Abstract
Trauma and chronic stress exposure are the strongest predictors of lifetime neuropsychiatric disease presentation. These disorders often have significant sex biases, with females having higher incidences of affective disorders such as major depression, anxiety, and PTSD. Understanding the mechanisms by which stress exposure heightens disease vulnerability is essential for developing novel interventions. Current rodent stress models consist of a battery of sensory, homeostatic, and psychological stressors that are ultimately integrated by corticotropin-releasing factor (CRF) neurons to trigger corticosteroid release. These stress paradigms, however, often differ between research groups in the type, timing, and duration of stressors utilized. These inconsistencies, along with the variability of individual animals’ perception and response to each stressor, present challenges for reproducibility and translational relevance. Here, we hypothesized that a more direct approach using chemogenetic activation of CRF neurons would recapitulate the effects of traditional stress paradigms and provide a high-throughput method for examining stress-relevant phenotypes. Using a transgenic approach to express the Gq-coupled Designer Receptor Exclusively Activated by Designer Drugs (DREADD) receptor hM3Dq in CRF-neurons, we found that the DREADD ligand clozapine-N-oxide (CNO) produced an acute and robust activation of the hypothalamic-pituitary-adrenal (HPA) axis, as predicted. Interestingly, chronic treatment with this method of direct CRF activation uncovered a novel sex-specific dissociation of glucocorticoid levels with stress-related outcomes. Despite hM3Dq-expressing females producing greater corticosterone levels in response to CNO than males, hM3Dq-expressing males showed significant typical physiological stress sensitivity with reductions in body and thymus weights. hM3Dq-expressing females while resistant to the physiological effects of chronic CRF activation, showed significant increases in baseline and fear-conditioned freezing behaviors. These data establish a novel mouse model for interrogating stress-relevant phenotypes and highlight sex-specific stress circuitry distinct for physiological and limbic control that may underlie disease risk.
Subject terms: Stress and resilience, Experimental organisms
Introduction
The ability to respond to environmental and homeostatic perturbations is critical for survival, and accordingly, the stress response is highly conserved across species [1–8]. In response to stress, corticotropin-releasing factor (CRF) neurons, including in the paraventricular nucleus of the hypothalamus (PVN), prefrontal cortex, hippocampus, basolateral and central amygdala (BLA, CeA), and bed nucleus of the stria terminalis (BNST), modulate physiological, behavioral, and endocrine responses [9–20]. Limbic and cortical afferents converge onto CRF neurons in the PVN to initiate the hypothalamic-pituitary-adrenal (HPA) stress response, resulting in glucocorticoid secretion from the adrenal gland into circulation [17, 21–26].
Chronic stress and activation of CRF neurons are strongly linked to neuropsychiatric disorder development [17, 19, 21, 27–38]. Rodent models are commonly used to investigate the mechanisms by which chronic stress exposure contributes to risk as they recapitulate many of the behavioral and physiological effects seen in humans. Most models involve a battery of psychological, sensory, and homeostatic stressors, and while effective in inducing stress responses, the type, duration, and timing of exposure varies widely across research labs and produces an array of behavioral and physiological changes that rely on the individual animal’s perception of and response to the stress, often resulting in variability between cohorts of animals even within a single lab [39–45]. Even widely used models, such as chronic variable or unpredictable stress, are often modified to fit the needs of individual groups. Sex differences in the efficacy of some models add an additional complication, hindering our collective ability to uncover important sex-specific mechanisms in stress-related disorders or rigorously assess novel treatment efficacy [46–50].
To minimize variability and create a high-throughput system for investigating stress-relevant disorders, we developed a mouse model using the Gq-coupled Designer Receptors Exclusively Activated by Designer Drugs (DREADD) hM3Dq to selectively activate all CRF neurons in a temporally defined window and used palatable cookie dough treats to administer CNO, eliminating the need for daily intraperitoneal (i.p.) injections [51]. The DREADD ligand clozapine-N-oxide (CNO) activates the canonical Gq pathway, triggering neuronal discharge [51, 52]. Viral injection is the most common method for DREADD expression; however, notable drawbacks include variability in injection site placement, labor cost, and latency of expression. To minimize this variance, we utilized a transgenic strategy in which CRF-Cre mice were crossed with mice expressing a floxed DREADD hM3Dq gene. Here, we show that chemogenetic CRF-neuron activation effectively initiates the HPA axis stress response and that repeated, chronic activation induces sex- and brain region-specific effects.
Methods
Detailed methods are presented in Supplementary Materials
Animals
Adult (10–20 weeks) male and female mice heterozygous for the CRF-Cre transgene and hM3Dq transgene (CRF-Cre+/- X DREADD+/-, defined as DREADD + ) or heterozygous for the CRF-Cre transgene and wild-type for the hM3Dq transgene (CRF-Cre+/- X DREADD-/-, defined as DREADD-) were used for DREADD studies. Adult (8–9 weeks) male and female C57BL/6 J mice were used for chronic multimodal stress. All animal experiments were approved by the University of Maryland Baltimore Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
CNO administration
For i.p. injections, clozapine N-oxide (CNO) dihydrochloride (Hello Bio, catalog #HB6149) was prepared in 0.9% saline at 1 mM. Cookie dough treats were prepared at 0.1 mg CNO/g dough (Transgenic Dough Diet, Bio-Serv catalog #S3472, sugar cookie (Pillsbury), or Reese’s Peanut Butter (Pillsbury)).
Hypothalamic-Pituitary-Adrenal response to CNO and acute restraint stress
HPA axis reactivity to an i.p. CNO injection or 15 min restraint was measured as previously described [53]. 10 uL of blood was collected from a tail snip at specified time points, and plasma corticosterone levels were measured by 125I-Corticosterone radioimmunoassay (MP Biomedicals, catalog #07120103).
Chronic multimodal stress
8–9 week male and female C57BL/6 J mice were subjected to stress for 14 days as described [54, 55]. Stress consisted of restraint in a 3D printed restraint tube (Ender 3 printer, Creality), in a cage tilted 30° while being subjected to white noise, strobe lights, and predator odor (fox urine, Trap Shack Company). These mice were only examined for changes in the von Frey filament test.
Immunohistochemistry and thymus collection
3 h after a single CNO injection, mice were anesthetized and transcardially perfused and the thymus was dissected out. c-Fos immunohistochemistry was performed using c-Fos (1:2500, Synaptic Systems #226–308) and anti-guinea pig (1:200, Alexa Fluor 568, Thermo Fisher, #11075) antibodies and Hoechst counterstain (1:2000, Thermo Fisher, #33342). c-Fos density was normalized to Hoechst for each section. For HA immunohistochemistry, sections were probed using HA (1:800, Cell Signaling Technologies, #3724) and anti-rabbit antibodies (1:1000, Alexa Fluor 594, Thermo Fisher #A-11012).
von Frey filament test
Mice were placed on a suspended wire mesh, and monofilaments of increasing diameter with forces ranging from 0.008 to 11.0 g (NC Medical, catalog #NC12775-01) were pressed against the hind paw skin. Responses (withdrawal/no withdrawal) were recorded until the foot was withdrawn for 5 consecutive trials.
Open-field testing
Mice were placed in a 24-inch x 24-inch open plexiglass box and allowed to explore freely for 10 mins. Perimeter was defined as 6 inches from any wall, and corners were defined by a 6-inch x 6-inch square. Center was defined as a 12-inch x 12-inch square in the arena center. Sessions were analyzed using Noldus Ethovision XT tracking software.
Fear-conditioning
Day 1: Mice were habituated to context A for 10 min followed by context B for 10 min. Day 2: mice were placed in context A for 5 min and a 30 sec baseline was collected with a 65 dB tone. An 80 dB tone (conditioned stimulus, CS) was presented for 30 sec co-terminating with a 1 sec 0.6 mA shock. 3 tone-shock pairings were presented. Days 3–7: Mice were placed in context B and a 30-sec baseline was collected. The CS tone was presented for 30 sec and the baseline – CS tone presentation was repeated for 15 trials with 30-sec intertrial intervals. Movement was measured using a piezoelectric accelerometer and recorded using SR-Lab software.
Statistical analysis
All data are presented ± SEM. Statistical measurements were performed in GraphPad Prism and RStudio, and figures were prepared in GraphPad Prism and BioRender. Details for all statistical tests are presented in figure legends and Tables S2 and S3. Outliers were determined using Grubbs’ test with alpha set to 0.05. All testing was conducted by experimenters blinded to treatment and genotype groups.
Results
The DREADD ligand clozapine-N-oxide effectively activates the HPA axis in CRF-Cre + /DREADD+ mice
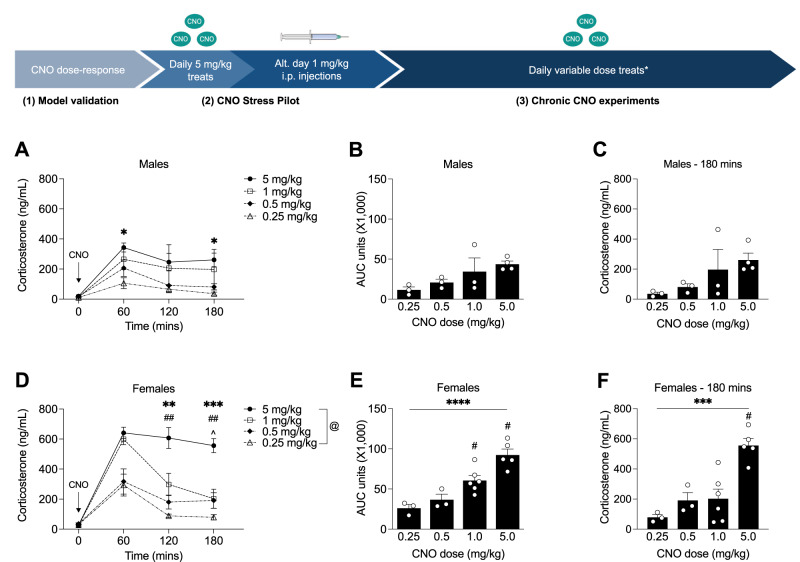
To validate that chemogenetic CRF neuron activation initiates an HPA stress axis hormonal response and to determine optimal CNO dosing to produce physiologically relevant responses, we injected CNO i.p. at doses from 0.25 to 5 mg/kg in adult male and female DREADD+ mice and measured plasma corticosterone levels. Males showed increased corticosterone in response to a 5 mg/kg dose compared to a 0.25 mg/kg dose (Fig. 1A) at 60 min and 120 min following CNO injection. Area under the curve analysis (Fig. 1B) showed a trend of increasing corticosterone release with increasing CNO dose that did not rise to the level of statistical significance, likely due to the small N’s and blunted HPA axis response of C57BL/6 J mice. We did not find a significant effect of CNO dose on the HPA response recovery timepoint in males (Fig. 1C). In females, a 5 mg/kg CNO dose induced higher corticosterone release at 120- and 180-mins post-injection (Fig. 1D). Area under the curve analysis (Fig. 1E) showed a significant effect of CNO dose on the total amount of corticosterone released and a significantly prolonged HPA response at the 5 mg/kg dose (Fig. 1F). We additionally confirmed that 1 mg/kg of CNO did not induce a corticosterone response in DREADD- males (Fig. S1A) or DREADD- females (Fig. S1B).
Fig. 1. CNO induces dose-responsive corticosterone release in CRF-Cre + /DREADD+ mice.
A Corticosterone levels were measured in response to 4 CNO doses in DREADD+ males (2-way RM ANOVA; Ftime(1.698,15.28) = 15.91, p < 0.001; Fdose(3,9) = 2.933, p = 0.092; Ftime*dose(9,27) = 1.278, p = 0.293; n = 3–4). Corticosterone was elevated in response to 5 mg/kg CNO compared to 0.25 mg/kg CNO at 60 mins (p = 0.020) and 180 mins (p = 0.041) post-injection. B Area under the curve analysis of total corticosterone release did not show a significant effect of CNO dose (1-way ANOVA; Fdose(3,9) = 2.998, p = 0.088; n = 3–4). C CNO dose did not significantly affect corticosterone levels at the HPA axis recovery timepoint in DREADD+ males (1-way ANOVA; Fdose(3,9) = 2.403; p = 0.135; n = 3–4). D Corticosterone responses in DREADD+ females were significantly affected by CNO dose (2-way RM ANOVA; Fdose(3,13) = 18.28, p < 0.0001; Ftime(2.066,26.86) = 55.10, p < 0.0001; Ftime*dose(9,39) = 6.684, p < 0.0001; 120 mins post-injection: 5 mg/kg vs 0.5 mg/kg p = 0.009; 5 mg/kg vs 0.25 mg/kg p = 0.005; 180 mins post-injection: 5 mg/kg vs 1 mg/kg, p = 0.007; 5 mg/kg vs. 0.5 mg/kg p = 0.013; 5 mg/kg vs 0.25 mg/kg p = 0.0008; n = 3–6). E CNO dosing significantly affected the total amount of corticosterone released in DREADD+ females (1-way ANOVA; Fdose(3,13) = 17.04, p < 0.0001; 0.25 mg/kg vs. 1.0 mg/kg p = 0.021; 0.25 mg/kg vs. 5.0 mg/kg p = 0.0001; 0.5 mg/kg vs. 5.0 mg/kg p = 0.0006, 1.0 mg/kg vs. 5.0 mg/kg p = 0.013; n = 3–6). F 5 mg/kg CNO significantly elevated corticosterone at the HPA axis recovery timepoint in DREADD+ females (1-way ANOVA; Fdose(3,13) = 13.51, p = 0.0003; 5 mg/kg vs 1.0 mg/kg p = 0.001; 5 mg/kg vs. 0.5 mg/kg p = 0.005; 5 mg/kg vs 0.25 mg/kg p = 0.0005; n = 3–6). (**** p < 0.0001, ***p < 0.001, **p < 0.01, ##p < 0.01, *p < 0.05, ^p < 0.05, #p < 0.05, @ main effect of genotype). *An example week of the variable-dose CNO treat paradigm is shown in Table S1.
Repeated high-dose CNO administration induces significant stress-like physiological changes in DREADD+ male mice
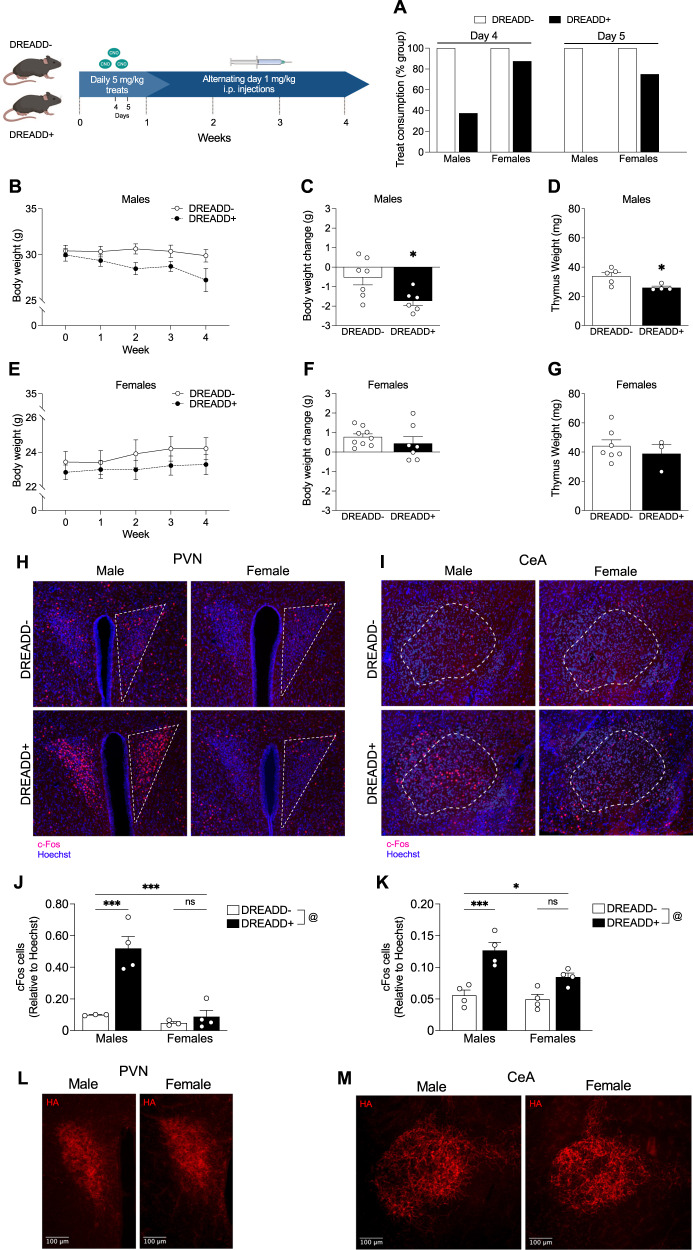
After confirming that CNO effectively induced acute stress-like corticosterone release in DREADD+ mice, we next wanted to determine if repeated CNO administration would replicate known stress-relevant physiological phenotypes. To minimize both experimenter time and handling stress to control animals, we used highly palatable cookie dough treats containing a measured dose of CNO. We administered a single 5 mg/kg CNO treat daily, and remarkably, by day 5, all male DREADD+ mice partially or fully stopped consuming CNO treats compared to 25% of female DREADD+ mice (Fig. 2A). To complete this initial study, we transitioned to 1 mg/kg CNO alternating day i.p. injections. Body weight loss and thymus atrophy are physiological hallmarks of severe stress, and we found that male DREADD+ mice showed a trend of decreasing body weight across 4 weeks (Fig. 2B) and lost significantly more weight (Fig. 2C) and had significantly smaller thymuses at the end of 4 weeks (Fig. 2D). Interestingly, female DREADD+ mice did not show body weight changes (Fig. 2E) or have significantly different body weights (Fig. 2F) or thymus weights (Fig. 2G) at the end of 4 weeks.
Fig. 2. DREADD+ males display physiological stress features and have increased c-Fos expression in the PVN and central amygdala following repeated high-dose CNO.
A 4 days after beginning CNO treat consumption, 62.5% of DREADD+ males (n = 7) partially or fully stopped consuming CNO treats compared to 12.5% of female DREADD+ mice (n = 9). 100% of DREADD+ males partially or fully stopped consuming the treats after 5 days compared to 25% of DREADD+ females. B Weekly body weight measurements across 4 weeks of CNO in male mice (2-way RM ANOVA; FTime(1.345,16.14) = 4.716, p = 0.039; Fgenotype(1,12) = 3.201, p = 0. 099; Ftime*genotype(4,48) = 2.480, p = 0.056; n = 7 per group). C 4 weeks of CNO significantly reduced body weight (unpaired t-test, t(11) = 2.638, p = 0.023; n = 6–7) and D thymus weights (unpaired t-test; t(11) = 2.638, p = 0.023, n = 4–5) in DREADD+ males compared to controls. E Weekly body weight measurements across 4 weeks of CNO in female mice (2-way RM ANOVA; Ftime(2.478,34.70) = 4.938, p = 0.009; Fgenotype(1,14) = 0.751, p = 0.401; Ftime*genotype(4,56) = 0.964, p = 0.435; n = 7–9). F 4 weeks of CNO did not induce overall body weight change (unpaired t-test, t(14) = 0.952, p = 0.357, n = 7–9) or (G) affect thymus weights (unpaired t-test; t(8)=0.721, p = 0.492; n = 3–7) in female DREADD+ mice compared to controls. H Representative images of c-Fos immunostaining in the PVN and (I) central amygdala. Dashed lines indicate region of interest used for quantification. J Quantification of c-Fos immunoreactivity in the PVN (2-way ANOVA; Fsex(1,10) = 22.77, p = 0.0008; Fgenotype(1,10) = 20.68, p = 0.001, Fsex*genotype(1,10) = 14.15, p = 0.004; n = 3–4). DREADD+ males had a significantly higher proportion of c-Fos immunoreactivity compared to controls (p = 0.0007) and DREADD+ females (p = 0.0003) while there were no significant differences in DREADD+ females compared to controls (p = 0.943). K Quantification of c-Fos immunoreactivity in the CeA (2-way ANOVA; Fsex(1,12) = 7.037, p = 0.021; Fgenotype(1,12) = 34.16, p < 0.0001; Fsex*genotype(1,12) = 3.831, p = 0.074). DREADD+ males had a significantly higher proportion of c-Fos immunoreactivity (p = 0.0007) compared to controls and DREADD+ females (p = 0.03) while there were no significant differences in c-Fos immunoreactivity between DREADD+ females and controls (p = 0.073). L Representative images of HA immunostaining in the PVN and (M) CeA in male and female DREADD+ mice. By visual inspection of expression patterns, no apparent differences were noted. (***p < 0.001, *p < 0.05, @ main effect of genotype).
To determine whether these sex-specific effects were due to sex differences in CRF neuron activation, we measured c-Fos immunoreactivity in both the PVN and central amygdala (CeA) in response to CNO, two regions with high CRF neuron densities (Fig. 2H, I). In the PVN, DREADD+ males had a significantly higher proportion of c-Fos immunoreactivity while DREADD+ females were not significantly different from controls (Fig. 2J). In the CeA, DREADD+ males again had a significantly higher proportion of c-Fos immunoreactivity while there were no significant differences in c-Fos immunoreactivity between DREADD+ females and controls (Fig. 2K). To confirm that the DREADD receptor is expressed similarly in CRF neurons between males and females, we performed immunohistochemistry using the HA-tag on hM3Dq. Based on visual inspection of expression patterns, no apparent differences were observed in either the PVN (Fig. 2L) or CeA (Fig. 2M).
9 weeks of variable lower-dose CNO does not induce a severe stress phenotype in DREADD+ mice
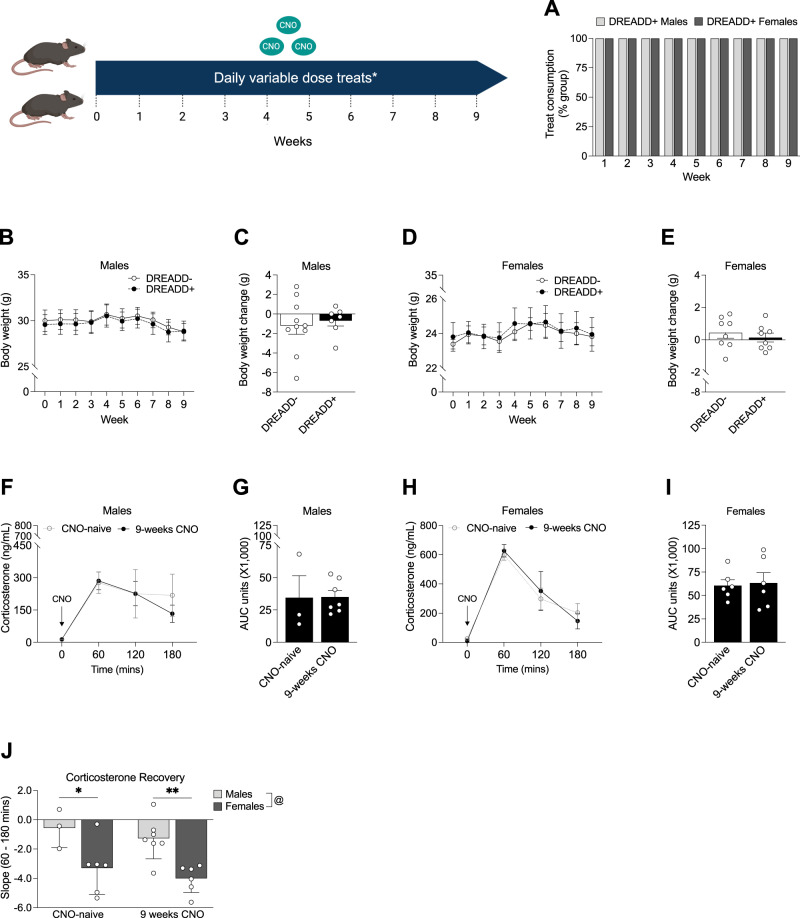
Our pilot study produced a more severe stress phenotype than we sought to model, so to avoid inducing this phenotype and to prevent DREADD+ animals from developing treat avoidance, we instead used a randomized lower-dose CNO treat paradigm consisting of 0.25 mg/kg, 0.5 mg/kg, and 1.0 mg/kg and 3 separate dough flavors to examine the effects of chronic CRF neuron activation (Table S1). Under this modified regimen, all DREADD+ mice of both sexes continued consuming the treats across 9 weeks of daily administration (Fig. 3A). In DREADD+ males, we found no significant changes in body weight across 9 weeks (Fig. 3B) and no overall body weight change after 9 weeks (Fig. 3C). Likewise, chronic CNO did not affect body weight in female DREADD+ mice (Fig. 3D) or induce overall body weight change after 9 weeks (Fig. 3E).
Fig. 3. Lower variable-dose chronic CNO does not induce a severe stress physiological phenotype or lead to CNO habituation.
*An example week of the variable-dose CNO treat paradigm is shown in Table S1. A 100% of male (n = 7) and female (n = 8) DREADD+ mice continued consuming daily CNO treats across 9 weeks. B Weekly body weight measurements across 9 weeks in DREADD- (n = 10) and DREADD+ (n = 7) males (2-way RM ANOVA; Ftime(1.091,16.36) = 5.050, p = 0.036, Fgenotype(1,15) = 0.043, p = .838; Ftime*genotype(9,135) = 0.162, p = 0.997). C Lower, variable dose CNO treats did not induce a greater overall body weight change in DREADD+ males compared to controls (unpaired t-test; t(15)=0.434, p = 0.670). D Weekly body weight measurements across 9 weeks in DREADD- (n = 8) and DREADD+ (n = 8) females (2-way RM ANOVA; Ftime(1.230,17.22) = 2.238, p = 0.15; Fgenotype(1,14) = 0.039, p = 0.847; Ftime*genotype(9,126) = 0.151, p = 0.998). E There were no differences in overall body weight change at the end of 9 weeks in DREADD+ females compared to controls (unpaired t-test; t(14)=0.684, p = 0.505; n = 8 per group). F 9 weeks of CNO did not affect HPA axis reactivity to an acute CNO injection (2-way RM ANOVA; FCNO chronicity(1,9) = 0.112, p = 0.746; Ftime (1.564, 14.08) = 13.09, p = 0.001; Ftime*CNO chronicity(3, 27) = 0.494, p = 0.690) or (G) total amount of corticosterone released following an acute CNO injection in DREADD+ males (n = 7) compared to CNO-naïve males (n = 3) (unpaired t-test; t(8) = 0.0467, p = 0.964). H 9 weeks of chronic CNO did not affect HPA axis reactivity to an acute CNO injection (2-way RM ANOVA; FCNO chronicity(1,10) = 0.002, p = 0.967; Ftime(1.686, 16.86) = 40.77, p < 0.0001; Ftime*CNO chronicity(3, 30) = 0.379, p = 0.769) or (I) total amount of corticosterone released in female DREADD+ mice (n = 6) compared to CNO-naïve females (n = 6) (unpaired t-test; t(10) = 0.221, p = 0.829). J Slope analysis of HPA axis corticosterone response from 60- to 120- mins post-injection. Female DREADD+ mice recovered faster from peak corticosterone levels than DREADD+ males when CNO-naïve (p = 0.014) and after 9 weeks of chronic CNO administration (p = 0.003) (2-way ANOVA; Fsex(1,18) = 18.49, p = 0.0004; FCNO chronicity(1,18) = 1.231, p = 0.282; Fsex*CNO chronicity(1,18) < 0.0001, p = 0.999). (*p < 0.05, @ main effect of sex).
We next measured plasma corticosterone levels following a single 1 mg/kg CNO injection after 9 weeks of chronic CNO to determine whether repeated activation of hM3Dq in CRF neurons induced CNO habituation. Male DREADD+ animals showed no change in their HPA axis response to a CNO injection compared to CNO-naïve animals that received a 1 mg/kg injection in our initial validation experiment (Figs. 1A and 3F) and no differences in total corticosterone released (Figs. 1B and 3G). Female DREADD+ animals also showed no change in their HPA axis response following chronic CNO compared to CNO-naïve animals (Figs. 1D and 3H) and showed no differences in total corticosterone released (Figs. 1E and 3I). Both CNO-naïve and chronically treated female DREADD+ mice had faster HPA axis recovery rates than CNO-naïve and chronically treated DREADD+ males (Fig. 3J).
Many studies using classical chronic stress models report locomotive changes which led us to perform an open-field test. We found no differences between DREADD+ males and controls in distance traveled (Fig. S2A), center time (Fig. S2B), total movement time (Fig. S2C), or average velocity (Fig. S2D). Likewise, we found no differences between DREADD+ females and controls in distance traveled (Fig. S2E), center time (Fig. S2F), total movement time (Fig. S2G), or average velocity (Fig. S2H).
Chronic CRF neuron activation increases stress reactivity in DREADD+ males, heightens fear responses in DREADD+ females, and increases tactile sensitivity in both sexes
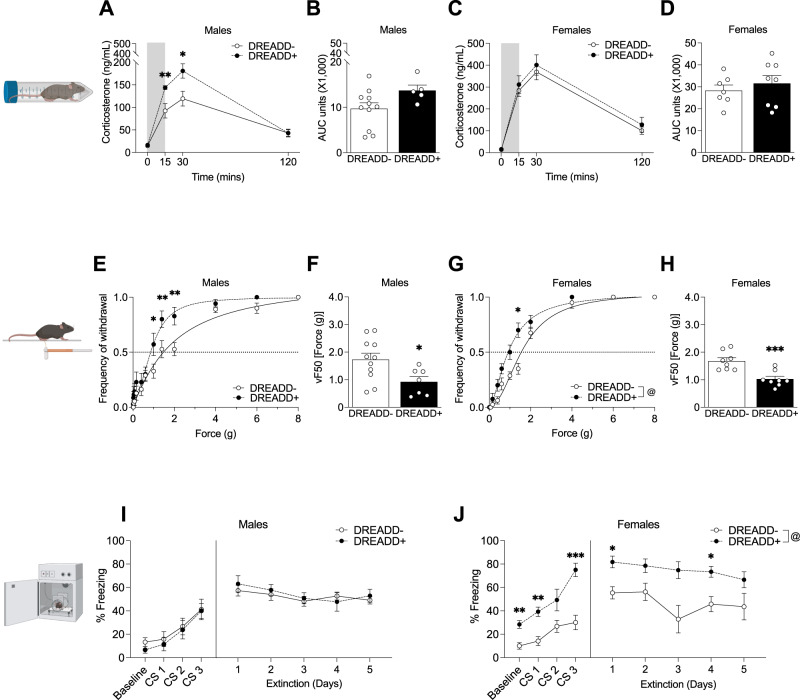
To determine whether chronic CRF activation altered HPA axis reactivity to an acute stressor, we measured plasma corticosterone levels in response to restraint stress. Male DREADD+ mice had an elevated HPA axis response to 15 min of restraint stress (Fig. 4A) following 9 weeks of CNO with a significant elevation in corticosterone levels 15 min and 30 min after restraint onset; however, we did not find a significant effect of chronic CNO on total corticosterone release (Fig. 4B). 9 weeks of CNO did not alter the HPA response to acute restraint stress in DREADD+ females (Fig. 4C) or affect total corticosterone released (Fig. 4D).
Fig. 4. Male DREADD+ mice have heightened HPA axis reactivity while female DREADD+ mice have heightened fear responses following chronic CNO.
A Male DREADD+ mice (n = 5) had an elevated HPA axis response to 15 min of restraint stress following 9 weeks of CNO (2-way RM ANOVA; Fgenotype(1,14) = 3.904, p = 0.068, Ftime(1.671,23.39) = 77.76, p < 0.0001; Ftime*genotype(3,42) = 5.130, p = 0.004) with a significant elevation in corticosterone levels 15 mins (p = 0.009) and 30 mins (p = 0.020) after restraint onset compared to controls (n = 11). B Area under the curve analysis did not show a significant difference in total corticosterone released in DREADD+ males following 9 weeks of chronic CNO (unpaired t-test; t(14) = 1.921, p = 0.075). C 9 weeks of chronic CNO did not alter the HPA axis response to acute restraint stress in DREADD+ females (n = 8) compared to controls (n = 7) (2-way RM ANOVA; Fgenotype(1,13) = 0.529, p = 0.480; Ftime(2.096,27.27) = 78.92, p < 0.0001; Ftime*genotype(3,39) = 0.167, p = 0.918) and (D) did not affect total corticosterone released (unpaired t-test; t(g13)=0.729, p = 0.479). E Using the von Frey filament test to measure tactile sensitivity, DREADD+ males (n = 7) showed a leftward shift of the paw withdrawal curve compared to controls (n = 11) (2-way RM ANOVA; Fgenotype(1,16) = 4.034, p = 0.062; Fforce(14,224) = 169.8, p < 0.0001; Fgenotype*force(14, 224) = 2.658, p = 0.001) with DREADD+ males having significantly more paw withdrawals at 1.0 g (p = 0.021), 1.4 g (p = 0.006), and 2.0 g (p = 0.001) of force. F DREADD+ males had a lower average force required for 50% paw withdrawal (VF50) compared to controls (unpaired t-test; t(16) = 2.489, p = 0.024). G DREADD+ females (n = 8) also showed a leftward shift of the paw withdrawal curve compared to controls (n = 8) (2-way RM ANOVA; Fgenotype(1,14) = 14.93, p = 0.002; Fforce(4.396,61.55) = 371.2, p < 0.0001; Fgenotype*force(14,196) = 5.182, p < 0.0001) with significantly more paw withdrawals at 1.4 g of force (p = 0.014), and (H) had lower VF50 (unpaired t-test; t(14) = 4.235, p = 0.0008) compared to controls. I Freezing behavior during auditory fear conditioning. Male DREADD+ mice (n = 7) showed no differences in freezing behavior during conditioning (2-way RM ANOVA; Fgenotype(1,16) = 0.287, p = 0.600; Ftrial(2.114,33.82) = 12.26, p < 0.0001; Fgenotype*trial(3,48) = 0.085, p = 0.968) or extinction (2-way RM ANOVA; Fgenotype(1,16) = 0.129, p = 0.725; Ftrial(2.441,39.05) = 5.210, p = 0.007; Ftrial*genotype(4,64) = 1.073, p = 0.377) compared to controls (n = 11) following 9 weeks of CNO. J Chronic CNO significantly elevated freezing behavior in female DREADD+ mice during conditioning (n = 8 per group; 2-way RM ANOVA; Fgenotype(1,14) = 23.36, p = 0.0003, Ftrial(2.170, 30.38) = 25.24, p < 0.0001; Fgenotype*trial(3,42) = 4.20, p = 0.011), with DREADD+ females freezing more at baseline (p = 0.003) and conditioning trials 1 (p = 0.002) and 3 (p = 0.0004). Chronic CNO also increased female DREADD+ freezing behavior (n = 6 per group) during the extinction trials (2-way RM ANOVA; Fgenotype(1,10) = 16.23, p = 0.002; Ftrial(2.092, 20.92) = 2.324, p = 0.121; Fgenotype*trial(4,40) = 0.780, p = 0.545), with significantly higher levels of freezing compared to controls during extinction trials 1 (p = 0.022) and 4 (p = 0.034). (***p < 0.001, **p < 0.01, *p < 0.05, @ main effect of genotype).
Altered sensory processing is associated with chronic stress states so we next used the Von Frey filament test to measure changes in tactile sensitivity. Male DREADD+ mice showed increased sensitivity compared to controls (Fig. 4E) and had lower force required for 50% paw withdrawal (VF50) (Fig. 4F). Likewise, DREADD+ females displayed increased sensitivity (Fig. 4G) and decreased VF50 compared to controls (Fig. 4H). These results replicate our findings that 2 weeks of chronic multimodal stress shifted the frequency of withdrawal curve leftward in both male and female mice (Fig. S3A, C) and decreased the VF50 in males and females (Fig. S3B, D) with females showing a greater percent decrease in the VF50 from baseline than males (Fig. S3E).
Chronic stress influences the acquisition and extinction of fear memories and given the key role of CeA CRF neurons in these processes, we performed auditory fear conditioning to determine whether chronic CNO affected fear memory acquisition and extinction in DREADD+ animals. Male DREADD+ mice showed no differences in freezing behavior during conditioning or extinction (Fig. 4I) following 9 weeks of CNO. In contrast, we found a significant effect of chronic CNO on freezing with DREADD+ females freezing more at baseline and conditioning trials 1 and 3, and extinction trials 1 and 4 (Fig. 4J).
Discussion
While utilization of animal models to study the lasting effects of chronic stress has provided important insight into mechanisms underlying disease risk, variability in outcomes between and within labs, as well as the high labor effort required for such studies, can often be an obstacle for the utilization and interpretation of these models [37, 39–45, 56–61]. We developed a model using transgenic mice expressing the Gq-coupled Designer Receptors Exclusively Activated by Designer Receptors (DREADD) hM3Dq in corticotropin-releasing factor (CRF) neurons, allowing us to activate the CRF system with the DREADD ligand clozapine-N-oxide (CNO) in a timing-selective and high-throughput manner [51, 52]. The goal of this approach was to administer ‘stress’ in a precisely controlled manner while reducing the inter-animal variability often found in chronic stress paradigms. Therefore, our studies used a chemogenetic approach designed to validate expected outcomes aligned findings from decades of stress research [12, 23, 25, 32, 37, 56–74].
We first utilized the HPA stress axis to validate CNO activation of hM3Dq-expressing CRF neurons. As predicted, CNO activated the HPA axis in DREADD+ mice, increasing corticosterone levels with a time course similar to an acute stressor [53, 75, 76]. The CNO activation of CRF neurons appeared dose-dependent. Interestingly, dose-dependent glucocorticoid production following modulation of CRF neuron activity has not been previously shown [77, 78]. Our results demonstrate that directly modulating CRF neuron activity is sufficient to alter the degree of HPA axis activation and ultimately direct corticosterone levels, highlighting a unique aspect of this model whereby the severity of ‘stress’ can be controlled by CNO dose. We also reproduced sex differences in peak corticosterone production that mirrored known sex differences in adult rodent HPA reactivity [79–84]. Interestingly, females had faster recovery rates than males at all doses tested except the highest.
Our ultimate goal was to model chronic stress with this chemogenetic approach. CNO is typically administered via intraperitoneal (i.p.) injection; however, this method produces animal stress and pain, including to control animals, and is not high throughput. Therefore, we developed CNO-containing cookie dough treats that could be administered efficiently, consumed within a defined timeframe, and be easily replicated across labs. As the bioavailability and metabolism of oral CNO was not clear, we began this pilot study with a daily dose of 5 mg/kg [85–87]. Interestingly, within the first 5 days of daily CNO treat consumption, all DREADD+ male mice, but only 25% of DREADD+ female mice, partially or fully stopped consuming the treats. To determine if this behavior reflected an anhedonic state resulting from the effects of repeated CRF activation, we administered palatable fruit-flavored sucrose pellets to the mice and found that all mice readily consumed these treats. This suggested that males learned the negative association faster or were more sensitive to the negative effects of CRF activation. Interestingly, this mirrors findings from conditioned taste aversion studies where male rodents developed an aversion to lithium chloride-containing saccharin faster than females and were slower to extinguish this aversion [88–92].
To continue this pilot study, we transitioned from oral CNO treats to alternate-day i.p. injections. After 4 weeks, males, but not females, lost body weight, a classic physiological sign of severe stress [70, 93–95]. Similarly, males, but not females, showed the classic stress phenotype of decreased thymus weight, consistent with known stress-induced thymus atrophy produced by elevated glucocorticoids [58, 96–98]. It was notable that despite having a lower corticosterone response to CNO than females, males developed CNO treat avoidance faster and displayed physiological features of severe stress. Glucocorticoids are primary mediators of stress effects, and many rodent studies model stress through direct corticosterone administration [99–106]. Our results, however, suggest an interesting sex-specific dissociation between glucocorticoid production and physiological outcomes, supporting possible cellular- and tissue-specific processes controlled by glucocorticoids.
Next, to examine possible sex differences in CRF neuron activation, we measured c-Fos immunoreactivity as a proxy for neuronal activity in 2 brain regions with significant CRF neuron populations, the PVN and central amygdala (CeA). 3 h following a CNO injection, male DREADD+ mice showed significantly more c-Fos immunoreactivity in both brain regions compared to DREADD+ females. Neuronal depolarization rapidly induces c-Fos expression, and persistent c-Fos immunoreactivity 3 h after CNO administration suggests either prolonged or increased neuronal activity in males in the PVN and CeA [107–109]. DREADD+ females appear to shut down CRF neuron activity more rapidly than males, though we cannot draw definitive conclusions without electrophysiological confirmation. This possibility is supported, however, by our results that females also recover faster from chemogenetic HPA axis activation at this same CNO dose. As we directly activated CRF neurons with CNO, it is not clear if these findings translate to all types of stress experiences.
Chronic stress is a critical underlying risk factor for future neuropsychiatric disease [31, 67, 70–72, 110, 111]. Our goal for developing this model was to titrate CRF activation to mimic chronic environmental stress without producing severe stress, and our 4-week pilot CNO data suggested that daily 5 mg/kg treats and even 1 mg/kg dosing every other day produced a more severe phenotype than we wanted to model. Therefore, we modified our CNO treat paradigm for a chronic study to include multiple flavors and 3 alternating CNO doses. Over 9 weeks of daily treatment, we found no changes in body weight, and all mice continued to consume daily CNO treats, supporting that we had identified a paradigm that was effective in both sexes and did not induce severe physiological stress phenotypes. Using an acute i.p. injection following 9 weeks of treatment, we validated that both male and female DREADD+ mice continued to respond to CNO in a nearly identical manner to CNO-naïve animals, indicating that DREADD-expressing CRF neurons remained responsive to CNO. Taken together with the more severe stress phenotypes induced by higher dose CNO in our pilot study, these results again highlight an advantage of this model where CNO dosing can induce a wide range of desired stress phenotypes.
Many neuropsychiatric diseases are characterized by altered HPA stress responses, and preclinical studies have repeatedly demonstrated that chronic stress alters HPA axis reactivity to acute stressors [112–122]. Our approach allowed us to ask whether these changes were encoded at the level of the CRF neurons themselves or by changes in afferent drive. In response to acute restraint stress following 9 weeks of chronic CRF activation, DREADD+ males showed a significant elevation in their HPA axis response compared to DREADD- males, but no difference was observed in females. These data suggest that the effects of chronic stress may be encoded upstream of CRF neurons. Indeed, chronic stress decreases PVN CRF neuron inhibition through both reduced GABAergic signaling and decreases endocannabinoid-mediated negative feedback, and increases glutamatergic and noradrenergic excitation [69, 73, 123–126].
Stress-mediated changes in sensory sensitivity are a common and translatable measure [127–129]. Therefore, we used the von Frey filament test to measure tactile sensitivity. We found that both male and female DREADD+ mice showed a leftward shift of the withdrawal curve and decreased VF50 following 9 weeks of CNO, indicating elevated tactile sensitivity. These results replicate our findings that both male and female mice showed leftward withdrawal curve shifts and decreased VF50 after 2 weeks of conventional multimodal stress. Chronic stress and enhanced sensory sensitivity have a bidirectional relationship, and the heightened tactile perception highlights a vulnerability of the somatosensory system to chronic stress.
We next utilized auditory fear conditioning to determine whether chronic CRF activation altered fear memory acquisition and extinction. Limbic CRF neurons, including in the CeA, have an important role in the formation and extinction of fear memories. [13, 15, 35, 36, 64, 65, 130–139]. Following 9 weeks of CNO, female DREADD+ mice froze significantly more than controls at baseline and across all conditioning and extinction trials, while male DREADD+ mice showed no differences in freezing compared to controls. Inbred mouse strains often do not show robust fear extinction using this paradigm of extinction trials, making it difficult to evaluate between groups [140–144]. However, the female-specific heightened freezing, even prior to foot shock, suggests that chronic CRF activation uniquely sensitizes fear responses in females and may reflect underlying female-specific vulnerability. Certainly, in humans, females are more than twice as likely as males to develop PTSD following traumatic events [145–151]. Interestingly, the sex differences in fear conditioning mirrored those seen in corticosterone production, i.e., females produced higher levels of corticosterone and showed heightened freezing. While glucocorticoids feedback negatively on PVN CRF neurons, they have a positive relationship with CeA CRF neurons [23, 152, 153]. Further studies are needed to dissect the mechanisms underlying the potential sex-specific sensitivities of CRF populations, including the PVN and CeA.
There are several limitations to this work that could be examined in future studies. First, the simultaneous activation of CRF-neurons in the brain for an extended period may not completely recapitulate the time course of circuit activity under a natural stress exposure [74, 109, 152, 154, 155]. While the majority of parvocellular PVN AVP neurons co-express CRF and therefore would be activated in our model, we did not examine AVP contributions or ACTH levels in these studies [156–158]. These studies largely focused on outcomes directly attributed to the PVN and CeA, however, additional CRF neuronal populations have important roles in orchestrating the stress response, including the bed nucleus of the stria terminalis (BNST) and the basolateral amygdala (BLA) [17–20, 138, 159]. Future work should examine the roles of these additional populations in the development of chronic stress-relevant phenotypes. Lastly, for several reasons outside our control (e.g., pandemic, vivarium parasite infestation), our experimental mouse numbers for some of the early pilot experiments were lower than a power calculation would recommend for detecting statistically significant sex differences. Direct examination of sex differences will be critical for future mechanistic studies. Our findings also highlight a need to re-examine the methods used for measuring stress-induced phenotypes. The robust differences we found in freezing and increased tactile sensitivity suggest that the addition of sensory tests to behavioral measures may enhance our ability to detect more nuanced and sex-specific phenotypes. Collectively, these results demonstrate the potential advantages of this model utilizing CNO to acutely or chronically activate hm3Dq-expressing CRF neurons to examine sex-specific stress-relevant phenotypes related to neuropsychiatric disorders.
Supplementary information
Acknowledgements
We thank Maxwell Madden for developing the design for the 3D-printed restraint tubes, Kate Matlin for her assistance with R, and Nickole Moon for her editorial feedback on the manuscript. Funding: These studies were supported by MH129495, MH108286, HD105771, and HD097093 to TLB.
Author contributions
KRM, MSB, and TLB conceived the studies and designed the experiments. KRM, MSB, LMF, RMR, HCZ, ABW, and EAK performed the experiments. KRM analyzed the data and prepared the figures. KRM and TLB wrote and edited the manuscript with input from all authors. TLB and SMT supervised the research.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01739-5.
References
- 1.Grant JK, Forrest APM, Symington T. The secretion of cortisol and corticosterone by the human adrenal cortex. Acta Endocrinologica (Nor) 1957;26:195–203. doi: 10.1530/acta.0.0260195. [DOI] [PubMed] [Google Scholar]
- 2.Brown KI. The validity of using plasma corticosterone as a measure of stress in the Turkey. Proc Soc Exp Biol Med. 1961;107:538–42. doi: 10.3181/00379727-107-26680. [DOI] [Google Scholar]
- 3.Triller H, Birmingham MK. Steroid production by incubated mouse adrenals: I. Characterization of steroid fractions. Gen Comp Endocrinol. 1965;5:618–23. doi: 10.1016/0016-6480(65)90081-X. [DOI] [PubMed] [Google Scholar]
- 4.Estergreen VL, Venkataseshu GK. Positive identification of corticosterone and cortisol in jugular plasma of dairy cattle. Steroids. 1967;10:83–92. doi: 10.1016/0039-128X(67)90072-4. [DOI] [PubMed] [Google Scholar]
- 5.Spiess J, Rivier J, Rivier C, Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci. 1981;78:6517–21. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivier J, Spiess J, Vale W. Characterization of rat hypothalamic corticotropin-releasing factor. Proc Natl Acad Sci. 1983;80:4851–5. doi: 10.1073/pnas.80.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esch F, Ling N, Bohlen P, Baird A, Benoit R, Guillemin R. Isolation and characterization of the bovine hypothalamic corticotropin-releasing factor. Biochemical Biophysical Res Commun. 1984;122:899–905. doi: 10.1016/0006-291X(84)91175-6. [DOI] [PubMed] [Google Scholar]
- 8.Stenzel-Poore MP, Heldwein KA, Stenzel P, Lee S, Vale WW. Characterization of the genomic corticotropin-releasing factor (CRF) gene from Xenopus laevis: two members of the CRF family exist in amphibians. Mol Endocrinol. 1992;6:1716–24. doi: 10.1210/mend.6.10.1448118. [DOI] [PubMed] [Google Scholar]
- 9.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 10.Rivier C, Vale W. Effects of corticotropin-releasing factor, neurohypophyseal peptides, and catecholamines on pituitary function. Fed Proc. 1985;44:189–95. [PubMed] [Google Scholar]
- 11.Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol. 2012;33:67–84. doi: 10.1016/j.yfrne.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deussing JM, Chen A. The corticotropin-releasing factor family: physiology of the stress response. Physiol Rev. 2018;98:2225–86. doi: 10.1152/physrev.00042.2017. [DOI] [PubMed] [Google Scholar]
- 13.Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann N. Y Acad Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- 14.Kalin NH, Takahashi LK, Chen F-L. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182–6. doi: 10.1016/0006-8993(94)91382-X. [DOI] [PubMed] [Google Scholar]
- 15.Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10:155–68. doi: 10.1615/CritRevNeurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- 16.Olschowka JA, O’Donohue TL, Mueller GP, Jacobowitz DM. The distribution of corticotropin releasing factor-like immunoreactive neurons in rat brain. Peptides. 1982;3:995–1015. doi: 10.1016/0196-9781(82)90071-7. [DOI] [PubMed] [Google Scholar]
- 17.Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 1983;3:1355–68. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–38. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- 19.Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic–pituitary–adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–34. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N. Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol. 1983;218:121–44. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- 22.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 24.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–66. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6:603–21. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemeroff CB. The Role of Corticotropin-Releasing Factor in the Pathogenesis of Major Depression. Pharmacopsychiatry. 1988;21:76–82. doi: 10.1055/s-2007-1014652. [DOI] [PubMed] [Google Scholar]
- 28.Nemeroff CB, Widerlöv E, Bissette G, Walléus H, Karlsson I, Eklund K, et al. Elevated Concentrations of CSF Corticotropin-Releasing Factor-Like Immunoreactivity in Depressed Patients. Science. 1984;226:1342–4. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 29.Bremner JD, Licinio J, Darnell A, Krystal JH, Owens MJ, Southwick SM, et al. Elevated CSF Corticotropin-Releasing Factor Concentrations in Posttraumatic Stress Disorder. Am J Psychiatry. 1997;154:624–9. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 31.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/S0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 32.Bale TL. Stress sensitivity and the development of affective disorders. Hormones Behav. 2006;50:529–33. doi: 10.1016/j.yhbeh.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 33.Blank T, Spiess J Corticotropin-Releasing Factor (CRF) and CRF-Related Peptides – a Linkage Between Stress and Anxiety. Stress - From Molecules to Behavior, John Wiley & Sons, Ltd; 2009. p. 151-65.
- 34.Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991;555:25–34. doi: 10.1016/0006-8993(91)90855-P. [DOI] [PubMed] [Google Scholar]
- 35.Van Bockstaele EJ, Colago EEO, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10:743–58. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- 36.Van Bockstaele EJ, Peoples J, Valentino RJ. Anatomic basis for differential regulation of the rostrolateral peri–locus coeruleus region by limbic afferents. Biol Psychiatry. 1999;46:1352–63. doi: 10.1016/S0006-3223(99)00213-9. [DOI] [PubMed] [Google Scholar]
- 37.Flak JN, Myers B, Solomon MB, McKlveen JM, Krause EG, Herman JP. Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. Eur J Neurosci. 2014;39:1903–11. doi: 10.1111/ejn.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, et al. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87:605–20. doi: 10.1016/j.neuron.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt MV, Sterlemann V, Ganea K, Liebl C, Alam S, Harbich D, et al. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology. 2007;32:417–29. doi: 10.1016/j.psyneuen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Jaggi AS, Bhatia N, Kumar N, Singh N, Anand P, Dhawan R. A review on animal models for screening potential anti-stress agents. Neurol Sci. 2011;32:993–1005. doi: 10.1007/s10072-011-0770-6. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt MV, Wang X-D, Meijer OC. Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology. 2011;214:131–40. doi: 10.1007/s00213-010-2096-0. [DOI] [PubMed] [Google Scholar]
- 43.Willner P. Reliability of the chronic mild stress model of depression: A user survey. Neurobiol Stress. 2017;6:68–77. doi: 10.1016/j.ynstr.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci Biobehav Rev. 2019;99:101–16. doi: 10.1016/j.neubiorev.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 45.Atrooz F, Alkadhi KA, Salim S. Understanding stress: Insights from rodent models. Curr Res Neurobiol. 2021;2:100013. doi: 10.1016/j.crneur.2021.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aloisi AM, Steenbergen HL, Van De Poll NE, Farabollini F. Sex-dependent effects of restraint on nociception and pituitary-adrenal hormones in the rat. Physiol Behav. 1994;55:789–93. doi: 10.1016/0031-9384(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 47.Dalla C, Pitychoutis PM, Kokras N, Papadopoulou-Daifoti Z Sex Differences in Response to Stress and Expression of Depressive-Like Behaviours in the Rat. In: Neill JC, Kulkarni J, editors. Biological Basis of Sex Differences in Psychopharmacology, Berlin, Heidelberg: Springer; 2011. p. 97–118. [DOI] [PubMed]
- 48.Franceschelli A, Herchick S, Thelen C, Papadopoulou-Daifoti Z, Pitychoutis PM. Sex differences in the chronic mild stress model of depression. Behavioural Pharmacol. 2014;25:372. doi: 10.1097/FBP.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 49.Johnson A, Rainville JR, Rivero-Ballon GN, Dhimitri K, Hodes GE. Testing the limits of sex differences using variable stress. Neuroscience. 2021;454:72–84. doi: 10.1016/j.neuroscience.2019.12.034. [DOI] [PubMed] [Google Scholar]
- 50.Lopez J, Bagot RC. Defining valid chronic stress models for depression with female rodents. Biol Psychiatry. 2021;90:226–35. doi: 10.1016/j.biopsych.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci. 2007;104:5163–8. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, et al. Remote control of neuronal activity in transgenic mice expressing evolved g protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison KE, Epperson CN, Sammel MD, Ewing G, Podcasy JS, Hantsoo L, et al. Preadolescent adversity programs a disrupted maternal stress reactivity in humans and mice. Biol Psychiatry. 2017;81:693–701. doi: 10.1016/j.biopsych.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hesselgrave N, Troppoli TA, Wulff AB, Cole AB, Thompson SM. Harnessing psilocybin: antidepressant-like behavioral and synaptic actions of psilocybin are independent of 5-HT2R activation in mice. Proc Natl Acad Sci. 2021;118:e2022489118. doi: 10.1073/pnas.2022489118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Troppoli TA, Zanos P, Georgiou P, Gould TD, Rudolph U, Thompson SM. Negative allosteric modulation of gamma-aminobutyric acid a receptors at α5 subunit–containing benzodiazepine sites reverses stress-induced anhedonia and weakened synaptic function in mice. Biol Psychiatry. 2022;92:216–26. doi: 10.1016/j.biopsych.2021.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol. 2009;517:156–65. doi: 10.1002/cne.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herman J. Neural control of chronic stress adaptation. Front Behav Neurosci. 2013;7:61. [DOI] [PMC free article] [PubMed]
- 58.Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC. Behavioral and endocrine change following chronic predatory stress. Physiol Behav. 1998;63:561–9. doi: 10.1016/S0031-9384(97)00508-8. [DOI] [PubMed] [Google Scholar]
- 59.Cabib S, Kempf E, Schleef C, Mele A, Puglisi-Allegra S. Different effects of acute and chronic stress on two dopamine-mediated behaviors in the mouse. Physiol Behav. 1988;43:223–7. doi: 10.1016/0031-9384(88)90242-9. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt MV, Sterlemann V, Müller MB. Chronic stress and individual vulnerability. Ann N. Y Acad Sci. 2008;1148:174–83. doi: 10.1196/annals.1410.017. [DOI] [PubMed] [Google Scholar]
- 61.Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci. 2011;31:4475–83. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Checkley S. The neuroendocrinology of depression and chronic stress. Br Med Bull. 1996;52:597–617. doi: 10.1093/oxfordjournals.bmb.a011570. [DOI] [PubMed] [Google Scholar]
- 63.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–4. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 64.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–52. doi: 10.1016/S0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 65.Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–3. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- 66.Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14:2579–84. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18:1413–20. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franco AJ, Chen C, Scullen T, Zsombok A, Salahudeen AA, Di S, et al. Sensitization of the Hypothalamic-Pituitary-Adrenal Axis in a Male Rat Chronic Stress Model. Endocrinology. 2016;157:2346–55. doi: 10.1210/en.2015-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tasker JG, Joëls M The Synaptic Physiology of the Central Nervous System Response to Stress. In: Russell J, Shipston M, editors. Neuroendocrinology of Stress, Chichester, UK: John Wiley & Sons, Ltd; 2015. p. 43–70.
- 70.Selye H. The general adaptation syndrome and the diseases of adaptation. J Clin Endocrinol Metab. 1946;6:117–230. doi: 10.1210/jcem-6-2-117. [DOI] [PubMed] [Google Scholar]
- 71.McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann N. Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 72.McEwen BS, Akil H. Revisiting the stress concept: implications for affective disorders. J Neurosci. 2020;40:12–21. doi: 10.1523/JNEUROSCI.0733-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bains JS, Cusulin JIW, Inoue W. Stress-related synaptic plasticity in the hypothalamus. Nat Rev Neurosci. 2015;16:377–88. doi: 10.1038/nrn3881. [DOI] [PubMed] [Google Scholar]
- 74.López JF, Akil H, Watson SJ. Neural circuits mediating stress. Biol Psychiatry. 1999;46:1461–71. doi: 10.1016/S0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- 75.Morrison KE, Cole AB, Kane PJ, Meadows VE, Thompson SM, Bale TL. Pubertal adversity alters chromatin dynamics and stress circuitry in the pregnant brain. Neuropsychopharmacology. 2020;45:1263–71. doi: 10.1038/s41386-020-0634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cole AB, Montgomery K, Bale TL, Thompson SM. What the hippocampus tells the HPA axis: Hippocampal output attenuates acute stress responses via disynaptic inhibition of CRF+ PVN neurons. Neurobiol Stress. 2022;20:100473. doi: 10.1016/j.ynstr.2022.100473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orth DN, Jackson RV, DeCherney GS, DeBold CR, Alexander AN, Island DP, et al. Effect of synthetic ovine corticotropin-releasing factor. Dose response of plasma adrenocorticotropin and cortisol. 1983. https://www.jci.org/articles/view/110804/scanned-page/590. Accessed 29 April 2023. [DOI] [PMC free article] [PubMed]
- 78.Veissier I, van Reenen CG, Andanson S, Leushuis IE. Adrenocorticotropic hormone and cortisol in calves after corticotropin-releasing hormone. J Anim Sci. 1999;77:2047–53. doi: 10.2527/1999.7782047x. [DOI] [PubMed] [Google Scholar]
- 79.Babb JA, Masini CV, Day HEW, Campeau S. Sex differences in activated CRF neurons within stress-related neurocircuitry and HPA axis hormones following restraint in rats. Neuroscience. 2013;234:40–52. doi: 10.1016/j.neuroscience.2012.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Hormones Behav. 1994;28:464–76. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 81.Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994;55:117–24. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 82.Bingaman EW, Magnuson DJ, Gray TS, Handa RJ. Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-lmmunoreactivity following gonadectomy. NEN. 1994;59:228–34. doi: 10.1159/000126663. [DOI] [PubMed] [Google Scholar]
- 83.Haas DA, George SR. Gonadal regulation of corticotropin-releasing factor immunoreactivity in hypothalamus. Brain Res Bull. 1988;20:361–7. doi: 10.1016/0361-9230(88)90065-2. [DOI] [PubMed] [Google Scholar]
- 84.Panagiotakopoulos L, Neigh GN. Development of the HPA axis: Where and when do sex differences manifest? Front Neuroendocrinol. 2014;35:285–302. doi: 10.1016/j.yfrne.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, et al. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–7. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roth BL. DREADDs for neuroscientists. Neuron. 2016;89:683–94. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jendryka M, Palchaudhuri M, Ursu D, van der Veen B, Liss B, Kätzel D, et al. Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci Rep. 2019;9:4522. doi: 10.1038/s41598-019-41088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chambers KC, Sengstake CB. Sexually dimorphic extinction of a conditioned taste aversion in rats. Anim Learn Behav. 1976;4:181–5. doi: 10.3758/BF03214032. [DOI] [PubMed] [Google Scholar]
- 89.Dacanay RJ, Mastropaolo JP, Olin DA, Riley AL. Sex differences in taste aversion learning: An analysis of the minimal effective dose. Neurobehavioral Toxicol Teratol. 1984;6:9–11. [PubMed] [Google Scholar]
- 90.Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97:229–38. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Randall-Thompson JF, Riley AL. Morphine-induced conditioned taste aversions: assessment of sexual dimorphism. Pharmacol Biochem Behav. 2003;76:373–81. doi: 10.1016/j.pbb.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 92.Bernanke A, Burnette E, Murphy J, Hernandez N, Zimmerman S, Walker QD, et al. Behavior and Fos activation reveal that male and female rats differentially assess affective valence during CTA learning and expression. PLOS ONE. 2021;16:e0260577. doi: 10.1371/journal.pone.0260577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levine AS, Morley JE. Stress-induced eating in rats. Am J Physiol. 1981;241:R72–76. doi: 10.1152/ajpregu.1981.241.1.R72. [DOI] [PubMed] [Google Scholar]
- 94.Michajlovskij N, Lichardus B, Kvetnanský R, Ponec J. Effect of acute and repeated immobilization stress on food and water intake, urine output and vasopressin changes in rats. Endocrinol Exp. 1988;22:143–57. [PubMed] [Google Scholar]
- 95.Vallès A, Martí O, García A, Armario A. Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am J Physiol-Regulatory, Integr Comp Physiol. 2000;279:R1138–R1144. doi: 10.1152/ajpregu.2000.279.3.R1138. [DOI] [PubMed] [Google Scholar]
- 96.Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–6. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 97.Magarin˜os AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-I. [DOI] [PubMed] [Google Scholar]
- 98.Tarcic N, Ovadia H, Weiss DW, Weidenfeld J. Restraint stress-induced thymic involution and cell apoptosis are dependent on endogenous glucocorticoids. J Neuroimmunol. 1998;82:40–46. doi: 10.1016/S0165-5728(97)00186-0. [DOI] [PubMed] [Google Scholar]
- 99.Ehlers CL, Chaplin RI, Kaneko WM. Effects of chronic corticosterone treatment on electrophysiological and behavioral measures in the rat. Psychoneuroendocrinology. 1992;17:691–9. doi: 10.1016/0306-4530(92)90028-6. [DOI] [PubMed] [Google Scholar]
- 100.Pavlovska-Teglia G, Stodulski G, Svendsen L, Dalton K, Hau J. Effect of oral corticosterone administration on locomotor development of neonatal and juvenile rats. Exp Physiol. 1995;80:469–75. doi: 10.1113/expphysiol.1995.sp003861. [DOI] [PubMed] [Google Scholar]
- 101.Kalynchuk LE, Gregus A, Boudreau D, Perrot-Sinal TS. Corticosterone increases depression-like behavior, with some effects on predator odor-induced defensive behavior, in male and female rats. Behav Neurosci. 2004;118:1365–77. doi: 10.1037/0735-7044.118.6.1365. [DOI] [PubMed] [Google Scholar]
- 102.Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behavioural Brain Res. 2005;156:105–14. doi: 10.1016/j.bbr.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 103.Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. Eur J Pharmacol. 2008;583:115–27. doi: 10.1016/j.ejphar.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 104.Kott JM, Mooney-Leber SM, Shoubah FA, Brummelte S. Effectiveness of different corticosterone administration methods to elevate corticosterone serum levels, induce depressive-like behavior, and affect neurogenesis levels in female rats. Neuroscience. 2016;312:201–14. doi: 10.1016/j.neuroscience.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 105.Mekiri M, Gardier AM, David DJ, Guilloux J-P. Chronic corticosterone administration effects on behavioral emotionality in female c57bl6 mice. Exp Clin Psychopharmacol. 2017;25:94–104. doi: 10.1037/pha0000112. [DOI] [PubMed] [Google Scholar]
- 106.Shahanoor Z, Sultana R, Baker MR, Romeo RD. Neuroendocrine stress reactivity of male C57BL/6N mice following chronic oral corticosterone exposure during adulthood or adolescence. Psychoneuroendocrinology. 2017;86:218–24. doi: 10.1016/j.psyneuen.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 107.Bullitt E. Expression of C-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–30. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- 108.Sharp FR, Sagar SM, Hicks K, Lowenstein D, Hisanaga K. c-fos mRNA, Fos, and Fos-related antigen induction by hypertonic saline and stress. J Neurosci. 1991;11:2321–31. doi: 10.1523/JNEUROSCI.11-08-02321.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- 110.Hodes GE, Epperson CN. Sex differences in vulnerability and resilience to stress across the life span. Biol Psychiatry. 2019;86:421–32. doi: 10.1016/j.biopsych.2019.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol. 2009;21:415–20. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Board F, Wadeson R, Persky H. Depressive affect and endocrine functions: blood levels of adrenal cortex and thyroid hormones in patients suffering from depressive reactions. AMA Arch Neurol Psychiatry. 1957;78:612–20. doi: 10.1001/archneurpsyc.1957.02330420072015. [DOI] [PubMed] [Google Scholar]
- 113.Gibbons JL, McHugh PR. Plasma cortisol in depressive illness. J Psychiatr Res. 1962;1:162–71. doi: 10.1016/0022-3956(62)90006-7. [DOI] [PubMed] [Google Scholar]
- 114.Carroll BJ, Martin FIR, Davies B. Pituitary-Adrenal function in depression. Lancet. 1968;291:1373–4. doi: 10.1016/S0140-6736(68)92072-2. [DOI] [PubMed] [Google Scholar]
- 115.Insel TR, Kalin NH, Guttmacher LB, Cohen RM, Murphy DL. The dexamethasone suppression test in patients with primary obsessive-compulsive disorder. Psychiatry Res. 1982;6:153–60. doi: 10.1016/0165-1781(82)90003-8. [DOI] [PubMed] [Google Scholar]
- 116.Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry. 1993;150:83–86. doi: 10.1176/ajp.150.1.83. [DOI] [PubMed] [Google Scholar]
- 117.Schreiber W, Lauer CJ, Krumrey K, Holsboer F, Krieg J-C. Dysregulation of the hypothalamic-pituitary-adrenocortical system in panic disorder. Neuropsychopharmacol. 1996;15:7–15. doi: 10.1016/0893-133X(95)00146-5. [DOI] [PubMed] [Google Scholar]
- 118.Plotsky PM, Owens MJ, Nemeroff CB. PSYCHONEUROENDOCRINOLOGY OF DEPRESSION: Hypothalamic-Pituitary-Adrenal Axis. Psychiatr Clin North Am. 1998;21:293–307. doi: 10.1016/S0193-953X(05)70006-X. [DOI] [PubMed] [Google Scholar]
- 119.Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin. 2002;25:341–68. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- 120.Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM. Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology. 2004;29:389–404. doi: 10.1016/S0306-4530(03)00052-0. [DOI] [PubMed] [Google Scholar]
- 121.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–7. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lentjes EGWM, Westenberg HGM. Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2007;32:215–26. doi: 10.1016/j.psyneuen.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 123.Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast Feedback Inhibition of the HPA Axis by Glucocorticoids Is Mediated by Endocannabinoid Signaling. Endocrinology. 2010;151:4811–9. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang Z, Chen C, Weiss GL, Fu X, Stelly CE, Sweeten BLW, et al. Stress-induced glucocorticoid desensitizes adrenoreceptors to gate the neuroendocrine response to somatic stress in male mice. Cell Rep. 2022;41:111509. doi: 10.1016/j.celrep.2022.111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schmidt M, Levine S, Oitzl MS, van der Mark M, Müller MB, Holsboer F, et al. Glucocorticoid receptor blockade disinhibits pituitary-adrenal activity during the stress hyporesponsive period of the mouse. Endocrinology. 2005;146:1458–64. doi: 10.1210/en.2004-1042. [DOI] [PubMed] [Google Scholar]
- 126.Herman JP, Tasker JG. Paraventricular Hypothalamic Mechanisms of Chronic Stress Adaptation. Frontiers in Endocrinology. 2016;7:147. [DOI] [PMC free article] [PubMed]
- 127.Benham G. The Highly Sensitive Person: Stress and physical symptom reports. Personal Individ Diff. 2006;40:1433–40. doi: 10.1016/j.paid.2005.11.021. [DOI] [Google Scholar]
- 128.Callahan ML, Storzbach D. Sensory sensitivity and posttraumatic stress disorder in blast exposed veterans with mild traumatic brain injury. Appl Neuropsychology: Adult. 2019;26:365–73. doi: 10.1080/23279095.2018.1433179. [DOI] [PubMed] [Google Scholar]
- 129.van Marle HJF, Hermans EJ, Qin S, Fernández G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66:649–55. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 130.Howerton AR, Roland AV, Fluharty JM, Marshall A, Chen A, Daniels D, et al. Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biological Psychiatry. 2014. 2014. 10.1016/j.biopsych.2013.10.013. [DOI] [PMC free article] [PubMed]
- 131.Homberg JR, Contet C. Deciphering the interaction of the corticotropin-releasing factor and serotonin brain systems in anxiety-related disorders. J Neurosci. 2009;29:13743–5. doi: 10.1523/JNEUROSCI.4362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koegler-Muly SM, Owens MJ, Ervin GN, Kilts CD, Nemeroff CB. Potential corticotropin-releasing factor pathways in the rat brain as determined by bilateral electrolytic lesions of the central amygdaloid nucleus and the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 1993;5:95–98. doi: 10.1111/j.1365-2826.1993.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 133.Kalin NH, Fox AS, Kovner R, Riedel MK, Fekete EM, Roseboom PH, et al. Overexpressing corticotropin-releasing factor in the primate amygdala increases anxious temperament and alters its neural circuit. Biol Psychiatry. 2016;80:345–55. doi: 10.1016/j.biopsych.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Swiergiel AH, Takahashi LK, Kalin NH. Attenuation of stress-induced behavior by antagonism of corticotropin-releasing factor receptors in the central amygdala in the rat. Brain Res. 1993;623:229–34. doi: 10.1016/0006-8993(93)91432-R. [DOI] [PubMed] [Google Scholar]
- 135.Curtis AL, Bello NT, Connolly KR, Valentino RJ. Corticotropin-releasing factor neurones of the central nucleus of the amygdala mediate locus coeruleus activation by cardiovascular stress. J Neuroendocrinol. 2002;14:667–82. doi: 10.1046/j.1365-2826.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- 136.Adamec RE, McKay D. Amygdala kindling, anxiety, and corticotrophin releasing factor (CRF) Physiol Behav. 1993;54:423–31. doi: 10.1016/0031-9384(93)90230-D. [DOI] [PubMed] [Google Scholar]
- 137.Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, et al. A central amygdala CRF circuit facilitates learning about weak threats. Neuron. 2017;93:164–78. doi: 10.1016/j.neuron.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–31. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 139.Jo YS, Namboodiri VMK, Stuber GD, Zweifel LS. Persistent activation of central amygdala CRF neurons helps drive the immediate fear extinction deficit. Nat Commun. 2020;11:422. doi: 10.1038/s41467-020-14393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson R-M, Saksida LM, et al. Impaired Fear Extinction Learning and Cortico-Amygdala Circuit Abnormalities in a Common Genetic Mouse Strain. J Neurosci. 2008;28:8074–85. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brinks V, de Kloet ER, Oitzl MS. Corticosterone facilitates extinction of fear memory in BALB/c mice but strengthens cue related fear in C57BL/6 mice. Exp Neurol. 2009;216:375–82. doi: 10.1016/j.expneurol.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 142.Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, et al. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci. 2012;109:16318–23. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Riddle MC, McKenna MC, Yoon YJ, Pattwell SS, Santos PMG, Casey BJ, et al. Caloric restriction enhances fear extinction learning in mice. Neuropsychopharmacol. 2013;38:930–7. doi: 10.1038/npp.2012.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu Z, Adler A, Li H, Pérez-Cuesta LM, Lai B, Li W, et al. Fear conditioning and extinction induce opposing changes in dendritic spine remodeling and somatic activity of layer 5 pyramidal neurons in the mouse motor cortex. Sci Rep. 2019;9:4619. doi: 10.1038/s41598-019-40549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ramikie TS, Ressler KJ. Mechanisms of sex differences in fear and posttraumatic stress disorder. Biol Psychiatry. 2018;83:876–85. doi: 10.1016/j.biopsych.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 146.Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen C-Y, Choi KW, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019;10:4558. doi: 10.1038/s41467-019-12576-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol Bull. 2006;132:959–92. [DOI] [PubMed]
- 148.Garza K, Jovanovic T. Impact of gender on child and adolescent PTSD. Curr Psychiatry Rep. 2017;19:87. doi: 10.1007/s11920-017-0830-6. [DOI] [PubMed] [Google Scholar]
- 149.Roeckner AR, Sogani S, Michopoulos V, Hinrichs R, van Rooij SJH, Rothbaum BO, et al. Sex-dependent risk factors for PTSD: a prospective structural MRI study. Neuropsychopharmacol. 2022;47:2213–20. doi: 10.1038/s41386-022-01452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Becker JB, Monteggia LM, Perrot-Sinal TS, Romeo RD, Taylor JR, Yehuda R, et al. Stress and disease: is being female a predisposing factor? J Neurosci. 2007;27:11851–5. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ravi M, Stevens JS, Michopoulos V. Neuroendocrine pathways underlying risk and resilience to PTSD in women. Front Neuroendocrinol. 2019;55:100790. doi: 10.1016/j.yfrne.2019.100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Walker CD, Tankosic P, Tilders FJ, Burlet A. Immunotargeted lesions of paraventricular CRF and AVP neurons in developing rats reveal the pattern of maturation of these systems and their functional importance. J Neuroendocrinol. 1997;9:25–41. doi: 10.1046/j.1365-2826.1997.00544.x. [DOI] [PubMed] [Google Scholar]
- 153.Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci. 2008;105:12004–9. doi: 10.1073/pnas.0803216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Swanson LW, Sawchenko PE, Lind RW Regulation of multiple peptides in CRF parvocellular neurosecretory neurons: implications for the stress response. In: Hökfelt T, Fuxe K, Pernow B, editors. Progress in Brain Research, 68, Elsevier; 1986. p. 169-90. [DOI] [PubMed]
- 155.Herman JP, Prewitt CM-F, Cullinan WE. Neuronal Circuit Regulation of the Hypothalamo-Pituitary-Adrenocortical Stress Axis. Crit Rev Neurobiol. 1996;10:371–94. [DOI] [PubMed]
- 156.Gillies GE, Linton EA, Lowry PJ. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–7. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- 157.Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci. 1984;81:1883–7. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ma X-M, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–32. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- 159.Birnie MT, Short AK, de Carvalho GB, Taniguchi L, Gunn BG, Pham AL, et al. Stress-induced plasticity of a CRH/GABA projection disrupts reward behaviors in mice. Nat Commun. 2023;14:1088. doi: 10.1038/s41467-023-36780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.