Abstract
Objective:
Childhood sexual abuse is the leading cause of posttraumatic stress disorder (PTSD) in women, and is a prominent cause of morbidity and loss of function for which limited treatments are available. Understanding the neurobiology of treatment response is important for developing new treatments. The purpose of this study was to assess neural correlates of personalized traumatic memories in women with childhood sexual abuse with and without PTSD, and to assess response to treatment.
Methods:
Women with childhood sexual abuse with (N = 28) and without (N = 17) PTSD underwent brain imaging with High-Resolution Positron Emission Tomography scanning with radiolabeled water for brain blood flow measurements during exposure to personalized traumatic scripts and memory encoding tasks. Women with PTSD were randomized to paroxetine or placebo followed by three months of double-blind treatment and repeat imaging with the same protocol.
Results:
Women with PTSD showed decreases in areas involved in the Default Mode Network (DMN), a network of brain areas usually active when the brain is at rest, hippocampus and visual processing areas with exposure to traumatic scripts at baseline while women without PTSD showed increased activation in superior frontal gyrus and other areas (p < 0.005). Treatment of women with PTSD with paroxetine resulted in increased anterior cingulate activation and brain areas involved in the DMN and visual processing with scripts compared to placebo (p < 0.005).
Conclusion:
PTSD related to childhood sexual abuse in women is associated with alterations in brain areas involved in memory and the stress response and treatment with paroxetine results in modulation of these areas.
1. Introduction
Posttraumatic Stress Disorder (PTSD) affects about 8% of Americans at some time in their lives (Kessler et al., 2005), is more common in civilians than in the military and is twice as common in women as in men (Kessler et al., 2005). The most common cause of PTSD in women is childhood sexual abuse (Kessler et al., 2005). Symptoms of PTSD, including intrusive memories, avoidance behaviors, sleep disturbance, and hyperarousal are the behavioral manifestation of stress-related changes in the brain (Bremner and Wittbrodt, 2020). The only medications approved by the Food and Drug Administration (FDA) for the treatment of PTSD are the Selective Serotonin Reuptake Inhibitor (SSRI) antidepressant medications, paroxetine and sertraline (Ballenger et al., 2000; Brady et al., 2000; Davis et al., 2016; Marshall et al., 2001; Stein et al., 2000; Tucker et al., 2001; Zohar et al., 2002). Evidence for their utility, however, is limited (Institute of Medicine, 2008). Understanding mechanisms in the brain underlying successful responses to treatment could be helpful in the development of new and better treatments (Bremner and Campanella, 2016; Bremner and Wittbrodt, 2020; Davis et al., 2016).
Brain imaging studies have outlined a network of brain areas mediating memory and the fear response in PTSD (Akiki et al., 2017; Boccia et al., 2016; Campanella and Bremner, 2016; Dixon et al., 2017; Etkin and Wager, 2007; Fitzgerald et al., 2018; Francati et al., 2007; Koch et al., 2016; Patel et al., 2012; Sartory et al., 2013; Thome et al., 2020; Wang et al., 2016). Brain imaging studies involving activation of PTSD symptoms using behavioral paradigms like trauma-related pictures, sounds and/or personalized scripts resulted in decreased function or failure of activation in the medial prefrontal cortex (mPFC)/anterior cingulate cortex (ACC) (Bremner et al., 1999a, 1999b; Dahlgren et al., 2018; Lanius et al., 2001, 2003; Lindauer et al., 2004; Offringa et al., 2013; Phan et al., 2006; Shin et al., 1997, 1999, 2004; Shin et al., 2001, 2005), ventromedial prefrontal cortex (Bluhm et al., 2012; Grupe et al., 2020; Milad et al., 2009; Phan et al., 2006; Rougemont-Bücking et al., 2011; Sanjuan et al., 2018), thalamus (Elman et al., 2018; Lanius et al., 2001, 2003; Schechter et al., 2012), fusiform gyrus (Cwik et al., 2017; Osuch et al., 2001), precuneus (Geuze et al., 2008a; Lanius et al., 2002; Werner et al., 2009), cuneus (Cortese et al., 2018; Cwik et al., 2017; Lindauer et al., 2004; Misaki et al., 2019; Morey et al., 2009; Ramage et al., 2013; Sun et al., 2019), visual association cortex (Bremner et al., 1999a; Gold et al., 2011; Lanius et al., 2001, 2003; Shin et al., 1997, 2004), temporal cortex (Chung et al., 2006; Geuze et al., 2008b; Werner et al., 2009), parietal cortex (Bremner et al., 1999a; Chung et al., 2006; Ke et al., 2016; Morey et al., 2008; Shin et al., 1997, 1999), parahippocampus (Sakamoto et al., 2005; Werner et al., 2009), hippocampus (Bremner et al., 1999a; Cisler et al., 2015b; Kim et al., 2012; Osuch et al., 2008; Yehuda et al., 2010, 2009), inferior frontal (Bremner et al., 1999a; Lanius et al., 2003; Sakamoto et al., 2005; Shin et al., 1997, 1999, 2001) middle and superior frontal gyrus, (Aupperle et al., 2012; Cisler et al., 2015b; Cohen et al., 2013; Cwik et al., 2017; Hou et al., 2007; Morey et al., 2009; Shin et al., 1997, 1999, 2004; Shin et al., 2001)
Activation of PTSD symptoms was associated with increased function in the insula (Bruce et al., 2013; Fonzo et al., 2010a; Kaczkurkin et al., 2017; King et al., 2009; Mazza et al., 2015; Misaki et al., 2019; Morey et al., 2015; Moser et al., 2013; Nicholson et al., 2016; Osuch et al., 2001; Simmons et al., 2008; Whalley et al., 2013), dorsal anterior cingulate cortex (dACC) (Bremner et al., 1999a, 1999b; Britton et al., 2005; Fonzo et al., 2010a, b; Herringa et al., 2013; Milad et al., 2009, 2007; Misaki et al., 2019; Piefke et al., 2007; Ramage et al., 2013; Rougemont-Bücking et al., 2011), motor cortex (Barkay et al., 2012; Bremner et al., 1999b; Cohen et al., 2013; Cwik et al., 2017; Naegeli et al., 2018), and posterior cingulate cortex (Bremner et al., 1999a, 1999b; Brinkmann et al., 2017; Ke et al., 2016; Lanius et al., 2001; Ramage et al., 2013; Shin et al., 1997, 2005). There was increased function in the amygdala with fear-related tasks including fear conditioning, exposure to fearful or angry faces, and exposure to pictures with traumatic content (Armony et al., 2005; Bremner et al., 2005; Dickie et al., 2008; Etkin and Wager, 2007; Felmingham et al., 2009; Lieberman et al., 2017; McLaughlin et al., 2014; Morey et al., 2009; Patel et al., 2016; Protopopescu et al., 2005; Shin et al., 2004, 2005; Stevens et al., 2014, 2017).
Studies of functional brain connectivity at rest show similar results in PTSD (Akiki et al., 2017; Etkin et al., 2011; Etkin and Wager, 2007; Fitzgerald et al., 2018; Koch et al., 2016; Patel et al., 2012; Sartory et al., 2013; Schulze et al., 2019; Thome et al., 2020; Wang et al., 2016). These studies show altered function/connectivity in the hippocampus (Abdallah et al., 2017; Admon et al., 2013; Bluhm et al., 2009; Chen and Etkin, 2013; Chen et al., 2019; Jin et al., 2014; Lazarov et al., 2017; Malivoire et al., 2018; Metz et al., 2019; Miller et al., 2017a; Rabellino et al., 2018a, 2018b; Stevens et al., 2014; Zhang et al., 2017), parahippocampus (Jin et al., 2014; Misaki et al., 2018), insula (Birn et al., 2014; Etkin et al., 2019; Harricharan et al., 2020; Ke et al., 2018; Koch et al., 2016; Liu et al., 2017; Nicholson et al., 2020; Sripada et al., 2012a; Tursich et al., 2015; Vanasse et al., 2019; Zhang et al., 2016b), amygdala (Admon et al., 2009; Barredo et al., 2018; Disner et al., 2018; Jin et al., 2014; Koch et al., 2016; Nicholson et al., 2019, 2017; Satterthwaite et al., 2016; Simmons et al., 2008; Stevens et al., 2013; Sun et al., 2020; Weng et al., 2019; Yan et al., 2013; Yuan et al., 2019; Zhang et al., 2016a; Zhu et al., 2017), medial prefrontal cortex (Akiki et al., 2018; Amad et al., 2019; Birn et al., 2014; Clausen et al., 2017b; Disner et al., 2018; Gong et al., 2014; Jin et al., 2014; Kennis et al., 2015; Liu et al., 2016; Misaki et al., 2018; Sadeh et al., 2015) and other brain areas involved in memory and visual and spatial processing (Olivé et al., 2018; Olson et al., 2019; Reuveni et al., 2016) (Bluhm et al., 2009; Disner et al., 2018; Fu et al., 2019; Gong et al., 2014; Jeon et al., 2020; Ke et al., 2017; Koch et al., 2016; Lanius et al., 2010; Liu et al., 2016, 2017; Miller et al., 2017a, 2017b; Qin et al., 2012; Shaw et al., 2002; Terpou et al., 2018; Wang et al., 2016; Weng et al., 2019; Yan et al., 2013; Yin et al., 2011; Zhang et al., 2017, 2016b, 2016c; Zhu et al., 2015). A number of studies have shown decreased conductivity in the default mode network (DMN), a network of brain regions that are active when the brain is not engaged, including ventromedial prefrontal cortex (vmPFC), dorsomedial PFC (dmPFC), posterior cingulate cortex (PCC), precuneus, medial temporal lobe, and medial and lateral parietal cortices (Akiki et al., 2017; Jin et al., 2017; Koch et al., 2016; Lei et al., 2015; Maron-Katz et al., 2020; Miller et al., 2017a, 2017b; Nicholson et al., 2020; Reuveni et al., 2016; Russman Block et al., 2017; Sripada et al., 2012b; Zhang et al., 2015; Zhu et al., 2019). Other studies showed altered connectivity in the salience network, which includes insula, parietal and lateral prefrontal cortex, and dACC (Koch et al., 2016; Misaki et al., 2018; Nicholson et al., 2020; Russman Block et al., 2017; Shang et al., 2014; Zhu et al., 2020).
Brain imaging studies have tracked the effects of successful treatment of PTSD on the brain (Bremner and Campanella, 2016; Campanella and Bremner, 2016). Studies showed increased connectivity in DMN with a variety of behavioral treatments (Bremner et al., 2017; Felmingham et al., 2007; Fonzo et al., 2017; King et al., 2016; Kluetsch et al., 2014; Lansing et al., 2005; Lee et al., 2019; Lindauer et al., 2008; Misaki et al., 2019; Nicholson et al., 2017; Pagani et al., 2007; Peres et al., 2007; Shou et al., 2017; Simmons et al., 2013; van Rooij et al., 2016; Yang et al., 2018; Zhu et al., 2018; Zotev et al., 2018). Open label studies and case reports with PET and SPECT showed increased medial prefrontal function in PTSD following treatment with the SSRI fluoxetine (Fernandez et al., 2001). Placebo-controlled studies of paroxetine in PTSD patients showed an increase in function in medial prefrontal, anterior cingulate (Fani et al., 2011) and orbitofrontal cortex (Zhu et al., 2015), and dorsolateral prefrontal cortex, and supplementary motor area (MacNamara et al., 2016) while decreased function was found in the medial temporal cortex with citalopram (Seedat et al., 2004). Other medication and neuromodulation treatment studies showed increased medial prefrontal function in PTSD (Metz et al., 2019) (Philip et al., 2018). (Wittbrodt et al., 2020, 2021).
Understanding the mechanism by which treatments act in the brain is potentially useful in developing new treatments for PTSD (Bremner and Campanella, 2016). The purpose of this study was to examine brain responses to personalized traumatic scripts in women with childhood sexual abuse with and without PTSD, and to examine neural correlates of response to treatment with paroxetine. This study built on our prior study of the effects of paroxetine versus placebo on men and women with PTSD from a variety of causes women (Fani et al., 2011) by looking at the effects of double-blind paroxetine treatment versus placebo on a more specific sample of women with PTSD related childhood sexual abuse that was independent from the sample in our earlier study, and comparing them to women with childhood sexual abuse without PTSD. We hypothesized based on prior studies involving behavioral, neuromodulation, and medication treatment of PTSD, including our prior study with paroxetine, that PTSD would be associated with a decrease in medial prefrontal cortex and other brain areas involved in the DMN at baseline in comparison to non-PTSD and that paroxetine would result in a greater increase in medial prefrontal cortex and other DMN areas in women with PTSD treated with paroxetine versus those treated with placebo.
2. Materials and methods
2.1. Institutional review board
The Emory University institutional review board provided approval for this study which is posted on ClinicalTrials.gov (ClinicalTrials.gov # NCT01681849). Verbal / written informed consent was provided by all participants before enrollment.
2.2. Participants
The participants were healthy women between the ages of 18 and 75 with a history of childhood sexual abuse. Women were included with childhood sexual abuse that began at age 13 or before, involving unwanted and repeated penetration or oral sex. Abused women were divided in those with and without PTSD. All women underwent brain imaging at baseline, and the women with PTSD were randomized to a double-blind study of paroxetine versus placebo followed by repeat brain imaging at three months while still on study medication. Fig. 1 presents the Consolidated Standards of Reporting Trials (CONSORT) for this study. Of the 135 individuals assessed for eligibility, 44 were excluded based on eligibility criteria and 91 consented to participate including 57 PTSD patients and 34 non-PTSD traumatized controls. Seven of the PTSD patients were excluded based on further screening and 22 lost interest or were lost to followup. Twenty-eight PTSD patients were randomized to paroxetine or placebo and scanned at baseline (15 paroxetine, 13 placebo). Post treatment scans were obtained on seven paroxetine and six placebo PTSD patients. Seventeen of the non-PTSD trauma controls lost interest or were lost to followup and 17 underwent the imaging protocol.
Fig. 1.
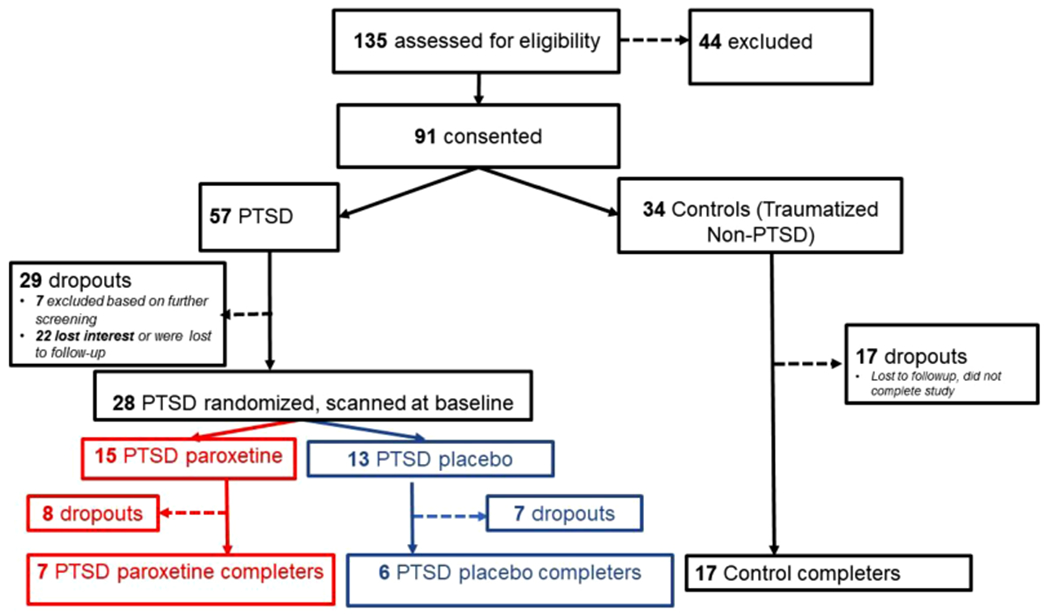
Consort diagram showing subject flow. Female subjects were screened and consented based on a history of early childhood sexual abuse and then divided into current PTSD and non-PTSD groups. Subsequent screening after consenting led to additional exclusions in the PTSD group and subjects who lost interest or were lost to followup in both groups. PTSD patients were then randomized to paroxetine or placebo and all subjects were scanned at baseline with personalized traumatic scripts and memory tasks. PTSD patients then underwent three months of double-blind treatment with paroxetine or placebo, during which time there were additional dropouts and patients lost to followup. Remaining patients were rescanned using the same protocol after three months.
The diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, bulimia, or anorexia, as defined by The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (APA, 2000), excluded individuals from participating. Further exclusion criteria were current pregnancy, traumatic brain injury, meningitis, or evidence or history of serious medical or neurological illness. Trained staff administered the Structured Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID) (First and Gibbon, 2004) for psychiatric diagnosis. The Clinician-Administered PTSD Scale for DSM-IV-TR (CAPS) was used to measure the severity of PTSD (Blake et al., 1995). The SCID and CAPS interviews were used to determine that PTSD was primarily related to childhood abuse. The Subjective Units of Distress Scale (SUDS) was used to measure behavioral responses to traumatic scripts in the scanner (Wolpe and Lazarus, 1966). Participants also completed the Early Trauma Inventory-Self Report-Short Form (ETI-SR-SF) (Bremner et al., 2007).
2.3. Study design
At the initial screening, a trained member of the research staff conducted a psychiatric interview and facilitated a written traumatic history of the participant. From there, the written traumatic experiences were converted into a 60-second script which was recorded by a member of the research team (Bremner et al., 1999a). Women with PTSD were randomized to receive three months of double-blind paroxetine 10–40 mg variable dose given once per day or placebo. Dosage was adjusted to be within the efficacious range based on prior studies in PTSD and to minimize side effects (Tucker et al., 2001). The study blinded assignment was kept by a research pharmacist who was not involved in the study.
2.4. Neuroimaging and analysis
During the second visit, participants underwent a high-resolution positron emission tomography (HR-PET) scan session consisting of auditory delivery of a series of both neutral and traumatic scripts. There were two neutral followed by two traumatic scripts delivered in fixed order. The neutral scripts were descriptions of nature designed to induce neutral-to-positive affective responses. Scripts were recorded by a female voice. The scripts were two minutes in length and divided into two scripts one minute in length to correspond to the scanning time of the two trauma related scans. All scripts were delivered using headphones as the participant lay supine in the HR-PET scanner. The scripts were delivered while the participant rested with eyes open. Twenty mCi of radiolabeled water ([15O]H2O) produced by an on-site cyclotron was injected intravenously five seconds before the beginning of the script followed by a 90 second HR-PET scan of the brain to measure brain blood perfusion. Regional cerebral blood flow was measured using the High Resolution Research Tomograph (HRRT) brain dedicated HR-PET device (CTI, Knoxville, TN) (Schmand et al., 1999). Following all scans, participants were asked to complete the Subjective Units of Distress Scale, rating their level of distress from 0 (no distress) to 100 (extreme distress).
HR-PET image analysis was completed similar to previous research (Wittbrodt et al., 2019) within the statistical parametrical mapping (SPM12; www.fil.ion.ucl.ac.uk/spm) suite. Scans were pre-processed by spatially normalizing to a mean intensity image across individual scans, transformed into a common anatomical space (SPM PET Template), smoothed using a three-dimensional Gaussian filter at 5-mm full width half maximum, and then normalized to whole-brain activity. First level (individual) models were computed using the neutral script and traumatic script conditions with the factor of scan pairs. The first level model was grand mean scaled, estimated, and contrasts computed for activation (trauma scripts – neutral scripts) and deactivation (neutral scripts – trauma scripts).
2.5. Statistical analysis
Comparison between groups was completed using a two-sample t-test or Mann-Whitney-Wilcoxon test for continuous and Fisher’s exact test for discrete variables, respectively. For assessments during the scripts, linear mixed-effects models were fit to the data (lme4; cran.r-project.org/web/packages/lme4) using between-participant fixed effects of PTSD versus non-PTSD group and within-participant fixed effects of script type (neutral, traumatic), random effect of participant, and baseline value as a covariate.
Regional brain blood flow changes between PTSD and non-PTSD and paroxetine versus placebo at baseline and after treatment were encoded similar to previous recommendations (Gläscher and Gitelman, 2008) resulting in t-statistic brain maps. For all types of analyses, a threshold of p < 0.005 (uncorrected) and a minimum voxel size of eleven was employed to minimize Type I and Type II errors in neuroimaging research (Lane et al., 1997). Significant cluster peaks were identified using the distance from the anterior commissure with x, y, and z coordinates transformed from Montreal Neurological Institute (MNI) space to those of the Talairach stereotaxic atlas (Talairach and Tournoux, 1988). Cluster peaks were identified using Brodmann Areas (BA) from the Talairach daemon (www.talairach.org). The a priori α level for non-brain imaging data was chosen at 0.05. All data are presented as mean ± SD.
3. Results
3.1. Demographics
Demographic variables including age, years of education, and body mass index were similar between PTSD and non-PTSD women. Early trauma exposure severity measured with the ETI-SR-SF was slightly higher in the PTSD group (Table 1). Early trauma, antidepressant usage and PTSD symptom levels at baseline are presented in Table 1. Comorbid diagnoses based on the SCID are presented in Table 2.
Table 1.
Co-Morbid Psychiatric Diagnoses in Traumatized Women with and without Posttraumatic Stress Disorder (PTSD).
| Measure | PTSD (n = 28) |
Non-PTSD (n 17) |
|---|---|---|
| Age (y) | 43 ± 8 | 41 ± 10 |
| Sex | 28 F | 17 F |
| BMI (kg·m − 2), | 31.4 ± 9.3 | 31.6 ± 8.0 |
| Race/Ethnicity | ||
| White/Caucasian | 12 (43%) | 8 (47%) |
| Black/AA | 12 (43%) | 8 (47%) |
| White Hispanic | 2 (7%) | 1 (6%) |
| Black Hispanic | 1 (4%) | 0 (0%) |
| Asian/mixed | 1 (4%) | 0 (0%) |
| Years of Education | 13 (3 SD) | 14 (2 SD) |
| Marital Status | ||
| Never Married | 11 (39%) | 6 (35%) |
| Married | 5 (18%) | 5 (29%) |
| Divorced | 9 (32%) | 6 (35%) |
| Separated | 2 (7%) | 0 (0%) |
| Widowed | 1 (4%) | 0 (0%) |
| Current Antidepressants | 7 (25%) | 5 (29%) |
| CAPS-Total Severity Score | 71 ± 19 | 24 ± 11 |
| ETI-SR-SF-Score | 17 ± 5 | 12 ± 6 |
AA=African-American; CAPS = Clinician-Administered PTSD Scale; ETI-SR-SF = Early Trauma Inventory-Self Report Short Form; Data expressed as numbers with percentages or means with standard deviations (SD).
Table 2.
Co-Morbid Psychiatric Disorders in Traumatized Women with and without Posttraumatic Stress Disorder.
| Measure | PTSD (n = 28) |
Non-PTSD (n = 17) |
|---|---|---|
| Major Depression | ||
| Current | 6 (21%) | 1 (6%) |
| Past | 5 (18%) | 4 (24%) |
| Dysthymia | ||
| Current | 5 (18%) | 1 (6%) |
| Past | 0 (0%) | 0 (0%) |
| Obsessive-Compulsive Disorder | ||
| Current | 1 (4%) | 1 (6%) |
| Past | 0 (0%) | 0 (0%) |
| PTSD | ||
| Current | 28 (100%) | 0 (0%) |
| Past | 0 (0%) | 4 (24%) |
| Social Phobia | ||
| Current | 1 (4%) | 0 (0%) |
| Past | 2 (7%) | 1 (6%) |
| Panic Disorder with AG | ||
| Current | 2 (7%) | 0 (0%) |
| Past | 0 (0%) | 2 (12%) |
| Panic Disorder without AG | ||
| Current | 1 (4%) | 0 (0%) |
| Past | 7 (25%) | 4 (24%) |
| Generalized Anxiety Disorder | ||
| Current | 2 (7%) | 0 (0%) |
| Past | 0 (0%) | 0 (0%) |
| Cocaine Dependence | ||
| Current | 0 (0%) | 0 (0%) |
| Past | 1 (4%) | 0 (0%) |
| Alcohol Dependence | ||
| Current | 0 (0%) | 0 (0%) |
| Past | 1 (4%) | 1 (6%) |
AA=African-American; AG=Agoraphobia; CAPS = Clinician-Administered PTSD Scale; ETI-SR-SF = Early Trauma Inventory-Self Report Short Form; Data expressed in means.
3.2. Psychometric measures during traumatic scripts
Exposure to traumatic scripts during the imaging session resulted in significant increases in distress measured by the SUDS (baseline to posttraumatic scripts) in both the traumatized PTSD (40 (33 SD) to 63 (37 SD), p = 0.007) and non-PTSD women (25 (23 SD) to 47 (29 SD), p = 0.016). There were no significant differences in the magnitude of increase in distress with traumatic scripts between traumatized women with and without PTSD.
3.3. Neuroimaging
Traumatized non-PTSD women showed activation with traumatic scrips in pre- and postcentral gyrus, frontal cortex (BA 9, 10, 45), precuneus, anterior cingulate (BA 32), cingulate (BA 31) and insula (Table 3). Decreases occurred in parts of the parietal and frontal cortex (Table 4). PTSD women also showed activation in pre- and post-central gyrus, cuneus, and frontal cortex (Table 5). Decreases were seen in fusiform gyrus, parahippocampus, and parts of the parietal cortex and frontal cortex (Table 6, Fig. 2). PTSD women had greater increases with traumatic scripts compared to non-PTSD in parts of the cingulate, frontal cortex and lentiform nucleus (Table 7). Non-PTSD women activated more (there was a relative decrease in activation in PTSD) in cerebellum, posterior cingulate (BA 30), parahippocampal gyrus, thalamus, parietal cortex, cuneus, precuneus, fusiform gyrus, left insula and temporal lobe (Table 8).
Table 3.
Increased Brain Activation with Traumatic Scripts in Traumatized Non-PTSD Women
Brain areas with significant (p < 0.005) activations during exposure to personalized trauma scripts in participants with posttraumatic stress disorder as measured with high-resolution positron emission tomography. Significant clusters are presented by size (number of voxels) and location (Brodmann area, cluster peak Talairach coordinates). Sub-cluster peaks are also identified.
| Voxel Number |
Brain Region | Brodmann Area |
X | Y | Z | Z Score |
|---|---|---|---|---|---|---|
| 1227 | L. Transverse Temporal Gyrus | 42 | −58 | −12 | 14 | 6.15 |
| L. Precentral Gyrus | 4 | −54 | −14 | 28 | 5.52 | |
| L. Postcentral Gyrus | 3 | −62 | −12 | 28 | 5.49 | |
| 749 | R. Precentral Gyrus | 4 | 62 | −8 | 26 | 5.41 |
| R. Precentral Gyrus | 6 | 58 | −14 | 42 | 3.73 | |
| R. Precentral Gyrus | 4 | 48 | −16 | 34 | 3.61 | |
| 1223 | R. Superior Frontal Gyrus | 9 | 14 | 54 | 20 | 4.85 |
| L. Medial Frontal Gyrus | 10 | −2 | 60 | 20 | 4.51 | |
| 97 | L. Middle Temporal Gyrus | 21 | −60 | −4 | −20 | 5.20 |
| L. Inferior Temporal Gyrus | 20 | −58 | −2 | −30 | 3.15 | |
| 438 | R. Precuneus | 31 | 16 | −52 | 32 | 4.56 |
| R. Precuneus | 31 | 6 | −62 | 26 | 4.49 | |
| R. Cingulate Gyrus | 31 | 2 | −52 | 28 | 3.53 | |
| 80 | L. Superior Temporal Gyrus | 38 | −40 | 16 | −34 | 4.53 |
| 35 | R. Inferior Frontal Gyrus | 45 | 62 | 18 | 10 | 4.46 |
| 45 | L. Declive | −16 | −68 | −18 | 4.45 | |
| 21 | L. Middle Temporal Gyrus | 21 | −50 | 6 | −34 | 4.09 |
| 62 | R. Superior Temporal Gyrus | 38 | 50 | 14 | −32 | 4.05 |
| 44 | R. Fusiform Gyrus | 20 | 64 | −4 | −26 | 3.89 |
| R. Middle Temporal Gyrus | 21 | 60 | 4 | −24 | 2.94 | |
| 30 | R. Inferior Temporal Gyrus | 21 | 70 | −16 | −20 | 3.78 |
| R. Middle Temporal Gyrus | 21 | 72 | −22 | −12 | 3.16 | |
| 55 | L. Insula | 13 | −34 | −2 | 12 | 3.58 |
| L. Claustrum | −38 | −10 | 4 | 2.79 | ||
| 53 | R. Declive | 20 | −66 | −22 | 3.48 | |
| 19 | R. Uvula | 26 | −84 | −26 | 3.38 | |
| 12 | R. Superior Frontal Gyrus | 8 | 16 | 42 | 46 | 3.31 |
| 17 | R. Anterior Cingulate Gyrus | 32 | 12 | 44 | 6 | 3.14 |
| 14 | L. Cingulate Gyrus | 31 | −4 | −38 | 36 | 3.08 |
| 11 | L. Insula | 22 | −42 | −26 | 0 | 2.87 |
Table 4.
Decreased Brain Activation with Traumatic Scripts in Traumatized Non-PTSD Women
Brain areas with significant (p < 0.005) deactivations during exposure to personalized trauma scripts in participants with posttraumatic stress disorder as measured with high-resolution positron emission tomography. Significant clusters are presented by size (number of voxels) and location (Brodmann area, cluster peak Talairach coordinates). Sub-cluster peaks are also identified.
| Voxel Number |
Brain Region | Brodmann Area |
X | Y | Z | Z Score |
|---|---|---|---|---|---|---|
| 470 | L. Middle Frontal Gyrus | 6 | −20 | 12 | 62 | 5.58 |
| L. Superior Frontal Gyrus | 6 | −22 | 2 | 70 | 4.38 | |
| L. Middle Frontal Gyrus | 6 | −34 | 6 | 62 | 4.01 | |
| 1200 | R. Supramarginal Gyrus | 40 | 56 | −48 | 32 | 5.15 |
| R. Inferior Parietal Lobule | 7 | 36 | −58 | 48 | 4.92 | |
| R. Inferior Parietal Lobule | 40 | 42 | −42 | 44 | 4.69 | |
| 1121 | R. Cerebellar Tonsil | 42 | −54 | −44 | 4.99 | |
| R. Inferior Temporal Gyrus | 20 | 62 | −52 | −16 | 4.88 | |
| R. Cerebellar Tonsil | 46 | −52 | −34 | 4.69 | ||
| 633 | R. Middle Frontal Gyrus | 46 | 56 | 32 | 22 | 4.84 |
| R. Middle Frontal Gyrus | 9 | 48 | 6 | 36 | 4.77 | |
| R. Middle Frontal Gyrus | 9 | 52 | 22 | 28 | 4.41 | |
| 658 | L. Superior Frontal Gyrus | 10 | −32 | 56 | 24 | 4.75 |
| L. Middle Frontal Gyrus | 9 | −48 | 30 | 30 | 4.36 | |
| L. Middle Frontal Gyrus | 8 | −46 | 26 | 42 | 4.35 | |
| 320 | L. Superior Frontal Gyrus | 10 | −34 | 62 | −6 | 4.68 |
| L. Superior Frontal Gyrus | 11 | −32 | 56 | −14 | 4.06 | |
| L. Superior Frontal Gyrus | 11 | −32 | 48 | −16 | 3.81 | |
| 669 | L. Inferior Parietal Lobule | 40 | −58 | −38 | 50 | 4.54 |
| L. Inferior Parietal Lobule | 40 | −44 | −46 | 38 | 4.29 | |
| L. Inferior Parietal Lobule | 40 | −44 | −48 | 48 | 4.28 | |
| 175 | L. Thalamus | −10 | −20 | 8 | 4.52 | |
| L. Thalamus | −6 | −12 | 6 | 3.55 | ||
| L. Thalamus | −4 | −20 | 0 | 3.26 | ||
| 221 | R. Middle Frontal Gyrus | 6 | 30 | −4 | 60 | 4.21 |
| R. Middle Frontal Gyrus | 6 | 30 | 8 | 62 | 4.17 | |
| R. Middle Frontal Gyrus | 6 | 36 | 14 | 62 | 2.88 | |
| 175 | L. Extra-Nuclear | 47 | −34 | 22 | −2 | 4.18 |
| L. Lentiform Nucleus | −24 | 18 | 0 | 3.42 | ||
| 566 | L. Cingulate Gyrus | 32 | −6 | 30 | 28 | 4.17 |
| R. Medial Frontal Gyrus | 8 | 8 | 22 | 46 | 3.92 | |
| L. Medial Frontal Gyrus | 8 | −4 | 32 | 38 | 3.87 | |
| 225 | R. Caudate | 18 | 16 | 4 | 4.04 | |
| R. Insula | 13 | 36 | 20 | 2 | 3.52 | |
| R. Inferior Frontal Gyrus | 47 | 36 | 28 | −4 | 3.39 | |
| 166 | R. Declive | 36 | −64 | −16 | 3.99 | |
| R. Inferior Temporal Gyrus | 37 | 48 | −64 | −4 | 3.26 | |
| R. Declive | 34 | −58 | −8 | 3.03 | ||
| 25 | R. Precentral Gyrus | 6 | 56 | −2 | 50 | 3.92 |
| 14 | R. Uncus | 28 | 18 | 2 | −30 | 3.86 |
| 42 | L. Medial Frontal Gyrus | 9 | −4 | 42 | 28 | 3.66 |
| L. Anterior Cingulate | 0 | 38 | 22 | 3.08 | ||
| L. Cerebellar Tonsil | −38 | −48 | −34 | 3.65 | ||
| L. Culmen | −28 | −54 | −16 | 3.61 | ||
| L. Cerebellar Tonsil | −38 | −50 | −44 | 3.46 | ||
| 87 | R. Nodule | 10 | −52 | −30 | 3.53 | |
| R. Nodule | 2 | −54 | −30 | 3.25 | ||
| R. Culmen | 0 | −60 | −22 | 3.18 | ||
| 30 | L. Cingulate Gyrus | 31 | −8 | −10 | 44 | 3.50 |
| 32 | R. Cingulate Gyrus | 31 | 14 | −22 | 42 | 3.49 |
| 16 | R. Postcentral Gyrus | 2 | 62 | −24 | 48 | 3.49 |
| 54 | R. Culmen | 26 | −40 | −22 | 3.48 | |
| R. Culmen | 24 | −48 | −20 | 2.88 | ||
| 26 | L. Culmen | −18 | −34 | −20 | 3.46 | |
| 17 | L. Cerebellar Tonsil | −22 | −58 | −44 | 3.45 | |
| 53 | R. Tuber | 48 | −76 | −30 | 3.42 | |
| 19 | R. Fusiform Gyrus | 19 | 52 | −74 | −14 | 3.42 |
| 19 | R. Inferior Occipital Gyrus | 18 | 40 | −90 | −10 | 3.36 |
| 61 | L. Uvula | −6 | −74 | −32 | 3.33 | |
| L. Inferior Semi-Lunar Lobule | −14 | −76 | −36 | 2.93 | ||
| 48 | R. Transverse Temporal Gyrus | 41 | 42 | −24 | 14 | 3.27 |
| 14 | L. Lentiform Nucleus | −28 | 2 | 0 | 3.27 | |
| 32 | L. Tuber | −34 | −64 | −30 | 3.26 | |
| 19 | L. Orbital Gyrus | 47 | −16 | 24 | −22 | 3.26 |
| 20 | L. Cuneus | 17 | −4 | −96 | 2 | 3.25 |
| 27 | R. Postcentral Gyrus | 3 | 32 | −30 | 62 | 3.23 |
| 41 | R. Middle Temporal Gyrus | 39 | 56 | −54 | 10 | 3.22 |
| R. Middle Temporal Gyrus | 21 | 60 | −46 | 6 | 2.94 | |
| 13 | L. Precuneus | 7 | −16 | −66 | 50 | 3.22 |
| 13 | L. Middle Frontal Gyrus | 6 | −44 | 2 | 58 | 3.15 |
| 32 | R. Paracentral Lobule | 5 | 12 | −36 | 54 | 3.15 |
| R. Paracentral Lobule | 5 | 16 | −32 | 48 | 3.07 | |
| 21 | L. Uvula | −10 | −62 | −34 | 3.14 | |
| 24 | R. Medial Frontal Gyrus | 6 | 2 | 2 | 58 | 3.13 |
| 10 | R. Culmen | 32 | −52 | −20 | 3.12 | |
| 47 | L. Declive | −40 | −68 | −18 | 3.11 | |
| 11 | R. Middle Temporal Gyrus | 21 | 58 | −30 | −4 | 3.11 |
| 14 | L. Middle Frontal Gyrus | 11 | −40 | 38 | −18 | 3.10 |
| 12 | L. Paracentral Gyrus | 6 | −8 | −30 | 52 | 3.08 |
| 14 | L. Culmen | −44 | −48 | −24 | 3.08 | |
| 13 | L. Cuneus | 18 | −16 | −94 | 10 | 3.07 |
| 19 | R. Inferior Frontal Gyrus | 45 | 48 | 14 | 14 | 3.05 |
| 33 | L. Superior Parietal Lobule | 7 | −32 | −60 | 46 | 3.04 |
| L. Superior Parietal Lobule | 7 | −28 | −50 | 40 | 2.85 | |
| 18 | R. Middle Frontal Gyrus | 10 | 40 | 62 | 2 | 2.96 |
| 18 | L. Cingulate Gyrus | 24 | −10 | 4 | 48 | 2.96 |
| 11 | R. Cuneus | 17 | 8 | −78 | 12 | 2.96 |
| 12 | R. Inferior Temporal Gyrus | 20 | 50 | −16 | −36 | 2.91 |
| 17 | L. Fusiform Gyrus | 20 | −50 | −34 | −22 | 2.88 |
| 28 | R. Middle Frontal Gyrus | 10 | 46 | 48 | 18 | 2.81 |
Table 5.
Increased Brain Activation with Traumatic Scripts in Women with PTSD
Brain areas with significant (p < 0.005) activations during exposure to personalized trauma scripts in participants with posttraumatic stress disorder as measured with high-resolution positron emission tomography. Significant clusters are presented by size (number of voxels) and location (Brodmann area, cluster peak Talairach coordinates). Sub-cluster peaks are also identified.
| Voxel Number |
Brain Region | Brodmann Area |
X | Y | Z | Z Score |
|---|---|---|---|---|---|---|
| 881 | L. Precentral Gyrus | 4 | −52 | −16 | 26 | 5.34 |
| L. Postcentral Gyrus | 43 | −66 | −12 | 16 | 5.22 | |
| L. Precentral Gyrus | 6 | −64 | −4 | 22 | 4.33 | |
| 705 | R. Precentral Gyrus | 6 | 64 | −10 | 30 | 5.17 |
| R. Postcentral Gyrus | 3 | 50 | −16 | 36 | 5.04 | |
| R. Postcentral Gyrus | 43 | 64 | −10 | 20 | 4.09 | |
| 89 | R. Cuneus | 19 | 10 | −90 | 40 | 4.34 |
| R. Cuneus | 19 | 6 | −86 | 34 | 3.69 | |
| 11 | L. Inferior Frontal Gyrus | 47 | −52 | 30 | −4 | 4.24 |
| 46 | R. Angular Gyrus | 39 | 54 | −70 | 36 | 4.16 |
| 57 | R. Cingulate Gyrus | 31 | 2 | −52 | 24 | 3.80 |
| 56 | R. Sup. Frontal Gyrus | 9 | 20 | 44 | 36 | 3.79 |
| 57 | L. Medial Frontal Gyrus | 10 | −6 | 60 | 12 | 3.66 |
| L. Medial Frontal Gyrus | −16 | 58 | 0 | 3.01 | ||
| 102 | L. Precuneus | 7 | −8 | −82 | 52 | 3.29 |
| R. Precuneus | 7 | 2 | −78 | 46 | 3.11 | |
| 36 | L. Superior Frontal Gyrus | 8 | −16 | 34 | 44 | 3.42 |
| 37 | L. Precuneus | 7 | 0 | −58 | 40 | 3.36 |
| 18 | R. Sup. Frontal Gyrus | 10 | 22 | 48 | −8 | 3.32 |
| 22 | L. Angular Gyrus | 39 | −46 | −72 | 38 | 3.23 |
| 17 | L. Anterior Cingulate | 32 | −10 | 38 | 10 | 3.19 |
| 58 | R. Medial Frontal Gyrus | 10 | 18 | 56 | 6 | 3.19 |
| R. Medial Frontal Gyrus | 10 | 16 | 62 | 0 | 3.11 | |
| 15 | R. Medial Frontal Gyrus | 10 | 6 | 50 | −10 | 3.14 |
| 13 | R. Declive | 20 | −62 | −22 | 3.02 | |
| 12 | L. Sup. Temporal Gyrus | 38 | −36 | 0 | −10 | 2.93 |
Table 6.
Decreased Brain Activation with Traumatic Scripts in Women with PTSD
Brain areas with significant (p < 0.005) deactivations during exposure to personalized trauma scripts in participants with posttraumatic stress disorder as measured with high-resolution positron emission tomography. Significant clusters are presented by size (number of voxels) and location (Brodmann area, cluster peak Talairach coordinates). Sub-cluster peaks are also identified.
| Voxel Number |
Brain Region | Brodmann Area |
X | Y | Z | Z Score |
|---|---|---|---|---|---|---|
| 11,124 | R. Fusiform Gyrus | 20 | 56 | −38 | −24 | 5.48 |
| L. Culmen | −38 | −54 | −24 | 5.40 | ||
| R. Tuber | 56 | −50 | −24 | 5.17 | ||
| 1686 | L. Middle Frontal Gyrus | 8 | −36 | 38 | 40 | 5.46 |
| L. Superior Frontal Gyrus | 9 | −32 | 50 | 30 | 5.09 | |
| L. Middle Frontal Gyrus | 10 | −40 | 54 | 8 | 4.92 | |
| 1344 | R. Inferior Parietal Lobule | 40 | 62 | −36 | 44 | 5.13 |
| R. Inferior Parietal Lobule | 40 | 46 | −38 | 44 | 4.64 | |
| R. Inferior Parietal Lobule | 40 | 64 | −40 | 36 | 4.04 | |
| 721 | R. Middle Frontal Gyrus | 46 | 48 | 32 | 16 | 5.04 |
| R. Middle Frontal Gyrus | 10 | 36 | 62 | −12 | 4.78 | |
| R. Middle Frontal Gyrus | 10 | 42 | 56 | −10 | 4.56 | |
| 363 | L. Cerebellar Tonsil | −2 | −54 | −34 | 5.01 | |
| L. Culmen | −2 | −46 | −20 | 3.47 | ||
| R. Cerebellar Tonsil | 16 | −50 | −38 | 3.25 | ||
| 299 | R. Superior Frontal Gyrus | 8 | 30 | 28 | 56 | 4.76 |
| R. Superior Frontal Gyrus | 8 | 30 | 44 | 44 | 4.01 | |
| R. Middle Frontal Gyrus | 6 | 30 | 8 | 64 | 3.78 | |
| 55 | L. Middle Temporal Gyrus | 21 | −44 | −34 | −4 | 4.67 |
| 519 | L. Insula | 13 | −32 | 22 | 0 | 4.60 |
| L. Lentiform Nucleus | −22 | 16 | −8 | 4.00 | ||
| L. Caudate | −14 | 18 | 6 | 3.71 | ||
| 651 | L. Precuneus | 19 | −28 | −66 | 42 | 4.43 |
| L. Inferior Parietal Lobule | 40 | −50 | −42 | 32 | 4.32 | |
| L. Supramarginal Gyrus | 40 | −60 | −48 | 32 | 3.94 | |
| 182 | R. Inferior Frontal Gyrus | 9 | 48 | 10 | 26 | 4.29 |
| R. Inferior Frontal Gyrus | 45 | 56 | 12 | 22 | 3.24 | |
| 379 | L. Middle Occipital Gyrus | 18 | −18 | −86 | 12 | 4.28 |
| L. Middle Occipital Gyrus | 19 | −32 | −88 | 16 | 3.97 | |
| L. Middle Occipital Gyrus | 19 | −32 | −84 | 8 | 3.89 | |
| 422 | R. Inferior Frontal Gyrus | 47 | 34 | 24 | −8 | 4.28 |
| R. Inferior Frontal Gyrus | 47 | 44 | 18 | 0 | 4.12 | |
| R. Inferior Frontal Gyrus | 47 | 34 | 26 | 0 | 4.00 | |
| 79 | L. Uvula | −10 | −74 | −32 | 4.28 | |
| 75 | R. Posterior Cingulate | 30 | 18 | −56 | 12 | 4.13 |
| 305 | R. Superior Frontal Gyrus | 8 | 4 | 16 | 48 | 3.89 |
| R. Superior Frontal Gyrus | 6 | 6 | 8 | 50 | 3.64 | |
| L. Medial Frontal Gyrus | 8 | 0 | 22 | 44 | 3.38 | |
| 61 | L. Thalamus | −4 | −2 | 4 | 3.89 | |
| 91 | L. Precentral Gyrus | 6 | −42 | −6 | 62 | 3.82 |
| L. Postcentral Gyrus | 3 | −44 | −20 | 64 | 3.26 | |
| L. Precentral Gyrus | 4 | −48 | −10 | 58 | 2.95 | |
| 33 | L. Uncus | 20 | −22 | −2 | −42 | 3.82 |
| L. Uncus | 36 | −18 | −8 | −36 | 2.98 | |
| 23 | L. Inferior Temporal Gyrus | 20 | −38 | −2 | −40 | 3.78 |
| 55 | L. Rectal Gyrus | 11 | −10 | 34 | −24 | 3.74 |
| 18 | R. Inferior Temporal Gyrus | 20 | 38 | −6 | −46 | 3.72 |
| 35 | R. Parahippocampal Gyrus | 30 | 24 | −36 | 4 | 3.71 |
| 19 | R. Caudate | 18 | 8 | 16 | 3.61 | |
| 33 | R. Superior Parietal Lobule | 7 | 38 | −66 | 52 | 3.56 |
| 22 | R. Lentiform Nucleus | 20 | −8 | −2 | 3.46 | |
| 56 | R. Uvula | 16 | −78 | −24 | 3.44 | |
| 48 | L. Middle Temporal Gyrus | 21 | −50 | −12 | −14 | 3.42 |
| 18 | R. Superior Temporal Gyrus | 38 | 34 | 14 | −44 | 3.42 |
| 48 | R. Orbital Gyrus | 47 | 18 | 18 | −26 | 3.42 |
| 10 | L. Insula | 13 | −32 | −26 | 14 | 3.32 |
| 29 | R. Insula | 13 | 44 | −20 | 12 | 3.31 |
| 31 | L. Inferior Parietal Lobule | 40 | −52 | −28 | 24 | 3.31 |
| 14 | R. Precentral Gyrus | 6 | 52 | −2 | 56 | 3.31 |
| 74 | R. Superior Temporal Gyrus | 22 | 52 | −4 | 6 | 3.31 |
| R. Superior Temporal Gyrus | 22 | 46 | −10 | −2 | 3.26 | |
| 10 | R. Precentral Gyrus | 6 | 36 | −10 | 70 | 3.29 |
| 35 | R. Middle Temporal Gyrus | 21 | 62 | −6 | −12 | 3.28 |
| 20 | R. Superior Temporal Gyrus | 22 | 60 | 6 | −4 | 3.27 |
| 25 | L. Inferior Parietal Lobule | 40 | −62 | −36 | 22 | 3.25 |
| L. Superior Temporal Gyrus | 42 | −62 | −30 | 12 | 2.91 | |
| 28 | R. Precuneus | 31 | 28 | −76 | 22 | 3.03 |
| 14 | R. Thalamus | 8 | −14 | 8 | 2.95 | |
| 12 | R. Superior Frontal | 11 | 26 | 50 | −18 | 2.94 |
| 28 | L. Cingulate Gyrus | 24 | −6 | −14 | 42 | 2.93 |
| 23 | L. Thalamus | −2 | −16 | 2 | 2.9 | |
| L. Thalamus | −10 | −18 | 8 | 2.88 | ||
| 16 | L. Middle Occipital Gyrus | 19 | −44 | −76 | 16 | 2.93 |
| L. Middle Occipital Gyrus | 19 | −42 | −76 | 8 | 2.78 | |
| 11 | R. Middle Frontal Gyrus | 11 | 32 | 36 | −20 | 2.93 |
| 28 | L. Cingulate Gyrus | 32 | −6 | 26 | 32 | 2.92 |
| 10 | R. Middle Frontal Gyrus | 10 | 26 | 62 | 22 | 2.92 |
Fig. 2.
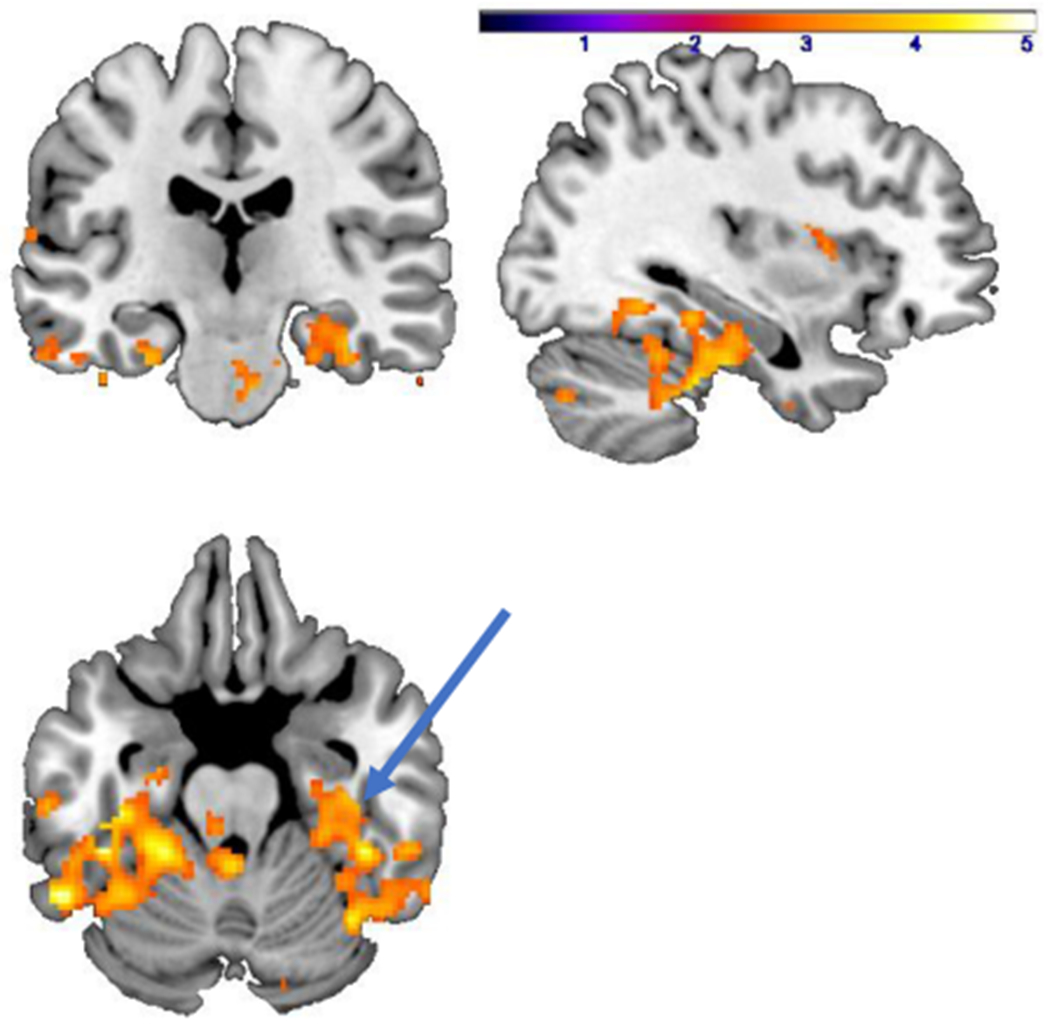
Areas representing decreased (p < 0.005) activation (yellow/orange) with traumatic scripts in PTSD patients at baseline (pre-treatment) compared to traumatized non-PTSD women showing decreased activation in right hippocampus (arrow). Color bar corresponds to z score.
Table 7.
Greater Increases in Blood Flow with Traumatic Scripts in Women with PTSD versus Traumatized Non-PTSD Women
Brain areas with significant (p < 0.005) activations during exposure to personalized trauma scripts in participants with posttraumatic stress disorder as measured with high-resolution positron emission tomography. Significant clusters are presented by size (number of voxels) and location (Brodmann area, cluster peak Talairach coordinates). Sub-cluster peaks are also identified.
| Voxel Number |
Brain Region | Brodmann Area |
X | Y | Z | Z Score |
|---|---|---|---|---|---|---|
| 35 | R. Lentiform Nucleus | 20 | −6 | 16 | 3.99 | |
| R. Lentiform Nucleus | 26 | −12 | 14 | 3.33 | ||
| 55 | R. Anterior Cingulate | 32 | 12 | 48 | −8 | 3.69 |
| 25 | L. Precentral Gyrus | 4 | −48 | −6 | 42 | 3.64 |
| 41 | L. Anterior Cingulate | 32 | −2 | 44 | 12 | 3.47 |
| 18 | L. Supramarginal Gyrus | 40 | −62 | −52 | 24 | 3.41 |
| 25 | R. Middle Frontal Gyrus | 8 | 50 | 14 | 40 | 3.41 |
| 13 | L. Medial Frontal Gyrus | 25 | −2 | 8 | −16 | 3.39 |
| 30 | L. Anterior Cingulate | 24 | −2 | 26 | 18 | 3.29 |
| L. Cingulate Gyrus | 32 | −4 | 30 | 26 | 2.87 | |
| 12 | R. Superior Frontal Gyrus | 11 | 24 | 58 | −20 | 3.29 |
| 21 | R. Supramarginal Gyrus | 40 | 58 | −46 | 34 | 3.28 |
| 10 | R. Precuneus | 19 | 46 | −78 | 40 | 3.19 |
| 12 | L. Caudate | −12 | −4 | 14 | 3.14 | |
| 17 | R. Subcallosal Gyrus | 25 | 12 | 24 | −10 | 3.09 |
| 20 | L. Medial Frontal Gyrus | 10 | −12 | 64 | −6 | 2.96 |
| 17 | L. Superior Frontal Gyrus | 11 | −30 | 50 | −16 | 2.95 |
| 15 | L. Middle Frontal Gyrus | 8 | −24 | 28 | 42 | 2.94 |
Table 8.
Greater Decreases in Blood Flow with Traumatic Scripts in Women with PTSD Versus Traumatized Non-PTSD Women
Brain areas with significant (p < 0.005) deactivations during exposure to personalized trauma scripts in participants with posttraumatic stress disorder as measured with high-resolution positron emission tomography. Significant clusters are presented by size (number of voxels) and location (Brodmann area, cluster peak Talairach coordinates). Sub-cluster peaks are also identified.
| Voxel Number |
Brain Region | Brodmann Area |
X | Y | Z | Z Score |
|---|---|---|---|---|---|---|
| 111 | R. Inferior Semi-Lunar Lobule | 34 | −76 | −38 | 4.75 | |
| R. Pyramis | 40 | −68 | −34 | 3.56 | ||
| 53 | L. Tuber | −54 | −52 | −22 | 3.98 | |
| L. Fusiform Gyrus | 20 | −52 | −42 | −22 | 3.04 | |
| 74 | R. Fusiform Gyrus | 36 | 44 | −38 | −20 | 3.82 |
| R. Fusiform Gyrus | 20 | 56 | −38 | −26 | 3.68 | |
| 76 | L. Declive | −20 | −68 | −18 | 3.69 | |
| 18 | L. Insula | 13 | −32 | 20 | 12 | 3.63 |
| 32 | L. Insula | 22 | −44 | −28 | −4 | 3.59 |
| 61 | L. Culmen | −2 | −38 | −22 | 3.56 | |
| 16 | R. Inferior Frontal Gyrus | 11 | 22 | 24 | −20 | 3.47 |
| 80 | R. Parahippocampal Gyrus | 30 | −22 | −16 | 3.47 | |
| R. Parahippocampal Gyrus | 36 | 30 | −30 | −14 | 3.04 | |
| R. Culmen | 32 | −32 | −22 | 2.84 | ||
| 31 | L. Claustrum | −32 | 2 | 10 | 3.41 | |
| 54 | R. Inferior Temporal Gyrus | 20 | 66 | −12 | −26 | 3.39 |
| 17 | R. Culmen | 16 | −24 | −26 | 3.37 | |
| 22 | R. Declive | 38 | −60 | −22 | 3.37 | |
| 43 | R. Cerebellar Tonsil | 36 | −38 | −34 | 3.33 | |
| 21 | L. Middle Temporal Gyrus | 21 | −54 | −12 | −12 | 3.29 |
| 14 | R. Inferior Parietal Lobule | 40 | 46 | −34 | 40 | 3.29 |
| 56 | L. Superior Temporal Gyrus | 38 | −38 | 14 | −36 | 3.28 |
| L. Superior Temporal Gyrus | 38 | −42 | 16 | −28 | 2.89 | |
| 13 | R. Middle Temporal Gyrus | 21 | 60 | 6 | −18 | 3.26 |
| 24 | L. Superior Temporal Gyrus | 22 | −48 | −4 | 4 | 3.25 |
| 48 | R. Posterior Cingulate | 30 | 22 | −62 | 6 | 3.22 |
| 14 | R. Middle Temporal Gyrus | 22 | 52 | −36 | 6 | 3.17 |
| 12 | R. Cuneus | 19 | 10 | −90 | 24 | 3.16 |
| 14 | R. Claustrum | 30 | 12 | 6 | 3.15 | |
| 15 | R. Precuneus | 31 | 6 | −70 | 20 | 3.14 |
| R. Cuneus | 18 | 10 | −74 | 26 | 2.67 | |
| 19 | R. Inferior Semi-Lunar Lobule | 2 | −60 | −40 | 3.09 | |
| L. Cerebellar Tonsil | −4 | −54 | −36 | 2.69 | ||
| 37 | R. Lingual Gyrus | 18 | 22 | −76 | −8 | 3.06 |
| 15 | L. Cuneus | 17 | −6 | −92 | 6 | 3.01 |
| 11 | L. Precentral Gyrus | 6 | −58 | −6 | 36 | 2.99 |
| 10 | L. Culmen | −36 | −46 | −20 | 2.89 | |
| 10 | L. Fusiform Gyrus | 37 | −44 | −54 | −14 | 2.89 |
| 10 | L. Parahippocampal Gyrus | 36 | −22 | −40 | −10 | 2.89 |
| 20 | R. Inferior Parietal Lobule | 40 | 62 | −30 | 36 | 2.88 |
| R. Inferior Parietal Lobule | 40 | 58 | −26 | 30 | 2.79 | |
| 14 | R. Lentiform Nucleus | 16 | −10 | 2 | 2.86 | |
| 15 | L. Thalamus | −16 | −28 | 4 | 2.85 | |
| 12 | R. Middle Temporal Gyrus | 20 | 42 | 4 | −44 | 2.83 |
3.4. Effects of treatment on PTSD symptoms, reactivity and brain function
Three months of treatment of women with PTSD resulted in a significant reduction in symptoms as measured by the CAPS for the paroxetine group (31 (10 SD) baseline versus 20 (10) post-treatment (p<0.05)) but not the placebo group (30 baseline (5 SD) versus 25 posttreatment (6 SD)). After three months of treatment there were not significant increases in distress with traumatic scripts in either the PTSD paroxetine group (16 (27 SD) to 51 (39 SD), p = 0.054) or the PTSD placebo group (14 (23 SD) to 32 (38 SD), p = 0.2). There were no significant differences in the magnitude of increase in distress with traumatic scripts between the PTSD paroxetine and PTSD placebo groups. With treatment of PTSD patients there were greater increases in the anterior cingulate (BA 24, 32) with exposure to traumatic scripts in the paroxetine compared to the placebo group (Fig. 3). Increases were also seen in the precuneus, middle and superior frontal gyri (BA 46, 10) and temporal cortex (BA 22, 39).
Fig. 3.
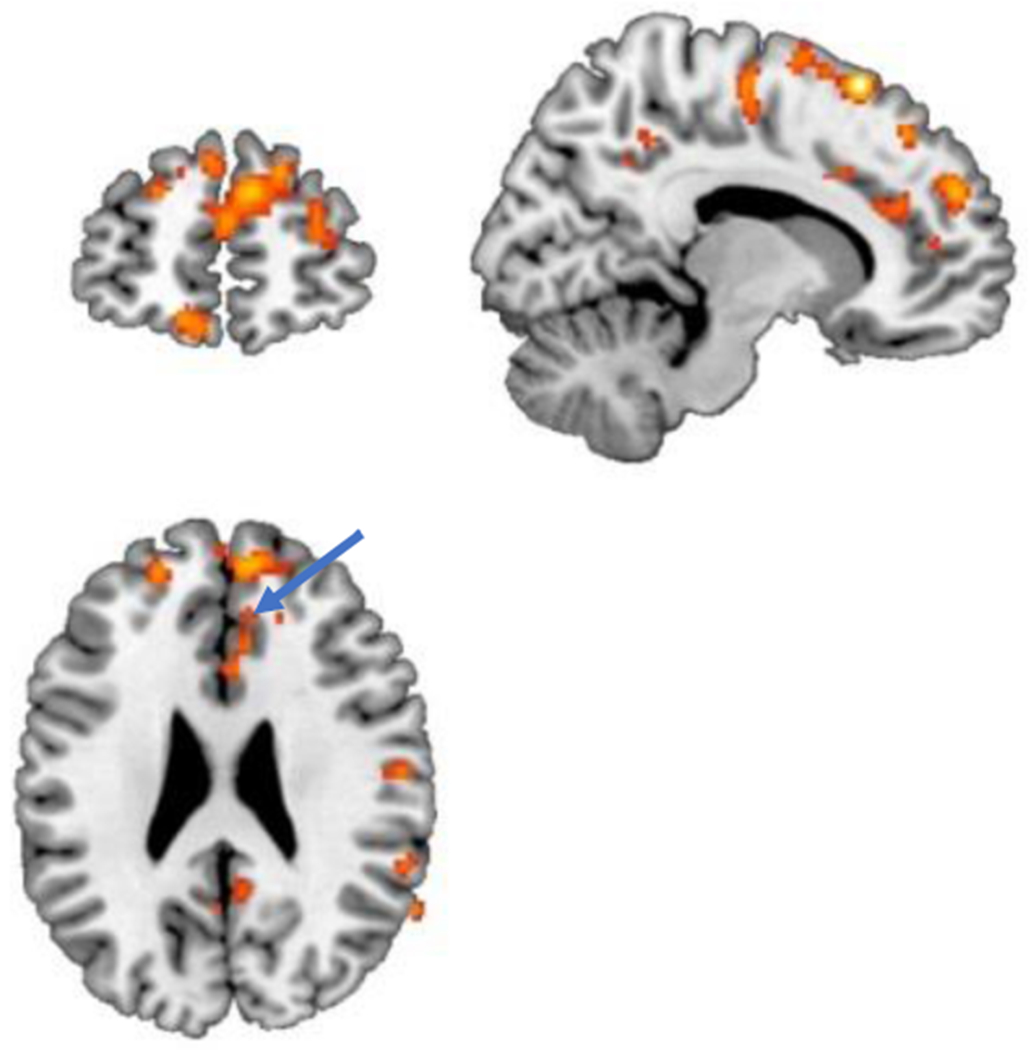
Areas representing greater (p < 0.005) activation (yellow/orange) with traumatic scripts in PTSD patients treated with paroxetine compared to PTSD patients treated with placebo after three months of treatment. There is increased activation in the anterior cingulate (BA 24, 32) (arrow). Increases were also seen in the precuneus, temporal cortex (BA 22, 39), and middle and superior frontal gyri (BA 46, 10).
4. Discussion
Women with childhood abuse-related PTSD in this study exposed to personalized traumatic scripts showed decreased function in parahippocampal gyrus, precuneus, cuneus, fusiform gyrus, insula, thalamus, posterior cingulate, dorsolateral prefrontal, parietal and temporal cortex compared to abused non-PTSD women. Treatment with paroxetine compared to placebo resulted in an improvement in symptoms of PTSD and increased function in anterior cingulate/medial prefrontal cortex, precuneus, dorsolateral prefrontal and temporal cortex.
The findings in this study are consistent with prior neuroimaging studies implicating several networks of interconnected brain areas with altered functional interconnectedness that respond to treatment in PTSD (Etkin et al., 2019). These include studies showing decreased connectivity in PTSD in the default mode network (DMN), a network of brain regions that are active when the brain is not engaged, including ventromedial prefrontal cortex (vmPFC), dorsomedial PFC (dmPFC), posterior cingulate cortex (PCC), precuneus, medial temporal lobe, and medial and lateral parietal cortices,(Akiki et al., 2017; Jin et al., 2017; Koch et al., 2016; Lei et al., 2015; Maron-Katz et al., 2020; Miller et al., 2017a, 2017b; Nicholson et al., 2020; Reuveni et al., 2016; Russman Block et al., 2017; Sripada et al., 2012b; Zhang et al., 2015; Zhu et al., 2019) in addition to related areas involved in visual processing including cuneus and fusiform gyrus as well as the hippocampus. Prior studies in PTSD have shown decreased function in precuneus (Geuze et al., 2008a; Lanius et al., 2002; Werner et al., 2009) and fusiform gyrus (Cwik et al., 2017; Osuch et al., 2001), and decreased connectivity in precuneus (Bluhm et al., 2009; Ke et al., 2017; Lanius et al., 2010; Liu et al., 2017; Yan et al., 2013; Yin et al., 2011), cuneus(Gong et al., 2014) and posterior cingulate (Bluhm et al., 2009; Ke et al., 2017; Miller et al., 2017a, 2017b; Qin et al., 2012) Studies also showed decreased connectivity between precuneus and posterior cingulate (Bluhm et al., 2009) as well as decreased connectivity between precuneus and other DMN brain regions. (Bluhm et al., 2009; Ke et al., 2017; Lanius et al., 2010; Liu et al., 2017; Yan et al., 2013; Yin et al., 2011) In addition to visual processing, which plays a critical role in assessment of threat, the precuneus plays a role in sense self, which may be relevant to disturbances in autobiographical memory in PTSD,(Germain et al., 2013; Geuze et al., 2008a; Klein and Ehlers, 2008; McNally et al., 1994; St Jacques et al., 2011, 2013; Thome et al., 2020) as well as lability of mood and other clinical aspects of PTSD. Fusiform gyrus is involved in memory for faces, which plays an obvious role in perception of threat. (Gur et al., 2002; Haxby et al., 1996)
PTSD was associated with decreases in other brain areas including dorsolateral prefrontal, parietal, and temporal cortex that were also responsive to treatment. Previous studies have shown decreases in parietal cortex function (Bremner et al., 1999a; Chung et al., 2006; Ke et al., 2016; Morey et al., 2008; Rauch et al., 1996; Sakamoto et al., 2005; Shin et al., 1997, 1999) and connectivity (Disner et al., 2018; Fu et al., 2019; Gong et al., 2014; Shaw et al., 2002; Weng et al., 2019; Zhang et al., 2017, 2016c; Zhu et al., 2015) in PTSD. The parietal cortex plays an important role in conceptualization of the self in space and time as well interpretation of visual information, both key to the stress response (Cornwell et al., 2007; Jonides et al., 1993; Luo et al., 2007; Selemon and Goldman-Rakic, 1988; van Rooij et al., 2015; Weber et al., 2005) Decreased function seen in PTSD in the temporal cortex (Chung et al., 2006; Geuze et al., 2008b; Werner et al., 2009), as well as connectivity,(Shang et al., 2014) may be relevant to alterations in memory in PTSD. Problems with memory and concentration may also be driven by decreased function in the dorsolateral prefrontal cortex (middle and superior frontal gyrus (BA 46, 10). (Aupperle et al., 2012; Cisler et al., 2015b; Cohen et al., 2013; Cwik et al., 2017; Hou et al., 2007; Morey et al., 2009; Sakamoto et al., 2005; Scheibel et al., 2015; Shin et al., 1997, 1999, 2004; Shin et al., 2001; Werner et al., 2009) or connectivity between this area and other brain regions in DMN. (Olivé et al., 2018; Olson et al., 2019; Reuveni et al., 2016) The insula is connected to these regions in salience networks and altered connectivity has been seen in this region as well. (Birn et al., 2014; Etkin et al., 2019; Harricharan et al., 2020; Ke et al., 2018; Koch et al., 2016; Liu et al., 2017; Nicholson et al., 2020; Sripada et al., 2012a; Tursich et al., 2015; Vanasse et al., 2019; Yin et al., 2011; Zhang et al., 2016b) The current study showed a decrease in function in several DMN and visual association regions that similar to prior studies of acoustic stimulation (Lee et al., 2019) and paroxetine (Fani et al., 2011; MacNamara et al., 2016) as treatments for PTSD reversed with treatment.
The current findings of decreased function in the hippocampus and parahippocampal gyrus are similar to other studies in PTSD. These studies showed decreased function (Bremner et al., 1999a; Cisler et al., 2015a, 2015b; Kim et al., 2012; Osuch et al., 2008; Yehuda et al., 2010, 2009), and connectivity in the hippocampus (Abdallah et al., 2017; Admon et al., 2013; Bluhm et al., 2009; Chen and Etkin, 2013; Chen et al., 2019; Jin et al., 2014; Lazarov et al., 2017; Malivoire et al., 2018; Metz et al., 2019; Miller et al., 2017a; Rabellino et al., 2018a, 2018b; Stevens et al., 2014; Zhang et al., 2017), as well as decreased function (Werner et al., 2009) and connectivity (Jin et al., 2014; Misaki et al., 2018) in the parahippocampal gyrus in PTSD. The hippocampus mediates consolidation of short term memory or explicit memory into long term memory. (Zola-Morgan and Squire, 1990) and is sensitive to stress (Sapolsky, 2003). The parahippocampal gyrus also plays an important role in memory. (Zola-Morgan et al., 1989) In addition to processing explicit memory the hippocampus, like the medial prefrontal cortex, plays a role in fear extinction and is involved in response to context-specific traumatic reminders (Ritov et al., 2014). In PTSD, a failure of hippocampal activation is commonly observed during trauma reminders (Bremner et al., 1999a), potentially as a result of alterations in this brain area (Karl et al., 2006) with associated declarative memory deficits (Samuelson, 2011). Additionally, PTSD disrupts a memory network consisting of the hippocampus, anterior cingulate, and orbitofrontal cortex, that is employed in the recall of emotionally valenced words (Bremner et al., 2003). Alterations in verbal declarative memory of the type mediated by the hippocampus and parahippocampus are associated with PTSD.(Bremner and Vermetten, 2012) Prior studies showed an improvement in verbal declarative memory(Fani et al., 2009; Vermetten et al., 2003) and increase in hippocampal volume. (Vermetten et al., 2003) following treatment with paroxetine in PTSD
Replicating prior studies, treatment with paroxetine resulted in an increase in anterior cingulate/medial prefrontal (BA 24, 32) function in women with PTSD. Multiple studies have shown a decrease in anterior cingulate/medial prefrontal function (Bluhm et al., 2012; Bremner et al., 1999a, 1999b, 2004; Britton et al., 2005; Clausen et al., 2017b; Dahlgren et al., 2018; Elzinga and Bremner, 2002; Fonzo et al., 2010a; Frewen et al., 2012; Gold et al., 2011; Grupe et al., 2020; Hopper et al., 2007; Hou et al., 2007; King et al., 2009; Lanius et al., 2001, 2004, 2003; Liberzon et al., 2003, 1999; Lindauer et al., 2004; New et al., 2009; Offringa et al., 2013; Phan et al., 2006; Pissiota et al., 2002; Shin et al., 1997, 1999, 2004; Shin et al., 2001, 2005; St Jacques et al., 2011, 2013; Yang et al., 2004) and connectivity (Akiki et al., 2018; Amad et al., 2019; Birn et al., 2014; Clausen et al., 2017b; Disner et al., 2018; Gong et al., 2014; Jin et al., 2014; Kennis et al., 2015; Liu et al., 2016; Misaki et al., 2018; Sadeh et al., 2015) in PTSD. Treatment with paroxetine (Fani et al., 2011; Zhu et al., 2015) transcutaneous vagal nerve stimulation (Wittbrodt et al., 2021) or hydrocortisone (Metz et al., 2019) in PTSD patients resulted in an increase in function in this region. Decreased function in this area may underlie deficient emotional regulation associated with traumatic remembrance (Etkin and Wager, 2007) due to a failure of inhibition of fear memories in the amygdala (Etkin et al., 2006). Increased anterior cingulate/medial prefrontal activity would facilitate inhibition of the amygdala leading to improved emotional regulation. This area is also part of the dorsal cognitive division, which is part of a distributed attentional network with reciprocal connections with the lateral prefrontal cortex, parietal cortex, and motor areas (Bush et al., 2000; Devinsky et al., 1995) that acts as a storage buffer of information before executing action (Heilbronner and Hayden, 2016) which confers advantage in fear extinction and/or emotional reappraisal (Clausen et al., 2017a; Etkin et al., 2011). These results suggest that paroxetine may have effects on brain circuits that span multiple aspects of emotional regulation and cognitive function.
The study has several limitations. The sample size is small, especially for the treatment portion, with only seven paroxetine completers and eight placebo completers. This fact limits generalizability of the findings. Therefore, the results should be considered as preliminary. The sample was limited to women with childhood sexual abuse-related PTSD and may not be generalizable to other PTSD populations. The scripts involved comparison of trauma scripts to neutral scripts, which does not separate trauma specific effects from general heightened emotional arousal, therefore it is not possible to determine if the brain effects are specific to trauma or just emotional experience in PTSD patients.
In summary this study showed that PTSD related to childhood sexual abuse in women was associated with a decrease in activation in medial prefrontal, Default Mode Network (DMN) and visual processing areas, and that treatment with paroxetine resulted in an improvement in PTSD symptoms as well as increased function in DMN and visual processing areas in addition to medial prefrontal cortex/anterior cingulate. Future studies are indicated to replicate and extend these findings. Modification of brain areas mediating emotion, memory, and the stress response may have implications for understanding successful treatment responses in PTSD.
Acknowledgments
This work was supported by research grant funding from NIH MH056120, MH076955, HL136205, HL109413, MH120262, DA048502, HL101398, HL077506, HL088726, HL125246, HL068630, AG026255, HL130025, MH067547, RR16917 and VA I01 RX003418. Paroxetine was provided free of charge by GlaxoSmithKline. We acknowledge and thank Margie Jones, C.N.M.T. and Steve Rhodes, R.N., for their assistance with imaging analysis, patient assessments, and clinical research.
Footnotes
Declaration of Competing Interest
No authors report potential conflicts of interests.
References
- Abdallah CG, Wrocklage KM, Averill CL, Akiki T, Schweinsburg B, Roy A, Martini B, Southwick SM, Krystal JH, Scott JC, 2017. Anterior hippocampal dysconnectivity in posttraumatic stress disorder: a dimensional and multimodal approach. Transl. Psychiatry 7, e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Leykin D, Lubin G, Engert V, Andrews J, Pruessner J, Hendler T, 2013. Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum. Brain Mapp 34, 2808–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, Hendler T, 2009. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proc. Natl. Acad. Sci. U. S. A 106, 14120–14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki TJ, Averill CL, Abdallah CG, 2017. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr. Psychiatry Rep 19, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki TJ, Averill CL, Wrocklage KM, Scott JC, Averill LA, Schweinsburg B, Alexander-Bloch A, Martini B, Southwick SM, Krystal JH, Abdallah CG, 2018. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Neuroimage 176, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amad A, Radua J, Vaiva G, Williams SC, Fovet T, 2019. Similarities between borderline personality disorder and post traumatic stress disorder: evidence from resting-state meta-analysis. Neurosci. Biobehav. Rev 105, 52–59. [DOI] [PubMed] [Google Scholar]
- APA, 2000. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press, Washington, D.C. [Google Scholar]
- Armony JL, Corbo V, Clement MH, Brunet A, 2005. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am. J. Psychiatry 162, 1961–1963. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Grimes EM, Simmons AN, Flagan T, Behrooznia M, Cissell SH, Twamley EW, Thorp SR, Norman SB, Paulus MP, Stein MB, 2012. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch. Gen. Psychiatry 69, 360–371. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ, Foa EB, Kessler RC, McFarlane AC, Shalev AY, 2000. Consensus statement on posttraumatic stress disorder from the International Consensus Group on Depression and Anxiety. J. Clin. Psychiatr 61, 60–66. [PubMed] [Google Scholar]
- Barkay G, Freedman N, Lester H, Louzoun Y, Sapoznikov D, Luckenbaugh D, Shalev AY, Chisin RG, Bonne O, 2012. Brain activation and heart rate during script-driven traumatic imagery in PTSD: preliminary findings. Psychiatry Res 204, 155–160. [DOI] [PubMed] [Google Scholar]
- Barredo J, Aiken E, van ’t Wout-Frank M, Greenberg BD, Carpenter LL, Philip NS, 2018. Network functional architecture and aberrant functional connectivity in post-traumatic stress disorder: a clinical application of network convergence. Brain Connect 8, 549–557. [DOI] [PubMed] [Google Scholar]
- Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ, 2014. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress. Anxiety 31, 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, 1995. The development of a clinician-administered PTSD scale. J. Trauma. Stress 8, 75–90. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Frewen PA, Coupland NC, Densmore M, Schore AN, Lanius RA, 2012. Neural correlates of self-reflection in post-traumatic stress disorder. Acta Psychiatr. Scand 125, 238–246. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, Neufeld RW, Theberge J, Lanius RA, 2009. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J. Psychiatr. Neurosci 34, 187–194. [PMC free article] [PubMed] [Google Scholar]
- Boccia M, D’Amico S, Bianchini F, Marano A, Giannini AM, Piccardi L, 2016. Different neural modifications underpin PTSD after different traumatic events: an fMRI meta-analytic study. Brain Imaging Behav 10, 226–237. [DOI] [PubMed] [Google Scholar]
- Brady KT, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM, 2000. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. J. Am. Med. Assoc 283, 1837–1844. [DOI] [PubMed] [Google Scholar]
- Bremner J, Bolus R, Mayer E, 2007. Psychometric properties of the Early Trauma Inventory-Self Report. J. Nerv. Ment. Dis 195, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Campanella C, 2016. Effects of psychotherapy for psychological trauma on PTSD symptoms and the brain. In: Bremner JD (Ed.), Posttraumatic Stress Disorder: From Neurobiology to Treatment. Wiley-Blackwell, Hoboken, N.J., pp. 413–420 [Google Scholar]
- Bremner JD, Mishra S, C C, Shah M, Kasher N, Evans S, Fani N, Shah AJ, Reiff C, Davis LL, Vaccarino V, Carmody J, 2017. A pilot study of the effects of Mindfulness-Based Stress Reduction on post-traumatic stress disorder symptoms and brain response to traumatic reminders of combat in Operation Enduring Freedom/Operation Iraqi Freedom combat veterans with post-traumatic stress disorder. Front. Psychiatry 8, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS, 1999a. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am. J. Psychiatry 156, 1787–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib L, Kaloupek D, Southwick SM, Soufer R, Charney DS, 1999b. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol. Psychiatry 45, 806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, 2012. The hippocampus and post-traumatic stress disorders. In: Bartsch T (Ed.), The Clinical Neurobiology of the Hippocampus: An integrative View. Oxford University Press, pp. 262–272. [Google Scholar]
- Bremner JD, Vermetten E, Nafzal N, Vythilingam M, 2004. Deficits in verbal declarative memory function in women with childhood sexual abuse-related posttraumatic stress disorder (PTSD). J. Nerv. Ment. Dis 192, 643–649. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS, 2005. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual abuse-related posttraumatic stress disorder. Psychol. Med 35, 791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, Soufer R, Charney DS, 2003. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol. Psychiatry 53, 879–889. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Wittbrodt MT, 2020. Stress, the brain, and trauma spectrum disorders. Int. Rev. Neurobiol 152, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann L, Buff C, Neumeister P, Tupak SV, Becker MP, Herrmann MJ, Straube T, 2017. Dissociation between amygdala and bed nucleus of the stria terminalis during threat anticipation in female post-traumatic stress disorder patients. Hum. Brain Mapp 38, 2190–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I, 2005. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol. Psychiatry 57, 832–840. [DOI] [PubMed] [Google Scholar]
- Bruce SE, Buchholz KR, Brown WJ, Yan L, Durbin A, Sheline YL, 2013. Altered emotional interference processing in the amygdala and insula in women with post-traumatic stress disorder. Neuroimage Clin 2, 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI, 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4, 215–222. [DOI] [PubMed] [Google Scholar]
- Campanella C, Bremner JD, 2016. Neuroimaging of PTSD. In: Bremner JD (Ed.), Posttraumatic Stress Disorder: From Neurobiology to Treatment. Wiley-Blackwell, Hoboken, New Jersey, pp. 291–320. [Google Scholar]
- Chen AC, Etkin A, 2013. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology 38, 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Zhang L, Ke J, Qi R, Xu Q, Zhong Y, Pan M, Li J, Lu GM, Chen F, 2019. Altered resting-state dorsal anterior cingulate cortex functional connectivity in patients with post-traumatic stress disorder. Aust. N. Z. J. Psychiatry 53, 68–79. [DOI] [PubMed] [Google Scholar]
- Chung YA, Kim SH, Chung SK, Chae JH, Yang DW, Sohn HS, Jeong J, 2006. Alterations in cerebral perfusion in posttraumatic stress disorder patients without re-exposure to accident-related stimuli. Clin. Neurophysiol 117, 637–642. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Bush K, James GA, Smitherman S, Kilts CD, 2015a. Decoding the traumatic memory among women with PTSD: implications for neurocircuitry models of PTSD and real-time fMRI neurofeedback. PLoS ONE 10, e0134717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Bush K, Steele JS, Lenow JK, Smitherman S, Kilts CD, 2015b. Brain and behavioral evidence for altered social learning mechanisms among women with assault-related posttraumatic stress disorder. J. Psychiatr. Res 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen AN, Francisco AJ, Thelen J, Bruce J, Martin LE, McDowd J, Simmons WK, Aupperle RL, 2017a. PTSD and cognitive symptoms relate to inhibition-related prefrontal activation and functional connectivity. Depress. Anxiety 34, 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen AN, Francisco AJ, Thelen J, Bruce J, Martin LE, McDowd J, Simmons WK, Aupperle RL, 2017b. PTSD and cognitive symptoms relate to inhibition-related prefrontal activation and functional connectivity. Depress. Anxiety 34, 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JE, Shalev H, Admon R, Hefetz S, Gasho CJ, Shachar LJ, Shelef I, Hendler T, Friedman A, 2013. Emotional brain rhythms and their impairment in post-traumatic patients. Hum. Brain Mapp 34, 1344–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Baas JM, Johnson L, Holroyd T, Carver FW, Lissek S, Grillon C, 2007. Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. Neuroimage 37, 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese BM, Schumann AY, Howell AN, McConnell PA, Yang QX, Uhde TW, 2018. Preliminary evidence for differential olfactory and trigeminal processing in combat veterans with and without PTSD. Neuroimage Clin 17, 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwik JC, Sartory G, Nuyken M, Schürholt B, Seitz RJ, 2017. Posterior and prefrontal contributions to the development posttraumatic stress disorder symptom severity: an fMRI study of symptom provocation in acute stress disorder. Eur. Arch. Psychiatry Clin. Neurosci 267, 495–505. [DOI] [PubMed] [Google Scholar]
- Dahlgren MK, Laifer LM, VanElzakker M, Offringa R, Hughes KC, Staples-Bradley LK, Dubois SJ, Lasko NB, Hinojosa CA, Orr SP, Pitman RK, Shin LM, 2018. Diminished medial prefrontal cortex activation during the recollection of stressful events is an acquired characteristic of PTSD. Psychol. Med 48, 1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis L, Hamner M, Bremner JD, 2016. Pharmacotherapy for PTSD: effects on PTSD symptoms and the brain. In: Bremner JD (Ed.), Posttraumatic Stress Disorder: From Neurobiology to Treatment. Wiley Blackwell, Hoboken, N,J, pp. 389–412. [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA, 1995. Contributions of anterior cingulate cortex to behaviour. Brain 118, 279–306. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Brunet A, Akerib V, Armony JL, 2008. An fMRI investigation of memory encoding in PTSD: influence of symptom severity. Neuropsychologia 46, 1522–1531. [DOI] [PubMed] [Google Scholar]
- Disner SG, Marquardt CA, Mueller BA, Burton PC, Sponheim SR, 2018. Spontaneous neural activity differences in posttraumatic stress disorder: a quantitative resting-state meta-analysis and fMRI validation. Hum. Brain Mapp 39, 837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Thiruchselvam R, Todd R, Christoff K, 2017. Emotion and the prefrontal cortex: an integrative review. Psychol. Bull 143, 1033–1081. [DOI] [PubMed] [Google Scholar]
- Elman I, Upadhyay J, Langleben DD, Albanese M, Becerra L, Borsook D, 2018. Reward and aversion processing in patients with post-traumatic stress disorder: functional neuroimaging with visual and thermal stimuli. Transl. Psychiatry 8, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD, 2002. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J. Affect. Disord 70, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, A MK, W W, Fonzo GA, Huemer J, Vértes PE, Patenaude B, Richiardi J, Goodkind MS, Keller CJ, Ramos-Cejudo J, Zaiko YV, Peng KK, Shpigel E, Longwell P, Toll RT, Thompson A, Zack S, Gonzalez B, Edelstein R, Chen J, Akingbade I, Weiss E, Hart R, Mann S, Durkin K, Baete SH, Boada FE, Genfi A, Autea J, Newman J, Oathes DJ, Lindley SE, Abu-Amara D, Arnow BA, Crossley N, Hallmayer J, Fossati S, Rothbaum BO, Marmar CR, Bullmore ET, O’Hara R, 2019. Using fMRI connectivity to define a treatment-resistant form of post-traumatic stress disorder. Sci. Transl. Med 11, eaal3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J, 2006. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51, 871–882. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD, 2007. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 164, 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Ashraf A, Afzal N, Jawed F, Kitayama N, Reed L, Bremner JD, 2011. Increased neural response to trauma scripts in posttraumatic stress disorder following paroxetine treatment: a pilot study. Neurosci. Lett 491, 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Kitayama N, Ashraf A, Reed L, Afzal N, Jawed F, Bremner JD, 2009. Neuropsychological functioning in patients with posttraumatic stress disorder following short-term paroxetine treatment. Psychopharmacol. Bull 42, 53–68. [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A, Bryant R, 2007. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol. Sci 18, 127–129. [DOI] [PubMed] [Google Scholar]
- Felmingham KL, Williams LM, Kemp AH, Rennie C, Gordon E, Bryant RA, 2009. Anterior cingulate activity to salient stimuli is modulated by autonomic arousal in posttraumatic stress disorder. Psychiatry Res 173, 59–62. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Pissiota A, Frans O, von Knorring L, Fischer H, Fredrikson M, 2001. Brain function in a patient with torture related post-traumatic stress disorder before and after fluoxetine treatment: a positron emission tomography provocation study. Neurosci. Lett 297, 101–104. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, 2004. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In: Segal MJHDL (Ed.), Comprehensive Handbook of Psychological Assessment. John Wiley & Sons Inc., Hoboken, NJ, US, pp. 134–143. [Google Scholar]
- Fitzgerald JM, DiGangi JA, Phan KL, 2018. Functional neuroanatomy of emotion and its regulation in PTSD. Harv. Rev. Psychiatry 26, 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Goodkind MS, Oathes DJ, Zaiko YV, Harvey M, Peng KK, Weiss ME, Thompson AL, Zack SE, Mills-Finnerty CE, Rosenberg BM, Edelstein R, Wright RN, Kole CA, Lindley SE, Arnow BA, Jo B, Gross JJ, Rothbaum BO, Etkin A, 2017. Selective effects of psychotherapy on frontopolar cortical function in PTSD. Am. J. Psychiatry 174, 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB, 2010a. Blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol. Psychiatry 68, 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB, 2010b. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol. Psychiatry 68, 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francati V, Vermetten E, Bremner JD, 2007. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress. Anxiety 24, 202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJ, Neufeld RW, Lane RD, Densmore M, Stevens TK, Lanius RA, 2012. Emotional numbing in posttraumatic stress disorder: a functional magnetic resonance imaging study. J. Clin. Psychiatr 73, 431–436. [DOI] [PubMed] [Google Scholar]
- Fu S, Ma X, Li C, Wang T, Li C, Bai Z, Hua K, Yin Y, Wu Y, Yu K, Liu M, Ke Q, Tian J, Jiang G, 2019. Aberrant regional homogeneity in post-traumatic stress disorder after traffic accident: a resting-state functional MRI study. Neuroimage Clin 24, 101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, James J, Insana S, Herringa RJ, Mammen O, Price J, Nofzinger EA, 2013. A window into the invisible wound of war: functional neuroimaging of REM sleep in returning combat veterans with PTSD. Psychiatry Res 211, 176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, de Kloet CS, Westenberg HG, 2008a. Precuneal activity during encoding in veterans with posttraumatic stress disorder. Prog. Brain Res 167, 293–297. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Ruf M, de Kloet CS, Westenberg HG, 2008b. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. J. Psychiatr. Res 42, 659–669. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Gitelman D, 2008. Contrast weights in flexible factorial design with multiple groups of subjects. SPM@ JISCMAIL. AC. UK: 1–12. [Google Scholar]
- Gold AL, Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Metzger LJ, Dougherty DD, Alpert NM, Fischman AJ, Pitman RK, 2011. Decreased regional cerebral blood flow in medial prefrontal cortex during trauma-unrelated stressful imagery in Vietnam veterans with PTSD. Psychol. Med 41, 2563–2572. [DOI] [PubMed] [Google Scholar]
- Gong Q, Li L, Du M, Pettersson-Yeo W, Crossley N, Yang X, Li J, Huang X, Mechelli A, 2014. Quantitative prediction of individual psychopathology in trauma survivors using resting-state fMRI. Neuropsychopharmacology 39, 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grape DW, Imhoff-Smith T, Wielgosz J, Nitschke JB, Davidson RJ, 2020. A common neural substrate for elevated PTSD symptoms and reduced pulse rate variability in combat-exposed veterans. Psychophysiology 57, e13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, Alsop D, Maldjian J, Gur RE, 2002. Brain activation during facial emotion processing. Neuroimage 16, 651–662. [DOI] [PubMed] [Google Scholar]
- Harricharan S, Nicholson AA, Thome J, Densmore M, McKinnon MC, Théberge J, Frewen PA, Neufeld RWJ, Lanius RA, 2020. PTSD and its dissociative subtype through the lens of the insula: anterior and posterior insula resting-state functional connectivity and its predictive validity using machine learning. Psychophysiology 57, e13472. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL, 1996. Face encoding and recognition in the human brain. Proc. Natl. Acad. Sci. U. S. A 93, 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Hayden BY, 2016. Dorsal Anterior Cingulate Cortex: a Bottom-Up View. Annu. Rev. Neurosci 39, 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Phillips ML, Fournier JC, Kronhaus DM, Germain A, 2013. Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans. Psychol. Med 43, 1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper JW, Frewen PA, van der Kolk BA, Lanius RA, 2007. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J. Trauma. Stress 20, 713–725. [DOI] [PubMed] [Google Scholar]
- Hou C, Liu J, Wang K, Li L, Liang M, He Z, Liu Y, Zhang Y, Li W, Jiang T, 2007. Brain responses to symptom provocation and trauma-related short-term memory recall in coal mining accident survivors with acute severe PTSD. Brain Res 1144, 165–174. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, 2008. Treatment of Posttraumatic Stress Disorder: An Assessment of the Evidence. The National Academies Press, Washington, DC. [Google Scholar]
- Jeon S, Lee YJ, Park I, Kim N, Kim S, Jun JY, Yoo SY, Lee SH, Kim SJ, 2020. Resting state functional connectivity of the thalamus in North Korean refugees with and without posttraumatic stress disorder. Sci Rep 10, 3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Jia H, Lanka P, Rangaprakash D, Li L, Liu T, Hu X, Deshpande G, 2017. Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Hum. Brain Mapp 38, 4479–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Qi R, Yin Y, Hu X, Duan L, Xu Q, Zhang Z, Zhong Y, Feng B, Xiang H, Gong Q, Liu Y, Lu G, Li L, 2014. Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychol. Med 44. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA, 1993. Spatial working memory in humans as revealed by PET. Nature 363, 623–625. [DOI] [PubMed] [Google Scholar]
- Kaczkurkin AN, Burton PC, Chazin SM, Manbeck AB, Espensen-Sturges T, Cooper SE, Sponheim SR, Lissek S, 2017. Neural substrates of overgeneralized conditioned fear in PTSD. Am. J. Psychiatry 174, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A, 2006. A meta-analysis of structural brain abnormalities in PTSD. Neurosci. Biobehav. Rev 30, 1004–1031. [DOI] [PubMed] [Google Scholar]
- Ke J, Chen F, Qi R, Xu Q, Zhong Y, Chen L, Li J, Zhang L, Lu GM, 2017. Post-traumatic stress influences local and remote functional connectivity: a resting-state functional magnetic resonance imaging study. Brain Imaging Behav 11, 1316–1325. [DOI] [PubMed] [Google Scholar]
- Ke J, Zhang L, Qi R, Li W, Hou C, Zhong Y, He Z, Li L, Lu G, 2016. A longitudinal fMRI investigation in acute post-traumatic stress disorder (PTSD). Acta Radiol 57, 1387–1395. [DOI] [PubMed] [Google Scholar]
- Ke J, Zhang L, Qi R, Xu Q, Zhong Y, Liu T, Li J, Lu G, Chen F, 2018. Typhoon-related post-traumatic stress disorder and trauma might lead to functional integration abnormalities in intra- and inter-resting state networks: a resting-state fmri independent component analysis. Cell. Physiol. Biochem 48, 99–110. [DOI] [PubMed] [Google Scholar]
- Kennis M, Rademaker AR, van Rooij SJ, Kahn RS, Geuze E, 2015. Resting state functional connectivity of the anterior cingulate cortex in veterans with and without post-traumatic stress disorder. Hum. Brain Mapp 36, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Kim SY, Chung YK, Kim BS, Lee SJ, Yoon JK, An YS, 2012. Resting cerebral glucose metabolism and perfusion patterns in women with posttraumatic stress disorder related to sexual assault. Psychiatry Res 201, 214–217. [DOI] [PubMed] [Google Scholar]
- King AP, Abelson JL, Britton JC, Phan KL, Taylor SF, Liberzon I, 2009. Medial prefrontal cortex and right insula activity predict plasma ACTH response to trauma recall. Neuroimage 47, 872–880. [DOI] [PubMed] [Google Scholar]
- King AP, Block SR, Sripada RK, Rauch S, Giardino N, Favorite T, Angstadt M, Kessler D, Welsh R, Liberzon I, 2016. Altered Default Mode Network (DMN) resting state functional connectivity following a Mindfulness-based Exposure Therapy for posttraumatic stress disorder (PTSD) in combat veterans of Afghanistan and Iraq. Depress. Anxiety 33, 289–299. [DOI] [PubMed] [Google Scholar]
- Klein B, Ehlers A, 2008. Reduced autobiographical memory specificity predicts depression and posttraumatic stress disorder after recent trauma. J. Consult. Clin. Psychol 76, 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluetsch RC, Ros T, Théberge J, Frewen PA, Calhoun VD, Schmahl C, Jetly R, Lanius RA, 2014. Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatr. Scand 130, 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M, 2016. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress. Anxiety 33, 592–605. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern CE, Schwartz GE, Davidson RJ, 1997. Neuroanatomical correlates of happiness, sadness, and disgust. Am. J. Psychiatry 154, 926–933. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, Neufeld RW, Williamson PC, Brimson M, 2010. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr. Scand 121, 33–40. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Gati JS, Menon RS, 2002. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol. Psychiatry 52, 305–311. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS, 2001. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am. J. Psychiatry 158, 1920–1922. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Neufeld RW, Gati JS, Menon RS, 2004. The nature of traumatic memories: a 4-T FMRI functional connectivity analysis. Am. J. Psychiatry 161, 36–44. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Hopper J, Densmore M, Boksman K, Gupta MA, Neufeld RW, Gati JS, Menon RS, 2003. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol. Psychiatry 53, 204–210. [DOI] [PubMed] [Google Scholar]
- Lansing K, Amen DG, Hanks C, Rudy L, 2005. High-resolution brain SPECT imaging and eye movement desensitization and reprocessing in police officers with PTSD. J. Neuropsychiatry Clin. Neurosci 17, 526–532. [DOI] [PubMed] [Google Scholar]
- Lazarov A, Zhu X, Suarez-Jimenez B, Rutherford BR, Neria Y, 2017. Resting-state functional connectivity of anterior and posterior hippocampus in posttraumatic stress disorder. J. Psychiatry Res 94, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Laurienti PJ, Burdette JH, Tegeler CL, Morgan AR, Simpson SL, Gerdes L, Tegeler CH, 2019. Functional brain network changes following use of an allostatic, closed-loop, acoustic stimulation neurotechnology for military-related traumatic stress. J. Neuroimaging 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei D, Li K, Li L, Chen F, Huang X, Lui S, Li J, Bi F, Gong Q, 2015. Disrupted functional brain connectome in patients with posttraumatic stress disorder. Radiology 276, 818–827. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Britton JC, Phan KL, 2003. Neural correlates of traumatic recall in posttraumatic stress disorder. Stress 6, 151–156. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM, 1999. Brain activation in PTSD in response to trauma-related stimuli. Biol. Psychiatry 45, 817–826. [DOI] [PubMed] [Google Scholar]
- Lieberman L, Gorka SM, DiGangi JA, Frederick A, Phan KL, 2017. Impact of posttraumatic stress symptom dimensions on amygdala reactivity to emotional faces. Biol. Psychiatry 79, 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer RJ, Booij J, Habraken JB, Uylings HB, Olff M, Carlier IV, den Heeten GJ, van Eck-Smit BL, Gersons BP, 2004. Cerebral blood flow changes during script-driven imagery in police officers with posttraumatic stress disorder. Biol. Psychiatry 56, 853–861. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Booij J, Habraken JB, van Meijel EP, Uylings HB, Olff M, Carlier IV, den Heeten GJ, van Eck-Smit BL, Gersons BP, 2008. Effects of psychotherapy on regional cerebral blood flow during trauma imagery in patients with post-traumatic stress disorder: a randomized clinical trial. Psychol. Med 38, 543–554. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li B, Feng N, Pu H, Zhang X, Lu H, Yin H, 2016. Perfusion deficits and functional connectivity alterations in memory-related regions of patients with post-traumatic stress disorder. PLoS ONE 11, e0156016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li L, Li B, Feng N, Li L, Zhang X, Lu H, Yin H, 2017. Decreased triple network connectivity in patients with recent onset post-traumatic stress disorder after a single prolonged trauma exposure. Sci. Rep 7, 12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Mitchell D, Jones M, Mondillo K, Vythilingam M, Blair RJ, 2007. Common regions of dorsal anterior cingulate and prefrontal-parietal cortices provide attentional control of distracters varying in emotionality and visibility. Neuroimage 38, 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Rabinak CA, Kennedy AE, Fitzgerald DA, Liberzon I, Stein MB, Phan KL, 2016. Emotion regulatory brain function and SSRI treatment in PTSD: neural correlates and predictors of change. Neuropsychopharmacology 41, 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malivoire BL, Girard TA, Patel R, Monson CM, 2018. Functional connectivity of hippocampal subregions in PTSD: relations with symptoms. BMC Psychiatry 18,129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron-Katz A, Zhang Y, Narayan M, Wu W, Toll RT, Naparstek S, De Los Angeles C, Longwell P, Shpigel E, Newman J, Abu-Amara D, Marmar C, Etkin A, 2020. Individual patterns of abnormality in resting-state functional connectivity reveal two data-driven PTSD subgroups. Am. J. Psychiatry 177, 244–253. [DOI] [PubMed] [Google Scholar]
- Marshall RD, Beebe KL, Oldham M, Zaninelli R, 2001. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. American Journal of Psychiatry 158, 1982–1988. [DOI] [PubMed] [Google Scholar]
- Mazza M, Tempesta D, Pino MC, Nigri A, Catalucci A, Guadagni V, Gallucci M, Iaria G, Ferrara M, 2015. Neural activity related to cognitive and emotional empathy in post-traumatic stress disorder. Behav. Brain Res 282. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA, 2014. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depress. Anxiety 31, 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ, Litz BT, Prassas A, Chin LM, Weathers FW, 1994. Emotional priming of autobiographical memory in posttraumatic stress disorder. Cognition and Emotion 8, 351–367. [Google Scholar]
- Metz S, Fleischer J, Grimm S, Gärnter M, Golde S, Duesenberg M, Roepke S, Wolf OT, Otte C, Wingenfeld K, 2019. Resting-state functional connectivity after hydrocortisone administration in patients with post-traumatic stress disorder and borderline personality disorder. Eur. Neuropsychopharmacol 29, 936–946. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL, 2009. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry 66, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL, 2007. A role for the human dorsal anterior cingulate cortex in fear expression. Biol. Psychiatry 62, 1191–1194. [DOI] [PubMed] [Google Scholar]
- Miller DR, Hayes SM, Hayes JP, Spielberg JM, Lafleche G, Verfaellie M, 2017a. Default mode network subsystems are differentially disrupted in posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DR, Logue MW, Wolf EJ, Maniates H, Robinson ME, Hayes JP, Stone A, Schichman S, McGlinchey RE, Milberg WP, 2017b. Posttraumatic stress disorder symptom severity is associated with reduced default mode network connectivity in individuals with elevated genetic risk for psychopathology. Depress. Anxiety 34, 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M, Phillips R, Zotev V, Won CK, Wurfel BE, Krueger F, Feldner M, Bodurka J, 2019. Brain activity mediators of PTSD symptom reduction during real-time fMRI amygdala neurofeedback emotional training. Neuroimage Clin 24, 102047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M, Phillips R, Zotev V, Wong CK, Wurfel BE, Krueger F, Feldner M, Bodurka J, 2018. Real-time fMRI amygdala neurofeedback positive emotional training normalized resting-state functional connectivity in combat veterans with and without PTSD: a connectome-wide investigation. Neuroimage Clin 20, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Dolcos F, Petty CM, Cooper DA, Pannu Hayes J, LaBar KS, McCarthy G, 2009. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. J. Psychiatr. Res 43, 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Dunsmoor JE, Haswell CC, Brown VM, Vora A, Weiner J, Stjepanovic D, Wagner HR, V.A. Mid-Atlantic MIRECC Workgroup, LaBar KS, 2015. Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Transl. Psychiatry 5, e700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Cooper DA, Labar KS, McCarthy G, 2008. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Res 162, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser DA, Aue T, Wang Z, Rusconi Serpa S, Favez N, Peterson BS, Schechter DS, 2013. Limbic brain responses in mothers with post-traumatic stress disorder and comorbid dissociation to video clips of their children. Stress 16, 493–502. [DOI] [PubMed] [Google Scholar]
- Naegeli C, Zeffiro T, Piccirelli M, Jaillard A, Weilenmann A, Hassanpour K, Schick M, Rufer M, Orr SP, Mueller-Pfeiffer C, 2018. Locus coeruleus activity mediates yyperresponsiveness in posttraumatic stress disorder. Biol. Psychiatry 83, 254–262. [DOI] [PubMed] [Google Scholar]
- New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, Tang CY, Charney DS, 2009. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol. Psychiatry 66, 656–664. [DOI] [PubMed] [Google Scholar]
- Nicholson AA, Densmore M, McKinnon MC, Neufeld RWJ, Frewen PA, Théberge J, Jetly R, Richardson JD, Lanius RA, 2019. Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: a multimodal neuroimaging approach. Psychol. Med 49, 2049–2059. [DOI] [PubMed] [Google Scholar]
- Nicholson AA, Friston KJ, Zeidman P, Harricharan S, McKinnon MC, Densmore M, Neufeld RWJ, Théberge J, Corrigan F, Jetly R, Spiegel D, Lanius RA, 2017. Dynamic causal modeling in PTSD and its dissociative subtype: bottom-up versus top-down processing within fear and emotion regulation circuitry. Hum. Brain Mapp 38, 5551–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Harricharan S, Densmore M, Neufeld RWJ, Ros T, McKinnon MC, Frewen PA, Théberge J, Jetly R, Pedlar D, Lanius RA, 2020. Classifying heterogenous presentations of PTSD via the default mode, central executive, and salience networks with machine learning. Neuroimage Clin 27, 102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AA, Sapru I, Densmore M, Frewen PA, Neufeld RWJ, Théberge J, McKinnon MC, Lanius RA, 2016. Unique insula subregion resting-state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Res. Neuroimaging 250, 61–72. [DOI] [PubMed] [Google Scholar]
- Offringa R, Brohawn KH, Staples LK, Dubois SJ, Hughes KC, Pfaff DL, VanElzakker MB, Davis FC, Shin LM, 2013. Diminished rostral anterior cingulate cortex activation during trauma-unrelated emotional interference in PTSD. Biol Mood Anxiety Disord 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivé I, Densmore M, Harricharan S, Théberge J, McKinnon MC, Lanius R, 2018. Superior colliculus resting state networks in post-traumatic stress disorder and its dissociative subtype. Hum. Brain Mapp 39, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EA, Kaiser RH, Pizzagalli DA, Rauch SL, Rosso IM, 2019. Regional prefrontal resting-state functional connectivity in posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuch EA, Benson B, Geraci M, Podell D, Herscovitch P, McCann UD, Post RM, 2001. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol. Psychiatry 50, 246–253. [DOI] [PubMed] [Google Scholar]
- Osuch EA, Willis MW, Bluhm R, Ursano RJ, Drevets WC, 2008. Neurophysiological responses to traumatic reminders in the acute aftermath of serious motor vehicle collisions using [15O]-H2O positron emission tomography. Biol. Psychiatry 64, 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani M, Hogberg G, Salmaso D, Nardo D, Sundin O, Jonsson C, Soares J, Aberg-Wistedt A, Jacobsson H, Larsson SA, Hallstrom T, 2007. Effects of EMDR psychotherapy on 99mTc-HMPAO distribution in occupation-related post-traumatic stress disorder. Nucl. Med. Commun 28, 757–765. [DOI] [PubMed] [Google Scholar]
- Patel R, Girard TA, Pukay-Martin N, Monson C, 2016. Preferential recruitment of the basolateral amygdala during memory encoding of negative scenes in posttraumatic stress disorder. Neurobiol. Learn. Mem 130, 170–176. [DOI] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA, 2012. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev 36, 2130–2142. [DOI] [PubMed] [Google Scholar]
- Peres JFP, Newberg AB, Mercante JP, Simao M, 2007. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol Med 37, 1481–1491. [DOI] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I, 2006. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch. Gen. Psychiatry 63, 184–192. [DOI] [PubMed] [Google Scholar]
- Philip NS, Barredo J, van ’t Wout-Frank M, Tyrka AR, Price LH, Carpenter LL, 2018. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol. Psychiatry 83, 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piefke M, Pestinger M, Arin T, Kohl B, Kastrau F, Schnitker R, Vohn R, Weber J, Ohnhaus M, Erli HJ, Perlitz V, Paar O, Petzold ER, Flatten G, 2007. The neurofunctional mechanisms of traumatic and non-traumatic memory in patients with acute PTSD following accident trauma. Neurocase 13, 342–357. [DOI] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Fernandez M, Von Knorring L, Fischer H, Fredrikson M, 2002. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur. Arch. Psychiatry Clin. Neurosci 252, 68–75. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D, Stern E, 2005. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol. Psychiatry 57, 464–473. [DOI] [PubMed] [Google Scholar]
- Qin LD, Wang Z, Sun YW, Wan JQ, Su SS, Zhou Y, Xu JR, 2012. A preliminary study of alterations in default network connectivity in post-traumatic stress disorder patients following recent trauma. Brain Res 1484, 50–56. [DOI] [PubMed] [Google Scholar]
- Rabellino D, Densmore M, Harricharan S, Jean T, McKinnon MC, Lanius RA, 2018a. Resting-state functional connectivity of the bed nucleus of the stria terminalis in post-traumatic stress disorder and its dissociative subtype. Hum. Brain Mapp 39, 1367–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D, Densmore M, Théberge J, McKinnon MC, Lanius RA, 2018b. The cerebellum after trauma: resting-state functional connectivity of the cerebellum in posttraumatic stress disorder and its dissociative subtype. Hum. Brain Mapp 39, 3354–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage AE, Laird AR, Eickhoff SB, Acheson A, Peterson AL, Williamson DE, Telch MJ, Fox PT, 2013. A coordinate-based meta-analytic model of trauma processing in posttraumatic stress disorder. Hum. Brain Mapp 34, 3392–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK, 1996. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch. Gen. Psychiatry 53, 380–387. [DOI] [PubMed] [Google Scholar]
- Reuveni I, Bonne O, Giesser R, Shragai T, Lazarovits G, Isserles M, Schreiber S, Bick AS, 2016. Anatomical and functional connectivity in the default mode network of post-traumatic stress disorder patients after civilian and military-related trauma. Hum. Brain Mapp 37, 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritov G, Ardi Z, Richter-Levin G, 2014. Differential activation of amygdala, dorsal and ventral hippocampus following an exposure to a reminder of underwater trauma. Front. Behav. Neurosci 8, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougemont-Bücking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR, 2011. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci. Ther 17, 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russman Block S, King AP, Sripada RK, Weissman DH, Welsh R, Liberzon I, 2017. Behavioral and neural correlates of disrupted orienting attention in posttraumatic stress disorder. Cognit. Affect. Behav. Neurosci 17, 422–436. [DOI] [PubMed] [Google Scholar]
- Sadeh N, JM S, Miller MW, Milberg WP, Salat DH, Amick MM, Fortier CB, McGlinchey RE, 2015. Neurobiological indicators of disinhibition in posttraumatic stress disorder. Hum. Brain Mapp 36, 3076–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, Shirouzu I, Yamasue H, Akiyama T, Kato N, 2005. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. Neuroimage 26, 813–821. [DOI] [PubMed] [Google Scholar]
- Samuelson KW, 2011. Post-traumatic stress disorder and declarative memory functioning: a review. Dialogues Clin. Neurosci 13, 346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuan PM, Andrews C, Claus ED, 2018. Abnormal target detection and novelty processing neural response in posttraumatic stress disorder. Progr. Neuropsychopharmacol. Biologic. Psychiatry 85, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, 2003. Stress and plasticity in the limbic system. Neurochem. Res 28, 1735–1742. [DOI] [PubMed] [Google Scholar]
- Sartory G, Cwik J, Knuppertz H, Schürholt B, Lebens M, Seitz RJ, Schulze R, 2013. In search of the trauma memory: a meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD). PLoS ONE 8, e58150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Cook PA, Bruce SE, Conway C, Mikkelsen E, Satchell E, Vandekar SN, Durbin T, Shinohara RT, Sheline YI, 2016. Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivity. Mol. Psychiatry 21, 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter DS, Moser DA, Wang Z, Marsh R, Hao X, Duan Y, Yu S, Gunter B, Murphy D, McCaw J, Kangarlu A, Willheim E, Myers MM, Hofer MA, Peterson BS, 2012. An fMRI study of the brain responses of traumatized mothers to viewing their toddlers during separation and play. Soc. Cogn. Affect. Neurosci 7, 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel RS, Pastorek NJ, Troyanskaya M, Kennedy JE, Steinberg JL, Newsome MR, Lin X, Levin HS, 2015. The suppression of brain activation in post-deployment military personnel with posttraumatic stress symptoms. Brain Imaging Behav 9, 513–526. [DOI] [PubMed] [Google Scholar]
- Schmand M, Wienhard K, Casey M, Eriksson L, Jones W, Reed J, Treffert J, Lenox M, Luk P, Bao J, 1999. Performance evaluation of a new LSO high resolution research tomograph-HRRT. In: Nuclear Science Symposium, 1999. Conference Record. 1999. IEEE. IEEE, pp. 1067–1071. [Google Scholar]
- Schulze L, Schulze A, Renneberg B, Schmahl C, Niedtfeld I, 2019. Neural correlates of affective disturbances: a comparative meta-analysis of negative affect processing in borderline personality disorder, major depressive disorder, and posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 220–232. [DOI] [PubMed] [Google Scholar]
- Seedat S, Warwick J, van Heerden B, Hugo C, Zungu-Dirwayi N, Van Kradenburg J, Stein DJ, 2004. Single photon emission computed tomography in posttraumatic stress disorder before and after treatment with a selective serotonin reuptake inhibitor. J. Affect. Disord 80, 45–53. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS, 1988. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J. Neurosci 8, 4049–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J, Lui S, Meng Y, Zhu H, Qiu C, Gong Q, Liao W, Zhang W, 2014. Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: a resting-state fMRI study. PLoS ONE 9, e96834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw ME, Strother SC, McFarlane AC, Morris P, Anderson J, Clark CR, Egan GF, 2002. Abnormal functional connectivity in posttraumatic stress disorder. Neuroimage 15, 661–674. [DOI] [PubMed] [Google Scholar]
- Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Maeklin ML, Pitman RK, 1997. Visual imagery and perception in posttraumatic stress disorder: a positron emission tomographic investigation. Arch. Gen. Psychiatry 54, 233–241. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK, 1999. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am. J. Psychiatry 156, 575–584. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Maeklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK, 2004. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch. Gen. Psychiatry 61, 168–176. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Maeklin ML, Lasko NB, Orr SP, Mclnerney SC, Rauch SL, 2001. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol. Psychiatry 50, 932–942. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Maeklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL, 2005. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch. Gen. Psychiatry 62, 273–281. [DOI] [PubMed] [Google Scholar]
- Shou H, Yang Z, Satterthwaite TD, Cook PA, Bruce SE, Shinohara RT, Rosenberg B, Sheline YI, 2017. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. Neuroimage Clin 14, 464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AN, Norman SB, Spadoni AD, Strigo IA, 2013. Neurosubstrates of remission following prolonged exposure therapy in veterans with posttraumatic stress disorder. Psychother. Psychosom 82, 382–389. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB, 2008. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol. Psychiatry 64, 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I, 2012a. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J. Psychiatr. Neurosci 37, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I, 2012b. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med 74, 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Botzung A, Miles A, Rubin DC, 2011. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. J. Psychiatr. Res 45, 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Kragel PA, Rubin DC, 2013. Neural networks supporting autobiographical memory retrieval in posttraumatic stress disorder. Cogn. Affect. Behav. Neurosci 13, 554–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Seedat S, van der Linden GJ, Zungu-Dirwayi N, 2000. Selective serotonin reuptake inhibitors in the treatment of posttraumatic stress disorder: a meta-analysis of randomized controlled trials. Int. Clin. Psychopharmacol 15, S31–S39. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, Reiser E, Ely TD, Dhanani R, Glover EM, Jovanovic T, Ressler KJ, 2014. PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proc. Natl. Acad. Sci. U. S. A 111, 3158–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ, 2013. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J. Psychiatr. Res 47, 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, Hudak LA, Jovanovic T, Rothbaum BO, Ressler KJ, 2017. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biol. Psychiatry 81, 1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Gold AL, Swanson CA, Haswell CC, Brown VM, Stjepanovic D, Mid-Atlantic MIRECC Workgroup, V.A., LaBar KS, Morey RA, 2020. Threat-induced anxiety during goal pursuit disrupts amygdala-prefrontal cortex connectivity in posttraumatic stress disorder. Transl. Psychiatry 10, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Phillips RD, Mulready HL, Zablonski ST, Turner JA, Turner MD, McClymond K, Nieuwsma JA, Morey RA, 2019. Resting-state brain fluctuation and functional connectivity dissociate moral injury from posttraumatic stress disorder. Depress. Anxiety 36, 442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System, An Approach to Cerebral Imaging. Georg Thieme, Stuttgart, Germany. [Google Scholar]
- Terpou BA, Densmore M, Théberge J, Frewen P, McKinnon MC, Lanius RA, 2018. Resting-state pulvinar-posterior parietal decoupling in PTSD and its dissociative subtype. Hum. Brain Mapp 39, 4228–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J, Terpou BA, McKinnon MC, Lanius RA, 2020. The neural correlates of trauma-related autobiographical memory in posttraumatic stress disorder: a meta-analysis. Depress. Anxiety 37, 321–345. [DOI] [PubMed] [Google Scholar]
- Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD, 2001. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled flexible-dosage trial. J. Clin. Psychiatr 62, 860–868. [DOI] [PubMed] [Google Scholar]
- Tursich M, Ros T, Frewen PA, Kluetsch RC, Calhoun VD, Lanius RA, 2015. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatr. Scand 132, 29–38. [DOI] [PubMed] [Google Scholar]
- van Rooij SJ, Kennis M, Vink M, Geuze E, 2016. Predicting treatment outcome in PTSD: a longitudinal functional MRI study on trauma-unrelated emotional processing. Neuropsychopharmacology 41, 1156–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJH, Geuze E, Kennis M, Rademaker AR, Vink M, 2015. Neural correlates of inhibition and contextual cue processing related to treatment response in PTSD. Neuropsychopharmacology 40, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanasse TJ, Franklin C, Salinas FS, Ramage AE, Calhoun VD, Robinson PC, Kok M, Peterson AL, Mintz J, Litz BT, Young-McCaughan S, Resick PA, Fox PT, Consortium, STRONG STAR, 2019. A resting-state network comparison of combat-related PTSD with combat-exposed and civilian controls. Soc. Cogn. Affect. Neurosci 14, 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD, 2003. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatry 54, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Liu J, Zhang J, Zhan W, Li L, Wu M, Huang H, Zhu H, Kemp GJ, Gong Q, 2016. Altered resting-state functional activity in posttraumatic stress disorder: a quantitative meta-analysis. Sci. Rep 6, 27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DL, Clark CR, McFarlane AC, Moores KA, Morris P, Egan GF, 2005. Abnormal frontal and parietal activity during working memory updating in post-traumatic stress disorder. Psychiatry Res. Neuroimaging 140, 27–44. [DOI] [PubMed] [Google Scholar]
- Weng Y, Qi R, Zhang L, Luo Y, Ke J, Xu Q, Zhong Y, Li J, Chen F, Cao Z, Lu G, 2019. Disturbed effective connectivity patterns in an intrinsic triple network model are associated with posttraumatic stress disorder. Neurol. Sci 40, 339–349. [DOI] [PubMed] [Google Scholar]
- Werner NS, Meindl T, Engel RR, Rosner R, Riedel M, Reiser M, Fast K, 2009. Hippocampal function during associative learning in patients with posttraumatic stress disorder. J. Psychiatr. Res 43, 309–318. [DOI] [PubMed] [Google Scholar]
- Whalley MG, Kroes MC, Huntley Z, Rugg MD, Davis SW, Brewin CR, 2013. An fMRI investigation of posttraumatic flashbacks. Brain Cogn 81, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittbrodt MT, Gurel NZ, Nye JA, Ladd S, Shandhi MMH, Huang M, Shah AJ, Pearce BD, Alam ZS, Rapaport MH, Murrah N, Ko YA, Haffer AA, Shallenberger LH, Vaccarino V, Inan OT, Bremner JD, 2020. Non-invasive vagal nerve stimulation decreases brain activity during trauma scripts. Brain Stimul 13, 1333–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittbrodt MT, Gurel NZ, Nye JA, Shandhi MH, Gazi AH, Shah AJ, Pearce BD, Murrah N, Ko YA, Shallenberger L, Inan OT, Bremner JD, 2021. Non-invasive cervical vagal nerve stimulation alters brain activity during traumatic stress in individuals with posttraumatic stress disorder. Psychosom. Med 83, 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittbrodt MT, Moazzami K, Lima BB, Alam ZS, Corry D, Hammadah M, Campanella C, Ward L, Quyyumi AA, Shah AJ, Vaccarino V, Nye JA, Bremner DJ, 2019. Early childhood trauma alters neurological responses to mental stress in patients with coronary artery disease. J. Affect. Disord 254, 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe J, Lazarus AA, 1966. Behavioral Therapy Techniques. Pergamon Press, New York. [Google Scholar]
- Yan X, Brown AD, Lazar M, Cressman VL, Henn-Haase C, Neylan TC, Shalev A, Wolkowitz OM, Hamilton SP, Yehuda R, Sodickson DK, Weiner MW, Marmar CR, 2013. Spontaneous brain activity in combat related PTSD. Neurosci. Lett 547, 1–5. [DOI] [PubMed] [Google Scholar]
- Yang P, Wu MT, Hsu CC, Ker JH, 2004. Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neurosci. Lett 370, 13–18. [DOI] [PubMed] [Google Scholar]
- Yang Z, Oathes DJ, Linn KA, Bruce SE, Satterthwaite TD, Cook PA, Satchell EK, Shou H, Sheline YI, 2018. Cognitive behavioral therapy is associated with enhanced cognitive control network activity in major depression and posttraumatic stress disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Golier JA, Bierer LM, Mikhno A, Pratchett LC, Burton CL, Makotkine I, Devanand DP, Pradhaban G, Harvey PD, Mann JJ, 2010. Hydrocortisone responsiveness in Gulf War veterans with PTSD: effects on ACTH, declarative memory hippocampal [(18)F]FDG uptake on PET. Psychiatry Res 184, 117–127. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Harvey PD, Golier JA, Newmark RE, Bowie CR, Wohltmann JJ, Grossman RA, Schmeidler J, Hazlett EA, Buchsbaum MS, 2009. Changes in relative glucose metabolic rate following cortisol administration in aging veterans with posttraumatic stress disorder: an FDG-PET neuroimaging study. J. Neuropsychiatry Clin. Neurosci 21, 132–143. [DOI] [PubMed] [Google Scholar]
- Yin Y, Li L, Jin C, Hu X, Duan L, Eyler LT, Gong Q, Song M, Jiang T, Liao M, Zhang Y, Li W, 2011. Abnormal baseline brain activity in posttraumatic stress disorder: a resting-state functional magnetic resonance imaging study. Neurosci. Lett 498, 185–189. [DOI] [PubMed] [Google Scholar]
- Yuan M, Pantazatos SP, Zhu H, Li Y, Miller JM, Rubin-Falcone H, Zanderigo F, Ren Z, Yuan C, Lui S, Gong Q, Qiu C, Zhang W, Mann JJ, 2019. Altered amygdala subregion-related circuits in treatment-naive post-traumatic stress disorder comorbid with major depressive disorder. Eur. Neuropsychopharmacol 29, 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang J, Wang L, Li R, Zhang W, 2016a. Altered resting-state functional connectivity of the amygdala in Chinese earthquake survivors. Biol. Psychiatry 65, 208–214. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Yin Y, Hu XL, Duan L, Qi R, Xu Q, Lu GM, Li LJ, 2017. Altered default mode network configuration in posttraumatic stress disorder after earthquake: a resting-stage functional magnetic resonance imaging study. Medicine (Baltimore) 96, e7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu F, Chen H, Li M, Duan X, Xie B, Chen H, 2015. Intranetwork and internetwork functional connectivity alterations in post-traumatic stress disorder. J. Affect. Disord 187, 114–121. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xie B, Chen H, Li M, Guo X, Chen H, 2016b. Disrupted resting-state insular subregions functional connectivity in post-traumatic stress disorder. Medicine (Baltimore) 95, e4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xie B, Chen H, Li M, Liu F, Chen H, 2016c. Abnormal functional connectivity density in post-traumatic stress disorder. Brain Topogr 29, 405–411. [DOI] [PubMed] [Google Scholar]
- Zhu H, Li Y, Yuan M, Ren Z, Yuan C, Meng Y, Wang J, Deng W, Qiu C, Huang X, Gong Q, Lui S, Zhang W, 2019. Increased functional segregation of brain network associated with symptomatology and sustained attention in chronic post-traumatic stress disorder. J. Affect. Disord 247, 183–191. [DOI] [PubMed] [Google Scholar]
- Zhu H, Qiu C, Meng Y, Cui H, Zhang Y, Huang X, Zhang J, Li T, Gong Q, Zhang W, Lui S, 2015. Altered spontaneous neuronal activity in chronic posttraumatic stress disorder patients before and after a 12-week paroxetine treatment. J. Affect. Disord 174, 257–264. [DOI] [PubMed] [Google Scholar]
- Zhu H, Yuan M, Qiu C, Ren Z, Li Y, Wang J, Huang X, Lui S, Gong Q, Zhang W, Zhang Y, 2020. Multivariate classification of earthquake survivors with post-traumatic stress disorder based on large-scale brain networks. Acta Psychiatr. Scand 141, 285–298. [DOI] [PubMed] [Google Scholar]
- Zhu X, Helpman L, Papini S, Schneier F, Markowitz JC, Van Meter PE, Lindquist MA, Wager TD, Neria Y, 2017. Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress. Anxiety 34, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Suarez-Jimenez B, Lazarov A, Helpman L, Papini S, Lowell A, Durosky A, Lindquist MA, Markowitz JC, Schneier F, Wager TD, Neria Y, 2018. Exposure-based therapy changes amygdala and hippocampus resting-state functional connectivity in patients with posttraumatic stress disorder. Depress. Anxiety 35, 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar J, Amital D, Miodownik C, Kotler M, Bleich A, Lane RM, Austin C, 2002. Double-blind placebo-controlled pilot study of sertraline in military veterans with posttraumatic stress disorder. J. Clin. Psychopharmacol 22, 190–195. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA, 1989. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J. Neurosci 9, 4355–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan SM, Squire LR, 1990. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science 250, 288–290. [DOI] [PubMed] [Google Scholar]
- Zotev V, Phillips R, Misaki M, Wong CK, Wurfel BE, Krueger F, Feldner M, Bodurka J, 2018. Real-time fMRI neurofeedback training of the amygdala activity with simultaneous EEG in veterans with combat-related PTSD. Neuroimage Clin 19, 106–121. [DOI] [PMC free article] [PubMed] [Google Scholar]


