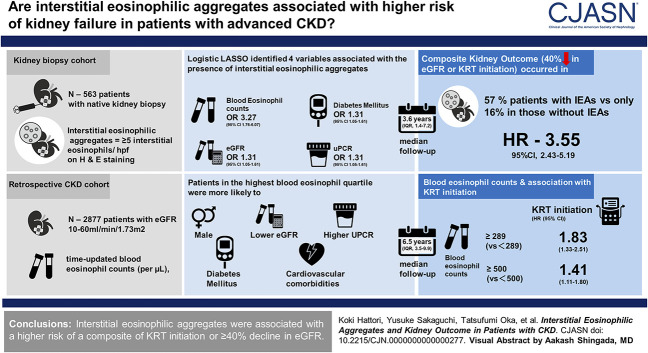
Visual Abstract
Keywords: CKD
Abstract
Background
Interstitial eosinophilic aggregates are observed in various kidney diseases, but their clinical implications remain unknown. We assessed the association between interstitial eosinophilic aggregates and kidney outcomes and further analyzed the association between blood eosinophil count, as a surrogate for interstitial eosinophilic aggregates, and the risk of kidney failure in patients with advanced CKD.
Methods
We analyzed datasets from two retrospective cohort studies: (1) the kidney biopsy cohort including 563 patients who underwent native kidney biopsy at Osaka University Hospital between 2009 and 2021 and (2) the retrospective CKD cohort including 2877 patients with an eGFR of 10–60 ml/min per 1.73 m2 referred to the nephrology outpatient center at Osaka University Hospital between 2005 and 2018. Interstitial eosinophilic aggregates were defined as ≥5 interstitial eosinophils in the high-power field on hematoxylin and eosin staining. This study outcome was initiation of KRT or ≥40% decline in eGFR.
Results
In the kidney biopsy cohort, interstitial eosinophilic aggregates were found in 17% of patients, most frequently in those with diabetic nephropathy (50%). Interstitial eosinophilic aggregates were associated with a higher rate of the composite kidney outcome after adjustment for clinical and histological variables (hazard ratio, 3.61; 95% confidence interval, 2.47 to 5.29; P < 0.001). LASSO revealed that blood eosinophil count was the strongest predictor of interstitial eosinophilic aggregates. In the retrospective CKD cohort, higher baseline and time-updated blood eosinophil counts were significantly associated with a higher rate of KRT initiation in Cox proportional hazards models and marginal structural models.
Conclusions
Interstitial eosinophilic aggregates were associated with a higher risk of a composite of KRT initiation or ≥40% decline in eGFR.
Podcast
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/CJASN/2023_12_08_CJN0000000000000277.mp3
Introduction
Eosinophils are multifunctional leukocytes that play a pivotal role in various pathophysiological processes, including allergic reactions, infections, autoimmunity, and malignancy.1 Eosinophils are also involved in tissue fibrosis and remodeling through type 2 immunity.2 For example, activated eosinophils exacerbate fibrosis in the lung,3 liver,4 and intestine5 and promote cardiac remodeling6 and atheroscrelosis.7,8 Population-based cohort studies have reported an elevated risk of cardiovascular events among patients with high levels of plasma eosinophilic cationic protein, a marker of eosinophil activity and degranulation.8,9
By contrast, the role of eosinophils in the pathogenesis of kidney disease has been poorly investigated. Eosinophilic infiltration in the kidney interstitium has been considered a particular finding in certain etiologies, such as drug-induced tubulointerstitial nephritis. However, it has become apparent that interstitial eosinophilic aggregates are observed in a broader spectrum of kidney diseases, including diabetic kidney disease, membranous nephropathy, IgA nephropathy, focal and segmental glomerulosclerosis, and membranoproliferative glomerulonephritis.10–12 Moreover, interstitial eosinophilic aggregates are closely correlated with the degree of interstitial fibrosis/tubular atrophy.11,12 Nevertheless, the effect of interstitial eosinophilic aggregates on kidney outcomes is unknown.
The primary aim of our study was to examine the association between interstitial eosinophilic aggregates and kidney outcomes. In addition, because we identified blood eosinophil count as a potent predictor of interstitial eosinophilic aggregates, we analyzed the association between blood eosinophil count, as a surrogate of interstitial eosinophilic aggregates, and the risk of kidney failure to gain more information about interstitial eosinophilic aggregates in advanced CKD.
Methods
We retrospectively analyzed (1) the association between interstitial eosinophilic aggregates and kidney outcome in the kidney biopsy cohort and (2) the association between blood eosinophil count and kidney outcome in the retrospective CKD cohort. This study was conducted in accordance with the principles of the Declaration of Helsinki. The Ethics Committee of Osaka University Hospital approved the study protocol and waived the need for written informed consent given the retrospective nature of the study (no: 20352, 22047, 22467).
The Kidney Biopsy Cohort
Study Population
Consecutive patients (aged 20 years and older) who underwent clinically indicated native kidney biopsy at the Department of Nephrology, Osaka University Hospital, between 2009 and 2021 were enrolled. Patients were excluded if the biopsy specimen was insufficient or if the staining quality was poor (fading of hematoxylin and eosin [H&E] staining). The follow-up period was from the date of the first kidney biopsy to the onset of kidney outcomes, death, or the date of the last hospital visit, based on the patients' electronic medical records.
Demographic and Clinical Variables
Demographic and clinical data on the date of the first kidney biopsy were obtained using an electronic data capture system developed at Osaka University Hospital. The electronic data capture system, which is integrated with the electronic medical records, can automatically extract individual patients' data.13–15 These data included age, sex, diabetes mellitus, eGFR, urinary protein-to-creatinine ratio (UPCR), blood eosinophil counts, and prescriptions (angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, loop diuretics, thiazide diuretics, mineralocorticoid receptor antagonists, proton pump inhibitors, histamine type-2 receptor antagonists, corticosteroids, and nonsteroidal anti-inflammatory drugs). eGFR was calculated using the equation for the Japanese population.16 Body weight and BP levels were measured before performing the biopsy.
Definition of Interstitial Eosinophilic Aggregates
Interstitial eosinophilic aggregates were defined as the presence of ≥5 interstitial eosinophils per high-power field (i.e., 40× objective lens and 10× ocular lens) on H&E staining (Figure 1).11 All specimens were converted to high-resolution digital data by virtual slide scanners and were evaluated by two nephrologists, who were unaware of patients' information, using image viewing software NDP.view2 (Hamamatsu Photonics Co., Ltd., Hamamatsu, Japan). A consensus between the two nephrologists was reached through repeated review of the specimens and discussion. Interobserver agreement for the diagnosis of interstitial eosinophilic aggregates was assessed using the Cohen kappa statistic.17
Figure 1.
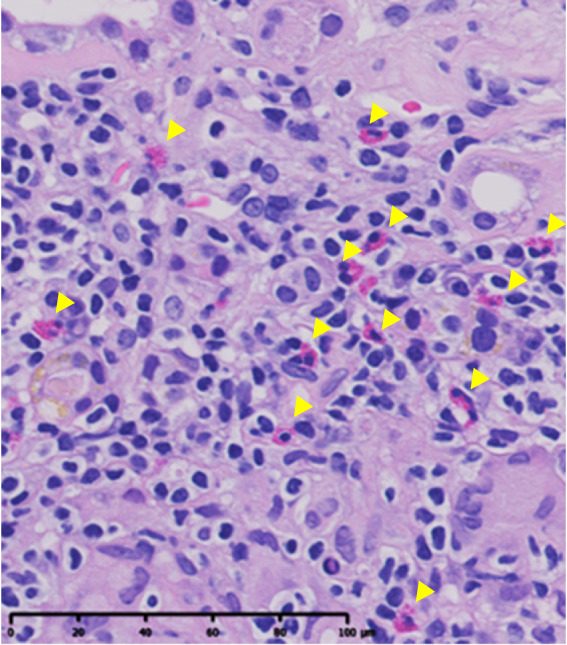
Interstitial eosinophilic aggregates. Interstitial eosinophilic aggregates are defined as the presence of at least five eosinophils (yellow arrowheads) per HPF in the kidney interstitium. H&E staining. Bar, 100 μm. H&E, hematoxylin and eosin; HPF, high-power field.
Evaluation of Histopathology
Clinicopathological diagnoses and histopathological findings were obtained from biopsy reports documented by an expert kidney pathologist independent of this study. These reports provided detailed information about biopsy findings, including the number of sclerotic glomeruli and the degree of interstitial fibrosis, tubular atrophy, and arteriosclerosis.
The histological chronicity score was calculated on the basis of the glomerulosclerosis, interstitial fibrosis, tubular atrophy, and arteriosclerosis. This score has been shown to be a significant predictor of the progression of kidney diseases in several large cohorts of patients with a variety of glomerular diseases.18–20 Glomerulosclerosis was scored on the basis of the proportion of globally and segmentally sclerotic glomeruli: 0, <10%; 1, 10%–25%; 2, 26%–50%; and 3, >50%. Interstitial fibrosis and tubular atrophy were scored on the basis of the percentage of the cortical tubulointerstitial area involved: 0, <5%; 1, 5%–24%; 2, 25%–49%; and 3, ≥50%. Arteriosclerosis was scored on the basis of the severity of arteriolar hyalinosis and intimal thickening (0, none or partial arteriolar hyalinosis and intimal/media thickness <1; 1, arteriolar wall hyalinosis comprising >50% of the arteriolar circumference or intimal/media thickness ≥1). The histological chronicity score ranges from 0 to 3 on the basis of the sum of the scores for glomerulosclerosis, interstitial fibrosis, tubular atrophy, and arteriosclerosis (score 0, 0–1; score 1, 2–4; score 2, 5–7; score 3, 8–10).
Study End Point
The study end point was the composite kidney outcome consisting of a ≥40% decline in eGFR from the first biopsy or an initiation of KRT. KRT was defined as maintenance dialysis or kidney transplantation. The dates of KRT initiation were ascertained through a chart review of the patients' electronic medical records by nephrologists.
The Retrospective CKD Cohort
Study Population
This retrospective cohort included all patients referred to the Department of Nephrology, Osaka University Hospital, from 2005 to 2018, who met the following criteria: (1) 20 years and older, (2) eGFR of 10–60 ml/min per 1.73 m2, and (3) not receiving KRT. Patients were excluded if (1) they were followed up for <1 year or (2) they had received corticosteroids. The follow-up period was from the first hospital visit to death, KRT initiation, loss to follow-up, or February 28, 2019, whichever occurred first.
Demographic and Clinical Variables
The details of the collected data are described in the Supplemental Methods. Data on demographics and comorbidities were extracted from a chart review of the patients' electronic medical records by nephrologists. Time series data on laboratory measurements and prescriptions were collected using the electronic data capture system as described in the kidney biopsy cohort.
Exposure and Study End Point
The exposure was time-updated blood eosinophil counts (per µl), which were categorized into quartiles. Blood eosinophil counts were calculated by multiplying the total white blood cell count by the percentage of eosinophils measured using an automated white blood cell differential counter.
The study end point was KRT initiation, defined as the initiation of maintenance dialysis or kidney transplantation. The dates of KRT initiation were ascertained through a chart review of the electronic medical records.
Statistical Analysis
The Kidney Biopsy Cohort
Baseline characteristics were presented as number (proportion), mean (SD), or median (interquartile range [IQR]).
Logistic LASSO was performed to identify variables associated with the presence of interstitial eosinophilic aggregates (Supplemental Methods).21–23 We used an adaptive method that performs multiple iterations of a cross-validation method to determine the tuning parameter λ for the penalty term. Using a cross-fit partialing-out method, we estimated standardized odds ratios, 95% confidence intervals (CIs), and P values for selected variables (STATA command “xpologit”).24
A Cox proportional hazards model was used to examine the association between interstitial eosinophilic aggregates and the outcome. We created two multivariable models: (1) a clinical model on the basis of the Kidney Failure Risk Equation,25,26 which included age, sex, eGFR, and UPCR at biopsy and (2) a clinical/histological model that included the histological chronicity score in addition to the covariates in the clinical model. Subgroup analyses were conducted on the basis of prespecified relevant clinical variables (age, sex, diabetes mellitus, eGFR, UPCR, blood eosinophil counts, and IgA nephropathy). Proportional hazard assumptions were checked on the basis of complementary log-log plots and scaled Schoenfeld residuals.
We performed several sensitivity analyses. First, (1) diabetes mellitus and (2) interstitial cell infiltration scores were added to the clinical/histological model. The interstitial cell infiltration score was determined on the basis of the degree of interstitial cell infiltration in the kidney (0, <5%; 1, 5%–24%; 2, 25%–49%; and 3, ≥50%). Second, we repeated the analysis after excluding any etiologies that contained <10 patients, as well as tubulointerstitial nephritis, ANCA-related vasculitis, and lupus nephritis.
The Retrospective CKD Cohort
The relationship between blood eosinophil count and eGFR was depicted using a restricted cubic spline curve with three knots (10th, 50th, and 90th percentiles of eGFR).
We analyzed the association between blood eosinophil counts and the outcome using (1) baseline Cox model, (2) time-average Cox model, (3) group-based trajectory model, and (4) marginal structural model. The detailed methodology of these statistical models is described in the Supplemental Methods. We used the marginal structural model to account for time-dependent confounding. This is because blood eosinophil count increases as eGFR declines. As a result, time-dependent confounding may occur owing to a potential bidirectional relationship between blood eosinophil count and kidney function for the development of kidney outcome.
We calculated E values, which represent the minimum strength of association that unmeasured confounders need to have with both exposures and outcomes to fully explain the observed association.27 E values are generally calculated from risk ratio (RR):
We applied approximation risk ratio calculated from hazard ratio (HR) as follows27:
Missing data in the retrospective CKD cohort were imputed using multiple imputation by chained equation (Supplemental Methods). Missing data during follow-up were imputed using the last observation carried forward method. There were no missing data in the kidney biopsy cohort.
Statistical analysis was performed using STATA/IC software (version 17.0; Stata Corp, College Station, TX). A P value of < 0.05 was considered statistically significant.
Results
Study Patients in the Kidney Biopsy Cohort
Among 643 patients who underwent native kidney biopsy, 80 were excluded because of insufficient biopsy specimens or poor H&E staining. Of the remaining 563 patients with 1596 kidney biopsy specimens, interstitial eosinophilic aggregates were found in 96 (17%) patients. The interobserver agreement for the diagnosis of interstitial eosinophilic aggregates was good (kappa statistic, 0.91).
Interstitial eosinophilic aggregates were observed predominantly in the cortex (86%), of which 80% were localized within interstitial fibrosis. The distribution of interstitial eosinophilic aggregates was focal in all cases. The number of lesions per patient was 1, 2–4, and ≥5 in 83%, 13%, and 4% of the patients with interstitial eosinophilic aggregates, respectively. The median (IQR) number of eosinophils per a single lesion was 7 (5–10).
Those with interstitial eosinophilic aggregates were older and had higher systolic BP levels and a higher prevalence of diabetes mellitus (Table 1). In addition, they had a lower eGFR, higher UPCR, higher blood eosinophil counts, and more frequently received angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, loop diuretics, and proton pump inhibitors.
Table 1.
Baseline characteristics according to interstitial eosinophilic aggregates in the kidney biopsy cohort
| Characteristics | Total | Without Interstitial Eosinophilic Aggregates | With Interstitial Eosinophilic Aggregates |
|---|---|---|---|
| N=563 | n=467 (83%) | n=96 (17%) | |
| Age, yr | 46 (19) | 45 (19) | 53 (19) |
| Male, No. (%) | 273 (48) | 218 (47) | 55 (57) |
| Systolic BP, mm Hg | 126 (22) | 125 (22) | 131 (23) |
| Diabetes mellitus, No. (%) | 82 (15) | 50 (11) | 32 (33) |
| BMI, kg/m2 | 23 (4) | 23 (4) | 23 (4) |
| eGFR, ml/min per 1.73 m2 | 65 (32) | 70 (31) | 45 (28) |
| UPCR, g/gCre | 1.1 (0.4–2.9) | 0.9 (0.3–2.3) | 2.4 (0.6–6.7) |
| Blood eosinophil counts, /µl | 129 (64–232) | 128 (62–220) | 160 (75–315) |
| ACEIs/ARBs, No. (%) | 174 (31) | 131 (28) | 43 (45) |
| Loop diuretics, No. (%) | 95 (17) | 65 (14) | 30 (31) |
| Proton pump inhibitors, No. (%) | 122 (22) | 89 (19) | 33 (34) |
| H2 blockers, No. (%) | 51 (9) | 44 (9) | 7 (7) |
| Corticosteroids, No. (%) | 80 (14) | 63 (13) | 17 (18) |
| Antibiotics, No. (%) | 20 (4) | 14 (3) | 6 (6) |
| NSAIDs, No. (%) | 7 (1) | 4 (1) | 3 (3) |
Data are presented as mean (SD), No. (%), or median (25th–75th). BMI, body mass index; UPCR, urinary protein-to-creatinine ratio; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; H2 blockers, histamine H2 receptor antagonists; NSAIDs, nonsteroidal anti-inflammatory drugs.
Histopathological Findings
Various kidney diseases were included in the kidney biopsy cohort, with IgA nephropathy being the most prevalent (39%) (Supplemental Table 1). Supplemental Figure 1 shows the proportion of interstitial eosinophilic aggregates and individual component of the histological chronicity score across clinicopathological diagnoses. Although interstitial eosinophilic aggregates were most frequently observed in patients with diabetic nephropathy (50%), they were also detected across a wide range of etiologies.
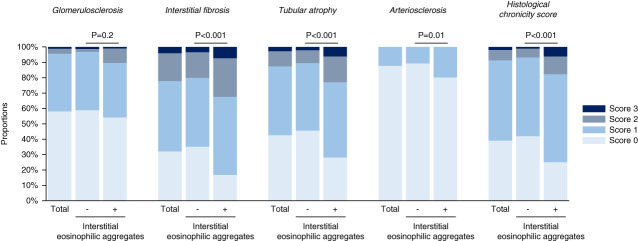
Compared with those without interstitial eosinophilic aggregates, those with interstitial eosinophilic aggregates showed higher scores for interstitial fibrosis, tubular atrophy, and arteriosclerosis, but not for glomerulosclerosis (Figure 2). A significant correlation was found between interstitial fibrosis and tubular atrophy scores (polychoric correlation coefficient, 0.75).
Figure 2.
Comparison of the individual components of the histological chronicity score between patients with and without interstitial eosinophilic aggregates. Patients with interstitial eosinophilic aggregates (n=96) had significantly higher scores for interstitial fibrosis, tubular atrophy, and arteriosclerosis, but not glomerulosclerosis, than those without interstitial eosinophilic aggregates (n=467). P values are based on the Mann–Whitney U test.
Blood Eosinophil Counts as a Predictor for Interstitial Eosinophilic Aggregates
Logistic LASSO identified four variables associated with the presence of interstitial eosinophilic aggregates: blood eosinophil count, diabetes mellitus, eGFR, and UPCR (Table 2). Among them, blood eosinophil count predicted most strongly the presence of interstitial eosinophilic aggregates (odds ratio, 3.27 per one SD higher blood eosinophil count; 95% CI, 1.76 to 6.07; P < 0.001).
Table 2.
Logistic LASSO for the presence of interstitial eosinophilic aggregates among 563 patients in the kidney biopsy cohort
| Selected Variable | Standardized ORa | 95% CI |
|---|---|---|
| Blood eosinophil counts | 3.27 | 1.76 to 6.07 |
| Diabetes mellitus | 1.31 | 1.05 to 1.61 |
| eGFR | 0.44 | 0.29 to 0.65 |
| UPCR | 1.72 | 1.28 to 2.29 |
Variables are standardized to a mean of zero and a SD of one.
Standardized ORs and 95% confidence intervals were estimated using a cross-fit partialing-out method. OR, odds ratio; CI, confidence interval; UPCR, urinary protein-to-creatinine ratio.
Per a one-SD higher each standardized variable.
Associations between Interstitial Eosinophilic Aggregates and Kidney Outcomes
During a median follow-up of 3.6 years (IQR, 1.4–7.2), the composite kidney outcome occurred in 66 (57%) of 96 patients with interstitial eosinophilic aggregates and in 77 (16%) of 467 patients without interstitial eosinophilic aggregates. There were 12 (13%) and 11 (2%) deaths in those with and without interstitial eosinophilic aggregates, respectively.
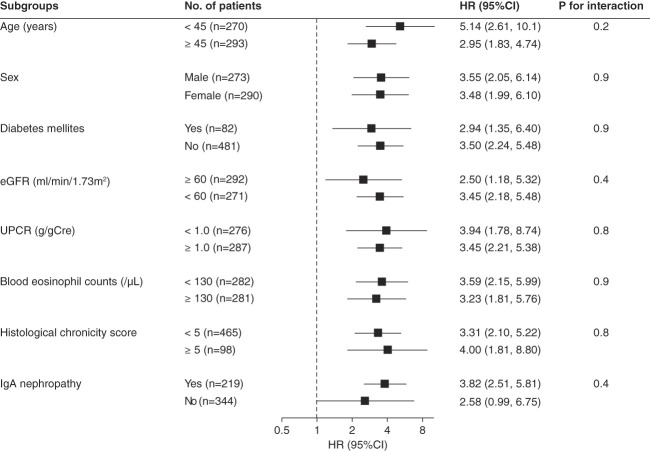
Interstitial eosinophilic aggregates were significantly associated with a higher rate of the kidney outcome in the clinical model (HR, 3.55; 95% CI, 2.43 to 5.19; P < 0.001; E value=4.17). The magnitude of this association was not substantially altered after further adjustment for the histological chronicity score (HR, 3.61; 95% CI, 2.47 to 5.29; P < 0.001; E value=4.22). Subgroup analyses showed that none of the prespecified covariates significantly modified the association between interstitial eosinophilic aggregates and kidney outcome (Figure 3).
Figure 3.
Subgroup analyses for the association between interstitial eosinophilic aggregates and kidney outcome. Models are adjusted for age, sex, eGFR, UPCR, and histological chronicity score. There are no significant effect modifications by the prespecified covariates for the association between interstitial eosinophilic aggregates and kidney outcome. CI, confidence interval; HR, hazard ratio; UPCR, urinary protein-to-creatinine ratio.
Sensitivity Analyses
First, the association between interstitial eosinophilic aggregates and kidney outcome remained largely unaltered after further adjustment for (1) diabetes mellitus and (2) the interstitial cell infiltration score in the clinical/histological model (Supplemental Table 2). Second, after excluding etiologies that involved fewer than ten patients as well as tubulointerstitial nephritis, ANCA-associated vasculitis, and lupus nephritis, interstitial eosinophilic aggregates were still significantly associated with a higher rate of kidney outcome (Supplemental Table 2).
The Retrospective CKD Cohort
Study Population
Among the 2889 patients who met the inclusion criteria, 2877 (99%) had available blood eosinophil count data (Supplemental Figure 2). The mean (SD) eGFR of these patients was 37 (14) ml/min per 1.73 m2. Patients in the highest blood eosinophil quartile were more likely to be male and have diabetes mellitus, cardiovascular comorbidities, lower eGFR, and higher UPCR (Supplemental Table 3). A monotonic negative correlation was observed between the blood eosinophil count and eGFR (Supplemental Figure 3).
Associations between Blood Eosinophil Counts and Kidney Outcome
Over the median follow-up of 6.5 years (IQR, 3.5–9.9), blood eosinophil counts were measured a median of 22 (IQR, 7–46) times per patient (4 [IQR, 2–6] times a year per patient). A total of 433 patients initiated KRT.
There was a dose-dependent association between blood eosinophil counts and the rate of KRT initiation (Table 3). Patients in the highest eosinophil quartile had a 2.12-fold (95% CI, 1.44 to 3.10; P < 0.001; E value=3.66) and 2.07-fold (95% CI, 1.40 to 3.09; P < 0.001; E value=3.56) higher rate of KRT initiation compared with those in the lowest eosinophil quartile, in the baseline and time-average Cox models, respectively (Table 3, Supplemental Table 4).
Table 3.
Associations between blood eosinophil count and kidney outcome in the retrospective CKD cohort
| Outcome: KRT Initiation | Baseline Cox Model | Time-Average Cox Model | Group-Based Trajectory Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood Eosinophil Count Category: (/µl) | Q1: <90 (n=688) | Q2: 90–170 (n=684) | Q3: 170–289 (n=736) | Q4: >289 (n=769) | Q1: ≤105 (n=696) | Q2: 106–183 (n=669) | Q3: 184–301 (n=723) | Q4: ≥302 (n=789) | Low (n=324) | Middle (n=1430) | High (n=1123) |
| No. of events | 71 | 79 | 139 | 144 | 62 | 93 | 141 | 134 | 26 | 196 | 208 |
| Incidence rate per 100 p-y (95% CI) | 1.4 (1.1 to 1.7) | 1.5 (1.2 to 1.9) | 2.7 (2.3 to 3.2) | 3.0 (2.5 to 3.5) | 1.1 (0.9 to 1.5) | 1.9 (1.5 to 2.3) | 2.9 (2.4 to 3.4) | 2.8 (2.4 to 3.3) | 1.0 (0.7 to 1.5) | 1.9 (1.6 to 2.1) | 3.0 (2.6 to 3.4) |
| HR (95% CI) | 1.00 (reference) | 1.18 (0.77 to 1.78) | 1.77 (1.20 to 2.61) | 2.12 (1.44 to 3.10) | 1.00 (reference) | 1.42 (0.95 to 2.11) | 1.98 (1.34 to 2.94) | 2.07 (1.40 to 3.09) | 1.00 (reference) | 1.74 (1.06 to 2.85) | 2.30 (1.38 to 3.84) |
The models are adjusted for age, sex, diabetes mellitus, body mass index, systolic BP, chronic respiratory diseases, cardiovascular comorbidities, history of catheterization, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, loop diuretics, thiazide diuretics, mineralocorticoid receptor antagonists, proton pump inhibitors, histamine H2 receptor antagonists, nonsteroidal anti-inflammatory drugs, hemoglobin, sodium, potassium, albumin, eGFR, C-reactive protein, urinary protein-to-creatinine ratio, and white blood cell count. p-y, person-years; CI, confidence interval; HR, hazard ratio.
The group-based trajectory model identified three distinct trajectories of blood eosinophil counts: low, middle, and high groups. The trajectories of the three groups were mostly constant (Supplemental Figure 4). The high-trajectory group showed a 2.30-fold (95% CI, 1.38 to 3.84; P = 0.001; E value=4.03) higher rate of KRT initiation than the low-trajectory group (Table 3).
In the marginal structural model, higher blood eosinophil counts (≥289/µl) were associated with a 1.83-fold (95% CI, 1.33 to 2.51; P < 0.001) higher rate of KRT initiation than lower blood eosinophil counts (<289/µl). A similar result was obtained when higher blood eosinophil counts were defined as ≥500/µl (Table 4).
Table 4.
Marginal structural models for the association between blood eosinophil counts and kidney outcome in the retrospective CKD cohort
| Blood Eosinophil Counts (/µl) | KRT Initiation |
|---|---|
| HR (95% CI) | |
| ≥289 (versus <289) | 1.83 (1.33 to 2.51) |
| ≥500 (versus <500) | 1.41 (1.11 to 1.80) |
The models are adjusted for the baseline and time-dependent covariates. Baseline covariates include age, sex, body mass index, systolic BP, diabetes mellitus, chronic respiratory diseases, cardiovascular comorbidities, history of catheterization, hemoglobin, albumin, eGFR, sodium, potassium, C-reactive protein, urinary protein-to-creatinine ratio, loop diuretics, thiazide diuretics, mineralocorticoid receptor antagonists, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and histamine H2 receptor antagonists. Time-dependent covariates include catheterization performed during follow-up, hemoglobin, albumin, eGFR, sodium, potassium, C-reactive protein, urinary protein-to-creatinine ratio, loop diuretics, thiazide diuretics, mineralocorticoid receptor antagonists, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, histamine H2 receptor antagonists, and corticosteroids. HR, hazard ratio; CI, confidence interval.
Discussion
The major findings of our study are as follows: (1) interstitial eosinophilic aggregates were found in 17% of native kidney biopsy samples comprising a diverse spectrum of kidney diseases; (2) patients with interstitial eosinophilic aggregates had more severe chronic histological lesions; (3) interstitial eosinophilic aggregates were associated with worse kidney outcomes; (4) LASSO identified blood eosinophil count as a potent predictor of interstitial eosinophilic aggregates; and (5) blood eosinophil count was significantly associated with a higher risk of kidney outcome in patients with advanced CKD. These findings highlight the clinical implications of interstitial eosinophilic aggregates, a previously overlooked histological feature, as a novel predictor of kidney disease prognosis.
Contrary to the previous belief that eosinophilic infiltrations are observed in limited etiologies such as drug-induced interstitial nephritis, eosinophilic granulomatosis with polyangiitis,28 and idiopathic hypereosinophilic syndrome,29,30 interstitial eosinophilic aggregates have been detected in various kidney diseases including diabetic nephropathy, IgA nephropathy, membranous nephropathy, FSGS, and membranoproliferative glomerulonephritis.11 In our study, interstitial eosinophilic aggregates were also found in nephrosclerosis and lupus nephritis. Therefore, nephrologists should remember that interstitial eosinophilic aggregates are commonly observed in a variety of etiologies. Particularly, diabetic nephropathy had the highest frequency of interstitial eosinophilic aggregates. Diabetes mellitus was also identified as an independent predictor of interstitial eosinophilic aggregates in LASSO. It is known that eotaxin, a potent eosinophilic chemokine, is constitutively expressed in the kidney.31 Notably, its expression is increased in diabetic nephropathy,32 which might contribute to the formation of interstitial eosinophilic aggregates. It is tempting to speculate that eotaxin expression is also upregulated in other kidney diseases. The precise mechanism of the formation of interstitial eosinophilic aggregates as well as their direct effect on kidney diseases should be elucidated by future basic studies.
We found that interstitial eosinophilic aggregates were correlated with histological chronicity scores. Particularly, interstitial eosinophilic aggregates were associated with more severe interstitial fibrosis and tubular atrophy. This may be explained by the fact that eosinophils promote tissue fibrosis through eosinophilic cytokines (IL-4, IL-5, IL-13, transforming growth factor-β, and platelet-derived growth factor) and mediators.3–5 It seems reasonable that interstitial eosinophilic aggregates were not associated with glomerulosclerosis because interstitial eosinophilic aggregates existed mostly in the tubulointerstitium. Eosinophils are known to exacerbate atherosclerosis through interaction with platelets,7,8 which may explain the significant association between interstitial eosinophilic aggregates and arteriolosclerosis in our study.
Owing to the observational study design, we cannot provide a decisive conclusion as to whether interstitial eosinophilic aggregates directly damage kidney tissues or just result from kidney inflammation and fibrosis as innocent spectators at a fire. Recently, animal studies have suggested that increased eosinophils play a role in tissue protection against heart and liver injury.33–35 Thus, it is also possible that interstitial eosinophilic aggregates in the kidney represent a protective response to an active disease status. Whether eosinophils play a protective or harmful role may depend on the target organs and the specific pathological context. Although beyond the scope of our study, the precise role of eosinophils in the progression of CKD requires further investigation.
LASSO revealed that the blood eosinophil count was a potent predictor of interstitial eosinophilic aggregates. Because blood eosinophil count and interstitial eosinophilic aggregates were both associated with eGFR, their correlation may be partly explained by confounding by eGFR. However, the association between blood eosinophil count and interstitial eosinophilic aggregates was independent of eGFR. Furthermore, blood eosinophil count was more strongly associated with interstitial eosinophilic aggregates than eGFR, suggesting that the link between higher blood eosinophil count and a higher likelihood of interstitial eosinophilic aggregates is not merely a reflection of deteriorated kidney function. Although further evidence is needed, it can be hypothesized that eosinophils accumulated in the kidney spill out into the bloodstream, or conversely, increased blood eosinophils may contribute to the formation of interstitial eosinophilic aggregates in the kidney.
Using blood eosinophil count as a surrogate for interstitial eosinophilic aggregates, we examined the association between blood eosinophil count and kidney outcomes to gain insight into interstitial eosinophilic aggregates among patients with advanced CKD. Although some studies have examined the association between blood eosinophil count and the risk of CKD progression,36,37 they were limited by small sample size, highly selective patient population, and insufficient adjustment for relevant confounders. More importantly, they did not consider longitudinal alterations in blood eosinophil count despite the fact that it increases as kidney function declines. In the retrospective CKD cohort, we demonstrated a significant association between blood eosinophil count and kidney outcomes using group-based trajectory modeling and marginal structural models, both of which captured longitudinal changes in blood eosinophil count. The marginal structural models revealed that this association was independent of time-dependent confounders, such as eGFR, in addition to various baseline confounders related to eosinophilia. In the Cox model, the E value for blood eosinophil count was 3.66, which was higher than the HRs for the two most important risk factors of CKD progression, eGFR and UPCR (1.61 [per 10 ml/min per 1.73 m2 lower] and 1.36 [per 1 g/gCre higher], respectively [Supplemental Table 4]). This implies that the significant association between blood eosinophil count and kidney outcome was not solely attributable to unmeasured confounders. Our findings suggest that eosinophils may play a role in the progression of CKD.
The strengths of our study include robust results regarding the association between interstitial eosinophilic aggregates and poor kidney prognosis. The retrospective CKD cohort had a large sample size, long follow-up period, clinically relevant end points, and <1% missing data on blood eosinophil counts, which were measured repeatedly within individuals. A variety of baseline and time-dependent covariates potentially related to eosinophilia were adjusted in the marginal structural model. Our study also has several limitations. First, the observational study design precluded causal inferences between the exposures and kidney outcome. Second, there may be residual confounding and unmeasured confounding between blood eosinophil count and kidney outcome. However, the possibility that unmeasured confounding factors fully explain the observed association may be unlikely given the magnitude of the E value, although high E values do not completely rule out unmeasured confounding.38 Third, because interstitial eosinophilic aggregates are most frequently observed in diabetic nephropathy, the prognostic value of interstitial eosinophilic aggregates might merely reflect the poor prognosis of patients with diabetic nephropathy. However, the association between interstitial eosinophilic aggregates and kidney outcomes persisted in the subgroup of patients without diabetes mellitus. Finally, because this was a single-center cohort study, external validation of our findings is required.
In conclusion, we showed the association between interstitial eosinophilic aggregates and a higher risk of kidney outcome. Because interstitial eosinophilic aggregates were observed in various kidney diseases, nephrologists should not dismiss this underappreciated histological feature. Blood eosinophil count, which may be used as a surrogate for interstitial eosinophilic aggregates, was also associated with worse kidney outcome. Although our findings need to be confirmed by other studies, interstitial eosinophilic aggregates may improve clinical management of patients with kidney diseases as a novel histological predictor of kidney outcomes. Further mechanistic studies are required to elucidate the exact role of eosinophils and their potential as therapeutic targets in CKD.
Supplementary Material
Footnotes
K.H. and Y.S. contributed equally to this work.
Disclosures
Y. Isaka reports consultancy for Kirin Co., Ltd. and Sanwa Kagaku Kenkyusyo Co., Ltd.; research funding from Kirin Co., Ltd.; advisory or leadership role for Kirin Co., Ltd. and Sanwa Kagaku Kenkyusyo Co., Ltd.; and speakers bureau for Astellas Pharma Inc., AstraZeneca plc, Kirin Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma, Otsuka Pharmaceutical Co., Ltd., and Sanwa Kagaku Kenkyusyo Co., Ltd. I. Matsui reports consultancy for Kyowa Hakko Kirin. Y. Sakaguchi reports research funding from Chugai pharmaceutical Co., Ltd., FUSO Pharmaceutical Industries, Ltd., Kissei pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Terumo Co., Ltd., and Torii pharmaceutical Co., Ltd., and speakers bureau for AstraZeneca plc, Fuso Pharmaceutical Industries, Ltd., Kirin Co., Ltd., Kissei Pharmaceutical Co., Ltd., Ono Pharma Healthcare Co., Ltd., and Torii Pharmaceutical Co., Ltd. All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
Conceptualization: Koki Hattori, Jun-Ya Kaimori, Tatsufumi Oka, Yusuke Sakaguchi.
Data curation: Yuta Asahina, Koki Hattori, Takayuki Kawaoka, Yusuke Sakaguchi.
Formal analysis: Koki Hattori.
Investigation: Yusuke Sakaguchi.
Methodology: Yuta Asahina, Koki Hattori, Takayuki Kawaoka, Tatsufumi Oka, Yusuke Sakaguchi.
Supervision: Yoshitaka Isaka, Jun-Ya Kaimori, Isao Matsui, Masayuki Mizui, Ryohei Yamamoto.
Writing – original draft: Koki Hattori, Yusuke Sakaguchi.
Writing – review & editing: Yuta Asahina, Yoshitaka Isaka, Jun-Ya Kaimori, Takayuki Kawaoka, Tatsufumi Oka.
Data Sharing Statement
The datasets used in this study are only available from the corresponding author on reasonable request.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B803.
Supplemental Table 1. Clinicopathological diagnosis in the kidney biopsy cohort.
Supplemental Table 2. Sensitivity analyses for the association between interstitial eosinophilic aggregates and kidney outcome.
Supplemental Table 3. Baseline characteristics according to blood eosinophil quartile in the retrospective CKD cohort.
Supplemental Table 4. Adjusted hazard ratios for kidney outcome in the baseline Cox proportional hazards model.
Supplemental Figure 1. Proportion of patients with interstitial eosinophilic aggregates and each component of the histological chronicity score (≥score 2) across etiologies.
Supplemental Figure 2. Flow diagram of the retrospective CKD cohort.
Supplemental Figure 3. A restricted cubic spline curve for the association between blood eosinophil counts and kidney function.
Supplemental Figure 4. Blood eosinophil count trajectories identified by a group-based trajectory model.
References
- 1.Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov. 2013;12(2):117–129. doi: 10.1038/nrd3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gieseck RL, III, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18(1):62–76. doi: 10.1038/nri.2017.90 [DOI] [PubMed] [Google Scholar]
- 3.Morimoto Y Hirahara K Kiuchi M, et al. Amphiregulin-producing pathogenic memory T helper 2 cells instruct eosinophils to secrete osteopontin and facilitate airway fibrosis. Immunity. 2018;49(1):134–150.e6. doi: 10.1016/j.immuni.2018.04.023 [DOI] [PubMed] [Google Scholar]
- 4.Reiman RM Thompson RW Feng CG, et al. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect Immun. 2006;74(3):1471–1479. doi: 10.1128/iai.74.3.1471-1479.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takemura N Kurashima Y Mori Y, et al. Eosinophil depletion suppresses radiation-induced small intestinal fibrosis. Sci Transl Med. 2018;10(429):eaan0333. doi: 10.1126/scitranslmed.aan0333 [DOI] [PubMed] [Google Scholar]
- 6.Diny NL Baldeviano GC Talor MV, et al. Eosinophil-derived IL-4 drives progression of myocarditis to inflammatory dilated cardiomyopathy. J Exp Med. 2017;214(4):943–957. doi: 10.1084/jem.20161702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marx C Novotny J Salbeck D, et al. Eosinophil-platelet interactions promote atherosclerosis and stabilize thrombosis with eosinophil extracellular traps. Blood. 2019;134(21):1859–1872. doi: 10.1182/blood.2019000518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uderhardt S Ackermann JA Fillep T, et al. Enzymatic lipid oxidation by eosinophils propagates coagulation, hemostasis, and thrombotic disease. J Exp Med. 2017;214(7):2121–2138. doi: 10.1084/jem.20161070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundström J Söderholm M Borné Y, et al. Eosinophil cationic protein, carotid plaque, and incidence of stroke. Stroke. 2017;48(10):2686–2692. doi: 10.1161/strokeaha.117.018450 [DOI] [PubMed] [Google Scholar]
- 10.Najafian B, Lusco MA, Alpers CE, Fogo AB. Approach to kidney biopsy: core curriculum 2022. Am J Kidney Dis. 2022;80(1):119–131. doi: 10.1053/j.ajkd.2021.08.024 [DOI] [PubMed] [Google Scholar]
- 11.Dai DF Sasaki K Lin MY, et al. Interstitial eosinophilic aggregates in diabetic nephropathy: allergy or not? Nephrol Dial Transplant. 2015;30(8):1370–1376. doi: 10.1093/ndt/gfv067 [DOI] [PubMed] [Google Scholar]
- 12.Wu WY Zhou XJ Sun PP, et al. Interstitial eosinophilic infiltration in diabetic nephropathy is indicative of poor prognosis, with no therapy benefit from steroid. J Diabetes. 2020;12(12):881–894. doi: 10.1111/1753-0407.13077 [DOI] [PubMed] [Google Scholar]
- 13.Oka T Sakaguchi Y Hattori K, et al. Mineralocorticoid receptor antagonist use and hard renal outcomes in real-world patients with chronic kidney disease. Hypertension. 2022;79(3):679–689. doi: 10.1161/hypertensionaha.121.18360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asahina Y Sakaguchi Y Kajimoto S, et al. Association of time-updated anion gap with risk of kidney failure in advanced CKD: a cohort study. Am J Kidney Dis. 2022;79(3):374–382. doi: 10.1053/j.ajkd.2021.05.022 [DOI] [PubMed] [Google Scholar]
- 15.Kajimoto S, Sakaguchi Y, Asahina Y, Kaimori JY, Isaka Y. Modulation of the association of hypobicarbonatemia and incident kidney failure with replacement therapy by venous pH: a cohort study. Am J Kidney Dis. 2021;77(1):35–43. doi: 10.1053/j.ajkd.2020.06.019 [DOI] [PubMed] [Google Scholar]
- 16.Matsuo S Imai E Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–992. doi: 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 17.McHugh M. Interrater reliability: the kappa statistic. Biochem Med. 2012;22(3):276–282. doi: 10.11613/bm.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X Luo F Chen R, et al. Use of histologic parameters to predict glomerular disease progression: findings from the China kidney biopsy cohort study. Am J Kidney Dis. 2023;81(4):416–424.e1. doi: 10.1053/j.ajkd.2022.08.021 [DOI] [PubMed] [Google Scholar]
- 19.Sethi S D’Agati VD Nast CC, et al. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int. 2017;91(4):787–789. doi: 10.1016/j.kint.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 20.Srivastava A Palsson R Kaze AD, et al. The prognostic value of histopathologic lesions in native kidney biopsy specimens: results from the Boston kidney biopsy cohort study. J Am Soc Nephrol. 2018;29(8):2213–2224. doi: 10.1681/ASN.2017121260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B (Methodological). 1996;58(1):267–288. doi: 10.1111/j.2517-6161.1996.tb02080.x [DOI] [Google Scholar]
- 22.Pavlou M, Ambler G, Seaman S, De Iorio M, Omar RZ. Review and evaluation of penalised regression methods for risk prediction in low-dimensional data with few events. Stat Med. 2016;35(7):1159–1177. doi: 10.1002/sim.6782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou H. The adaptive lasso and its oracle properties. J Am Stat Assoc. 2006;101(476):1418–1429. doi: 10.1198/016214506000000735 [DOI] [Google Scholar]
- 24.Chernozhukov V Chetverikov D Demirer M, et al. Double/debiased machine learning for treatment and structural parameters. Econom J. 2018;21(1):C1–C68. doi: 10.1111/ectj.12097 [DOI] [Google Scholar]
- 25.Tangri N Stevens LA Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553–1559. doi: 10.1001/jama.2011.451 [DOI] [PubMed] [Google Scholar]
- 26.Tangri N Grams ME Levey AS, et al. Multinational assessment of accuracy of equations for predicting risk of kidney failure: a meta-analysis. JAMA. 2016;315(2):164–174. doi: 10.1001/jama.2015.18202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linden A, Mathur M, VanderWeele TJ. Conducting sensitivity analysis for unmeasured confounding in observational studies using E-values: the evalue package. Stata J. 2020;20(1):162–175. doi: 10.1177/1536867x20909696 [DOI] [Google Scholar]
- 28.Durel CA Sinico RA Teixeira V, et al. Renal involvement in eosinophilic granulomatosis with polyangiitis (EGPA): a multicentric retrospective study of 63 biopsy-proven cases. Rheumatology (Oxford). 2021;60(1):359–365. doi: 10.1093/rheumatology/keaa416 [DOI] [PubMed] [Google Scholar]
- 29.Dong JH, Xu ST, Xu F, Zhou YC, Li Z, Li SJ. Clinical and morphologic spectrum of renal involvement in idiopathic hypereosinophilic syndrome. Clin Exp Nephrol. 2021;25(3):270–278. doi: 10.1007/s10157-020-02012-5 [DOI] [PubMed] [Google Scholar]
- 30.Shehwaro N, Langlois AL, Gueutin V, Izzedine H. Renal involvement in idiopathic hypereosinophic syndrome. Clin Kidney J. 2013;6(3):272–276. doi: 10.1093/ckj/sft046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2(4):449–456. doi: 10.1038/nm0496-449 [DOI] [PubMed] [Google Scholar]
- 32.Araújo LS Torquato BGS da Silva CA, et al. Renal expression of cytokines and chemokines in diabetic nephropathy. BMC Nephrol. 2020;21(1):308. doi: 10.1186/s12882-020-01960-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J Yang C Liu T, et al. Eosinophils improve cardiac function after myocardial infarction. Nat Commun. 2020;11(1):6396. doi: 10.1038/s41467-020-19297-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang C Li J Deng Z, et al. Eosinophils protect pressure overload- and β-adrenoreceptor agonist-induced cardiac hypertrophy. Cardiovasc Res. 2023;119(1):195–212. doi: 10.1093/cvr/cvac060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y Yang Y Wang M, et al. Eosinophils attenuate hepatic ischemia-reperfusion injury in mice through ST2-dependent IL-13 production. Sci Transl Med. 2021;13(579):eabb6576. doi: 10.1126/scitranslmed.abb6576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal R, Light RP. Patterns and prognostic value of total and differential leukocyte count in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(6):1393–1399. doi: 10.2215/CJN.10521110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tariq A Okamato K Tariq A, et al. Eosinophilia and risk of incident end stage kidney disease. BMC Nephrol. 2020;21(1):14. doi: 10.1186/s12882-020-1685-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaster T, Eggertsen CM, Støvring H, Ehrenstein V, Petersen I. Quantifying the impact of unmeasured confounding in observational studies with the E value. BMJ Med. 2023;2(1):e000366. doi: 10.1136/bmjmed-2022-000366 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in this study are only available from the corresponding author on reasonable request.