Abstract
Many strains of Helicobacter pylori are naturally competent for transformation in vitro. Since there is a high degree of genetic variation among H. pylori strains, we sought to determine whether mechanisms of DNA exchange other than transformation exist in these organisms. Studies were done with H. pylori cells that each were resistant to two different antibiotics; the procedure used involved mating of cells on plates or in broth, in the absence or presence of DNase. In each experiment, such matings produced progeny with the markers of both parents. Examination of the full resistance profile and random arbitrarily primed DNA PCR (RAPD-PCR) profiles of the progeny indicated that DNA transfer was bidirectional. DNase treatment reduced but did not eliminate transfer; only the presence of both DNase and a membrane separating the cells did so. For progeny derived from matings in the presence of DNase, antibiotic resistance and RAPD profiles indicated that transfer was unidirectional. DNase-treated cell-free supernatants also did not transform, ruling out transduction. These experiments indicate that both a DNase-sensitive mechanism (transformation) and a DNase-resistant conjugation-like mechanism involving cell-to-cell contact may contribute to DNA transfer between H. pylori cells.
Helicobacter pylori, a gram-negative bacterium that colonizes the human stomach, has been recognized as the causative agent of chronic gastritis and as playing a role in peptic ulcer disease and gastric cancer (5). H. pylori strains are highly diverse at the genetic level (1, 4, 20), including a high rate of point mutations in conserved genes such as ureB (14) and flaA (29), mosaicism within genes such as vacA (3), and the presence of nonconserved DNA fragments, in particular the cag pathogenicity island (2, 7, 8, 31). Other factors that lead to further diversity among H. pylori strains are the presence of insertion sequences (7, 12) and plasmids (16) and variability of gene order (13). The genetic diversity of H. pylori has clinical significance, since markers of strains with enhanced virulence have been identified (3, 8, 22, 31). Epidemiological studies have used techniques like restriction fragment length polymorphism and randomly amplified polymorphic DNA PCR (RAPD-PCR) to exploit the heterogeneity for studies on transmission and intrafamilial clustering of strains (1, 25, 32).
Many H. pylori strains are known to be naturally competent for transformation in vitro (21, 28, 30, 31, 34), but the mechanisms for genetic exchange among H. pylori strains are not well understood. Horizontal DNA transfer within the reservoir for H. pylori, i.e., the stomach of primates (in particular, humans), would contribute to the development of genetic diversity (17), and the mosaicism of vacA, for example (3), would be consistent with its occurrence in vivo. Recognition of the mechanisms involved in genetic exchange helps us understand the adaptation of H. pylori to changing environments and may shed light on the clinically important development of antibiotic resistance (24, 33). We therefore sought to determine whether we could define conditions in which H. pylori strains exchange DNA, calculate the frequency of the events, and begin to characterize mechanisms involved.
MATERIALS AND METHODS
Bacterial strains.
The H. pylori strains used in this study are listed in Table 1. Kanamycin resistance (Kanr) was obtained in strains 84-183 and HPK1 by natural transformation with plasmid pMK180 (15), which carries a Kanr cassette (aph1) within H. pylori ureB, allowing its incorporation in the bacterial chromosomal DNA. The integration of aph1 into the ureB locus was confirmed by PCR for each of these strains. In addition, the strains were shown to have a urease-negative phenotype. HPK5 was rendered Kanr by electroporation with pHP1, a hybrid H. pylori-E. coli plasmid, which carries a Kanr cassette (aph1) (15a), and selection on Brucella agar (BBL Microbiology Systems, Cockeysville, Md.) containing 10% newborn calf serum (Intergen, New York, N.Y.) and 25 μg of kanamycin per ml. Plasmid preparations confirmed that these strains did not contain any plasmids other than pHP1 and one uncharacterized plasmid (30) in HPK5. We selected strains that were streptomycin, spectinomycin, or rifampin resistant (Strepr, Specr, or Rifr) by plating large numbers (approximately 1010) of bacteria on Brucella or Trypticase soy agar (TSA) medium containing streptomycin (10 μg/ml), spectinomycin (10 μg/ml), or rifampin (0.5 μg/ml), respectively. Analysis of the presence of vacA, iceA, and cagA genetic markers was done by PCR using appropriate primers as previously described (3, 22, 31).
TABLE 1.
H. pylori strains used in this study
| Strain | Genotype
|
Phenotype
|
||||
|---|---|---|---|---|---|---|
| cagA | vacA | iceA | IS605 | Strepr Kanr | Specr Rifr | |
| Wild types (origin) | ||||||
| 84-183 (United States) | + | s1b/m1 | 2 | + | − | − |
| HPK1 (Japan) | − | s1a/m1 | 1 | + | − | − |
| HPK5 (Japan) | + | s1a/m1 | 1 | + | − | − |
| Mutants (description) | ||||||
| 84-183 (Strepr KanrureB::Kanr) | + | − | ||||
| 84-183 (Specr Rifr) | − | + | ||||
| HPK1 (Strepr KanrureB::Kanr) | + | − | ||||
| HPK1 (Specr Rifr) | − | + | ||||
| HPK5 (Strepr pHP1 Kanr) | + | − | ||||
| HPK5 (Specr Rifr) | − | + | ||||
Media and growth conditions.
Bacterial cells were grown in 5% CO2 at 37°C on solid or in liquid media. The solid media consisted of TSA–5% sheep blood plates (BBL) and Brucella-serum (BS) plates, prepared with Brucella agar (BBL) and 10% newborn calf serum. The liquid medium consisted of brucella broth (BB) with 10% newborn calf serum.
Electroporation.
Bacterial cells grown on five TSA plates for 24 h were harvested into 40 ml of sterile water and then centrifuged twice for 15 min each time at 5,000 × g, and the supernatant was discarded. The cells were resuspended in 14 ml of electroporation buffer (15% glycerol, 10% sucrose), centrifuged for 15 min at 5,000 × g, resuspended in 1 ml of electroporation buffer, and centrifuged at 7,000 × g for 10 min. After the cells were resuspended in 300 μl of electroporation buffer, 50 and 1 μl of H2O containing 2.5 μg of pHP1 were transferred to electroporation cuvettes (0.1-cm gap) on ice. Electroporation was done at 1.8 kV and 200 and 25 μF; then 800 μl of cold BB was added, and cells were placed on ice (34). The cells were inoculated to TSA plates, incubated overnight at 37°C in 5% CO2, then transferred to BS plates containing kanamycin, and incubated for 4 days for growth of transformants.
DNA exchange on solid medium.
Each mating experiment involved two strains (here represented as A and B) with mutually exclusive antibiotic resistance markers. After 48 h of growth on two TSA plates each, cells of each strain were harvested and suspended in 1 ml of 0.9% saline. The cells were centrifuged for 5 min at 8,500 × g, the supernatant was discarded, and the cells were resuspended in 250 μl of 0.9% saline and then spotted in 25-μl aliquots on three TSA plates in the following order: plate 1, separate single spots of strain A and strain B; plates 2 and 3, three spots of strain A and strain B together. On plate 3, 20 μl of DNase I (1 mg/ml; 3,500 U/mg; Amersham Life Sciences) dissolved in 1 ml of 20 mM Tris-Cl (pH 7.5)–1 mM MgCl2 was added to each of the three spots to reach a final DNase I concentration of 285 μg/ml. The DNase I was added to the aliquot of strain A before the addition of strain B and was equally distributed. After the plates were incubated overnight (37°C, 5% CO2), the bacteria were harvested, and each spot was suspended in 1 ml of 0.9% saline in a separate tube. The suspensions from plate 1 were serially diluted, and 100 μl of 10−5, 10−6, and 10−7 dilutions was inoculated on TSA plates without antibiotics; 250 μl of the undiluted sample was plated on BS plates containing 20 μg of both streptomycin and spectinomycin per ml (BSSS plates) to assess for spontaneous mutants. Undiluted suspensions of each individual spot of plates 2 and 3 were inoculated as follows: 250 μl on a TSA plate without antibiotics and 250, 100, and 25 μl on three BSSS plates. All plates were incubated for 72 h, after which colonies were counted. Single colonies on the BSSS plates were subcultured to a TSA plate without antibiotics and two BS plates containing either 25 μg of kanamycin per ml (BSK) or 0.5 μg of rifampin per ml (BSR).
DNA exchange using cell culture supernatants.
Experiments using supernatants as a DNA source were done as described above, with the following modifications. After the harvested cells of strain A or B were centrifuged, the supernatants were passaged through a filter with a 0.2-μm pore size (Life Sciences Products, Inc.) and then centrifuged for an additional 3 min at 11,500 × g. As an alternative method for filtration, the supernatants of strain A or B were mixed with 50 μl of chloroform and then centrifuged at 11,500 × g for 3 min. The two methods were found to be equally effective in clearing any remaining cells from the supernatant, and the results obtained were the same. Aliquots (25 μl) of strain A were spotted on TSA plates together with 25 μl of the treated supernatants of strain B, and vice versa. These experiments were done in the presence and absence of DNase I, as described above.
DNA exchange in liquid medium.
Experiments in broth were carried out as on plates, with the following modifications. The strains were harvested after 48 h of growth on four TSA plates each and suspended in 1.5 ml of BB; 250-μl aliquots of these suspensions were added to wells of a 24-well tissue culture plate (Costar, Cambridge, Mass.) in the following order. The first two wells contained strains A and B separately. Wells 3 to 6 contained both strains together. Well 3 contained both strains without further addition. Well 4 also contained 160 μl of the 1-mg/ml DNase I solution, reaching a final concentration of 285 μg/ml. Wells 5 and 6 contained both strains, but separated by Transwell7 6.5-mm tissue culture inserts with a 0.1-μm-pore-size polycarbonate membrane (Costar). In well 5, there was no further addition. In well 6, 80 μl of DNase I was added to each side of the membrane. BB then was added to all wells to obtain a final volume of 700 μl per well, and the plates were incubated overnight at 37°C in 5% CO2. The bacteria then were harvested, spun for 5 min at 8,500 × g, and resuspended in 0.9% saline; the membrane-separated samples of wells 5 and 6 were handled separately. Cells from each of the suspensions then were inoculated to nonselective and selective plates exactly as described above.
RAPD-PCR.
Chromosomal DNA was prepared from bacteria harvested after 48 h of growth on TSA plates as described previously (35). DNA concentrations were measured by fluorometry (Dynaquant 200; Hoefer), and 100 ng was used as the template for RAPD-PCR. Primers 9355 and d11344 were used, and results were analyzed by gel electrophoresis as described previously (1).
RESULTS
Genetic exchange occurs between strains of H. pylori on plates.
In initial experiments, three H. pylori strains [Table 1; HPK1 (Specr Rifr), HPK5 (Strepr pHP1 Kanr), and 84-183 (Strepr Kanr)] with the indicated antibiotic resistance markers were grown overnight both separately and paired together on TSA plates in three different combinations, and then passed to BSSS plates. Doubly resistant progeny were observed on every occasion when strains were grown together but were never observed when the strains were grown separately (data not shown), excluding the possibility that our observations were the result of spontaneous mutation.
Effect of DNase I on genetic exchange.
As before, HPK1 (Specr Rifr) and HPK5 (Strepr pHP1 Kanr) cells grown separately did not yield any doubly resistant progeny (Table 2). When the two strains were grown together, the number of doubly resistant CFU was 112 ± 22 (mean ± standard error of the mean [SEM])/107 bacterial pairs as determined from three replicate experiments (Table 2). Addition of DNase I decreased this number by 89% to 12 ± 5 CFU/107 bacterial pairs. However, in none of the experiments was the presence of DNase I able to prevent the development of doubly resistant mutants. Similar results were obtained for other combinations as well (Table 3). In the presence of DNase I, doubly resistant progeny were obtained after the mating of each of the combinations of two different strains and also when isolates of the same strain that were differently marked were grown together (Table 3). The relative percentage of doubly resistant mutants observed from matings in the presence versus the absence of DNase I varied only slightly for the different combinations (between 10 and 13%). Thus, both DNase-sensitive and -resistant events were observed, and these occurred at stable frequencies under the experimental conditions used.
TABLE 2.
Effect of DNase I treatment on the frequency of recombination between two H. pylori strains in plate matings
| Mating conditions
|
Specr Strepr CFUb
|
Relative %c | ||
|---|---|---|---|---|
| Strain(s)a | Treatment with DNase (285 μg/ml) | No. | No./107 bacterial pairs | |
| A alone | − | 0 | 0 | 0 |
| B alone | − | 0 | 0 | 0 |
| A + B | − | 941 ± 307 | 112 ± 22 | 100 |
| A + B | + | 101 ± 69 | 12 ± 5 | 11d |
Strain A = HPK1 (Specr Rifr); strain B = HPK5 (Strepr pHP1 Kanr).
Mean ± SEM from three replicate experiments.
Relative to A + B, no DNase.
P < 0.01 in comparison with A + B without DNase (Mann-Whitney test).
TABLE 3.
Variation in the second H. pylori strain has little effect on the frequency of recombinants after plate matings with HPK1 (Specr Rifr)
| Second strain | Specr Strepr CFU/107 bacterial pairsa
|
Relative %b | |
|---|---|---|---|
| DNase I absent | DNase I present | ||
| HPK1 (Strepr Kanr) | 169 ± 61 | 22 ± 14 | 13 |
| HPK5 (Strepr pHP1 Kanr) | 112 ± 22 | 12 ± 5 | 11 |
| 84-183 (Strepr Kanr) | 110 ± 24 | 11 ± 6 | 10 |
Mean ± SEM from two to four replicate experiments.
Comparison of results in presence versus absence of DNase I (285 μg/ml).
Effect of DNase I on natural transformation with chromosomal DNA.
Addition of 2 μg of chromosomal H. pylori DNA from strain 60190 bearing a chloramphenicol resistance marker to 108 HPK1 or HPK5 bacteria on TSA plates resulted in 199 ± 62 (mean ± SEM of 16 experiments) chloramphenicol-resistant colonies. However, in parallel experiments, addition of 285 μg of DNase I per ml prevented development of chloramphenicol-resistant colonies. Thus, the presence of progeny after DNase treatments in the cell mating studies was not due to low DNase activity but suggested that the DNase resistance might be due to cell-cell contact.
Genetic exchange in broth and effect of DNase I.
To examine genetic exchange in broth, cells of HPK1 (Specr Rifr) and HPK5 (Strepr pHP1 Kanr) were incubated together overnight in wells containing BB in the presence or absence of a 0.1-μm-pore-size membrane that prohibited cell-to-cell contact. In the absence of membrane separation and DNase I, the frequency of Specr Strepr recombinants was 22 ± 4/107 bacterial pairs (Table 4). Membrane separation of the strains reduced the frequency of recombinants to 70% of the value without the membrane. The addition of DNase I to strains not separated by a membrane reduced the frequency to 6%, similar to that observed in the plate matings, but was not able to completely prevent the development of doubly resistant progeny. However, the addition of DNase I to membrane-separated strains abolished the development of doubly resistant progeny (Table 4).
TABLE 4.
Frequency of recombinants after H. pylori matings in 24-well tissue culture plates
| Strain(s)a | Mating conditions
|
Specr Strepr CFUb
|
Relative %c | ||
|---|---|---|---|---|---|
| Membrane (0.1-μm pore size) | Treatment with DNase (285 μg/ml) | No. | No./107 bacterial pairs | ||
| A alone | − | − | 0 | 0 | 0 |
| B alone | − | − | 0 | 0 | 0 |
| A + B | − | − | 102 ± 13 | 22 ± 4 | 100 |
| A + B | − | + | 5.8 ± 2.9 | 1.3 ± 0.4 | 6d |
| A + B | + | − | 68 ± 4 | 16 ± 5 | 70e |
| A + B | + | + | 0 | 0 | 0 |
Strain A = HPK1 (Specr Rifr); strain B = HPK5 (Strepr pHP1 Kanr).
Mean ± SEM from three replicate experiments.
Relative to A + B, no membrane, no DNase.
P < 0.05 in comparison with A + B, no membrane or DNase (Mann-Whitney test).
P > 0.05 in comparison with A + B, no membrane or DNase (Mann-Whitney test).
Genetic exchange using culture supernatants and cells.
We next examined whether the DNase-resistant transfer might be due to transducing bacteriophages. Addition of 25 μl of cell-free culture supernatant of either HPK1 (Specr Rifr) or HPK5 (Strepr pHP1 Kanr) transformed the reciprocal strain at a frequency of 67 ± 24 or 10 ± 2 per 107 bacteria, respectively (Table 5). However, for both combinations, the addition of DNase I totally abolished the development of doubly resistant mutants, supporting the concept that bacteriophages are not responsible for the observed DNA transfer process, at least for the strains studied. To confirm that the DNase-resistant recombination was not due to protein-coated DNA or DNA present in membrane blebs, additional experiments were performed with supernatants which were neither filtered nor chloroform treated. The results were the same as those observed with filtered and chloroform-treated supernatants. To exclude the possibility that the results were due to a phage induced by the coculturing of strains, additional experiments were performed with supernatants from cocultures of strains A and B. The results were again the same (data not shown).
TABLE 5.
Effect of DNase I treatment on the frequency of recombinants in which either H. pylori cells or culture supernatants were used as exogenous DNA source
| HPK1 (Specr Rifr) | HPK5 (Strepr pHP1 Kanr) | Treatment with DNase I (285 μg/ml) | Specr/Strepr CFU/107 bacterial pairsa | Relative %b |
|---|---|---|---|---|
| Cellsc | —e | − | 0 | 0 |
| Supernatantd | — | − | 0 | 0 |
| — | Cells | − | 0 | 0 |
| — | Supernatant | − | 0 | 0 |
| Cells | Cells | − | 112 ± 22 | 100 |
| Cells | Cells | + | 12 ± 5 | 11f |
| Supernatant | Cells | − | 67 ± 24 | 60g |
| Supernatant | Cells | + | 0 | 0 |
| Cells | Supernatant | − | 10 ± 2 | 9f |
| Cells | Supernatant | + | 0 | 0 |
Mean ± SEM of three replicate experiments.
In comparison to cell-cell mating in the absence of DNase I.
H. pylori cells from 48-h cultures on two TSA plates were resuspended in 250 μl of 0.9% saline, and 25 μl of the suspension was used as the source of cells.
H. pylori cells from 48-h cultures on two TSA plates were suspended in 1 ml of 0.9% saline. The suspension was centrifuged at 8,500 × g for 5 min. The supernatant was saved and either passed through a 0.2-μm-pore-size filter or treated with 5% CHCl3.
—, no exogenous DNA source.
P < 0.01 compared to cell-cell mating in the absence of DNase (Mann-Whitney test).
P > 0.05 compared to cell-cell mating in the absence of DNase (Mann-Whitney test).
Directional transfer analysis, as assessed by additional antibiotic resistance marker selection.
Finally, we sought to assess the direction of DNA transfer between the parental strains to yield particular progeny. The Specr Strepr progeny from the experiments mating HPK1 (Specr Rifr) with either HPK5 (Strepr pHP1 Kanr) or 84-183 (Strepr Kanr) were examined by transfer to BSK or BSR plates to determine the directionality of DNA transfer. In the absence of DNase, whether the matings were done on plates or in broth, 55% (89 of 160 colonies) of the resultant Specr Strepr progeny grew on BSK and the remaining 45% grew on BSR; none of the progeny grew on both. These data are consistent with bidirectional DNA transfer between the two parental strains. In contrast, for matings conducted in the presence of DNase I, the Specr Strepr progeny were invariably (160 of 160 colonies) resistant to rifampin and sensitive to kanamycin (Kans), consistent with unidirectional DNA transfer from either HPK5 (Strepr pHP1 Kanr) or 84-183 (Strepr Kanr) to HPK1 (Specr Rifr). When HPK1 (Specr Rifr) was mated with HPK1 (Strepr pHP1 Kanr), in the absence of DNase I, 26% (21 of 80) of the Specr Strepr colonies were Rifr and Kans, whereas the remaining 74% were Rifs and Kanr. However, in the presence of DNase I, all of the 81 Specr Strepr colonies were Rifs and Kanr. These data again are consistent with unidirectional transfer in the presence of DNase I.
Directional transfer analysis, as assessed by RAPD-PCR.
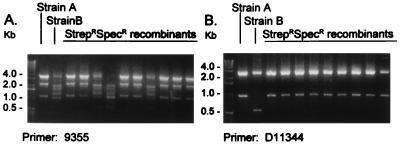
For the mating of HPK1 (Specr Rifr) and HPK5 (Strepr pHP1 Kanr), doubly resistant progeny also were analyzed by RAPD-PCR. Progeny from matings in the absence of DNase I showed patterns that resembled one or the other of the two parental strains, again consistent with bidirectional DNA transfer (Fig. 1A). However, the progeny from matings conducted in the presence of DNase I invariably showed a pattern resembling HPK1, but not HPK5, consistent with unidirectional DNA transfer from HPK5 to HPK1 (Fig. 1B).
FIG. 1.
RAPD-PCR of DNA isolated from Strepr Specr progeny resulting from coculture of HPK1 (Specr Rifr) (strain A) and HPK5 (Strepr pHP1 Kanr) (strain B). (A) Progeny obtained in the absence of DNase I, as analyzed with primer 9355 (1). Some of the progeny show the profile of each of the parental strains, consistent with bidirectional DNA transfer. (B) Progeny obtained in the presence of 285 μg of DNase I per ml, as analyzed with primer d11344 (1). All progeny have the same profile as HPK1 (Specr Rifr), consistent with unidirectional DNA transfer from HPK5 to HPK1.
DISCUSSION
Although several H. pylori strains have been shown to be naturally competent for in vitro uptake of free chromosomal DNA (21, 30, 34), our results demonstrate that in vitro culture of H. pylori strains together can result in the exchange of DNA. In our experiments, this exchange occurred at a frequency of approximately 10−5 to 10−6 bacterial pairs on plates and in broth, respectively. Analysis of doubly resistant progeny both by resistance to secondary antibiotic markers and by RAPD-PCR confirmed that this process of DNA transfer is bidirectional, which is consistent with natural transformation.
Although conducting the matings in the presence of DNase I resulted in a considerable decrease in the number of recombinants, in none of the experiments did it prevent development of doubly resistant progeny. To ensure that we destroyed all free DNA, we used a concentration of DNase I (285 μg/ml) that was two- to sevenfold higher than in previous analogous experiments performed with other naturally competent bacteria (9, 10, 27) and that in our experiments completely prevented transformation by free H. pylori chromosomal DNA. The remaining progeny in the presence of DNase conceivably could have been due to spontaneous mutation; however, we included controls for spontaneous mutation in every experiment, and this event was not observed. Furthermore, in the liquid culture experiments with DNase I, mutants were never observed if the strains were separated by a membrane, whereas cell-to-cell contact allowed the unidirectional development of mutants. These observations exclude spontaneous mutation as an explanation for our findings. We also showed that addition of DNase I changed DNA transfer from a bidirectional to a unidirectional phenomenon. Because of the possibility that the doubly resistant progeny in the matings in which DNase I was included might result from residual transformation, we performed control experiments in broth in which the strains either were allowed direct contact or were separated by a membrane that allowed passage of DNA but not of intact bacteria. These experiments confirmed that in the presence of DNase I, the development of doubly resistant progeny required cell-to-cell contact. By definition, this indicates a conjugative process (18, 19), but we have not defined the specific mechanism of conjugation in H. pylori. That the frequencies for three different combinations of strains were approximately the same, including the matings of HPK1 versus itself, suggests that there is no protective mechanism present in donor strains. A similar process of DNase-resistant transformation not involving conjugal plasmids has been described for Neisseria gonorrhoeae (6).
The development of transformants during incubation with H. pylori culture supernatants has to our knowledge not been shown previously, and we believe that it results from the lysis of cells in culture (23), leading to the release of free DNA. The ability of DNase to eliminate this event supports that hypothesis. Since bacteriophages have been identified in H. pylori (11, 26), we also performed experiments to determine whether they might be involved in horizontal gene transfer. The failure of cell-free supernatants to induce doubly resistant progeny in the presence of DNase I indicates that bacteriophages are not responsible for the observed DNA transfer process, at least for the strains studied. We cannot rule out the possibility that cell-to-cell contact induces a lysogenic phage, which then transforms the recipient cells. However, given that we could not find DNase-resistant transformation by supernatants alone (obtained either from culture of single strains or from cocultured strains), if such induction did occur, it requires cell-to-cell contact and in essence is a conjugation-like activity.
In summary, our results demonstrate that genetic exchange can occur among H. pylori cells during growth in vitro at a neutral pH. This genetic exchange in vitro mainly occurs by a DNase I-sensitive mechanism, which is bidirectional and therefore consistent with transformation. This event is likely to occur by the uptake of free DNA from lysed cells. However, genetic exchange also takes place by a DNase I-resistant mechanism, which is unidirectional and requires cell-to-cell contact, observations that suggest that conjugation can occur in H. pylori. This phenomenon may be involved in the adaptation to environmental stress in the acidic milieu of the stomach, where free chromosomal DNA has a short half-life, and may contribute to the heterogeneity among H. pylori strains.
ACKNOWLEDGMENTS
This study was supported in part by grant RO1 DK50837 from the National Institutes of Health and by the Medical Research Service of the Department of Veterans Affairs, the Iris and Homer Akers Fellowship in Infectious Diseases, and the Stichting van Helten of the Royal Dutch Academy of Sciences.
REFERENCES
- 1.Akopyanz N S, Bukanov N O, Westblom T U, Kresovich S, Berg D E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992;20:5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akopyanz N S, Kersulyte D, Berg D E. CagII, a new multigene locus associated with virulence in Helicobacter pylori. Gut. 1995;37:A1. [Google Scholar]
- 3.Atherton J C, Cao P, Peek R M, Tummuru M K R, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 4.Blaser M J. Genetic basis for heterogeneity of Helicobacter pylori. In: Hunt R H, Tytgat G N J, editors. Helicobacter pylori. Basic mechanisms to clinical cure. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 33–39. [Google Scholar]
- 5.Blaser M J, Parsonnet J. Parasitism by the slow bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest. 1994;94:4–6. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catlin B W. Cell-to-cell transmission of chromosomal loci in Neisseria gonorrhoeae. In: Strieps U, editor. Genetic exchange: a celebration and a new generation. Proceedings of the 25th Wind River Conference. New York, N.Y: Marcel Dekker; 1981. pp. 310–325. [Google Scholar]
- 7.Censini S, Lange C, Xiang Z, Crabtree J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. Cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factor. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, Rappuoli R. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danner G B, Smith H O, Narang S A. Construction of DNA recognition sites active in Haemophilus transformation. Proc Natl Acad Sci USA. 1982;79:2393–2397. doi: 10.1073/pnas.79.7.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frosch M, Meyer T F. Transformation-mediated exchange of virulence determinants by co-cultivation of Neisseria. FEMS Microbiol Lett. 1992;100:345–350. doi: 10.1111/j.1574-6968.1992.tb14062.x. [DOI] [PubMed] [Google Scholar]
- 11.Heintschel von Heineg E, Nalik H P, Schmid E N. Characterization of a Helicobacter pylori phage (HP1) J Med Microbiol. 1993;38:245–249. doi: 10.1099/00222615-38-4-245. [DOI] [PubMed] [Google Scholar]
- 12.Höök-Nikanne J, Tummuru M K R, Kersulyte D, Berg D, Blaser M J. IS605, a novel chimaeric transposable element, suitable for typing Helicobacter pylori strains. Gastroenterology. 1996;110:A135. [Google Scholar]
- 13.Jiang Q, Hiratsuka K, Taylor D E. Variability of gene order in different Helicobacter pylori strains contributes to genome diversity. Mol Microbiol. 1996;20:833–842. doi: 10.1111/j.1365-2958.1996.tb02521.x. [DOI] [PubMed] [Google Scholar]
- 14.Kansau I, Raymond J, Bingen E, Courcoux P, Kalach N, Bergeret M, Briami N, Dupont C, Labigne A. Genotyping of Helicobacter pylori isolates by sequencing of PCR products and comparison with the RAPD technique. Res Microbiol. 1996;147:661–669. doi: 10.1016/0923-2508(96)84023-x. [DOI] [PubMed] [Google Scholar]
- 15.Karita M, Tummuru M K R, Wirth H P, Blaser M J. Effect of growth phase and acid shock on Helicobacter pylori cagA expression. Infect Immun. 1996;64:4501–4507. doi: 10.1128/iai.64.11.4501-4507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Kleanthous, H. Unpublished data.
- 16.Kleanthous H, Clayton C L, Tabaqchali S. Characterization of a plasmid from Helicobacter pylori encoding a replication protein common to plasmids in gram-positive bacteria. Mol Microbiol. 1991;5:2377–2389. doi: 10.1111/j.1365-2958.1991.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence J G, Roth J R. Selfish operons: horizontal gene transfer may drive the evolution of gene clusters. Genetics. 1996;143:1843–1860. doi: 10.1093/genetics/143.4.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lederberg J, Tatum E. Gene recombination in E. coli. Nature. 1946;158:558. doi: 10.1038/158558a0. [DOI] [PubMed] [Google Scholar]
- 19.Lederberg J. Sex compatibility in E. coli. Genetics. 1952;37:720–730. doi: 10.1093/genetics/37.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logan R P H, Berg D E. Genetic diversity of Helicobacter pylori. Lancet. 1996;348:1462–1463. doi: 10.1016/s0140-6736(05)65885-0. [DOI] [PubMed] [Google Scholar]
- 21.Nedenskov P, Sorensen, Bukholm G, Bovre K. Natural competence for genetic transformation in Campylobacter pylori. J Infect Dis. 1990;161:365–366. doi: 10.1093/infdis/161.2.365. [DOI] [PubMed] [Google Scholar]
- 22.Peek R M, Thompson S A, Atherton J C, Blaser M J, Miller G G. Expression of ice A, a novel ulcer-associated H. pylori gene, is induced by contact with gastric epithelial cells and is associated with enhanced mucosal IL-8. Gut. 1996;39:A71. [Google Scholar]
- 23.Phadnis S H, Parlow M H, Levy M, Ilver D, Caulkins C M, Connors J B, Dunn B E. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect Immun. 1996;64:905–912. doi: 10.1128/iai.64.3.905-912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rautelin H, Seppälä K, Renkunen O V, Vainio U, Kosunen T U. Role of metronidazole resistance in therapy of Helicobacter pylori infections. Antimicrob Agents Chemother. 1992;36:163–166. doi: 10.1128/aac.36.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riccardi V M, Rotter J I. Familial Helicobacter pylori infection. Societal factors, human genetics, and bacterial genetics. Ann Intern Med. 1994;120:1043–1045. doi: 10.7326/0003-4819-120-12-199406150-00012. [DOI] [PubMed] [Google Scholar]
- 26.Schmid E N, von Recklinghausen G, Ansorg R. Bacteriophages in Helicobacter (Campylobacter) pylori. J Med Microbiol. 1990;32:101–104. doi: 10.1099/00222615-32-2-101. [DOI] [PubMed] [Google Scholar]
- 27.Scocca J J, Poland R L, Zoon K C. Specificity in deoxyribonucleic acid uptake by transformable Haemophilus influenzae. J Bacteriol. 1974;118:369–373. doi: 10.1128/jb.118.2.369-373.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segal E D, Tompkins L S. Transformation of Helicobacter pylori by electroporation. BioTechniques. 1993;14:225–226. [PubMed] [Google Scholar]
- 29.Suerbaum S, Josenhans C, Labigne A. Cloning and genetic characterization of the Helicobacter pylori and Helicobacter mustelae flaB flagellin genes and construction of H. pylori flaA- and flaB-negative mutants by electroporation-mediated allelic exchange. J Bacteriol. 1993;175:3278–3288. doi: 10.1128/jb.175.11.3278-3288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuda M, Karita M, Nakazawa T. Genetic transformation in Helicobacter pylori. Microbiol Immunol. 1993;37:85–89. doi: 10.1111/j.1348-0421.1993.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 31.Tummuru M K R, Cover T L, Blaser M J. Cloning and expression of a high molecular mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799–1809. doi: 10.1128/iai.61.5.1799-1809.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Hulst R W M, Rauws E A J, Köycü B, Keller J J, ten Kate F J W, Dankert J, Tytgat G N J, van der Ende A. Helicobacter pylori reinfection is virtually absent after successful eradication. J Infect Dis. 1997;176:196–200. doi: 10.1086/514023. [DOI] [PubMed] [Google Scholar]
- 33.Versalovic J, Shortridge D, Kibler K, Griffy M V, Beyer J, Flamm R K, Tanaka S K, Graham D Y, Go M F. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob Agents Chemother. 1996;40:477–480. doi: 10.1128/aac.40.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Roos K P, Taylor D E. Transformation of Helicobacter pylori by chromosomal metronidazole resistance and by a plasmid with a selectable chloramphenicol resistance marker. J Gen Microbiol. 1993;139:2485–2493. doi: 10.1099/00221287-139-10-2485. [DOI] [PubMed] [Google Scholar]
- 35.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons; 1995. pp. 2.4.1–2.4.5. [DOI] [PubMed] [Google Scholar]