Abstract
Chronic Obstructive Pulmonary Disease (COPD) is a progressive, age-dependent, and unmet chronic inflammatory disease of the peripheral airways, leading to difficulty in exhalation. Several biomarkers have been tested in general towards the resolution for a long time, but no apparent success was achieved. Ongoing therapies of COPD have only symptomatic relief but no cure. Reactive oxygen species (ROS) are highly reactive species which include oxygen radicals and nonradical derivatives, and are the prominent players in COPD. They are produced as natural byproducts of cellular metabolism, but their levels can vary due to exposure to indoor air pollution, occupational pollution, and environmental pollutants such as cigarette smoke. In COPD, the lungs are continuously exposed to high levels of ROS thus leading to oxidative stress. ROS can cause damage to cells, proteins, lipids, and DNA which further contributes to the chronic inflammation in COPD and exacerbates the disease condition. Excessive ROS production can overwhelm cellular antioxidant systems and act as signaling molecules that regulate cellular processes, including antioxidant defense mechanisms involving glutathione and sirtuins which further leads to cellular apoptosis, cellular senescence, inflammation, and sarcopenia. In this review paper, we focused on COPD from different perspectives including potential markers and different cellular processes such as apoptosis, cellular senescence, inflammation, sirtuins, and sarcopenia, and tried to connect the dots between them so that novel therapeutic strategies to evaluate and target the possible underlying mechanisms in COPD could be explored.
1. Introduction
COPD is an obstructive airway condition wherein a person feels difficulty exhaling the lungs’ air. As per the Global Initiative for Chronic Obstructive Lung Disease (GOLD), a person having forced expiratory volume 1% (FEV1%) < 0.7 is regarded as COPD. COPD is the third leading cause of the death in USA and second leading cause of death in India as per World Health Organiation (WHO) 2019 data.
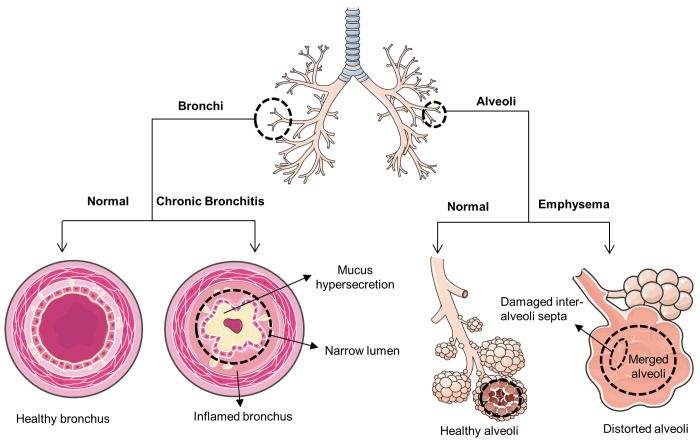
Emphysema and chronic bronchitis are two such chronic conditions of the lungs that are commonly associated with COPD. Alveoli are the primary sites that are affected in emphysema. Interalveolar septa and elastic tissues of alveoli get destroyed due to protease–antiprotease imbalance, namely elastase-α1-antitrypsin (AAT).1 Bronchial tubes, on the other hand, are a cause of concern in chronic bronchitis. Inflammation and remodeling of the bronchial tubes, chronic cough, and hypermucus secretion are the hallmarks of chronic bronchitis. Mucus plugging or mucus pooling is the prominent cause of air obstruction, which further impairs mucociliary clearance and is mainly observed due to hyperplasia and hypertrophy of goblet cells and submucosal glands, respectively. In short, emphysema deals with structural issues, whereas chronic bronchitis deals with mucus issues (Figure 1).
Figure 1.
| Schematic representation of COPD.
COPD develops and is diagnosed later in life, typically in those over the age of 65. COPD has also been observed in younger patients, those under the age of 55. In these cases, the severity distribution and disease progression are comparable to those reported in older patients.2 Numerous factors have been reported so far, such as indoor air pollution, outdoor air pollution, and genetic factors such as deficiency of the α1-antitrypsin (AAT) gene, a person’s lifestyle, and many more. Cigarette smoke, on the other hand, is the major cause of COPD in which good lifestyle roles in curbing COPD’s underlying cause are even less. ROS generated from cigarette smoke destroys the cilia due to which cilia fails to push the mucus.3 In turn, mucus buildup results in impaired mucociliary clearance, greater work of breathing, dyspnea, deteriorating airway resistance, and an inability to exercise. In severe situations, this stretchy mucus can create mucus plugs that are difficult to remove from the airways and are likely to lead to infection or localized atelectasis.4,5 Millions of people have been diagnosed with COPD, but millions are unaware of having COPD.
Mitochondria are the major source of ROS in the mammalian cells.6 In COPD, mitochondrial dysfunction can occur due to oxidative stress. The increased production of ROS and reduced antioxidant defenses in COPD lead to mitochondrial dysfunction. As a result, apoptosis can be dysregulated and leads to increased cell death in lung tissues. The deterioration of lung parenchyma and the development of emphysema, a common feature of COPD, can be attributed to excessive apoptosis of lung epithelial and endothelial cells, which further spills systemically as chronic inflammation. This chronic inflammation along with fasting, physical inactivity, and malnutrition, leads to the development of sarcopenia which reduces the respiratory function and exercise capacity in individuals with COPD.7 In addition to apoptosis, oxidative stress also leads to nondividing senescent cells (SNCs). These SNCs further secretes senescence-associated secretory phenotypes (SASP) factors such as cytokines, chemokines, growth factors, and proteases, which further remodels the extracellular matrix (ECM), induces inflammation and fibrosis, triggers unwanted cell death, and directly mimics the process of natural aging.8
This Review will discuss the mechanism underlying the mitochondrial dysfunction, reduced antioxidant machinery, apoptosis, cellular senescence, DNA-associated inflammation, antiaging markers, and muscle wasting through a deeper understanding of the supporting literature and will try to develop the interplay between these components so that novel therapeutic strategies to curb COPD could be explored.
2. Mitochondria and Cytoplasm Interplay
2.1. Reactive Oxygen Species (ROS) Generation
Mitochondria are not only the powerhouse of a cell but also the site of ROS generation. ROS generation in mitochondria is a natural process that usually happens due to oxidative phosphorylation that gives rise to ATP production within a cell. In general, ROS species such as superoxide (O2•–) and hydrogen peroxide (H2O2) get neutralized by superoxide dismutase and glutathione peroxidase using glutathione as a cofactor. Under stress conditions of exaggerated ROS such as cigarette smoke and many more, functionalities of the mitochondrial enzymes get impaired, and mitochondrial GSH (mGSH) gets reduced. As a result, ROS does not get neutralized by the mitochondrial machinery, leading to mitochondrial dysfunction and, eventually, cell death6,9 (Figure 2).
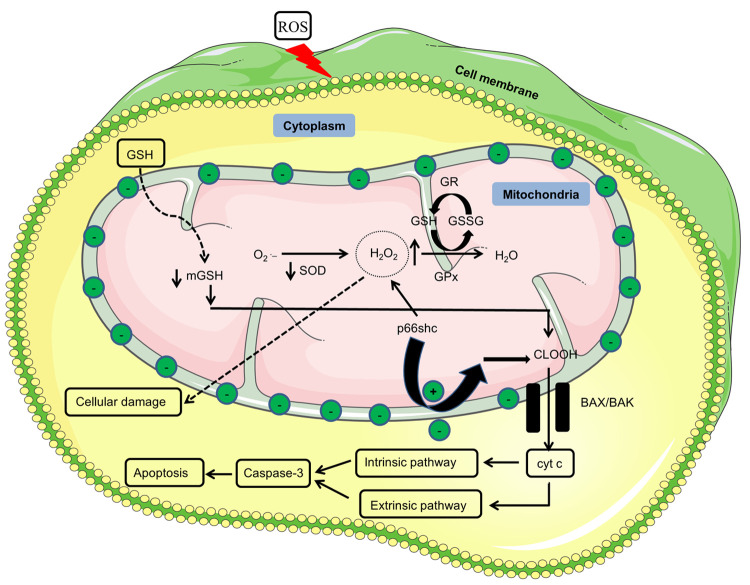
Figure 2.
| Mitochondria functionality. Reduced GSH due to ROS is translocated into the mitochondria, where it acts as a cofactor for GPx for the conversion of hydrogen peroxide into water. Due to reduced levels of GSH, H2O2 keeps on accumulating in the mitochondria, which diffuses from the mitochondria and starts causing cellular damage. Adaptor protein p66shc under stress conditions translocates into the mitochondria and starts producing H2O2 on its own in the absence of superoxide dismutase, which further leads to higher levels of H2O2. Stress conditions generated due to lower mitochondrial glutathione levels cause the oxidation of the cardiolipin–cytochrome c complex. As a result, cytochrome c diffuses through the mitochondria via BAX/BAK created pores and starts the apoptosis via caspase-3. P66shc also reduces the membrane potential to release cyt c into the cytosol. Upper head arrows show increased expression, while lower head arrows represent a decreased expression. Green circles with negative symbol represents membrane negative potential whereas green circles with plus symbol presents reduction in the membrane potential. O2•–, superoxide; SOD, superoxide dismutase; H2O2, hydrogen peroxide; GPx, glutathione peroxidase; GSH, glutathione; GSSG, glutathione disulfide; CLOOH, oxidized cardiolipin; BAX, B-cell lymphoma-2(Bcl-2)-associated X protein; BAK, Bcl-2 antagonist/killer; cty c, cytochrome c; p66shc, 66 kDa adaptor protein and member of the Src homologous-collagen homologue.
2.2. Glutathione and Its Role
Glutathione (GSH) is a potent thiol-based antioxidant that normalizes the ROS levels in mitochondria. GSH is also essential for cell growth and other metabolic activities. GSH is synthesized in the cytoplasm via the γ-glutamyl cycle utilizing Adenosine triphosphate (ATP). Apart from mitochondria, GSH is also localized in the endoplasmic reticulum (ER) and nucleus. In the mitochondria and the nucleus, GSH is present in the reduced form, where it accounts for 10–15% of the total GSH pool and functions as redox stabilizer and DNA repair moiety, respectively.10,11 However, in ER, GSH is present in the oxidized form, where it forms disulfide bond in proteins and helps in protein folding.12
Mitochondria do not have the machinery for the GSH synthesis, as GSH is synthesized exclusively in the cytosol. So, in order to neutralize ROS to normal levels, GSH is imported from the cytoplasm. GSH is anionic at physiological pH, and mitochondria, too, have a negative membrane potential because of oxidative phosphorylation. As a result, GSH cannot cross the mitochondrial membrane on its own. Therefore, it is transported by electroneutral antiport carriers or polypeptides such as 2-oxoglutarate and dicarboxylate to neutralize the ROS in the mitochondria. Imported GSH is regarded as mitochondrial GSH (mGSH). Mitochondrial enzymes such as glutathione peroxidase prevent lipid peroxidation and oxidative damage to the cell components by converting H2O2 into the water using GSH as a cofactor. Disulfide GSH (GSSG) formed after conversion of H2O2 into the water cannot cross the mitochondrial membrane, so it gets converted back to GSH by GSH reductase (GR) using nicotinamide adenine dinucleotide phosphate (NADPH) as a coenzyme11,13−18 which is further reused as a cofactor in H2O2 neutralization (Figure 2).
Therefore, the role of gluathione is of utmost importance in COPD as it acts as a free radical scavenger and detoxifies the harmful molecules which contribute to the inflammation in the airways and also prevents the lung damage exacerbations. Additionally, it maintains the fluidity of the airway mucus to allow for the propulsion of the foreign substances, thus reducing infection susceptibility.
2.3. Apoptosis
Apoptosis or programmed cell death is the cascade of events that leads to dysfunctional cells’ death. Caspases are known to be critical enzymes involved in apoptosis. Caspases are further categorized into initiator caspases and executioner or effector caspases. Caspases-2, 8, 9, and 10 are among the initiator caspases that initiate apoptosis, whereas caspases-3, 6, and 7 are among the executioner caspases that executes apoptosis.19−21 Apoptosis, as a whole, works in two different pathways, namely, the intrinsic pathway and the extrinsic pathway.
Intrinsic or the mitochondrial pathway of apoptosis is a mitochondrial associated event involving the mitochondrial outer membrane’s permeabilization. As mitochondria lack catalase enzyme for the metabolism of H2O2. So, it mainly utilizes imported GSH for the breakdown of H2O2 via glutathione peroxidase.11 Under normal circumstances, GSH neutralizes ROS. Under stress conditions of cigarette smoke and exaggerated ROS, the amount of cholesterol increases, which may decrease the membrane fluidity and increase the membrane rigidity.22 As a result, GSH may not be imported much from the cytosol to compensate for the mitochondria’s ROS. Due to this, H2O2 does not get neutralized by glutathione peroxidase. As a result, accumulated H2O2 diffuses into the cytosol and starts damaging the DNA and cell components through the Fenton process.23 Another phenomenon is also reported that involves adaptor protein p66shc. Under normal circumstances, p66shc resides in the cytosol, but under stress conditions, it translocates into the mitochondria and generates H2O2 in the absence of superoxide through cytochrome c oxidation.24,25 It is also believed that p66shc drops the membrane potential to release cytochrome c into the cytoplasm26 (Figure 2).
Lower mGSH loosens the electrostatic bond between positively charged cytochrome c at physiological pH and anionic phospholipid, cardiolipin, in the inner mitochondrial membrane. Cardiolipin provides fluidity and stability to the mitochondrial membrane, but once cardiolipin is oxidized due to lower mGSH. Association between cardiolipin and cytochrome c gets broken. As a result, cytochrome c diffuses into the cytoplasm. Cardiolipin is also observed to be oxidized by phospholipase A2, cytochrome c and cardiolipin complex itself, and ROS. Calcium also interferes with the electrostatic interaction between the cardiolipin and cytochrome c, resulting in the detachment of cytochrome c from the cardiolipin. Cytochrome c detachment results in the disruption of the electron transport chain, which causes the burst in ROS, thus further amplifies the oxidative stress loop.27−31
B-cells lymphoma protein-2 (Bcl-2) keeps the pore-forming proteins such as BAX/BAK in the cytosol under normal conditions. Under an apoptotic environment, tumor suppressor protein, p53, blocks activity of Bcl-2 protein and recruits BAX/BAK in the mitochondria.32 BAX/BAK translocates into the outer mitochondrial membrane and forms a pore through which cytochrome c gets released into the cytosol33,34 (Figure 2). Cytochrome c, along with apoptotic protease activating factor-1 (Apaf-1) and ATP, activates caspase-9, thus forming a complex known as the apoptosome. Apoptosome further activates executioner caspase-3,35 which leads to the activation of proteases and nucleases, leading to cell fragmentation and other structural changes such as flipping of phosphatidylserine on the outer leaflet of cells, which serves as a signal for phagocytes to perform opsonization. This phosphatidylserine is usually detected under early apoptosis by flow cytometry using Annexin V staining.36
In the extrinsic or death-receptor pathway, the apoptotic signal is associated with an extracellular ligand binding to a transmembrane receptor. A death-inducing signaling complex (DISC) is formed after receiving the apoptotic signal, which further activates caspase-8. Caspase-8 then activates caspase-3, which serves as the same function as caspase-3 does in the intrinsic pathway11 (Figure 2). Thus, caspase-3 is the common caspase in both apoptotic pathways. Caspase-8 also turns truncated BID (tBID) into the proapoptotic protein BID. BID further activates BAX and BAK. In this way, caspase-8 cross-talks with an intrinsic pathway.20,21
Apoptosis dysregulation has been linked to the etiology of COPD. Apoptosis normally maintains tissue integrity by removing damaged cells, but in COPD, this integrity is disrupted by an imbalance between cell survival and cell death, which results in the exaggerated death of structural cells like fibroblasts and epithelial cells in the lungs. This results in the disruption of normal lung tissue repair and matrix remodeling processes, which causes the progressive loss of lung function that is typically observed in COPD. The main factor for the deregulation of apoptosis is increased ROS-mediated oxidative stress. Understanding apoptosis and its dysregulation is crucial for identifying the precise apoptotic pathway components that can be targeted as therapeutic targets to reduce lung tissue damage and slow the progression of COPD.
3. Cellular Senescence-Associated Aging Markers
Cellular senescence is a condition of permanent cell cycle arrest in response to irreparable damage, typically DNA damage. Senescent cells (SNCs) gets accumulated over time and damage the nearby tissues, which are associated with the features of natural aging, by releasing the metalloproteinases, cytokines, growth factors, chemokines, and many more factors, collectively known as senescent-associated secretory phenotype (SASP)37−42 (Figure 3). Senolysis, or the selective removal of SNCs, increases “healthspan”, or the amount of time an organism can survive without developing chronic illnesses of aging. It is now being thought of as a potential treatment option for diseases of aging to interfere with the pro-aging effects of SNCs, either by completely deleting SNCs or by turning off the SNC secretory machinery.
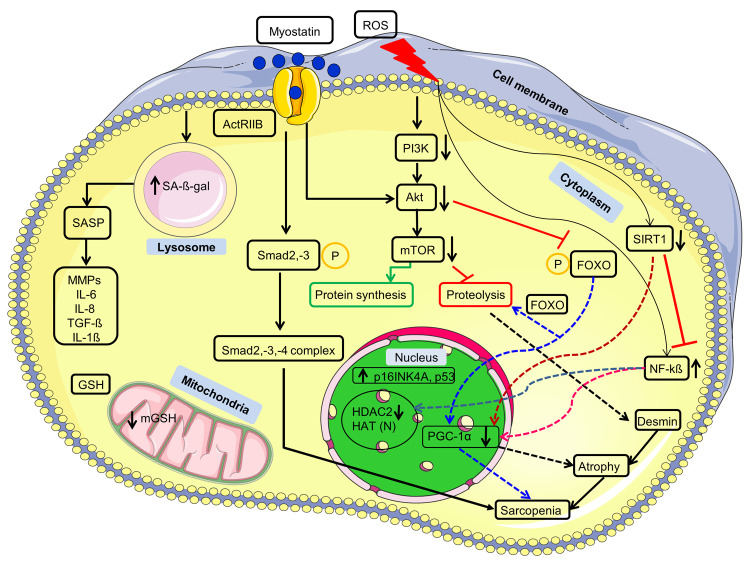
Figure 3.
| Interplay of signaling pathway and COPD biomarkers. Myostatin on binding to its receptor ActRIIB causes the phosphorylation of the Smad2 and Smad3, which further recruits Smad4 and forms a trio-complex which via gene transcription in the nucleus results in sarcopenia. On the other hand, myostatin binding also reduces the Akt activity, which usually keeps the FOXO in the cytosol by phosphorylating it under normal conditions. FOXO on dephosphorylation due to reduced Akt activity translocates into the nucleus and transcribes the genes involved in sarcopenia via proteolysis. Activated FOXO also reduces the expression of PGC-1α, which via atrophy leads to sarcopenia. ROS causes a reduction in the levels of SIRT1 and the endogenous antioxidant GSH. SIRT1 inhibits NF-kß signaling under normal conditions, but under stress conditions, it is unable to do so; thus, NF-kß translocates into the nucleus and starts its downstream effects, such as a reduction in the PGC-1α expression. SIRT1 under normal circumstances increases the PGC-1α expression, but under stress conditions, it cannot do so; thus, the expression of PGC-1α gets suppressed. ROS also increases the lysosomal SA-ß-gal, and tumor suppressors p16INK4A and p53 levels associated with cellular senescence, which further secrete SASP. ROS mediated inflammation is caused by reduction in the levels of HDAC2 under stress conditions via activated NF-kß. ROS also reduces the level of PI3K/Akt/mTOR pathway expression, typically involved in protein synthesis. However, under stress conditions, protein synthesis is reduced and causes the skeletal muscle protein desmin’s proteolysis, which is involved in muscle wasting via atrophy. Upper head arrows show increased expression, while lower head arrows represent the decreased expression. Different colored arrows are used to indicate the paths of different markers and their outcomes. N represents no significant change. ActRIIB, activin A receptor, type IIB; PI3K, phosphatidylinositol 3-kinase; Akt, serine/threonine protein kinase B; mTOR, mammalian target of rapamycin; NF-kß, nuclear factor kappa light chain enhancer of activated B cells; SASP, senescence associated secretory phenotype; HDAC2, histone deacetylase 2; HAT, histone acetyltransferase; p16INK4A, cyclin-dependent kinase inhibitor 2A; p53, 53 kDa tumor protein; PGC-1α, peroxisome proliferator-activated receptor c coactivator 1; SIRT1, sirtuin 1; Smad2, mothers against decapentaplegic homologue 2; FOXO, forkhead box protein O; GSH, glutathione.
3.1. Cyclin-Dependent Kinase Inhibitor 2A or P16INK4A
Universal markers to detect SNCs have not been reported so far. Tumor suppressors such as P16INK4A is known to be an inhibitor of cyclin-dependent kinases (CDKs) such as CDK4/6, which are essential for the cell to enter from one phase to another phase of the cell cycle. These tumor suppressors promote wound healing and inhibit the abnormal cells from dividing and entering into S phase from the G1 phase of the cell cycle by binding to cyclin-D.43−51
SNCs diminish the stem cell pool in COPD. As a result, the alveolar type I cells with a gaseous exchange role do not renew once they get damaged under stress condition of cigarette smoke. Around 10-fold increase was observed in the expression of p16 mRNA in p16+/+ mice compared to p16–/– mice upon four months of cigarette smoke exposure. It was found that p16 was increased by 30% in COPD patient lungs compared to the healthy patients across interstitial, alveolar, and bronchial samples, resulting in a reduced number of alveolar type II cells. Thus, it was observed found that p16–/– mice promote the stem cell pool and further increase alveolar regeneration.51 Human bronchial epithelial cells (BEAS2B) showed a 5-fold increase in the p16 mRNA expression after induction with 0.5% cigarette smoke extract (CSE). Thus, apart from natural senescence, external stress factors such as cigarette smoke also trigger cellular senescence rather than natural phenomena.52
Other tumor suppressors, such as p19ARF (tumor suppressor in mouse) and p14ARF (tumor suppressor in humans), are also reported for cellular senescence. In mice, around 4-fold increase in the expression of p19ARF was reported using cigarettes smoke exposure in the epithelial cells of mice lungs.53 More extensive research is still needed to understand its potential in cellular senescence. So far, no significant information about p14ARF has been reported in COPD patients and in BEAS2B cell lines by using cigarette smoke exposure.
3.2. Senescence-Associated β-Galactosidase (SA-ß-gal) Activity
SA-ß-gal is another biomarker used to stain SNCs both in vitro and in vivo. SA-ß-gal is a hydrolase enzyme of the lysosome, which shows its optimal activity at pH 6 while, on the other hand, peak enzymatic activity at pH 4–4.5 of ß-galactosidase was reported for its common isoforms.42
Four months cigarette smoke-exposed mice showed a ∼1.4 fold increase in the SA-ß-gal activity in lung homogenate against room air-exposed mice.51 BEAS2B cell showed a 2- and 4-fold increase in the SA-ß-gal activity for 5% and 10% CSE exposure, respectively54,55 while on the other hand, lung fibroblast cells from COPD patients showed 2-fold higher SA-ß-gal activity against healthy patients.56 The levels of SA-ß-gal are not significantly increased as P16INK4A even after more extended duration studies of cigarette smoke, thus indicating that other cell cycle arrest points need to be checked for better insights into cellular senescence associated with SA-ß-gal activity.
Senolytics must have negligible off-target effects in non-SNCs in order to be considered safe. Though the possibility of therapeutically targeting SNCs is just beginning to become apparent, our knowledge of how SNCs may actively contribute to aging and age-related disorders is still in its infancy. Traditional thinking has not viewed aging as an illness per se, and there have been no studies done to date that have specifically examined how various treatments affect aging as a whole.
However, a number of technical obstacles must be removed in order to make therapies a reality. First off, cellular senescence is not always harmful and can assist a number of positive processes, such as the prevention of tumors, the speedy healing of wounds, the development of embryos57−59 and tissues,60 and the stimulation of pancreatic-ß cells61 ability to secrete insulin as they get older. Second, despite the fact that numerous moieties have been found to eradicate SNCs in vivo, some of these substances have unfavorable side effects, like thrombocytopenia.62 Third, the vulnerabilities of SNCs from various tissue origins vary, necessitating the development of special compounds that not only target the right cell type in vivo but also accumulate in the tissue where these SNCs are found. Finally, as SNCs would persist even after treatment, it would be necessary to continuously ingest chemicals that inhibit the SNC secretome. Because these compounds have been found to be immunosuppressive,63 long-term use of them could pose safety issues.
4. Inflammatory Markers
4.1. Histone Acetylation and Deacetylation
Histone belongs to the class of basic proteins that gives a structural role to the DNA. Nearly six feet long DNA wraps around the histone octamer thus forming the nucleosome assembly64,65 and further condenses to form chromatin. DNA with its negatively charged phosphate group winds tightly around the positively charged histone lysine residues, thus acting as a barrier for transcription. Histone acetylation and deacetylation are associated with gene regulation. Histone acetyltransferase (HAT) and histone deacetylase (HDAC) are the two major nuclear enzymes that are involved in gene transcription and gene silencing, respectively,66,67 and thus are involved in modifying the expression of the genes involved in inflammation by regulating the chromatin structure.68
HAT reduces the positive charge on the histone lysine by transferring its acetyl group, resulting in DNA unwrapping around the histones. As a result, DNA gets relaxed. Thus, gene transcription sites on the relaxed DNA are then accessible to the RNA polymerase and the transcription factors, whereas HDAC performs opposite functions of compressing the DNA around the histone so that gene transcription sites are not accessible to RNA polymerase for transcription. Resistance towards corticosteroids in COPD is mainly contributed to the reduced expression of HDAC2. Peroxynitrite (ONOO–) formed from the combination of superoxide radical (O2•–) and nitric oxide (NO) from cigarette smoke leads to the nitration of the tyrosine residue of HDAC2, resulting in the inactivation of the HDAC2 and thus the relaxation of the DNA.69−71
In COPD patients, peripheral lung tissues showed around 7-fold lower HDAC2 levels against healthy patients. These levels further decrease with increasing COPD severity. This reduced HDAC2 trend was also observed in the alveolar macrophages, and bronchial biopsy samples from COPD patients.72
Reduced HDAC2 expression was also observed even after 4 h in BEAS2B cell lines after induction with 5% cigarette smoke extract (CSE), and a similar trend also followed in rat COPD model of lipopolysaccharide (LPS) and cigarette smoke exposure model for 30 days.73−75 HAT, on the other hand, did not show significant differences among all the stages of COPD and even in alveolar macrophages and bronchial biopsies samples from COPD patients.72 Thus, decreased HDAC2 expression is associated with increased expression of inflammatory genes via NF-kß pathway71 (Figure 3).
4.2. Granulocyte-Colony Stimulating Factor (G-CSF)
G-CSF is known to be the principal regulator involved in the development, production, and release of neutrophils from the bone marrow.76 Neutrophils are short-lived, terminally differentiated, and abundantly present leukocytes in humans. For bacterial and fungal infections, neutrophils are considered as the first-line defense support to the body.77
Interleukin 6 (IL-6), along with IL-23, triggers the release of stromal IL-17 from Th-17 cells. This IL-17, in turn, releases neutrophils from the G-CSF. G-CSF deficient mice model showed reduced lung tissue destruction, systemic inflammation, bone osteoporosis, reduced airway inflammation, and attenuated right heart hypertrophy. Bronchoalveolar lavage fluid (BALF) from COPD patients showed around a 3-fold increase in the G-CSF levels.78 Neutrophils, on arrival at the site of inflammation, release their genomic DNA, which increases the viscosity and viscoelasticity of the sputum, which causes airway obstruction and thus impairs the mucociliary clearance. This form of genomic DNA extrusion serves as a trap known as neutrophil extracellular traps (NETs), which traps and kills bacteria.79 It also plays prominent roles in inflammatory diseases.80 NETosis (NET formation) is associated with gasdermin D, neutrophil elastase, peptidylarginine deaminase 4 (PAD4), and and myeloperoxidase (MPO). Thus, these four constituents of NETs are the targets for inflammatory conditions.81−83 Thus, G-CSF and neutrophils’ play prominent in inflammation77 and may serve as a distinguished marker in differentiating the different stages of COPD.
5. Oxidative Stress Associated Antiaging Markers
5.1. Sirtuins (SIRTs)
Sirtuins or SIRTs are nicotinamide adenine dinucleotide (NAD) dependent protein deacetylases that mainly belongs to class III HDACs.84,85 SIRTs are known for their antioxidant and oxidative stress antagonism involved in longevity, mitochondrial functions, metabolism, and DNA damage repair.86
Seven SIRTs family members have been reported in the literature and are known for their different roles. SIRT1, 3, and 5 are anti-ROS agents, whereas SIRT2, 6, and 7 are involved in the modulation of oxidative stress genes and metabolism. Interestingly, SIRT4 has both ROS inducing and antioxidant properties as well.87
SIRT1 is a well-studied member of the mammalian SIRTs family. They are found in both nuclei as well as in the cytoplasm.88,89 They are known for their role in cellular senescence, development, cell death, calorie restriction, DNA repair, and longevity.87,90 Increased matrix metalloproteinase-9 (MMP-9) mRNA expression was directly correlated with reduced SIRT1 in emphysema.91 SIRT1 also promotes muscle mass, growth, maintenance, and repair. Attenuation in the NF-kß transcription activity was also observed by deacetylating the p53 subunit of NF-kß at lysine 310 residue.92 The 2-fold decrease in the levels of SIRT1 was seen in the COPD serum patients and rat lung tissues from the LPS and cigarette smoke-induced model which was strongly correlated with the stress condition generated in COPD93,94 and levels may increase with disease severity. To confirm the role of SIRT1 as an oxidative stress antagonist, BEAS2B cells were pretreated with buthionine sulfoximine (BSO), which is an intracellular glutathione depeletor, before induction with cigarette smoke extract. As anticipated, SIRT1 levels were decreased by 7-fold than the normal, thus proved the fact that SIRT1 has an antioxidant role as well95 (Figure 3). SIRT1 also increases the expression of PGC-1α which further results in the mitochondrial and peroxisome biogenesis87 (Figure 3).
SIRT2 is found in the cytoplasm and nucleus.96 It is expressed in the organs related to metabolism, such as the brain, kidneys, liver, testes, pancreases, and mice’s fat tissues. It has been found that SIRT2 has a role in cell’s fuel detection, and its expressions are based on the cell’s energy state: suppressed in high energy state while expressed in low energy state.97 Under stress conditions, SIRT2 deacetylases and activates the key enzymes of pentose phosphate pathway involved in NADPH production as NADPH is essential to combat the oxidative stress by keeping GSH in reduced form.87,98
SIRT3 is a nucleus sirtuin, but it gets translocated to the mitochondria under stress conditions of DNA damage, where it gets activated by the mitochondrial peptidase, thus designated as the mitochondrial sirtuin as it is localized in its active form in mitochondria.99 SIRT3 is also believed to have antitumor activities through ROS modulation. SIRT3, on the other hand, is also known for repairing the mitochondrial DNA by replenishing the activity of human 8-oxoguanine-DNA glycosylase 1 (OGG1) that repairs the damaged DNA.100 In antioxidant and redox signaling (ARS), SIRT3 is known as a genotoxic and oxidative stress antagonist.87
SIRT4 is designated as the mitochondrial sirtuin, whose primary function is to ribosylate the adenosine diphosphate (ADP).96,101 SIRT4 may have enhanced roles in the kidneys, brain, liver, and heart, as it is predominantly found in these organ tissues.102 All of the sirtuins except SIRT4 have a role in mitigating the mitochondrial stress through the calorie restriction process. Knockout and overexpression of SIRT4 decreased and increased the ROS levels in angiotensin II-induced cardiac hypertrophy in mice. It was also found that SIRT4 reduced the activity of SOD2 as it inhibits the binding of SOD2 to SIRT3.103 In one of the studies conducted in 2010, SIRT4 knockdown resulted in enhanced oxygen consumption and fatty acid oxidation, possibly in primary mouse hepatocytes, by regulating the expression of SIRT1. As fatty acid oxidation leads to mitochondrial ROS and is also linked to the kidney damage in diabetes.104,105 Due to its role as an oxidative metabolism, SIRT4 is being evaluated as a biomarker in coronary artery disease, but more studies need to be conducted to verify this finding. More robust studies are needed to clarify the role of SIRT4 in ARS.
SIRT5 is a mitochondrial sirtuin where it demalonylates, deacetylate, and desuccinylates various proteins.96,106 They are known for their roles in regulating oxidative stress, a mediator in the apoptosis pathway, detoxification, energy production, and cellular metabolism.107 SIRT5 is involved in converting ammonia into nontoxic urea by increasing the activity of carbamoyl phosphate synthetase (CPS1), which is involved in the detoxification of ammonia in the urea cycle. As ammonia is a ROS inducer and involved in decreasing the antioxidant GSH content. Increased levels of ammonia were seen in SIRT5 knockout mice during fasting, thus indicating its essential role in ARS.108−110
SIRT6 is designated as the nuclear sirtuin whose primary function is to deacetylase the lysine 9 and 56 residues on histone 3 abbreviated as H3K9 and H3K56.111−116 The first evidence of SIRT6 in regulating mammalian aging was observed in SIRT6 knockout mice.87,96 SIRT6 overexpression curbs the cellular senescence and inhibits fibrosis in COPD.117 Reduced SIRT6 expression was found in the lung homogenate. SIRT6 overexpression inhibits cigarette smoke-induced senescence in human bronchial epithelial cells. It was also seen that SIRT6 overexpression induces autophagy through the attenuation of the insulin-like growth factor-Akt-mammalian target of rapamycin (IGF-Akt-mTOR) signaling. Thus, implying that increased expression of the IGF-Akt-mTOR pathway leads to insufficient autophagic elimination of dysfunctional cellular components, which may be involved in COPD development, thus making the role of SIRT6 important in mitigating the development and progression of COPD.117,118 SIRT6 not only works as chromatin regulator and recruiter of transcription factors such as NF-kß but also involved in DNA repair, gene expression, and glucose homeostasis.115,119−122
The last member of the sirtuin family, SIRT7, is designated as the nucleoli sirtuin. This protein directly attaches to the histones and is involved in the positive regulation of the transcription of rDNA (rDNA).96 SIRT7 is differentially expressed in all tissues with enhanced expression in higher metabolic activities and reduced expression as the age progresses.123,124 Very little is known about the role of SIRT7 as it is a recent area of research and needs more robust studies to understand its substrates on which it acts and its proper mechanism of action. Although, the past few pieces of research indicated its minor role in the regulation of oxidative stress.87 Further studies are needed to explore the deeper understanding of SIRT7.
6. Sarcopenia Markers
Sarco in Greek is “flesh”, and -penia is “deficiency”. So, sarcopenia refers to muscle wasting or the gradual loss of skeletal muscle with age. The possible reason for the muscle mass is excessive proteolysis with reduced protein synthesis. It is predominantly observed in older people or due to factors such as fasting or malnutrition. Sarcopenia is also associated with specific disease conditions such as COPD, diabetes, renal failure, cardiac failure, cancer-associated cachexia, and many more.7
6.1. Myostatin and Myogenin
Myostatin, a member of the transforming growth factor-beta (TGF-ß) family, is expressed predominantly in skeletal muscles and expressed in low levels in cardiac and adipose tissues.125 Myostatin is known as an autocrine inhibitor for normal muscle growth. Enhanced levels of myostatin and its homologue activin A causes the rapid loss of muscle mass.125−127 Myostatin is the negative regulator of satellite cells which are the muscle stem cells required in repair and regeneration of the muscle after injury.128−131 Myostatin on binding to its receptor, activin A receptor, type IIB (ActRIIB) on muscle cells activates Smad2 and Smad3 by phosphorylation. Activated Smad2 and Smad3 complexes recruit Smad4 in their complexes and form the trio-complex. This complex translocates into the nucleus and starts the transcription of genes, resulting in muscle wasting. PI3K/Akt/mTOR pathway promotes protein synthesis and prevents proteolysis, but myostatin on binding to its receptors on muscle cells reduces the PI3K/Akt/mTOR pathway expression. Akt keeps forkhead box protein O (FOXO) in the cytosol by phosphorylating it. However, due to reduced activity of Akt, FOXO gets dephosphorylated and translocates into the nucleus and start the transcription of the Atrogin 1, muscle-specific RING-finger 1(MURF 1), autophagy-related genes (ATGs) which via the ubiquitin-proteasome system (UPS) and autophagy promotes the muscle wasting7,125 (Figure 3). NF-kß is also known as the potent inducer of myostatin or the inhibitor of myogenesis.7 Although myostatin is a skeletal muscle marker but a 2-fold decrease in the serum of COPD patients was also reported.132 A 5-fold decrease in the myostatin levels in COPD patients’ diaphragm was reported thus indicating its potential of muscle wasting in COPD patients.133 Around 10-fold decreases in myostatin levels in gastrocnemius muscle (lower leg muscle) were reported in the 16-week cigarette smoke-induced rat model by immunohistochemistry. In contrast, a 4-fold decrease in myogenin was observed in the gastrocnemius muscle, which is the transcription factor involved in the development of muscles/myogenesis.134 These findings are a proof of concept that muscle wasting is predominantly present in COPD and thus represents a therapeutic potential in treating COPD.
6.2. Forkhead Box Protein O (FOXO)
Forkhead box protein O and FOXOs are the transcription factors involved in regulating oxidative stress resistance, cell-cycle progression, and apoptosis. The expression of FOXO is majorly regulated by Akt-mediated phosphorylation in response to growth factors.135 Akt inactivates the FOXO1 and -3 by phosphorylating it, and as a result, FOXO1 and -3 remain sequestered from the nucleus in the cytosol (Figure 3) to prevent the transcription of the muscle-specific E3 ubiquitin ligases gene.136,137 A 4-fold increase in the FOXO1 in quadriceps femoris mRNA expression was observed in COPD patients associated with muscle loss via proteolysis, which ultimately leads to muscle atrophy.138 Cigarette smoke condensate (CSC) induced autophagy leads to around a 7-fold increase in the levels of acetylated FOXO3a. Smokers’ lung and mice lungs exposed to cigarette smoke also showed increased expression of acetylated FOXO3a.139,140 FOXOs are mediators in the pathway of oxidative stress, as other pathways or factors may be associated with its activation. More extensive research is needed to obtain more precise information about the FOXO proteins in COPD.
6.3. PGC1α
The peroxisome proliferator-activated receptor c coactivator 1 (PGC1) is a family of transcription factors involved in metabolism. The family consists of three members known as PGC1α, PGC1ß, and the PGC related coactivator (PRC). Among three members, PGC1α protein (encoded by PPARGC1A) is the well-studied member who is known for mitochondrial biogenesis and increased respiration for the detoxification of ROS, gluconeogenesis, and adaptive thermogenesis.141−144
The primary organelle that supports the functions of the mitochondria during oxidative metabolism is the peroxisome. Peroxisomes are known for the metabolism of complex fatty acids that cannot be metabolized by mitochondria. They do not fully metabolize the fatty acids, but they export the short-chained fatty acids to the mitochondria for complete breakdown. Thus, mitochondria and peroxisome aids each other in oxidative metabolism.145 Muscle cell PGC1α enhances the SOD2 and glutathione peroxidase expression to remove the superoxide and hydrogen peroxide, respectively.142 Elevated PGC1α levels enhance peroxisome and mitochondrial biogenesis, increasing the number of mitochondria and ROS-associated detoxification enzymes. In particular, PGC1α acts as a coactivator for nuclear respiratory factor 1 (NRF1) which is a regulator of antioxidant response elements (AREs) which encodes antioxidant associated enzymes.146,147 In other words, PGC1α gives increased respiration to tackle the ROS and helps attenuates the process of aging.148 Reduced levels of PGC1α is associated with atrophy by activating NF-kß and FOXO transcription factors (Figure 3), which in turn accelerates protein degradation induced by fasting or denervation.149,150 PGC1α also activates the expression of the mitochondrial sirtuins, such as SIRT3.151,152 Deacetylation and methylation increase the activity of PGC1α while, on the other hand; acetylation and sumoylation decrease its activity. There is growing evidence of organelle remodeling via PGC1α. It is believed that the mitochondria and the peroxisomes that are newly produced from the biogenesis via PGC-1α have different intrinsic compositions and properties as compared to the original organelles.148 Enhanced levels of PGC-1α in muscles could delay the kick-off of the aging process linked with sarcopenia. PGC1α is a recent area of research working towards the mitigation of the mitochondrial ROS. PGC1α could be one of the markers to resolve sarcopenia associated with COPD.
6.4. Desmin
Desmin proteins are the intermediate filaments of the sarcomere architecture that are required for the mechanochemical signaling, efficient force transmission, and maintenance of cell integrity within the myocytes. Proteolysis causes the collapse of the muscle architecture, thus reducing the force generation ability and the force transmission capacity along and across the muscle fiber. As a result, muscles become weak and prone to damage.153,154 Desmin associated atrophy can be explained with two ubiquitin ligases, such as ubiquitin tripartite motif-containing protein 32 (TRIM32) and MURF1. MURF1 catalyzes the breakdown of the thick myosin filament’s proteins, whereas TRIM32 catalyzes the Z-band and the desmin cytoskeleton’s breakdown. TRIM32 reduces the PI3K/Akt/mTOR pathway expression, thus triggering the proteolysis via FOXO, ultimately leading to atrophy.7 Other muscle proteins might also be prone to proteolysis under stress/diseased conditions, but not much work is reported in that area. Desmin could be one of the potential markers to study the sarcopenia associated tremor in COPD.
7. Therapeutic Interplay of Potential Markers
COPD is an age-specific disease, and its underlying cause is still not fully understood. Current medications are symptomatic relievers. Maintaining the ROS equilibrium while treating COPD could be a breakthrough in the disease’s management. Therapies such as antioxidants or the factors that can raise the downregulated expression of the nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) under diseased conditions would be beneficial. Different strategies were devised to target the dysfunctional mitochondria, such as targeting mitochondria based on its biophysical properties of high membrane negative potential, targeting locations within the mitochondria that are specific for particular enzymes that convert prodrugs into active drugs, and targeting the transporter-based delivery of prodrugs.155 Antioxidant-based mitochondrial targeting may prevent cardiolipin oxidation, thereby maintaining cardiolipin–cytochrome c association and further preventing apoptosis (Figure 2). Heat-shock proteins (HSP) such as HSP27 prevent the release of the cytochrome c into the cytoplasm and prevent its downstream effects.29,156,157 Therapies that can address these issues will be able to control apoptosis and the underlying ROS that are common in age-related illnesses.
Senotherapies are being evaluated to prevent the proaging effects associated with SNCs. Senotherapies are classified into three categories: senolytics to selectively kills the SNCs, senomorphics that modulate the SNC associated morphology and functions, delayed progression of young cells to SNCs, and the immune system based clearance of SNCs.158 Senotherapies are based mostly on the optimistic premise that the harmful effects of SASPs connected to SNCs might be eliminated and there will be no risk of tumor escape from senescence once SNCs are eliminated which is otherwise possible if SNCs are allowed to linger indefinitely, and clearance of SNCs extends the healthspan which is a global measure of aging in mice41,159 thus it is suggested that senotherapies retards aging and reduces the age-related illnesses.42
Anti-inflammatory therapies cannot reverse COPD. Poor clinical response was observed for corticosteroids in COPD patients.160 Bronchodilators also fail to target the underlying inflammatory cause of COPD patients. The magnitude of inflammation in COPD progresses with the disease stage with a higher number of lymphocytes, neutrophils, and macrophages.161 This inflammation “spillover” into the systemic circulation from the peripheral airways, thus giving rise to the systemic inflammation, which is associated with several comorbidities such as cardiovascular disease.162 The lower respiratory tract of severe COPD patients is colonized with Streptococcus pneumoniae and Haemophilus influenzae, and this bacterial colonization is associated with defective phagocytosis of bacteria and thus may be the cause of persistent inflammation and immune response in COPD.163 Targeting specific cytokines gave disappointing results in COPD patients. Emphysema escalates the lung aging, thus giving rise to the faulty endogenous antiaging mechanism involving sirtuins and FOXO.164 Thus, targeting oxidative stress could have the therapeutic potential against the inflammatory factors such as increased expression of NF-kß, p38 mitogen-activated protein kinase (p38 MAPK), a higher amount of autoantibodies165 against carbonylated proteins166 and reduced aging factors such as reduced expression of SIRT1, HDAC2 (Figure 3), and the reduced expression of antiproteases that leads to fibrosis and emphysema with overexpressed transforming growth factor-beta (TGF-ß).
Reduced expression of proteins associated with atrophy leads to a decreased muscle mass. Proteins such as SIRT1, PGC1α149,150 and many more are among those that induce during exercise and promote hypertrophy, and maintains the skeletal muscle mass. Therapies that block the wasting of these proteins or increase the level of these proteins could be promising to prevent COPD associated sarcopenia (Figure 3). Myostatin–activin A-growth differentiation factor 11 (GDF11) signaling inhibitions could have therapeutic potential in muscle wasting associated diseases. Antagonists such as follistatin167,168 soluble forms of activin A receptor, type IIB (ActRIIB)169 myostatin antibodies that can block its receptors170 and many more could be the potential antagonists for myostatin or activin A. The loss of cytoskeletal proteins, such as desmin, is activated by phosphorylation. The agents that can block desmin phosphorylation could help stop atrophy by preventing the collapse of the desmin and the myofibrils (Figure 3).7,171 During fasting, TRIM32 reduces the expression of the PI3K/Akt/FOXO signaling pathway that is associated with protein synthesis and prevents the proteolysis.171,172 Thus, TRIM32 inhibitors could prevent the cytoskeletal collapse associated with desmin and myofibrils, but so far, no success has been attained in developing the TRIM32 inhibitor. Such inhibitors, once developed, may have good potential for COPD treatment.
8. Conclusion and Future Directions
COPD has become a burden for older people and economy. Current COPD treatments, including bronchodilators, vaccines, corticosteroids, phosphodiesterases (PDEs), and long-term antibiotics, give symptomatic relief but cannot halt the progression of COPD due to it is progressive nature. Due to age factor, targeting, reduced antioxidant capacity, increased cellular senescence, and enhanced sarcopenia would be among those factors that may be beneficial in treating COPD. Daily chore or exercise is decisive for COPD patients as there are some essential biomarkers such as PGC-1α whose levels get increased during exercise thus, giving beneficial downstream effects by raising the levels of NRF-1 factors associated with endogenous antioxidant mechanisms. Patients with heart, joint, liver, kidney, brain, and gastrointestinal (GI) tract problems should also be mandatedly checked for COPD using a pulmonary function test (PFT) to validate the health of the lungs in order to lessen the worldwide burden of COPD. This strategy would be ideal for detecting COPD at an early stage since early stage COPD treatments, such as corticosteroids, are most effective; however, as the disease progresses, these treatments lose their effectiveness. The early detection and treatment of COPD may be possible with these strategies.
Acknowledgments
The authors extend their thanks to the late Prof. Rinti Banerjee for providing the opportunity to initiate this review paper and Prof. Sanjeeva Srivastava to carry forward this review paper. Also, G.S. acknowledges Indian Institute of Technology Bombay for providing the platform to dissiminate the scientific knowledge to the global research community. G.S. also thanks Debarghya Pratim Gupta and Amrita Mukhjerjee from Protoemics Lab IIT Bombay for reviewing the manuscript and giving critical input to make the manuscript comprehendible for the readers. The authors also acknowledge Servier Medical Art. Parts of the graphical abstract and table of contents graphic, Figure 1, Figure 2, and Figure 3 were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
The authors declare no competing financial interest.
Author Status
⊥ Prof. Rinti Banerjee passed away in July 2021 due to post-COVID-19 complications.
This paper published ASAP on November 20, 2023. Additonal corrections were received and a new version reposted on November 27, 2023.
References
- Lee K. H.; Lee J.; Jeong J.; Woo J.; Lee C. H.; Yoo C. G. Cigarette Smoke Extract Enhances Neutrophil Elastase-Induced IL-8 Production via Proteinase-Activated Receptor-2 Upregulation in Human Bronchial Epithelial Cells. Exp. Mol. Med. 2018, 50 (7), 1. 10.1038/s12276-018-0114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Salcedo P.; Divo M.; Casanova C.; Pinto-Plata V.; De-Torres J. P.; Cote C.; Cabrera C.; Zagaceta J.; Rodriguez-Roisin R.; Zulueta J. J.; Marin J. M.; Celli B. Disease Progression in Young Patients with COPD: Rethinking the Fletcher and Peto Model. Eur. Respir. J. 2014, 44 (2), 324–331. 10.1183/09031936.00208613. [DOI] [PubMed] [Google Scholar]
- Tilley A. E.; Walters M. S.; Shaykhiev R.; Crystal R. G. Cilia Dysfunction in Lung Disease. Annu. Rev. Physiol. 2015, 77 (646), 379–406. 10.1146/annurev-physiol-021014-071931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahy J. V.; Dickey B. F. Airway Mucus Function and Dysfunction. N Engl J Med 2010, 363, 2233. 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randell S. H.; Boucher R. C. Effective Mucus Clearance Is Essential for Respiratory Health. Am J Respir Cell Mol Biol 2006, 35 (1), 20–28. 10.1165/rcmb.2006-0082SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S.; Nathan J. A.; Goldberg A. L. Muscle Wasting in Disease: Molecular Mechanisms and Promising Therapies. Nat. Rev. Drug Discovery 2015, 14 (1), 58–74. 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- Childs B. G.; Gluscevic M.; Baker D. J.; Laberge R. M.; Marquess D.; Dananberg J.; Van Deursen J. M. Senescent Cells: An Emerging Target for Diseases of Ageing. Nat. Rev. Drug Discovery 2017, 16 (10), 718–735. 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumayao R.; Newsholme P.; McMorrow T. The Role of Cystinosin in the Intermediary Thiol Metabolism and Redox Homeostasis in Kidney Proximal Tubular Cells. Antioxidants (Basel, Switzerland) 2018, 7 (12), 179. 10.3390/antiox7120179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M.; Leibfritz D.; Moncol J.; Cronin M. T. D.; Mazur M.; Telser J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39 (1), 44–84. 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Marí M.; Morales A.; Colell A.; García-Ruiz C.; Ferná Ndez-Checa J. C. Mitochondrial Glutathione, a Key Survival Antioxidant. Antioxid. Redox Signal. 2009, 11 (11), 2685–2700. 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C.; Sinskey A. J.; Lodish H. F. Oxidized Redox State of Glutathione in the Endoplasmic Reticulum. Science (80-.). 1992, 257 (5076), 1496–1502. 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Lash L. H. Evidence for Mitochondrial Uptake of Glutathione by Dicarboxylate and 2-Oxoglutarate Carriers 1. J. Pharmacol. Exp. Ther. 1998, 285 (2), 608–618. [PubMed] [Google Scholar]
- Chen Z.; Putt D. A.; Lash L. H. Enrichment and Functional Reconstitution of Glutathione Transport Activity from Rabbit Kidney Mitochondria: Further Evidence for the Role of the Dicarboxylate and 2-Oxoglutarate Carriers in Mitochondrial Glutathione Transport. Arch. Biochem. Biophys. 2000, 373 (1), 193–202. 10.1006/abbi.1999.1527. [DOI] [PubMed] [Google Scholar]
- Coll O.; Colell A.; García-Ruiz C.; Kaplowitz N.; Fernández-Checa J. C. Sensitivity of the 2-Oxoglutarate Carrier to Alcohol Intake Contributes to Mitochondrial Glutathione Depletion. Hepatology 2003, 38 (3), 692–702. 10.1053/jhep.2003.50351. [DOI] [PubMed] [Google Scholar]
- García-Ruiz C.; Morales A.; Colell A.; Rodes J.; Yi J. R.; Kaplowitz N.; Fernandez-Checa J. C. Evidence That the Rat Hepatic Mitochondrial Carrier Is Distinct from the Sinusoidal and Canalicular Transporters for Reduced Glutathione: EXPRESSION STUDIES IN XENOPUS LAEVIS OOCYTES. J. Biol. Chem. 1995, 270 (27), 15946–15949. 10.1074/jbc.270.27.15946. [DOI] [PubMed] [Google Scholar]
- Lash L. H. Mitochondrial Glutathione Transport: Physiological, Pathological and Toxicological Implications. Chem. Biol. Interact. 2006, 163 (1–2), 54–67. 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J.; Lai J. C. K.; Meister A. High-Affinity Transport of Glutathione Is Part of a Multicomponent System Essential for Mitochondrial Function. Proc. Natl. Acad. Sci. U. S. A. 1990, 87 (18), 7185. 10.1073/pnas.87.18.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldin M. P.; Goncharov T. M.; Goltseve Y. V.; Wallach D. Involvement of MACH, a Novel MORT1/FADD-Interacting Protease, in Fas/APO-1- and TNF Receptor-Induced Cell Death. Cell 1996, 85 (6), 803–815. 10.1016/S0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Muzio M.; Chinnaiyan A. M.; Kischkel F. C.; O’Rourke K.; et al. FLICE, A Novel FADD-Homologous ICE/CED-3-like Protease, Is Recruited to the CD95 (Fas/APO-1) Death-Inducing Signaling Complex. Cell 1996, 85, 817–827. 10.1016/S0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B. Caspases: The Enzymes of Death. Essays Biochem. 2003, 39, 25–40. 10.1042/bse0390025. [DOI] [PubMed] [Google Scholar]
- Srinivasa Rao C.; Emmanuel Subash Y. The Effect of Chronic Tobacco Smoking and Chewing on the Lipid Profile. J. Clin. Diagn. Res. 2013, 7 (1), 31–34. 10.7860/JCDR/2012/5086.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J. A.; Chin S. M.; Linn S. Toxic DNA Damage by Hydrogen Peroxide through the Fenton Reaction in Vivo and in Vitro. Science 1988, 240 (4852), 640–642. 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Giorgio M.; Migliaccio E.; Orsini F.; Paolucci D.; Moroni M.; Contursi C.; Pelliccia G.; Luzi L.; Minucci S.; Marcaccio M.; Pinton P.; Rizzuto R.; Bernardi P.; Paolucci F.; Pelicci P. G. Electron Transfer between Cytochrome c and P66Shc Generates Reactive Oxygen Species That Trigger Mitochondrial Apoptosis. Cell 2005, 122 (2), 221–233. 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Ribas V.; García-Ruiz C.; Fernández-Checa J. C. Glutathione and Mitochondria. Front. Pharmacol. 2014, 5, 151. 10.3389/fphar.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimov E. R. The Role of P66shc in Oxidative Stress and Apoptosis. Acta Naturae 2010, 2 (4), 44. 10.32607/20758251-2010-2-4-44-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan V. E.; Tyurin V. A.; Jiang J.; Tyurina Y. Y.; Ritov V. B.; Amoscato A. A.; Osipov A. N.; Belikova N. A.; Kapralov A. A.; Kini V.; Vlasova I. I.; Zhao Q.; Zou M.; Di P.; Svistunenko D. A.; Kurnikov I. V.; Borisenko G. G. Cytochrome c Acts as a Cardiolipin Oxygenase Required for Release of Proapoptotic Factors. Nat. Chem. Biol. 2005, 1 (4), 223–232. 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- Ott M.; Robertson J. D.; Gogvadze V.; Zhivotovsky B.; Orrenius S. Cytochrome c Release from Mitochondria Proceeds by a Two-Step Process. Proc. Natl. Acad. Sci. U. S. A. 2002, 99 (3), 1259–1263. 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ow Y. L. P.; Green D. R.; Hao Z.; Mak T. W. Cytochrome c: Functions beyond Respiration. Nat. Rev. Mol. Cell Biol. 2008, 9 (7), 532–542. 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- Mootha V. K.; Wei M. C.; Buttle K. F.; Scorrano L.; Panoutsakopoulou V.; Mannella C. A.; Korsmeyer S. J. A Reversible Component of Mitochondrial Respiratory Dysfunction in Apoptosis Can Be Rescued by Exogenous Cytochrome C. EMBO J. 2001, 20 (4), 661–671. 10.1093/emboj/20.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Wang Z. B.; Xu J. X. Effect of Cytochrome c on the Generation and Elimination of O2*- and H2O2 in Mitochondria. J. Biol. Chem. 2003, 278 (4), 2356–2360. 10.1074/jbc.M209681200. [DOI] [PubMed] [Google Scholar]
- Hemann M. T.; Lowe S. W. The P53-Bcl-2 Connection. Cell Death Differ. 2006, 13 (8), 1256–1259. 10.1038/sj.cdd.4401962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Pinedo C.; Guío-Carrión A.; Goldstein J. C.; Fitzgerald P.; Newmeyer D. D.; Green D. R. Different Mitochondrial Intermembrane Space Proteins Are Released during Apoptosis in a Manner That Is Coordinately Initiated but Can Vary in Duration. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (31), 11573–11578. 10.1073/pnas.0603007103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L.; Ashiya M.; Buttle K.; Weiler S.; Oakes S. A.; Mannella C. A.; Korsmeyer S. J. A Distinct Pathway Remodels Mitochondrial Cristae and Mobilizes Cytochrome c during Apoptosis. Dev. Cell 2002, 2 (1), 55–67. 10.1016/S1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Li P.; Nijhawan D.; Budihardjo I.; Srinivasula S. M.; Ahmad M.; Alnemri E. S.; Wang X. Cytochrome c and DATP-Dependent Formation of Apaf-1/Caspase-9 Complex Initiates an Apoptotic Protease Cascade. Cell 1997, 91 (4), 479–489. 10.1016/S0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Lakshmanan I.; Batra S. K. Protocol for Apoptosis Assay by Flow Cytometry Using Annexin V Staining Method Materials and Reagents. BIO-PROTOCOL 2013, 3, e374. 10.21769/BioProtoc.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Espín D.; Serrano M. Cellular Senescence: From Physiology to Pathology. Nat. Rev. Mol. Cell Biol. 2014, 15 (7), 482–496. 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- Van Deursen J. M. The Role of Senescent Cells in Ageing. Nature 2014, 509 (7501), 439–446. 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless N. E.; Sherr C. J. Forging a Signature of in Vivo Senescence. Nat. Rev. Cancer 2015, 15 (7), 397–408. 10.1038/nrc3960. [DOI] [PubMed] [Google Scholar]
- Baker D. J.; Childs B. G.; Durik M.; Wijers M. E.; Sieben C. J.; Zhong J.; Saltness R. A.; Jeganathan K. B.; Verzosa G. C.; Pezeshki A.-M.; Khazaie K.; Miller J. D.; Van Deursen J. M. Naturally Occurring P16 Ink4a-Positive Cells Shorten Healthy Lifespan HHS Public Access. Nature 2016, 530 (7589), 184–189. 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs B. G.; Gluscevic M.; Baker D. J.; Laberge R. M.; Marquess D.; Dananberg J.; Van Deursen J. M. Senescent Cells: An Emerging Target for Diseases of Ageing. Nat. Rev. Drug Discovery 2017, 16 (10), 718–735. 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M.; Seeger W.; Voswinckel R. Senescence-Associated Secretory Phenotype and Its Possible Role in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2014, 51 (3), 323–333. 10.1165/rcmb.2013-0382PS. [DOI] [PubMed] [Google Scholar]
- Campisi J. Senescent Cells, Tumor Suppression, and Organismal Aging: Good Citizens, Bad Neighbors. Cell 2005, 120 (4), 513–522. 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ogrodnik M.; Miwa S.; Tchkonia T.; Tiniakos D.; Wilson C. L.; Lahat A.; Day C. P.; Burt A.; Palmer A.; Anstee Q. M.; Grellscheid S. N.; Hoeijmakers J. H. J.; Barnhoorn S.; Mann D. A.; Bird T. G.; Vermeij W. P.; Kirkland J. L.; Passos J. F.; Von Zglinicki T.; Jurk D. Cellular Senescence Drives Age-Dependent Hepatic Steatosis. Nat. Commun. 2017 81 2017, 8 (1), 1–12. 10.1038/ncomms15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T.; Zhu Y.; Van Deursen J.; Campisi J.; Kirkland J. L. Cellular Senescence and the Senescent Secretory Phenotype: Therapeutic Opportunities. J. Clin. Invest. 2013, 123 (3), 966–972. 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J.; D’Adda Di Fagagna F. Cellular Senescence: When Bad Things Happen to Good Cells. Nat. Rev. Mol. Cell Biol. 2007, 8 (9), 729–740. 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Lebrasseur N. K.; Tchkonia T.; Kirkland J. L. Cellular Senescence and the Biology of Aging, Disease, and Frailty. Nestle Nutr. Inst. Workshop Ser. 2015, 83, 11–18. 10.1159/000382054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanArsdale T.; Boshoff C.; Arndt K. T.; Abraham R. T. Molecular Pathways: Targeting the Cyclin D-CDK4/6 Axis for Cancer Treatment. Clin. Cancer Res. 2015, 21 (13), 2905–2910. 10.1158/1078-0432.CCR-14-0816. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Johnson S. M.; Fedoriw Y.; Rogers A. B.; Yuan H.; Krishnamurthy J.; Sharpless N. E. Expression of P16INK4a Prevents Cancer and Promotes Aging in Lymphocytes. Blood 2011, 117 (12), 3257. 10.1182/blood-2010-09-304402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottage C. T.; Peterson N.; Kearley J.; Berlin A.; Xiong X.; Huntley A.; Zhao W.; Brown C.; Migneault A.; Zerrouki K.; Criner G.; Kolbeck R.; Connor J.; Lemaire R. Targeting P16-Induced Senescence Prevents Cigarette Smoke-Induced Emphysema by Promoting IGF1/Akt1 Signaling in Mice. Commun Biol 2019, 2, 307. 10.1038/s42003-019-0532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X.-Y.; Li Y.-Y.; Huang C.; Li J.; Yao H.-W. AMP-Activated Protein Kinase Reduces Inflammatory Responses and Cellular Senescence in Pulmonary Emphysema. Oncotarget 2017, 8 (14), 22513–22523. 10.18632/oncotarget.15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa R.; Sato T.; Suzuki Y.; Baskoro H.; Kawaguchi K.; Sugimoto M. P19Arf Exacerbates Cigarette Smoke-Induced Pulmonary Dysfunction. Biomolecules 2020, 10 (3), 462. 10.3390/biom10030462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodas M.; Pehote G.; Silverberg D.; Gulbins E.; Vij N. Autophagy Augmentation Alleviates Cigarette Smoke-Induced CFTR-Dysfunction, Ceramide-Accumulation and COPD-Emphysema Pathogenesis. Free Radic. Biol. Med. 2019, 131, 81–97. 10.1016/j.freeradbiomed.2018.11.023. [DOI] [PubMed] [Google Scholar]
- Vij N.; Chandramani-Shivalingappa P.; Van Westphal C.; Hole R.; Bodas M. Cigarette Smoke-Induced Autophagy Impairment Accelerates Lung Aging, COPD-Emphysema Exacerbations and Pathogenesis. Am. J. Physiol. Cell Physiol. 2018, 314 (1), C73–C87. 10.1152/ajpcell.00110.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldhuis R. R.; de Vries M.; Timens W.; van den Berge M.; Demaria M.; Oliver B. G. G.; Heijink I. H.; Brandsma C. A. Link between Increased Cellular Senescence and Extracellular Matrix Changes in COPD. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319 (1), L48–L60. 10.1152/ajplung.00028.2020. [DOI] [PubMed] [Google Scholar]
- Storer M.; Mas A.; Robert-Moreno A.; Pecoraro M.; Ortells M. C.; Di Giacomo V.; Yosef R.; Pilpel N.; Krizhanovsky V.; Sharpe J.; Keyes W. M. Senescence Is a Developmental Mechanism That Contributes to Embryonic Growth and Patterning. Cell 2013, 155 (5), 1119. 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- Mosteiro L.; Pantoja C.; Alcazar N.; Marión R. M.; Chondronasiou D.; Rovira M.; Fernandez-Marcos P. J.; Muñoz-Martin M.; Blanco-Aparicio C.; Pastor J.; Gómez-López G.; De Martino A.; Blasco M. A.; Abad M.; Serrano M. Tissue Damage and Senescence Provide Critical Signals for Cellular Reprogramming in Vivo. Science 2016, 354 (6315), aaf4445. 10.1126/science.aaf4445. [DOI] [PubMed] [Google Scholar]
- Muñoz-Espín D.; Cañamero M.; Maraver A.; Gómez-López G.; Contreras J.; Murillo-Cuesta S.; Rodríguez-Baeza A.; Varela-Nieto I.; Ruberte J.; Collado M.; Serrano M. Programmed Cell Senescence during Mammalian Embryonic Development. Cell 2013, 155 (5), 1104. 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Li T.; Kon N.; Jiang L.; Tan M.; Ludwig T.; Zhao Y.; Baer R.; Gu W. Tumor Suppression in the Absence of P53-Mediated Cell-Cycle Arrest, Apoptosis, and Senescence. Cell 2012, 149 (6), 1269–1283. 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman A.; Klochendler A.; Azazmeh N.; Gabai Y.; Horwitz E.; Anzi S.; Swisa A.; Condiotti R.; Granit R. Z.; Nevo Y.; Fixler Y.; Shreibman D.; Zamir A.; Tornovsky-Babeay S.; Dai C.; Glaser B.; Powers A. C.; Shapiro A. M. J.; Magnuson M. A.; Dor Y.; Ben-Porath I. P16(Ink4a)-Induced Senescence of Pancreatic Beta Cells Enhances Insulin Secretion. Nat. Med. 2016, 22 (4), 412–420. 10.1038/nm.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin C. M.; Hann C. L.; Garon E. B.; Ribeiro De Oliveira M.; Bonomi P. D.; Camidge D. R.; Chu Q.; Giaccone G.; Khaira D.; Ramalingam S. S.; Ranson M. R.; Dive C.; McKeegan E. M.; Chyla B. J.; Dowell B. L.; Chakravartty A.; Nolan C. E.; Rudersdorf N.; Busman T. A.; Mabry M. H.; Krivoshik A. P.; Humerickhouse R. A.; Shapiro G. I.; Gandhi L. Phase II Study of Single-Agent Navitoclax (ABT-263) and Biomarker Correlates in Patients with Relapsed Small Cell Lung Cancer. Clin. Cancer Res. 2012, 18 (11), 3163–3169. 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge R. M.; Sun Y.; Orjalo A. V.; Patil C. K.; Freund A.; Zhou L.; Curran S. C.; Davalos A. R.; Wilson-Edell K. A.; Liu S.; Limbad C.; Demaria M.; Li P.; Hubbard G. B.; Ikeno Y.; Javors M.; Desprez P. Y.; Benz C. C.; Kapahi P.; Nelson P. S.; Campisi J. MTOR Regulates the Pro-Tumorigenic Senescence-Associated Secretory Phenotype by Promoting IL1A Translation. Nat. Cell Biol. 2015, 17 (8), 1049–1061. 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truss M.; Bartsch J.; Mows C.; Chavez S.; Beato M. Chromatin Structure of the MMTV Promoter and Its Changes during Hormonal Induction. Cell. Mol. Neurobiol. 1996, 16 (2), 85–101. 10.1007/BF02088169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M.; Eisfeld K. Transcription Factor Access to Chromatin. Nucleic Acids Res. 1997, 25 (18), 3559–3563. 10.1093/nar/25.18.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ura K.; Kurumizaka H.; Dimitrov S.; Almouzni G.; Wolffe A. P. Histone Acetylation: Influence on Transcription, Nucleosome Mobility and Positioning, and Linker Histone-Dependent Transcriptional Repression. EMBO J. 1997, 16 (8), 2096–2107. 10.1093/emboj/16.8.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P. Sinful Repression. Nat. 1997 3876628 1997, 387 (6628), 16–17. 10.1038/387016a0. [DOI] [PubMed] [Google Scholar]
- Urnov F. D.; Wolffe A. P. Chromatin Remodeling and Transcriptional Activation: The Cast (in Order of Appearance). Oncogene 2001, 20 (24), 2991–3006. 10.1038/sj.onc.1204323. [DOI] [PubMed] [Google Scholar]
- Workman J. L.; Buchman A. R. Multiple Functions of Nucleosomes and Regulatory Factors in Transcription. Trends Biochem. Sci. 1993, 18 (3), 90–95. 10.1016/0968-0004(93)90160-O. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. New Anti-Inflammatory Targets for Chronic Obstructive Pulmonary Disease. Nat. Rev. Drug Discovery 2013, 12 (7), 543–559. 10.1038/nrd4025. [DOI] [PubMed] [Google Scholar]
- Ito K.; Barnes P. J.; Adcock I. M. Glucocorticoid Receptor Recruitment of Histone Deacetylase 2 Inhibits Interleukin-1beta-Induced Histone H4 Acetylation on Lysines 8 and 12. Mol. Cell. Biol. 2000, 20 (18), 6891–6903. 10.1128/MCB.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K.; Ito M.; Elliott W. M.; Cosio B.; Caramori G.; Kon O. M.; Barczyk A.; Hayashi S.; Adcock I. M.; Hogg J. C.; Barnes P. J. Decreased Histone Deacetylase Activity in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2005, 352 (19), 1967–1976. 10.1056/NEJMoa041892. [DOI] [PubMed] [Google Scholar]
- Hu L.; Liu F.; Li L.; Zhang L.; Yan C.; Li Q.; Qiu J.; Dong J.; Sun J.; Zhang H. Effects of Icariin on Cell Injury and Glucocorticoid Resistance in BEAS-2B Cells Exposed to Cigarette Smoke Extract. Exp. Ther. Med. 2020, 20 (1), 283–292. 10.3892/etm.2020.8702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Li X.; Qin Y.; Cheng J.; Hao G.; Jin R.; Zhu C. Jinwei Tang Modulates HDAC2 Expression in a Rat Model of COPD. Exp. Ther. Med. 2018, 15 (3), 2604. 10.3892/etm.2018.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L.; Gao Z.; Huang F.; Huang S.; Zhang R.; Ma D.; Wu Q.; Li F.; Chen H.; Wang J. Erythromycin Enhances the Anti-Inflammatory Activity of Budesonide in COPD Rat Model. Int. J. Clin. Exp. Med. 2015, 8 (12), 22217. [PMC free article] [PubMed] [Google Scholar]
- Manz M. G.; Boettcher S. Emergency Granulopoiesis. Nat. Rev. Immunol. 2014, 14 (5), 302–314. 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- Németh T.; Sperandio M.; Mócsai A. Neutrophils as Emerging Therapeutic Targets. Nat. Rev. Drug Discovery 2020, 19 (4), 253–275. 10.1038/s41573-019-0054-z. [DOI] [PubMed] [Google Scholar]
- Tsantikos E.; Lau M.; Castelino C. M. N.; Maxwell M. J.; Passey S. L.; Hansen M. J.; McGregor N. E.; Sims N. A.; Steinfort D. P.; Irving L. B.; Anderson G. P.; Hibbs M. L. Granulocyte-CSF Links Destructive Inflammation and Comorbidities in Obstructive Lung Disease. J. Clin. Invest. 2018, 128 (6), 2406–2418. 10.1172/JCI98224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V.; Reichard U.; Goosmann C.; Fauler B.; Uhlemann Y.; Weiss D. S.; Weinrauch Y.; Zychlinsky A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303 (5663), 1532–1535. 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Daniel C.; Leppkes M.; Muñoz L. E.; Schley G.; Schett G.; Herrmann M. Extracellular DNA Traps in Inflammation, Injury and Healing. Nat. Rev. Nephrol. 2019, 15 (9), 559–575. 10.1038/s41581-019-0163-2. [DOI] [PubMed] [Google Scholar]
- Porto B. N.; Stein R. T. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing?. Front. Immunol. 2016, 7 (AUG), 311. 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger G.; Choidas A.; Burn G. L.; Habenberger P.; Di Lucrezia R.; Kordes S.; Menninger S.; Eickhoff J.; Nussbaumer P.; Klebl B.; Krüger R.; Herzig A.; Zychlinsky A. Gasdermin D Plays a Vital Role in the Generation of Neutrophil Extracellular Traps. Sci. Immunol. 2018, 3 (26), eaar6689. 10.1126/sciimmunol.aar6689. [DOI] [PubMed] [Google Scholar]
- Chen K. W.; Monteleone M.; Boucher D.; Sollberger G.; Ramnath D.; Condon N. D.; von Pein J. B.; Broz P.; Sweet M. J.; Schroder K. Noncanonical Inflammasome Signaling Elicits Gasdermin D-Dependent Neutrophil Extracellular Traps. Sci. Immunol. 2018, 3 (26), eaar6676. 10.1126/sciimmunol.aar6676. [DOI] [PubMed] [Google Scholar]
- Frye R. A. Phylogenetic Classification of Prokaryotic and Eukaryotic Sir2-like Proteins. Biochem. Biophys. Res. Commun. 2000, 273 (2), 793–798. 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- Singh C. K.; Nihal M.; Ahmad N. Histone Deacetylase Inhibitory Approaches for the Management of Osteoarthritis. Am. J. Pathol. 2016, 186 (10), 2555. 10.1016/j.ajpath.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T.; Guarente L. Sirtuins at a Glance. J. Cell Sci. 2011, 124 (6), 833–838. 10.1242/jcs.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh C. K.; Chhabra G.; Ndiaye M. A.; Garcia-Peterson L. M.; MacK N. J.; Ahmad N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28 (8), 643. 10.1089/ars.2017.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai W.; Zhang X. Nucleus or Cytoplasm? The Mysterious Case of SIRT1’s Subcellular Localization. Cell Cycle 2016, 15 (24), 3337. 10.1080/15384101.2016.1237170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilking M. J.; Singh C.; Nihal M.; Zhong W.; Ahmad N. SIRT1 Deacetylase Is Overexpressed in Human Melanoma and Its Small Molecule Inhibition Imparts Anti-Proliferative Response via P53 Activation. Arch. Biochem. Biophys. 2014, 563, 94–100. 10.1016/j.abb.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.; Auwerx J. Protein Deacetylation by SIRT1: An Emerging Key Post-Translational Modification in Metabolic Regulation. Pharmacol. Res. 2010, 62 (1), 35–41. 10.1016/j.phrs.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H.; Hwang J. W.; Sundar I. K.; Friedman A. E.; McBurney M. W.; Guarente L.; Gu W.; Kinnula V. L.; Rahman I. SIRT1 Redresses the Imbalance of Tissue Inhibitor of Matrix Metalloproteinase-1 and Matrix Metalloproteinase-9 in the Development of Mouse Emphysema and Human COPD. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2013, 305 (9), L615. 10.1152/ajplung.00249.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Zhang W.; Pan H.; Feldser H. G.; Lainez E.; Miller C.; Leung S.; Zhong Z.; Zhao H.; Sweitzer S.; Considine T.; Riera T.; Suri V.; White B.; Ellis J. L.; Vlasuk G. P.; Loh C. SIRT1 Activators Suppress Inflammatory Responses through Promotion of P65 Deacetylation and Inhibition of NF-ΚB Activity. PLoS One 2012, 7 (9), e46364. 10.1371/journal.pone.0046364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S.; Papaioannou A. I.; Papaporfyriou A.; Baker J. R.; Vuppusetty C.; Loukides S.; Barnes P. J.; Ito K. Decreased Serum Sirtuin-1 in COPD. Chest 2017, 152 (2), 343–352. 10.1016/j.chest.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z.; Zhang W.; Qiao J.; He B. Melatonin Attenuates Airway Inflammation via SIRT1 Dependent Inhibition of NLRP3 Inflammasome and IL-1β in Rats with COPD. Int. Immunopharmacol. 2018, 62, 23–28. 10.1016/j.intimp.2018.06.033. [DOI] [PubMed] [Google Scholar]
- Yanagisawa S.; Baker J. R.; Vuppusetty C.; Koga T.; Colley T.; Fenwick P.; Donnelly L. E.; Barnes P. J.; Ito K. The Dynamic Shuttling of SIRT1 between Cytoplasm and Nuclei in Bronchial Epithelial Cells by Single and Repeated Cigarette Smoke Exposure. PLoS ONE 2018, 13, e0193921. 10.1371/journal.pone.0193921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska W.; Sikora E.; Bielak-Zmijewska A. Sirtuins, a Promising Target in Slowing down the Ageing Process. Biogerontology 2017, 18 (4), 447. 10.1007/s10522-017-9685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes P.; Fleming Outeiro T.; Cavadas C. Emerging Role of Sirtuin 2 in the Regulation of Mammalian Metabolism. Trends Pharmacol. Sci. 2015, 36 (11), 756–768. 10.1016/j.tips.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Wang Y. P.; Zhou L. S.; Zhao Y. Z.; Wang S. W.; Chen L. L.; Liu L. X.; Ling Z. Q.; Hu F. J.; Sun Y. P.; Zhang J. Y.; Yang C.; Yang Y.; Xiong Y.; Guan K. L.; Ye D. Regulation of G6PD Acetylation by SIRT2 and KAT9Modulates NADPH Homeostasis and Cell Survival during Oxidative Stress. EMBO J. 2014, 33 (12), 1304. 10.1002/embj.201387224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahara T.; Bonasio R.; Narendra V.; Reinberg D. SIRT3 Functions in the Nucleus in the Control of Stress-Related Gene Expression. Mol. Cell. Biol. 2012, 32 (24), 5022–5034. 10.1128/MCB.00822-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.; Ren X.; Gowda A. S. P.; Shan Y.; Zhang L.; Yuan Y. S.; Patel R.; Wu H.; Huber-Keener K.; Yang J. W.; Liu D.; Spratt T. E.; Yang J. M. Interaction of Sirt3 with OGG1 Contributes to Repair of Mitochondrial DNA and Protects from Apoptotic Cell Death under Oxidative Stress. Cell Death Dis. 2013, 4 (7), e731. 10.1038/cddis.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. A.; Huynh F. K.; Fisher-Wellman K.; Stuart J. D.; Peterson B. S.; Douros J. D.; Wagner G. R.; Thompson J. W.; Madsen A. S.; Green M. F.; Sivley R. M.; Ilkayeva O. R.; Stevens R. D.; Backos D. S.; Capra J. A.; Olsen C. A.; Campbell J. E.; Muoio D. M.; Grimsrud P. A.; Hirschey M. D. SIRT4 Is a Lysine Deacylase That Controls Leucine Metabolism and Insulin Secretion. Cell Metab. 2017, 25 (4), 838–855. 10.1016/j.cmet.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis M. C.; Mostoslavsky R.; Haigis K. M.; Fahie K.; Christodoulou D. C.; Murphy A. J. J.; Valenzuela D. M.; Yancopoulos G. D.; Karow M.; Blander G.; Wolberger C.; Prolla T. A.; Weindruch R.; Alt F. W.; Guarente L. SIRT4 Inhibits Glutamate Dehydrogenase and Opposes the Effects of Calorie Restriction in Pancreatic Beta Cells. Cell 2006, 126 (5), 941–954. 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Luo Y. X.; Tang X.; An X. Z.; Xie X. M.; Chen X. F.; Zhao X.; Hao D. L.; Chen H. Z.; Liu D. P. SIRT4 Accelerates Ang II-Induced Pathological Cardiac Hypertrophy by Inhibiting Manganese Superoxide Dismutase Activity. Eur. Heart J. 2016, 38 (18), 1389–1398. 10.1093/eurheartj/ehw138. [DOI] [PubMed] [Google Scholar]
- Nasrin N.; Wu X.; Fortier E.; Feng Y.; Baré O. C.; Chen S.; Ren X.; Wu Z.; Streeper R. S.; Bordone L. SIRT4 Regulates Fatty Acid Oxidation and Mitochondrial Gene Expression in Liver and Muscle Cells. J. Biol. Chem. 2010, 285 (42), 31995–32002. 10.1074/jbc.M110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosca M. G.; Vazquez E. J.; Chen Q.; Kerner J.; Kern T. S.; Hoppel C. L. Oxidation of Fatty Acids Is the Source of Increased Mitochondrial Reactive Oxygen Species Production in Kidney Cortical Tubules in Early Diabetes. Diabetes 2012, 61 (8), 2074–2083. 10.2337/db11-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J.; Zhou Y.; Su X.; Yu J. J.; Khan S.; Jiang H.; Kim J.; Woo J.; Kim J. H.; Choi B. H.; He B.; Chen W.; Zhang S.; Cerione R. A.; Auwerx J.; Hao Q.; Lin H. Sirt5 Is a NAD-Dependent Protein Lysine Demalonylase and Desuccinylase. Science (80-.). 2011, 334 (6057), 806–809. 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.; Che W.; Zheng C.; Liu W.; Wen J.; Fu H.; Tang K.; Zhang J.; Xu Y. SIRT5: A Safeguard against Oxidative Stress-Induced Apoptosis in Cardiomyocytes. Cell. Physiol. Biochem. 2013, 32 (4), 1050–1059. 10.1159/000354505. [DOI] [PubMed] [Google Scholar]
- Bobermin L. D.; Wartchow K. M.; Flores M. P.; Leite M. C.; Quincozes-Santos A.; Gonçalves C. A. Ammonia-Induced Oxidative Damage in Neurons Is Prevented by Resveratrol and Lipoic Acid with Participation of Heme Oxygenase 1. Neurotoxicology 2015, 49, 28–35. 10.1016/j.neuro.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Nakagawa T.; Lomb D. J.; Haigis M. C.; Guarente L. SIRT5 Deacetylates Carbamoyl Phosphate Synthetase 1 and Regulates the Urea Cycle. Cell 2009, 137 (3), 560–570. 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura M.; Nakamura Y.; Tanaka D.; Zhuang X.; Fujita Y.; Obara A.; Hamasaki A.; Hosokawa M.; Inagaki N. Overexpression of SIRT5 Confirms Its Involvement in Deacetylation and Activation of Carbamoyl Phosphate Synthetase 1. Biochem. Biophys. Res. Commun. 2010, 393 (1), 73–78. 10.1016/j.bbrc.2010.01.081. [DOI] [PubMed] [Google Scholar]
- Michishita E.; McCord R. A.; Berber E.; Kioi M.; Padilla-Nash H.; Damian M.; Cheung P.; Kusumoto R.; Kawahara T. L. A.; Barrett J. C.; Chang H. Y.; Bohr V. A.; Ried T.; Gozani O.; Chua K. F. SIRT6 Is a Histone H3 Lysine 9 Deacetylase That Modulates Telomeric Chromatin. Nature 2008, 452 (7186), 492–496. 10.1038/nature06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E.; McCord R. A.; Boxer L. D.; Barber M. F.; Hong T.; Gozani O.; Chua K. F. Cell Cycle-Dependent Deacetylation of Telomeric Histone H3 Lysine K56 by Human SIRT6. Cell Cycle 2009, 8 (16), 2664–2666. 10.4161/cc.8.16.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E.; Park J. Y.; Burneskis J. M.; Barrett J. C.; Horikawa I. Evolutionarily Conserved and Nonconserved Cellular Localizations and Functions of Human SIRT Proteins. Mol. Biol. Cell 2005, 16 (10), 4623. 10.1091/mbc.e05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennen R. I.; Bua D. J.; Wright W. E.; Chua K. F. SIRT6 Is Required for Maintenance of Telomere Position Effect in Human Cells. Nat. Commun. 2011, 2 (1), 433. 10.1038/ncomms1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennen R. I.; Chua K. F. Chromatin Regulation and Genome Maintenance by Mammalian SIRT6. Trends Biochem. Sci. 2011, 36 (1), 39–46. 10.1016/j.tibs.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meter M.; Mao Z.; Gorbunova V.; Seluanov A. Repairing Split Ends: SIRT6, Mono-ADP Ribosylation and DNA Repair. Aging (Albany. NY). 2011, 3 (9), 829–835. 10.18632/aging.100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun P. Role of Sirtuins in Chronic Obstructive Pulmonary Disease. Arch. Pharm. Res. 2015, 38 (1), 1–10. 10.1007/s12272-014-0494-2. [DOI] [PubMed] [Google Scholar]
- Takasaka N.; Araya J.; Hara H.; Ito S.; Kobayashi K.; Kurita Y.; Wakui H.; Yoshii Y.; Yumino Y.; Fujii S.; Minagawa S.; Tsurushige C.; Kojima J.; Numata T.; Shimizu K.; Kawaishi M.; Kaneko Y.; Kamiya N.; Hirano J.; Odaka M.; Morikawa T.; Nishimura S. L.; Nakayama K.; Kuwano K. Autophagy Induction by SIRT6 through Attenuation of Insulin-like Growth Factor Signaling Is Involved in the Regulation of Human Bronchial Epithelial Cell Senescence. J. Immunol. 2014, 192 (3), 958–968. 10.4049/jimmunol.1302341. [DOI] [PubMed] [Google Scholar]
- Finkel T.; Deng C. X.; Mostoslavsky R. Recent Progress in the Biology and Physiology of Sirtuins. Nature 2009, 460 (7255), 587–591. 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G.; Su L.; Singhal S.; Liu X. Emerging Roles of SIRT6 on Telomere Maintenance, DNA Repair, Metabolism and Mammalian Aging. Mol. Cell. Biochem. 2012, 364 (1–2), 345–350. 10.1007/s11010-012-1236-8. [DOI] [PubMed] [Google Scholar]
- Michan S.; Sinclair D. Sirtuins in Mammals: Insights into Their Biological Function. Biochem. J. 2007, 404 (1), 1–13. 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toiber D.; Sebastian C.; Mostoslavsky R. Characterization of Nuclear Sirtuins: Molecular Mechanisms and Physiological Relevance. Handb. Exp. Pharmacol. 2011, 206, 189–224. 10.1007/978-3-642-21631-2_9. [DOI] [PubMed] [Google Scholar]
- Ford E.; Voit R.; Liszt G.; Magin C.; Grummt I.; Guarente L. Mammalian Sir2 Homolog SIRT7 Is an Activator of RNA Polymerase I Transcription. Genes Dev. 2006, 20 (9), 1075–1080. 10.1101/gad.1399706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S.; Anwar T.; Kiran M.; Ramakrishna G. Sirtuin 7 in Cell Proliferation, Stress and Disease: Rise of the Seventh Sirtuin!. Cell. Signal. 2015, 27 (3), 673–682. 10.1016/j.cellsig.2014.11.026. [DOI] [PubMed] [Google Scholar]
- Han H. Q.; Zhou X.; Mitch W. E.; Goldberg A. L. Myostatin/Activin Pathway Antagonism: Molecular Basis and Therapeutic Potential. Int. J. Biochem. Cell Biol. 2013, 45 (10), 2333–2347. 10.1016/j.biocel.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Sartori R.; Milan G.; Patron M.; Mammucari C.; Blaauw B.; Abraham R.; Sandri M. Smad2 and 3 Transcription Factors Control Muscle Mass in Adulthood. Am. J. Physiol. Cell Physiol. 2009, 296 (6), C1248. 10.1152/ajpcell.00104.2009. [DOI] [PubMed] [Google Scholar]
- Trendelenburg A. U.; Meyer A.; Rohner D.; Boyle J.; Hatakeyama S.; Glass D. J. Myostatin Reduces Akt/TORC1/P70S6K Signaling, Inhibiting Myoblast Differentiation and Myotube Size. Am. J. Physiol. Cell Physiol. 2009, 296 (6), C1258. 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- McCroskery S.; Thomas M.; Maxwell L.; Sharma M.; Kambadur R. Myostatin Negatively Regulates Satellite Cell Activation and Self-Renewal. J. Cell Biol. 2003, 162 (6), 1135–1147. 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAURO A. Satellite Cell of Skeletal Muscle Fibers. J. Biophys. Biochem. Cytol. 1961, 9 (2), 493–495. 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan J.; Rando T. A. Stem Cells in Postnatal Myogenesis: Molecular Mechanisms of Satellite Cell Quiescence, Activation and Replenishment. Trends Cell Biol. 2005, 15 (12), 666–673. 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kuang S.; Gillespie M. A.; Rudnicki M. A. Niche Regulation of Muscle Satellite Cell Self-Renewal and Differentiation. Cell Stem Cell 2008, 2 (1), 22–31. 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Ju C. R.; Chen R. C. Serum Myostatin Levels and Skeletal Muscle Wasting in Chronic Obstructive Pulmonary Disease. Respir. Med. 2012, 106 (1), 102–108. 10.1016/j.rmed.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Testelmans D.; Crul T.; Maes K.; Agten A.; Crombach M.; Decramer M.; Gayan-Ramirez G. Atrophy and Hypertrophy Signalling in the Diaphragm of Patients with COPD. Eur. Respir. J. 2010, 35 (3), 549–556. 10.1183/09031936.00091108. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Cao L.; Wang Z.; Feng H.; Cai X.; Xu M.; Li M.; Yu N.; Yin Y.; Wang W.; Kang J. Salidroside Mitigates Skeletal Muscle Atrophy in Rats with Cigarette Smoke-Induced COPD by up-Regulating Myogenin and down-Regulating Myostatin Expression. Biosci. Rep. 2019, 39 (11), BSR20190440. 10.1042/BSR20190440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M. E.; Brunet A. FOXO Transcription Factors. Curr. Biol. 2007, 17 (4), R113. 10.1016/j.cub.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Stitt T. N.; Drujan D.; Clarke B. A.; Panaro F.; Timofeyva Y.; Kline W. O.; Gonzalez M.; Yancopoulos G. D.; Glass D. J. The IGF-1/PI3K/Akt Pathway Prevents Expression of Muscle Atrophy-Induced Ubiquitin Ligases by Inhibiting FOXO Transcription Factors. Mol. Cell 2004, 14 (3), 395–403. 10.1016/S1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- Sandri M.; Sandri C.; Gilbert A.; Skurk C.; Calabria E.; Picard A.; Walsh K.; Schiaffino S.; Lecker S. H.; Goldberg A. L. Foxo Transcription Factors Induce the Atrophy-Related Ubiquitin Ligase Atrogin-1 and Cause Skeletal Muscle Atrophy. Cell 2004, 117 (3), 399–412. 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet M.; Russell A. P.; Léger B.; Debigaré R.; Joanisse D. R.; Caron M. A.; LeBlanc P.; Maltais F. Muscle Atrophy and Hypertrophy Signaling in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007, 176 (3), 261–269. 10.1164/rccm.200605-704OC. [DOI] [PubMed] [Google Scholar]
- Hwang J.; Rajendrasozhan S.; Yao H.; Chung S.; Sundar I. K.; Huyck H. L.; Pryhuber G. S.; Kinnula V. L.; Rahman I. FoxO3 Deficiency Leads to Increased Susceptibility to Cigarette Smoke-Induced Inflammation, Airspace Enlargement, and Chronic Obstructive Pulmonary Disease. J. Immunol. 2011, 187 (2), 987. 10.4049/jimmunol.1001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Yin N.; Xuan L. L.; Yao C. S.; Meng A. M.; Hou Q. Vam3, a Derivative of Resveratrol, Attenuates Cigarette Smoke-Induced Autophagy. Acta Pharmacol. Sin. 2012, 33 (7), 888–896. 10.1038/aps.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C.; Spiegelman B. M. Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1 Coactivators, Energy Homeostasis, and Metabolism. Endocr. Rev. 2006, 27 (7), 728–735. 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- St-Pierre J.; Lin J.; Krauss S.; Tarr P. T.; Yang R.; Newgard C. B.; Spiegelman B. M. Bioenergetic Analysis of Peroxisome Proliferator-Activated Receptor γ Coactivators 1α and 1β (PGC-1α and PGC-1β) in Muscle Cells. J. Biol. Chem. 2003, 278 (29), 26597–26603. 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- St-Pierre J.; Drori S.; Uldry M.; Silvaggi J. M.; Rhee J.; Jäger S.; Handschin C.; Zheng K.; Lin J.; Yang W.; Simon D. K.; Bachoo R.; Spiegelman B. M. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell 2006, 127 (2), 397–408. 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Valle I.; Álvarez-Barrientos A.; Arza E.; Lamas S.; Monsalve M. PGC-1alpha Regulates the Mitochondrial Antioxidant Defense System in Vascular Endothelial Cells. Cardiovasc. Res. 2005, 66 (3), 562–573. 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Schrader M.; Yoon Y. Mitochondria and Peroxisomes: Are the ‘Big Brother’ and the ‘Little Sister’ Closer than Assumed?. BioEssays 2007, 29 (11), 1105–1114. 10.1002/bies.20659. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Puigserver P.; Andersson U.; Zhang C.; Adelmant G.; Mootha V.; Troy A.; Cinti S.; Lowell B.; Scarpulla R. C.; Spiegelman B. M. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell 1999, 98 (1), 115–124. 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Chepelev N. L.; Enikanolaiye M. I.; Chepelev L. L.; Almohaisen A.; Chen Q.; Scoggan K. A.; Coughlan M. C.; Cao X. L.; Jin X.; Willmore W. G. Bisphenol A Activates the Nrf1/2-Antioxidant Response Element Pathway in HEK 293 Cells. Chem. Res. Toxicol. 2013, 26 (3), 498–506. 10.1021/tx400036v. [DOI] [PubMed] [Google Scholar]
- Austin S.; St-Pierre J. PGC1α and Mitochondrial Metabolism-Emerging Concepts and Relevance in Ageing and Neurodegenerative Disorders. J. Cell Sci. 2012, 125 (21), 4963–4971. 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- Sandri M.; Lin J.; Handschin C.; Yang W.; Arany Z. P.; Lecker S. H.; Goldberg A. L.; Spiegelman B. M. PGC-1alpha Protects Skeletal Muscle from Atrophy by Suppressing FoxO3 Action and Atrophy-Specific Gene Transcription. Proc. Natl. Acad. Sci. U. S. A. 2006, 103 (44), 16260–16265. 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault J. J.; Jespersen J. G.; Goldberg A. L. Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1alpha or 1beta Overexpression Inhibits Muscle Protein Degradation, Induction of Ubiquitin Ligases, and Disuse Atrophy. J. Biol. Chem. 2010, 285 (25), 19460–19471. 10.1074/jbc.M110.113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houten S. M.; Auwerx J. PGC-1alpha: Turbocharging Mitochondria. Cell 2004, 119 (1), 5–7. 10.1016/j.cell.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Giralt A.; Hondares E.; Villena J. A.; Ribas F.; Díaz-Delfín J.; Giralt M.; Iglesias R.; Villarroya F. Peroxisome Proliferator-Activated Receptor-γ Coactivator-1α Controls Transcription of the Sirt3 Gene, an Essential Component of the Thermogenic Brown Adipocyte Phenotype. J. Biol. Chem. 2011, 286 (19), 16958. 10.1074/jbc.M110.202390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering R. M.; O’Neill A.; Muriel J. M.; Prosser B. L.; Strong J.; Bloch R. J. Physiology, Structure, and Susceptibility to Injury of Skeletal Muscle in Mice Lacking Keratin 19-Based and Desmin-Based Intermediate Filaments. Am. J. Physiol. - Cell Physiol. 2011, 300 (4), C803. 10.1152/ajpcell.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzuca-Nassr G. N.; Vitzel K. F.; Mancilla-Solorza E.; Márquez J. L. Sarcomere Structure: The Importance of Desmin Protein in Muscle Atrophy. Int. J. Morphol. 2018, 36 (2), 576–583. 10.4067/S0717-95022018000200576. [DOI] [Google Scholar]
- Sheu S. S.; Nauduri D.; Anders M. W. Targeting Antioxidants to Mitochondria: A New Therapeutic Direction. Biochim. Biophys. Acta 2006, 1762 (2), 256–265. 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Paul C.; Manero F.; Gonin S.; Kretz-Remy C.; Virot S.; Arrigo A.-P. Hsp27 as a Negative Regulator of Cytochrome c Release. Mol. Cell. Biol. 2002, 22 (3), 816. 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruey J. M.; Ducasse C.; Bonniaud P.; Ravagnan L.; Susin S. A.; Diaz-Latoud C.; Gurbuxani S.; Arrigo A. P.; Kroemer G.; Solary E.; Garrido C. Hsp27 Negatively Regulates Cell Death by Interacting with Cytochrome C. Nat. Cell Biol. 2000, 2 (9), 645–652. 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Kim E. C.; Kim J. R. Senotherapeutics: Emerging Strategy for Healthy Aging and Age-Related Disease. BMB Rep. 2019, 52 (1), 47–55. 10.5483/BMBRep.2019.52.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. J.; Wijshake T.; Tchkonia T.; Lebrasseur N. K.; Childs B. G.; Van De Sluis B.; Kirkland J. L.; Van Deursen J. M. Clearance of P16Ink4a-Positive Senescent Cells Delays Ageing-Associated Disorders. Nature 2011, 479 (7372), 232–236. 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpitt S. V.; Rogers D. F.; Shah P.; De Matos C.; Russell R. E. K.; Donnelly L. E.; Barnes P. J. Impaired Inhibition by Dexamethasone of Cytokine Release by Alveolar Macrophages from Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2003, 167 (1), 24–31. 10.1164/rccm.200204-298OC. [DOI] [PubMed] [Google Scholar]
- Hogg J. C.; Chu F.; Utokaparch S.; Woods R.; Elliott W. M.; Buzatu L.; Cherniack R. M.; Rogers R. M.; Sciurba F. C.; Coxson H. O.; Paré P. D. The Nature of Small-Airway Obstruction in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2004, 350 (26), 2645–2653. 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. Chronic Obstructive Pulmonary Disease: Effects beyond the Lungs. PLoS Med. 2010, 7 (3), e1000220. 10.1371/journal.pmed.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. E.; Finney-Hayward T. K.; Quint J. K.; Thomas C. M. R.; Tudhope S. J.; Wedzicha J. A.; Barnes P. J.; Donnelly L. E. Defective Macrophage Phagocytosis of Bacteria in COPD. Eur. Respir. J. 2010, 35 (5), 1039–1047. 10.1183/09031936.00036709. [DOI] [PubMed] [Google Scholar]
- Nakamaru Y.; Vuppusetty C.; Wada H.; Milne J. C.; Ito M.; Rossios C.; Elliot M.; Hogg J.; Kharitonov S.; Goto H.; Bemis J. E.; Elliott P.; Barnes P. J.; Ito K. A Protein Deacetylase SIRT1 Is a Negative Regulator of Metalloproteinase-9. FASEB J. 2009, 23 (9), 2810–2819. 10.1096/fj.08-125468. [DOI] [PubMed] [Google Scholar]
- Donovan C.; Hansbro P. M. IL-33 in Chronic Respiratory Disease: From Preclinical to Clinical Studies. ACS Pharmacol. Transl. Sci. 2020, 3 (1), 56–62. 10.1021/acsptsci.9b00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham P. A.; Caramori G.; Casolari P.; Papi A. A.; Edwards M.; Shamji B.; Triantaphyllopoulos K.; Hussain F.; Pinart M.; Khan Y.; Heinemann L.; Stevens L.; Yeadon M.; Barnes P. J.; Chung K. F.; Adcock I. M. Oxidative Stress-Induced Antibodies to Carbonyl-Modified Protein Correlate with Severity of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2011, 184 (7), 796–802. 10.1164/rccm.201010-1605OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J.; McPherron A. C. Regulation of Myostatin Activity and Muscle Growth. Proc. Natl. Acad. Sci. U. S. A. 2001, 98 (16), 9306–9311. 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota J.; Handy C. R.; Haidet A. M.; Montgomery C. L.; Eagle A.; Rodino-Klapac L. R.; Tucker D.; Shilling C. J.; Therlfall W. R.; Walker C. M.; Weisbrode S. E.; Janssen P. M. L.; Clark K. R.; Sahenk Z.; Mendell J. R.; Kaspar B. K. Follistatin Gene Delivery Enhances Muscle Growth and Strength in Nonhuman Primates. Sci. Transl. Med. 2009, 1 (6), 6ra15. 10.1126/scitranslmed.3000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.; Wang J. L.; Lu J.; Song Y.; Kwak K. S.; Jiao Q.; Rosenfeld R.; Chen Q.; Boone T.; Simonet W. S.; Lacey D. L.; Goldberg A. L.; Han H. Q. Reversal of Cancer Cachexia and Muscle Wasting by ActRIIB Antagonism Leads to Prolonged Survival. Cell 2010, 142 (4), 531–543. 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Lach-Trifilieff E.; Minetti G. C.; Sheppard K.; Ibebunjo C.; Feige J. N.; Hartmann S.; Brachat S.; Rivet H.; Koelbing C.; Morvan F.; Hatakeyama S.; Glass D. J. An Antibody Blocking Activin Type II Receptors Induces Strong Skeletal Muscle Hypertrophy and Protects from Atrophy. Mol. Cell. Biol. 2014, 34 (4), 606–618. 10.1128/MCB.01307-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S.; Lee D.; Zhai B.; Gygi S. P.; Goldberg A. L. Trim32 Reduces PI3K-Akt-FoxO Signaling in Muscle Atrophy by Promoting Plakoglobin-PI3K Dissociation. J. Cell Biol. 2014, 204 (5), 747–758. 10.1083/jcb.201304167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M. Signaling in Muscle Atrophy and Hypertrophy. Physiology (Bethesda). 2008, 23 (3), 160–170. 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]