Abstract
Purpose:
Mismatch repair deficiency (dMMR)/microsatellite instability-high (MSI-H) are positive predictive markers for immune checkpoint inhibitors. However, data on the activity of nivolumab in advanced dMMR/MSI-H rare cancers and more accurate biomarkers are worth exploring.
Patients and Methods:
We conducted a multicenter phase II, open-label, single-arm clinical trial to explore the effectiveness and safety of nivolumab monotherapy in patients with advanced rare cancers with dMMR/MSI-H, in parallel with immune phenotype analysis, to explore new biomarkers. A Bayesian adaptive design was applied. Characterization of peripheral blood mononuclear cells (PBMC) was characterized by multicolor flow cytometric analysis and CyTOF using samples collected before and after the intervention. The dMMR was identified by the complete loss of MLH1/MSH2/MSH6/PMS2.
Results:
From May 2018 to March 2021, 242 patients were screened, and 11 patients were enrolled, of whom 10 were included in the full analysis. Median follow-up was 24.7 months (interquartile range, 12.4–31.5). Objective response rate was 60% [95% confidence interval (CI), 26.2–87.8] by central assessment and 70% (95% CI, 34.8–93.3) by local investigators. Median progression-free survival was 10.1 months (95% CI, 0.9–11.1). No treatment-related adverse events of grade 3 or higher were observed. Patients with a tumor mutation burden of ≥10/Mb showed a 100% response rate (95% CI, 47.8–100). Responders had increased T-bet+ PD-1+ CD4+ T cells in PBMC compared with nonresponders (P < 0.05).
Conclusions:
The trial met its primary endpoint with nivolumab, demonstrating clinical benefit in advanced dMMR/MSI-H rare solid cancers. Besides, the proportion of T-bet+ PD-1+ CD4+ T-cells may serve as a novel predictive biomarker.
Translational Relevance.
Checkpoint inhibitors have made tremendous improvements in outcomes for mismatch repair deficiency (dMMR)/microsatellite instability-high (MSI-H) rare cancers. However, there is still room to seek true predictive biomarkers for this population. In this single-arm phase II open-label clinical trial of patients with advanced rare cancer harboring dMMR or MSI-H, nivolumab treatment showed that 60% of the patients achieved a partial response. Responders had increased T-bet+ PD-1+ CD4+ T cells in their peripheral blood compared with nonresponders.
The trial met its primary endpoint, suggesting a clinical benefit of nivolumab for advanced dMMR/MSI-H rare solid cancers. Besides, the proportion of T-bet+ PD-1+ CD4+ T-cells may serve as a novel predictive biomarker.
Introduction
Mismatch repair (MMR) gene defects lead to an inability to rectify DNA replication errors, causing the accumulation of point mutations and resulting in high microsatellite instability (MSI-H). Mismatch repair gene deficiency (dMMR) affects one of the MMR genes, such as MLH1, PMS2, MSH2, or MSH6 (1), and can arise from either an inherited genetic mutation or an occasional genetic alteration. Tumors with a marked increase in somatic mutations (2) and a higher neoantigen load show an increased CD3-positive, CD8-positive, and programmed death-1 (PD-1)-expressing tumor-infiltrating lymphocytes (TIL) and programmed death ligand-1 (PD-L1)-expressing intraepithelial and peritumoral immune cells, compared with microsatellite-stable cancers (3).
Approximately 2% to 4% of all diagnosed cancers (4–7) share this dMMR/MSI-H characteristic but vary across different cancer types, including endometrial, gastric, and colorectal cancers, with a relatively high frequency. There is little evidence for other cancer types with dMMR/MSI-H, and among these are rare cancers, defined as cancer types with an incidence rate of less than 6 per 100,000 people. Because each rare cancer population is small, clinicopathologic and molecular data are limited, leading to a poor prognosis, in contrast to common cancers in which drug development is active. Therefore, platform studies, such as basket trials, have emerged as methods for evaluating drugs in a tumor-agnostic manner.
The first tumor-agnostic approval was the humanized monoclonal anti–PD-1 antibody pembrolizumab for MSI-H solid tumors (8). In a non-randomized, open-label, multi-cohort, phase II KEYNOTE-158 study of multiple types of advanced cancer, pembrolizumab demonstrated an objective response rate (ORR) of 34.3% (8).
Nivolumab monotherapy has also shown activity across a number of cancer types, leading to pharmaceutical approval such as dMMR/MSI-H colorectal cancers and a recent approval for cancer of unknown primary by the Japanese Pharmaceuticals and Medical Devices Agency (9). The activity of dMMR in noncolorectal cancers was explored using the basket trial NCI-MATCH (EAY131) sub-protocol (10). This study included some patients with rare cancer. However, due to the lack of further data on these minor populations and the absence of a tumor-agnostic molecular background other than dMMR/MSI-H, nivolumab's efficacy as a reliable predictive biomarker across pan-cancers is yet to be fully explored. Therefore, to further investigate the efficacy and safety of nivolumab monotherapy for dMMR/MSI-H rare cancers and to understand the molecular background of the immune microenvironment in these patients, we conducted a prospective phase II clinical trial for this rare population.
Patients and Methods
Study design
This multicenter, open-label, single-arm phase II trial was conducted at three sites in Japan among the MASTER KEY Project (11) institutions.
Patients
Patients had histologically confirmed metastatic or unresectable advanced rare cancers, defined as an annual incidence of less than 6 per 100,000 population, with dMMR and/or MSI-H. The inclusion criteria were as follows: age ≥ 16 years, at least one measurable lesion as defined by the RECIST version 1.1 (12), disease progression after standard chemotherapy, Eastern Cooperative Oncology Group performance status (PS) of 0 or 1, and adequate organ function. There were no limitations on the number of previous treatments, and previous treatment with immune checkpoint inhibitors (ICI) was not allowed. For cancers with no applicable standard of care, patients without previous treatment were enrolled. Full details of all the inclusion criteria are provided in the protocol. The exclusion criteria were multiple primary malignancies requiring systemic pharmacotherapy, interstitial lung diseases, active or chronic relapsed autoimmune disease, systemic infections, and testing positive for HIV antibody, HTLV-1 antibody, HBs antigen, or HCV RNA. The representativeness of study participants with regard to factors such as sex, age, and ethnicity compared with the literature is shown in Supplementary Table S4.
Procedures
Patients received 240 mg nivolumab intravenously on day one of a 2-week cycle until disease progression or unacceptable adverse events occurred. Laboratory tests and electrocardiography were performed according to the protocol and as needed, according to clinical symptoms. CT scans were planned at baseline, week 8, week 16, and week 24; every 12 weeks beyond that; protocol treatment discontinuation; and as needed, according to clinical symptoms. No dose adjustments were performed.
The antitumor response was assessed according to RECIST (version 1.1; ref. 12). All evaluations of the treatment response were performed by an independent central radiological committee and local investigators. Adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 4.03. All adverse events were monitored and recorded. The association between adverse events and nivolumab treatment was assessed by the investigators at each site.
dMMR/MSI and tumor mutation burden testing
Tumors were determined to be dMMR when at least one of the four MMR proteins was absent on IHC (MLH1/MSH2/MSH6/PMS2). MSI was evaluated using commercially available MSI assay kits (SRL Inc.). Central testing for dMMR was performed in this study, but patients who were determined to have dMMR/MSI-H locally were also eligible. Tumor mutation burden (TMB) was tested using commercially available targeted DNA sequencing tests in patients who provided their consent for testing.
Outcomes
The primary endpoint was ORR, which was evaluated using an independent central review. It was defined as the proportion of patients with complete response (CR) or partial response (PR) as the best overall response. CR and PR were confirmed at least 4 weeks after the initial evaluation. Secondary endpoints were ORR by local investigators, disease control rate (DCR) by independent central review, progression-free survival (PFS) by local investigators, overall survival (OS), and safety. PFS was defined as the duration from trial registration to the date of disease progression or death from any cause. OS was defined as the duration from trial registration to the date of death from any cause. DCR was defined as the proportion of patients with CR, PR, or stable disease (SD).
Statistical analysis
The efficacy analysis included patients who received at least one dose of nivolumab and met key eligibility criteria. We initially planned to enroll 5 to 15 patients, which was determined on the basis of the Bayesian design (13). According to prior distributions based on the Bayesian design, we assumed a mean of 5% for the no-effect response rate and used the beta distribution with shape parameters of 10 and 190 [Beta (10, 190)] based on investigator assessments. We also assumed a mean response rate of 30% for nivolumab, based on other clinical trial results in patients with rare solid cancers who previously received standard chemotherapy and then used beta (0.6, 1.4). Two responders were required to achieve a posterior probability >95%, and the response rate to nivolumab was >5% in 5 to 11 patients (or 3 responders in 12 to 15 patients). This design controls the type-I error rate at the target level of <0.10.
Analyses of ORR, DCR, PFS, and OS were performed in the intention-to-treat population. Safety analyses were performed in patients who received one or more doses of nivolumab. The 95% confidence intervals (CI) for the ORR by independent central review, the 95% CI for the ORR by the local investigator, and the DCR were estimated using the Clopper and Pearson methods. Median PFS and OS were estimated using the Kaplan–Meier method, and the Brookmeyer and Crowley method was used to calculate 95% CIs. Survivors and those lost to follow-up were censored on the last confirmed survival date. Subgroup analyses by sex, PS, cancer type, and TMB were performed for all endpoints. Data were analyzed at the cutoff date (March 30, 2022). All statistical analyses in the clinical outcome part were performed using SAS version 9.4 (SAS Institute). For immune biomarker analysis, Weltch's unpaired t test (responders vs. nonresponders at the baseline) or paired t test (pre- vs. post-nivolumab treatment) was used to compare the means of two groups. P values < 0.05 were considered to be statistically significant. Analysis was performed using Graphpad Prism 8 (GraphPad Software Inc.).
Translation research procedures
Blood sample collection for biomarker research
Blood samples were collected before and after nivolumab treatment, after obtaining written informed consent (Supplementary Fig. S1). Peripheral blood mononuclear cells (PBMC) were isolated from the blood using BD Vacutainer CPT tubes (BD Biosciences) and suspended and cryopreserved in CELLBANKER1 (Zenoaq) until use.
Immune phenotype analysis
The antibodies used in this study are listed in Supplementary Table S1. PBMCs were stained for viability using the LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit (Invitrogen). Surface markers were stained with PBS containing 2% FBS. Intracellular (nuclear) staining was performed using the Foxp3/Transcription Factor Staining Kit (eBioscience), according to the manufacturer's instructions. All data were collected using a Symphony A3 cytometer (BD Biosciences) and analyzed using FlowJo ver. 10 software (BD Biosciences).
Ethical consideration
The study was approved by the institutional review board of each participating institution and conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. Written informed consent was obtained from all the patients before enrollment in the study. This trial was listed in the clinical trial registry as jRCT2091220344.
Data availability
Raw data for this study were generated at National Cancer Center Hospital (NCCH), Tokyo. The data generated in this study are not publicly available due to restrictions of information that could compromise patient privacy and/or consent but are available upon reasonable request from the corresponding author. Please contact the corresponding author for requests of deidentified raw data.
Results
Between April 2018 and March 2021, 242 patients were screened, and 11 received the protocol treatment. One patient was excluded from the primary analysis due to the absence of a measurable lesion at baseline (Supplementary Fig. S2). Baseline characteristics of the 11 enrolled patients are presented in Table 1. The most common cancer type was soft-tissue sarcoma (N = 3). All the patients had a history of at least one pharmacotherapy treatment. Nine patients were identified as having dMMR, and two were enrolled based solely on their MSI-H status. Five patients tested positive for both dMMR and MSI-H.
Table 1.
Patient characteristics.
| N = 11 (%) | |
|---|---|
| Age | |
| Median (range) | 70 (54–78) |
| Sex | |
| Female | 8 (73) |
| Male | 3 (27) |
| PS | |
| 0 | 3 (27) |
| 1 | 8 (73) |
| Disease condition | |
| Primary advanced | 5 (45) |
| Relapsed | 6 (55) |
| Distant metastasis | |
| Yes | 9 (82) |
| No | 2 (18) |
| History of surgery | |
| Yes | 8 (73) |
| No | 3 (27) |
| Prior lines of therapy | |
| 0 | 0 (0) |
| 1 | 4 (36) |
| 2 | 3 (27) |
| ≥3 | 4 (36) |
| Cancer types | |
| Soft-tissue sarcoma | 3 (27) |
| High-grade sarcoma with rhabdomyoblastic differentiation | 1 |
| Leiomyosarcoma | 1 |
| Solitary fibrous tumor | 1 |
| Extramammary Paget's disease | 1 (9.1) |
| Dedifferentiated carcinoma of endometrium | 1 (9.1) |
| Endometrial carcinosarcoma | 1 (9.1) |
| Glioblastoma | 2 (18) |
| Neuroendocrine carcinoma | 1 (9.1) |
| Small intestine adenocarcinoma | 1 (9.1) |
| Peritoneal carcinoma | 1 (9.1) |
The median number of nivolumab cycles was 23 (range, 2–77). The median duration of follow-up was 24.7 months (interquartile range, 12.71–31.54) at the data cut-off date, and 3 patients were still receiving nivolumab on this date.
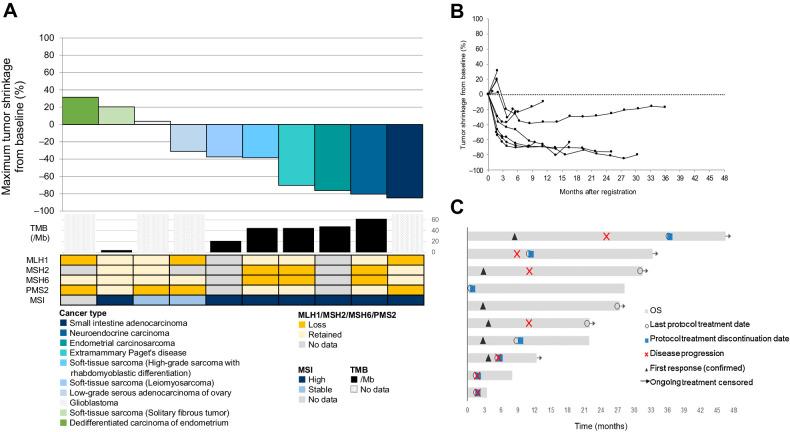
Among the 10 intention-to-treat patients, 1 patient (10%) had CR, 5 (50%) had PR, and 1 (10%) had SD. The ORR, as determined by an independent central review, was 60% (95% CI, 26.2–87.8; Supplementary Table S2). This trial met the primary endpoint of this study. The DCR was 70% (95% CI, 34.8–93.3; Fig. 1A–C). Seven (70%; 95% CI, 34.8–93.3) of 10 patients had an objective response according to the local investigator assessment.
Figure 1.
A, Waterfall plot of tumor response, showing the best percentage change in target lesion size as assessed by an independent central review. Cancer types are represented by different colors. The waterfall plot below shows the tumor tissue TMB (mutations/Mb), tumor tissue dMMR status (protein expression of MLH1, MSH2, MSH6, and PMS2), and tumor tissue MSI status for each patient. Patients with no data for either TMB, MMR, or MSI are shown in gray (for MMR and MSI) or shaded gray (for TMB). For the patient who achieved CR, the target lesions were lymph nodes. Thus, although the sum of target lesions did not reach 0 mm, the ORR was determined as “CR”. B, Spider plot of tumor response, showing longitudinal changes in target lesion size from baseline, assessed by an independent central review. C, Swimmer's plot of tumor response, showing time to response and duration of response.
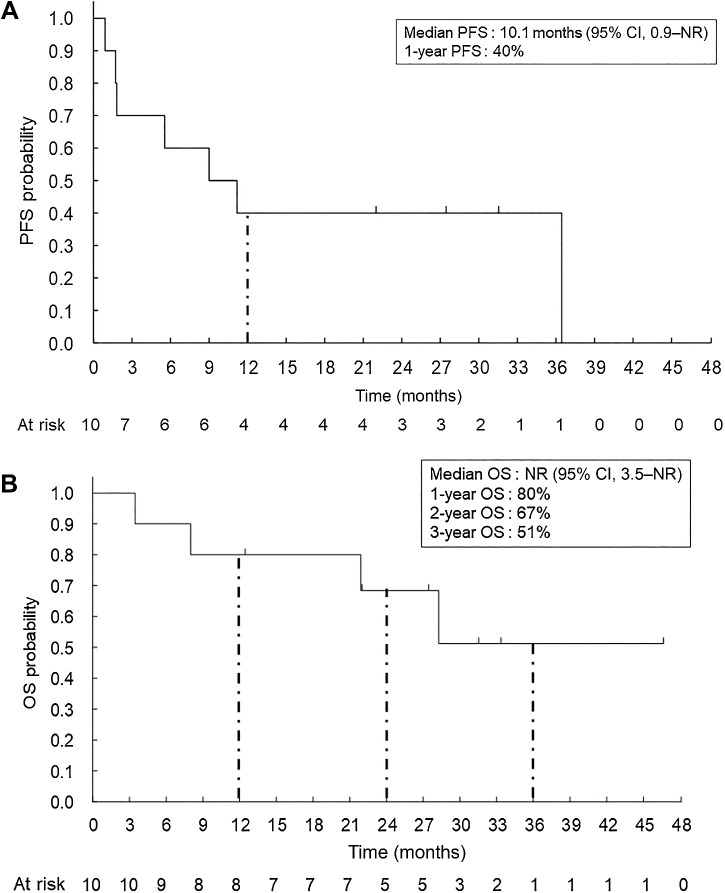
The median PFS was 10.1 months [95% CI, 0.9–not reached (NR)], and the median OS was not reached (95% CI, 3.5–NR; Fig. 2A and B). The probability of a 12-month OS was 80% (95% CI, 70.9–94.6).
Figure 2.
PFS and OS. Kaplan–Meier analysis for PFS (A) and OS (B) was performed according to the investigator assessments for the full analysis set (N = 10). One-year PFS rate is also shown. One-year OS, 2-year OS, and 3-year OS are also indicated.
The results of subgroup analyses are shown in Supplementary Table S3. All 5 patients (100%; 95% CI, 47.8–100) with a TMB ≥10/Mb achieved an objective response, whereas 1 patient with a TMB of <10/Mb did not achieve a response.
Among the 11 patients who received at least one dose of nivolumab, the major treatment-related adverse events of any grade were diarrhea [3 (27.3%)], rash [2 (18.2%)], increased aspartate aminotransferase [2 (18.2%)], and musculoskeletal and connective tissue disorders [2 (18.2%)]. No treatment-related grade 3–4 adverse events were observed. One grade 4 adverse event of hyperglycemia [1 (9.1%)] was observed but was not related to treatment (Table 2). Two (18.2%) patients discontinued treatment because of adverse events, including tumor pain and pneumonitis. No treatment-related deaths occurred during the study.
Table 2.
Treatment-related and immune-related adverse events.
| Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) | Any grade, n (%) | |
|---|---|---|---|---|---|
| Diarrhea | 3 (27.3) | 0 | 0 | 0 | 3 (27.3) |
| Rash | 2 (18.2) | 0 | 0 | 0 | 2 (18.2) |
| AST increased | 2 (18.2) | 0 | 0 | 0 | 2 (18.2) |
| Musculoskeletal stiffness | 2 (18.2) | 0 | 0 | 0 | 2 (18.2) |
| Nausea | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Vomiting | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Stomatitis | 0 | 1 (9.1) | 0 | 0 | 1 (9.1) |
| Pruritus | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Malaise | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Pyrexia | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Decreased appetite | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| ALT increased | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| White blood cell count decreased | 0 | 1 (9.1) | 0 | 0 | 1 (9.1) |
| Neutrophil count decreased | 0 | 1 (9.1) | 0 | 0 | 1 (9.1) |
| Lymphocyte count decreased | 0 | 1 (9.1) | 0 | 0 | 1 (9.1) |
| Anemia | 0 | 1 (9.1) | 0 | 0 | 1 (9.1) |
| Pneumonitis | 0 | 1 (9.1) | 0 | 0 | 1 (9.1) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Biomarker analysis
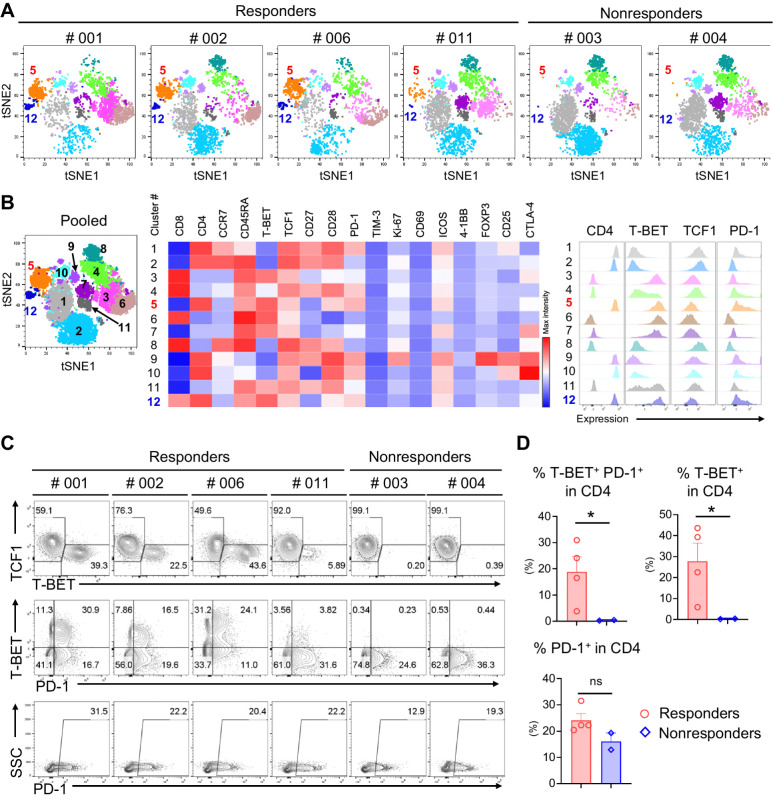
PBMCs were subjected to immune phenotypic analysis using multicolor flow cytometry (FCM) to determine the expression of 21 different molecules (Supplementary Table S1; Supplementary Fig. S3). Six samples were available from pretreatment patients for analysis: 4 responders (PR) and 2 nonresponders (PD). CD45+ CD3+ lymphocytes were examined using high-dimensional clustering of the t-SNE algorithm based on 17 downstream paramerters (14), which generated 12 clusters on a dimensionally reduced map (Fig. 3A and B; Supplementary Fig. S4). Clusters #5 (CD4+ T-bet+ TCF-7− PD-1++) and #12 (CD4+ T-bet+ TCF1− PD-1+) were highly enriched in responders compared with a lack of these clusters population in nonresponders. Further analysis of molecular expression levels by flow plot clearly demonstrated increased T-bet+ TCF-7− and T-bet+ PD-1+ populations in CD4+ T cells among responders, whereas a complete lack of the corresponding population was observed in nonresponders (Fig. 3C and D). Interestingly, the total PD-1 expression was similar among all patients, suggesting an important role for T-BET+ CD4+ T cells or T-BET+ PD-1+ CD4+ T cells in response to PD-1 blockade (Fig. 3C and D). On the basis of the results obtained for the CD4+ T cells, we applied the same analysis to the CD8+ T-cell population. Similar to the CD4+ T-cell population, the T-BET+ TCF1− and T-BET+ PD-1+ populations increased in responders but there was no statistical significance between responders and nonresponders (Supplementary Fig. S5A and S5B). In contrast to the CD4+ population, some T-BET+ T cells were detectable in nonresponders despite a lack of PD-1 expression in this population. T-BET is a member of the T-box family of transcription factors that regulates linage commitment in CD4 Th lymphocytes by activating the hallmark type-1 helper T cell (Th1) cytokine, IFNγ, which is critical for infection or tumor control (15, 16). It is known that T-BET is associated with much differentiated T-cell subsets such as effector memory T cells (T-eff). In contrast, TCF1 (encoded by the TCF7 gene) is highly expressed by naïve T cells and has a critical role in T-cell memory formation (17). TCF-1 induces the expression of Gata-3 and inhibits Th1 fate by suppressing IFNγ expression. Therefore, PD-1 expressing T-BET+ TCF1− T cells which are associated with good responses in this cohort potentially indicates preexisting antitumor T-cell responses.
Figure 3.
Immune profiling of PBMCs from patients at the baseline. t-SNE plots based on the multicolor FCM analysis of PBMCs at the baseline showing distinctly isolated 12 clusters (A and B). Cluster #5 (CD4+ T-BET+ TCF1− PD-1+ as shown in the mid heat map and the right histograms of B) and Cluster #12 (CD4+ T-BET+ TCF1− PD-1++) were highly enriched among the responders. Flow plots showed enriched T-BET+ or T-BET+ PD-1+ cells among responders and complete lack of these populations among nonresponders (C and D, top). PD-1 expression alone did not predict the responses (C and D, bottom). *, P < 0.05; ns, not significant.
To further elucidate the immune response after nivolumab treatment, four available pre- and posttreatment sample sets were subjected to FCM analysis as described above (Supplementary Fig. S3). PD-1 was no longer detectable after nivolumab administration, confirming the successful blockade of PD-1 by nivolumab (Supplementary Fig. S6A). There was a trend of T-cell differentiation: decreased CD45RA+ CD27+ naïve T cells and increased CD45RA− CD27+ central memory T cells (Supplementary Fig. S6B). In addition, there were trends of increased CD56+ NK cells and decreased CD19+ B cells after nivolumab therapy (Supplementary Fig. S6C). Although we were unable to identify biomarkers for response, as all available sample sets were collected from responders, we did confirm the successful blockade and immune responses to nivolumab therapy.
Discussion
In this prospective trial, we have confirmed the antitumor activity of nivolumab in patients with metastatic or unresectable advanced rare cancers harboring dMMR and/or MSI-H, who have no further standard treatment options. The trial achieved an ORR of 60% (95% CI, 26.2–87.8) and DCR of 70% (95% CI, 34.8–93.3), thus meeting its endpoint. In addition, the median PFS was 10.1 months (95% CI, 0.9–NR) and the median OS was not reached on the cutoff date. All treatment-related adverse events were manageable, and no treatment-related deaths were recorded.
Because the patient population was extremely small, it was important to use a nationwide network (11) and a rather simple screening method. Accordingly, IHC testing was used to detect MMR. In addition, we applied the Bayesian statistical method, which is one of the strengths of this study. Bayesian methods allow us to quantify and interpret the therapeutic effects of investigational drugs based on probability statements of the posterior distribution (18), thus facilitating trials with limited sample size (19). In contrast to clinical trials for common populations that evaluate clinical efficacy using the frequentist approach, the Bayesian approach allows us to estimate the posterior probability of the response rate exceeding a fixed threshold of the null response rate.
The response rate in this study (60%) compares well with previously reported single-arm phase II studies, such as the KEYNOTE-158 (8) and NCI-MATCH (EAY131; ref. 10) trials. Both trials included dMMR/MSI-H non-colorectal cancers, resulting in an overall response rate of 34.3% and 36%, respectively. Compared with other clinical trials of dMMR noncolorectal cancers, our cohort had a higher proportion of sarcoma patients, with two of the three showing a response. This highlights the need to test the MMR/MSI status of these types of cancer. In addition, our trial checked for three biomarkers of interest when applying ICIs: MMR, MSI, and TMB, as previous trials lacked information on all three. All patients with MSI-H status and TMB > 10 mut/Mb responded to nivolumab. However, 3 patients showing intratumoral discordance between biomarkers did not respond to nivolumab. A large retrospective study assessed the relationship between TMB and ICI efficacy in patients with previously treated advanced solid tumors enrolled in 12 trials, resulting in an ORR of 31.4% for TMB-H patients (20). Together, these data imply that approximately 70% of patients may not benefit from ICI despite the tumor being dMMR, MSI-H, or TMB. Each of these biomarkers provides distinct information about the genomic characteristics of a tumor and can have implications for prognosis, treatment selection, and response to immunotherapy. However, these biomarkers individually may not be sufficient to accurately predict efficacy.
In addition to tumor-intrinsic factors, the host immune status could serve as a potential biomarker for predicting responses to ICI (21). We applied highly multiplexed FCM analysis to identify the immune phenotypes of PBMCs and found that increased T-bet+ PD-1+ in CD4+ T cells at baseline could be a potential predictive biomarker for a preferable response to ICI. Interestingly, in our patient cohort, PD-1 expression alone could not serve as a predictive marker, suggesting a role for the T-bet-expressing T-cell population in response to PD-1 blockade. T-bet is a member of the T-box family of transcription factors that is critical for driving CD4+ Th1 differentiation by suppressing Th2/Th17 differentiation (22, 23). Although prior studies have demonstrated that CD8+ T cells are crucial for effective antitumor responses (24, 25), Th1 cells also play a distinct role in promoting the priming and differentiation of naïve CD8 T cells into tumor-reactive cytotoxic T cells during antigen presentation by producing cytokines such as IFNγ and IL2. They also contribute to the maturation and activation of DCs, leading to the DC licensing process (26). Indeed, a study by Alspach and colleagues (27) demonstrated that successful tumor rejection with ICIs also requires CD4+ T cells, which recruit and activate CD8+ T cells. They also found that ICIs were most effective when combined with a vaccine that incorporated targets of both CD8+ and CD4+ T cells.
Another important point is that our findings were obtained using PBMC samples. Markers from peripheral blood are much more affordable than those from tumors or TILs. In addition, our findings support a previous report that systemic CD4 immunity is a key contributor to ICI therapy. Interestingly, it has been reported that the ability of PD-1 blockade to rescue preexisting TILs from exhaustion might be limited, demonstrating that expanded TIL clones post-therapy arose from novel clonotypes recruited from the periphery (28). Taken together, our findings suggest that preexisting peripheral T-bet+ PD-1+ CD4+ T cells are potentially tumor-reactive and that invigorating this population by PD-1 blockade could induce antitumor responses. Therefore, it can be considered a predictive biomarker in advanced dMMR/MSI-H rare solid cancers.
The limitations of this study include its small sample size. For clinical trials targeting extremely small populations, a nationwide genomic analysis system (29) and a network of platform studies (11) to focus on these small fractions will be useful.
Nivolumab demonstrated high clinical benefit in advanced dMMR/MSI-H rare solid cancers. The specifically enriched T-bet+ PD-1+ CD4+ T cells observed at baseline of the responders supports the favorable clinical response of nivolumab treatment, although it has yet to be fully explored to make conclusive evidence as a predictive marker, due to the limited patient number. Further studies in a larger validation cohort would be required.
Supplementary Material
Supplementary Figure S1: Translation Research biomarker analysis procedure
Supplementary Figure S2: Disposition of Patients in the Study
Supplementary Figure S3: Gating strategy for immune phenotyping analysis.
Supplementary Figure S4: Heat map representation of each parameter in the tSNE plots.
Supplementary Figure S5: T-BET and PD-1 expression on the CD8+ T cells at the base line (related with the main figure 3).
Supplementary Figure S6: Immune responses after nivolumab treatment.
Supplementary Table S1 Antibodies used for immune phenotype analysis of peripheral blood mononuclear cells
Supplementary Table 2: Response rate assessed by independent central review.
Supplementary Table S3: Subgroup analysis of the response rate
Supplementary Table S4: Representativeness of Study Participants
Acknowledgments
This project supported by the Clinical Research Support Office, NCCH, Tokyo (sponsor), and funded by Ono Pharmaceutical Co., Ltd. (funder).
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Selected Articles from This Issue, p. 4991
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
H.S. Okuma reports other support from Ono Pharmaceutical during the conduct of the study. K. Watanabe reports grants from Ono Pharmaceutical during the conduct of the study; K. Watanabe also reports to be one of the founders and shareholders of Sustainable Cell Therapeutics. K. Tsuchihashi reports personal fees from Ono Pharma during the conduct of the study. R. Machida reports other support from Ono Pharmaceutical during the conduct of the study. A. Hirakawa reports personal fees from Ono Pharmaceutical, Astellas, AbbVie, Kissei Pharmaceutical, Nippon Shinyaku, Novartis, Kyowa Kirin, Chugai Pharmaceutical, Taiho Pharmaceutical, and Takeda Pharmaceuticals outside the submitted work. M. Kanai reports personal fees from Chugai Pharmaceutical, as well as other support from Therabiopharam outside the submitted work. M. Kamikura reports other support from Ono Pharmaceutical during the conduct of the study. K. Anjo reports other support from Ono Pharmaceutical during the conduct of the study. A. Hiramitsu reports other support from Ono Pharmaceutical during the conduct of the study. S. Sekine reports grants from AstraZeneca and Roche, as well as personal fees from MSD outside the submitted work. N. Okita reports grants from Ono Pharmaceutical during the conduct of the study. H. Mano reports grants from Ono Pharmaceutical, Daiichi Sankyo, PFDeNA, and Konica-Minolta, as well as personal fees from CureGene outside the submitted work. H. Nishikawa reports grants and other support from Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharmaceutical, and Merck Sharp and Dohme (MSD); other support from Amgen, Sustainable Cell Therapeutics, and Cellian-Biclo; and grants from Taiho Pharmaceutical, Daiichi Sankyo, Kyowa Kirin, Zenyaku Kogyo, Oncolys BioPharma, Debiopharma, Asahi-Kasei, Sysmex, Fujifilm, SRL, Astellas Pharmaceutical, Sumitomo Dainippon Pharma, Becton Dickinson outside the submitted work. K. Nakamura reports grants from Ono Pharmaceutical during the conduct of the study, as well as personal fees from Takeda, Chugai, AstraZeneca, Lilly, and Taiho outside the submitted work. K. Yonemori reports grants from Ono during the conduct of the study, as well as personal fees from Pfizer, Eisai, AstraZeneca, Novartis, Taiho, MSD, Eli Lilly, Daiichi Sankyo, Takeda, Ono, Chugai, and Fujifilm outside the submitted work. K. Yonemuri also reports consulting or advisory role from Chugai Pharma, Ono Pharmaceutical, Novartis, Eisai, and OncXerna Therapeutics, as well as research funding to the institution from Ono Pharmaceutical, MSD, Daiichi Sankyo/AstraZeneca, AstraZeneca/MedImmune, Taiho Pharmaceutical, Pfizer, Novartis, Takeda, Chugai Pharma, Sanofi, Seattle Genetics, Eisai, Lilly, Genmab, Boehringer Ingelheim, Kyowa Hakko Kirrin, Haihe Pharmaceutical, Nihonkayaku. No disclosures were reported by the other authors.
Disclaimer
The sponsor (Clinical Research Support Office, NCCH) had a role in study design and conduct of the study including data collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The funder (Ono Pharmaceutical Co., Ltd.) had no role in the conduct of the study: data collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Authors' Contributions
H.S. Okuma: Conceptualization, investigation, writing–original draft. K. Watanabe: Conceptualization, data curation. K. Tsuchihashi: Resources, investigation. R. Machida: Formal analysis. R. Sadachi: Formal analysis. A. Hirakawa: Formal analysis. H. Ariyama: Investigation. M. Kanai: Investigation. M. Kamikura: Data management. K. Anjo: Data monitoring. A. Hiramitsu: Data monitoring. S. Sekine: Investigation. N. Okita: Project administration. H. Mano: Supervision, funding acquisition. H. Nishikawa: Supervision. K. Nakamura: Resources, supervision, project administration. K. Yonemori: Conceptualization, supervision, writing–review and editing.
References
- 1. Pakish JB, Zhang Q, Chen Z, Liang H, Chisholm GB, Yuan Y, et al. Immune microenvironment in microsatellite-instable endometrial cancers: hereditary or sporadic origin matters. Clin Cancer Res 2017;23:4473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol 2015;1:1319–23. [DOI] [PubMed] [Google Scholar]
- 4. Huang RSP, Haberberger J, Severson E, Duncan DL, Hemmerich A, Edgerly C, et al. A pan-cancer analysis of PD-L1 immunohistochemistry and gene amplification, tumor mutation burden, and microsatellite instability in 48,782 cases. Mod Pathol 2021;34:252–63. [DOI] [PubMed] [Google Scholar]
- 5. Latham A, Srinivasan P, Kemel Y, Shia J, Bandlamudi C, Mandelker D, et al. Microsatellite instability is associated with the presence of lynch syndrome pan-cancer. J Clin Oncol 2019;37:286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cortes-Ciriano I, Lee S, Park WY, Kim TM, Park PJ. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun 2017;8:15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen HZ, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol 2017;2017:PO.17.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord J-P, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020;38:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanizaki J, Yonemori K, Akiyoshi K, Minami H, Ueda H, Takiguchi Y, et al. Open-label phase II study of the efficacy of nivolumab for cancer of unknown primary. Ann Oncol 2022;33:216–26. [DOI] [PubMed] [Google Scholar]
- 10. Azad NS, Gray RJ, Overman MJ, Schoenfeld JD, Mitchell EP, Zwiebel JA, et al. Nivolumab is effective in mismatch repair–deficient noncolorectal cancers: results from Arm Z1D-A subprotocol of the NCI-MATCH (EAY131) Study. J Clin Oncol 2020;38:214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okuma HS, Yonemori K, Narita SN, Sukigara T, Hirakawa A, Shimizu T, et al. MASTER KEY project: powering clinical development for rare cancers through a platform trial. Clin Pharmacol Ther 2020;108:596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 13. Thall PF, Simon R. Practical Bayesian guidelines for Phase IIB clinical trials. Biometrics 1994;50:337–49. [PubMed] [Google Scholar]
- 14. van der Maaten L, Hinton G. Visualizing data using t-SNE. J Mach Learn Res 2008;9:2579–605. [Google Scholar]
- 15. Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000;100:655–69. [DOI] [PubMed] [Google Scholar]
- 16. Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFNγ production in CD4 and CD8 T cells. Science 2002;295:338–42. [DOI] [PubMed] [Google Scholar]
- 17. Escobar G, Mangani D, Anderson AC. T cell factor 1: a master regulator of the T-cell response in disease. Sci Immunol 2020;5:eabb9726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirakawa A, Sato H, Igeta M, Fujikawa K, Daimon T, Teramukai S. Regulatory issues and the potential use of Bayesian approaches for early drug approval systems in Japan. Pharm Stat 2022;21:691–5. [DOI] [PubMed] [Google Scholar]
- 19. Nishikawa T, Hasegawa K, Matsumoto K, Mori M, Hirashima Y, Takehara K, et al. Trastuzumab deruxtecan for human epidermal growth factor receptor 2-expressing advanced or recurrent uterine carcinosarcoma (NCCH1615): the STATICE trial. J Clin Oncol 2023;41;2789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cristescu R, Aurora-Garg D, Albright A, Xu L, Liu XQ, Loboda A, et al. Tumor mutational burden predicts the efficacy of pembrolizumab monotherapy: a pan-tumor retrospective analysis of participants with advanced solid tumors. J Immunother Cancer 2022;10:e003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol 2020;21:1346–58. [DOI] [PubMed] [Google Scholar]
- 22. Saravia J, Chapman NM, Chi H. Helper T-cell differentiation. Cell Mol Immunol 2019;16:634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Basu A, Ramamoorthi G, Albert G, Gallen C, Beyer A, Snyder C, et al. Differentiation and regulation of T(H) cells: a balancing act for cancer immunotherapy. Front Immunol 2021;12:669474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, et al. Defining T-cell states associated with response to checkpoint immunotherapy in melanoma. Cell 2018;175:998–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1(-)CD8(+) tumor-infiltrating T cells. Immunity 2019;50:181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zuazo M, Arasanz H, Bocanegra A, Fernandez G, Chocarro L, Vera R, et al. Systemic CD4 immunity as a key contributor to PD-L1/PD-1 blockade immunotherapy efficacy. Front Immunol 2020;11:586907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M, Luoma AM, et al. MHC-II neoantigens shape tumor immunity and response to immunotherapy. Nature 2019;574:696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med 2019;25:1251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kohno T, Kato M, Kohsaka S, Sudo T, Tamai I, Shiraishi Y, et al. C-CAT: the national datacenter for cancer genomic medicine in Japan. Cancer Discov 2022;12:2509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Translation Research biomarker analysis procedure
Supplementary Figure S2: Disposition of Patients in the Study
Supplementary Figure S3: Gating strategy for immune phenotyping analysis.
Supplementary Figure S4: Heat map representation of each parameter in the tSNE plots.
Supplementary Figure S5: T-BET and PD-1 expression on the CD8+ T cells at the base line (related with the main figure 3).
Supplementary Figure S6: Immune responses after nivolumab treatment.
Supplementary Table S1 Antibodies used for immune phenotype analysis of peripheral blood mononuclear cells
Supplementary Table 2: Response rate assessed by independent central review.
Supplementary Table S3: Subgroup analysis of the response rate
Supplementary Table S4: Representativeness of Study Participants
Data Availability Statement
Raw data for this study were generated at National Cancer Center Hospital (NCCH), Tokyo. The data generated in this study are not publicly available due to restrictions of information that could compromise patient privacy and/or consent but are available upon reasonable request from the corresponding author. Please contact the corresponding author for requests of deidentified raw data.