Highlights
-
•
Patients with fibromyalgia underwent a three-week exercise therapy on inpatient basis.
-
•
The three-week exercise therapy improved symptoms of fibromyalgia and patients motor ability.
-
•
Alterations of functional connectivity in sensorimotor cortices were related to improved symptoms of fibromyalgia.
-
•
Alterations of functional connectivity in the mesocortico-limbic system were related to improved motor ability of patients with fibromyalgia.
-
•
Alterations of functional connectivity by exercise therapy can be involved in the improvement of symptoms of fibromyalgia and patients’ motor ability.
Keywords: Fibromyalgia, Exercise therapy, Resting-state fMRI, Functional connectivity, Mesocortico-limbic system
Abstract
Background
Fibromyalgia (FM) is a chronic pain syndrome characterized by widespread pain, tenderness, and fatigue. Patients with FM have no effective medication so far, and their activity of daily living and quality of life are remarkably impaired. Therefore, new therapeutic approaches are awaited. Recently, exercise therapy has been gathering much attention as a promising treatment for FM. However, the underlying mechanisms are not fully understood, particularly, in the central nervous system, including the brain. Therefore, we investigated functional connectivity changes and their relationship with clinical improvement in patients with FM after exercise therapy to investigate the underlying mechanisms in the brain using resting-state fMRI (rs-fMRI) and functional connectivity (FC) analysis.
Methods
Seventeen patients with FM participated in this study. They underwent a 3-week exercise therapy on in-patient basis and a 5-min rs-fMRI scan before and after the exercise therapy. We compared the FC strength of sensorimotor regions and the mesocortico-limbic system between two scans. We also performed a multiple regression analysis to examine the relationship between pre-post differences in FC strength and improvement of patients’ clinical symptoms or motor abilities.
Results
Patients with FM showed significant improvement in clinical symptoms and motor abilities. They also showed a significant pre-post difference in FC of the anterior cingulate cortex and a significant correlation between pre-post FC changes and improvement of clinical symptoms and motor abilities. Although sensorimotor regions tended to be related to the improvement of general disease severity and depression, brain regions belonging to the mesocortico-limbic system tended to be related to the improvement of motor abilities.
Conclusion
Our 3-week exercise therapy could ameliorate clinical symptoms and motor abilities of patients with FM, and lead to FC changes in sensorimotor regions and brain regions belonging to the mesocortico-limbic system. Furthermore, these changes were related to improvement of clinical symptoms and motor abilities. Our findings suggest that, as predicted by previous animal studies, spontaneous brain activities modified by exercise therapy, including the mesocortico-limbic system, improve clinical symptoms in patients with FM.
1. Introduction
Fibromyalgia (FM) is a chronic pain syndrome. The primary symptoms of this syndrome are muscle, joint, and bone pain (Wolfe et al., 2010). It is also accompanied by tenderness, fatigue, and other symptoms (Weir et al., 2006). It mainly affects women between 40 and 60 years of age (Wolfe et al., 1995). Its prevalence rate is approximately 2% (Wolfe et al., 1995). The activity of daily living (ADL) and quality of life (QOL) are remarkably impaired in patients with FM. However, many cases are refractory to multiple therapeutic approaches (Carville et al., 2008). Therefore, new therapeutic approaches are awaited.
Recently, exercise therapy has been attracting much attention as a promising treatment for FM (Busch et al., 2011, Masquelier and D’haeyere, 2021). This therapy is thought to be based on exercise-induced hypoalgesia (EIH). EIH is a phenomenon that pain sensitivity temporarily decreases after a single bout of physical exercise (Rice et al., 2019). The phenomenology and physiology of EIH have been extensively studied in animals as well as in pain-free and chronic pain populations (Rice et al., 2019). Although EIH has been observed in patients with various chronic pain conditions, including FM (Meeus et al., 2010, Staud et al., 2010, Kosek et al., 2013, Christensen et al., 2017), the physiological mechanisms of EIH remain incompletely understood. So far, it is thought that the interaction between the opioid and endocannabinoid systems has a critical role in EIH (Koltyn et al., 2014). The interaction between the opioid and serotonergic systems also seems to be essential for EIH (Lima et al., 2017). Furthermore, some studies suggest that the immune system (Light et al., 2009) and autonomic nervous system (Malfliet et al., 2018) are involved in EIH.
Meanwhile, the effectiveness of exercise therapy on chronic pain has achieved a consensus. A meta-analysis of 24 randomized controlled trials for patients with chronic low back pain (Malfliet et al., 2019), neck pain (Sterling et al., 2019), and osteoarthritis (Rice et al., 2019) revealed that exercise therapy improves their pain and dysfunction. Moreover, for patients with FM, four Cochrane systematic reviews have suggested that exercise therapy can effectively improve QOL, pain, tiredness, bodily function, and muscle stiffness (Bidonde et al., 2014, Bidonde et al., 2017, Bidonde et al., 2019, Kim et al., 2019). In addition, in the EULAR revised recommendations for managing fibromyalgia, exercise therapy, particularly aerobic exercise and strength training, are recommended (Macfarlane et al., 2017). However, as with the physiological mechanisms of EIH, the underlying mechanisms of exercise therapy for chronic pain are not fully understood. Several studies pointed out the importance of cognitive approaches, such as education and cognitive behavioral therapy (Elbers et al., 2018, Wood and Hendrick, 2019, Marris et al., 2021), and behavioral medicine approaches (Malfliet et al., 2019, Sterling et al., 2019, Rice et al., 2019) in combination with exercise therapy. These findings suggest that some other factors besides EIH may contribute to the effectiveness of exercise therapy in the treatment of chronic pain. Therefore, investigating the underlying mechanisms of exercise therapy itself, as well as that of EIH, is necessary.
In recent years, the combination of resting-state functional MRI (rs-fMRI) and functional connectivity (FC) analysis has frequently been used as a tool for investigating functional brain alterations in patients with a wide variety of diseases (Lee et al., 2013; Filippi et al., 2019). This approach focuses on spontaneous brain activity and functional coupling between distant brain regions. Using this approach, many groups reported functional alterations in patients with chronic pain and the relationship between symptoms of chronic pain, such as pain intensity and anxiety, and FC strength (Thorp et al., 2018; Pfannmöller and Lotze, 2019). In particular, several previous studies reported that FC between the medial prefrontal cortex (mPFC) or the posterior cingulate cortex (PCC) and insula is closely related to the development and maintenance of FM (Napadow et al., 2010, Ichesco et al., 2014; Čeko et al., 2020). On the other hand, only one study examined the underlying mechanisms of exercise therapy for patients with FM using this approach, by comparing pre-post differences in functional connectivity and clinical symptoms in the same group of FM patients (Flodin et al., 2015). Therefore, we attempted to elucidate the underlying mechanisms of exercise therapy in patients with FM from the brain function perspective by using rs-fMRI and FC analysis.
2. Methods
2.1. Participants
Seventeen patients with FM participated in this study (58.2 ± 13.7 years old). All of them were females. They were diagnosed by pain specialists based on the American College of Rheumatology 1990 criteria for fibromyalgia (Wolfe et al., 1990). All participants gave informed written consent before study enrollment. This study was approved by the Institutional Review Board of Osaka University Hospital (approval numbers: 13212-9 and 19245) and was conducted by the Declaration of Helsinki and related guidelines.
2.2. Exercise therapy and assessment
The patients underwent a 3-week exercise therapy on inpatient basis. This exercise therapy consisted of aerobic exercise, range of motion, and strength training. Exercise intensities for each patient were adjusted to an 11–15 Borg scale for strength training and a 60% heart rate max calculated by Karvonen formula for aerobic exercise and strength training. Physical therapists instructed and supervised each patient throughout the 3-week therapy.
To confirm the effect of the exercise therapy on patients’ condition, we assessed the clinical condition of each patient before and after the therapy by using the following clinical measures: the Japanese version of the Fibromyalgia Impact Questionnaire (JFIQ) (Burckhardt et al., 1991; Bennett, 2005, Osada et al., 2011), pain intensity, Pain Disability Assessment Scale (PDAS) (Yamashiro et al., 2011), Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snaith, 1983; Kitamura, 1993; Matsudaira et al., 2009), Pain Catastrophizing Scale (PCS) (Sullivan et al., 1995, Matsuoka and Sakano, 2007), EuroQol 5 dimensions 3-level (EQ-5D-3L) (EuroQol Group, 1990, Tsuchiya et al., 2002), Pain Self-Efficacy Questionnaire (PSEQ) (Nicholas, 2007, Adachi et al., 2014), and Athens Insomnia Scale (AIS) (Soldatos et al., 2000, Okajima et al., 2013). Moreover, to confirm motor ability improvement by the therapy, we assessed the following motor ability indices (Table 1): walking speed, cadence, stride, daily step counts, one-leg standing time, ergometer cycling intensity, and ergometer cycling time.
Table 1.
Motor ability indices.
| Index name | Definition | Evaluation content |
|---|---|---|
| walking speed | walking distance per unit of time | walking ability |
| cadence | frequency of steps | walking ability |
| stride | distance between consecutive steps | walking ability |
| daily step counts | the number of steps a day | general endurance and activity |
| one-leg standing time | the duration that one can stand on one leg | lower-limb muscle strength and balance ability |
| ergometer cycling intensity | maximum workload at target motor intensity | lower-limb muscle strength and general endurance |
| ergometer cycling time | the duration that one can continue to cycling at target motor intensity | lower-limb muscle strength and general endurance |
Note: “target motor intensity” was set at 60% of heart rate maximum calculated according to the Karvonen formula.
2.3. fMRI data acquisition and analysis
MRI imaging was performed at Osaka University Hospital using a 3-Tesla scanner (General Electric Discovery750, Waukesha, Wisconsin, United States). Anatomical images were acquired with a high-resolution 3D T1-weighted image sequence (1 × 1 × 1 mm3 voxel size). One hundred fifty volumes of functional images were obtained using a single-shot gradient-echo echo-planar imaging sequence. Scanning parameters were as follows: repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, the number of slices = 40, matrix size = 64 × 64, field of view = 220 × 220 mm, slice thickness = 3.5 mm without gap. The slice acquisition order was interleaved (bottom-to-up). Participants were instructed to be awake and keep their eyes closed during the 5-minute rs-fMRI scan. Participants’ arousal was orally confirmed just before and after an rs-fMRI scan by MRI operators, and whether each participant had been able to be awake during an rs-fMRI scan was additionally confirmed after the entire MRI scanning session.
Rs-fMRI data were processed with SPM12 (version 6906, https://www.fil.ion.ucl.ac.uk/spm/), MATLAB R2017b (Mathworks Inc. Sherborn, MA), and the Conn toolbox (version 17f; https://www.nitrc.org/projects/conn; RRID: SCR_009550; Whitfield-Gabrieli & Nieto-Castanon, 2012). The preprocessing consisted of realignment, unwrapping, slice-timing correction, normalization to Montreal Neurological Institute (MNI) space, and smoothing with an 8-mm full-width at half-maximum Gaussian kernel. Then, we performed denoising with multiple regression to remove noise related to body movement and non-neural physiological activities. In this step, nuisance regressors were six realign parameters and five principal components of fMRI signals from voxels corresponding to white matter and cerebrospinal fluid made by CompCor (Behzadi et al., 2007). We also applied scrubbing to the data to remove outliers resulting from head movements (Power et al., 2014). Subsequently, we used a band-pass filter (0.008 < f < 0.09 Hz) to remove the remaining noises from fMRI signals.
In each participant, FC maps were calculated using seed-based correlation analysis. The seed regions of this study were as follows: the anterior cingulate cortex (ACC), PCC, mPFC, anterior insular cortex (aINS), sensorimotor cortex, corresponding to the lateral part of the sensorimotor network, amygdala (AMG), and nucleus accumbens (NAc). In previous studies, these regions are thought to be related to pain in FM and EIH. The seed templates of these regions were predefined and built into the Conn toolbox. In addition to the FC analysis, we performed amplitude of low-frequency fluctuations (ALFF) (Yang et al., 2007), fractional ALFF (fALFF) (Zou et al., 2008), and local correlation (LCOR) (Deshpande et al., 2009) analysis to investigate spontaneous brain activity.
2.4. Statistics
For the comparison in clinical indices and motor abilities before and after the 3-week exercise therapy, we performed paired t-tests with significance level of 0.05. In addition, the Bonferroni correction was applied in each statistical comparison for clinical indices (the number of comparisons was eight) and motor abilities (that was seven).
For the second-level analysis for ALFF, fALFF, LCOR, and FC, the threshold at the voxel level was set at uncorrected p < 0.001, and the cluster level threshold was set at false discovery rate (FDR)-corrected p < 0.05. Because statistical comparisons were conducted for the FC of 11 seed regions (mPFC, PCC, ACC, Left/Right aINS, Left/Right sensorimotor cortex, Left/Right AMG, and Left/Right NAc), we applied the Bonferroni correction to the cluster-level threshold. We also performed multiple regression analysis to explore the relationship between pre-post differences in FC and clinical and motor improvement. In this analysis, we chose JFIQ and HADS-D scores among clinical indices as covariates because the former is the index that reflects the total severity of FM symptoms, and we assumed that malfunctioning of the mesocortico-limbic system was directly linked to depression, the latter index. Moreover, we chose walking speed, daily step counts, and ergometer cycling time among the indices of motor ability as covariates for multiple regression analysis because they reflect bodily function, ADL, and exercise tolerance, respectively. For this analysis, the threshold at the voxel level was set at uncorrected p < 0.001, and the cluster level threshold was set at FDR-corrected p < 0.05. The Bonferroni correction was applied to the cluster-level threshold in each regression analysis for clinical indices (2 indices and 11 seeds) and motor abilities (3 indices and 11 seeds).
3. Results
3.1. Demographics and clinical improvement
Two patients dropped out of this study. In addition, clinical indices were lacking in the other two patients. As a result, motor ability and rs-fMRI data from 15 patients (59.5 ± 13.7 years old), and clinical indices from 13 patients (58.1 ± 15.0 years old) were analyzed. Clinical and motor ability indices are shown in Table 2. After the exercise therapy, JFIQ, HADS-A, and HADS-D significantly improved (all Ps < 0.00625 after the Bonferroni correction). Furthermore, walking speed, cadence, stride, daily step counts, one-leg standing time, ergometer cycling intensity, and ergometer cycling time, significantly improved (all Ps < 0.00714 after the Bonferroni correction).
Table 2.
Clinical and motor ability indices before and after exercise therapy.
| Index name | Before ET (Mean ± S.D.) | After ET (Mean ± S.D.) | P value |
|---|---|---|---|
| Clinical indices | |||
| JFIQ | 63.5 ± 19.5 | 50.5 ± 21.0 | 0.001858* |
| PDAS | 28.8 ± 14.8 | 20.6 ± 15.8 | 0.020507 |
| HADS Anxiety | 7.8 ± 5.2 | 6.1 ± 4.3 | 0.003056* |
| HADS Depression | 7.6 ± 3.2 | 4.5 ± 3.5 | 0.000904* |
| PCS | 30.5 ± 10.4 | 27.8 ± 13.8 | 0.219374 |
| EQ-5D-3L | 0.503 ± 0.177 | 0.597 ± 0.144 | 0.024169 |
| PSEQ | 25.8 ± 13.6 | 28.6 ± 14.3 | 0.220170 |
| AIS | 11.8 ± 4.5 | 9.6 ± 4.3 | 0.094338 |
| Motor ability | |||
| Walking speed (m/s) | 0.97 ± 0.48 | 1.28 ± 0.46 | 0.000340† |
| Cadence (step/s) | 1.8 ± 0.5 | 2.0 ± 0.5 | 0.001209† |
| Stride (m) | 1.04 ± 0.36 | 1.23 ± 0.32 | 0.003060† |
| Daily step counts | 3885.5 ± 2528.5 | 5900.9 ± 3488.5 | 0.001745† |
| One-leg standing time (s; up to 60 s) | 24.8 ± 25.5 | 28.3 ± 25.2 | 0.004155† |
| Ergometer cycling intensity (watts) | 23.6 ± 13.3 | 34.8 ± 13.5 | 0.002532† |
| Ergometer cycling time (s) | 10.9 ± 7.6 | 18.3 ± 9.1 | 0.000268† |
ET = exercise therapy; JFIQ = Japanese version of the Fibromyalgia Impact Questionnaire; PDAS = Pain Disability Assessment Scale; HADS = Hospital Anxiety and Depression Scale; PCS = Pain Catastrophizing Scale, PSEQ = Pain Self-Efficacy Questionnaire; AIS = Athens Insomnia Scale. Asterisks (*) mean statistically significant after the Bonferroni correction (since it was below 0.00625 = 0.05/8). Daggers (†) mean statistically significant after the Bonferroni correction (since it was below 0.00714 = 0.05/7).
3.2. Functional connectivity and other indices for rs-fMRI data
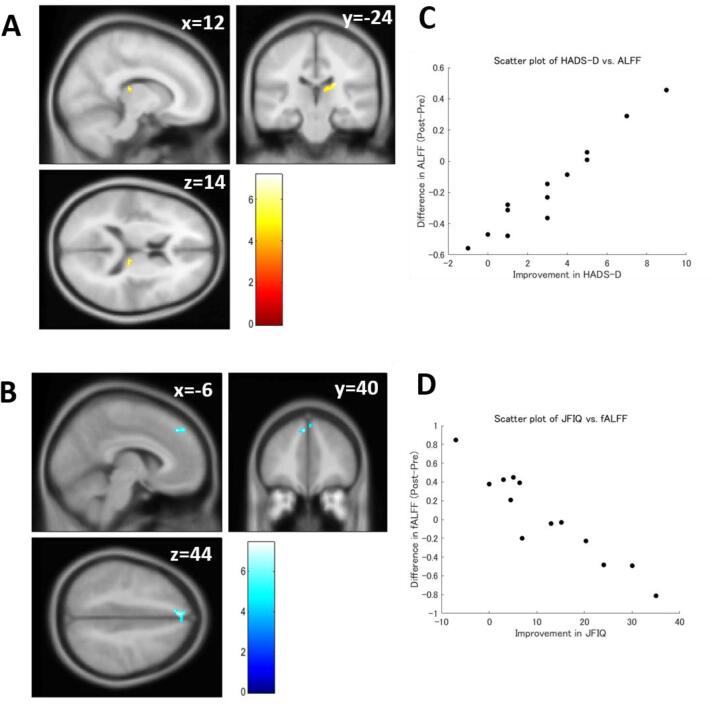
In paired t-tests, ALFF, fALFF, and LCOR did not show significant pre-post differences. In multiple regression analyses, ALFF showed a significant negative correlation with HADS-D, and fALFF showed a significant positive correlation with JFIQ (Table 3 and Fig. 1). However, these correlations were not significant after the Bonferroni correction. LCOR was significantly correlated with neither clinical indices nor motor ability.
Table 3.
Results of multiple regression analysis for ALFF and fALFF.
| Covariate | Correlation type | Cluster size (voxels) | Cluster p-value (FDR) | Peak voxel location |
Brain regions | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| ALFF | |||||||
| HADS-D | Positive | 55 | 0.0346 | 18 | −28 | 18 | Right thalamus |
| fALFF | |||||||
| JFIQ | Negative | 40 | 0.0287 | −6 | 40 | 40 | Left SFG, Right SFG, Left FP |
ALFF = Amplitude of Low-Frequency Fluctuations; fALFF = fractional ALFF; JFIQ = Japanese version of the Fibromyalgia Impact Questionnaire; HADS-D = Hospital Anxiety and Depression Scale Depression subscale; SFG = superior frontal gyrus; FP = frontal pole.
Fig. 1.
Results of the multiple regression analysis for ALFF and fALFF. (A) Brain regions that showed a significant positive correlation between pre-post ALFF change and improvement of Hospital Anxiety and Depression Scale Depression subscale (HADS-D) score. (B) Brain regions that showed a significant negative correlation between pre-post fALFF change and improvement of Japanese version of the Fibromyalgia Impact Questionnaire (JFIQ) score. (C) A scatter plot of HADS-D vs. pre-post ALFF change. (D) A scatter plot of JFIQ vs. pre-post fALFF change. ALFF = Amplitude of Low-Frequency Fluctuations; fALFF = fractional ALFF.
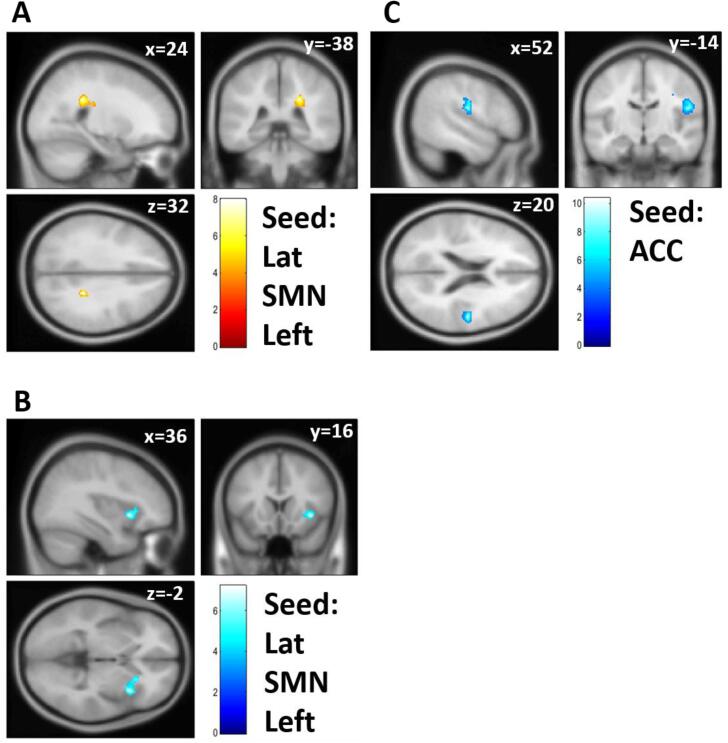
FC analysis showed significant FC differences before and after the 3-week exercise therapy (Table 4 and Fig. 2). FC between the ACC and operculum regions significantly decreased after exercise therapy. FC of the left sensorimotor cortex with the caudate nucleus significantly increased, and that with the aINS decreased after exercise therapy. These pre-post differences were statistically significant even after applying the Bonferroni correction for the cluster-level threshold, except for FC between the left sensorimotor cortex and aINS.
Table 4.
Results of paired t-test for functional connectivity.
| Seed | Cluster size (voxels) | Cluster p-value (FDR) | Peak voxel location |
Brain regions | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Post > Pre ET | ||||||
| Lateral SMN Left | 219 | 0.00426* | 24 | −38 | –32 | Right thalamus, Right caudate |
| Pre > Post ET | ||||||
| ACC | 284 | 0.00104* | 52 | −14 | 20 | Right CO, Right PreCG, Right PostCG, Right PO |
| Lateral SMN Left | 205 | 0.00948 | 36 | 16 | −2 | Right IC, Right FO, Right Caudate, Right Putamen, Right Orbitofrontal gyrus |
ET = exercise therapy; ACC = anterior cingulate cortex; SMN = sensorimotor network; FDR = false discovery rate; CO = central operculum; PreCG = precentral gyrus; PostCG = postcentral gyrus; PO = posterior operculum; IC = insular cortex; FO = frontal operculum. Asterisks (*) mean statistically significant after the Bonferroni correction (since it was below 0.00455 = 0.05/11).
Fig. 2.
Functional connectivity (FC) showed a significant difference between pre- and post-exercise therapy. (A) FC between the left lateral part of the sensorimotor network (SMN) and the right caudate/thalamus after the exercise therapy was stronger than that before the exercise therapy. (B) FC between the left lateral part of the SMN and the right anterior insula was weaker after the exercise therapy than before. (C) FC between the ACC and right operculum was weaker than before the exercise therapy. Lat = lateral; SMN = sensorimotor network; ACC = anterior cingulate cortex.
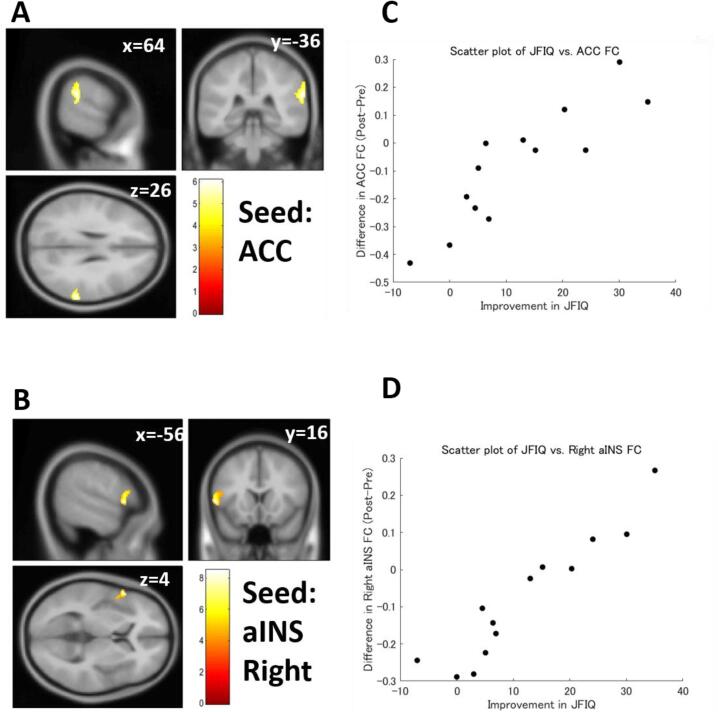
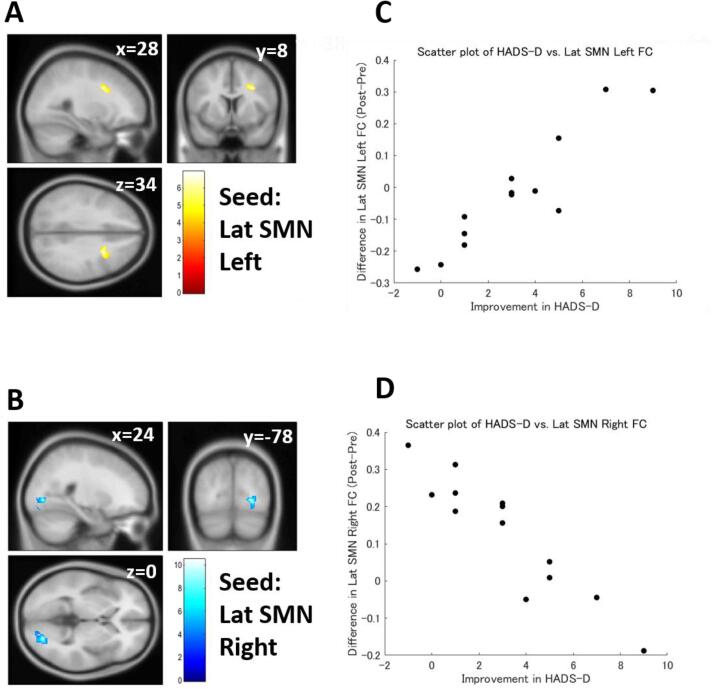
Multiple regression analysis revealed that differences in FC strength were significantly correlated with symptomatic improvement in patients with FM (Table 5). The pre-post difference in the JFIQ score was positively correlated with pre-post differences in ACC FC strength and right aINS FC strength (Fig. 3). The pre-post difference in the HADS-D score was also correlated with the pre-post difference in the lateral part of the sensorimotor network (SMN) (Fig. 4) and ACC. However, the correlations did not reach significance after the Bonferroni correction, except for the correlation between HADS-D and the right side of the lateral SMN FC.
Table 5.
Results of multiple regression analyses for functional connectivity.
| Covariate | Seed | Correlation type | Cluster size (voxels) | Cluster p-value (FDR) | Peak voxel location |
Brain regions | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| JFIQ | ACC | Positive | 188 | 0.00548 | 64 | −36 | 26 | Right SMG, Right PT, Right PO |
| Right aINS |
Positive | 155 | 0.02736 | −56 | 16 | 4 | Left IFG, Left PreCG | |
| HADS-D | Lateral SMN Left | Positive | 166 | 0.00827 | 28 | 8 | 34 | Right MFG |
| Lateral SMN Right | Negative | 252 | 0.000781* | 24 | −78 | 0 | Right OFusG, Right iLOC | |
| ACC | Positive | 149 | 0.018912 | 28 | 32 | −10 | Right FOrb, Right FP, Right IC | |
| 126 | 0.019591 | −62 | −54 | −18 | Left toITG | |||
| Negative | 120 | 0.039719 | 30 | 64 | 16 | Right FP | ||
JFIQ = Japanese version of the Fibromyalgia Impact Questionnaire; HADS-D = Hospital Anxiety and Depression Scale Depression Subscale; ACC = anterior cingulate cortex; SMN = sensorimotor network; SMG = supramarginal gyrus; PT = planum temporale; PO = posterior operculum; IFG = inferior frontal gyrus; PreCG = precentral gyrus; MFG = middle frontal gyrus; OFusG = occipital fusiform gyrus; iLOC = inferior lateral occipital cortex; FOrb = orbitofrontal gyrus; FP = frontal pole; IC = insular cortex; toITG = temporooccipital part of inferior temporal gyrus. An asterisk (*) means statistically significant after the Bonferroni correction (since it was below 0.00227 = 0.05/22).
Fig. 3.
Functional connectivity (FC) correlated with Japanese version of the Fibromyalgia Impact Questionnaire (JFIQ) score. (A) FC between the anterior cingulate cortex (ACC) and the right supramarginal gyrus showed a significant positive correlation with the JFIQ score. (B) FC between right anterior insula (aINS) and the left inferior frontal gyrus/precentral gyrus also showed a significant positive correlation with the JFIQ score. (C) A scatter plot of JFIQ score vs. FC of the ACC. (D) A scatter plot of JFIQ score vs. FC of the right aINS. Color bars indicate t-values.
Fig. 4.
Functional connectivity (FC) correlated with Hospital Anxiety and Depression Scale Depression Subscale (HADS-D) score. (A) FC between the left lateral part of the sensorimotor network (SMN) and middle frontal gyrus showed a significant positive correlation with the HADS-D score. (B) FC between the right lateral part of the SMN and the right occipital fusiform gyrus showed a significant negative correlation with the HADS-D score. (C) A scatter plot of HADS-D score vs. FC of the left lateral part of SMN. (D) A scatter plot of HADS-D score vs. FC of the right lateral part of SMN. Lat = lateral; Color bars indicate t-values.
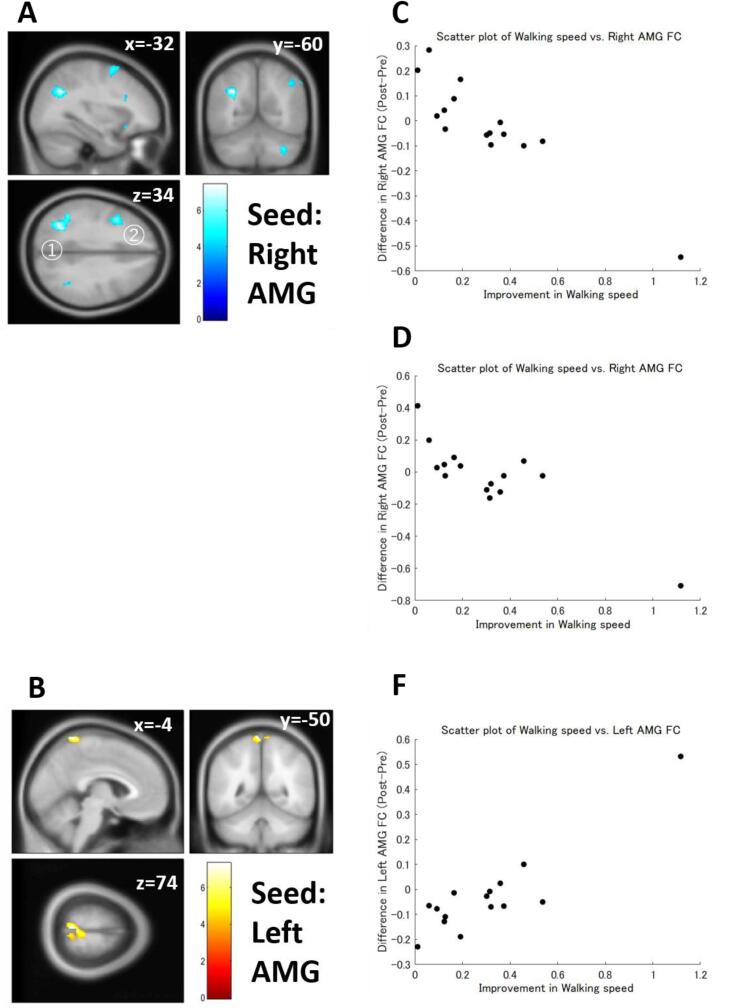
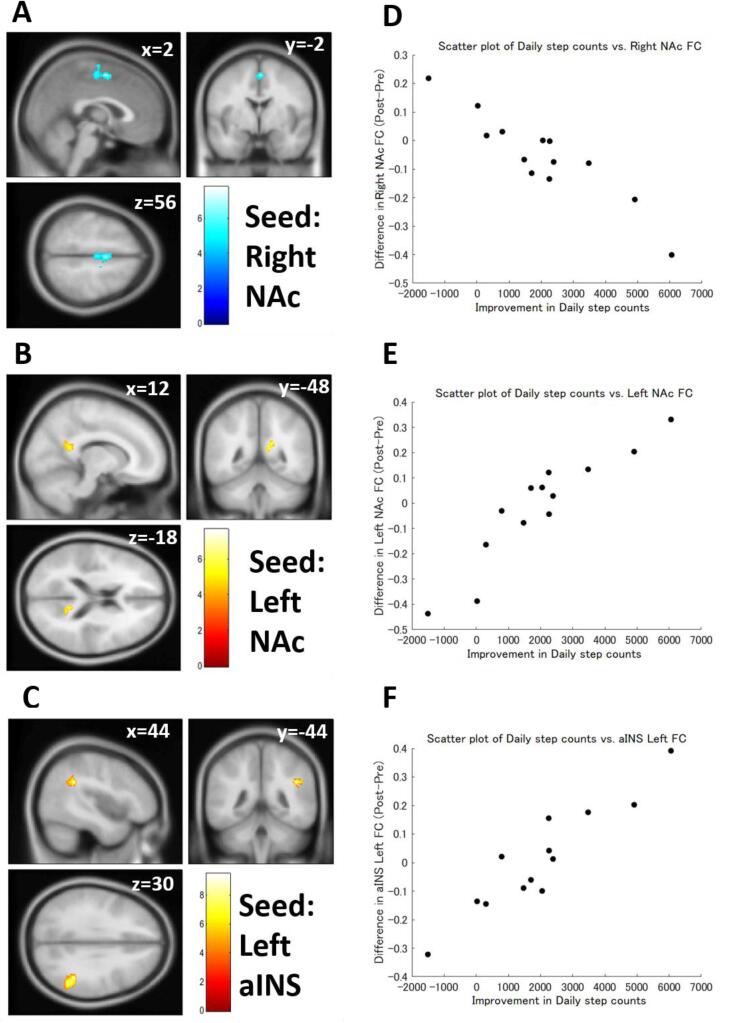
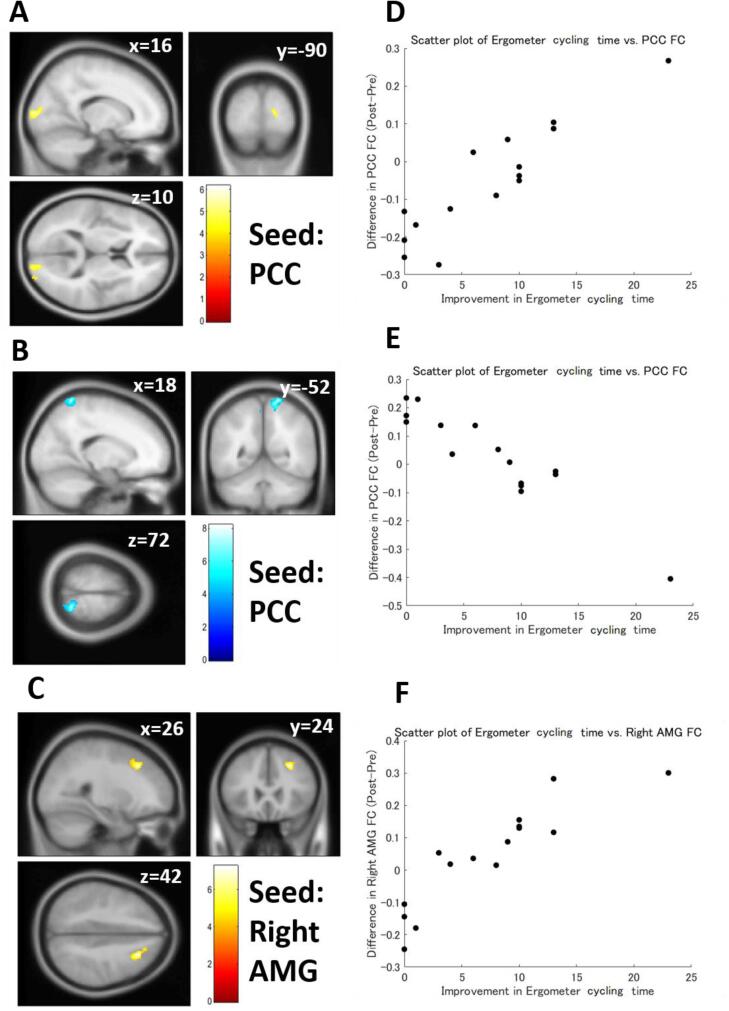
Multiple regression analysis also revealed that differences in FC strength were significantly correlated with improved exercise capacity in patients with FM (Table 6). Improvement of walking speed was negatively correlated with the pre-post differences in the AMG (Fig. 5A, 5C, and 5D) and PCC FC strength. The left AMG was also positively correlated with walking speed improvement (Fig. 5B and 5F). An increase in daily step counts was negatively correlated with the pre-post differences in the right NAc (Fig. 6A and 6D) and positively correlated with the pre-post differences in the left NAc FC (Fig. 6B and 6E) and the left aINS FC strength (Fig. 6C and 6F). Ergometer cycling time improvement was positively correlated with the pre-post difference in the right AMG (Fig. 7C and 7F), mPFC, PCC (Fig. 7A and 7D), and ACC FC strength and negatively correlated with the pre-post difference in PCC (Fig. 7B and 7E) and the left aINS FC strength. The correlations between walking speed and right AMG FC, daily step counts and left aINS FC, and ergometer cycling time and PCC FC were still significant even after the Bonferroni correction.
Table 6.
Results of multiple regression analyses for functional connectivity.
| Covariate | Seed | Correlation type | Cluster size (voxels) | Cluster p-value (FDR) | Peak voxel location |
Brain regions | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Walking speed | Right AMG | Negative | 341 | 0.000327* | –32 | −60 | 34 | Left sLOC, Left AG |
| 245 | 0.001808 | −40 | 10 | 36 | Left MFG, Left PreCG | |||
| 220 | 0.002368 | −66 | −26 | −2 | Left pMTG, Left pSTG | |||
| 175 | 0.005284 | −34 | 6 | 60 | Left MFG | |||
| 174 | 0.005284 | 34 | −64 | −40 | Cerebellum | |||
| 130 | 0.016959 | 68 | −24 | −14 | Right pMTG, Right toMTG | |||
| 126 | 0.016959 | –22 | 20 | −16 | Left Orbitofrontal gyrus | |||
| 115 | 0.021478 | 56 | −58 | 46 | Right AG, Right sLOC | |||
| Left AMG | Positive | 223 | 0.002073 | −4 | −50 | 74 | Right PostCG, Left PostCG, Left SPL, Precuneus, Right PreCG, Left PreCG, Right SPL | |
| Negative | 147 | 0.04921 | −26 | 24 | −16 | Left orbitofrontal gyrus | ||
| Daily step counts | Right NAc | Negative | 196 | 0.004006 | 2 | −2 | 56 | Right SMA, Left SMA, Right PreCG, Left PreCG |
| 186 | 0.004006 | −38 | 14 | 6 | Left FO, Left IC, Left FOrb | |||
| Left NAc | Positive | 115 | 0.048617 | 16 | −52 | 22 | Precuneus | |
| Left AMG | Negative | 142 | 0.025791 | −66 | –32 | −12 | Left pMTG | |
| 111 | 0.029456 | −24 | 14 | −20 | Left orbitofrontal gyrus, Left TP, Left IC | |||
| 106 | 0.029456 | –32 | 32 | −12 | Left orbitofrontal gyrus, Left FP | |||
| ACC | Positive | 185 | 0.007309 | −16 | –32 | −8 | Brainstem, PC, Left pPaHC, Hipp, Thalamus, Cerebellum, Right pPaHC | |
| Left aINS | Positive | 229 | 0.001069* | 44 | −44 | 30 | Right AG, Right pSMG | |
| Ergometer cycling time | Right AMG | Positive | 188 | 0.006603 | 26 | 24 | 42 | Right MFG, Right SFG, Right FP |
| 182 | 0.006603 | 44 | −74 | 32 | Right sLOC, Right AG | |||
| mPFC | Positive | 222 | 0.004757 | 34 | −66 | −40 | Cerebellum | |
| PCC | Positive | 204 | 0.006302 | 26 | −98 | 16 | Right OP | |
| Negative | 356 | 0.000062* | 18 | −52 | 72 | Right SPL, Precuneus, Left PostCG, Right PostCG, Right sLOC, Left PreCG | ||
| ACC | Positive | 216 | 0.004057 | 10 | −90 | 4 | Right OP, Right ICC, Right LG | |
| 135 | 0.020982 | −24 | 44 | −2 | mPFC | |||
| Left aINS | Negative | 143 | 0.017686 | −48 | −82 | −2 | Left iLOC | |
sLOC = superior lateral occipital cortex; AG = angular gyrus; MFG = middle frontal gyrus; PreCG = precentral gyrus; pMTG = posterior part of middle temporal gyrus; toMTG = temporooccipital part of middle temporal gyrus; PostCG = postcentral gyrus; SPL = superior parietal lobule; SMA = supplementary motor area; FO = frontal operculum; IC = insular cortex; FOrb = orbitofrontal gyrus; TP = temporal pole; FP = frontal pole; pPaHC = posterior part of parahippocampal cortex; Hipp = hippocampus; pSMG = posterior supramarginal gyrus; SFG = superior frontal gyrus; OP = occipital pole; ICC = intracalcarine cortex; LG = lingual gyrus. Asterisks (*) mean statistically significant after the Bonferroni correction (since it was below 0.001515 = 0.05/33).
Fig. 5.
Functional connectivity (FC) correlated with walking speed. (A) FC between the right amygdala (AMG) and the left lateral occipital cortex (LOC) (A①) or the left middle frontal gyrus (MFG) (A②) showed a significant negative correlation with walking speed. (B) FC between the left AMG and the postcentral gyrus showed a significant positive correlation with walking speed. (C) A scatter plot of walking speed vs. the right AMG-LOC FC. (D) A scatter plot of walking speed vs. the right AMG-MFG FC. (F) A scatter plot of walking speed vs. the left AMG FC. Color bars indicate t-values.
Fig. 6.
Functional connectivity (FC) correlated with daily step counts. (A) FC between the right nucleus accumbens (NAc) and the supplementary motor area/precentral gyrus showed a significant negative correlation with daily step counts. (B) FC between the left NAc and the precuneus showed a significant positive correlation with daily step counts. (C) FC between the left anterior insula (aINS) and the supramarginal gyrus showed a significant positive correlation with daily step counts. (D) A scatter plot of daily step counts vs. the right NAc FC. (E) A scatter plot of daily step counts vs. the left NAc FC. (F) A scatter plot of daily step counts vs. the left aINS FC. Color bars indicate t-values.
Fig. 7.
Functional connectivity (FC) correlated with ergometer cycling time. (A) FC between the posterior cingulate cortex (PCC) and the occipital pole showed a significant positive correlation with ergometer cycling time. (B) FC between the PCC and the precuneus showed a significant negative correlation with ergometer cycling time. (C) FC between the right amygdala (AMG) and the middle frontal gyrus showed a significant positive correlation with ergometr cycling time. (D) A scatter plot of the ergometer cycling time vs. the PCC-occipital pole FC. (E) A scatter plot of the ergometer cycling time vs. the PCC-precuneus FC. (F) A scatter plot of the ergometer cycling time vs. the right AMG FC. Color bars indicate t-values.
4. Discussion
In this study, we examined the effect of the 3-week exercise therapy on FM and its neural basis using rs-fMRI and functional connectivity analysis. We found that our 3-week exercise therapy improved not only motor abilities but also several clinical symptoms of patients with FM. Moreover, the degrees of improvement in those indices were significantly correlated with the pre-post differences in FC of brain regions that are considered to be related to pain chronification, including the NAc and AMG. These results indicate that exercise therapy can ameliorate symptoms of FM, and they suggest that its effect results from functional alterations of the brain, including the mesocortico-limbic system.
4.1. Effect of the 3-week exercise therapy on symptoms of FM
Our 3-week exercise therapy was able to improve not only motor abilities but also symptoms of FM. In particular, exercise therapy significantly improved depression and anxiety in patients with FM, suggesting that exercise therapy normalizes the aberrant functioning of the mesocortico-limbic system.
The mesocortico-limbic system consists of the reward system, AMG, hippocampus, and mPFC (Klein et al., 2019). Under chronic pain or chronic stress condition, the reward system is suppressed, and the AMG in excessive excitability due to fear and anxiety suppresses mPFC and NAc activities (Radley, 2012). In previous animal studies, two weeks of voluntary wheel running decreased pain behavior in partial sciatic nerve ligation (PSL) model mice, which is accompanied by activation of dopamine neurons in the ventral tegmental area (VTA) projecting to the NAc (brain reward system) (Kami et al., 2018). Moreover, the voluntary running activated the basomedial amygdala neurons that project to the NAc to promote goal-directed behavior (Kami et al., 2020), while it suppressed gamma-aminobutyric acidergic neurons in the central AMG, an output nucleus of the AMG, to reduce fear response to painful stimuli (Kami et al., 2020). It has been reported that the mesocortico-limbic system of patients or animals suffering chronic pain is malfunctioning (Baliki et al., 2010, Baliki et al., 2012 1 Vachon-Presseau et al., 2016). Especially, the activity of the NAc is depressed (Ji et al., 2010, Lee et al., 2015). As the findings of animal studies have indicated (Kami et al., 2018, Kami et al., 2020), exercise may activate the reward system and normalize the function of the mesocortico-limbic system, the center of which is the NAc. Moreover, exercise enables us to take positive, goal-directed behavior, while suppressing negative fear response, such as freezing (immobility). Our result suggests that the same mechanism acts in patients with FM.
4.2. Pre-post differences in functional connectivity
In this study, FC analysis revealed that the sensorimotor cortex and ACC changed their functional relations after the 3-week exercise therapy. FC between the left sensorimotor cortex and right thalamus after exercise therapy was stronger than those before the exercise therapy. In contrast, FC between the left sensorimotor cortex and the right aINS before exercise therapy was stronger than those after exercise therapy. In addition, FC between the ACC and the ventral part of the right sensorimotor cortex before exercise therapy was stronger than those after exercise therapy. Interestingly, the strengths of these FC after exercise therapy were around zero, suggesting that exercise therapy normalized aberrant FC alterations in patients with FM.
FC that showed significant pre-post differences mainly consisted of the SMN, that is, the sensorimotor cortex, and the salience network, in particular, the ACC and the aINS. These networks correspond to the lateral and medial systems of the pain matrix, respectively. It is thought that the former has a role in processing the sensory aspect of pain and the latter in processing the affective aspect of pain (Ingvar, 1999; Brooks & Tracey, 2005). These regions were reported to show higher responsiveness to experimental pain stimuli in patients with FM (Gracely et al., 2002). These two networks are functionally connected in healthy adults, although their spatial patterns are highly heterogeneous (Hegarty et al., 2020). In patients with chronic pain, FC strength between the sensorimotor cortex and insula was increased compared to healthy controls (Kutch et al., 2017). Moreover, patients with low back pain showed that the salience network had stronger functional connections to a specific area in the primary somatosensory cortex representing the back than healthy controls. This functional connection was strengthened by the maneuvers exacerbating pain (Kim et al., 2019). In contrast to low back pain, pain in FM occurs all over the body. Therefore, the FC of the salience network is probably uniform across the sensorimotor cortices in patients with FM.
Although functional connections between the SMN and the salience network changed after the 3-week exercise therapy, those changes did not directly link to improved clinical symptoms or motor abilities. It is unclear how SMN-salience network FC change is involved in symptomatic improvement by exercise therapy in patients with FM. Therefore, it is necessary to investigate this issue in future studies.
Relationship between changes in functional connectivity and improvement of clinical symptoms and motor abilities after the 3-week exercise therapy for patients with FM
Changes in FC strength of sensorimotor cortices, aINS and ACC were related to improved FM symptoms. In particular, FC strength between the right sensorimotor cortex and the fusiform gyrus was negatively correlated with the improvement of HADS-D. Although these regions are not generally considered to be related to depression, a previous study reported hypoconnectivity between the fusiform gyrus and sensorimotor areas in patients with major depressive disorder (Chen et al., 2021). Furthermore, a gray matter volume decrease (Shi et al., 2016) and abnormal dopamine function (Albrecht et al., 2016) in this area were previously reported in patients with FM. The fusiform gyrus is involved in emotional regulation (Fonville et al., 2013, Harry et al., 2013). Taken together, this finding can shed light on the involvement of the fusiform gyrus in the depressive symptoms of patients with FM.
In contrast to FM symptoms, changes in FC strength of the mesocortico-limbic system, such as the ACC, aINS, AMG, and NAc, were related to improved motor ability. This finding suggests that exercise therapy not only directly but also indirectly improves motor ability in patients with FM via cognitive and motivational process. Although the mesocortico-limbic system (reward system; from VTA to NAc) is related to reward and motivation rather than motor function (Klein et al., 2019), the NAc projects to motor-related structures, and is thought to be involved in goal-directed behaviors (LeDoux & Daw, 2018; Namburi et al., 2015; Beyeler et al., 2016). Furthermore, as mentioned above, exercise may activate the reward system and normalize the function of the mesocortico-limbic system (Kami et al.,2020), making patients with FM take positive and goal-directed behavior. Therefore, the relationship between improved motor ability and FC changes in the mesocortico-limbic system after exercise therapy can reflect this process. This interpretation is in line with a previous finding that depression evolves in the background of pain-induced disability (Fishbain et al., 1997).
In this study, AMG-angular gyrus (AG) FC, aINS-AG/supramarginal gyrus (SMG) FC, and PCC-sensorimotor cortex FC were significantly correlated to the improvement of motor abilities by the 3-week exercise therapy. However, as mentioned above, these FC are less likely to be directly involved in motor function except for the sensorimotor cortex. On the other hand, these regions have been thought to be related to the development and maintenance of chronic pain (Yang et al., 2019). The AMG is known to have a critical role in emotion (Šimić et al., 2021). A previous meta-analysis revealed that pain with concomitant depression was associated with the right AMG (Zheng et al., 2022). The angular gyrus has a role in integrating multimodal sensory information (Seghier, 2013), and this region is considered a part of the default mode network (DMN) (Raichle, 2015). Although studies showing that AMG-AG FC is directly related to pain processing are scarce, several studies pointed out that this FC is involved in the regulation of emotions. For example, a previous study reported that gray matter volumes of the AMG and AG in patients with mild cognitive impairment were significantly correlated with their financial capacity (Giannouli & Tsolaki, 2019). The authors interpreted that this result suggested the importance of emotion regulation in a financial capacity. Another study reported that AMG-AG FC was increased by imagined music performance (Tanaka & Kirino, 2021), suggesting that this FC is related to emotion regulation, because music evokes emotion. Moreover, a previous study reported that music listening decreased pain intensity and spontaneous AG activity in patients with FM, and they were significantly correlated (Garza-Villarreal et al., 2015). As with this study, our 3-week exercise therapy could improve motor ability and FM symptoms via normalization of emotion regulation system.
The aINS is known to be a part of the salience network (Seeley, 2019). The inferior parietal lobule, including the AG and SMG, is known to be a part of the default mode network (Raichle, 2015). The aberrant functional interaction between them has been reported in patients with FM. DMN-aINS FC strength was correlated to pain intensity in patients with FM (Napadow et al., 2010, Ichesco et al., 2014; Čeko et al., 2020), and Pregabalin administration decreased DMN-aINS FC (Harris et al., 2013). Moreover, a previous study reported that pre-treatment aINS-AG FC was significantly correlated with the pre-post difference in pain severity (Argaman et al., 2022). Taken together, the aINS-AG/SMG FC change by the exercise therapy could be a part of the underlying mechanism of exercise-induced improvement of FM symptoms.
PCC is one of the central regions of the DMN (Raichle, 2015). The sensorimotor cortex is a part of the SMN (Smith et al., 2009). These two regions receive sensory information from the thalamus. Previous studies have reported PCC FC alteration in patients with chronic pain (Cifre, 2012, Duke Han et al., 2013, Kucyi et al., 2014, Hemington et al., 2016). In patients with acquired upper limb amputation, FC between the DMN and missing hand region in the SI increased compared to healthy controls (Makin et al., 2015). Furthermore, in patients with chronic low back pain, the increase in pain intensity by specific maneuvers was related to the change in FC strength between the DMN and the primary somatosensory cortex (Kim et al., 2019). Patients with comorbid migraine and insomnia showed increased PCC-precentral gyrus FC compared to healthy controls (Chou et al., 2021). Patients with FM experience sleep disturbance (Harding, 1998), and pain is closely related to insomnia (Affleck et al., 1996). Moreover, a previous study revealed that insomnia causes hyperalgesia and physical inactivity, which may lead to depression (Bigatti et al., 2008). Although not statistically significant, patients tended to improve their insomnia in the present study. Therefore, considering these previous findings, the relationship between the pre-post difference in PCC-sensorimotor cortex FC and the improvement of ergometer cycling time suggests that our 3-week exercise therapy improved sleep quality and physical performance, which in turn lead to improved FM symptoms.
As mentioned above, we found that FC of several brain regions was related to improvement of FM symptoms and motor ability. However, they did not show significant differences in their strengths before and after the exercise therapy. It suggests that FC change among certain brain regions is critical to the improvement of FM symptoms and motor ability in patients with FM even though FC change induced by the exercise therapy is too small to detect in a pre-post comparison. Moreover, as we hypothesized, brain regions related to emotion or reward processing comprised FC related to clinical improvement in patients with FM. Of course, there is another possibility that slight FC change occurred secondarily from the improvement of FM symptoms and motor ability induced by the exercise therapy. Our study cannot distinguish them due to methodological limitations. To test those possibilities, further studies with larger sample size to perform statistical analysis for evaluating causal relationship or interventions manipulating directly FC strength are necessary.
Limitations
We should consider several limitations in this study. Firstly, the sample size of this study was small. In recent years, it has been revealed that FC strength is unstable (Noble et al., 2017), and the influences of diseases on FC are less than expected (Yamashita et al., 2019). Thus, results obtained from studies with a small sample size are vulnerable, and the power of tests is low. Therefore, results and the subsequently derived interpretations should be taken with caution. However, to our knowledge, only one study has investigated the neural mechanisms underlying exercise therapy in FM patients so far. Therefore, we are confident that our study is meaningful and valuable. In the future, conducting clinical studies with a larger sample size is necessary. Secondly, the lack of any control groups is also a critical limitation of this study. Therefore, we cannot conclude in this study that exercise therapy caused changes in brain function and improved symptoms and physical performance. Clinical randomized trials are necessary to confirm the effect of exercise therapy on symptoms and brain activities in patients with FM. Thirdly, our results do not indicate any causal relationship between FC strength and FM symptoms. As widely accepted/recognized, correlations do not ensure the presence of causal relationships. Therefore, we cannot conclude any causal relationship between exercise therapy, FM symptoms, and FC strength. To fully understand the underlying mechanisms of exercise therapy on FM symptoms and spontaneous brain activity, statistical approaches that can evaluate the causal relationship, such as structural equation modeling, should be applied. Finally, we did not examine how long the effect of the 3-week exercise therapy lasts in this study. Our study was able to demonstrate that the 3-week exercise therapy alleviated symptoms of FM. However, we did not examine how long this effect will last. If exercise therapy is widely applied in clinical practice to treat patients with FM, this point should be examined in future studies.
Conclusion
Using rs-fMRI and FC analysis, we examined whether our 3-week exercise therapy can improve FM symptoms and what FC changes exercise therapy causes in patients with FM. Patients with FM showed significant improvement in motor abilities and clinical symptoms of FM after the 3-week exercise therapy. Moreover, pre-post differences in several FC were significantly correlated with clinical improvement of these patients, such as the general severity of FM symptoms and depression. FC changes in sensorimotor regions were related to the improvement of FM symptoms, whereas FC changes in the mesocortico-limbic system were related to improvements in motor ability. Although our findings are apparently counterintuitive, they are consistent with our hypothesis that exercise can improve the behavioral disturbance of patients with FM through functional normalization of the mesocortico-limbic system, which may lead to the improvement of FM symptoms. Although there are some limitations, our study provides intriguing evidence that the malfunctioning of the mesocortico-limbic system is involved in the development of FM, and exercise therapy can normalize the functioning of this system, changing patients’ behavior in a positive way. It will in turn improve the clinical symptoms of FM.
CRediT authorship contribution statement
Shigeyuki Kan: Software, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Visualization. Nobuko Fujita: Methodology, Investigation, Resources, Writing – review & editing, Project administration, Funding acquisition. Masahiko Shibata: Writing – review & editing, Project administration. Kenji Miki: Investigation, Resources, Writing – review & editing. Masao Yukioka: Resources, Writing – review & editing. Emiko Senba: Conceptualization, Methodology, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors give special thanks to Hiroyuki Tarewaki, Yoichiro Ikushima, Keiko Tanaka, and Masaaki Kawahara for performing MRI and Ikuyo Kurata, Kiyoka Enomoto, Yoshiko Miyake, Riho Kato, and Seiichi Osako for assistance in collecting patient data.
Funding
This work was supported by JSPS KAKENHI Grant Number 16K09002.
Data availability
Data will be made available on request.
References
- Adachi T., Nakae A., Maruo T., Shi K., Shibata M., Maeda L., Saitoh Y., Sasaki J. Validation of the Japanese version of the pain self-efficacy questionnaire in Japanese patients with chronic pain. Pain Med. 2014;15(8):1405–1417. doi: 10.1111/pme.12446. Epub 2014 Apr 9 PMID: 24717053. [DOI] [PubMed] [Google Scholar]
- Affleck G., Urrows S., Tennen H., Higgins P., Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68(2–3):363–368. doi: 10.1016/s0304-3959(96)03226-5. PMID: 9121825. [DOI] [PubMed] [Google Scholar]
- Albrecht D.S., MacKie P.J., Kareken D.A., Hutchins G.D., Chumin E.J., Christian B.T., Yoder K.K. Differential dopamine function in fibromyalgia. Brain Imaging Behav. 2016;10(3):829–839. doi: 10.1007/s11682-015-9459-4. PMID: 26497890; PMCID: PMC4842344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaman Y., Granovsky Y., Sprecher E., Sinai A., Yarnitsky D., Weissman-Fogel I. Resting-state functional connectivity predicts motor cortex stimulation-dependent pain relief in fibromyalgia syndrome patients. Sci. Rep. 2022;12(1):17135. doi: 10.1038/s41598-022-21557-x. PMID: 36224244; PMCID: PMC9556524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Geha P.Y., Fields H.L., Apkarian A.V. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66(1):149–160. doi: 10.1016/j.neuron.2010.03.002. PMID: 20399736; PMCID: PMC2873199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Petre B., Torbey S., Herrmann K.M., Huang L., Schnitzer T.J., Fields H.L., Apkarian A.V. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012 1;15(8):1117–1119. doi: 10.1038/nn.3153. PMID: 22751038; PMCID: PMC3411898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin. Exp. Rheumatol. 2005;23(5 Suppl 39) S154–62, PMID: 16273800. [PubMed] [Google Scholar]
- Bidonde J., Busch A.J., Webber S.C., Schachter C.L., Danyliw A., Overend T.J., Richards R.S., Rader T. Aquatic exercise training for fibromyalgia. Cochrane Database Syst. Rev. 2014 doi: 10.1002/14651858.CD011336. PMID: 25350761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A., Namburi P., Glober G.F., Simonnet C., Calhoon G.G., Conyers G.F., Luck R., Wildes C.P., Tye K.M. Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron. 2016;90(2):348–361. doi: 10.1016/j.neuron.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidonde J., Busch A.J., Schachter C.L., Overend T.J., Kim S.Y., Góes S.M., Boden C., Foulds H.J. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst. Rev. 2017 doi: 10.1002/14651858.CD012700. PMID: 28636204; PMCID: PMC6481524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidonde J., Busch A.J., Schachter C.L., Webber S.C., Musselman K.E., Overend T.J., Góes S.M., Dal Bello-Haas V., Boden C. Mixed exercise training for adults with fibromyalgia. Cochrane Database Syst. Rev. 2019 doi: 10.1002/14651858.CD013340. PMID: 31124142; PMCID: PMC6931522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigatti S.M., Hernandez A.M., Cronan T.A., Rand K.L. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum. 2008;59(7):961–967. doi: 10.1002/art.23828. PMID: 18576297; PMCID: PMC3691959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J., Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathways. J. Anat. 2005;207(1):19–33. doi: 10.1111/j.1469-7580.2005.00428.x. PMID: 16011543; PMCID: PMC1571498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt C.S., Clark S.R., Bennett R.M. The fibromyalgia impact questionnaire: development and validation. J. Rheumatol. 1991;18(5):728–733. PMID: 1865419. [PubMed] [Google Scholar]
- Busch A.J., Webber S.C., Brachaniec M., Bidonde J., Bello-Haas V.D., Danyliw A.D., Overend T.J., Richards R.S., Sawant A., Schachter C.L. Exercise therapy for fibromyalgia. Curr. Pain Headache Rep. 2011;15(5):358–367. doi: 10.1007/s11916-011-0214-2. PMID: 21725900; PMCID: PMC3165132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carville S.F., Arendt-Nielsen S., Bliddal H., Blotman F., Branco J.C., Buskila D., Da Silva J.A.P., Danneskiold-Samsøe B., Dincer F., Henriksson C., Henriksson K.G., Kosek E., Longley K., McCarthy G.M., Perrot S., Puszczewicz M., Sarzi-Puttini P., Silman A., Späth M., Choy E.H. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann. Rheum. Dis. 2008;67(4):536–541. doi: 10.1136/ard.2007.071522. [DOI] [PubMed] [Google Scholar]
- Čeko M., Frangos E., Gracely J., Richards E., Wang B., Schweinhardt P., Catherine Bushnell M. Default mode network changes in fibromyalgia patients are largely dependent on current clinical pain. NeuroImage. 2020;216:116877. doi: 10.1016/j.neuroimage.2020.116877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Liu Z., Zuo J., Xi C., Long Y., Li M.D., Ouyang X., Yang J. Decreased cortical folding of the fusiform gyrus and its hypoconnectivity with sensorimotor areas in major depressive disorder. J. Affect. Disord. 2021;1(295):657–664. doi: 10.1016/j.jad.2021.08.148. Epub 2021 Sep 3 PMID: 34509781. [DOI] [PubMed] [Google Scholar]
- Chou K.H., Kuo C.Y., Liang C.S., Lee P.L., Tsai C.K., Tsai C.L., Huang M.H., Hsu Y.C., Lin G.Y., Lin Y.K., Lin C.P., Yang F.C. Shared patterns of brain functional connectivity for the comorbidity between migraine and insomnia. Biomedicines. 2021;9(10):1420. doi: 10.3390/biomedicines9101420. PMID: 34680538; PMCID: PMC8533078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S.W., Hirata R.P., Graven-Nielsen T. Altered pain sensitivity and axioscapular muscle activity in neck pain patients compared with healthy controls. Eur. J. Pain. 2017;21(10):1763–1771. doi: 10.1002/ejp.1088. Epub 2017 Aug 27 PMID: 28845598. [DOI] [PubMed] [Google Scholar]
- Cifre I, Sitges C, Fraiman D, Muñoz MÁ, Balenzuela P, González-Roldán A, Martínez-Jauand M, Birbaumer N, Chialvo DR, Montoya P. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med. 2012;74(1):55-62. doi: 10.1097/PSY.0b013e3182408f04. Epub 2011 Dec 30. PMID: 22210242. [DOI] [PubMed]
- Deshpande G., LaConte S., Peltier S., Hu X. Integrated local correlation: a new measure of local coherence in fMRI data. Hum. Brain Mapp. 2009;30(1):13–23. doi: 10.1002/hbm.20482. PMID: 17979117; PMCID: PMC6870773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke Han S., Buchman A.S., Arfanakis K., Fleischman D.A., Bennett D.A. Functional connectivity networks associated with chronic musculoskeletal pain in old age. Int. J. Geriatr. Psychiatry. 2013;28(8):858–867. doi: 10.1002/gps.3898. Epub 2012 Nov 5. PMID: 23124844; PMCID: PMC3594549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers S., Wittink H., Pool J.J.M., Smeets R.J.E.M. The effectiveness of generic self-management interventions for patients with chronic musculoskeletal pain on physical function, self-efficacy, pain intensity and physical activity: A systematic review and meta-analysis. Eur. J. Pain. 2018;22(9):1577–1596. doi: 10.1002/ejp.1253. Epub 2018 Jun 27. PMID: 29845678; PMCID: PMC6175326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EuroQol Group EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. PMID: 10109801. [DOI] [PubMed] [Google Scholar]
- Filippi M., Spinelli E.G., Cividini C., Agosta F. Resting state dynamic functional connectivity in neurodegenerative conditions: A review of magnetic resonance imaging findings. Frontiers in neuroscience. 2019;13:657. doi: 10.3389/fnins.2019.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbain D.A., Cutler R., Rosomoff H.L., Rosomoff R.S. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. The Clinical journal of pain. 1997;13(2):116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Fonville L., Giampietro V., Surguladze S., Williams S., Tchanturia K. Increased BOLD signal in the fusiform gyrus during implicit emotion processing in anorexia nervosa. Neuroimage Clin. 2013;7(4):266–273. doi: 10.1016/j.nicl.2013.12.002. PMID: 24501698; PMCID: PMC3913832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Villarreal E.A., Jiang Z., Vuust P., Alcauter S., Vase L., Pasaye E.H., Cavazos-Rodriguez R., Brattico E., Jensen T.S., Barrios F.A. Music reduces pain and increases resting state fMRI BOLD signal amplitude in the left angular gyrus in fibromyalgia patients. Front. Psychol. 2015;22(6):1051. doi: 10.3389/fpsyg.2015.01051. PMID: 26257695; PMCID: PMC4510313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannouli V., Tsolaki M. Are left angular gyrus and amygdala volumes important for financial capacity in mild cognitive impairment? Hell. J. Nucl. Med. 2019 PMID: 30877733. [PubMed] [Google Scholar]
- Gracely R.H., Petzke F., Wolf J.M., Clauw D.J. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46(5):1333–1343. doi: 10.1002/art.10225. PMID: 12115241. [DOI] [PubMed] [Google Scholar]
- Harding S.M. Sleep in fibromyalgia patients: subjective and objective findings. Am. J. Med. Sci. 1998;315(6):367–376. doi: 10.1097/00000441-199806000-00005. PMID: 9638893. [DOI] [PubMed] [Google Scholar]
- Harris R.E., Napadow V., Huggins J.P., Pauer L., Kim J., Hampson J., Sundgren P.C., Foerster B., Petrou M., Schmidt-Wilcke T., Clauw D.J. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119(6):1453–1464. doi: 10.1097/ALN.0000000000000017. PMID: 24343290. [DOI] [PubMed] [Google Scholar]
- Harry B., Williams M.A., Davis C., Kim J. Emotional expressions evoke a differential response in the fusiform face area. Front. Hum. Neurosci. 2013;28(7):692. doi: 10.3389/fnhum.2013.00692. PMID: 24194707; PMCID: PMC3809557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty A.K., Yani M.S., Albishi A., Michener L.A., Kutch J.J. Salience network functional connectivity is spatially heterogeneous across sensorimotor cortex in healthy humans. Neuroimage. 2020;221:117177. doi: 10.1016/j.neuroimage.2020.117177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemington K.S., Wu Q., Kucyi A., Inman R.D., Davis K.D. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct. Funct. 2016;221(8):4203–4219. doi: 10.1007/s00429-015-1161-1. Epub 2015 Dec 15 PMID: 26669874. [DOI] [PubMed] [Google Scholar]
- Ichesco E., Schmidt-Wilcke T., Bhavsar R., Clauw D.J., Peltier S.J., Kim J., Napadow V., Hampson J.P., Kairys A.E., Williams D.A., Harris R.E. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. J. Pain. 2014;15(8):815–826.e1. doi: 10.1016/j.jpain.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar M. Pain and functional imaging. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1999;354(1387):1347–1358. doi: 10.1098/rstb.1999.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G., Sun H., Fu Y., Li Z., Pais-Vieira M., Galhardo V., Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J. Neurosci. 2010;30(15):5451–5464. doi: 10.1523/JNEUROSCI.0225-10.2010. PMID: 20392966; PMCID: PMC2868074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami K., Tajima F., Senba E. Activation of mesolimbic reward system via laterodorsal tegmental nucleus and hypothalamus in exercise-induced hypoalgesia. Sci. Rep. 2018;8(1):11540. doi: 10.1038/s41598-018-29915-4. PMID: 30069057; PMCID: PMC6070570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kami K., Tajima F., Senba E. Plastic changes in amygdala subregions by voluntary running contribute to exercise-induced hypoalgesia in neuropathic pain model mice. Mol. Pain. 2020;16 doi: 10.1177/1744806920971377. 1744806920971377, PMID: 33297861; PMCID: PMC7734490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.Y., Busch, A.J., Overend, T.J., Schachter, C.L., van der Spuy, I., Boden, C., Góes, S.M., Foulds, H.J., Bidonde, J. Flexibility exercise training for adults with fibromyalgia. Cochrane Database Syst. Rev. 2019;9(9):CD013419. 10.1002/14651858.CD013419. PMID: 31476271; PMCID: PMC6718217. [DOI] [PMC free article] [PubMed]
- Kim J., Mawla I., Kong J., Lee J., Gerber J., Ortiz A., Kim H., Chan S.T., Loggia M.L., Wasan A.D., Edwards R.R., Gollub R.L., Rosen B.R., Napadow V. Somatotopically specific primary somatosensory connectivity to salience and default mode networks encodes clinical pain. Pain. 2019;160(7):1594–1605. doi: 10.1097/j.pain.0000000000001541. PMID: 30839429; PMCID: PMC6586503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T. The Hospital Anxiety and Depression Scale (HADS). Archives of Psychiatric Diagnostics and Clinical Evaluation. 1993;4:371–372. [Google Scholar]
- Klein M.O., Battagello D.S., Cardoso A.R., Hauser D.N., Bittencourt J.C., Correa R.G. Dopamine: functions, signaling, and association with neurological diseases. Cell. Mol. Neurobiol. 2019;39(1):31–59. doi: 10.1007/s10571-018-0632-3. Epub 2018 Nov 16 PMID: 30446950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltyn K.F., Brellenthin A.G., Cook D.B., Sehgal N., Hillard C. Mechanisms of exercise-induced hypoalgesia. J. Pain. 2014;15(12):1294–1304. doi: 10.1016/j.jpain.2014.09.006. PMID: 25261342; PMCID: PMC4302052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek E., Roos E.M., Ageberg E., Nilsdotter A. Increased pain sensitivity but normal function of exercise induced analgesia in hip and knee osteoarthritis–treatment effects of neuromuscular exercise and total joint replacement. Osteoarthritis Cartilage. 2013;21(9):1299–1307. doi: 10.1016/j.joca.2013.06.019. PMID: 23973144. [DOI] [PubMed] [Google Scholar]
- Kucyi A., Moayedi M., Weissman-Fogel I., Goldberg M.B., Freeman B.V., Tenenbaum H.C., Davis K.D. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J. Neurosci. 2014;34(11):3969–3975. doi: 10.1523/JNEUROSCI.5055-13.2014. PMID: 24623774; PMCID: PMC6705280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutch J.J., Ichesco E., Hampson J.P., Labus J.S., Farmer M.A., Martucci K.T., Ness T.J., Deutsch G., Apkarian A.V., Mackey S.C., Klumpp D.J., Schaeffer A.J., Rodriguez L.V., Kreder K.J., Buchwald D., Andriole G.L., Lai H.H., Mullins C., Kusek J.W., Landis J.R., Mayer E.A., Clemens J.Q., Clauw D.J., Harris R.E. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain. 2017;158(10):1979–1991. doi: 10.1097/j.pain.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J., Daw N.D. Surviving threats: neural circuit and computational implications of a new taxonomy of defensive behaviour. Nat. Rev. Neurosci. 2018;19(5):269–282. doi: 10.1038/nrn.2018.22. Epub 2018 Mar 29 PMID: 29593300. [DOI] [PubMed] [Google Scholar]
- Lee M., Manders T.R., Eberle S.E., Su C., D'amour J., Yang R., Lin H.Y., Deisseroth K., Froemke R.C., Wang J. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J. Neurosci. 2015;35(13):5247–5259. doi: 10.1523/JNEUROSCI.3494-14.2015. PMID: 25834050; PMCID: PMC4380998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, White AT, Hughen RW, Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain. 2009 Oct;10(10):1099-112. doi: 10.1016/j.jpain.2009.06.003. Epub 2009 Jul 31. PMID: 19647494; PMCID: PMC2757484. [DOI] [PMC free article] [PubMed]
- Lee M.H., Smyser C.D., Shimony J.S. Resting-state fMRI: a review of methods and clinical applications. AJNR. American journal of neuroradiology. 2013;34(10):1866–1872. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima L.V., Abner T.S.S., Sluka K.A. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena: exercise pain and analgesia. J. Physiol. 2017;595(13):4141–4150. doi: 10.1113/JP273355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane G.J., Kronisch C., Dean L.E., Atzeni F., Häuser W., Fluß E., Choy E., Kosek E., Amris K., Branco J., Dincer F., Leino-Arjas P., Longley K., McCarthy G.M., Makri S., Perrot S., Sarzi-Puttini P., Taylor A., Jones G.T. EULAR revised recommendations for the management of fibromyalgia. Annals of the rheumatic diseases. 2017;76(2):318–328. doi: 10.1136/annrheumdis-2016-209724. [DOI] [PubMed] [Google Scholar]
- Makin T.R., Filippini N., Duff E.P., Henderson Slater D., Tracey I., Johansen-Berg H. Network-level reorganisation of functional connectivity following arm amputation. Neuroimage. 2015;114:217–225. doi: 10.1016/j.neuroimage.2015.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfliet A., Pas R., Brouns R., De Win J., Hatem S.M., Meeus M., Ickmans K., van Hooff R.J., Nijs J. Cerebral blood flow and heart rate variability in chronic fatigue syndrome: a randomized cross-over study. Pain Physician. 2018;21(1):E13–E24. PMID: 29357332. [PubMed] [Google Scholar]
- Malfliet A., Ickmans K., Huysmans E., Coppieters I., Willaert W., Bogaert W.V., Rheel E., Bilterys T., Wilgen P.V., Nijs J. Best evidence rehabilitation for chronic pain part 3: Low Back Pain. J. Clin. Med. 2019;8(7):1063. doi: 10.3390/jcm8071063. PMID: 31331087; PMCID: PMC6679058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marris D., Theophanous K., Cabezon P., Dunlap Z., Donaldson M. The impact of combining pain education strategies with physical therapy interventions for patients with chronic pain: A systematic review and meta-analysis of randomized controlled trials. Physiother. Theory Pract. 2021;37(4):461–472. doi: 10.1080/09593985.2019.1633714. Epub 2019 Jun 28 PMID: 31250682. [DOI] [PubMed] [Google Scholar]
- Masquelier E., D’haeyere J. Physical activity in the treatment of fibromyalgia. Joint Bone Spine. 2021;88(5):105202. doi: 10.1016/j.jbspin.2021.105202. [DOI] [PubMed] [Google Scholar]
- Matsudaira T., Igarashi H., Kikuchi H., Kano R., Mitoma H., Ohuchi K., Kitamura T. Factor structure of the Hospital Anxiety and Depression Scale in Japanese psychiatric outpatient and student populations. Health Qual. Life Outcomes. 2009;17(7):42. doi: 10.1186/1477-7525-7-42. PMID: 19445722; PMCID: PMC2687424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka H., Sakano Y. Assessment of cognitive aspect of pain: Development, reliability, and validation of Japanese version of Pain Catastrophizing Scale. Jpn. J. Psychosom. Med. 2007;47(2):95–102. [Google Scholar]
- Meeus M., Roussel N.A., Truijen S., Nijs J. Reduced pressure pain thresholds in response to exercise in chronic fatigue syndrome but not in chronic low back pain: an experimental study. J. Rehabil. Med. 2010;42(9):884–890. doi: 10.2340/16501977-0595. PMID: 20878051. [DOI] [PubMed] [Google Scholar]
- Namburi P., Beyeler A., Yorozu S., Calhoon G.G., Halbert S.A., Wichmann R., Holden S.S., Mertens K.L., Anahtar M., Felix-Ortiz A.C., Wickersham I.R., Gray J.M., Tye K.M. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520(7549):675–678. doi: 10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V., LaCount L., Park K., As-Sanie S., Clauw D.J., Harris R.E. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–2555. doi: 10.1002/art.27497. PMID: 20506181; PMCID: PMC2921024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas M.K. The pain self-efficacy questionnaire: Taking pain into account. Eur. J. Pain. 2007;11(2):153–163. doi: 10.1016/j.ejpain.2005.12.008. Epub 2006 Jan 30 PMID: 16446108. [DOI] [PubMed] [Google Scholar]
- Noble S., Spann M.N., Tokoglu F., Shen X., Constable R.T., Scheinost D. Influences on the test-retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cereb. Cortex. 2017;27(11):5415–5429. doi: 10.1093/cercor/bhx230. PMID: 28968754; PMCID: PMC6248395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima I., Nakajima S., Kobayashi M., Inoue Y. Development and validation of the Japanese version of the Athens Insomnia Scale. Psychiatry Clin. Neurosci. 2013;67(6):420–425. doi: 10.1111/pcn.12073. Epub 2013 Aug 5 PMID: 23910517. [DOI] [PubMed] [Google Scholar]
- Osada K., Oka H., Isomura T., Nakamura I., Tominaga K., Takahashi S., Kojima A., Nishioka K. Development of the Japanese version of the Fibromyalgia Impact Questionnaire (JFIQ): psychometric assessments of reliability and validity. Int. J. Rheum. Dis. 2011;14(1):74–80. doi: 10.1111/j.1756-185X.2010.01585.x. PMID: 21303485. [DOI] [PubMed] [Google Scholar]
- Pfannmöller J., Lotze M. Review on biomarkers in the resting-state networks of chronic pain patients. Brain and cognition. 2019;131:4–9. doi: 10.1016/j.bandc.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;1(84):320–341. doi: 10.1016/j.neuroimage.2013.08.048. Epub 2013 Aug 29. PMID: 23994314; PMCID: PMC3849338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J.J. Toward a limbic cortical inhibitory network: implications for hypothalamic-pituitary-adrenal responses following chronic stress. Front. Behav. Neurosci. 2012;29(6):7. doi: 10.3389/fnbeh.2012.00007. PMID: 22479241; PMCID: PMC3314944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. The brain's default mode network. Annu. Rev. Neurosci. 2015;8(38):433–447. doi: 10.1146/annurev-neuro-071013-014030. Epub 2015 May 4 PMID: 25938726. [DOI] [PubMed] [Google Scholar]
- Rice D., McNair P., Huysmans E., Letzen J., Finan P. Best evidence rehabilitation for chronic pain part 5: Osteoarthritis. J. Clin. Med. 2019;8(11):1769. doi: 10.3390/jcm8111769. PMID: 31652929; PMCID: PMC6912819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D., Nijs J., Kosek E., Wideman T., Hasenbring M.I., Koltyn K., Graven-Nielsen T., Polli A. Exercise-induced hypoalgesia in pain-free and chronic pain populations: state of the art and future directions. J. Pain. 2019;20(11):1249–1266. doi: 10.1016/j.jpain.2019.03.005. Epub 2019 Mar 21 PMID: 30904519. [DOI] [PubMed] [Google Scholar]
- Seeley W.W. The Salience Network: A Neural System For Perceiving And Responding To Homeostatic Demands. J. Neurosci. 2019;39(50):9878–9882. doi: 10.1523/JNEUROSCI.1138-17.2019. Epub 2019 Nov 1. PMID: 31676604; PMCID: PMC6978945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19(1):43–61. doi: 10.1177/1073858412440596. Epub 2012 Apr 30. PMID: 22547530; PMCID: PMC4107834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Yuan C., Dai Z., Ma H., Sheng L. Gray matter abnormalities associated with fibromyalgia: a meta-analysis of voxel-based morphometric studies. Semin. Arthritis Rheum. 2016;46(3):330–337. doi: 10.1016/j.semarthrit.2016.06.002. Epub 2016 Jun 8 PMID: 27989500. [DOI] [PubMed] [Google Scholar]
- Šimić G., Tkalčić M., Vukić V., Mulc D., Španić E., Šagud M., Olucha-Bordonau F.E., Vukšić M., Hof R. Understanding emotions: origins and roles of the amygdala. Biomolecules. 2021;11(6):823. doi: 10.3390/biom11060823. PMID: 34072960; PMCID: PMC8228195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. PNAS. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldatos C.R., Dikeos D.G., Paparrigopoulos T.J. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000;48(6):555–560. doi: 10.1016/s0022-3999(00)00095-7. PMID: 11033374. [DOI] [PubMed] [Google Scholar]
- Staud R., Robinson M.E., Weyl E.E., Price D.D. Pain variability in fibromyalgia is related to activity and rest: role of peripheral tissue impulse input. J. Pain. 2010;11(12):1376–1383. doi: 10.1016/j.jpain.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling M., de Zoete R.M.J., Coppieters I., Farrell S.F. Best evidence rehabilitation for chronic pain part 4: Neck Pain. J. Clin. Med. 2019;8(8):1219. doi: 10.3390/jcm8081219. PMID: 31443149; PMCID: PMC6723111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M., Bishop S., Pivik J. The pain catastrophizing scale: development and validation. Psychol. Assess. 1995;7:524–532. doi: 10.1037/1040-3590.7.4.524. [DOI] [Google Scholar]
- Tanaka S., Kirino E. Increased functional connectivity of the angular gyrus during imagined music performance. Front. Hum. Neurosci. 2019;18(13):92. doi: 10.3389/fnhum.2019.00092. Erratum. In: Front Hum Neurosci. 2021 Jul 07;15:716376. PMID: 30936827; PMCID: PMC6431621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp S.L., Suchy T., Vadivelu N., Helander E.M., Urman R.D., Kaye A.D. Functional connectivity alterations: Novel therapy and future implications in chronic pain management. Pain physician. 2018;21(3):E207–E214. [PubMed] [Google Scholar]
- Tsuchiya A., Ikeda S., Ikegami N., Nishimura S., Sakai I., Fukuda T., Hamashima C., Hisashige A., Tamura M. Estimating an EQ-5D population value set: the case of Japan. Health Econ. 2002;11(4):341–353. doi: 10.1002/hec.673. PMID: 12007165. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E., Tétreault P., Petre B., Huang L., Berger S.E., Torbey S., Baria A.T., Mansour A.R., Hashmi J.A., Griffith J.W., Comasco E., Schnitzer T.J., Baliki M.N., Apkarian A.V. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016;139(7):1958–1970. doi: 10.1093/brain/aww100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir P.T., Harlan G.A., Nkoy F.L., Jones S.S., Hegmann K.T., Gren L.H., Lyon J.L. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th Revision codes. J. Clin. Rheumatol. 2006;12(3):124–128. doi: 10.1097/01.rhu.0000221817.46231.18. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. Epub 2012 Jul 19 PMID: 22642651. [DOI] [PubMed] [Google Scholar]
- Wolfe F., Smythe H.A., Yunus M.B., Bennett R.M., Bombardier C., Goldenberg D.L., Tugwell P., Campbell S.M., Abeles M., Clark P., Fam A.G., Farber S.J., Fiechtner J.J., Michael Franklin C., Gatter R.A., Hamaty D., Lessard J., Lichtbroun A.S., Masi A.T., Mccain G.A., John Reynolds W., Romano T.J., Jon Russell I., Sheon R.P. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- Wolfe F., Ross K., Anderson J., Russell I.J., Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38(1):19–28. doi: 10.1002/art.1780380104. PMID: 7818567. [DOI] [PubMed] [Google Scholar]
- Wolfe F., Clauw D.J., Fitzcharles M.-A., Goldenberg D.L., Katz R.S., Mease P., Russell A.S., Russell I.J., Winfield J.B., Yunus M.B. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- Wood L., Hendrick P.A. A systematic review and meta-analysis of pain neuroscience education for chronic low back pain: Short-and long-term outcomes of pain and disability. Eur. J. Pain. 2019;23(2):234–249. doi: 10.1002/ejp.1314. Epub 2018 Oct 14 PMID: 30178503. [DOI] [PubMed] [Google Scholar]
- Yamashiro K., Arimura T., Iwaki R., Jensen M.P., Kubo C., Hosoi M. A multidimensional measure of pain interference: reliability and validity of the pain disability assessment scale. Clin. J. Pain. 2011;27(4):338–343. doi: 10.1097/AJP.0b013e318204858a. PMID: 21178590. [DOI] [PubMed] [Google Scholar]
- Yamashita A., Yahata N., Itahashi T., Lisi G., Yamada T., Ichikawa N., Takamura M., Yoshihara Y., Kunimatsu A., Okada N., Yamagata H., Matsuo K., Hashimoto R., Okada G.o., Sakai Y., Morimoto J., Narumoto J., Shimada Y., Kasai K., Kato N., Takahashi H., Okamoto Y., Tanaka S.C., Kawato M., Yamashita O., Imamizu H., Macleod M.R. Harmonization of resting-state functional MRI data across multiple imaging sites via the separation of site differences into sampling bias and measurement bias. PLoS Biol. 2019;17(4):e3000042. doi: 10.1371/journal.pbio.3000042. PMID: 30998673; PMCID: PMC6472734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Chang M.C. Chronic pain: structural and functional changes in brain structures and associated negative affective states. Int. J. Mol. Sci. 2019;20(13):3130. doi: 10.3390/ijms20133130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Long X.Y., Yang Y., Yan H., Zhu C.Z., Zhou X.P., Zang Y.F., Gong Q.Y. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36(1):144–152. doi: 10.1016/j.neuroimage.2007.01.054. Epub 2007 Mar 12 PMID: 17434757. [DOI] [PubMed] [Google Scholar]
- Zheng C.J., Van Drunen S., Egorova-Brumley N. Neural correlates of co-occurring pain and depression: an activation-likelihood estimation (ALE) meta-analysis and systematic review. Transl. Psychiatry. 2022;12(1):196. doi: 10.1038/s41398-022-01949-3. PMID: 35545623; PMCID: PMC9095719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. PMID: 6880820. [DOI] [PubMed] [Google Scholar]
- Zou Q.H., Zhu C.Z., Yang Y., Zuo X.N., Long X.Y., Cao Q.J., Wang Y.F., Zang Y.F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods. 2008;172(1):137–141. doi: 10.1016/j.jneumeth.2008.04.012. Epub 2008 Apr 22. PMID: 18501969; PMCID: PMC3902859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.