Abstract
Objectives:
This study aimed to analyze characteristics of mpox hospitalization in a Brazilian cohort, further exploring the impact of HIV on mpox-related outcomes and hospitalization.
Design:
We conducted a descriptive analysis, comparing characteristics of individuals diagnosed with mpox according to hospitalization and HIV status, and described the mpox cases among those living with HIV.
Methods:
This was a single-center, prospective cohort study conducted at a major infectious diseases referral center in Rio de Janeiro, Brazil, that enrolled participants older than 18 years of age diagnosed with mpox. Information was collected on standardized forms, including data on sociodemographic, behavioral, clinical and laboratory characteristics. For comparisons, we used chi-squared, Fisher's exact and the Moods median tests whenever appropriate.
Results:
From June to December, 2022, we enrolled 418 individuals diagnosed with mpox, of whom 52% were people with HIV (PWH). PWH presented more frequently with fever, anogenital lesions and proctitis. The overall hospitalization rate was 10.5% (n = 43), especially for pain control. Among hospitalized participants, PWH had more proctitis and required invasive support. Mpox severity was related to poor HIV continuum of care outcomes and low CD4+ cell counts. All deaths (n = 2) occurred in PWH with CD4+ less than 50 cells/μl.
Conclusion:
HIV-related immunosuppression likely impacts mpox clinical outcomes. This is of special concern in settings of poor adherence and late presentation to care related to socioeconomic inequalities, such as Brazil. The HIV continuum of care must be taken into account when responding to the mpox outbreak.
Keywords: AIDS, Chlamydia trachomatis, HIV, mpox, Neisseria gonorrheae, sexually transmitted infections, syphilis
Background
The 2022 multinational mpox outbreak in nonendemic countries led to its declaration as a health emergency in July 2022, reaching the global number of 90 618 cases and 157 deaths by 27 September 2023, with Brazil being the second most affected country [1,2]. Cisgender MSM were disproportionately burdened by this outbreak; 30–50% of individuals diagnosed with mpox were living with HIV [3–11]. Generally, people with HIV (PWH) and mpox were older and more often presented with concomitant sexually transmitted infections (STIs), anogenital lesions and proctitis [4,8,9,12]. Although initial mpox descriptions reported no substantial differences in hospitalizations or clinical severity according to HIV status, these individuals were mostly virologically stable and without severe, immunosuppression [3,4,7,10,13].
Recent studies, however, suggest that uncontrolled HIV might impact on mpox-related outcomes, especially if CD4+ cell counts are below 200 cells/μl, suggesting an association between advanced HIV disease and severe mpox presentation [5,8,9,14–17]. This is of special concern in Latin America – the region accounted for 55% of PWH with mpox and CD4+ counts lower than 350 cells/μl; access to prevention and experimental mpox treatment were quite limited [15,18]. Overall, mpox-related hospitalization rates during the 2022 multinational outbreak are up to 10%, mostly related to pain control, secondary bacterial infections, urologic or proctologic complications, odynophagia, keratitis and, rarely, encephalitis, pulmonary involvement or myocarditis [4,5,7,15,17,19–22]. Brazil reported up to 50% of mpox cases and at least three deaths among PWH [4,23]. Despite the country's longstanding commitment to universal access to antiretroviral therapy (ART), structural barriers remain, resulting in a high prevalence of HIV late presentation to care and poor ART adherence. These aspects have worsened after the COVID-19 pandemic and contributed to the high mpox burden [24–26].
This study aimed to characterize mpox-related hospitalizations and further explore the impact of HIV status on mpox outcomes among individuals enrolled at the Evandro Chagas National Institute of Infectious Diseases (INI/Fiocruz), a major referral center for mpox in Rio de Janeiro, Brazil.
Methods
Study design and participants
This was a single-center, prospective cohort study that enrolled participants older than 18 years diagnosed with mpox from 12 June to 31 December 2022, at INI/Fiocruz. We offered mpox virus quantitative real-time PCR (qPCR) testing to all individuals with suspected infection, which was performed at the Mpox Reference Laboratory at the Oswaldo Cruz Institute/Fiocruz. A positive result on qPCR from any swab specimens led to case confirmation. Follow-up period was 28 days or until resolution of mpox lesions. Individuals requiring hospitalization were followed at the INI-Fiocruz Inpatient Care Unit; if hospitalization occurred elsewhere, data were collected retrospectively. Procedures of INI's mpox cohort have been previously described [4].
Procedures
We prospectively collected sociodemographic, epidemiological, behavior, clinical and laboratory data onto a standardized case report form. Information was gathered on birth date, gender identity and race according to self-report. In Brazil, smallpox vaccination was compulsory until 1975, so for these analyses we considered those born before 1975 as vaccinated. Systemic signs and symptoms included the presence of fever, asthenia, adenomegaly, myalgia, arthralgia and/or headache. For hospitalized participants, invasive support included mechanical ventilatory support, vasoactive drugs, use of nasoenteric tube or indwelling urinary catheters, need for hemodialysis or either arterial or venous central line. We included data on reasons for hospitalization, length of hospitalization and clinical outcomes. Tecovirimat was requested according to the Ministry of Health's protocol for compassionate use, upon availability, if the individual met criteria of a severe clinical course [27].
At the initial assessment, we offered HIV rapid test (3rd generation) performed according to the Brazilian Ministry of Health algorithm [28]; HIV-RNA viral load; rapid Treponema pallidum test for syphilis screening with subsequent confirmation with nontreponemal testing [veneral disease research laboratory (VDRL)]; molecular detection of Chlamydia trachomatis and Neisseria gonorrheae in rectal swabs (Abbott Real Time Platform); hepatitis B surface antigen rapid test; anti-HCV rapid test for hepatitis C. Active syphilis was defined as VDRL titers equal to or higher than 1 : 8.
For PWH, we assessed CD4+ count (cells/μl) and HIV-RNA viral loads records closest to the onset of mpox symptoms, obtained from either clinical charts or the Brazilian Laboratory Control System database (SISCEL). We adapted a cross-sectional cascade of HIV care including the following stages: HIV-diagnosed (previously known HIV infection at mpox diagnosis); linked to HIV care (at least one record of HIV-related care appointment, laboratory examinations or ART prescription after HIV diagnosis and before mpox assessment); retained in HIV care (ART prescription in the last 6 months or at least two records of HIV-RNA viral load or CD4+ cell count in the last year); on ART (at least one dispensation of antiretroviral drugs in the last 6 months, obtained from the Brazilian Antiretroviral Logistics database – SICLOM); and virologically suppressed (HIV-RNA viral load ≤200 copies/ml) [29].
Statistical analysis
We compared study characteristics of confirmed mpox cases according to HIV status and, among PWH, by hospitalization status (PWH hospitalized vs. PWH not hospitalized). We used the chi-squared test or Fisher's exact test for qualitative variables and the Moods median test for quantitative variables. We performed a sub-analysis among PWH to compare clinical outcomes according to CD4+ count strata and to describe HIV continuum of care outcomes by hospitalization status. All analyses were performed in R-Project (4.2.1).
Ethical considerations
This study was approved by the Ethics Review Board at INI-Fiocruz (CAAE #61290422.0.0000.5262). Participants provided written informed consent.
Results
From June 12 to 31 December 2022, 418 participants had a confirmed mpox diagnosis. Overall, median age was 33 years [interquartile range (IQR) 28–40]; 91.6% self-identified as cisgender men, 5.7% as cisgender women and 2.6% as travesti or transgender women (TGW). The majority were black or pardo (60.5%, n = 207/342), 38.6% were white (n = 132/342) and 0.9% were indigenous (n = 3/342). Most participants had studied until primary education (60.2%, n = 213/352), 6% had secondary education (n = 21/352) and 33.8% went to postsecondary education (n = 119/352). Among the cisgender men, 87% were MSM (n = 336/362).
Data on HIV serostatus were available for 409 participants, of whom 52.1% were PWH, mostly with previous HIV diagnosis. Compared with HIV-negative individuals, PWH diagnosed with mpox were older [35 (IQR 30–40) vs. 31 (IQR 26–38), P < 0.01] and most frequently self-reported being cisgender men (95.8 vs. 87.3%, P < 0.01), travesti or TGW (3.3 vs. 2%, P < 0.01) (Table 1). Among those who were negative for HIV, PrEP use was reported by 33% (Table 1).
Table 1.
Sociodemographic, behavioral and clinical characteristics of mpox cases according to HIV status (N = 409).
| HIV− (N = 196)a [n (%)] | HIV+ (N = 213)a [n (%)] | Total (N = 409)a [n (%)] | P valueb | |
| Age (median, IQR) | 31 (26–38) | 35 (30–40) | 33 (28–40) | <0.01 |
| Age range | <0.01 | |||
| 18–24 years | 34/196 (17.4%) | 12/213 (5.6%) | 46/409 (11.2%) | |
| 25–29 years | 49/196 (25%) | 33/213 (15.5%) | 82/409 (20.1%) | |
| 30–39 years | 71/196 (36.2%) | 105/213 (49.3%) | 176/409 (43%) | |
| ≥40 years | 42/196 (21.4%) | 63/213 (29.6%) | 105/409 (25.7%) | |
| Gender identity | <0.01 | |||
| Cisgender men | 171/196 (87.3%) | 204/213 (95.8%) | 375/409 (91.7%) | |
| Cisgender women | 21/196 (10.7%) | 2/213 (0.9%) | 23/409 (5.6%) | |
| Nonbinary | 0/196 | 0/213 | 0/409 | |
| Transgender men | 0/196 | 0/213 | 0/409 | |
| Travesti or transgender women | 4/196 (2%) | 7/213 (3.3%) | 11/409 (2.7%) | |
| Race | 0.65 | |||
| Black | 53/166 (31.9%) | 52/175 (29.7%) | 105/341 (30.8%) | |
| Pardo or mixed | 45/166 (27.1%) | 57/175 (32.6%) | 102/341 (29.9%) | |
| White | 67/166 (40.4%) | 64/175 (36.6%) | 131/341 (38.4%) | |
| Indigenous | 1/166 (0.6%) | 2/175 (1.1%) | 3/341 (0.9%) | |
| Education | 0.19 | |||
| Primary | 110/169 (65.1%) | 100/180 (55.6%) | 210/349 (60.2%) | |
| Secondary | 9/169 (5.3%) | 12/180 (6.6%) | 21/349 (6%) | |
| Post secondary | 50/169 (29.6%) | 68/180 (37.8%) | 118/349 (33.8%) | |
| MSM | 144/165 (87.3%) | 190/193 (98.4%) | 334/358 (93.3%) | <0.01 |
| Current sex work | 6/178 (3.4%) | 6/190 (3.2%) | 12/368 (3.3%) | 0.91 |
| PrEP use | 64/194 (32.9%) | NA | NA | |
| Reported sex in the last 30 days | 175/186 (94.1%) | 175/197 (88.8%) | 350/383 (91.4%) | 0.07 |
| Time from symptoms onset to initial assessment (median, IQR) | 6 (4, 9) | 6.0 (4, 10) | 6.0 (4, 9) | 0.52 |
| Median number of sex partners in the last 30 days (IQR) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.54 |
| Reported anal sex in the last 30 daysc | 78/175 (44.6%) | 88/175 (50.3%) | 166/350 (47.4%) | 0.28 |
| Sex contact with potential mpox cased | 35/171 (20.5%) | 35/173 (20.2%) | 70/344 (20.3%) | 0.58 |
| Home contact with potential mpox casese | 20/169 (11.9%) | 12/172 (7%) | 32/341 (9.4%) | 0.12 |
| Probable vaccinated for smallpox | 18/196 (9.2%) | 19/213 (8.9%) | 37/409 (9%) | 0.97 |
| Active syphilis | 18/180 (10%) | 59/198 (29.8%) | 77/383 (20.1%) | <0.01 |
| Anorectal gonorrhea | 11/141 (7.8%) | 15/164 (9.15%) | 26/305 (8.52%) | 0.69 |
| Anorectal Chlamydia | 12/141 (8.5%) | 18/164 (11%) | 30/305 (9.8%) | 0.47 |
| Any bacterial STI | 39/180 (21.7%) | 84/198 (42.4%) | 123/378 (32.5%) | <0.01 |
| Hepatitis B | 2/181 (1.1%) | 3/189 (1.6%) | 5/370 (1.4%) | >0.99 |
| Hepatitis C | 5/187 (2.7%) | 19/194 (9.8%) | 23/381 (6%) | <0.01 |
| Systemic symptoms or signs | 157/186 (75.3%) | 187/207 (84.1%) | 344/393 (79.8%) | 0.07 |
| Fever | 107/192 (55.7%) | 150/211 (71.1%) | 257/403 (63.7%) | <0.01 |
| Anogenital lesions | 143/193 (74.1%) | 170/207 (82.1%) | 313/400 (78.3%) | 0.05 |
| Clinical signs of proctitis | 37/194 (19.1%) | 63/212 (29.7%) | 100/406 (24.6%) | 0.01 |
| Rectal swab | 0.01 | |||
| PCR MPXV detectable | 80/121 (66.1%) | 131/163 (80.4%) | 211/284 (75.3%) | |
| PCR MPXV not detectable | 41/121 (33.9%) | 32/163 (19.6%) | 73/284 (24.7%) | |
| Required hospitalization | 19/196 (9.7%) | 24/213 (11.3%) | 43/409 (10.5%) | 0.60 |
| Death | 0/196 | 2/213 (1%) | 2/409 (0.5%) | 0.50 |
IQR, interquartile range; PrEP, pre-exposure prophylaxis.
n (%); median (IQR).
Fisher's exact test; Wilcoxon rank sum test; Pearson's chi-squared test.
Both receptive and insertive anal sex.
Reported sexual contact with someone who was suspect case or diagnosis with mpox or presenting mpox-like lesions in the 30 days previous the study entry.
Reported living in the same household of suspected/confirmed mpox case in the 30 days previous the study entry.
Compared with HIV-negative participants, more PWH reported being MSM (98.4 vs. 87.3%, P < 0.01); had active syphilis (29.8 vs. 10%, P < 0.01), hepatitis C (9.8 vs. 2.7%, P < 0.1) or any bacterial STI (42.4 vs. 21.7%, P < 0.01) (Table 1); presented more frequently with fever (71.1 vs. 55.7%, P < 0.01), anogenital lesions (82.1 vs. 74.1%, P = 0.05) and proctitis (29.7 vs. 19.1%, P = 0.01) and more often had a positive MPXV qPCR in rectal swabs (80.37 vs. 66.12%, P = 0.01).
Overall, 10.5% (n = 43) of participants were hospitalized because of mpox (39 at INI-Fiocruz Inpatient Care Unit), 55.8% were PWH, with a median age of 31 years old (IQR 28–39); 93% were cisgender men, 50% self-identified as black/pardo and 53.5% had primary education. The hospitalization rate during follow-up was not different based on HIV status although 34.9% had been admitted at the time of the initial mpox assessment. No participants reported immunosuppression related to drugs or other medical conditions. Median interval between symptoms onset and hospitalization was 9 days (IQR 7–12) and length of hospitalization was 7.5 days (IQR 5–12). The most common reason for hospitalization was pain control (90.7%), often requiring opioids use (67.5%), followed by secondary bacterial infections (55.8%). The majority of hospitalized participants presented with fever (69.1%) and/or anogenital lesions (67.4%). PWH presented more frequently with proctitis [58.33 vs. 26.32%, P = 0.04)] and required invasive support (34.8 vs. 0, P = 0.01) (Table 2).
Table 2.
Sociodemographic, laboratorial, and clinical information on mpox-related hospitalized participants according to HIV status (N = 43).
| HIV − (N = 19)a | HIV + (N = 24)a | Total (N = 43)a | P valueb | |
| Median age (IQR) | 30 (25–36) | 35 (30–42) | 31 (28–40) | 0.08 |
| Age range | 0.20 | |||
| 18–24 years | 3/19 (15.8%) | 1/24 (4.2%) | 4/43 (9.3%) | |
| 25–29 years | 6/19 (31.6%) | 3/24 (12.5%) | 9/43 (20.9%) | |
| 30–39 years | 7/19 (36.8%) | 12/24 (50%) | 19/43 (44.2%) | |
| ≥40 years | 3/19 (15.8%) | 8/24 (33.3%) | 11/43 (25.6%) | |
| Gender identity | 0.08 | |||
| Cisgender men | 16/19 (84.2%) | 24/24 (100%) | 40/43 (93%) | |
| Cisgender women | 3/19 (15.8%) | 0/24 | 3/43 (7%) | |
| Race | >0.99 | |||
| White | 7/18 (38.9%) | 9/21 (42.9%) | 16/39 (41.1%) | |
| Pardo or mixed | 5/18 (27.8%) | 5/21 (23.8%) | 10/39 (25.6%) | |
| Black | 6/18 (33.3%) | 7/21 (33.3%) | 13/39 (33.3%) | |
| Education | 0.13 | |||
| Primary | 13/19 (68.4%) | 10/24 (41.7%) | 23/43 (53.5%) | |
| Secondary | 1/19 (5.3%) | 1/24 (4.1%) | 2/43 (4.6%) | |
| Postsecondary | 5/19 (26.3%) | 13/24 (54.2%) | 18/43 (41.9%) | |
| Fever | 14/19 (73.7%) | 15/23 (65.2%) | 29/42 (69%) | 0.55 |
| Anogenital lesions | 9/19 (47.4%) | 20/24 (83.3%) | 29/43 (67.4%) | 0.01 |
| Time from symptoms onset to hospitalization (days, IQR) | 9 (8–11) | 8 (7–14) | 9 (7–12) | >0.99 |
| Length of hospitalization (days, IQR) | 7 (5–10) | 8 (4–14) | 7.5 (5–12) | 0.56 |
| Reason of hospitalization | ||||
| Pain control | 18/19 (94.7%) | 21/24 (87.5%) | 39/43 (90.7%) | 0.62 |
| Urological complication | 7/19 (36.8%) | 10/24 (41.7%) | 16/43 (37.21%) | 0.75 |
| Proctitis | 5/19 (26.3%) | 14/24 (58.3%) | 18/43 (41.9%) | 0.04 |
| Superbacterial infection | 11/19 (57.9%) | 13/24 (54.2%) | 24/43 (55.8%) | 0.81 |
| Ophthalmologic complications | 2/19 (10.5%) | 1/24 (4.2%) | 3/43 (7%) | 0.58 |
| Neuropsychiatric symptoms | 4/19 (21.1%) | 5/24 (20.8%) | 9/43 (20.9%) | >0.99 |
| Odynophagia | 6/19 (31.6%) | 5/24 (20.8%) | 11/43 (25.6%) | 0.49 |
| Need for invasive supportc | 0/19 | 8/23 (34.8%) | 8/42 (19.1%) | 0.01 |
| Urological or bowel obstruction | 1/19 (5.3%) | 3/23 (13%) | 4/42 (9.5%) | 0.61 |
| Use of opioids for pain control | 14/17 (82.4%) | 13/23 (56.5%) | 27/40 (67.5%) | 0.09 |
| Death | 0/19 | 2/24 (8.3%) | 2/43 (4.7%) | 0.50 |
IQR, interquartile range.
n (%); median (IQR).
Fisher's exact test; Wilcoxon rank sum test; Pearson's chi-squared test.
Invasive support included need for mechanical ventilation, vasoactive drugs, hemodialysis, venous or arterial lines and/or vesical catheterization.
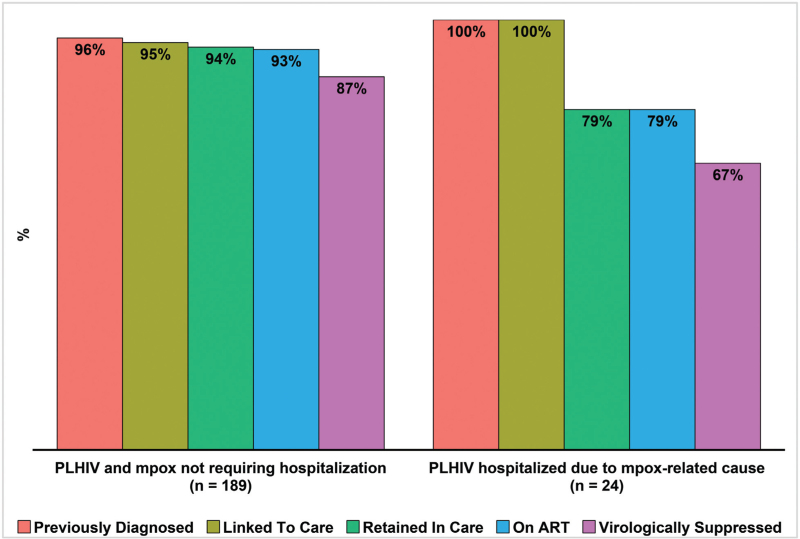
When comparing to PWH and mpox who have not required inpatient care (n = 189), those hospitalized (n = 24) reported less frequent sex contact one month prior to symptoms onset (66.7 vs. 91.5%, P < 0.01) and presented worse outcomes related to the HIV care continuum, with poorer rates of retention (79.2 vs. 94.7%, P = 0.01), regular ART use (79.2 vs. 94.1%, P = 0.02) and virological suppression (66.7 vs. 87.7%, P = 0.01), in addition to showing lower CD4+ cell counts [median 448 (IQR 174–850) vs. 630 (IQR 487–889), P = 0.02] and less frequently presenting CD4+ counts higher than 350 cells/μl (58.3 vs. 91.8%, P < 0.01) (Table 3 and Fig. 1).
Table 3.
Sociodemographic, behavioral and clinical characteristics of mpox participants coinfected with HIV according to hospitalization during mpox follow-up (N = 213).
| Not hospitalized, (N = 189)a | Hospitalized (N = 24)a | Totala (N = 213) | P valueb | |
| Median age (IQR) | 35 (30–40) | 35 (30–42) | 35 (30–40) | 0.75 |
| Age range | 0.83 | |||
| 18–24 years | 11/189 (5.8%) | 1/24 (4.2%) | 12/213 (5.6%) | |
| 25–29 years | 30/189 (15.9%) | 3/24 (12.5%) | 33/213 (15.5%) | |
| 30–39 years | 93/189 (49.2%) | 12/24 (50%) | 105/213 (49.3%) | |
| ≥40 years | 55/189 (29.1%) | 8/24 (33.3%) | 63/213 (29.6%) | |
| Gender identity | >0.99 | |||
| Cisgender men | 180/189 (95.2%) | 24/24 (100%) | 204/213 (95.8%) | |
| Cisgender women | 2/189 (1.1%) | 0/24 | 2/213 (0.9%) | |
| Travesti or TGW | 7/189 (3.7%) | 0/24 | 7/213 (3.3%) | |
| Race | 0.75 | |||
| White | 55/154 (35.7%) | 9/21 (42.9%) | 64/175 (36.6%) | |
| Indigenous | 2/154 (1.3%) | 0/21 | 2/175 (1.1%) | |
| Pardo or mixed | 52/154 (33.8%) | 5/21 (23.8%) | 57/175 (32.6%) | |
| Black | 45/154 (29.2%) | 7/21 (33.3%) | 52/175 (29.7%) | |
| Education | 0.22 | |||
| Primary | 90/156 (57.7%) | 10/24 (41.7%) | 100/180 (55%) | |
| Secondary | 11/156 (7.1%) | 1/24 (4.2%) | 12/180 (7.6%) | |
| Postsecondary | 55/156 (35.3%) | 13/24 (54.2%) | 68/180 (38%) | |
| Reported sexual contact 30 days before symptoms onset | 161/176 (91.5%) | 14/21 (66.7%) | 175/197 (88.8%) | <0.01 |
| Probably vaccinated for smallpox | 15/189 (7.9%) | 4/24 (16.7%) | 19/213 (8.9%) | 0.12 |
| HIV-RNA viral load | 0.04 | |||
| <40 copies/ml | 143/188 (76.1%) | 13/24 (54.2%) | 156/212 (73.6%) | |
| 41–200 copies/ml | 21/188 (11.2%) | 3/24 (12.5%) | 24/212 (11.3%) | |
| 201–1000 copies/ml | 6/188 (3.2%) | 2/24 (8.3%) | 8/212 (3.8%) | |
| >1000 copies/ml | 18/188 (9.5%) | 6/24 (25%) | 24/212 (11.3%) | |
| Median CD4+ (cells/μl, IQR) | 630 (487, 889) | 448 (174, 850) | 624 (463, 889) | 0.02 |
| CD4+ range (cells/μl) | <0.01 | |||
| ≤50 | 1/170 (0.6%) | 4/24 (16.7%) | 5/194 (2.6%) | |
| 51–100 | 0/170 | 1/24 (4.2%) | 1/194 (0.5%) | |
| 101–200 | 2/170 (1.2%) | 3/24 (12.5%) | 5/194 (2.6%) | |
| 201–350 | 11/170 (6.5%) | 2/24 (8.3%) | 13/194 (6.7%) | |
| >350 | 156/170 (91.7%) | 14/24 (58.3%) | 170/194 (87.6%) | |
| ART status | ||||
| Never initiated | 5/187 (2.7%) | 1/24 (4.2%) | 6/211 (2.8%) | 0.02 |
| On ART, regular adherencec | 166/187 (88.8%) | 17/24 (70.8%) | 183/211 (86.8%) | |
| On ART, irregular adherencec | 13/187 (6.9%) | 3/24 (12.5%) | 16/211 (7.6%) | |
| No ART in last 6 monthsc | 3/187 (1.6%) | 3/24 (12.5%) | 6/211 (2.8%) | |
ART, antiretroviral therapy; IQR, interquartile range.
n (%); median (IQR).
Fisher's exact test; Wilcoxon rank sum test; Pearson's chi-squared test.
Self-reported information on ART adherence referring to the 6-month period prior to mpox first assessment. Regular ART adherence was considered if the participant reports taking at least five pills per week in the recall time.
Fig. 1.
HIV continuum of care outcomes among mpox-confirmed participants, according to hospitalization status during follow-up.
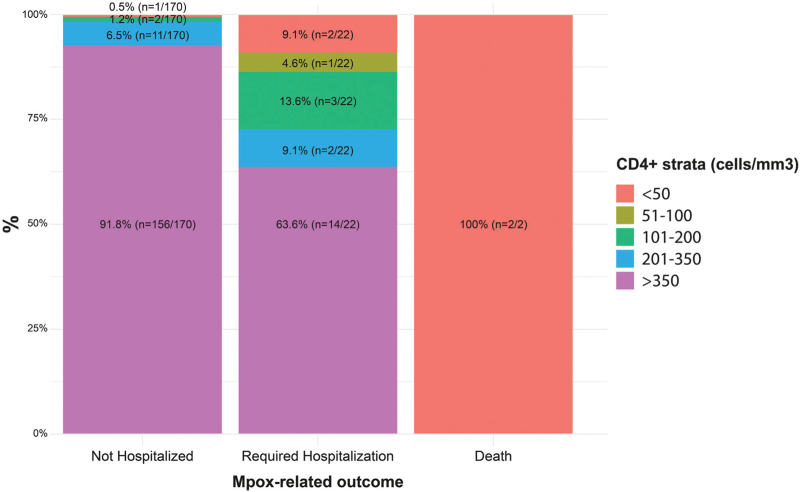
Among the 24 PWH hospitalized because of mpox, 11 had detectable HIV-RNA viral load (higher than 40 copies/ ml), all of them presented mucosal involvement, frequently requiring intravenous antibiotics (n = 8), analgesics (n = 10) or opioids (n = 8). Among them, eight had CD4+ cell counts below 200 cells/μl and most frequently experienced worse mpox-related outcomes, including hospitalization and death (Fig. 2). Concomitant opportunistic infections were identified: tuberculosis (n = 4), histoplasmosis (n = 1), cytomegalovirus infection (n = 1), oropharyngeal candidiasis (n = 1), Kaposi's sarcoma (n = 1). All participants with deep tissue involvement had CD4+ counts below 50 cells/μl (n = 4). Two participants, both with CD4+ counts below 50 cells/μl, had respiratory complications [pleural effusion (n = 1); pulmonary nodules (n = 1)]. All deaths occurred among PWH (n = 2), all of them with poor ART adherence and CD4+ counts below 50 cells/μl. Both developed bowel and urologic obstruction, sepsis and refractory shock, requiring mechanical ventilation and use of vasoactive drugs.
Fig. 2.
Outcomes during follow-up stratified by CD4+ cell count.
Discussion
Our findings contribute to further characterize the mpox outbreak in Latin America, focusing on individuals living with HIV with different levels of immunosuppression. HIV prevalence was in line with global data [12]. In our study, mpox individuals showed a high frequency of a concomitant STI and hepatitis C virus (HCV). A recent systematic review showed an association between syphilis coinfection and HIV among individuals diagnosed with mpox, despite a significant amount of missing data due to variations in access to STI testing across different settings [12,30]. Our elevated syphilis prevalence contrasts with international data, including other Latin American countries but is compatible with the alarming epidemiological syphilis scenario in Brazil [5,8,10,31]. Such findings might be related to shared sexual networks or higher risk of HIV acquisition in the context of a concomitant STI, and underscores the importance of mpox assessment as an opportunity to expand other STI diagnosis [32,33].
Despite presenting more often with fever, anogenital lesions and proctitis, PWH in our cohort showed similar proportions of hospitalization as individuals not living with HIV, which is still controversial in available global data [5,9,10,14]. Reasons for admission were quite similar to other cohorts, being mainly associated with pain control, bacterial superinfection, or management of urologic and proctological complications [17,34]. Evidence shows that, among PWH, those with CD4+ counts lower than 200 cells/μl or unsuppressed HIV-RNA viral load are more prone to developing mpox-related complications, including hospitalization, intensive care support and death [15–17]. Mpox-related complications in individuals with CD4+ cell counts below 200 cells/μl can lead to fulminant clinical course coalescing necrotizing skin and soft tissue lesions, as well as pulmonary involvement [15]. There is still a gap regarding the best practices related to time for ART initiation or resumption, given the risk of Immune Reconstitution Inflammatory Syndrome, as well as the duration of mpox-specific antiviral treatment [15,16]. Special attention must be given to potential concurrent opportunistic infections [15].
The global health response to mpox is marked by inequities related to a focus on Northern countries and scarce access to preventive and treatment alternatives in Latin America and Africa. Moreover, mpox's description as an HIV-related opportunistic infection and its emergence as a global threat underscores the concern regarding the poorer HIV cascade of care outcomes [15,35]. Our findings suggest that PWH with mpox requiring hospitalization performed poorly in HIV continuum of care when compared with those followed only at an outpatient unit, with significantly lower ART adherence. Likewise, a recent report on mpox-related deaths in the United States unveiled structural inequities mostly affecting black and people experiencing homelessness [16]; most of whom had a late HIV diagnosis, and no previous ART use, as opposed to our scenario [16]. Notably, in our cohort, the most concerning outcomes were concentrated in the distal end of the HIV continuum of care, suggesting that poor ART adherence plays a greater role than late diagnosis.
In Brazil, access to HIV prevention and treatment is guaranteed by the Unified Health System (SUS), reinforcing its principles of equity, universality and integrality [36], and allowing consistent improvement in HIV-related outcomes. By 2021, Brazil had made steady progress in achieving UNAIDS 95–95–95 goals, with an estimate of 89% PWH diagnosed, of whom 82% were on regular ART and, among those, 95% were virologically suppressed [29]. Nevertheless, the country faces a concentrated HIV epidemic, mirroring structural gender and racial inequities: MSM and TGW are still the most vulnerable populations, and, despite the overall reduction in AIDS-related mortality since 2011, it has increased among Black individuals [37–39]. Moreover, the HIV continuum of care among sexual and gender minorities is significantly worse than among the overall population of PWH [38,40]. This might be related to HIV stigma; lack of social support or access to basic rights, such as housing, food and education; and barriers to access to HIV care, representing ultimately a proxy for further socioeconomic disparities [41–45].
A lack of sustainable improvement of the HIV-continuum of care outcomes may be related to the last government's conservative policies, which reduced HIV program budget, deepened violence against the LGBTQIA+ community and reinforced HIV stigma [36,46,47]. Furthermore, the COVID-19 pandemic disrupted healthcare services access [24,29,39]. In this sense, socioeconomic disparities and HIV-related immunosuppression synergically contributed to the overlapping of an uncontrolled HIV epidemics in Rio de Janeiro, Brazil, with a high burden of mpox cases [48].
Our study had limitations. It was a single-center study located in the second Brazilian state most affected by the mpox outbreak, thus results might not be generalizable to other contexts. Nevertheless, they are in line with data from international cohorts and case series published so far. By December 2022, mpox vaccination was not available in Brazil in the private or public sectors, remaining mostly unavailable, and no participants with mpox in our cohort were vaccinated abroad. Information on previous smallpox vaccination was subject to recall bias, and we opted to use birth date as a proxy, which could have overestimated smallpox vaccination. The low numbers of individuals with advanced HIV disease jeopardizes the possibility of establishing stronger associations, especially in the context of a global decline in number of cases, including in Brazil after a period of sustained community transmission. Most participants who required hospitalization had access to our center's inpatient care unit, allowing consistent and detailed data collection.
In conclusion, despite a usual benign clinical course, mpox-related hospitalizations during the 2022 multinational outbreak were concerning. Our findings suggest that HIV-related immunosuppression may lead to severe complications, especially in the context of ART discontinuation and poor HIV care continuum outcomes. In Brazil, the combination of public health emergencies – mpox and AIDS – unveils a landscape marked by structural inequities that reinforce a cycle of neglected diseases, affecting the most vulnerable population, increasing stigma and discrimination. Tackling social determinants of health is critical to improve ART adherence and HIV-related outcomes. Strategies to revert the negative impact of COVID-19 pandemics on HIV care are urgent. In Brazil, strengthening SUS is key to ensuring adequate responses to epidemics and outbreaks.
Acknowledgements
We thank all health professionals involved in both inpatient and ambulatory care of individuals diagnosed with mpox, and all participants who contributed to this study.
Funding: Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz (INI-Fiocruz).
Authors’ contributions: M.S.T.S., B.G., V.G.V. and S.W. conceived and designed the study. B.G., C.C. and T.S.T. conceived and supervised current analysis and manuscript preparation. B.G., V.G.V., M.S.T.S., B.H., S.W. and E.P.N. developed the case report form. M.O.B., M.B.M., P.P.S.R., P.S.M. and A.E.G. performed data collection. E.M.P., T.S.T., C.C., M.S.T.S., R.I.M. and F.C.S.L. coordinated data acquisition and performed statistical analyses. M.S.T.S., M.O.B., I.C.F.T., E.P.N., M.B.M., P.P.S.R., P.S.M., H.B.A. and A.E.G. performed clinical evaluations. B.G., B.H., V.G.V., S.W. and E.P.N. were involved in revising the manuscript for important intellectual content. All authors reviewed and approved the final manuscript. B.G. had full access to study data and had final responsibility for the decision to submit for publication.
Conflicts of interest
There are no conflicts of interest.
References
- 1. WHO. 2022-23 Mpox Outbreak: Global Trends [Internet]. 2023. Available at: https://worldhealthorg.shinyapps.io/mpx_global/. [Accessed 10 October 2023] [Google Scholar]
- 2.Zarocostas J. Monkeypox PHEIC decision hoped to spur the world to act. Lancet 2022; 400:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. SHARE-net Clinical Group. Monkeypox virus infection in humans across 16 countries — April-June 2022. N Engl J Med 2022; 387:679–691. [DOI] [PubMed] [Google Scholar]
- 4.Silva MST, Coutinho C, Torres TS, Peixoto E, Ismério R, Lessa F, et al. INI-Fiocruz Mpox Study Group. Ambulatory and hospitalized patients with suspected and confirmed mpox: an observational cohort study from Brazil. Lancet Reg Health Am 2022; 17:100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel A, Bilinska J, Tam JCH, Da Silva Fontoura D, Mason CY, Daunt A, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ 2022; 378:e072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girometti N, Byrne R, Bracchi M, Heskin J, McOwan A, Tittle V, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis 2022; 22:1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarín-Vicente EJ, Alemany A, Agud-Dios M, Ubals M, Suñer C, Antón A, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet 2022; 400:661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Núnez I, García-Grimshaw M, Ceballos-Liceaga S, Toledo-Salinas C, Carbajal-Sandoval G. Epidemiological and clinical characteristics of patients with human monkeypox infection in Mexico: a nationwide observational study. Lancet Reg Health Am 2022; 17:100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran KG, Eberly K, Russell OO, Snyder RE, Phillips EK, Tang EC, et al. Monkeypox, HIV, and STI Team. HIV and sexually transmitted infections among persons with monkeypox — eight U.S. Jurisdictions, May 17-July 22, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann C, Jessen H, Wyen C, Grunwald S, Noe S, Teichmann J, et al. Clinical characteristics of monkeypox virus infections among men with and without HIV: a large outbreak cohort in Germany. HIV Med 2022; 24:389–397. [DOI] [PubMed] [Google Scholar]
- 11.Girometti N, Ogoina D, Tan DHS, Pozniak A, Klein MB. Intersecting HIV and mpox epidemics: more questions than answers. J Int AIDS Soc 2022; 25:e26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin H, Shin H, Rahmati M, Koyanagi A, Jacob L, Smith L, et al. Comparison of clinical manifestations in mpox patients living with HIV versus without HIV: a systematic review and meta-analysis. J Med Virol 2023; 95:e28713. [DOI] [PubMed] [Google Scholar]
- 13.Vivancos-Gallego MJ, Sánchez-Conde M, Rodríguez-Domínguez M, Fernandez-Gonzalez P, Martínez-García L, Garcia-Mouronte E, et al. Human monkeypox in people with HIV: transmission, clinical features, and outcome. Open Forum Infect Dis 2022; 9:ofac557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chastain DB, Motoa G, Ortiz-Martínez Y, Gharamti A, Henao-Martínez AF. Characteristics and clinical manifestations of monkeypox among people with and without HIV in the United States: a retrospective cohort. AIDS 2023; 37:611–616. [DOI] [PubMed] [Google Scholar]
- 15.Mitjà O, Alemany A, Marks M, Lezama Mora JI, Rodríguez-Aldama JC, Torres Silva MS, et al. SHARE-NET writing group. Mpox in people with advanced HIV infection: a global case series. Lancet 2023; 401:939–949. [DOI] [PubMed] [Google Scholar]
- 16.Riser AP, Hanley A, Cima M, Lewis L, Saadeh K, Alarcón J, et al. Epidemiologic and clinical features of Mpox-associated deaths — United States, May 10, 2022-March 7, 2023. MMWR Morb Mortal Wkly Rep 2023; 72:404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MJ, Cash-Goldwasser S, Marx GE, Schrodt CA, Kimball A, Padgett K, et al. CDC Severe Monkeypox Investigations Team. Severe monkeypox in hospitalized patients — United States, August 10-October 10, 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1412–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheffer M, Paiva VSF, Barberia LG, Russo G. Monkeypox in Brazil between stigma, politics, and structural shortcomings: have we not been here before?. Lancet Reg Health Am 2022; 17:100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gessain A, Nakoune E, Yazdanpanah Y. Monkeypox. N Engl J Med 2022; 387:1783–1793. [DOI] [PubMed] [Google Scholar]
- 20.Mitjà O, Ogoina D, Titanji BK, Galvan C, Muyembe JJ, Marks M, et al. Monkeypox. Lancet 2022; 401:60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mailhe M, Beaumont AL, Thy M, Le Pluart D, Perrineau S, Houhou-Fidouh N, et al. Clinical characteristics of ambulatory and hospitalized patients with monkeypox virus infection: an observational cohort study. Clin Microbiol Infect 2023; 29:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angelo KM, Smith T, Camprubí-Ferrer D, Balerdi-Sarasola L, Díaz Menéndez M, Servera-Negre G, et al. Epidemiological and clinical characteristics of patients with monkeypox in the GeoSentinel Network: a cross-sectional study. Lancet Infect Dis 2022; 23:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascom ARP, Souza IN de, Krummenauer A, Duarte MMS, Sallas J, Rohlfs DB, et al. Características epidemiológicas e clínicas dos casos de monkeypox no Brasil em 2022: estudo transversal. Epidemiol Serv Saúde 2022; 31:e2022851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bocage AE, Coelho LE, Lake JE, Clark JL, Torres TS, Jalil EM, et al. The Impact of COVID-19 on HIV Care in Rio de Janeiro, Brazil 2019-2021: Disparities by Age and Gender. AIDS Behav 2023; 27:2629–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pascom ARP, Meireles MV, Benzaken AS. Sociodemographic determinants of attrition in the HIV continuum of care in Brazil, in 2016. Medicine (Baltimore) 2018; 97 (1s suppl 1):S69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. Relatório de Monitoramento Clínico do HIV 2020 [Internet]. Brasília/DF: Ministério da Saúde; 2020. Available at: https://www.gov.br/aids/pt-br/central-de-conteudo/publicacoes/2020/relatorio-de-monitoramento-clinico-do-hiv-2020/view. [Accessed 10 October 2023] [Google Scholar]
- 27. Ministério da Saúde. Monkeypox: orientações técnicas para assistência à saúde [Internet]. Ministério da Saúde; 2022. Available at: https://www.gov.br/saude/pt-br/campanhas-da-saude/2022/variola-dos-macacos/publicacoes/protocolos/monkeypox-orientacoes-tecnicas-para-a-assistencia-a-saude/view. [Accessed 28 May 2023] [Google Scholar]
- 28. Ministério da Saúde. Manual Técnico para o Diagnóstico da Infecção pelo HIV em Adultos e Crianças [Internet]. Ministério da Saúde; 2018. Available at: https://www.gov.br/aids/pt-br/centrais-de-conteudo/publicacoes/2018/manual_tecnico_hiv_27_11_2018_web.pdf/view. [Accessed 16 August 2023] [Google Scholar]
- 29. Brasil. Relatório de Monitoramento Clínico do HIV 2021 [Internet]. Ministério da Saúde; 2022. Available at: http://antigo.aids.gov.br/pt-br/pub/2022/relatorio-de-monitoramento-clinico-do-hiv-2021. [Accessed 10 October 2023] [Google Scholar]
- 30.Ong JJ, Fu H, Baggaley RC, Wi TE, Tucker JD, Smith MK, et al. Missed opportunities for sexually transmitted infections testing for HIV preexposure prophylaxis users: a systematic review. J Intern AIDS Soc 2021; 24:e25673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ministério da Saúde. Boletim Epidemiológico de Sífilis [Internet]. Ministério da Saúde; 2022. Available at: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2022/boletim-epidemiologico-de-sifilis-numero-especial-out-2022/view. [Accessed 10 November 2023] [Google Scholar]
- 32.Stewart J, Bartkus M, Sperring H, Ruiz-Mercado G, Johnson S, Pierre C. Monkeypox vaccination strategy and missed opportunities in STI and HIV prevention: an urban sexual health clinic's experience during a public health emergency. Open Forum Infect Dis 2023; 10:ofad006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gandhi M, Spinelli MA, Mayer KH. Addressing the sexually transmitted infection and HIV syndemic. JAMA 2019; 321:1356–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Oliveira-Júnior JM, Tenório MDL, dos Santos Caduda S, Santana RRR, Martins-Filho PR. Reasons for hospitalization of patients with monkeypox: a quantitative evidence synthesis. Infection [Internet]. Available at: https://link.springer.com/10.1007/s15010-022-01937-1. [Accessed 23 November 2023] [Google Scholar]
- 35.O'Shea J, Daskalakis D, Brooks JT. The emergence of mpox as an HIV-related opportunistic infection. Lancet 2023; 401:1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castro MC, Massuda A, Almeida G, Menezes-Filho NA, Andrade MV, de Souza Noronha KVM, et al. Brazil's unified health system: the first 30 years and prospects for the future. Lancet 2019; 394:345–356. [DOI] [PubMed] [Google Scholar]
- 37.Castro R, Ribeiro-Alves M, Corrêa RG, Derrico M, Lemos K, Grangeiro JR, et al. The Men Who Have Sex with Men HIV Care Cascade in Rio de Janeiro, Brazil. Paraskevis D, organizador. PLoS One 2016; 11:e0157309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jalil EM, Wilson EC, Luz PM, Velasque L, Moreira RI, Castro CV, et al. HIV testing and the care continuum among transgender women: population estimates from Rio de Janeiro, Brazil. J Int AIDS Soc 2017; 20:21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ministério da Saúde. Boletim Epidemiológico de HIV/Aids. Ministério da Saúde; 2022. [Google Scholar]
- 40.Chow JY, Konda KA, Borquez A, Caballero P, Silva-Santisteban A, Klausner JD, et al. Peru's HIV care continuum among men who have sex with men and transgender women: opportunities to optimize treatment and prevention. Int J STD AIDS 2016; 27:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, Tsai AC. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc 2013; 16: (3 Suppl 2): 18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almeida-Brasil CC, Moodie EEM, McLinden T, Hamelin AM, Walmsley SL, Rourke SB, et al. Medication nonadherence, multitablet regimens, and food insecurity are key experiences in the pathway to incomplete HIV suppression. AIDS 2018; 32:1323–1332. [DOI] [PubMed] [Google Scholar]
- 43.Daltro ACB, Almeida CS, Unfried AGC, de Aquino TR, Travassos AGÁ. Virological failure and adherence to antiretroviral therapy in adolescents and young adults living with human immunodeficiency virus. Tropical Med Int Health 2023; 28:162–174. [DOI] [PubMed] [Google Scholar]
- 44.Rodrigues A, Struchiner CJ, Coelho LE, Veloso VG, Grinsztejn B, Luz PM. Late initiation of antiretroviral therapy: inequalities by educational level despite universal access to care and treatment. BMC Public Health 2021; 21:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hovhannisyan L, Coelho LE, Velasque L, De Boni RB, Clark J, Cardoso SW, et al. Multilevel analysis of individual and neighborhood characteristics associated with viral suppression among adults with HIV in Rio de Janeiro, Brazil. AIDS Behav 2022; 26:947–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor L. Brazil's general election will decide the future of world's largest public health system. BMJ 2022; o2601. [DOI] [PubMed] [Google Scholar]
- 47.Agostini R, Rocha F, Melo E, Maksud I. A resposta brasileira à epidemia de HIV/AIDS em tempos de crise. Ciênc saúde coletiva 2019; 24:4599–4604. [DOI] [PubMed] [Google Scholar]
- 48.Paula AAD, Pires DF, Alves Filho P, Lemos KRVD, Veloso VG, Grinsztejn B, et al. Perfis de mortalidade em pessoas vivendo com HIV/aids: comparação entre o Rio de Janeiro e as demais unidades da federação entre 1999 e 2015. Rev bras epidemiol 2020; 23:e200017. [DOI] [PubMed] [Google Scholar]