Summary
Background
Chlorine-based disinfectants, such as bleach, are commonly used for cleaning in healthcare settings to prevent the transmission of nosocomial pathogens. To enhance the efficacy of disinfection, ultraviolet-C (UV-C) light systems have been proposed to supplement standard cleaning procedures. As bleach decomposes in UV light, we hypothesised that the use of UV-C light as an adjunct to manual cleaning with bleach, may decrease the efficacy of disinfection instead.
Methods
In the laboratory, stainless steel sheets and plastic keyboards were inoculated with Pseudomonas aeruginosa (∼106 CFU/ml) and subjected to treatment with either UV-C light only, bleach only or a combination of UV-C light and bleach. The residual bioburden (CFU/ml) was quantified through conventional microbiological techniques. Results were compared to non-exposed control surfaces and against each treatment strategy.
Results
On tested surfaces, there were statistically significant reductions in P. aeruginosa when surfaces were treated with UV-C light only (>2.5 log10 reduction), bleach only (>5.6 log10 reduction) and a combination of UV-C light and bleach (>6.3 log10 reduction) compared to positive control (P < 0.001, all treatment strategies). No significant differences were observed when surfaces were treated with the addition of UV-C light to bleach compared to treatment with bleach alone.
Conclusion
There was no difference in the efficacy of disinfection against P. aeruginosa with the combined treatment strategy of UV-C light and bleach compared to bleach alone under laboratory conditions. Further studies are warranted to elucidate the effectiveness of this technology on other healthcare-associated pathogens.
Keywords: Bleach, Cleaning, Ultraviolet-C light, Disinfection
Introduction
Improved surface cleaning and disinfection is crucial to prevent the risk of transmission of nosocomial pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), Clostridioides difficile and Pseudomonas aeruginosa [[1], [2], [3], [4], [5]]. Cleaning is defined as the physical removal of organic material on soiled surfaces (e.g. using a cloth to remove dirt) while disinfection refers to the killing or inactivation of most pathogenic microorganisms on inanimate surfaces [6]. For disinfection to be effective, it must be preceded by cleaning.
Recently, ultraviolet light-C (UV-C) devices have been advocated for use in hospitals as it may potentially enhance the efficacy of disinfection [[7], [8], [9]]. When used at specific wavelengths, UV-C light causes cellular damage and prevents the replication of microorganisms by breaking molecular bonds and forming photo dimeric lesions in DNA and RNA [10,11]. As the removal of visible soil is essential for disinfection to be effective, UV light disinfection technology is suggested as a supplement to, rather than a replacement for standard cleaning processes [5,12].
Evidence supporting the use of UV radiation as an adjunct to manual cleaning is generally of poor quality. Most clinical studies which observed a reduction in HAIs with the implementation of UV light systems are before-and-after studies which are inherently subject to multiple biases introduced by the lack of blinding, randomisation and a control group [[13], [14], [15], [16]]. The highest quality of evidence stems from a cluster-randomised, multicentre controlled trial which compared the effect of four different terminal room disinfection strategies on the acquisition and infection with multidrug-resistant organisms (MDROs) [17]. The study concluded that the addition of UV-C provided an enhanced strategy for terminal room disinfection and reduced the risk of acquisition of pathogens. Specifically, the trial demonstrated a statistically significant reduction in the incidence of pathogen transmission when the treatment of UV-C light was combined with quaternary ammonium disinfectant compared to the use of quaternary ammonium disinfectant alone. In contrast, the trial observed no difference in the incidence of C. difficile infections with the combined treatment of UV-C and bleach compared to the use of bleach alone [17].
A statistical analysis (Appendix A) was carried out to ascertain whether the observed lack of effect for the combined treatment of UV-C and bleach was due to a type II error, as was suggested in the paper, or whether an interaction exists between the two treatment modalities. A statistically significant evidence of effect modification was found, implying that this was not a chance finding.
The analysis suggests that there is not a simple additive effect from combining chemical disinfectants and UV-C. There is a biologically plausible explanation for the apparent lack of effect or negative effect when UV-C was used in addition to bleach. When exposed to UV light, the active ingredient in bleach, sodium hypochlorite (NaOCl) decomposes into sodium chloride (NaCl) and oxygen (O2) via photolysis [18,19]. If the treatments occurred simultaneously, it is possible that exposure to UV light resulted in the decomposition of bleach before a bactericidal or sporicidal effect could occur. Hence, it is hypothesised that if UV light was used as an adjunct to manual cleaning with bleach, it could possibly decrease the efficacy of the disinfection process, as opposed to improving it.
Clearly, the post-hoc analysis of clinical trial data alone is not enough to demonstrate that the use of UV-C as an adjunct to manual cleaning with bleach is unsafe. This was the rationale for conducting this investigation, which aims to assess the in-vitro efficacy of adjunctive UV-C radiation with bleach. The controlled conditions in the laboratory will allow for variables to be isolated effectively, enabling a precise interaction between UV-C and bleach to be established.
Materials and methods
Preparation of Pseudomonas aeruginosa
Stationary phase cultures (∼107 CFU/ml) of Pseudomonas aeruginosa PAO1 were prepared prior to experiments. P. aeruginosa is responsible for a wide range of nosocomial infections among critically ill patients [20]. The World Health Organization has classified it as a “Priority 1” pathogen, in which there is a critical need for the development of new antibiotics to treat this organism [[21], [22], [23], [24]]. Apart from antimicrobial resistance, P. aeruginosa is resistant to some disinfectants such as quaternary ammonium compounds and biguanides. These were the rationales for using P. aeruginosa in this study.
UV device
The UV device used in this investigation is the Cross-Linker® CL-508 (UVItec, Cambridge, UK). Previous studies have established that vegetative bacteria is vulnerable to doses of UV-C light at a wavelength of 254 nm and intensity of 0.120 J/cm2 [2,8,[25], [26], [27]]. Hence, preliminary experiments (Appendix B) were conducted to determine the efficacy of the UV device for killing P. aeruginosa at this specific wavelength and intensity.
Preparation of surfaces
Stainless steel
The area of contamination (4 cm x 4 cm) on stainless steel was defined using a pencil. This area was then divided into four sections, with each section (2 cm x 2 cm) representing one sample point.
Keyboard
On each keyboard part, 4 keys were defined as the area of contamination (3.8 cm x 3.8 cm). Each key (1.9 cm x 1.9 cm), including the top and sides were considered to be one sample point. There were a total of four sample points on each surface. Prior to each experiment, the surfaces were cleaned using warm water, left to air-dry and subsequently disinfected with 70% ethanol [28]. To determine the efficacy of this cleaning method, sterile swab samples were obtained and streaked on nutrient and blood agar plates. No bacterial colonies were observed upon sampling. In addition, a negative control was always present in parallel to experiments carried out to ensure consistency of the cleaning protocol.
Contamination of surfaces
A vial of stationary-phase P. aeruginosa was thawed from -80 °C and washed twice with PBS before being suspended in 1ml of 3% Bovine Serum Albumin (BSA). BSA is recommended for use as an interfering substance to mimic organic soiling when investigating the efficacy of disinfectants [29]. Preliminary experiments (Appendix C) established that 3% was the ideal concentration of BSA to emulate dirty conditions in this investigation. The resulting bacterial suspension was diluted 10-fold to achieve a concentration of ∼106 CFU/ml. In each experiment, the concentration of inoculum was confirmed by serial dilutions and plating to obtain viable counts.
Stainless steel
Using a pipette, 160 uL of bacterial suspension containing approximately 106 CFU/ml was inoculated onto stainless steel. Using a sterile L-shaped spreader, the inoculum was evenly spread out to cover the area of contamination. The liquid was then left to dry for one hour.
Keyboard
An intranasal mucosal atomisation device was used to disperse 200 uL of bacterial suspension containing approximately 106 CFU/ml onto the defined area of contamination on the keyboard. The liquid was then left to dry for one hour. Due to the composition of keys and indentations present on the surfaces of keyboards, a different inoculation process was used.
Disinfection of surfaces
Arm 1: Negative Control
The surface was not contaminated with bacteria.
Arm 2: Positive Control
The surface was contaminated but left untreated.
Arm 3: UV-C light only
The surface was placed in the UV device, and exposed to UV-C light at 254 nm and 0.120 J/cm2 for 1 cycle (65 seconds).
Arm 4: Bleach only
The surface was wiped (four strokes up and down) with bleach disinfectant wipes, containing 0.55% sodium hypochlorite (Clorox Healthcare® Bleach Germicidal Wipes, Clorox, Oakland, CA), The same product was used in the randomised controlled trial [17] described earlier.
Arm 5: Bleach and UV-C light
The surface was wiped (four strokes up and down) with bleach wipes. Approximately 2 minutes later, the surface was exposed to UV-C light at 254 nm and 0.120 J/cm2 for 1 cycle (65 seconds).
Quantification of bacteria from surfaces
A pre-moistened sterile cotton-tipped swab was used to sample each sample point immediately after disinfection. Each swab was placed in 10 ml of media and vortexed for 3 minutes to release the bacteria and homogenise the solution [30]. The culture was then incubated for 18 hours at 37 °C rotating at 250 rpm in air. Serial 10-fold dilutions of the resulting suspension were made and inoculated on to nutrient agar plates. The plates were incubated at 37 °C for a further 24 hours before counting the number of colonies and determining the viable cell count (CFU/ml). There were four sample points on each test surface, with the mean from each experiment used for analyses.
Statistical analysis
Data distribution, statistical analysis and graph production was performed using GraphPad Prism (version 8.4.1). The data were tested for normality using Shapiro-Wilk test. As data showed normal distribution, parametric tests were used for analyses. A one-way analysis of variance (ANOVA) and post-hoc Tukey test was used to compare the effectiveness of each method of disinfection against the positive control and each other. All experiments were performed in triplicates and P 0.05 was considered statistically significant.
The laboratory protocol can be referred to in Appendix D.
Results
Reduction of P. aeruginosa on stainless steel sheets
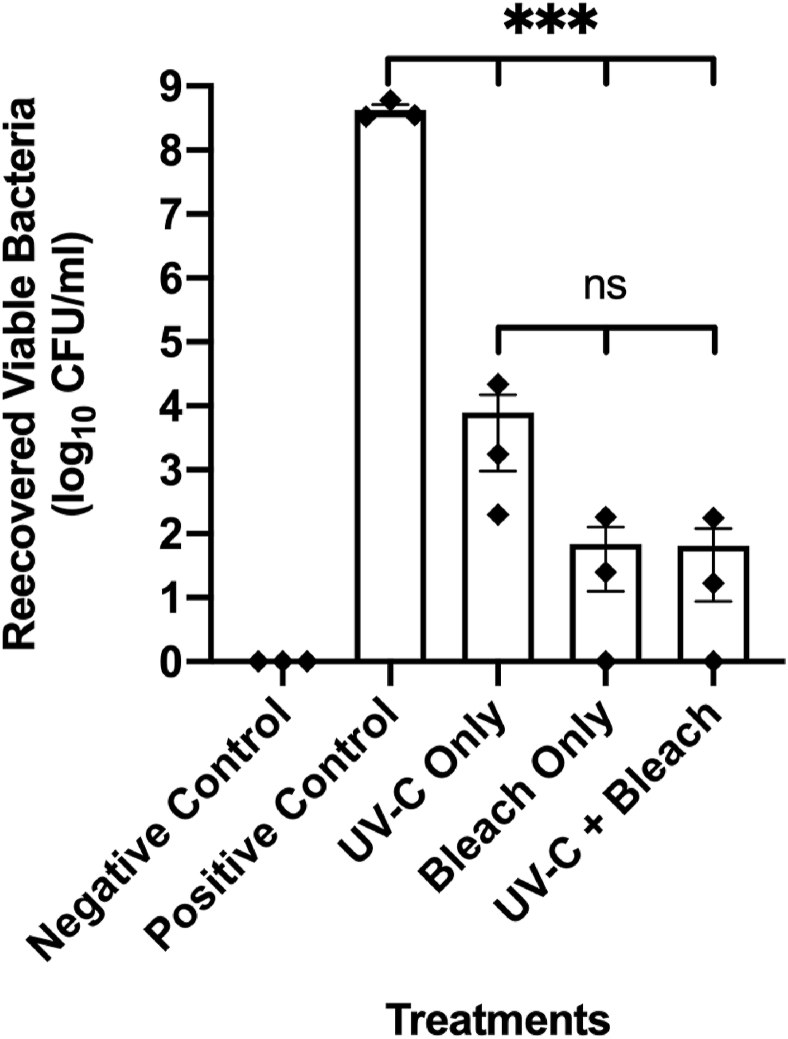
On stainless steel sheets that were inoculated with P. aeruginosa suspended in 3% BSA, there were statistically significant reductions in bacteria when treated with UV-C (4.73 log10 reduction), bleach (6.79 log10 reduction), and a combination of UV-C and bleach (6.83 log10 reduction) compared to positive control with P < 0.001 for all treatments (Figure 1). Disinfection with bleach wipes resulted in a lower number of recovered viable bacteria compared to irradiation with UV-C only, though this did not reach statistical significance. No differences were observed between treatments with bleach and the combination of UV-C and bleach.
Figure 1.
Reduction of P. aeruginosa on Stainless Steel Sheets. The graph shows the number of viable bacteria recovered from stainless steel sheets following treatment with UV-C light only, bleach only and a combination of UV-C light and bleach (Mean ± standard error of mean; n=3). Asterisks depict statistically significant differences (∗∗∗, P 0.001; ns, not significant).
Reduction of P. aeruginosa on keyboards
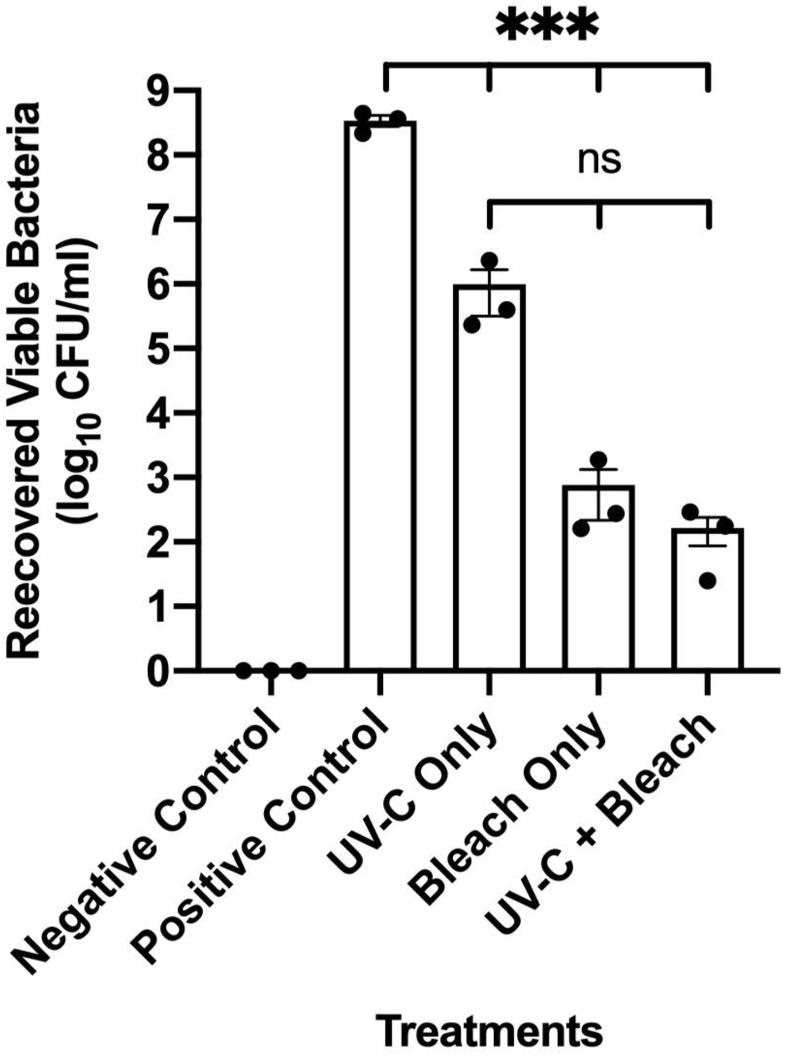
On keyboards that were inoculated with P. aeruginosa suspended in 3% BSA, there were statistically significant reductions in bacteria when treated with UV-C (2.54 log10 reduction), bleach (5.65 log10 reduction) and a combination of UV-C and bleach (6.32 log10 reduction) compared to positive control with P < 0.001 for all treatments (Figure 2). Treatment with bleach wipes resulted in a lower number of recovered viable bacteria compared to disinfection with UV-C light, but this was not statistically significant. There was no significant difference between the number of viable bacteria when keyboards were treated with the addition of UV-C light to bleach compared to bleach alone.
Figure 2.
Reduction of P. aeruginosa on Keyboards. The graph shows the number of viable bacteria recovered from keyboards following treatment with UV-C light only, bleach only and a combination of UV-C light and bleach. (Mean ± standard error of mean; n=3). Asterisks depict statistically significant differences (∗∗∗, P 0.001; ns, not significant).
Discussion
Optimal cleaning and disinfection of patient rooms is crucial to reduce and prevent the transmission of pathogens in the health care environment [1,5]. Sodium hypochlorite, more commonly known as bleach is a common chemical agent used for cleaning and disinfection of environmental surfaces. As with most chemical disinfectants used in clinical settings, bleach enables cleaning and disinfection to be completed in 1-step, rather than requiring 2 independent steps [31]. Hence, a separate pre-cleaning procedure is not needed unless gross contamination is present.
The microbicidal activity of bleach is largely attributed to undissociated hypochlorous acid (HOCl) (Eq. (1)), though the precise mechanism of this activity has not been fully elucidated [32]. The stability of sodium hypochlorite is an important issue which should be recognised when using bleach. Its stability depends on the concentration of solution, pH, temperature and exposure to UV light [18,19]. Of particular relevance, UV light catalyses photochemical reactions which results in the decomposition of sodium hypochlorite (Eq. (2)) and hypochlorous acid (Eq. (3)). Thus, as aforementioned, the use of adjunctive UV radiation as a supplement to manual cleaning with bleach is hypothesised to be ineffective.
| (1) |
| (2) |
| (3) |
On stainless steel sheets and keyboards that were inoculated with P. aeruginosa suspended in 3% BSA, statistically significant reductions in bacteria compared to positive control were observed for all treatment strategies. Even in the absence of manual cleaning, the use of UV-C alone demonstrated a statistically significant reduction in P. aeruginosa with 4.73 log10 reductions on stainless steel and 2.54 log10 reductions on keyboards. Similarly, several in-vitro studies have demonstrated that UV-C light devices can effectively reduce pathogenic bacteria in the presence of simulated organic load without prior cleaning [28,33]. Moore et al. found that exposure to UV-C light for 60 seconds reduced methicillin-sensitive Staphylococcus aureus (MSSA) and P. aeruginosa to below detectable levels (i.e. achieved a 6 log10 reduction) [28]. In another study by Nerandzic et al., the application of UV-C resulted in 2–3 log10 reductions in MRSA and 4–5 log10 reductions in VRE in the presence of organic challenge [33].
However, UV-C is less effective at eliminating bacteria in healthcare settings compared to non-clinical, experimental conditions. To exemplify, a prospective cohort study demonstrated that the implementation of UV light systems before standard terminal cleaning of rooms where the patient was infected or colonised with VRE, C. difficile or Acinetobacter only produced a 1.35 log10 reduction in the CFUs of target pathogens [27]. These same pathogens have shown reductions by 2–5 log10 values in the laboratory [25,33]. Another study reported that without mechanical cleaning, the use of UV-C did not show a decrease in the number of viable bacteria on 72% of computer keyboards located in hospital wards [34]. These findings suggest that traditional cleaning of surfaces is still essential prior to the implementation of UV-C devices.
Based on the results from this study and the studies cited above, it is apparent that there is a need for the development of appropriate testing standards for automated disinfection systems. Currently, BSA is recommended for use as an interfering substance to mimic organic soiling when evaluating the efficacy of liquid disinfectants [29]. The European Standards recommend varying the concentration of BSA to emulate ‘clean’ and ‘dirty’ conditions [29]. However, this does not seem to be a good representation of dirt and debris on surfaces when evaluating the efficacy of UV-C devices under experimental conditions. Hence, suitable testing standards should be established to allow results from in-vitro studies to be extrapolated to clinical settings.
In addition, the efficacy of UV-C depends on the distance between the UV-C source and the exposed surfaces, the angle of irradiation and the presence of shadowing [35]. These factors may contribute to the reduced efficacy when using UV-C devices to disinfect hospital rooms compared to laboratory experiments, where contaminated surfaces are placed in a small UV-C chamber, as exemplified in our study. Using other UV-C devices with different designs may also influence the outcome.
This study demonstrated that manual cleaning with bleach disinfectant wipes resulted in a greater reduction of P. aeruginosa compared to disinfection with UV-C alone. The recovery of viable bacteria from surfaces that were cleaned with bleach was approximately 2–3 log10 CFU/ml less than surfaces that were treated with UV-C, though this did not reach statistical significance. Nonetheless, this implies that the removal of organic material improves the efficacy of disinfection. This finding is not surprising and is consistent with current understanding of decontamination strategies, where cleaning should precede disinfection [6].
Of particular importance, no differences were observed in the recovery of viable bacteria when UV-C was used in addition to bleach compared to the use of bleach alone. Thus, the results contradicted our initial hypothesis that the combination of treatment strategies would decrease the efficacy of disinfection. There is a possible explanation for this observed phenomenon. The biocidal effects of bleach depend on its contact time. Chlorine-based disinfectants such as bleach can kill vegetative bacteria, including P. aeruginosa within 30 seconds [36,37]. In this investigation, the time between cleaning with bleach and exposure to UV-C light would have taken 2 minutes, at the very least. Hence, even if exposure to UV-C light resulted in the decomposition of bleach, it would not have affected the disinfection process because bleach would have already exerted its microbicidal properties.
However, other organisms such as spore-forming bacteria and certain viruses require a longer inactivation period [38]. For example, the CDC recommends a contact time of ≥10 minutes for bleach to inactivate C. difficile spores [6]. If UV-C was implemented immediately after cleaning with bleach on surfaces contaminated with C. difficile, this may have resulted in the decomposition of bleach before a sporicidal effect could occur. This is important because C. difficile produces spores that are resistant to killing by commonly used hospital disinfectants such as standard quaternary ammonium compounds [39]. Only highly concentrated chlorine-based disinfectants, such as a 1:10 dilution of bleach is able to exert sporicidal effects [39]. Due to limited expertise and time constraints of an undergraduate project, C. difficile was not experimented with in this study. However, future studies should be carried out to assess the impact of UV-C on pathogens which require a longer inactivation period.
Interestingly, the use of UV-C radiation as a supplement to cleaning with bleach did not improve the efficacy of disinfection either. This finding contradicts the majority of clinical before-and-after studies which have demonstrated a positive effect with a combined treatment strategy [[13], [14], [15], [16]]. Admittedly, in this investigation, the UV-C device was only used for one cycle, with an irradiation time of 65 seconds. Previous experimental studies have demonstrated that the duration of exposure is an important determinant for the performance of UV-C disinfection technology [25,28,33,40]. For instance, Moore et al. [28] observed that when irradiation exposure was increased from 3 minutes to 6 minutes, there was an additional 3 log10 reductions in viral DNA of Adenovirus. In another study, the killing efficacy of UV-C decreased from more than 4 log10 to 3 log10 for MRSA and from 5 log10 to 4 log10 on VRE with irradiation exposure of 20 minutes compared to 10 minutes [40]. Although the heterogeneity among studies due to the type of bacteria and quantification methods prevent direct comparisons to be made, it should still be acknowledged that a longer duration of exposure could have possibly resulted in a different outcome. In addition, the UV-C irradiation dose used in this study was based on the irradiance value specified by the manufacturer. As the UV-C light output deteriorates over time, the wavelength should ideally be tested to ensure that an accurate dose is used [41]. Thus, an undetected decline in the UV-C output may have also affected the outcome of this study.
It is noted that there are other potential factors which may have influenced the results of this study. As the efficacy of UV-C disinfection systems varies with the type of contaminated surface, the use of different materials may result in different outcomes [28]. The impact of other chlorine-based disinfectants, such as sodium dichloroisocyanurate, should also be considered [42].
There were several limitations in the quantification method which was used in the study. Firstly, a cotton-tipped swab was used to sample the surfaces. The recovery and release of bacteria from cotton swabs are often poor, with several studies observing a recovery rate of less than 25% [43]. The use of flocked swabs, which feature perpendicular nylon fibres are proven to have a better recovery rate, providing a more accurate reflection of the amount of microbial load on surfaces [44]. However, due to financial constraints of the project, flocked swabs were not used in this study.
In this study, the swabs were incubated in media for 18 hours prior to dilution plating. This is because with immediate plating of suspensions, no bacterial colonies were detected, even from surfaces which were contaminated but not subjected to any of the disinfection strategies (positive control). Due to the growth of bacteria introduced by the overnight incubation process, the number of viable bacteria recovered from surfaces is inflated. However, as reasonable differences were observed in the number of bacteria between the different study arms, it was assumed that this quantification method was suitable. Furthermore, methods were standardised and all samples were subjected to the same process, thus reducing the likelihood of systematic bias.
A neutraliser was not added to media because trace amounts of bleach that may have been transferred onto the cotton swab would have been diluted many folds and dissipated in media. However, there is a small possibility that the biocidal effect of bleach was overestimated. Lastly, as there was only a single investigator carrying out experiments, blinding was not feasible. Hence, this study is subject to sampling bias.
Finally, it is important to acknowledge that a statistically significant decrease in bioburden would not necessarily translate to impact clinically important outcomes. Currently, there seems to be no clear evidence regarding the acceptable levels of residual microbial load in order to prevent the transmission of infections [3]. Considering that chemical disinfectants and automated technology used in patient rooms are for the purpose of disinfection and not sterilisation, it must be accepted that some residual bioburden will always be present [6]. Benchmarks that correlate with decreases in pathogen acquisition should therefore be established to facilitate the interpretation of log reduction of pathogens when evaluating the efficacy of these devices.
Conclusion
The novelty of the present study is the demonstration of the in-vitro susceptibility of P. aeruginosa when treated with a combination of UV-C and bleach. No significant differences were observed in the number of recovered viable bacteria with the combined treatment strategy compared to the use of bleach alone. Further studies are required to elucidate the effectiveness of this technology on pathogens which require a longer contact time with the use of bleach, such as C. difficile. This study also highlights the need for a universal set of testing guidelines to be developed for the evaluation of UV-C disinfection systems.
Conflict of interest statement
None.
Funding source
This work was supported by the University of Edinburgh Medical School.
Credit author statement
Nur Shazlin Shek Daud: Conceptualization, Methodology, Investigation, Project Administration, Writing – Original Draft, Writing – Review and Editing.
Mark Dunn: Conceptualization, Methodology, Writing – Review and Editing, Supervision.
Olga Lucia Moncayo-Nieto: Conceptualization, Methodology, Writing – Review and Editing, Supervision.
Alasdair Hay: Conceptualization, Methodology, Writing – Review and Editing, Supervision.
Acknowledgements
We would like to thank Professor David Dockrell for the opportunity to be part of the Dockrell Lab Group and Jennifer Marshall for the guidance provided in carrying out laboratory experiments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.infpip.2023.100307.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Weber D.J., Anderson D., Rutala W.A. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis. 2013;26:338–344. doi: 10.1097/QCO.0b013e3283630f04. [DOI] [PubMed] [Google Scholar]
- 2.Han J.H., Sullivan N., Leas B.F., Pegues D.A., Kaczmarek J.L., Umscheid C.A. Cleaning hospital room surfaces to prevent health care-associated infections: A technical brief. Ann Intern Med. 2015;163:598–607. doi: 10.7326/M15-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carling P.C., Bartley J.M. Evaluating hygienic cleaning in health care settings: What you do not know can harm your patients. Am J Infect Control. 2010;38:S41–S50. doi: 10.1016/j.ajic.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Weber D.J., Rutala W.A., Miller M.B., Huslage K., Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38:S25–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- 5.Dancer S.J. Controlling hospital-acquired infection: Focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014;27:665–690. doi: 10.1128/CMR.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutala W.A., Weber D.J. 2008. Guideline for disinfection and sterilisation in healthcare facilities (2008)https://www.cdc.gov/infectioncontrol/guidelines/disinfection/ [Google Scholar]
- 7.Carling P. Methods for assessing the adequacy of practice and improving room disinfection. Am J Infect Control. 2013;41:S20–S25. doi: 10.1016/j.ajic.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Otter J.A., Yezli S., Perl T.M., Barbut F., French G.L. The role of “no-touch” automated room disinfection systems in infection prevention and control. J Hosp Infect. 2013;83:1–13. doi: 10.1016/j.jhin.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Boyce J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control. 2016;5:1–10. doi: 10.1186/s13756-016-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conner-Kerr T.A., Sullivan P.K., Gaillard J., Franklin M.E., Jones R.M. The effects of ultraviolet radiation on antibiotic-resistant bacteria in vitro. Ostomy/Wound Manag. 1998;44:50—56. [PubMed] [Google Scholar]
- 11.Beck S.E., Rodriguez R.A., Hawkins M.A., Hargy T.M., Larason T.C., Linden K.G. Comparison of UV-induced inactivation and RNA damage in MS2 phage across the germicidal UV spectrum. Appl Environ Microbiol. 2016;82:1468–1474. doi: 10.1128/AEM.02773-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutala W.A., Weber D.J. Monitoring and improving the effectiveness of surface cleaning and disinfection. Am J Infect Control. 2016;44:e69–e76. doi: 10.1016/j.ajic.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Haas J.P., Menz J., Dusza S., Montecalvo M.A. Implementation and impact of ultraviolet environmental disinfection in an acute care setting. Am J Infect Control. 2014;42:586–590. doi: 10.1016/j.ajic.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Miller R., Simmons S., Dale C., Stachowiak J., Stibich M. Utilization and impact of a pulsed-xenon ultraviolet room disinfection system and multidisciplinary care team on Clostridium difficile in a long-term acute care facility. Am J Infect Control. 2015;43:1350–1353. doi: 10.1016/j.ajic.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Vianna P.G., Dale C.R., Simmons S., Stibich M., Licitra C.M. Impact of pulsed xenon ultraviolet light on hospital-acquired infection rates in a community hospital. Am J Infect Control. 2016;44:299–303. doi: 10.1016/j.ajic.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Green C., Pamplin J.C., Chafin K.N., Murray C.K., Yun H.C. Pulsed-xenon ultraviolet light disinfection in a burn unit: Impact on environmental bioburden, multidrug-resistant organism acquisition and healthcare associated infections. Burns. 2017;43:388–396. doi: 10.1016/j.burns.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Anderson D.J., Chen L.F., Weber D.J., Moehring R.W., Lewis S.S., Triplett P.F., et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): a cluster-randomised, multicentre, crossover study. Lancet. 2017;389:805–814. doi: 10.1016/S0140-6736(16)31588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill Brothers Chemical Co . 1923. Product profile - sodium hypochlorite NaOCl.http://hillbrothers.com/pdf/Product-Profiles/Sodium-Hypochlorite-Stability.pdf [Google Scholar]
- 19.OxyChem . vol. 7. Occident Chem Corp; 2014. https://www.oxy.com/globalassets/documents/chemicals/products/other-essentials/sodium-chlorite-handbook.pdf (OxyChem Sodium hypochlorite handbook). [Google Scholar]
- 20.Moore L.S.P., Freeman R., Gilchrist M.J., Gharbi M., Brannigan E.T., Donaldson H., et al. Homogeneity of antimicrobial policy, yet heterogeneity of antimicrobial resistance: Antimicrobial non-susceptibility among 108717 clinical isolates from primary, secondary and tertiary care patients in London. J Antimicrob Chemother. 2014;69:3409–3422. doi: 10.1093/jac/dku307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lister P.D., Wolter D.J., Hanson N.D. Antibacterial-resistant Pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsch E.B., Tam V.H. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev Pharmacoecon Outcomes Res. 2010;10:441–451. doi: 10.1586/erp.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hancock R.E.W., Speert D.P. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and impact on treatment. Drug Resist Updates. 2000;3:247–255. doi: 10.1054/drup.2000.0152. [DOI] [PubMed] [Google Scholar]
- 24.WHO . WHO, Press; 2017. Prioritization of pathogens. [Google Scholar]
- 25.Rutala W.A., Gergen M.F., Weber D.J. Room Decontamination with UV Radiation. Infect Control Hosp Epidemiol. 2010;31:1025–1029. doi: 10.1086/656244. [DOI] [PubMed] [Google Scholar]
- 26.Nerandzic M.M., Cadnum J.L., Eckart K.E., Donskey C.J. Evaluation of a hand-held far-ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens. BMC Infect Dis. 2012;12 doi: 10.1186/1471-2334-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson D.J., Gergen M.F., Smathers E., Sexton D.J., Chen L.F., Weber D.J., et al. Decontamination of Targeted Pathogens from Patient Rooms Using an Automated Ultraviolet-C-Emitting Device. Infect Control Hosp Epidemiol. 2013;34:466–471. doi: 10.1086/670215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore G., Ali S., Cloutman-Green E.A., Bradley C.R., Wilkinson M.A.C., Hartley J.C., et al. Use of UV-C radiation to disinfect non-critical patient care items: A laboratory assessment of the Nanoclave Cabinet. BMC Infect Dis. 2012;12 doi: 10.1186/1471-2334-12-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lourenço C., Macdonald T.J., Gavriilidis A., Allan E., Macrobert A.J., Parkin I.P. Effects of bovine serum albumin on light activated antimicrobial surfaces. RSC Adv. 2018;8:34252–34258. doi: 10.1039/c8ra04361b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.PHE . Microbiology Services Food Water and Environmental Microbiology Standard Method About Public Health England. Vol. 22. 2013. Detection and enumeration of bacteria in swabs and other environmental samples; pp. 1–23. [Google Scholar]
- 31.Rutala W.A., Weber D.J. Selection of the Ideal Disinfectant. Infect Control Hosp Epidemiol. 2014;35:855–865. doi: 10.1086/676877. [DOI] [PubMed] [Google Scholar]
- 32.Rutala W.A., Weber D.J. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin Microbiol Rev. 1997;10:597–610. doi: 10.1128/cmr.10.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nerandzic M.M., Cadnum J.L., Pultz M., Donskey C.J. Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect Dis. 2010;10:197. doi: 10.1186/1471-2334-10-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeney C.P., Dancer S.J. Can hospital computers be disinfected using a hand-held UV light source? J Hosp Infect. 2009;72:92–94. doi: 10.1016/j.jhin.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Boyce J.M., Donskey C.J. Understanding ultraviolet light surface decontamination in hospital rooms: A primer. Infect Control Hosp Epidemiol. 2019;40:1030–1035. doi: 10.1017/ice.2019.161. [DOI] [PubMed] [Google Scholar]
- 36.Shih M., Marshall F.J., Rosen S. The bactericidal efficiency of sodium hypochlorite as an endodontic irrigant. Oral Surgery. Oral Med Oral Pathol. 1970;29:613–619. doi: 10.1016/0030-4220(70)90473-1. [DOI] [PubMed] [Google Scholar]
- 37.CloroxPro. Bleach Germicidal Disinfectants n.d. https://www.cloroxpro.com/products/clorox-healthcare/bleach-germicidal-disinfectants/(accessed January 3, 2020).
- 38.Babb J.R., Bradley C.R., Ayliffe G.A.J. Sporicidal activity of glutaraldehydes and hypochlorites and other factors influencing their selection for the treatment of medical equipment. J Hosp Infect. 1980;1:63–75. doi: 10.1016/0195-6701(80)90033-X. [DOI] [PubMed] [Google Scholar]
- 39.Gerding D.N., Muto C.A., Owens R.C., Jr. Measures to Control and Prevent Clostridium difficile Infection. Clin Infect Dis. 2008;46:S43–S49. doi: 10.1086/521861. [DOI] [PubMed] [Google Scholar]
- 40.Nerandzic M.M., Fisher C.W., Donskey C.J. Sorting through the wealth of options: Comparative evaluation of two ultraviolet disinfection systems. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grist S.M., Geldert A., Gopal A., Su A., Balch H.B., Herr A.E. Current Understanding of Ultraviolet-C Decontamination of N95 Filtering Facepiece Respirators. Appl Biosaf. 2021;26:90–102. doi: 10.1089/apb.20.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clasen T., Edmondson P. Sodium dichloroisocyanurate (NaDCC) tablets as an alternative to sodium hypochlorite for the routine treatment of drinking water at the household level. Int J Hyg Environ Health. 2006;209:173–181. doi: 10.1016/j.ijheh.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Moore G., Griffith C. Problems associated with traditional hygiene swabbing: The need for in-house standardization. J Appl Microbiol. 2007;103:1090–1103. doi: 10.1111/j.1365-2672.2007.03330.x. [DOI] [PubMed] [Google Scholar]
- 44.Hedin G., Rynbäck J., Loré B. New technique to take samples from environmental surfaces using flocked nylon swabs. J Hosp Infect. 2010;75:314–317. doi: 10.1016/j.jhin.2010.02.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.