Abstract
The effects of mitragynine on anxiety-related behaviours in the open-field and elevated plus-maze tests were evaluated. Male Sprague–Dawley rats were orally treated with mitragynine (10, 20 and 40 mg/kg) or diazepam (10 mg/kg) 60 min before behavioural testing. Mitragynine doses used in this study were selected on the basis of approximately human equivalent doses with reference to our previous literature reports. Acute administration of mitragynine (10, 20 and 40 mg/kg) or diazepam (10 mg/kg) increased central zone and open arms exploration in the open-field and elevated plus-maze tests respectively. These anxiolytic-like effects of mitragynine were effectively antagonized by intraperitoneal administration of naloxone (2 mg/kg), flumazenil (10 mg/kg), sulpiride (0.5 mg/kg) or SCH 23390 (0.02 mg/kg) 15 min before mitragynine treatments. These findings reveal that the acute administration of mitragynine produces anxiolytic-like effects and this could be possibly attributed to the interactions among opioidergic, GABAergic and dopaminergic systems in brain regions involved in anxiety.
Keywords: Anxiolytic, Mitragynine, Open-field, Elevated plus-maze
Introduction
Anxiety disorders are the most prevalent forms of mental illnesses [26]. Various classes of medications are currently in use to treat these disorders, with benzodiazepines being the commonest [40]. However, their use is limited by undesired side effects such as sedation, myorelaxation and development of dependence [20]. The use of alternative medicine, mostly herbal remedies, to treat mental disorders has increased significantly over the past few years as these are taken to be effective with fewer side effects than conventional drugs [3]. Mitragyna speciosa (Rubiaceae) is an indigenous tree growing in Southeast Asian countries, particularly in Malaysia and Thailand. The leaves of this plant are known as ‘Ketum’ or “Kratom” by indigenous people of Malaysia and Thailand. The leaves have been traditionally used by locals for their opium- and coca-like effects in enhancing work tolerance under intense tropical heat [11]. In addition, the leaves were also used to relieve pain, reduce coughing, treat diarrhoea, and to mitigate morphine withdrawal symptoms [46]. Over 25 alkaloids have been isolated from the mature leaves of Mitragyna speciosa with mitragynine being the most dominant active alkaloid in this plant [4]. Earlier studies reported the role of supraspinal opioid receptors and both descending noradrenergic and serotonergic systems in the antinociceptive effect of mitragynine [24, 25]. In addition, mitragynine suppressed the electrically stimulated contraction of guinea-pig ileum and mouse vas deferens through the activation of μ-, κ- and δ-opioid receptors [46, 47]. Recently, mitragynine has also been shown to produce an antidepressant-like effect in forced swimming and tail suspension tests [8] and decrease novel environment stress in mice [14]. Similarly, mitragynine attenuated stress-related behaviours in morphine-withdrawn zebrafish tested in a novel tank diving test [18]. It is well known that administration of opioid agonists induces anxiolytic-like effects in laboratory animals [33, 48] through interactions among opioidergic, γ-aminobutyric acid (GABA)ergic and dopaminergic systems in the brain [19, 39]. In view of these findings, mitragynine being a psychotropic drug could have a beneficial effect on the prevention and management of stress-induced disorders such as anxiety. Therefore, the present study was conducted to evaluate the effect of mitragynine on anxiety-related behaviours using open-field (OF) and elevated plus-maze (EPM) tests. The possible involvements of opioidergic, GABAergic and dopaminergic systems in the observed effects of mitragynine were also investigated.
Results and discussion
Effects of mitragynine on anxiety-like behaviours in the OF and EPM tests
As shown in Fig. 1a and b, acute oral administration of mitragynine (10, 20 and 40 mg/kg) or diazepam (10 mg/kg) induced a significant increase in the number of entries (CZE) (Fig. 1a) and the percentage time spent in the central zone (%CZT) (Fig. 1b) of the OF, when compared to the control group (p < 0.05). Neither mitragynine nor diazepam significantly changed the overall locomotor activity (total distance travelled) (Fig. 1c). In the EPM test, acute administrations of mitragynine (10, 20 and 40 mg/kg) as well as diazepam (10 mg/kg,) significantly increased (p < 0.05) the percentage of open arm entries (%OAE) and the time spent on open arms (%OAT) of the plus-maze, when compare to the control group (Fig. 2a, b). On the other hand, acute administration of mitragynine or diazepam did not significantly affect the total number of arm visits in the elevated plus-maze test (Fig. 2c). As shown in Figs. 1 and 2, rats treated with 40 mg/kg mitragynine demonstrated highly significant (p < 0.01) changes in central zone and open arm exploration in the OF and the EPM tests respectively, therefore, this dose was chosen for the subsequent antagonism studies.
Fig. 1.
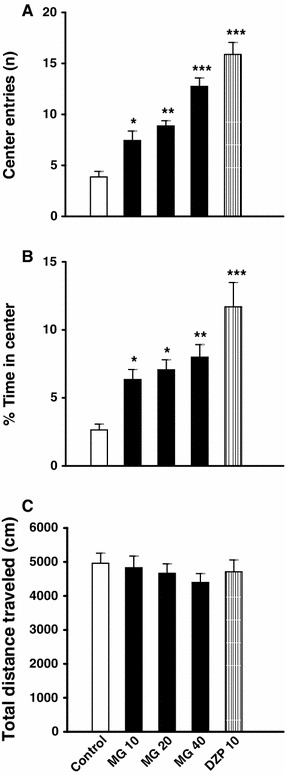
Effects of acute administration of mitragynine and diazepam on the number of entries into the central zone (a), the percentage of time spent in centre (b) and the total distance travelled (c) in the open-field. Animals were orally treated with Tween 20 (5 ml/kg), mitragynine (10, 20 and 40 mg/kg) or diazepam (10 mg/kg) 60 min before testing. Each bar represents mean ± SEM. n = 8. *p < 0.05, **p < 0.01 and ***p < 0.001 when compared to control group
Fig. 2.
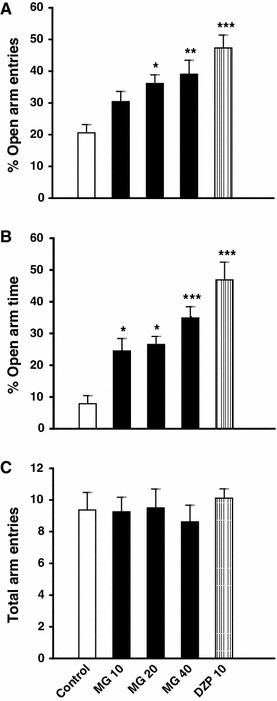
Effects of acute oral administration of mitragynine and diazepam on the number of entries into the open arms (a), the percentage of time spent on open arms (b) and the total arm entries (c) in the elevated plus maze. Animals were orally treated with Tween 20 (5 ml/kg), mitragynine (10, 20 and 40 mg/kg) or diazepam (10 mg/kg) 60 min before testing. Each bar represents mean ± SEM. n = 8. *p < 0.05, **p < 0.01 and ***p < 0.001 when compared to control group
The role of the opioidergic system in the anxiolytic-like effect of mitragynine in the EPM test
Administration of naloxone alone did not significantly change the plus-maze activity when compared to the control group (Fig. 3a, b). However, a significant decrease in open arm activities was observed when naloxone was injected 15 min before oral or intraperitoneal administrations of mitragynine and morphine, respectively (p < 0.05; Fig. 3a, b). Open arm exploration was significantly decreased in rats pretreated with naloxone (2 mg/kg) when compared to rats treated with mitragynine or morphine alone (Fig. 3a, b). However, acute administration of morphine did not significantly affect the total number of arm visits in the EPM test (Fig. 3c).
Fig. 3.
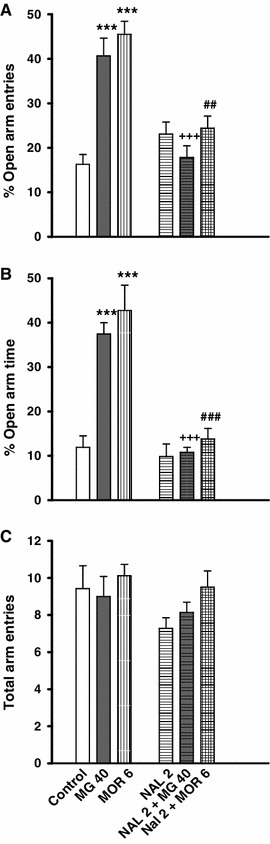
The effect of naloxone (NAL) on anxiolytic-like behaviours induced by mitragynine (MG) and morphine (MOR) in rats. The animals received a pre-test injection of saline (1 ml/kg) or NAL (2 mg/kg) 15 min before MG (40 mg/kg) or MOR (6 mg/kg) treatments. Control (5 ml/kg) and NAL groups received saline (1 ml/kg) 15 min before treatments. The anxolytic-like behaviour indices include changes in %OAE (a), %OAT (b) and the total arm entries (c). Each bar represents mean ± SEM. ***p < 0.001 when compared to control group; +++ p < 0.001 when compared to MG group; ## p < 0.01 and ### p < 0.001 when compared to MOR group
The role of the GABAergic system in the anxiolytic-like effect of mitragynine in the EPM test
Acute oral administration of mitragynine (40 mg/kg) or diazepam (10 mg/kg) demonstrated a significant increase in the %OAE and %OAT of the plus-maze when compared to the control group (p < 0.001; Fig. 4a, b). These effects were significantly antagonized when flumazenil was injected 15 min before the oral administrations of mitragynine and diazepam (Fig. 4a, b). Acute intraperitoneal administration of flumazenil alone did not produce significant changes in the plus-maze activities when compared to the control group (Fig. 4a–c).
Fig. 4.
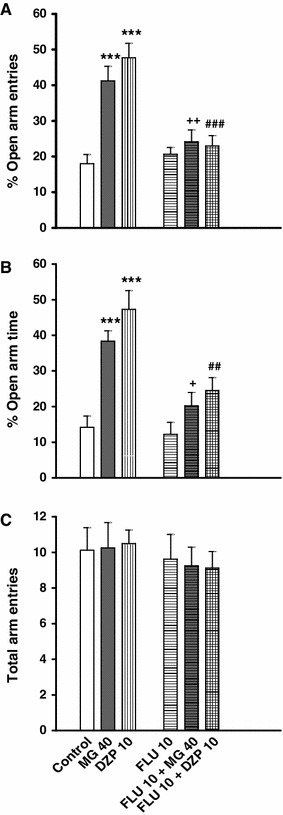
The effect of flumazenil (FLU) on anxiolytic-like behaviours induced by mitragynine (MG) and diazepam (DZP) in rats. The animals received a pre-test injection of saline (1 ml/kg) or FLU (10 mg/kg) 15 min before MG (40 mg/kg) or DZP (10 mg/kg) treatments. Control (5 ml/kg) and FLU groups received saline (1 ml/kg) 15 min before treatments. The anxolytic-like behaviour indices include changes in %OAE (a), %OAT (b) and the total arm entries (c). Each bar represents mean ± SEM. ***p < 0.001 when compared to control group; + p < 0.05 and ++ p < 0.01 when compared to MG group; ## p < 0.01 and ### p < 0.001 when compared to DZP group
The role of the dopaminergic system in the anxiolytic-like effect of mitragynine in the EPM test
As shown in Fig. 5a, b, acute oral administration of mitragynine (40 mg/kg) or apomorphine (0.5 mg/kg) demonstrated a significant increase in the %OAE and %OAT of the plus-maze when compared to the control group (p < 0.05; Fig. 5a, b). In addition, acute intraperitoneal administration of sulpiride or SCH 23390 alone did not produce significant changes in the plus-maze activities when compared to the control group (Fig. 5a–c). However, open arm exploration was significantly decreased when sulpiride or SCH 23390 were injected 15 min before the oral or intraperitoneal administrations of mitragynine and apomorphine, respectively (p < 0.05; Fig. 5a, b).
Fig. 5.

The effect of sulpiride (SLP) and SCH 23390 (SCH) on anxiolytic-like behaviours induced by mitragynine (MG) and apomorphine (APO) in rats. The animals received a pre-test injection of saline (1 ml/kg), SLP (0.5 mg/kg) or SCH (0.02 mg/kg) 15 min before MG (40 mg/kg) or APO (0.5 mg/kg) treatments. Control (5 ml/kg), SLP and SCH groups received saline (1 ml/kg) 15 min before treatments. The anxiolytic-like behaviour indices include changes in %OAE (a), %OAT (b) and the total arm entries (c). Each bar represents mean ± SEM. ***p < 0.001 when compared to control group; + p < 0.05 and +++ p < 0.001 when compared to MG group; ## p < 0.01 and ### p < 0.001 when compared to APO group
OF and EPM tests have been widely used to assess anxiety-related behaviour in rodents [15, 29]. The OF test is based upon conflict between the innate tendency of rodents to explore novel environments and aversion to open spaces. The CZE and %CZT have been well established as a measure of anxiety state in the OF test [34]. Our results demonstrated that acute oral administration of mitragynine (10, 20 and 40 mg/kg) produced anxiolytic-like effects in the OF test, as shown by the increase in central zone activities. Similar effects were also observed following the oral administration of diazepam (10 mg/kg). These anxiolytic-like effects were also reinforced by the behavioural findings from the EPM model, which relies upon conflict between exploration and aversion to elevated open places. It has been previously reported that anxiolytics such as diazepam increase open-arm exploration in the EPM test [32]. In this study, mitragynine at three different doses (10, 20 and 40 mg/kg) demonstrated anxiolytic-like effects, and a similar effect was observed in the rat group administered diazepam (10 mg/kg). This is the first reported anxiolytic-like effects of mitragynine in rats at doses equivalent to the human dose derived from the informal consumption of air ketum [31, 44]. Whilst mitragynine is a psychotropic drug and the principal alkaloid in ketum, this anxiolytic-like effect may have some basis in the informal use of ketum to mitigate opiate withdrawal symptoms, but this warrants further studies.
Antagonism of these activities using naloxone in this study appeared to mitigate the anxiolytic-like effect of mitragynine as well as that of morphine in the EPM test. These findings are consistent with previous studies reporting mitragynine activation of opioid receptors, with high affinity for both μ- and κ- followed by δ-opioid receptor subtypes [2, 42]. Together, these findings strongly implicate the role of the opioidergic system in the anxiolytic-like effects of mitragynine. Several lines of evidence indicate the role of opioid receptors in the behavioural response to anxiety. Microinjections of morphine into the ventral hippocampus or the nucleus accumbens decreased aversion to the open arms of the plus-maze, suggesting a potential role of μ-opioid receptors in the anxiolytic-like effect of morphine [48]. Previous studies have also reported that activation of κ- and δ-opioid receptors produce anxiolytic-like behaviour in rodents [33, 41]. Privette and Terrian [33] reported anxiolytic-like effects in rats following intraperitoneal administrations of κ-opioid receptor agonists U-50,488 H and U-69,593. These effects were significantly antagonized after pretreatment with naloxone. Similarly, bilateral administration of enkephalin into rat dorsal hippocampus has also shown to induce an anxiolytic-like effect via the activation of δ-opioid receptors [41].
It has been previously reported that opioid control of anxiety is mediated by indirect interactions with GABAergic [39, 43] and dopaminergic pathways [19, 36] in brain regions involved in fear and anxiety such as the amygdala, hypothalamus, hippocampus and midbrain areas. A study by Sasaki and colleagues [39] evaluated the interaction between opioidergic and GABAergic systems. In this study, administration of morphine to wild-type mice has been shown to enhance the anxiolytic-like effects of muscimol, a selective GABAA agonist, in EPM tests, by raising its binding in posterior thalamic, mediodorsal thalamic and amygdaloid areas. However, these effects were not observed in μ-opioid receptor knockout mice. These findings suggest the crucial role of μ-opioid receptors in modulating anxiolytic behaviour regulated by GABAergic neurotransmission. Results from the same study have also shown that the anxiolytic-like effects observed following morphine administration to wild-type mice were effectively antagonized by naloxone as well as bicucullin, GABAA antagonist. These results further confirm the role of interactions between opioidergic and GABAergic systems in modulating anxiety behaviours [39]. In another study, the interaction between κ-opioid and GABAergic systems was examined using the Vogel conflict model in mice. The anticonflict effect of diazepam was significantly abolished by pretreatment with naloxone or nor-binaltorphimine, the selective κ-opioid receptor antagonist, suggesting the involvement of the κ-opioid system in the anxiolytic-like effects of diazepam [43]. Although the exact mechanism of interaction between opioidergic and GABAergic systems is unknown, the co-location of GABA and opiate receptors in the central nervous system, and the existence of possible cross-reactivity and common pathways of intracellular transduction may play a key role in this interaction [27]. Earlier studies have also reported the indirect modulation of dopamine cells by opioid agonists and endogenous opioid peptides [5, 6]. Stimulation of μ-opioid receptors, located presynaptically on the descending GABAergic axon terminals and interneurons innervating dopamine cells, enhances dopamine release in the ventral tegmental area by removing tonic inhibitory regulation of GABAergic input [17, 19]. In view of the opioid agonistic effect of mitragynine and the key role of GABAergic and dopaminergic systems in modulating anxiety behaviours [23, 28], the possible involvement of GABAergic and dopaminergic systems in mitragynine anxiolytic-like effects were investigated in this study. Our results demonstrated that pretreatment with flumazenil significantly reversed the anxiolytic-like effects of mitragynine and diazepam in the EPM test. Similarly, pretreatment with sulpiride or SCH 23390 significantly abolished the anxiolytic-like effects of mitragynine and apomorphine. These findings suggest the involvement of GABAergic and dopaminergic systems in the anxiolytic-like effect of mitragynine. It can therefore be assumed that mitragynine may produce anxiolytic-like effects by indirect modulation of GABAergic and dopaminergic systems through the activation of opioid receptors in brain regions involved in anxiety such as amygdala, hypothalamus, hippocampus and midbrain areas.
Mitragynine, at the doses employed (10, 20 and 40 mg/kg) did not show any sedative effect as evidenced by the total distance travelled in the OF, or the number of total arm entries in the EPM test. Our previous studies have shown that similar doses of mitragynine did not produce any muscle relaxant effect in the rota-rod test [14] and proved the wide therapeutic index of mitragynine compared to the alkaloid extract of Mitragyna speciosa leaves [37]. An earlier study by Macko and colleagues [22] has shown that single oral administration of 806 mg/kg mitragynine failed to produce toxicity signs in rats. Similarly, sub-acute administration of 50 mg/kg/day mitragynine for 5 days a week for 6 weeks did not show any toxic signs in rats [22]. In addition, our recent toxicity study has also shown that oral administration of 1 and 10 mg/kg mitragynine for 28 days did not exhibit toxicity signs in rats. However, administration of 100 mg/kg mitragynine for the same period of time produced significant toxicity as demonstrated by histopathological changes in the liver, kidney and brain as well as biochemical and haematological changes [38]. The probability of mitragynine causing dependence in humans is not well documented. A recent study reported the informal use of ketum to manage opiate withdrawal symptoms and reduce addiction to other drugs. Ketum users described it as affordable, easily available and having no serious side effects despite prolonged use [44]. In support of these findings, the most recent in vitro study has shown a low tendency of mitragynine to develop dependence and tolerance compared to morphine [16]. Human neuroblastoma cells treated with mitragynine demonstrated significantly fewer changes in cyclic adenosine monophosphate cAMP level and μ-opioid receptor expression during cell differentiation compared to morphine-treated cells. In addition, cells treated with both mitragynine and morphine demonstrated significantly fewer changes in cAMP level and μ-opioid receptor expression compared to morphine treatment alone, suggesting a possible role of mitragynine in avoiding the development of dependence and tolerance after chronic administration of morphine [16]. In another study, administration of morphine (5 mg/kg) for 9 days produced significant increases in cAMP and cAMP response element binding (CREB) expression in the thalamus and cortex of mice brains due to the development of cAMP transmission tolerance. However, no significant changes in these protein expressions were observed in mice treated with mitragynine (15 and 25 mg/kg) and morphine (5 mg/kg) for the same period of time. These results further support the role of mitragynine in preventing the development of tolerance following chronic administration of morphine [7]. Taken together, these findings tend to suggest that mitragynine is relatively safe and devoid of unwanted side effects such as the development of dependence and tolerance.
Experiments
Animals
Male Sprague-Dawley rats (weighing 170–200 g) were obtained from the Animal Research and Service Centre (ARASC), Universiti Sains Malaysia (USM). Rats were housed in polypropylene cages (40 × 34 × 17 cm) in groups of six for at least 1 week prior to behavioural testing, with free access to the standard commercial food pellets (Gold Coin Feed Mills Sdn Bhd, Malaysia) and water ad libitum. Rats were housed in a temperature-controlled room at 24 ± 1 °C and maintained on a reversed 12-h light/dark cycle (with lights off at 07:00 h). Behavioural tests were performed during the dark phase of the light cycle (09:00 to 12:00 h). The experimental protocols for care and use of laboratory animals described in this study were guided and approved by the Animal Ethics Committee, USM [USM/2010/58 (200)].
Drugs
Mitragynine was purified from the crude alkaloid extract of Mitragyna speciosa leaves as we previously described [1]. Morphine sulphate was provided by Hospital Universiti Sains Malaysia (Kelantan, Malaysia). Diazepam (GABAA receptor agonist) was purchased from Lipomed, Switzerland. Flumazenil (GABAA receptor antagonist), apomorphine hydrochloride (dopamine D1 and D2 receptor agonist), SCH23390 (dopamine D1 receptor antagonist, (R)-(+)-7-chloro-8-hydroxy-3-methyl–1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride), sulpiride (dopamine D2 receptor antagonist) and naloxone hydrochloride dehydrate (opioid receptor antagonist) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All drugs were dissolved in sterile saline (0.9 % NaCl) just before the experiment, except for mitragynine and diazepam which were dissolved in 20 % (v/v) Tween 20 (ChemPur, Germany).
Treatments
The selection of mitragynine doses used in this study was based on recently published data on the informal use of ketum drink in the Northern Peninsular of Malaysia. Ketum users were found to consume 0.3–5.7 mg/kg/day mitragynine in ketum drink to wean off the opioid withdrawal symptoms [31, 44]. This translates to an equivalent dose range of 1.85–35.15 mg/kg mitragynine in rats [35]. Therefore, in the present study we administered three different doses of mitragynine (10, 20 and 40 mg/kg) orally to rats to examine their effects on anxiety behaviours. Prior to the experiments, animals were transferred to the testing room and habituated for 1 h. After each testing session, the apparatus was thoroughly cleaned with 70 % ethanol to eliminate the residual odours and traces of the previously tested animal. Differences in the behavioural data observed after the administration of 20 % Tween 20 or saline to rats were compared and found not significant (data not shown), which justified our choice to include only the Tween 20 group as control for comparison.
Experiment 1: effects of mitragynine on anxiety-like behaviours in the open-field (OF) and elevated plus-maze (EPM) tests
Five experimental groups (n = 8, each) were orally treated with different doses of mitragynine (10, 20 and 40 mg/kg), diazepam (10 mg/kg) or Tween 20 (5 ml/kg) 60 min before the OF test [14]; Popic et al. [32]. Immediately following the OF test, the animal was transferred to the EPM.
Experiment 2: the role of the opioidergic system in the anxiolytic-like effect of mitragynine in the EPM test
In this experiment, 48 rats were randomly divided into six experimental groups (n = 8, each). They were orally treated with mitragynine (40 mg/kg) or Tween 20 (5 ml/kg) 60 min before the EPM test. Morphine (6 mg/kg) and naloxone (2 mg/kg) were intraperitoneally administered 30 min before the test [30, 50]. To evaluate the possible involvement of the opioidergic system in the anxiolytic-like effects of mitragynine, two experimental groups were intraperitoneally administered naloxone (2 mg/kg) 15 min before mitragynine and morphine treatments [33]. Rats treated with mitragynine, control, morphine and naloxone were intraperitoneally administered saline solution (1 ml/kg) 15 min before oral or intraperitoneal treatments.
Experiment 3: the role of the GABAergic system in the anxiolytic-like effect of mitragynine in the EPM test
Forty-eight rats were used in this experiment. Animals were randomly divided into six experimental groups (n = 8, each). They were orally treated with mitragynine (40 mg/kg), diazepam (10 mg/kg) or Tween 20 (5 ml/kg) 60 min before the EPM test. Flumazenil (10 mg/kg) was intraperitoneally administered 15 min before the test [12]. To evaluate the possible involvement of the GABAergic system in the anxiolytic-like effects of mitragynine, two experimental groups were intraperitoneally administered flumazenil (10 mg/kg) 15 min before mitragynine and diazepam treatments [43]. Rats treated with mitragynine, control, diazepam and flumazenil were intraperitoneally administered saline solution (1 ml/kg) 15 min before oral or intraperitoneal treatments.
Experiment 4: the role of the dopaminergic system in the anxiolytic-like effect of mitragynine in the EPM test
In this experiment, 72 rats were randomly divided into nine groups (n = 8, each). Animals were orally treated with mitragynine (40 mg/kg) or Tween 20 (5 ml/kg) 60 min before the EPM test. Apomorphine (0.5 mg/kg), sulpiride (0.5 mg/kg) and SCH 23390 (0.02 mg/kg) were intraperitoneally administered 15 min before the plus-maze test [9]. To evaluate the putative involvement of the dopaminergic system in the anxiolytic-like effects of mitragynine, four experimental groups were intraperitoneally administered sulpiride (0.5 mg/kg) and SCH 23390 (0.02 mg/kg) 15 min before mitragynine and apomorphine treatments [9, 49]. Rats treated with mitragynine, control, apomorphine, sulpiride and SCH 23390 were intraperitoneally administered saline solution (1 ml/kg) 15 min before oral or intraperitoneal treatments.
Behavioural testing
OF test
The OF apparatus (Panlab, Spain) consisted of a black-painted wood arena (45 × 45 cm) with 50-cm high walls. The arena was virtually divided by software (ActitTrack, Panlab, Spain) into central and peripheral zones. Rats were individually placed at the centre of the arena and allowed to freely explore the apparatus for 5 min [29]. Activities in the central zone, including the number of entries (CZE) and the percentage of time spent in the central zone (%CZT) were automatically recorded by infrared beams and taken as a measure of anxiety [10]. The overall motor activity during the OF test was considered as the total distance travelled as previously described [13].
EPM test
The test was performed as described elsewhere [45]. The plus-maze (Columbus Instruments, Ohio, USA) consisted of two open (50 × 10 cm) and two closed (50 × 10 × 40 cm) arms, extending from a central platform (10 × 10 cm). The apparatus was made of stainless steel and elevated to a height of 50 cm above the floor. Rats were individually placed at the centre of the maze, facing an open arm and allowed to freely explore the maze for 5 min. The time spent on the open and closed arms and the number of entries made into each arm was recorded using a video camera (Sony, DCR-SX44E). Arm entries were defined as entry of all four paws into an arm. The percentage of time spent on open arms (%OAT) (100× time on open arms/total time spent in all arms) and the percentage of open arm entries (%OAE) (100× number of open arm entries/total entries into all arms) were used as a measure of anxiety [15]. The number of total arm entries was used as a measure of spontaneous locomotor activity [21].
Statistical analysis
Statistical analysis was performed with SigmaPlot 11.0 software, using one-way analysis of variance (ANOVA) followed by Duncan’s post hoc test for multiple comparisons. Data that did not pass normality or homogeneity of variance were analysed using a Kruskal–Wallis test and Dunnʼs post hoc test for multiple comparisons. Results were expressed as mean ± standard error of mean (SEM). p values <0.05 were considered statistically significant.
Conclusions
In conclusion, acute oral dosages of mitragynine (10, 20 and 40 mg/kg) produced anxiolytic-like effects in the OF and EPM tests similar to diazepam at the doses tested. These effects could be mediated via interactions among opioidergic, GABAergic and dopaminergic systems in brain regions involved in anxiety. However, this finding warrants further investigation and the identification of the exact mechanisms and brain regions involved in the anxiolytic-like effect of mitragynine are worth pursuing.
Acknowledgments
We thank Ms. Vemala Devi for her advice and help with the statistical analysis. This work was made possible by the support of a Ministry of Science, Technology and Innovation (MOSTI) Research Grant and USM Research University Grant.
References
- 1.Beng GT, Hamdan MR, Siddiqui MJ, Mordi MN, Mansor SM. A simple and cost effective isolation and purification protocol of mitragynine from Mitragyna speciosa Korth (ketum) leaves. Mal J Anal Sci. 2011;15:54–60. [Google Scholar]
- 2.Boyer EW, Babu KM, Adkins JE, McCurdy CR, Halpern JH. Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth) Addiction. 2008;103:1048–1050. doi: 10.1111/j.1360-0443.2008.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlini EA. Plants and the central nervous system. Pharmacol Biochem Behav. 2003;75:501–512. doi: 10.1016/S0091-3057(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 4.Chittrakarn S, Sawangjaroen K, Prasettho S, Janchawee B, Keawpradub N. Inhibitory effects of kratom leaf extract (Mitragyna speciosa Korth) on the rat gastrointestinal tract. J Ethnopharmacol. 2008;116:173–178. doi: 10.1016/j.jep.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Dilts RP, Kalivas PW. Autoradiographic localization of mu-opioid and neurotensin receptors within the mesolimbic dopamine system. Brain Res. 1989;488:311–327. doi: 10.1016/0006-8993(89)90723-3. [DOI] [PubMed] [Google Scholar]
- 6.Dilts RP, Kalivas PW. Autoradiographic localization of delta opioid receptors within the mesocorticolimbic dopamine system using radioiodinated [2-d-penicillamine, 5-d-penicillamine] enkephalin (125I-DPDPE) Synapse. 1990;6:121–132. doi: 10.1002/syn.890060203. [DOI] [PubMed] [Google Scholar]
- 7.Fakurazi S, Abdul Rahman S, Taufik Hidayat M, Ithnin H, Moklas MAA, Arulselvan P. The combination of mitragynine and morphine prevents the development of morphine tolerance in mice. Molecules. 2013;18:666–681. doi: 10.3390/molecules18010666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farah Idayu N, Taufik Hidayat M, Moklas MA, Sharida F, Nurul Raudzah AR, Shamima AR, Apryani E. Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa Korth in mice model of depression. Phytomed. 2011;218:402–407. doi: 10.1016/j.phymed.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Garcia AM, Martinez R, Brandão ML, Morato S. Effects of apomorphine on rat behavior in the elevated plus-maze. Physiol Behav. 2005;85:440–447. doi: 10.1016/j.physbeh.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Charoenphandhu N, Teerapornpuntakit J, Lapmanee S, Dorkkam N, Krishnamra N, Charoenphandhu J. Long-term swimming in an inescapable stressful environment attenuates the stimulatory effect of endurance swimming on duodenal calcium absorption in rats. J Physiol Sci. 2011;61:473–486. doi: 10.1007/s12576-011-0168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grewal KS. Observations on the pharmacology of mitragynine. J Pharmacol Exp Ther. 1932;46:251–271. [Google Scholar]
- 12.Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci USA. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grundmann O, Nakajima J, Seo S, Butterweck V. Anti-anxiety effects of Apocynum venetum L. in the elevated plus maze test. J Ethnopharmacol. 2007;110:406–411. doi: 10.1016/j.jep.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 14.Hazim AI, Mustapha M, Mansor SM. The effects on motor behaviour and short-term memory tasks in mice following an acute administration of Mitragyna speciosa alkaloid extract and mitragynine. J Med Plants Res. 2011;5:5810–5817. [Google Scholar]
- 15.Hellión-Ibarrola MC, Ibarrola DA, Montalbetti Y, Kennedy ML, Heinichen O, Campuzano M, Tortoriello J, Fernández S, Wasowski C, Marder M, De Lima TC, Mora S. The anxiolytic-like effects of Aloysia polystachya (Griseb.) Moldenke (Verbenaceae) in mice. J Ethnopharmacol. 2006;105:400–408. doi: 10.1016/j.jep.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Jamil MF, Subki MF, Lan TM, Majid MI, Adenan MI. The effect of mitragynine on cAMP formation and mRNA expression of mu-opioid receptors mediated by chronic morphine treatment in SK-N-SH neuroblastoma cell. J Ethnopharmacol. 2013;148:135–143. doi: 10.1016/j.jep.2013.03.078. [DOI] [PubMed] [Google Scholar]
- 17.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khor BS, Amar JM, Adenan MI, Chong SA. Mitragynine attenuates withdrawal syndrome in morphine-withdrawn zebrafish. PLoS One. 2011;6:e28340. doi: 10.1371/journal.pone.0028340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klitenick MA, DeWitte P, Kalivas PW. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J Neurosci. 1992;12:2623–2632. doi: 10.1523/JNEUROSCI.12-07-02623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langen B, Egerland U, Bernöster K, Dost R, Unverferth K, Rundfeldt C. Characterization in rats of the anxiolytic potential of ELB139 [1-(4-chlorophenyl)-4-piperidin-1-yl-1, 5-dihydro-imidazol-2-on], a new agonist at the benzodiazepine binding site of the GABAA receptor. J Pharmacol Exp Ther. 2005;314:717–724. doi: 10.1124/jpet.105.084681. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacol (Berl) 2010;207:535–545. doi: 10.1007/s00213-009-1684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macko E, Weisbach JA, Douglas B. Some observations on the pharmacology of mitragynine. Arch Int Pharmacodyn Ther. 1972;198:145–161. [PubMed] [Google Scholar]
- 23.Maruyama K, Shimoju R, Ohkubo R, Maruyama H, Kurosawa M. Tactile skin stimulation increases dopamine release in the nucleus accumbens in rats. J Physiol Sci. 2012;62:259–266. doi: 10.1007/s12576-012-0205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto K, Mizowaki M, Suchitra T, Murakami Y, Takayama H, Sakai S, Aimi N, Watanabe H. Central antinociceptive effects of mitragynine in mice: contribution of descending noradrenergic and serotonergic systems. Eur J Pharmacol. 1996;317:75–81. doi: 10.1016/S0014-2999(96)00714-5. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto K, Mizowaki M, Suchitra T, Takayama H, Sakai S, Aimi N, Watanabe H. Antinociceptive action of mitragynine in mice: evidence for the involvement of supraspinal opioid receptors. Life Sci. 1996;59:1149–1155. doi: 10.1016/0024-3205(96)00432-8. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzaki M, Matsushita H, Tomizawa K, Matsui H. Oxytocin: a therapeutic target for mental disorders. J Physiol Sci. 2012;62:441–444. doi: 10.1007/s12576-012-0232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Megarbane B, Gueye P, Baud F. Interactions between benzodiazepines and opioids. Ann Med Interne (Paris) 2003;154:S64–S72. [PubMed] [Google Scholar]
- 28.Nikolaus S, Antke C, Beu M, Müller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders—results from in vivo imaging studies. Rev Neurosci. 2010;21:119–139. doi: 10.1515/REVNEURO.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- 29.Okajima D, Kudo G, Yokota H. Antidepressant-like behavior in brain-specific angiogenesis inhibitor 2-deficient mice. J Physiol Sci. 2011;61:47–54. doi: 10.1007/s12576-010-0120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozdemir E, Gursoy S, Bagcivan I. The effects of serotonin/norepinephrine reuptake inhibitors and serotonin receptor agonist on morphine analgesia and tolerance in rats. J Physiol Sci. 2012;62:317–323. doi: 10.1007/s12576-012-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parthasarathy S, Ramanathan S, Murugaiyah V, Hamdan MR, Said MI, Lai CS, Mansor SM. A simple HPLC-DAD method for the detection and quantification of psychotropic mitragynine in Mitragyna speciosa (ketum) and its products for application in forensic investigation. Forensic Sci Int. 2013;226:183–187. doi: 10.1016/j.forsciint.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Popik P, Kostakis E, Krawczyk M, Nowak G, Szewczyk B, Krieter P, Chen Z, Russek SJ, Gibbs TT, Farb DH, Skolnick P, Lippa AS, Basile AS. The anxioselective agent 7-(2-chloropyridin-4-yl) pyrazolo-[1,5-a]-pyrimidin-3-yl](pyridin-2-yl)methanone (DOV 51892) is more efficacious than diazepam at enhancing GABA-gated currents at alpha1 subunit-containing GABAA receptors. J Pharmacol Exp Ther. 2006;319:244–252. doi: 10.1124/jpet.106.107201. [DOI] [PubMed] [Google Scholar]
- 33.Privette TH, Terrian DM. Kappa opioid agonists produce anxiolytic-like behavior on the elevated plus-maze. Psychopharmacol. 1995;118:444–450. doi: 10.1007/BF02245945. [DOI] [PubMed] [Google Scholar]
- 34.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- 35.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 36.Rezayof A, Hosseini SS, Zarrindast MR. Effects of morphine on rat behaviour in the elevated plus maze: the role of central amygdala dopamine receptors. Behav Brain Res. 2009;202:171–178. doi: 10.1016/j.bbr.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 37.Sabetghadam A, Navaratnam V, Mansor SM. Dose-response relationship, acute toxicity, and therapeutic index between the alkaloid extract of Mitragyna speciosa and its main active compound mitragynine in mice. Drug Dev Res. 2013;74:23–30. doi: 10.1002/ddr.21052. [DOI] [Google Scholar]
- 38.Sabetghadam A, Ramanathan S, Sasidharan S, Mansor SM. Subchronic exposure to mitragynine, the principal alkaloid of Mitragyna speciosa, in rats. J Ethnopharmacol. 2013;146:815–823. doi: 10.1016/j.jep.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki K, Fan LW, Tien LT, Ma T, Loh HH, Ho IK. The interaction of morphine and gamma-aminobutyric acid (GABA)ergic systems in anxiolytic behavior: using mu-opioid receptor knockout mice. Brain Res Bull. 2002;57:689–694. doi: 10.1016/S0361-9230(01)00785-7. [DOI] [PubMed] [Google Scholar]
- 40.Shader RI, Greenblatt DJ. Use of benzodiazepines in anxiety disorders. N Engl J Med. 1993;328:1398–1405. doi: 10.1056/NEJM199305133281907. [DOI] [PubMed] [Google Scholar]
- 41.Solati J, Zarrindast M, Salari AA. Dorsal hippocampal opioidergic system modulates anxiety-like behaviors in adult male Wistar rats. Psych Clin Neurosci. 2010;64:634–641. doi: 10.1111/j.1440-1819.2010.02143.x. [DOI] [PubMed] [Google Scholar]
- 42.Taufik Hidayat M, Apryani E, Nabishah B, Moklas MAA, Sharida F, Farhan MA. Determination of mitragynine bound opioid receptors. Adv Med Dent Sci. 2010;3:65–70. [Google Scholar]
- 43.Tsuda M, Suzuki T, Misawa M, Nagase H. Involvement of the opioid system in the anxiolytic effect of diazepam in mice. Eur J Pharmacol. 1996;307:7–14. doi: 10.1016/0014-2999(96)00219-1. [DOI] [PubMed] [Google Scholar]
- 44.Vicknasingam B, Narayanan S, Beng GT, Mansor SM. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21:283–288. doi: 10.1016/j.drugpo.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe K, Yano S, Horie S, Yamamoto LT. Inhibitory effect of mitragynine, an alkaloid with analgesic effect from Thai medicinal plant Mitragyna speciosa, on electrically stimulated contraction of isolated guinea-pig ileum through the opioid receptor. Life Sci. 1997;60:933–942. doi: 10.1016/S0024-3205(97)00023-4. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto LT, Horie S, Takayama H, Aimi N, Sakai S, Yano S, Shan J, Pang PK, Ponglux D, Watanabe K. Opioid receptor agonistic characteristics of mitragynine pseudoindoxyl in comparison with mitragynine derived from Thai medicinal plant Mitragyna speciosa . Gen Pharmacol. 1999;33:73–81. doi: 10.1016/S0306-3623(98)00265-1. [DOI] [PubMed] [Google Scholar]
- 48.Zarrindast MR, Babapoor-Farrokhran S, Babapoor-Farrokhran S, Rezayof A. Involvement of opioidergic system of the ventral hippocampus, the nucleus accumbens or the central amygdala in anxiety-related behavior. Life Sci. 2008;82:1175–1181. doi: 10.1016/j.lfs.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 49.Zarrindast MR, Esfahani DE, Oryan S, Nasehi M, Nami MT. Effects of dopamine receptor agonist and antagonists on cholestasis-induced anxiolytic-like behaviors in rats. Eur J Pharmacol. 2013;702:25–31. doi: 10.1016/j.ejphar.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Zarrindast MR, Rostami P, Zarei M, Roohbakhsh A. Intracerebroventricular effects of histaminergic agents on morphine-induced anxiolysis in the elevated plus-maze in rats. Basic Clin Pharmacol Toxicol. 2005;97:276–281. doi: 10.1111/j.1742-7843.2005.pto_116.x. [DOI] [PubMed] [Google Scholar]


