Abstract
Objective
Colorectal cancer (CRC) is a leading cause of cancer-related deaths, with the majority of cases initiated by inactivation of the APC tumour suppressor. This results in the constitutive activation of canonical WNT pathway transcriptional effector ß-catenin, along with induction of WNT feedback inhibitors, including the extracellular palmitoleoyl-protein carboxylesterase NOTUM which antagonises WNT-FZD receptor-ligand interactions. Here, we sought to evaluate the effects of NOTUM activity on CRC as a function of driver mutation landscape.
Design
Mouse and human colon organoids engineered with combinations of CRC driver mutations were used for Notum genetic gain-of-function and loss-of-function studies. In vitro assays, in vivo endoscope-guided orthotopic organoid implantation assays and transcriptomic profiling were employed to characterise the effects of Notum activity. Small molecule inhibitors of Notum activity were used in preclinical therapeutic proof-of-principle studies targeting oncogenic Notum activity.
Results
NOTUM retains tumour suppressive activity in APC-null adenomas despite constitutive ß-catenin activity. Strikingly, on progression to adenocarcinoma with P53 loss, NOTUM becomes an obligate oncogene. These phenotypes are Wnt-independent, resulting from differential activity of NOTUM on glypican 1 and 4 in early-stage versus late-stage disease, respectively. Ultimately, preclinical mouse models and human organoid cultures demonstrate that pharmacological inhibition of NOTUM is highly effective in arresting primary adenocarcinoma growth and inhibiting metastatic colonisation of distal organs.
Conclusions
Our findings that a single agent targeting the extracellular enzyme NOTUM is effective in treating highly aggressive, metastatic adenocarcinomas in preclinical mouse models and human organoids make NOTUM and its glypican targets therapeutic vulnerabilities in advanced CRC.
Keywords: COLORECTAL CANCER, ONCOGENES
WHAT IS ALREADY KNOWN ON THIS TOPIC.
NOTUM is an extracellular palmitoleoyl-protein carboxylesterase that antagonises canonical Wnt signalling in mammals.
NOTUM can enzymatically cleave glypicans in Drosophila melanogaster and mammalian cells.
In Drosophila, NOTUM acts on cell-surface glypicans to modulate numerous signal transduction pathways at the receptor-ligand level.
Pharmacological NOTUM inhibition in the mammalian gut enhances Wnt signalling and stem cell activity.
WHAT THIS STUDY ADDS
NOTUM has tumour suppressive activity in APC-mutant colon tumouroids and adenomas despite canonical Wnt/B- catenin activation.
APC and P53 inactivation synergise during the transition from adenoma to adenocarcinoma to confer oncogenic activity on NOTUM.
Activity of glypicans 1 and 4 can account for differential effects of NOTUM activity in early-stage versus late-stage disease, respectively.
Pharmacological NOTUM inhibition is efficacious in a preclinical animal model of metastatic colon cancer.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The finding that pharmacological NOTUM inhibition is effective at inhibiting metastatic colon cancer in preclinical models harbouring both APC and P53 mutations provides an impetus for further probing the molecular mechanisms of NOTUM function and evaluating potential for NOTUM inhibition in treating human disease.
Introduction
Colorectal cancer (CRC) is the third-leading cause of cancer-related deaths in the USA, with 5-year survival rates around 10%. The majority (>80%) of CRC is driven by loss-of-function (LOF) mutations in the tumour suppressor APC,1–3 which results in the constitutive activation of the canonical WNT pathway effector ß-catenin.4 5 APC loss, however, also activates feedback inhibitors of canonical WNT signalling, including the extracellular palmitoleoyl-protein carboxylesterase NOTUM which inhibits WNT signalling at the ligand/receptor level in normal intestinal epithelium. WNT inhibition by NOTUM is proposed to occur through two distinct mechanisms. First, studies in Drosophila revealed that NOTUM can enzymatically cleave the Glypicans Dally and Dally-like protein (Dlp) from the cell surface.6–8 These glypicans potentiate WNT receptor-ligand interactions, and thus NOTUM activity antagonises canonical WNT.6–8 NOTUM cleavage of mammalian glypicans was subsequently confirmed.9 A second model for NOTUM antagonism of WNT signalling is that NOTUM directly cleaves the palmitoleate tails from WNT ligands, thus rendering them incapable of interaction with their Fzd receptors.10
Both models posit that the ability of NOTUM to antagonise WNT activity occurs at the level of receptor-ligand interaction. Therefore, inactivation of APC is predicted to render APC-null cells insensitive to the extracellular activity of NOTUM. Consistent with this, recent studies investigating the role of NOTUM in APC-null intestinal stem cell clones in mice suggest that its ability to attenuate WNT activity confers a competitive advantage to APC-null cells over their wild-type neighbours.11 12
We evaluated NOTUM function in genetically defined mouse models of CRC and made two striking observations. First, NOTUM retains potent, cell-autonomous tumour suppressive activity in APC-null epithelial cells, despite constitutive downstream activation of ß-catenin. Second, on subsequent inactivation of the P53 tumour suppressor during the adenoma-to-adenocarcinoma transition, NOTUM activity paradoxically becomes highly oncogenic. Mechanistically, we found that differential activity of glypicans GPC1 and GPC4 in APC-null adenomas and advanced colorectal adenocarcinomas, respectively, can account for the tumour suppressor-to-oncogene switch of NOTUM. Ultimately, preclinical mouse models of metastatic CRC demonstrate that pharmacological inhibition of NOTUM is highly effective in arresting primary adenocarcinoma growth and inhibiting metastatic colonisation of distal organs.
Methods
Mice studies
The mouse strains used in this study are from the following sources: Apcflx/flx mice were originally described by Shibata et al (PMID: 9311916).13 The Lgr5EGFP-IRES-CreERT2 (JAX strain 008875), KrasLSL-G12D (JAX strain 008179), C57BL/6J (JAX strain 000664) and transgenic Villin-CreER (JAX strain 033019) mice were obtained from The Jackson Laboratory (Bar Harbor, Maine, USA). All mice were bred and maintained on a C57BL/6J genetic background.
Human studies
Human normal and tumour organoids were established and cultured using previously established protocols.14
Detailed methods used in this study can be found in online supplemental method section.
gutjnl-2022-329140supp005.pdf (145.2KB, pdf)
Results
Oncogenic NOTUM activity in advanced colorectal adenocarcinoma
The majority of CRCs exhibit APC inactivation, and APC reactivation in established tumours is sufficient to arrest tumour growth, restore differentiation and re-establish normal tissue architecture despite the presence of oncogenic Trp53 and Kras mutations.15 We, therefore, reasoned that the immediate effects of APC inactivation may represent unexplored therapeutic vulnerabilities in later stages of colorectal adenocarcinoma. To this end, we performed transcriptome profiling of colon organoids immediately after APC inactivation and cross-referenced gene expression changes to the transcriptomes of several mouse models of colon cancer, including an inflammation-induced CRC model (azoxymethane (AOM)/dextran sodium sulfate (DSS)), a model of familial adenomatous polyposis (the ApcMin/+ mouse), and an implantation-based model of metastatic adenocarcinoma using Apc/Trp53/KrasG12D/Smad4-mutant tumour organoids16 (APKS tumouroids) (figure 1A). Of the roughly 150 genes whose expression is acutely induced on APC loss and remains elevated in these models (online supplemental table 1), the palmitoleoyl-protein carboxylesterase Notum stood out as it has previously been implicated in initiation of adenoma formation.12
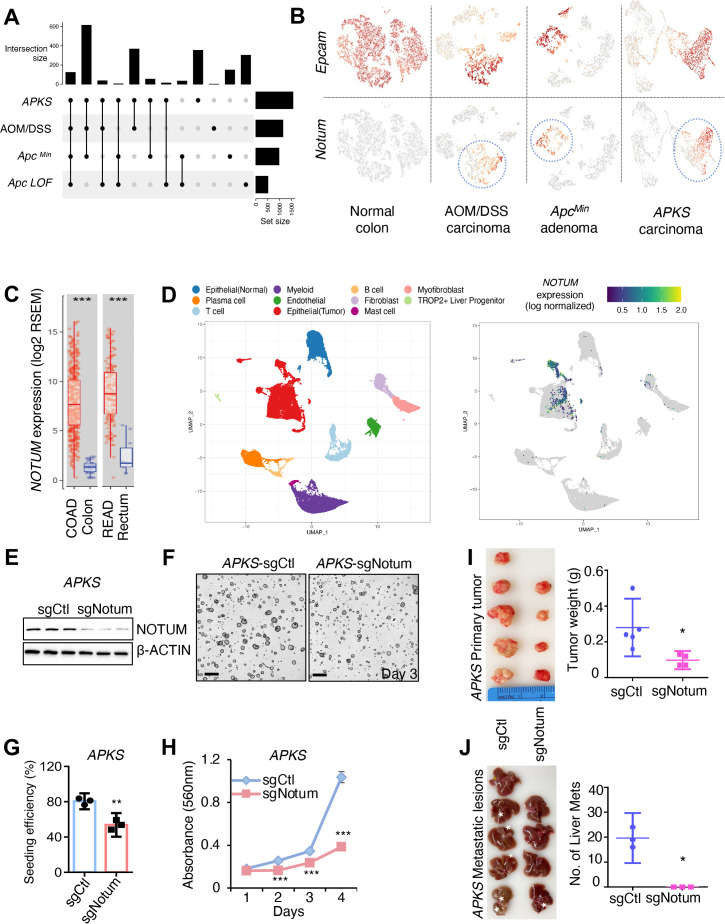
Figure 1.
Notum is a cell-autonomous oncogene in colorectal adenocarcinoma. (A) UpSet plot showing common differentially expressed genes (p<0.05, log2FC>1) in tumour cells from mouse models of CRC relative to normal colonic epithelium. APKS: adenocarcinoma resulting from orthotopic implantation of Apc/Trp53/Kras/Smad4 quadruple-mutant tumouroids into syngeneic mouse colon. Azoxymethane/dextran sodium sulfate (AOM/DSS): inflammation-induced colon adenocarcinoma. ApcMin/+ : colon adenoma induced by spontaneous loss of Apc heterozygosity in the Apcmin/+ mouse model. Apc LOF: colonic epithelium after acute loss-of-function of Apc in an Apcflox/flox::Villin-CreER mouse 5 days after tamoxifen-induced Apc inactivation. (B) Uniform manifold approximation projections (UMAP) of single cell RNA sequencing (scRNAseq) datasets from mouse tumour models in (A), with epithelial/carcinoma cells expressing Epcam (top). Notum expression (bottom) is restricted to epithelial/carcinoma cells (circled). (C) Human NOTUM expression in the Cancer Genome Atlas (TCGA) colon (COAD) and rectal (READ) adenocarcinoma datasets. (D) Left: UMAP representation the cell populations of human CRC primary tumour and adjacent normal epithelium. Cells are coloured according to their cell types. (n=17). Right: UMAP showing NOTUM expression restricted to human carcinoma cells. (E) Western blot analysis for NOTUM in mouse APKS tumouroids infected with control sgRNA (sgCtl) or Notum sgRNA(sgNotum). β-ACTIN was used as a loading control. (F) Images of mouse APKS tumouroids from (E) 3 days after seeding (n=3 technical replicates, with one representative of three independent experiments shown). Scale bar: 200 µm. (G) Clonal seeding efficiency of single APKS cells from (E/F). (H) MTT proliferation assays of APKS mouse tumouroids (n=3 technical replicates, with one representative of three independent experiments shown). (I) Left: Primary tumours formed 8 weeks after orthotopic injection of APKS tumouroids into the distal colon of syngeneic recipient mice (n=5 in sgCtl group, n=4 in sgNotum group). Right: Weight of primary tumours from the left. (J) Left: Livers of the mice bearing primary APKS tumours from (I). White asterisks show metastatic liver lesions. Right: The number of macrometastases visible in the liver of mice from (I). For all panels: *p<0.05, **p<0.01, ***p<0.001, Student’s t-test. See online supplemental figures 8,9 for validation of driver mutations and sgRNA knockdown. AOM/DSS, azoxymethane/dextran sodium sulfate; CRC, colorectal cancer.
gutjnl-2022-329140supp001.xlsx (28.5KB, xlsx)
gutjnl-2022-329140supp004.pdf (17.4MB, pdf)
High Notum expression was confirmed in mouse tumours, where its expression is limited to epithelial/tumour cells (figure 1B and online supplemental figure 1AB). We also confirmed strong activation of Notum in response to acute genetic ablation Apc or activation ß-catenin in colonic epithelium in vivo, consistent with Notum being a known direct ß-catenin target gene17 (online supplemental figure 1C). Further, NOTUM expression is significantly elevated in colon and rectal cancers in the human Cancer Genome Atlas3 (TCGA colon adenocarcinoma (COAD) and rectal adenocarcinoma (READ), respectively) relative to normal tissue (figure 1C). We also examined single cell transcriptome profiles of primary human COAD18 and confirmed that NOTUM expression is largely restricted to carcinoma cells and almost entirely absent in the tumour microenvironment (figure 1D).
Finally, examining a group of paired human primary tumours and their cognate liver metastases18 revealed further increases in NOTUM expression in the metastatic lesions (online supplemental figure 1D). These findings confirm published findings that NOTUM expression is induced in response to APC inactivation, and demonstrate that it remains elevated throughout the transition from adenoma to invasive adenocarcinoma, and becomes even further upregulated in metastatic lesions.
We initially evaluated NOTUM function in a mouse model of invasive and metastatically competent COAD: APKS tumouroids.19 On introduction of gRNAs targeting Notum, tumouroid cultures grew slower and had significantly diminished clonal tumouroid seeding efficiencies (the ability of a single cell to seed a new tumouroid, a proxy for tumour-initiating capacity) (figure 1E–H, online supplemental figure 1E,F). Conversely, gain-of-function (GOF) experiments in Apc/Trp53 (AP), Apc/Trp53/Kras (APK) and APKS tumouroids revealed that NOTUM overexpression can accelerate proliferation across this allelic series (online supplemental figure 1G,H). To test whether NOTUM has similar oncogenic activity in vivo, we performed both subcutaneous and orthotopic tumour formation20 assays with APKS tumouroids and NOTUM LOF. These assays revealed NOTUM LOF results in significant decreases in primary tumour growth, and, in the orthotopic model, a complete block of macrometastases in the liver (figure 1I,J and online supplemental figure 1I–M). These experiments were performed with bulk tumouroid cultures receiving gRNAs targeting Notum. Attempts at establishing subclonal cultures with biallelic Notum inactivation failed. One hypomorphic subclonal line exhibiting an approximately 80% reduction in NOTUM protein levels was stabilised (referred to as APKSHypo). This line grew poorly in vitro and failed to generate tumours in vivo (online supplemental figure 1N,O). Taken together, these data suggest that Notum is an obligate oncogene in colorectal adenocarcinoma.
NOTUM maintains tumour suppressive activity on APC inactivation
The oncogenic activity of NOTUM in these CRC models was surprising and counterintuitive given that NOTUM is an established negative regulator of canonical WNT signalling in the intestinal epithelium.10 12 We thus returned to re-evaluate NOTUM function in normal mouse organoids. NOTUM LOF in bulk wild-type colon organoid culture via CRISPR/Cas9 resulted dramatic increases in clonal organoid seeding efficiency, organoid growth, and ß-catenin target gene expression (figure 2A–C and online supplemental figure 2A), and NOTUM overexpression had converse effects (figure 2D,E and online supplemental figure 2B,C), consistent with the literature indicating that NOTUM negatively regulates the canonical WNT pathway in the normal intestinal stem cell compartment.10 21
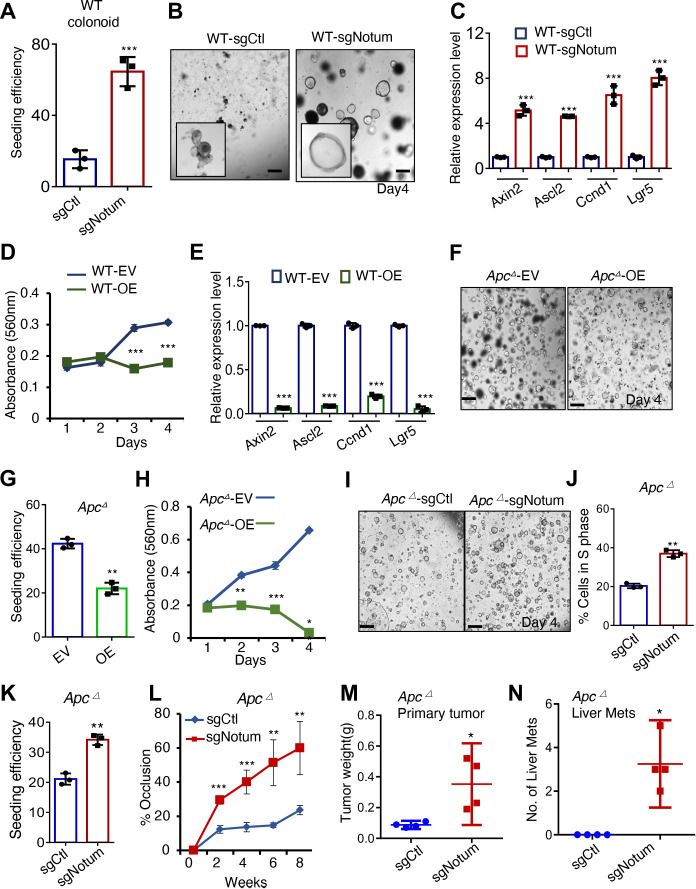
Figure 2.
NOTUM has potent tumour suppressive activity in normal and APC-mutant mouse colon tumouroids. (A) Clonal seeding efficiency of wild-type (WT) mouse colon organoids infected with control sgRNA (sgCtl) or Notum sgRNA (sgNotum) (n=3 technical replicates). (B) Images of WT mouse organoids infected with control sgRNA (sgCtl) or Notum sgRNA (sgNotum) 4 days after seeding (n=3 technical replicates). Scale bar: 100 µm. (C) QRT-PCR analysis of canonical Wnt/B-CATENIN pathway target genes including Axin2, Ascl2, Ccnd1 and Lgr5 in organoids from (B). (D) MTT proliferation assays of WT organoids infected with empty lentiviral vector (EV) or Notum overexpression vector (OE) (n=3 technical replicates). (E) Relative expression level of Wnt pathway target genes including Axin2, Ascl2, Ccnd1 and Lgr5 in WT organoids infected with EV or Notum OE (n=3 technical replicates, with one represent). (F) Images of Apc mutant mouse tumouroids infected with EV or vector overexpressing Notum 4 days after seeding (n=3 technical replicates, with one representative of three independent experiments shown). Scale bar: 200 µm. (G) Seeding efficiency of Apc mutant tumouroids from (F). (H) MTT proliferation assays of Apc mutant tumouroids from (F) (n=3 technical replicates, with one representative of three independent experiments shown). (I) Images of Apc mutant mouse tumouroid cultures infected with control sgRNA (sgCtl) or Notum sgRNA (sgNotum) 4 days after seeding (n=3 biological replicates). Scale bar: 200 µm. (J) The percentage of cells in S-phase (EdU+) in cultures from (I). (K) Clonal seeding efficiency of cells from tumouroids in (I). (L) Percent lumen occlusion was measured after orthotopic implantation of Apc-mutant tumouroids infected with control sgRNA (sgCtl) or Notum sgRNA (sgNotum) (n=4 biological replicates). (M) Weight of primary, orthotopic Apc mutant mouse colon tumours with or without Notum loss of function (n=4 biological replicates). (N) The number of macroscopically observable liver metastases in mice harbouring orthotopic from (L, M). For all panels: *p<0.05, **p<0.01, ***p<0.001, Student’s t-test. See (online supplemental figure 8,9) for validation of driver mutations, sgRNA knockdown and overexpression.
We performed analogous experiments in adenomatous tumouroids with inactivating Apc mutations introduced by CRISPR/Cas9 (Apc ∆). Remarkably, NOTUM GOF in Apc ∆ tumouroids resulted in decreased clonal seeding efficiency and proliferation, and NOTUM LOF had converse effects (figure 2F–K and online supplemental figure 2D,E). We confirmed these findings in a second model: tumouroids from Apcflox/flox::Villin-CreER mice after Cre activation (online supplemental figure 2F,G). Given that APC loss stabilises ß-catenin, these findings are unexpected, as NOTUM-mediated Wnt antagonism is expected to occur at the receptor-ligand interaction.12 Recent studies suggest that NOTUM induction in an Apc ∆ intestinal stem cell confers a competitive advantage over surrounding wild-type epithelial cells via juxtacrine/paracrine activity.11 12 We, therefore, asked how NOTUM loss influences tumourigenesis of Apc ∆ tumouroids in vivo. Endoscope-guided orthotopic implantation of Apc ∆ tumouroids confirmed that NOTUM retains potent tumour suppressive activity in the absence of APC function, with Apc ∆ tumouroids forming only small adenomas in the colonic epithelium. In contrast, loss of NOTUM dramatically increased primary tumour size and promoted metastasis (figure 2L–N and online supplemental figure 2H–L). Thus, NOTUM retains potent tumour suppressive activity in adenomatous lesions driven by APC inactivation despite constitutive ß-catenin activation.
Because of the surprising nature of these findings, we sought to validate them using an orthogonal approach: pharmacological inhibition of NOTUM activity in vivo in adenomas formed by inactivation of Apc in epithelial stem cells using Lgr5CreER .22 Tumours were allowed to form for 20 days after Tamoxifen induction in Lgr5CreER::Apcflx/flx mice, then animals were treated daily with the small molecule NOTUM catalytic inhibitor ABC99,8 23 or vehicle control for four additional weeks (online supplemental figure 3A). At the experimental endpoint, mice treated with NOTUM inhibitor had more frequent and proliferative adenomas relative to controls, confirming CRISPR-Cas9 organoid experiments indicating that NOTUM retains tumour suppressive activity in APC-null lesions (online supplemental figure 3B–E).
Novel tumour suppressive and oncogenic functions of NOTUM require extracellular enzymatic activity
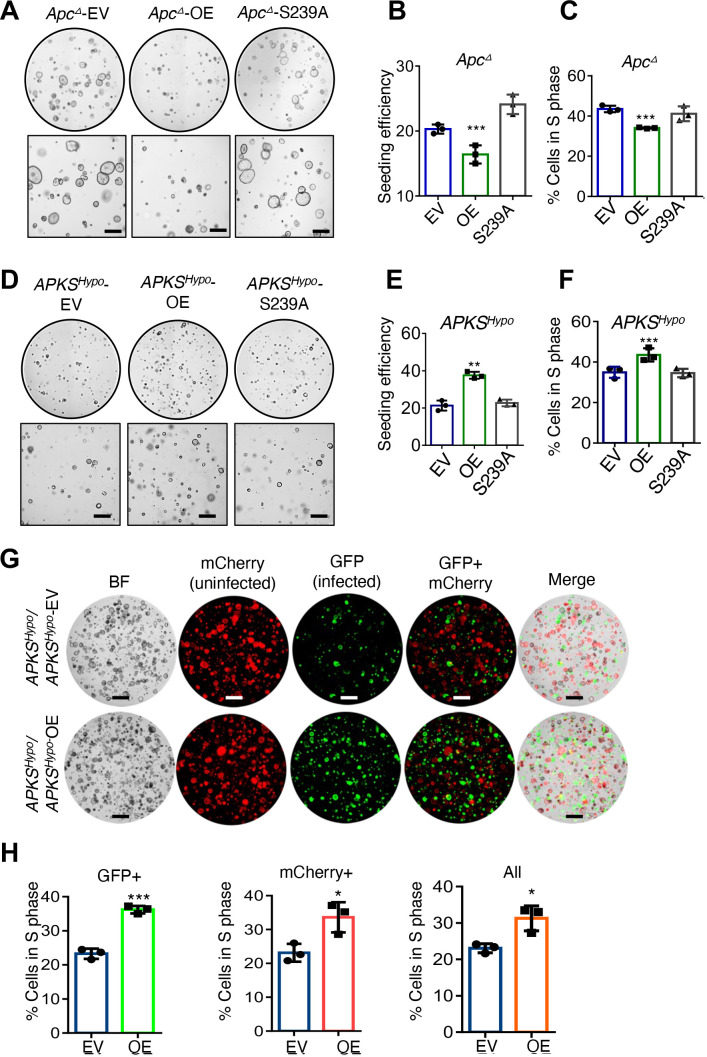
Because our findings in Apc ∆ tumouroids, and more aggressive AP/APK/APKS tumouroids were unexpected based on models of NOTUM function in mammals, we next tested whether NOTUM’s tumour suppressive activity in Apc ∆ tumouroids and oncogenic activity in AP/APK/APKS tumouroids require enzymatic activity. We overexpressed wild-type or catalytically dead (S239A)10 NOTUM in mouse Apc ∆ tumouroids and found that NOTUM S239A was unable to suppress proliferation and clonal seeding efficiency (figure 3A–C and (online supplemental figure 4A). Conversely, we overexpressed wild-type and S239A NOTUM in the hypomorphic APKS subclone (online supplemental figure 1O), and found that, while wild-type NOTUM increased tumouroid seeding efficiency and proliferation, catalytically dead NOTUM did not (figure 3D–F and online supplemental figure 4B). Thus, catalytic activity is required for both NOTUM’s tumour suppressive activity in Apc ∆ tumouroids and for oncogenic activity in APKS tumouroids.
Figure 3.
Tumour suppressive and oncogenic functions of NOTUM require catalytic activity and occur via paracrine/juxtacrine signalling. (A) Brightfield images of Apc-mutant mouse tumouroids infected with empty vector (EV), vector expressing wild-type NOTUM (OE) or catalytically dead NOTUM with Ser239Ala mutation (S239A) (n=3 technical replicates). Scale bar: 100 µm. (B) Clonal seed efficiency from single cells in (A). (C) S-phase/EdU incorporation assays in tumouroids from (A). (D) Brightfield images of the mouse APKS subclone with hypomorphic NOTUM mutation from online supplemental figure 1N,O (APKSHypo ) infected with EV, vector expressing wild-type NOTUM (OE), or catalytically dead NOTUM with Ser239Ala mutation (S239A) (n=3 technical replicates). Scale bar: 100 µm. (E) Clonal seed efficiency from single cells in (D). (F) EdU incorporation assays in APKSHypo organoid from (D). (G) Images of APKSHypo mouse tumouroids, with tumouroids infected with an mCherry-expressing vector and cocultured with tumouroids infected with a vector expressing GFP only (EV) or expressing GFP and NOTUM (OE) (n=3 technical replicates). Scale bar: 100 µm. (H) EdU assays in the cultures in (G), quantifying the percentage of cells in S phase in the GFP+or mCherry+populations or the overall population. *p<0.05,**p<0.01, ***p<0.001, Student’s t-test. see online supplemental figures 8,9 for validation of driver mutations, sgRNA knockdown and overexpression.
We next asked whether NOTUM’s tumour suppressive and oncogenic activities in Apc ∆ and APKS tumouroids, respectively, occur in a paracrine/juxtacrine manner. We GFP-tagged mouse Apc ∆ tumouroids overexpressing NOTUM or an empty vector and cocultured them with unlabeled Apc ∆ tumouroids. Both GFP+cells overexpressing NOTUM and GFP-negative cells proliferated slower than empty vector controls, confirming that NOTUM’s tumour suppressive activity in the absence of functional APC occurs in a paracrine/juxtacrine manner (online supplemental figure 4C,D). We performed an analogous experiment in Notum hypomorphic APKS tumouroids, this time tagging half the culture with mCherry and the other half with a vector expressing GFP and NOTUM or GFP-only. As with Apc ∆ tumouroids, we found that GFP+, NOTUM overexpressing cells were able to drive the proliferation of mCherry+cells non-cell-autonomously, as well as the cell-autonomous proliferation of GFP+APKS cells (figure 3G,H). We, therefore, conclude that the novel oncogenic activity of NOTUM requires catalytic activity and occurs extracellularly.
Apc and Trp53 inactivation synergise to confer oncogenic activity on NOTUM
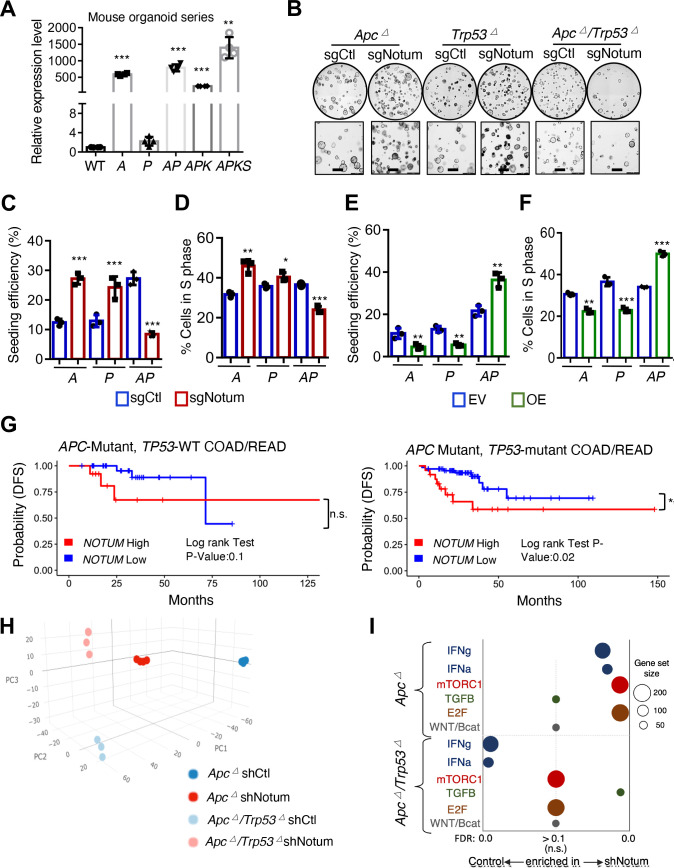
Having established that NOTUM enzymatic activity is tumour suppressive in wild-type and Apc ∆ tumouroids and is oncogenic in AP/APK/APKS tumouroids, we reasoned that a genetic switch must underly the reversal of NOTUM function, and that P53 inactivation may be responsible. Notum expression is strongly upregulated on Apc inactivation, and remains elevated on accumulation of oncogenic Trp53, Kras, and Smad4 mutations (figure 4A). In contrast, inactivation of Trp53 alone had little effect on Notum expression. We assayed the effects of Notum LOF and GOF in Apc ∆, Trp53 ∆ and AP tumouroids and found, remarkably, that NOTUM activity remains tumour suppressive in both Apc ∆ and Trp53 ∆ tumouroids, but together these mutations synergise to confer oncogenic activity on NOTUM (figure 4B–F and online supplemental figure 5A–C).
Figure 4.
APC and P53 loss synergise to convert NOTUM from a tumour suppressor to an oncoprotein. (A) Relative Notum transcript levels in WT mouse colon organoids, and mouse tumouroids harbouring Apc loss of function (A), Trp53 loss of function (P), both (AP), AP with KrasG12D mutation (APK) and APK with Smad4 loss of function (APKS) (n=3 technical replicates). (B) Brightfield image of A, P and AP mouse tumouroids infected with control sgRNA (sgCtl) or Notum sgRNA (sgNotum) (n=3). Scale bar: 100 µm. (C) Clonal seeding efficiency of A, P and AP cells from (B). (D) S-phase progression (EdU+) assays in A, P and AP tumouroids from (b) (n=3 technical replicates with one representative of three independent experiments shown). (E) Clonal seeding efficiency of A, P and AP cells infected with NOTUM overexpressing lentivirus (OE) or empty vector control (EV) (n=3 technical replicates). (F) S-phase progression (EdU+) assays in A, P and AP tumouroids as in (E). (G) Analysis of disease-free survival (DFS) in human colon and rectal adenocarcinoma patients (COAD, READ) for which gene expression data is available in the Cancer Genome Atlas (TCGA). Left panel shows DFS for APC mutant, TP53 wild-type patients (n=46), binned on highest and lowest quartile of NOTUM expression (Q1 vs Q4). Right panel shows DFS for APC/TP53 double mutant COAD/READ patients (n=93), binned on highest and lowest quartile of NOTUM expression (Q1 vs Q4). (H) Principal component analysis (PCA) of transcriptome profiles from Apc mutant or Apc/Trp53 mutant mouse cultures with or without shRNA-mediated knockdown of Notum (n=4 biological replicates). (I). Gene set enrichment analysis of transcriptome profiles from (H). For all panels: *p<0.05, **p<0.01, ***p<0.001, Student’s t-test. See online supplemental figure 8,9 for validation of driver mutations, shRNA/sgRNA knockdown and overexpression.
Given the striking nature of these findings, we asked whether evidence exists in human CRCs for mutational background-dependent oncogenic NOTUM activity. We pooled patient data from COAD and READ from TCGA, and stratified patient disease-free survival based on NOTUM expression (top vs bottom 50% or quartile 1 vs quartile 4) in patients that have APC mutations but wild-type TP53, or those harbouring both APC and TP53 mutations. Consistent with our experimental findings, high NOTUM expression predicts significant decreases in disease-free survival only in TP53-mutant tumours and not in TP53 wild-type tumours (figure 4G and online supplemental figure 5D). These differences are likely underappreciated since P53 function can be inhibited independent of genetic mutation (eg, MDM2 overexpression).24 Given that APC and TP53 are the two most frequently mutated genes in CRC and often coincident in advanced tumours,3 these findings have clinical implications for advanced colorectal adenocarcinoma.
To delineate the molecular mechanisms underlying NOTUM’s tumour suppressive-to-oncogenic switch, we performed transcriptome profiling of Apc ∆ and AP cultures, with or without NOTUM LOF (figure 4H). The pathways most differentially affected by NOTUM LOF between Apc ∆ and AP mutant cultures included mTORC1 and E2F signalling (induced by NOTUM LOF in Apc ∆ but not AP), Interferon α/γ signalling (induced by NOTUM LOF in Apc ∆ suppressed in AP), and TGFß (induced by NOTUM LOF in AP, not in Apc ∆) (figure 4I, online supplemental figure 4E and online supplemental table 2). Importantly, canonical WNT/ß-catenin signalling was unaltered in either Apc ∆ or AP cultures on NOTUM inhibition (p=0.975 and 0.885, respectively, figure 4I,).
Figure 5.
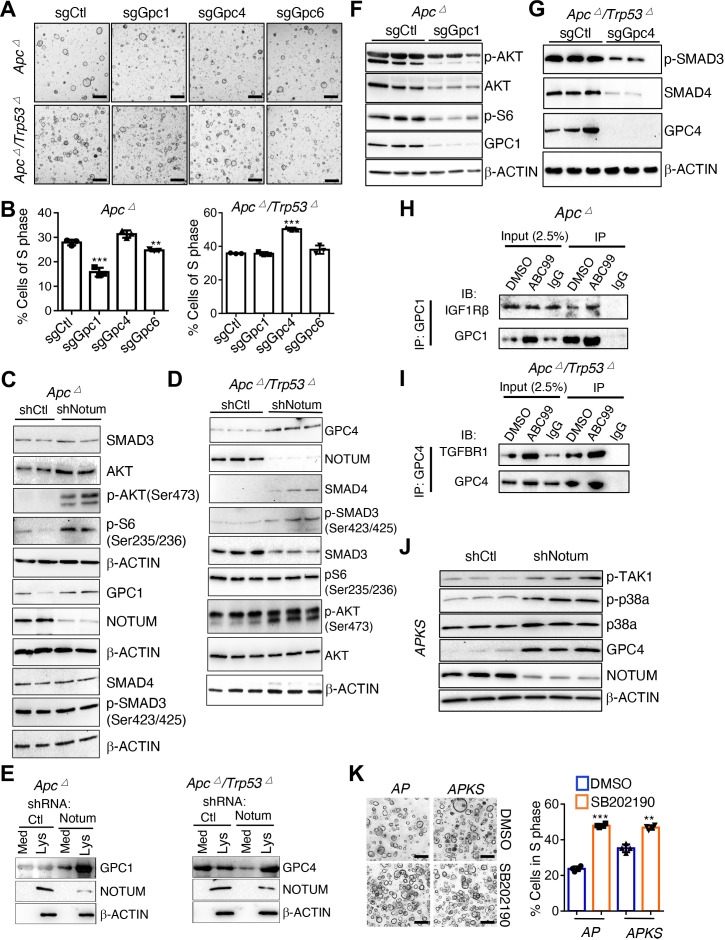
Glypicans mediate the differential effects of NOTUM activity in Apc mutant vs Apc/Trp53 mutant mouse tumouroids. (A) Brightfield image of Apc mutant mouse tumouroids (A) and Apc/Trp53 double mutant tumouroids (AP) infected with control sgRNA (sgCtl), Gpc1 sgRNA (sgGpc1), Gpc4 sgRNA (sgGpc4), Gpc6 sgRNA (sgGpc6) (n=3 technical replicates). Scale bar: 100 µm. (B) EdU assays quantifying fraction of cells in S-phase from cultures shown in (A) (n=3 technical replicates). (C) Western blotting in Apc mutants infected with control shRNA (shCtl) or Notum shRNA (shNotum). β-ACTIN was used as a loading control. Note multiple β-ACTIN blots, one for each of three identical replicate blots. (D) Western blotting in Apc/Trp53 mutant tumouroids infected with control shRNA (shCtl) or Notum shRNA (shNotum). β-ACTIN was used as a loading control. (E) Western blotting for GPC1 (left) and GPC4 (right) using N-terminal antibodies recognising the extracellular domain of indicated glypicans in the media supernatant (Med) or cell lysate (Lys) of Apc mutant or Apc/Trp53 double mutant tumouroids infected with control shRNA (shCtl) or Notum shRNA (shNotum). β-ACTIN was used as a loading control. (F) Western blotting in Apc mutant tumouroids infected with control sgRNA (sgCtl) or Gpc1 sgRNA (sgGpc1). β-ACTIN was used as a loading control. (G) Western blotting in Apc/Trp53 mutant tumouroids infected with control sgRNA (sgCtl) or Gpc4 sgRNA (sgGpc4). β-ACTIN was used as a loading control. (H) Coimmunoprecipitation of IGF1Rb with anti-GPC1 antibody in Apc mutant tumouroids in the presence or absence of the small molecule NOTUM inhibitor ABC99. (I) Coimmunoprecipitation of TGFβR1 with anti-GPC4 antibody in Apc/Trp53 double mutant tumouroids in the presence or absence of the small molecule NOTUM inhibitor ABC99. (J) Western blotting assessing non-canonical TGFβ-TAK1-p38a pathway activity in APKS tumouroids in response to NOTUM inhibition. β-ACTIN was used as a loading control. (K) AP and APKS tumouroids treated with the p38 inhibitor SB202190, with EdU-based quantification of S-phase (n=3 technical replicates). Scale bar: 100 µm. For all panels: **p<0.01, ***p<0.001, Student’s t-test. See online supplemental figures 8,9 for validation of driver mutations, shRNA/sgRNA knockdown and overexpression.
gutjnl-2022-329140supp002.xlsx (10.2KB, xlsx)
The transcriptional pathways effected by NOTUM inhibition reside downstream of cell-surface receptors, many of which are influenced by the activity of Glypicans, a family of proteoglycans that can be cleaved from the cell surface by NOTUM activity.7 9 Glypicans have been implicated in the regulation of WNT, TGFβ, IGF, FGF, VEGF, Hh, HGF and BMP receptor activity, primarily in studies carried out in Drosophila.25–27 In mammals, how each of the six paralogous orthologs of Drosophila glypicans (mammalian Gpc1-6) influence activity of these receptors and in what context remains poorly understood.
We examined the expression of the mammalian Glypicans in mouse and human tumours and tumouroids and found Gpc/GPC1 and Gpc/GPC4 to be the dominantly expressed family members (online supplemental figure 6A–D). Given the models established in the literature, we hypothesised that on cleavage of their glycosyl-phosphatidylinositol (GPI) anchor by NOTUM, GPCs released from the cell surface may either be neutralised or negatively regulate receptor activity due to their ability to compete with cell surface-bound receptors for their cognate ligands.
The tumour suppressive versus oncogenic functions of NOTUM can be accounted for by differential GPC activity
We tested whether genetic inactivation of Gpc1/4 could phenocopy NOTUM activity. Interestingly, while GPC1/4 protein levels were both detectible in mouse Apc ∆ and AP tumouroids (online supplemental figure 6E), GPC1 exhibited oncogenic activity only in Apc ∆ cultures, with no effect in AP tumouroids. Conversely, GPC4 exhibited tumour suppressive activity only in AP tumouroids, with no effect in Apc ∆ tumouroids (figure 5A–B, online supplemental figure 6F–H). Gpc6, whose expression was low but detectable, had little to no effect in either genotype (figure 5A–B).
To link NOTUM function, GPC protein and signalling pathway activity, we knocked down Notum in mouse Apc ∆ and AP tumouroids and examined pathway activity and cell-associated GPC protein levels. Notum knockdown in Apc ∆ tumouroids lead to increased mTORC1 signalling coupled with an accumulation of GPC1 protein, with no apparent changes to TGFß pathway activity (figure 5C). In contrast, Notum knockdown in AP tumouroids lead to increased TGFß pathway activity and an accumulation of GPC4 protein, with no apparent effect on mTORC1 activity (figure 5D). These experiments assayed GPC protein levels in whole cell lysates and support a model whereby Notum LOF prevents cleavage of GPCs from the cell surface leading to their accumulation, as previously reported.7 9 To further probe this possibility, we examined levels of free N-terminal GPC1 and GPC4 in the media supernatant of Apc ∆ and AP tumouroids after NOTUM inhibition. NOTUM LOF in Apc ∆ tumouroids increased cell associated GPC1 relative to GPC1 in the supernatant (figure 5E). Conversely, Notum LOF in AP tumouroids led to increased cell associated GPC4 concomitant to decreased GPC4 in the supernatant (figure 5E). Interestingly, NOTUM protein itself was associated with the cell lysate and not supernatant, a finding that, in conjunction with the establish extracellular enzymatic effects of NOTUM, suggests that NOTUM is physically associated with the extracellular cell surface (figure 5E).
To further test whether the effects of GPC manipulation are consistent with NOTUM cleaving GPCs 1 and 4 from the surface of Apc ∆ and AP tumouroids, respectively, we analysed the effects of GPC inactivation on the pathways differentially effected by NOTUM activity. Indeed, GPC1 inactivation in mouse Apc ∆ tumouroids inhibited mTORC1 activity, and GPC4 inactivation in mouse AP tumouroids inhibited TGFß activity, consistent with the effects of NOTUM manipulation (figure 5F–G). Finally, we sought to confirm direct interaction between GPC1 or GPC4 and the receptors upstream of the affected signal transduction pathways. Immunoprecipitation of GPC1 in Apc ∆ tumouroids coimmunoprecipitated IGF1Rß, an IGF receptor upstream of mTORC1 activation implicated in colon cancer and chemotherapeutic resistance28 29 (figure 5H). In AP tumouroids, immunoprecipitation of GPC4 coimmunoprecipitated TGFßR1 upstream of the TGFß pathway (figure 5I). Addition the small molecule NOTUM inhibitor ABC99,8 23 appeared to potentiate these interactions (figure 5H–I).
Taken together, these findings support a model whereby NOTUM exerts its tumour suppressive effects in Apc ∆ cells by suppression of oncogenic GPC1 activation of mTORC1. In contrast, on Trp53 inactivation NOTUM exerts oncogenic effects via inhibition of tumour suppressive GPC4 activation of TGFß activity. However, we also observed oncogenic effects of NOTUM in aggressive APKS mouse tumouroids and tumours, where the canonical TGFß pathway is not functional due to inactivating mutations in Smad4 (an established suppressor of colon cancer metastasis).30 31 We, therefore, interrogated the effects of NOTUM on non-canonical TGFß pathway activity mediated through TAK1- p38a signal transduction. Consistent with our findings in AP tumouroids, NOTUM inhibition resulted in increased GPC4 in APKS cell lysate (figure 5J). Further consistent with NOTUM’s effects as a negative regulator of TGFß signalling, Notum inhibition in APKS tumouroids resulted in increased phosphorylation of TGFß-Activated Kinase (TAK1) and downstream p38a phosphorylation (figure 5J). The role of p38a activity in cancer is pleiotropic and context-dependent, including evidence for both oncogenic and tumour suppressive effects (the latter through cell cycle arrest, differentiation, senescence, apoptosis, particularly in KRAS-activated cancers).32–34 We observed that p38a inhibition in both AP and APKS tumouroids increased proliferation and inhibited cell death (figure 5K and online supplemental figure 6I). In APKS tumouroids, NOTUM inhibition induced apoptosis, and this was reversed by concomitant inhibition of p38a (online supplemental figure 6I). These findings support the notion that NOTUM may exert oncogenic effects in adenocarcinoma, at least in part, via inhibition of tumour suppressive activity of the non-canonical TGFß pathway.
Pharmacological NOTUM inhibition is efficacious in a preclinical animal model of metastatic colon cancer
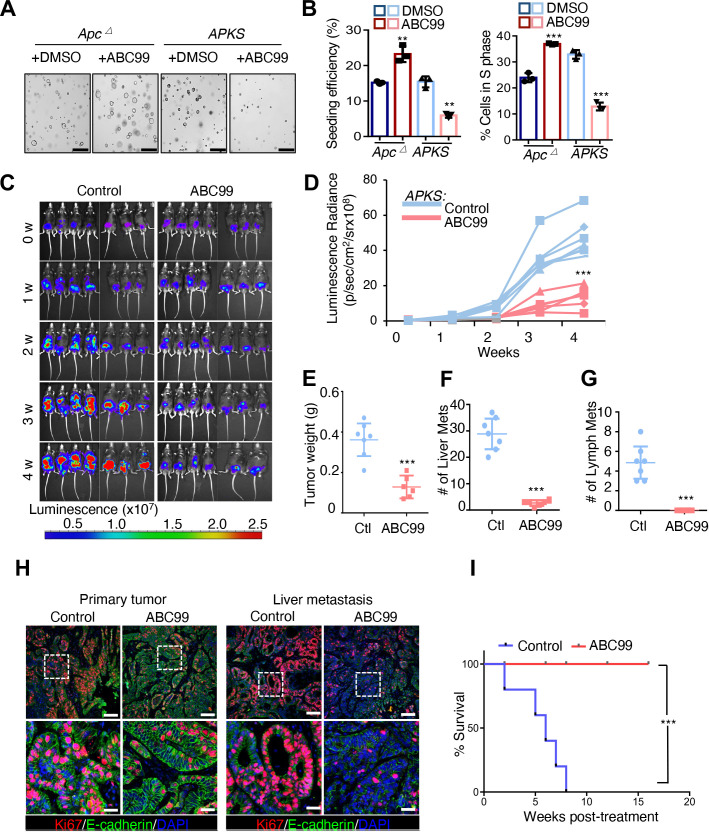
Ultimately, we asked whether small molecule inhibition of NOTUM represents a viable therapeutic strategy in advanced CRCs harbouring inactivating mutations in Apc and Trp53. ABC99 is a selective NOTUM inhibitor with in vivo efficacy.21 23 We initially confirmed that ABC99 treatment phenocopies genetic inhibition of NOTUM in Apc ∆ and APKS tumouroids (figure 6A,B and online supplemental figure 7A,B). Next, we orthotopically implanted APKS tumouroids into the colonic mucosa of syngeneic mice via endoscope-guided injection and allowed tumours to engraft and grow for 1 month, after which mice were randomly assigned to a vehicle control or ABC99 group. An absence of difference in tumour size was confirmed between the randomised groups at baseline (online supplemental figure 7C,D). Mice were then treated with ABC99 or vehicle control for an additional 4 weeks. ABC99 treatment largely arrested primary tumour growth, with dramatic decreases in both BLI and tumour weight relative to control animals (figure 6C–E). Remarkably, NOTUM inhibition nearly completely inhibited liver and lymph node metastases in this highly aggressive CRC model (figure 6F and G and online supplemental figure 6E,F), and ABC99-treated primary and rare metastatic lesions exhibited notable inhibition of cell proliferation at the termination of the experiment (figure 6H). We generated another cohort of mice with orthoptic APKS tumours, allowed them to engraft for 1 month as before, then began ABC99 or vehicle control treatment and monitored survival up to 4 months. Remarkably, while the of vehicle control-treated mice became moribund over this time course, none of the ABC99-treated cohort succumbed to disease for the duration of this experiment (figure 6I).
Figure 6.
Small molecule inhibition of NOTUM inhibits tumour growth and metastasis in a mouse model of colorectal cancer. (A) Brightfield image of Apc mutant and APKS mouse tumouroids treated with the small molecule inhibitor of NOTUM, ABC99 or vehicle control (DMSO) (n=3 technical). Scale bar: 100 µm. (B) Single cell clonal seeding efficiency and EdU assay quantifying fraction of cells in S phase from tumouroids in (A) (n=3 technical replicates). (C) Bioluminescent imaging (BLI) of mice after endoscope-guided orthotopic implantation of APKS mouse tumouroids into the colonic mucosa. Mice were treated with ABC99 or vehicle (corn oil) control after 4 weeks of engraftment and tumour growth. Mice are treated for indicated number of weeks (week 0=4 weeks post-implantation) (n=7 mice). (D) Quantification of luminescence radiance from (C) at indicated time points (n=7 mice). (E–G) Quantification of primary tumour weight (E), number of macrometastases in the liver (F), and number of lymph node metastases (G) in mice from (C) at the termination of the experiment (8 weeks total, 4 weeks post-treatment). (H) Immunofluorescence staining for KI67 and E-CADHERIN in primary tumour and liver metastases from tumours in (C) (n=7 mice). Nuclei are visualised by DAPI staining. Scale bar of top panels: 200 µm. Lower panels: 50 µm. (I) Kaplan-Meier survival analysis for mice harbouring orthotopic APKS tumours as in (C), treated with ABC99 or vehicle (corn oil) control for 16 weeks following an initial 4-week period of engraftment and tumour growth (total experimental time course of 5 months) (n=10 mice). For all panels, **p<0.01, ***p<0.001, Student’s t-test.
Finally, we asked whether pharmacological NOTUM inhibition had similar efficacy in human CRC tumouroids. Treatment of APC/TP53 mutant human colon tumouroids with ABC99 inhibited clonal tumouroid initiation capacity and proliferation, confirming that NOTUM vulnerability translates into humans (online supplemental figure 6G–I). Ultimately, these findings identify NOTUM as a tractable therapeutic target in advanced colorectal adenocarcinoma harbouring inactivating mutations in both APC and TP53.
Discussion
Advanced CRC remains a leading cause of cancer-related deaths globally, with few efficacious therapeutic options available once the primary tumour has disseminated. While it is well established that APC inactivation is likely the initiating event in the vast majority of CRC, how the events downstream of APC inactivation influence later stages of tumourigenesis after accumulation of additional oncogenic mutations remains unclear. Recent data suggest that APC inactivation is required for tumour maintenance in more advanced disease.15 Activation of the extracellular palmitoleoyl-protein carboxylesterase NOTUM has long been observed downstream of APC inactivation, and Notum is recognised as a direct ß-catenin transcriptional target gene.17
In the context of intestinal tumourigenesis, induction of Notum expression downstream of APC inactivation might be viewed as a futile attempt of the transformed cell to attenuate oncogenic ß-catenin transcriptional activity. Indeed, a recent study put forth the hypothesis that the robust induction of Notum expression in an intestinal epithelial stem cell that has inactivated APC gives that Apc-null clone a competitive advantage over its Apc-wild-type neighbours via NOTUM-mediated juxtacrine inhibition of canonical WNT signalling.11 12 On the surface, these findings appear contradictory with our current observations. However, these findings can potentially be reconciled if the anti-proliferative effects of NOTUM are stronger on wild-type versus Apc-null stem cells, which is reasonable to assume given the exquisite dependence of wild-type stem cells on WNT ligand/receptor interactions. In this context, a Notum-null clone would have a proliferative advantage over its wild-type neighbours. However, on reaching some critical size at which the adenomatous lesion is no longer constrained by neighbouring wild-type epithelial cells (as in our organoid cultures, orthotopic implantation model and mouse genetic model of Apc inactivation in Lgr5+stem cells), the cell-autonomous tumour suppressive role of Notum in an Apc-null clone, which has previously not been addressed, becomes evident. The unexpected finding that NOTUM retains potent tumour suppressive activity in adenomatous Apc-null cells is not explicable by NOTUM’s attenuation of WNT pathway activity, nor is its’ switch to oncogenic activity on progression to adenocarcinoma with subsequent P53 inactivation.
Instead, the literature points to a potentially much broader role for NOTUM in the regulation of numerous signal transduction cascades via its ability to cleave cell surface Glypicans. Glypicans are a family of proteoglycans that have expanded to six paralogous orthologs in mammals (Gpc1-6).35 Most current models for Glypican function indicate that these proteoglycans, when anchored to the cell surface, act to potentiate receptor-ligand interactions.7 25–27 NOTUM can cleave GPCs at their GPI linkage to the cell surface,7 9 and once cleaved from the cell surface, N-terminal GPCs can now act as competitive inhibitors of the same signalling pathways they potentiate when surface-bound, via ligand competition. Ultimately, the function of mammalian Glypicans is poorly understood, and their role in CRC remains unstudied.
In the current study, gene expression changes downstream of Notum LOF are context-dependent in Apc ∆ vs Apc/Trp53-mutant cells, with clear induction of mTORC1 activity in response to Notum inactivation in Apc-mutant cells, and activation of TGFß activity in Apc/Trp53 double mutants. This finding is consistent with the observed functions of GPC1 and GPC4 in Apc-mutant and Apc/Trp53 double mutant cultures, respectively, as well as with the model that Glypicans potentiate signal transduction at the receptor-ligand level, and the negative regulation of this interaction by NOTUM (figure 7). While these downstream pathways likely contribute to the tumour suppressive and oncogenic effects of NOTUM activity in Apc ∆ and Apc/Trp53 mutant cultures, respectively, there remains a possibility that GPC1 and GPC4 also act on additional signalling pathways, and that additional Glypicans may be affected by NOTUM activity during the ontogeny of colorectal adenocarcinomas. Nonetheless, despite the potential for pleiotropic functions of NOTUM in colon cancer, the clear and compelling finding that extracellular enzymatic activity of NOTUM is required for adenocarcinoma maintenance and progression, coupled with the finding that small molecule inhibition of NOTUM activity is sufficient to arrest tumour progression and metastasis in immune-competent mouse models of invasive COAD indicate that NOTUM is a potentially tractable therapeutic target in this deadly disease.
Figure 7.
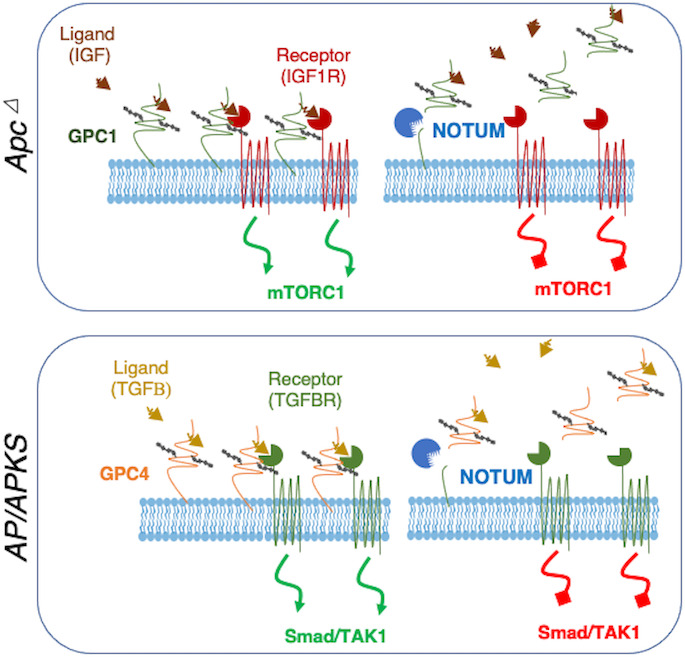
Model for NOTUM tumour suppressive-to-oncogenic switch during the adenoma-adenocarcinoma transition. In early adenomas resulting from APC inactivation, NOTUM exerts tumour suppressive activity through cleavage of oncogenic glypican 1 from the cell surface. Here, GPC1 acts as an oncogene by potentiating IGF1R activity upstream of mTORC1, and NOTUM antagonises this oncogenic axis (TOP). In contrast, on transition to adenocarcinoma with inactivation of P53 (and on accrual of additional driver mutations), NOTUM functions as an obligate oncogene by inhibiting tumour suppressive TGFßR activity via inactivating cleavage of Glypican 4 from the cell surface, which normally potentiates TGFßR activity upstream of TAK1/Smad signalling.
gutjnl-2022-329140supp003.xlsx (10.4KB, xlsx)
Footnotes
Contributors: YT, CL and NL designed and performed all experiments unless noted otherwise below. XW and KNE generated the mouse APK, APKS organoid and human iPS derived AP organoid. ZC and MAB performed TCGA data analysis. JR performed single cell and RNA bulk sequence data analysis. XM and SA-T performed organoid orthotopic injection and technical support. CL, NL and YT wrote the manuscript with editorial support from MAB, EBJ, BK and ZY. CL and NL are the guarantors of the study and take ultimate responsibility for all data and contents.
Funding: This work was funded by NCI R01CA168654 (CL), a grant from the Abramson Cancer Center (CL), and a grant from the Institute for Regenerative Medicine at the University of Pennsylvania (NL, CL and BK). NL was supported by NCI R50CA22184. This work used core facilities of the NIDDK P30 Center for Molecular Studies in Digestive and Liver Diseases at the University of Pennsylvania (P30DK050306).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The mouse transcriptome sequencing datasets generated during the current study is available in the NCBI Gene Expression Omnibus (GEO) with GSE198759, 198758, 198757. Human single cell transcriptomic data is from doi.org/10.1101/2022.09.13.506996.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
Human colorectal cancer and normal colon tissues were obtained previously from patients undergoing elective surgery at Hospital of the University of Pennsylvania with written informed consent under the protocol approved by the University of Pennsylvania Institutional Review Board (Protocol number 827759), as described in doi.org/10.1101/2022.09.13.506996. All experimental procedures involving mice were conducted in compliance with the guidelines and regulations approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
References
- 1. Miyaki M, Seki M, Okamoto M, et al. Genetic changes and histopathological types in colorectal tumors from patients with familial adenomatous Polyposis. Cancer Res 1990;50:7166–73. [PubMed] [Google Scholar]
- 2. Nakamura Y, Nishisho I, Kinzler KW, et al. Mutations of the APC (adenomatous Polyposis coli) gene in FAP (familial Polyposis coli) patients and in sporadic colorectal tumors. Tohoku J Exp Med 1992;168:141–7. 10.1620/tjem.168.141 [DOI] [PubMed] [Google Scholar]
- 3. Cancer Genome Atlas Network . Comprehensive molecular characterization of human colon and Rectal cancer. Nature 2012;487:330–7. 10.1038/nature11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-Catenin-TCF signaling in colon cancer by mutations in beta-Catenin or APC. Science 1997;275:1787–90. 10.1126/science.275.5307.1787 [DOI] [PubMed] [Google Scholar]
- 5. Korinek V, Barker N, Morin PJ, et al. Constitutive transcriptional activation by a beta-Catenin-TCF complex in APC-/- colon carcinoma. Science 1997;275:1784–7. 10.1126/science.275.5307.1784 [DOI] [PubMed] [Google Scholar]
- 6. Han C, Yan D, Belenkaya TY, et al. Drosophila Glypicans dally and dally-like shape the extracellular Wingless Morphogen gradient in the wing disc. Development 2005;132:667–79. 10.1242/dev.01636 [DOI] [PubMed] [Google Scholar]
- 7. Kreuger J, Perez L, Giraldez AJ, et al. Opposing activities of dally-like Glypican at high and low levels of Wingless Morphogen activity. Dev Cell 2004;7:503–12. 10.1016/j.devcel.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 8. Tu R, Duan B, Song X, et al. Dlp-mediated HH and WNT signaling interdependence is critical in the niche for Germline stem cell progeny differentiation. Sci Adv 2020;6:eaaz0480. 10.1126/sciadv.aaz0480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Traister A, Shi W, Filmus J. Mammalian Notum induces the release of Glypicans and other GPI-anchored proteins from the cell surface. Biochem J 2008;410:503–11. 10.1042/BJ20070511 [DOI] [PubMed] [Google Scholar]
- 10. Kakugawa S, Langton PF, Zebisch M, et al. Notum Deacylates WNT proteins to suppress signalling activity. Nature 2015;519:187–92. 10.1038/nature14259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Neerven SM, de Groot NE, Nijman LE, et al. Apc-mutant cells act as Supercompetitors in intestinal tumour initiation. Nature 2021;594:436–41. 10.1038/s41586-021-03558-4 [DOI] [PubMed] [Google Scholar]
- 12. Flanagan DJ, Pentinmikko N, Luopajärvi K, et al. NOTUM from APC-mutant cells biases Clonal competition to initiate cancer. Nature 2021;594:430–5. 10.1038/s41586-021-03525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shibata H, Toyama K, Shioya H, et al. Rapid colorectal adenoma formation initiated by conditional targeting of the APC gene. Science 1997;278:120–3. 10.1126/science.278.5335.120 [DOI] [PubMed] [Google Scholar]
- 14. Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial Organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 2011;141:1762–72. 10.1053/j.gastro.2011.07.050 [DOI] [PubMed] [Google Scholar]
- 15. Dow LE, O’Rourke KP, Simon J, et al. Apc restoration promotes cellular differentiation and Reestablishes crypt homeostasis in colorectal cancer. Cell 2015;161:1539–52. 10.1016/j.cell.2015.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Sousa e Melo F, Kurtova AV, Harnoss JM, et al. A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature 2017;543:676–80. 10.1038/nature21713 [DOI] [PubMed] [Google Scholar]
- 17. Watanabe K, Biesinger J, Salmans ML, et al. Integrative chip-Seq/Microarray analysis identifies a Ctnnb1 target signature enriched in intestinal stem cells and colon cancer. PLoS ONE 2014;9:e92317. 10.1371/journal.pone.0092317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li N, Zhu Q, Tian Y, et al. Mapping and modeling human colorectal carcinoma interactions with the tumor microenvironment. Cancer Biology [Preprint] 2022. 10.1101/2022.09.13.506996 [DOI] [PMC free article] [PubMed]
- 19. Jackson EL, Willis N, Mercer K, et al. Analysis of lung tumor initiation and progression using conditional expression of Oncogenic K-Ras. Genes Dev 2001;15:3243–8. 10.1101/gad.943001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roper J, Tammela T, Cetinbas NM, et al. Corrigendum: in vivo genome editing and Organoid transplantation models of colorectal cancer and metastasis. Nat Biotechnol 2017;35:1211. 10.1038/nbt1217-1211a [DOI] [PubMed] [Google Scholar]
- 21. Pentinmikko N, Iqbal S, Mana M, et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 2019;571:398–402. 10.1038/s41586-019-1383-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009;457:608–11. 10.1038/nature07602 [DOI] [PubMed] [Google Scholar]
- 23. Suciu RM, Cognetta AB, Potter ZE, et al. Selective irreversible inhibitors of the WNT-Deacylating enzyme NOTUM developed by activity-based protein profiling. ACS Med Chem Lett 2018;9:563–8. 10.1021/acsmedchemlett.8b00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abdel-Fattah G, Yoffe B, Krishnan B, et al. Mdm2/P53 protein expression in the development of colorectal adenocarcinoma. J Gastrointest Surg 2000;4:109–14. 10.1016/s1091-255x(00)80041-4 [DOI] [PubMed] [Google Scholar]
- 25. Yan D, Lin X. Opposing roles for Glypicans in hedgehog signalling. Nat Cell Biol 2008;10:761–3. 10.1038/ncb0708-761 [DOI] [PubMed] [Google Scholar]
- 26. Jen Y-H, Musacchio M, Lander AD. Glypican-1 controls brain size through regulation of fibroblast growth factor signaling in early Neurogenesis. Neural Dev 2009;4:33. 10.1186/1749-8104-4-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan J, Ho M. Role of Glypican-1 in regulating multiple cellular signaling pathways. Am J Physiol Cell Physiol 2021;321:C846–58. 10.1152/ajpcell.00290.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharon C, Baranwal S, Patel NJ, et al. Inhibition of insulin-like growth factor receptor/AKT/mammalian target of rapamycin axis targets colorectal cancer stem cells by attenuating Mevalonate-Isoprenoid pathway in vitro and in vivo. Oncotarget 2015;6:15332–47. 10.18632/oncotarget.3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vigneri PG, Tirrò E, Pennisi MS, et al. The insulin/IGF system in colorectal cancer development and resistance to therapy. Front Oncol 2015;5:230.:230. 10.3389/fonc.2015.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maitra A, Molberg K, Albores-Saavedra J, et al. Loss of Dpc4 expression in Colonic adenocarcinomas correlates with the presence of metastatic disease. Am J Pathol 2000;157:1105–11. 10.1016/S0002-9440(10)64625-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang B, Halder SK, Kashikar ND, et al. Antimetastatic role of Smad4 signaling in colorectal cancer. Gastroenterology 2010;138:969–80. 10.1053/j.gastro.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dolado I, Swat A, Ajenjo N, et al. P38Alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell 2007;11:191–205. 10.1016/j.ccr.2006.12.013 [DOI] [PubMed] [Google Scholar]
- 33. Ellinger-Ziegelbauer H, Kelly K, Siebenlist U. Cell cycle arrest and reversion of Ras-induced transformation by a conditionally activated form of mitogen-activated protein kinase kinase kinase 3. Mol Cell Biol 1999;19:3857–68. 10.1128/MCB.19.5.3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li S-P, Junttila MR, Han J, et al. P38 mitogen-activated protein kinase pathway suppresses cell survival by inducing dephosphorylation of mitogen-activated protein/extracellular signal-regulated kinase kinase1,2. Cancer Res 2003;63:3473–7. [PubMed] [Google Scholar]
- 35. Filmus J, Capurro M, Rast J. Glypicans. Genome Biol 2008;9:224. 10.1186/gb-2008-9-5-224 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2022-329140supp005.pdf (145.2KB, pdf)
gutjnl-2022-329140supp001.xlsx (28.5KB, xlsx)
gutjnl-2022-329140supp004.pdf (17.4MB, pdf)
gutjnl-2022-329140supp002.xlsx (10.2KB, xlsx)
gutjnl-2022-329140supp003.xlsx (10.4KB, xlsx)
Data Availability Statement
Data are available on reasonable request. The mouse transcriptome sequencing datasets generated during the current study is available in the NCBI Gene Expression Omnibus (GEO) with GSE198759, 198758, 198757. Human single cell transcriptomic data is from doi.org/10.1101/2022.09.13.506996.