Abstract
Objective
Spectacle lenses with highly aspherical lenslets (HAL) and slightly aspherical lenslets (SAL) showed effective myopia control. This study was to investigate their effects on macular choroidal thickness (ChT) in myopic children.
Methods
Exploratory analysis from a 2-year, double-masked, randomised trial. 170 children aged 8–13 years with myopia between −0.75D and −4.75D, astigmatism of 1.50D or less, and anisometropia of 1.00D or less were recruited. Participants were randomly assigned in a 1:1:1 ratio to receive HAL, SAL or single vision spectacle lenses (SVL). The subfoveal, parafoveal and perifoveal ChT were evaluated every 6 months.
Results
154 participants completed all examinations. The ChT showed significant changes over time in all three groups in all regions (all p<0.05). The ChTs continuously decreased in the SVL group (ranging from −20.75 (SD 22.34) μm to −12.18 (22.57) μm after 2 years in different regions). Compared with the SVL group, ChT in the SAL group decreased less (ranging from −16.49 (21.27) μm to −5.29 (18.15) μm). In the HAL group, ChT increased in the first year and then decreased in the second year (ranging from −0.30 (27.54) μm to 8.92 (23.97) μm after two years). The perifoveal ChT decreased less than the parafoveal ChT, and the superior region decreased the least.
Conclusions
The ChT of the macula decreased after 2 years of myopia progression with SVL. Wearing spectacle lenses with aspherical lenslets reduced or abolished the ChT thinning and HAL had a more pronounced effect.
Trial registration number
ChiCTR1800017683.
Keywords: Choroid, Clinical Trial, Optics and Refraction
What is already known on this topic
The introduction of myopic defocus in the myopic children for a short time resulted in a significant thickening of choroid thickness and shortening of axial length, but the changes restored after removal of defocus.
What this study adds
Wearing spectacle lenses with aspherical lenslets for 2 years can inhibit the thinning of the choroid and slow down axial length elongation with a dose-dependent effect.
How this study might affect research, practice or policy
Changes of choroid thickness can be used as a short-term indicator to reflect the long-term effectiveness of myopia control lenses.
Introduction
The highly vascular choroid, located between the retinal pigment epithelium and the sclera, supplies oxygen and nutrients to the outer retina and plays an essential role in vision-guided eye growth. The choroid is hypothesised to influence visually guided eye growth via two mechanisms. The first is a change in choroidal thickness (ChT) that pushes the retina close to the focal plane.1 2 The second is a change in choroidal blood flow, which can initiate a cascade of signalling molecules or growth factors from the retina to the sclera and induce remodelling of the extracellular matrix resulting in alterations in eye growth.1 2
The placement of positive or negative lenses in front of the eyes to induce myopic or hyperopic defocus is a commonly used method to induce experimental hyperopia or myopia in animal models. Notably, axial elongation with the visual-guided pattern generated by defocus lenses has been significantly correlated with changes in ChT.3–7 Previous animal studies showed rapid, temporary and reversible changes in ChT and axial length (AL) after inducing hyperopic or myopic defocus in chicks,3–6 8 guinea pigs,9 marmosets,10 and macaque monkeys.11 Wildsoet and Wallman also found that a higher defocus magnitude (±15 dioptrers (D) vs ±6 D) induced more significant changes in ChT and AL in chicks.5
Similar observations have been reported in humans. For young myopic and emmetropic human adults, a +2 D myopic defocus significantly increased the ChT in as little as 10 min (reverse changes in 30 min for −2 D hyperopic defocus) and sustained it for the entire defocus time.12 Two hours of defocus induced by +3 D and −3 D lenses increase and decrease subfoveal ChT (SF ChT) in myopic children, respectively. These changes gradually revert after removing the lenses.13 Many recent studies in humans focused on short-term defocus on ChT (such as 2 hours) and found that the ChT reverted to baseline value once the defocus was removed.12 13 Therefore, long-term observation to determine the real impact of defocus on ChT and its relationship with axial elongation is needed.
Recent studies on myopia control based on myopic defocus have involved orthokeratology (OK),14 bifocal and multifocal soft contact lenses,15 and specially designed spectacles.16 OK lens wearing for 1 week caused a rapid increase in the ChT17 that was sustained for the entire wearing period.7 18 However, after the cessation of lens wearing, the ChT quickly reverted to the baseline thickness.19 In those wearing bifocal soft contact lenses, the ChT showed no significant changes after lens wearing for 30 min.20 Few studies have reported the effects of long-term wearing of specific spectacles with myopic defocus on ChT in children.
We have recently investigated the efficacy of two new myopia control spectacle lenses with lenslets with different asphericity and found that both spectacle lenses with highly aspherical lenslets (HAL) and spectacle lenses with slightly aspherical lenslets (SAL) effectively reduced the rate of myopia progression and axial elongation compared with single vision spectacle lenses (SVL) over 2 years, with the highest efficacy for HAL.21 At each study visit, we also collected optical coherence tomography (OCT) images (see the Method section for further details), and the purpose of this paper is to evaluate the effects of spectacle lenses with aspherical lenslets on ChT. We hypothesised that the changes in ChT would be related to the myopia control efficacy of the test lenses, and HAL would have a stronger effect.
Methods
Study design
This 2-year, double-blind, randomised clinical trial was conducted at the Eye Hospital of Wenzhou Medical University. Participants were followed every 6 months for 2 years. The details of the study have been published previously,21 22 and the changes in ChT were an exploratory outcome in the study. Written informed consent and assent were obtained from the subjects, and their parents or guardians after verbal and written explanations of the objectives and possible consequences of the study were provided. The inclusion criteria were age 8–13 years, spherical equivalent refraction between −0.75 D and −4.75 D, astigmatism of not more than 1.50 D, anisometropia of not more than 1.00 D, no strabismus or other ocular disease, and no myopia control history. Participants were randomised in a 1:1:1 ratio to receive HAL, SAL or SVL.21 22
Measurements
To exclude the effects of accommodation on refraction, cycloplegia was performed with two drops of 1% cyclopentolate, with an interval of 5 min between drops; measurements were performed at least 30 min after administration of the second drop. AL was measured by Lenstar ocular biometry (LS900, Haag-Streit International, Koeniz, Switzerland). Five individual measurements with no more than 0.02 mm differences were obtained and averaged.
All participating children underwent macular scans using swept-source OCT (Topcon Corporation, Tokyo, Japan). The instrument’s follow-up mode was activated, and built-in software was used to segment layers and construct topographic maps. The ‘follow-up’ mode means that the machine defines the first image as the benchmark, and all the subsequent shots of the same subject are positioned according to the features in the first image, therefore, the images can be captured at the same position in the fundus in all visits. The SF ChT was measured three times with a 9 mm line scan composed of 128 single A-scans. Twelve-line radial scans centred on the fovea, 6 mm in length and separated by 15°, were obtained three times. Each radial OCT image was constructed from an average of 16 scans. A central 6×6 mm circular region was partitioned automatically according to the Early Treatment Diabetic Retinopathy Study (ETDRS).23 The nine regions were classified as the central foveal, 3 mm nasal (N3), 3 mm superior (S3), 3 mm temporal (T3), 3 mm inferior (I3), 6 mm nasal (N6), 6 mm superior (S6), 6 mm temporal (T6) and 6 mm inferior (I6) regions. The scan length was adjusted by the instrument based on AL and refractive error of the individual participants at each visit. The ChT was determined as the thickness between the outer retinal pigment epithelium and the inner choroidoscleral interface. The average ChT of each region was calculated by integrated software. To avoid the influence of variability in anatomical features on automatic techniques, manual corrections for boundaries (retinal-choroidal and choroidal-scleral interfaces) were conducted by two masked and well-trained independent observers. The two observes made manual correction in all OCT images in every participant. The images were reanalysed if the differences between the results of the two observers were more than 10 µm. The repeatability of the ChT measurements of all visits in different regions between two observes was shown in online supplemental figure 1; they showed good repeatability with mean differences from −0.99 µm to 1.19 µm, the smallest 95% limits of agreement −8.59 to 6.60 µm were at SF ChT, and the largest 95% limits of agreement −9.89 to 10.49 µm were at T6 ChT. The results of the three measurements obtained by two observers were averaged.
bjo-2022-321815supp001.pdf (205.1KB, pdf)
Statistical analysis
IBM SPSS Statistics for Windows, V.24.0 (IBM, Released 2016,) was used for data analysis. Only right-eye data were included in the analysis. Repeated-measures analysis of variance was computed to assess the impact of time (5 visits), region (SF and 9 ETDRS regions, 10 regions in total) and group (3 groups) on the ChT. The relationship between the change in AL and baseline SF ChT or between the change in AL and the change in SF ChT was tested using Pearson correlation analysis. Values p<0.05 were considered to be statistically significant difference.
Results
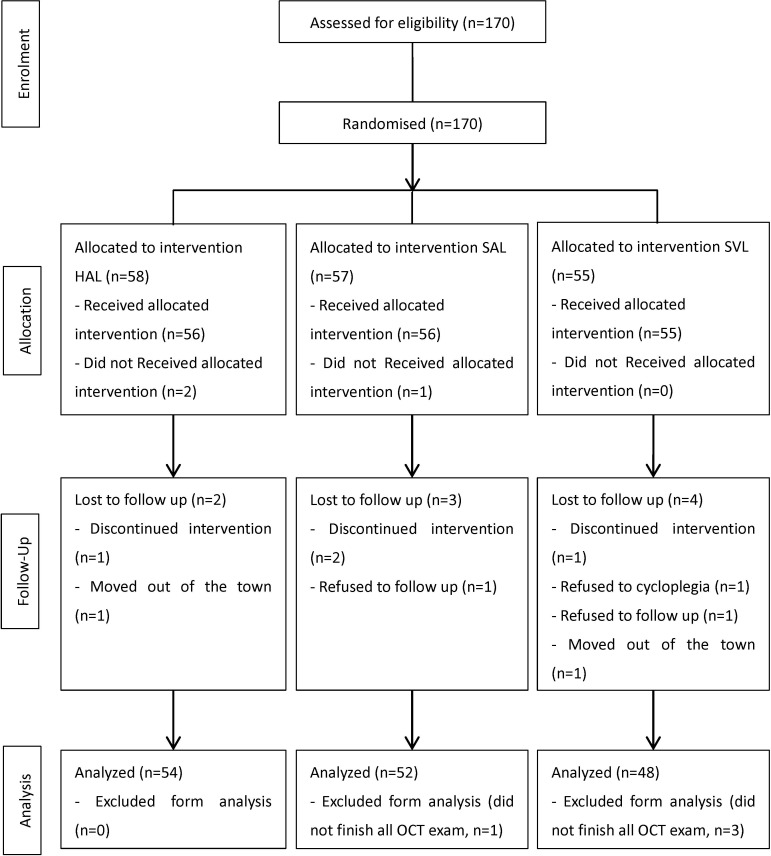
This study initially included 170 participants, but 3 discontinued at baseline and 9 were lost to follow-up, due to changes in spectacles or OK lenses use (n=4), relocation (n=2), follow-up refusal (n=3), cycloplegia refusal (n=1), intermittent exotropia (n=1) and history of progressive addition lenses use (n=1). In addition, one participant missed the 18-month visit but came back for the 24-month visit. Of the 157 participants who attended the complete 2-year visit, 3 participants did not have complete OCT data. Consequently, 154 participants were included in the analysis (figure 1). The 18-month follow-up was scheduled for February 2020, during the COVID-19 epidemic in China. Therefore, the 18-month follow-up was delayed to March to April 2020, resulting in delays of 17 (SD 19) days, the same in the three groups (F 2, 151 = 0.90, p=0.91). In the meantime, participants experienced home-school courses, which involved near-work without outdoor activities.
Figure 1.
Consolidated Standards of Reporting Trials flow diagram of the study. HAL, highly aspherical lenslets; OCT, optical coherence tomography; SAL, slightly aspherical lenslets; SVL, single vision spectacle lenses.
There was significant sex (p=0.03) and baseline AL (p=0.03) differences among the groups (table 1). The SAL group contained more girls and had a shorter AL than the other two groups. There were no differences in baseline SF ChT or ChT values in the ETDRS regions among the three groups before and after adjustment for sex and AL (all p>0.05, table 2).
Table 1.
Baseline characteristics and al elongation of participants
| Parameters | HAL (n=54) | SAL (n=52) | SVL (n=48) |
| Age | 10.65 (1.15) | 10.25 (1.19) | 10.38 (1.21) |
| Gender (M/F) | 26/28 | 17/35 | 28/20 |
| SER (D) | −2.70 (1.02) | −2.28 (0.96) | −2.46 (0.90) |
| AL (mm) | 24.76 (0.68) | 24.44 (0.76) | 24.77 (0.67) |
| 2 year AL elongation (mm) | 0.34 (0.25) | 0.50 (0.23) | 0.68 (0.24) |
Data are expressed as the mean (SD).
AL, axial length; D, dioptres; F, female; HAL, spectacle lenses with highly aspherical lenslets; M, male; SAL, spectacle lenses with slightly aspherical lenslets; SER, spherical equivalent of refraction; SVL, single-vision spectacle lenses.
Table 2.
Choroidal thickness over time
| Regions | Baseline | 6 Months | 12 Months | 18 Months | 24 Months | ||||||||||
| HAL | SAL | SVL | HAL | SAL | SVL | HAL | SAL | SVL | HAL | SAL | SVL | HAL | SAL | SVL | |
| SF ChT | 223 (59) | 239 (64) | 220 (47) | 227 (62) | 236 (67) | 212 (43) | 230 (61) | 230 (61) | 204 (46) | 224 (63) | 223 (60) | 197 (47) | 230 (64) | 228 (57) | 205 (46) |
| C ChT | 231 (57) | 248 (64) | 228 (44) | 241 (61) | 250 (65) | 224 (43) | 244 (61) | 245 (60) | 217 (46) | 236 (62) | 235 (60) | 208 (45) | 232 (63) | 231 (57) | 207 (47) |
| N3 ChT | 205 (53) | 221 (60) | 202 (45) | 215 (57) | 224 (60) | 200 (43) | 217 (56) | 220 (56) | 193 (45) | 210 (58) | 212 (56) | 186 (45) | 208 (57) | 210 (53) | 187 (47) |
| S3 ChT | 237 (58) | 252 (58) | 233 (44) | 249 (63) | 258 (59) | 231 (43) | 253 (62) | 254 (55) | 225 (45) | 246 (63) | 246 (54) | 216 (44) | 242 (63) | 241 (50) | 216 (46) |
| T3 ChT | 246 (56) | 261 (61) | 242 (43) | 254 (61) | 265 (63) | 239 (43) | 258 (58) | 259 (57) | 232 (46) | 251 (61) | 250 (58) | 223 (45) | 246 (62) | 245 (55) | 222 (48) |
| I3 ChT | 233 (54) | 254 (62) | 231 (47) | 242 (56) | 258 (65) | 230 (48) | 246 (56) | 254 (58) | 223 (51) | 239 (57) | 246 (61) | 215 (50) | 234 (57) | 240 (57) | 214 (52) |
| N6 ChT | 167 (45) | 181 (53) | 165 (42) | 177 (49) | 185 (52) | 165 (40) | 177 (48) | 183 (50) | 159 (42) | 168 (50) | 172 (49) | 150 (41) | 167 (50) | 172 (47) | 152 (44) |
| S6 ChT | 238 (54) | 249 (52) | 235 (46) | 252 (59) | 258 (52) | 234 (45) | 256 (57) | 256 (49) | 231 (49) | 249 (58) | 247 (48) | 222 (48) | 247 (59) | 244 (46) | 223 (49) |
| T6 ChT | 252 (52) | 264 (55) | 248 (42) | 261 (56) | 272 (56) | 247 (43) | 264 (54) | 265 (49) | 239 (44) | 255 (53) | 257 (52) | 229 (44) | 253 (57) | 253 (50) | 229 (46) |
| I6 ChT | 226 (50) | 249 (57) | 226 (46) | 235 (53) | 256 (60) | 225 (47) | 240 (53) | 254 (54) | 219 (49) | 232 (53) | 244 (57) | 210 (49) | 229 (53) | 239 (53) | 210 (50) |
Data are expressed as mean (SD).
AL, axial length; C, Central 1 mm; ChT, choroidal thickness; HAL, spectacle lenses with highly aspherical lenslets; I3, 3 mm inferior; I6, 6 mm inferior; N3, 3 mm nasal; N6, 6 mm superior; S3, 3 mm superior; S6, 6 mm superior; SAL, spectacle lenses with slightly aspherical lenslets; SF, subfoveal; SVL, single vision spectacle lenses; T3, 3 mm temporal; T6, 6 mm temporal.
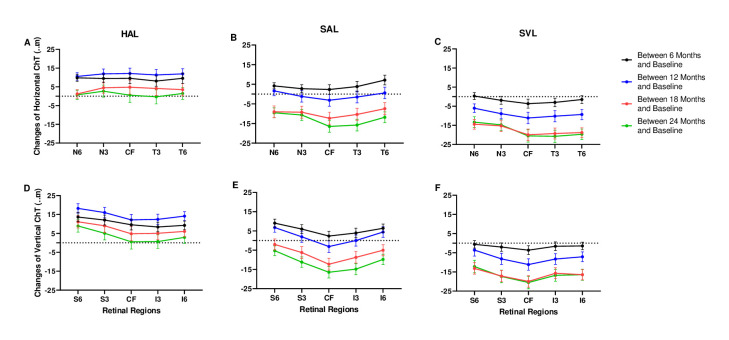
Significant interaction was found between time and group (p<0.001), and between time and region (p<0.001), but not between group and region (p>0.99). The ChT showed significant changes over time in all three group in all regions (figures 2 and 3, table 2). The SVL group showed the largest consistent decrease ChT in all regions (all p<0.05, figure 2C,F). The ChT in the SAL group increased during the first 6 months and then decreased until the end of follow-up (p<0.05 in all regions, figure 2B,E). The ChT in the HAL group increased in the first year and decrease in the second year (p<0.05 in all regions, figure 2A,D). The ChTs of the outer regions decreased less than the inner regions, and the ChTs of the superior regions decreased the least in the parafovea and perifovea in all three groups (all p<0.05). This ChT change pattern of different regions did not differ between the three groups as there was no significant interaction between group and region.
Figure 2.
ChT changes in horizontal (A–C) and vertical (D–F) regions in the three groups. Changes in ChT between baseline and 6 months are shown in black, changes between 6 months and 12 months are in blue, changes between 12 months and 18 months are in red, changes between 18 months and 24 months are in green. Error bars represent one SE of the mean. CF, central foveal; ChT, choroid thickness; HAL, spectacle lenses with highly aspherical lenslets; I3, 3 mm inferior region; I6, 6 mm inferior region; N3, 3 mm nasal region; N6, 6 mm nasal region; S3, 3 mm superior region; S6, 6 mm superior region; SAL,spectacle lenses with slightly aspherical lenslets; SVL, single vision spectacle lenses; T3, 3 mm temporal region; T6, 6 mm temporal region.
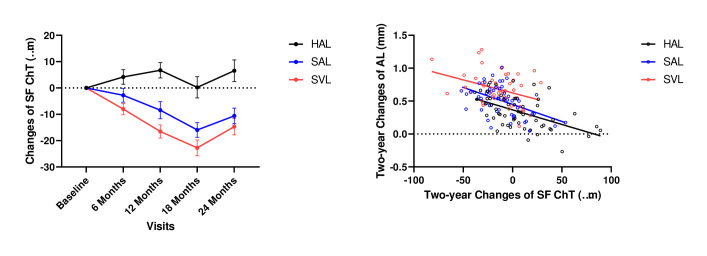
Figure 3.
Changes in subfoveal ChT (A) and correlation with axial length (AL) in the three groups (B). Changes in ChT and correlation with AL are shown in black for the HAL group, in blue for SAL group and in red for SVL group. Error bars represent one SE of the mean. ChT, choroidal thickness; HAL, spectacle lenses with highly aspherical lenslets; SAL, spectacle lenses with slightly aspherical lenslets; SF, subfoveal; SVL, single vision spectacle lenses.
Two-year AL elongation was significantly different among groups (F 2, 151 = 25.64, p<0.001, table 1), occurred significantly faster in the SVL group than in the HAL (p<0.001) and SAL (p=0.001) groups, and participants in the SAL group exhibited a faster elongation than those in the HAL group (p=0.002). Changes in the SF ChT were negatively correlated with changes in AL in all groups (r=−0.53, p<0.001 in the HAL group; r=−0.48, p<0.001 in the SAL group; r=−0.36, p=0.01 in the SVL group, figure 3). The 2-year change in AL was not correlated with baseline SF ChTs in any group (r=−0.15, p=0.29 in the HAL group; r=0.22, p=0.12 in the SAL group; r=0.02, p=0.92 in the SVL group).
Discussion
The main purpose of this study was to determine the long-term influence of spectacle lenses with aspherical lenslets on macular ChT. The ChT was decreased in the control SVL group, while the ChT in the HAL group did not decrease despite AL elongation after 2 years; the ChT in the SAL group also decreased, but to a lesser degree than that in the SVL group. There were significant negative correlations between the changes in AL and SF ChT in all groups.
Previous animal and clinical studies showed that short-term myopic defocus increased ChT in a transient and reversible way. Our study detected long-term changes in ChT and showed similar results. Over 2 years of lens wearing, SAL had little effect on increasing the ChT; furthermore, the choroid started to thin after the 6-month visit. Therefore, lenslets with slight asphericity may not be sufficient to prevent thinning of the ChT caused by axial elongation in the long term, and there may be a minimum lenslet asphericity required to prevent thinning. Lenslets with higher asphericity had a greater effect on preventing the ChT thinning. Among participants wearing HAL for 1 year, the ChT increased more than 10 µm (range: 11–16 µm) in all regions. However, in the second year, all the regions started to thin and finally showed no difference from the values at baseline. We assume that the retina gradually adapted and became less sensitive to defocus signals; therefore, the effect on choroidal thickening and myopia control reduced with time.
There were significant differences in the changes in the ChTs among the different regions. The lack of a significant interaction for group and region means the concentric rings design of lenslets had a similar influence on different regions of the choroid. The trend of change among regions was consistent across all three groups. The outer regions decreased less than the inner regions, and the superior regions showed the smallest decreases. Previous studies showed that when compared with emmetropic or hyperopic children, myopic children had the fewest changes in the superior region of the choroid.24 25 Studies on myopia progression also showed that the smallest decrease in ChT occurred in the superior regions.25 26 The reasons for this minor change in the superior ChT during myopia development and progression remain unknown. The sensitivities of different choroidal regions to myopic or hyperopic defocus can be further tested to determine the reasons for this phenomenon in the superior ChT.
The changes in the ChT at 18 months and 24 months were not consistent with those in the first year. The ChT revealed more decrease between the 12-month and the 18-month visits than in the first year, but less decrease between the 18-month and the 24-month visits (figure 2). One reason for this inconsistent change was a relatively long period of 18-month visit (7 months) and a shorter period of 24-month visit (5 months) because of COVID-19. Another hypothesis is that 1 month of near-work without outdoor activities before the 18-month visit led to transient but reversible thinning. Ghosh et al 27 and Woodman et al 28 found a decrease in the ChT with short-term accommodation that quickly reversed after relaxation of the accommodation. One month of near-work in our study (homeschooling from February to the date of visit29 may have induced additional thinning of ChT besides myopia progression at the 18-month visit, but this additional thinning may have reversed after participants stopped homeschooling (the end of May 2020,29 so the degree of thinning at the 24-month follow-up was less than other visits). The AL also showed the most elongation between 12 months and 18 months compared with other periods (0.12 mm, 0.15 mm, 0.21 mm between 12 months and 18 months, 0.34 mm, 0.50 mm, 0.68 mm for 2 years in the HAL, SAL and SVL group, respectively).
Both two test lenses in our study showed effective myopia control. Interestingly, after 2 years, the AL of three participants in the HAL group was shortened (−0.04 mm, −0.09 mm and −0.27 mm). Short-term myopic defocus induced by positive lenses worn for 1 hour significantly shortened the AL by approximately 10 µm, equal to the changes in ChT.30 In our study, after accounting for the changes in the ChT and the central corneal thickness, three participants still had AL shortening of more than 0.02 mm, which was more than the measurement error range and diurnal fluctuations. It indicates that the changes in ChT cannot fully explain the effect of HAL and SAL on myopia control.
The OCT examinations were done after two drops of cyclopentolate in this study, while previous studies showed this cycloplegic agent would alter ChT. Bahar et al 31 found that ChT decreased by 1 µm after 1% cyclopentolate. Ye et al 32 also found cyclopentolate caused a 3 µm decrease in ChT. We did not explore the influence of cycloplegia on the ChT, but all OCT exams were done at least 30 min after that last drop of cyclopentolate in all visits. On the other hand, participants were randomly assigned to three groups, and ChT had no difference among groups at baseline. We believe cycloplegia had the same impact on each ChT measurement and did not affect the results.
A limitation of this study is that the ChT measurement times were inconsistent between the visits. The times varied between 9:00 and 16:oo hours, and therefore, there is a possibility of diurnal fluctuations on ChT (±10 µm).33 As this study is a randomised and long-term follow-up design, we consider this limitation has little impact on the overall results and conclusions.
Conclusions
The macular ChT in myopic children had a lower degree of thinning or an increase after the use of spectacles lenses with aspherical lenslets for 2 years compared with the use of SVL, and the HAL had a better effect. For all groups, the outer regions experienced less thinning than the inner regions, and the superior choroidal regions had the most significant increases (HAL) or most minor decreases (SAL and SVL) in thickness compared with all other regions. More attention should be given to the properties of the superior choroid in the clinic rather than only the SF choroid. One month of homeschooling during the COVID-19 epidemic led to transient but reversible thinning of the choroid, and associations of near-work with ChT and myopia progression should be further explored.
Footnotes
YH and XL contributed equally.
HC and JB contributed equally.
Presented at: An abstract summarising the results at one year was presented online at the Association for Research in Vision and Ophthalmology, 2021.
Contributors: JB and HC are guarantors. YH and XL share cofirst authorship. Concept and design: JB and HC. Acquisition, analysis, or interpretation of data: YH, XL, JW, JH, FZ, JZ, JB and HC. Drafting of the manuscript: YH. Critical revision of the manuscript for important intellectual content: Huang, XL, JW, JH, FZ, JZ, AY, DPS, HC and JB. Statistical analysis: YH, XL, JB. Obtained funding: YH, JB and HC. Administrative, technical or material support: JB, YH, XL and YH. Supervision: JB and HC.
Funding: This work was supported by the Basic Scientific Research Project of Wenzhou (grant number Y2020343), the Medical and Health Science and Technology Project of Zhejiang Provincial Health Commission of China (grant number 2022PY072) and a collaborative research project with Essilor International (Wenzhou Medical University grant numbers 95016010, 95020005).
Competing interests: AY and DPS are employees of Essilor International. This company supplied the study devices.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by Ethics Committee of the Eye Hospital of Wenzhou Medical University approved this study (Y2018-054). Participants gave informed consent to participate in the study before taking part.
References
- 1. Summers JA. The choroid as a sclera growth regulator. Exp Eye Res 2013;114:120–7. 10.1016/j.exer.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res 2010;29:144–68. 10.1016/j.preteyeres.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beresford JA, Crewther SG, Kiely PM, et al. Comparison of refractive state and circumferential morphology of retina, choroid, and sclera in chick models of experimentally induced ametropia. Optom Vis Sci 2001;78:40–9. 10.1097/00006324-200101010-00013 [DOI] [PubMed] [Google Scholar]
- 4. Nickla DL, Totonelly K. Choroidal thickness predicts ocular growth in normal chicks but not in eyes with experimentally altered growth. Clin Exp Optom 2015;98:564–70. 10.1111/cxo.12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res 1995;35:1175–94. 10.1016/0042-6989(94)00233-C [DOI] [PubMed] [Google Scholar]
- 6. Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vision Res 1995;35:37–50. 10.1016/0042-6989(94)E0049-Q [DOI] [PubMed] [Google Scholar]
- 7. Tideman JWL, Polling JR, Jaddoe VWV, et al. Environmental risk factors can reduce axial length elongation and myopia incidence in 6- to 9-year-old children. Ophthalmology 2019;126:127–36. 10.1016/j.ophtha.2018.06.029 [DOI] [PubMed] [Google Scholar]
- 8. Nava DR, Antony B, Zhang LI, et al. Novel method using 3-dimensional segmentation in spectral domain-optical coherence tomography imaging in the chick reveals defocus-induced regional and time-sensitive asymmetries in the choroidal thickness. Vis Neurosci 2016;33:E010. 10.1017/S0952523816000067 [DOI] [PubMed] [Google Scholar]
- 9. Howlett MHC, McFadden SA. Spectacle lens compensation in the pigmented guinea pig. Vision Res 2009;49:219–27. 10.1016/j.visres.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 10. Troilo D, Nickla DL, Wildsoet CF. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci 2000;41:1249–58. [PubMed] [Google Scholar]
- 11. Hung LF, Wallman J, Smith EL. Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci 2000;41:1259–69. [PubMed] [Google Scholar]
- 12. Chiang ST-H, Phillips JR, Backhouse S. Effect of retinal image defocus on the thickness of the human choroid. Ophthalmic Physiol Opt 2015;35:405–13. 10.1111/opo.12218 [DOI] [PubMed] [Google Scholar]
- 13. Wang D, Chun RKM, Liu M, et al. Optical Defocus rapidly changes choroidal thickness in schoolchildren. PLoS One 2016;11:e0161535. 10.1371/journal.pone.0161535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mutti DO, Sinnott LT, Reuter KS, et al. Peripheral refraction and eye lengths in myopic children in the bifocal lenses in Nearsighted kids (blink) study. Transl Vis Sci Technol 2019;8:17. 10.1167/tvst.8.2.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berntsen DA, Kramer CE. Peripheral defocus with spherical and multifocal soft contact lenses. Optom Vis Sci 2013;90:1215–24. 10.1097/OPX.0000000000000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lam CSY, Tang WC, Tse DY-Y, et al. Defocus incorporated multiple segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. Br J Ophthalmol 2020;104:363–8. 10.1136/bjophthalmol-2018-313739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Z, Xue F, Zhou J, et al. Effects of Orthokeratology on choroidal thickness and axial length. Optom Vis Sci 2016;93:1064–71. 10.1097/OPX.0000000000000894 [DOI] [PubMed] [Google Scholar]
- 18. Li Z, Cui D, Hu Y, et al. Choroidal thickness and axial length changes in myopic children treated with orthokeratology. Cont Lens Anterior Eye 2017;40:417–23. 10.1016/j.clae.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 19. Lau JK, Wan K, Cheung S-W, et al. Weekly changes in axial length and choroidal thickness in children during and following Orthokeratology treatment with different compression factors. Transl Vis Sci Technol 2019;8:9. 10.1167/tvst.8.4.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Breher K, García García M, Ohlendorf A, et al. The effect of the optical design of multifocal contact lenses on choroidal thickness. PLoS One 2018;13:e0207637. 10.1371/journal.pone.0207637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bao J, Huang Y, Li X, et al. Spectacle lenses with aspherical lenslets for myopia control vs single-vision spectacle lenses: a randomized clinical trial. JAMA Ophthalmol 2022;140:472–8. 10.1001/jamaophthalmol.2022.0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bao J, Yang A, Huang Y, et al. One-year myopia control efficacy of spectacle lenses with Aspherical Lenslets. Br J Ophthalmol 2022;106:1171–6. 10.1136/bjophthalmol-2020-318367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hwang YH, Kim YY. Macular thickness and volume of myopic eyes measured using spectral-domain optical coherence tomography. Clin Exp Optom 2012;95:492–8. 10.1111/j.1444-0938.2012.00749.x [DOI] [PubMed] [Google Scholar]
- 24. Read SA, Alonso-Caneiro D, Vincent SJ, et al. Longitudinal changes in choroidal thickness and eye growth in childhood. Invest Ophthalmol Vis Sci 2015;56:3103–12. 10.1167/iovs.15-16446 [DOI] [PubMed] [Google Scholar]
- 25. Deng J, Li X, Jin J, et al. Distribution pattern of choroidal thickness at the posterior pole in Chinese children with myopia. Invest Ophthalmol Vis Sci 2018;59:1577–86. 10.1167/iovs.17-22748 [DOI] [PubMed] [Google Scholar]
- 26. Jin P, Zou H, Xu X, et al. Longitudinal changes in choroidal and retinal thicknesses in children with myopic shift. Retina 2019;39:1091–9. 10.1097/IAE.0000000000002090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghosh A, Collins MJ, Read SA, et al. Axial elongation associated with biomechanical factors during near work. Optom Vis Sci 2014;91:322–9. 10.1097/OPX.0000000000000166 [DOI] [PubMed] [Google Scholar]
- 28. Woodman EC, Read SA, Collins MJ. Axial length and choroidal thickness changes accompanying prolonged accommodation in myopes and emmetropes. Vision Res 2012;72:34–41. 10.1016/j.visres.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 29. Zhang H, Guo L-W, Gao Y-Y, et al. The impact of the COVID-19 pandemic on pediatric clinical practice in Wenzhou, China: a retrospective study. Front Pediatr 2020;8:585629. 10.3389/fped.2020.585629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Read SA, Collins MJ, Sander BP. Human optical axial length and defocus. Invest Ophthalmol Vis Sci 2010;51:6262–9. 10.1167/iovs.10-5457 [DOI] [PubMed] [Google Scholar]
- 31. Bahar A, Pekel G. The effects of pharmacological accommodation and cycloplegia on axial length and choroidal thickness. Arq Bras Oftalmol 2021;84:107-112. 10.5935/0004-2749.20210015 [DOI] [PubMed] [Google Scholar]
- 32. Ye L, Li S, Shi Y, et al. Comparisons of atropine versus cyclopentolate cycloplegia in myopic children. Clin Exp Optom 2021;104:143–50. 10.1111/cxo.13128 [DOI] [PubMed] [Google Scholar]
- 33. Chakraborty R, Read SA, Collins MJ. Hyperopic defocus and diurnal changes in human choroid and axial length. Optom Vis Sci 2013;90:1187–98. 10.1097/OPX.0000000000000035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjo-2022-321815supp001.pdf (205.1KB, pdf)
Data Availability Statement
No data are available.