Significance
Many enzymes that utilize oxygen and hydrogen peroxide generate powerfully oxidizing reactive intermediates that have the potential to damage the protein scaffold when reaction with substrate fails. A longstanding question about these enzymes is how they avoid self-destruction during catalysis. The cytochrome P450 monooxygenase from Priestia megaterium solves this problem by deactivating the reactive intermediate using electron transfer along a chain of tryptophan and tyrosine residues. When this chain is disrupted, the enzyme completes many fewer catalytic cycles before it is deactivated. Redox-active tryptophan/tyrosine chains could provide a general means of extending the functional lifetimes of oxidizing enzymes.
Keywords: cytochrome P450, tryptophan, tyrosine, total turnover number, protection
Abstract
Powerfully oxidizing enzymes need protective mechanisms to prevent self-destruction. The flavocytochrome P450 BM3 from Priestia megaterium (P450BM3) is a self-sufficient monooxygenase that hydroxylates fatty acid substrates using O2 and NADPH as co-substrates. Hydroxylation of long-chain fatty acids (≥C14) is well coupled to O2 and NADPH consumption, but shorter chains (≤C12) are more poorly coupled. Hydroxylation of p-nitrophenoxydodecanoic acid by P450BM3 produces a spectrophotometrically detectable product wherein the coupling of NADPH consumption to product formation is just 10%. Moreover, the rate of NADPH consumption is 1.8 times that of O2 consumption, indicating that an oxidase uncoupling pathway is operative. Measurements of the total number of enzyme turnovers before inactivation (TTN) indicate that higher NADPH concentrations increase TTN. At lower NADPH levels, added ascorbate increases TTN, while a W96H mutation leads to a decrease. The W96 residue is about 7 Å from the P450BM3 heme and serves as a gateway residue in a tryptophan/tyrosine (W/Y) hole transport chain from the heme to a surface tyrosine residue. The data indicate that two oxidase pathways protect the enzyme from damage by intercepting the powerfully oxidizing enzyme intermediate (Compound I) and returning it to its resting state. At high NADPH concentrations, reducing equivalents from the flavoprotein are delivered to Compound I by the usual reductase pathway. When NADPH is not abundant, however, oxidizing equivalents from Compound I can traverse a W/Y chain, arriving at the enzyme surface where they are scavenged by reductants. Ubiquitous tryptophan/tyrosine chains in highly oxidizing enzymes likely perform similar protective functions.
The oxygenation of Earth’s atmosphere by cyanobacteria some 2.4 billion years ago provided a powerful and highly reactive energy source for living organisms (1). This toxic atmospheric pollutant was likely fatal to many anaerobic organisms that had evolved in the prior anoxic environment. The evolutionary challenge was to exploit the power of this new oxidant while mitigating deleterious side effects (2, 3). Prime examples of adaptation to the oxygen atmosphere are the cytochromes P450 (P450). These heme-thiolate proteins are members of a superfamily of enzymes that consume NAD(P)H to catalyze the incorporation of one oxygen atom from O2 into an organic substrate, with the second oxygen atom released in a water molecule (4). The oldest P450s from the CYP51 family are involved in steroid synthesis (5) and are thought to have arisen early in the oxygenation of the atmosphere (6, 7). Today, more than 2,000 CYP families (enzymes within a family have >40% sequence identity) among all branches of life (8, 9) perform an extraordinarily diverse array of biological functions (10).
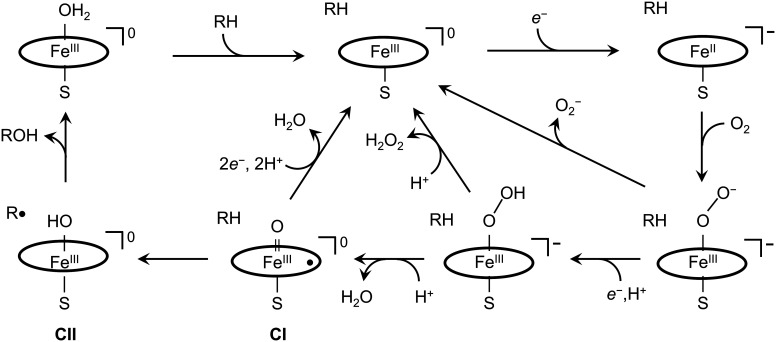
The canonical P450 catalytic mechanism involves no fewer than 6 carefully choreographed steps, including substrate binding and delivery of reducing equivalents (Fig. 1) (4). The key intermediate in catalysis is Compound I (CI) (11), a ferryl-porphyrin radical (FeIV(O)P•) that abstracts a hydrogen atom from the substrate (RH), producing FeIV(OH)P (Compound II, CII) and a substrate radical (R•). Hydroxyl rebound to R• forms the product (ROH) and regenerates the resting ferric enzyme.
Fig. 1.
Reaction pathways in cytochrome P450 catalysis. The peripheral pathway involves productive substrate (RH) hydroxylation using reducing equivalents (e−) sourced from NAD(P)H through a reductase. The three shunt pathways intercept intermediates producing superoxide, hydrogen peroxide, or water.
In the exhaustively studied bacterial enzyme from Pseudomonas putida (CYP101A1), d-camphor is hydroxylated with nearly perfect regio- and stereoselectivity, consuming one molecule each of NADPH and O2 and producing d-5-exo-hydroxycamphor and water (12). When an alternative substrate is used (e.g., norcamphor), however, selectivity is degraded and the tight stoichiometric coupling between NADPH and O2 consumption in relation to product formation is compromised (12–14). Indeed, subsequent studies have demonstrated that uncoupled catalysis is a common feature in many P450s (15). Three uncoupling pathways (shunts) identified in the P450 cycle (Fig. 1) produce superoxide, hydrogen peroxide, or water instead of hydroxylated substrate. We have suggested that electron transport chains in P450 composed of tryptophan (W) and tyrosine (Y) residues participate in the water-producing oxidase shunt to protect the enzyme from damage when substrate hydroxylation is impaired (16–21).
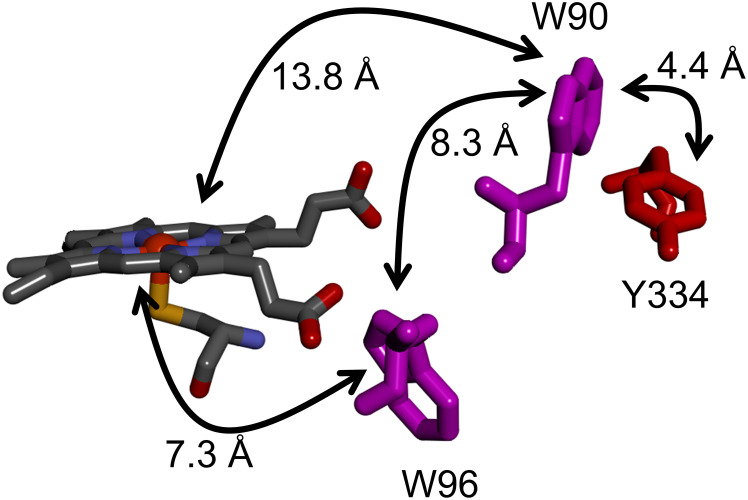
We chose cytochrome P450BM3 from Bacillus megaterium (now Priestia megaterium) (CYP102A1) to explore whether W/Y chains protect the enzyme from damage during catalysis. P450BM3 is a self-sufficient hydroxylase comprised of heme and reductase domains fused in a single polypeptide (22). Interestingly, sequence alignments and structural analyses indicate that both P450BM3 domains are more closely related to eukaryotic microsomal analogues than to prokaryotic enzymes (23–25). Catalysis is initiated by the addition of the substrate and NADPH to an enzyme solution in aerated buffer. Although the natural P450BM3 substrate is not known with certainty, the enzyme efficiently catalyzes hydroxylation of long-chain fatty acids at the ω-1, ω-2, and ω-3 positions (26). Analysis of the P450BM3 heme domain (27) revealed a potential electron-transport (ETr) chain comprised of W96/W90/Y334 provides a conduit for oxidizing equivalents (holes) to escape from the heme and migrate to the enzyme surface (Fig. 2) where they can be scavenged by cellular reductants (20). Using a spectroscopic probe of P450BM3 catalysis, we have examined the survival of the wild-type enzyme and several mutants to explore the potential protective role of this ETr chain.
Fig. 2.
Structural model of the W/Y electron transport chain in P450BM3 extending from the heme to the surface exposed Y334 residue. Electron transfer distances are taken from PDB ID 2IJ2, ref. 21. Sidechain solvent accessibilities are W96, 0.5%; W90, 34%; Y334, 32% (SI Appendix).
Results
The substrate in our investigations of P450BM3 catalysis is p-nitrophenoxydodecanoic acid (12-pNCA) (28). Hydroxylation of 12-pNCA at the ω-1 position produces an unstable hemiacetal that releases a spectrophotometrically detectable product, p-nitrophenolate. In prior work, we examined 12-pNCA oxidation kinetics and coupling efficiency catalyzed by wild-type (WT) P450BM3 and 3 mutant enzymes (W96H, W96H|W90F, W96H|W90F|Y334F). The activities of the mutant enzymes, defined by the ratio of Michaelis–Menten parameters kcat/KM, differed from that of WT by less than 50%. The coupling efficiency, defined as the yield of hydroxylated product divided by NADPH consumed, was (10 ± 2)% for all four enzymes. Overall, mutation of the residues in the ETr chain had very little impact on P450BM3 kinetics or product yield.
The superoxide and peroxide shunt pathways (Fig. 1) seemed the likely avenues for P450BM3 uncoupling with the 12-pNCA substrate. Analysis of H2O2 produced during enzyme turnover, however, revealed that it amounted to less than 10% of the NADPH consumed, indicating a minor uncoupling role for the superoxide and peroxide shunts. By elimination, then, the oxidase shunt in which two electrons and two protons are delivered to CI likely accounts for most of the uncoupling in P450BM3 catalyzed 12-pNCA oxidation. A critical distinction between the oxidase shunt and the superoxide and peroxide shunts is the ratio of NADPH to O2 consumption: 2:1 for the oxidase pathway and 1:1 for superoxide and peroxide. We measured rates of O2 and NADPH consumption during catalysis of 12-pNCA oxidation by P450BM3 and found a ratio of 1.8, confirming a dominant role for the oxidase uncoupling pathway.
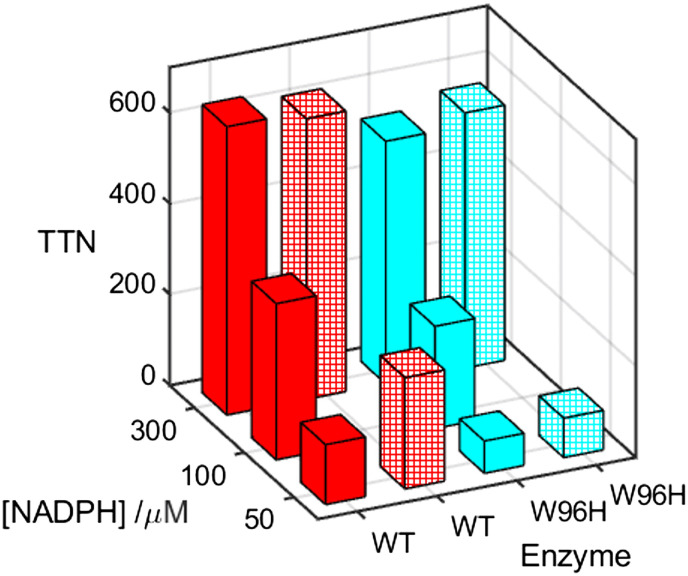
If uncoupled turnover through the oxidase shunt pathway protects the enzyme from damage in the event of impaired substrate oxidation, then its effectiveness should be apparent in the total number of turnovers (TTN) the enzyme performs before it is inactivated. In our prior investigation, we found that TTN values in W96H, W96H/W90F, and W96H/W90F/Y334 mutants were 50 to 70% of WT values (21). We have found that TTNs for P450BM3 catalyzed oxidation of 12-pNCA are a sensitive function of the NADPH concentration. With 300 μM NADPH, the enzyme catalyzes 635 ± 9 turnovers before inactivation. With 50 μM NADPH, however, the enzyme is inactivated after just 132 ± 9 turnovers (Table 1 and Fig. 3). If the W96|W90|Y334 ETr pathway is active in protecting the enzyme by shuttling holes to the enzyme surface, then mutating the first residue in the pathway should negatively impact TTN. At high NADPH concentration, introduction of the W96H mutation has a relatively minor effect on TTN, decreasing to 84% of the WT value. As the NADPH concentration is lowered, however, the impact of the W96H mutation increases such that when [NADPH] = 50 μM, TTN of the mutant is just 54% of that for WT.
Table 1.
Total turnover numbers (TTN) for WT and W96H catalyzed oxidation of 12-pNCA
| Enzyme |
[NADPH] /μM ([Asc] = 0) |
[NADPH] /μM ([Asc] = 100 μM) |
|||
|---|---|---|---|---|---|
| 50 | 100 | 300 | 50 | 300 | |
| WT P450BM3 | 132 ± 5 | 344 ± 20* | 635 ± 9 | 245 ± 24 | 618 ± 19 |
| W96H P450BM3 | 71 ± 4 | 225 ± 3* | 534 ± 7 | 87 ± 9 | 562 ± 24 |
*Ref. 21.
Fig. 3.
Total turnover numbers (TTN) for WT (red) and W96H (cyan) P450BM3 catalyzed aerobic oxidation of 12-pNCA (25 μM) at three NADPH concentrations in the absence (solid bars) and presence of 100 μM ascorbate (patterned bars).
The oxidase shunt pathway requires reducing equivalents to convert CI back to the resting ferric state of the enzyme. We explored the effect on TTN of added ascorbate (100 μM) for WT and W96H P450BM3. At high NADPH concentration, ascorbate has little impact on TTN for both WT and W96H enzymes. At low NADPH concentration, however, ascorbate nearly doubles TTN in the WT enzyme. This result contrasts with that of the W96H mutant where 100 μM ascorbate produces only a modest increase in TTN.
Discussion
Although the natural substrate for P450BM3 has not been identified definitively, the coupling efficiencies for hydroxylation of straight-chain fatty acids range from 34 ± 7% for lauric acid to 93 ± 3% for palmitic acid (29, 30). These coupling efficiencies parallel the dissociation constants of the acids (lauric acid, 43 μM; palmitic acid, 0.1 μM). The coupling efficiency for the 12-pNCA substrate is just 12%, indicating that introduction of the aromatic group at the end of the 12-carbon fatty acid further disrupts substrate binding. The ratio of NADPH to O2 consumption rates (1.8) strongly indicates that most of the uncoupling with 12-pNCA proceeds through the oxidase shunt.
The dependence of TTN on NADPH concentration implicates the natural electron transfer (ET) route from the reductase domain to the heme domain in the oxidase shunt pathway. The P450BM3 enzyme is an unusual homodimer in which the reductase domain of one monomer delivers electrons to the heme domain of the other (31, 32). This redox model contrasts with that of the more common P450s that obtain reducing equivalents from one or more separate redox partners (33). For these enzymes, low-driving-force interprotein ET is typically rate limiting in catalysis (34, 35). Elimination of the bimolecular step in P450BM3 produces relatively rapid delivery of reducing equivalents (k > 102 s−1) (25, 36, 37), making this enzyme among the fastest in the P450 superfamily. That the W96H mutation has little impact on enzyme survival at high NADPH concentration reflects the efficiency of electron delivery from the reductase domain. When the NADPH concentration is lowered to 50 μM, electron delivery from the reductase domain slows and TTN decreases. Under these conditions, the W96 residue is much more important for enzyme survival. When the NADPH concentration is low, addition of ascorbate produces a substantial increase in TTN for the WT enzyme but has little effect on the W96H mutant. These observations are consistent with a second oxidase shunt pathway involving the W96|W90|Y334 ETr chain.
We suggest that when CI fails to abstract a hydrogen atom from the substrate, W96 reduces the porphyrin radical, and the hole further migrates via W90 to the enzyme surface, forming a Y334 radical. NADPH is a two-electron reductant and likely could not readily reduce a tyrosine radical. Ascorbate, on the other hand, can reduce the tyrosine radical and protect the enzyme from damage. The W96H mutation disrupts this ETr pathway so that addition of ascorbate during enzyme catalysis does not substantially improve TTN. W96 appears to be the critical residue in the second oxidase shunt pathway. It is a highly conserved residue in CYP102, appearing in all but one of 250 sequences (SI Appendix). It is noteworthy that in bacterial P450 enzymes, a histidine residue appears more commonly at this location. In eukaryotes, however, tryptophan typically occupies this site (38). The other two sites in the putative W96|W90|Y334 ETr pathway are far less well conserved (W90, 20%; Y334 65%), implying that multiple hole migration routes to the surface might be available.
Concluding Remarks
Uncoupled substrate hydroxylation has been characterized in many cytochromes P450, and the oxidase shunt pathway frequently is involved (12, 13, 39–42). Our TTN determinations demonstrate that the oxidase shunt protects P450BM3 from inactivation during catalysis. Two oxidase pathways appear to operate in P450BM3. The first pathway exploits ET from the reductase domain to the heme. The uncommon fusion of reductase and heme domains in P450BM3 renders this intraprotein ET pathway particularly effective when the NADPH concentration is high. At low NADPH concentrations, a second protective pathway utilizing W96 becomes important. The biological significance of the two pathways depends on the intracellular concentration of NADPH in Priestia megaterium. Estimates of intracellular NADPH concentrations in bacterial cells fall in the 20- to 300-μM range (43–47). A similarly wide variation has been reported for eukaryotic cells (48–51). This broad concentration range depends on growth conditions, cell type, and analytical method and spans the regions of dominance for the two pathways. When NADPH is abundant, protection can be afforded by the reductase. When NADPH is scarce, the W96 pathway can protect P450BM3 by extracting reducing equivalents from the high intracellular concentration of glutathione (43) and delivering them to CI and CII before either highly oxidizing intermediate can damage the enzyme.
The fusion of reductase and oxidase domains into a single polypeptide in P450BM3 is rare. The usual delivery of electrons to cytochromes P450 involves reaction with a separate reductase. Bimolecular reduction of CI and CII by an enzyme diffusing in the cytoplasm or in a lipid membrane will be much less efficient than the intraprotein electron delivery in P450BM3. In cytochromes P450 lacking a fused reductase domain, W/Y hole transport pathways likely will assume a greater role in protecting the enzyme from damage during catalysis.
Our studies provide evidence that a hole transport pathway through W96 can extend the functional lifetime of P450BM3. A single protection event can, in principle, double the functional lifetime of an enzyme. The data, however, do not tell us whether the W96 pathway is an evolutionary adaption in Priestia megaterium or simply an in vitro curiosity. Recent studies have indicated that high enzyme replacement rates reduce an organism’s productivity (52). Detailed analysis of Lactococcus lactis growth revealed that protein synthesis and turnover consume at least half of cellular ATP (53). Comparison of two Saccharomyces cerevisiae strains demonstrated that biomass yields were lower in the strain with greater protein turnover (54, 55). The energetic burden that high protein turnover imposes on a cell suggests that protective mechanisms that extend enzyme lifetime provide a selective advantage to an organism. Many other oxidizing enzymes have highly reactive intermediates that potentially endanger their viability. Our analysis of protein structures found that W/Y chains are common in enzymes that utilize O2 as a substrate (16, 20). Results with P450BM3 support the hypothesis (16–20) that redox-active W/Y chains extend the functional lifetimes of oxidizing enzymes.
Materials and Methods
Wild-type and mutant proteins used in this study were prepared by heterologous expression in E. coli using standard procedures that have been described previously (21) and are described in further detail in SI Appendix. The amino acid and nucleotide sequences for wild-type P450BM3, as well as the primers used for site-directed mutants, are listed in SI Appendix. All proteins were purified by ion-exchange chromatography.
Total turnover numbers were evaluated spectroscopically by quantifying the total amount of p-nitrophenolate produced by P450BM3 with the 12-pNCA substrate. Reaction products were periodically separated from the enzyme by ultrafiltration. Reactions were continued until catalysis ceased (SI Appendix). Kinetics of O2 consumption were measured using a NeoFox optical oxygen sensing system (Ocean Optics, Largo, FL). NADPH consumption kinetics were measured spectrophotometrically. Hydrogen peroxide produced in P450BM3 catalysis was determined using an Abcam (Cambridge, UK) peroxide assay kit (ab272537). Calibration details are provided in SI Appendix.
Additional experimental details are provided in SI Appendix.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (PDF)
Acknowledgments
We thank Maryann Morales and Jill Clinton for invaluable laboratory assistance and many helpful discussions during this research. Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH under award number R01DK019038 (H.B.G. and J.R.W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional support for this work was provided by the Arnold and Mabel Beckman Foundation (H.B.G. and J.R.W.).
Author contributions
H.B.G. and J.R.W. designed research; R.R. and Y.S. performed research; R.R., H.B.G., and J.R.W. analyzed data; and R.R., H.B.G., and J.R.W. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: M.T.G., University of California, Irvine; and S.G.S., University of Illinois Urbana-Champaign.
Contributor Information
Harry B. Gray, Email: hbgray@caltech.edu.
Jay R. Winkler, Email: winklerj@caltech.edu.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information.
Supporting Information
References
- 1.Olejarz J., Iwasa Y., Knoll A. H., Nowak M. A., The Great Oxygenation Event as a consequence of ecological dynamics modulated by planetary change. Nat. Commun. 12, 3985 (2021), 10.1038/s41467-021-23286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer W. W., Hemp J., Valentine J. S., How did life survive Earth’s great oxygenation? Curr. Opin. Chem. Biol. 31, 166–178 (2016), 10.1016/j.cbpa.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Klinman J. P., How do enzymes activate oxygen without inactivating themselves? Acc. Chem. Res. 40, 325–333 (2007), 10.1021/ar6000507. [DOI] [PubMed] [Google Scholar]
- 4.Denisov I. G., Makris T. M., Sligar S. G., Schlichting I., Structure and chemistry of cytochrome P450. Chem. Rev. 105, 2253–2277 (2005), 10.1021/cr0307143. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida Y., et al. , Structural and evolutionary studies on sterol 14-demethylase P450 (CYP51), the most conserved P450 monooxygenase: II. Evolutionary analysis of protein and gene structures. J. Biochem. 122, 1122–1128 (1997), 10.1093/oxfordjournals.jbchem.a021870. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen B., Fletcher I. R., Brocks J. J., Kilburn M. R., Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455, 1101–1104 (2008), 10.1038/nature07381. [DOI] [PubMed] [Google Scholar]
- 7.Waldbauer J. R., Newman D. K., Summons R. E., Microaerobic steroid biosynthesis and the molecular fossil record of Archean life. Proc. Natl. Acad. Sci. U.S.A. 108, 13409–13414 (2011), 10.1073/pnas.1104160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson D. R., Cytochrome P450 diversity in the tree of life. Biochim. Biophy. Acta Proteins Proteom. 1866, 141–154 (2018), 10.1016/j.bbapap.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parvez M., et al. , Molecular evolutionary dynamics of cytochrome P450 monooxygenases across kingdoms: Special focus on mycobacterial P450s. Sci. Rep. 6, 33099 (2016), 10.1038/srep33099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werck-Reichhart D., Feyereisen R., Cytochromes P450: A success story. Gen. Biol. 1, REVIEWS3003 (2000), 10.1186/gb-2000-1-6-reviews3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittle J., Green M. T., Cytochrome P450 compound I: Capture, characterization, and C-H bond activation kinetics. Science 330, 933–937 (2010), 10.1126/science.1193478. [DOI] [PubMed] [Google Scholar]
- 12.Atkins W. M., Sligar S. G., Metabolic switching in cyctochrome P-450cam: Deuterium isotope effects on regiospecificity and the monooxygenase/oxidase ratio. J. Am. Chem. Soc. 109, 3754–3760 (1987), 10.1021/ja00246a038. [DOI] [Google Scholar]
- 13.Atkins W. M., Sligar S. G., Deuterium isotope effects in norcamphor metabolism by cytochrome P-450cam: Kinetic evidence for the two-electron reduction of a high-valent iron-oxo intermediate. Biochemistry 27, 1610–1616 (1988), 10.1021/bi00405a033. [DOI] [PubMed] [Google Scholar]
- 14.Kadkhodayan S., Coulter E. D., Maryniak D. M., Bryson T. A., Dawson J. H., Uncoupling oxygen transfer and electron transfer in the oxygenation of camphor analogues by cytochrome P450-CAM: Direct observation of an intermolecular isotope effect for substrate C-H activation. J. Biol. Chem. 270, 28042–28048 (1995), 10.1074/jbc.270.47.28042. [DOI] [PubMed] [Google Scholar]
- 15.Grinkova Y. V., Denisov I. G., McLean M. A., Sligar S. G., Oxidase uncoupling in heme monooxygenases: Human cytochrome P450 CYP3A4 in Nanodiscs. Biochem. Biophys. Res. Commun. 430, 1223–1227 (2013), 10.1016/j.bbrc.2012.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray H. B., Winkler J. R., Hole hopping through tyrosine/tryptophan chains protects proteins from oxidative damage. Proc. Natl. Acad. Sci. U.S.A. 112, 10920–10925 (2015), 10.1073/pnas.1512704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler J. R., Gray H. B., Electron flow through biological molecules: Does hole hopping protect proteins from oxidative damage? Quart. Rev. Biophys. 48, 411–420 (2015), 10.1017/s0033583515000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray H. B., Winkler J. R., The rise of radicals in bioinorganic chemistry. Isr. J. Chem. 56, 640–648 (2016), 10.1002/ijch.201600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray H. B., Winkler J. R., Living with oxygen. Acc. Chem. Res. 51, 1850–1857 (2018), 10.1021/acs.accounts.8b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray H. B., Winkler J. R., Functional and protective hole hopping in metalloenzymes. Chem. Sci. 12, 13988–14003 (2021), 10.1039/D1SC04286F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravanfar R., Sheng Y., Gray H. B., Winkler J. R., Tryptophan-96 in cytochrome P450 BM3 plays a key role in enzyme survival. FEBS Lett. 597, 59–64 (2023), 10.1002/1873-3468.14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narhi L. O., Fulco A. J., Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J. Biol. Chem. 261, 7160–7169 (1986). [PubMed] [Google Scholar]
- 23.Porter T. D., An unusual yet strongly conserved flavoprotein reductase in bacteria and mammals. Trends Biochem. Sci. 16, 154–158 (1991), 10.1016/0968-0004(91)90059-5. [DOI] [PubMed] [Google Scholar]
- 24.Lewis D. F. V., Watson E., Lake B. G., Evolution of the cytochrome P450 superfamily: Sequence alignments and pharmacogenetics. Mutat. Res. 410, 245–270 (1998), 10.1016/S1383-5742(97)00040-9. [DOI] [PubMed] [Google Scholar]
- 25.Whitehouse C. J. C., Bell S. G., Wong L. L., P450BM3 (CYP102A1): Connecting the dots. Chem. Soc. Rev. 41, 1218–1260 (2012), 10.1039/c1cs15192d. [DOI] [PubMed] [Google Scholar]
- 26.Miura Y., Fulco A. J., ω-1, ω-2 and ω-3 Hydroxylation of long-chain fatty acids, amides and alcohols by a soluble enzyme system from Bacillus megaterium. Biochim. Biophys. Acta Lipids Lipid Metab. 388, 305–317 (1975), 10.1016/0005-2760(75)90089-2. [DOI] [PubMed] [Google Scholar]
- 27.Girvan H. M., et al. , Structural and spectroscopic characterization of P450 BM3 mutants with unprecedented P450 heme iron ligand sets - New heme ligation states influence conformational equilibria in P450 BM3. J. Biol. Chem. 282, 564–572 (2007), 10.1074/jbc.M607949200. [DOI] [PubMed] [Google Scholar]
- 28.Schwaneberg U., Schmidt-Dannert C., Schmitt J., Schmid R. D., A Continuous spectrophotometric assay for P450 BM-3, a fatty acid hydroxylating enzyme, and its mutant F87A. Anal. Biochem. 269, 359–366 (1999), 10.1006/abio.1999.4047. [DOI] [PubMed] [Google Scholar]
- 29.Cryle M. J., De Voss J. J., Facile determination of the absolute stereochemistry of hydroxy fatty acids by GC: Application to the analysis of fatty acid oxidation by a P450BM3 mutant. Tetrahedron 18, 547–551 (2007), 10.1016/j.tetasy.2007.01.034. [DOI] [Google Scholar]
- 30.Cryle M. J., De Voss J. J., The role of the conserved threonine in P450BM3 oxygen activation: Substrate-determined hydroxylation activity of the Thr268Ala mutant. ChemBioChem 9, 261–266 (2008), 10.1002/cbic.200700537. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H., et al. , The full-length cytochrome P450 enzyme CYP102A1 dimerizes at its reductase domains and has flexible heme domains for efficient catalysis. J. Biol. Chem. 293, 7727–7736 (2018), 10.1074/jbc.RA117.000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su M., Chakraborty S., Osawa Y., Zhang H., Cryo-EM reveals the architecture of the dimeric cytochrome P450 CYP102A1 enzyme and conformational changes required for redox partner recognition. J. Biol. Chem. 295, 1637–1645 (2020), 10.1074/jbc.RA119.011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook D. J., Finnigan J. D., Cook K., Black G. W., Charnock S. J., “Chapter Five–Cytochromes P450: History, classes, catalytic mechanism, and industrial application” in Advances in Protein Chemistry and Structural Biology, Christov C. Z., Ed. (Academic Press, 2016), vol. 105, pp. 105–126. [DOI] [PubMed] [Google Scholar]
- 34.Purdy M. M., Koo L. S., Ortiz de Montellano P. R., Klinman J. P., Steady-state kinetic investigation of cytochrome P450cam: Interaction with redox partners and reaction with molecular oxygen. Biochemistry 43, 271–281 (2004), 10.1021/bi0356045. [DOI] [PubMed] [Google Scholar]
- 35.Purdy M. M., Koo L. S., Ortiz de Montellano P. R., Klinman J. P., Mechanism of O2 activation by cytochrome P450cam studied by isotope effects and transient state kinetics. Biochemistry 45, 15793–15806 (2006), 10.1021/bi061726w. [DOI] [PubMed] [Google Scholar]
- 36.Sevrioukova I., Shaffer C., Ballou D. P., Peterson J. A., Equilibrium and transient state spectrophotometric studies of the mechanism of reduction of the flavoprotein domain of P450BM-3. Biochemistry 35, 7058–7068 (1996), 10.1021/bi960060a. [DOI] [PubMed] [Google Scholar]
- 37.Hazzard J. T., Govindaraj S., Poulos T. L., Tollin G., Electron transfer between the FMN and heme domains of cytochrome P450BM-3: Effects of substrate and CO. J. Biol. Chem. 272, 7922–7926 (1997), 10.1074/jbc.272.12.7922. [DOI] [PubMed] [Google Scholar]
- 38.Sørensen M. L. H., et al. , Hole hopping through cytochrome P450. J. Phys. Chem. B 124, 3065–3073 (2020), 10.1021/acs.jpcb.9b09414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Matteis F., Ballou D. P., Coon M. J., Estabrook R. W., Haines D. C., Peroxidase-like activity of uncoupled cytochrome P450 Studies with bilirubin and toxicological implications of uncoupling. Biochem. Pharmacol. 84, 374–382 (2012), 10.1016/j.bcp.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Loida P. J., Sligar S. G., Molecular recognition in cytochrome P-450: Mechanism for the control of uncoupling reactions. Biochemistry 32, 11530–11538 (1993), 10.1021/bi00094a009. [DOI] [PubMed] [Google Scholar]
- 41.Yeom H., Sligar S. G., Oxygen activation by cytochrome P450BM-3: Effects of mutating an active site acidic residue. Arch. Biochem. Biophys. 337, 209–216 (1997), 10.1006/abbi.1996.9763. [DOI] [PubMed] [Google Scholar]
- 42.Pompon D., Rabbit liver cytochrome P-450 LM2: Roles of substrates, inhibitors, and cytochrome b5 in modulating the partition between productive and abortive mechanisms. Biochemistry 26, 6429–6435 (1987), 10.1021/bi00394a020. [DOI] [PubMed] [Google Scholar]
- 43.Bennett B. D., et al. , Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 5, 593–599 (2009), 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.García-Calvo L., et al. , Central carbon metabolite profiling reveals vector-associated differences in the recombinant protein production host Escherichia coli BL21. Front. Chem. Engineering 5, (2023), 10.3389/fceng.2023.1142226. [DOI] [Google Scholar]
- 45.Røst L. M., Shafaei A., Fuchino K., Bruheim P., Zwitterionic HILIC tandem mass spectrometry with isotope dilution for rapid, sensitive and robust quantification of pyridine nucleotides in biological extracts. J. Chromatography B 1144, 122078 (2020), 10.1016/j.jchromb.2020.122078. [DOI] [PubMed] [Google Scholar]
- 46.Thorfinnsdottir L. B., García-Calvo L., Bø G. H., Bruheim P., Røst L. M., Optimized fast filtration-based sampling and extraction enables precise and absolute quantification of the Escherichia coli central carbon metabolome. Metabolites 13, 150 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka K., et al. , Quantification of NAD(P)H in cyanobacterial cells by a phenol extraction method. Photosynth. Res. 148, 57–66 (2021), 10.1007/s11120-021-00835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar K., Bruheim P., Large dependency of intracellular NAD and CoA pools on cultivation conditions in Saccharomyces cerevisiae. BMC Res. Notes 14, 372 (2021), 10.1186/s13104-021-05783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu W., Wang L., Li C., Hui S., Rabinowitz J. D., Extraction and quantitation of nicotinamide adenine dinucleotide redox cofactors. Antioxid. Redox Signal. 28, 167–179 (2018), 10.1089/ars.2017.7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seifar R. M., et al. , Quantitative analysis of intracellular coenzymes in Saccharomyces cerevisiae using ion pair reversed phase ultra high performance liquid chromatography tandem mass spectrometry. J. Chromatography A 1311, 115–120 (2013), 10.1016/j.chroma.2013.08.076. [DOI] [PubMed] [Google Scholar]
- 51.Tao R., et al. , Genetically encoded fluorescent sensors reveal dynamic regulation of NADPH metabolism. Nat. Methods 14, 720–728 (2017), 10.1038/nmeth.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanson A. D., et al. , The number of catalytic cycles in an enzyme’s lifetime and why it matters to metabolic engineering. Proc. Natl. Acad. Sci. U.S.A. 118, e2023348118 (2021), 10.1073/pnas.2023348118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lahtvee P.-J., Seiman A., Arike L., Adamberg K., Vilu R., Protein turnover forms one of the highest maintenance costs in Lactococcus lactis. Microbiol. 160, 1501–1512 (2014), 10.1099/mic.0.078089-0. [DOI] [PubMed] [Google Scholar]
- 54.Hong K.-K., Hou J., Shoaie S., Nielsen J., Bordel S., Dynamic 13C-labeling experiments prove important differences in protein turnover rate between two Saccharomyces cerevisiae strains. FEMS Yeast Res. 12, 741–747 (2012), 10.1111/j.1567-1364.2012.00823.x. [DOI] [PubMed] [Google Scholar]
- 55.Canelas A. B., et al. , Integrated multilaboratory systems biology reveals differences in protein metabolism between two reference yeast strains. Nat. Commun. 1, 145 (2010), 10.1038/ncomms1150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (PDF)
Data Availability Statement
All study data are included in the article and/or supporting information.