Abstract
Opioid use disorder (OUD) is a major current cause of morbidity and mortality. Long-term exposure to short-acting opioids (MOP-r agonists such as heroin or fentanyl) results in complex pathophysiological changes to neuroimmune and neuroinflammatory functions, affected in part by peripheral mechanisms (e.g., cytokines in blood), and by neuroendocrine systems such as the hypothalamic-pituitary-adrenal (HPA) stress axis. There are important findings from preclinical models, but their role in the trajectory and outcomes of OUD in humans is not well understood. The goal of this narrative review is to examine available data on immune and inflammatory functions in persons with OUD, and to identify major areas for future research. Peripheral blood biomarker studies revealed a pro-inflammatory state in persons with OUD in withdrawal or early abstinence, consistent with available postmortem brain studies (which show glial activation) and diffusion tensor imaging studies (indicating white matter disruptions), with gradual abstinence-associated recovery. The mechanistic roles of these neuroimmune and neuroinflammatory changes in the trajectory of OUD (including recovery and medication management) cannot be examined practically with postmortem data. Collection of longitudinal data in larger-scale human cohorts would allow examination of these mechanisms associated with OUD stage and progression. Given the heterogeneity in presentation of OUD, a precision medicine approach integrating multi-omic peripheral biomarkers and comprehensive phenotyping, including neuroimaging, can be beneficial in risk stratification, and individually optimized selection of interventions for individuals who will benefit, and assessments under refractory therapy.
Keywords: opioid, immune, inflammation, opioid use disorder, neuroimaging, biomarker
Introduction
Causing high rates of morbidity and mortality, opioid use disorders (OUD) continue to cause escalating public health concerns (Brown et al., 2022, Garcia et al., 2022, Kulkarni et al., 2019, Mattson et al., 2021, Wakeman et al., 2020). Acute mortality due to non-medical use of abused opioids (primarily agonists at μ-opioid receptors, MOP-r) is driven by respiratory depression mediated by brainstem mechanisms (Baldo and Rose, 2022). Preclinical studies and a relatively small number of studies in humans with OUD further implicate immune and inflammatory alterations after MOP-r agonist exposure, detected in the periphery and in the CNS. However, the roles of these immune and inflammatory mechanisms in the trajectory and morbidity of OUD in humans are not well understood. The goal of this review is to examine and conceptualize human data in this field, and to identify major areas for future research.
Organization of the Review:
Initial sections provide background and context for the trajectory and phenomenology of OUD and its interaction with immune and inflammatory functions (with effects in vitro and in vivo). We then consider core issues in translation of results between rodent models and human studies.
The literature supporting a role for the immune system in humans with OUD will be organized in five major sections with accompanying Tables:
Functional changes in peripheral blood leukocytes and in blood cytokines
mRNA and transcriptomics from peripheral blood leukocytes
Postmortem brain studies, including immune and inflammatory changes
Effects of pharmacological interventions on immune or inflammatory targets
Mechanisms that can interact with immune and inflammatory functions as part of the phenomenology of OUD.
We will conclude the review with a synthesis of the major future areas for human research.
Background and context
Trajectory and phenomenology of opioid use disorders in humans – potential effects on immune and inflammatory processes:
The trajectory of OUD includes initial exposure to mu-opioid receptor (MOP-r) agonists, followed by escalation and heavy use, and withdrawal that occur in cycles. Early exposure to MOP-r agonists can be iatrogenic (for example with prescription opioids such as oxycodone, for the treatment of pain), or happen in the context of illicit drug use. As the disease progresses, many persons with OUD use heroin or synthetic compounds such as fentanyl by the intravenous route (Comer and Cahill, 2019). The standard of care for OUD includes chronic treatment with MOP-r ligands, especially methadone (an orally available MOP-r agonist) or buprenorphine (a MOP-r partial agonist and KOP-r antagonist) (Kreek et al., 2019, Strang et al., 2020). Acute and chronic exposure to MOP-r agonists can have immune and inflammatory effects at different stages in the OUD trajectory.
Persons in the active stage of severe OUD usually self-administer short-acting compounds (e.g., heroin or fentanyl analogs) several times per day, whereas maintenance medications cause a stable level of activation (Kreek, et al., 2019, Strang et al., 2020). Opioid use and withdrawal cause robust hypothalamic-pituitary-adrenal (HPA)-axis activation, resulting in high levels of systemic glucocorticoids (Cami et al., 1992), which can potentially affect a large number of downstream immune and inflammatory processes (Cain and Cidlowski, 2017, McGregor et al., 2016). Therefore, through their chronic drug use in which persons with OUD can be exposed for years, there are long-term disruptions in their HPA-axis leading to downstream changes in immune and inflammatory processes. Different stages in OUD (e.g., active use, withdrawal, medication maintenance with methadone) are thought to be associated with different immune profiles. For example, persons who were actively using heroin exhibited decreased natural killer (NK) cell activity compared to healthy controls, but persons in stable methadone maintenance were indistinguishable from controls (Novick et al., 1989). Other studies in humans also showed that during the early post-withdrawal period (e.g., approximately 15 days), there were elevations in some cytokines (e.g., IL-6 and RANTES), that normalized over more extended post-withdrawal periods (e.g., 1 year) (Re et al., 2022). These studies therefore show that immune profiles change dynamically over the course of active use and recovery and recovery with medication maintenance.
Adding to the direct acute or chronic immune perturbations of abused MOP-r agonists is the broader phenomenology of OUD in humans. Unlike standard designs in laboratory rodents, humans with OUD are exposed to infections [e.g., human immunodeficiency virus (HIV) and hepatitis C virus (HCV)] as a result of risky behaviors including syringe sharing and unsafe sex. In addition, the often years-long course of OUD can include poly-drug and alcohol exposure (Butelman et al., 2021, Hassan and Le Foll, 2019), disruptions in sleep patterns (Mukherjee et al., 2021), poor nutrition , and increases in socioeconomic stressors (Chavez and Rigg, 2020, Forati et al., 2021). Stress exposure, sex differences, psychiatric and medical comorbidities, and sleep disruptions can each have immune and inflammatory consequences, based on in vitro and in vivo studies, as well as in rodent studies (Aubry et al., 2022, Costi et al., 2021, Deonaraine et al., 2020, Langstengel and Yaggi, 2022, Rodas et al., 2021, Wang et al., 2022). Therefore, these interacting mechanisms, as well as sex differences, can potentially contribute to individual differences in immune and inflammatory – mediated functions in humans with OUD, as also shown in some rodent models (Hofford et al., 2019, Lucerne et al., 2021, Parekh et al., 2023).
Effects of MOP-r agonists on immune or inflammatory functions in vitro:
MOP agonists act through their canonical target, MOP-r, to directly affect immune and inflammatory processes in leukocytes (either from humans or from rodent models) (Eisenstein, 2019, Machelska and Celik, 2020, McCarthy et al., 2001, Novick, et al., 1989, Wybran et al., 1979). Some studies suggest that exogenous opioids may also produce immunomodulatory effects by acting through toll-like receptor 4 (TLR4), enriched on myeloid cells (i.e., monocytes and neutrophils), but the impact of this alternative (non MOP-r) mechanism has not been examined extensively in persons with OUD (Grace et al., 2015).
In vitro studies often involve the extraction of a subset of leukocytes (peripheral blood mononuclear cells, PBMCs), followed by stimulation with exogenous compounds such as lipopolysaccharide (LPS, which act primarily as ligands of TLR4) or phytohemagglutinin (PHA). Early studies reported that beta-endorphin (one of the major endogenous MOP-r neuropeptide) affected responsiveness of human PBMC cultures in vitro, including an increase in natural killer cell activity (Kay et al., 1984, Mandler et al., 1986, Sharp et al., 1987). Later studies also showed effects of MOP-r agonists on release of several cytokines from PBMC cultures, including transforming growth factor-β, and TNF-α (Chao et al., 1992, Chao et al., 1993). Other in vitro studies also show that MOP-r receptors and specific cytokine receptors (e.g., CCR5) can form functional heterodimers in the same cells (Huang et al., 2021, Kong et al., 2022, Szabo et al., 2002). This demonstrates complex interactions between MOP-r and cytokine receptors, their cognate ligands and immune cell activity.
Long-term MOP-r agonist-induced changes to immune or inflammatory functions can be mediated by epigenetic changes (e.g., DNA methylation or histone modifications), resulting in changes in mRNA transcription and protein levels. For example, chronic exposure to MOP-r agonists in T-cells in vitro can cause long-term changes in IL-2 expression, mediated in part by histone modifications (Wang et al., 2007). This can be a bidirectional process, as exposure to a cytokine (IL-4) can upregulate expression of mRNA for OPRM1, the gene encoding MOP-r, also in part through histone modifications (Kraus et al., 2010).
Overall, the immune effects of MOP-r studies in vitro are highly dependent on the types of cells studied, and also on multiple methodological conditions, including incubation and challenge parameters (Peterson et al., 1998). This variability underscores the importance of in vivo studies (either in preclinical species or humans) in examining pathophysiological mechanisms that underlie OUD (see text below).
Effects of MOP-r agonists on immune or inflammatory functions in vivo:
Early studies showed that injection of MOP-r agonists to rodents can cause diverse immunosuppressive effects, including decreasing the release of the cytokine interferon following a chemical challenge (Bryant et al., 1987, Geber et al., 1977, Shavit et al., 1986). MOP-r receptors have been detected in human T lymphocytes (Wybran, et al., 1979), supporting potential mediation of these effects by opioid receptors on peripheral immune cells. Importantly, immune effects of MOP-r agonists in vivo can be mediated indirectly through the HPA axis (through corticosteroid release with its own immunosuppressant effects), as shown in rodents (Bryant et al., 1991). This mechanism can be especially relevant during advanced stages of OUD, when cycles of dependence and withdrawal can cause increased and dysregulated adrenal secretion of corticosteroids (see also below). Among the earlier studies, fresh blood collected from persons in methadone maintenance therapy showed decreased production of reactive oxygen species after a challenge with a chemical activator, phorbol ester (Peterson et al., 1989). Other studies in persons in the active stage of OUD or in withdrawal showed other immunosuppressive effects, such as decreases in peripheral blood T-lymphocyte proliferation after stimulation with PHA (Govitrapong et al., 1998).
persons with OUD had immune cell distributions different than controls’, especially showing increases in regulatory T cells (Tregs) with a “fragile” profile (i.e., they have decreased immunosuppressive function) (Zhu et al., 2023). This study also reported that opioid withdrawal was positively correlated with increased migration of regulatory T cells into the brains of opioid-exposed mice (Zhu, et al., 2023).
The immune effects of MOP-r agonists in vivo can be mediated by several interacting mechanisms, including through different types of leukocytes, and also by direct effects on glia, based on studies from human samples, or in rodent models (Cuitavi et al., 2023, Machelska and Celik, 2020). Furthermore, acute opioid withdrawal causes hyperactivation of the HPA-axis (Kreek and Koob, 1998, Ueno et al., 2011). Peripheral immune and inflammatory disruptions can result in increased migration of specific types of leukocytes (especially monocytes and macrophages) into the CNS, in part due to changes in functional properties of the blood-brain barrier, as detected in rodent models (Wohleb et al., 2014). Also, when these macrophages penetrate into the brain parenchyma, they can embody properties of residing microglia, as shown in mice (Wohleb et al., 2013). Such migration of immune cells can further exacerbate immune and inflammatory changes in the CNS.
Aside from direct action on immune cells, MOP-r activation can also affect the functional status of different glial cells, as examined in rodent models (Avey et al., 2018). Some glia including astrocytes have important structural and functional interactions with neurons, therefore MOP-r agonists have been shown to affect glial-neuronal signaling in rodent brain (Kruyer et al., 2022, Kruyer et al., 2020). Microglia, the resident immune cells in the brain, can express MOP-r, based on mouse studies (Maduna et al., 2018). Therefore, in vivo exposure to MOP-r agonists could result in CNS inflammation by acting directly on microglia (Maduna, et al., 2018, Reiss et al., 2020). Also, changes in the expression of several genes in oligodendrocytes (the glia responsible for myelination in the brain) were detected in the nucleus accumbens (NAc) after exposure to MOP-r agonists in rats (Reiner et al., 2022). It is unclear if this effect is caused by MOP-r expressed in oligodendrocytes, or indirectly by changes in cytokines, neuronal or other glial function. Nevertheless, at a morphological level, chronic exposure to the MOP-r agonist oxycodone in rats resulted in several white matter changes, including deformation of axonal tracts (Fan et al., 2018).
Of relevance to potential effects in oligodendrocytes and myelinaton, recent diffusion tensor imaging studies indicate that persons with OUD have white matter impairments, both whole brain and in specific tracts (Gaudreault et al., 2022, King et al., 2022, Zhu et al., 2022), and that recovery and abstinence are associated with normalization and potential remyelination (Wang et al., 2011, Zhu, et al., 2022).
Translational considerations:
Among the difficulties in translational studies of OUD are the greater variability in duration, amounts and patterns of opioid exposure in humans versus designs typically used in preclinical species (primarily rodents) (Ahmed et al., 2000, Butelman, et al., 2021, Zernig et al., 2007). For example, exposure drug patterns in humans with severe OUD can extend for several years and can involve extensive poly-drug exposure (e.g., to cocaine and alcohol) (Butelman et al., 2019, Butelman, et al., 2021). Such patterns, and their trajectory throughout the lifespan, are inherently difficult to model in preclinical species. Several regulatory functions for the effects of OUD may also differ between rodents and humans. For example, promoters of several genes (important for the regulation of transcription of mRNA), can differ functionally in rodents and humans (Dermitzakis and Clark, 2002, Hagai et al., 2018, Rockman et al., 2005). Other regulatory elements, including long noncoding RNA and gene enhancers (which affect gene transcription), can also differ between rodents and humans (Fullard et al., 2019, Gao and Qian, 2019, Sarropoulos et al., 2019). Other relevant mechanisms that can differ between humans and commonly used nocturnal species, such as rats or mice, include the sleep cycle and entrained circadian hypothalamic-pituitary-adrenal hormonal systems (e.g., ACTH and corticosteroids), which affect immune status (Baldo et al., 2013, McKillop and Vyazovskiy, 2020, Yasenkov and Deboer, 2012). Also, potential differences have been reported in microglial function in humans versus rodents (Smith and Dragunow, 2014).
Literature findings in persons with OUD:
Section 1: Peripheral mechanisms in blood, including leukocytes and cytokines
Table 1 summarizes major studies that have examined cytokines and peripheral cellular biomarkers in persons with OUD. Early studies examining immune cell responsivity in persons with active stage OUD showed primarily a decreased response, consistent with peripheral immunosuppression (Brown et al., 1974, Eisenstein, 2019, Govitrapong, et al., 1998, Novick, et al., 1989). More recently, several studies showed that the pro-inflammatory cytokines TNF-alpha (Shi and Pamer, 2011), TGF-alpha and IL-8 were increased in the blood of persons with OUD (Table 1), especially during early abstinence (e.g., within 1 week) (Piepenbrink et al., 2016, Purohit et al., 2022, Salarian et al., 2018). Some, but not all, studies found that such proinflammatory changes were decreased with more prolonged abstinence, and also during stable long-term MAT (Novick, et al., 1989, Re, et al., 2022, Wang et al., 2018, Zhang et al., 2021).
Section 1 (Table 1):
Peripheral mechanisms in blood, including leukocytes and cytokines
| In-vivo Sample (Dx, N, age, sex) Stage in OUD |
Target | Direction of effect, compared to healthy controls | Other patient characteristics and notes | Citation |
|---|---|---|---|---|
| Males with OUD (n=48), mean age=32 Within 24 h of last opioid exposure |
Serum cytokine panel | ↑TNF-α, ↑IL-10 ↓IL-6 |
(Purohit, et al., 2022) | |
| Heroin users (n=81), mean age=35, males Abstinence periods ranged: 7 days – 12 mo |
Plasma cytokine panel (27 targets) | ↑IL-17A, ↑IL-6 ↑IL-10, ↑IL-1ra, ↑IL-4, ↑RANTES High at early abstinence, then decrease over time ↑IL-7, ↑IL-17A and other cytokines High at early abstinence and remained high |
(Re, et al., 2022) | |
| Males with OUD in acute (7–14 days; n=10) or protracted (1 year; n=21) abstinence, mean age=37 | Plasma cytokine panel | ↑IL-4, ↑G-CSF, ↑IL-7, ↑IL-10, ↑IL-17A, IL-2 In acute abstinence |
Some poly-drug use (esp. methamphetamine) Several of the cytokine changes normalized by 1 year abstinence |
(Zhang, et al., 2021) |
| Heroin IVDU (n=19), mean age=28, 68%male Injected heroin within 3 days of study |
Plasma and cellular panel | ↑TNF-α, ↑TGF-alpha, ↑IL-8 | Poly-drug use reported (e.g., cocaine). In syringe exchange program |
(Piepenbrink, et al., 2016) |
| Males with heroin addiction (n=90), age range 18–45, in buprenorphine treatment. |
Neutrophil and platelet: lymphocyte ratios | Ratios increased, indicating greater systemic inflammation | Ratios correlated with duration of addiction | (Cicek et al., 2018) |
| Males with OUD (n=20), mean age 36 In methadone maintenance. |
TNF-α levels before and in early methadone maintenance | ↑TNF-α at early abstinence, decreases over 2 weeks of methadone. | All OUD patients were also tobacco smokers | (Salarian, et al., 2018) |
| OUD patients (n=47), mean age 37, 87%males In methadone maintenance. | Plasma cytokine panel and cognitive tests over 12 weeks of MMT | Negative correlation between TNF-α levels and visual index, and IL-6 and verbal memory index | Inflammatory markers and verbal memory tended to recover during MMT. | (Wang, et al., 2018) |
| Persons with OUD (n=33), mean age= 40, 90% male Blood samples obtained at the onset of methadone maintenance, and up to 12 weeks. |
Plasma cytokine panel | ↑TNF-α, ↑TIL-8 | TNF-α levels remained elevated for at least 12 weeks of MMT. | (Chen et al., 2012) |
| Males with OUD (n=34), mean age=40. Samples obtained after at least 1 month of methadone maintenance. |
Serum cytokine panel | ↑IL-1β, ↑IL-6, ↑IL-8 |
(Chan et al., 2015) | |
| Males with OUD (n=20), age range: 21–40 | immune stimulation of blood samples. | ↑IFN-γ, ↑IL-10 Production after stimulation | Phytohemagglutinin or lipopolysaccharide stimulation | (Azarang et al., 2007) |
| Males with OUD (n=19) and in heroin withdrawal (n=17). Mean ages 22–26. | immune stimulation of blood samples. | ↓T-cell activation after stimulation in OUD. Decreases in CD4/CD8 ratios observed in early withdrawal. |
Phytohemagglutinin stimulation | (Govitrapong, et al., 1998) |
| Males with OUD (n=11 heroin users, n=11 in methadone maintenance. Mean ages ranged 29–41 | measurement of natural killer (NK) cell activity in vitro | ↓NK activity in active heroin users | Recovery of NK activity in MMT | (Novick, et al., 1989) |
| Persons with OUD (n=38), age at least 21. Sex unspecified. Recruited during admission to methadone maintenance. |
immune stimulation of blood samples in vitro. | ↓T-cell activation after stimulation. | (Brown, et al., 1974) |
Dx: diagnosis; MMT: methadone maintenance therapy
Comparison to preclinical studies:
A larger number of studies in rodents examined peripheral responses in leukocytes from sites other than blood (including spleen, thymus and peritoneum) (Pacifici et al., 1993, Peng et al., 2000), and detected an immunosuppressive effect caused by opioid withdrawal (Franchi et al., 2017, Rahim et al., 2004). Since leukocytes from these other sites are not commonly studied in humans with OUD due to ethical and practical reasons, it is unclear if all aspects of immune and inflammatory function are shared by humans with OUD and rodent models. Interestingly, in rodent models, chronic MOP-r agonist exposure increases blood to brain passage of some peripheral cytokines (e.g., IL-2) but not others (TNF-alpha) (Lynch and Banks, 2008). This result points to potentially highly specific patterns of blood-brain-barrier disruption following chronic MOP-r agonist exposure, but it is unknown whether these patterns are shared between humans and rodents.
Section 2: mRNA and transcriptomic changes in peripheral blood leukocytes:
Studies using transcriptomics (e.g., RNAseq) in peripheral leukocytes (Table 2) identified a complex array of genes in immune and cytokine pathways that were upregulated or downregulated in patients with OUD compared to controls (Dai et al., 2022). One study also examined men with OUD at acute (7–14 days) versus prolonged abstinence (approximately 1 year) (Zhang, et al., 2021) and found expression changes in mRNA of several cytokine and chemokine genes. This study also found changes in pathways including leukocyte trans-endothelial migration (possibly indicating blood-brain barrier dysfunction) and chemokine signaling (Zhang, et al., 2021). The recent development of droplet-based single cell RNA sequencing (scRNA-seq) approaches allows for single cell resolution profiling of gene expression in blood. A study (Karagiannis et al., 2020) explored the immune modulatory effect of opioids on PBMCs from 7 opioid-dependent individuals and 7 age/sex matched non-dependent controls. The study demonstrated that chronic opioid exposure either from opioid dependent individuals or after 24h in vitro morphine exposure caused widespread suppression of antiviral genes upon ex vivo endotoxin challenge, and also in interferon-stimulated genes. Mechanisms for this effect could include two major interferon signaling inhibitors (Karagiannis, et al., 2020) Overall, this study indicated that opioid exposure suppresses the antiviral program, predisposing the body’s defense response to potential infection.
Section 2 (Table 2):
mRNA and transcriptomic changes in peripheral blood leukocytes
| In-vivo Sample (Dx, N, age, sex) Stage in OUD |
Target(s) | Direction of effects, compared to healthy controls | Other patient characteristics and notes | Citation |
|---|---|---|---|---|
| Males with OUD (n=7), age 40–50 Stage in OUD not clearly delineated. |
Total RNA from PBC, analyzed with RNAseq | Differentially expressed genes (DEG, including IL-22) in immune systems, and also several miRNA | (Dai, et al., 2022) | |
| Males with OUD in early (7–14 days; n=10) or protracted (1 year; n=21) abstinence, mean age=37 | RNAseq from PBC and also exosomes | Several immune-related pathways were affected in persons with OUD | (Zhang, et al., 2021) | |
| Age/sex matched with/out OUD (n=14), mean age 24–45 | Single cell RNA seq from PBMCs, exposed to LPS and control PBMCs exposed to morphine | Suppression of antiviral genes and interferon-stimulated genes (ISGs) in opioid-dependent PBMCs and of antiviral response under endotoxin stimulation except for CD8+ T cells and monocytes. | (Karagiannis, et al., 2020) |
DEG: differentially expressed genes; LPS: lipopolysaccharide endotoxin; PBMC: peripheral blood mononuclear cells
Comparison to preclinical studies:
In rats examined after a 5-day morphine exposure protocol, mRNA microarrays in PBMC revealed an increase in expression of some immune genes (e.g., IL-1R) and a decrease in others (e.g., CCL2, also known as monocyte chemoattractant protein 1, MCP-1), during early withdrawal (Desjardins et al., 2008). Citing similar limitations in bulk sequencing from peripheral blood from humans, cell-type specific analyses are important to account for the heterogeneity of gene expression patterns, and remain to be performed in animal models. Altogether these results from both humans with OUD and animal models of OUD highlight the robust changes in gene expression in PBMCs following MOP-r agonist exposure. They also suggest a consequential effect in altering susceptibility to infection in humans. Future investigation is necessary in both humans and animal models to determine the extent and significance of cell specificity in transcriptional dysregulation in OUD.
Section 3: Postmortem brain studies in humans with OUD
Post-mortem studies are of great value because they allow for diverse molecular, tissue and neuroanatomical analyses of CNS samples. Table 3 summarizes brain post-mortem studies in persons with OUD that have focused on neuroimmune and inflammatory mechanisms. One study using semi-quantitative immunohistochemical methods (i.e., using antibodies directed at specific proteins) detected increased levels of TNF-alpha and IL-6 immunoreactivity in neurons and glia in OUD decedents (Neri et al., 2013). This result is consistent with an inflammatory state, as observed in peripheral biomarker studies mentioned above.
Section 3 (Table 3):
Postmortem brain studies in humans with OUD
| In-vivo Sample (Dx, N, age, sex) | Target | Effects, compared to controls | Notes | Citation |
|---|---|---|---|---|
| Post-mortem brain of persons with OUD (n=20), mean age 47, 50% male | RNAseq of DLPFC and NAc | Several neuroinflammatory modules affected, including TNF-α and cytokine signaling. | (Seney, et al., 2021) | |
| Post-mortem males with opioid abuse (n=30), mean age=52. | RNAseq of Ventral midbrain | Several immune-related modules (e.g., cytokine response) affected. | (Saad, et al., 2019) | |
| Post-mortem males with OUD from microarray database (n=22), age range 15–35. | Expression microarray of NAc | Several immune-related gene modules affected in OUD, including IL4 as a hub gene. | (Zhang et al., 2020) | |
| Post mortem brain from persons who died of heroin-induced causes (n=45), 93% male. | Measured expression of cytokine panel in slices of several brain areas. | ↑expression in several cytokines, especially: TNF-α, IL6 and IL15, CD68 microglia marker. |
Immunohistochemistry based on antibody labeling of cytokines. | (Neri, et al., 2013) |
| Post-mortem from opioid overdose, (n=45 cases, n=50 controls), 88% male | Single nucleus RNA seq on ventral midbrain | Widespread glial activation associated with expression of genes involved in inflammation, immune response, and downregulated synaptic function | (Wei, et al., 2023) | |
| iPSCs stimulated forebrain organoids with individuals with OUD, (n=3), 100% male | Single nucleus RNA seq of organoids exposed to oxycodone or buprenorphine | Buprenorphine displayed significant influence on transcription regulation in glial cells; oxycodone induced type I interferon signaling via STAT1 in most cell types | (Ho, et al., 2022) |
DLPFC: dorsolateral prefrontal cortex; NAc: Nucleus accumbens; iPSCs: induced pluripotent stem cells
Other recent studies using microarray and RNAseq technologies have examined a larger set of targets that may be affected in the post-mortem brains of persons with OUD (Saad et al., 2019, Seney et al., 2021). These studies detected activation of several immune pathways including those related to pro-inflammatory responses and TNF-α signaling, in areas including the prefrontal cortex and nucleus accumbens, involved primarily in executive functions and reward processing, respectively. Of note, some of these studies used bulk brain tissue and then predicted differential cell expression in neurons and glia using cell deconvolution analyses (Saad, et al., 2019, Seney, et al., 2021).
One study that used snRNAseq to examine gene expression from the ventral midbrain tissue samples from individuals who had died of opioid overdoses found evidence of widespread glial cell activation, including microglia and astrocytes (Wei et al., 2023). This glial activation was associated with changes in the expression of genes involved in inflammation, immune response, and synaptic function. In another study, scRNAseq was used to investigate the effects of oxycodone and buprenorphine exposure on brain organoids generated from induced pluripotent stem cells (iPSCs) from individuals with OUD (Ho et al., 2022). The results showed that oxycodone and buprenorphine elicited distinct transcriptional effects, in which oxycodone treatment evoked changes in gene expression involved in inflammation, oxidative stress, and mitochondrial function, whereas buprenorphine led to changes in synaptic function and neuroplasticity.
Comparison to preclinical studies and conclusions:
Mouse and rat models provide a controlled source of brain tissues for examination of immune and inflammatory changes after opioid exposure, including examination after specific times of exposure. Prior reviews of preclinical literature have noted differences in designs and methodology in many rodent studies, and concluded that complex pro- and anti-inflammatory changes may occur in the brain of opioid-exposed subjects (Hofford, et al., 2019). At a cell and protein level, repeated exposure to mu-opioid agonists in rodents can result in neuroinflammatory features, including microgliosis and astrogliosis and an increase in cytokine levels, including TNF-α (Zamani et al., 2023). Also, , mice who underwent 14 days of oxycodone self-administration showed changes in mRNA expression of numerous inflammatory and immune genes in bulk tissue of dorsal or ventral striatum, including CCR5, which is thought to be a co-receptor for MOP-r (Zhang et al., 2017). Another study in rats found that 17 days of i.v. fentanyl-self-administration resulted in changes in several immune and inflammatory genes in the hippocampus, NAc and to some extent in the prefrontal cortex (Ezeomah et al., 2020). Single nucleus RNAseq was was also used to investigate cell-type specific gene expression changes in the NAc of rats who self-administered morphine (Reiner, et al., 2022). Many of the differentially expressed genes are involved in synaptic transmission, ion transport and G-protein coupled receptor signaling and transcription factor activation, suggesting that morphine-induced changes in gene expression may be regulated by transcriptional mechanisms (Reiner, et al., 2022).
Overall, these results from human post-mortem studies and animal models highlight neuro-immune cell specificity in transcriptional responses following chronic MOP-r agonist exposure. However, future investigation is required to determine the extent and significance of neuroimmune cell specificity, interaction between glial immune cells and neurons, and possible brain and spinal cord regional differences in OUD.
Section 4: Effects of pharmacological interventions on immune or inflammatory targets:
Thus far, only a small number of studies have directly examined the effects of pharmacological interventions on neuroimmune and neuroinflammatory targets in persons with OUD (summary in Table 4). The antibiotic minocycline, known to decrease microglial activation, caused a decrease in oxycodone-induced subjective effects in non-dependent users (Mogali et al., 2021), but not in opioid withdrawal signs or craving in persons on medication maintenance therapy (Arout et al., 2019). Ibudilast, an anti-inflammatory agent that decreases microglial cell activation and may act through TLR4 receptors (Grace et al., 2014), caused a decrease in some subjective effects of oxycodone in opioid-detoxified volunteers, and in opioid withdrawal ratings, in opioid-dependent volunteers (Cooper et al., 2016, Metz et al., 2017). The effects of commonly used non-steroidal anti-inflammatory agents on the peripheral immune and cytokine markers in persons with OUD has not been investigated extensively (Li et al., 2021). Furthermore, the effects of major clinically available agents which modulate specific cytokine systems have not been examined extensively in persons with OUD.
Section 4 (Table 4):
Effects of pharmacological interventions on immune or inflammatory targets
| In-vivo Sample Dx, N, age, sex |
Intervention and target | Effect | Citation |
|---|---|---|---|
| Recreational opioid users (n=12), mean age=36, 83% males. non-dependent |
Minocycline (immune modulator; 100 or 200 mg PO) | No effect on respiratory effects of oxycodone. Decreased some positive subjective effects of oxycodone. | (Mogali, et al., 2021) |
| Persons with OUD (n=20), ages 23–56, 70–80% males Dependent in MMT or buprenorphine treatment |
Minocycline (200 mg PO, 15 days) | No effect on withdrawal severity or opioid craving. Improvement in cognitive performance (Go / No go task) | (Arout, et al., 2019) |
| Males with OUD (n=11), mean age=45 Post-detoxification, non-dependent |
Ibudilast (50 mg PO, BID) | Ibudilast decreased liking and wanting of oxycodone, and decreased break-point for one dose of oxycodone (15 mg po). | (Metz, et al., 2017) |
| Morphine-maintained persons with OUD (n=10 group) dependent |
Ibudilast (glial modulator; 20 or 40 mg PO, BID) | No consistent effect of ibudilast on oxycodone-induced subjective profile | (Cooper et al., 2017) |
| Morphine-maintained persons with OUD (n=31), mean age range 38–40, 80–90% male. |
Ibudilast (20 or 40 mg PO, BID) | Overall SOWS and COWS withdrawal scores did not differ. Selected subjective rating of withdrawal decreased. | (Cooper, et al., 2016) |
| Non-dependent opioid users (n=17; 15 men); mean age=35 | Pioglitazone (glial modulator PPAR-gamma agonist; 15 or 45 mg PO, daily for 14–16 days) | No effect on measures of oxycodone abuse liability. | (Jones et al., 2018) |
SOWS: Subjective Opioid Withdrawal Scale; COWS: Clinical Opioid Withdrawal Scale
Comparison to preclinical studies and conclusions:
There is a larger number of preclinical studies on the effects of immune and inflammatory modulators on the rewarding effects of MOP-r agonists (see reviews) (Hofford, et al., 2019, Wu and Li, 2020)], showing some inconsistencies with the human literature described above. For example, minocycline decreased opioid withdrawal signs in rodents (Liu et al., 2021). Also, pioglitazone decreased withdrawal and relapse-like operant responding in mice (de Guglielmo et al., 2017). An antagonist for CCR5 (a potential MOP-r co-receptor) caused a decrease in oxycodone-induced place preference and relapse-like operant responding in rats (Iriah et al., 2021), but clinical studies of this target are not currently available.
Section 5: Mechanisms that can interact with immune and inflammatory functions as part of the phenomenology of OUD
Table 5 summarizes studies on major interacting mechanisms that could also affect immune and inflammatory functions in OUD. Firstly, there are known sex differences in immune and inflammatory function that could be due to hormonal or other factors, including genomic and epigenetic factors (Chlamydas et al., 2022, Pujantell and Altfeld, 2022). Of note, many genes in immune and inflammatory pathways are on the human X-chromosome. Therefore, the regulatory responses of these genes to opioid exposure could be, in part, sexually dimorphic. We have recently shown that overdose mortality due to synthetic opioids and heroin is robustly higher in men versus women, even taking into account different levels of misuse (Butelman et al., 2023). It is unknown if neuro-glial or cytokine changes over the course of OUD are involved in this sex difference.
Section 5 (Table 5):
Mechanisms that can interact with immune and inflammatory functions as part of the phenomenology of OUD
| Mechanism | Molecular Target(s) | Effect | Source (central vs. peripheral) | Citation |
|---|---|---|---|---|
| Sex | ||||
| Diverse immune-related genes and micro-RNA are X-linked in humans | Genes Include: CD40L, CD99, CRFL2, CXCR3, GMCSFR, IL2RG, IL3RA, IL13RA2, IL9R, IL13RA1, IRAK1, TLR7, TLR8 |
Sex as a complex mediating variable in different neuroimmune aspects of OUD. | Potentially both, depending on mechanism and contextual variables. | (Libert, et al., 2010) |
| Neurosteroid effects on innate immune function in macrophages | Likely TLR4 | Allopreganolone (positive modulator of GABA-receptor) has sex-specific immunomodulatory effects after LPS in vitro exposure | In vitro study | (Balan et al., 2022) |
| Sex affects cytokine responses to LPS. | Likely TLR4 and other mechanisms (IL6, IL-10, TNF-α) | Female cells respond to LPS with higher IL-10 and lower TNF-α than males. | In vitro study | (Rodas, et al., 2021) |
| Differential transcriptional effects of X-liked in genes in leukocytes | TLR4 and other mechanisms | Leukocytes from males and females show sex-specific changes in transcription of 54 genes, after LPS. | In vitro LPS stimulation | (Stein et al., 2021) |
| i.v. LPS and hyperalgesia | TLR4 and other mechanisms | fMRI responses to inflammatory pain in rACC differ between sexes | Central responses | (Karshikoff et al., 2016, Karshikoff et al., 2015) |
| Poly-drug Use | ||||
| Smoking status affects cytokine responses to LPS. | Likely TLR4 and other mechanisms (IL6, IL-10, TNF-α) | Smoking status Predicts differential cytokine responsiveness to LPS stimulation | In vitro study | (Rodas, et al., 2021) |
| Microglial imaging in persons with AUD | Effects of chronic alcohol on microglial function. | Brain TSPO binding lower in persons with AUD, but only if they were stratified by genotype | Brain PET study with TSPO radiotracer (11C-PBR-28) | (Kalk et al., 2017, Kim, et al., 2018) |
| Stress exposure | ||||
| Acute social stress in males n=44 (age 21–65). | Acute psychological stress effects on HPA axis and neuroimmune function |
Acute social stress causes ↓TNF-α and ↓IL-6 after LPS in vitro stimulation | Potentially central and hypothalamic, based on social stress exposure | (Wirtz, et al., 2007) |
| PTSD and cytokine levels (n=28, 50% females, mean age 41–42). | Chronic PTSD affects peripheral cytokines | ↑levels of several pro-inflammatory cytokines in PTSD vs. controls: esp. TNF-α, IFN-γ, IL-6 and IL-1β | Unclear | (Hoge, et al., 2009) |
| Sleep and Circadian Rhythm | ||||
| Simulated night shift work effects on cytokines (n=10, 90% male, mean age 27). | 4 days of simulated night shift | Disruption in circadian cycle of cytokines in response to stimulation | Propose several central and peripheral mechanisms | (Cuesta, et al., 2016) |
| Chronic insomnia and cytokine function (n=11 young adults, 55% male). | 4-day sleep study | Disruption of cytokine release in persons with insomnia (↑IL-6 and TNF-α, as well as cortisol in the daytime, compared to controls). | Diverse mechanisms possible (including HPA axis disruption) | (Vgontzas, et al., 2002) |
AUD: Alcohol use disorder
HPA: hypothalamo-pituitary adrenal axis
LPS: Lipopolysaccharide (experimental TLR4 agonist stimulus)
rACC: rostral anterior cingulate cortex
TSPO: 18KDa-translocator protein; marker for activated microglia
Persons with OUD often have a history of using other substances (e.g., psychostimulants), alcohol and nicotine, each with its own potential effects on immune function (Ahearn et al., 2021, Alfonso-Loeches et al., 2010, Fox et al., 2012, Redwine et al., 2003, Rodas, et al., 2021). Common patterns of poly-drug use (e.g., opioids and cocaine) may also cause more severe neurotoxicity than opioid use alone (Cunha-Oliveira et al., 2010).
Sleep disturbances, common in persons with OUD (including insomnia during withdrawal)(Sharkey et al., 2011), also affect neuroinflammatory function (Cuesta et al., 2016, Furman et al., 2019, Redwine, et al., 2003, Vgontzas et al., 2002). Stress exposure and post-traumatic stress disorder, common in persons with OUD, can similarly have prominent effects on peripheral neuroimmune biomarkers (Hoge et al., 2009, Pfau et al., 2019, von Känel et al., 2007, Wirtz et al., 2007). Other co-morbid conditions that can interact with immune status in persons with OUD include nutritional deficiencies (Chavez and Rigg, 2020).
Comparison to preclinical studies and conclusions:
Numerous preclinical studies (primarily in mice or rats) also show sex differences in neuroimmune and inflammatory functions and mechanisms (Libert et al., 2010, Pujantell and Altfeld, 2022). However, mice and humans do not have identical sets of inflammatory and immune genes on the X chromosome (Libert, et al., 2010), potentially limiting cross-species examinations. Sleep disruption also results in changes in immune parameters and mRNA expression in rodents (Archer and Oster, 2015, Brager et al., 2013). Neuroimmune effects of poly-drug exposure (e.g., cocaine and other drugs) have also been detected in rodents (Hofford, et al., 2019, Wu and Li, 2020).
Overall conclusions and future research directions in persons with OUD
Most studies of acute abstinence or withdrawal in persons with OUD detect a peripheral pro-inflammatory state, and this is consistent with postmortem brain studies. Furthermore, neuroimaging studies in persons with OUD also show white matter changes in major tracts, which may recover with abstinence. As can be seen from Tables 1 and 2, the majority of subjects with OUD in these studies were men. Therefore comparative studies in men and women remain critically necessary, especially due to potential sex differences in inflammatory and immune regulation (Libert, et al., 2010).
The direct pharmacological manipulation of neuroimmune or neuroinflammatory function has only been examined with a very limited number of targets in persons with OUD (Table 4). Several other pharmacological targets could be selected (e.g., CCR5 and TNF-α-alpha) (Chen et al., 2007, Iriah, et al., 2021, Lee et al., 2021). Such interventional studies could more directly examine the causality of these processes at specific stages of OUD trajectory and recovery. Some immune/inflammatory pharmacological agents that modulated MOP-r agonist-induced effects in rodent models did not do so in humans (de Guglielmo, et al., 2017), suggesting further need for proof-of-mechanism studies in humans. Furthermore, pharmacological intervention studies can benefit from parallel neuroimaging readouts (especially with PET, fMRI and diffusion tensor imaging) to examine the functional status of these molecular targets, signal processing, and brain white matter at specific stages of OUD and recovery (Gaudreault, et al., 2022, Kim et al., 2018, Raval et al., 2022). Such combined pharmacological-neuroimaging studies could eventually guide novel mechanism-based prognostic or therapeutic approaches for OUD.
Major co-morbid conditions including poly-drug exposure, sleep disturbances and stress exposure (and post-traumatic stress disorders) can each have immune and inflammatory sequelae (Daskalakis et al., 2016, Marchese et al., 2022). Therefore, as differences in their status could underlie apparent discrepancies between studies (and inter-individual differences), these major mechanisms should be studied as covariates in persons with OUD. Such co-morbid and interacting factors are difficult to interrogate in preclinical models of OUD.
Peripheral blood biomarker studies will continue to be important in the study of persons with OUD. These studies could take a multi-level approach, including transcriptomics (e.g., RNA seq) and epigenetics of immune and inflammatory genes, neuroendocrine HPA status, as well as cytokine quantification. Longitudinal studies at specific stages in OUD trajectory may also provide critical advantages for the control of individual differences in diverse interacting mechanisms (e.g., stress exposure, co-infections, and sleep disruptions), and to examine the potential process of recovery.
Future studies should evaluate the functional status of the blood-brain-barrier at specific stages of OUD trajectory and recovery, with parallel biomarker and neuroimaging studies. These studies could interrogate the potential causality of blood-brain-barrier disruption on OUD, including the impact of leukocyte infiltration or altered cytokine permeability into the CNS.
Future studies could examine whether variability in OUD trajectory is associated with polymorphisms in immune or inflammatory genes, or genes regulating corticosteroid function (Levran et al., 2014). There is also growing evidence of shared genetic liabilities between OUD with other clinical conditions (Seney, et al., 2021). In addition to infectious diseases, chronic pain, other substance use disorders, and other neuropsychiatric illnesses also have shared genetic liabilities with OUD (Sanchez-Roige et al., 2021). Interestingly, polygenic risk score analyses for prescription opioid misuse have revealed widespread associations with cardiometabolic conditions that do have an inflammatory component (e.g., ischemic heart disease, stroke) (Sanchez-Roige, et al., 2021).
Bulk RNA transcriptomics employs a “whole genome” based approach to study immune mechanisms driving differential gene expression in OUD. This unbiased approach integrates genomics, computational biology, and systems biology to identify overlap in differentially expressed genes and transcription factors from peripheral blood and in post-mortem brains with OUD. However, a limitation of bulk RNA transcriptomic studies is the assumption that transcripts from the tissue are homogeneous (Munsky et al., 2012, Raj and van Oudenaarden, 2008, Stegle et al., 2015).
Overall, there is a major need for “omic” studies of peripheral blood biomarkers, in parallel with neuroimaging studies (including white matter investigations), to characterize neuroinflammatory and neuroimmune mechanisms systematically at different stages over the course of OUD and recovery. This will contribute toward a precision medicine approach to risk stratification for prevention and mechanistically-based therapeutic efforts, focusing on these potentially important molecular targets that have not been exploited to date in the treatment of opioid use disorders.
Figure 1:
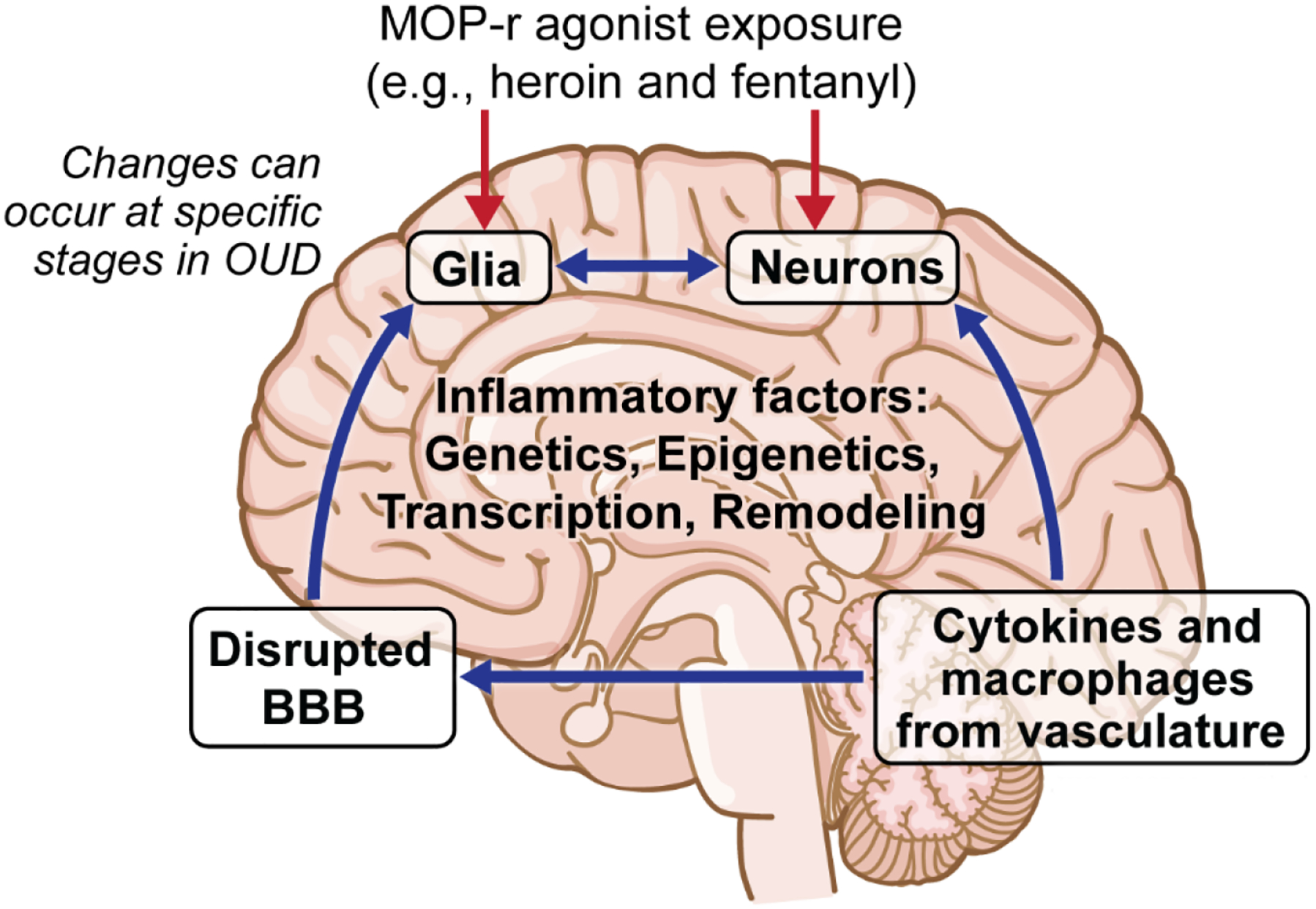
Schematic of major postulated immune interactions with CNS in persons with opioid use disorders (OUD). The four main interactive compartments affected in OUD can be summarized as 1) Neuronal populations in different parts of the brain, 2) Central glia that can functionally interact with neurons, 3) Changes in blood-brain barrier structure and function resulting in pathological permeability to peripheral macrophages cells and cytokines, and 4) Changes in peripheral immune cells and their release of soluble mediators, including cytokines.
Figure 2:
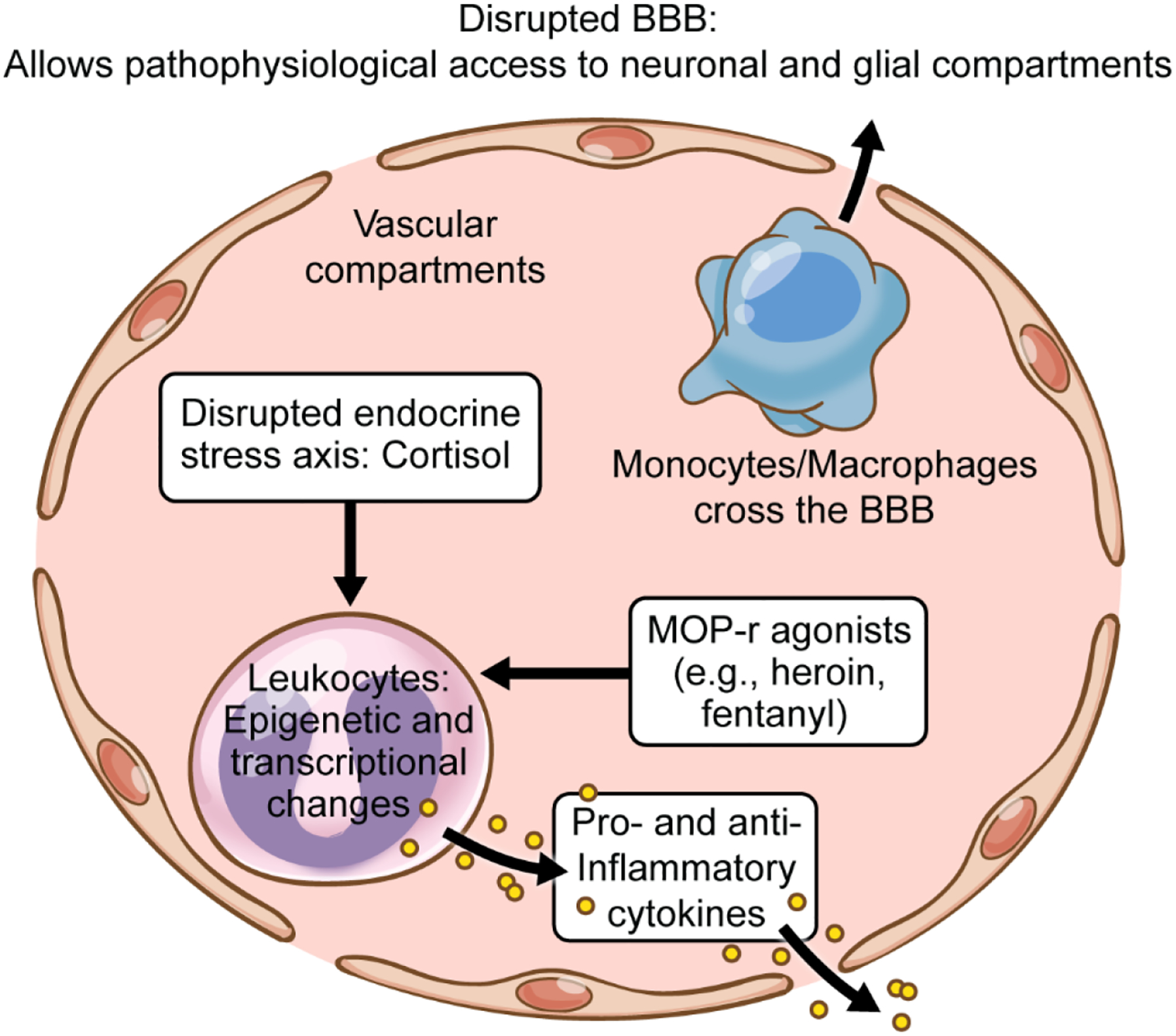
Schematic of main vascular compartments, blood-brain barrier (BBB), and their potential changes in OUD. These changes can be caused directly by actions of self-administered mu-opioid receptor (MOP-r) agonists on immune cells and by neuroendocrine changes in the stress axis (e.g., affecting cortisol levels and its diverse downstream targets).
Highlights:
Opioid use disorder (OUD) is a major current cause of morbidity and mortality
Neuroimmune and neuroinflammatory mechanisms are changed in OUD
These mechanisms can include peripheral components (cytokines and leukocytes).
Glia, neurons and blood-brain barrier can also be affected by inflammation in OUD.
Longitudinal multiomic biomarker / neuroimaging studies are necessary in OUD.
Immune and inflammatory processes involved in OUD.
Cytokines:
Diverse group of signaling proteins, which can be released by leukocytes into blood, or by glia in the CNS. Cytokines act on specific receptors and can have pleiotropic immune effects. Cytokines include several major families, including interleukins, chemokines, growth factors, interferons, and tumor-necrosis factor (TNF)-related proteins. See summary below.
Interleukins (IL):
A large group of signaling proteins that have pleiotropic effects on immune functions. Specific interleukins can have either pro- or anti-inflammatory effects.
Chemokines:
A large group of proteins involved primarily in the guidance of cell migration (chemotaxis).
Growth Factors:
Growth factors modulate the proliferation of diverse immune cell types (including cells that release other cytokines), as part of the immune response.
Interferons (IFN):
A group of proteins that are involved in intra-cellular and extracellular responses to infection or inflammation.
Tumor Necrosis Factor (TNF):
TNF-related proteins that are released at the site of inflammation of infection, can enhance several adaptive immune responses, and also result in tissue necrosis.
Glia:
Non-neuronal cells in the CNS (4 main types: astrocytes, microglia, oligo-progenitor cells and oligodendrocytes). Glia have complex and dynamic interactions with neurons.
Astrocytes:
glial cells with diverse roles in the brain, including regulation of neuronal maturation and function.
Microglia:
resident immune cells of the brain, also involved in synaptic maintenance and pruning.
Oligodendrocytes:
glial cells which regulate central neuronal myelination, thus affecting signal transmission.
Oligodendrocyte progenitor cells:
precursor cells to oligodendrocytes.
Leukocytes (white blood cells):
immune cells with differing functions. These can include the release of antibodies and also soluble signaling molecules, the cytokines. Some leukocytes can migrate into the CNS during inflammatory conditions.
Peripheral blood mononuclear cells (PBMC):
A subset of leukocytes found in blood, bone marrow and spleen that have a single nucleus. PBMCs include monocytes and macrophages, T cells, B cells and natural killer (NK) cells.
Acknowledgements:
This work was supported by NIDA U01DA053625 (ERB), NIDA 1RO1DA048301-01A1 (RZG) and NIDA 1RO1DA049547 (NAK), R01MH104559, R01DA047880, R01AG067025 (PR), R01MH127820 (SJR) and CTSA KL2TR004421-CN (CN).
The authors are grateful to Jill K. Gregory MFA, CMI, for the expert illustrations.
Abbreviations:
- BBB
blood-brain barrier
- CNS
Central nervous system
- DTI
diffusion tensor imaging, measuring brain white matter anatomical connectivity
- Dx
Diagnosis
- HPA
hypothalamic – pituitary - adrenal axis, ultimately controlling the release of the main glucocorticoid cortisol in humans. Cortisol can affect the transcription of numerous immune and inflammatory genes
- IVDU
intravenous drug user
- KOP-r
kappa-opioid receptor
- LPS
lipopolysaccharide; bacterial surface molecule often used as an experimental immune stimulator for blood leukocytes, acts through toll-like TLR4 receptors
- MAT
medication-assisted therapy
- MMT
methadone maintenance therapy
- MOP-r
mu-opioid receptor
- NK
natural killer cells
- OPRM1
gene encoding MOP-r, the main target of abused opioids
- OUD
Opioid use disorder
- PBMC
Peripheral blood mononuclear cells
- PHA
Phytohemagglutinin; plant-derived molecule often used as an in vitro experimental immune stimulator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Interest: The authors have not interests to disclose.
Statement on use of generative AI: During the preparation of this work the authors did not use generative AI in the writing process.
References
- Ahearn OC, Watson MN, Rawls SM (2021), Chemokines, cytokines and substance use disorders. Drug Alcohol Depend 220:108511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF (2000), Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacol 22:413–421. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C (2010), Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci 30:8285–8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer SN, Oster H (2015), How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. J Sleep Res 24:476–493. [DOI] [PubMed] [Google Scholar]
- Arout CA, Waters AJ, MacLean RR, Compton P, Sofuoglu M (2019), Minocycline does not affect experimental pain or addiction-related outcomes in opioid maintained patients. Psychopharmacology 236:2857–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry AV, Joseph Burnett C, Goodwin NL, Li L, Navarrete J, Zhang Y, Tsai V, Durand-de Cuttoli R, et al. (2022), Sex differences in appetitive and reactive aggression. Neuropsychopharmacol 47:1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avey D, Sankararaman S, Yim AKY, Barve R, Milbrandt J, Mitra RD (2018), Single-Cell RNA-Seq Uncovers a Robust Transcriptional Response to Morphine by Glia. Cell reports 24:3619–3629.e3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarang A, Mahmoodi M, Rajabalian S, Shekari MA, Nosratabadi J, Rezaei N (2007), T-helper 1 and 2 serum cytokine assay in chronic opioid addicts. Eur Cytokine Netw 18:210–214. [DOI] [PubMed] [Google Scholar]
- Balan I, Aurelian L, Williams KS, Campbell B, Meeker RB, Morrow AL (2022), Inhibition of human macrophage activation via pregnane neurosteroid interactions with toll-like receptors: Sex differences and structural requirements. Frontiers in immunology 13:940095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Hanlon EC, Obermeyer W, Bremer Q, Paletz E, Benca RM (2013), Upregulation of Gene Expression in Reward-Modulatory Striatal Opioid Systems by Sleep Loss. Neuropsychopharmacol 38:2578–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Rose MA (2022), Mechanisms of opioid-induced respiratory depression. Archives of Toxicology. [DOI] [PubMed] [Google Scholar]
- Brager AJ, Ehlen JC, Castanon-Cervantes O, Natarajan D, Delisser P, Davidson AJ, Paul KN (2013), Sleep loss and the inflammatory response in mice under chronic environmental circadian disruption. PloS one 8:e63752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KG, Chen CY, Dong D, Lake KJ, Butelman ER (2022), Has the United States Reached a Plateau in Overdoses Caused by Synthetic Opioids After the Onset of the COVID-19 Pandemic? Examination of Centers for Disease Control and Prevention Data to November 2021. Frontiers in psychiatry https://www.frontiersin.org/articles/10.3389/fpsyt.2022.947603/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Stimmel B, Taub RN, Kochwa S, Rosenfield RE (1974), Immunologic Dysfunction in Heroin Addicts. Archives of Internal Medicine 134:1001–1006. [PubMed] [Google Scholar]
- Bryant HU, Bernton EW, Holaday JW (1987), Immunosuppressive effects of chronic morphine treatment in mice. Life sciences 41:1731–1738. [DOI] [PubMed] [Google Scholar]
- Bryant HU, Bernton EW, Kenner JR, Holaday JW (1991), Role of adrenal cortical activation in the immunosuppressive effects of chronic morphine treatment. Endocrinology 128:3253–3258. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Chen CY, Brown KG, Kreek MJ (2019), Escalation of drug use in persons dually diagnosed with opioid and cocaine dependence: Gender comparison and dimensional predictors. Drug Alcohol Depend 205:107657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Chen CY, Brown KG, Lake KJ, Kreek MJ (2021), Age of onset of heaviest use of cannabis or alcohol in persons with severe opioid or cocaine use disorders. Drug Alcohol Depend 226:108834. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Huang Y, Epstein DH, Shaham Y, Goldstein RZ, Volkow ND, Alia-Klein N (2023), Overdose mortality rates for opioids and stimulant drugs are substantially higher in men than in women: statelevel analysis. Neuropsychopharmacol 10.1038/s41386-023-01601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DW, Cidlowski JA (2017), Immune regulation by glucocorticoids. Nature Reviews Immunology 17:233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cami J, Gilabert M, San L, de la Torre R (1992), Hypercortisolism after opioid discontinuation in rapid detoxification of heroin addicts. British journal of addiction 87:1145–1151. [DOI] [PubMed] [Google Scholar]
- Chan YY, Yang SN, Lin JC, Chang JL, Lin JG, Lo WY (2015), Inflammatory response in heroin addicts undergoing methadone maintenance treatment. Psychiatry research 226:230–234. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Molitor TW, Zhou Y, Murtaugh MP, Tsang M, Peterson PK (1992), Morphine potentiates transforming growth factor-beta release from human peripheral blood mononuclear cell cultures. The Journal of pharmacology and experimental therapeutics 262:19–24. [PubMed] [Google Scholar]
- Chao CC, Molitor TW, Close K, Hu S, Peterson PK (1993), Morphine inhibits the release of tumor necrosis factor in human peripheral blood mononuclear cell cultures. International journal of immunopharmacology 15:447–453. [DOI] [PubMed] [Google Scholar]
- Chavez MN, Rigg KK (2020), Nutritional implications of opioid use disorder: A guide for drug treatment providers. Psychology of Addictive Behaviors 34:699–707. [DOI] [PubMed] [Google Scholar]
- Chen S-L, Lee S-Y, Tao P-L, Chang Y-H, Chen S-H, Chu C-H, Chen PS, Lee IH, et al. (2012), Dextromethorphan Attenuated Inflammation and Combined Opioid Use in Humans Undergoing Methadone Maintenance Treatment. Journal of Neuroimmune Pharmacology 7:1025–1033. [DOI] [PubMed] [Google Scholar]
- Chen X, Geller EB, Rogers TJ, Adler MW (2007), Rapid heterologous desensitization of antinociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend 88:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlamydas S, Markouli M, Strepkos D, Piperi C (2022), Epigenetic mechanisms regulate sex-specific bias in disease manifestations. J Mol Med (Berl) 100:1111–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicek E, Demirel B, Kirac AS, Eren I (2018), Increased neutrophil-lymphocyte and platelet-lymphocyte ratios in male heroin addicts: a prospective controlled study. Clin Psychopharmacol Neurosci 16:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Cahill CM (2019), Fentanyl: Receptor pharmacology, abuse potential, and implications for treatment. Neuroscience and biobehavioral reviews 106:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Johnson KW, Pavlicova M, Glass A, Vosburg SK, Sullivan MA, Manubay JM, Martinez DM, et al. (2016), The effects of ibudilast, a glial activation inhibitor, on opioid withdrawal symptoms in opioid-dependent volunteers. Addict Biol 21:895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Johnson KW, Vosburg SK, Sullivan MA, Manubay J, Martinez D, Jones JD, Saccone PA, et al. (2017), Effects of ibudilast on oxycodone-induced analgesia and subjective effects in opioid-dependent volunteers. Drug Alc Depend 178:340–347. [DOI] [PubMed] [Google Scholar]
- Costi S, Morris LS, Collins A, Fernandez NF, Patel M, Xie H, Kim-Schulze S, Stern ER, et al. (2021), Peripheral immune cell reactivity and neural response to reward in patients with depression and anhedonia. Translational psychiatry 11:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta M, Boudreau P, Dubeau-Laramée G, Cermakian N, Boivin DB (2016), Simulated Night Shift Disrupts Circadian Rhythms of Immune Functions in Humans. Journal of immunology (Baltimore, Md : 1950) 196:2466–2475. [DOI] [PubMed] [Google Scholar]
- Cuitavi J, Andrés-Herrera P, Meseguer D, Campos-Jurado Y, Lorente JD, Caruana H, Hipólito L (2023), Focal mu-opioid receptor activation promotes neuroinflammation and microglial activation in the mesocorticolimbic system: Alterations induced by inflammatory pain. Glia. [DOI] [PubMed] [Google Scholar]
- Cunha-Oliveira T, Rego AC, Garrido J, Borges F, Macedo T, Oliveira CR (2010), Neurotoxicity of heroin-cocaine combinations in rat cortical neurons. Toxicology 276:11–17. [DOI] [PubMed] [Google Scholar]
- Dai Q, Pu SS, Yang X, Li C, He Y, Liu X, Wang G (2022), Whole Transcriptome Sequencing of Peripheral Blood Shows That Immunity/GnRH/PI3K-Akt Pathways Are Associated With Opioid Use Disorder. Frontiers in psychiatry 13:893303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis NP, Cohen H, Nievergelt CM, Baker DG, Buxbaum JD, Russo SJ, Yehuda R (2016), New translational perspectives for blood-based biomarkers of PTSD: From glucocorticoid to immune mediators of stress susceptibility. Experimental neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guglielmo G, Kallupi M, Scuppa G, Demopulos G, Gaitanaris G, Ciccocioppo R (2017), Pioglitazone attenuates the opioid withdrawal and vulnerability to relapse to heroin seeking in rodents. Psychopharmacology 234:223–234. [DOI] [PubMed] [Google Scholar]
- Deonaraine KK, Wang Q, Cheng H, Chan KL, Lin HY, Liu K, Parise LF, Cathomas F, et al. (2020), Sex-specific peripheral and central responses to stress-induced depression and treatment in a mouse model. Journal of neuroscience research 98:2541–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermitzakis ET, Clark AG (2002), Evolution of Transcription Factor Binding Sites in Mammalian Gene Regulatory Regions: Conservation and Turnover. Molecular biology and evolution 19:1114–1121. [DOI] [PubMed] [Google Scholar]
- Desjardins S, Belkai E, Crete D, Cordonnier L, Scherrmann JM, Noble F, Marie-Claire C (2008), Effects of chronic morphine and morphine withdrawal on gene expression in rat peripheral blood mononuclear cells. Neuropharmacology 55:1347–1354. [DOI] [PubMed] [Google Scholar]
- Eisenstein TK (2019), The Role of Opioid Receptors in Immune System Function. Frontiers in immunology 10:2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeomah C, Cunningham KA, Stutz SJ, Fox RG, Bukreyeva N, Dineley KT, Paessler S, Cisneros IE (2020), Fentanyl self-administration impacts brain immune responses in male Sprague-Dawley rats. Brain, behavior, and immunity 87:725–738. [DOI] [PubMed] [Google Scholar]
- Fan R, Schrott LM, Arnold T, Snelling S, Rao M, Graham D, Cornelius A, Korneeva NL (2018), Chronic oxycodone induces axonal degeneration in rat brain. BMC Neurosci 19:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forati AM, Ghose R, Mantsch JR (2021), Examining Opioid Overdose Deaths across Communities Defined by Racial Composition: a Multiscale Geographically Weighted Regression Approach. Journal of urban health : bulletin of the New York Academy of Medicine 98:551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, D’Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, Sinha R (2012), Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol 27:156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi S, Amodeo G, Gandolla M, Moschetti G, Panerai AE, Sacerdote P (2017), Effect of Tapentadol on Splenic Cytokine Production in Mice. Anesthesia and analgesia 124:986–995. [DOI] [PubMed] [Google Scholar]
- Fullard JF, Rahman S, Roussos P (2019), Genetic Variation in Long-Range Enhancers. Current topics in behavioral neurosciences 42:35–50. [DOI] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, et al. (2019), Chronic inflammation in the etiology of disease across the life span. Nature medicine 25:1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Qian J (2019), EnhancerAtlas 2.0: an updated resource with enhancer annotation in 586 tissue/cell types across nine species. Nucleic acids research 48:D58–D64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GP, Stringfellow EJ, DiGennaro C, Poellinger N, Wood J, Wakeman S, Jalali MS (2022), Opioid overdose decedent characteristics during COVID-19. Annals of medicine 54:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreault PO, King SG, Malaker P, Alia-Klein N, Goldstein RZ (2022), Whole-brain white matter abnormalities in human cocaine and heroin use disorders: association with craving, recency, and cumulative use. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geber WF, Lefkowitz SS, Hung CY (1977), Duration of interferon inhibition following single and multiple injections of morphine. J Toxicol Environ Health 2:577–582. [DOI] [PubMed] [Google Scholar]
- Govitrapong P, Suttitum T, Kotchabhakdi N, Uneklabh T (1998), Alterations of immune functions in heroin addicts and heroin withdrawal subjects. The Journal of pharmacology and experimental therapeutics 286:883–889. [PubMed] [Google Scholar]
- Grace PM, Maier SF, Watkins LR (2015), Opioid-induced central immune signaling: implications for opioid analgesia. Headache 55:475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Ramos KM, Rodgers KM, Wang X, Hutchinson MR, Lewis MT, Morgan KN, Kroll JL, et al. (2014), Activation of adult rat CNS endothelial cells by opioid-induced toll-like receptor 4 (TLR4) signaling induces proinflammatory, biochemical, morphological, and behavioral sequelae. Neuroscience 280:299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagai T, Chen X, Miragaia RJ, Rostom R, Gomes T, Kunowska N, Henriksson J, Park J-E, et al. (2018), Gene expression variability across cells and species shapes innate immunity. Nature 563:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AN, Le Foll B (2019), Polydrug use disorders in individuals with opioid use disorder. Drug Alc Depend. [DOI] [PubMed] [Google Scholar]
- Ho MF, Zhang C, Moon I, Zhu X, Coombes BJ, Biernacka J, Skime M, Oesterle TS, et al. (2022), Single cell transcriptomics reveals distinct transcriptional responses to oxycodone and buprenorphine by iPSC-derived brain organoids from patients with opioid use disorder. Molecular psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford RS, Russo SJ, Kiraly DD (2019), Neuroimmune mechanisms of psychostimulant and opioid use disorders. The European journal of neuroscience 50:2562–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM (2009), Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depression and anxiety 26:447–455. [DOI] [PubMed] [Google Scholar]
- Huang B, Wang H, Zheng Y, Li M, Kang G, Barreto-de-Souza V, Nassehi N, Knapp PE, et al. (2021), Structure-Based Design and Development of Chemical Probes Targeting Putative MOR-CCR5 Heterodimers to Inhibit Opioid Exacerbated HIV-1 Infectivity. Journal of medicinal chemistry 64:7702–7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriah SC, Borges C, Shalev U, Cai X, Madularu D, Kulkarni PP, Ferris CF (2021), The utility of maraviroc, an antiretroviral agent used to treat HIV, as treatment for opioid abuse? Data from MRI and behavioural testing in rats. Journal of psychiatry & neuroscience : JPN 46:E548–e558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Bisaga A, Metz VE, Manubay JM, Mogali S, Ciccocioppo R, Madera G, Doernberg M, et al. (2018), The PPARgamma Agonist Pioglitazone Fails to Alter the Abuse Potential of Heroin, But Does Reduce Heroin Craving and Anxiety. Journal of psychoactive drugs:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalk NJ, Guo Q, Owen D, Cherian R, Erritzoe D, Gilmour A, Ribeiro AS, McGonigle J, et al. (2017), Decreased hippocampal translocator protein (18 kDa) expression in alcohol dependence: a [(11)C]PBR28 PET study. Translational psychiatry 7:e996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis TT, Cleary JP Jr., Gok B, Henderson AJ, Martin NG, Yajima M, Nelson EC, Cheng CS (2020), Single cell transcriptomics reveals opioid usage evokes widespread suppression of antiviral gene program. Nature communications 11:2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karshikoff B, Jensen KB, Kosek E, Kalpouzos G, Soop A, Ingvar M, Olgart Höglund C, Lekander M, et al. (2016), Why sickness hurts: A central mechanism for pain induced by peripheral inflammation. Brain, behavior, and immunity 57:38–46. [DOI] [PubMed] [Google Scholar]
- Karshikoff B, Lekander M, Soop A, Lindstedt F, Ingvar M, Kosek E, Olgart Höglund C, Axelsson J (2015), Modality and sex differences in pain sensitivity during human endotoxemia. Brain, behavior, and immunity 46:35–43. [DOI] [PubMed] [Google Scholar]
- Kay N, Allen J, Morley JE (1984), Endorphins stimulate normal human peripheral blood lymphocyte natural killer activity. Life sciences 35:53–59. [DOI] [PubMed] [Google Scholar]
- Kim SW, Wiers CE, Tyler R, Shokri-Kojori E, Jang YJ, Zehra A, Freeman C, Ramirez V, et al. (2018), Influence of alcoholism and cholesterol on TSPO binding in brain: PET [(11)C]PBR28 studies in humans and rodents. Neuropsychopharmacol 43:1832–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SG, Gaudreault PO, Malaker P, Kim JW, Alia-Klein N, Xu J, Goldstein RZ (2022), Prefrontal-habenular microstructural impairments in human cocaine and heroin addiction. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Shata MTM, Brown JL, Lyons MS, Sherman KE, Blackard JT (2022), The synthetic opioid fentanyl increases HIV replication and chemokine co-receptor expression in vitro. J Neurovirol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J, Lehmann L, Börner C, Höllt V (2010), Epigenetic mechanisms involved in the induction of the mu opioid receptor gene in Jurkat T cells in response to interleukin-4. Molecular Immunology 48:257–263. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF (1998), Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend 51:23–47. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Reed B, Butelman ER (2019), Current status of opioid addiction treatment and related preclinical research. Science advances 5:eaax9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruyer A, Angelis A, Garcia-Keller C, Li H, Kalivas PW (2022), Plasticity in astrocyte subpopulations regulates heroin relapse. Science advances 8:eabo7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruyer A, Chioma VC, Kalivas PW (2020), The Opioid-Addicted Tetrapartite Synapse. Biological psychiatry 87:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A, Anderson AG, Merullo DP, Konopka G (2019), Beyond bulk: a review of single cell transcriptomics methodologies and applications. Curr Opin Biotechnol 58:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langstengel J, Yaggi HK (2022), Sleep Deficiency and Opioid Use Disorder: Trajectory, Mechanisms, and Interventions. Clin Chest Med 43:e1–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Mansur RB, Brietzke E, Kapogiannis D, Delgado-Peraza F, Boutilier JJ, Chan TCY, Carmona NE, et al. (2021), Peripheral inflammatory biomarkers define biotypes of bipolar depression. Molecular psychiatry 26:3395–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Peles E, Randesi M, Li Y, Rotrosen J, Ott J, Adelson M, Kreek MJ (2014), Stress-related genes and heroin addiction: a role for a functional FKBP5 haplotype. Psychoneuroendocrinology 45:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chang Y, Song S, Losina E, Costenbader KH, Laidlaw TM (2021), Impact of reported NSAID “allergies” on opioid use disorder in back pain. Journal of Allergy and Clinical Immunology 147:1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert C, Dejager L, Pinheiro I (2010), The X chromosome in immune functions: when a chromosome makes the difference. Nature Reviews Immunology 10:594–604. [DOI] [PubMed] [Google Scholar]
- Liu Q, Min T, Dong J, Wang X (2021), Minocycline alleviates the symptoms of morphine withdrawal via the CaMKII-Ras-ERK signaling pathway. Neuroscience letters 752:135825. [DOI] [PubMed] [Google Scholar]
- Lucerne KE, Osman A, Meckel KR, Kiraly DD (2021), Contributions of neuroimmune and gut-brain signaling to vulnerability of developing substance use disorders. Neuropharmacology 192:108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JL, Banks WA (2008), Opiate modulation of IL-1α, IL-2, and TNF-α transport across the blood–brain barrier. Brain, behavior, and immunity 22:1096–1102. [DOI] [PubMed] [Google Scholar]
- Machelska H, Celik M (2020), Opioid Receptors in Immune and Glial Cells-Implications for Pain Control. Frontiers in immunology 11:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduna T, Audouard E, Dembélé D, Mouzaoui N, Reiss D, Massotte D, Gaveriaux-Ruff C (2018), Microglia Express Mu Opioid Receptor: Insights From Transcriptomics and Fluorescent Reporter Mice. Frontiers in psychiatry 9:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler RN, Biddison WE, Mandler R, Serrate SA (1986), beta-Endorphin augments the cytolytic activity and interferon production of natural killer cells. Journal of immunology (Baltimore, Md : 1950) 136:934–939. [PubMed] [Google Scholar]
- Marchese S, Cancelmo L, Diab O, Cahn L, Aaronson C, Daskalakis NP, Schaffer J, Horn SR, et al. (2022), Altered gene expression and PTSD symptom dimensions in World Trade Center responders. Molecular psychiatry 27:2225–2246. [DOI] [PubMed] [Google Scholar]
- Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL, Trends and Geographic Patterns in Drug and Synthetic Opioid Overdose Deaths — United States, 2013–2019, MMWR Morbidity and mortality weekly report, 2021, pp. 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ (2001), Opioids, opioid receptors, and the immune response. Drug Alcohol Depend 62:111–123. [DOI] [PubMed] [Google Scholar]
- McGregor BA, Murphy KM, Albano DL, Ceballos RM (2016), Stress, cortisol, and B lymphocytes: a novel approach to understanding academic stress and immune function. Stress (Amsterdam, Netherlands) 19:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillop LE, Vyazovskiy VV (2020), Sleep and ageing: from human studies to rodent models. Current Opinion in Physiology 15:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz VE, Jones JD, Manubay J, Sullivan M, Mogali S, Segoshi A, Madera G, Johnson KW, et al. (2017), Effects of Ibudilast on the Subjective, Reinforcing and Analgesic Effects of Oxycodone in Recently Detoxified Adults with Opioid Dependence. Neuropsychopharmacol 42:1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogali S, Askalsky P, Madera G, Jones JD, Comer SD (2021), Minocycline attenuates oxycodone-induced positive subjective responses in non-dependent, recreational opioid users. Pharmacology, biochemistry, and behavior 209:173241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee D, Stankoski DM, Tilden SE, Huhn AS, Bixler EO, Kong L, Meyer RE, Deneke E, et al. (2021), Reregulation of Cortisol Levels and Sleep in Patients with Prescription Opioid Use Disorder During Long-Term Residential Treatment. Drug Alc Depend:108931. [DOI] [PubMed] [Google Scholar]
- Munsky B, Neuert G, van Oudenaarden A (2012), Using gene expression noise to understand gene regulation. Science (New York, NY) 336:183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri M, Panata L, Bacci M, Fiore C, Riezzo I, Turillazzi E, Fineschi V (2013), Cytokines, chaperones and neuroinflammatory responses in heroin-related death: what can we learn from different patterns of cellular expression? International journal of molecular sciences 14:19831–19845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick DM, Ochshorn M, Ghali V, Croxson TS, Mercer WD, Chiorazzi N, Kreek MJ (1989), Natural killer cell activity and lymphocyte subsets in parenteral heroin abusers and long-term methadone maintenance patients. The Journal of pharmacology and experimental therapeutics 250:606–610. [PubMed] [Google Scholar]
- Pacifici R, Di Carlo S, Bacosi A, Zuccaro P (1993), Macrophage functions in drugs of abuse-treated mice. Int J Immunopharmacol 15:711–716. [DOI] [PubMed] [Google Scholar]
- Parekh SV, Adams LO, Barkell GA, Lysle DT (2023), Sex-differences in anxiety, neuroinflammatory markers, and enhanced fear learning following chronic heroin withdrawal. Psychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Mosser DM, Adler MW, Rogers TJ, Meissler JJ Jr, Eisenstein TK (2000), Morphine enhances interleukin-12 and the production of other pro-inflammatory cytokines in mouse peritoneal macrophages. Journal of Leukocyte Biology 68:723–728. [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Brummitt C, Pentel P, Bullock M, Simpson M, Hitt J, Sharp B (1989), Suppression of human peripheral blood mononuclear cell function by methadone and morphine. The Journal of infectious diseases 159:480–487. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Molitor TW, Chao CC (1998), The opioid–cytokine connection. Journal of neuroimmunology 83:63–69. [DOI] [PubMed] [Google Scholar]
- Pfau ML, Menard C, Cathomas F, Desland F, Kana V, Chan KL, Shimo Y, LeClair K, et al. (2019), Role of Monocyte-Derived MicroRNA106b~25 in Resilience to Social Stress. Biological psychiatry 86:474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepenbrink MS, Samuel M, Zheng B, Carter B, Fucile C, Bunce C, Kiebala M, Khan AA, et al. (2016), Humoral Dysregulation Associated with Increased Systemic Inflammation among Injection Heroin Users. PloS one 11:e0158641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujantell M, Altfeld M (2022), Consequences of sex differences in Type I IFN responses for the regulation of antiviral immunity. Frontiers in immunology 13:986840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit P, Roy D, Dwivedi S, Nebhinani N, Sharma P (2022), Association of miR-155, miR-187 and Inflammatory Cytokines IL-6, IL-10 and TNF-α in Chronic Opium Abusers. Inflammation 45:554–566. [DOI] [PubMed] [Google Scholar]
- Rahim RT, Feng P, Meissler JJ, Rogers TJ, Zhang L, Adler MW, Eisenstein TK (2004), Paradoxes of immunosuppression in mouse models of withdrawal. Journal of neuroimmunology 147:114–120. [DOI] [PubMed] [Google Scholar]
- Raj A, van Oudenaarden A (2008), Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval NR, Wetherill RR, Wiers CE, Dubroff JG, Hillmer AT (2022), Positron Emission Tomography of Neuroimmune Responses in Humans: Insights and Intricacies. Seminars in nuclear medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re GF, Jia J, Xu Y, Zhang Z, Xie ZR, Kong D, Lu D, Li Y, et al. (2022), Dynamics and correlations in multiplex immune profiling reveal persistent immune inflammation in male drug users after withdrawal. International immunopharmacology 107:108696. [DOI] [PubMed] [Google Scholar]
- Redwine L, Dang J, Hall M, Irwin M (2003), Disordered sleep, nocturnal cytokines, and immunity in alcoholics. Psychosom Med 65:75–85. [DOI] [PubMed] [Google Scholar]
- Reiner BC, Zhang Y, Stein LM, Perea ED, Arauco-Shapiro G, Ben Nathan J, Ragnini K, Hayes MR, et al. (2022), Single nucleus transcriptomic analysis of rat nucleus accumbens reveals cell type-specific patterns of gene expression associated with volitional morphine intake. Translational psychiatry 12:374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss D, Maduna T, Maurin H, Audouard E, Gaveriaux-Ruff C (2020), Mu opioid receptor in microglia contributes to morphine analgesic tolerance, hyperalgesia, and withdrawal in mice. Journal of neuroscience research. [DOI] [PubMed] [Google Scholar]
- Rockman MV, Hahn MW, Soranzo N, Zimprich F, Goldstein DB, Wray GA (2005), Ancient and recent positive selection transformed opioid cis-regulation in humans. PLoS biology 3:e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodas L, Martínez S, Riera-Sampol A, Moir HJ, Tauler P (2021), Blood Cell In Vitro Cytokine Production in Response to Lipopolysaccharide Stimulation in a Healthy Population: Effects of Age, Sex, and Smoking. Cells 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad MH, Rumschlag M, Guerra MH, Savonen CL, Jaster AM, Olson PD, Alazizi A, Luca F, et al. (2019), Differentially expressed gene networks, biomarkers, long noncoding RNAs, and shared responses with cocaine identified in the midbrains of human opioid abusers. Scientific reports 9:1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salarian A, Kadkhodaee M, Zahmatkesh M, Seifi B, Bakhshi E, Akhondzadeh S, Adeli S, Askari H, et al. (2018), Opioid Use Disorder Induces Oxidative Stress and Inflammation: The Attenuating Effect of Methadone Maintenance Treatment. Iran J Psychiatry 13:46–54. [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige S, Fontanillas P, Jennings MV, Bianchi SB, Huang Y, Hatoum AS, Sealock J, Davis LK, et al. (2021), Genome-wide association study of problematic opioid prescription use in 132,113 23andMe research participants of European ancestry. Molecular psychiatry:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarropoulos I, Marin R, Cardoso-Moreira M, Kaessmann H (2019), Developmental dynamics of lncRNAs across mammalian organs and species. Nature 571:510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Kim S-M, Glausier JR, Hildebrand MA, Xue X, Zong W, Wang J, Shelton MA, et al. (2021), Transcriptional Alterations in Dorsolateral Prefrontal Cortex and Nucleus Accumbens Implicate Neuroinflammation and Synaptic Remodeling in Opioid Use Disorder. Biological psychiatry 90:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey KM, Kurth ME, Anderson BJ, Corso RP, Millman RP, Stein MD (2011), Assessing sleep in opioid dependence: a comparison of subjective ratings, sleep diaries, and home polysomnography in methadone maintenance patients. Drug Alcohol Depend 113:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp BM, Tsukayama DT, Gekker G, Keane WF, Peterson PK (1987), Beta-endorphin stimulates human polymorphonuclear leukocyte superoxide production via a stereoselective opiate receptor. The Journal of pharmacology and experimental therapeutics 242:579–582. [PubMed] [Google Scholar]
- Shavit Y, Terman GW, Lewis JW, Zane CJ, Gale RP, Liebeskind JC (1986), Effects of footshock stress and morphine on natural killer lymphocytes in rats: studies of tolerance and cross-tolerance. Brain research 372:382–385. [DOI] [PubMed] [Google Scholar]
- Shi C, Pamer EG (2011), Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11:762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Dragunow M (2014), The human side of microglia. Trends in neurosciences 37:125–135. [DOI] [PubMed] [Google Scholar]
- Stegle O, Teichmann SA, Marioni JC (2015), Computational and analytical challenges in single-cell transcriptomics. Nat Rev Genet 16:133–145. [DOI] [PubMed] [Google Scholar]
- Stein MM, Conery M, Magnaye KM, Clay SM, Billstrand C, Nicolae R, Naughton K, Ober C, et al. (2021), Sex-specific differences in peripheral blood leukocyte transcriptional response to LPS are enriched for HLA region and X chromosome genes. Scientific reports 11:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, Marshall BDL, Tyndall M, et al. (2020), Opioid use disorder. Nature Reviews Disease Primers DOI: 10.1038/s41572-019-0137-5. [DOI] [PubMed] [Google Scholar]
- Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, Marshall BDL, Tyndall M, et al. (2020), Opioid use disorder. Nature Reviews Disease Primers https://www.nature.com/articles/s41572-019-0137-5. [DOI] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ (2002), Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci USA 99:10276–10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Maeda T, Kiguchi N, Kobayashi Y, Ozaki M, Kishioka S (2011), Availability of serum corticosterone level for quantitative evaluation of morphine withdrawal in mice. Drug discoveries & therapeutics 5:71–75. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin HM, Vela-Bueno A, Kales A, et al. (2002), Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism 51:887–892. [DOI] [PubMed] [Google Scholar]
- von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U (2007), Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. Journal of psychiatric research 41:744–752. [DOI] [PubMed] [Google Scholar]
- Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, Azocar F, Sanghavi DM (2020), Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA Netw Open 3:e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Barke RA, Roy S (2007), Transcriptional and epigenetic regulation of interleukin-2 gene in activated T cells by morphine. The Journal of biological chemistry 282:7164–7171. [DOI] [PubMed] [Google Scholar]
- Wang TY, Lee SY, Chang YH, Chen SL, Chen PS, Chu CH, Huang SY, Tzeng NS, et al. (2018), Correlation of cytokines, BDNF levels, and memory function in patients with opioid use disorder undergoing methadone maintenance treatment. Drug Alcohol Depend 191:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li W, Li Q, Yang W, Zhu J, Wang W (2011), White matter impairment in heroin addicts undergoing methadone maintenance treatment and prolonged abstinence: a preliminary DTI study. Neuroscience letters 494:49–53. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao M, Li P, Wu C, Lv Y, Jiang Y (2022), Gene–environment interaction between circadian clock gene polymorphisms and job stress on the risk of sleep disturbances. Psychopharmacology 239:3337–3344. [DOI] [PubMed] [Google Scholar]
- Wei J, Lambert TY, Valada A, Patel N, Walker K, Lenders J, Schmidt CJ, Iskhakova M, et al. (2023), Single Nucleus Transcriptomics Reveals Pervasive Glial Activation in Opioid Overdose Cases. bioRxiv:2023.2003.2007.531400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz PH, Känel Rv, Emini L, Suter T, Fontana A, Ehlert U (2007), Variations in anticipatory cognitive stress appraisal and differential proinflammatory cytokine expression in response to acute stress. Brain, behavior, and immunity 21:851–859. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF (2014), Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci 34:2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF (2013), Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 33:13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Li JX (2020), Toll-Like Receptor 4 Signaling and Drug Addiction. Frontiers in pharmacology 11:603445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J, Appelboom T, Famaey J-P, Govaerts A (1979), Suggestive evidence for receptors for morphine and methionine-enkephalin on normal human blood T lymphocytes. The Journal of Immunology 123:1068–1070. [PubMed] [Google Scholar]
- Yasenkov R, Deboer T (2012) Chapter 12 - Circadian modulation of sleep in rodents. In: Progress in brain research, vol. 199 (Kalsbeek A, Merrow M, Roenneberg T, Foster RG, eds), pp. 203–218. Elsevier. [DOI] [PubMed] [Google Scholar]
- Zamani N, Osgoei LT, Aliaghaei A, Zamani N, Hassanian-Moghaddam H (2023), Chronic exposure to methadone induces activated microglia and astrocyte and cell death in the cerebellum of adult male rats. Metab Brain Dis 38:323–338. [DOI] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, et al. (2007), Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology 80:65–119. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yu H, Bai R, Ma C (2020), Identification and Characterization of Biomarkers and Their Role in Opioid Addiction by Integrated Bioinformatics Analysis. Frontiers in neuroscience 14:608349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang Y, Levran O, Randesi M, Yuferov V, Zhao C, Kreek MJ (2017), Alterations of expression of inflammation/immune-related genes in the dorsal and ventral striatum of adult C57BL/6J mice following chronic oxycodone self-administration: a RNA sequencing study. Psychopharmacology 234:2259–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wu H, Peng Q, Xie Z, Chen F, Ma Y, Zhang Y, Zhou Y, et al. (2021), Integration of Molecular Inflammatory Interactome Analyses Reveals Dynamics of Circulating Cytokines and Extracellular Vesicle Long Non-Coding RNAs and mRNAs in Heroin Addicts During Acute and Protracted Withdrawal. Frontiers in immunology 12:730300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yan X, Lyu Z, Wang S, Chen J, Wang W, Li Q, Li W (2022), Characteristics of White Matter Integrity During Different Phases of Abstinence in Heroin Use Disorders: A Diffusion Tensor Imaging Study. Journal of addiction medicine 16:541–548. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Yan P, Wang R, Lai J, Tang H, Xiao X, Yu R, Bao X, et al. (2023), Opioid-induced fragile-like regulatory T cells contribute to withdrawal. Cell 186:591–606.e523. [DOI] [PubMed] [Google Scholar]


