Abstract
Importance:
DNA methylation (DNAm) provides a plausible mechanism by which adverse exposures become embodied and contribute to health inequities, due to its role in genome regulation and responsiveness to social and biophysical exposures tied to societal context. However, scant epigenome-wide association studies (EWAS) have included structural and lifecourse measures of exposure, especially in relation to structural discrimination.
Objective:
Our study tests the hypothesis that DNAm is a mechanism by which racial discrimination, economic adversity, and air pollution become biologically embodied.
Design:
A series of cross-sectional EWAS, conducted in My Body My Story (MBMS, biological specimens collected 2008–2010, DNAm assayed in 2021); and the Multi Ethnic Study of Atherosclerosis (MESA; biological specimens collected 2010–2012, DNAm assayed in 2012–2013); using new georeferenced social exposure data for both studies (generated in 2022).
Setting:
MBMS was recruited from four community health centers in Boston; MESA was recruited from four field sites in: Baltimore, MD; Forsyth County, NC; New York City, NY; and St. Paul, MN.
Participants:
Two population-based samples of US-born Black non-Hispanic (Black NH), white non-Hispanic (white NH), and Hispanic individuals (MBMS; n=224 Black NH and 69 white NH) and (MESA; n=229 Black NH, n=555 white NH and n=191 Hispanic).
Exposures:
Eight social exposures encompassing racial discrimination, economic adversity, and air pollution.
Main outcome:
Genome-wide changes in DNAm, as measured using the Illumina EPIC BeadChip (MBMS; using frozen blood spots) and Illumina 450k BeadChip (MESA; using purified monocytes). Our hypothesis was formulated after data collection.
Results:
We observed the strongest associations with traffic-related air pollution (measured via black carbon and nitrogen oxides exposure), with evidence from both studies suggesting that air pollution exposure may induce epigenetic changes related to inflammatory processes. We also found suggestive associations of DNAm variation with measures of structural racial discrimination (e.g., for Black NH participants, born in a Jim Crow state; adult exposure to racialized economic residential segregation) situated in genes with plausible links to effects on health.
Conclusions and Relevance:
Overall, this work suggests that DNAm is a biological mechanism through which structural racism and air pollution become embodied and may lead to health inequities.
Introduction
Recent advances enabling large population-based epigenetic studies are permitting researchers to test hypotheses linking socially-patterned exposures, gene regulation, and health inequities1–3. DNA methylation (DNAm) is a plausible biological mechanism by which adverse social exposures may become embodied4,5, because 1) it plays an active role in genome regulation6–8, 2) it changes in response to environmental exposures1,9 and internal human physiology like ageing2 and inflammation10, and 3) induced changes can be long-lasting11–14. There is a growing literature reporting associations between DNAm and environmental factors to which social groups are unequally exposed; a recent review15 found associations between DNAm and measures of socioeconomic position (SEP), including income, education, occupation, and neighbourhood measures; and illustrated timing and duration of exposure is important. Exposure to toxins, including air pollution, is often inequitable between social groups16,17. A number of EWAS have identified associations with particulate matter18–21 and oxides of nitrogen (NOx)21,22; although there is little replication between studies, and some studies have failed to find effects of particulate matter22,23, NOx23, and residential proximity to roadways24. Two EWAS have each found two (non-overlapping) DNAm sites associated with experience of racial discrimination, one in first generation Ghanaian migrants living in Europe3, and one in African American women25; given population and migration differences the lack of replication is perhaps not surprising.
However, no EWAS has yet examined associations between DNAm and exposure to racial discrimination and economic adversity, both at individual and structural levels, and measured at different points in the lifecourse, in the same group of people. This is important because it is not clear if the different timing, duration, and levels of these adverse exposures are embodied in different ways involving differing biological pathways. Supporting attention to these issues is a growing body of research documenting how exposure to health-affecting factors such as toxins, quality healthcare, education, fresh food, and green spaces are determined by the way dominant social groups have structured society, which in turn results in health inequities between dominant social groups and groups they have minoritized4,26. Structural racism (the totality of ways in which society discriminates against racialized groups27), for example, results in people of colour often disproportionately bearing the burden of adverse exposures and economic hardship4,28, thus driving racialized health inequities29. Associations between structural racism and cardiovascular health have been shown for discriminatory housing policies and continuing neighbourhood racial segregation30,31; with the historical legacy of slavery32; and with state-level institutional domains33. Associations have also been shown for diabetes outcomes in the US34 and globally35.
Guided by the ecosocial theory of disease distribution4,5, we tested the hypothesis that DNAm is a biological mechanism by which embodiment of structural racial discrimination, economic hardship, and air pollution may occur. We tested our study hypothesis using data from US-born participants in two US population based studies with similar exposure data: our primary study, the My Body My Story study (MBMS), and the Multi-Ethnic Study of Atherosclerosis study (MESA), which we use for evidence triangulation36 due to differences between the two study populations.
Methods
Participants
This study utilises biological specimens obtained in 2010–2012 from MBMS and MESA, two US population-based studies that contain similar data on the study exposures. In 2021–2022, the study team newly conducted epigenetic assays for MBMS and added new georeferenced social exposure data. Full study descriptions are in the supplementary materials. Our analyses comprised 293 participants (224 Black and 69 white) from MBMS; and 975 participants from MESA who were US-born (229 Black, 555 white NH, additionally including 191 Hispanic).
Social exposures
We tested the relationship between DNAm and eight variables relating to exposure to racial discrimination (both structural and self-reported), economic hardship, and air pollution; these are described in detail in Supplementary Table 2.
DNA methylation
For detailed description of DNA extraction and DNAm data generation, please see the Supplementary materials. Briefly, for MBMS DNA was extracted from frozen blood spots in 2021, and data were generated using the Illumina Infinium MethylationEPIC Beadchip. For MESA, DNA was extracted from purified monocytes in 2012–2013 and data were generated using the Illumina Infinium HumanMethylation450 BeadChip. We used DNAm beta values for both studies, which measure DNAm on a scale of 0 (0% methylation) to 1 (100% methylation).
Participant stratification
EWAS were stratified by self-reported membership of racialized groups, for two reasons. Firstly, for most of our exposures, different constructs are represented between the racialized groups; for example, being born in a Jim Crow state means something very different for individuals who identify as Black versus white. Secondly, stratification prevents potential confounding by racialized group due to exposure and a degree of genetic differences between groups. Racialized groups are social constructs that are changeable and dependent on local context37; they are important to our research question because group membership is pertinent to the experience of social inequities perpetuated by structural racial discrimination.
EWAS
All EWAS were conducted using linear regression models implemented using the R package meffil38. Many exposures had low levels of missing data, complete case numbers for each EWAS can be found in Table 3. EWAS details can be found in the Supplementary materials; briefly, we adjusted for age, reported gender (MBMS)/sex (MESA), smoking status, blood cell count proportions, and batch effects.
Table 3:
Summary of the number of DNAm sites passing the genome-wide threshold in each individual EWAS in MBMS (threshold 2.4e-7) and MESA (threshold 9e-8). The list of specific DNAm sites passing the genome-wide threshold can be found in supplementary table 4.
| MBMS | MESA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Black NH | white NH | Black NH | white NH | Hispanic | ||||||
| N | N sites | N | N sites | N | N sites | N | N sites | N | N sites | |
| Birth in a Jim Crow state | 224 | 1 | NA1 | NA1 | 225 | 0 | 549 | 532 | 186 | 0 |
| Parent’s highest education (high vs low) | 64 | 0 | 33 | 0 | 117 | 0 | 288 | 0 | NA3 | NA3 |
| Parent’s highest education (high vs mid) | 129 | 0 | 49 | 0 | 128 | 0 | 384 | 0 | NA3 | NA3 |
| Participant’s education (high vs low) | 63 | 1 | 36 | 0 | 54 | 0 | 142 | 0 | 51 | 0 |
| Participant’s education (high vs mid) | 190 | 0 | 61 | 0 | 202 | 0 | 534 | 0 | 156 | 0 |
| Household poverty to income ratio | 190 | 0 | 66 | 0 | 218 | 0 | 528 | 0 | 180 | 0 |
| Racialized economic segregation | 224 | 0 | 69 | 0 | 223 | 1 | 545 | 1 | 174 | 0 |
| Black carbon | 224 | 0 | 69 | 0 | 211 | 12 | 526 | 7 | 175 | 1 |
| Nitrogen oxides | 219 | 0 | 69 | 0 | 211 | 22 | 526 | 4 | 175 | 1 |
| EOD5 (1–2 vs 0) | 82 | 0 | 59 | 0 | ||||||
| EOD5 (1–2 vs 3+) | 192 | 0 | 34 | 0 | ||||||
| MDS6 (1–2 vs 0) | 204 | 1 | 554 | 0 | 184 | 0 | ||||
| MDS6 (1–2 vs 3+) | 97 | 0 | NA4 | NA4 | 60 | 0 | ||||
The EWAS was not run for Jim Crow birth state for white NH participants in MBMS, due to small cell numbers.
See text; these 53 sites were driven by air pollution differences between individuals born and not born in a Jim Crow state.
The two EWAS for parental education were not run for Hispanic participants in MESA, due to small cell numbers.
The EWAS was not run for MDS (score of 1–2 vs 3+) for white NH participants in MESA, as no participants had a score of 3 or more.
EOD – Experiences of Discrimination scale.
MDS – Major Discrimination Scale
Sensitivity analysis
In MESA we conducted a sensitivity analysis to test whether our results were influenced by population stratification; details are in the Supplementary materials. Additional sensitivity analysis restricted the MESA analysis to participants recruited from the Baltimore and New York sites, because these cities bear the greatest similarity to the Boston area in terms of geographical location, city environment, and social histories.
Meta-analysis
We meta-analysed associations with air pollution within MBMS and within MESA because air pollution is the only exposure we tested that we would hypothesize to have the same meaning, and therefore biological effect, for all individuals. We used METAL39 to meta-analyse effect sizes and standard errors of the EWAS summary statistics of each racialized group, for black carbon/LAC and NOx.
Functional relevance of sites passing the genome-wide threshold
For DNAm sites associated with an exposure, we used the UCSC genome browser to identify genomic regions. For sites within known genes, we used GeneCards (https://www.genecards.org/) and literature searches to identify putative gene functions. We used the EWAS Catalog to determine if associations between DNAm sites and other traits had been reported in previous studies. Where multiple DNAm sites were associated with an exposure we performed gene set enrichment analysis using the missMethyl R package40.
Biological enrichments of top sites
Following each EWAS we performed analyses to ascertain whether DNAm sites associated with our exposures indicate effects on particular biological pathways, processes or functions. Details are in the Supplementary materials; briefly, we conducted gene set enrichment analyses, and for enrichments of tissue-specific chromatin states, genomic regions and transcription factor binding sites (TFBS).
Lookup of associations in a priori specified genomic locations
We hypothesised a priori that our EWAS would detect DNAm sites that have been robustly associated with our study exposures, or factors that might relate to our exposures, in previous studies. See Supplementary materials for details.
Results
Participant characteristics
Both cohorts include racialized groups that are underrepresented in epigenetic studies. Beyond this, substantial differences existed between the racialized groups within and across MBMS and MESA. Overall, MBMS participants were on average 21 years younger than MESA participants, had less variability in exposure to air pollution, and far more were current smokers. In both studies, Black NH compared to white NH participants had higher BMI, rates of smoking, impoverishment, lower education, rates of self-reported exposure to racial discrimination, and were more likely to be born in a Jim Crow state and live in a neighbourhood with extreme concentrations of low-income persons of colour. In MESA, Hispanic participants reported the lowest levels of personal and parental education.
EWAS results and biological interpretation
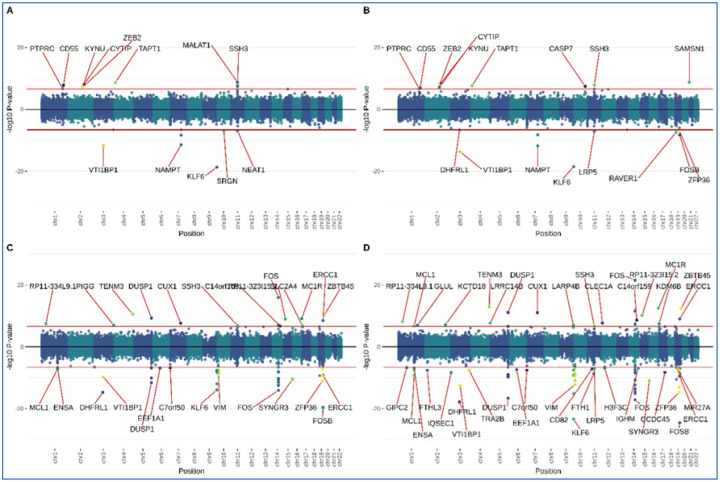
In MBMS, among the Black NH participants one DNAm site, in ZNF286B, was associated with being born in a Jim Crow state. Another DNAm site, PLXND1, was associated with participants having less than high school education. Among white NH participants, no associations passed the genome-wide threshold. See Table 2 for details of gene functions; Table 3 for numbers of associated EWAS sites; and Supplementary figures 1 and 2 for Miami plots. In MESA, two DNAm sites were associated with racialized economic segregation – one in Black NH participants (in FUT6) and one in white NH participants (a CpG previously associated with BMI); and in Black NH participants one DNAm site (in PDE4D) was associated with an MDS score of 0. The majority of associations in MESA were related to air pollution exposure – among Black NH participants, 12 sites with LAC and 22 sites with NOx. Notably, many of these sites are clustered in genes with putative roles in immune responses and are known to interact with one another, including KLF6, MIR23A, FOS, FOSB, ZFP36 and DUSP1. Among the MESA white NH participants, four DNAm sites were associated with both LAC and NOx, and an additional 3 uniquely associated with LAC. Associations of 53 DNAm sites with birth in a Jim Crow state were the result of confounding by air pollution (see Supplementary Materials). Among Hispanic participants, one site was associated with LAC (NPNT) and one with NOx (ADPRHL1). See Table 3 for numbers of associations for all EWAS performed and Supplementary Figures 3–5 for corresponding Miami plots.
Table 2:
Putative functions of genes in which the top exposure-associated DNAm sites sit; with details of how many sites within that gene were identified, and in which main analysis EWAS they were identified.
| Gene | Chr | Functional relevance | MBMS | MESA | |||
|---|---|---|---|---|---|---|---|
| Black NH | White NH | Black NH | White NH | Hispanic | |||
| ZNF286B | 17 | A pseudogene, which is predicted to be involved in regulation of RNA polymerase 2 (Pol II)-mediated transcription (Pol II transcribes protein-coding genes into mRNA41). | Born in a Jim Crow state: 1 | ||||
| PLXND1 | 3 | Encodes a cell receptor involved in axonal guidance, migration of endothelial cells, and regulates atherosclerotic plaque deposition42. | <HS education: 1 | ||||
| FUT6 | 19 | A Golgi stack membrane protein that is involved in basophil-mediated allergic inflammation43. | residential racialized economic segregation: 1 | ||||
| KLF6 | 10 | A transcriptional activator and tumour suppressor, which regulates macrophage inflammatory responses44. | LAC: 2 NOx: 3 | LAC: 1 NOx: 1 | |||
| FOS | 14 | FOS is an early-response gene, and is a subunit of the AP-1 transcription factor complex, which regulates gene expression involved in lung injury, repair and transformation45, as well as regulating many cytokine genes and T-cell differentiation46,47. | LAC: 1 NOx: 7 | ||||
| FOSB | 19 | FOSB is another subunit of AP-1. | LAC: 1 NOx: 1 | ||||
| ZFP36 | 19 | ZFP36 encodes a protein (TTP) that is a key regulator of post-transcriptional regulation, which has roles in immune and inflammatory responses48. | NOx: 2 | ||||
| DUSP1 | 5 | DUSP1 is a gene that regulates airway inflammation; DUSPl’s key mechanism of inflammation modulation may be via modulating the actions of the protein TTP encoded by the ZFP36 gene49. | LAC: 1 NOx: 1 | ||||
| VIM | 10 | Encodes a filament protein responsible for integrity of cell shape and cytoplasm. Pathogens can attach to this protein on the cell surface. Putative involvement regulating innate immune response to lung injury and irritation50 | NOx: 1 | ||||
| PDE4D | 5 | PDE4s, including PDE4D, have roles in cell signalling, as well as regulating inflammatory responses51. | MDS: 1 | ||||
| MALAT1 | 11 | Metastasis associated lung adenocarcinoma transcript 1, IncRNA that acts as transcriptional regulator; upregulation linked to cancerous tissues and proliferation and metastasis of tumour cells | LAC: 1 | ||||
| CYTIP | 2 | Modulates activation of ARF (ADP-ribosylation factor) genes, which regulate vesicle budding, tethering and cytoskeleton organization. Dysregulation of ARFs may be involved in cancer cell migration and invasion. | LAC: 1 NOx: 1 | ||||
| ZEB2 | 2 | DNA-binding transcriptional repressor involved in the transforming growth factor-P (TGF-P) signalling pathway that interacts with activated SMADs. May be related to small cell lung cancer52. | LAC: 1 NOx: 1 | ||||
| PTPRC | 1 | A receptor-type PTP that is an essential regulator of T- and B-cell antigen receptor signalling. | LAC: 1 NOx: 1 | ||||
| NPNT | 4 | An extracellular matrix protein that has roles in kidney development and carcinogenesis53. | LAC: 1 | ||||
| ADPRHL1 | 13 | a protein encoding a pseudoenzyme involved in cardiogenesis54. | NOx: 1 | ||||
MESA subgroup analysis
The main impact of removing the Minnesota and Forsyth County sites (which both had very low levels of air pollution) was to remove the confounding structure between air pollution and Jim Crow birth state among white NH participants. It also increased the similarity of air pollution associations between the Black NH and white NH participants; for example, of the 19 DNAm sites associated with NOx among white NH participants, 12 passed the genome-wide threshold in the Black NH participant EWAS. Numbers of associated sites are in Table 4. Miami plots for this MESA subgroup can be found in Supplementary figures 6, 7 and 8.
Table 4:
Summary of EWAS results for MESA subgroup analysis.
| Black NH | white NH | Hispanic | ||||
|---|---|---|---|---|---|---|
| N | N sites | N | N sites | N | N sites | |
| Birth in a Jim Crow state | 221 | 0 | 237 | 0 | NA1 | NA1 |
| Parent’s highest education (high vs low) | 115 | 0 | 134 | 0 | NA2 | NA2 |
| Parent’s highest education (high vs mid) | 125 | 0 | 164 | 0 | NA2 | NA2 |
| Participant’s education (high vs low) | 54 | 0 | 55 | 0 | NA2 | NA2 |
| Participant’s education (high vs mid) | 198 | 0 | 227 | 0 | NA2 | NA2 |
| Household poverty:income ratio | 214 | 0 | 227 | 1 | 67 | 0 |
| Racialized economic segregation | 219 | 1 | 233 | 0 | 59 | 0 |
| Light Absorption Coefficient | 208 | 10 | 231 | 6 | 57 | 0 |
| Nitrogen oxides | 208 | 20 | 231 | 19 | 57 | 0 |
| Major Discrimination Scale (1–2 vs 0) | 200 | 1 | 236 | 0 | 67 | 0 |
| Major Discrimination Scale (1–2 vs 3+) | 94 | 0 | NA3 | NA3 | NA3 | NA3 |
The EWAS for Jim Crow birth state was not run for Hispanic participants due to small cell numbers.
The EWAS for parental and participant education were not run for Hispanic participants, due to small cell numbers.
The EWAS was not run for MDS (score of 1–2 vs 3+) for white NH and Hispanic participants in MESA, as no participants had a score of 3 or more.
Meta-analysis
Meta-analysis in MBMS did not yield any sites passing the genome-wide threshold. In MESA we see approximately similar numbers of associations as with the Black NH subgroup (17 for LAC and 18 for NOx); see Supplementary Table 3. When we restricted to participants recruited at the Baltimore and New York sites, a much larger number of DNAm sites passed the genome-wide threshold (51 for LAC and 79 for NOx); this may be because Minnesota and Forsyth County sites had very low variance in pollution levels. The MESA sensitivity meta-analysis identified multiple associations linked to DUSP1, FOS, KLF6, MCL1, and VIM; genes that have putative roles in inflammation and immunity.
Biological enrichments of exposure associations
Gene ontology
We observed no evidence for gene set enrichments for any Gene Ontology terms among the top 100 sites of the main EWAS we conducted. However, we did observe that the 22 sites associated with NOx above the genome-wide threshold among MESA Black NH participants were enriched for the gene ontology terms ‘response to glucocorticoid’ and ‘response to corticosteroid’ (FDR>0.05). We also observed that the MESA meta-analysis of NOx among all participants was associated with 13 Gene Ontology terms (FDR>0.05) related to blood-based immune response.
EWAS catalog
We observed a number of relevant enrichments among sites identified in our EWAS. Details of the associations (p < 0.05, Fisher’s exact test) can be found in the Supplementary Materials and Supplementary figures 6–16. Briefly, in MBMS, we see enrichment for inflammation for both NOx and LAC EWAS among Black NH participants, and in the NOx meta-analysis. In MESA, we observed consistent enrichment for infection and cancer among Black NH and white NH participants, and also in the meta-analyses. We observed enrichment for inflammation among Hispanic participants. We also found among both MESA Black NH and Hispanic participants, the racialized economic segregation EWAS was enriched for neurological traits. Among both the white NH and Hispanic participants, household poverty to income ratio EWAS was enriched for SEP and education. In the MESA subgroup analysis, enrichment for prenatal exposures was observed for the Jim Crow birth state EWAS among the Black NH and Hispanic participants.
Enrichment for genomic features
When we looked at enrichment of genomic locations of the top 100 sites (p < 0.05, Fisher’s exact test), we found that among MBMS Black NH participants, NOx was the only exposure with associated CpGs being located in active genomic regions (please see Supplementary materials for details). In the MBMS meta-analyses, NOx was enriched for regions related to gene promoters. Among MESA Black NH participants, we observe enrichment for regions related to transcription and genome regulation in the LAC and NOx EWAS. We also observed enrichment relating to transcription regulation for the birth in a Jim Crow state EWAS. Among MESA white NH participants, we observed enrichment for transcription regulation for both measures of air pollution. Among MESA Hispanic participants, LAC exposure shows some associations with active genomic regions. When we restrict MESA to the New York and Baltimore sites, we see a similar set of enrichments; and in the MESA meta-analyses we see consistent enrichment related to transcription regulation and promotors. Notably, genomic feature enrichments for NOx among both MBMS and MESA Black NH participants involved similar genomic locations (CpG islands and shores) and chromatin states (related to promotors), as well as 6 of a possible 9 TFBS.
Lookup of associations in a priori specified genomic locations
We did not observe any associations in our EWAS results for sites identified in previous EWAS of related exposures.
Discussion
The series of EWAS we conducted on a range of adverse exposures at different levels and at different points in the lifecourse, drawing on two different population-based studies with similar exposure data, provide evidence that DNAm may be a biological pathway by which societal context shapes health inequities. This work has shown for the first time associations between DNAm and multiple levels of structural discrimination, in genes that are biologically plausible routes of embodiment involving gene regulation, including inflammation. Additionally, our EWAS and meta-analyses of air pollution showed clear association between two road traffic-related measures of air pollution, and DNAm of multiple CpGs in multiple genes that have been consistently associated with inflammation and infection, suggesting that the material environment people live may induce inflammatory changes. Our study has added to the existing literature on air pollution; there are few EWAS studies looking at NOx (n=3), and none so far looking at black carbon. In total, this work highlights the need for researchers to consider multiple levels of discrimination and adversity across the lifecourse, especially structural inequities in the material world in which people live, to fully elucidate drivers and biological mechanisms of inequitable health.
Associations detected at the genome-wide level in MBMS related more closely to early-life exposures (being born in a Jim Crow state, and low educational attainment); in MESA they related more to current experiences and exposures (air pollution, racialized economic segregation, and experiences of discrimination), possibly reflecting the relatively older age of the MESA participants. The much stronger associations with air pollution in MESA compared to MBMS could potentially be due to: (1) the use of purified monocytes in MESA, with a single cell type making associations easier to detect; (2) less variation in exposure to air pollution in MBMS compared MESA; (3) longer duration of air pollution exposure in MESA (due to older age of the participants); or (4) reduced statistical power in MBMS, due to lower quantities of DNA.
Notably, inflammation was the predominant pathway indicated in the air pollution analyses, both via putative gene functions and enrichment analyses. These findings underscore that while there is a large psychosocial literature on inflammation being a mechanism by which discrimination harms health28,55,56, it is also critical to consider inequities in biophysical exposures in the material world as an important driver of this inflammation. Overall, air pollution sites tend to be enriched for inflammation in MBMS and infection in MESA; this could represent different mechanisms of the same process due to the different blood cell types sampled in the two cohorts; with monocytes being specialised in infection prevention, and neutrophils (the highest proportion cell in whole blood) being specialised in inflammatory responses.
Our study identified a greater number of associations with air pollution measures than previous work in MESA20,57; this is likely due to the fact that we do not adjust for recruitment site (which would reduce variation in the exposure because exposure is location-dependent); and previous analyses have adjusted for racialized group membership, which is also associated with air pollution exposure; this may have masked the effects that we have detected. This joins other research that has demonstrated the importance of considering spatial effects of air pollution58.
A limitation of our study is that we cannot infer causality. Although it would be possible to conduct Mendelian randomization instrumenting cis-mQTLs, we did not conduct this analysis because we think the results would be highly speculative. Additionally, the MESA sample we used may have been subject to selection bias, because (1) individuals who had experienced prior cardiovascular events were excluded from recruitment, and (2) a number of participants died between Exam 1 and Exam 5. If adversity and discrimination are associated with these cardiovascular events and mortality, associations could be biased in MESA.
Conclusions
We think this work provides direction for future epigenetic studies to consider the role of inequitable adverse social and biophysical exposures across the lifecourse, including but not limited to structural discrimination. Our results suggest inflammation may be a key biological pathway by which inequities become embodied, in our case driven primarily by exposure to air pollution, and not self-reported racial discrimination. These findings accordingly suggest that attention to how social inequities shape biophysical as well as social exposures is crucial for understanding how societal inequities can become embodied, via pathways involving DNAm.
Supplementary Material
Figure 1:
MESA air pollution meta-analysis miami plots. A: MESA full cohort LAC meta-analysis. B: MESA full cohort NOx meta-analysis. C: MESA subgroup LAC meta-analysis. D: MESA subgroup NOx meta-analysis.
Table 1:
Characteristics of MBMS and MESA participants.
| Variable | MBMS: Black NH | MBMS: white NH | MESA: Black NH | MESA: white NH | MESA: Hispanic | |
|---|---|---|---|---|---|---|
| Total N | 224 | 69 | 229 | 555 | 191 | |
| Sociodemographic characteristics | ||||||
| Age: mean (SD) | 49.02 (7.8) | 48.7 (8.3) | 71 (8.9) | 70.1 (9.5) | 68.5 (8.9) | |
| Gender: N (%) women | 135 (60.3%) | 49 (71%) | 133 (58.1%) | 264 (47.6%) | 86 (45%) | |
| BMI: mean (SD) | 32.1 (7.7) | 29.7 (7.2) | 30.6 (5.7) | 28.7 (5.3) | 30.8 (5.5) | |
| Smoking: N (%) | Current | 115 (51.3%) | 24 (34.8%) | 31 (13.7%) | 44 (8%) | 16 (8.6%) |
| Former | 31 (13.8%) | 23 (33.3%) | 101 (44.9%) | 262 (47.7%) | 80 (43%) | |
| Never | 78 (34.8%) | 22 (31.9%) | 93 (41.5%) | 243 (44.3%) | 90 (48.4%) | |
| Missing | 0 | 0 | 4 (1.7%) | 6 (1.1%) | 5 (2.6%) | |
| Childhood exposure to racialized and economic adversity: | ||||||
| Born in a Jim Crow state1: N (%) yes | 71 (31.7%) | 2 (3%) | 165 (72.1%) | 166 (29.9%) | 19 (9.9%) | |
| Parent’s highest education: N (%) | <High school | 29 (18.4%) | 8 (14%) | 95 (42.2%) | 161 (29.3%) | 129 (69.7%) |
| >= High school and <4yr college | 94 (59.5%) | 24 (42.1%) | 106 (47.3%) | 258 (47%) | 51 (27.6%) | |
| 4+ years college | 35 (22.2%) | 25 (43.9%) | 24 (10.7%) | 130 (23.7%) | 5 (2.7%) | |
| Missing | 66 (29.5%) | 12 (17.4%) | 4 (1.7%) | 6 (1.1%) | 6 (3.1%) | |
| Participant’s education: N (%) | <High school | 34 (15.2%) | 8 (11.6%) | 23 (10%) | 21 (3.8%) | 35 (18.3%) |
| >= High school and <4yr college | 161 (71.9%) | 33 (47.8%) | 175 (76.4%) | 413 (74.4%) | 140 (73.3%) | |
| 4+ years college | 29 (12.9%) | 28 (40.6%) | 31 (13.5%) | 121 (21.8%) | 16 (8.4%) | |
| Missing: | 0 | 0 | 0 | 0 | 0 | |
| Adult exposure to racialized and economic adversity: | ||||||
| Household income to poverty ratio2: mean (SD) | 2.2 (2.2) | 2.9 (2.3) | 3.9 (2.3) | 4.8 (2.9) | 3.3 (2.1) | |
| Missing | 34 (15.2%) | 3 (4.3%) | 9 (3.9%) | 24 (4.3%) | 7 (3.7%) | |
| Index of Concentration at the Extremes for racialized economic segregation3: mean(SD) | -0.07 (0.2) | 0.19 (0.2) | -0.11 (0.2) | 0.16 (0.2) | 0.09 (0.2) | |
| Missing | 0 | 0 | 2 (0.9%) | 4 (0.7%) | 12 (6.3%) | |
| Black carbon (μg/m3): mean (SD) | 0.64 (0.1) | 0.63 (0.17) | ||||
| Missing | 0 | 0 | ||||
| Light absorption coefficient (10−5/m): mean (SD) | 0.89 (0.35) | 0.6 (0.3) | 0.7 (0.4) | |||
| Missing | 14 (6.1%) | 23 (4.1%) | 11 (5.6%) | |||
| Pollution Proximity Index4 (scale of 0–5): mean (SD) | 4.3 (1.1) | 3.9 (1.4) | ||||
| Missing | 5 (2.2%) | 0 | ||||
| Oxides of nitrogen (NOx, parts per billion): mean (SD) | 31.9 (16.2) | 21.55 (12.2) | 27 (16.4) | |||
| Missing | 14 (6.1%) | 23 (4.1%) | 11 (5.6%) | |||
| Experiences of Discrimination (EOD, N of domains)5: N (%) | 0 | 30 (13.4%) | 35 (50.7%) | |||
| 1–2 | 52 (23.2%) | 24 (34.8%) | ||||
| 3+ | 140 (62.5%) | 10 (14.5%) | ||||
| Missing | 2 (0.9%) | 0 | ||||
| Major Discrimination Scale (MDS, N of domains)6: N (%) | 0 | 129 (56.6%) | 534 (96.4%) | 131 (68.6%) | ||
| 1–2 | 79 (34.6%) | 20 (3.6%) | 53 (27.7%) | |||
| 3+ | 20 (8.8%) | 0 | 7 (3.7%) | |||
| Missing | 1 (0.4%) | 1 (0.2%) | 0 | |||
| Predicted cell count proportions | ||||||
| B Cell | 0.08 (0.02) | 0.06(0.01) | 0.04 (0.03) | 0.03 (0.02) | 0.03 (0.02) | |
| CD4+T cells | 0.17 (0.05) | 0.15 (0.04) | 0.04 (0.02) | 0.03 (0.03) | 0.03 (0.01) | |
| CD8+T cells | 0.005 (0.02) | 0.002 (0.01) | 0.002 (0.006) | 0.0007 (0.003) | 0.0006 (0.003) | |
| Monocytes | 0.124 (0.02) | 0.116 (0.02) | 0.9 (0.05) | 0.91 (0.04) | 0.92 (0.04) | |
| Neutrophils | 0.55 (0.1) | 0.62 (0.08) | 0.0003 (0.002) | 0.0008 (0.005) | 0.0009 (0.007) | |
| Natural Killer | 0.1 (0.04) | 0.09 (0.04) | 0.015 (0.01) | 0.012 (0.01) | 0.01 (0.01) | |
| Eosinophils | 0.007 (0.02) | 0.003 (0.009) | 0.01(0.01) | 0.01(0.01) | 0.01 (0.01) | |
Jim Crow states are the 21 US states (plus the District of Columbia) which permitted legal racial discrimination prior to the 1964 US Civil Rights Act.
Participants’ ratio of household income in 2010 dollars to the US 2010 poverty line given household composition.
Census tract measure of economic and racialized segregation, scored from −1 to 1
NOx measurements were used to construct a weighted score of roadway pollution
Validated self-report questionnaire measuring the number of domains of exposure to racial discrimination. Score range 0–9, categorised into 0, 1–2, 3
Validated self-report questionnaire measuring the number of domains of exposure to racial discrimination; combined with the attribution aspect from EDS (everyday discrimination scale) to enable comparability between EOD and MDS. Score range 0–5, categorised into 0, 1–2, 3+
Key points.
Question:
Could DNAm be a mechanism by which adversity becomes embodied?
Findings:
Traffic-related air pollution exposure may induce epigenetic changes related to inflammatory processes; and there are suggestive associations with measures of structural racism.
Meaning:
DNAm may be a biological mechanism through which structural racism and air pollution become biologically embodied.
Acknowledgements
We thank, with written permission: (1) Nykesha Johnson for her insightful comments on this manuscript (2) Linda V. Nguyen (Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA; May 1, 2023) for her contributions to extracting the DNA from the MBMS blood spots and preparing it for shipment to the Bristol team members for analysis, (3) Emily Wright, PhD (Harvard TH Chan School of Public Health; May 1, 2023), for her assistance, when a doctoral student, with linking area-based data to the MBMS study participant records; and (4) Karen Ho (May 17, 2023), (5) Louise Falk (May 18, 2023), (6) Sophie FitzGibbon (May 24, 2023), and (7) Catherine Slack (May 19, 2023) for their role in generating the MBMS Illumina data.
We are extremely grateful to all the MBMS participants who took part in the original MBMS study, the staff at the four participating community health centers, and the researchers who conducted participant recruitment and interviews. We are likewise grateful to all the MESA participants and investigators whose data and dedicated work enabled us to have incorporate MESA data into our research project, including Amanda Gassett, MS for providing us with the air pollution dataset used from MESA Air (written permission: July 20, 2023); a full list of participating MESA investigators and institutions can be found at: http://www.mesa-nhlbi.org. This paper has been reviewed and approved by the MESA Publications and Presentations Committee (DATE).
Funding
The work for this study was supported by the National Institutes of Health, National Institute of Minority and Health Disparities [grant number grant R01MD014304 to N.K. and C.R., as MPIs]. Additionally, GDS, CR, KT, MS, SHW work within the MRC Integrative Epidemiology Unit at the University of Bristol, which is supported by the Medical Research Council (MC_UU_00011/1,3, & 5 and MC_UU-00032/1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Prior funding supporting our work:
a) The prior MBMS study was funded by the National Institutes of Health/National Institute on Aging (R01AG027122) and generation of the black carbon data for this study was supported by a pilot grant from the Harvard NIEHS Center (P30 ES000002).
b) No additional grant funding to MESA was obtained for the conduct of this study whose Data Use Agreement (G638) was approved by MESA (on 11/22/2019) to use pre-existing MESA data.
Support for generating these pre-existing MESA data is provided by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1-TR-001881, and DK063491. The MESA Epigenomics & Transcriptomics Studies were funded by NIH grants 1R01HL101250, 1RF1AG054474, R01HL126477, R01DK101921, and R01HL135009.
This publication was developed under the Science to Achieve Results (STAR) research assistance agreements, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication.
Funding for MESA SHARe genotyping was provided by NHLBI Contract N02-HL-6-4278. Genotyping was performed at the Broad Institute of Harvard and MIT (Boston, Massachusetts, USA) and at Affymetrix (Santa Clara, California, USA) using the Affymetrix Genome-Wide Human SNP Array 6.0.
Funding Statement
The work for this study was supported by the National Institutes of Health, National Institute of Minority and Health Disparities [grant number grant R01MD014304 to N.K. and C.R., as MPIs]. Additionally, GDS, CR, KT, MS, SHW work within the MRC Integrative Epidemiology Unit at the University of Bristol, which is supported by the Medical Research Council (MC_UU_00011/1,3, & 5 and MC_UU-00032/1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Ethics approval
The study protocol, involving use of both the MBMS and MESA data, was approved by the Harvard T.H. Chan School of Public Health Office of Human Research Administration (Protocol # IRB19-0524; June 10, 2019).
The original MBMS study protocol, implemented in accordance with the Helsinki Declaration of 1975, as revised in 2000, was approved by the Harvard School of Public Health Office of Human Research Administration (protocol #11950-127, which covered 3 of the 4 health centers through reciprocal IRB agreements), and was also separately approved by the fourth community health center’s Institutional Review Board. All participants provided written informed consent.
Information regarding the MESA protocols and their IRB approvals and other information, is available at: www.mesa-nhlbi.org.
Conflict of interest
The authors declare no conflict of interest.
Data sharing
This study (NIH Grant number R01MD014304) relied on three sources of data, each of which is subject to distinct data sharing stipulations: (1) the non-public data from the “My Body, My Story” (MBMS) study; (2) the non-public data from the Multi-Ethnic Study of Atherosclerosis (MESA; data use agreement G638); and (3) the public de-identified data from the US Census, the American Community Survey, and the State Policy Liberalism Index. We provide descriptions of these data sharing stipulations and access to these data below; this information is also available at: https://www.hsph.harvard.edu/nancy-krieger/data-sharing-resources/
ICE metrics relating to racial composition, income distribution, and housing tenure that were derived from sources in the public domain i.e. the US Census and the American Community Survey are available at the census tract level now on GitHub.
Code used to construct the variables is available on GitHub and here.
The State Policy Liberalism Index data used in our study is also publicly available and can be obtained from the Harvard Dataverse. Reference: Caughey, Devin; Warshaw, Christopher, 2014, “The Dynamics of State Policy Liberalism, 1936–2014”, http://dx.doi.org/10.7910/DVN/ZXZMJB Dataverse [Distributor] V1 [Version].
De-identified data from the My Body My Story study used for this project will be made available only for purposes approved by the study PI, as stipulated by the study’s informed consent protocol. The application form to obtain these data will be made available via this website after completion of this project in late Fall 2024.
Data from the Multi-Ethnic Study of Atherosclerosis (MESA) must be obtained directly from the MESA website via their application protocol.
The scripts to run the EWAS and downstream analyses are available on GitHub.
EWAS summary statistics will be uploaded to the EWAS catalog website upon publication.
References
- 1.Martin EM, Fry RC. Environmental Influences on the Epigenome: Exposure- Associated DNA Methylation in Human Populations. Annu Rev Public Health. 2018;39:309–333. [DOI] [PubMed] [Google Scholar]
- 2.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19(6):371–384. [DOI] [PubMed] [Google Scholar]
- 3.van der Laan LC, Meeks KAC, Chilunga FP, et al. Epigenome-wide association study for perceived discrimination among sub-Saharan African migrants in Europe - the RODAM study. Sci Rep. 2020;10(1):4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krieger N. Ecosocial Theory, Embodied Truths, and the People’s Health. New York: Oxford University Press; 2021. [Google Scholar]
- 5.Krieger N. Methods for the scientific study of discrimination and health: an ecosocial approach. Am J Public Health. 2012;102(5):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaluscha S, Domcke S, Wirbelauer C, et al. Evidence that direct inhibition of transcription factor binding is the prevailing mode of gene and repeat repression by DNA methylation. Nat Genet. 2022;54(12):1895–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell JT, Pai AA, Pickrell JK, et al. DNA methylation patterns associate with genetic and gene expression variation in HapMap cell lines. Genome Biol. 2011;12(1):R10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutierrez-Arcelus M, Lappalainen T, Montgomery SB, et al. Passive and active DNA methylation and the interplay with genetic variation in gene regulation. Elife. 2013;2:e00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiansen C, Castillo-Fernandez JE, Domingo-Relloso A, et al. Novel DNA methylation signatures of tobacco smoking with trans-ethnic effects. Clin Epigenetics. 2021;13(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wielscher M, Mandaviya PR, Kuehnel B, et al. DNA methylation signature of chronic low-grade inflammation and its role in cardio-respiratory diseases. Nat Commun. 2022;13(1):2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugden K, Hannon EJ, Arseneault L, et al. Establishing a generalized polyepigenetic biomarker for tobacco smoking. Transl Psychiatry. 2019;9(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai PC, Glastonbury CA, Eliot MN, et al. Smoking induces coordinated DNA methylation and gene expression changes in adipose tissue with consequences for metabolic health. Clin Epigenetics. 2018;10(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richmond RC, Suderman M, Langdon R, Relton CL, Davey Smith G. DNA methylation as a marker for prenatal smoke exposure in adults. Int J Epidemiol. 2018;47(4):1120–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watkins SH, Iles-Caven Y, Pembrey M, Golding J, Suderman M. Grandmaternal smoking during pregnancy is associated with differential DNA methylation in peripheral blood of their grandchildren. Eur J Hum Genet. 2022;30(12):1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerutti J, Lussier AA, Zhu Y, Liu J, Dunn EC. Associations between indicators of socioeconomic position and DNA methylation: a scoping review. Clin Epigenetics. 2021;13(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger N, Waterman PD, Gryparis A, Coull BA. Black carbon exposure, socioeconomic and racial/ethnic spatial polarization, and the Index of Concentration at the Extremes (ICE). Health Place. 2015;34:215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Liu P, Schwartz J, et al. Disparities in ambient nitrogen dioxide pollution in the United States. Proc Natl Acad Sci U S A. 2023;120(16):e2208450120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panni T, Mehta AJ, Schwartz JD, et al. Genome-Wide Analysis of DNA Methylation and Fine Particulate Matter Air Pollution in Three Study Populations: KORA F3, KORA F4, and the Normative Aging Study. Environ Health Perspect. 2016;124(7):983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruzieva O, Xu CJ, Yousefi P, et al. Prenatal Particulate Air Pollution and DNA Methylation in Newborns: An Epigenome-Wide Meta-Analysis. Environ Health Perspect. 2019;127(5):57012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi GC, Liu Y, MacDonald JW, et al. Epigenome-wide analysis of long-term air pollution exposure and DNA methylation in monocytes: results from the Multi-Ethnic Study of Atherosclerosis. Epigenetics. 2022;17(3):297–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gondalia R, Baldassari A, Holliday KM, et al. Methylome-wide association study provides evidence of particulate matter air pollution-associated DNA methylation. Environ Int. 2019;132:104723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de F. C. Lichtenfels AJ, van der Plaat DA, de Jong K, et al. Long-term Air Pollution Exposure, Genome-wide DNA Methylation and Lung Function in the LifeLines Cohort Study. Environ Health Perspect. 2018;126(2):027004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eze IC, Jeong A, Schaffner E, et al. Genome-Wide DNA Methylation in Peripheral Blood and Long-Term Exposure to Source-Specific Transportation Noise and Air Pollution: The SAPALDIA Study. Environ Health Perspect. 2020;128(6):67003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kingsley SL, Eliot MN, Whitsel EA, et al. Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environ Int. 2016;92–93:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barcelona de Mendoza V, Huang Y, Crusto CA, Sun YV, Taylor JY. Perceived Racial Discrimination and DNA Methylation Among African American Women in the InterGEN Study. Biol Res Nurs. 2018;20(2):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Havranek EP, Mujahid MS, Barr DA, et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;132(9):873–898. [DOI] [PubMed] [Google Scholar]
- 27.Krieger N. Discrimination and health inequities. Int J Health Serv. 2014;44(4):643–710. [DOI] [PubMed] [Google Scholar]
- 28.Williams DR, Lawrence JA, Davis BA. Racism and Health: Evidence and Needed Research. Annu Rev Public Health. 2019;40:105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey ZD, Krieger N, Agenor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389(10077):1453–1463. [DOI] [PubMed] [Google Scholar]
- 30.Sistrunk C, Tolbert N, Sanchez-Pino MD, et al. Impact of Federal, State, and Local Housing Policies on Disparities in Cardiovascular Disease in Black/African American Men and Women: From Policy to Pathways to Biology. Front Cardiovasc Med. 2022;9:756734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Circulation. 2015;131(2):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramer MR, Black NC, Matthews SA, James SA. The legacy of slavery and contemporary declines in heart disease mortality in the U.S. South. SSM Popul Health. 2017;3:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukachko A, Hatzenbuehler ML, Keyes KM. Structural racism and myocardial infarction in the United States. Soc Sci Med. 2014;103:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egede LE, Campbell JA, Walker RJ, Linde S. Structural Racism as an Upstream Social Determinant of Diabetes Outcomes: A Scoping Review. Diabetes Care. 2023;46(4):667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal S, Wade AN, Mbanya JC, et al. The role of structural racism and geographical inequity in diabetes outcomes. Lancet. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawlor DA, Tilling K, Davey Smith G. Triangulation in aetiological epidemiology. Int J Epidemiol. 2016;45(6):1866–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liebler CA, Porter SR, Fernandez LE, Noon JM, Ennis SR. America’s Churning Races: Race and Ethnicity Response Changes Between Census 2000 and the 2010 Census. Demography. 2017;54(1):259–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Min JL, Hemani G, Davey Smith G, Relton C, Suderman M. Meffil: efficient normalization and analysis of very large DNA methylation datasets. Bioinformatics. 2018;34(23):3983–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phipson B, Maksimovic J, Oshlack A. missMethyl: an R package for analyzing data from Illumina’s HumanMethylation450 platform. Bioinformatics. 2016;32(2):286–288. [DOI] [PubMed] [Google Scholar]
- 41.Sainsbury S, Bernecky C, Cramer P. Structural basis of transcription initiation by RNA polymerase II. Nat Rev Mol Cell Biol. 2015;16(3):129–143. [DOI] [PubMed] [Google Scholar]
- 42.Mehta V, Pang KL, Rozbesky D, et al. The guidance receptor plexin D1 is a mechanosensor in endothelial cells. Nature. 2020;578(7794):290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puan KJ, San Luis B, Yusof N, et al. FUT6 deficiency compromises basophil function by selectively abrogating their sialyl-Lewis x expression. Commun Biol. 2021;4(1):832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim GD, Das R, Goduni L, McClellan S, Hazlett LD, Mahabeleshwar GH. Kruppel-like Factor 6 Promotes Macrophage-mediated Inflammation by Suppressing B Cell Leukemia/Lymphoma 6 Expression. J Biol Chem. 2016;291(40):21271–21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy SP, Mossman BT. Role and regulation of activator protein-1 in toxicant-induced responses of the lung. Am J Physiol Lung Cell Mol Physiol. 2002;283(6):L1161–1178. [DOI] [PubMed] [Google Scholar]
- 46.Zenz R, Eferl R, Scheinecker C, et al. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res Ther. 2008;10(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou L, Xue C, Chen Z, Jiang W, He S, Zhang X. c-Fos is a mechanosensor that regulates inflammatory responses and lung barrier dysfunction during ventilator-induced acute lung injury. BMC Pulm Med. 2022;22(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makita S, Takatori H, Nakajima H. Post-Transcriptional Regulation of Immune Responses and Inflammatory Diseases by RNA-Binding ZFP36 Family Proteins. Front Immunol. 2021;12:711633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smallie T, Ross EA, Ammit AJ, et al. Dual-Specificity Phosphatase 1 and Tristetraprolin Cooperate To Regulate Macrophage Responses to Lipopolysaccharide. J Immunol. 2015;195(1):277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.dos Santos G, Rogel MR, Baker MA, et al. Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun. 2015;6:6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H, Zuo J, Tang W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front Pharmacol. 2018;9:1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang T, Chen X, Qiao W, Kong L, Sun D, Li Z. Transcription factor E2F1 promotes EMT by regulating ZEB2 in small cell lung cancer. BMC Cancer. 2017;17(1):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magnussen SN, Toraskar J, Hadler-Olsen E, Steigedal TS, Svineng G. Nephronectin as a Matrix Effector in Cancer. Cancers (Basel). 2021;13(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith SJ, Towers N, Saldanha JW, et al. The cardiac-restricted protein ADP-ribosylhydrolase-like 1 is essential for heart chamber outgrowth and acts on muscle actin filament assembly. Dev Biol. 2016;416(2):373–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simons RL, Lei MK, Klopack E, Beach SRH, Gibbons FX, Philibert RA. The effects of social adversity, discrimination, and health risk behaviors on the accelerated aging of African Americans: Further support for the weathering hypothesis. Soc Sci Med. 2021;282:113169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cuevas AG, Ong AD, Carvalho K, et al. Discrimination and systemic inflammation: A critical review and synthesis. Brain Behav Immun. 2020;89:465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chi GC, Liu Y, MacDonald JW, et al. Long-term outdoor air pollution and DNA methylation in circulating monocytes: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health. 2016;15(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abed Al Ahad M, Demsar U, Sullivan F, Kulu H. The spatial-temporal effect of air pollution on individuals’ reported health and its variation by ethnic groups in the United Kingdom: a multilevel longitudinal analysis. BMC Public Health. 2023;23(1):897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study (NIH Grant number R01MD014304) relied on three sources of data, each of which is subject to distinct data sharing stipulations: (1) the non-public data from the “My Body, My Story” (MBMS) study; (2) the non-public data from the Multi-Ethnic Study of Atherosclerosis (MESA; data use agreement G638); and (3) the public de-identified data from the US Census, the American Community Survey, and the State Policy Liberalism Index. We provide descriptions of these data sharing stipulations and access to these data below; this information is also available at: https://www.hsph.harvard.edu/nancy-krieger/data-sharing-resources/
ICE metrics relating to racial composition, income distribution, and housing tenure that were derived from sources in the public domain i.e. the US Census and the American Community Survey are available at the census tract level now on GitHub.
Code used to construct the variables is available on GitHub and here.
The State Policy Liberalism Index data used in our study is also publicly available and can be obtained from the Harvard Dataverse. Reference: Caughey, Devin; Warshaw, Christopher, 2014, “The Dynamics of State Policy Liberalism, 1936–2014”, http://dx.doi.org/10.7910/DVN/ZXZMJB Dataverse [Distributor] V1 [Version].
De-identified data from the My Body My Story study used for this project will be made available only for purposes approved by the study PI, as stipulated by the study’s informed consent protocol. The application form to obtain these data will be made available via this website after completion of this project in late Fall 2024.
Data from the Multi-Ethnic Study of Atherosclerosis (MESA) must be obtained directly from the MESA website via their application protocol.
The scripts to run the EWAS and downstream analyses are available on GitHub.
EWAS summary statistics will be uploaded to the EWAS catalog website upon publication.