Abstract
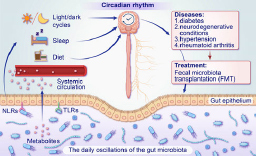
Circadian rhythms influence various aspects of the biology and physiology of the host, such as food intake and sleep/wake cycles. In recent years, an increasing amount of genetic and epidemiological data has shown that the light/dark cycle is the main cue that regulates circadian rhythms. Other factors, including sleep/wake cycles and food intake, have necessary effects on the composition and rhythms of the gut microbiota. Interestingly, the gut microbiota can affect the circadian rhythm of hosts in turn through contact-dependent and contact-independent mechanisms. Furthermore, the gut microbiota has been shown to regulate the sleep/wake cycles through gut-brain-microbiota interaction. In addition to diabetes, the gut microbiota can also intervene in the progression of neuro- degenerative diseases through the gut-brain-microbiota interaction, and also in other diseases such as hypertension and rheumatoid arthritis, where it is thought to have a spare therapeutic potential. Even though fecal microbiota transplantation has good potential for treating many diseases, the risk of spreading intestinal pathogens should not be ignored.
Keywords: circadian rhythm, the gut microbiota, metabolism, gut-brain-microbiota interaction, fecal microbiota transplantation
Introduction
Jeffrey Hall, Michael Rosbash, and Michael Young have been collectively honored with the 2017 Nobel Prize in Physiology or Medicine for their breakthrough discoveries regarding the regulation of the circadian rhythm. Michael Rosbash has put forward the theory that the internal clock plays a crucial role in influencing various aspects of our health, including the aging process, diabetes, and chronic diseases (Burki 2017). Earth’s rotation results in day/dark cycles, known as the circadian rhythm. Under conditions of continuous exposure to either light or darkness, circadian rhythms can maintain consistent oscillations over 24 hours. The suprachiasmatic nucleus (SCN) at the bottom of the hypothalamus receives external daytime information (light/dark cycles) through the retina and synchronizes this information (Hastings et al. 2020). The SCN directly controls the central biological clock, as well as the peripheral clock in the surrounding tissues (such as the gastrointestinal tract and liver) (Voigt et al. 2016; Astiz et al. 2019; Blume et al. 2019). Interestingly, feeding can impact peripheral clocks independent of the SCN and influence the expression of key clock genes (Patke et al. 2020; Taleb and Karpowicz 2022).
The majority of human microbiota exists in the gastrointestinal tract, accounting for 97% of the total, with the colon being the primary site, housing a rich diversity of Firmicutes and Bacteroidetes (Sender et al. 2016; Rinninella et al. 2019). The gut microbiota (GM) mediates a wide range of physiological functions, and any disruption in its composition can lead to the development of diseases. Furthermore, the metabolites produced by the GM can influence the endocrine and nervous systems through the gut-brain-microbiota (GBM) interaction, which explains the close association between GM and metabolic, cardiovascular diseases, and central nervous system diseases (Rahman et al. 2022). In this review, we have summarized several factors contributing to disruptions in host circadian rhythms, such as light/dark cycles, sleep patterns, and dietary impact on GM. Conversely, the GM can also regulate host circadian rhythms, including sleep/ wake cycles, through the GBM. Additionally, we have discussed the therapeutic potential of targeting GM in treating metabolic disorders, neurological diseases, hypertension, and rheumatoid arthritis. We provide the latest insights into these treatment strategies.
What is the molecular clock?
At the molecular level, circadian oscillations are generated by a complex network of genes called “clock genes”. Circadian locomotor output cycles kaput (CLOCK) and brain and muscle aryl hydrocarbon receptor nuclear translocator protein-1 (BMAL1, also known as ARNTL) serve as the central genes regulating the circadian rhythm in mammals. They are pivotal in orchestrating the biological processes that follow a 24-hour cycle. CLOCK and BMAL1 proteins come together to form a heterodimer, often called the “positive arm”, in the cytoplasm. This heterodimer then drives the transcription of clock-controlled genes, including period circadian regulators (PERs), cryptochrome circadian regulators (CRYs), reverse erythroblastosis virus (REV-ERBs), retinoic acid receptor-related orphan receptor (RORs), nuclear factor interleukin-3-regulated protein (NFIL3), and D-box binding protein (DBP). Significantly, PER and CRY proteins can assemble into dimers, which subsequently inhibit the transcriptional activity of the CLOCK/BMAL1 complex. This exemplifies the fundamental mechanism of negative feedback regulation within the biological clock gene network (Angelousi et al. 2019; Stanton et al. 2022). The network is shown in Fig. 1.
Fig. 1.
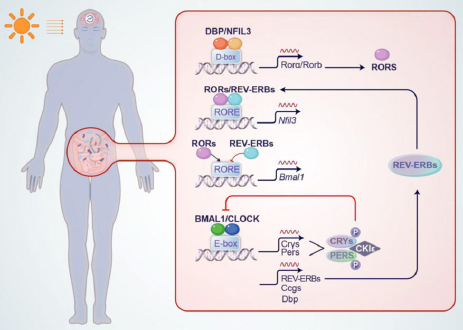
The network of circadian rhythms.
The circadian mechanism consists of a histone acetyltransferase activator composed of CLOCK/BMAL1 and E-box motifs (CACGTG) to regulate transcription genes, such as CRYs, PERs, DBP, REV-ERBs, and other clock-controlled genes (CCGs). Among them, CLOCK/BMAL1 positively regulates the expression of other circadian regulators like PERS and CRYs. In the presence of light during the daytime, PER and CRY proteins form dimers and bind to the CLOCK /BMAL1 complex located on the E-box, ultimately inhibiting transcription. In contrast, during the nighttime, the de-dimerization of PER and CRY proteins occurs, leading to the reduction of inhibition towards the Bmal1/ CLOCK factors. Casein Kinase 1 ε (CK1ε) is responsible for phosphorylating the PER and CRY proteins, which promotes their degradation. This is the first circadian transcription-translation feedback loop. RORs and REV-ERBs comprise the second circadian loop by competitively binding to the RORE element and regulating BMAL1 expression. RORs can activate BMAL1, while REV-ERB acts as a repressor of BMAL1 transcription. Additionally, DBP and NFIL3 form a heterodimer and bind to the D-box to activate the transcription of RORs.
The daily oscillations of the GM
The gut, primarily the colon, is home to the majority of the microbiome, hosting over 100 trillion bacteria from a diverse range of more than 1,000 species (Weger et al. 2019). The GM predominantly comprises Firmicutes and Bacteroidetes, followed by Proteobacteria, Actinobacteria, and Acidobacteria, which show interindividual variation (Rinninella et al. 2019). In both humans and mice, Firmicutes were predominant during the day, while Bacteroidetes were more prevalent at night (Liang et al. 2015; Reitmeier et al. 2020). Continuous exposure to light in mice leads to a reduction in the diversity of GM and alterations in the abundance of various taxa. Specifically, there is an observed increase in the abundance of Ruminococcus torques and a decrease in the abundance of Lactobacillus johnsonii (Deaver et al. 2018). However, even without exposure to the light/dark cycle, fluctuations in environmental factors such as nutrient accessibility, dietary habits, host-produced antimicrobial peptides, and autoantibodies can impact the microbiota (Wollmuth and Angert 2023)
In photosynthetic bacteria, such as cyanobacteria, a circadian clock mechanism has been specifically identified. The circadian rhythmicity is maintained by three central clock genes: kaiA, kaiB, and kaiC (Bhadra et al. 2017). Nevertheless, in non-photosynthetic bacteria, especially those significant for human health, there are only few examples describing the presence of circadian rhythm in bacteria (Table I). Fortunately, Diallo et al. (2022) made significant progress in the field of circadian rhythms in non-photosynthetic bacteria last year. They identified RadA as the homolog of KaiC in Escherichia coli by bioinformatics analysis. RadA expression exhibited a circadian pattern lasting at least 3 days, reaching its highest point in the morning. At the same time, the circadian oscillations of gene expression were absent in E. coli radA mutants (Diallo et al. 2022). The forthcoming research may unveil more GM with circadian rhythms and shed light on their underlying mechanisms. This could enable us to investigate whether the intrinsic circadian rhythms in GM hold potential as a therapeutic approach in metabolism.
Table I.
Evidences for bacterial circadian rhythms relevant to human health.
| Bacteria | Circadian components/time-dependent host response | References |
|---|---|---|
| Streptococcus pneumoniae | dependent on BMAL1 in Clara cells enhanced clearance at ZT12 | Gibbs et al. 2014 |
| Chlamydia trachomatis | enhanced clearance at ZT15 | Lundy et al. 2019 |
| Helicobacter pylori | enhanced lymphocyte migration to lymph nodes at ZT7 | Druzd et al. 2017 |
| Enterobacter aerogenes | swarming and motility rhythms | Paulose et al. 2016 |
| Pseudomonas putida | a putative kaiC homolog was found in the genome of Pseudomonas putida KT2440 by bioinformatics analyses | Soriano et al. 2010 |
| Escherichia coli | receptors for blue light | Gomelsky and Klug 2002 |
| Legionella pneumophila | kaiB and kaiC-encoding genes | Loza-Correa et al. 2010 |
| Salmonella Thyphimurium | enhanced clearance at ZT16 | Bellet et al. 2013 |
| Listeria monocytogenes | enhanced clearance at ZT8 dependent on BMAL1 in Ly6Chi monocytes | Nguyen et al. 2013 |
ZT – the time (hour) for receiving light
Two inseparable ropes: circadian rhythms and the GM
The complex interplay between the host’s GM and circadian rhythms is subject to various factors, and alterations in one component can significantly influence the other, ultimately impacting the host’s sleep, metabolism, and other related processes (Pearson et al. 2020). The gut-brain axis (GBA), a two-way link between the GM and the brain, functions through neural, immune, and endocrine pathways. In the past 15 years, our understanding of the traditional GBA has evolved into a systems biology perspective of the gut-brain-microbiota (GBM) interaction. The two main barriers to GBM are the intestinal barrier and the blood-brain barrier (BBB). Both barriers are dynamic, and factors including the GM, inflammatory signals, and stress all have the capability to modulate their permeability (Asadi et al. 2022; Mayer et al. 2022).
Impact of circadian rhythms on the GM
Here, our primary focus is exploring the impact of various internal and external factors, such as light/dark (LD) cycles, sleep patterns, and dietary habits, on GM.
Light/dark cycles
The LD cycles are the most crucial stimulus for regulating the internal clock of mammals, including humans. With the rapid pace of modern life, our lifestyles have undergone significant changes. Factors such as increased exposure to artificial light at night, disrupted eating habits, jet lag, and night shift work have become common. Unfortunately, these aspects can disrupt the natural LD cycle, impacting our circadian rhythms. Disrupted circadian rhythms have been associated with various adverse effects, such as metabolic dysregulation. (Sinturel et al. 2020; Lee et al. 2021).
In the study conducted by Zhen et al. (2023), the researchers explored the impact of different LD cycles (LD light hour/dark hour) on the interconnected rhythms of GM, hypothalamic, and hepatic clock genes, as well as immunity and metabolism. They employed multi-omics approaches to investigate four irregular LD cycles (LD0/24, LD24/0, LD8/16, LD16/8) along with a normal LD cycle (LD12/12). Their findings revealed that irregular LD cycles disrupted the rhythmicity of central clock genes while having minimal effects on the diurnal expression of peripheral clock genes in the liver, including Bmal1. Interestingly, certain GM species such as Limosilactobacillus, Actinomyces, Veillonella, Prevotella and Campylobacter were found to have the ability to regulate hepatic circadian rhythms even under irregular LD cycles. Furthermore, the study demonstrated that GM could influence immune and metabolic disorders caused by circadian dysregulation. These insights offer potential targets for developing probiotics specifically tailored for individuals with circadian disruption, such as shift workers (Zhen et al. 2023).
Constant darkness, an extreme alteration of the LD cycle, often brings to mind melatonin (MT), as it is primarily produced during the nighttime. MT is an “arm” of the biological clock, as it reacts to signals from the SCN. The rhythm of MT secretion provides insights into the state of the clock’s phase (i.e., the internal time of the clock relative to external time) and amplitude (Arendt 2019). Increasing evidence suggests that MT can influence the typical composition and quantity of the gut bacterial population, especially in various pathological states such as inflammatory bowel diseases. Following MT administration, the ratio of Firmicutes (such as Ruminococcaceae and Coprococcus), Bifidobacterium and Lactobacillus increased, Proteobacteria and Streptococcus spp. decreased (Kim et al. 2020; Jing et al. 2022). Additionally, MT possesses robust antioxidative properties, effectively eliminating reactive oxygen species. Its lipophilic nature allows it to readily interact with the brain and the GM through the BBB (Liu et al. 2023).
Sleep
Sleep problems such as jet lag, delayed bedtimes, and sleep fragmentation often occur in contemporary people, and these phenomena always result in circadian disruption. Sleep fragmentation is associated with increased mean blood pressure in mice and changes in the composition of the GM. The Bacteroi- detes ratio was decreased, while the Proteobacteria ratio was increased. Moreover, midsleep fragmentation was also characterized by lower alpha diversity (Maki et al. 2020), and the disturbance of the GM caused by short sleep is related to lower expression of HD5 (Shimizu et al. 2023). Recently, many studies have identified that disturbed sleep has the potential to impact the balance and stability of GM. Liu et al. (2020) conducted a study that simulated an irregular sleep/wake cycle in young adults representative of the contemporary population. They defined microbial taxa from their fecal samples by 16S rRNA gene amplicon sequencing, and they found that the functions of the microbes were enriched during the irregular sleep/wake cycle rather than the relative abundances of the microbes (Liu et al. 2020).
Diet
While the SCN serves as the central regulator of the circadian system, peripheral clocks in organs can become disconnected from SCN control due to external factors, such as food intake (Kolbe et al. 2019). The arrangement and makeup of the GM can adapt quickly to shifts in macronutrients within 24 to 48 hours, demonstrating a remarkable level of flexibility. However, these adaptations may only be temporary and last for shorter durations. On the other hand, more lasting changes to the GM composition may necessitate a more extended period of adherence to a specific dietary pattern (Romaní-Pérez et al. 2021). Animal-based diets increase the quantity of bacteria such as Alistipes spp. and Bilophila spp. because of bile tolerance. Diets with a high intake of fiber and carbohydrates increase the quantity of Bifidobacterium spp., Bacteroidetes and Akkermansia muciniphila. Diets high in fat can increase the propagation of Firmicutes and Proteobacteria (Schmalle and Lorentz 2020; Choi et al. 2021; Gutierrez Lopez et al. 2021). It was found that a high-fat diet can decrease the alpha diversity of the GM and lead to a decrease in the number of microbial species exhibiting diel oscillation patterns of relative abundance (Frazier et al. 2022). Although we hold the view that a high-fat diet is not favorable for overall health, it is worth noting that mice subjected to a high-fat diet but restricted to nighttime feeding during their active phase exhibit a noteworthy rise in the diversity of gut bacterial species displaying diel patterns of relative abundance, in contrast to mice on an unrestricted high-fat diet. This highlights the potential significance of feeding time and frequency on the composition of GM. Moreover, it is crucial to acknowledge that mistimed eating can have detrimental effects on metabolic well-being (Challet 2019; Wollmuth and Angert 2023).
The role of the GM in the regulation of circadian rhythms. Mechanisms of GM regulation of circadian rhythms
In the small and large intestines, the absence of GM in germ-free (GF) or antibiotic-treated mice can alter the expression of circadian genes or reduce the number of genes that display rhythmic expression. Schmalle and Lorentz (2020) showed that antibiotic-treated or GF mice exhibit reduced expression of Bmal1 and Cry1 and increased expression of Per1 and Per2 in the intestinal epithelium under normal LD cycles. In contrast, the absence of GM increases the number of genes with rhythmic expression in the liver. Moreover, many serum metabolites exhibit daily rhythms, and the presence or absence of GM can influence these rhythms. Several metabolites lose their rhythmicity in the serum of GF and antibiotic-treated mice.
There are two primary mechanisms by which the GM can influence host circadian rhythms: contact-dependent and contact-independent. Contact-dependent mechanisms involve direct interactions between gut bacteria and gastrointestinal cells, leading to the activation of pattern recognition receptors like NOD-like receptors (NLRs) and Toll-like receptors (TLRs) (Bishehsari et al. 2020). TLRs can detect bacterial metabolites and incorporate them into the rhythmic processes of c-Jun N-terminal kinase (JNK) and inhibitor of nuclear factor kappa-B kinase subunit beta (IKKβ). This integration helps inhibit PPARa-mediated activation of REV-ERBa (Liang and FitzGerald 2017). This signaling rhythm then triggers the periodic corticosterone production by intestinal epithelial cells (IECs) in the ileum. However, if no microbiota is present, the constitutive expression of PPARa causes the IEC clock to malfunction, leading to excessive production of corticosterone (hypercortisolism) (Henao-Mejia et al. 2013). Contactindependent mechanisms, on the other hand, rely on small molecular metabolites produced by the GM, such as bile acids and short-chain fatty acids (SCFAs), to act as mediators (Bishehsari et al. 2020). The metabolites of the GM influence the host’s circadian rhythm, such as lactate, SCFAs, and MT (Paulose and Cassone 2016). SCFAs, mainly acetate, propionate, and butyrate, should be noted (Leone et al. 2015; Parkar et al. 2019). Recently, Fawad et al. (2022) discovered that PER2::LUC enteroids exposed to SCFAs (such as acetate, isovaleric acid, propionate, butyrate) resulted in significant phase delays in the abundance of PER2::LUC compared to untreated controls in mouse enteroids. Their study demonstrated that SCFAs generated by host microbes directly alter host circadian rhythms ex vivo without involving other intestinal cell types, such as immune and vascular. Specific SCFAs induce distinct patterns of circadian entrainment in intestinal epithelial cells, affecting parameters like circadian rhythm amplitude and phase shift magnitude while maintaining a roughly 24-hour period. A similar phenomenon was observed in human enteroids, colonoids exposed to microbial metabolites basolaterally, and a human-transformed colonic cell line (Caco-2). Furthermore, SCFA alters the host clock through HDAC inhibition (Fawad et al. 2022).
The GM can influence circadian rhythms via the GBM
As previously discussed, circadian rhythm disruptions, such as LD cycle and dietary patterns, can significantly impact the GM. Disruptions in LD cycle can influence the sleep state to some extent (Sgro et al. 2022), and the GM possesses the ability to react to sleep/wake cycle via the GBM. The GM and their metabolites can impact the neurons of the enteric nervous system and interact with the afferent pathways of the vagal nerve, which in turn affects the neural circuits involved in sleep/wake regulation. Additionally, immune mediators derived from the gut can be transmitted to the brain through the bloodstream and afferent vagal pathways, influencing sleep. For instance, lipopolysaccharides (LPS) and SCFAs can modulate immune cell responses and interact with inflammatory homeostasis, triggering microglia activation that, in turn, affects sleep/wake regulation. Apart from LPS and SCFAs, the regulation of the sleep/wake cycle is also influenced by other metabolites, including serotonin (5-HT), orexin, and histamine. Their collective actions contribute to the overall orchestration of the sleep/wake cycle (Wang et al. 2022).
GM, a new frontier in human disease treatment
As we all know, unhealthy dietary practices could contribute to the development of metabolic disorders, and diet is the most critical determinant in shaping the configuration of the GM. One of the most prominent illustrations of an unhealthy eating pattern is diabetes. The presence of Akkermansia, Bifidobacterium, Roseburia, Bacteroides and Faecalibacterium showed a negative association with type 2 diabetes (T2D). On the other hand, the presence of Ruminococcus, Fusobacterium, and Blautia genera exhibited a positive association with T2D (Gurung et al. 2020). In addition, notable disparities in the composition of GM have been observed between individuals with prediabetes and those with diabetes (Wu et al. 2020).
In recent years, there has been a surge of interest in exploring the role of the GM, often referred to as the “forgotten organ”, in various diseases. This has led to many studies investigating the potential use of GM-based therapies for treating diabetes. As an illustration, fecal microbiota transplantation (FMT) is a technique that involves transferring healthy fecal microbes from a donor to the gastrointestinal tract of a patient in order to modify the GM and address the disease (Huda et al. 2021). Scientific reports indicate that FMT can improve plasma metabolic parameters, including enhanced peripheral and hepatic insulin sensitivity (Antushevich 2020). However, in a double-anonymized study involving 22 obese patients who received FMT capsules derived from a single lean donor, no significant changes in mean BMI were observed after 12 weeks.
Nonetheless, the study revealed a sustained decrease in taurocholic acid stool levels, and the patients’ bile acid profiles began to resemble those of the donor more closely (Allegretti et al. 2020). This indicates that the utilization of FMT alone is insufficient for the treatment of diabetes and the reduction of body weight in diabetic patients. Moreover, we are still in the early stages of understanding how GM can be harnessed to effectively prevent the onset and progression of diabetes (Iatcu et al. 2021).
FMT has shown promising results not only in treating diabetes but also in addressing cancer and psychiatric disorders (Antushevich 2020), and GBM can provide a plausible explanation for this treatment. In recent years, the investigation of the GBM has brought to light valuable insights into epilepsy and neurode-generative conditions, including Alzheimer’s disease (AD) and Parkinson’s disease (PD). For instance, it has been shown that FMT improves both motor and non-motor symptoms in patients with PD (Cheng et al. 2022). Moreover, exogenous MT can be utilized as an additional treatment to enhance cognition in patients with AD, potentially due to its ability to modulate the composition of GM (Chen et al. 2021). It is worth noting that these diseases are closely associated with circadian rhythms. The circadian clock genes in the brain, beyond being confined to the SCN, are involved in controlling a range of brain functions, including synaptic conduction of neurons (McMartin et al. 2021). This implies that the GBM facilitates the intricate bidirectional association between the GM and circadian rhythms. Overall, FMT is considered a safe therapeutic approach, although occasional short-term adverse reactions, such as diarrhea, may occur. However, some potential risks should be taken into account. The transmission of opportunistic pathogens or viruses through filtered or liquefied fecal matter directly or indirectly administered into the colon via capsules. The U.S. Food and Drug Administration (FDA) has reported two cases of infections caused by E. coli producing extended-spectrum beta-lactamase (ESBL), one of which resulted in death, and six cases of infections caused by E. coli producing Shiga toxin, one of which also resulted in death. These two cases were potentially transmitted through donor fecal matter, but the fecal matter was not screened for ESBL (Gupta et al. 2021; Park and Seo 2021). Therefore, prior to FMT treatment, it is crucial to handle these risk factors with caution, emphasizing the need for the development of comprehensive, advanced, and efficient screening methods.
Additionally, other diseases, such as 1) hypertension and 2) rheumatoid arthritis (RA), have also been shown to be closely associated with dysbiosis of the GM. 1) The GM can communicate through the enteric nervous system with the brain. Dysbiosis associated with intestinal epithelial barrier dysfunction can trigger systemic inflammation and activate mechanisms related to blood pressure regulation, such as the renin-angiotensin-aldosterone system, the autonomic nervous system, and the immune system (O’Donnell et al. 2023). This mechanism suggests that GM is likely a key factor involved in blood pressure regulation. Modulating the GM in hypertensive patients through appropriate means may enhance the effectiveness of antihypertensive medications. 2) Disease-modifying anti-rheumatic drugs, such as sulfasalazine, etanercept, and methotrexate, have been shown to modulate the GM and alleviate symptoms beneficially (Zhao et al. 2022). Furthermore, Zeng et al. (2021) successfully treated a case of refractory RA with FMT, providing strong evidence for the influential role of GM in immune system regulation.
Conclusions
In this review, we described that the circadian rhythm-GM interaction is a mutual feedback system where one participant’s actions elicit a reaction from the other. Given the significant associations between circadian variations in GM and the onset of physiology and diseases, it is possible that GM could serve as a promising diagnostic tool for monitoring disease progression in humans. We have summarized the mechanisms underlying the involvement of GM in certain metabolic and neurological disorders and discussed the advantages and challenges of treatment options, including FMT. A study based on drosophila models found that even with minimal changes in the rhythmicity of their gut microbiota after timed feeding, this is entirely different from the phenomenon observed in mammalian GM that follows a day/night cycle – moreover, timed feeding compromised the flies’ responses to stressors. These findings were somewhat surprising, yet there is compelling evidence that GM plays a significant role in tempering the response of the gut clock to fluctuations in the day/night cycle. As a result, it facilitates the harmonization of cir-cadian rhythms between the gut and the brain, ensuring their synchronization (Zhang et al. 2023). The conclusions we have discussed above also serve as a reminder that it is essential to highlight that there is a future need to address the challenge of effectively tracking the dynamic fluctuations in the GM and distinguishing between changes induced by dietary factors and those influenced by external circadian rhythms in order to understand their implications in disease better.
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Allegretti JR, Kassam Z, Mullish BH, Chiang A, Carrellas M, Hurtado J, Marchesi JR, McDonald JAK, Pechlivanis A, Barker GF, et al Effects of fecal microbiota transplantation with oral capsules in obese patients Clin Gastroenterol Hepatol 2020 NaNl8(4):855.:e2 doi: 10.1016/j.cgh.2019.07.006. . . . ; ( ): - . . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Angelousi A, Kassi E, Ansari-Nasiri N, Randeva H, Kaltsas G, Chrousos G Clock genes and cancer development in particular in endocrine tissues Endocr Relat Cancer 2019 NaN26(6):R305. doi: 10.1530/ERC-19-0094. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Antushevich H Fecal microbiota transplantation in disease therapy Clin Chim Acta 2020 NaN503:90. doi: 10.1016/j.cca.2019.12.010. . . . ; : –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Arendt J Melatonin: Countering chaotic time cues Front Endocrinol 2019 NaN10:391. doi: 10.3389/fendo.2019.00391. . . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi A, Shadab Mehr N, Mohamadi MH, Shokri F, Heidary M, Sadeghifard N, Khoshnood S Obesity and gut-microbiota-brain axis: A narrative review J Clin Lab Anal 2022 NaN36(5):e24420 doi: 10.1002/jcla.24420. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astiz M, Heyde I, Oster H Mechanisms of communication in the mammalian circadian timing system Int J Mol Sci 2019 NaN20(2):343. doi: 10.3390/ijms20020343. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, Edwards RA, Sahar S, Dandekar S, Baldi P, et al Circadian clock regulates the host response to Salmonella Proc Natl Acad Sci USA 2013 NaN110(24):9897. doi: 10.1073/pnas.1120636110. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra U, Thakkar N, Das P, Pal Bhadra M Evolution of circadian rhythms: From bacteria to human Sleep Med 2017 NaN35:49. doi: 10.1016/j.sleep.2017.04.008. . . . ; : –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Bishehsari F, Voigt RM, Keshavarzian A Circadian rhythms and the gut microbiota: From the metabolic syndrome to cancer Nat Rev Endocrinol 2020 NaN16(12):731. doi: 10.1038/s41574-020-00427-4. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume C, Garbazza C, Spitschan M Effects of light on human circadian rhythms, sleep and mood Somnologie 2019 NaN23(3):147. doi: 10.1007/s11818-019-00215-x. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki T Nobel Prize awarded for discoveries in circadian rhythm Lancet 2017 NaN390(10104):e25 doi: 10.1016/S0140-6736(17)32661-2. . . . ; ( ): . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Challet E The circadian regulation of food intake Nat Rev Endocrinol 2019 NaN15(7):393. doi: 10.1038/s41574-019-0210-x. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Chen Q, Wu J, Dong X, Yin H, Shi X, Su S, Che B, Li Y, Yang J Gut flora-targeted photobiomodulation therapy improves senile dementia in an Aß-induced Alzheimer’s disease animal model J Photochem Photobiol B 2021 NaN216:112152 doi: 10.1016/j.jphotobiol.2021.112152. . . . ; : . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Cheng WY, Ho YS, Chang RC Linking circadian rhythms to microbiome-gut-brain axis in aging-associated neurodegenerative diseases Ageing Res Rev 2022 NaN78:101620 doi: 10.1016/j.arr.2022.101620. . . . ; : . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Choi H, Rao MC, Chang EB Gut microbiota as a transducer of dietary cues to regulate host circadian rhythms and metabolism Nat Rev Gastroenterol Hepatol 2021 NaN18(10):679. doi: 10.1038/s41575-021-00452-2. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaver JA, Eum SY, Toborek M Circadian disruption changes gut microbiome taxa and functional gene composition Front Microbiol 2018 NaN9:737. doi: 10.3389/fmicb.2018.00737. . . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo AB, Mezouar S, Boumaza A, Fiammingo O, Coiffard B, Pontarotti P, Desnues B, Mege JL RadA,a key gene of the circadian rhythm of Escherichia coli Int J Mol Sci 2022 NaN23(11):6136. doi: 10.3390/ijms23116136. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzd D, Matveeva O, Ince L, Harrison U, He W, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, et al Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses Immunity 2017 NaN46(1):120. doi: 10.1016/j.immuni.2016.12.011. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawad JA, Luzader DH, Hanson GF, Moutinho TJ, Jr, McKinney CA, Mitchell PG, Brown-Steinke K, Kumar A, Park M, Lee S, et al Histone deacetylase inhibition by gut microbe-generated shortchain fatty acids entrains intestinal epithelial circadian rhythms Gastroenterology 2022 NaN163(5):1377.:e11 doi: 10.1053/j.gastro.2022.07.051. . . . ; ( ): –. . . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier K, Kambal A, Zale EA, Pierre JF, Hubert N, Miyoshi S, Miyoshi J, Ringus DL, Harris D, Yang K, et al High-fat diet disrupts REG3γ and gut microbial rhythms promoting metabolic dysfunction Cell Host Microbe 2022 NaN30(6):809.:e6 doi: 10.1016/j.chom.2022.03.030. . . . ; ( ): –. . . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Ince L, Matthews L, Mei J, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, et al An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action Nat Med 2014 NaN20(8):919. doi: 10.1038/nm.3599. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomelsky M, Klug G BLUF: A novel FAD-binding domain involved in sensory transduction in microorganisms Trends Biochem Sci 2002 NaN27(10):497. doi: 10.1016/S0968-0004(02)02181-3. . . . ; ( ): –. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Gupta S, Mullish BH, Allegretti JR Fecal microbiota transplantation: The evolving risk landscape Am J Gastroenterol 2021 NaN116(4):647. doi: 10.14309/ajg.0000000000001075. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N Role of gut microbiota in type 2 diabetes pathophysiology EBioMedicine 2020 NaN51:102590 doi: 10.1016/j.ebiom.2019.11.051. . . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez Lopez DE, Lashinger LM, Weinstock GM, Bray MS Circadian rhythms and the gut microbiome synchronize the host’s metabolic response to diet Cell Metab 2021 NaN33(5):873. doi: 10.1016/j.cmet.2021.03.015. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Hastings MH, Smyllie NJ, Patton AP Molecular-genetic manipulation of the suprachiasmatic nucleus circadian clock J Mol Biol 2020 NaN432(12):3639. doi: 10.1016/j.jmb.2020.01.019. . . . ; ( ): –. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J, Strowig T, Flavell RA Microbiota keep the intestinal clock ticking Cell 2013 NaN153(4):741. doi: 10.1016/j.cell.2013.04.043. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Huda MN, Kim M, Bennett BJ Modulating the microbiota as a therapeutic intervention for type 2 diabetes Front Endocrinol 2021 NaN12:632335 doi: 10.3389/fendo.2021.632335. . . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatcu CO, Steen A, Covasa M Gut microbiota and complications of type-2 diabetes Nutrients 2021 NaN14(1):166. doi: 10.3390/nu14010166. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing W, Zhu M, Wang F, Zhao X, Dong S, Xu Y, Wang S, Yang J, Wang K, Liu W Hyaluronic acid-melatonin nanoparticles improve the dysregulated intestinal barrier, microbiome and immune response in mice with dextran sodium sulfate-induced colitis J Biomed Nanotechnol 2022 NaN18(1):175. doi: 10.1166/jbn.2022.3232. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Kim SW, Kim S, Son M, Cheon JH, Park YS Melatonin controls microbiota in colitis by goblet cell differentiation and antimicrobial peptide production through Toll-like receptor 4 signalling Sci Rep 2020 NaN10(1):2232. doi: 10.1038/s41598-020-59314-7. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe I, Brehm N, Oster H Interplay of central and peripheral circadian clocks in energy metabolism regulation J Neuroendocrinol 2019 NaN31(5):e12659 doi: 10.1111/jne.12659. . . . ; ( ): . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Lee Y, Field JM, Sehgal A Circadian rhythms, disease and chronotherapy J Biol Rhythms 2021 NaN36(6):503. doi: 10.1177/07487304211044301. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, Pierre JF, Heneghan AF, Nadimpalli A, Hubert N, et al Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism Cell Host Microbe 2015 NaN17(5):681. doi: 10.1016/j.chom.2015.03.006. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Bushman FD, FitzGerald GA Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock Proc Natl Acad Sci USA 2015 NaN112(33):10479. doi: 10.1073/pnas.1501305112. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Fitz Gerald GA Timing the microbes: The circadian rhythm of the gut microbiome J Biol Rhythms 2017 NaN32(6):505. doi: 10.1177/0748730417729066. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Liu S, Cheng L, Liu Y, Zhan S, Wu Z, Zhang X Relationship between dietary polyphenols and gut microbiota: New clues to improve cognitive disorders, mood disorders and circadian rhythms Foods 2023 NaN12(6):1309. doi: 10.3390/foods12061309. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wei ZY, Chen J, Chen K, Mao X, Liu Q, Sun Y, Zhang Z, Zhang Y, Dan Z, et al Acute sleep-wake cycle shift results in community alteration of human gut microbiome mSphere 2020 NaN5(1):e00914–19 doi: 10.1128/mSphere.00914-19. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loza-Correa M, Gomez-Valero L, Buchrieser C Circadian clock proteins in prokaryotes: Hidden rhythms? Front Microbiol 2010 NaN1:130. doi: 10.3389/fmicb.2010.00130. . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy SR, Ahmad T, Simoneaux T, Benyeogor I, Robinson Y, George Z, Ellerson D, Kirlin W, Omosun T, Eko FO, et al Effect of time of day of infection on chlamydia infectivity and pathogenesis Sci Rep 2019 NaN9(1):11405 doi: 10.1038/s41598-019-47878-y. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki KA, Burke LA, Calik MW, Watanabe-Chailland M, Sweeney D, Romick-Rosendale LE, Green SJ, Fink AM Sleep fragmentation increases blood pressure and is associated with alterations in the gut microbiome and fecal metabolome in rats Physiol Genomics 2020 NaN52(7):280. doi: 10.1152/physiolgenomics.00039.2020. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Nance K, Chen S The gut-brain axis Annu Rev Med 2022 NaN73:439. doi: 10.1146/annurev-med-042320-014032. . . . ; : –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- McMartin L, Kiraly M, Heller HC, Madison DV, Ruby NF Disruption of circadian timing increases synaptic inhibition and reduces cholinergic responsiveness in the dentate gyrus Hippocampus 2021 NaN31(4):422. doi: 10.1002/hipo.23301. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A Circadian gene Bmallregulates diurnal oscillations of Ly6Chi inflammatory monocytes Science 2013 NaN341(6153):1483. doi: 10.1126/science.1240636. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell JA, Zheng T, Meric G, Marques FZ The gut microbiome and hypertension Nat Rev Nephrol 2023 NaN19(3):153. doi: 10.1038/s41581-022-00654-0. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Park SY, Seo GS Fecal microbiota transplantation: Is it safe? Clin Endosc 2021 NaN54(2):157. doi: 10.5946/ce.2021.072. . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkar SG, Kalsbeek A, Cheeseman JF Potential role for the gut microbiota in modulating host circadian rhythms and metabolic health Microorganisms 2019 NaN7(2):41. doi: 10.3390/microorganisms7020041. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patke A, Young MW, Axelrod S Molecular mechanisms and physiological importance of circadian rhythms Nat Rev Mol Cell Biol 2020 NaN21(2):67. doi: 10.1038/s41580-019-0179-2. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Paulose JK, Cassone VM The melatonin-sensitive circadian clock of the enteric bacterium Enterobacter aerogenes Gut Microbes 2016 NaN7(5):424. doi: 10.1080/19490976.2016.1208892. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulose JK, Wright JM, Patel AG, Cassone VM Human gut bacteria are sensitive to melatonin and express endogenous circadian rhythmicity PLoS One 2016 NaN11(1):e0146643 doi: 10.1371/journal.pone.0146643. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JA, Wong FS, Wen L Crosstalk between circadian rhythms and the microbiota Immunology 2020 NaN161(4):278. doi: 10.1111/imm.13278. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Islam F, -Or-Rashid MH, Mamun AA, Rahaman MS, Islam MM, Meem AFK, Sutradhar PR, Mitra S, Mimi AA, et al The gut microbiota (microbiome) in cardiovascular disease and its therapeutic regulation Front Cell Infect Microbiol 2022 NaN 20;12:903570 doi: 10.3389/fcimb.2022.903570. . . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitmeier S, Kiessling S, Clavel T, List M, Almeida EL, Ghosh TS, Neuhaus K, Grallert H, Linseisen J, Skurk T, et al Arrhythmic gut microbiome signatures predict risk of type 2 diabetes Cell Host Microbe 2020 NaN28(2):258.:e6 doi: 10.1016/j.chom.2020.06.004. . . . ; ( ): –. . . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases Microorganisms 2019 NaN7(1):14. doi: 10.3390/microorganisms7010014. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaní-Pérez M, Bullich-Vilarrubias C, López-Almela I, Liébana-García R, Olivares M, Sanz Y The microbiota and the gut-brain axis in controlling food intake and energy homeostasis Int J Mol Sci 2021 NaN22(11):5830. doi: 10.3390/ijms22115830. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalle V, Lorentz A Role of the microbiota in circadian rhythms of the host Chronobiol Int 2020 NaN37(3):301. doi: 10.1080/07420528.2020.1726374. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R Revised estimates for the number of human and bacteria cells in the body PLoS Biol 2016 NaN14(8):e1002533 doi: 10.1371/journal.pbio.1002533. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgro M, Kodila ZN, Brady RD, Reichelt AC, Mychaisuk R, Yamakawa GR Synchronizing our clocks as we age: the influence of the brain-gut-immune axis on the sleep-wake cycle across the lifespan Sleep 2022 NaN45(3):zsab268 doi: 10.1093/sleep/zsab268. . . . ; ( ): . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Yamamura R, Yokoi Y, Ayabe T, Ukawa S, Nakamura K, Okada E, Imae A, Nakagawa T, Tamakoshi A, et al Shorter sleep time relates to lower human defensin 5 secretion and compositional disturbance of the intestinal microbiota accompanied by decreased short-chain fatty acid production Gut Microbes 2023 NaN15(1):2190306 doi: 10.1080/19490976.2023.2190306. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinturel F, Petrenko V, Dibner C Circadian clocks make metabolism run J Mol Biol 2020 NaN432(12):3680. doi: 10.1016/j.jmb.2020.01.018. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Soriano MI, Roibás B, García AB, Espinosa-Urgel M Evidence of circadian rhythms in non-photosynthetic bacteria? J Circadian Rhythms 2010 NaN8:8. doi: 10.1186/1740-3391-8-8. . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton D, Justin HS, Reitzel AM Step in time: Conservation of circadian clock genes in animal evolution Integr Comp Biol 2022 NaN62(6):1503. doi: 10.1093/icb/icac140. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Taleb Z, Karpowicz P Circadian regulation of digestive and metabolic tissues Am J Physiol Cell Physiol 2022 NaN323(2):C306. doi: 10.1152/ajpcell.00166.2022. . . . ; ( ): –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Voigt RM, Forsyth CB, Green SJ, Engen PA, Keshavarzian A Circadian rhythm and the gut microbiome Int Rev Neurobiol 2016;131:193. doi: 10.1016/bs.irn.2016.07.002. . . . ; : –. . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang Z, Lu T, Chen W, Yan W, Yuan K, Shi L, Liu X, Zhou X, Shi J, et al The microbiota-gut-brain axis in sleep disorders Sleep Med Rev 2022 NaN65:101691 doi: 10.1016/j.smrv.2022.101691. . . . ; : . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Weger BD, Gobet C, Yeung J, Martin E, Jimenez S, Betrisey B, Foata F, Berger B, Balvay A, Foussier A, et al The mouse microbiome is required for sex-specific diurnal rhythms of gene expression and metabolism Cell Metab 2019 NaN29(2):362.:e8 doi: 10.1016/j.cmet.2018.09.023. . . . ; ( ): –. . . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth EM, Angert ER Microbial circadian clocks: Hostmicrobe interplay in diel cycles BMC Microbiol 2023 NaN23(1):124. doi: 10.1186/s12866-023-02839-4. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Tremaroli V, Schmidt C, Lundqvist A, Olsson LM, Krämer M, Gummesson A, Perkins R, Bergström G, Bäckhed F The gut microbiota in prediabetes and diabetes: A population-based crosssectional study Cell Metab 2020 NaN32(3):379.:e3 doi: 10.1016/j.cmet.2020.06.011. . . . ; ( ): –. . . https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Zeng J, Peng L, Zheng W, Huang F, Zhang N, Wu D, Yang Y Fecal microbiota transplantation for rheumatoid arthritis: A case report Clin Case Rep 2020 NaN9(2):906. doi: 10.1002/ccr3.3677. . . . ; ( ): –. . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li Y, Barber AF, Noya SB, Williams JA, Li F, Daniel SG, Bittinger K, Fang J, Sehgal A The microbiome stabilizes circadian rhythms in the gut Proc Natl Acad Sci USA 2023 NaN120(5):e2217532120 doi: 10.1073/pnas.2217532120. . . . ; ( ): . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Wei Y, Zhu Y, Xie Z, Hai Q, Li Z, Qin D Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities Front Immunol 2022 NaN13:1007165 doi: 10.3389/fimmu.2022.1007165. . . . ; : . https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Wang Y, He F, Chen Y, Hu L, Ge L, Wang Y, Wei W, Rahmat A, Loor JJ, et al Homeostatic crosstalk among gut microbiome, hypothalamic and hepatic circadian clock oscillations, immunity and metabolism in response to different light-dark cycles: A multiomics study J Pineal Res 2023 NaN75(2):e12892 doi: 10.1111/jpi.12892. . . . ; ( ): . https://doi.org/ [DOI] [PubMed] [Google Scholar]


