Abstract
Summary
We evaluated whether older adults who received kyphoplasty had reduced risk of mortality compared to those who did not. In unmatched analyses, those receiving kyphoplasty were at reduced risk of death but after matching on age and medical complications, patients who received kyphoplasty were at increased risk of death.
Purpose
In previous observational studies, kyphoplasty for treatment of osteoporotic vertebral fractures has been associated with decreased mortality compared to conservative management. The purpose of this research was to determine whether older adults who received kyphoplasty had reduced risk of mortality compared to matched patients who did not.
Methods
Retrospective cohort study of US Medicare enrollees with osteoporotic vertebral fractures between 2017–2019 comparing patients who underwent kyphoplasty to those who did not. We identified 2 control groups a priori: 1) non-augmented patients who met inclusion criteria (group 1); 2) propensity-matched patients on demographic and clinical variables (group 2). We then identified additional control groups using matching for medical complications (group 3) and age + comorbidities (group 4). We calculated hazard ratios (HRs) and 95% confidence intervals (95% CIs) associated with mortality.
Results
A total of 235,317 patients (mean (± standard deviation) age 81.1 ± 8.3 years; 85.8% female) were analyzed. In the primary analyses, those who received kyphoplasty were at reduced risk of death compared to those who did not: adjusted HR (95% CI) in group 1 = 0.84 (0.82, 0.87); and in group 2 = 0.88 (0.85, 0.91). However, in post hoc analyses, patients who received kyphoplasty were at increased risk of death: adjusted HR (95% CI) in group 3 = 1.32 (1.25, 1.41) and 1.81 (1.58, 2.09) in group 4.
Conclusion
An apparent benefit of kyphoplasty on mortality among patients with vertebral fractures was not present after rigorous propensity matching, illustrating the importance of comparing similar individuals when evaluating observational data.
Keywords: Kyphoplasty, Mortality, Osteoporosis, Propensity score, Spine
Introduction
Osteoporotic vertebral fractures are common in older adults, with an estimated prevalence of approximately 20% among North American adults over 50 years [1]. They are associated with functional limitations, decreased quality of life [2], increased risk of subsequent osteoporotic fractures [3, 4], and mortality [5].
Treatment options are limited and include use of analgesics, physical therapy [6], exercise, and education [7]. Vertebroplasty, a percutaneous injection of polymethyl-methacrylate into the affected vertebral body, is associated with small improvements in pain and function among older adults [8] but it is not endorsed by major medical societies [9, 10] because it showed little benefit compared to sham procedures in randomized trials [11]. Kyphoplasty, a similar augmentation procedure, has been shown in unblinded studies to improve back pain, function, and quality of life compared to non-surgical management [12, 13]. However, no randomized trials have compared kyphoplasty to sham procedures, and a recent analysis we conducted in a younger cohort showed that kyphoplasty was associated with increased fills of opioids and spine-related healthcare costs [14].
A recent meta-analysis reported that patients who received vertebral augmentation (either vertebroplasty or kyphoplasty) were at reduced risk of death compared to patients who received non-surgical management [15]. However, the included studies may have been affected by immortal time bias [16, 17] in which the follow-up time for survival starts at the event (osteoporotic vertebral fracture) rather than at the intervention (kyphoplasty). Thus, subjects who received kyphoplasty must have lived long enough after the osteoporotic vertebral fractures and have been healthy enough to have undergone kyphoplasty, whereas subjects who died shortly after their vertebral fractures did not have an opportunity to undergo kyphoplasty and were automatically placed in the non-surgical management group. This methodologic flaw may have biased results in favor of kyphoplasty [18, 19].
Our purpose was to determine whether kyphoplasty was associated with decreased risk of death by comparing mortality in patients who received kyphoplasty to carefully selected control patients who did not. We hypothesized that mortality rates would differ depending on which control groups were used. In the analysis that relied on adjustment, rather than matching, to account for selection bias among those who did and did not receive kyphoplasty, we hypothesized that patients who received kyphoplasty would have reduced rates of mortality compared to those who did not receive augmentation. We hypothesized that when we matched on patient demographic and clinical factors, we would observe similar death rates between the two groups.
Materials and Methods
Patient Population
We identified enrollees in the Centers for Medicare and Medicaid Services (CMS) who had International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) diagnosis codes [20] (eTable 1) from 2017–2019 that potentially indicated thoracic or lumbar osteoporotic vertebral fractures (“index fractures”). We did not include cervical vertebral fractures because the majority are not osteoporotic [21, 22]. Because some thoracic or lumbar vertebral fracture diagnosis codes were not specific to osteoporotic fractures, we also required that patients had at least one diagnosis code for osteoporosis within the year prior through the six months after their index fractures. To limit the sample to those with osteoporotic fractures, we excluded patients with neoplasms, intraspinal abscesses, inflammatory spondylosis, osteomyelitis, or transportation/spinal cord injuries in the year prior to the fracture. We excluded patients who were not enrolled in CMS ≥ 1 year before their index fracture and excluded individuals who were enrolled in health maintenance organization (HMO) plans in addition to their CMS insurance because we did not have access to HMO claims. Because we wanted to identify fills of opioid prescriptions, we excluded patients not enrolled in Part D (drug) plans (Fig. 1).
Fig. 1.
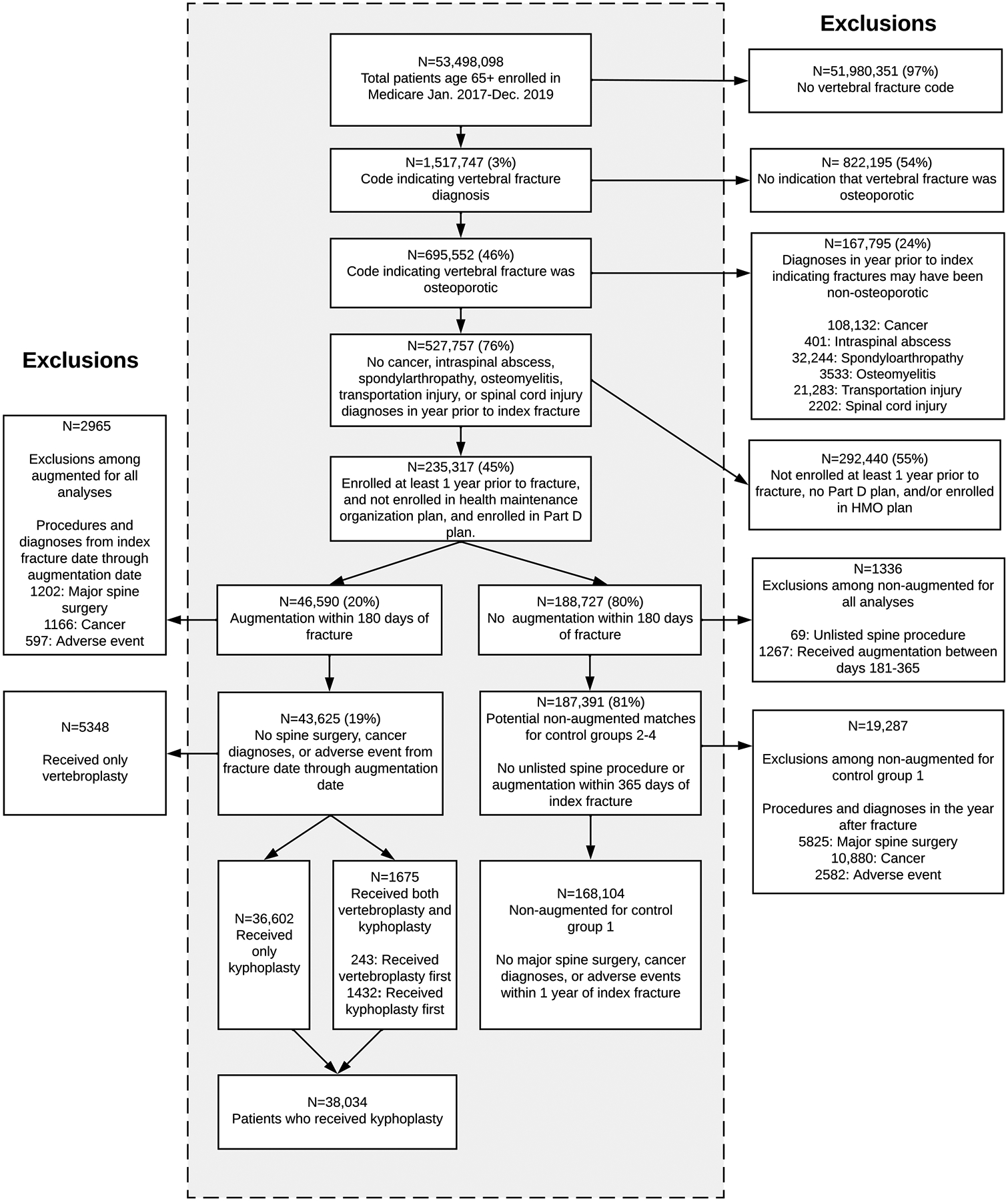
Flow of participants in analysis
Kyphoplasty identification
We identified patients with codes indicating kyphoplasty from the day of the index fracture through 180 days later and compared them to patients who did not receive augmentation (either kyphoplasty or vertebroplasty). We excluded patients who received kyphoplasty or vertebroplasty > 180 days after the index fracture or had unlisted spine procedures that may have included vertebral augmentation. We also excluded patients who had major spine surgery, cancer diagnoses, or adverse events such as cauda equina syndrome, spinal cord injuries, or spinal artery compression syndromes from the index fracture dates through the kyphoplasty dates (Fig. 1). In matched analyses, we identified a “pseudo-kyphoplasty” date for patients who did not receive augmentation procedures, defined as the index fracture date plus the number of days between the matched pair’s fracture date and the date of kyphoplasty. We did this so we could begin to assess mortality on the same day relative to the index fracture for those who did and did not receive kyphoplasty.
Non-augmented control groups: primary analyses
We identified two control groups of patients who did not receive augmentation procedures to compare with the group of patients who had kyphoplasty (eTable 2). Control group 1 was an “unmatched” group consisting of patients who met the inclusion criteria above, with the additional stipulations that patients could not have had major spine surgery, cancer diagnoses, or adverse events related to the index fracture within 1 year of the index fracture and did not undergo augmentation within 180 days of the index fracture.
Control group 2 used 1:1 propensity score matching to account for the fact that patients who underwent kyphoplasty were likely different from patients who did not receive kyphoplasty. This group was chosen a priori as the primary comparator to patients who received kyphoplasty. We used logistic regressions that included age, sex, patient race, year of index fracture, admission for the index fracture, fulfillment of pain medications (opioids, non-steroidal anti-inflammatory drugs (NSAIDs), muscle relaxers, gabapentin, benzodiazepines, pregabalin) 1–90 days prior to the index fracture, spinal region of the index fracture, whether the state paid the individual’s monthly premium for coverage (a metric for patient socioeconomic status), number of inpatient and outpatient encounters in the 1–365 days prior to the index fracture, region of residence, frailty score [23], whether the patient had major medical complications [24] (eTable 2) in the year prior to the index fracture, and Charlson co-morbidity score [25] to determine each individual’s propensity to receive kyphoplasty. Because we did not have indications of pain severity, control group 2 also matched exactly on fills of opioids and receipt of cross-sectional imaging, both of which are associated with greater pain severity [26, 27]. We matched patients who underwent kyphoplasty and who filled ≥ 1 opioid prescription between the day of the index fracture and the day before kyphoplasty to patients who did not receive augmentation but also filled ≥ 1 prescription for an opioid in the analogous period between the index fracture and the day before their pseudo-kyphoplasty. We performed a similar matching strategy for patients who did not fill opioids during this time window. We also matched patients who underwent kyphoplasty who received imaging from six weeks before the index fracture through the day before kyphoplasty to patients who did not receive kyphoplasty but received imaging during the same time. We performed similar matching for patients who did not receive imaging during this time.
Non-augmented control groups: secondary analyses
The analysis of control group 2 showed that individuals who did not receive kyphoplasty were more likely than those who did to experience major medical complications after their index fracture dates but before the pseudo-kyphoplasty dates. This suggested that patients who might have been candidates for kyphoplasty had been deemed unsafe to undergo the procedure due to concerning medical conditions, leading to “confounding by contraindication[28].” Specifically, confounding could occur if these complications were associated both with kyphoplasty (patients with complications would be less likely to be offered kyphoplasty than those without) and death (patients with these complications would be at increased risk of imminent death).
This concern motivated two post hoc secondary analyses. Control group 3 had the same requirements as group 2, but also matched exactly on whether patients had diagnosis codes for major health complications (eTable 2) that occurred after the date of the index fracture but before the date of kyphoplasty/pseudo-kyphoplasty. Control group 4 had the same requirements as control group 3 and also matched exactly for age (± 1 year) and number of comorbidities (categorized as 0, 1, 2–3, 4–5, and ≥ 6).
Outcomes
For control group 1, we began follow-up at the time of the index fracture and assessed mortality at any time after the index fracture date, emulating prior studies [15, 29, 30]. For control groups 2–4, we began ascertainment of deaths on the day of kyphoplasty/pseudo-kyphoplasty. The primary outcome using time-to-event analysis was death at any time during follow-up. We also evaluated deaths at 30 days, 6 months, and 12 months.
Statistical Analysis
Following propensity score matching, we calculated descriptive statistics for the matched variables. We created Kaplan–Meier curves and graphed the conditional probability of death among those who did and did not receive kyphoplasty to visualize unadjusted mortality. We used Cox proportional hazards regression to determine hazard ratios (HRs) and 95% confidence intervals (95% CIs), adjusted for a priori identified potential confounders: age, sex, race, year of fracture, admission for fracture, pre-fracture fills of pain medications, level of fracture, state buy-in of Medicare, healthcare utilization prior to fracture, Charlson comorbidity score derived from the year prior to fracture, and frailty score.
We used SAS version 9.4 (Cary, NC) and two-sided p-values < 0.05 were considered statistically significant.
Results
A total of 168,104 individuals who did not receive augmentation procedures met our inclusion criteria for the unmatched (control group 1) analysis and were compared to 38,034 (18.5%) patients who received kyphoplasty within 180 days (Fig. 1). Characteristics of these individuals are shown in Table 1. Those who received kyphoplasty tended to be younger, were more likely to have identified as white and to have filled pain medications prior to their fractures. Those who received kyphoplasty were at reduced risk of death starting at day 1 throughout follow-up, with an adjusted HR (95% CI) of 0.84 (0.82, 0.87) for deaths at any time (Fig. 2a; Table 2). When we examined the conditional probability of death over time (eFigure 1), deaths among patients who did not receive kyphoplasty were much higher than those who received kyphoplasty in the first ~ 100 days of follow-up, after which the conditional probability of death was similar.
Table 1.
Comparison of patients who received kyphoplasty to those who received no augmentation (control group 1)
| Variable | No Augmentation n = 168,104 (83%) | Kyphoplasty n = 38,034 (17%) | Total n = 206,138 |
|---|---|---|---|
| Age (Median, IQR) | 81.3 (74.4, 87.8) | 80.5 (74.4, 86.4) | 81.1 (74.4, 87.5) |
| Female | 146,007 (87) | 30,850 (81) | 176,857 (86) |
| Patient race | |||
| Unknown | 1287 (0.8) | 229 (0.6) | 1516 (0.7) |
| White | 150,937 (90) | 35,480 (93) | 186,417 (90) |
| Black | 3913 (2.3) | 530 (1.4) | 4443 (2.2) |
| Other | 2642 (1.6) | 413 (1.1) | 3055 (1.5) |
| Asian | 5181 (3.1) | 785 (2.1) | 5966 (2.9) |
| Hispanic | 3403 (2.0) | 483 (1.3) | 3886 (1.9) |
| North American Native | 741 (0.4) | 114 (0.3) | 855 (0.4) |
| Year of Fracture | |||
| 2017 | 60,816 (36) | 14,050 (37) | 74,866 (36) |
| 2018 | 54,758 (33) | 12,899 (34) | 67,657 (33) |
| 2019 | 52,530 (31) | 11,085 (29) | 63,615 (31) |
| Charlson Co-morbidity Score (Median, IQR) | 2.0 (1, 3) | 2.0 (1, 3) | 2.0 (1, 3) |
| 0 | 40,065 (24) | 8815 (23) | 48,880 (24) |
| 1 | 38,403 (23) | 8974 (24) | 47,377 (23) |
| 2–3 | 50,155 (30) | 11,354 (30) | 61,509 (30) |
| 4–5 | 24,118 (14) | 5337 (14) | 29,455 (14) |
| 6 + | 15,363 (9) | 3554 (9) | 18,917 (9) |
| Individual Comorbidities in year prior to fracture | |||
| Congestive heart failure | 42,235 (25) | 9603 (25) | 51,838 (25) |
| Dementia | 27,197 (16) | 4042 (11) | 31,239 (15) |
| Renal failure | 31,105 (19) | 6928 (18) | 38,033 (18) |
| Weight loss | 16,321 (10) | 3096 (8) | 19,417 (9) |
| Hemiplegia | 2480 (1.5) | 468 (1.2) | 2948 (1.4) |
| Alcohol abuse | 798 (0.5) | 236 (0.6) | 1034 (0.5) |
| Cardiac arrhythmias | 62,690 (37) | 14,916 (39) | 77,606 (38) |
| Chronic pulmonary disease | 54,032 (32) | 13,220 (35) | 67,252 (33) |
| Coagulopathy | 8263 (4.9) | 2135 (5.6) | 10,398 (5.0) |
| Complicated diabetes | 25,244 (15) | 6362 (17) | 31,606 (15) |
| Deficiency anemia | 47,309 (28) | 10,417 (27) | 57,726 (28) |
| Fluid/electrolyte disorders | 37,125 (22) | 8419 (22) | 45,544 (22) |
| Liver disease | 9016 (5) | 2348 (6) | 11,364 (6) |
| Peripheral vascular disease | 46,799 (28) | 9710 (26) | 56,509 (27) |
| Mood disorders | 39,329 (23) | 8301 (22) | 47,630 (23) |
| Pulmonary circulation disorders | 11,593 (7) | 2822 (7) | 14,415 (7) |
| HIV/AIDS | 169 (0.1) | 29 (0.1) | 198 (0.1) |
| Hypertension | 126,858 (75) | 29,730 (78) | 156,588 (76) |
| Myocardial infarction | 8371 (5) | 1871 (5) | 10,242 (5) |
| Cardiovascular disease | 33,686 (20) | 7810 (21) | 41,496 (20) |
| Rheumatic disease | 16,185 (10) | 3598 (9) | 19,783 (10) |
| Peptic ulcer disease | 3643 (2.2) | 928 (2.4) | 4571 (2.2) |
| Diabetes without complications | 38,191 (23) | 9429 (25) | 47,620 (23) |
| Frailty Scorea (Median, IQR) | 9.5 (4.8, 16.9) | 9.1 (4.7, 15.7) | 9.5 (4.8, 16.6) |
| Intermediate/High Frail Score | 124,565 (74) | 27,880 (73) | 152,445 (74) |
| Admitted for fracture | 38,785 (23) | 9307 (24) | 48,092 (23) |
| Filled ≥ 1 medication from 90 days prior—fracture date | |||
| Opioid | 43,018 (26) | 14,936 (39) | 57,954 (28) |
| Muscle relaxer | 13,381 (8) | 5100 (13) | 18,481 (9) |
| Gabapentin | 11,907 (7) | 2625 (7) | 14,532 (7) |
| Benzodiazepine | 16,185 (10) | 4525 (12) | 20,710 (10) |
| NSAID | 10,875 (6) | 3086 (8) | 13,961 (7) |
| Pregabalin | 13,558 (8) | 3009 (8) | 16,567 (8) |
| Inpatient encounters in year prior to fracture (Median, IQR) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) |
| Outpatient encounters in year prior to fracture (Median, IQR) | 4 (2, 10) | 5 (2, 10) | 4 (2, 10) |
| Fracture Level | |||
| Lumbar | 69,113 (41) | 18,486 (49) | 87,599 (43) |
| Thoracic | 60,600 (36) | 12,399 (33) | 72,999 (35) |
| Thoracolumbar or Unspecified | 38,391 (23) | 7149 (19) | 45,540 (22) |
| Patient had buy-in from state b | 37,350 (22) | 6027 (16) | 43,377 (21) |
| Days from fracture to kyphoplasty | – | 13 (4, 31) | 13 (4, 31) |
| Days of follow-up after fracture (Median, IQR) | 456 (203, 746) | 487 (243, 761) | 456 (213, 761) |
Frail score derived from Gilbert et al.[23]
Patients whose states (including state Medicaid programs) paid their monthly premiums
HIV/AIDS Human immunodeficiency virus/acquired immunodeficiency syndrome, IQR Interquartile range, NSAID Non-steroidal anti-inflammatory drug
Fig. 2.
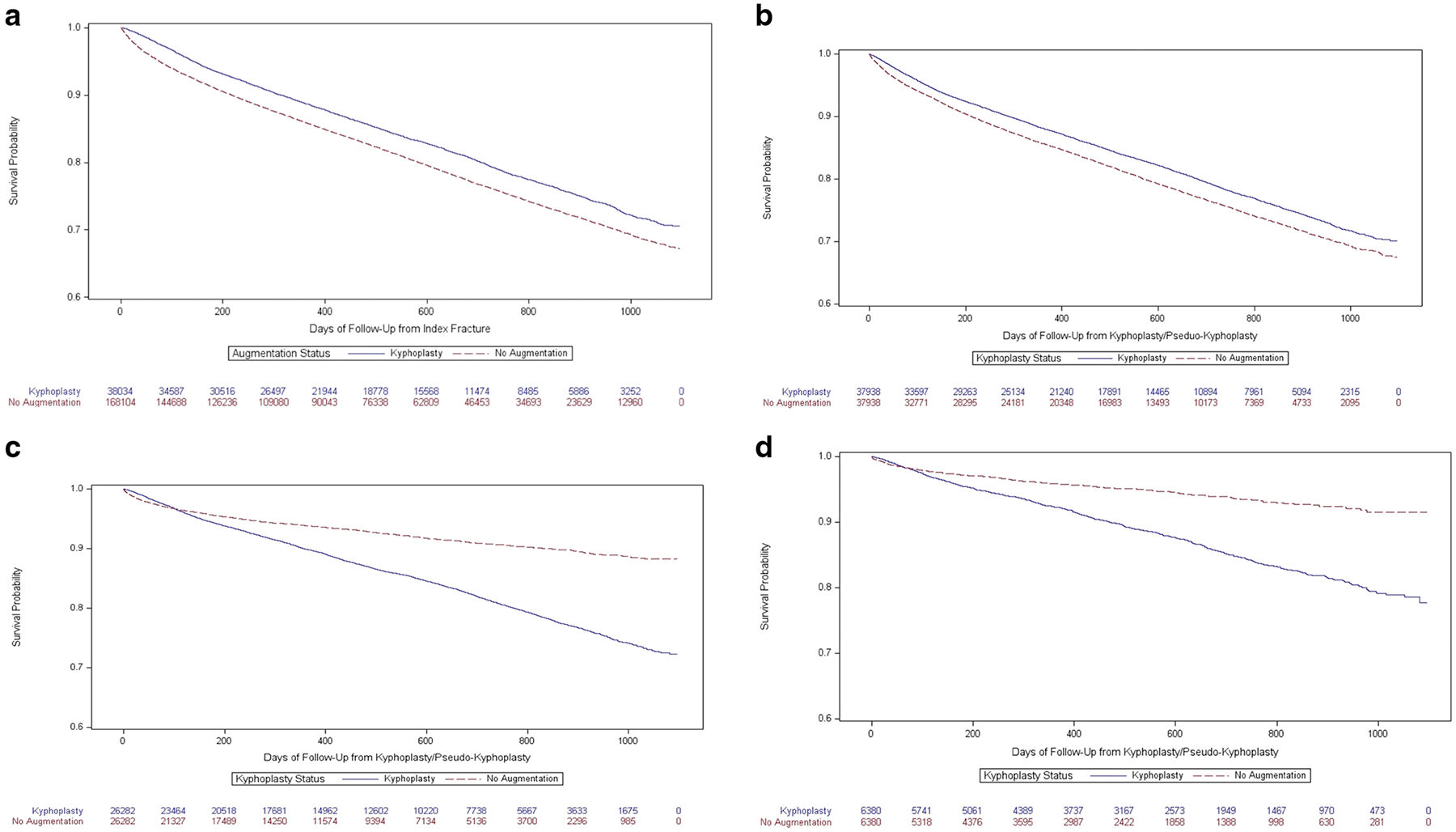
a-d Kaplan Meier curves comparing mortality among patients who received kyphoplasty to those who did not among 4 different matched groups
Table 2.
Numbers, percentages, and hazard ratios associated with 30-day, 6-month, 12-month, and any time mortality among patients who received kyphoplasty versus those who did not receive augmentation
| CONTROL GROUP 1 | CONTROL GROUP 2 | CONTROL GROUP 3 | CONTROL GROUP 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Augmentation n = 168,104 |
Kyphoplasty n = 38,034 |
Adjusteda hazard ratio (95% CI) | No Augmentation n = 37,938 |
Kyphoplasty n = 37,938 |
Adjusteda hazard ratio (95% CI) | No Augmentation n = 26,282 |
Kyphoplasty n = 26,282 |
Adjusteda hazard ratio (95% CI) | No Augmentation n = 6380 |
Kyphoplasty n = 6380 |
Adjusteda hazard ratio (95% CI) | |
| Death within 30 days | 4383 (2.6) | 278 (0.7) | 0.29 (0.26, 0.33) | 960 (2.5) | 464 (1.2) | 0.53 (0.47, 0.59) | 445 (1.7) | 224 (0.9) | 0.35 (0.29, 0.41) | 65 (1.0) | 44 (0.7) | 0.45 (0.30, 0.68) |
| Median (IQR) time to death (days) | 13 (7–21)b | 18 (12–24)b | – | 13 (6–22)c | 17 (10–24)c | – | 11 (4, 18)c | 17 (10, 24)c | – | 9 (2, 21)c | 19.5 (9.5, 25.5c | – |
| Death within 6 months | 14,150 (8.4) | 2291 (6.0) | 0.71 (0.67, 0.74) | 3195 (8.4) | 2536 (6.7) | 0.82 (0.78, 0.86) | 1059 (4.0) | 1420 (5.4) | 0.87 (0.80, 0.94) | 165 (2.6) | 273 (4.3) | 1.25 (1.03, 1.53) |
| Median (IQR) time to death (days) | 60 (23–113)b | 94 (52–134)b | – | 62 (24–119)c | 80 (41–125)c | – | 41 (13–94)c | 87 (45–128)c | – | 41 (14, 95)c | 89 (47, 130)c | – |
| Death within 1 year | 20,845 (12.4) | 3781 (9.9) | 0.78 (0.75–0.81) | 4769 (12.6) | 3946 (10.4) | 0.84 (0.81, 0.88) | 1340 (5.1) | 2293 (8.7) | 1.06 (0.99, 1.13) | 217 (3.4) | 424 (6.7) | 1.45 (1.23, 1.72) |
| Median (IQR) time to death (days) | 110 (39–217)b | 148 (78–245)b | – | 118 (40–217)c | 131 (61–235)c | – | 65 (19–164)c | 139 (69–244)c | – | 75 (24, 177)c | 135 (66, 235)c | – |
| Death any time | 31,977 (19.0) | 6339 (16.7) | 0.84 (0.82, 0.87) | 7134 (18.8) | 6323 (16.7) | 0.88 (0.85, 0.91) | 1684 (6.4) | 3850 (14.7) | 1.32 (1.25, 1.41) | 283 (4.4) | 748 (11.7) | 1.81 (1.58, 2.09) |
| Median (IQR) time to death (days) | 225 (72–480)b | 284 (124–524)b | – | 217 (72–461)c | 259 (102–500)c | – | 105 (27, 288)c | 285 (115, 522)c | – | 132 (32, 342)c | 306 (115, 541)c | – |
Adjusted for age, sex, race, year of fracture, admission for fracture, pre-fracture fills of opioids, muscle relaxants, gabapentin, benzodiazepine, pregabalin, level of fracture, state buy-in of Medicare, healthcare utilization prior to fracture, Charlson comorbidity score derived from the year prior to fracture, and frailty score
Time to death in control group 1 measured from fracture
Time to death in control groups 2–4 measured from augmentation/pseudo-augmentation 95% CI 95% confidence interval, IQR Interquartile range
For the matched (control groups 2–4) analyses, a total of 187,391 individuals who did not receive augmentation procedures met the inclusion criteria (Fig. 1). For control group 2, 37,938 (99.7%) individuals who received kyphoplasty were propensity-matched to controls who had the same pattern of filling opioid prescriptions and receiving advanced imaging prior to kyphoplasty/pseudo-kyphoplasty. Patients who had kyphoplasty were slightly younger, less frail, less likely to have been admitted for the index fracture and had fewer complications in the time between the index fracture and their kyphoplasties than those who did not receive kyphoplasty (Table 3). Like control group 1, those who received kyphoplasty were at reduced risk of death throughout follow-up (Fig. 2b; Table 2) but again we noted in the conditional probability plot that the majority of the differences in death rates occurred in the first ~ 100 days of follow-up, after which rates of death were similar (eFigure 2).
Table 3.
Comparison of patients who received kyphoplasty to propensity matched patients who did not receive augmentation. Exact match on opioids and advanced imaging between fracture and kyphoplasty/pseudo-kyphoplasty (control group 2)
| Variable | No Augmentation n = 37,938 (50%) n (%) |
Kyphoplasty n = 37,938 (50%) n (%) |
Total n = 75,876 |
|---|---|---|---|
| Age (Median, IQR) | 80.7 (74.1, 87.2) | 80.5 (74.4, 86.4) | 80.6 (74.2, 86.8) |
| Female | 31,726 (84) | 30,823 (81) | 62,549 (82) |
| Patient race | |||
| Unknown | 210 (1) | 229 (1) | 439 (1) |
| White | 35,159 (93) | 35,384 (93) | 70,543 (93) |
| Black | 640 (2) | 530 (1) | 1170 (2) |
| Other | 466 (1) | 413 (1) | 879 (1) |
| Asian | 853 (2) | 785 (2) | 1638 (2) |
| Hispanic | 497 (1) | 483 (1) | 980 (1) |
| North American Native | 113 (0) | 114 (0) | 227 (0) |
| Year of Fracture | |||
| 2017 | 13,762 (36) | 14,007 (37) | 27,769 (37) |
| 2018 | 12,909 (34) | 12,864 (34) | 25,773 (34) |
| 2019 | 11,267 (30) | 11,067 (29) | 22,334 (29) |
| Charlson Co-morbidity Score (Median, IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| 0 | 8715 (23) | 8800 (23) | 17,515 (23) |
| 1 | 8653 (23) | 8949 (24) | 17,602 (23) |
| 2–3 | 11,451 (30) | 11,323 (30) | 22,774 (30) |
| 4–5 | 5504 (15) | 5321 (14) | 10,825 (14) |
| 6 + | 3615 (10) | 3545 (9) | 7160 (9) |
| Comorbidities in year prior to fracture | |||
| Congestive heart failure | 9628 (25) | 9578 (25) | 19,206 (25) |
| Dementia | 4702 (12) | 4041 (11) | 8743 (12) |
| Renal failure | 6989 (18) | 6914 (18) | 13,903 (18) |
| Weight loss | 3128 (8) | 3089 (8) | 6217 (8) |
| Hemiplegia | 515 (1) | 468 (1) | 983 (1) |
| Alcohol abuse | 208 (1) | 234 (1) | 442 (1) |
| Cardiac arrhythmias | 14,891 (39) | 14,870 (39) | 29,761 (39) |
| Chronic pulmonary disease | 12,848 (34) | 13,174 (35) | 26,022 (34) |
| Coagulopathy | 2126 (6) | 2119 (6) | 4245 (6) |
| Complicated diabetes | 6297 (17) | 6338 (17) | 12,635 (17) |
| Deficiency anemia | 10,768 (28) | 10,393 (27) | 21,161 (28) |
| Fluid/electrolyte disorders | 8618 (23) | 8398 (22) | 17,016 (22) |
| Liver disease | 2284 (6) | 2332 (6) | 4616 (6) |
| Peripheral vascular disease | 10,132 (27) | 9693 (26) | 19,825 (26) |
| Mood disorders | 8729 (23) | 8290 (22) | 17,019 (22) |
| Pulmonary circulation disorders | 2746 (7) | 2816 (7) | 5562 (7) |
| HIV/AIDS | 29 (0) | 29 (0) | 58 (0) |
| Hypertension | 29,654 (78) | 29,644 (78) | 59,298 (78) |
| Myocardial infarction | 1961 (5) | 1868 (5) | 3829 (5) |
| Cardiovascular disease | 8203 (22) | 7784 (21) | 15,987 (21) |
| Rheumatic disease | 3763 (10) | 3598 (9) | 7361 (10) |
| Peptic ulcer disease | 896 (2) | 924 (2) | 1820 (2) |
| Diabetes without complications | 9402 (25) | 9394 (25) | 18,796 (25) |
| Frailty Score a | 9.7 (5.0–16.8) | 9.1 (4.7–15.7) | 9.4 (4.8–16.2) |
| Intermediate/High Frail Score | 28,506 (75) | 27,8218 (73) | 56,327 (74) |
| Admitted for fracture | 11,243 (30) | 9307 (25) | 20,550 (27) |
| Filled ≥ 1 medication from 90 days prior—fracture date | |||
| Opioid | 15,001 (40) | 14,840 (39) | 29,841 (39) |
| Muscle relaxer | 4738 (12) | 5038 (13) | 9776 (13) |
| Gabapentin | 2897 (8) | 2620 (7) | 5517 (7) |
| Benzodiazepine | 4576 (12) | 4511 (12) | 9087 (12) |
| NSAID | 3056 (8) | 3057 (8) | 6113 (8) |
| Pregabalin | 3167 (8) | 2999 (8) | 6166 (8) |
| Inpatient encounters in year prior to fracture (Median, IQR) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) |
| Outpatient encounters in year prior to fracture (Median, IQR) | 5 (2, 10) | 5 (2, 10) | 5 (2, 10) |
| Fracture Level | |||
| Lumbar | 18,804 (50) | 18,403 (49) | 37,207 (49) |
| Thoracic | 12,484 (33) | 12,387 (33) | 24,871 (33) |
| Thoracolumbar or Unspecified | 6650 (18) | 7148 (19) | 13,798 (18) |
| Patient had buy-in from state b | 6778 (18) | 6026 (16) | 12,804 (17) |
| Received advanced imaging in from 6 weeks prior to index fracture through kyphoplasty/pseudo-kyphoplasty | 35,782 (94) | 35,781 (94) | 71,562 (94) |
| Filled prescription for opioid from index fracture through kyphoplasty/pseudo-kyphoplasty | 14,381 (38) | 14,381 (38) | 28,762 (38) |
| Complications between index fracture and kyphoplasty/pseudo-kyphoplasty | |||
| Cardiac arrest | 60 (0) | 35 (0) | 95 (0) |
| Acute myocardial infarction | 506 (1) | 318 (1) | 824 (1) |
| Respiratory failure | 612 (1) | 381 (1) | 993 (1) |
| Pulmonary embolism | 288 (1) | 295 (1) | 583 (1) |
| Pneumonia | 2003 (5) | 1602 (4) | 3605 (5) |
| Stroke | 1171 (3) | 686 (2) | 1857 (2) |
| CPR | 19 (0) | 9 (0) | 28 (0) |
| Intubation | 164 (0) | 70 (0) | 234 (0) |
| Ventilator | 223 (1) | 85 (0) | 308 (0) |
| Deep vein thrombosis | 452 (1) | 378 (1) | 830 (1) |
| Hip fracture | 1438 (4) | 460 (1) | 1898 (3) |
| Paralysis | 350 (1) | 160 (0) | 510 (1) |
| Weight Loss | 2019 (5) | 1541 (4) | 3560 (5) |
| Liver Disease | 1046 (3) | 1072 (3) | 2118 (3) |
| HIV/AIDS | 11 (0) | 15 (0) | 26 (0) |
| Coagulopathy | 1288 (3) | 1250 (3) | 2538 (3) |
| Fluid and electrolyte disorders | 6578 (17) | 5980 (16) | 12,558 (17) |
| Sepsis | 986 (3) | 561 (1) | 1547 (2) |
| Gastrointestinal bleed | 400 (1) | 366 (1) | 766 (1) |
| Any complication | 11,485 (30) | 10,176 (27) | 21,661 (29) |
| Days from fracture to kyphoplasty (Median, IQR) | – | 13 (4, 31) | 13 (4, 31) |
| Days of follow-up after kyphoplasty/pseudo-kyphoplasty (Median, IQR) | 442 (196, 726) | 467 (217, 747) | 453 (207, 734) |
| Death prior to matched pair receiving kyphoplasty | 0 | – | 0 |
Frail score derived from Gilbert et al.[23]
Patients whose states (including state Medicaid programs) paid their monthly premiums
HIV/AIDS Human immunodeficiency virus/acquired immunodeficiency syndrome, IQR Interquartile range, NSAID Non-steroidal anti-inflammatory drug
Post hoc analyses applied more stringent propensity matching to account for medical complications between the index fracture and kyphoplasty/pseudo-kyphoplasty, comorbidities, and age. For control group 3, which used the same matching as control group 2 and included exact matching on medical complications, 26,282 (69.1%) patients who received kyphoplasty were matched to patients who did not. Descriptive characteristics of the matched individuals (Table 4) show that patients who received kyphoplasty were older on average, had more comorbidities, and were frailer. When we examined mortality rates, patients who received kyphoplasty were at lower risk of death in the beginning of follow-up, but after about a year they were at greater risk of death (Fig. 2c). Overall, patients who received kyphoplasty were at 32% (95% CI: 25%−41%) increased risk of death compared to those who did not (Table 2). The conditional probability of death was greater in those who did not receive kyphoplasty through the first weeks of follow-up but later became greater in those who received kyphoplasty (eFigure 3).
Table 4.
Comparison of patients who received kyphoplasty to propensity matched patients who did not receive augmentation. Exact match on opioids, advanced imaging, and diagnoses of complications between fracture and kyphoplasty/pseudo-kyphoplasty (control group 3)
| Variable | No Augmentation n = 26,282 (50%) n (%) |
Kyphoplasty n = 26,282 (50%) n (%) |
Total n = 52,564 |
|---|---|---|---|
| Age (Median, IQR) | 78.9 (72.8, 85.7) | 80.5 (74.5, 86.4) | 79.8 (73.6, 86.1) |
| Female | 22,506 (86) | 21,801 (83) | 44,307 (84) |
| Patient race | |||
| Unknown | 237 (1) | 157 (1) | 394 (1) |
| White | 24,214 (92) | 24,384 (93) | 48,598 (92) |
| Black | 365 (1) | 397 (2) | 762 (1) |
| Other | 386 (1) | 317 (1) | 703 (1) |
| Asian | 614 (2) | 571 (2) | 1185 (2) |
| Hispanic | 390 (1) | 367 (1) | 757 (1) |
| North American Native | 76 (0) | 89 (0) | 165 (0) |
| Year of Fracture | |||
| 2017 | 6249 (24) | 9673 (37) | 15,922 (30) |
| 2018 | 8321 (32) | 8823 (34) | 17,144 (33) |
| 2019 | 11,712 (45) | 7786 (30) | 19,498 (37) |
| Charlson Co-morbidity Score (Median, IQR) | 1 (0, 3) | 2 (1, 3) | 1 (0, 3) |
| 0 | 8772 (33) | 6459 (25) | 15,231 (29) |
| 1 | 6773 (26) | 6361 (24) | 13,134 (25) |
| 2–3 | 6873 (26) | 7812 (30) | 14,685 (28) |
| 4–5 | 2608 (10) | 3477 (13) | 6085 (12) |
| 6 + | 1256 (5) | 2173 (8) | 3429 (7) |
| Comorbidities in year prior to fracture | |||
| Congestive heart failure | 4381 (17) | 6198 (24) | 10,579 (20) |
| Dementia | 2411 (9) | 2979 (11) | 5390 (10) |
| Renal failure | 3417 (13) | 4597 (17) | 8014 (15) |
| Weight loss | 1356 (5) | 2016 (8) | 3372 (6) |
| Hemiplegia | 153 (1) | 275 (1) | 428 (1) |
| Alcohol abuse | 60 (0) | 108 (0) | 168 (0) |
| Cardiac arrhythmias | 7902 (30) | 9732 (37) | 17,634 (34) |
| Chronic pulmonary disease | 6690 (25) | 8596 (33) | 15,286 (29) |
| Coagulopathy | 624 (2) | 1164 (4) | 1788 (3) |
| Complicated diabetes | 3385 (13) | 4122 (16) | 7507 (14) |
| Deficiency anemia | 5474 (21) | 6874 (26) | 12,348 (23) |
| Fluid/electrolyte disorders | 3659 (14) | 5390 (21) | 9049 (17) |
| Liver disease | 866 (3) | 1386 (5) | 2252 (4) |
| Peripheral vascular disease | 5373 (20) | 6521 (25) | 11,894 (23) |
| Mood disorders | 5075 (19) | 5765 (22) | 10,840 (21) |
| Pulmonary circulation disorders | 1092 (4) | 1676 (6) | 2768 (5) |
| HIV/AIDS | 0 | 12 (0) | 12 (0) |
| Hypertension | 18,605 (71) | 20,128 (77) | 38,733 (74) |
| Myocardial infarction | 797 (3) | 1163 (4) | 1960 (4) |
| Cardiovascular disease | 3926 (15) | 5148 (20) | 9074 (17) |
| Rheumatic disease | 2307 (9) | 2447 (9) | 4754 (9) |
| Peptic ulcer disease | 447 (2) | 608 (2) | 1055 (2) |
| Diabetes without complications | 5274 (20) | 6280 (24) | 11,554 (22) |
| Frailty Score a | 7.5 (3.8, 12.9) | 8.9 (4.6, 15.3) | 8.2 (4.1, 14.1) |
| Intermediate/High Frail Score | 17,490 (67) | 19,068 (73) | 36,558 (70) |
| Admitted for fracture | 4766 (18) | 5127 (20) | 9893 (19) |
| Filled ≥ 1 medication from 90 days prior—fracture date | |||
| Opioid | 8813 (34) | 9571 (36) | 18,384 (35) |
| Muscle relaxer | 3328 (13) | 3182 (12) | 6510 (12) |
| Gabapentin | 1642 (6) | 1837 (7) | 3479 (7) |
| Benzodiazepine | 2677 (10) | 3054 (12) | 5731 (11) |
| NSAID | 2276 (9) | 2115 (8) | 4391 (8) |
| Pregabalin | 1788 (7) | 2088 (8) | 3876 (7) |
| Inpatient encounters in year prior to fracture (Median, IQR) | 0 (0, 0) | 0 (0, 1) | 0 (0, 0.5) |
| Outpatient encounters in year prior to fracture (Median, IQR) | 4 (1, 9) | 5 (2, 10) | 4 (2, 9) |
| Fracture Level | |||
| Lumbar | 13,267 (50) | 12,187 (46) | 25,454 (48) |
| Thoracic | 8521 (32) | 8903 (34) | 17,424 (33) |
| Thoracolumbar or Unspecified | 4494 (17) | 5192 (20) | 9686 (18) |
| Patient had buy-in from state b | 4078 (17) | 4327 (16) | 8405 (16) |
| Received advanced imaging in from 6 weeks prior to fracture through kyphoplasty/pseudo-kyphoplasty | 24,184 (92) | 24,184 (92) | 48,368 (92) |
| Filled prescription for opioid from fracture to kyphoplasty/pseudo-kyphoplasty | 8321 (32) | 8321 (32) | 16,642 (32) |
| Complications between index fracture and kyphoplasty/ pseudo-kyphoplasty | |||
| Cardiac arrest | 0 | 0 | 0 |
| Acute myocardial infarction | 31 (0) | 31 (0) | 62 (0) |
| Respiratory failure | 23 (0) | 23 (0) | 46 (0) |
| Pulmonary embolism | 23 (0) | 23 (0) | 46 (0) |
| Pneumonia | 283 (1) | 283 (1) | 566 (1) |
| Stroke | 141 (1) | 141 (1) | 282 (1) |
| CPR | 0 | 0 | 0 |
| Intubation | 0 | 0 | 0 |
| Ventilator | 0 | 0 | 0 |
| Deep vein thrombosis | 37 (0) | 37 (0) | 74 (0) |
| Hip fracture | 116 (0) | 116 (0) | 232 (0) |
| Paralysis | 18 (0) | 18 (0) | 36 (0) |
| Weight Loss | 416 (2) | 416 (2) | 832 (2) |
| Liver Disease | 225 (1) | 225 (1) | 450 (1) |
| HIV/AIDS | 0 | 0 | 0 |
| Coagulopathy | 194 (1) | 194 (1) | 388 (1) |
| Fluid and electrolyte disorders | 2568 (10) | 2568 (10) | 5136 (10) |
| Sepsis | 57 (0) | 57 (0) | 114 (0) |
| Gastrointestinal bleed | 43 (0) | 43 (0) | 86 (0) |
| Any complication | 3619 (14) | 3619 (14) | 7238 (14) |
| Days from fracture to kyphoplasty (Median, IQR) | – | 12 (4, 29) | 12 (4, 29) |
| Days of follow-up after kyphoplasty/pseudo-kyphoplasty (Median, IQR) | 341 (142, 629) | 478 (229, 755) | 408 (178, 698) |
| Death prior to matched pair receiving kyphoplasty | 0 | – | 0 |
Frail score derived from Gilbert et al.[23]
Patients whose states (including state Medicaid programs) paid their monthly premiums
HIV/AIDS Human immunodeficiency virus/acquired immunodeficiency syndrome, IQR Interquartile range, NSAID Non-steroidal anti-inflammatory drug
Finally, in control group 4, which included the same matching criteria as control group 3 with the added stipulation of close matching on age and comorbidities, 6380 (16.8%) kyphoplasty patients matched to non-augmented controls. Characteristics of these individuals (eTable 3) indicate that, as expected, those who did and did not receive kyphoplasty were very similar in age, number of comorbidities, and most specific comorbidities, but the group that received kyphoplasty was somewhat frailer and more likely to have been admitted for the index fracture. In our analyses of mortality in control group 4, we found that patients who received kyphoplasty were at reduced risk of death in the first 30 days after kyphoplasty/pseudo-kyphoplasty (adjusted HR (95% CI): 0.45 (0.30, 0.68)) but at increased risk of death at all subsequent timepoints, including over total follow-up (1.81 (1.58, 2.09)) (Fig. 2d; Table 2; eFigure 4).
Discussion
This study provides important evidence that the composition of patients in the control groups of observational studies examining the effectiveness of an intervention can meaningfully alter the effect estimates. In particular, analysis strategies need to account for time-varying factors that may be important confounders. We found that in unmatched analyses assessing mortality after osteoporotic vertebral fractures, patients who received kyphoplasty were at decreased risk of death. However, when we used increasingly careful matching to account for the fact that those who received kyphoplasty were healthier than those who did not, we found an increased overall risk of death among those who received kyphoplasty. These differences in results depending on matching technique highlight the importance of choosing proper control groups in observational studies.
Our findings differ from those of a 2020 meta-analysis of observational studies [15]. The authors included 7 studies, 4 of which [31–34] included patients who received vertebroplasty, not kyphoplasty. The largest of the studies on kyphoplasty included over 2 million osteoporotic vertebral fracture patients enrolled in Medicare from 2005–2014 by Ong et al. [29]. These authors determined that those who received kyphoplasty had a 19% (95% CI: 19–19%) lower adjusted 10-year mortality risk than the cohort that did not receive augmentation procedures, which was defined in a similar way as our control group 1. Furthermore, their method of assessing death from the date of the index fracture date was similar to our analyses of control group 1, in which we found a decreased adjusted risk of death at any time (16% (13%−18%)). However, by assessing death beginning at the osteoporotic vertebral fracture and allowing kyphoplasty to have occurred up to 1 year later, this study was subject to immortal time bias. Those who received kyphoplasty had to have survived long enough to receive it by design, but those who died soon after their fractures and were less likely to have been healthy enough to have been offered kyphoplasty were placed in the non-surgical management group.
Since the 2020 meta-analysis, a study by Lohan et al. also examined mortality among those who received kyphoplasty at one institution compared to those who did not receive augmentation procedures at a different institution [35]. These authors concluded that there was no difference in adjusted mortality between those who received kyphoplasty and those who were treated conservatively.
Confounding by indication can occur when treatments are influenced by patient or clinician impressions of disease severity or prognosis. We anticipated addressing this in the primary analyses using control group 2, which matched > 99% of individuals who received kyphoplasty to controls and showed a beneficial effect of kyphoplasty on mortality, but it occurred considerably earlier than expected. The posited mechanisms [15] in which kyphoplasty may reduce mortality include improvement in pulmonary function by reducing kyphosis [36, 37] and providing pain relief [38] allowing patients to resume their usual activities more readily compared to conservative treatment. We thought it unlikely that these factors would lead to death within 1 month in those who did not receive kyphoplasty, but instead might lead to differential mortality rates over months or years. Additionally, matched controls in control group 2 had notably higher comorbidities and medical complications occurring prior to the date of pseudo-kyphoplasty. With more stringent matching, lower proportions of those who received kyphoplasty matched to controls but the direction of effect of kyphoplasty reversed, indicating a detrimental effect on mortality. This likely reflects confounding by contraindication, a type of bias in which the indications for not receiving a treatment are influenced by disease severity or prognosis. This may have manifested as pre-procedural concerns that some patients could not tolerate kyphoplasty, leading to them preferentially not being offered the procedure. Our findings demonstrate how confounding by contraindication can bias effect estimates in observational studies, but also illustrate how such biases can be detected and accounted for, even using administrative data.
Our findings also indicate that, by selecting patients who are most likely to tolerate kyphoplasty, providers may be inadvertently selecting those patients who are most likely to survive in general. However, once this selection is accounted for by rigorously matching such patients to controls, kyphoplasty is actually associated with increased mortality risk. Since no blinded randomized control trials comparing kyphoplasty to sham controls have been conducted, the only evidence evaluating mortality rates have compared kyphoplasty to non-surgical management. In these two studies, researchers found equal rates of mortality in the kyphoplasty and non-surgical management groups [12, 39] but death rates were very low in both studies and neither was powered to detect differences in mortality.
Our study included a large, geographically representative population of older adults with osteoporotic vertebral compression fractures in the United States. Furthermore, as our control groups became more similar to patients who received kyphoplasty, we found a dose–response relationship of an increased risk of mortality among those who received kyphoplasty. Our study had some limitations, however. While we applied many methods including exclusions, propensity matching and exact matching of carefully selected variables, we cannot be certain that our results are not affected by residual confounding, as the patients who underwent kyphoplasty may have been meaningfully different from those who did not. Additionally, analyses of control groups 3 and 4 were not planned a priori.
Conclusions
In analyses with limited matching for age, comorbidities, and complications, we found that kyphoplasty was associated with a reduced risk of death, which might be due to the kyphoplasty, but could be attributed to confounding. After incrementally more rigorous matching for potential confounders including age, comorbidities, pain medications, imaging, and major medical complications that occurred prior to kyphoplasty/pseudo-kyphoplasty, we found that receipt of kyphoplasty was associated with a decreasing benefit that turned into a statistically significantly increased risk of death after 30 days. These findings illustrate how studies of the effects of treatment using administrative data can detect and account for sources of confounding, including confounding by contraindication. Our work also emphasizes how difficult it can be to analyze and interpret observational studies and that healthcare providers, policy makers and patients must be extremely cautious about concluding that an intervention conveys a benefit in an event as rare as mortality without supporting evidence from RCTs.
Supplementary Material
Acknowledgements
We would like to acknowledge Dr. Brook I. Martin for his help with obtaining the data used for these analyses.
Funding
This work was supported by the University of Washington Clinical Learning, Evidence, And Research (CLEAR) Center for Musculoskeletal Disorders, Administrative, Methodologic and Resource Cores and NIAMS/NIH grant P30AR072572. The funding source had no role in the study design, collection, analysis and interpretation of the data, writing of the report, or the decision to submit this article for publication.
Competing interests
Drs. Gold, Suri, O’Reilly, and Heagerty do not have competing interests. David F. Kalmes discloses that he has ownership/stock in Kypheze, LLC; patents involved in spine augmentation; and has received research support and royalties from Medtronic. Dr. Jeffrey G Jarvik reports royalties as a book co-editor from Springer Publishing and travel reimbursement for Faculty Board of Review from GE-Association of University Radiologists Radiology Research Academic Fellowship (GERRAF) and royalties as a chapter author from Wolters Kluwer/UpToDate.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00198-023-06796-6.
Statement of Human and Animal Rights In accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments, this study was determined to be exempt from review by the University of Washington Institutional Review Board.
References
- 1.Ballane G, Cauley JA, Luckey MM, El-Hajj Fuleihan G (2017) Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int 28:1531–1542. 10.1007/s00198-017-3909-3 [DOI] [PubMed] [Google Scholar]
- 2.Suzuki N, Ogikubo O, Hansson T (2009) The prognosis for pain, disability, activities of daily living and quality of life after an acute osteoporotic vertebral body fracture: its relation to fracture level, type of fracture and grade of fracture deformation. Eur Spine J 18:77–88. 10.1007/s00586-008-0847-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balasubramanian A, Zhang J, Chen L, Wenkert D, Daigle SG, Grauer A, Curtis JR (2019) Risk of subsequent fracture after prior fracture among older women. Osteoporos Int 30:79–92. 10.1007/s00198-018-4732-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inose H, Kato T, Ichimura S et al. (2021) Risk factors for subsequent vertebral fracture after acute osteoporotic vertebral fractures. Eur Spine J 30:2698–2707. 10.1007/s00586-021-06741-3 [DOI] [PubMed] [Google Scholar]
- 5.Jalava T, Sarna S, Pylkkanen L et al. (2003) Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 18:1254–1260. 10.1359/jbmr.2003.18.7.1254 [DOI] [PubMed] [Google Scholar]
- 6.LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, Siris ES (2022) The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 10.1007/s00198-021-05900-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto D, Alshahrani M, Chapurlat R et al. (2022) The global approach to rehabilitation following an osteoporotic fragility fracture: A review of the rehabilitation working group of the International Osteoporosis Foundation (IOF) committee of scientific advisors. Osteoporos Int 33:527–540. 10.1007/s00198-021-06240-7 [DOI] [PubMed] [Google Scholar]
- 8.Chou R, Fu R, Dana T, Pappas M, Hart E, Mauer KM (2021) Interventional Treatments for Acute and Chronic Pain: Systematic Review. In Reviews ACE (ed) Report No: 21-EHC030 Rockville (MD) [PubMed] [Google Scholar]
- 9.Ebeling PR, Akesson K, Bauer DC et al. (2019) The Efficacy and Safety of Vertebral Augmentation: A Second ASBMR Task Force Report. J Bone Miner Res 34:3–21. 10.1002/jbmr.3653 [DOI] [PubMed] [Google Scholar]
- 10.McGuire R (2011) AAOS Clinical Practice Guideline: the Treatment of Symptomatic Osteoporotic Spinal Compression Fractures. J Am Acad Orthop Surg 19:183–184. 10.5435/00124635-201103000-00008 [DOI] [PubMed] [Google Scholar]
- 11.Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, Graves S, Staples MP, Murphy B (2009) A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 361:557–568. 10.1056/NEJMoa0900429 [DOI] [PubMed] [Google Scholar]
- 12.Berenson J, Pflugmacher R, Jarzem P, Zonder J, Schechtman K, Tillman JB, Bastian L, Ashraf T, Vrionis F, Evaluation CPF, I, (2011) Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 12:225–235. 10.1016/S1470-2045(11)70008-0 [DOI] [PubMed] [Google Scholar]
- 13.Garfin SR, Buckley RA, Ledlie J, Balloon Kyphoplasty Outcomes G (2006) Balloon kyphoplasty for symptomatic vertebral body compression fractures results in rapid, significant, and sustained improvements in back pain, function, and quality of life for elderly patients. Spine (Phila Pa 1976) 31:2213–2220. 10.1097/01.brs.0000232803.71640.ba [DOI] [PubMed] [Google Scholar]
- 14.Gold LS, O’Reilly MK, Heagerty PJ, Jarvik JG (2021) Complications and healthcare utilization in commercially-insured osteoporotic vertebral compression fracture patients: a comparison of kyphoplasty versus propensity-matched controls. Spine J 21:1347–1354. 10.1016/j.spinee.2021.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinde K, Maingard J, Hirsch JA, Phan K, Asadi H, Chandra RV (2020) Mortality Outcomes of Vertebral Augmentation (Vertebroplasty and/or Balloon Kyphoplasty) for Osteoporotic Vertebral Compression Fractures: A Systematic Review and Meta-Analysis. Radiology 295:96–103. 10.1148/radiol.2020191294 [DOI] [PubMed] [Google Scholar]
- 16.Levesque LE, Hanley JA, Kezouh A, Suissa S (2010) Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 340:b5087. 10.1136/bmj.b5087 [DOI] [PubMed] [Google Scholar]
- 17.O’Reilly MK, Heagerty PJ, Gold LS, Kallmes DF, Jarvik JG (2020) Augmented Reality. AJNR Am J Neuroradiol 41:E67–E68. 10.3174/ajnr.A6587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyrer F, Bhaskaran K, Rutherford MJ (2022) Immortal time bias for life-long conditions in retrospective observational studies using electronic health records. BMC Med Res Methodol 22:86. 10.1186/s12874-022-01581-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadav K, Lewis RJ (2021) Immortal Time Bias in Observational Studies. JAMA 325:686–687. 10.1001/jama.2020.9151 [DOI] [PubMed] [Google Scholar]
- 20.(1990) ICD-9-CM. International Classification of Diseases, 9th revision, Clinical Modification. 3d edition, volumes 1, 2 and 3. Official authorized addendum effective October 1, 1990--HCFA. J Am Med Rec Assoc 61:suppl 1–35 [PubMed] [Google Scholar]
- 21.Marcon RM, Cristante AF, Teixeira WJ, Narasaki DK, Oliveira RP, de Barros Filho TE (2013) Fractures of the cervical spine Clinics (Sao Paulo) 68:1455–1461. 10.6061/clinics/2013(11)12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torlincasi AM, Waseem M (2020) Cervical Injury. Treasure Island (FL) [Google Scholar]
- 23.Gilbert T, Neuburger J, Kraindler J et al. (2018) Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 391:1775–1782. 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edidin AA, Ong KL, Lau E, Kurtz SM (2015) Morbidity and Mortality After Vertebral Fractures: Comparison of Vertebral Augmentation and Nonoperative Management in the Medicare Population. Spine (Phila Pa 1976) 40:1228–1241. 10.1097/BRS.0000000000000992 [DOI] [PubMed] [Google Scholar]
- 25.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V (2011) Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173:676–682. 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 26.Ashworth J, Green DJ, Dunn KM, Jordan KP (2013) Opioid use among low back pain patients in primary care: Is opioid prescription associated with disability at 6-month follow-up? Pain 154:1038–1044. 10.1016/j.pain.2013.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarvik JG, Gold LS, Comstock BA et al. (2015) Association of early imaging for back pain with clinical outcomes in older adults. JAMA 313:1143–1153. 10.1001/jama.2015.1871 [DOI] [PubMed] [Google Scholar]
- 28.Feenstra H, Grobbee RE, in’t Veld BA, Stricker BH (2001) Confounding by contraindication in a nationwide cohort study of risk for death in patients taking ibopamine. Ann Intern Med 134:569–572. 10.7326/0003-4819-134-7-200104030-00010 [DOI] [PubMed] [Google Scholar]
- 29.Ong KL, Beall DP, Frohbergh M, Lau E, Hirsch JA (2018) Were VCF patients at higher risk of mortality following the 2009 publication of the vertebroplasty “sham” trials? Osteoporos Int 29:375–383. 10.1007/s00198-017-4281-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lange A, Kasperk C, Alvares L, Sauermann S, Braun S (2014) Survival and cost comparison of kyphoplasty and percutaneous vertebroplasty using German claims data. Spine (Phila Pa 1976) 39:318–326. 10.1097/BRS.0000000000000135 [DOI] [PubMed] [Google Scholar]
- 31.Diamond TH, Bryant C, Browne L, Clark WA (2006) Clinical outcomes after acute osteoporotic vertebral fractures: a 2-year non-randomised trial comparing percutaneous vertebroplasty with conservative therapy. Med J Aust 184:113–117. 10.5694/j.1326-5377.2006.tb00148.x [DOI] [PubMed] [Google Scholar]
- 32.Gerling MC, Eubanks JD, Patel R, Whang PG, Bohlman HH, Ahn NU (2011) Cement augmentation of refractory osteoporotic vertebral compression fractures: survivorship analysis. Spine (Phila Pa 1976) 36:E1266–1269. 10.1097/BRS.0b013e31820a0b3f [DOI] [PubMed] [Google Scholar]
- 33.Lin JH, Chien LN, Tsai WL, Chen LY, Chiang YH, Hsieh YC (2017) Early vertebroplasty associated with a lower risk of mortality and respiratory failure in aged patients with painful vertebral compression fractures: a population-based cohort study in Taiwan. Spine J 17:1310–1318. 10.1016/j.spinee.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 34.McDonald RJ, Achenbach SJ, Atkinson EJ, Gray LA, Cloft HJ, Melton LJ 3rd, Kallmes DF (2011) Mortality in the vertebroplasty population. AJNR Am J Neuroradiol 32:1818–1823. 10.3174/ajnr.A2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotan R, Smorgick Y, Anekstein Y, Rudik O, Prosso I, Hershkovich O (2022) Kyphoplasty for Elderly Patients With Vertebral Compression Fractures-Do We Save Lives? Mortality Rates Analysis Comparison in a Long-Term Follow-Up Cohort. Global Spine J 12:1443–1448. 10.1177/2192568220982282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voggenreiter G (2005) Balloon kyphoplasty is effective in deformity correction of osteoporotic vertebral compression fractures. Spine (Phila Pa 1976) 30:2806–2812. 10.1097/01.brs.0000190885.85675.a0 [DOI] [PubMed] [Google Scholar]
- 37.Harrison RA, Siminoski K, Vethanayagam D, Majumdar SR (2007) Osteoporosis-related kyphosis and impairments in pulmonary function: a systematic review. J Bone Miner Res 22:447–457. 10.1359/jbmr.061202 [DOI] [PubMed] [Google Scholar]
- 38.Yuan WH, Hsu HC, Lai KL (2016) Vertebroplasty and balloon kyphoplasty versus conservative treatment for osteoporotic vertebral compression fractures: A meta-analysis. Medicine (Baltimore) 95:e4491. 10.1097/MD.0000000000004491 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


