Abstract
BACKGROUND
Alcohol use disorder has a detrimental impact on global health and new treatment targets are needed. Preclinical studies show attenuating effects of glucagon-like peptide-1 (GLP-1) agonists on addiction-related behaviors in rodents and nonhuman primates. Some trials have shown an effect of GLP-1 agonism on reward processes in humans; however, results from clinical studies remain inconclusive.
METHODS
This is a predefined secondary analysis of a double-blind, randomized, placebo-controlled trial evaluating the GLP-1 agonist dulaglutide as a therapy for smoking cessation. The main objective was to assess differences in alcohol consumption after 12 weeks of treatment with dulaglutide compared to placebo. The effect of dulaglutide on alcohol consumption was analyzed using a multivariable generalized linear model.
RESULTS
In the primary analysis, participants out of the cohort (n = 255) who reported drinking alcohol at baseline and who completed 12 weeks of treatment (n = 151; placebo n = 75, dulaglutide n = 76) were included. The median age was 42 (IQR 33–53) with 61% (n = 92) females. At week 12, participants receiving dulaglutide drank 29% less (relative effect = 0.71, 95% CI 0.52–0.97, P = 0.04) than participants receiving placebo. Changes in alcohol consumption were not correlated with smoking status at week 12.
CONCLUSION
These results provide evidence that dulaglutide reduces alcohol intake in humans and contribute to the growing body of literature promoting the use of GLP-1 agonists in treatment of substance use disorders.
TRIAL REGISTRATION
ClinicalTrials.gov NCT03204396.
FUNDING
Swiss National Foundation, Gottfried Julia Bangerter-Rhyner Foundation, Goldschmidt-Jacobson Foundation, Hemmi Foundation, University of Basel, University Hospital Basel, Swiss Academy of Medical Science.
Keywords: Endocrinology
Keywords: Addiction, Neuroendocrine regulation
Introduction
Alcohol use disorder (AUD) affects approximately 5% of people worldwide and is considered a key risk factor for noncommunicable disease, such as a variety of cancers, neuropsychiatric disorders, and liver cirrhosis (1). In 2020, the estimated prevalence of substance use disorders (SUDs), i.e., drug use, other than consumption of alcohol, which leads to dependence and/or requires treatment, was at 0.76% among the population aged 15–64, with an increasing trend between 2016 and 2019. However, when taking into account the past 15 years, SUD prevalence remained relatively stable (2).
There are currently 4 approved pharmacological treatments for AUD, and though these substances display favorable effects in some studies (3), they do not seem to work effectively for all individuals affected by AUD. Considering the complex nature of the disease and the heterogeneity of affected patients (4), it becomes evident that researchers should focus on the identification of new concepts and targets in treating AUD and SUD.
Glucagon-like peptide-1 (GLP-1) agonists have been used in the treatment of type 2 diabetes and obesity due to their incretin and anorexigenic effects. The fact that they reduce appetite has led researchers to investigate their effect on brain circuits that regulate the motivational properties of drugs of abuse. GLP-1–producing neurons in the CNS (5) project to sites of the mesolimbic reward system, which express GLP-1 receptors (6). In 2013, Egecioglu et al. found that the GLP-1 agonist Exendin-4 reduces alcohol intake and alcohol-seeking behavior in rodents and that it attenuated the effect of alcohol on the mesolimbic system (7). These results were largely reproduced using liraglutide (8), dulaglutide (9), and semaglutide (10). Similarly, GLP-1 agonists (exenatide and liraglutide) reduced alcohol consumption in nonhuman primates (11). Furthermore, GLP-1 agonism has been found to attenuate reward induced by other substances, e.g., psychostimulants (12–14) and nicotine (15, 16).
The sole randomized trial in patients with AUD found no group difference regarding reduction in heavy drinking days, but functional MRI (fMRI) cue reactivity and dopamine transporter availability were significantly decreased in the exenatide group compared with placebo (17). Another research group identified a GLP-1 receptor gene variant associated with higher occurrences of AUD and pathological drinking patterns (18). To date, clinical trials have not shown a reduction in cocaine use under GLP-1 agonists, but interestingly, acute cocaine administration was found to reduce GLP-1 serum levels (19, 20). Taken together, these studies suggest that GLP-1 does play an important role in reward-related processes in humans and should be further investigated.
We previously conducted a randomized placebo-controlled trial that investigated whether the GLP-1 agonist dulaglutide increased abstinence rates from nicotine during smoking cessation (21). Smoking abstinence rates at the end of the trial intervention did not differ significantly between the treatment groups. However, whether dulaglutide had an effect on alcohol consumption in this population was unknown. The current study is a predefined secondary analysis aimed to explore whether dulaglutide affects alcohol intake in patients treated for smoking cessation. We hypothesized that 12-week treatment with dulaglutide would lead to a reduction in weekly alcohol intake. This would contribute to the body of evidence promoting the use of GLP-1 agonists in treatment of addictive disorders.
Results
Baseline participant characteristics.
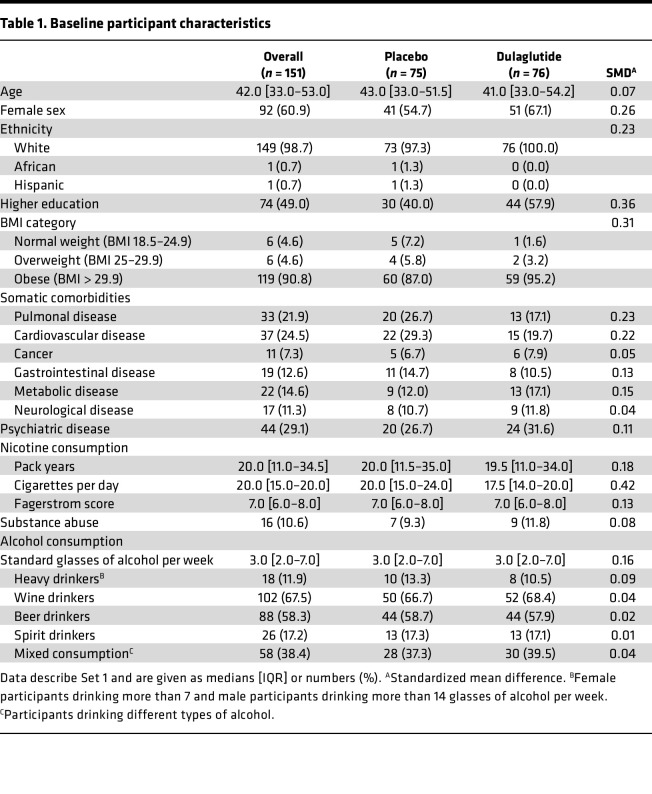
Of the full set of 255 participants, 151 (59.2%) consumed alcohol at baseline. Baseline characteristics of the full set and according to alcohol consumption are shown in Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.170419DS1 Of the participants consuming alcohol at baseline (Set 1), 75 were randomized to placebo and 76 to dulaglutide. This subset was composed of 60.9% females (n = 92), with a median age of 42 (IQR 33–53) years. Baseline patient characteristics were comparable between groups. Participants in both groups had a median of 20 (placebo: IQR 11.5–35.0; dulaglutide: IQR 11.0–34.0) pack years. Median cigarettes smoked per day in the placebo and dulaglutide groups were 20 (IQR 15–24) and 17.4 (IQR 14–20), respectively. The median Fagerstrom score was 7 (IQR 6–8) in both groups, indicating a high cigarette dependence (22, 23). In both treatment groups, the median alcohol consumption at baseline was 3 (IQR 2–7) standard glasses per week, 67.5% (n = 102) of the participants drank wine, 58.3% (n = 88) drank beer, and 17.2% (n = 26) consumed spirits. One-third (38.4%, n = 58) reported drinking more than one type of alcohol. Twelve percent (n = 18) were classified as heavy drinkers, i.e., females who drink more than 7 and males who drink more than 14 glasses of alcohol per week (24). Detailed information on baseline participant characteristics from Set 1 is shown in Table 1.
Table 1. Baseline participant characteristics.
Alcohol consumption.
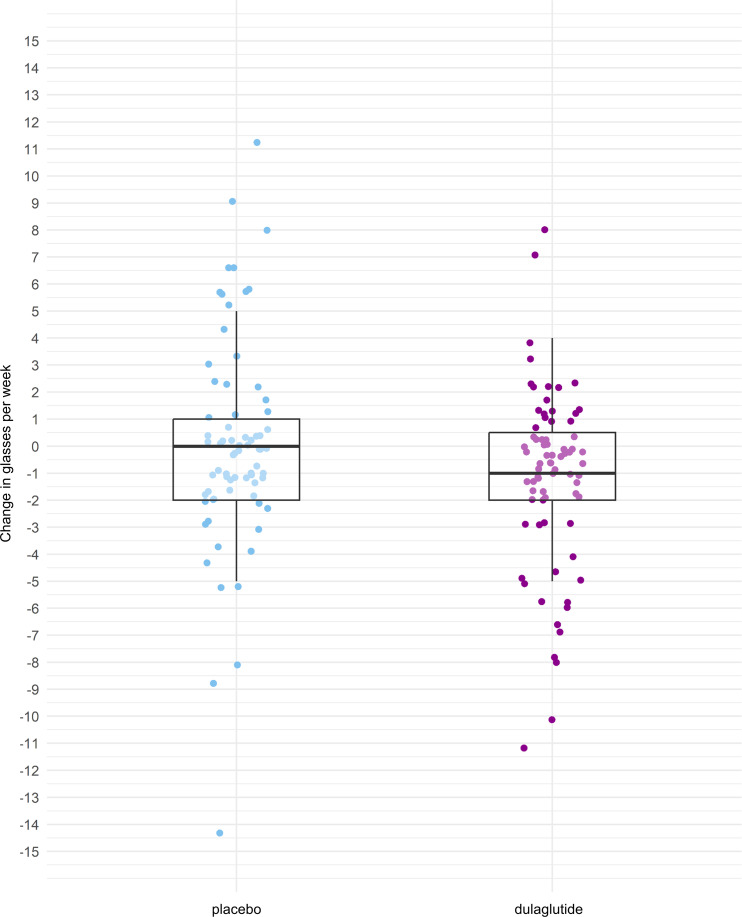
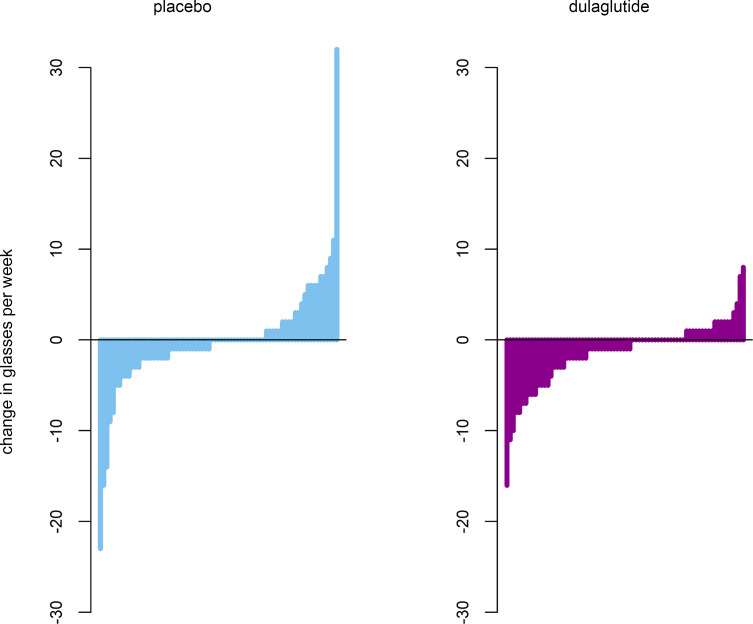
The mean change in weekly alcohol consumption after 12 weeks was –1.4 (SD 3.7) glasses in the dulaglutide group and –0.1 (SD 6.3) glasses in the placebo group. Summary statistics on changes in weekly alcohol consumption according to treatment group are shown in Figure 1. Changes in weekly alcohol consumption from baseline to week 12 and according to treatment group are shown for each participant in Figure 2. At week 12, participants in the dulaglutide group drank an estimated 29% less (baseline alcohol intake adjusted relative effect = 0.71, 95% CI 0.52–0.97, P = 0.04) than participants in the placebo group. There was no interaction between baseline alcohol consumption and treatment (P = 0.2), meaning that the effect of dulaglutide on alcohol consumption at week 12 did not depend on baseline alcohol consumption. When additionally adjusted for level of education, as suggested by selection via quasi-Akaike Information Criterion (qAIC), the relative effect size of dulaglutide increased to 0.64 (95% CI 0.47–0.86, P = 0.004), meaning that patients in the dulaglutide group drank 36% less at week 12 than participants in the placebo group.
Figure 1. Changes in weekly alcohol consumption.
Changes in glasses of alcohol consumed per week from baseline to week 12 according to treatment group. The thick lines represent medians (placebo 0, dulaglutide –1); box indicates the IQR (placebo –2 to 1, dulaglutide –2.25 to 0.25). Whiskers include all data points within the range of 1.5 times the IQR. Note that the figure shows descriptive summary statistics of the data and that dots represent individual data points. Outliers have been removed for better visualization (placebo: +32, –15, –23; dulaglutide: –16).
Figure 2. Changes in weekly alcohol consumption.
Changes in glasses of alcohol consumed per week from baseline to week 12 for each participant according to treatment group. The bars represent individual data points.
Subgroup analysis of heavy drinkers.
In the subgroup of heavy drinkers (10 in the placebo group, 8 in the dulaglutide group), no group difference regarding changes in alcohol consumption was observed (P = 0.5).
Alcohol consumption and smoking cessation.
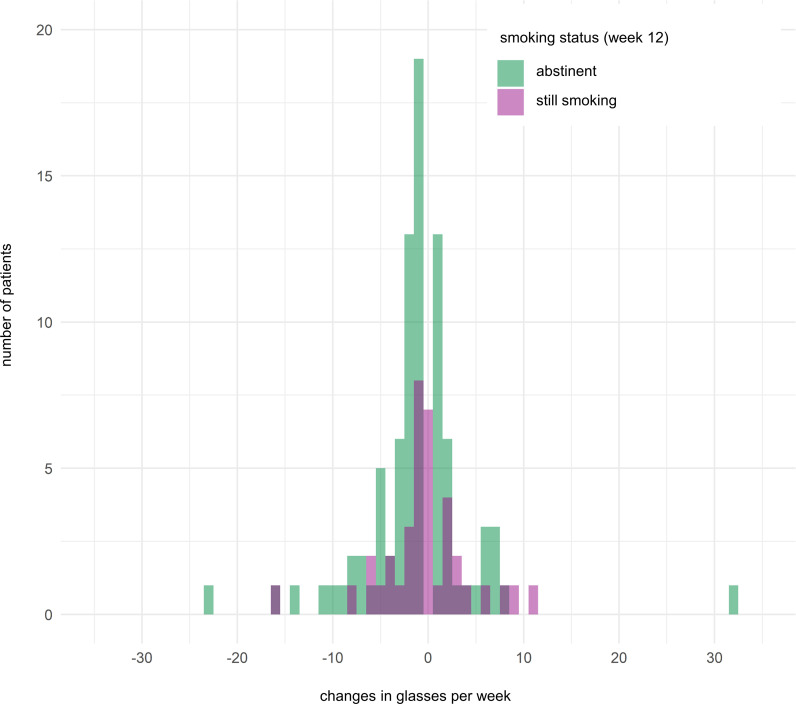
Changes in weekly alcohol consumption from baseline to week 12 and smoking status at week 12 were not correlated (correlation coefficient 0.045, 95% CI –0.116 to 0.203, P = 0.58; Figure 3).
Figure 3. Changes in alcohol consumption according to smoking status.
Changes in glasses of alcohol consumed per week from baseline to week 12 according to smoking status at week 12. Each bar represents the number of patients drinking a certain number of glasses alcohol per week.
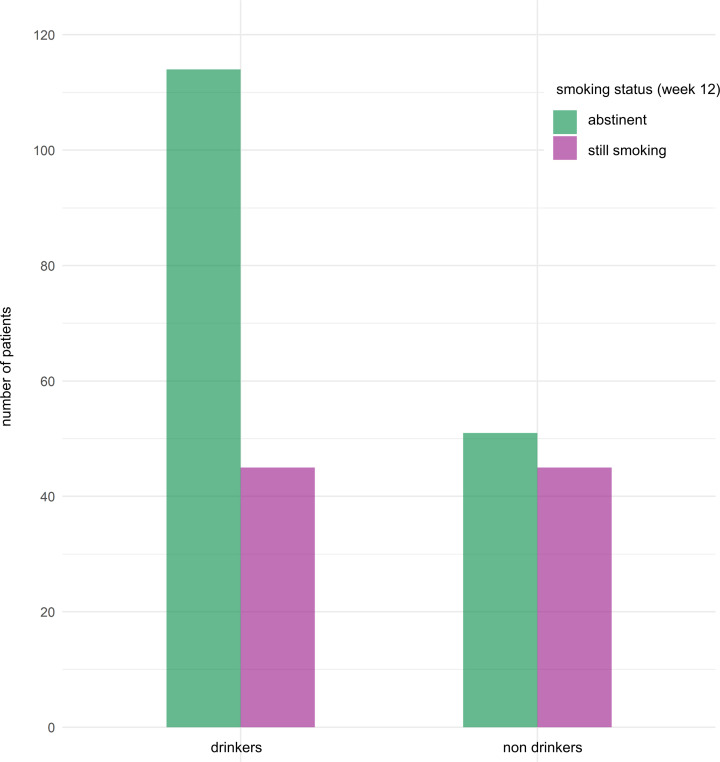
Abstinence rates from cigarettes at week 12 among alcohol consumers were higher compared with nondrinkers (n = 114/159 [72%] vs. 51/96 [53%]; difference in proportions: –0.19 [95% CI –0.32 to –0.06]; P = 0.004) (Figure 4).
Figure 4. Smoking abstinence rates.
Smoking abstinence rates at week 12 according to baseline alcohol consumption. The bars represent absolute numbers of patients.
New alcohol drinkers.
From the baseline nondrinkers (n = 96), 13 participants started consuming alcohol during the study, of which 7 had been randomized to placebo and 6 to dulaglutide. One-third (30.8%, n = 4) of this subset was female. The smoking abstinence rate at week 12 was 53.8% (n = 7).
Drug consumption.
At baseline, 10 participants in the placebo group and 12 participants in the dulaglutide group reported consuming drugs (i.e., drugs such as cannabis, benzodiazepines, opioids, psychostimulants, or others), of which 4 in the placebo and 2 in the dulaglutide group quit. Four participants started consuming drugs in each treatment group during the study. There was no evidence to support the hypothesis that the intake of GLP-1 agonists leads to changes in the consumption of drugs (P = 0.6).
Discussion
The main finding of the present study is that participants treated for smoking cessation drink significantly less alcohol after 12 weeks of treatment with dulaglutide compared with placebo, without correlation to smoking status. Our results thus support the hypothesized effect of GLP-1 agonists on alcohol intake in humans.
The role of GLP-1 in reward-related processes has been demonstrated in preclinical studies. GLP-1 receptors are present in the mesolimbic reward system (6) and their activation attenuates alcohol-induced reward and reduces alcohol intake in animals (7, 8, 11, 25). Translating these findings into humans has proven difficult. Treatment with exenatide for 26 weeks compared to placebo did not lead to a greater reduction in heavy drinking days in a randomized controlled trial of patients with AUD. It did, however, decrease fMRI cue reactivity and central dopamine transporter availability (17); hence, there is evidence that GLP-1 agonism affects the human reward system. A potent placebo response, which is often the case in trials for addiction treatment (26), might have reduced the effect size in the above-mentioned study (17) as well as in our main trial (21). The strong placebo response in the study by Kruse-Klausen et al. may in part be explained by the fact that all participants received standard AUD behavioral treatment sessions every second week throughout the trial (17). Indeed, in the main analysis of the present study, participants in both groups achieved unusually high abstinence rates from cigarettes (63% in the dulaglutide group, 65% in the placebo group), independent of treatment allocation. The fact that we found a significant group difference in alcohol intake at week 12 might be explained by participants’ focus on smoking cessation rather than on reducing their drinking, and participants did not suffer from AUD as a primary disorder. This might also explain why results from animal studies cannot be extrapolated to humans. Animals, unlike participants in clinical trials, have no intrinsic motivation to reduce consumption of alcohol and thus display no placebo effect.
The population of the present study was predominantly obese (BMI > 29.9 kg/m2; 90.8% overall, 87.0% in the placebo group, 95.2% in the dulaglutide group). Interestingly, although Kruse-Klausen et al. found no overall group difference regarding heavy drinking days in AUD patients in the above-mentioned randomized controlled trial, subgroup analyses revealed that exenatide significantly reduced heavy drinking days in obese individuals (defined as BMI > 30 kg/m2) (17). Furthermore, exenatide inhibited brain responses to food cues in obese individuals, but not in lean individuals in a cross-sectional study (27). GLP-1 agonists might therefore affect the reward system differently in obese patients compared with lean individuals.
Participants in our study received standard smoking cessation therapy, i.e., behavioral counselling and varenicline. Behavioral counselling is a form of psychotherapy implemented in treatment of SUD, including AUD. Varenicline has been found to reduce alcohol intake, especially in smokers, but also independently of smoking status (28). These concomitant therapies might have affected alcohol intake in our population. However, all participants received varenicline and behavioral counselling regardless of treatment allocation, and thus we consider group differences in alcohol consumption to be due to the effect of treatment allocation.
In pursuit of alternative reinforcers, patients quitting smoking may increase their consumption of alcohol (29). Furthermore, alcohol may lessen withdrawal symptoms from nicotine (30); hence, one could hypothesize that participants who try to quit smoking would not only drink more alcohol for reward effects, but also curb withdrawal symptoms. Preclinical data suggest that GLP-1 can attenuate withdrawal, as experiments with GLP-1 agonists reduced withdrawal-induced anxiety in rodents (31, 32). If the same were true in humans, GLP-1 agonists would diminish the urge to consume alcohol when experiencing withdrawal symptoms. In the present study, there was no increase in alcohol consumption in the placebo group. One possible explanation might be that participants in both groups received a concomitant therapy with varenicline, which, as mentioned above, has also been reported to reduce alcohol consumption (28, 33). In type 2 diabetes and obesity, GLP-1 agonists are prescribed due to their effect on appetite and homeostatic feeding, and clinical studies report a reduction in fluid intake (34, 35). We did not assess calorie or fluid intake and thus cannot rule out that these mechanisms had an effect on our results. However, 2 facts lead us to believe that the reduction in alcohol consumption under dulaglutide is not solely due to a reduction in overall energy and fluid intake. First, in rodents, GLP-1 agonists reduce consumption of substances that have no caloric value, i.e., psychostimulants; and second, different preclinical studies have shown a decrease in alcohol intake independent of fluid or calorie intake (8, 25, 36). Furthermore, changes in consumption of alcohol and weight changes were not correlated (see supplemental material).
Gastrointestinal symptoms are common side effects of GLP-1 agonists (37–40) and might have affected alcohol intake. However, as expected, these symptoms were only transient and more common in the dulaglutide group at week 2, but comparable at week 12, when alcohol consumption was assessed (21).
Smoking and drinking are closely associated (41–43). It has been hypothesized that nicotine deprivation leads to a higher intake of alcohol in pursuit of alternative reinforcers (29) and attenuates withdrawal symptoms (44). Accordingly, acute nicotine deprivation has been reported to increase the urge to consume alcohol and actual alcohol intake (30). However, other studies indicate that smoking cessation does not lead to increased alcohol intake (45–47); some data even suggest that reducing nicotine consumption may have beneficial effects on alcohol consumption (48). Our finding that smoking status at week 12 was not correlated with changes in alcohol consumption is in line with the majority of literature. However, in contrast to the studies reviewed, most of our participants were not heavy drinkers or AUD patients. Furthermore, high abstinence rates from nicotine in the placebo group might have masked a correlation. Alcohol is a well-established smoking trigger (49, 50). Heavy and binge drinkers are less likely to quit smoking, especially in the short term (51–54). The influence of moderate alcohol consumption on smoking cessation success remains elusive; some studies found no difference in quit rates among moderate and nondrinkers (41, 53–55). In other trials though, and in accordance with our study, moderate drinkers displayed higher abstinence rates compared with those who did not drink (52, 56). Nondrinkers were found to have fewer smokers in their social network (52). Thus, it has been hypothesized that those who start smoking despite their environment not actively enabling them are those who find smoking more rewarding (51). Indeed, smoking patterns of nondrinkers have been described as “heavy, automatic, and characterized by a sense of loss of control” (52).
In analogy to the effects on alcohol-mediated reward, in preclinical studies, GLP-1 agonism has been found to also attenuate reward induced by other substances, i.e., psychostimulants (12–14, 57), nicotine (15, 16), and to some extent opiates, though data on the latter are contradictory (58–60). An experimental study testing the effects of exenatide on cocaine consumption in 13 non–treatment-seeking individuals suffering from cocaine use disorder failed to show a reduction in cocaine self-administration and found no changes in subjective cocaine-related effects (19). However, cocaine consumption has been found to decrease GLP-1 serum levels in humans, and the authors hypothesized that this mechanism leads to sustained cocaine consumption (19, 20). To elucidate the exact mechanisms, and to investigate whether GLP-1 plays a role in addiction to other drugs of abuse, such as cannabis or opioids, more data are needed. In our analysis we did not find an effect of dulaglutide on the consumption of drugs, probably because the number of participants consuming drugs (n = 30) was too small.
The present study has limitations. First, our participants did not per se suffer from AUD or SUD and the subgroup of heavy drinkers was too small to provide valuable results. Whether our findings can be extended to heavy- or binge-drinking smokers or nonsmoking AUD patients, and how smoking status influences the effect of GLP-1 agonists on alcohol intake needs to be investigated. Second, our primary endpoint of alcohol consumption is self-reported and therefore subjective. However, we were not able to assess objective measurements to quantify alcohol intake, such as biomarkers, in the present study. Furthermore, we did not assess drinking patterns, such as percent heavy-drinking days, abstinence, time spent drinking, or the context in which our participants drank. Third, in our study, participants received dulaglutide for only 12 weeks. Preclinical data show that alcohol intake was reduced for 3 weeks after discontinuation of dulaglutide in male but not female rats (9). Whether termination of treatment leads to a rebound effect in humans or whether the effects are sustained, and whether sex plays a role in posttreatment effects will need to be explored in future studies.
In conclusion, our analysis showed an effect of a GLP-1 agonist on alcohol intake in humans and our results thus strengthen the rationale for implementing GLP-1 agonists in pharmacological AUD treatment. However, it has to be considered that AUD is characterized by a wide range of drinking behaviors and prevalent comorbid psychiatric disorders, including substance abuse. Thus, treatment strategies need to be multidimensional and personalized, taking into account psychosocial components. To identify the GLP-1 agonist’s role in the treatment of AUD and SUD, further studies are needed, elucidating which patients would benefit and whether GLP-1 agonists improve long-term abstinence.
Methods
Further details can be found in Supplemental Methods.
Trial design and participants
This is a predefined secondary analysis of a single-center, randomized, double-blind, placebo-controlled, parallel-group trial conducted at the University Hospital Basel, Switzerland, from June 2017 to June 2022, investigating the effect of a 12-week treatment with dulaglutide on smoking abstinence when added to standard smoking cessation therapy (i.e., varenicline plus behavioral counselling). A detailed description of the study methodology has been published elsewhere (61). In brief, the study included 255 smokers with at least moderate cigarette dependence (defined by a Fagerstrom score of ≥5 points; ref. 22), aged 18 to 75 years, who were willing to quit smoking and willing to undergo treatment with varenicline. Main exclusion criteria were pregnancy, preexisting treatment with GLP-1 agonists, severe renal insufficiency (defined as estimated glomerular filtration rate <30 mL/min/1.73 m²), and unstable psychiatric conditions.
Study outcomes
The primary outcome in this secondary analysis was the difference in total consumption of standard glasses of alcohol per week after 12 weeks of treatment with dulaglutide compared to placebo. Secondary outcomes were correlation between changes in consumption of alcohol and smoking status at week 12, differences in smoking abstinence rates at week 12 between baseline alcohol drinkers and nondrinkers, number and characteristics of participants who started consuming alcohol from baseline to week 12, and changes in consumption of other drugs (i.e., cannabis, benzodiazepines, opiates, psychostimulants, and others).
Randomization, study medication, and standard of care
Participants were 1:1 randomized according to a computer-generated randomization list (randomly selected, varying block sizes; no stratification). Participants received a once-weekly, subcutaneous injection of either dulaglutide or placebo. Dulaglutide was injected at an initial dose of 0.75 mg/0.5 mL in the first week and then increased to 1.5 mg/0.5 mL in the following weeks until the end of treatment. The placebo intervention was a 0.5 mL injection of 0.9% NaCl. The standard of care included behavioral counselling according to individual needs and a 12-week treatment with the nicotinic receptor partial agonist varenicline, which was uptitrated to a daily dose of 2 mg.
Study procedure and assessments
At baseline, data on demographics, comorbidities, including psychiatric diseases and substance abuse, and consumption of alcohol and nicotine were collected via face-to-face interview and a short physical examination was performed. Data on consumption of alcohol, nicotine, and other drugs were also assessed at week 12. Alcohol consumption was assessed with a standardized questionnaire by asking for the number of standard glasses of each type of alcohol (i.e., wine, beer, and spirits) consumed per week on average, analogous to the timeline followback method (62). Standard glasses in Switzerland are defined as 3 dL of beer, 1 dL of wine, or 0.3 dL of spirits, i.e., drinks containing roughly 10 g of pure ethanol (63). Smoking abstinence at week 12 was defined as self-reported 7-day smoking abstinence and end-expiratory exhaled carbon monoxide measurements of 10 ppm or less. For more details, we refer to the study protocol (61).
Statistics
Analysis sets.
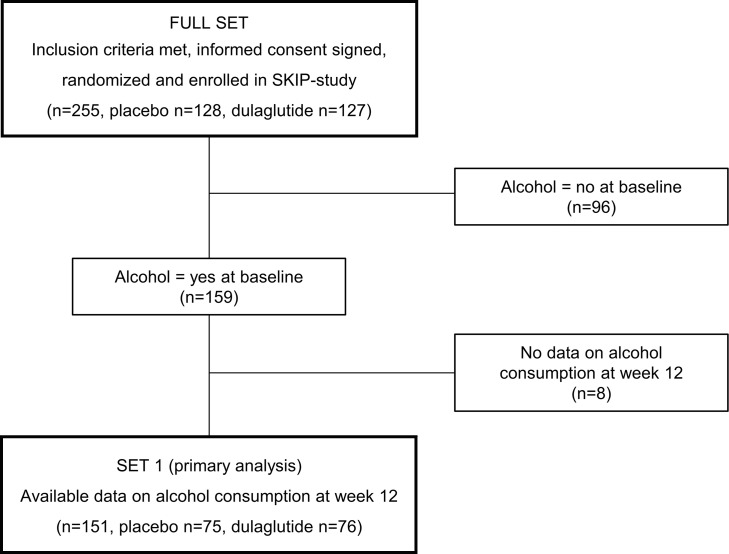
The full analysis set consisted of 255 participants. According to alcohol consumption at baseline, participants were then divided into alcohol “consumers” and “nonconsumers” (159 and 96 participants, respectively). For the primary outcome, only alcohol consumers with available data on alcohol consumption at week 12 were included (Set 1, n = 151; Figure 5).
Figure 5. Study flowchart.
Study flowchart showing participant selection. The SKIP study is described in Lengsfeld et al. (61).
Statistical methods.
Baseline patient characteristics are described using descriptive statistics. Discrete variables are expressed as frequencies (n) and percentages (%). Continuous variables are expressed as mean and SD or median and IQR, depending on the data distribution.
The primary outcome, i.e., differences in total amount of alcohol intake in glasses per week at week 12, were analyzed using a generalized linear model with treatment (dulaglutide vs. placebo) and baseline alcohol consumption as predictors (basic model). A quasipoisson error distribution was used to account for overdispersion. The interaction between treatment and baseline alcohol consumption was examined by adding the interaction term to the basic model. The basic model for the primary outcome was further adjusted for education. To assess group differences in changes in alcohol consumption from baseline to week 12 in the subgroup of heavy drinkers, Wilcoxon’s signed-rank test was used. The correlation between changes in consumption of alcohol and smoking status at week 12 was analyzed by calculating the point biserial correlation coefficient. Differences in smoking abstinence rates between baseline consumers and nonconsumers were analyzed using the χ2 test. Characteristics of patients who started drinking were summarized using descriptive statistics. Fisher’s exact test was used to test for group differences in drug consumption. For all analyses, a P value of less than 0.05 was considered significant. Detailed statistical methods, supplemental analyses, and detailed outputs can be found in the supplemental material. For statistical analyses, the statistics program R v2022.02.1 was used (64).
Study approval
All participants provided written consent. The study was registered on ClinicalTrials.gov (NCT03204396) and conducted according to the principles of the Declaration of Helsinki. The study protocol was approved by the Ethical Committee Northwest and Central Switzerland (EKNZ 2017-00286) and the national agency for the authorization and supervision of therapeutic products (Swissmedic 2017DR2066).
Data availability
The data sets used in this analysis are available upon reasonable request. Furthermore, we may share related documents, including the study protocol and the statistical analysis plan. Data will be available with the publication of our manuscript on receipt of a request detailing the study hypothesis and statistical analysis plan. All requests should be sent to the corresponding author. Values for all data points in graphs are reported in the Supporting Data Values file. The steering committee of this study will discuss all requests and decide based on the scientific rigor of the proposal whether data sharing is appropriate. All applicants will be asked to sign a data access agreement.
Author contributions
LP analyzed and interpreted the data, did the literature search, and wrote the manuscript. SM and LP planned, performed, and interpreted the data analysis. DRV gave important inputs to the statistical analysis plan and supervised the statistical analyses. SM reviewed the manuscript. MCC, TB, and AM were involved in the study design. SL and CB contributed to data collection. MCC gave input to the study design. BW designed the study, wrote the protocol, collected, analyzed and interpreted data, and supervised all steps of the conduct of the study. All authors edited and approved the final manuscript.
Supplementary Material
Acknowledgments
We are grateful to our participants for taking part in the trial. We thank the support staff and study and laboratory personnel at the University Hospital Basel, especially Nina Hutter, Joyce Santos de Jesus, Fabienne Baur, Milica Popovic, Cihan Atila, Karin Wild, Silke Purschke, Vanessa Grassedonio, Klaus Ehrlich, Patrick Simon, Uta Engler, Silvia Caviola, Nathalie Klaus, and Nicole Salvisberg and all members of the clinical neuroendocrinology research team for their most helpful support during the study. This study was supported by funds from the Swiss National Foundation (grant PZ00P3_193206), the Gottfried Julia Bangerter-Rhyner Foundation, the Goldschmidt-Jacobson Foundation, the Hemmi Foundation, the University of Basel, University Hospital Basel, and the Swiss Academy of Medical Science.
Version 1. 11/22/2023
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2023, Probst et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: JCI Insight. 2023;8(22):e170419.https://doi.org/10.1172/jci.insight.170419.
Contributor Information
Sophie Monnerat, Email: sophie.monnerat@usb.ch.
Deborah R. Vogt, Email: deborah.vogt@usb.ch.
Sophia Lengsfeld, Email: SophiaPitschna.Lengsfeld@usb.ch.
Thilo Burkard, Email: thilo.burkard@usb.ch.
Andrea Meienberg, Email: andrea.meienberg@usb.ch.
Cemile Bathelt, Email: cemile.bathelt@usb.ch.
Mirjam Christ-Crain, Email: mirjam.christ@usb.ch.
Bettina Winzeler, Email: bettina.winzeler@usb.ch.
References
- 1. World Health Organization. Global Status Report on Alcohol and Health 2018. https://www.who.int/publications/i/item/9789241565639 Accessed October 10, 2023.
- 2. United Nations Office on Drugs and Crime. World Drug Report 2022. https://www.unodc.org/unodc/data-and-analysis/world-drug-report-2022.html Accessed October 10, 2023.
- 3.Witkiewitz K, et al. Advances in the science and treatment of alcohol use disorder. Sci Adv. 2019;5(9):eaax4043. doi: 10.1126/sciadv.aax4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litten RZ, et al. Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res. 2015;39(4):579–584. doi: 10.1111/acer.12669. [DOI] [PubMed] [Google Scholar]
- 5.Merchenthaler I, et al. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403(2):261–280. doi: 10.1002/(SICI)1096-9861(19990111)403:2<261::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Alhadeff AL, et al. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153(2):647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egecioglu E, et al. The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology. 2013;38(8):1259–1270. doi: 10.1016/j.psyneuen.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Vallöf D, et al. The glucagon-like peptide 1 receptor agonist liraglutide attenuates the reinforcing properties of alcohol in rodents. Addict Biol. 2016;21(2):422–437. doi: 10.1111/adb.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallöf D, et al. Long-term treatment with a glucagon-like peptide-1 receptor agonist reduces ethanol intake in male and female rats. Transl Psychiatry. 2020;10(1):238. doi: 10.1038/s41398-020-00923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aranäs C, et al. Semaglutide reduces alcohol intake and relapse-like drinking in male and female rats. EBioMedicine. 2023;93:104642. doi: 10.1016/j.ebiom.2023.104642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomsen M, et al. Effects of glucagon-like peptide 1 analogs on alcohol intake in alcohol-preferring vervet monkeys. Psychopharmacology (Berl) 2019;236(2):603–611. doi: 10.1007/s00213-018-5089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egecioglu E, et al. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One. 2013;8(7):e69010. doi: 10.1371/journal.pone.0069010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez NS, et al. Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology. 2018;43(10):2000–2008. doi: 10.1038/s41386-018-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erreger K, et al. Exendin-4 decreases amphetamine-induced locomotor activity. Physiol Behav. 2012;106(4):574–578. doi: 10.1016/j.physbeh.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egecioglu E, et al. The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One. 2013;8(10):e77284. doi: 10.1371/journal.pone.0077284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuesta LM, et al. GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci. 2017;20(5):708–716. doi: 10.1038/nn.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klausen MK, et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight. 2022;7(19):e159863. doi: 10.1172/jci.insight.159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suchankova P, et al. The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Transl Psychiatry. 2015;5(6):e583. doi: 10.1038/tp.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angarita GA, et al. Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder. Drug Alcohol Depend. 2021;221:108614. doi: 10.1016/j.drugalcdep.2021.108614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouhlal S, et al. Acute effects of intravenous cocaine administration on serum concentrations of ghrelin, amylin, glucagon-like peptide-1, insulin, leptin and peptide YY and relationships with cardiorespiratory and subjective responses. Drug Alcohol Depend. 2017;180:68–75. doi: 10.1016/j.drugalcdep.2017.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lengsfeld S, et al. Effect of dulaglutide in promoting abstinence during smoking cessation: a single-centre, randomized, double-blind, placebo-controlled, parallel group trial. EClinicalMedicine. 2023;57:101865. doi: 10.1016/j.eclinm.2023.101865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res. 2012;14(1):75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 23.Heatherton TF, et al. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 24. National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking Accessed October 10, 2023.
- 25.Shirazi RH, et al. Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS One. 2013;8(4):e61965. doi: 10.1371/journal.pone.0061965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litten RZ, et al. The placebo effect in clinical trials for alcohol dependence: an exploratory analysis of 51 naltrexone and acamprosate studies. Alcohol Clin Exp Res. 2013;37(12):2128–2137. doi: 10.1111/acer.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eldor R, et al. Discordance between central (brain) and pancreatic action of exenatide in lean and obese subjects. Diabetes Care. 2016;39(10):1804–1810. doi: 10.2337/dc15-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erwin BL, Slaton RM. Varenicline in the treatment of alcohol use disorders. Ann Pharmacother. 2014;48(11):1445–1455. doi: 10.1177/1060028014545806. [DOI] [PubMed] [Google Scholar]
- 29.Vuchinich RE, Tucker JA. Behavioral theories of choice as a framework for studying drinking behavior. J Abnorm Psychol. 1983;92(4):408–416. doi: 10.1037/0021-843X.92.4.408. [DOI] [PubMed] [Google Scholar]
- 30.Palfai TP, et al. Effects of nicotine deprivation on alcohol-related information processing and drinking behavior. J Abnorm Psychol. 2000;109(1):96–105. doi: 10.1037/0021-843X.109.1.96. [DOI] [PubMed] [Google Scholar]
- 31.Sharma AN, et al. Glucagon-like peptide-1 (GLP-1) receptor agonist prevents development of tolerance to anti-anxiety effect of ethanol and withdrawal-induced anxiety in rats. Metab Brain Dis. 2015;30(3):719–730. doi: 10.1007/s11011-014-9627-z. [DOI] [PubMed] [Google Scholar]
- 32.Listos J, et al. Linagliptin, a selective dipeptidyl peptidase-4 inhibitor, reduces physical and behavioral effects of morphine withdrawal. Molecules. 2022;27(8):2478. doi: 10.3390/molecules27082478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oon-arom A, et al. Efficacy and acceptability of varenicline for alcoholism: a systematic review and meta-analysis of randomized-controlled trials. Drug Alcohol Depend. 2019;205:107631. doi: 10.1016/j.drugalcdep.2019.107631. [DOI] [PubMed] [Google Scholar]
- 34.Winzeler B, et al. Effects of glucagon-like peptide-1 receptor agonists on fluid intake in healthy volunteers. Endocrine. 2020;70(2):292–298. doi: 10.1007/s12020-020-02394-2. [DOI] [PubMed] [Google Scholar]
- 35.Winzeler B, et al. A randomized controlled trial of the GLP-1 receptor agonist dulaglutide in primary polydipsia. J Clin Invest. 2021;131(20):e151800. doi: 10.1172/JCI151800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomsen M, et al. The glucagon-like peptide 1 receptor agonist Exendin-4 decreases relapse-like drinking in socially housed mice. Pharmacol Biochem Behav. 2017;160:14–20. doi: 10.1016/j.pbb.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Wysham C, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1) Diabetes Care. 2014;37(8):2159–2167. doi: 10.2337/dc13-2760. [DOI] [PubMed] [Google Scholar]
- 38.Giorgino F, et al. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2) Diabetes Care. 2015;38(12):2241–2249. doi: 10.2337/dc14-1625. [DOI] [PubMed] [Google Scholar]
- 39.Umpierrez G, et al. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3) Diabetes Care. 2014;37(8):2168–2176. doi: 10.2337/dc13-2759. [DOI] [PubMed] [Google Scholar]
- 40.Blonde L, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385(9982):2057–2066. doi: 10.1016/S0140-6736(15)60936-9. [DOI] [PubMed] [Google Scholar]
- 41.Dawson DA. Drinking as a risk factor for sustained smoking. Drug Alcohol Depend. 2000;59(3):235–249. doi: 10.1016/S0376-8716(99)00130-1. [DOI] [PubMed] [Google Scholar]
- 42.Weinberger AH, et al. A review of epidemiologic research on smoking behavior among persons with alcohol and illicit substance use disorders. Prev Med. 2016;92:148–159. doi: 10.1016/j.ypmed.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant BF, et al. Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry. 2015;72(8):757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning in C57BL/6J mice depend on both dose and duration of treatment. Psychopharmacology (Berl) 2008;196(3):483–495. doi: 10.1007/s00213-007-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahler CW, et al. Quitting smoking and change in alcohol consumption in the international tobacco control (ITC) four country survey. Drug Alcohol Depend. 2010;110(1–2):101–107. doi: 10.1016/j.drugalcdep.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalman D, et al. Addressing tobacco use disorder in smokers in early remission from alcohol dependence: the case for integrating smoking cessation services in substance use disorder treatment programs. Clin Psychol Rev. 2010;30(1):12–24. doi: 10.1016/j.cpr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gulliver SB, et al. Smoking cessation and alcohol abstinence: what do the data tell us? Alcohol Res Health. 2006;29(3):208–212. [PMC free article] [PubMed] [Google Scholar]
- 48.Philibert R, et al. Alcohol use intensity decreases in response to successful smoking cessation therapy. Genes (Basel) 2022;13(1):2. doi: 10.3390/genes13010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahler CW, et al. Acute effects of low and high dose alcohol on smoking lapse behavior in a laboratory analogue task. Psychopharmacology (Berl) 2014;231(24):4649–4657. doi: 10.1007/s00213-014-3613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sayette MA, et al. The effects of alcohol on cigarette craving in heavy smokers and tobacco chippers. Psychol Addict Behav. 2005;19(3):263–270. doi: 10.1037/0893-164X.19.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kahler CW, et al. Alcohol consumption and quitting smoking in the international tobacco control (ITC) four country survey. Drug Alcohol Depend. 2009;100(3):214–220. doi: 10.1016/j.drugalcdep.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cook JW, et al. Relations of alcohol consumption with smoking cessation milestones and tobacco dependence. J Consult Clin Psychol. 2012;80(6):1075–1085. doi: 10.1037/a0029931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaye JT, et al. Searching for personalized medicine for binge drinking smokers: smoking cessation using varenicline, nicotine patch, or combination nicotine replacement therapy. J Stud Alcohol Drugs. 2020;81(4):426–435. doi: 10.15288/jsad.2020.81.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lynch KL, et al. Level of alcohol consumption and successful smoking cessation. Nicotine Tob Res. 2019;21(8):1058–1064. doi: 10.1093/ntr/nty142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho RKS, et al. Pattern and determinants of alcohol and tobacco co-use and its relationship with smoking cessation in Hong Kong. Tob Prev Cessat. 2021;7:21. doi: 10.18332/tpc/132288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sobell MB, et al. Dual Recoveries From Alcohol and Smoking Problems. In: Fertig JB, Allen JP, eds. Alcohol and Tobacco: From Basic Science to Clinical Practice. National Institute on Alcohol Abuse and Alcoholism; 1995:207–224. [Google Scholar]
- 57.Sørensen G, et al. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol Behav. 2015;149:262–268. doi: 10.1016/j.physbeh.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bornebusch AB, et al. Glucagon-like peptide-1 receptor agonist treatment does not reduce abuse-related effects of opioid drugs. eNeuro. 2019;6(2):ENEURO.0443-18.2019. doi: 10.1523/ENEURO.0443-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, et al. Activation of GLP-1 receptors attenuates oxycodone taking and seeking without compromising the antinociceptive effects of oxycodone in rats. Neuropsychopharmacology. 2020;45(3):451–461. doi: 10.1038/s41386-019-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Douton JE, et al. Glucagon-like peptide-1 receptor agonist, exendin-4, reduces reinstatement of heroin-seeking behavior in rats. Behav Pharmacol. 2021;32(4):265–277. doi: 10.1097/FBP.0000000000000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lengsfeld S, et al. Glucagon-like peptide-1 analogues: a new way to quit smoking? (SKIP)—a structured summary of a study protocol for a randomized controlled study. Trials. 2023;24(1):284. doi: 10.1186/s13063-023-07164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sobell LC, et al. Timeline Followback Method (Drugs, Cigarettes and Marihuana). Paper presented at: 30th Annual Meeting of the Association for Advancement of Behavior Therapy; November 21–24, 1996; New York, New York, USA. https://www.nova.edu/gsc/forms/TLFBDrugCigMJoverview.pdf Accessed October 10, 2023.
- 63. Bundesamt für Gesundheit. The Standard Glass of Alcohol. https://www.alcohol-facts.ch/de/das-standardglas-alkohol Accessed October 10, 2023.
- 64. R Core Team (2022). R: a language and environment for statistical computing. https://www.r-project.org/ Accessed October 10, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used in this analysis are available upon reasonable request. Furthermore, we may share related documents, including the study protocol and the statistical analysis plan. Data will be available with the publication of our manuscript on receipt of a request detailing the study hypothesis and statistical analysis plan. All requests should be sent to the corresponding author. Values for all data points in graphs are reported in the Supporting Data Values file. The steering committee of this study will discuss all requests and decide based on the scientific rigor of the proposal whether data sharing is appropriate. All applicants will be asked to sign a data access agreement.