Abstract
Purpose:
It is essential that clinicians have evidence-based benchmarks to support accurate diagnosis and clinical decision making. Recent studies report poor reliability for diagnostic judgments and identifying mechanisms of impairment from videofluoroscopy (VFSS). Establishing VFSS reference values for healthy swallowing would help resolve such discrepancies. Steele et al. (2019) released preliminary reference data for quantitative VFSS measures in healthy adults aged < 60 years. Here, we extend that work to provide reference percentiles for VFSS measures across a larger age span.
Method:
Data for 16 VFSS parameters were collected from 78 healthy adults aged 21–82 years (39 male). Participants swallowed three comfortable sips each of thin, slightly, mildly, moderately, and extremely thick barium (20% w/v). VFSS recordings were analyzed in duplicate by trained raters, blind to participant and task, using the Analysis of Swallowing Physiology: Events, Kinematics and Timing (ASPEKT) Method. Reference percentiles (p2.5, 5, 25, 50, 75, 95, and 97.5) were determined as per Clinical and Laboratory Standards Institute EP28-A3c guidelines.
Results:
We present VFSS reference percentile tables, by consistency, for (a) timing parameters (swallow reaction time; the hyoid burst–to–upper esophageal sphincter (UES)-opening interval; UES opening duration; time–to–laryngeal vestibule closure (LVC); and LVC duration) and (b) anatomically scaled pixel-based measures of maximum UES diameter, pharyngeal area at maximum pharyngeal constriction and rest, residue (vallecular, pyriform, other pharyngeal locations, total), and hyoid kinematics (X, Y, XY coordinates of peak position; speed). Clinical decision limits are proposed to demarcate atypical values of potential clinical concern.
Conclusion:
These updated reference percentiles and proposed clinical decision limits are intended to support interpretation and reliability for VFSS assessment data.
Supplemental Material:
Swallowing impairment (dysphagia) is a common sequela of many age-related disease and injury processes, and is estimated to affect 6.7% of hospital admissions in the United States annually (Altman et al., 2010; Clave & Shaker, 2015) with an annual attributable cost of $547 million (Patel et al., 2018). Early and accurate identification and monitoring of dysphagia is therefore paramount to optimize patient outcomes and health care utilization and to mitigate the development of negative health-related outcomes. Assessment of swallowing typically begins with a clinical “bedside” swallowing examination (CBSE), in which the clinician obtains a patient history, performs an oral mechanism exam, and observes oral intake of different foods and liquids (McCullough et al., 1999). Although the CBSE is a cornerstone of clinical practice, it has been criticized for its lack of standardization, its reliance on subjective impressions, and the inability to directly visualize bolus flow to confirm the presence of impairments in swallowing safety and/or efficiency (McCullough et al., 2001, 2005). Instrumental swallowing assessments, involving either dynamic videofluoroscopic swallowing studies (VFSSs) or fiberoptic endoscopic evaluation of swallowing, are widely accepted as “gold” standard approaches for dysphagia diagnosis. However, although such methods afford direct visualization of bolus flow, even these procedures are criticized for a lack of standards and poor interrater agreement for clinical interpretation (Plowman & Humbert, 2018; Swan et al., 2019). Recently, an international, multidisciplinary expert panel summarized the current state of VFSS practice as follows: “there is a lack of up-to-date, comprehensive, evidence-based information on the diagnosis of oropharyngeal dysphagia, …high-quality guidance would serve to improve standardization, permit reliable intrapatient evaluation of repeat examinations, and allow for interpatient examination comparisons within and between institutions.” (Martin-Harris et al., 2021).
Several recent studies have demonstrated suboptimal reliability and accuracy of clinician's diagnostic judgments from VFSS exams (Plowman & Humbert, 2018), and in the identification of primary mechanisms of swallowing impairment (Vose et al., 2018). Such differences of opinion when identifying mechanisms of swallowing impairment lead to wide variability in treatment recommendations (Vose et al., 2018). A paucity of available VFSS reference data has been identified as one factor that contributes to discrepancies in clinician judgments regarding the presence or absence of abnormal swallowing physiology.
There have been prior efforts to establish normative reference values for VFSS measures of swallowing, most notably led by the work of Leonard and Kendall (e.g., K. Kendall, McKenzie, & Leonard, 2000; K. A. Kendall, McKenzie, Leonard, et al., 2000; Leonard et al., 2000), whose seminal work using a sample of 60 healthy adults aged 18–78 years proposed definitions for key events in the swallowing sequence and established mean values and standard deviations for timing and millimeter-based structural displacement measures for three volumes of liquid swallow (1, 3, and 20 cc) and a 3-cc paste consistency bolus. Since that time, the International Dysphagia Diet Standardisation Initiative (IDDSI) Framework was introduced as a new taxonomy for defining and labelling different consistencies of food and liquids used in dysphagia management (Cichero et al., 2017, 2020). Beginning in May 2016, our lab began a major project to develop a comprehensive set of reference values for quantitative VFSS measures of healthy swallowing physiology across the range from thin to extremely thick liquids, as defined by the IDDSI Framework. As part of that project, we developed a standard operating procedure for obtaining quantitative VFSS measures known as the ASPEKT (Analysis of Swallowing Physiology: Events, Kinematics and Timing) Method. We published a preliminary set of ASPEKT reference values in 2019, based on data for an array of 20% w/v barium stimuli, prepared with a commercially available xanthan-gum thickener (Steele et al., 2019). That first paper included frequencies for categorical and ordinal parameters, and means and standard deviations for continuous parameters from a sex-balanced sample of healthy adults aged 21–59 years. A subsequent paper provided preliminary reference values for measures of hyoid kinematics for the same data set (Smaoui et al., 2021). Here, we extend that work to provide reference values for VFSS measures across a larger age span, including a cohort of individuals aged 60–82 years.
Medical diagnostic testing routinely involves the comparison of patient values to reference values from healthy individuals to identify clinically relevant differences. Best practice methods for establishing reference intervals to guide clinical diagnostics exist in other fields, most notably in laboratory medicine. According to these methods, the healthy reference interval is defined as the range of values falling between the 2.5th and 97.5th percentiles (p2.5, p97.5) of the reference distribution, bounding the central 95% of the measured values (Ozarda, 2016). Prior studies reporting reference values for quantitative VFSS measures, including our own prior paper, have tended to report means and standard deviations (e.g., Donohue et al., 2022; K. Kendall, McKenzie, & Leonard, 2000; Steele et al., 2019) rather than defining the healthy reference interval; where thresholds for differentiating normal values from values of clinical concern have been proposed, these have tended to be 2-SD boundaries from the mean (e.g., Leonard, 2019; Waito et al., 2020). For this paper, we have partnered with experts from laboratory medicine to apply well-established best practice methods from that discipline (Adeli et al., 2017; Ceriotti, 2009; Ceriotti & Henny, 2008; Ceriotti et al., 2009; Ozarda, 2016) to the novel context of swallowing. The result of this collaboration is shared here: a revised set of preliminary reference values for quantitative VFSS measures of swallowing physiology, which document the 2.5th, 50th, and 97.5th percentiles, for healthy adults across a broader age range, from 21 to 82 years, including the original sample described previously (Steele et al., 2019) and an additional cohort of healthy older adults aged 60–82 years. In the Discussion section of this article, we address frequently asked questions about the ASPEKT Method, outline limitations of this current iteration of reference values, and discuss future directions.
Method
Ethics Approval and Study Registration
The protocol for this project received human subjects' ethics approval from the local institutional review board (University Health Network Coordinated Approval Process for Clinical Research Protocol 15–9431). All participants provided signed informed consent. The project was classified by the National Institutes of Health as a Basic Experimental Study in Humans involving a clinical trial exploring the impact of bolus consistency on swallowing; therefore, the protocol was also registered with ClinicalTrials.gov (NCT04114617).
Participants
This study was conducted in a sample of adults with no reported symptoms of dysphagia, and no reported difficulties with motor speech, gastroesophageal, or neurological function, sinusitis, or altered taste. Individuals who reported a medical history of congenital stroke, neurodegenerative disease diagnoses, head and neck cancer, major surgery or radiation to the oropharynx or neck, or Type 1 diabetes were excluded, along with participants who reported known allergies to stimulus ingredients and women who were currently pregnant or breastfeeding. The original publication (Steele et al., 2019) described a sample of 40 participants under age 60 years; for the purpose of expanding the age range to inform reference interval calculation, a new sample of 40 participants over the age of 60 years was recruited.
Videofluoroscopy
Data for both the previously published under age 60 years cohort and for the new over 60 years cohort were collected between May 2016 and July 2019 using the same methods, including continuous lateral view fluoroscopy without magnification. The fluoroscope was a Toshiba Ultimax ADR-1000 (Toshiba America Medical Systems Inc), and recordings were captured at 30 frames per second on the KayPENTAX Digital Swallow Workstation (KayPentax). The boundaries of the field of view were defined as the lips anteriorly, the pharyngeal wall posteriorly, the nasopharynx superiorly, and just below the upper esophageal sphincter (UES) inferiorly.
The VFSS protocol comprised a series of 27 boluses, beginning with three boluses of thin liquid barium and then progressing to four sets of thickened liquid stimuli, presented in order of ascending thickness (i.e., slightly, mildly, moderately, and extremely thick liquids). All stimuli were prepared in a 20% w/v barium concentration using E-Z-PAQUE 96% w/w barium sulfate powder (Bracco Diagnostics) and bottled water (Nestlé Pure Life). The thickened liquids were prepared according to standard recipes (Barbon & Steele, 2019). Each set of thickened liquids comprised six boluses, arranged in two blocks: three boluses prepared with a xanthan-gum thickener (ThickenUp Clear powder, Nestlé Health Science), and three prepared with a starch thickener (ThickenUp powder, Nestlé Health Science). Within each consistency, the xanthan-gum and starch thickener stimuli were matched for flow using the IDDSI Flow Test (Hanson et al., 2019), but were known to have different viscosities (Ong et al., 2018). Participants were randomly assigned to one of two counterbalanced orders of thickener blocks, such that half of the sample completed the xanthan-gum thickened stimuli for a given consistency first, followed by the starch thickened stimuli of the same consistency, while the other half of the sample completed these blocks in the reverse order. This article only reports data for the xanthan-gum thickened stimuli, which were chosen as the reference condition; the effect of thickener (and associated differences in viscosity) within consistency will be the focus of a future planned analysis.
For each data collection session, a set of prelabeled cups (120-ml capacity) was prefilled with a 40-ml volume of each stimulus and covered with lids to prevent spillage. The cups were weighed on an Ohaus digital balance (Model PA1502 analytical scale: capacity = 1.5 kg, readability = 0.01 g) and placed in serving order in a muffin tray. All stimuli were served at room temperature, not more than 3 hr after preparation. Participants self-administered the stimuli, taking comfortable sips of the thin, slightly and mildly thick liquids, and comfortable teaspoons-full of the moderately and extremely thick liquids. Participants were instructed to swallow when they felt ready, without waiting for a cue. After each bolus was taken, the cup was placed back in the muffin tray, and the lid was replaced to prevent spillage of any leftover liquid. Cup weights were repeated after the protocol to enable the calculation of sip mass and the derivation of sip volume.
VFSS Rating
The VFSS recordings were postprocessed and rated using a standard operating procedure known as the ASPEKT Method (Steele et al., 2019). Each full-length VFSS recording was first reviewed to identify the time codes associated with onset/offset of the x-ray for bolus contained in the recording. The identified boundaries were then used to splice the recording into smaller video clips, each containing the swallows associated with a single bolus. The spliced bolus-level video clips, stripped of audio information, were labeled with a random file number, and assigned for rating in duplicate, in randomly compiled batches of 150 videos. Ratings were performed using ImageJ software (National Institutes of Health, https://imagej.nih.gov). All raters had previously completed a training program and had demonstrated knowledge of definitions, competency in rating procedures, and interrater agreement with other team members on a training set of 20 video recordings. As described below, a rigorous process was followed for flagging and resolving discrepancies across duplicate ratings.
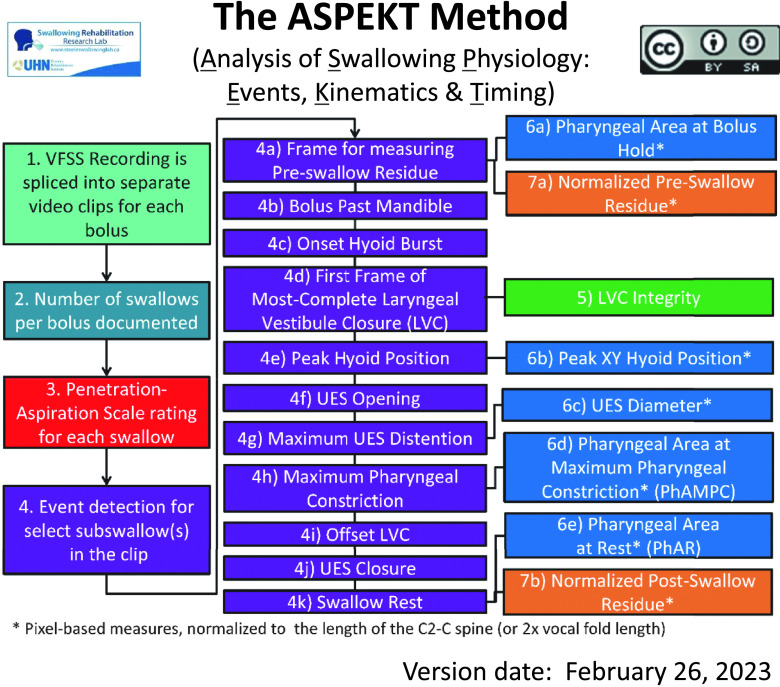
Figure 1 shows the steps of the ASPEKT method of VFSS rating (Steele et al., 2019), which yields a parsimonious set of quantitative VFSS measures:
Figure 1.
Flowchart illustrating the rating steps in the ASPEKT Method (Analysis of Swallowing Physiology: Events, Kinematics and Timing). VFSS = videofluoroscopic swallowing study; UES = upper esophageal sphincter.
-
a) Swallowing safety is indexed using:
the Penetration–Aspiration Scale (PAS; Rosenbek et al., 1996) score for the initial swallow of each bolus;
in the event of a single bolus for which there are multiple swallows, the worst (i.e., highest) PAS score across all of those swallows; and
the degree of laryngeal vestibule closure (LVC) integrity on every swallow (i.e., complete closure, partial closure, or incomplete closure; Vose & Humbert, 2019);
in the event of multiple swallows for a bolus, the worst LVC integrity across all of those swallows.
b) Swallowing efficiency is characterized based on the number of swallows per bolus and anatomically referenced pixel-based measures of residue (valleculae, pyriform sinuses, other pharyngeal locations and in total), before and after each swallow.
c) Timing measures (in frames) are calculated between a series of key events for the initial swallow of each bolus, as illustrated in Figure 1, Step 4 and defined in Table 1: bolus passing mandible (BPM), hyoid burst onset (HYB), upper esophageal sphincter opening (UESO), the first frame of most-complete LVC, LVC offset (LVCOff), and UES closure (UESC). The resulting timing measures include swallow reaction time (i.e., HYB-BPM), the hyoid burst–to–UES-opening interval (i.e., UESO–HYB), UES opening duration (i.e., UESC–UESO); time-to-LVC (i.e., LVC–HYB), and LVC duration (i.e., LVCOff–LVC). Additional key frames are also indexed to facilitate subsequent kinematic measures as follows: maximum UES opening (UESmax), maximum pharyngeal constriction (MPC), and swallow rest (SR). The frame of peak XY hyoid position (peak XY hyoid) can also be indexed during this stage, or, as described below, identified through inspection of hyoid position time histories from frame-by-frame tracking.
d) Kinematic measures of maximum UES diameter and pharyngeal area (at MPC and at rest) are made using pixel-based tracing of bolus area or anatomical area, normalized to the length of the C2–C4 cervical spine (Molfenter & Steele, 2014). Similarly, measures of peak hyoid position (X, Y, and XY coordinates) and hyoid speed (i.e., rate of change in position along the XY axis, between the frames of HYB and peak XY position) are made using pixel-based tracing of structural position, relative to the anterior inferior corner of the C4 vertebrae and normalized using the C2–C4 cervical spine scalar. For the current project, hyoid position was tracked on every frame, beginning five frames before HYB until 5 frames after reaching a plateau at peak position, and the position-time histories were inspected to identify the first frame of peak position along the XY axis, from which position data were taken for analysis.
Table 1.
Definitions for key events and timing measures.
| Event or parameter | Definition |
|---|---|
| Bolus passing mandible (BPM) | The first frame where the leading edge of the bolus touches or crosses the shadow of the ramus of mandible. In cases where the bolus was considered to have escaped prematurely from the mouth into the pharynx, the first frame showing bolus material at or below the ramus of mandible was counted as the BPM frame. When a double mandible shadow was seen on the lateral view image, the lower edge of the more superior ramus was used as the landmark. |
| Hyoid burst onset (HYB) | The first anterior–superior “jump” of the hyoid that is associated with a swallow. This event has previously been referred to using the terminology “onset of maximal hyoid excursion” or “onset of the pharyngeal response” (Robbins et al., 1992). |
| Upper esophageal sphincter opening (UESO) | The first frame where the leading edge of the bolus (or, in rare cases, air) passes through the upper esophageal sphincter (UES). The UES is a narrow segment or region that typically lies between C4 and C6; the narrowest opening seen between C4 and C6 during a swallow is marked as the location of the sphincter (Leonard et al., 2004). In addition, recognizing that the UES moves superiorly during the swallow (Kahrilas et al., 1992), the narrowest portion may be located above C4. The superior boundary of the tracheal air column can be used as a guide to decide where the location of the UES is during pharyngeal shortening. |
| First frame of most-complete laryngeal vestibule closure (LVC) | The first frame where there is maximum approximation of the arytenoids to the laryngeal surface of the epiglottis. Complete closure of the laryngeal vestibule (i.e., a seal between epiglottis and arytenoids leaving no visible airspace) may or may not be present. |
| Laryngeal vestibule closure offset (LVCOff) | The first frame where there is visible opening (white space) of the laryngeal vestibule. This requires some separation of the tissues or of the arytenoids from the inferior surface of the epiglottis, but complete opening is not required. The leaf of the epiglottis may still be in a downward position. This event cannot be identified in cases of incomplete LVC. |
| Upper esophageal sphincter closure (UESC) | The first frame where the UES achieves closure behind the bolus tail. This does not require closure of the entire UES segment, simply closure at a single point along the segment. |
| Maximum UES opening (UESmax) | The frame where the distention of the upper esophageal sphincter has the widest width (i.e., widest lumen width and/or bolus column). This frame is not used in any timing measures but is used for measuring the diameter of UES opening. |
| Maximum pharyngeal constriction (MPC) | The first frame showing maximum obliteration of the pharynx (i.e., the smallest bolus area and/or airspace in the pharynx). This event typically occurs between UESO and LVCOff, before the upper pharynx begins to relax, and before the laryngeal air column begins to descend. This frame is not used in any timing measures but is used for measuring pharyngeal area at maximum pharyngeal constriction (PhAMPC). |
| Swallow rest | The terminal event of each swallow, identified as the first frame showing the pyriform sinuses at their lowest position, relative to the spine, prior to any hyoid burst or laryngeal elevation for a subsequent clearing or piecemeal swallow. For the terminal swallow of a bolus, this event is further defined as occurring within 30 frames (approximately 1 s) of UESC, prior to any nonswallow events such as coughing, talking, or UES reopening. This frame is not used in any timing measures but is used for measuring postswallow pharyngeal residue and pharyngeal area at rest. |
| Peak XY hyoid position | The first frame showing the hyoid at peak position, i.e., the greatest distance measured along the XY hypotenuse from the anterior inferior corner of the C4 vertebra (Smaoui et al., 2021). |
| Swallow reaction time | The interval between BPM and HYB. This parameter has gone under a variety of different names in previous literature including “pharyngeal delay time” (Logemann et al., 2000, 2002), “duration of stage transition” (Robbins et al., 1992), and “swallow response time” (Power et al., 2007). |
| Hyoid burst–to–UES opening | The interval between HYB and UESO. This parameter has previously gone under the name “pharyngeal swallow duration” (Palmer, 1998) and “pharyngeal response time” (Rademaker et al., 1994). |
| UES opening duration | The interval between UESO and UESC. |
| Time–to–most complete LVC | The interval between HYB and LVC. This parameter has gone under the name LVC Reaction Time in previous literature (Guedes et al., 2017; Humbert et al., 2018; Steele et al., 2019). |
| LVC duration | The interval between LVC and LVCOff. |
Interrater Agreement
All ratings were performed in duplicate, by raters who were blinded to each other's ratings, and then inspected for interrater agreement. The same interrater agreement procedures described in the original manuscript (Steele et al., 2019) were carried forward for the expanded sample of older participants. ASPEKT Method rating occurs in two phases: Steps 1–4 in Figure 1 are completed first, and differences in frame identification are resolved before continuing, to ensure that raters use the same frames for the subsequent pixel-based ratings.
For this project, the data set, including boluses for both the xanthan-gum and starch-thickened liquids contained ratings for a total of 2,076 boluses. Prespecified thresholds were used to flag cases where there was disagreement between raters that required review and resolution in a consensus meeting, attended by three trained raters. Over the 5-year course of the project, thresholds were adjusted to ensure review of at least the top 10% of the discrepancy distribution for all parameters. Additional details, together with frequencies for absolute rater differences are shown in Supplemental Material S1 (categorical parameters and event identification) and Supplemental Material S2 (timing and pixel-based parameters). Preconsensus interrater agreement was strong for all timing parameters. For pixel-based measures, several preconsensus intra-class correlations (ICCs) fell below .5; this is not unexpected, given that these measures involve ratios requiring measurement of multiple components, thereby increasing the opportunity for variability. In cases where disagreement fell below the thresholds specified for triggering review, rules were used to select the rating of record. For frame identification, the earlier rated frame for BPM, HYB, LVC, and UESO was selected, whereas the later frame was chosen as the frame of record for MPC, UESMax, UESC, LVCoff, and SR. For rater differences on pixel-based measures that did not require resolution, the smaller of the two rating values was taken as the rating of record.
Hyoid measures were the only parameters not collected in duplicate in this project, and where interrater agreement was not calculated. The nature of frame-by-frame hyoid tracking across a series of consecutive frames (beginning prior to HYB and continuing until several frames after a plateau at peak position is reached) yields highly reliable measures, with only small variations across successive frames. Our method involves inspection and plotting of point measures, frame by frame, to identify the onset and peak position frames. This makes it easy to identify point measures that deviate from a smooth movement path across successive frames, representing cases where review may be needed.
Sip Volume
Explorations of typical sip volume were conducted within consistency using each participant's mean sip volume across task repetitions. Univariate general linear model analyses of variance were performed in SPSS Version 29, to identify main effects of sex, a continuous covariate of age, and their interaction, with post hoc Cohen's d calculations of effect size for significant pairwise comparisons. p values less than .05 were considered statistically significant.
Categorical Parameters
For the categorical parameters of number of swallows per bolus, PAS score (Rosenbek et al., 1996), and LVC integrity, participant worst (i.e., highest) scores across all boluses were captured for each consistency. Frequency tables for different scores were developed. Chi-square tests were performed to explore any differences in score frequencies by consistency.
Reference Interval Calculation
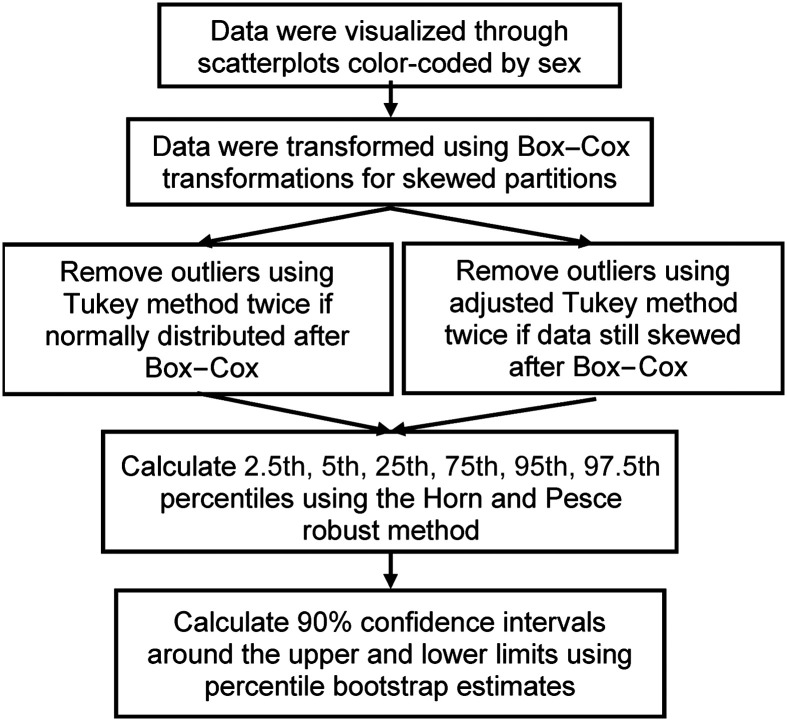
Reference interval calculations were completed using R Statistical Programming (Version 4.2.1), as illustrated in Figure 2 (Adeli et al., 2017). All timing measure calculations were performed in units of frames; the results were subsequently converted into milliseconds, rounded to the closest frame (33-ms units), based on a frame rate of 30 frames per second. Prior to reference interval calculation, participant mean values across task repetitions by IDDSI consistency level were calculated for each parameter. Pharyngeal area at rest was the only exception, for which participant results were averaged across all IDDSI consistency levels, given that rest state of the pharynx after swallowing is not expected to vary across bolus consistencies (Smaoui et al., 2023). Data were plotted by age and sex to visually inspect any apparent age- and/or sex-specific effects. Data were then transformed using the Box-Cox transformation, and extreme outliers were removed in two iterations using the Tukey method for normal distributions or the adjusted Tukey method for skewed distributions, as determined by the Shapiro–Wilk test (Adeli et al., 2017). Reference interval boundaries, defined as the 2.5th and 97.5th percentiles (with 90% confidence intervals), were estimated using the robust method of Horn and Pesce (Horn et al., 1998), as recommended by the Clinical and Laboratory Standards Institute EP28-A3c guidelines when the sample size is less than 120 individuals (Horowitz, 2010). Additional percentiles (i.e., 5th, 25th, 75th, and 95th) and median values were also estimated. For parameters containing any negative values, a constant equal to the minimum was added prior to percentile calculation and then adjusted in final estimates. In some cases, the strongly predominant score for residue measures was a value of zero; this meant that the robust method was unable to estimate some percentile values with confidence. In these situations, the minimum and maximum scores were documented, together with the frequency of nonzero values.
Figure 2.
Flowchart illustrating the data analysis steps followed to obtain estimates of reference percentiles.
Results
Participant Demographics
In total, 80 participants consented to participate in the study. The sample spanned ages 21–82 years, including 41 women (Mage = 51 years, Mdn = 54 years, range: 25–82 years) and 39 men (Mage = 51 years, Mdn = 58 years, range: 21–82 years). The self-reported racial profile of the sample, in descending order of frequency, included 61 individuals reporting racial heritage as White, 14 Asian, three Black/African American, one Native Hawaiian/Pacific Islander, and one Alaskan/American Indian. Three participants reported Hispanic/Latino ethnicity. As reported in the original paper (Steele et al., 2019), VFSS data were unavailable for two participants under age 60 years, resulting in a final data set from 78 participants (39 women and 39 men).
Sip Volume
Grand mean values for sip volume, by consistency, were 13 ml for thin liquids, 11 ml for slightly and mildly thick liquids, and 5 ml for the moderately and extremely thick liquids (which were served by teaspoon). Table 2 shows descriptive statistics for sip volume by sex, for each bolus consistency. Male participants took significantly larger sips than women for the thin, mildly thick, and extremely thick liquids. There was no significant effect of age, or of Sex × Age interaction on sip volume.
Table 2.
Descriptive statistics for sip volume in ml, by consistency and sex.
| Consistency | Sex | M | 95% Confidence interval lower bound | 95% Confidence interval upper bound | SD | F | p value | Cohen's d | Effect size interpretation |
|---|---|---|---|---|---|---|---|---|---|
| Level 0 thin | Female | 10.7 | 9.0 | 12.4 | 4.9 | 6.39 | .02 | 0.51 | Medium |
| Male | 15.6 | 12.8 | 18.4 | 8.2 | |||||
| Level 1 slightly thick | Female | 9.2 | 7.9 | 10.5 | 4.0 | 3.26 | .09 | 0.36 | Small |
| Male | 11.8 | 9.8 | 13.8 | 6.1 | |||||
| Level 2 mildly thick | Female | 8.9 | 7.6 | 10.2 | 4.0 | 5.13 | .04 | 0.39 | Small |
| Male | 11.7 | 9.7 | 13.8 | 6.1 | |||||
| Level 3 moderately thick | Female | 4.4 | 3.6 | 5.2 | 2.5 | 4.59 | .05 | 0.17 | Negligible |
| Male | 4.9 | 4.3 | 5.5 | 1.8 | |||||
| Level 4 extremely thick | Female | 4.7 | 4.0 | 5.5 | 2.2 | 5.13 | .04 | 0.28 | Small |
| Male | 5.6 | 4.9 | 6.3 | 2.2 |
Categorical Parameters
Three of the parameters of interest behave as categorical rather than continuous parameters: (a) number of swallows per bolus; (b) PAS scores (Steele & Grace-Martin, 2017); and (c) LVC integrity. For these parameters, score frequencies by consistency can be found in Tables 2–4. Additionally, Appendix Table A1 provides guidance for clinicians, proposing scores of potential clinical concern based on score frequencies.
Table 4.
Participant level frequencies for worst Penetration–Aspiration Scale (PAS) scores seen, by consistency.
| Consistency | PAS = 1 | PAS = 2 | PAS = 3 | PAS = 4 | PAS = 5 |
|---|---|---|---|---|---|
| Level 0 thin | 72% | 19% | 1% | 1% | 7% |
| Level 1 slightly thick | 83% | 16% | 0% | 0% | 1% |
| Level 2 mildly thick | 88% | 8% | 1% | 0% | 3% |
| Level 3 moderately thick | 97% | 3% | 0% | 0% | 0% |
| Level 4 extremely thick | 96% | 4% | 0% | 0% | 0% |
| Overall | 87.4% | 9.7% | 0.5% | 0.3% | 2.1% |
Note. Key to PAS scores: 1 = no airway invasion; 2 = penetration into upper laryngeal vestibule with ejection; 3 = penetration into upper laryngeal vestibule without ejection; 4 = penetration to the level of the true vocal folds with ejection; 5 = penetration to the level of the true vocal folds without ejection.
Number of swallows per bolus. Table 3 shows participant level frequencies for the highest number of swallows seen per bolus, by consistency. Single swallows accounted for 80.7% of the data overall; a second swallow was seen on 15.1% of boluses. Patterns of three or four swallows per bolus were rare, occurring on only 3.6% and 0.5% of the boluses, respectively. Chi-square tests found no significant differences overall or by sex in the frequencies of secondary, tertiary, quaternary, or the pooled category of multiple swallows per bolus across consistencies, or between thin versus thicker liquid consistencies.
Table 3.
Participant-level frequencies for the maximum number of swallows per bolus, by consistency.
| Consistency | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Level 0 thin | 68% | 24% | 8% | 0% |
| Level 1 slightly thick | 83% | 14% | 3% | 0% |
| Level 2 mildly thick | 83% | 14% | 1% | 1% |
| Level 3 moderately thick | 84% | 12% | 4% | 0% |
| Level 4 extremely thick | 84% | 12% | 3% | 1% |
| Overall | 80.7% | 15.1% | 3.6% | 0.5% |
PAS. As shown in Table 4, the mode of a participant's worst PAS scores for all consistencies was a score of 1 (no airway invasion), representing 87.4% of the data overall. Scores of 2 (penetration into the upper laryngeal vestibule with ejection) and 4 (penetration to the level of the true vocal folds with ejection) accounted for a further 10% of the data overall. These results are consistent with previous papers showing occasional scores of 2 in individuals with healthy swallowing (Daggett et al., 2006; Molfenter & Steele, 2013). Scores of 3 and 5 (i.e., penetration into the upper laryngeal vestibule and to the level of the true vocal folds, respectively, without ejection) were seen in the remaining 2.6% of cases overall. Scores of 3, 4, and 5 did not occur on the moderately and extremely thick liquids. A chi-square test found a significant difference in the frequency of PAS scores of 3, 4, and 5 by consistency: χ2(df 1) = 16.93, p < .001, with higher frequencies on thinner consistencies. Chi-square tests within consistency found no significant sex differences in the frequency of scores of 3 and 5, indicating penetration without ejection.
LVC integrity. Table 5 shows the frequencies of complete and partial LVC by consistency. Complete LVC was seen in 98.2% of cases overall. Partial LVC was noted on 3%–4% of the thin, slightly thick, and mildly thick consistencies, but on none of the moderately or extremely thick boluses. Chi-square tests found no significant sex differences in the frequency of partial LVC by consistency. There were no occurrences of incomplete LVC in the data set.
Table 5.
Participant-level frequencies for laryngeal vestibule closure (LVC) patterns, by consistency.
| Consistency | Complete | Partial | Incomplete |
|---|---|---|---|
| Level 0 thin | 96% | 4% | 0% |
| Level 1 slightly thick | 97% | 3% | 0% |
| Level 2 mildly thick | 97% | 3% | 0% |
| Level 3 moderately thick | 100% | 0% | 0% |
| Level 4 extremely thick | 100% | 0% | 0% |
| Overall | 98.2% | 1.8% | 0% |
Quantitative Parameters
Appendix Tables A1–A6, which can be found at the end of this article, contain reference interval values for all quantitative parameters, organized by IDDSI Level for easy clinical reference. Each table outlines the median and the 2.5th and 97.5th percentiles for each parameter. Proposed clinical decision limits, demarcating atypical values of potential clinical concern, are shown in the far right columns of each table. These will be explained further in the Discussion section below. Supplemental Material S3 provides additional details, including reference interval boundaries (the 2.5th, 5th, 25th, 50th, 75th, 95th, and 97.5th percentiles, with 90% confidence intervals) by consistency for each parameter, together with the number of data points used in reference interval calculation after outlier removal. Scatter plots showing parameter value distribution by sex and age are also included (Supplemental Material S4). For residue measures, it should be noted that both the modal and median scores were zero in many cases (i.e., no residue), hindering estimation of percentiles; in these cases, minima and maxima are reported together with the frequency (percentage) of nonzero values.
Discussion
In this article, we provide updated reference intervals for quantitative VFSS measures of swallowing in adults without dysphagia, based on analysis using the ASPEKT Method. Use of this method for research involves the following elements intended to promote rigor and transparency: clear operational definitions for the VFSS features to be rated; training of raters to a high level of interrater agreement with regular recalibration; rigorous duplicate rating with raters blinded to each other's decisions and to bolus consistency and volume; randomization of video clips to remove contextual bias related to task position in the examination sequence; and consensus-based resolution of discrepancies exceeding predefined tolerance limits (Steele et al., 2019).
Readers who are familiar with the original manuscript may notice that several of the parameters reported in that paper are not reported here. These include ordinal ratings of bolus location on the key frames of HYB (i.e., “bolus location at swallow onset”) and LVC; residue reported using pixel-based area measures of %-full (relative to the space where residue is located); and residue measures reported using the Normalized Residue Ratio Scale (Pearson et al., 2013). We have decided not to carry forward the bolus location measures in the ASPEKT Method, due to the fact that the original paper showed relatively equal distribution across pharyngeal locations for the bolus location at swallow onset. A ceiling effect was also observed on bolus location at LVC, with 60% of boluses already in the UES, rendering moot the potential clinical concern of an unusually low bolus position at LVC. The Normalized Residue Ratio Scale (NRRS) was included in the previous manuscript for the purposes of comparison, but due to questions regarding validity of the measures of spatial housing area that inform both the %-full measures and the NRRS, we have chosen a different equation for calculating residue severity in the ASPEKT Method, namely, the %(C2–4)2 equation (Steele, Peladeau-Pigeon, Nagy, et al., 2020).
One important thing for readers to note is that the reported measures of central tendency and dispersion for continuous parameters have changed from means and standard deviations in the previous paper (Steele et al., 2019), to medians and the boundaries of the reference interval in this article. One consequence of this shift is that is more challenging to compare the reported reference interval values to means and standard deviations reported by others (e.g., Donohue et al., 2022; K. Kendall, McKenzie, & Leonard, 2000). However, the shift is deliberate, motivated both by best practice guidelines for establishing reference intervals using percentiles (Clinical and Laboratory Standards Institute, 2010), and by the fact that the distributions of many of the VFSS parameters captured by the ASPEKT Method display marked skews. For example, the most common measures expected for residue and pharyngeal area at MPC in healthy swallowing fall close to zero, resulting in positive skews. Conversely, values for maximum UES diameter or LVC duration display negative skews, such that values approaching zero would be unexpected in healthy individuals and might represent clinical concern. These distributional characteristics need to be considered when comparing values across participant groups or within participants over time. Defining the healthy reference interval is a first step toward equipping future analyses with appropriate knowledge of expected data distributions for these parameters. In the subsections that follow, we discuss the results and constraints of the current project that will need consideration in future studies.
Sample Size, Sex, and Age Considerations
At the time of project inception, we targeted a sample size of 80 participants with a plan for comparing quantitative VFSS measures across two age-based cohorts of 40 participants (under and over 60 years of age), with sex balanced within age cohort. The sample size calculation was based on a power analysis using prior data showing age-group differences of 200–400 ms in hyoid movement duration, depending on the consistency of liquid studied (Steele et al., 2012). Over time, as we came to appreciate the distribution of the data across the age continuum (see Supplemental Material S4), it became clear that age 60 years was an arbitrary point for dividing the sample into two age cohorts. As we have learned more about best practices for reference interval calculation, we have come to appreciate that the process often requires the iterative collection of data across multiple samples. The initial determination of reference interval limits requires a sample of at least 40 individuals, while samples of ≥ 120 individuals are preferred to apply the nonparametric method for establishing confidence intervals around those reference interval limits (Adeli et al., 2017; Lahti et al., 2004). The sample analyzed for this study included 78 individuals, half male and half female, without stratification by age cohort. This sample is adequate for initial estimation of reference interval limits; however, it does not meet the threshold for confidence interval calculation using nonparametric methods; in such cases, it is acceptable to use the robust method, as an alternative for deriving meaningful estimates (Horn et al., 1998). Furthermore, given that age was not sampled in a representative manner across subdivisions such as quartiles or decades, we decided that it was inappropriate to attempt partitioning of the currently available data into age-based subgroups or to perform analysis of age as a continuous covariate. Inspection of the scatter plots (see Supplemental Material S4) did not show any obvious separation of the data by sex; therefore, reference interval estimations were not partitioned by sex. These limitations will need to be addressed through the future collection and analysis of additional data using the same methods. Despite these limitations, we feel that it is important to share reference intervals now, based on the available data for this sample of 78 participants. We also hope to advocate for a shift from the use of means and standard deviations to the use of percentile-based reference interval limits as the basis for classifying VFSS results as falling within or outside the ranges expected in healthy swallowing.
One option for assembling larger and sufficiently powered data sets for establishing reference values with confidence would be to pool similar data collected across different studies in so-called “big data” initiatives. For example, the recent publication by Donohue et al. (2022) contains reference value data in the form of means and standard deviations for timing measures on thin liquid barium swallows from a similarly sized sample of 70 community dwelling adults aged 21–87 years (31 male). Similarly, the historic data collected by Leonard and Kendall (K. Kendall, McKenzie, & Leonard, 2000) might provide an opportunity for data pooling. However, before such initiatives can be undertaken, it is important to confirm that the data are truly comparable, and that differences in elements such as participant demographics, barium concentration, stimulus consistency, bolus administration, rating definitions, and methods of summarizing data across task repetitions do not account for variations in results. Donohue et al. (2022) compared timing measure data from their participants to those of the original Steele et al. (2019) study using a modified Cohen's d statistic and identified apparently significant differences for several parameters across studies. Close inspection of the two studies suggests several potential sources of these differences, including differences in participant age, bolus volume, rating definitions (particularly for hyoid movement onset), and summarization of data across task repetitions (i.e., across rather than within bolus volume conditions).
Protocol Considerations
In laboratory medicine, it is recognized that several factors related to the procedures used for data collection and data handling may influence reference values, including pre-examination preparation instructions; time of day of testing; body posture during testing; posttesting sample handling; routine, traceable analytical methods; quality control; and the statistical method(s) by which reference limits are calculated, including the appropriate treatment of outlier values (Adeli et al., 2017; Ceriotti, 2009; Ceriotti & Henny, 2008; Ceriotti et al., 2009; Lahti et al., 2004). This project was designed to use a strict standard VFSS protocol to control for the influence of methodological factors such as barium concentration, participant instructions and cueing, and sip-size. At this stage, the magnitude of differences that may be attributable to these factors remains unclear and we cannot comment regarding the generalizability of the resulting reference values to other protocols. Furthermore, our protocol intentionally included three repetitions of each task with the goal of incorporating normal, within-participant variability in swallowing behavior into the determination of reference values. We acknowledge that the stimulus consistencies were served in blocks of ascending thickness, rather than in a completely randomized sequence; although this may represent a clinically salient order of bolus presentation, order effects cannot be ruled out. Apparent differences in reference values across consistency may include the influence of both protocol-related and physiological fatigue for the thickest consistencies that were served last. A priority for future studies will be to explore the impact of variations in VFSS protocol on reference values.
Bolus Properties
A core premise in dysphagia management is the idea that swallowing physiology varies in response to factors such as bolus volume or consistency (Steele et al., 2015), making intentional manipulations of these factors available as potential clinical interventions. This study provides reference interval data for swallowing of thin liquids and of slightly, mildly, moderately, and extremely thick liquids prepared with a xanthan-gum thickener to meet the flow levels defined for these consistencies by the IDDSI (Cichero et al., 2017, 2020; Hanson et al., 2019). Data were also collected with a starch thickener, but these trials were included for the purpose of answering a separate research question that is not explored in this article. Although statistical comparisons of continuous VFSS parameters across the difference consistencies of the xanthan-gum thickened stimuli are not presented in the manuscript, inspection of the numbers in the reference tables makes it apparent that there are some stimulus effects, most notably prolongation of several timing parameters with thicker consistencies (Gandhi et al., 2023). We encourage readers to remain mindful of the fact that the thin, slightly and mildly thick liquids in this study were consumed by comfortable cup sip, whereas the moderately and extremely thick liquids were served by comfortable spoon-full, using a 5-cc capacity teaspoon. Thus, further research is required to tease apart the potentially confounding effect of smaller bolus volumes on the reference values for the moderately and extremely thick liquids. Similarly, the generalizability of the reference interval data for the thinner consistencies to alternate volumes should not be presumed.
Contrast Media
Another potentially important source of variation in quantitative VFSS measures lies in the barium stimuli or other contrast media that are used in videofluoroscopy. In this study, all stimuli were prepared in a 20% w/v concentration using E-Z-PAQUE powdered barium sulfate. The choice of this barium product was dictated by regulatory and access limitations to other barium products in Canada at the time of data collection. Studies suggest that some VFSS parameters may differ both across barium concentration and across barium products (Dantas et al., 1989; Fink & Ross, 2009; Steele et al., 2022). Whether the magnitude of such differences is large enough to affect reference interval calculations, or to shift parameter values across proposed clinical decision limits remains unknown. A top priority for our research team moving forward is to determine whether the reference intervals from this article generalize to VFSS measures collected using Bracco's Varibar products, a Federal Drug Administration–approved line of barium products intended specifically for oropharyngeal imaging that is widely used in the United States, and which has very recently been approved for use in Canada. Future studies will also be needed to determine whether VFSS parameters vary between barium stimuli and alternative iodine-based contrast media such as diatrizoate, iohexol, or iopamidol.
Potential Use Scenarios for the ASPEKT Reference Tables
At this time, we envision three potential use scenarios for the ASPEKT Reference Tables (see Appendix Tables A2–A6).
Guiding the interpretation of VFSS exams in clinical practice. Clinicians might compare values in their patients to the values in the Appendix Tables, to determine whether a patient's values fall outside the range of values expected in healthy swallowing. We have recently published two articles illustrating this approach to profiling individual patients' swallowing, in case series of patients with dysphagia following traumatic spinal cord injury (Valenzano et al., 2023) and supratentorial ischemic stroke (Smaoui, Peladeau-Pigeon, Mancopes, et al., 2022). When using the reference tables to guide the interpretation of individual patient-level VFSS findings, it is important to bear in mind the previously stated cautions regarding generalizability of these reference values to data collected using different methods (e.g., bolus volumes, cued swallows, brands, and concentrations of contrast media).
In addition to delineating the 2.5th and 97.5th percentile boundaries of the healthy reference interval, each table in the Appendix includes a column on the far right proposing thresholds, or clinical decision limits, to demarcate values of potential clinical concern. Clinical decision limits are thresholds along the distribution of a given parameter that differentiate values for individuals who are symptomatic for a suspected condition from values for healthy individuals without that condition. Clinical decision limits can be used as the basis for diagnosis and for recommending specific interventions (Ceriotti, 2009). For example, blood tests can be used to confirm a diagnosis of Type 1 diabetes, based on a fasting plasma glucose of ≥ 7.0 mmol/L or a glycated hemoglobin (A1C) of ≥ 6.5% (Diabetes Canada Clinical Practice Guidelines Expert Committee et al., 2018). Similarly, prediabetes, an increased risk for developing diabetes, can be diagnosed based on an A1C of 6.0%–6.4% (Diabetes Canada Clinical Practice Guidelines Expert Committee et al., 2018). Decision limits can also be used to measure the effectiveness of interventions for returning biomarker measures to a state consistent with that seen in healthy individuals.
Currently, decision limits have not been established to confirm dysphagia diagnosis, detect emerging risk for dysphagia, or measure dysphagia treatment outcomes. We propose use of either the 25th or the 75th percentiles of the healthy reference distribution as preliminary clinical decision limits for differentiating healthy swallowing from potential dysphagia, depending on directionality of the parameter in question. We recommend that clinicians use the term atypical to describe values that fall beyond these initial clinical decision limits, recognizing that up to one quarter of adults with healthy swallowing will show values beyond one of these limits. The 25th and 75th percentiles are arguably lenient thresholds for identifying values of potential clinical concern, but the alternative of defining values of concern only as those outside the healthy reference interval is arguably overly restrictive. For the time being, we believe it is clinically prudent to begin with the more lenient thresholds, with the added consideration that heightened clinical concern would be warranted for a person who shows atypical values on multiple boluses, across multiple consistencies, and/or on multiple parameters. Over time, we expect that future studies will identify alternative clinical decision limits that achieve optimal sensitivity and specificity for separating cases of concern from cases of no concern using receiver operating characteristic curves. We also expect that different thresholds may be needed to define boundaries of concern for different clinical populations.
One additional comment to note is that the ASPEKT Method was primarily developed as a research method for the comprehensive characterization of swallowing; time constraints in routine clinical practice are likely to make it challenging to complete the full ASPEKT analysis for all patients. To address this concern, we have developed an abbreviated method known as the ASPEKT-C Method (ASPEKT for use in Clinical Practice). The ASPEKT-C Method adopts a stepwise decision-making algorithm to identify impairments in swallowing safety and efficiency, and to confirm whether atypical values in the most likely underlying mechanistic parameters explain those impairments. Full instructions and scoring sheets can be accessed on our lab website (https://steeleswallowinglab.ca/srrl/best-practice/vfss-analysis/).
Determining the frequency of atypical values in research samples with specific diagnoses. A second anticipated use of the reference tables in the Appendix is to support researchers in characterizing the profiles of dysphagia in specific clinical cohorts (sometimes referred to as “phenotypes”), based on the frequency of atypical values for specific VFSS parameters. When the frequency of parameter values beyond the proposed clinical decision limits exceeds 25% in a particular patient group, this may suggest a distinguishing feature of dysphagia in that condition. Examples of this use scenario can be found in several recent publications from our lab, including analyses in preliminary samples of adults with amyotrophic lateral sclerosis (ALS; Waito et al., 2020), Parkinson's disease (PD; Gandhi et al., 2021), oropharyngeal cancer treated with radiation (Barbon et al., 2020), and chronic obstructive pulmonary disease (Mancopes et al., 2020). We have also used the frequency of atypical values in odds ratio analyses and logistic regression models to explore mechanisms of impairment leading to penetration and aspiration (Smaoui et al., 2022; Steele, Peladeau-Pigeon, Barrett, et al., 2020). One limitation of this work to date has been the challenge of recruiting large samples of patients at similar stages of disease progression or severity. Additionally, considerable heterogeneity in both severity and mechanisms of impairment may be expected across patients who share in common a diagnosis.
Comparison of parameter values across groups. The third anticipated use of the reference interval data in this article is use in research to identify significant differences in parameter values across study samples or groups. In our own lab, we have used this approach to compare timing measures in patients with ALS and PD versus healthy controls (Gandhi et al., 2023) and to measure the impact of slightly thick liquids on penetration and aspiration in patients with oropharyngeal cancer (Barbon et al., 2022). A further example of this use scenario is found in the previously mentioned study by Donohue et al. (2022), who used published reference values to calculate effect sizes for across-study differences in timing measures. As noted, this comparison showed differences in some measures that may be attributable to differences in methods. By describing our data collection, data processing, rating, and analysis methods in rigorous detail, we hope to limit the potentially confounding effects of methodological differences in such comparisons. We also encourage researchers to recognize the skewed nature of data distributions for many quantitative VFSS measures and to consider using nonparametric rather than parametric statistics when determining the magnitude of group differences.
Our work to date comparing ASPEKT parameters across groups of research participants has highlighted the importance of understanding the variability seen in healthy swallowing, and of clearly distinguishing disease-related changes in swallowing from those that occur in healthy aging (Gandhi et al., 2021, 2023; Mancopes et al., 2021, 2020). To achieve these goals, substantially larger clinical cohorts will be needed, together with control for age and sex, and explorations of the need to partition data, based on factors such as disease duration and severity. One particularly interesting finding that has emerged from exploring changes in swallowing across the age continuum, and from comparing swallowing in adults with specific medical diagnoses to age-matched controls, is the fact that not all age-related changes occur in the direction of impairment. It is true many of the changes in timing measures that are seen with healthy aging involve prolongation, suggesting slowing of movement. One such change is a longer swallow reaction time (i.e., the interval between BPM and HYB), which is often interpreted to reflect a delay in initiation of the pharyngeal swallow and represents a potential clinical concern. However, analysis of thin liquid swallowing data from the current data set also shows significant age-related prolongation of the durations of LVC closure and UES opening. These changes would appear to be opposite to the direction of impairment, suggesting that airway protection and UES opening are being prolonged in a compensatory fashion to allow for slower bolus transit. For the majority of parameters (except pharyngeal area at rest), we have proposed clinical decision limits on only one side of the distribution of values, thereby indicating the directionality of atypical values that suggest possible clinical concern.
Conclusions
According to the Institute of Medicine of the National Academy of Sciences, diagnostic errors are exceedingly common, and are costly and detrimental to patients and families (Ball & Balogh, 2016). The current standard of care for swallowing assessment shows poor consensus with respect to identifying abnormal swallowing physiology and mechanisms of impairment (Plowman & Humbert, 2018; Vose et al., 2018). Our hope is that the preliminary reference values provided in this article will serve as a valuable resource for clinicians and researchers, to aid in identifying swallowing pathophysiology that deviates from values expected in healthy adults. Future steps in this line of research will include validation of these reference data in a new and larger sample of healthy adults, as well as the collection of data from individuals with clinical conditions where dysphagia contributes to morbidity to guide the determination of clinical decision limits for key physiologic parameters. In so doing, we hope to move the field toward more precise dysphagia diagnostics and to facilitate both the quantification and evidence-based interpretation of the presence, nature, and severity of swallowing impairment to guide the selection of targeted interventions and inform standards of dysphagia care.
Author Contributions
Catriona M. Steele: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft. Mark Bayley: Resources, Writing – review & editing. Mary Kathryn Bohn: Formal analysis, Writing – review & editing. Victoria Higgins: Formal analysis, Writing – review & editing. Melanie Peladeau-Pigeon: Data curation, Investigation, Project administration, Software, Validation, Writing – review & editing. Vathany Kulasingam: Formal analysis, Supervision, Writing – review & editing.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to ethical/legal restrictions. Inquiries regarding access to the data should be directed to the corresponding author.
Supplementary Material
Acknowledgments
Funding for this project came from an operating grant to the first author (C. M. Steele) from the National Institute of Deafness and Other Communication Disorders (R01DC011020). The authors gratefully acknowledge the following individuals for their help with data collection, videofluoroscopy rating, and data management across the course of the project: Andrea Bandini, Carly A. E. Barbon, Emily Barrett, Kristyn Emmerzael, Pooja Gandhi, Brittany Guida, Andrea Guran, Renata Mancopes, Ashwini M. Namasivayam-MacDonald, Weslania V. Nascimento, Vanessa Panes, Sana Smaoui, Todd Reesor, Michelle M. Simmons, Danielle Sutton, Melanie S. Tapson, Teresa J. Valenzano, Ashley A. Waito, and Talia S. Wolkin.
Appendix
ASPEKT Method Reference Values (Version Date April 8, 2023)
Table A1.
ASPEKT method reference values for categorical parameters (number of swallows per bolus; Penetration–Aspiration Scale scores; and LVC integrity) by consistency.
| Parameter name | Consistency | Mode | Atypical | Proposed scores of clinical concern |
|---|---|---|---|---|
| Number of swallows per bolus | Thin | 1 | 2 or more | 3 or higher |
| Slightly thick | ||||
| Mildly thick | ||||
| Moderately thick | ||||
| Extremely thick | ||||
| Penetration–Aspiration Scale scores | Thin | 1 | 2 or 4 | 3, 5, or higher |
| Slightly thick | ||||
| Mildly thick | ||||
| Moderately thick | ||||
| Extremely thick | ||||
| Laryngeal vestibule closure (LVC) Integrity |
Thin | Complete | Partial | Incomplete |
| Slightly thick | ||||
| Mildly thick | ||||
| Moderately thick | ||||
| Extremely thick |
Table A2.
ASPEKT method reference values for quantitative VFSS parameters on IDDSI Level 0 thin liquids.
| Parameter name (unit) | Healthy reference interval |
Proposed clinical decision limit(s) |
|||
|---|---|---|---|---|---|
| 2.5%ile (p2.5) | 50%ile (p50) | 97.5%ile (p97.5) | Percentile | Threshold | |
| Timing measures: ϕ | |||||
| Swallow reaction time (bolus passing mandible to hyoid burst) (ms) | −33 | 133 | 967 | p75 | > 400 |
| Hyoid burst–to–UES-opening interval (ms) | 33 | 100 | 200 | p75 | > 133 |
| Upper esophageal sphincter (UES) opening duration (ms) | 400 | 467 | 634 | p25 | < 434 |
| Time-to-LVC (time from hyoid burst to LVC) (ms) | 33 | 133 | 267 | p75 | > 167 |
| Laryngeal vestibule closure (LVC) duration (ms) | 300 | 467 | 934 | p25 | < 400 |
| Pixel-based measures: | |||||
| Vallecular residue %(C2–4)2 | 0.0 | 0.1 | 1.8 | p75 | > 0.7 |
| Pyriform sinus residue %(C2–4)2 | 0.0§ | 0.0 | 1.9 | p75§ | > 0.5 |
| Other pharyngeal residue %(C2–4)2 | 0.0§ | 0.0 | 1.3§ | p75§ | > 0.3 |
| Total pharyngeal residue %(C2–4)2 | 0.0 | 0.2 | 4.5 | p75 | > 1.7 |
| Maximum UES diameter %(C2–4)2 | 9 | 21 | 32 | p25 | < 17 |
| Pharyngeal area at maximum pharyngeal constriction (PhAMPC) %(C2–4)2 | 0.0 | 1.0 | 6.2 | p75 | > 2.7 |
| Pharyngeal area at rest %(C2–4)2 | 37 | 59 | 92 | p25 | < 51 |
| p75 | > 70 | ||||
| Hyoid peak position: X coordinate %(C2–4)* | 122 | 142 | 182 | p25 | < 134 |
| Hyoid peak position: Y coordinate %(C2–4)* | 40 | 88 | 136 | p25 | < 73 |
| Hyoid peak position: XY coordinate %(C2–4)* | 148 | 172 | 197 | p25 | < 163 |
| Hyoid Speed: XY %(C2–4)/sϒ | 71 | 122 | 180 | p25 | < 103 |
Timing parameters are derived from frame-based measurement and converted into milliseconds.
We selected the frame showing the furthest hyoid XY position relative to the anterior–inferior corner of C4 as the hyoid peak position. Values reported are showing the X, Y, and XY coordinates of hyoid on that frame.
Rate of change in hyoid position from burst onset to hyoid peak XY position divided by duration of the hyoid burst movement from burst onset to peak position.
There were insufficient examples of pyriform sinus residue and other pharyngeal residue on slightly thick liquid swallows in the healthy reference data set to identify these percentiles with confidence. For the 2.5th and 97.5th percentiles, minimum and maximum values are substituted, respectively. We have proposed clinical decision limits based on other consistencies.
Table A3.
ASPEKT method reference values for quantitative VFSS parameters on IDDSI Level 1 slightly thick liquids.
| Parameter name (unit) | Healthy reference interval |
Proposed clinical decision limit(s) |
||||
|---|---|---|---|---|---|---|
| 2.5%ile (p2.5) | 50%ile (p50) | 97.5%ile (p97.5) | Percentile | Threshold | ||
| Timing measures: ϕ | ||||||
| Swallow reaction time (bolus passing mandible to hyoid burst) (ms) | −67 | 133 | 934 | p75 | > 400 | |
| Hyoid burst–to–UES-opening interval (ms) | 33 | 133 | 200 | p75 | > 167 | |
| Upper esophageal sphincter (UES) opening duration (ms) | 334 | 467 | 634 | p25 | < 400 | |
| Time-to-LVC (time from hyoid burst to LVC) (ms) | −100 | 133 | 367 | p75 | > 234 | |
| Laryngeal vestibule closure (LVC) duration (ms) | 300 | 467 | 767 | p25 | < 400 | |
| Pixel-based measures: | ||||||
| Vallecular residue %(C2–4)2 | 0.0 | 0.1 | 2.8 | p75 | > 1.0 | |
| Pyriform sinus residue %(C2–4)2 | 0.0§ | 0.0 | 1.9 | p75 | > 0.6 | |
| Other pharyngeal residue %(C2–4)2 | 0.0§ | 0.0 | 0.0§ | p75§ | > 0.3 | |
| Total pharyngeal residue %(C2–4)2 | 0.0 | 0.3 | 5.1 | p75 | > 1.9 | |
| Maximum UES diameter %(C2–4)2 | 9 | 19 | 31 | p25 | < 15 | |
| Pharyngeal area at maximum pharyngeal constriction (PhAMPC) %(C2–4)2 | 0.0 | 1.2 | 5.0 | p75 | > 2.5 | |
| Pharyngeal area at rest %(C2–4)2 | 37 | 59 | 92 | p25 | < 51 | |
| p75 | > 70 | |||||
| Hyoid peak position: X coordinate %(C2–4)* | 119 | 141 | 181 | p25 | < 132 | |
| Hyoid peak position: Y coordinate %(C2–4)* | 39 | 88 | 143 | p25 | < 70 | |
| Hyoid peak position: XY coordinate %(C2–4)* | 144 | 168 | 206 | p25 | < 159 | |
| Hyoid speed: XY %(C2–4)/sϒ | 67 | 113 | 209 | p25 | < 94 | |
Timing parameters are derived from a frame-based measurement and converted into milliseconds.
We selected the frame showing the furthest hyoid XY position relative to the anterior–inferior corner of C4 as the hyoid peak position. Values reported are showing the X, Y, and XY coordinates of hyoid on that frame.
Rate of change in hyoid position from burst onset to hyoid peak XY position divided by duration of the hyoid burst movement from burst onset to peak position.
There were insufficient examples of pyriform sinus residue and other pharyngeal residue on slightly thick liquid swallows in the healthy reference data set to identify these percentiles with confidence. For the 2.5th percentile, minimum values are substituted. We have proposed clinical decision limits based on other consistencies.
Table A4.
ASPEKT method reference values for quantitative VFSS parameters on IDDSI Level 2 mildly thick liquids.
| Parameter name (unit) | Healthy reference interval |
Proposed clinical decision limit(s) |
|||
|---|---|---|---|---|---|
| 2.5%ile (p2.5) | 50%ile (p50) | 97.5%ile (p97.5) | Percentile | Threshold | |
| Timing measures: ϕ | |||||
| Swallow reaction time (bolus passing mandible to hyoid burst) (ms) | −33 | 167 | 1301 | p75 | > 534 |
| Hyoid burst–to–UES-opening interval (ms) | 33 | 133 | 234 | p75 | > 167 |
| Upper esophageal sphincter (UES) opening duration (ms) | 334 | 467 | 634 | p25 | < 400 |
| Time-to-LVC (time from hyoid burst to LVC) (ms) | −33 | 133 | 367 | p75 | > 200 |
| Laryngeal vestibule closure (LVC) duration (ms) | 300 | 467 | 734 | p25 | < 400 |
| Pixel-based measures: | |||||
| Vallecular residue %(C2–4)2 | 0.0 | 0.1 | 3.0 | p75 | > 1.1 |
| Pyriform sinus residue %(C2–4)2 | 0.0 | 0.1 | 1.1 | p75 | > 0.4 |
| Other pharyngeal residue %(C2–4)2 | 0.0§ | 0.4 | 1.2 | p75 | > 0.6 |
| Total pharyngeal residue %(C2–4)2 | 0.0 | 0.6 | 5.3 | p75 | > 2.2 |
| Maximum UES diameter %(C2–4)2 | 9 | 19 | 30 | p25 | < 15 |
| Pharyngeal area at maximum pharyngeal constriction (PhAMPC) %(C2–4)2 | 0.0 | 1.2 | 7.5 | p75 | > 3.3 |
| Pharyngeal area at rest %(C2–4)2 | 37 | 59 | 92 | p25 | < 51 |
| p75 | > 70 | ||||
| Hyoid peak position: X coordinate %(C2–4)* | 121 | 143 | 179 | p25 | < 134 |
| Hyoid peak position: Y coordinate %(C2–4)* | 52 | 90 | 140 | p25 | < 77 |
| Hyoid peak position: XY coordinate %(C2–4)* | 143 | 173 | 201 | p25 | < 161 |
| Hyoid speed: XY %(C2–4)/sϒ | 73 | 114 | 189 | p25 | < 96 |
Timing parameters are derived a frame-based measurement and converted into milliseconds.
We selected the frame showing the furthest hyoid XY position relative to the anterior–inferior corner of C4 as the hyoid peak position. Values reported are showing the X, Y, and XY coordinates of hyoid on that frame.
Rate of change in hyoid position from burst onset to hyoid peak XY position divided by duration of the hyoid burst movement from burst onset to peak position.
There were insufficient examples of other pharyngeal residue on mildly thick liquid swallows in the healthy reference data set to identify the 2.5th percentile with confidence. The minimum value is shown instead.
Table A5.
ASPEKT method reference values for quantitative VFSS parameters on IDDSI Level 3 moderately thick liquids/liquidized foods.
| Parameter name (unit) | Healthy reference interval |
Proposed clinical decision limit(s) |
|||
|---|---|---|---|---|---|
| 2.5%ile (p2.5) | 50%ile (p50) | 97.5%ile (p97.5) | Percentile | Threshold | |
| Timing measures: ϕ | |||||
| Swallow reaction time (bolus passing mandible to hyoid burst) (ms) | −67 | 267 | 1468 | p75 | > 667 |
| Hyoid burst–to–UES-opening interval (ms) | 67 | 167 | 267 | p75 | > 200 |
| Upper esophageal sphincter (UES) opening duration (ms) | 300 | 400 | 534 | p25 | < 367 |
| Time-to-LVC (time from hyoid burst to LVC) (ms) | 0 | 167 | 300 | p75 | > 200 |
| Laryngeal vestibule closure (LVC) duration (ms) | 300 | 434 | 734 | p25 | < 400 |
| Pixel-based measures: | |||||
| Vallecular residue %(C2–4)2 | 0.0§ | 0.0 | 1.8 | p75 | > 0.6 |
| Pyriform sinus residue %(C2–4)2 | 0.0§ | 0.0 | 1.4 | p75 | > 0.5 |
| Other pharyngeal residue %(C2–4)2 | 0.0§ | 0.2 | 1.8§ | p75 | > 0.6 |
| Total pharyngeal residue %(C2–4)2 | 0.0 | 0.2 | 4.3 | p75 | > 1.6 |
| Maximum UES diameter %(C2–4)2 | 8 | 15 | 26 | p25 | < 12 |
| Pharyngeal area at maximum pharyngeal constriction (PhAMPC) %(C2–4)2 | 0.0 | 0.7 | 4.9 | p75 | > 2.1 |
| Pharyngeal area at rest %(C2–4)2 | 37 | 59 | 92 | p25 | < 51 |
| p75 | > 70 | ||||
| Hyoid peak position: X coordinate %(C2–4)* | 120 | 140 | 176 | p25 | < 132 |
| Hyoid peak position: Y coordinate %(C2–4)* | 47 | 88 | 130 | p25 | < 72 |
| Hyoid peak position: XY coordinate %(C2–4)* | 139 | 170 | 200 | p25 | < 158 |
| Hyoid speed: XY %(C2–4)/sϒ | 61 | 104 | 157 | p25 | < 89 |
Timing parameters are derived from a frame-based measurement and converted into milliseconds.
We selected the frame showing the furthest hyoid XY position relative to the anterior–inferior corner of C4 as the hyoid peak position. Values reported are showing the X, Y, and XY coordinates of hyoid on that frame.
Rate of change in hyoid position from burst onset to hyoid peak XY position divided by duration of the hyoid burst movement from burst onset to peak position.
There were insufficient examples of residue on moderately thick liquid swallows in the healthy reference data set to identify these percentiles with confidence. For the 2.5th percentile, minimum values are substituted. For the 95th percentile, values for mildly thick liquid are substituted. We have proposed clinical decision limits based on other consistencies.
Table A6.
ASPEKT method reference values for quantitative VFSS parameters on IDDSI Level 4 extremely thick liquids/pureed foods.
| Parameter name (unit) | Healthy reference interval |
Proposed clinical decision limit(s) |
|||
|---|---|---|---|---|---|
| 2.5%ile (p2.5) | 50%ile (p50) | 97.5%ile (p97.5) | Percentile | Threshold | |
| Timing measures: ϕ | |||||
| Swallow reaction time (bolus passing mandible to hyoid burst) (ms) | −67 | 367 | 1701 | p75 | > 801 |
| Hyoid burst–to–UES-opening interval (ms) | 67 | 167 | 267 | p75 | > 200 |
| Upper esophageal sphincter (UES) opening duration (ms) | 300 | 400 | 534 | p25 | < 367 |
| Time-to-LVC (time from hyoid burst to LVC) (ms) | 33 | 133 | 267 | p75 | > 167 |
| Laryngeal vestibule closure (LVC) duration (ms) | 334 | 434 | 634 | p25 | < 400 |
| Pixel-based measures: | |||||
| Vallecular residue %(C2–4)2 | 0.0 | 0.0 | 1.5 | p75 | > 0.5 |
| Pyriform sinus residue %(C2–4)2 | 0.0§ | 0.0 | 1.4§ | p75 | > 0.5§ |
| Other pharyngeal residue %(C2–4)2 | 0.0§ | 0.1 | 1.1§ | p75 | > 0.6§ |
| Total pharyngeal residue %(C2–4)2 | 0.0 | 0.1 | 4.2 | p75 | > 1.5 |
| Maximum UES diameter %(C2–4)2 | 9 | 16 | 26 | p25 | < 14 |
| Pharyngeal area at maximum pharyngeal constriction (PhAMPC) %(C2–4)2 | 0.0 | 0.2 | 3.8 | p75 | > 1.4 |
| Pharyngeal area at rest %(C2–4)2 | 37 | 59 | 92 | p25 | < 51 |
| p75 | > 70 | ||||
| Hyoid peak position: X coordinate %(C2–4)* | 120 | 143 | 177 | p25 | < 134 |
| Hyoid peak position: Y coordinate %(C2–4)* | 42 | 87 | 133 | p25 | < 72 |
| Hyoid peak position: XY coordinate %(C2–4)* | 144 | 168 | 202 | p25 | < 160 |
| Hyoid speed: XY %(C2–4)/sϒ | 67 | 105 | 203 | p25 | < 89 |
Timing parameters are derived a frame-based measurement and converted into milliseconds.
We selected the frame showing the furthest hyoid XY position relative to the anterior–inferior corner of C4 as the hyoid peak position. Values reported are showing the X, Y, and XY coordinates of hyoid on that frame.
Rate of change in hyoid position from burst onset to hyoid peak XY position divided by duration of the hyoid burst movement from burst onset to peak position.
There were insufficient examples of residue on extremely thick liquid swallows in the healthy reference data set to identify these percentiles with confidence. For the 2.5th percentile, minimum values are substituted. For the 95th percentile, values for moderately thick liquid are substituted. We have proposed clinical decision limits based on other consistencies.
Funding Statement
Funding for this project came from an operating grant to the first author (C. M. Steele) from the National Institute of Deafness and Other Communication Disorders (R01DC011020).
References
- Adeli, K., Higgins, V., Trajcevski, K., & White-Al Habeeb, N. (2017). The Canadian laboratory initiative on pediatric reference intervals: A CALIPER white paper. Critical Reviews in Clinical Laboratory Sciences, 54(6), 358–413. 10.1080/10408363.2017.1379945 [DOI] [PubMed] [Google Scholar]
- Altman, K. W., Yu, G. P., & Schaefer, S. D. (2010). Consequence of dysphagia in the hospitalized patient. Archives of Otolaryngology—Head & Neck Surgery, 136(8), 784–789. 10.1001/archoto.2010.129 [DOI] [PubMed] [Google Scholar]
- Ball, J. R., & Balogh, E. (2016). Improving diagnosis in health care: Highlights of a report from the National Academies of sciences, engineering, and medicine. Annals of Internal Medicine, 164(1), 59–61. 10.7326/M15-2256 [DOI] [PubMed] [Google Scholar]
- Barbon, C. E. A., Chepeha, D. B., Hope, A. J., Peladeau-Pigeon, M., Waito, A. A., & Steele, C. M. (2020). Mechanisms of impaired swallowing on thin liquids following radiation treatment for oropharyngeal cancer. Journal of Speech, Language, and Hearing Research, 63(9), 2870–2879. 10.1044/2020_JSLHR-19-00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbon, C. E. A., Chepeha, D. B., Hope, A. J., Peladeau-Pigeon, M., Waito, A. A., & Steele, C. M. (2022). Determining the impact of thickened liquids on swallowing in patients undergoing irradiation for oropharynx cancer. Otolaryngology—Head & Neck Surgery, 166(3), 511–514. 10.1177/01945998211010435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbon, C. E. A., & Steele, C. M. (2019). Characterizing the flow of thickened barium and non-barium liquid recipes using the IDDSI flow test. Dysphagia, 34(1), 73–79. 10.1007/s00455-018-9915-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriotti, F. (2009). Common reference intervals: The IFCC position. Clinical Biochemistry, 42(4–5), Article 297. 10.1016/j.clinbiochem.2008.09.017 [DOI] [PubMed] [Google Scholar]
- Ceriotti, F., & Henny, J. (2008). “Are my laboratory results normal?” Considerations to be made concerning reference intervals and decision limits. The Journal of the International Federation of Clinical Chemistry and Laboratory Medicine, 19(2), 106–114. https://www.ncbi.nlm.nih.gov/pubmed/27683305 [PMC free article] [PubMed] [Google Scholar]
- Ceriotti, F., Hinzmann, R., & Panteghini, M. (2009). Reference intervals: The way forward. Annals of Clinical Biochemistry, 46(1), 8–17. 10.1258/acb.2008.008170 [DOI] [PubMed] [Google Scholar]
- Cichero, J. A. Y., Lam, P. T. L., Chen, J., Dantas, R. O., Duivestein, J., Hanson, B., Kayashita, J., Pillay, M., Riquelme, L. F., Steele, C. M., & Vanderwegen, J. (2020). Release of updated International Dysphagia Diet Standardisation Initiative framework (IDDSI 2.0). Journal of Texture Studies, 51(1), 195–196. 10.1111/jtxs.12481 [DOI] [PubMed] [Google Scholar]
- Cichero, J. A. Y., Lam, P., Steele, C. M., Hanson, B., Chen, J., Dantas, R. O., Duivestein, J., Kayashita, J., Lecko, C., Murray, J., Pillay, M., Riquelme, L., & Stanschus, S. (2017). Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: The IDDSI framework. Dysphagia, 32(2), 293–314. 10.1007/s00455-016-9758-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clave, P., & Shaker, R. (2015). Dysphagia: Current reality and scope of the problem. Nature Reviews Gastroenterology Hepatology, 12(5), 259–270. 10.1038/nrgastro.2015.49 [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute. (2010). EP28-A3c: Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved guideline (3rd ed.). https://clsi.org/standards/products/method-evaluation/documents/ep28/
- Daggett, A., Logemann, J., Rademaker, A., & Pauloski, B. (2006). Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia, 21(4), 270–274. 10.1007/s00455-006-9051-6 [DOI] [PubMed] [Google Scholar]
- Dantas, R. O., Dodds, W. J., Massey, B. T., & Kern, M. K. (1989). The effect of high- vs low-density barium preparations on the quantitative features of swallowing. American Journal of Roentgenology, 153(6), 1191–1195. 10.2214/ajr.153.6.1191 [DOI] [PubMed] [Google Scholar]
- Diabetes Canada Clinical Practice Guidelines Expert Committee, Punthakee, Z., Goldenberg, R., & Katz, P. (2018). Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Canadian Journal of Diabetes, 42(Suppl. 1), S10–S15. 10.1016/j.jcjd.2017.10.003 [DOI] [PubMed] [Google Scholar]
- Donohue, C., Khalifa, Y., Mao, S., Perera, S., Sejdic, E., & Coyle, J. L. (2022). Establishing reference values for temporal kinematic swallow events across the lifespan in healthy community dwelling adults using high-resolution cervical auscultation. Dysphagia, 37(3), 664–675. 10.1007/s00455-021-10317-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink, T. A., & Ross, J. B. (2009). Are we testing a true thin liquid? Dysphagia, 24(3), 285–289. 10.1007/s00455-008-9203-y [DOI] [PubMed] [Google Scholar]
- Gandhi, P., Mancopes, R., Sutton, D., Plowman, E. K., & Steele, C. M. (2021). The frequency of atypical and extreme values for pharyngeal phase swallowing measures in mild Parkinson disease compared to healthy aging. Journal of Speech, Language, and Hearing Research, 64(8), 3032–3050. 10.1044/2021_JSLHR-21-00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi, P., Plowman, E. K., & Steele, C. M. (2023). Differences in pharyngeal swallow event timing: Healthy aging, Parkinson disease and amyotrophic lateral sclerosis. Laryngoscope Investigative Otolaryngology, 8(2), 466–477. 10.1002/lio2.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes, R., Azola, A., Macrae, P., Sunday, K., Mejia, V., Vose, A., & Humbert, I. A. (2017). Examination of swallowing maneuver training and transfer of practiced behaviors to laryngeal vestibule kinematics in functional swallowing of healthy adults. Physiology & Behavior, 174, 155–161. 10.1016/j.physbeh.2017.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, B., Steele, C. M., Lam, P., & Cichero, J. A. Y. (2019). Fluid testing methods recommended by IDDSI. Dysphagia, 34(5), 716–717. 10.1007/s00455-018-9957-9 [DOI] [PubMed] [Google Scholar]
- Horn, P. S., Pesce, A. J., & Copeland, B. E. (1998). A robust approach to reference interval estimation and evaluation. Clinical Chemistry, 44(3), 622–631. https://www.ncbi.nlm.nih.gov/pubmed/9510871 [PubMed] [Google Scholar]
- Humbert, I. A., Sunday, K. L., Karagiorgos, E., Vose, A. K., Gould, F., Greene, L., Azola A., Tolar A., & Rivet A. (2018). Swallowing kinematic differences across frozen, mixed, and ultrathin liquid boluses in healthy adults: Age, sex, and normal variability. Journal of Speech, Language, and Hearing Research, 61(7), 1544–1559. 10.1044/2018_JSLHR-S-17-0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahrilas, P. J., Logemann, J. A., Lin, S., & Ergun, G. A. (1992). Pharyngeal clearance during swallowing: A combined manometric and videofluoroscopic study. Gastroenterology, 103(1), 128–136. 10.1016/0016-5085(92)91105-D [DOI] [PubMed] [Google Scholar]
- Kendall, K. A., McKenzie, S., Leonard, R. J., Goncalves, M. I., & Walker, A. (2000). Timing of events in normal swallowing: A videofluoroscopic study. Dysphagia, 15(2), 74–83. 10.1007/s004550010004 [DOI] [PubMed] [Google Scholar]
- Kendall, K., McKenzie, S., & Leonard, R. (2000). Dynamic swallow study: Objective measures and normative data. In Leonard R. & Kendall K. (Eds.), Dysphagia assessment and treatment planning: A team approach (pp. 101–160). Singular. [Google Scholar]
- Lahti, A., Petersen, P. H., Boyd, J. C., Rustad, P., Laake, P., & Solberg, H. E. (2004). Partitioning of nongaussian-distributed biochemical reference data into subgroups. Clinical Chemistry, 50(5), 891–900. 10.1373/clinchem.2003.027953 [DOI] [PubMed] [Google Scholar]
- Leonard, R. (2019). Predicting aspiration risk in patients with dysphagia: Evidence from fluoroscopy. Laryngoscope Investigative Otolaryngology, 4(1), 83–88. 10.1002/lio2.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, R., Kendall, K. A., & McKenzie, S. (2004). Structural displacements affecting pharyngeal constriction in nondysphagic elderly and nonelderly adults. Dysphagia, 19(2), 133–141. 10.1007/s00455-003-0508-6 [DOI] [PubMed] [Google Scholar]
- Leonard, R. J., Kendall, K. A., McKenzie, S., Goncalves, M. I., & Walker, A. (2000). Structural displacements in normal swallowing: A videofluoroscopic study. Dysphagia, 15(3), 146–152. 10.1007/s004550010017 [DOI] [PubMed] [Google Scholar]
- Logemann, J. A., Pauloski, B. R., Rademaker, A. W., Colangelo, L. A., Kahrilas, P. J., & Smith, C. H. (2000). Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. Journal of Speech, Language, and Hearing Research, 43, 1264–1274. 10.1044/jslhr.4305.1264 [DOI] [PubMed] [Google Scholar]
- Logemann, J. A., Pauloski, B. R., Rademaker, A. W., & Kahrilas, P. J. (2002). Oropharyngeal swallow in younger and older women: Videofluoroscopic analysis. Journal of Speech, Language, and Hearing Research, 45, 434–445. 10.1044/1092-4388(2002/034) [DOI] [PubMed] [Google Scholar]
- Mancopes, R., Gandhi, P., Smaoui, S., & Steele, C. M. (2021). Which physiological swallowing parameters change with healthy aging? OBM Geriatrics, 5(1), Article 153. 10.21926/obm.geriatr.2101153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancopes, R., Peladeau-Pigeon, M., Barrett, E., Guran, A., Smaoui, S., Pasqualoto, A. S., & Steele, C. M. (2020). Quantitative videofluoroscopic analysis of swallowing physiology and function in individuals with chronic obstructive pulmonary disease. Journal of Speech, Language, and Hearing Research, 63(11), 3643–3658. 10.1044/2020_JSLHR-20-00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Harris, B., Bonilha, H. S., Brodsky, M. B., Francis, D. O., Fynes, M. M., Martino, R., O'Rourke, A. K., Rogus-Pulia, N. M., Spinazzi, N. A., & Zarzour, J. (2021). The modified barium swallow study for oropharyngeal dysphagia: Recommendations from an interdisciplinary expert panel. Perspectives of the ASHA Special Interest Groups, 6(3), 610–619. 10.1044/2021_PERSP-20-00303 [DOI] [Google Scholar]
- McCullough, G. H., Rosenbek, J. C., Wertz, R. T., McCoy, S., Mann, G., & McCullough, K. (2005). Utility of clinical swallowing examination measures for detecting aspiration post-stroke. Journal of Speech, Language, and Hearing Research, 48(6), 1280–1293. 10.1044/1092-4388(2005/089) [DOI] [PubMed] [Google Scholar]
- McCullough, G. H., Wertz, R. T., & Rosenbek, J. C. (2001). Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. Journal of Communication Disorders, 34(1–2), 55–72. 10.1016/s0021-9924(00)00041-1 [DOI] [PubMed] [Google Scholar]
- McCullough, G. H., Wertz, R. T., Rosenbek, J. C., & Dinneen, C. (1999). Clinicians' preferences and practices in conducting clinical/bedside and videofluoroscopic swallowing examinations in an adult, neurogenic population. American Journal of Speech Language Pathology, 8, 149–163. 10.1044/1058-0360.0802.149 [DOI] [Google Scholar]
- Molfenter, S. M., & Steele, C. M. (2013). Variation in temporal measures of swallowing: Sex and volume effects. Dysphagia, 28(2), 226–233. 10.1007/s00455-012-9437-6 [DOI] [PubMed] [Google Scholar]
- Molfenter, S. M., & Steele, C. M. (2014). Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. Journal of Speech, Language, and Hearing Research, 57(3), 768–778. 10.1044/2014_JSLHR-S-13-0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, J. J.-X., Steele, C. M., & Duizer, L. M. (2018). Sensory characteristics of liquids thickened with commercial thickeners to levels specified in the International Dysphagia Diet Standardization Initiative (IDDSI) framework. Food Hydrocolloids, 79, 208–217. 10.1016/j.foodhyd.2017.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozarda, Y. (2016). Reference intervals: Current status, recent developments and future considerations. Biochemia Medica, 26(1), 5–16. 10.11613/BM.2016.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, J. B. (1998). Bolus aggregation in the oropharynx does not depend on gravity. Archives of Physical Medicine and Rehabilitation, 79, 691–696. 10.1016/S0003-9993(98)90046-6 [DOI] [PubMed] [Google Scholar]
- Patel, D. A., Krishnaswami, S., Steger, E., Conover, E., Vaezi, M. F., Ciucci, M. R., & Francis, D. O. (2018). Economic and survival burden of dysphagia among inpatients in the United States. Diseases of the Esophagus, 31(1), 1–7. 10.1093/dote/dox131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson, W. G., Jr., Molfenter, S. M., Smith, Z. M., & Steele, C. M. (2013). Image-based measurement of post-swallow residue: The normalized residue ratio scale. Dysphagia, 28(2), 167–177. 10.1007/s00455-012-9426-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman, E. K., & Humbert, I. A. (2018). Elucidating inconsistencies in dysphagia diagnostics: Redefining normal. International Journal of Speech Language Pathology, 20(3), 310–317. 10.1080/17549507.2018.1461931 [DOI] [PubMed] [Google Scholar]
- Power, M. L., Hamdy, S., Singh, S., Tyrrell, P. J., Turnbull, I., & Thompson, D. G. (2007). Deglutitive laryngeal closure in stroke patients. Journal of Neurology, Neurosurgery, & Psychiatry, 78(2), 141–146. 10.1136/jnnp.2006.101857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademaker, A. W., Pauloski, B. R., Logemann, J. A., & Shanahan, T. K. (1994). Oropharyngeal swallow efficiency as a representative measure of swallowing function. Journal of Speech and Hearing Research, 37, 314–325. 10.1044/jshr.3702.314 [DOI] [PubMed] [Google Scholar]
- Robbins, J., Hamilton, J. W., Lof, G. L., & Kempster, G. B. (1992). Oropharyngeal swallowing in normal adults of different ages. Gastroenterology, 103(3), 823–829. 10.1016/0016-5085(92)90013-O [DOI] [PubMed] [Google Scholar]
- Rosenbek, J. C., Robbins, J. A., Roecker, E. B., Coyle, J. L., & Wood, J. L. (1996). A penetration–aspiration scale. Dysphagia, 11(2), 93–98. 10.1007/BF00417897 [DOI] [PubMed] [Google Scholar]
- Smaoui, S., Mancopes, R., Simmons, M. M., Peladeau-Pigeon, M., & Steele, C. M. (2023). The influence of sex, age, and repeated measurement on pixel-based measures of pharyngeal area at rest. Journal of Speech, Language, and Hearing Research, 66(3), 863–871. 10.1044/2022_JSLHR-22-00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaoui, S., Peladeau-Pigeon, M., Mancopes, R., Sutton, D., Richardson, D., & Steele, C. M. (2022). Profiles of swallowing impairment in a cohort of patients with reduced tongue strength within 3 months of cerebral ischemic stroke. Journal of Speech, Language, and Hearing Research, 65(7), 2399–2411. 10.1044/2022_JSLHR-21-00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaoui, S., Peladeau-Pigeon, M., & Steele, C. M. (2021). Variations in hyoid kinematics across liquid consistencies in healthy swallowing. Journal of Speech, Language, and Hearing Research, 64(1), 51–58. 10.1044/2020_JSLHR-20-00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaoui, S., Peladeau-Pigeon, M., & Steele, C. M. (2022). Determining the relationship between hyoid bone kinematics and airway protection in swallowing. Journal of Speech, Language, and Hearing Research, 65(2), 419–430. 10.1044/2021_JSLHR-21-00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, C. M., Alsanei, W. A., Ayanikalath, S., Barbon, C. E., Chen, J., Cichero, J. A., Coutts, K., Dantas, R. O., Duivestein, J., Giosa, L., Hanson, B., Lam, P., Lecko, C., Leigh, C., Nagy, A., Namasivayam, A. M., Nascimento, W. V., Odendaal, I., Smith, C. H., & Wang, H. (2015). The influence of food texture and liquid consistency modification on swallowing physiology and function: A systematic review. Dysphagia, 30(1), 2–26. 10.1007/s00455-014-9578-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, C. M., Barrett, E., & Peladeau-Pigeon, M. (2022). Which videofluoroscopy parameters are susceptible to the influence of differences in barium product and concentration? American Journal of Speech-Language Pathology, 31(5), 2145–2158. 10.1044/2022_AJSLP-22-00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, C. M., & Grace-Martin, K. (2017). Reflections on clinical and statistical use of the Penetration–Aspiration Scale. Dysphagia, 32(5), 601–616. 10.1007/s00455-017-9809-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, C. M., Peladeau-Pigeon, M., Barbon, C. A. E., Guida, B. T., Namasivayam-MacDonald, A. M., Nascimento, W. V., Smaoui, S., Tapson, M. S., Valenzano, T. J., Waito, A. A., & Wolkin, T. S. (2019). Reference values for healthy swallowing across the range from thin to extremely thick liquids. Journal of Speech, Language, and Hearing Research, 62(5), 1338–1363. 10.1044/2019_JSLHR-S-18-0448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, C. M., Peladeau-Pigeon, M., Barrett, E., & Wolkin, T. S. (2020). The risk of penetration–aspiration related to residue in the pharynx. American Journal of Speech Language Pathology, 29(3), 1608–1617. 10.1044/2020_AJSLP-20-00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, C. M., Peladeau-Pigeon, M., Nagy, A., & Waito, A. A. (2020). Measurement of pharyngeal residue from lateral view videofluoroscopic images. Journal of Speech, Language, and Hearing Research, 63(5), 1404–1415. 10.1044/2020_JSLHR-19-00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele, C. M., Sasse, C., & Bressmann, T. (2012). Tongue-pressure and hyoid movement timing in healthy liquid swallowing. International Journal of Language & Communication Disorders, 47(1), 77–83. 10.1111/j.1460-6984.2011.00082.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan, K., Cordier, R., Brown, T., & Speyer, R. (2019). Psychometric properties of visuoperceptual measures of videofluoroscopic and fibre-endoscopic evaluations of swallowing: A systematic review. Dysphagia, 34(1), 2–33. 10.1007/s00455-018-9918-3 [DOI] [PubMed] [Google Scholar]
- Valenzano, T. J., Smaoui, S., Peladeau-Pigeon, M., Barbon, C. E. A., Craven, B. C., & Steele, C. M. (2023). Using reference values to identify profiles of swallowing impairment in a case series of individuals with traumatic spinal cord injury. American Journal of Speech Language Pathology, 32(2), 688–700. 10.1044/2022_AJSLP-22-00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose, A. K., & Humbert, I. (2019). “Hidden in plain sight”: A descriptive review of laryngeal vestibule closure. Dysphagia, 34(3), 281–289. 10.1007/s00455-018-9928-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose, A. K., Kesneck, S., Sunday, K., Plowman, E., & Humbert, I. (2018). A survey of clinician decision making when identifying swallowing impairments and determining treatment. Journal of Speech, Language, and Hearing Research, 61(11), 2735–2756. 10.1044/2018_JSLHR-S-17-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waito, A. A., Plowman, E. K., Barbon, C. E. A., Peladeau-Pigeon, M., Tabor-Gray, L., Magennis, K., Robison, R., & Steele, C. M. (2020). A cross-sectional, quantitative videofluoroscopic analysis of swallowing physiology and function in individuals with amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 63(4), 948–962. 10.1044/2020_JSLHR-19-00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to ethical/legal restrictions. Inquiries regarding access to the data should be directed to the corresponding author.



 This work is licensed under a
This work is licensed under a