.
FIG. 2.
Influence of hvrA mutations on NifA-dependent expression of nifH-lacZ. Mutant strains M3-42I, M3-42II, and M3-47 contain the hvrA insertion or deletion mutations outlined in the legend to Fig. 1, respectively, in the background of the parental strain W49I/R372I [nifA1(Con) nifH-lacZYA]. β-Galactosidase activities expressed in mU per mg of protein (nmoles of o-nitrophenol × min−1 × mg of protein−1) were determined as described previously (6). (A) Cells were grown under phototrophic conditions in the presence of ammonium (7.5 mM) or serine (9.5 mM) as nitrogen source. Data shown are means of at least three independent experiments measured as triplicates, and the standard error in each case was less than 20%. (B) Cells were grown either aerobically or microaerobically (for details, see text) with serine (9.5 mM) as the nitrogen source. β-Galactosidase activities shown are the averages of two independent experiments measured as triplicates.
Since R. capsulatus NifA belongs to the class of interdomain linker-containing NifA proteins, which were shown to be regulated by oxygen (7), the effect of hvrA mutations on this regulation was studied (Fig. 2B). All strains were cultivated in RCV medium containing serine under either aerobic or microaerobic conditions. Aerobic growth was achieved by growing 20-ml cultures in 500-ml flasks with shaking (150 rpm). For growth under microaerobic conditions, 70-ml cultures were incubated in 100-ml flasks with shaking (150 rpm). After aerobic or microaerobic growth only negligible β-galactosidase activities were detected for the parental strain W49I/R372I. In contrast, all mutant strains expressed the nifH-lacZ fusion in the presence of oxygen. No significant differences in the level of β-galactosidase activities could be observed between high- and low-oxygen cultures (Fig. 2B), whereas strictly anaerobic conditions resulted in a further, about 40-fold increase of nifH expression (Fig. 2A).
No significant differences in ammonium or oxygen control between the hvrA insertion mutant M3-47 and the hvrA-hvrB-Orf5 deletion mutants M3-42I and M3-42II were obvious, indicating that neither hvrB nor Orf5 contributed to the observed phenotype. In conclusion, HvrA is not only involved in low-light activation of the photosynthetic apparatus (3) but also influences the modulation of NifA activity by oxygen and ammonium.
Induction of active nitrogenase is paralleled by an increase in pigment synthesis.
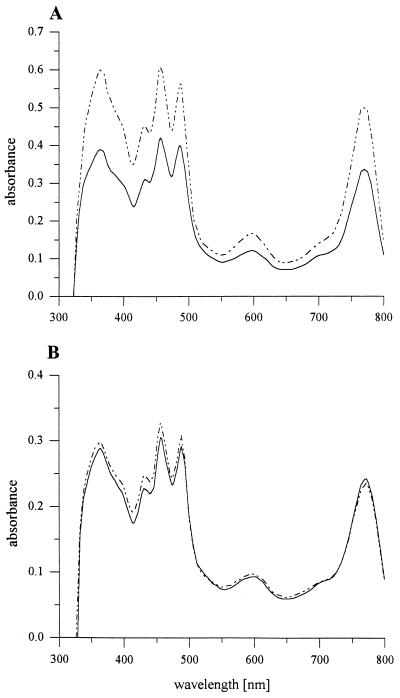
To fulfill the high energy demand imposed by nitrogen fixation, photosynthetic bacteria can use photosynthesis to create ATP and low potential redox equivalents. This energetic link between photosynthesis and nitrogen fixation was reflected by a significantly increased pigment content found in nitrogen-fixing cultures of R. capsulatus wild type B10S compared to ammonium-grown cells (Fig. 3A). In contrast, mutant strain M3-47 [nifA(Con) nifH-lacZ hvrA::Ω-Km] lacked the ability to increase photopigment synthesis under nitrogenase-derepressing conditions (Fig. 3B).
FIG. 3.
Spectral analysis of photopigments of R. capsulatus wild-type B10S (A) and mutant strain M3-47 (B). M3-47 contains the hvrA mutation outlined in the legend to Fig. 1 in the background of strain W49I/R372I [nifA(Con) nifH-lacZYA]. Cells were grown for 48 h to late exponential phase (optical density at 660 nm, 1.0) under nitrogenase-repressing (15 mM ammonium, solid lines) or -derepressing (9.5 mM serine, hatched lines) high-light conditions (approximately 10,000 lx). BChla was extracted from equal amounts of cells, and spectral analysis was carried out as described previously (16).
To determine whether the observed differences in phenotypes were a consequence of hvrA disruption or constitutive expression of nifA or were due to lack of an active, energy-consuming nitrogenase, we measured BChla content and nitrogenase activities of mutant strains affected in each of the corresponding genes (Table 2). Neither a single hvrA mutation (B10S-47) nor a constitutively expressed nifA gene (W49I) affected the increase of BChla content under nitrogenase-derepressing conditions compared to nitrogen-replete conditions. In contrast, R. capsulatus R372I carrying a chromosomal nifH-lacZ fusion showed no increase in BChla content. Since the integration of the nifH-lacZ fusion into the chromosome abolished the expression of nifHDK and thus prevents synthesis of nitrogenase apoproteins, it was tempting to assume that the lack of active nitrogenase was the major reason for the observed phenotype. To test this hypothesis, strains carrying lesions in nifR1 and nifR4 encoding NtrC and ς54, two major regulatory components necessary for nif gene expression, were analyzed. In addition, a nifQ mutant strain, which is able to synthesize active nitrogenase only in the presence of 1 mM Na2MoO4 (25), was tested. As shown in Table 2, the ability to synthesize active nitrogenase was directly paralleled with the increase of BChla content, whereas HvrA did not influence this regulation. These results indicated that the high demand for ATP and/or redox equivalents of nitrogenase, which are necessary to reduce nitrogen to ammonia, are involved in the up-regulation of the photosynthetic apparatus.
TABLE 2.
BChla content and nitrogenase activities of R. capsulatus wild type and mutant strains
| Strain | Relevant genotype | BChla contenta
|
Nitro- genase activityb | |
|---|---|---|---|---|
| NH4+ | Serine | |||
| B10S | Wild type | 13.0 ± 1.4 | 24.0 ± 2.3 | 66 |
| B10S + Moc | Wild type | 10.0 ± 1.7 | 18.0 ± 0.5 | 40 |
| M3-47 | nifA1(Con) nifH-lacZYA hvrA::Ω-Km | 13.4 ± 1.7 | 8.9 ± 1.1 | 0 |
| B10S-47 | hvrA::Ω-Km | 17.1 ± 1.2 | 28.0 ± 1.0 | 84 |
| W49I | nifA1(Con) | 12.0 ± 2.0 | 28.0 ± 1.0 | 64 |
| R372I | nifH-lacZYA | 13.8 ± 0.2 | 15.0 ± 0.5 | 0 |
| W49I/R372I | nifA1(Con) nifH-lacZYA | 9.7 ± 0.7 | 8.0 ± 1.0 | 0 |
| PBK2I | nifR1::Ω-Km | 17.4 ± 0.8 | 15.4 ± 2.2 | 0 |
| KSIIIAII | nifR4::Km | 10.2 ± 0.4 | 9.5 ± 0.1 | 0 |
| R477I | nifQ::Km | 8.3 ± 1.3 | 8.9 ± 0.8 | 2 |
| R477I + Moc | nifQ::Km | 7.9 ± 1.3 | 14.5 ± 1.2 | 56 |
Cells were cultivated in RCV medium supplemented with either NH4+ (7.5 mM) or serine (9.5 mM) as nitrogen source under phototrophic growth conditions (for details, see the legend to Fig. 3). BChla (μg/mg of protein) was quantified as described previously (16). Data given are means of at least four independent assays measured as duplicates.
Nitrogenase activity (nmoles of ethylene × min−1 × mg of protein−1) of serine-grown cells was determined by the acetylene reduction assay (17).
The growth medium was supplemented with 1 mM Na2MoO4.
It is worth noting that the hvrA mutant strain B10S-47 exhibited an increased specific nitrogenase activity compared to the wild type (Table 2). This would be in line with the finding that nifH expression was also significantly increased under nitrogenase-derepressing conditions in hvrA mutant strains (Fig. 2). These results indicate that HvrA might directly or indirectly act as a general negative effector of the nitrogen fixation process.
Conclusions.
Disruption of the hvrA gene allowed NifA-dependent nif gene expression in the presence of ammonium and oxygen, but an hvrA mutation did not affect photosynthetic growth or nitrogenase activity under the high-light conditions used in this study. Therefore, it is unlikely that the observed phenotype was due to altered transport of ammonium or to a reduced photosynthetic capacity resulting in an altered energy charge of the cells. One might speculate that HvrA modulates the activity of a not yet identified signal-transducing cascade involved in the posttranslational regulation of NifA. Since HvrA belongs to the class of histone-like, DNA-binding proteins, it seems more likely that HvrA either affects binding of NifA to its upstream activator sequence or influences DNA bending between the upstream activator sequence and the ς54-RNA polymerase binding site. However, since it is known that histone-like proteins are involved in the regulation of a large number of genes in other bacterial species, it could not be excluded that indirect effects are responsible for the observed phenotypes.
Acknowledgments
This work was supported by financial grants from the Deutsche Forschungsgemeinschaft, Fonds der Chemischen Industrie, and Bundesministerium für Forschung und Technologie.
REFERENCES
- 1.Bauer C E. Regulation of photosynthesis gene expression. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1221–1234. [Google Scholar]
- 2.Buggy J J, Sganga M W, Bauer C E. Nucleotide sequence and characterization of the Rhodobacter capsulatus hvrB gene: HvrB is an activator of S-adenosyl-l-homocysteine hydrolase expression and is a member of the LysR family. J Bacteriol. 1994;176:61–69. doi: 10.1128/jb.176.1.61-69.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buggy J J, Sganga M W, Bauer C E. Characterization of a light-responding trans-activator responsible for differentially controlling reaction center and light-harvesting-I gene expression in Rhodobacter capsulatus. J Bacteriol. 1994;176:6936–6943. doi: 10.1128/jb.176.22.6936-6943.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colonna-Romano S, Arnold W, Schlüter A, Boistard P, Pühler A, Priefer U B. An Fnr-like protein encoded in Rhizobium leguminosarum biovar viciae shows structural and functional homology to Rhizobium meliloti FixK. Mol Gen Genet. 1990;223:138–147. doi: 10.1007/BF00315806. [DOI] [PubMed] [Google Scholar]
- 5.Cullen P J, Foster-Hartnett D, Gabbert K K, Kranz R G. Structure and expression of the alternative sigma factor, RpoN, in Rhodobacter capsulatus; physiological relevance of an autoactivated nifU2-rpoN superoperon. Mol Microbiol. 1994;11:51–65. doi: 10.1111/j.1365-2958.1994.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 6.Elsen S, Richaud P, Colbeau A, Vignais P M. Sequence analysis and interposon mutagenesis of the hupT gene, which encodes a sensor protein involved in repression of hydrogenase synthesis in Rhodobacter capsulatus. J Bacteriol. 1993;175:7404–7412. doi: 10.1128/jb.175.22.7404-7412.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer H-M. Genetic regulation of nitrogen fixation in rhizobia. Microbiol Rev. 1994;58:352–386. doi: 10.1128/mr.58.3.352-386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster-Hartnett D, Cullen P J, Gabbert K K, Kranz R G. Sequence, genetic, and lacZ fusion analyses of a nifR3-ntrB-ntrC operon in Rhodobacter capsulatus. Mol Microbiol. 1993;8:903–914. doi: 10.1111/j.1365-2958.1993.tb01636.x. [DOI] [PubMed] [Google Scholar]
- 9.Foster-Hartnett D, Cullen P J, Monika E M, Kranz R G. A new type of NtrC transcriptional activator. J Bacteriol. 1994;176:6175–6187. doi: 10.1128/jb.176.20.6175-6187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster-Hartnett D, Kranz R G. Analysis of the promoters and upstream sequences of nifA1 and nifA2 in Rhodobacter capsulatus; activation requires ntrC but not rpoN. Mol Microbiol. 1992;6:1049–1060. doi: 10.1111/j.1365-2958.1992.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 11.Foster-Hartnett D, Kranz R G. The Rhodobacter capsulatus glnB gene is regulated by NtrC at tandem rpoN-independent promoters. J Bacteriol. 1994;176:5171–5176. doi: 10.1128/jb.176.16.5171-5176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hübner P, Masepohl B, Klipp W, Bickle T A. nif gene expression studies in Rhodobacter capsulatus: ntrC-independent repression by high ammonium concentrations. Mol Microbiol. 1993;10:123–132. doi: 10.1111/j.1365-2958.1993.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 13.Hübner P, Willison J C, Vignais P M, Bickle T A. Expression of regulatory nif genes in Rhodobacter capsulatus. J Bacteriol. 1991;173:2993–2999. doi: 10.1128/jb.173.9.2993-2999.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones R, Haselkorn R. The DNA sequence of the Rhodobacter capsulatus ntrA, ntrB and ntrC gene analogues required for nitrogen fixation. Mol Gen Genet. 1989;215:507–516. doi: 10.1007/BF00427050. [DOI] [PubMed] [Google Scholar]
- 15.Joshi H M, Tabita F R. A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc Natl Acad Sci USA. 1996;93:14515–14520. doi: 10.1073/pnas.93.25.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kern M, Klemme J-H. Inhibition of bacteriochlorophyll biosynthesis by gabaculin (3-amino,2,3-dihydrobenzoic acid) and presence of an enzyme of the C5-pathway of δ-aminolevulinate synthesis in Chloroflexus aurantiacus. Z Naturforsch. 1989;44c:77–80. [Google Scholar]
- 17.Kern M, Koch H-G, Klemme J-H. EDTA activation of H2 photoproduction by Rhodospirillum rubrum. Appl Microbiol Biotechnol. 1992;37:496–500. [Google Scholar]
- 18.Klipp W, Masepohl B, Pühler A. Identification and mapping of nitrogen fixation genes of Rhodobacter capsulatus: duplication of a nifA-nifB region. J Bacteriol. 1988;170:693–699. doi: 10.1128/jb.170.2.693-699.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kranz R G, Foster-Hartnett D. Transcriptional regulatory cascade of nitrogen-fixation genes in anoxygenic photosynthetic bacteria: oxygen- and nitrogen-responsive factors. Mol Microbiol. 1990;4:1793–1800. doi: 10.1111/j.1365-2958.1990.tb02027.x. [DOI] [PubMed] [Google Scholar]
- 20.Kranz R G, Pace V M, Caldicott I M. Inactivation, sequence, and lacZ fusion analysis of a regulatory locus required for repression of nitrogen fixation genes in Rhodobacter capsulatus. J Bacteriol. 1990;172:53–62. doi: 10.1128/jb.172.1.53-62.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masepohl B, Klipp W. Organization and regulation of genes encoding the molybdenum nitrogenase and the alternative nitrogenase in Rhodobacter capsulatus. Arch Microbiol. 1996;165:80–90. [Google Scholar]
- 22.Masepohl B, Klipp W, Pühler A. Genetic characterization and sequence analysis of the duplicated nifA/nifB gene region of Rhodobacter capsulatus. Mol Gen Genet. 1988;212:27–37. doi: 10.1007/BF00322441. [DOI] [PubMed] [Google Scholar]
- 23.Masepohl B, Krey R, Klipp W. The draTG gene region of Rhodobacter capsulatus is required for post-translational regulation of both the molybdenum and the alternative nitrogenase. J Gen Microbiol. 1993;139:2667–2675. doi: 10.1099/00221287-139-11-2667. [DOI] [PubMed] [Google Scholar]
- 24.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 351–355. [Google Scholar]
- 25.Moreno-Vivian C, Hennecke S, Pühler A, Klipp W. Open reading frame 5 (ORF5), encoding a ferredoxinlike protein, and nifQ are cotranscribed with nifE, nifN, nifX, and ORF4 in Rhodobacter capsulatus. J Bacteriol. 1989;171:2591–2598. doi: 10.1128/jb.171.5.2591-2598.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosley C S, Suzuki J Y, Bauer C E. Identification and molecular genetic characterization of a sensor kinase responsible for coordinately regulating light harvesting and reaction center gene expression in response to anaerobiosis. J Bacteriol. 1994;176:7566–7573. doi: 10.1128/jb.176.24.7566-7573.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian Y, Tabita F R. A global signal transduction system regulates aerobic and anaerobic CO2 fixation in Rhodobacter sphaeroides. J Bacteriol. 1996;178:12–18. doi: 10.1128/jb.178.1.12-18.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sganga M W, Bauer C E. Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell. 1992;68:945–954. doi: 10.1016/0092-8674(92)90037-d. [DOI] [PubMed] [Google Scholar]
- 29.Willison J C, Pierrard J, Hübner P. Sequence and transcript analysis of the nitrogenase structural gene operon (nifHDK) of Rhodobacter capsulatus: evidence for intramolecular processing of nifHDK mRNA. Gene. 1993;133:39–46. doi: 10.1016/0378-1119(93)90222-o. [DOI] [PubMed] [Google Scholar]