Abstract
Objective
To identify the best evidence on the efficacy of pharmacological interventions in reducing fatigue in people with inflammatory rheumatic and musculoskeletal diseases (I-RMDs) and to summarise their safety in the identified studies to inform European Alliance of Associations for Rheumatology recommendations for the management of fatigue in people with I-RMDs.
Methods
Systematic review of adults with I-RMDs conducted according to the Cochrane Handbook. Search strategy ran in Medline, Embase, Cochrane Library, CINAHL Complete, PEDro, OTseeker and PsycINFO. Only randomised controlled trials (RCTs) or controlled clinical trials were eligible. Assessment of risk of bias, data extraction and synthesis performed by two reviewers independently and in duplicate. Data pooled in statistical meta-analyses.
Results
From 4151 records, 455 were selected for full-text review, 99 fulfilled the inclusion criteria and 19 RCTs were included in meta-analyses. Adalimumab was superior to placebo in reducing fatigue at 12 and 52 weeks in rheumatoid arthritis (RA) (n=3 and 2 RCTs; mean difference (MD)= −3.03, p<0.001; MD=−2.25, p=0.03, respectively). Golimumab (n=2 RCTs; 24 weeks: MD=−5.27, p<0.001), baricitinib (n=2 RCTs; 24 weeks: MD=−4.06, p<0.001), sarilumab (n=2 RCTs; 24 weeks: MD=−3.15, p<0.001), tocilizumab (n=3 RCTs; 24 weeks: MD=−3.69, p<0.001) and tofacitinib (n=3 RCTs; 12 weeks: MD=−4.44, p<0.001) were also superior to placebo in reducing fatigue in RA. A dose/effect relationship was observed for sarilumab, tocilizumab and tofacitinib. In spondyloarthritis (excluding psoriatic arthritis), secukinumab was superior to placebo in reducing fatigue at 16 weeks (n=2 RCTs; MD=−4.15, p<0.001), with a dose/effect relationship also observed. The narrative results of the RCTs not included in the meta-analysis indicated that several other pharmacological interventions were efficacious in reducing fatigue, with reassuring safety results.
Conclusions
Several pharmacological interventions are efficacious and generally safe for managing fatigue in people with I-RMDs.
Keywords: Outcome Assessment, Health Care; Inflammation; Autoimmune Diseases; Arthritis; Biological Therapy
WHAT IS ALREADY KNOWN ON THIS TOPIC
Fatigue is one of the most common and debilitating symptoms of inflammatory rheumatic and musculoskeletal diseases (I-RMDs); however, interventions to manage fatigue are complex and challenging to implement.
Evidence regarding the effects of pharmacological interventions on fatigue in all I-RMDs has never been systematically assessed.
WHAT THIS STUDY ADDS
This systematic review reinforces the importance of pharmacological interventions, especially biologics, for fatigue in people with I-RMDs, suggesting that control of inflammatory disease activity coadjuvates the reduction of fatigue levels.
There is a strong evidence that pharmacological interventions, particularly biologics, are efficacious and safe in reducing fatigue. In some cases (eg, sarilumab, tocilizumab, tofacitinib and secukinumab), a dose/effect relationship was observed.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
It can be communicated to patients that several pharmacological interventions are also effective and safe for the management of fatigue in people with I-RMD.
Future research should examine the efficacy and safety of interventions in specific inflammatory RMDs where evidence for fatigue management is still scarce (e.g.eg, systemic sclerosis, idiopathic inflammatory myopathies, and giant cell arteritis).
Introduction
Inflammatory rheumatic and musculoskeletal diseases (I-RMDs) include a set of chronic, inflammatory and autoimmune conditions, such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), axial spondyloarthritis (axSpA), gout, systemic lupus erythematosus (SLE), systemic sclerosis (SSc), Sjogren’s syndrome (SjS), idiopathic inflammatory myopathies (IIM), vasculitis and undifferentiated arthritis, among others. I-RMDs can affect the joints, bones, cartilage, ligaments, tendons, muscles, glands, skin and other organs or systems. They are typically chronic conditions that impose a heavy burden on people’s life and can impair daily self-care and quality of life. I-RMDs often require complex treatment regimens, which, if started early, reduce the risk of long-term structural damage, the need for surgeries and the number of complications.1
Fatigue is one of the most common and can be one of the most debilitating symptoms of I-RMD.2 An international consensus statement has proposed that it should be measured in all RA clinical trials,3 and international delegates at Outcome Measures in Rheumatology eighth meeting endorsed fatigue as an addition to the ‘core set’ of outcome measures for all future RA studies.3 Nevertheless, there is still a significant unmet need in the management of fatigue, which is mainly due to the lack of evidence on the cost-effectiveness of providing fatigue therapies using different treatment modalities, the lack of training available for healthcare professionals to provide evidence-based fatigue therapies,2 4 5 and the complexity of fatigue itself, since it is a multidimensional symptom that varies from patient-to-patient and over time,6 making it more difficult to manage effectively.
There is evidence that pharmacological interventions, including biological therapies, can improve inflammation, disease activity and function in I-RMDs, and fatigue has increasingly been included as a secondary outcome of I-RMDs clinical trials. However, no systematic review (SR) has established the evidence for the pharmacological management of fatigue in all I-RMD, although few SRs are available in specific conditions.7–9
Several European Alliance of Associations for Rheumatology (EULAR) recommendations for the management of people with specific I-RMDs have pointed out the relevance of pharmacological interventions in the management of the condition, including fatigue.10–16 However, these recommendations are either disease-specific or focusing on specific therapeutic groups (eg, certain biological drugs), and lack an integrated view of the overall evidence for fatigue management in the wider context of all I-RMD. The current conceptual models of mechanisms and factors that can cause and maintain fatigue, and how to measure and assess them, are integrative aspects of fatigue management and need a holistic rather than fragmented view if they are to be widely implemented in clinical practice.17
To inform the task force responsible for the 2023 EULAR recommendations for the management of fatigue in people with I-RMD, we performed an SR that aimed to identify and evaluate the evidence on the efficacy of pharmacological interventions in reducing fatigue in people with I-RMD and to describe their safety, if reported, in the included studies.
Methods
This SR was conducted according to the Cochrane Handbook18 and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.19
The steering group of the EULAR task force (BF, EJFS, ED and PMM) established and published the SR protocol in PROSPERO (CRD42021282899). Although this protocol refers to all interventions to manage fatigue, the interventions were subsequently divided into pharmacological and non-pharmacological, and two SRs were generated given the high number of included studies. The SR for non-pharmacological interventions was published elsewhere.
The outlined research questions, as approved by the task force at the first meeting, were:
Which pharmacological interventions are efficacious in reducing fatigue in people with I-RMD?
Which pharmacological interventions are safe in reducing fatigue in people with I-RMD?
These questions were framed and structured according to the EULAR standardised operating procedures20 using the ‘Patients, Intervention, Comparator or Control, Outcome, Type of study’ format, as follows:
Participants
A study was eligible for inclusion if the included participants were adults (aged 18 years or over) with I-RMD, specifically, RA, axSpA, peripheral SpA, PsA, gout, SLE, SSc, SjS, IIM (dermatomyositis, polymyositis, immune-mediated necrotising myopathy, antisynthetase syndrome, inclusion body myositis) and primary systemic vasculitis (large-vessel vasculitis: giant cell arteritis (GCA) (and the related condition polymyalgia rheumatica), Takayasu’s arteritis; medium-vessel vasculitis: polyarteritis nodosa; small-vessel vasculitis limited to the antineutrophilic cytoplasmic antibody (ANCA)-associated vasculitis: granulomatosis with polyangiitis (GPA, previously named Wegener’s granulomatosis), microscopic polyangiitis (MPA) and eosinophilic GPA (previously named Churg-Strauss); and variable-vessel vasculitis: Behçet syndrome, also known as Behçet disease). Only studies in which patients were formally diagnosed with I-RMDs or who satisfied internationally accepted disease classification criteria were included to maximise accuracy.21–24 Studies focusing on information regarding people with other concomitant diseases were summarised separately and by subgroups, whenever possible.
Interventions
Regarding the eligible interventions, all pharmacological interventions were included. Pharmacological interventions were classified as medicinal products in accordance with the EU Directive 2001/83/EEC (EU 2001),25 which states: ‘any substance or combination of substances which may be used in or administered to human beings either with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis’.25
Comparator or control
The comparator was placebo or usual care (standard care).
Outcomes
Regarding outcomes, the core concept was fatigue. Fatigue is a complex, multifaceted phenomenon. Importantly, most people have experienced fatigue during their everyday life, but qualitative research suggests differences between fatigue associated with chronic diseases and ‘usual’ or premorbid fatigue. The most distinguishing features of fatigue associated with chronic diseases include the perception of fatigue as having no obvious ‘explanation’, a lack of improvement with rest, variability in severity, unpredictability and the experience of profound or overwhelming fatigue.26 In that sense, we accepted self-reported fatigue scores using quantitative and validated measures, such as Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F),27 Rheumatoid Arthritis Impact of Disease-Fatigue,28 29 Fatigue-Visual Analogue Scale (VAS),30 36-Item Short Form Survey (SF-36) vitality scale,31 the Multidimensional Assessment of Fatigue,32 Profile of Mood States-subscale fatigue,33 Checklist Individual Strength,34 Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ),35 36 BRAF Numerical Rating Scales for severity, effect and coping,35 36 among others.
Type of study
Only SRs and randomised controlled trials (RCTs) or controlled clinical trials were eligible because they are considered the most robust study designs and represent the strongest evidence.37 The studies integrating SRs were extracted for joint analysis with the remaining primary studies. SRs were not analysed.
Regarding the context, there were no constraints.
Search strategy and study selection
A search strategy was run by one of the authors (EJFS) in Medline through PubMed, Embase, Cochrane Library, CINAHL Complete, PEDro, OTseeker and PsycINFO. The start date was the date of inception of the database, and the end date was 27 December 2021. Studies published in English, French, Portuguese, Spanish and Turkish language, with no restriction on the publication date, were considered for inclusion. Details on complete search strategies are provided in online supplemental material S1.
rmdopen-2023-003349supp001.pdf (88.6KB, pdf)
All identified citations were uploaded into an EndNote V.X9 (Clarivate Analytics, Pennsylvania, USA) library and the duplicates removed. Titles and abstracts were screened by two independent reviewers (BF and EJFS) to assess eligibility criteria. The full articles were retrieved for all studies that met or had insufficient information to assess the inclusion criteria, and two reviewers (BF and EJFS) independently examined them in detail. Any disagreements between the reviewers were resolved through discussion or adjudication by a third reviewer (PMM). The study selection was performed using Rayyan.
Risk of bias (quality) assessment
Two reviewers (BF and EJFS) assessed the risk of bias in each included study using the Cochrane Collaboration’s tool for RCTs.38 Any disagreements between the reviewers were resolved through discussion or adjudication by a third reviewer (PMM).
Data extraction and synthesis
Data were extracted from the selected reports by the same two independent reviewers (BF and EJFS), and disagreements were discussed until consensus was achieved, or with adjudication by the third reviewer (PMM), whenever necessary. Authors of papers were contacted to request missing or additional data, where required.
Studies were pooled for statistical meta-analysis using Review Manager V.5.2.8. and SPSS Statistics, V.28 (IBM), if the needed statistics were available. Effect sizes were expressed as mean differences (MD) or final postintervention standardised MDs (SMD), and their 95% CIs were calculated. MD is the difference between effect estimates for intervention and control on a specific scale. Because pooling of the MD from individual RCTs is done after weighting the values for precision, this pooled MD is also known as the weighted MD. The selection of SMD was determined primarily because all studies reported the outcome using different scales/metrics.18 We imputed SD where necessary according to sections 6.5.2.2 and 6.5.2.3 of the Cochrane Handbook.18 Heterogeneity was assessed statistically using the standard χ2 and I² tests. For a value of I² equal to 0%, we assume no heterogeneity between studies (homogeneity), around 25% low heterogeneity, around 50% moderate heterogeneity and around or greater than 75% high heterogeneity.39 Statistical analyses were performed using random effects models only in the presence of moderate to high heterogeneity (I²>50%) and, in their absence, fixed effect models were used instead.40 41 Where statistical pooling was not possible, the findings were presented in narrative form, including tables and figures, where appropriate. Subgroup analyses were conducted if sufficient data was provided, with subanalyses being based on different diseases categories and pharmacological doses. Sensitivity analyses were conducted to test decisions made. At last, the level of evidence was assigned for each intervention using the 2011 Oxford Centre for Evidence Based Medicine Levels of Evidence.37
Results
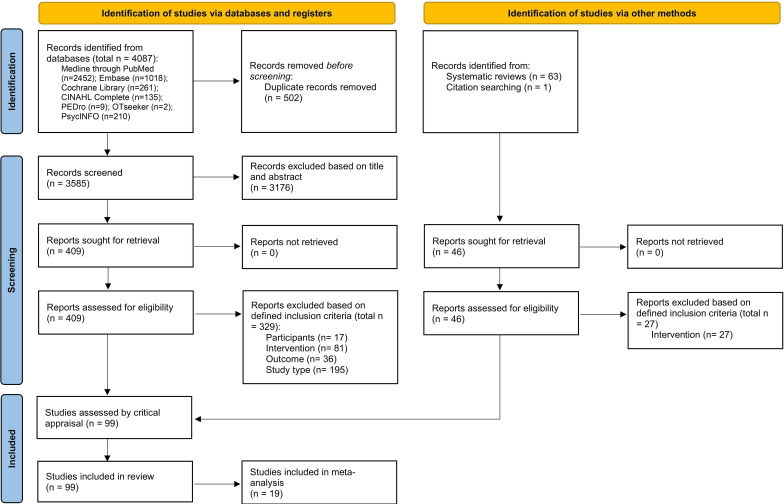
Out of a total of 4151 records (3585 non-duplicate records, 502 duplicate records, 63 SRs and 1 record obtained by citation searching), 455 were selected for full-text review, and 99 studies fulfilled the inclusion criteria and were included in this SR. Of these, 19 RCTs were included in the meta-analysis. There was no need to contact the authors of the papers to request additional information. The results of the searches are shown in a flow diagram (figure 1).
Figure 1.
Flow chart of the study selection and inclusion process.
Methodological quality
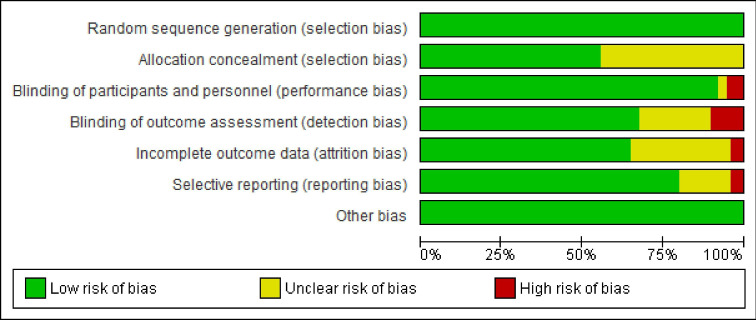
The critical appraisal of results for each study are summarised in figure 2 and online supplemental file 2. There was agreement among the reviewers to include all the studies that were appraised. In general, the RCTs included were of high quality, corresponding to level 1 of evidence according to the 2011 Oxford Centre for Evidence Based Medicine Levels of Evidence.37 All RCTs complied with a random sequence generation. Regarding allocation concealment, there was an unclear risk of bias in almost 50% of the studies but this was probably due to a reporting bias or/and poor reporting. On several occasions, the authors implied that they complied with the allocation concealment without referring to it specifically and without reporting the description of the procedure. There were residual issues with participant, personnel, and outcome assessment blinding, but overall, these attributes were met, which is expected given that these were RCTs of pharmacological interventions.
Figure 2.
Risk of bias summary graph for included clinical trials. Review authors’ judgements about each risk of bias item presented as percentages across all included studies using the Cochrane RoB tool.
rmdopen-2023-003349supp002.pdf (118.1KB, pdf)
Characteristics of included studies and interventions
Study characteristics are detailed in online supplemental file 3. Pharmacological interventions where fatigue was included as an outcome were tested in the following I-RMD among the 99 RCTs: RA (n=50),42–91 SpA (excluding PsA) (n=13),92–104 SjS (n=15),105–119 PsA (n=10),120–129 SLE (n=8),130–137 SSc (n=1),138 IIM (n=1)139 and GCA (n=1).140
rmdopen-2023-003349supp003.pdf (869.2KB, pdf)
The summary of findings integrating all included RCTs, the interventions tested per I-RMD and their impact on outcome is presented in table 1. Overall, we found pharmacological interventions to be efficacious in reducing fatigue. The most commonly tested pharmacological interventions were biologicals (82%) and the most common comparator was placebo (95%). The most used time points for outcome assessment were 12, 16, 24, 36, 48 and 52 weeks.
Table 1.
Summary of findings
| Disease | Intervention | Drug class/type | No of RCTs | Impact on outcome | References |
| Rheumatoid arthritis | Prednisone | Glucocorticoids | 1 | Reduced fatigue | 42 |
| 1 | No difference | 44 | |||
| Tocilizumab | bDMARD | 4 | Reduced fatigue | 45–48 | |
| Etanercept | bDMARD | 3 | Reduced fatigue | 43 49 50 | |
| Certolizumab pegol | bDMARD | 4 | Reduced fatigue | 51–54 | |
| Adalimumab | bDMARD | 4 | Reduced fatigue | 55–58 | |
| Rituximab | bDMARD | 5 | Reduced fatigue | 59–63 | |
| Canakinumab | bDMARD | 1 | Unclear | 64 | |
| Tofacitinib | tsDMARD | 6 | Reduced fatigue | 65–69 91 | |
| 1 | Unclear | 70 | |||
| Abatacept | bDMARD | 4 | Reduced fatigue | 71–74 | |
| Golimumab | bDMARD | 4 | Reduced fatigue | 75–78 | |
| Anti-TNF | bDMARD | 1 | Reduced fatigue | 79 | |
| Filgotinib | tsDMARD | 2 | Reduced fatigue | 80 81 | |
| Sarilumab | bDMARD | 2 | Reduced fatigue | 82 83 | |
| 1 | No difference | 84 | |||
| Baricitinib | tsDMARD | 5 | Reduced fatigue | 85–89 | |
| Upadacitinib | tsDMARD | 1 | Reduced fatigue | 90 | |
| Systemic lupus erythematosus | Dehydroepiandrosterone | Other | 1 | No difference | 130 |
| Hydroxychloroquine | csDMARD | 1 | No difference | 131 | |
| Abatacept | bDMARD | 1 | Reduced fatigue | 132 | |
| Belimumab | bDMARD | 2 | Reduced fatigue | 133 134 | |
| Blisibimod | bDMARD | 1 | Reduced fatigue | 135 | |
| Tabalumab | bDMARD | 1 | No difference | 136 | |
| N-Acetylcysteine | Other | 1 | Reduced fatigue | 137 | |
| Psoriatic arthritis | Certolizumab Pegol | bDMARD | 1 | Reduced fatigue | 120 |
| Adalimumab | bDMARD | 2 | Reduced fatigue | 121 122 | |
| Secukinumab | bDMARD | 1 | Reduced fatigue | 123 | |
| Ustekinumab | bDMARD | 1 | Reduced fatigue | 124 | |
| Infliximab | bDMARD | 1 | Reduced fatigue | 125 | |
| Upadacitinib | tsDMARD | 2 | Reduced fatigue | 126 127 | |
| Tofacitinib | tsDMARD | 2 | Reduced fatigue | 128 129 | |
| Sjogren’s syndrome | Rituximab | bDMARD | 3 | No difference | 106–108 |
| 1 | Reduced fatigue | 105 | |||
| Infliximab | bDMARD | 1 | No difference | 109 | |
| Dehydroepiandrosterone | Other | 2 | No difference | 110 111 | |
| Gammalinolenic acid | Other | 1 | No difference | 112 | |
| Doxycycline | Other | 1 | Increased fatigue | 113 | |
| RSLV-132 | Other | 1 | Reduced fatigue | 114 | |
| Interleukin-1 receptor antagonist | bDMARD | 1 | No difference | 115 | |
| Total glucosides of peony | Other | 1 | Reduced fatigue | 116 | |
| Hydroxychloroquine | csDMARD | 2 | No difference | 117 118 | |
| Ianalumab | bDMARD | 1 | Reduced fatigue | 119 | |
| Spondyloarthritis (excluding psoriatic arthritis) |
Etanercept | bDMARD | 3 | Reduced fatigue | 92 93 95 |
| 2 | No difference | 94 96 | |||
| Certolizumab pegol | bDMARD | 1 | Reduced fatigue | 97 | |
| Adalimumab | bDMARD | 1 | Reduced fatigue | 98 | |
| Secukinumab | bDMARD | 3 | Reduced fatigue | 99–101 | |
| Probiotic therapy | Other | 1 | No difference | 102 | |
| Ixekizumab | bDMARD | 1 | No difference | 103 | |
| Infliximab | bDMARD | 1 | Reduced fatigue | 104 | |
| Systemic sclerosis | Tocilizumab | bDMARD | 1 | No difference | 138 |
| Inflammatory myopathies | Creatine supplements | Other | 1 | No difference | 139 |
| Giant cell arteritis | Tocilizumab | bDMARD | 1 | Reduced fatigue | 140 |
Green colour indicates ‘reduced fatigue’, yellow indicates ‘no difference’ and red indicates ‘increased fatigue’ when compared with the control group.
Reduced fatigue—there was a statistically significant decrease in the fatigue outcome between study arms in the original studies or/and the difference has moderate magnitude; No difference—there was no statistically significant difference in the fatigue outcome between study arms in the original studies; Unclear—there was a statistically significant difference in the fatigue outcome between study arms in the original studies and the difference has a weak magnitude and the results are from individual RCTs; Increased fatigue—there is a statistically significant increase in the fatigue outcome between study arms in the original studies.
bDMARD, biological disease-modifying antirheumatic drug; csDMARD, conventional synthetic DMARD; RCT, randomised controlled trial; tsDMARD, targeted synthetic DMARD.
Meta-analysis and narrative synthesis
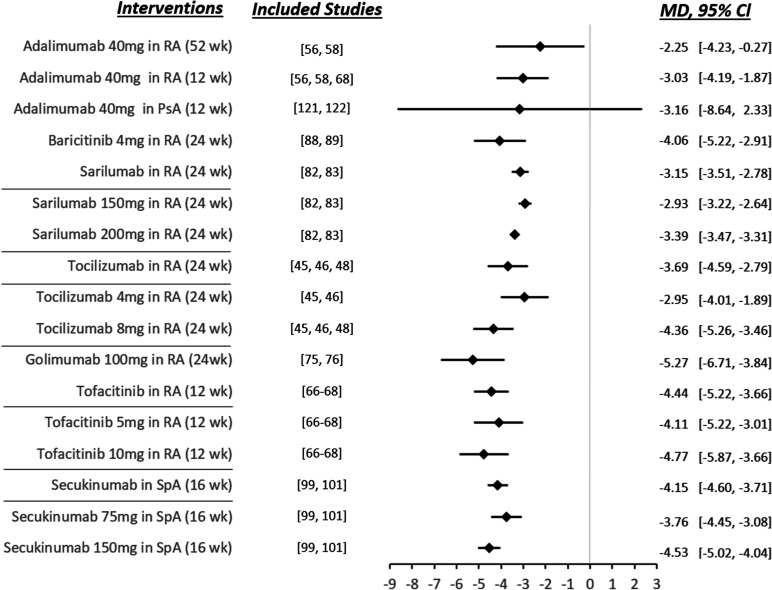
Meta-analyses of the results are detailed in online supplemental file 4. The summary of the meta-analyses was grouped into a single forest plot (figure 3).
Figure 3.
Meta-analyses summary. The values shown are mean differences in overall fatigue levels and their 95% CIs from the comparison of the identified pharmacological intervention versus placebo. A negative value indicates a reduction in fatigue levels. MD, mean difference; RA, rheumatoid arthritis; SpA, spondyloarthritis.
rmdopen-2023-003349supp004.pdf (162KB, pdf)
Fatigue was a secondary outcome in approximately two-thirds of studies, as disease-specific treatment response measures of drug efficacy were commonly the primary outcome in the RCTs (with results being consistent between studies irrespective of fatigue being the primary or secondary endpoint).
The main fatigue scale/instrument used in the studies integrated in the meta-analysis comparisons was the FACIT-F. The only exception was the comparison between Etanercept 50 mg and placebo in SpA (excluding PsA) at 12 weeks, which used the Multidimensional Fatigue Inventory (MFI) and the Fatigue Severity Scale (FSS).
The meta-analysis showed that adalimumab was superior to placebo in reducing fatigue at 52 and 12 weeks in RA (MD=−2.25, 95% CI −4.23 to −0.27, p=0.03; MD=−3.03, 95% CI −4.19 to −1.87, p<0.001, respectively). Adalimumab was not superior in PsA (MD=−3.16, 95% CI −8.64 to 2.33, p=0.26). Golimumab (MD=−5.27, 95% CI −6.71 to −3.84, p<0.001, at 24 weeks), baricitinib (MD=−4.06, 95% CI −5.22 to −2.91, p<0.001, at 24 weeks), sarilumab (MD=−3.15, 95% CI −3.51 to −2.78, p<0.001, at 24 weeks), tocilizumab (MD=−3.69, 95% CI −4.59 to −2.79, p<0.001, at 24 weeks) and tofacitinib (MD=−4.44, 95% CI −5.22 to −3.66, p<0.001, at 12 weeks) were also superior to placebo in reducing fatigue in RA. Subgroup meta-analyses revealed a dose/effect relationship for sarilumab, tocilizumab and tofacitinib, that is, the higher the dose, the greater the reduction in fatigue measures. In SpA (excluding PsA), secukinumab was superior to placebo in reducing fatigue at 16 weeks (MD=−4.15, 95% CI −4.60 to −3.71, p<0.001), with a dose/effect relationship also being observed. Finally, in SpA (excluding PsA), etanercept was not superior to placebo in reducing fatigue at 12 weeks (SMD=3.07, 95% CI −3.86 to 10.01, p=0.39).
It should be noted that in 6 of the meta-analyses performed there was no heterogeneity (I2=0%) (adalimumab 40 mg in RA at 52 weeks and at 12 weeks, baricitinib 4 mg in RA at 24 weeks, tocilizumab 4 and 8 mg in RA at 24 weeks, golimumab 100 mg in RA at 24 weeks, and tofacitinib 5 and 10 mg in RA at 12 weeks). In 4 of the meta-analyses, we found high heterogeneity (I2>75%) (adalimumab 40 mg in PsA at 12 weeks, sarilumab 150 and 200 mg in RA at 24 weeks, secukinumab 75 and 150 mg in SpA (excluding PsA) at 16 weeks, and etanercept 50 mg in SpA (excluding PsA) at 12 weeks).
As mentioned above, the narrative results of the RCTs not included in the meta-analysis were globally integrated in table 1 and showed that many of the investigated pharmacological interventions, including other biologics not included in the meta-analysis, were efficacious in reducing fatigue. However, in some specific I-RMDs, the evidence was still limited and did not allow us to draw strong conclusions (eg, SSc, IIM and GCA).
Regarding the safety of pharmacological interventions, most studies reported that they were well tolerated or similar to placebo.44 50 56 57 59 60 62 64 65 71 73 77 78 80 82 86 88 91–94 97 98 101 102 105 108 111 112 114 115 117–120 122–124 131–133 135 136 138 139 A few studies reported more adverse events in the intervention arm,45 51 106 107 109 116 125 130 137 and a minority of studies in the placebo arm.46 121 However, detailed safety information was not available in 42 RCTs.42 43 47–49 52–55 58 61 63 66–70 72 74–76 79 81 83 85 87 89 90 95 96 99 100 103 104 113 126–129 134 140
Discussion
This SR shows strong evidence that pharmacological interventions, especially biologics, are efficacious in reducing fatigue in people with I-RMDs, suggesting that control of inflammatory disease activity coadjuvates the reduction of fatigue levels. This trend was observed after evaluating several specific I-RMDs, namely RA (n=49),42–91 SpA (excluding PsA) (n=13),92–104 SjS (n=15),105–119 PsA (n=10),120–129 SLE (n=8),130–137 SSc (n=1),138 IIM (n=1)139 and GCA (n=1).140
Safety results were reassuring and in line with known safety profiles and summaries of product characteristics of the respective pharmacological intervention. However, safety information was often lacking in the retrieved studies and mentioning safety in detail in future fatigue intervention studies is advisable.
Regarding the quality of the included studies, most of them were of high quality, as mentioned previously, corresponding to level 1 of evidence according to the 2011 Oxford Centre for Evidence Based Medicine Levels of Evidence.37 Among bias items, high risk was more frequently present in the 'blinding' items (participants, personnel and outcome assessment), however, this was only observed in 10 of the 99 included RCTs.
Among the 99 studies that fulfilled inclusion criteria, fatigue was evaluated with one scale in 92 studies and two scales in 7. The assessment scale of fatigue was the FACIT-F in more than half of the studies (57 studies), followed by Fatigue-VAS (24 studies), MFI (7 studies), Fatigue Assessment Scale (FAS; 5 studies), Bath Ankylosing Spondylitis Disease Activity Index-fatigue item (5 studies), FSS (1 study), SF-36 fatigue item (1 study), Chalder Fatigue score (1 study), Brief fatigue index (BFI; 1 study), BRAF-MDQ (1 study), asking fatigue for presence and severity compared with previous visit (1 study), Health Assessment Questionnaire-fatigue item (1 study) and Profile of Fatigue (1 study). Although the lack of a gold standard measurement for fatigue and the evaluation of fatigue with 13 different measurements represent study limitations, causing difficulties in meta-analysis integration, the use of the FACIT-F in most of the studies is reassuring in terms of overall data assessment. In the future, agreement on a standardised scale for the assessment of fatigue will allow easier data pooling and better generalisability of results.
Regarding the limitations of this SR, it should be noted that the safety analysis of pharmacological interventions was restricted to RCTs and did not encompass observational studies, which affects the quality of the safety component of the SR. The task force made this decision because the safety profile of the studied drugs is already well documented in other disease-specific safety SRs.141 142 Including observational studies within the scope of the current task force would have required the inclusion of a substantially larger number of articles, resulting in duplicated data without substantial additional value. Another limitation is that we could only perform a meta-analysis on 19 out of the 99 included RCTs, primarily due to the insufficient number of studies available for specific comparisons in the meta-analysis; in a few remaining cases, the necessary data for pooling was unavailable. Finally, the small number of studies and the small number of participants in some studies may have resulted in lack of statistical power and non-significant/wider CIs despite potentially clinically relevant effect sizes.
In conclusion, in this review, we collected the existing evidence on the efficacy of pharmacological interventions in reducing fatigue in people I-RMDs, with fatigue having been evaluated either as a primary or secondary outcome measure. This has important clinical implications, because it is evidence-based information that can be communicated to patients and used to inform patient management. To reduce clinical heterogeneity, each I-RMD was evaluated separately and grouped according to pharmacological intervention. This SR provides robust evidence on the efficacy and safety of several pharmacological interventions in the majority of I-RMDs. However, in some specific I-RMDs (eg, SSc, IIM, and GCA), the evidence is still limited, and future well-designed pharmacological intervention studies should investigate their role in managing fatigue in these conditions.
Acknowledgments
This study was previously presented at EULAR 2023 - Annual European Congress of Rheumatology, and its abstract published in: BF, ES, ED. POS1087 Efficacy of pharmacological interventions: a systematic review informing the 2023 EULAR recommendations for the management of fatigue in people with inflammatory rheumatic and musculoskeletal diseases. Annals of the rheumatic diseases. 2023;82:867-867.
Footnotes
Twitter: @EduardoJFSantos, @pedrommcmachado
BF and EJFS contributed equally.
Collaborators: The EULAR taskforce on Recommendations for the management of fatigue in people with inflammatory rheumatic diseases: Anna Molto (France) anna.molto@aphp.fr Bayram Farisogullari (Turkey) bayramfarisogullari@gmail.com Caroline Feldthusen (Sweden) caroline.feldthusen@gu.se Claire Harris (UK) claire.harris10@nhs.net Corinna Elling-Audersch (Germany) ellingiaudersch@aol.com Deirdre Conolly (Ireland) connoldm@tcd.ie Eduardo Santos (Portugal) ejf.santos87@gmail.com Elena Elefante (Italy) elena.elefante87@gmail.com Emma Dures (UK) emma2.dures@uwe.ac.uk Fernando Estévez-López (USA, Spain) fer@estevez-lopez.com Ilaria Bini (Italy) dottssa.ilariabini.psi@gmail.com Jette Primdahl (Denmark) jprimdahl@danskgigthospital.dk Kirsten Hoeper (Germany) hoeper.kirsten@mh-hannover.de Marie Urban (UK) urban.zing.101@hotmail.co.uk Mart van de Laar (Netherlands) m.a.f.j.vandelaar@utwente.nl Marta Redondo (Spain) mredondo@ucjc.edu Pedro Machado (UK) p.machado@ucl.ac.uk Peter Böhm (Germany) peboehm@gmx.de Raj Amarnani (UK) raj.amarnani@nhs.net Rhys Hayward (UK) rhys.hayward@nhs.net Rinie Geenen (Netherlands) r.geenen@uu.nl Simona Rednic (Romania) srednic.umfcluj@gmail.com Susanne Pettersson (Sweden) susanne.pettersson@sll.se Tanja Thomsen (Denmark) tanja.thomsen@regionh.dk Till Uhlig (Norway) tillmann.uhlig@medisin.uio.no Valentin Ritschl (Austria) valentin.ritschl@meduniwien.ac.at.
Contributors: All authors are members of the EULAR’s task force for the development of 2023 EULAR Recommendations for the management of fatigue in people with inflammatory rheumatic diseases. EJFS and BF were the fellows. ED was the convenor. PMM was the methodologist and is responsible for the overall content as guarantor. All authors have contributed to the work, read and finally approved the manuscript for submission.
Funding: This study was funded by the European Alliance of Associations for Rheumatology-EULAR (Project HPR052: EULAR points to consider/recommendations for the management of fatigue inpeople with inflammatory rheumatic diseases).
Competing interests: PMM has received consulting/speaker's fees from Abbvie, BMS, Celgene, Eli Lilly, Galapagos,
Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche and UCB, all unrelated to this manuscript, and is supported by the National Institute for Health Research (NIHR), University College London Hospitals (UCLH), Biomedical Research Centre (BRC).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: The EULAR taskforce on Recommendations for the management of fatigue in people withinflammatory rheumatic diseases, Anna Molto, Bayram Farisogullari, Caroline Feldthusen, Claire Harris, Corinna Elling-Audersch, Deirdre Conolly, Eduardo Santos, Elena Elefante, Emma Dures, Fernando Estévez-López, Ilaria Bini, Jette Primdahl, Kirsten Hoeper, Marie Urban, Mart van de Laar, Marta Redondo, Pedro Machado, Peter Böhm, Raj Amarnani, Rhys Hayward, Rinie Geenen, Simona Rednic, Susanne Pettersson, Tanja Thomsen, Till Uhlig, and Valentin Ritschl
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.WHO Scientific Group on the Burden of Musculoskeletal Conditions at the Start of the New Millennium . The burden of musculoskeletal conditions at the start of the new millenium: report of a WHO scientific group. Geneve: World Health Organization; 2003. [Google Scholar]
- 2.Santos EJF, Duarte C, da Silva JAP, et al. The impact of fatigue in rheumatoid arthritis and the challenges of its assessment. Rheumatology (Oxford) 2019;58:v3–9. 10.1093/rheumatology/kez351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirwan JR, Minnock P, Adebajo A, et al. Patient perspective: fatigue as a recommended patient centered outcome measure in rheumatoid arthritis. J Rheumatol 2007;34:1174–7. [PubMed] [Google Scholar]
- 4.Hewlett S, Cockshott Z, Byron M, et al. Patients' perceptions of fatigue in rheumatoid arthritis: overwhelming, uncontrollable, ignored. Arthritis Rheum 2005;53:697–702. 10.1002/art.21450 [DOI] [PubMed] [Google Scholar]
- 5.Dures E, Rooke C, Hammond A, et al. Training and delivery of a novel fatigue intervention: a qualitative study of rheumatology health-care professionals' experiences. Rheumatol Adv Pract 2019;3:rkz032. 10.1093/rap/rkz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewlett S, Chalder T, Choy E, et al. Fatigue in rheumatoid arthritis: time for a conceptual model. Rheumatology 2011;50:1004–6. 10.1093/rheumatology/keq282 [DOI] [PubMed] [Google Scholar]
- 7.Almeida C, Choy EHS, Hewlett S, et al. Biologic interventions for fatigue in rheumatoid arthritis. Cochrane Database Syst Rev 2016;2016:CD008334. 10.1002/14651858.CD008334.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choy EH. Effect of Biologics and targeted synthetic disease-modifying anti-rheumatic drugs on fatigue in rheumatoid arthritis. Rheumatology (Oxford) 2019;58:v51–5. 10.1093/rheumatology/kez389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reygaerts T, Mitrovic S, Fautrel B, et al. Effect of Biologics on fatigue in Psoriatic arthritis: a systematic literature review with meta-analysis. Joint Bone Spine 2018;85:405–10. 10.1016/j.jbspin.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 10.Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3–15. 10.1136/annrheumdis-2015-207524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy G, Roodenrijs NMT, Welsing PMJ, et al. EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis 2022;81:20–33. 10.1136/annrheumdis-2021-220973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of Psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos-Casals M, Brito-Zerón P, Bombardieri S, et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann Rheum Dis 2020;79:3–18.:3. 10.1136/annrheumdis-2019-216114 [DOI] [PubMed] [Google Scholar]
- 14.Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. 10.1136/annrheumdis-2019-215089 [DOI] [PubMed] [Google Scholar]
- 15.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying Antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 16.Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017;76:1327–39. 10.1136/annrheumdis-2016-209909 [DOI] [PubMed] [Google Scholar]
- 17.Dures E, Cramp F, Hackett K, et al. Fatigue in inflammatory arthritis. Best Practice & Research Clinical Rheumatology 2020;34:101526. 10.1016/j.berh.2020.101526 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for systematic reviews of interventions version 6.0. 2019. 10.1002/9781119536604 [DOI]
- 19.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:71.:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Heijde D, Aletaha D, Carmona L, et al. Update of the EULAR standardised operating procedures for EULAR-endorsed recommendations. Ann Rheum Dis 2015;74:8–13. 10.1136/annrheumdis-2014-206350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 22.Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: an American college of rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 23.Taylor W, Gladman D, Helliwell P, et al. Classification criteria for Psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73. 10.1002/art.21972 [DOI] [PubMed] [Google Scholar]
- 24.Rudwaleit M, van der Heijde D, Landewe R, et al. The development of assessment of Spondyloarthritis International society classification criteria for axial Spondyloarthritis (part II): validation and final selection. Annals of the Rheumatic Diseases 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 25.Union E. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the community code relating to medicinal products for human use. Official Journal of the European Communities No L-311/67 of 28 November 2001 2001. [Google Scholar]
- 26.Davies K, Dures E, Ng W-F. Fatigue in inflammatory rheumatic diseases: Current knowledge and areas for future research. Nat Rev Rheumatol 2021;17:651–64. 10.1038/s41584-021-00692-1 [DOI] [PubMed] [Google Scholar]
- 27.Cella D, Lai J-S, Chang C-H, et al. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer 2002;94:528–38. 10.1002/cncr.10245 [DOI] [PubMed] [Google Scholar]
- 28.Gossec L, Dougados M, Rincheval N, et al. Elaboration of the preliminary rheumatoid arthritis impact of disease (RAID) score: a EULAR initiative. Ann Rheum Dis 2009;68:1680–5. 10.1136/ard.2008.100271 [DOI] [PubMed] [Google Scholar]
- 29.Gossec L, Paternotte S, Aanerud GJ, et al. Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: a EULAR initiative. Ann Rheum Dis 2011;70:935–42. 10.1136/ard.2010.142901 [DOI] [PubMed] [Google Scholar]
- 30.Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol 1996;23:1407–17. [PubMed] [Google Scholar]
- 31.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Medical Care 1992;30:473–83. 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 32.Belza BL, Henke CJ, Yelin EH, et al. Correlates of fatigue in older adults with rheumatoid arthritis. Nurs Res 1993;42:93–9. [PubMed] [Google Scholar]
- 33.Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. J Rheumatol 1995;22:639–43. [PubMed] [Google Scholar]
- 34.Vercoulen JH, Swanink CM, Fennis JF, et al. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res 1994;38:383–92. 10.1016/0022-3999(94)90099-x [DOI] [PubMed] [Google Scholar]
- 35.Nicklin J, Cramp F, Kirwan J, et al. Collaboration with patients in the design of patient-reported outcome measures: capturing the experience of fatigue in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62:1552–8. 10.1002/acr.20264 [DOI] [PubMed] [Google Scholar]
- 36.Nicklin J, Cramp F, Kirwan J, et al. Measuring fatigue in rheumatoid arthritis: a cross-sectional study to evaluate the Bristol rheumatoid arthritis fatigue multi-dimensional questionnaire, visual analog scales, and numerical rating scales. Arthritis Care Res (Hoboken) 2010;62:1559–68. 10.1002/acr.20282 [DOI] [PubMed] [Google Scholar]
- 37.OCEBM Levels of Evidence Working Group . The Oxford levels of evidence. Oxford centre for evidence-based medicine (updated in 2012). 2009. Available: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
- 38.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos E, Cardoso D, Apóstolo J. How to measure and explore heterogeneity in a meta-analysis: key methodological strategies. Revista de Enfermagem Referência 2022;6:e21077. [Google Scholar]
- 40.Tufanaru C, Munn Z, Stephenson M, et al. Fixed or random effects meta-analysis? common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc 2015;13:196–207. 10.1097/XEB.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 41.Tufanaru C, Munn Z, Aromataris E, et al. Chapter 3: systematic reviews of effectiveness. In: Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer’s Manual. The Joanna Briggs Institute, 2017. [Google Scholar]
- 42.Alten R, Grahn A, Holt RJ, et al. Delayed-release prednisone improves fatigue and health-related quality of life: findings from the CAPRA-2 double-blind randomised study in rheumatoid arthritis. RMD Open 2015;1:e000134. 10.1136/rmdopen-2015-000134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreland LW, Genovese MC, Sato R, et al. Effect of Etanercept on fatigue in patients with recent or established rheumatoid arthritis. Arthritis Rheum 2006;55:287–93. 10.1002/art.21838 [DOI] [PubMed] [Google Scholar]
- 44.Pincus T, Swearingen CJ, Luta G, et al. Efficacy of prednisone 1-4 mg/day in patients with rheumatoid arthritis: a randomised, double-blind, placebo controlled withdrawal clinical trial. Ann Rheum Dis 2009;68:1715–20. 10.1136/ard.2008.095539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of Interleukin-6 receptor inhibition with Tocilizumab in patients with rheumatoid arthritis. Lancet 2008;371:99. 10.1016/S0140-6736(08)60453-5 [DOI] [PubMed] [Google Scholar]
- 46.Strand V, Burmester GR, Ogale S, et al. Improvements in health-related quality of life after treatment with Tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors. Rheumatology (Oxford) 2012;51:1860–9. 10.1093/rheumatology/kes131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strand V, Michalska M, Birchwood C, et al. Impact of Tocilizumab administered intravenously or subcutaneously on patient-reported quality-of-life outcomes in patients with rheumatoid arthritis. RMD Open 2018;4:e000602. 10.1136/rmdopen-2017-000602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teitsma XM, Jacobs JWG, Welsing PMJ, et al. Patient-reported outcomes in newly diagnosed early rheumatoid arthritis patients treated to target with a Tocilizumab- or methotrexate-based strategy. Rheumatology (Oxford) 2017;56:2179–89. 10.1093/rheumatology/kex319 [DOI] [PubMed] [Google Scholar]
- 49.Bae S-C, Gun SC, Mok CC, et al. Improved health outcomes with Etanercept versus usual DMARD therapy in an Asian population with established rheumatoid arthritis. BMC Musculoskelet Disord 2013;14:13. 10.1186/1471-2474-14-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kekow J, Moots RJ, Emery P, et al. Patient-reported outcomes improve with Etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial. Ann Rheum Dis 2010;69:222–5. 10.1136/ard.2008.102509 [DOI] [PubMed] [Google Scholar]
- 51.Fleischmann R, Vencovsky J, van Vollenhoven RF, et al. Efficacy and safety of Certolizumab Pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying Antirheumatic therapy: the Fast4Ward study. Ann Rheum Dis 2009;68:805–11. 10.1136/ard.2008.099291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pope J, Bingham CO, Fleischmann RM, et al. Impact of Certolizumab Pegol on patient-reported outcomes in rheumatoid arthritis and correlation with clinical measures of disease activity. Arthritis Res Ther 2015;17:343. 10.1186/s13075-015-0849-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strand V, Mease P, Burmester GR, et al. Rapid and sustained improvements in health-related quality of life, fatigue, and other patient-reported outcomes in rheumatoid arthritis patients treated with Certolizumab Pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial. Arthritis Res Ther 2009;11:R170. 10.1186/ar2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strand V, Smolen JS, van Vollenhoven RF, et al. Certolizumab Pegol plus methotrexate provides broad relief from the burden of rheumatoid arthritis: analysis of patient-reported outcomes from the RAPID 2 trial. Ann Rheum Dis 2011;70:996–1002. 10.1136/ard.2010.143586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mittendorf T, Dietz B, Sterz R, et al. Improvement and longterm maintenance of quality of life during treatment with Adalimumab in severe rheumatoid arthritis. J Rheumatol 2007;34:2343–50. [PubMed] [Google Scholar]
- 56.Strand V, Rentz AM, Cifaldi MA, et al. Health-related quality of life outcomes of Adalimumab for patients with early rheumatoid arthritis: results from a randomized multicenter study. J Rheumatol 2012;39:63–72. 10.3899/jrheum.101161 [DOI] [PubMed] [Google Scholar]
- 57.Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha Monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003;48:35–45. 10.1002/art.10697 [DOI] [PubMed] [Google Scholar]
- 58.Yount S, Sorensen MV, Cella D, et al. Adalimumab plus methotrexate or standard therapy is more effective than methotrexate or standard therapies alone in the treatment of fatigue in patients with active, inadequately treated rheumatoid arthritis. Clin Exp Rheumatol 2007;25:838–46. [PubMed] [Google Scholar]
- 59.Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006;54:2793–806. 10.1002/art.22025 [DOI] [PubMed] [Google Scholar]
- 60.Emery P, Fleischmann R, Filipowicz-Sosnowska A, et al. The efficacy and safety of Rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 2006;54:1390–400. 10.1002/art.21778 [DOI] [PubMed] [Google Scholar]
- 61.Keystone E, Burmester GR, Furie R, et al. Improvement in patient-reported outcomes in a Rituximab trial in patients with severe rheumatoid arthritis refractory to anti-tumor necrosis factor therapy. Arthritis Rheum 2008;59:785–93. 10.1002/art.23715 [DOI] [PubMed] [Google Scholar]
- 62.Mease PJ, Revicki DA, Szechinski J, et al. Improved health-related quality of life for patients with active rheumatoid arthritis receiving Rituximab: results of the dose-ranging assessment: International clinical evaluation of Rituximab in rheumatoid arthritis (DANCER) trial. J Rheumatol 2008;35:20–30. [PubMed] [Google Scholar]
- 63.Rigby W, Ferraccioli G, Greenwald M, et al. Effect of Rituximab on physical function and quality of life in patients with rheumatoid arthritis previously untreated with methotrexate. Arthritis Care Res (Hoboken) 2011;63:711–20. 10.1002/acr.20419 [DOI] [PubMed] [Google Scholar]
- 64.Alten R, Gomez-Reino J, Durez P, et al. Efficacy and safety of the human anti-IL-1Β Monoclonal antibody Canakinumab in rheumatoid arthritis: results of a 12-week, phase II, dose-finding study. BMC Musculoskelet Disord 2011;12:153. 10.1186/1471-2474-12-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bird P, Hall S, Nash P, et al. Treatment outcomes in patients with Seropositive versus Seronegative rheumatoid arthritis in phase III randomised clinical trials of tofacitinib. RMD Open 2019;5:e000742. 10.1136/rmdopen-2018-000742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strand V, Burmester GR, Zerbini CAF, et al. Tofacitinib with methotrexate in third-line treatment of patients with active rheumatoid arthritis: patient-reported outcomes from a phase III trial. Arthritis Care & Research 2015;67:475–83. 10.1002/acr.22453 [DOI] [PubMed] [Google Scholar]
- 67.Strand V, Kremer JM, Gruben D, et al. Tofacitinib in combination with conventional disease-modifying Antirheumatic drugs in patients with active rheumatoid arthritis: patient-reported outcomes from a phase III randomized controlled trial. Arthritis Care Res (Hoboken) 2017;69:592–8. 10.1002/acr.23004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strand V, van Vollenhoven RF, Lee EB, et al. Tofacitinib or Adalimumab versus placebo: patient-reported outcomes from a phase 3 study of active rheumatoid arthritis. Rheumatology 2016;55:1031–41. 10.1093/rheumatology/kev442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strand V, van der Heijde D, Tanaka Y, et al. Tofacitinib in combination with methotrexate in patients with rheumatoid arthritis: patient-reported outcomes from the 24-month phase 3 ORAL scan study. Clin Exp Rheumatol 2020;38:848–57. [PubMed] [Google Scholar]
- 70.Li Z, An Y, Su H, et al. Tofacitinib with conventional synthetic disease-modifying Antirheumatic drugs in Chinese patients with rheumatoid arthritis: patient-reported outcomes from a phase 3 randomized controlled trial. Int J Rheum Dis 2018;21:402–14. 10.1111/1756-185X.13244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Genovese MC, Schiff M, Luggen M, et al. Efficacy and safety of the selective Co-stimulation modulator Abatacept following 2 years of treatment in patients with rheumatoid arthritis and an inadequate response to anti-tumour necrosis factor therapy. Ann Rheum Dis 2008;67:547–54. 10.1136/ard.2007.074773 [DOI] [PubMed] [Google Scholar]
- 72.Russell AS, Wallenstein GV, Li T, et al. Abatacept improves both the physical and mental health of patients with rheumatoid arthritis who have inadequate response to methotrexate treatment. Ann Rheum Dis 2007;66:189–94. 10.1136/ard.2006.057018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wells G, Li T, Maxwell L, et al. Determining the minimal clinically important differences in activity, fatigue, and sleep quality in patients with rheumatoid arthritis. J Rheumatol 2007;34:280–9. [PubMed] [Google Scholar]
- 74.Westhovens R, Cole JC, Li T, et al. Improved health-related quality of life for rheumatoid arthritis patients treated with Abatacept who have inadequate response to anti-TNF therapy in a double-blind, placebo-controlled, Multicentre randomized clinical trial. Rheumatology (Oxford) 2006;45:1238–46. 10.1093/rheumatology/kel066 [DOI] [PubMed] [Google Scholar]
- 75.Bingham CO, Weinblatt M, Han C, et al. The effect of intravenous Golimumab on health-related quality of life in rheumatoid arthritis: 24-week results of the phase III GO-FURTHER trial. J Rheumatol 2014;41:1067–76. 10.3899/jrheum.130864 [DOI] [PubMed] [Google Scholar]
- 76.Genovese MC, Han C, Keystone EC, et al. Effect of Golimumab on patient-reported outcomes in rheumatoid arthritis: results from the GO-FORWARD study. J Rheumatol 2012;39:1185–91. 10.3899/jrheum.111195 [DOI] [PubMed] [Google Scholar]
- 77.Li Z, Zhang F, Kay J, et al. Efficacy and safety results from a phase 3, randomized, placebo-controlled trial of subcutaneous Golimumab in Chinese patients with active rheumatoid arthritis despite methotrexate therapy. Int J Rheum Dis 2016;19:1143–56. 10.1111/1756-185X.12723 [DOI] [PubMed] [Google Scholar]
- 78.Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors. Lancet 2009:210–21. [DOI] [PubMed] [Google Scholar]
- 79.Ghiti Moghadam M, Ten Klooster PM, Vonkeman HE, et al. Impact of stopping tumor necrosis factor inhibitors on rheumatoid arthritis patients' burden of disease. Arthritis Care Res (Hoboken) 2018;70:516–24. 10.1002/acr.23315 [DOI] [PubMed] [Google Scholar]
- 80.Genovese MC, Kalunian K, Gottenberg J-E, et al. Effect of Filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying Antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA 2019;322:315–25. 10.1001/jama.2019.9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Genovese M, Westhovens R, Meuleners L, et al. Effect of Filgotinib, a selective JAK 1 inhibitor, with and without methotrexate in patients with rheumatoid arthritis: patient-reported outcomes. Arthritis Res Ther 2018;20:57. 10.1186/s13075-018-1541-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strand V, Kosinski M, Chen C-I, et al. Sarilumab plus methotrexate improves patient-reported outcomes in patients with active rheumatoid arthritis and inadequate responses to methotrexate: results of a phase III trial. Arthritis Res Ther 2016;18:198. 10.1186/s13075-016-1096-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strand V, Reaney M, Chen C-I, et al. Sarilumab improves patient-reported outcomes in rheumatoid arthritis patients with inadequate response/intolerance to tumour necrosis factor inhibitors. RMD Open 2017;3:e000416. 10.1136/rmdopen-2016-000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strand V, Gossec L, Proudfoot CWJ, et al. Patient-reported outcomes from a randomized phase III trial of Sarilumab monotherapy versus Adalimumab monotherapy in patients with rheumatoid arthritis. Arthritis Res Ther 2018;20:129. 10.1186/s13075-018-1614-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keystone EC, Taylor PC, Tanaka Y, et al. Patient-reported outcomes from a phase 3 study of Baricitinib versus placebo or Adalimumab in rheumatoid arthritis: secondary analyses from the RA-BEAM study. Ann Rheum Dis 2017;76:1853–61. 10.1136/annrheumdis-2017-211259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Z, Hu J, Bao C, et al. Baricitinib in patients with rheumatoid arthritis with inadequate response to methotrexate: results from a phase 3 study. Clin Exp Rheumatol 2020;38:732–41. [PubMed] [Google Scholar]
- 87.Li Z-G, Hu J-K, Li X-P, et al. Rapid onset of efficacy of Baricitinib in Chinese patients with moderate to severe rheumatoid arthritis: results from study RA-BALANCE. Adv Ther 2021;38:772–81. 10.1007/s12325-020-01572-y [DOI] [PubMed] [Google Scholar]
- 88.Schiff M, Takeuchi T, Fleischmann R, et al. Patient-reported outcomes of Baricitinib in patients with rheumatoid arthritis and no or limited prior disease-modifying Antirheumatic drug treatment. Arthritis Res Ther 2017;19:208. 10.1186/s13075-017-1410-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smolen JS, Kremer JM, Gaich CL, et al. Patient-reported outcomes from a randomised phase III study of Baricitinib in patients with rheumatoid arthritis and an inadequate response to biological agents (RA-BEACON). Ann Rheum Dis 2017;76:694–700. 10.1136/annrheumdis-2016-209821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Strand V, Pope J, Tundia N, et al. Upadacitinib improves patient-reported outcomes in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying Antirheumatic drugs: results from SELECT-NEXT. Arthritis Res Ther 2019;21:272. 10.1186/s13075-019-2037-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burmester GR, Blanco R, Charles-Schoeman C, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013;381:451–60. 10.1016/S0140-6736(12)61424-X [DOI] [PubMed] [Google Scholar]
- 92.Braun J, McHugh N, Singh A, et al. Improvement in patient-reported outcomes for patients with Ankylosing Spondylitis treated with Etanercept 50 mg once-weekly and 25 mg twice-weekly. Rheumatology (Oxford) 2007;46:999–1004. 10.1093/rheumatology/kem069 [DOI] [PubMed] [Google Scholar]
- 93.Calin A, Dijkmans BAC, Emery P, et al. Outcomes of a Multicentre randomised clinical trial of Etanercept to treat Ankylosing Spondylitis. Ann Rheum Dis 2004;63:1594–600. 10.1136/ard.2004.020875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dougados M, Tsai W-C, Saaibi DL, et al. Evaluation of health outcomes with Etanercept treatment in patients with early Nonradiographic axial Spondyloarthritis. J Rheumatol 2015;42:1835–41. 10.3899/jrheum.141313 [DOI] [PubMed] [Google Scholar]
- 95.Hammoudeh M, Zack DJ, Li W, et al. Associations between inflammation, nocturnal back pain and fatigue in Ankylosing Spondylitis and improvements with Etanercept therapy. J Int Med Res 2013;41:1150–9. 10.1177/0300060513488501 [DOI] [PubMed] [Google Scholar]
- 96.Wanders AJB, Gorman JD, Davis JC, et al. Responsiveness and Discriminative capacity of the assessments in Ankylosing Spondylitis disease-controlling Antirheumatic therapy core set and other outcome measures in a trial of Etanercept in Ankylosing Spondylitis. Arthritis Rheum 2004;51:1–8. 10.1002/art.20075 [DOI] [PubMed] [Google Scholar]
- 97.Sieper J, Kivitz A, van Tubergen A, et al. Impact of Certolizumab Pegol on patient-reported outcomes in patients with axial Spondyloarthritis. Arthritis Care Res (Hoboken) 2015;67:1475–80. 10.1002/acr.22594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Revicki DA, Luo MP, Wordsworth P, et al. Adalimumab reduces pain, fatigue, and stiffness in patients with Ankylosing Spondylitis: results from the Adalimumab trial evaluating long-term safety and efficacy for Ankylosing Spondylitis (ATLAS). J Rheumatol 2008;35:1346–53. [PubMed] [Google Scholar]
- 99.Deodhar AA, Dougados M, Baeten DL, et al. Effect of Secukinumab on patient-reported outcomes in patients with active Ankylosing Spondylitis: A phase III randomized trial (MEASURE 1). Arthritis Rheumatol 2016;68:2901–10. 10.1002/art.39805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deodhar A, Conaghan PG, Kvien TK, et al. Secukinumab provides rapid and persistent relief in pain and fatigue symptoms in patients with Ankylosing Spondylitis irrespective of baseline C-reactive protein levels or prior tumour necrosis factor inhibitor therapy: 2-year data from the MEASURE 2 study. Clin Exp Rheumatol 2019;37:260–9. [PubMed] [Google Scholar]
- 101.Marzo-Ortega H, Sieper J, Kivitz A, et al. Secukinumab and sustained improvement in signs and symptoms of patients with active Ankylosing Spondylitis through two years: results from a phase III study. Arthritis Care Res (Hoboken) 2017;69:1020–9. 10.1002/acr.23233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jenks K, Stebbings S, Burton J, et al. Probiotic therapy for the treatment of Spondyloarthritis: a randomized controlled trial. J Rheumatol 2010;37:2118–25. 10.3899/jrheum.100193 [DOI] [PubMed] [Google Scholar]
- 103.Deodhar A, Mease P, Rahman P, et al. Ixekizumab improves patient-reported outcomes in non-radiographic axial Spondyloarthritis: results from the coast-X trial. Rheumatol Ther 2021;8:135–50. 10.1007/s40744-020-00254-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Braun J, van der Heijde D, Doyle MK, et al. Improvement in hemoglobin levels in patients with Ankylosing Spondylitis treated with Infliximab. Arthritis Rheum 2009;61:1032–6. 10.1002/art.24865 [DOI] [PubMed] [Google Scholar]
- 105.Devauchelle-Pensec V, Mariette X, Jousse-Joulin S, et al. Treatment of primary Sjögren syndrome with Rituximab: a randomized trial. Ann Intern Med 2014;160:233–42. 10.7326/M13-1085 [DOI] [PubMed] [Google Scholar]
- 106.Bowman SJ, Everett CC, O’Dwyer JL, et al. Randomized controlled trial of Rituximab and cost-effectiveness analysis in treating fatigue and oral dryness in primary Sjögren's syndrome. Arthritis Rheumatol 2017;69:1440–50. 10.1002/art.40093 [DOI] [PubMed] [Google Scholar]
- 107.Dass S, Bowman SJ, Vital EM, et al. Reduction of fatigue in Sjögren syndrome with Rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann Rheum Dis 2008;67:1541–4. 10.1136/ard.2007.083865 [DOI] [PubMed] [Google Scholar]
- 108.Meijer JM, Meiners PM, Vissink A, et al. Effectiveness of Rituximab treatment in primary Sjögren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2010;62:960–8. 10.1002/art.27314 [DOI] [PubMed] [Google Scholar]
- 109.Mariette X, Ravaud P, Steinfeld S, et al. Inefficacy of Infliximab in primary Sjögren’s syndrome: results of the randomized, controlled trial of Remicade in primary Sjögren’s syndrome (TRIPSS). Arthritis Rheum 2004;50:1270–6. 10.1002/art.20146 [DOI] [PubMed] [Google Scholar]
- 110.Hartkamp A, Geenen R, Godaert GLR, et al. Effect of Dehydroepiandrosterone administration on fatigue, well-being, and functioning in women with primary Sjögren syndrome: a randomised controlled trial. Ann Rheum Dis 2008;67:91–7. 10.1136/ard.2007.071563 [DOI] [PubMed] [Google Scholar]
- 111.Virkki LM, Porola P, Forsblad-d’Elia H, et al. Dehydroepiandrosterone (DHEA) substitution treatment for severe fatigue in DHEA-deficient patients with primary Sjögren’s syndrome. Arthritis Care Res (Hoboken) 2010;62:118–24. 10.1002/acr.20022 [DOI] [PubMed] [Google Scholar]
- 112.Theander E, Horrobin DF, Jacobsson LTH, et al. Gammalinolenic acid treatment of fatigue associated with primary Sjögren’s syndrome. Scandinavian Journal of Rheumatology 2002;31:72–9. 10.1080/03009740252937577 [DOI] [PubMed] [Google Scholar]
- 113.Seitsalo H, Niemelä RK, Marinescu-Gava M, et al. Effectiveness of low-dose Doxycycline (LDD) on clinical symptoms of Sjögren’s syndrome: a randomized, double-blind, placebo controlled cross-over study. J Negat Results Biomed 2007;6:11. 10.1186/1477-5751-6-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Posada J, Valadkhan S, Burge D, et al. Improvement of severe fatigue following Nuclease therapy in patients with primary Sjögren’s syndrome: a randomized clinical trial. Arthritis Rheumatol 2021;73:143–50. 10.1002/art.41489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Norheim KB, Harboe E, Gøransson LG, et al. Interleukin-1 inhibition and fatigue in primary Sjögren’s syndrome--a double blind, randomised clinical trial. PLoS ONE 2012;7:e30123. 10.1371/journal.pone.0030123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu X, Li X, Li X, et al. The efficacy and safety of total Glucosides of Peony in the treatment of primary Sjögren’s syndrome: a multi-center, randomized, double-blinded, placebo-controlled clinical trial. Clin Rheumatol 2019;38:657–64. 10.1007/s10067-018-4315-8 [DOI] [PubMed] [Google Scholar]
- 117.Gottenberg J-E, Ravaud P, Puéchal X, et al. Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: the JOQUER randomized clinical trial. JAMA 2014;312:249–58. 10.1001/jama.2014.7682 [DOI] [PubMed] [Google Scholar]
- 118.Kruize AA, Hené RJ, Kallenberg CG, et al. Hydroxychloroquine treatment for primary Sjögren’s syndrome: a two year double blind crossover trial. Ann Rheum Dis 1993;52:360–4. 10.1136/ard.52.5.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dörner T, Posch MG, Li Y, et al. Treatment of primary Sjögren’s syndrome with Ianalumab (Vay736) targeting B cells by BAFF receptor blockade coupled with enhanced, antibody-dependent cellular cytotoxicity. Ann Rheum Dis 2019;78:641–7. 10.1136/annrheumdis-2018-214720 [DOI] [PubMed] [Google Scholar]
- 120.Gladman D, Fleischmann R, Coteur G, et al. Effect of Certolizumab Pegol on multiple facets of Psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken) 2014;66:1085–92. 10.1002/acr.22256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Genovese MC, Mease PJ, Thomson GTD, et al. Safety and efficacy of Adalimumab in treatment of patients with Psoriatic arthritis who had failed disease modifying Antirheumatic drug therapy. J Rheumatol 2007;34:1040–50. [PubMed] [Google Scholar]
- 122.Gladman DD, Mease PJ, Cifaldi MA, et al. Adalimumab improves joint-related and skin-related functional impairment in patients with Psoriatic arthritis: patient-reported outcomes of the Adalimumab effectiveness in Psoriatic arthritis trial. Annals of the Rheumatic Diseases 2007;66:163–8. 10.1136/ard.2006.057901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Strand V, Mease P, Gossec L, et al. Secukinumab improves patient-reported outcomes in subjects with active Psoriatic arthritis: results from a randomised phase III trial. Ann Rheum Dis 2017;76:203–7. 10.1136/annrheumdis-2015-209055 [DOI] [PubMed] [Google Scholar]
- 124.Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 P40 Monoclonal antibody, Ustekinumab, in patients with active Psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3. Ann Rheum Dis 2014;73:990–9. 10.1136/annrheumdis-2013-204655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Baranauskaite A, Raffayová H, Kungurov NV, et al. Infliximab plus methotrexate is superior to methotrexate alone in the treatment of Psoriatic arthritis in methotrexate-naive patients: the RESPOND study. Ann Rheum Dis 2012;71:541–8. 10.1136/ard.2011.152223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Strand V, Van den Bosch F, Ranza R, et al. Patient-reported outcomes in Psoriatic arthritis patients with an inadequate response to biologic disease-modifying Antirheumatic drugs: SELECT-PSA 2. Rheumatol Ther 2021;8:1827–44. 10.1007/s40744-021-00377-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Strand V, Mease PJ, Soriano ER, et al. Improvement in patient-reported outcomes in patients with Psoriatic arthritis treated with Upadacitinib versus placebo or Adalimumab: results from SELECT-PSA 1. Rheumatol Ther 2021;8:1789–808. 10.1007/s40744-021-00379-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Strand V, de Vlam K, Covarrubias-Cobos JA, et al. Tofacitinib or Adalimumab versus placebo: patient-reported outcomes from OPAL broaden-a phase III study of active Psoriatic arthritis in patients with an inadequate response to conventional synthetic disease-modifying Antirheumatic drugs. RMD Open 2019;5:e000806. 10.1136/rmdopen-2018-000806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Strand V, de Vlam K, Covarrubias-Cobos JA, et al. Effect of tofacitinib on patient-reported outcomes in patients with active Psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors in the phase III, randomised controlled trial: OPAL beyond. RMD Open 2019;5:e000808. 10.1136/rmdopen-2018-000808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hartkamp A, Geenen R, Godaert GLR, et al. Effects of Dehydroepiandrosterone on fatigue and well-being in women with quiescent systemic lupus erythematosus: a randomised controlled trial. Ann Rheum Dis 2010;69:1144–7. 10.1136/ard.2009.117036 [DOI] [PubMed] [Google Scholar]
- 131.Yokogawa N, Eto H, Tanikawa A, et al. Effects of hydroxychloroquine in patients with cutaneous lupus erythematosus: A multicenter, double-blind, randomized, parallel-group trial. Arthritis Rheumatol 2017;69:791–9. 10.1002/art.40018 [DOI] [PubMed] [Google Scholar]
- 132.Merrill JT, Burgos-Vargas R, Westhovens R, et al. The efficacy and safety of Abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2010;62:3077–87. 10.1002/art.27601 [DOI] [PubMed] [Google Scholar]
- 133.Stohl W, Schwarting A, Okada M, et al. Efficacy and safety of subcutaneous Belimumab in systemic lupus erythematosus: A fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol 2017;69:1016–27. 10.1002/art.40049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Strand V, Levy RA, Cervera R, et al. Improvements in health-related quality of life with Belimumab, a B-lymphocyte Stimulator-specific inhibitor, in patients with autoantibody-positive systemic lupus erythematosus from the randomised controlled BLISS trials. Ann Rheum Dis 2014;73:838–44. 10.1136/annrheumdis-2012-202865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Petri MA, Martin RS, Scheinberg MA, et al. Assessments of fatigue and disease activity in patients with systemic lupus erythematosus enrolled in the phase 2 clinical trial with Blisibimod. Lupus 2017;26:27–37. 10.1177/0961203316654767 [DOI] [PubMed] [Google Scholar]
- 136.Merrill JT, van Vollenhoven RF, Buyon JP, et al. Efficacy and safety of subcutaneous Tabalumab, a Monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, Multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2016;75:332–40. 10.1136/annrheumdis-2015-207654 [DOI] [PubMed] [Google Scholar]
- 137.Lai Z-W, Hanczko R, Bonilla E, et al. N-Acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2012;64:2937–46. 10.1002/art.34502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khanna D, Denton CP, Jahreis A, et al. Safety and efficacy of subcutaneous Tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 2016;387:2630–40. 10.1016/S0140-6736(16)00232-4 [DOI] [PubMed] [Google Scholar]
- 139.Chung Y-L, Alexanderson H, Pipitone N, et al. Creatine supplements in patients with idiopathic inflammatory Myopathies who are clinically weak after conventional pharmacologic treatment: six-month, double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2007;57:694–702. 10.1002/art.22687 [DOI] [PubMed] [Google Scholar]
- 140.Strand V, Dimonaco S, Tuckwell K, et al. Health-related quality of life in patients with giant cell arteritis treated with Tocilizumab in a phase 3 randomised controlled trial. Arthritis Res Ther 2019;21:64. 10.1186/s13075-019-1837-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sepriano A, Kerschbaumer A, Bergstra SA, et al. Safety of synthetic and biological Dmards: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2023;82:107–18. 10.1136/ard-2022-223357 [DOI] [PubMed] [Google Scholar]
- 142.Webers C, Ortolan A, Sepriano A, et al. Efficacy and safety of biological Dmards: a systematic literature review informing the 2022 update of the ASAS-EULAR recommendations for the management of axial Spondyloarthritis. Ann Rheum Dis 2023;82:130–41. 10.1136/ard-2022-223298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003349supp001.pdf (88.6KB, pdf)
rmdopen-2023-003349supp002.pdf (118.1KB, pdf)
rmdopen-2023-003349supp003.pdf (869.2KB, pdf)
rmdopen-2023-003349supp004.pdf (162KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.