Abstract
Aberrant autophagic activity is observed in osteoarthritic joints. Vitamin D was shown to alleviate not only osteoarthritis severity, but also autophagy process. However, the influence of vitamin D on autophagy in knee osteoarthritis (KOA) remains ambiguous. This study aimed to determine the effect of vitamin D2 on serum levels of autophagosome protein LC3A in patients with KOA and whether LC3A levels were correlated with serum 25‐hydroxyvitamin D (25(OH)D) and clinical outcomes of patients with KOA. A total of 165 patients with KOA and 25 healthy controls were recruited. Vitamin D2 (ergocalciferol) was administered to patients with KOA at a weekly dosage of 40,000 IU. Serum LC3A, knee pain and functional scores, muscle strength, physical performance, and biochemical parameters were examined before and after 6 months of vitamin D2 supplementation. Serum LC3A levels were significantly higher in patients with KOA than healthy controls. In patients with KOA, vitamin D2 supplementation significantly decreased serum LC3A levels. Furthermore, baseline levels of serum LC3A were significantly associated with radiographic severity, pain and functional scores, total cholesterol, hs‐CRP, IL‐6, protein carbonyl, and serum 25(OH)D. After adjusting for established confounders, independent relationships among serum LC3A and radiographic severity, pain and functional scores, total cholesterol, hs‐CRP, IL‐6, protein carbonyl, and serum 25(OH)D were also observed. Vitamin D2 supplementation was shown to not only decrease serum levels of LC3A, inflammatory markers, as well as oxidative stress, but also improve muscle strength and physical performance in patients with KOA.
Abbreviations
- 25(OH)D
25‐hydroxyvitamin D
- 6MWT
6‐min walk test
- ACR
American College of Rheumatology
- ASMM
appendicular skeletal muscle mass
- ATG
autophagy‐related protein
- BMI
body mass index
- ELISA
enzyme‐linked immunosorbent assay
- FBG
fasting blood glucose
- GFP
green fluorescent protein
- HOMA‐IR
homeostasis model assessment
- hs‐CRP
high sensitivity‐C reactive protein
- IL‐6
interleukin‐6
- KL
Kellgren‐Lawrence
- KOA
knee osteoarthritis
- LC3
microtubule‐associated protein‐1 light chain 3
- LC3A
microtubule‐associated protein 1A/1B light chain 3A
- MAP1/LC3A
microtubule‐associated protein 1A/1B light chain 3A
- PPARγ
peroxisome proliferator‐activated receptor‐γ
- PTH
parathyroid hormone
- RANKL
receptor activator of nuclear factor kappa‐B ligand
- STS
sit‐to‐stand test
- TUGT
timed up and go test
- VAS
visual analog scale
- VDR
vitamin D receptor
- WC
waist circumference
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Aberrant autophagic activity is observed in osteoarthritic joints. Vitamin D was shown to alleviate not only osteoarthritis severity, but also autophagy process.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study aimed to determine the effect of vitamin D2 on serum levels of autophagosome protein LC3A in patients with knee osteoarthritis (KOA) and whether LC3A levels were correlated with serum 25‐hydroxyvitamin D (25(OH)D) and clinical outcomes of patients with KOA.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Vitamin D2 supplementation was observed to have a dual effect on KOA by decreasing serum levels of autophagosome protein, inflammatory markers, as well as oxidative stress and by improving muscle strength and physical performance in patients with KOA.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
A deeper comprehension of the role of vitamin D in autophagy signaling could potentially reveal novel prospects for its therapeutic application in the treatment of KOA. The effectiveness, safety, and accessibility of vitamin D provides a strong foundation for understanding its use in clinical practices.
INTRODUCTION
Knee osteoarthritis (KOA) is a degenerative joint disease caused by a variety of factors. In the articular cartilage of KOA, the number of chondrocytes and their capacity to rebuild the extracellular matrix and adapt adequately to stress have decreased. 1 Despite the fact that pain is the primary symptom of KOA, the devastating consequences of this chronic condition cannot be alleviated by the use of conventional therapies, including pain palliation and alterations to one's way of life. For those whose symptoms have not responded to more conservative methods of care, joint replacement surgery is often the last resort for enhancing their quality of life. 2 Despite ongoing investigations into markers and the development of innovative treatments, such as platelet‐rich plasma, bone marrow mesenchymal stem cells, and autologous microfragmented adipose tissue, 3 , 4 challenges on the natural course of disease still exist. 5 From that viewpoint, it is conceivable that better understanding what causes joint degeneration might pave the way for development of disease‐modifying drugs and discovery of potential biomarkers to aid in early diagnosis, both of which might prolong the time before the patients become severely disabled.
Autophagy is a homeostatic process through which cells recycle broken or redundant parts of themselves inside the cytoplasm. 6 As a primary adaptive mechanism in the body, autophagy has been suggested to protect against the pathogenesis of several pathological conditions, including cancer, infection, neurodegeneration, aging, and cardiovascular disease. 7 In fact, the articular cartilage of the knees does not have the blood vessels, nerves, lymphatic vessels, or connective tissue which would normally provide oxygen and nutrition to the cartilage. 8 Because of this, autophagy is essential for metabolism, and this is particularly true in cells with a slow turnover rate. 9 In the context of KOA, previous studies have uncovered that cartilage deterioration was associated with aberrant autophagy levels. 10 More specifically, gene expressions of autophagy markers have been observed to be downregulated in human osteoarthritic joints. 11 , 12 , 13 The aforementioned findings highlight the significant involvement of autophagy in KOA pathogenesis. When viewed from this perspective, it is important to note that possible molecules known to play a possible part in autophagy have considerable promise as both diagnostic biomarkers and therapeutic targets for KOA.
One of several molecules known to be implicated in autophagy, vitamin D, is drawing attention as a potential protective molecule against the onset and progression of KOA. In addition to vitamin D's role in bone health – especially KOA, 14 it has been shown to have effects on autophagy in a variety of pathological conditions via both genomic and nongenomic pathways. 15 This finding lends further support to the notion that autophagy levels could be modulable by vitamin D and might possibly be predictive of cartilage deterioration in KOA. Despite extensive research on vitamin D's links to the radiographic severity and clinical outcomes of KOA, 16 , 17 , 18 its effect on autophagy process in patients with KOA has yet to be determined. Accordingly, this study aimed to determine whether vitamin D supplement could alter serum levels of an autophagy marker, microtubule‐associated protein 1A/1B light chain 3A (MAP1/LC3A), also known as LC3A, in patients with KOA and to examine clinical correlations between serum LC3A levels, serum vitamin D, biochemical parameters, indicators of muscular strength, and physical performance, as well as inflammatory markers in patients with KOA.
METHODS
The study protocol was approved by the Ethical Committee on Human Research of the Faculty of Dentistry/Faculty of Pharmacy, Mahidol University (IRB No. MU‐DT/py‐IRB2022PY072) and carried out in compliance with the Declaration of Helsinki guidelines. All participants provided their written informed consent before being included in the study. This trial has been registered in the Thai Clinical Trials Registry (TCTR) under the registration number: TCTR20230901001.
Study participants
In this prospective, single‐site, single‐arm, nonrandomized interventional trial, a total of 165 patients with KOA were recruited from the outpatient clinic of the Department of Orthopedics at King Chulalongkorn Memorial Hospital. The inclusion criteria were defined as individuals aged 50 to 80 years, who had been diagnosed with bilateral primary KOA based on the American College of Rheumatology criteria, 2 and who exhibited serum 25‐hydroxyvitamin D (25(OH)D) levels below 30 ng/mL defined as insufficient – particularly Thai patients with KOA 16 and Asian populations. 19 Using the Kellgren‐Lawrence (KL) system, radiographic severity was classified in a non‐observer‐controlled manner. The radiographic severity of KOA was ranked on a scale from 0 to 4: grade 0 (no X‐ray changes), grade 1 (doubtful narrowing of joint space and possible osteophyte lipping), grade 2 (definite osteophytes and possible joint space narrowing), grade 3 (moderate multiple osteophytes, definite narrowing of joint space, bone sclerosis, and possible deformity of bone contour), and grade 4 (large osteophytes, marked joint space narrowing, severe sclerosis, and clear deformity of bone contour). 20 The exclusion criteria for this study were defined as follows: the presence of secondary OA caused by a known disorder; any recent arthroscopy, surgery, or joint injection in the target knee within the past 6 months; a history of knee joint replacement; the presence of any serious systemic disease, cardiovascular disease, diabetes mellitus, liver disorders, renal disorders, thyroid disorders, or other chronic inflammatory diseases; pregnancy or lactation; smoking; alcohol consumption; the use of lipid‐lowering medications, omega‐3‐fatty acids, antioxidant supplements, or vitamin supplementations containing vitamin D, glucosamine or chondroitin in the past 30 days; and the use of non‐steroidal anti‐inflammatory drugs within 2 weeks prior to and during the intervention. As per the trial's protocol, the administration of non‐steroidal anti‐inflammatory drugs was prohibited. Besides this, patients who were unable to be contacted for further evaluation or who received treatment beyond the scope of clinical palliative cares were excluded from the study.
Intervention
A schematic representation of the flow of subjects through the current trial is illustrated in Figure 1. According to the guidelines provided by the Endocrine Society, 21 it has been recommended that adults take a weekly dose of 50,000 IU of vitamin D2 for a duration of 8 weeks in order to consistently maintain serum 25(OH)D levels above 30 ng/mL. In the context of Thailand, vitamin D2 (ergocalciferol) is accessible in the form of 20,000 IU per capsule. Consequently, participants were instructed to consume a dosage of 40,000 IU of vitamin D2, specifically in the form of two capsules containing 20,000 IU of ergocalciferol each, sourced from the British Dispensary in Bangkok, Thailand. This regimen was maintained for a duration of 6 months. To mitigate non‐compliance, biweekly telephone calls were conducted, consisting of assessments lasting between 5 and 8 min. In order to evaluate the adherence of participants to the intervention, they were requested to return their depleted pill containers upon completion of the study. Adherence was quantified by dividing the number of pills consumed by the expected number of pills to be taken throughout the study and subsequently expressing this ratio as a percentage. Given that the primary objective of this single‐arm trial was to gather preliminary evidence on the effect of vitamin D supplementation on serum levels of LC3A, an autophagy marker, in patients with KOA, the utilization of placebo controls is generally deemed unnecessary for this study.
FIGURE 1.
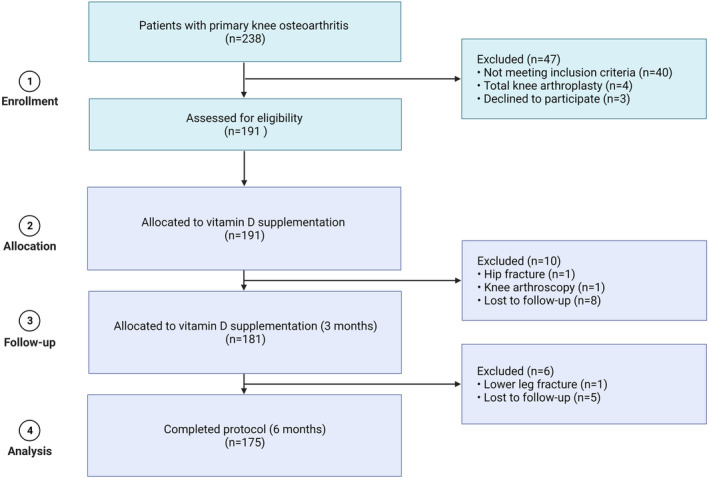
Flowchart of patient inclusion.
At enrollment and after 6 months of intervention, body composition measurements and clinical outcome assessments, such as knee pain, physical disability, muscle strength, as well as physical performance were performed, and blood samples were collected from patients with KOA.
Study outcomes
The primary exploratory outcome determined the effect of vitamin D on serum levels of autophagosome protein LC3A in patients with KOA. The second exploratory outcome evaluated the associations between serum LC3A levels, serum 25(OH)D levels, and clinical parameters of patients with KOA.
Methodologic approaches used to mitigate bias
Further details are provided in Supplementary Material S1.
Clinical outcome measurements
Clinical outcomes were all evaluated by healthcare professionals who were blinded to the study purpose and hypotheses, as well as the interventions administered to the study participants. All laboratory assessments were conducted at baseline and after 6 months of vitamin D2 supplementation.
Body composition
Standard measurement techniques were used to determine body composition, height, weight, and waist circumference. Body mass index (BMI) was calculated by dividing body weight in kilograms (kg) by height in meters squared (m2). The bioelectrical impedance analysis (BC‐418 Segmental Body Composition Analyzer; Tanita Corporation) was used for body composition analyses, including appendicular skeletal muscle mass (ASMM), percentage of total fat mass (%fat), and fat mass. The ASMM was calculated as the sum of skeletal muscle mass of the arms and legs in kg, as described previously. 16
Knee pain and physical disability
A 10‐cm visual analog scale (VAS) is generally used to measure the severity of pain, in which scores of 0 and 10 indicate no discomfort and maximum pain, respectively. The Western Ontario and MacMaster University (WOMAC) index, a self‐administered questionnaire consisting of 24 items, is commonly used to evaluate the severity of pain, stiffness, and physical disability. On a scale of 0–4, the test questions are scored as follows: none (0), mild (1), moderate (2), severe (3), and extreme (4). The scores for each subscale are summed up, with a possible score range of 0–20 for pain, 0–8 for stiffness, and 0–68 for physical function. The total WOMAC score is the sum of the three subscales, as described previously. 16
Muscle strength
Muscle strength consisting of grip strength, knee flexors, and extensor strength was assessed using a grip strength dynamometer (Takei Scientific Instruments Ltd.) and a handheld dynamometer (Hoggan Scientific LLC), as described previously. 16
Physical performances
Physical performance was evaluated using gait speed, timed up and go test (TUGT), sit‐to‐stand test (STS), and 6‐min walk test (6MWT). For the gait speed test, the time required to walk 5 m at a typical pace was measured using a conventional stopwatch, excluding the first and final 50 cm. The TUGT was calculated based on the amount of time required to stand up from a sitting position, walk 3 m, turn around, walk back, and sit down again. STS was an additional physical performance test in which patients were instructed to stand and sit as rapidly as possible from a standard‐height chair (45 cm) with their arms crossed over their chests. The length of time it took the patient to complete this sequence of movements was also recorded. The 6MWT was the last physical performance test used to assess how far the participants could walk in only 6 min, as described previously. 16
Laboratory methods
Fasting venous blood samples were collected from patients with KOA at the beginning and after 6 months of vitamin D supplementation and subsequently centrifuged to collect serum. The serum samples were immediately stored at −20°C for later use. Biochemical markers including fasting blood glucose (FBG), lipid profiles (total cholesterol, triglyceride, LDL‐cholesterol, and HDL‐cholesterol), calcium, phosphorus, and high‐sensitivity C‐reactive protein (hs‐CRP) were measured using a Roche Hitachi 912 chemistry analyzer (Roche Diagnostics). Blood concentrations of 25(OH)D, parathyroid hormone (PTH), and insulin were measured using chemiluminescent immunoassay (Roche Diagnostics GmbH). The limit of detection of vitamin D assay was 4.0 ng/mL, intra‐assay coefficient of variation (CV) was 3.4%, and interassay CV was 7.6%. Insulin resistance was determined based on homeostasis model assessment (HOMA‐IR) with the following formula: fasting serum insulin (μU/mL) × fasting plasma glucose (mg/dL)/405. Serum levels of protein carbonyl were measured using spectrophotometer, following the protocol established by Castegna et al., 22 as described previously. 16 Serum LC3A and IL‐6 levels were measured quantitatively using a commercially available sandwich ELISA kit (R&D Systems). Further details on quantitation of serum LC3A levels are provided in Supplementary Material S2.
Statistical analyses
Descriptive statistics were used to provide a summary of the data pertaining to baseline characteristics of study participants. Counts and proportions were used for categorical variables, and the statistical significance was determined using either Pearson's chi‐square test or Fisher's exact test, as appropriate. Continuous variables were described using the median and interquartile range (IQR; Q1–Q3), and the Mann–Whitney U test was used to evaluate any statistical differences. The normality of the data distribution was assessed using the one‐sample Kolmogorov–Smirnov test. Statistical differences in serum LC3A levels, serum 25(OH)D levels, and biochemical parameters at baseline and after treatment with vitamin D2 supplement were assessed using Wilcoxon signed‐rank test for matched data due to non‐normal distribution of the variable; paired sample t‐test for matched samples was used to assess the mean difference and establish the 95% confidence interval (CI). Associations of baseline levels of serum LC3A and baseline levels of serum 25(OH)D and various clinical parameters, including radiographic severity, indicators of body composition, muscle strength, as well as physical performance, and biochemical parameters were initially assessed using simple linear regression – specifically Spearman's rho correlation coefficients (r). The strength of the correlations was categorized as weak, moderate, or strong based on the corresponding r values of less than 0.3, 0.3–0.5, and greater than or equal to 0.5, respectively. 23 Following this, multivariate models, specifically multiple linear regression with forward stepwise methods, were used to adjust for potential confounding variables including age, gender, and BMI.
All analyses were deemed statistically significant if the p value was less than 0.05 (2‐tailed test) with a 95% CI. As a prespecified analysis plan is not available in this study, no adjustments for multiplicity were performed, and the interpretation of the various comparisons should be viewed as exploratory. Statistical analyses were all executed using the statistical package for social sciences version 26.0 (SPSS) and GraphPad Prism version 9.4.1.
RESULTS
Baseline and on‐treatment characteristics of study participants
The detailed demographic characteristics of patients with KOA both prior to and following 6 months of vitamin D2 supplementation are summarized in Table 1. When comparing clinical characteristics of patients with KOA before and after a 6‐month period of vitamin D2 supplementation, no statistically significant differences were observed in body composition (BMI, ASMM, %fat, and fat mass), some metabolic markers (FBG, insulin, HOMA‐IR, total cholesterol, and triglyceride), and a biochemical parameter (phosphorus). However, vitamin D2 supplementation for a period of 6 months resulted in significant improvements in muscle strength (grip strength and knee extension force), enhanced physical performance (gait speed, TUGT, STS, and 6MWT), and increased circulating calcium levels in patients with KOA (p < 0.001, p < 0.001, p = 0.008, p = 0.023, p < 0.001, p < 0.001, and p = 0.033, respectively). In addition to this, vitamin D2 supplementation significantly decreased knee pain score (VAS; p = 0.003), metabolic markers (LDL‐cholesterol and HDL‐cholesterol), circulating PTH levels (p < 0.001), circulating levels of inflammatory markers (hs‐CRP and IL‐6; p = 0.014 and p = 0.014, respectively), and oxidative stress (protein carbonyl; p = 0.010, respectively). Additionally, patients with KOA who received vitamin D2 supplementation for a duration of 6 months exhibited a notable elevation in their serum 25(OH)D levels compared to their baseline levels prior to commencing the supplementation (p < 0.001). On the basis of radiographic severity, over half of the patients with KOA (56.97%) were classified as having KL grade 2–3.
TABLE 1.
Demographic and clinical characteristics of patients with KOA before and after vitamin D2 supplementation.
| Variables | Patients with KOA (n = 165) | p value | |
|---|---|---|---|
| Vitamin D2 supplementation | |||
| Before intervention | After intervention | ||
| Gender (F/M) | 149 (90.30%):16 (9.70%) | 149 (90.30%):16 (9.70%) | 1.000 |
| Age (years) | 65.00 (60.00, 69.00) | 65.00 (60.00, 69.00) | 1.000 |
| Body composition | |||
| BMI (kg/m2) | 24.97 (22.95, 28.31) | 24.75 (22.73, 28.09) | 0.061 |
| ASMM (kg) | 16.58 (15.08, 20.08) | 16.50 (15.09, 19.91) | 0.169 |
| Percentage of fat (%) | 35.87 (30.78, 40.06) | 33.73 (28.64, 37.92) | 0.001 |
| Fat mass (kg) | 21.44 (17.39, 27.93) | 19.71 (16.11, 25.75) | 0.001 |
| Radiographic severity | |||
| KL grade 0 | 0 (0.00%) | 0 (0.00%) | 1.000 |
| KL grade 1 | 0 (0.00%) | 0 (0.00%) | 1.000 |
| KL grade 2 | 43 (26.06%) | 43 (26.06%) | 1.000 |
| KL grade 3 | 51 (30.91%) | 51 (30.91%) | 1.000 |
| KL grade 4 | 71 (43.03%) | 71 (43.03%) | 1.000 |
| Pain and physical disability | |||
| VAS (0–10) | 3.00 (2.00, 5.00) | 2.59 (2.00, 5.00) | 0.003 |
| WOMAC (0–10) | 3.00 (2.00, 4.00) | 2.74 (2.00, 4.00) | 0.395 |
| Muscle strength | |||
| Grip strength (kg) | 17.02 (18.74, 26.06) | 17.67 (19.39, 26.71) | <0.001 |
| Knee extension force (N) | 256.01 (202.88, 309.14) | 358.61 (310.57, 406.65) | <0.001 |
| Physical performance | |||
| Gait speed (m/s) | 0.82 (0.78, 1.14) | 1.00 (0.96, 1.32) | 0.002 |
| TUGT (s) | 9.30 (8.11, 11.51) | 8.14 (7.13, 10.17) | <0.001 |
| STS (s) | 12.33 (11.57, 18.17) | 10.74 (9.80, 16.76) | <0.001 |
| 6MWT (m) | 374.02 (318.09, 424.35) | 424.00 (369.14, 473.26) | <0.001 |
| Metabolic markers | |||
| FBG (mg/dL) | 95.84 (86.45, 109.67) | 96.27 (84.56, 112.42) | 0.589 |
| Insulin (μIU/mL) | 4.00 (2.00, 9.00) | 4.77 (2.00, 9.00) | 0.197 |
| HOMA‐IR | 0.94 (0.36, 2.32) | 1.15 (0.39, 2.71) | 0.117 |
| Total cholesterol (mg/dL) | 210.44 (185.78, 238.10) | 211.40 (184.86, 240.94) | 0.659 |
| Triglyceride (mg/dL) | 116.84 (89.19, 163.49) | 114.20 (82.36, 165.04) | 0.134 |
| LDL‐cholesterol (mg/dL) | 131.22 (110.77, 160.07) | 123.44 (102.82, 152.46) | <0.001 |
| HDL‐cholesterol (mg/dL) | 53.62 (46.00, 64.00) | 55.72 (46.00, 64.00) | 0.006 |
| Biochemical markers | |||
| PTH (pg/mL) | 49.57 (37.84, 68.56) | 43.00 (26.90, 66.36) | <0.001 |
| Calcium (mg/dL) | 9.19 (9.00, 9.50) | 9.28 (9.00, 9.50) | 0.033 |
| Phosphorus (mg/dL) | 3.50 (3.40, 4.00) | 3.67 (3.40, 4.00) | 0.083 |
| hs‐CRP (mg/L) | 1.70 (0.43, 4.00) | 1.06 (0.18, 3.76) | 0.014 |
| IL‐6 (pg/mL) | 22.36 (1.65, 43.09) | 20.58 (15.95, 25.23) | 0.014 |
| Protein carbonyl (nmol/mg) | 0.66 (0.43, 1.15) | 0.57 (0.43, 0.97) | 0.010 |
| 25(OH)D (ng/mL) | 20.84 (17.52, 23.94) | 32.25 (26.87, 37.41) | <0.001 |
| LC3A (pg/mL) | 65.04 (49.20, 87.54) | 60.04 (45.37, 82.20) | <0.001 |
Note: Data are represented as either median interquartile range (IQR) or Q1, Q3) for continuous variables or percentages for categorical variables. Bold values denote statistically significant at p‐ value less than 0.05 (two‐tailed).
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; 6MWT, 6‐min walk test; ASMM, appendicular skeletal muscle mass; BMI, body mass index; FBG, fasting blood glucose; HOMA‐IR, Homeostatic Model Assessment‐Insulin Resistance; hs‐CRP, high‐sensitivity C‐reactive protein; KL, Kellgren‐Lawrence; KOA, knee osteoarthritis; IL‐6, interleukin‐6; LC3A, microtubule‐associated protein 1A/1B light chain 3A; PTH, parathyroid hormone; STS, sit‐to‐stand test; TUGT, timed up and go test; VAS, visual analog scale; WOMAC, Western Ontario and MacMaster University.
In comparisons of demographic and clinical characteristics of patients with KOA before vitamin D2 supplementation and healthy controls (Table 2), no statistically significant differences were observed in gender ratio, median age, and body composition markers (BMI, ASMM, %fat, and fat mass). As expected, WOMAC score and indicators of muscle strength (grip strength) and physical performance (TUGT and 6MWT) were significantly higher in patients with KOA than that in healthy controls (p = 0.007, p = 0.021, p < 0.001, and p = 0.018, respectively), whereas STS, an additional indicator of physical performance, was significantly lower in patients with KOA than those in healthy controls (p = 0.001). These findings imply that patients with KOA exhibited significantly lower levels of physical functioning in comparison to healthy controls.
TABLE 2.
Baseline characteristics of patients with KOA with pre‐vitamin D2 supplementation and healthy controls.
| Variables | Patients with KOA before vitamin D2 supplementation (n = 165) | Healthy controls (n = 25) | p value |
|---|---|---|---|
| Gender (F/M) | 149 (90.30%):16 (9.70%) | 23 (92.00%):2 (8.00%) | 0.831 |
| Age (years) | 65.00 (60.00, 69.00) | 65.00 (53.50, 71.00) | 0.925 |
| Body composition | |||
| BMI (kg/m2) | 24.97 (22.95, 28.31) | 23.60 (21.10, 25.80) | 0.249 |
| ASMM (kg) | 16.58 (15.08, 20.08) | 14.20 (13.00, 16.80) | 0.134 |
| Percentage of fat (%) | 35.87 (30.78, 40.06) | 35.10 (27.45, 40.80) | 0.265 |
| Fat mass (kg) | 21.44 (17.39, 27.93) | 21.50 (16.30, 25.50) | 0.864 |
| Radiographic severity | |||
| KL grade 0 | 0 (0.00%) | N/A | N/A |
| KL grade 1 | 0 (0.00%) | N/A | N/A |
| KL grade 2 | 43 (26.06%) | N/A | N/A |
| KL grade 3 | 51 (30.91%) | N/A | N/A |
| KL grade 4 | 71 (43.03%) | N/A | N/A |
| Pain and physical disability | |||
| VAS (0–10) | 3.00 (2.00, 5.00) | N/A | N/A |
| WOMAC (0–10) | 3.00 (2.00, 4.00) | 1.33 (0.10, 2.67) | 0.007 |
| Muscle strength | |||
| Grip strength (kg) | 17.02 (18.74, 26.06) | 19.50 (17.40, 25.50) | 0.021 |
| Knee extension force (N) | 256.01 (202.88, 309.14) | N/A | N/A |
| Physical performance | |||
| Gait speed (m/s) | 0.82 (0.78, 1.14) | 0.95 (0.79, 1.16) | 0.053 |
| TUGT (s) | 9.30 (8.11, 11.51) | 6.84 (5.89, 8.09) | <0.001 |
| STS (s) | 12.33 (11.57, 18.17) | 10.81 (9.28, 15.84) | 0.001 |
| 6MWT (m) | 374.02 (318.09, 424.35) | 380 (285.00, 404.00) | 0.018 |
| Metabolic markers | |||
| FBG (mg/dL) | 95.84 (86.45, 109.67) | N/A | N/A |
| Insulin (μIU/mL) | 4.00 (2.00, 9.00) | N/A | N/A |
| HOMA‐IR | 0.94 (0.36, 2.32) | N/A | N/A |
| Total cholesterol (mg/dL) | 210.44 (185.78, 238.10) | N/A | N/A |
| Triglyceride (mg/dL) | 116.84 (89.19, 163.49) | N/A | N/A |
| LDL‐cholesterol (mg/dL) | 131.22 (110.77, 160.07) | N/A | N/A |
| HDL‐cholesterol (mg/dL) | 53.62 (46.00, 64.00) | N/A | N/A |
| Biochemical markers | |||
| PTH (pg/mL) | 49.57 (37.84, 68.56) | N/A | N/A |
| Calcium (mg/dL) | 9.19 (9.00, 9.50) | N/A | N/A |
| Phosphorus (mg/dL) | 3.50 (3.40, 4.00) | N/A | N/A |
| hs‐CRP (mg/L) | 1.06 (0.18, 3.76) | N/A | N/A |
| IL‐6 (pg/mL) | 20.58 (15.95, 25.23) | N/A | N/A |
| Protein carbonyl (nmol/mg) | 0.66 (0.43, 1.15) | N/A | N/A |
| 25(OH)D (ng/mL) | 20.84 (17.52, 23.94) | N/A | N/A |
| LC3A (pg/mL) | 65.04 (49.20, 87.54) | 39.70 (34.20, 56.37) | <0.001 |
Note: Data are represented as either median interquartile range (IQR) or Q1, Q3 for continuous variables or percentages for categorical variables. Bold values denote statistically significant at p value less than 0.05 (two‐tailed).
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; 6MWT, 6‐min walk test; ASMM, appendicular skeletal muscle mass; BMI, body mass index; FBG, fasting blood glucose; HOMA‐IR, Homeostatic Model Assessment‐Insulin Resistance; hs‐CRP, high‐sensitivity C‐reactive protein; KL, Kellgren‐Lawrence; KOA, knee osteoarthritis; IL‐6, interleukin‐6; LC3A, microtubule‐associated protein 1A/1B light chain 3A; PTH, parathyroid hormone; STS, sit‐to‐stand test; TUGT, timed up and go test; VAS, visual analog scale; WOMAC, Western Ontario and MacMaster University.
LC3A levels in patients with KOA with and without vitamin D2 supplementation
Patients with KOA without vitamin D2 supplementation showed serum LC3A levels at a median of 65.04 pg/mL (IQR: 49.20–87.54). Following vitamin D2 supplementation, serum LC3A levels were reduced to a median of 60.04 pg/mL (IQR: 45.37–82.20). Difference in serum LC3A levels between pre‐ and post‐vitamin D2 supplementation groups was statistically significant (p = 0.0155, 95% CI: −8.333 to −2.333; Figure 2a,b). Compared to healthy controls, serum LC3A levels were significantly increased in patients with KOA with vitamin D2 supplementation at baseline and 6 months (p < 0.0001, 95% CI: −32.00 to −12.67; p = 0.0009, 95% CI: −27.33 to −8.00; respectively; Figure 2c).
FIGURE 2.
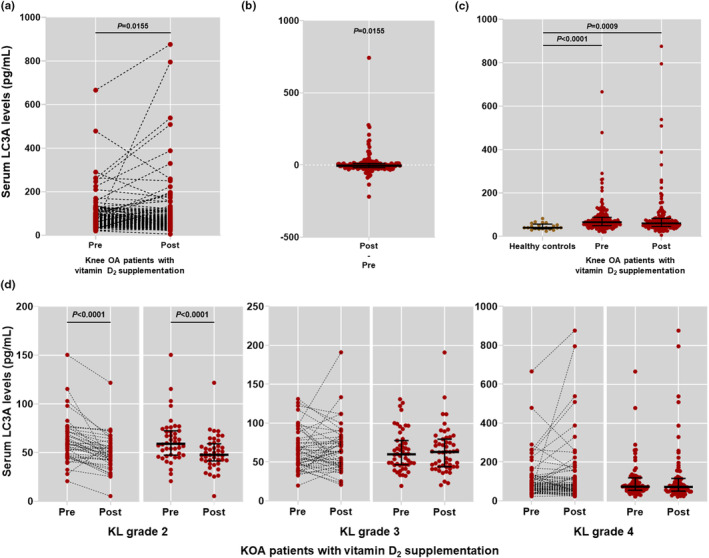
Serum levels of autophagosome protein LC3A in study participants. (a) Comparison of serum LC3A levels in patients with KOA with pre‐ and post‐vitamin D2 supplementation. (b) Graph differences of serum LC3A levels between before and after vitamin D2 supplementation in patients with KOA. (c) Comparison of serum LC3A levels in patients with KOA with pre‐ and post‐vitamin D2 supplementation and healthy controls. (d) Comparison of serum LC3A levels among patients with KOA with different KL grades before and after 6 months vitamin D2 supplementation. KL, Kellgren‐Lawrence; KOA, knee osteoarthritis; OA, osteoarthritis.
In stratified analysis by the disease severity classified by KL grades, patients with KOA with KL grade 2 had a significant decrease in serum LC3A levels after 6 months of vitamin D2 supplementation, as compared to their pre‐supplementation levels (p < 0.0001, 95% CI: −16.67 to −5.00; Figure 2d). On the other hand, there were no significant differences in serum LC3A levels at baseline and after a 6‐month period of supplementation among patients with KOA with higher KL grades (Figure 2d).
Correlations between circulating LC3A levels and clinical parameters of patients with KOA
Associations between serum LC3A baseline levels and outcome parameters of patients with KOA prior to receiving vitamin D supplementation are detailed in Figure 3a. Spearman's rho correlation analysis uncovered that serum LC3A baseline levels were positively associated with radiographic severity (KL grade: r = 0.379, p < 0.001, 95% CI: 0.24–0.51), scores of knee pain (VAS: r = 0.458, p < 0.001, 95% CI: 0.32–0.58) and physical disability (WOMAC: r = 0.242, p = 0.004, 95% CI: 0.07–0.40), metabolic parameter (total cholesterol: r = 0.493, p < 0.001, 95% CI: 0.36–0.60), and inflammatory markers (hs‐CRP: r = 0.493, p < 0.001, 95% CI: 0.50–0.70; IL‐6: r = 0.626, p < 0.001, 95% CI: 0.51–0.72; Figure 3b–g). On the other hand, serum LC3A levels were found to be inversely correlated with oxidative stress (protein carbonyl: r = −0.200, p = 0.013, 95% CI: −0.35 to −0.01) and serum 25(OH)D levels in patients with KOA (r = −0.506, p < 0.001, 95% CI: −0.61 to −0.38; Figure 3h,i).
FIGURE 3.
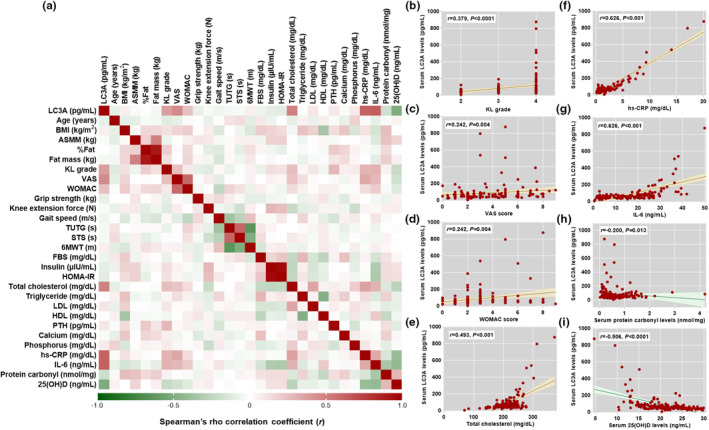
Correlation between serum LC3A levels and clinical parameters in patients with KOA. (a) Heatmap of Spearman's rho correlations between serum LC3A levels and clinical parameters of patients with KOA. (b) Scatter plot displaying significant correlation between serum LC3A levels and KL grade. (c) Scatter plot displaying significant correlation between serum LC3A levels and knee pain (VAS score). (d) Scatter plot of significant correlation between serum LC3A levels and physical disability (WOMAC score). (e) Scatter plot of significant correlation between serum LC3A levels and total cholesterol. (f) Scatter plot of significant correlation between serum LC3A levels and hs‐CRP. (g) Scatter plot of significant correlation between serum LC3A levels and IL‐6. (h) Scatter plot of significant correlation between serum LC3A levels and protein carbonyl. (i) Scatter plot of significant correlation between serum LC3A levels and 25(OH)D. 6MWT, 6‐min walk test; ASMM, appendicular skeletal muscle mass; BMI, body mass index; FBS, fetal bovine serum; HOMA‐IR, insulin resistance homeostasis model assessment; KL, Kellgren‐Lawrence; KOA, knee osteoarthritis; STS, sit‐to‐stand test; TUGT, timed up and go test; VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities.
To determine whether serum LC3A levels were independently associated with clinical parameters of patients with KOA, multivariate logistic regression analysis was further executed. After adjusting for confounding factors, including age, gender, and BMI, serum LC3A levels were observed to be independently associated with KL grade (β‐coefficient = 22.67, 95% CI: 9.70–35.65, p = 0.001), VAS score (β‐coefficient = 4.91, 95% CI: 3.46–13.28, p = 0.029), WOMAC score (β‐coefficient = 4.00, 95% CI: 3.58–13.59, p = 0.002), total cholesterol (β‐coefficient = 0.25, 95% CI: 0.19–0.68, p = 0.026), hs‐CRP (β‐coefficient = 36.72, 95% CI: 34.45–38.89, p < 0.001), IL‐6 (β‐coefficient = −2.05, 95% CI: −5.57 to 1.46, p = 0.034), protein carbonyl (β‐coefficient = 20.13, 95% CI: 2.28–39.98, p = 0.037), and serum 25(OH)D levels (β‐coefficient = −11.61, 95% CI: −14.62 to −8.62, p < 0.0001; Table 3).
TABLE 3.
Multivariate linear regression analysis of independent association between serum LC3A levels and clinical parameters of patients with KOA before vitamin D2 supplementation.
| Variables | Serum LC3A levels | p value* |
|---|---|---|
| β‐coefficient (95% CI) | ||
| Radiographic severity | ||
| KL grade | 22.67 (9.70 to 35.65) | 0.001 |
| Pain and physical disability | ||
| VAS | 4.91 (3.46 to 13.28) | 0.029 |
| WOMAC | 4.00 (3.58 to 13.59) | 0.020 |
| Metabolic markers | ||
| Total cholesterol | 0.25 (0.19 to 0.68) | 0.026 |
| Biochemical markers | ||
| hs‐CRP | 36.72 (34.45 to 38.89) | <0.001 |
| IL‐6 | −2.05 (−5.57 to −1.46) | 0.034 |
| Protein carbonyl | 20.13 (2.28, 39.98) | 0.037 |
| 25(OH)D | −11.61, (−14.62 to −8.62) | <0.001 |
Note: Bold values denote statistically significant at p value less than 0.05 (two‐tailed).
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; CI, confidence interval; hs‐CRP, high‐sensitivity C‐reactive protein; KL, Kellgren‐Lawrence; KOA, knee osteoarthritis; IL‐6, interleukin‐6; LC3A, microtubule‐associated protein 1A/1B light chain 3A; VAS, visual analog scale; WOMAC, Western Ontario and MacMaster University.
p‐values were all adjusted by established confounders including age, gender, and BMI.
DISCUSSION
Autophagy has been known to be linked to osteoarthritis, leading to recent breakthroughs in our understanding of the mechanisms behind alterations in cellular and environmental physiology in KOA. 24 It has been well‐recognized that cartilage and chondrocytes in arthritic joints are particularly vulnerable to the slow, cumulative effects of mechanical and oxidative stress. These include deterioration in cartilage homeostasis, decreased antioxidative forces, abnormal inflammatory responses, and altered gene and protein expressions. 25 From this viewpoint, adaptive mechanisms that enable a restoration of cellular injury, such as autophagy, are crucial for the maintenance of chondrocyte survival and function. In the light of this presumption, the current study compared the levels of LC3A, an autophagosome protein, in the blood of healthy controls, as well as those of patients with KOA before and after vitamin D2 supplementation. The possible correlations between serum LC3A levels and clinical parameters including pain score, mobility, physical performance, and biochemical parameters were also determined. Our results provide credence to the idea that vitamin D might have a significant importance in autophagy via decreasing levels of autophagosome protein LC3A in the blood of patients with KOA. Besides this, it appears to have positive effects on physical performance and reduction of inflammation in patients with KOA.
Autophagy is a highly conserved catalytic metabolism. Through the autophagic pathway, human cells eliminate or sequester cellular contents to lysosomes for degradation. 6 This complicated system of autophagy includes induction or initiation, nucleation, elongation, maturation, and degradation. It is initiated by the assembly of autophagy‐related (ATG) proteins and signaling molecules to form an autophagosome. Microtubule‐associated protein‐1 light chain 3‐I (LC3A), which has been synthesized as a precursor (LC3), and cleaved by ATG4B to its cytosolic free‐form, is converted to autophagosome membrane‐associated form (LC3B). The LC3B precisely targets the elongation and subsequent fusion with lysosomes for degradation. 26 The elongation is a critical step in the formation of complete autophagosomes, and the conversion of LC3A to LC3B is a key factor in the elongation process. 27 Regarding this, it has been suggested that LC3 quantities (LC3A, LC3B, or LC3B/LC3A ratio) are commonly used as a proxy for the autophagy flux. 28 Based on this premise, our study measured serum levels of LC3A and demonstrated that serum LC3A levels in patients with KOA were significantly greater than those in healthy control. Besides this, patients with KOA with vitamin D2 supplementation had significantly lower serum LC3A levels. Upregulated levels of autophagosome protein, LC3A, could be explained from the pathological features of KOA, such as the disease severity, the patient's age, zoning of cartilage, and the location of the pathology. Evidence from animal studies support the hypothesis that expression of autophagy varied with KOA severity, with increased autophagy at the beginning of the disease process and a deliberate decrease as the disease progresses. 29 , 30 Consistently, Cheng et al. 31 uncovered that autophagy was enhanced in the early stages and diminished in the late stages of experimental OA in SW1353 human chondrosarcoma cells. In the context of aging, autophagy activation was detected in knee articular cartilage from young mice when compared to the older ones. With increasing age, expression of apoptotic marker, poly (ADP‐ribose) polymerase p85, was upregulated, whereas expressions of autophagy‐related genes including ATG5 and LC3 and cartilage cellularity were both decreased. 32 More specifically, a clinical study unveiled that clusters of chondrocytes residing in the middle zone of KOA cartilage from patients showed robust expressions of Beclin‐1 and LC3. 33 In addition to this, the result derived from GFP‐LC3 transfection showed that OA chondrocytes were more simply susceptible to autophagy compared to chondrocytes from healthy individuals. 34 These results were consistent with previous reports denoting that expression of autophagy marker was upregulated in OA chondrocytes and cartilage, particularly during the initial degenerative phase, to counteract against apoptosis and reactive oxygen species (ROS). With gradual cartilage degradation, declined autophagy levels were observed to be associated with cell deaths. 12 , 35 , 36
Current evidence suggests that vitamin D deficiency has been implicated in the pathogenesis of KOA. 37 In details, the findings of several studies indicate a potential preventive role of vitamin D in KOA. 38 , 39 , 40 Besides this, the findings from several longitudinal studies 16 , 41 , 42 and systematic reviews 43 are promising, which provide encouragement for further studies. However, numerous randomized clinical trials that examined the therapeutic benefits of vitamin D supplementation in treating KOA did not find any conclusive evidence of its effectiveness. 44 , 45 It is apparent that inconsistent results on the effect of vitamin D supplementation on KOA may be attributed to several factors, such as KOA severity, baseline level of serum vitamin D, the duration of treatment, the dosage of vitamin D administered, and study design. The degradation of articular cartilage, however, is expected to be more amenable to reversal via novel therapeutic mechanisms. Interestingly, vitamin D was recognized to have a protective effect against a wide range of diseases. 15 In terms of bone‐related cells, vitamin D has been shown to induce autophagy by increasing expression of RANKL in osteoclasts, 46 restoring autophagic flux through vitamin D receptor and peroxisome proliferator‐activated receptor‐γ in synovial fibroblasts, 47 activating AMPK/mTOR signaling in chondrocytes, 48 , 49 and enhancing autophagic flux as well as attenuating inflammatory response in rat KOA chondrocytes. 50 , 51 In contrast to the aforementioned findings derived from animal models, our clinical study uncovered that vitamin D reduced serum levels of LC3A as an autophagy marker in patients with KOA. This contrasting result could be attributable to methodological differences between animal and clinical studies.
In addition to quantification of serum LC3A levels, other biochemical measurements in patients with KOA were further performed in the present study. Our results uncovered significant correlations of serum LC3A with markers of inflammation and oxidative stress in patients with KOA. It has been recognized that oxidative stress and inflammation play a role in the development of KOA. 52 It has been shown that excessive production of ROS‐induced oxidative stress and inflammation has the potential to disrupt the regulation of autophagy, ultimately leading to cellular damage and cartilage destruction. 51 , 52 Based on prior studies, 51 , 52 it has been observed that vitamin D exhibited a protective effect against ROS production and inflammation. The aforementioned findings might help to explain why significant associations of serum LC3A with serum 25(OH)D and markers of inflammation and oxidative stress were observed in this study.
Despite the fact that the foregoing findings support the therapeutic potential of autophagy in KOA, several barriers must be addressed before autophagy regulators may be used clinically for the prevention or treatment of KOA. First, given that autophagy is not the only process that regulates cartilage deterioration of KOA, vitamin D2 therapy aimed at altering autophagy levels may not be adequate to halt the disease's progression and improve clinical outcomes of patients with KOA. Another limitation of the study is that ergocalciferol was solely used in the present study. Furthermore, the inherent limitations of this study are its nonrandomized design and the absence of a placebo control, which both hinder the ability to draw inferences from comparisons. For that reason, it is appropriate to interpret the findings of the study as exploratory in nature. Additional limitation of our study pertains to the lack of sufficient information provided on healthy controls, which hinders a comprehensive comparison with patients with KOA with post‐vitamin D2 supplementation. However, given that the primary objective of this study was to determine the effect of vitamin D supplementation on serum LC3A levels in patients with KOA, we refrained from gathering demographic data of healthy controls following a 6‐month duration for comparing their demographic data with patients with KOA who underwent post‐supplementation. Additionally, because vitamin D has a dual role, both activating and inhibiting, depending on cell targets, it is important to keep in mind that the influence of autophagy differs across tissues and cells. In support of this, several studies unveiled the KOA chondrocytes with the early state showed both upregulated and downregulated levels of autophagosome proteins, whereas expressions of autophagosome proteins were drastically decreased in KOA chondrocytes with the late stage. 29 , 31 , 34 These previous findings support our results revealing that vitamin D was effective in reducing elevated levels of LC3A to the baseline, which might be used to restore bone homeostasis in patients with KOA with the early stage.
To the best of our knowledge, this study is the first to demonstrate that vitamin D supplementation significantly reduced serum levels of autophagosome protein, LC3A, in patients with KOA. Alongside this, serum levels of LC3A were found to be significantly correlated with knee pain, physical function, and joint inflammation in patients with KOA. From this, it is important to note that new possibilities for using vitamin D to treat KOA might be gleaned from a better understanding of its role in autophagy signaling. Attributable to the effectiveness, safety, and accessibility of vitamin D, there is a solid basis for elucidating its use in clinical practices. These considerations highlight the need for further study to determine how autophagy regulator generates effective, precise, and optimal adjustments to autophagy levels in patients with KOA.
AUTHOR CONTRIBUTIONS
W.S. and W.U. wrote the manuscript. W.U. designed the research. W.S. performed the research. W.S., J.J., U.C., and W.U. analyzed the data. J.J., T.T., S.H., and W.U. contributed reagents, materials, and analytical tools.
FUNDING INFORMATION
This work was supported by the National Research Council of Thailand (NRCT; N42A650217) and the Royal Golden Jubilee (RGJ) PhD Programme Year 2020 (NRCT5‐RGJ63012‐145).
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
ETHICS STATEMENT
This study was endorsed by the Ethical Committee on Human Research of the Faculty of Dentistry/Faculty of Pharmacy, Mahidol University (IRB No. MU‐DT/py‐IRB2022PY072). This study follows the principles of the Declaration of Helsinki. All patients who participated in this study provided informed consent.
Supporting information
Data S1
Data S2
ACKNOWLEDGMENTS
The authors would like to express sincere gratitude to Central Research Unit (CRU), Faculty of Pharmacy, Mahidol University and Center of Excellence in Osteoarthritis and Musculoskeleton, Faculty of Medicine and King Chulalongkorn Memorial Hospital for the generous support in research project.
Saengsiwaritt W, Jittikoon J, Chaikledkaew U, Tawonsawatruk T, Honsawek S, Udomsinprasert W. Effect of vitamin D supplementation on circulating level of autophagosome protein LC3A, inflammation, and physical performance in knee osteoarthritis. Clin Transl Sci. 2023;16:2543‐2556. doi: 10.1111/cts.13646
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
REFERENCES
- 1. Martel‐Pelletier J, Barr AJ, Cicuttini FM, et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. [DOI] [PubMed] [Google Scholar]
- 2. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the Management of Osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020;72(2):149‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Udomsinprasert W, Panon K, Preechanukul S, Jittikoon J, Jinawath A, Honsawek S. Diagnostic value of Interleukin‐34 as a novel biomarker for severity of knee osteoarthritis. Cartilage. 2021;13(2_suppl):1174S‐1184S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Udomsinprasert W, Manoy P, Yuktanandana P, Tanavalee A, Anomasiri W, Honsawek S. Decreased serum adiponectin reflects low vitamin D, high interleukin 6, and poor physical performance in knee osteoarthritis. Arch Immunol Ther Exp. 2020;68(3):16. [DOI] [PubMed] [Google Scholar]
- 5. Primorac D, Molnar V, Rod E, et al. Knee osteoarthritis: a review of pathogenesis and state‐of‐the‐art non‐operative therapeutic considerations. Genes. 2020;11(8):854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mizushima N, Levine B. Autophagy in human diseases. N Engl J Med. 2020;383(16):1564‐1576. [DOI] [PubMed] [Google Scholar]
- 7. Levine B, Kroemer G. Biological functions of autophagy genes: a disease perspective. Cell. 2019;176(1–2):11‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luo P, Gao F, Niu D, et al. The role of autophagy in chondrocyte metabolism and osteoarthritis: a comprehensive research review. Biomed Res Int. 2019;2019:5171602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kao WC, Chen JC, Liu PC, et al. The role of autophagy in osteoarthritic cartilage. Biomolecules. 2022;12(10):1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu SY, Du YC, Yue CF. Sirt7 protects chondrocytes degeneration in osteoarthritis via autophagy activation. Eur Rev Med Pharmacol Sci. 2020;24(18):9246‐9255. [DOI] [PubMed] [Google Scholar]
- 12. Sasaki H, Takayama K, Matsushita T, et al. Autophagy modulates osteoarthritis‐related gene expression in human chondrocytes. Arthritis Rheum. 2012;64(6):1920‐1928. [DOI] [PubMed] [Google Scholar]
- 13. Zhong G, Long H, Ma S, Shunhan Y, Li J, Yao J. miRNA‐335‐5p relieves chondrocyte inflammation by activating autophagy in osteoarthritis. Life Sci. 2019;226:164‐172. [DOI] [PubMed] [Google Scholar]
- 14. McAlindon T, LaValley M, Schneider E, et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA. 2013;309(2):155‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhutia SK. Vitamin D in autophagy signaling for health and diseases: insights on potential mechanisms and future perspectives. J Nutr Biochem. 2022;99:108841. [DOI] [PubMed] [Google Scholar]
- 16. Manoy P, Yuktanandana P, Tanavalee A, et al. Vitamin D supplementation improves quality of life and physical performance in osteoarthritis patients. Nutrients. 2017;9:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heidari B, Babaei M. Therapeutic and preventive potential of vitamin D supplementation in knee osteoarthritis. ACR Open Rheumatol. 2019;1(5):318‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Amirkhizi F, Ghoreishy SM, Baker E, Hamedi‐Shahraki S, Asghari S. The association of vitamin D status with oxidative stress biomarkers and matrix metalloproteinases in patients with knee osteoarthritis. Front Nutr. 2023;10:1101516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siddiqee MH, Bhattacharjee B, Siddiqi UR, MeshbahurRahman M. High prevalence of vitamin D deficiency among the south Asian adults: a systematic review and meta‐analysis. BMC Public Health. 2021;21(1):1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren‐Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886‐1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holick MF, Binkley NC, Bischoff‐Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911‐1930. [DOI] [PubMed] [Google Scholar]
- 22. Castegna A, Drake J, Pocernich C, et al. Protein carbonyl levels—an assessment of protein oxidation. In: Hensley K, Floyd RA, eds. Methods in Biological Oxidative Stress. Methods in Pharmacology and Toxicology. Humana Press; 2003:161‐168. [Google Scholar]
- 23. Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763‐1768. [DOI] [PubMed] [Google Scholar]
- 24. Aigner T, Söder S, Gebhard PM, McAlinden A, Haag J. Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis–structure, chaos and senescence. Nat Clin Pract Rheumatol. 2007;3(7):391‐399. [DOI] [PubMed] [Google Scholar]
- 25. Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage‐bone crosstalk. Nat Rev Rheumatol. 2016;12(11):632‐644. [DOI] [PubMed] [Google Scholar]
- 26. Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO J. 2021;40(19):e108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shen HM, Codogno P. Autophagic cell death: loch ness monster or endangered species? Autophagy. 2011;7(5):457‐465. [DOI] [PubMed] [Google Scholar]
- 28. Rocha FAC, Ali SA. Soluble biomarkers in osteoarthritis in 2022: year in review. Osteoarthr Cartil. 2022;31(2):167‐176. [DOI] [PubMed] [Google Scholar]
- 29. Zhang SL, Zhang KS, Wang JF, et al. Corresponding changes of autophagy‐related genes and proteins in different stages of knee osteoarthritis: an animal model study. Orthop Surg. 2022;14(3):595‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang M, Zhang J, Lu L, et al. Enhancement of chondrocyte autophagy is an early response in the degenerative cartilage of the temporomandibular joint to biomechanical dental stimulation. Apoptosis. 2013;18(4):423‐434. [DOI] [PubMed] [Google Scholar]
- 31. Cheng NT, Meng H, Ma LF, et al. Role of autophagy in the progression of osteoarthritis: the autophagy inhibitor, 3‐methyladenine, aggravates the severity of experimental osteoarthritis. Int J Mol Med. 2017;39(5):1224‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caramés B, Olmer M, Kiosses WB, Lotz MK. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 2015;67(6):1568‐1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging‐related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang J, Wang W, Zhang H, et al. The dual role of autophagy in chondrocyte responses in the pathogenesis of articular cartilage degeneration in osteoarthritis. Int J Mol Med. 2013;32(6):1311‐1318. [DOI] [PubMed] [Google Scholar]
- 35. Li YS, Zhang FJ, Zeng C, et al. Autophagy in osteoarthritis. Joint Bone Spine. 2016;83(2):143‐148. [DOI] [PubMed] [Google Scholar]
- 36. Almonte‐Becerril M, Navarro‐Garcia F, Gonzalez‐Robles A, Vega‐Lopez MA, Lavalle C, Kouri JB. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of osteoarthritis within an experimental model. Apoptosis. 2010;15(5):631‐638. [DOI] [PubMed] [Google Scholar]
- 37. Vaishya R, Vijay V, Lama P, Agarwal A. Does vitamin D deficiency influence the incidence and progression of knee osteoarthritis?—a literature review. J Clin Orthop Trauma. 2019;10(1):9‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bischoff‐Ferrari HA, Zhang Y, Kiel DP, Felson DT. Positive association between serum 25‐hydroxyvitamin D level and bone density in osteoarthritis. Arthritis Rheum. 2005;53(6):821‐826. [DOI] [PubMed] [Google Scholar]
- 39. Heidari B, Javadian Y, Babaei M, Yousef‐Ghahari B. Restorative effect of vitamin D deficiency on knee pain and quadriceps muscle strength in knee osteoarthritis. Acta Med Iran. 2015;53(8):466‐470. [PubMed] [Google Scholar]
- 40. Sanghi D, Mishra A, Sharma AC, et al. Does vitamin D improve osteoarthritis of the knee: a randomized controlled pilot trial. Clin Orthop Relat Res. 2013;471(11):3556‐3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao ZX, He Y, Peng LH, et al. Does vitamin D improve symptomatic and structural outcomes in knee osteoarthritis? A systematic review and meta‐analysis. Aging Clin Exp Res. 2021;33(9):2393‐2403. [DOI] [PubMed] [Google Scholar]
- 42. Alsubiaee KM, Alkhathlan KT, Omair A, Alenezi FM. The effects of vitamin D supplementation in patients with knee osteoarthritis: uncontrolled open label clinical trial. J Arthritis. 2016;5:222. doi: 10.4172/2167-7921.1000222 [DOI] [Google Scholar]
- 43. Arden NK, Cro S, Sheard S, et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: a randomised controlled trial. Osteoarthr Cartil. 2016;24(11):1858‐1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ji L, Gao J, Kong R, Gao Y, Ji X, Zhao D. Autophagy exerts pivotal roles in regulatory effects of 1α,25‐(OH)2D3 on the osteoclastogenesis. Biochem Biophys Res Commun. 2019;511(4):869‐874. [DOI] [PubMed] [Google Scholar]
- 45. Wang W, Li C, Zhang Z, Zhang Y. Arsenic trioxide in synergy with vitamin D rescues the defective VDR‐PPAR‐γ functional module of autophagy in rheumatoid arthritis. PPAR Res. 2019;2019:6403504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kong C, Wang C, Shi Y, Yan L, Xu J, Qi W. Active vitamin D activates chondrocyte autophagy to reduce osteoarthritis via mediating the AMPK‐mTOR signaling pathway. Biochem Cell Biol. 2020;98(3):434‐442. [DOI] [PubMed] [Google Scholar]
- 47. Caramés B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71(4):575‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xue JF, Shi ZM, Zou J, Li XL. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother. 2017;89:1252‐1261. [DOI] [PubMed] [Google Scholar]
- 49. Jhun J, Woo JS, Kwon JY, et al. Vitamin D attenuates pain and cartilage destruction in OA animals via enhancing Autophagic flux and attenuating inflammatory cell death. Immune Netw. 2022;22(4):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74(4):324‐329. [DOI] [PubMed] [Google Scholar]
- 51. Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int. 2007;27(4):339‐344. [DOI] [PubMed] [Google Scholar]
- 52. Regan E, Flannelly J, Bowler R, et al. Extracellular superoxide dismutase and oxidant damage in osteoarthritis. Arthritis Rheum. 2005;52(11):3479‐3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.


