Significance
Individuals differ considerably in their motivation and ability to tackle physical or cognitive challenges (e.g., professional athletes, scholars), and these differences are implicated in psychiatric symptoms. Although the subjective experience of effort is ubiquitous, the processes underlying effort-based decision making are not fully understood, including the relationship between individual differences in willingness to exert cognitive and physical effort. We developed a task that measures effort-based decisions indirectly using patch foraging. We found that cognitive and physical effort avoidance (i.e., costs) were positively correlated, suggesting these are processed in common terms. However, cognitive and physical effort measures appeared to have distinct relationships to self-report measures of motivation and affect, suggesting these may be useful in dissociating factors underlying psychiatric symptoms.
Keywords: cognitive control, effort-based decision-making, foraging, motivation, computational psychiatry
Abstract
Effort-based decisions, in which people weigh potential future rewards against effort costs required to achieve those rewards involve both cognitive and physical effort, though the mechanistic relationship between them is not yet understood. Here, we use an individual differences approach to isolate and measure the computational processes underlying effort-based decisions and test the association between cognitive and physical domains. Patch foraging is an ecologically valid reward rate maximization problem with well-developed theoretical tools. We developed the Effort Foraging Task, which embedded cognitive or physical effort into patch foraging, to quantify the cost of both cognitive and physical effort indirectly, by their effects on foraging choices. Participants chose between harvesting a depleting patch, or traveling to a new patch that was costly in time and effort. Participants’ exit thresholds (reflecting the reward they expected to receive by harvesting when they chose to travel to a new patch) were sensitive to cognitive and physical effort demands, allowing us to quantify the perceived effort cost in monetary terms. The indirect sequential choice style revealed effort-seeking behavior in a minority of participants (preferring high over low effort) that has apparently been missed by many previous approaches. Individual differences in cognitive and physical effort costs were positively correlated, suggesting that these are perceived and processed in common. We used canonical correlation analysis to probe the relationship of task measures to self-reported affect and motivation, and found correlations of cognitive effort with anxiety, cognitive function, behavioral activation, and self-efficacy, but no similar correlations with physical effort.
People make effort-based decisions every day, weighing the potential rewards associated with an action against the effort it requires. These decisions can involve cognitive effort, physical effort, or both. Economic utility theory has been productively applied in effort-based decision-making research: People seek to maximize reward while minimizing effort, which can be accomplished by computing an “expected value” of effort (1–5). In these theories, effort is described as costly, reducing the value of rewards. Evidence from cognitive psychology and neuroscience shows that people consistently factor effort into their decisions but that individuals approach tradeoffs between rewards and cognitive(6, 7) and physical (8, 9) effort differently. Effort-related behaviors range from effort seeking (e.g., running a marathon, writing a book) to effort avoiding (e.g., sedentary behavior, academic procrastination). There is much to learn about the trait and state factors that lead to effort seeking versus avoiding. Advancing knowledge of effort-based decision making is important because of evidence demonstrating proxies of cognitive and physical effort costs are related to psychiatric symptoms, including negative symptoms of schizophrenia (10–12). Physical (13–16) and cognitive (16–20) effort avoidance has been found to be increased in clinical depression. Depression is heterogeneous across symptom domains (presence of apathy, anhedonia, anxiety, cognitive function symptoms, and others). Relatively little is known about the shared versus distinct contributions of cognitive and physical effort costs to specific affect and motivation symptoms. Understanding this structure would be valuable for identifying “subtypes” of depression, transdiagnostic approaches, and precision psychiatry. Psychiatric symptom relationships to physical and cognitive effort decision making have mostly been studied separately (though see refs. 16 and 18). Here, we address this gap by measuring cognitive and physical effort avoidance and self-reported motivation and affect within an individual in a large online sample.
The reinforcement learning literature has been concerned with the extent to which there is a common representation of “value” that integrates different kinds of rewards (e.g., food, money, refs. 21, 22, 23). To what extent is there also a common representation of “cost” that integrates different domains of effort and other costs associated with an activity (i.e., cognitive and physical effort, and time costs, refs. 16 and 24)? Relatedly, how are effort costs common or different across the wide range of tasks that can be considered “physical” or “cognitive”? Do cognitive and physical effort constitute separate domains or is there a different organizational principle?
Human and animal research suggests that cognitive and physical effort-based decisions are controlled by shared neural populations (24–28). Individual differences provide a window into the relationship between different domains of cost. Do individuals who avoid cognitive effort more also avoid physical effort more? Research is limited about the relationship between individual differences in cognitive and physical effort costs. Lopez-Gamundi and Wardle (49) found a positive relationship (r 0.43) between the percentage of “hard task choices” in the cognitive (task-switching task) and physical (rapid key-pressing task) versions of the Effort Expenditure for Rewards Task [this finding was replicated in ref. 29, r 0.35).
We offer an approach to these questions by making use of a variant of a decision task from the foraging literature, which offers a distinct approach to studying how organisms integrate rewards and costs. The study of foraging using serial decision problems originated in ethology and is noteworthy for strong theoretical foundations and ecological validity (30). More recently, it has been increasingly adopted in psychology, neuroscience, and neuroeconomics (31–33), but compared to other aspects of reinforcement learning, has not widely been used in studies of individual differences. In classic ecological contexts, foraging tasks study how organisms optimize some fitness objective (e.g., maximizing reward rate), while balancing rewards (e.g., food) and costs (especially time). Foraging-style tasks have proven to be valuable in understanding decision making in formally rigorous terms, and relating it to underlying neural mechanisms, across a variety of species, including rodents (34, 35), nonhuman primates (36, 37) and humans (38–41). However, most human foraging tasks have manipulated rewards and time costs, but not effort costs. Here, we expand the study of human patch foraging to include effort.
The Effort Foraging Task.
In the present study, we developed an “Effort Foraging Task” designed to leverage the strengths of the patch foraging paradigm (i.e., ecologically valid serial decisions, well-developed formal frameworks, and common neural and cognitive substrates across a range of settings, refs. 30, 31, 32, 35, 38, 42 and 43). The task had two variants—cognitive and physical—which we used to fit a computational model to individual participants’ behavior to estimate the costs associated with each form of effort. Within this model, we evaluated the correlation between the estimated individual differences in cognitive and physical effort costs.
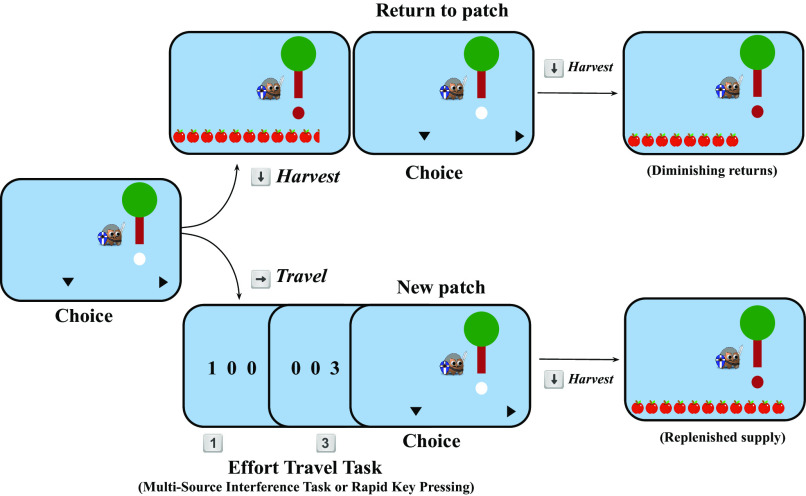
The Effort Foraging Task adapts a version of the computerized patch-foraging task developed by Constantino and Daw (44), adding travel costs in the form of cognitively and physically effortful tasks. In a standard patch foraging task, the forager visits a “patch” which can be harvested to yield rewards (here, a simulated orchard with apple trees). Within a given patch, the marginal return (apples) associated with each successive harvest decreases over time. At any point, the forager can travel to a new patch, which has replenished rewards, but it takes time to travel there. Deciding when to leave a depleting patch in a foraging environment involves tradeoffs between harvesting rewards available from the current patch, and the time spent traveling to a different (but richer) one. For this reason, the level at which the forager decides to exit the current patch (i.e., their “exit threshold” or the number of apples they have last received before quitting) reflects the reward they are willing to forgo by leaving that patch and spending the time to travel to another. In these respects, the exit threshold reveals the point of equivalence in the tradeoff between the cost of harvesting with diminishing rewards and the time cost of traveling to a new patch.
These considerations are formalized in the Marginal Value Theorem (45), which asserts that a simple threshold policy maximizes reward rate. The forager simply needs to maintain an estimate of the average reward rate in the environment and exit a patch when the instantaneous reward rate falls below the long-run average.
| [1] |
According to (Eq. 1), reward rate is maximized when the exit threshold (, the reward level at which the forager exits) is equal to the long-run average reward rate, which includes the sum of all rewards () minus sum of (nontime) costs incurred in the environment (, e.g., energy spent extracting rewards or traveling to the next patch), divided by the total number of harvest periods (the time cost normalized by the harvest time, total time/harvest time).
Constantino and Daw (44) found that human participants playing a virtual foraging game used a threshold exit strategy consistent with the Marginal Value Theorem, which explained behavior better than other reinforcement learning models (e.g., temporal difference learning). Furthermore, they found that exit thresholds (the reward expected to receive by harvesting when participants chose to travel to a new patch) shifted reliably and in predicted ways when the environment changed (e.g., when travel time and/or reward depletion was experimentally manipulated). For example, when the travel time between patches was increased, participants’ exit thresholds decreased, reflecting the increased opportunity cost of travel time and an overall decrease in average reward rate.
For the Effort Foraging Task, rather than manipulating travel time, we manipulated travel costs by varying the effort—cognitive or physical—required to travel between patches and compared exit thresholds in high versus low effort conditions. In line with MVT, which predicts an increase in travel costs to result in a lower exit threshold, we predicted that contexts with higher effort costs would, in general, decrease participants’ estimates of average reward rate, leading the exit threshold to be lower; that is, a greater willingness to accept diminishing rewards to avoid effortful travel. Conversely, some participants might instead exhibit effort-seeking, i.e., treat the low effort task as more costly, exiting at a lower threshold in low compared to high effort conditions (as seen in ref. 46). Accordingly, we used the difference in exit threshold between high and low effort conditions to infer the perceived costs of travel.
More specifically, we used participants’ decision thresholds to create a computational model based on the Marginal Value Theorem to quantify the added cost of high compared to low effort conditions in this task. Using this model, we found that most participants avoided the high effort tasks, treating them as costly, while some participants sought high-effort tasks, treating them as valued. We directly fit the correlation between individual differences in cognitive and physical effort costs in the same currency (money) and found a moderate positive correlation.
In developing the task, we conducted four experiments (complete details in SI Appendix, Text 5). Experiment 1 (N 678) is the focus of our results as it has the largest sample size and is the most efficient version to administer (16 min of main task time per effort type). Experiments 2–4 were conducted largely to validate results and rule out confounds. Specifically, Experiment 2 (N 116) serves to demonstrate the generalizability of the method to multiple cognitive effort tasks exercising multiple cognitive processes (interference control, in Experiment 1, working memory in Experiment 2); Experiment 3 (N 43) verifies that standard patch-foraging manipulations are effective in the novel effort context (thresholds sensitive to patch richness); and Experiment 4 (N 71) addresses the possibility that effort avoidance might be confounded by differences in subjective travel duration perception across effort levels.
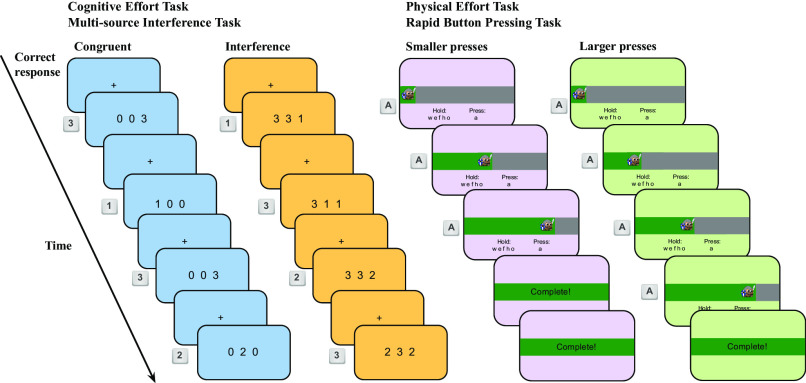
Effort level was manipulated block-wise (Fig. 1, Experiment 1, 4-min blocks). In Experiment 1, the cognitive effort variant required performing trials of the Multi-Source Interference Task [MSIT, Fig. 2, ref. 47]. The high effort condition required completing interference trials (demanding more cognitive effort), and the low effort condition required completing congruent trials (demanding relatively less cognitive effort). The physical variant of the task required participants to rapidly press a key to reach a new patch (Fig. 2, Right, based on previous research demonstrating that rapid key-pressing is physically effortful and costly, ref. 8). The high effort condition required participants to press the key the maximum number of times they could in the time allotted (individually determined in a preceding calibration phase), and the low effort condition required half that number of key-presses. Travel time (i.e., time to complete the MSIT or key-pressing tasks) was fixed and the same across both variants of the task and the high and low effort conditions of each. We predicted effort-avoiding participants would have a lower patch leaving threshold (in units of apples) in the high effort conditions compared to the low effort conditions, since travel (effort) costs were greater in the former. The measure of effort cost for an individual was the differential travel cost of the more effortful condition (incongruent MSIT, or Larger Number of Presses) compared to the less effortful condition (congruent MSIT, or Smaller Number of Presses).
Fig. 1.
Foraging trial diagram. On each trial, participants chose to harvest the tree they were at (down arrow key) or travel to a new tree (right arrow key), during the travel they completed an effortful task, after which they arrived at a new patch with a replenished supply of apples.
Fig. 2.
Effort travel tasks Experiment 1. Left: Cognitive Effort, Multi-source Interference Task. Participants identified which number was the oddball in a list of three numbers. The background color differed for the high effort (interference trials, orange) and low effort (congruent trials, blue) conditions. They responded with the “1,” “2,” and “3” keys. Interference trials have a competing distractor response for which the oddball target is flanked by the distractor and in the spatial position of the distractor. The correct response for each example screen is displayed on the left of that example screen. Right panel: Physical Effort, Rapid Key-pressing Task. Participants rapidly pressed the “a” key while holding down the “w,” “e,” “f,” “h,” and “o” keys. Pressing the “a” key moved the avatar rightward and filled up the gray horizontal bar with green. When participants reached the goal number of presses, “Complete!” appeared in the horizontal bar and participants waited for the remainder of the travel time. The background color differed for the high effort (smaller presses, purple) and low effort (larger presses, green) conditions.
Theorized advantages compared to previous tasks.
The problem of demand characteristics, which cue participants to the purpose of the study and change how participants behave (48), can be problematic for assessing participants’ motivation. For this reason, there has been a shift from relying strictly on self-reports toward using laboratory tasks. Most effort decision making tasks directly ask participants to choose between low effort/low reward and high effort/high reward options (7, 8, 49–51). Assessing preferences in this way is still subject to demand characteristics, which may reduce construct and convergent validity (52–55). In addition, decisions that explicitly trade off numeric quantities (56, 57) are susceptible to idiosyncratic arithmetic heuristics (58). We developed the Effort Foraging Task to measure preferences indirectly, based on the effect of effort requirements on foraging behavior. We theorized that by measuring effort preferences indirectly, we could get closer to preferences that directly drive behavior, and thus increase task validity.
Direct tasks, such as the Cognitive Effort Discounting Paradigm (7) or the Effort Expenditure for Rewards Task (8) may engage real-world economic considerations (e.g., that one should be paid more to work more), which may obscure or interfere with effort seeking behaviors that may otherwise occur in naturalistic settings. This is consistent with the observation that effort seeking is not reported in direct tasks (i.e., reverse effort discounting, preferring to do a more demanding task for less money over a less demanding task for more money refs. 7 and 8. Interestingly, in the results reported below, we consistently found a subset of participants who appeared to be effort-seeking in this task, in line with the idea that secondary demand characteristics obscure or interfere with effort seeking behavior in explicit tasks. Another potential problem is that some existing tasks have involved hypothetical choices, or choices separated in time from a later realization of cognitive effort (7, 49). In the Effort Foraging Task, participants experience the effort directly and immediately, after each choice to travel.
Another concern common to previous studies is the presentation of two options simultaneously, as this may distort choices or complicate their interpretation (6, 7, 49). Research in intertemporal choice has shown that rodents and primates (including humans) are less impulsive decision makers when they are making serial rather than simultaneous choices. It has been hypothesized that this is because serial decision making is more ecologically valid (evaluating each single option in isolation and choosing to accept or reject it and search for another, refs. 35, 42, and 43). The Effort Foraging Task is serial, as participants decide to either accept the current harvest value or reject it and travel to a new patch (see also refs. 56 and 57). Formal analysis of these foraging decisions using the Marginal Value Theorem provides a theoretically motivated and quantitatively rigorous approach to measuring effort costs.
Results
The primary dependent variable in our analyses was exit thresholds (the expected reward for harvesting when participants chose to travel), which reflect the point when the cost of leaving just offsets the benefits of reaching a replenished patch (which is increasing as the current patch yields progressively less). In line with the Marginal Value Theorem (1), we assumed that participants set their exit thresholds to maximize the rate of rewards minus costs per time step. As all of the reward rate variables are observed, this allows us to solve for the subjective cost () that best rationalizes the observed exit behavior. We implemented a computational model based on the Marginal Value Theorem and fit it to participants’ exit thresholds to quantify the relative increase in travel cost between the high and low effort conditions for each effort type (Analysis Methods, Hierarchical Bayesian Marginal Value Theorem model).
Summary of Results.
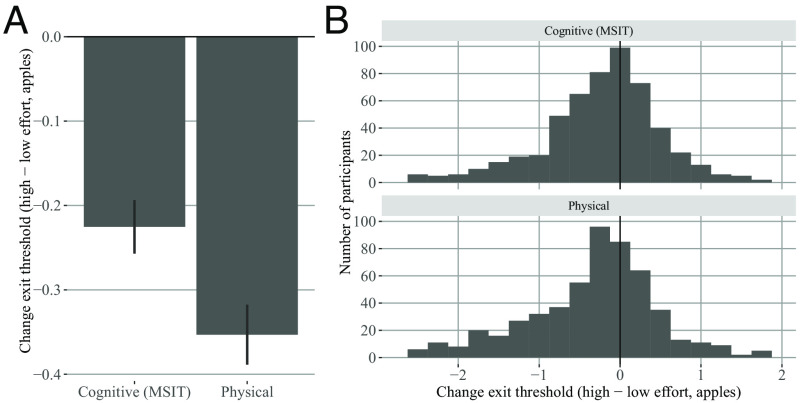
We found that differences in foraging decisions (viz., exit thresholds) are a useful indirect measure of motivation to exert both cognitive and physical effort. Consistent with our prediction, average exit threshold was lower in the higher travel effort than the lower travel effort conditions (Fig. 3). Participants (Experiment 1, N 537) opted to stay longer in a patch, accepting diminishing rewards, in the high travel effort conditions to avoid the increased cost of travel. Results from Experiments 2–4 confirmed that participants’ behavior remains similar across manipulations of cognitive effort type [Experiment 2 (N-Back)], environment richness [Experiment 3 (Richness)], and with explicit travel time instructions (Experiment 4). Fits of the Marginal Value Theorem model to trial-by-trial behavior [Experiment 1 (MSIT)] further confirmed the presence of high effort costs and revealed an interesting mixture of effort-avoiding and effort-seeking participants. We found that cognitive and physical effort costs were moderately positively correlated, but that only cognitive effort cost was strongly correlated with self-report measures related to motivation and affect.
Fig. 3.
Change in exit thresholds by effort condition. Experiment 1 (MSIT). (A) y-axis: Group-level mean change in exit threshold for cognitive and physical effort. x-axis: effort type. As predicted, on average participants exhibited lower exit thresholds in the high relative to low effort conditions. Error bars indicate SEM. (B) Individual variation in change in exit threshold. Top row: Histogram of participants mean change in exit threshold for cognitive high effort relative to cognitive low effort. Bottom row: Mean change in exit threshold for physical high effort relative to physical low effort. Most participants were effort-avoiding (negative change in threshold), whereas some participants showed indifference to effort condition (near zero) or were effort-seeking (positive change in threshold).
We used a data-driven approach—canonical correlation analysis—to look for shared versus distinct relationships between cognitive and physical effort task behavior and self-report surveys of motivation and affect. Results support external validity of the cognitive effort measures and extend the picture of the interrelationships of effort decision-making variables to include anxiety, cognitive function, behavioral activation, and self-efficacy.
Change in Exit Threshold by Effort Condition.
As a model-agnostic metric of high effort cost, we used the change in exit threshold from low to high effort conditions. For each participant, we computed the average exit threshold per condition (see overall threshold results in SI Appendix, Figs. S1 and S2), and the difference between them (high effort–low effort mean threshold). We expected this value to be negative, reflecting effort avoidance. If threshold increased for a participant, this suggested effort seeking. Across participants, we found a mix of effort avoidance, effort seeking, and indifference to effort (values close to zero) (Fig. 3, Right). We computed the group average change in threshold (Fig. 3) and used linear mixed-effects regression to test whether change in exit thresholds significantly differed from zero. As predicted, on average, participants exited trees later in the high relative to low effort conditions (mixed-effects regression: interference–congruent MSIT, apples, df 460.071, , , Larger - Smaller Number of Presses apples, df 474.041, F 87.326, P0.001). We estimated the split-half reliability of the change in exit threshold measure, which was for cognitive effort, and 0.82 for physical effort (details in SI Appendix, Text 2). As a model-agnostic estimate of the relationship between cognitive and physical effort cost, we measured the correlation of the change in exit threshold across cognitive vs. physical effort conditions and found a significant positive correlation (r 0.203, t 4.78, df 535, P0.001). Next, we used the foraging behavior to formally quantify the additional cost of the high effort tasks using a model based on the Marginal Value Theorem.
Hierarchical Bayesian Marginal Value Theorem Model to Estimate Effort Costs for an Individual.
We fit a hierarchical Bayesian logistic model based on the Marginal Value Theorem (45), which predicted harvest versus exit decisions by comparing expected reward on the next harvest against the average reward rate (Analysis Methods, Hierarchical Bayesian Marginal Value Theorem model). We defined the reward rate in terms of known reward rate values of the foraging environment per effort condition per participant (apples earned, time cost incurred, number of patches visited), as well as the unknown reward rate value (the cost of travel). The cost of travel in high effort blocks was expressed as the marginal increase in cost of travel from low to high effort. Defining this cost as a difference measure controls for any additional biases individual participants may have (such as differences in the subjective value of the reward) which are common to both conditions. The dependent individual differences measures in this task were the inferred cognitive and physical effort cost parameters. The other model parameters were the travel costs in the cognitive and physical low effort conditions, and the inverse temperature applied to the softmax function (higher values indicate less noisy effects of rewards and thresholds on choices).
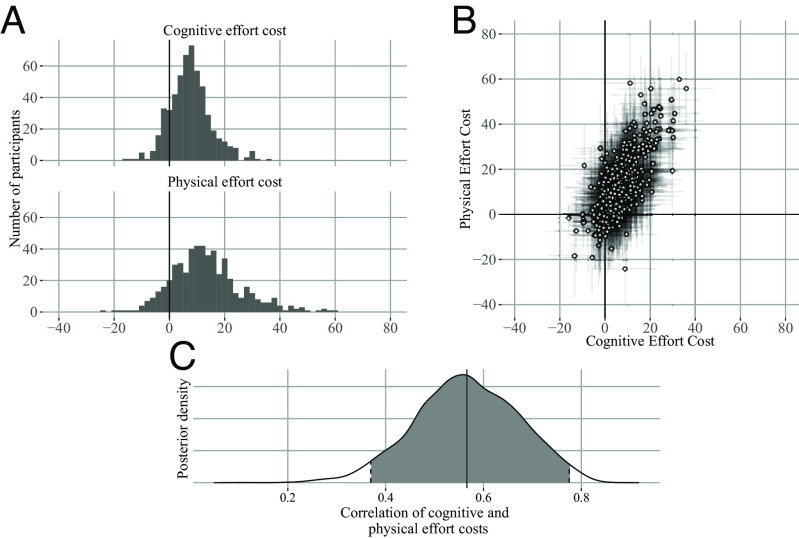
Consistent with the model-agnostic change in threshold metric, the group-level posterior parameter fit indicated that both high-effort tasks were costly on average (SI Appendix, Table S1). There was a range of individual differences (Fig. 4), cost was positive for most participants, some participants were indifferent to the effort manipulation (cost near zero), and some participants had a negative cost (cognitive, N 78, 14.5% of sample, physical, N 67, 12.5% of sample), suggesting that effort was valued.
Fig. 4.
Correlation between individual differences in cognitive and physical effort costs. Experiment 1 (MSIT), (A) Individual differences in the high effort travel costs (expressed as the additional cost of the high relative to the low effort condition). Paralleling the pattern of exit thresholds, most participants experienced the high effort conditions as effortful (positive cost), whereas some participants were insensitive to the effort manipulation (cost near zero) and others were effort-seeking (negative cost). (B) x-axis: Individual differences in cognitive effort costs, y-axis: Individual differences in physical effort costs. Error bars indicate 80% HDI. (C) Posterior distribution of correlation between high effort cost for cognitive and physical effort. Cognitive and physical effort costs are positively correlated (correlation 0.566, 95% HDI 0.355–0.766).
Relationship between Cognitive and Physical Effort Costs.
We estimated the correlation across participants between the cognitive and physical high effort costs (again, each estimated as reflecting the additional cost of high effort relative to the low baseline) using the participant-level covariance matrix when fitting the MVT model. We found a moderate positive relationship (mean correlation 0.566, 95% HDI 0.355–0.766, Fig. 4). This suggests a potential common representation for costs of different types used in effort-based decision making. We confirmed this correlation was not driven by the subset of participants who showed negative effort costs by refitting the model omitting participants with negative cognitive or physical effort cost (SI Appendix, Fig. S3).
Travel Task Performance Relationship to Effort Costs.
For cognitive effort, we tested whether cognitive task performance contributed to the cognitive effort cost measured by foraging choices (see individual differences in MSIT performance in SI Appendix, Fig. S4 and relationship between cognitive and physical effort costs and travel task performance in SI Appendix, Fig. S5). We regressed the difference in (log transformed) MSIT error rate and (log transformed) reaction time onto cognitive effort costs. We found that the difference in error rate significantly predicted cognitive effort cost (estimate 15.313, SE 3.684, t 4.156, P0.001), indicating that participants with higher costs performed worse on the MSIT. However, the reaction time interference effect did not predict cognitive effort cost (estimate 4.861, SE 3.802, t 1.278, P 0.202). In this regression, the intercept was not significantly different from zero, suggesting that the effort costs measured are performance-related (estimate 4.861, SE 3.802, t 1.278, P 0.202, compared to an intercept-only model estimate 7.548, SE 0.323, t 23.38, ). We see the same qualitative result using robust regression. This finding suggests participants may adaptively calibrate their effort costs according to their error rates, and/or that effort costs and error rates are both affected by more general task engagement or motivation (Discussion).
Next, we investigated the analogous relationships for physical effort. We regressed two measures of performance, the percentage of uncompleted presses in the smaller and larger presses condition, and the required number of presses determined in the calibration phase, onto physical effort costs. We found no relationship with physical effort costs and the percentage of uncompleted presses in the smaller (estimate 0.387, SE 0.234, , ) nor the larger (estimate 0.112, SE 0.140, , ) presses condition, nor to the required presses (estimate 0.018, SE 0.088, , ). The physical effort cost effect remained controlling for all these variables (intercept estimate 13.20, SE 2.08, , ). One potentially important difference is that the physical effort requirement was individually calibrated such that completion rates were near ceiling (SI Appendix, Fig. S4). Last, we correlated the error rates across the high-effort cognitive and physical effort tasks and found a weak positive correlation (, df 535, ).
Relationship to Self-reported Motivation and Affect.
We conducted an exploratory canonical correlation analysis (CCA), to test the external validity of our measure, and to investigate the shared versus distinct symptom associations of cognitive and physical effort sensitivity (with data from Experiment 1 participants who completed the survey and passed attention checks; see SI Appendix, Fig. S6, details in Analysis Methods, Canonical correlation analysis). The high dimensionality of both the survey and task measures poses a multiple comparisons issue for correlation or regression analyses. In previous work, we and others have used dimensionality reduction techniques such as factor analysis to summarize key dimensions of survey data prior to regressing them on individual task measures (59). Here, we build on that approach by using CCA, a dimensionality reduction technique that simultaneously performs dimension reduction on two domains of data, to identify summaries of each domain that maximally relate to one another (here, the relationship between surveys and task behavior measures, ref. 60). The dependent variables () were all the self-report summary scores (SI Appendix, Table S2). The predictor variables () were all the task behavior measures of interest: (log transformed) error rate on congruent and incongruent trials, cognitive and physical effort costs, and overall threshold. Including multiple task behavior measures allowed us to explore the structure of multivariate relationships between (potentially partially correlated) task measures such as effort costs and task performance, and on the other hand, several proxies of real-world behavior. This approach has the benefit of increasing sensitivity (by making use of all the measures simultaneously), while reducing the risks of multiple comparisons (by treating all of these factors in a single omnibus analysis).
CCA revealed five dimensions, one of which was significant (summarized in Table 1, for completeness all coefficients for the first two dimensions displayed in SI Appendix, Fig. S7, Wilks’ Lamda (61), using F-approximation, dimension 1: correlation 0.283, stat 0.819, F-approx 1.538, df1 55, df2 1919.899, , dimension 2: correlation 0.257, stat 0.891, F-approx 1.222, df1 40, df2 1575.487, , dimension 3: 0.161, dimension 4: 0.130, dimension 5: 0.063, for dimensions 3 to 5). To interpret which task behavior and/or self-report measures contributed most strongly to the significant dimension, we highlighted those that had a coefficient greater than 0.5 along each dimension (Table 1). On the task measure side, the first dimension was most closely associated with increased MSIT Error Rates but decreased Cognitive Effort Cost (despite that these were weakly positively associated). On the survey side, this dimension was most closely associated with decreased (self-reported) Cognitive Function, increased Behavioral Activation, decreased Anxiety, and decreased Self-efficacy. Overall, Dimension 1 may capture a pattern of individual differences ranging from cautious/error-averse attentiveness to error-prone inattentiveness to the tasks (similar to ref. 62). This is reflected (on the self-report side) in a spectrum from high anxiety to high behavioral activation, which is related on the task side with increasing error rates.
Table 1.
Canonical correlation analysis result summary Experiment 1 (MSIT)
| Task behavior (coefficient) | Self-reports (coefficient) |
|---|---|
| Congruent Error Rate (0.66) | Cognitive Function (0.75) |
| Interference Error Rate (0.58) | Behavioral Activation (0.74) |
| Cognitive Effort Cost (0.52) | Anxiety (0.62) |
| Self-efficacy (0.51) |
For each dimension coefficients larger than 0.5 are displayed in column 1 (for task behavior variables) and in column 2 (for self-reports). Arrows indicate positive or negative coefficients. Dimension 1 (canonical correlation coefficient 0.283, ) reflected increased MSIT Error Rates and decreased Cognitive Effort Cost. The associated self-report coefficients in Dimension 1 were decreased Cognitive Function, increased Behavioral Activation, decreased Anxiety, and decreased Self-efficacy.
Intriguingly, this dimension is also associated with decreased cognitive effort costs but not physical effort costs [if anything, the physical effort coefficient has an opposite loading (0.251), SI Appendix, Fig. S7]. This suggests Dimension 1 does not explain the positive correlation between cognitive and physical effort. The finding that cognitive (but not physical) effort loaded strongly on this factor may also suggest a dissociation between cognitive and physical effort cost relationships to affective and motivational symptoms. Overall threshold did not load onto Dimension 1 either, despite previously hypothesized associations of measured self-reports with individual differences in subjective reward rate (SI Appendix, Text 1). Examining the direction of the correlations with cognitive effort cost, the negative correlation with behavioral activation is consistent with real-world motivation. However, it is unclear why cognitive effort cost would have a positive correlation to cognitive function and self-efficacy.
Validation Experiments.
In addition to the main experiment [Experiment 1 (MSIT)], we tested three other versions (Experiments 2–4) to validate key findings (see SI Appendix, Text 5).
Generalizability of cognitive effort manipulation.
In Experiment 2 (N-Back), we tested a different form of effort as the travel manipulation ( included; see exclusions, quantitative cutoffs, and number of outlier participants in SI Appendix, Table S3). Specifically, we compared foraging behavior when the travel task was the 3-Back versus 1-Back level of the N-Back task (63). As predicted, on average across participants exited trees later in the high (3-Back) relative to low (1-Back) cognitive effort conditions; linear mixed-effects regression estimate for N-Back (3-Back to 1-Back): apples, df 75.981, F 30.339, P 0.001, physical (smaller–larger) , df 75.170, F 27.151, P 0.001. The MVT model fit also indicated a positive high effort cost (SI Appendix, Table S4). Although much smaller in sample size, Experiment 2 did not replicate the correlation observed in Experiment 1 between cognitive and physical effort cost as estimated by the MVT model (SI Appendix, Fig. S8). The highest density interval (HDI) was very wide (0.38 to 0.45) suggesting Experiment 2 () may have been underpowered to detect a correlation similar in size to that seen in Experiment 1. Another possibility is that the size of any correlation may have been reduced by a selection bias in Experiment 2 (i.e., admission to university, ref. 64, SI Appendix, Text 5.A.4).
Collateral predictions of the Marginal Value Theorem.
In Experiment 3 (Richness), we tested adherence to classic predictions of the Marginal Value Theorem, not tested in Experiment 1, by manipulating patch richness. Leaner environments (yielding lower overall mean reward rate) should be associated with lower exit thresholds relative to richer environments. To test for this effect, we compared two levels of reward richness, by adjusting the mean of a normal distribution used to draw the initial yield of a patch. As predicted, we found that participants lowered their exit thresholds in the lean compared to rich conditions (Richness contrast; sum sq. 0.788, mean sq. 0.788, DenDF 27.95, , ).
Explicit instruction travel time is fixed.
Research shows that time perception is subjective and that a more demanding task can make subjective time estimates more variable and less accurate (65). If subjective travel time estimates were generally longer in the high effort condition, that could contribute to effort avoidance (though higher cognitive load can also shorten subjective time estimates, refs. 66 and 67). In Experiments 1–3, we did not instruct participants that the travel time was fixed regardless of the effort condition, leaving open the possibility that participants’ subjective time estimates differed between travel task conditions, and contributed to the observed effect. In Experiment 4, we evaluated this possibility by including explicit instructions that the travel time was fixed across all conditions. The results replicated the findings of effort avoidance in Experiment 1. As predicted, on average, participants still exited trees later in the high relative to low effort conditions for both cognitive and physical effort (linear mixed-effects regression estimate for MSIT (Interference–Congruent): apples, df 49.50, , , physical (smaller–larger) , df 47.72, , , see SI Appendix, Fig. S9).
Discussion
We developed the Effort Foraging Task to quantify the costs of cognitive and physical effort at the level of the individual. Participants played a computer game in which they could forage for virtual apples in a patch with diminishing returns or abandon that patch for a new (initially) richer patch at the expense of time and effort. Participants completed blocks of the task in which the travel cost was either cognitive or physical effort, each at one of two difficulty levels (high and low effort). We measured their exit threshold as the number of apples the participant could have expected to get on their next harvest on trials in which they decided to travel instead. We found that on average participants lowered their exit threshold (staying longer, accepting diminishing returns) in the high relative to low effort conditions, consistent with the high effort task having a monetary cost.
Further analyses in Experiment 1 demonstrated that these cognitive effort costs are correlated with differences in error rates between the low and high cognitive effort tasks, suggesting that the costs may at least partially reflect error avoidance, and/or that effort costs and error rates are both affected by more general task engagement. Expected Value of Control model simulations (68) demonstrated the problem of identifiability of effort costs versus other factors that contribute to cognitive effort allocation: skill and reward sensitivity. That is, if someone avoids effort (i.e., restricts allocation of cognitive effort to a demanding task), this could reflect a higher cost of effort, but it could also reflect poorer ability and/or weaker incentives. These individual differences would impact choices in the foraging task and impact or otherwise index performance on the cognitive effort travel task. To address these potential confounds, we employed a multidimensional, canonical correlation analysis, offer a richer view on the interrelationship between multiple variables.
Cognitive and Physical Effort Relationship.
Our design measures cognitive and physical effort costs on a common scale, revealing a significant and substantial correlation between them (though we did not replicate this finding in the smaller sample of undergraduates in Experiment 2, possibly due to sample size or differences in population). This suggests that a common mechanism may compute costs across multiple domains, consistent with research showing that; i) overlapping in brain areas is involved in cognitive and physical effort decisions using human neuroimaging 24, 28, ii) intermixing choices about cognitive and physical effort affects choices for both effort types (57), iii) cognitive fatigue impacts physical effort exertion and vice versa (69, 70).
Indeed, one set of mechanisms that may be shared between the domains in our study, and contribute to their relationship, is the cognitive and neural mechanisms for foraging decisions themselves. Spatial foraging is hypothesized to be an evolutionary antecedent of abstract cognitive search processes such as memory search (71). As such, shared processes for foraging (both external and mental) have been proposed to be a core component of cognition (31, 32, 38, 39, 72–74). Hills et al. (38) demonstrated, for instance, a causal connection between spatial and mental foraging; they manipulated the distribution of resources in a spatial foraging task and observed that this affected behavior on a subsequent mental foraging task (involving a word search puzzle). This interplay between the cognitive and physical domains offers an intriguing explanation for the observed relationship between individual differences in cognitive and physical effort costs and an interesting set of hypotheses about interaction across the domains to pursue in future work with the Effort Foraging Task.
Foraging provides an ideal framework to understand interactions between action costs (e.g., cognitive and physical effort costs, time costs, and factors such as risk and uncertainty, refs. 75 and 76) as they are all at play in naturalistic foraging behaviors. For example, exerting greater physical effort (e.g., vigor, ref. 77) can reduce time costs, and exerting greater cognitive effort (i.e., planning an efficient action) can reduce both physical effort and time costs. The present study moves in this direction by adding a cognitive or physical effort task requirement to travel in a virtual patch foraging environment with human participants. To directly test one aspect of interplay between cognitive and physical effort decisions, the task could be adapted to intermix cognitive and physical blocks. To ascertain the effect exposure to one domain on decision making has on the other, a study could compare effort type order effects (cognitive before physical versus physical before cognitive), as dissociated from the effect of overall experiment time and fatigue (double length cognitive, and double length physical).
Generalizability.
The Effort Foraging Task as well as existing alternatives are constrained to a limited set of pragmatic laboratory tasks which may not be representative of the putative broader domains of cognitive and physical effort. Experiment 2 offers some evidence for the generality of our approach across distinct operationalizations of cognitive effort, since it produced broadly similar results (e.g., predominantly effort avoidance, some effort-seeking) using a different putatively cognitively effortful task (MSIT vs. N-Back) than Experiment 1. However, the extent to which this holds across the much wider range of tasks that can be considered physical or cognitive (78), and whether such different tasks track each other in terms such as individual differences or external correlations in effort avoidance, remains an important question for future research. It should be noted, however, that Experiment 2 did not replicate the cognitive and physical effort cost correlation, which may be due to sample size or differences in population. Within-participant comparisons using a broad range of cognitive and physical travel tasks could speak to the domain generality of cognitive versus physical effort costs. Intriguingly, research using the Cognitive Effort Discounting Paradigm found correlations between effort avoidance for two distinct cognitive control domains (working memory and speech comprehension during background noise) suggesting a task general component of cognitive effort costs (79).
Future studies should test the hypothesized advantages of the Effort Foraging Task over existing tasks in terms of predictive validity. These results also leave open how broad or narrow the observed external validity is beyond the collection of self-reports measured. Future studies should also clarify whether the high internal reliability using a split half approach reflects trait or state influences. Longitudinal applications of this task could be used to characterize trait-like versus state-dependent contributions to behavior. State variables such as time of day, arousal level, hunger, affect, the presence of psychiatric symptoms, and others may all contribute to effort-based decision making. Understanding state-dependent influences could prove valuable in identifying tractable tools to promote effort exertion in daily life. Relatedly, test–retest reliability should be measured in a replication study; here, we found the split-half reliability to be above 0.8 for both effort costs.
Effort Seeking.
In all the experiments reported here, we consistently observed a subset of participants who exhibited negative effort costs (i.e., a preference for the high effort option over the low effort option) for both the cognitive and physical effort conditions, suggestive of effort seeking. We hypothesized this emerged because our indirect sequential choice style, as this is not commonly seen in direct tasks (but see ref. 46). Both cognitive and physical effort seeking occur frequently in real world behaviors and have been linked in the psychological literature to “need for cognition” and “learned industriousness” among other constructs. Evidence shows instances in which effort requirements add value to a reward (as opposed to discounting rewards, reviewed in ref. 80). Although this comprised a minority of participants in our experiments (14.5% cognitive, 12.5% physical in Experiment 1), it nevertheless suggests the need for extensions to existing utility models (e.g., the Expected Value of Control framework). Cognitive effort seeking, prima facie, indicates positive value assigned to exerting cognitive effort, which may reflect—directly or indirectly—longer-term value attached to information-seeking and learning that yield better future performance (81–84). In the same vein, physical effort seeking may reflect value attached to future performance improvements, for example, via physical learning (e.g., skill acquisition, strength building), and information seeking in physical space. In both cases, effort seeking may also have to do with boredom, which may hold a disutility that encourages application of effort (e.g., ref. 85). This can be adaptive because effort expenditure can help to obtain rewards, in which case doing nothing carries an opportunity cost. Individual differences in the value assigned to novel information/learning (e.g., information bonus, ref. 86) may also have contributed to the behavior of participants with negative effort costs, for whom the information/learning value would be higher in the high effort conditions, leading to a preference for the high effort conditions. Each of these factors likely comprise their own dimensions of individual variation (e.g., boredom aversion, information bonuses) that were not measured in our experiments. An additional variable which might contribute to effort avoidance versus effort seeking is distortions in subjective time perception (65–67). Specifically, if subjective times in the low effort conditions were exaggerated for some participants (e.g., if for them, greater engagement leads to a perception that less time has passed), this could lead them to perceive a net increase in the travel cost relative to the high effort conditions, promoting effort seeking. However, in Experiment 4, we confirmed that the main effect of effort avoidance was conserved even when participants were explicitly instructed that the travel time was fixed. Future research is needed to further investigate the factors that drive effort seeking in this task and others.
Testing Effort Cost Theories.
Further work using the Effort Foraging Task may be useful in testing alternative accounts of the basis of effort costs (i.e., opportunity cost, processing, and metabolic accounts, refs. 87, 88, 89). For example, to test opportunity cost accounts, an experiment could manipulate whether a low effort alternative task is available (e.g., browsing social media instead of completing the Effort Foraging Task for money). Opportunity cost accounts would predict that the cognitive effort cost measured by foraging behavior would be higher during periods in which an alternative was on offer (87). To test cost of processing accounts, the travel task could involve multitasking. By these accounts, participants should treat multitask sets that recruit more shared representations as more costly than sets that recruit more separated representations (88).
Relationship to Self-reported Motivation and Affect.
CCA revealed interrelationships between Effort Foraging Task variables and self-report proxies of real-world motivation and psychiatric symptoms such as cognitive function, anxiety, behavioral activation, and self-efficacy. CCA results offer preliminary evidence for a distinction between cognitive and physical effort costs and affective and motivational symptoms. This suggests cognitive- versus physical-effort decision measures may prove useful in capturing subtypes of depression, which should be explored in major depression using clinician rated (vs. self-reported) symptoms.
One significant dimension was identified that loaded on multiple cognitive task variables and self-report measures (Tab. 1). This dimension plausibly captures something like cautious or compliant attentiveness to the tasks: Specifically, on the task side, it reflects increased error rates, which, on the self-report side, are correlated with movement along a spectrum from anxiety to behavioral activation. However, this dimension also dissociates cognitive effort sensitivity (which is decreasing in this dimension) from error rates (which is increasing in this dimension) and more tentatively physical effort sensitivity (which does not load highly on this dimension, and if anything is increasing). These dissociations also argue against the possibility that variation in this type of task engagement (62) drives the univariate correlation between cognitive and physical effort costs. Altogether, Dimension 1 suggests a more complex profile than simply nonspecific attentional engagement. These rather nuanced results may be enabled by our relatively careful exclusion of grossly inattentive participants (both through attention check items in the survey and task behavior-based exclusions), who can otherwise drive uninformative correlations and obscure more informative relationships (90).
Sensibly, we found that individuals who reported better cognitive function in the past week (and/or reported fewer cognitive difficulties on the survey), and higher self-efficacy, exhibited better performance in the cognitive effort travel tasks in Dimension 1. However, the positive association between such cognitive function, self-efficacy, and cognitive effort cost in Dimension 1 is contrary to our predictions. It may be that the subjective evaluation of cognitive function and self-efficacy are more related to error proneness than to cognitive effort cost. Alternatively, it may be that participants who are experiencing worse cognitive function and lower self-efficacy are less sensitive to the cognitive effort task demand differences. Last, while we did not have any a priori hypotheses about anxiety’s relationship to effort costs, it figures strongly in predicting both effort costs and error rates in Dimension 1, meriting further investigation into how effort costs might relate to symptoms of anxiety. The CCA is an exploratory analysis and factor structure identified should be replicated in a confirmatory sample in future work. Informed by these results, future work could go beyond self-report and use ecological momentary assessment measures (52, 91, 92).
Effort-based decision making has considerable importance in daily life. Critical questions remain about how to disentangle aspects of motivation for effort, how these aspects are represented in the brain, and the role they play in real-world behaviors. The cognitive computational study of motivation has the potential to help people reach their goals by identifying the mechanisms of motivation and ways to enhance motivation toward what matters most to an individual.
Materials and Methods
The Effort Foraging Task adapts a version of the patch foraging paradigm by embedding cognitive and physical effort costs in between patches (here, a simulated orchard with apple trees). The logic of the task is described in the Introduction section “The Effort Foraging Task” and complete details on the task and analysis are described below. On each trial an image of a tree appeared on the screen, representing an immediately available source of reward. Participants could choose to harvest that patch (tree) or travel to a new, replenished patch (Fig. 1). When a tree was harvested, it “shook” and apples were displayed under it (apples were displayed in a single, left justified, row). Reward depleted within a patch such that the more times a tree was harvested the fewer apples it produced. When participants choose to exit the patch, they had to “travel” which consisted of completing a cognitively or physically effortful task. Participants had a fixed amount of time to collect apples (money). Therefore, they must balance the diminishing returns associated with staying at a patch with the travel costs required to reach a new, replenished patch.
Block-wise Manipulation.
Patches were presented block-wise. We manipulated two factors that defined a block: effort type (cognitive and physical) and effort level (low and high). Each block type was tested twice, making 8 blocks total. The total duration of the block was fixed (4 min). Participants had a self-paced break between blocks. Participants were instructed that the time in a block was fixed at 4 min and that they had to decide how to spend their time between harvesting and traveling. The cognitive and physical variants of the task were completed separately (i.e., all cognitive effort blocks were completed in sequence, as were all physical effort blocks). Participants did not know when playing the first effort variant that there would be a second variant upcoming in the experiment. The order of cognitive and physical effort variants of the task was counterbalanced across participants. Within blocks of an effort type, each effort level was tested once during the first half and once during the second half. Given that constraint, the effort level was fully counterbalanced, resulting in eight possible block orders. Which of the block orders was used was randomly selected for each participant. Participants were explicitly instructed about which travel task they had to perform in a particular block and that they could use the background color to know the travel task. Additional task details can be found in SI Appendix, Text 10 and complete instructions can be found in SI Appendix, Text 11.
Task Environment.
The only difference between blocks was the effort travel task, all other variables of the foraging environment were fixed (SI Appendix, Table S5). The time it took to harvest the tree (2 s) or travel to a new tree (8.33 s) was fixed, regardless of reaction times. The apple yield of the first visit to a patch was drawn from a normal distribution (, maximum 20 apples). Each following yield was the product of the previous yield and the depletion rate. On each harvest trial the depletion rate was randomly sampled from a beta distribution ( 14.909, 2.033) and was on average 0.88 (though the yield would not deplete below 0.5 apples).
Travel Tasks.
Participants’ bonus earnings were not influenced by their travel task performance. In the main task, we did not set a performance criterion because that would have complicated the interpretation of the foraging behavior (which would then require estimating not just effort costs but, for example, subjective efficacy estimates per participant). However, training established the expectation that participants try to be accurate by tasking participants with completing a set of miniblocks with high accuracy.
Multi-Source Interference Task.
We used the Multi-Source Interference Task as the cognitive effort task. This task includes multiple types of interference effects—Stroop, Flanker, and Simon effects—and is simple to administer with a standard keyboard without the need to learn novel key mappings (47). In this task, participants identify the oddball out of three numbers by pressing either the 1, 2, or 3 keys, the trial could either be congruent (i.e., 1 0 0, press 1), or have interference (i.e., 3 3 1, press 1). Interference trials have a competing distractor response (here, 3), for which the oddball target is flanked by the distractor and in the spatial position of the distractor (here, 1 is in the third position). Participants completed 6 trials per travel for a total of 7.5 s of task time. If participants made two errors in a row, they saw an attention check (black dot). Participants were instructed to avoid seeing the black dot.
Rapid Key-pressing Task.
Participants performed rapid key-pressing as part of foraging task during travel between trees (7.5 s, Fig. 2; see training methods in SI Appendix, Text 9). In the task, participants rapidly pressed the keyboard with their nondominant pinky finger. All participants were right-handed and used their left pinky finger to press (the “a” key). Each press filled a horizontal bar that indicated progress toward the goal number of presses. There were two conditions referred to as the “Larger number of presses” and “Smaller number of presses.” Travel time was fixed, so if participants reached the goal presses before the travel duration they waited and saw the message Completed! on screen. If they failed to complete the goal number of presses, a black dot appeared on the screen. Participants were instructed to avoid seeing the black dot. To ensure within reason that participants used their nondominant pinky finger throughout the task, they were required to press “hold keys” to occupy other fingers.
Overview of Experiment.
Experiment 1 was conducted over a 90-min session. Participants gave electronic informed consent to participate in the study. All tasks and surveys were presented using the jsPsych library for JavaScript (93) and served with using NivTurk software (94) using the Flask software package for Python. Participants began the experiment with self-report surveys, followed by the foraging training, the main foraging task, and lastly a debrief survey including demographics. Based on recent theoretical work (95), we created a battery of surveys to capture trait need for cognition, and effortful control, and state motivation and affect (i.e., current symptoms of apathy, anhedonia, depression, anxiety, see SI Appendix, Table S2).
Participants.
A total of 678 Prolific participants (18–56 y, mean 24.5 y 6.7, 307 female, 365 male, and 6 prefer not to answer; race and Hispanic or Latino ethnicity reported in SI Appendix, Table S6) volunteered for the study. The study was approved by the Princeton University Institutional Review Board and participants were recruited from the Prolific platform for the large online sample. Participants were compensated with $8.33 for one hour a performance bonus up to $4 (Prolific bonus mean $3.52, SD 0.78, range $0.35–4). The total number of apples harvested in the Effort Foraging Task were converted into real money at the end of the experiment, with each apple being worth fractions of a cent (0.009 cents per apple). The conversion factor was set using pilot data, such that the best-performing participant (earned the most apples) would make the maximum bonus. To accommodate both the physical effort task (completed with the nondominant pinky finger) and the foraging task within standard keyboard layout, all participants were right-handed. Participants completed foraging decisions with their right hand and effort travel tasks with their left hand.
Analysis Methods.
Hierarchical Bayesian Marginal Value Theorem model.
Constantino and Daw (44) investigated trial-by-trial learning in the patch foraging task by predicting stay-or-exit choices using a softmax (noisy) version of a Marginal Value Theorem (45) threshold rule for each stay-or-exit choice. As threshold, the model used a dynamic reward rate estimate given by a running average over obtained rewards and experienced delays. They showed that this model outperformed other candidate learning rules, notably temporal difference learning.
For the present study, because we are investigating individual differences in effort costs at the condition level, we simplified that model to a factorial one in which the MVT threshold is instead taken as fixed per-condition, determined by the overall rewards and delays in each condition and a per-condition effort-cost parameter. Thus, the model omits trial-by-trial learning of the threshold, and instead formally absorbs any such variation into the softmax choice stochasticity. We believe this simplification is warranted because the condition-wise effort costs of interest aggregate over per-trial threshold variability and because we encouraged asymptotic behavior through extensive pretraining and using a stable foraging environment throughout. Also, we have found that learning effects are more easily estimated in a different class of foraging tasks, prey-selection tasks (57, 96–98), because trial-to-trial prey encounters are independent, whereas the decaying reward dynamics in patch foraging tasks correlate offers across trials.
In these respects, this model is intended as descriptive rather than as a process model. Indeed, our approach of solving for the effort costs that rationalize asymptotic behavior is also compatible with alternative assumptions about the way the decision variables are computed (e.g., prospectively, as an intertemporal choice between anticipated future rewards minus costs from harvesting vs. traveling).
First, we computed known reward rate values of the foraging environment per effort condition per participant: total rewards harvested (), number of harvest periods ( block duration/harvest time), and total times travelled (see foraging environment parameters in SI Appendix, Table S5). Then, we solved for the unknown component of average reward rate; the cost of travel (). We estimated the cost of the high effort task () for an individual by predicting harvest versus exit decisions using a hierarchical Bayesian logistic model (Eqs. 2–5). For each foraging trial, the model compares the expected reward on the next harvest [, defined as the average of the previous harvest and the product of the previous harvest with the mean depletion rate (0.88)] against the overall average reward rate for a block type (), using a softmax function (with inverse temperature parameter, ) to make a choice (harvest or exit). The cost of travel in high effort blocks () was expressed as the marginal increase in cost of travel () from low to high effort. Defining this cost as a difference measure controls for any additional biases individual participants may have which are common to both conditions (i.e., consistently high exit thresholds for some participants and low thresholds for others). We used () as the dependent measure of the effort cost for an individual.
For each effort level (low and high) and effort type (cognitive and physical), we predicted choices to stay or exit a patch:
| [2] |
where,
| [3] |
and,
| [4] |
where,
| [5] |
There were five parameters in the model, the inverse temperature (, which controls the noise of the softmax choice function, with lower values indicating more noisy effects of rewards and thresholds on choices), the cognitive low () and high effort costs (), and the physical low ( and high effort costs (). The model included a full covariance matrix of the parameters (5-by-5 matrix) which consists of a correlation matrix and a scale (SD) matrix. Parameters were drawn from a multivariate Gaussian distribution. We used the covariance matrix to estimate the correlation between individual differences in high cognitive and physical effort costs.
The prior distributions were , , . The prior on the correlation matrix was unbiased as to the presence or absence of a correlation (LKJ Correlation Distribution prior 1, ref. 99). Individual participant parameters and their group-level distributions were estimated using Markov Chain Monte Carlo sampling, implemented in Stan with the CmdStanR package (4,000 samples, 2,000 warm-up samples, across 4 chains, Stan Development Team, ref. 100). We also simulated the MVT model to estimate the best exit threshold with respect to reward and time given the foraging environment parameters (SI Appendix, Table S7 and Text 6).
Canonical correlation analysis.
To leverage the strength of our data in having many detailed individual differences measures of theoretically related constructs, we used Canonical Correlation analysis to perform a many to many correlation (we used the cc function from the CCA package in the R language, ref. 101). The task measures included were cognitive effort cost, interference and congruent trial error rate [transformed as log(2-correct)], physical effort cost, and overall exit threshold (estimated in log apples over all low effort blocks by participant using linear mixed-effects regression, discussed in SI Appendix, Text 1). The self-report measures were a combination of trait and symptom state measures of motivation for cognitive and physical effort (SI Appendix, Text 4 and Table S2). The trait self-reports were the need for cognition (102), behavioral inhibition and behavioral activation (103), and effortful control (Adult-Temperament Questionnaire, ref. 104). The symptom self-reports were cognitive function (PROMIS, ref. 105), apathy (Apathy Motivation Index, ref. 106), anhedonia (Snaith Hamilton Pleasure Scale, ref. 107), physical fatigue (PROMIS Fatigue, ref. 105), general self-efficacy (PROMIS, ref. 105), anxiety (Generalized Anxiety Disorder-7, ref. 108), and depression (Patient Health Questionnaire-9, ref. 109). For any measures with subscales, self-report scores were the combined overall averages.
Exclusion criteria.
Participants completed the study on their own outside of the laboratory. To ensure data quality, we used task behavior to constrain our sample to participants who completed the experiment in earnest (SI Appendix, Text 7 and Table S8 shows the Experiment 1 exclusion criteria, the quantitative cutoffs, and the number of outlier participants). The exclusion criteria were not completing the experiment, missing the response deadline on many harvesting trials, poor cognitive or physical travel task performance, too few exit trials in a condition, and very large changes in exit threshold from low to high cognitive and physical effort conditions. Participants with very large shifts in thresholds produced strong outliers in our Marginal Value Theorem model, many of whom had very few exit trials in one condition.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank Sara Constantino for providing base task code for the in-laboratory experiments and Evan Russek for providing base task code for the remote experiments; Allison Burton, Temitope Oshinowo, and Connor Lawhead for collecting the in laboratory data; Elizabeth Tong, Temitope Oshinowo, and Jeremy Lee for adapting and developing online task code; Kevin Lloyd for providing the base code for the optimal threshold simulations; Sebastien Bouret for helpful comments regarding the relationship between cognitive and physical effort costs; Yoel Sanchez Araujo, Sam Zorowitz, Ben Singer, and Dave Turner for assistance with cluster computing for model fitting; and Lindsay Hunter for feedback on the manuscript. This project was made possible through the support of a grant from the John Templeton Foundation (62454 and 57876), the NSF Graduate Research Fellowship Program, and the NIH (T32MH065214, T32DA007261, and R01MH124849). The opinions expressed in this publication are those of the authors and do not necessarily reflect the views of the John Templeton Foundation.
Author contributions
L.A.B., T.O., J.R.L., E.T., A.R.B., A.S., J.D.C., and N.D.D. designed research; L.A.B., T.O., J.R.L., E.T., and A.R.B. performed research; L.A.B. and N.D.D. contributed new reagents/analytic tools; L.A.B., T.O., J.D.C., and N.D.D. analyzed data; and L.A.B., T.O., A.S., J.D.C., and N.D.D. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
The Effort Foraging Task code, the analysis code, and data are openly available at the Open Science Framework repository https://osf.io/a4r2e/ (110).
Supporting Information
References
- 1.Shenhav A., Botvinick M. M., Cohen J. D.,The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 79, 217–240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shenhav A., et al. ,Toward a rational and mechanistic account of mental effort. Annu. Rev. Neurosci. 40, 99–124 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Rigoux L., Guigon E.,A model of reward- and effort-based optimal decision making and motor control. PLoS Comput. Biol. 8, e1002716 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salamone J. D., Correa M., Yang J.-H., Rotolo R., Presby R.,Dopamine, effort-based choice, and behavioral economics: Basic and translational research. Front. Behav. Neurosci. 12, 52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walton M. E., Rudebeck P. H., Bannerman D. M., Rushworth M. F. S.,Calculating the cost of acting in frontal cortex. Ann. N. Y. Acad. Sci. 1104, 340–356 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kool W., McGuire J. T., Rosen Z. B., Botvinick M. M.,Decision making and the avoidance of cognitive demand. J. Exp. Psychol. Gen. 139, 665–682 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westbrook A., Kester D., Braver T. S.,What is the subjective cost of cognitive effort? Load, trait, and aging effects revealed by economic preference. PLoS ONE 8, e68210 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treadway M. T., Buckholtz J. W., Schwartzman A. N., Lambert W. E., Zald D. H.,Worth the ‘EEfRT’ the effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS ONE 4, e6598 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treadway M. T., et al. ,Dopaminergic mechanisms of individual differences in human effort-based decision-making. J. Neurosci. 32, 6170–6176 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culbreth A., Westbrook A., Barch D.,Negative symptoms are associated with an increased subjective cost of cognitive effort. J. Abnorm. Psychol. 125, 528–536 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf D. H., et al. ,Amotivation in schizophrenia: Integrated assessment with behavioral, clinical, and imaging measures. Schizophr. Bull. 40, 1328–1337 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barch D. M., Treadway M. T., Schoen N.,Effort, anhedonia, and function in schizophrenia: Reduced effort allocation predicts amotivation and functional impairment. J. Abnorm. Psychol. 123, 387–397 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treadway M. T., Bossaller N., Shelton R. C., Zald D. H.,Effort-based decision-making in major depressive disorder: A translational model of motivational anhedonia. J. Abnorm. Psychol. 121, 553–558 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cléry-Melin M.-L., et al. ,Why don’t you try harder? An investigation of effort production in major depression. PLoS ONE 6, e23178 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X.-H., et al. ,Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Res. 220, 874–882 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Vinckier F., et al. ,Elevated effort cost identified by computational modeling as a distinctive feature explaining multiple behaviors in patients with depression. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 7, 1158–1169 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Hershenberg R., et al. ,Diminished effort on a progressive ratio task in both unipolar and bipolar depression. J. Affect. Disord. 196, 97–100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran T., Hagen A. E. F., Hollenstein T., Bowie C. R.,Physical- and cognitive-effort-based decision-making in depression: Relationships to symptoms and functioning. Clin. Psychol. Sci. 9, 53–67 (2021). [Google Scholar]
- 19.Patzelt E. H., Kool W., Millner A. J., Gershman S. J.,The transdiagnostic structure of mental effort avoidance. Sci. Rep. 9, 1689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westbrook A., et al. ,Economic choice and heart rate fractal scaling indicate that cognitive effort is reduced by depression and boosted by sad mood. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy D. J., Glimcher P. W.,The root of all value: A neural common currency for choice. Curr. Opin. Neurobiol. 22, 1027–1038 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy D. J., Glimcher P. W.,Comparing apples and oranges: Using reward-specific and reward-general subjective value representation in the brain. J. Neurosci. 31, 14693–14707 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chib V. S., Rangel A., Shimojo S., O’Doherty J. P.,Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J. Neurosci. 29, 12315–12320 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liane Schmidt M., Lebreton M.-L.C.-M., Daunizeau J., Pessiglione M.,Neural mechanisms underlying motivation of mental versus physical effort. PLoS Biol. 10, e1001266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borderies N., Bornert P., Gilardeau S., Bouret S.,Pharmacological evidence for the implication of noradrenaline in effort. PLoS Biol. 18, e3000793 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bornert P., Bouret S.,Locus coeruleus neurons encode the subjective difficulty of triggering and executing actions. PLoS Biol. 19, e3001487 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Gamundi P., et al. ,The neural basis of effort valuation: A meta-analysis of functional magnetic resonance imaging studies. Neurosci. Biobehav. Rev. 131, 1275–1287 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Chong T., et al. ,Neurocomputational mechanisms underlying subjective valuation of effort costs. PLoS Biol. 15, e1002598 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran T., Hagen A. E. F., Hollenstein T., Bowie C. R.,Physical- and cognitive-effort-based decision-making in depression: Relationships to symptoms and functioning. Clin. Psychol. Sci. 9, 53–67 (2020). [Google Scholar]
- 30.Stephens D. W., Krebs J. R., Foraging Theory (Princeton University Press, 1986), vol. 1. [Google Scholar]
- 31.Search C., Evolution, Algorithms, and the Brain (The MIT Press, 2012). [Google Scholar]
- 32.Hills T. T., et al. ,Exploration versus exploitation in space, mind, and society. Trends Cogn. Sci. 19, 46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayden B. Y., Niv Y.,The case against economic values in the orbitofrontal cortex (or anywhere else in the brain). Behav. Neurosci. 135, 192–201 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Kane G. A., et al. ,Rat anterior cingulate cortex continuously signals decision variables in a patch foraging task. J. Neurosci. 42, 5730–5744 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter E. C., David Redish A.,Rats value time differently on equivalent foraging and delay-discounting tasks. J. Exp. Psychol. Gen. 145, 1093–1101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayden B. Y., Pearson J. M., Platt M. L.,Neuronal basis of sequential foraging decisions in a patchy environment. Nat. Neurosci. 14, 933–939 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayden B. Y.,Economic choice: The foraging perspective. Curr. Opin. Behav. Sci. 24, 1–6 (2018). [Google Scholar]
- 38.Hills T. T., Todd P. M., Goldstone R. L.,Search in external and internal spaces: Evidence for generalized cognitive search processes. Psychol. Sci. 19, 802–808 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Hills T. T., Jones M. N., Todd P. M.,Optimal foraging in semantic memory. Psychol. Rev. 119, 431–440 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Kolling N., Behrens T. E. J., Mars R. B., Rushworth M. F. S.,Neural mechanisms of foraging. Science 336, 95–98 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenow J. K., Constantino S. M., Daw N. D., Phelps E. A.,Chronic and acute stress promote overexploitation in serial decision making. J. Neurosci. 37, 5681–5689 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanchard T. C., Hayden B. Y.,Monkeys are more patient in a foraging task than in a standard intertemporal choice task. PLoS ONE 10, e0117057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter E. C., Pedersen E. J., McCullough M. E.,Reassessing intertemporal choice: Human decision-making is more optimal in a foraging task than in a self-control task. Front. Psychol. 6, 95 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.S. M. Constantino, N. D. Daw, Learning the opportunity cost of time in a patch-foraging task. Cogn. Affect. Behav. Neurosci. 15, 837–853 (2015). [DOI] [PMC free article] [PubMed]
- 45.Charnov E. L.,Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 9, 129–136 (1976). [DOI] [PubMed] [Google Scholar]
- 46.Sayali C., Badre D.,Neural systems of cognitive demand avoidance. Neuropsychologia 123, 41–54 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bush G., Shin L. M.,The Multi-Source Interference Task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat. Protoc. 1, 308–313 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Orne M. T.,On the social psychology of the psychological experiment: With particular reference to demand characteristics and their implications. Am. Psychol. 17, 776–783 (1962). [Google Scholar]
- 49.Lopez-Gamundi P., Wardle M. C.,The cognitive effort expenditure for rewards task (C-EEfRT): A novel measure of willingness to expend cognitive effort. Psychol. Assess. 30, 1237–1248 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Kurniawan I. T., et al. ,Choosing to make an effort: The role of striatum in signaling physical effort of a chosen action. J. Neurophysiol. 104, 313–321 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gold J. M., et al. ,Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol. Psychiat. 74, 130–136 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.A. Strobel et al., Dispositional cognitive effort investment and behavioral demand avoidance: Are they related? PLoS ONE 15, e0239817 (2020). [DOI] [PMC free article] [PubMed]
- 53.I. Juvina et al., “Measuring individual differences in cognitive effort avoidance” in Proceedings of the 40th Annual Conference of the Cognitive Science Society, T. Rogers, M. Rau, X. Zhu, C. W. Kalish, Eds. (Austin, TX, 2018), pp. 1886–1891.
- 54.Mækelæ M. J., et al. ,Is it cognitive effort you measure? Comparing three task paradigms to the Need for Cognition scale. PLoS ONE 18, e0290177 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wp H., et al. ,Effort-based decision-making paradigms for clinical trials in schizophrenia: Part 2—External validity and correlates. Schizophr. Bull. 41, 1055–1065 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Heron C., et al. ,Distinct effects of apathy and dopamine on effort-based decision-making in Parkinson’s disease. Brain 141, 1455–1469 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toro-Serey C., Kane G. A., McGuire J. T.,Choices favoring cognitive effort in a foraging environment decrease when multiple forms of effort and delay are interleaved. Cogn. Affect. Behav. Neurosci. 22, 509–532 (2022). [DOI] [PubMed] [Google Scholar]
- 58.Marzilli K. M., Ericson J. M., White D. L., Cohen J. D.,Money earlier or later? Simple heuristics explain intertemporal choices better than delay discounting does. Psychol. Sci. 26, 826–833 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gillan C. M., Kosinski M., Whelan R., Phelps E. A., Daw N. D.,Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. eLife 5, e11305 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H.-T., et al. ,Finding the needle in a high-dimensional haystack: Canonical correlation analysis for neuroscientists. Neuroimage 216, 116745 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Wilks S. S.,On the independence of k sets of normally distributed statistical variables. Econometrica 3, 309–326 (1935). [Google Scholar]
- 62.Moutoussis M., et al. ,Decision-making ability, psychopathology, and brain connectivity. Neuron 109, 2025–2040.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nystrom L. E., et al. ,Working memory for letters, shapes, and locations: fMRI evidence against stimulus-based regional organization in human prefrontal cortex. Neuroimage 11, 424–446 (2000). [DOI] [PubMed] [Google Scholar]
- 64.Hernán M. A., Monge S.,Selection bias due to conditioning on a collider. BMJ 381, 1135 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Block R. A., Hancock P. A., Zakay D.,How cognitive load affects duration judgments: A meta-analytic review. Acta Physiol. (Oxf.) 134, 330–343 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Khan A., Sharma N. K., Dixit S.,Effect of cognitive load and paradigm on time perception. J. Indian Acad. Appl. Psychol. 32, 37–42 (2006). [Google Scholar]
- 67.F. Block, H. Gellersen, “The impact of cognitive load on the perception of time” in Proceedings of the 6th Nordic Conference on Human-Computer Interaction: Extending Boundaries, NordiCHI ’10 (Association for Computing Machinery, New York, NY, 2010), pp. 607–610.
- 68.S. Musslick, J. D. Cohen, A. Shenhav. “Estimating the costs of cognitive control from task performance: Theoretical validation and potential pitfalls” in Proceedings of the 40th Annual Meeting of the Cognitive Science Society, CogSci 2018 (2018), p. 6.
- 69.Marcora S. M., Staiano W., Manning V.,Mental fatigue impairs physical performance in humans. J. Appl. Physiol. (1985) 106, 857–864 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Giboin L.-S., Wolff W.,The effect of ego depletion or mental fatigue on subsequent physical endurance performance: A meta-analysis. Perform. Enhancement Health 7, 100150 (2019). [Google Scholar]
- 71.Hills T. T.,Animal foraging and the evolution of goal-directed cognition. Cogn. Sci. 30, 3–41 (2006). [DOI] [PubMed] [Google Scholar]
- 72.Pirolli P., Card S.,Information foraging. Psychol. Rev. 106, 643–675 (1999). [Google Scholar]
- 73.Wolfe J. M.,When is it time to move to the next raspberry bush? Foraging rules in human visual search. J. Vis. 13, 10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilke A., Hutchinson J. M. C., Todd P. M., Czienskowski U.,Fishing for the right words: Decision rules for human foraging behavior in internal search tasks. Cogn. Sci. 33, 497–529 (2009). [DOI] [PubMed] [Google Scholar]
- 75.Kilpatrick Z. P., Davidson J. D., El Hady A.,Uncertainty drives deviations in normative foraging decision strategies. J. R. Soc. Interface 18, 20210337 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ross Otto A., Daw N. D.,The opportunity cost of time modulates cognitive effort. Neuropsychologia 123, 92–105 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Niv Y., Daw N. D., Joel D., Dayan P.,Tonic dopamine: Opportunity costs and the control of response vigor. Psychopharmacology 191, 507–520 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Neisser U., Cognitive Psychology (Appleton-Century-Crofts, East Norwalk, CT, 1967). [Google Scholar]
- 79.Crawford J. L., Eisenstein S. A., Peelle J. E., Braver T. S.,Domain-general cognitive motivation: Evidence from economic decision-making—Final Registered Report. Cogn. Res. 7, 23 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Inzlicht M., Shenhav A., Olivola C. Y.,The effort paradox: Effort is both costly and valued. Trends Cogn. Sci. 22, 337–349 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.A. Geana, R. Wilson, N. D. Daw, J. Cohen, “Information-seeking, learning and the marginal value theorem: A normative approach to adaptive exploration” in CogSci 2016 (2016).
- 82.Agrawal M., Mattar M. G., Cohen J. D., Daw N. D.,The temporal dynamics of opportunity costs: A normative account of cognitive fatigue and boredom. Psychol. Rev. 129, 564–585 (2022). [DOI] [PubMed] [Google Scholar]
- 83.J. A. Masís, S. Musslick, J. Cohen, “The value of learning and cognitive control allocation” in Proceedings of the Annual Meeting of the Cognitive Science Society (2021).
- 84.Sayali C., Heling E., Cools R.,Learning progress mediates the link between cognitive effort and task engagement. Cognition 236, 105418 (2023). [DOI] [PubMed] [Google Scholar]
- 85.Agrawal M., Mattar M. G., Cohen J. D., Daw N. D.,The temporal dynamics of opportunity costs: A normative account of cognitive fatigue and boredom. Psychol. Rev. 129, 564–585 (2022). [DOI] [PubMed] [Google Scholar]
- 86.Wilson R. C., Geana A., White J. M., Ludvig E. A., Cohen J. D.,Humans use directed and random exploration to solve the explore-exploit dilemma. J. Exp. Psychol. Gen. 143, 2074–2081 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kurzban R., Duckworth A., Kable J. W., Myers J.,An opportunity cost model of subjective effort and task performance. Behav. Brain Sci. 36, 661–679 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Musslick S., Cohen J. D.,Rationalizing constraints on the capacity for cognitive control. Trends Cogn. Sci. 25, 757–775 (2021). [DOI] [PubMed] [Google Scholar]
- 89.Baumeister R. F., Heatherton T. F.,Self-regulation failure: An overview. Psychol. Inq. 7, 1–15 (1996). [Google Scholar]
- 90.Zorowitz S., Solis J., Niv Y., Bennett D.,Inattentive responding can induce spurious associations between task behaviour and symptom measures. Nat. Hum. Behav. 7, 1667–1681 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crawford J. L., English T., Braver T. S.,Cognitive effort-based decision-making across experimental and daily life indices in younger and older adults. J. Gerontol.: Ser. B 78, 40–50 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krönke K.-M., et al. ,Real-life self-control is predicted by parietal activity during preference decision making: A brain decoding analysis. Cogn. Affect. Behav. Neurosci. 21, 936–947 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Leeuw J. R.,jsPsych: A JavaScript library for creating behavioral experiments in a Web browser. Behav. Res. Methods 47, 1–12 (2015). [DOI] [PubMed] [Google Scholar]
- 94.S. Zorowitz, D. Bennett, NivTurk (v1.2-prolific). Zenodo. 10.5281/zenodo.6609218. Accessed 1 June 2021. [DOI]
- 95.Grahek I., Shenhav A., Musslick S., Krebs R. M., Koster E. H. W.,Motivation and cognitive control in depression. Neurosci. Biobehav. Rev. 102, 371–381 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krebs R. M., Boehler C. N., Woldorff M. G.,The influence of reward associations on conflict processing in the Stroop task. Cognition 117, 341–347 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blanchard T. C., Hayden B. Y.,Neurons in dorsal anterior cingulate cortex signal postdecisional variables in a foraging task. J. Neurosci. 34, 646–655 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garrett N., Daw N. D.,Biased belief updating and suboptimal choice in foraging decisions. Nat. Commun. 11, 3417 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lewandowski D., Kurowicka D., Joe H.,Generating random correlation matrices based on vines and extended onion method. J. Multivar. Anal. 100, 1989–2001 (2009). [Google Scholar]
- 100.J. Gabry, R. Češnovar, A. Johnson, cmdstanr: R Interface to ‘CmdStan’. https://mc-stan.org/cmdstanr/, https://discourse.mc-stan.org. (2023).
- 101.I. González, S. Déjean, CCA: Canonical Correlation Analysis, R package version 1.2.1, https://CRAN.R-project.org/package=CCA (2021).
- 102.Cacioppo J. T., Petty R. E., Kao C. F.,The efficient assessment of need for cognition. J. Pers. Assess. 48, 306–307 (1984). [DOI] [PubMed] [Google Scholar]
- 103.Carver C. S., White T. L.,Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J. Pers. Soc. Psychol. 67, 319–333 (1994). [Google Scholar]
- 104.Evans D. E., Rothbart M. K.,Developing a model for adult temperament. J. Res. Pers. 41, 868–888 (2007). [Google Scholar]
- 105.Cella D., et al. ,The patient-reported outcomes measurement information system (PROMIS). Med. Care 45, S3–S11 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ang Y.-S., Lockwood P., Apps M. A. J., Muhammed K., Husain M.,Distinct subtypes of apathy revealed by the apathy motivation index. PLoS ONE 12, e0169938 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Snaith R. P., et al. ,A scale for the assessment of hedonic tone the Snaith–Hamilton pleasure scale. Br. J. Psychiatry 167, 99–103 (1995). [DOI] [PubMed] [Google Scholar]
- 108.Spitzer R. L., Kroenke K., Williams J. B. W., Löwe B.,A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 166, 1092–1097 (2006). [DOI] [PubMed] [Google Scholar]
- 109.Kroenke K., Spitzer R. L., Williams J. B.,The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.L. A. Bustamante, T. Oshinowo, J. R. Lee, E. Tong, Effort Foraging Task. Open Science Framework. https://osf.io/a4r2e/. 19 Deposited December 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
The Effort Foraging Task code, the analysis code, and data are openly available at the Open Science Framework repository https://osf.io/a4r2e/ (110).