Abstract
Background
Ampullary or papillary carcinoma is a malignant tumor arising from the mucosa in the region of the major duodenal papilla, also known as the ampulla of Vater. Uniform treatment recommendations are lacking both for the adjuvant situation and for palliative care.
Methods
A selective literature search was carried out in PubMed in order to identify the most informative publications concerning the epidemiology, clinico-pathological background, and surgical and medical treatment of this condition.
Results
Ampullary carcinoma has an incidence of 0.5 to 0.9 per 100 000 persons and a poor prognosis, with a 5-year survival rate of 41% to 45% for locally confined and 4% to 7% for metastatic disease. Most such tumors are of an intestinal or a pancreaticobiliary immunohistochemical subtype; the latter has a worse prognosis (median survival, 72–80 vs. 33–41 months). Targeted treatment is not yet available for either subtype, nor is there enough scientific evidence available for the formulation of specific therapeutic recommendations in either the adjuvant or the palliative situation. The treatment of choice for ampullary carcinoma is radical oncological resection of the head of the pancreas with systematic lymphadenectomy. Five-year overall survival is between 10% and 75% depending on the stage. No definitive recommendation for adjuvant therapy can be given. Palliative therapy can be oriented to the published treatment strategies for cancer of the colon, pancreas, and bile duct.
Conclusion
The current state of the evidence on the treatment of ampullary carcinoma is poor. Therapeutic decisions should be discussed in an interdisciplinary tumor board and should, in our opinion, take the histological subtype into account.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.aerzteblatt.de. The deadline for submission is 26 October 2024.
The ampulla of Vater is a mucosal protuberance in the descending duodenum, which includes the joint confluence of the common bile duct and the pancreatic duct in the major duodenal papilla. Malignant neoplasms of the mucosa in this region are known as ampullary or papillary cancers (Figure 1). Somewhat broader in scope is the term periampullary cancers, which also includes distal bile duct carcinomas and cancers of the head of the pancreas. In recent years histopathologic, immunohistochemical, and molecular genetic analyses have characterized ampullary cancer in more details and it became possible to define subtypes. The treatment of choice is resection by means of pylorus preserving resection of the head of the pancreas in the early stages and systemic therapy in irresectable tumors. Because the tumor is so rare, no consistent therapeutic recommendations exist so far, and neither do adjuvant therapeutic concepts. This article aims to provide on this background a compact overview of what is currently known about the pathology and therapeutic options in ampullary cancer.
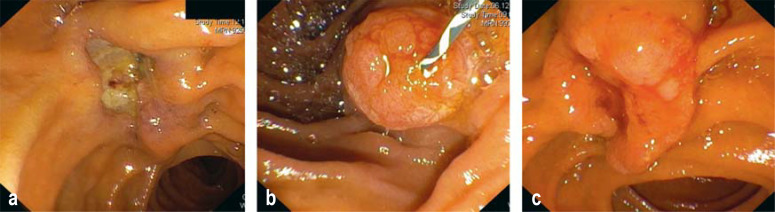
Figure 1.
Example image of papillary/ampullary carcinomas
a) Ulcerating tumor growth; stage: during exploratory laparoscopy, liver metastases were confirmed, immunohistochemically pancreaticobiliary phenotype
b) Polypoid tumor growth; after resection pT1b, pN0 (0/15), L0, V0, G2, R0; pancreaticobiliary subtype
c) Polypoid-ulcerating tumor growth; after resection pT3a, pN0 (0/22), L0, V0, Pn0, local R0, intestinal type
Method
We carried out a selective literature search in PubMed, using the search terms “ampullary carcinoma”, “ampullary cancer”, “adenocarcinoma of the ampulla of Vater”. We searched for prospective and large retrospective clinical studies and in the subject areas of epidemiology and molecular pathology with as large a number of cases as possible. To capture the incidence we interrogated the database at the center for cancer registry data at the Robert Koch-Institute (RKI).
Epidemiology and prognosis
The ICD-10 code for ampullary carcinomas is C24.1; the cancer data from the RKI have to date not reported a separate incidence. For extrahepatic cholangiocarcinomas (together with C24.0, C22.8, and C22.9) the incidence in Germany in 2015 was 2.9/100 000 in men and 1.8/100 000 in women (1). When we interrogated the database at the RKI center for cancer registry data for code 24.1 and the time period 2010–2019, we found a constant incidence between 0.76 and 0.91/100 000 for men and between 0.46 and 0.53/100 000 for women; the incidence in both sexes rises with increasing age (eTables 1 and 2). A large analysis of the US SEER database in 2009 showed an incidence of about 0.5/100 000 between 1970 and 2010 (2). The age when people originally became ill was between 40 and 70 years in both men and women, and about 50% of patients presented at an advanced—that is, metastasized or non-resectable—stage (3). Five-year survival rates varied between 10% and 75% in the localized stage and 4.7% in the metastatic stage (2–4). This means that at an early stage the prognosis is slightly better than for pancreatic carcinoma, which can be explained with the fact that the diagnosis is often made earlier—when fewer lymph nodes are involved—and the R0 rate is higher (5).
eTable 1. Age specific incidence and number of cases of ampullary carcinoma.
| Study period | Age specific incidence rates | Incidence of case numbers | ||||
| Men | Women | Total | Men | Women | Total | |
| 2010 | 0.77 | 0.53 | 0.64 | 455 | 397 | 852 |
| 2011 | 0.82 | 0.50 | 0.64 | 481 | 406 | 887 |
| 2012 | 0.81 | 0.50 | 0.63 | 479 | 366 | 845 |
| 2013 | 0.91 | 0.51 | 0.69 | 546 | 397 | 943 |
| 2014 | 0.86 | 0.50 | 0.66 | 528 | 393 | 921 |
| 2015 | 0.85 | 0.53 | 0.68 | 530 | 438 | 968 |
| 2016 | 0.76 | 0.46 | 0.60 | 497 | 388 | 885 |
| 2017 | 0.81 | 0.50 | 0.64 | 531 | 411 | 942 |
| 2018 | 0.78 | 0.52 | 0.64 | 513 | 429 | 942 |
| 2019 | 0.80 | 0.51 | 0.64 | 530 | 398 | 928 |
| 2015–2019 | 0.80 | 0.51 | 0.64 | 2601 | 2064 | 4665 |
| 2017–2019 | 0.80 | 0.51 | 0.64 | 1574 | 1238 | 2812 |
Source: Incidence data were reported by the center for cancer registry data at the Robert Koch-Institute (RKI)
eTable 2. Age standardized incidence of ampullary carcinoma.
|
2015–2019
Age (years) |
Age standardized incidence rate | ||
| Men | Women | Total | |
| 0–14 | 0.0 | 0.0 | 0.0 |
| 15–19 | 0.0 | 0.0 | 0.0 |
| 20–24 | 0.0 | 0.0 | 0.0 |
| 25–29 | 0.0 | 0.0 | 0.0 |
| 30–34 | 0.1 | 0.0 | 0.0 |
| 35–39 | 0.1 | 0.0 | 0.1 |
| 40–44 | 0.2 | 0.2 | 0.2 |
| 45–49 | 0.4 | 0.2 | 0.3 |
| 50–54 | 0.9 | 0.5 | 0.7 |
| 55–59 | 1.2 | 0.7 | 1.0 |
| 60–64 | 2.3 | 1.2 | 1.7 |
| 65–69 | 3.0 | 2.1 | 2.5 |
| 70–74 | 4.7 | 3.2 | 3.9 |
| 75–79 | 5.8 | 3.8 | 4.6 |
| 80–84 | 6.6 | 4.2 | 5.2 |
| 85 plus | 5.7 | 3.6 | 4.2 |
Source: Incidence data were obtained from the center for cancer registry data at the Robert Koch-Institute (RKI)
Histopathology
The above-mentioned analysis of the SEER database of 5625 cases of ampullary carcinoma found primarily adenocarcinoma as the underlying histologic diagnosis. The anatomical localization in close proximity to the epithelia of the common bile duct, pancreatic duct, and duodenum means that all three cell types may be implicated as the origin of adenocarcinomas. On this basis it has become customary to characterize ampullary cancers histopathologically in greater detail and classify them into an intestinal and pancreaticobiliary subtype. Further subtyping between biliary and pancreatic origin is histopathologically not possible.
The intestinal subtype is often found in addition to parts of a duodenal adenoma and shows histomorphologic parallels to colon cancer, whereas the pancreaticobiliary subtype resembles the growth pattern in pancreatic cancers and cholangiocarcinoma (Figure 2) (6). On histomorphology alone it is not always possible to distinguish the tumor entities, so that in support immunohistochemical markers can be stained. Here, the intestinal subtype often expresses marker known from colon cancer, such as CDX2 and CK20, whereas the pancreaticobiliary subtype expresses markers known from cholangiocarcinoma and pancreatic cancer, such as CK7 (7). To date, no standards exist in the immunohistochemical differentiation, and the prognostic validity of individual immunohistochemical makers is still under discussion (8). It also should be mentioned that up to 20% of ampullary cancers include both subtypes and are described as “mixed type,” with no clear diagnostic criteria existing as yet (8, 9).
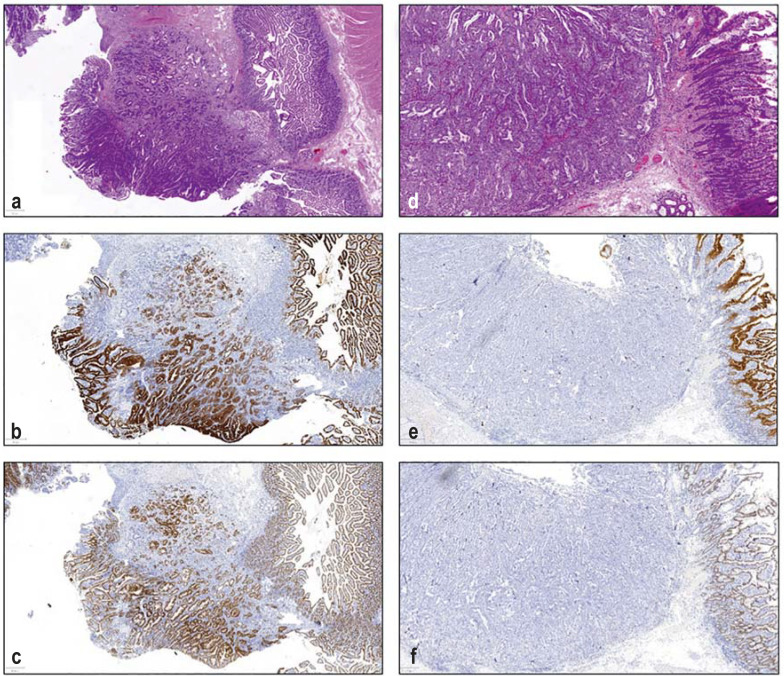
Figure 2.
Histology specimens of ampullary carcinomas
Left: Example of the intestinal subtype with HE stain (a) and with strong expression of CK 20 (b) and CDX2 (c)
Right: Example of a biliary subtype in HE (d) and with lacking expression of CK 20 (e) and CDX2 (f)
The great clinical relevance of subtyping is reflected in a relevant effect on the prognosis: survival in pancreaticobiliary carcinoma seems notably worse (median 33–41 months) than for the intestinal subtype (72–80 months) (6, 10). Interestingly, survival for the pancreaticobiliary subtype was notably better than for pancreatic cancer, even after the stages were matched (16 months) (6).
Molecular genetics
Thanks to the increasing availability of next generation sequencing (NGS) in recent years it became possible to analyze the molecular genetics of ampullary cancers in more detail. In the largest “whole exome” based studies to date, pancreaticobiliary cancers most often showed mutations in the genes KRAS and TP53, whereas the intestinal subtype mostly had increased pathogenic mutations in the APC gene too (11, 12). The Table provides an overview of the most commonly mutated genes. It should be borne in mind that because of the named mutations, no potential targets exist for the currently available therapeutic options on the market. To a lesser extent, pathogenic mutations were found in the genes ERBB2, PIK3CA, BRAF, and JAK3, which might provide potential application points for targeted therapies, so that NGS based panel diagnostics seems justified for ampullary cancers in the palliative setting. Furthermore, in 3% of cases, microsatellite instability was detected, so that immunohistochemical diagnostic testing in this regard should always be undertaken in order to review the option of therapy with pembrolizumab (11).
Table. Common genetic alterations, based on the data of two large whole exome based studies (11, 12), modified from (37).
| Gene | Intestinal subtype | Pancreaticobiliary subtype |
| TP53 | 39–64 % | 67–71 % |
| KRAS | 39–46 % | 65–67 % |
| APC | 41–49 % | – |
| CTNNB1 | 26 % | 15 % |
| PIK3CA | 26 % | 13 % |
| SMAD4 | 20 % | 18–20 % |
| CDKN2A | – | 16 % |
| ERBB3 | – | 14 % |
| ARID2 | 18 % | – |
Surgical treatment
The treatment of choice in ampullary carcinoma is radical oncological resection of the head of the pancreas with systematic lymphadenectomy. This includes removal of the gall bladder and the common bile duct, starting directly cranially from the branching off of the cystic duct, the duodenum, and the first jejunal loop after the ligament of Treitz and the head of the pancreas within it as an en bloc resection. The locoregional lymph nodes alongside the hepatoduodenal ligament, the superior margin of the pancreas right down to the celiac artery and in the triangle between portal vein, splenic/lienal artery, and splenic/lienal vein complete the resectate. Of importance is radical resection as pylorus preserving pancreatoduodectomy (PPPD) or Whipple procedure. A recent analysis of SEER data showed that in some 80% of patients a locally advanced or node positive carcinoma was present, which required a radical oncologic approach. The 5-year overall survival rates depending on the tumor stages range from below 10% and 75%. A prognostic assessment of this only curative method is done on the basis of the postoperative histologic result.
The role of resection of metachronous metastases is not clear at this time and was studied by Saedon et al in a meta-analysis (13). They ascertained that the data are currently inconclusive. For this reason—and analogous to the approach in solid organ metastases of primary gastrointestinal, urologic, and gynecologic tumors when carefully selected—an indication for metastasectomy or interventional therapy especially at the oligometastatic stage will be of great interest as an area of clinical research in the coming years. In ampullary carcinoma, resection of metastases can be discussed in the individual case, especially for the intestinal subtype (14).
It should be borne in mind that at the local stage of the pancreaticobiliary subtype, the differentiation between pancreatic cancer, distal cholangiocarcinoma, or ampullary carcinoma can in some cases not be achieved with certainty (15). In these cases, interdisciplinary discussion regarding neoadjuvant therapy analogous to the German S3 guideline is of great clinical importance.
Adjuvant therapy
The poor prognosis even at the early resectable stage prompts the question of whether adjuvant therapeutic concepts can potentially improve the disease course. Firstly it is worth drawing attention to the fact that none of the large studies that summarize the current therapeutic standard in bile duct and pancreatic cancers included patients with ampullary carcinoma (16–18). The first prospective study of the subject came from Japan in 2002. This study included patients with different pancreaticobiliary malignancies (n=508). The researchers studied adjuvant administration of mitomycin C and fluorouracil (5-FU), which in the subgroup of ampullary carcinomas (n=56) did not lead to a significant survival advantage (19).
The second and so far largest prospective study was the ESPAC-3 Trial, published in 2015, which included 428 patients with periampullary carcinomas (a total of 297 ampullary carcinomas). This study showed prolonged survival for chemotherapy using 5-FU or gemcitabine (43–35 months), but the advantage did not reach significance (p=0.25) (20). In patients with ampullary carcinoma the gemcitabine group survived longest (70.8 months) compared with the groups treated with F-FU (57.8) and watchful waiting only (40.6). Analysis by histologic subtype did not significantly change responsiveness to therapy. In the multivariate analysis adjuvant therapy was associated with an improved prognosis. Although these data hint at effectiveness of chemotherapy using gemcitabine, the study was ultimately formally negative.
The observations of these prospective studies contrast with multiple retrospective analyses that partly also include the histologic subtype. A study from Munich, for example, which included 112 patients, showed an improved prognosis for gemcitabine based therapy for the pancreaticobiliary subtype (32 versus 13 months), whereas gemcitabine seemed to have a negative effect on survival in the intestinal type (35 versus 112 months) (21). This observation is echoed by a large (n=976) international multicenter matched cohort analysis published in 2020: in this study it also seemed that only the pancreaticobiliary subtype benefited from gemcitabine based adjuvant chemotherapy (22). A similar conclusion was reached in a European multicenter study (also published in 2020) that included 214 patients (pancreaticobiliary: 140, intestinal: 74). In this study, adjuvant therapy showed a survival advantage for patients with the pancreaticobiliary (85 versus 65 months) but not the intestinal subtype (23). The heterogeneity of the adjuvant therapeutic concepts makes the interpretation of the data difficult, though (47% gemcitabine mono, 20% unclear regimens, 9% radiochemotherapy, 9% gemcitabine/oxaliplatin, 8% capecitabine, 7% FOLFOX).
By contrast, a retrospective multicenter study from the USA, which included 357 patients, did not show a relevant survival advantage (24). It needs to be borne in mind, however, that the groups who received chemotherapy were small (pancreaticobiliary: 31, intestinal: 34) and the therapeutic concepts diverged too. 15/34 patients with the intestinal subtype received gemcitabine. A recently published study from Korea showed in the subtype specific analysis a survival advantage for 5-FU based adjuvant therapy for the intestinal (n=31) versus the pancreaticobiliary and mixed type (n=29), but a control group was lacking (25).
The largest study to date (n=4190), based on data from the US National Cancer Database, compared adjuvant chemotherapy (21%) and radiochemotherapy (16%) and follow-up/aftercare (63%), with a survival advantage found for the adjuvant concepts, especially in patients with locally advanced tumors (N+, T3/T4, G3). This study did not give details on the histologic subtype and the therapeutic regimens were not defined in detail, which means that there is a limit to what conclusions can be drawn (26).
Some smaller retrospective studies also indicated a potential survival advantage for adjuvant 5-FU based radiochemotherapy in node positive patients (21, 27–30). The same is true for a meta-analysis published in 2015, which included 10 retrospective studies and 3361 patients. The study showed a survival advantage for radiochemotherapy in node positive and locally advanced tumors, but the interpretation is hampered by the inclusion of heterogeneous therapeutic concepts and study populations (31). In the only prospective study to date, which included 93 patients with periampullary carcinomas, no significant survival advantage was found in the context of the EORTC 40891 Trial (63% versus 67% 2-year survival rate) (32). The importance of adjuvant radiochemotherapy in ampullary carcinoma therefore remains unclear. Its use can be discussed especially in advanced cases with R1 resection.
On the background of the included data it is unfortunately not possible to give unequivocal recommendations regarding adjuvant therapy, and the treatment may have to be discussed individually in a tumor board. The Figure shows a possible approach, which we think is pragmatic. Because of the formally negative results of the BILCAP Study, the algorithm is aligned to current standards in pancreatic and colon carcinomas by considering the performance status and tumor stage (16). In view of the better prognosis compared with pancreatic carcinoma a slightly less aggressive approach was selected for the pancreaticobiliary subtype. The use of mFOLFIRINOX should—in view of the lacking data for this therapy to date in ampullary carcinoma as well as in view of potentially long lasting polyneuropathy—certainly be discussed, although it is recommended by some centers (33).
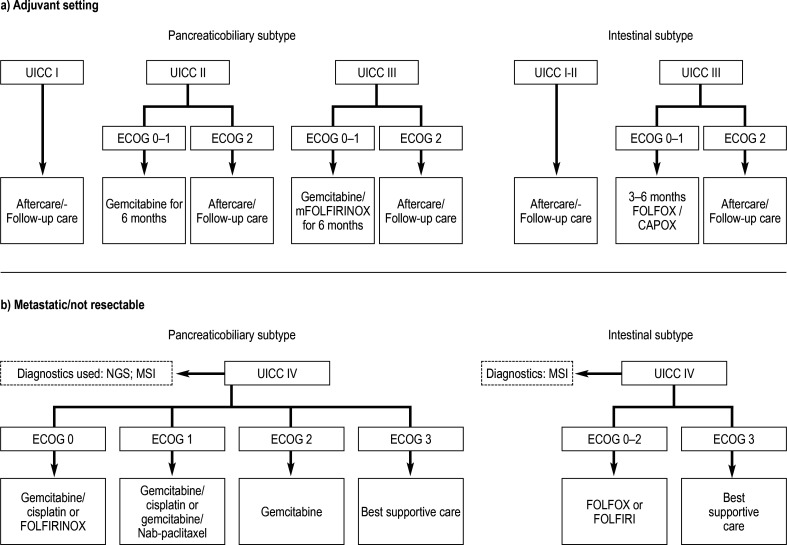
Figure.
Possible therapeutic algorithm for the management of patients with ampullary carcinoma in the adjuvant and palliative setting in first-line therapy. Stage III is defined by confirmation of positive lymph nodes or T4 situation without confirmation of distant metastases (classification of the Union internationale contre le cancer [UICC], 8th edition).
*At stage III and in the R1 situation, fluorouracil (5-FU) based radiochemotherapy can be discussed.
Therapeutic schemes: CAPOX: capecitabine/oxaliplatin; FOLFOX: folic acid, fluorouracil and oxaliplatin; FOLFIRI: folic acid, fluorouracil, irinotecan;
mFOLFIRINOX: oxaliplatin, leucovorin, irinotecan, fluorouracil; FOLFIRINOX: fluorouracil/leucovorin, irinotecan, and oxaliplatin
Ca, capecitabine; ECOG, Performance Status der Eastern Cooperative Oncology Group; NGS, next generation sequencing; MSI, microsatellite instability
Palliative therapy
The data regarding ampullary carcinoma in the palliative setting is even sparser than in the adjuvant setting. The Landmark ABC-02 Trial of cholangiocarcinoma published in 2010 by Valle et al, implementing therapy based on gemcitabine and cisplatin, did include patients with ampullary carcinoma, but their number was so small—20 out of a of 410 in the total cohort—that no profound conclusions could be drawn from this (34). The situation was similar in the ABC-06 TRIAL, published in 2021, which tested FOLFOX as second-line treatment in cholangiocarcinoma versus best supportive care (35). In 2009 a phase II study was published which showed in 30 patients effectiveness of CAPOX in periampullary tumors. For n=12 ampullary carcinomas not further subtyped, this study does not allow for practice-relevant conclusions either (36). Furthermore, several small studies with heterogeneous populations without consideration of the subtype exist, but these are not sufficient to generate hypotheses (37–40).
In sum, on the basis of current data no recommendation can be made, with the consequence that—in our opinion—only a pragmatic approach remains, to characterize the patients in the palliative setting histopathologically and molecular genetically, and to establish systemic therapy analogous to colon or pancreatic cancer or cholangiocarcinoma. The Figure shows a possible algorithm for first-line therapy.
Conclusion
Ampullary carcinoma is a histologically and molecular pathologically heterogeneous tumor whose clinical management continues to be largely inconsistent because of the lack of large randomized trials. In the overall view of the named data, no evidence based recommendation regarding adjuvant and palliative treatment can be made. There are reasons to assume that therapy in the adjuvant setting, especially at an advanced stage, yields a prognostic advantage and that treatment is justified in these cases. In addition to the stage the subtype can be considered in deciding on a therapeutic regimen, and treatment can be administered analogous to pancreatic cancer, cholangiocarcinoma, or colon carcinoma. Large retrospective and randomized prospective trials in this area are urgently needed. In the palliative setting, molecular genetic and immunohistochemical analysis regarding microsatellite instability seems sensible. Subsequently—in our opinion—treatment can be administered pragmatically, analogous to therapy of the subtype, but the data for the palliative setting are even sparser than for the adjuvant setting and randomized trials are required here too. An individual differentiated discussion of each case in the interdisciplinary tumor board is inevitable.
Questions on the article in issue 43/2023:
The Diagnosis and Treatment of Ampullary Carcinoma
The submission deadline is 26 October 2024. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Approximately what is the incidence of ampullary carcinoma?
0.01/100 000 persons
0.5/100 000 persons
5.4/100 000 persons
15.3/100 000 persons
32.1/100 000 persons
Question 2
What is the 5-year survival rate for the metastatic stage of ampullary carcinoma?
4–7 %
18–25 %
41–45 %
55–60 %
65–71 %
Question 3
Two large “whole exome” based studies investigated genetic alterations in intestinal and pancreaticobiliary ampullary carcinomas. Which genes were most often found to be mutated in the pancreaticobiliary subtype, at over 60% each?
APC and CTNNB1
PIK3CA und SMAD4
ARID2 and APC
ERBB3 and ARID2
TP53 and KRAS
Question 4
For which therapy was prolonged survival shown at various stages for the pancreaticobiliary subtype of ampullary carcinoma but not for the intestinal subtype?
Radiotherapy
HER2-antobody therapy
Gemcitabine based therapy
Lomustine based therapy
Stem cell transplantation
Question 5
Which combination of active substances is known by the acronym “FOLFOX”?
Folic acid, fluorouracil, oxalate
Folic acid, fluorouracil, oxaliplatin
Folic acid, fluoroquinolone, oxaliplatin
Folic acid, fluoride, oxalate
Folic acid, fluoride, oxaliplatin
Question 6
What percentage of ampullary carcinomas is a mixed type consisting of the pancreaticobiliary and intestinal subtypes?
5 %
10 %
20 %
35 %
55 %
Question 7
Which procedure is referred to as PPPD in the text?
Pancreatic duct-preserving resection of the pancreas
Resection of the pylorus with preservation of the head of the pancreas
Resection of the pylorus and head of the pancreas
Pylorus preserving resection of the head of the pancreas
Partial resection of the pylorus while preserving the head of the pancreas
Question 8
What drains in most cases in the major duodenal papilla (papilla/ampulla of Vater)?
The accessory pancreatic duct and common bile duct
The common bile duct and the pancreatic duct
The hepatic duct and pancreatic duct
The accessory pancreatic duct and hepatic duct
The hepatic duct and common bile duct
Question 9
According to data from the cancer registry of the RKI, how does the incidence of ampullary carcinoma evolve as regards sex and age?
The incidence is higher in men and rises with age only in women.
The incidence is higher in women and rises with age in both sexes.
The incidence is higher in men and rises with age in both sexes.
The incidence is the same in both sexes and rises with age in both sexes.
The incidence is higher in women but rises with age in men and aligns with the incidence in women.
Question 10
In ampullary carcinoma, which genetic alteration should be tested for so as to consider the option of treatment with pembrolizumab?
BRAF deletion
KRAS duplication
Mutations in theAPC gene
Microsatellite instability
TP53 duplication
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Footnotes
Conflict of interest statement
DW received advisory fees from BMS and lecture honoraria from AstraZeneva and Servier.
JT received advisory fees and/or lecture honoraria from Amgen, AstraZeneca, Bioprojet, Bayer Healthcare, Bristol Myers-Squibb, Eisai, Ipsen, Merck, Serono, Merck Sharp & Dome, Lill Imclone, Onkowissen TV, PCI Biotech, Roche, streammedup, and Servier.
FS declares that no conflict of interest exists.
References
- 1.Kaatsch P, Spix C, Katalinic A, Hentschel S, Luttmann S, Stegmaier C. Krebs in Deutschland 2013/2014. In: Robert Koch-Institut; die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V., editor. 11th edition. Berlin: 2017. www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2017/krebs_in_deutschland_2017.pdf?__blob=publicationFile (last accessed on 1 November 2022) [Google Scholar]
- 2.Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009;100:598–605. doi: 10.1002/jso.21374. [DOI] [PubMed] [Google Scholar]
- 3.Rostain F, Hamza S, Drouillard A, Faivre J, Bouvier AM, Lepage C. Trends in incidence and management of cancer of the ampulla of vater. World J Gastroenterol. 2014;20:10144–10150. doi: 10.3748/wjg.v20.i29.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N, Chen ZY, Yang Z, Shang HZ, Shi GJ. Development and verification of prognostic nomogram for ampullary carcinoma based on the SEER database. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1197626. 1197626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sommerville CAM, Limongelli P, Pai M, et al. Survival analysis after pancreatic resection for ampullary and pancreatic head carcinoma: an analysis of clinicopathological factors. J Surg Oncol. 2009;100:651–656. doi: 10.1002/jso.21390. [DOI] [PubMed] [Google Scholar]
- 6.Reid MD, Balci S, Ohike N, et al. Ampullary carcinoma is often of mixed or hybrid histologic type: an analysis of reproducibility and clinical relevance of classification as pancreatobiliary versus intestinal in 232 cases. Mod Pathol. 2016;29:1575–1585. doi: 10.1038/modpathol.2016.124. [DOI] [PubMed] [Google Scholar]
- 7.Kumari N, Prabha K, Singh RK, Baitha DK, Krishnani N. Intestinal and pancreatobiliary differentiation in periampullary carcinoma: the role of immunohistochemistry. Hum Pathol. 2013;44:2213–2219. doi: 10.1016/j.humpath.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Xue Y, Reid MD, Balci S, et al. Immunohistochemical classification of ampullary carcinomas. Am J Surg Pathol. 2017;41:865–876. doi: 10.1097/PAS.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 9.Kohler I, Jacob D, Budzies J, et al. Phenotypic and genotypic characterization of carcinoma of the papilla of Vater has prognostic and putative therapeutic implications. Am J Clin Pathol. 2011;135:202–211. doi: 10.1309/AJCPCTCUQSYI89YT. [DOI] [PubMed] [Google Scholar]
- 10.Williams JL, Chan CK, Toste PA, et al. Association of histopathologic phenotype of periampullary adenocarcinomas with survival. JAMA Surg. 2017;152:82–88. doi: 10.1001/jamasurg.2016.3466. [DOI] [PubMed] [Google Scholar]
- 11.Gingras MC, Covington KR, Chang DK, et al. Ampullary cancers harbor ELF3 tumor suppressor gene mutations and exhibit frequent WNT dysregulation. Cell Rep. 2016;14:907–919. doi: 10.1016/j.celrep.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yachida S, Wood LD, Suzuki M, et al. Genomic sequencing identifies ELF3 as a driver of ampullary carcinoma. Cancer Cell. 2016;29:229–240. doi: 10.1016/j.ccell.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saedon M, Maroulis I, Brooks A, et al. Metastasectomy of pancreatic and periampullary adenocarcinoma to solid organ: the current evidence. JBUON. 2018;23:1648–1654. [PubMed] [Google Scholar]
- 14.De Jong MC, Tsai S, Cameron JL, et al. Safety and efficacy of curative intent surgery for peri-ampullary liver metastasis. J Surg Oncol. 2010;102:256–263. doi: 10.1002/jso.21610. [DOI] [PubMed] [Google Scholar]
- 15.van Roessel S, Soer EC, Daamen LA, et al. Preoperative misdiagnosis of pancreatic and periampullary cancer in patients undergoing pancreatoduodenectomy: a multicentre retrospective cohort study. Eur J Surg Oncol. 2021;47:2525–2532. doi: 10.1016/j.ejso.2021.03.228. [DOI] [PubMed] [Google Scholar]
- 16.Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 17.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- 18.Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 19.Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95:1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 20.Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: The ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 21.Schiergens TS, Reu S, Neumann J, et al. Histomorphologic and molecular phenotypes predict gemcitabine response and overall survival in adenocarcinoma of the ampulla of Vater. Surg (United States) 2015;158:151–161. doi: 10.1016/j.surg.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Moekotte AL, Malleo G, van Roessel S, et al. Gemcitabine-based adjuvant chemotherapy in subtypes of ampullary adenocarcinoma: international propensity score-matched cohort study. Br J Surg. 2020;107:1171–1182. doi: 10.1002/bjs.11555. [DOI] [PubMed] [Google Scholar]
- 23.Bolm L, Ohrner K, Nappo G, et al. Adjuvant therapy is associated with improved overall survival in patients with pancreatobiliary or mixed subtype ampullary cancer after pancreatoduodenectomy—a multicenter cohort study. Pancreatology. 2020;20:433–441. doi: 10.1016/j.pan.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Ecker BL, Vollmer CM, Behrman SW, et al. Role of adjuvant multimodality therapy after curative-intent resection of ampullary carcinoma. JAMA Surg. 2019;154:706–714. doi: 10.1001/jamasurg.2019.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang J, Lee W, Shin J, et al. Controversial benefit of 5-fluorouracil/leucovorin-based adjuvant chemotherapy for ampullary cancer: a propensity score-matched analysis. Langenbecks Arch Surg. 2022;407:1091–1097. doi: 10.1007/s00423-021-02414-3. [DOI] [PubMed] [Google Scholar]
- 26.Nassour I, Hynan LS, Christie A, et al. Association of adjuvant therapy with improved survival in ampullary cancer: a national cohort study. J Gastrointest Surg. 2018;22:695–702. doi: 10.1007/s11605-017-3624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatia S, Miller RC, Haddock MG, Donohue JH, Krishnan S. Adjuvant therapy for ampullary carcinomas: the mayo clinic experience. Int J Radiat Oncol Biol Phys. 2006;66:514–519. doi: 10.1016/j.ijrobp.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 28.Narang AK, Miller RC, Hsu CC, et al. Evaluation of adjuvant chemoradiation therapy for ampullary adenocarcinoma: the Johns Hopkins Hospital—mayo clinic collaborative study. Radiat Oncol. 2011;6 doi: 10.1186/1748-717X-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha HR, Oh DY, Kim TY, et al. Survival outcomes according to adjuvant treatment and prognostic factors including host immune markers in patients with curatively resected ampulla of vater cancer. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151406. e0151406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palta M, Patel P, Broadwater G, et al. Carcinoma of the ampulla of vater: patterns of failure following resection and benefit of chemoradiotherapy. Ann Surg Oncol. 2012;19:1535–1540. doi: 10.1245/s10434-011-2117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon J, Kim BH, Kim K, Chie EK, Ha SW. Survival benefit of adjuvant chemoradiotherapy in patients with ampulla of vater cancer: a systematic review and meta-analysis. Ann Surg. 2015;262:47–52. doi: 10.1097/SLA.0000000000001182. [DOI] [PubMed] [Google Scholar]
- 32.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel M, Uboha NV. Treatment approach to adenocarcinoma of the ampulla of vater. Curr Treat Options Oncol. 2021;22 doi: 10.1007/s11864-021-00894-5. [DOI] [PubMed] [Google Scholar]
- 34.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 35.Lamarca A, Palmer DH, Wasan HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690–701. doi: 10.1016/S1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overman MJ, Varadhachary GR, Kopetz S, et al. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of vater. J Clin Oncol. 2009;27:2598–2603. doi: 10.1200/JCO.2008.19.7145. [DOI] [PubMed] [Google Scholar]
- 37.Kim ST, Lee J, Lee KT, et al. The efficacy of frontline platinum-based combination chemotherapy in advanced adenocarcinoma of the ampulla of Vater. Med Oncol. 2010;27:1149–1154. doi: 10.1007/s12032-009-9351-4. [DOI] [PubMed] [Google Scholar]
- 38.Cereda S, Passoni P, Reni M, et al. The cisplatin, epirubicin, 5-fluorouracil, gemcitabine (PEFG) regimen in advanced biliary tract adenocarcinoma. Cancer. 2010;116:2208–2214. doi: 10.1002/cncr.24970. [DOI] [PubMed] [Google Scholar]
- 39.André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339–1343. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 40.Gibson MK, Holcroft CA, Kvols LK, Haller D. Phase II study of 5-Fluorouracil, Doxorubicin, and Mitomycin C for metastatic small bowel adenocarcinoma. Oncologist. 2005;10:132–137. doi: 10.1634/theoncologist.10-2-132. [DOI] [PubMed] [Google Scholar]