Abstract
Background
Postoperative complications occur in up to 43% of patients after surgery, resulting in increased morbidity and economic burden. Prehabilitation has the potential to increase patients’ preoperative health status and thereby improve postoperative outcomes. However, reported results of prehabilitation are contradictory. The objective of this systematic review is to evaluate the effects of prehabilitation on postoperative outcomes (postoperative complications, hospital length of stay, pain at postoperative day 1) in patients undergoing elective surgery.
Methods
The authors performed a systematic review and meta-analysis of RCTs published between January 2006 and June 2023 comparing prehabilitation programmes lasting ≥14 days to ‘standard of care’ (SOC) and reporting postoperative complications according to the Clavien–Dindo classification. Database searches were conducted in PubMed, CINAHL, EMBASE, PsycINFO. The primary outcome examined was the effect of uni- or multimodal prehabilitation on 30-day complications. Secondary outcomes were length of ICU and hospital stay (LOS) and reported pain scores.
Results
Twenty-five studies (including 2090 patients randomized in a 1:1 ratio) met the inclusion criteria. Average methodological study quality was moderate. There was no difference between prehabilitation and SOC groups in regard to occurrence of postoperative complications (OR = 1.02, 95% c.i. 0.93 to 1.13; P = 0.10; I2 = 34%), total hospital LOS (−0.13 days; 95% c.i. −0.56 to 0.28; P = 0.53; I2 = 21%) or reported postoperative pain. The ICU LOS was significantly shorter in the prehabilitation group (−0.57 days; 95% c.i. −1.10 to −0.04; P = 0.03; I2 = 46%). Separate comparison of uni- and multimodal prehabilitation showed no difference for either intervention.
Conclusion
Prehabilitation reduces ICU LOS compared with SOC in elective surgery patients but has no effect on overall complication rates or total LOS, regardless of modality. Prehabilitation programs need standardization and specific targeting of those patients most likely to benefit.
Postoperative complications occur in up to 43% of cases. The efficacy of prehabilitation to prevent this outcome is still debated and warrants a meta-analysis. All prehab programmes longer than 14 days for all surgery types were included; 2090 patients were analysed. Prehab reduces ICU LOS compared with SOC in elective surgery patients but has no effect on overall complications or total LOS, regardless of modality.
Introduction
Postoperative complications occur after up to 43.5% of elective surgical procedures1,2, resulting in prolonged hospitalization, delayed functional recovery3 and worse oncological prognosis4. In addition to the negative impact on patients, postoperative complications represent a significant financial burden for healthcare systems5, with an estimated additional cost of more than $145 billion for the USA alone6,7. This financial burden varies according to the severity of the complications8 and the additional length of hospital stay (LOS).
Considerable progress has been made over the past 20 years to reduce the rate of postoperative complications due to the development of rehabilitation care pathways, such as ERAS® (Enhanced Recovery After Surgery)9. While patient care initially focused on the postoperative period to improve patients’ outcome, clinical teams proposed to focus on preoperative care as well to improve patients' resilience to surgical stress10–12. This preoperative management, named ‘prehabilitation’, was initiated in 2005.
The prehabilitation concept traditionally encompasses interventions such as nutritional support and physical therapy (PT)13–15. In addition, there is increasing evidence to suggest that other types of prehabilitation interventions may be beneficial, such as behavioural16 or cognitive training17. While prehabilitation programmes were first implemented 20 years ago as a unimodal approach (mainly with physical exercise18), prehabilitation groups and experts have increasingly promoted multimodal prehabilitation programmes, targeting at least physical exercise, nutrition and anxiety13,19,20. However, results from recent meta-analyses based on trials run between 1966 and 2018 are divergent regarding the effectiveness of prehabilitation on postoperative outcomes, particularly on LOS21–26 and postoperative complications27. These meta-analyses or systematic reviews suffer from several biases: they either focused on specific surgical areas/disciplines or on particular modalities (uni- or multimodal prehabilitation separately). In addition, several recent studies of interest on the effectiveness of prehabilitation have since been published17,28,29. A recent umbrella review suggests that prehabilitation may improve postoperative outcomes but suffers from several limitations25: 78% of the included reviews focused on unimodal prehabilitation and 25% included programmes with unreported duration or shorter than 2 weeks. Due to these divergent results on prehabilitation efficacy, there is currently no gold standard for prehabilitation as its modality, content and duration are not well-defined and vary widely across studies. The existing variability across studies and over time highlights the need for meta-analysis to understand the effects of various prehabilitation modalities on patient outcomes after surgery.
The main objective of this work is to evaluate the efficacy of either uni- or multimodal prehabilitation interventions compared to the ‘standard of care’ (SOC) in patients undergoing elective surgeries to prevent 30-day postoperative complications, measured using the Clavien–Dindo classification. Secondary objectives were to evaluate the influence of prehabilitation interventions on hospital and ICU LOS and on postoperative pain intensity. The impact of uni- or multimodal prehabilitation interventions on these outcomes was furthermore explored in subgroup analyses.
Methods
Protocol publication
This systematic review was conducted in accordance with the methods of the Cochrane Collaboration30, reported according to the PRISMA list31 and to the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) guidelines32. It was also registered in PROSPERO (International Prospective Register of Systematic Reviews, CRD42021236385).
Eligibility criteria
We included all RCTs published in English that investigated the effectiveness of uni- and multimodal prehabilitation programmes longer than 14 days before surgery compared with preoperative SOC on occurrence of postoperative complications according to the Clavien–Dindo classification up to 30 days after surgery, and/or hospital and ICU LOS, and/or pain at postoperative day one measured by the visual analogue scale (VAS) as primary or secondary outcomes. All types of elective surgical procedures were included. Studies involving paediatric patients (<18 years old) or with <20 patients were excluded.
Outcome measures
The primary outcome was the incidence of 30-day postoperative complications measured by the Clavien–Dindo classification (Table S1)33. Secondary outcomes were hospital and ICU LOS, measured in days, and postoperative pain measured by the VAS on the first postoperative day (D1).
Search strategy
The search strategy focused on data published in English between 1 January 2006 (corresponding to the beginning of the prehabilitation concept) and 13 June 2023 in the following databases: PubMed, CINAHL, EMBASE and PsycINFO. A preliminary keyword search was performed in PubMed. A second search was performed using medical subject headings (MeSH) terms. After ensuring that the equation using a combination of MeSH terms and Text Words yielded the most relevant studies, two researchers entered this equation into the four databases on 13 June 2023. The detailed equation of the MeSH terms is available online.
Study selection process
Article selection was performed using COVIDENCE systematic review software (Veritas Health Innovation, Melbourne Australia)34. The two lead authors (B.C. and A.C.) independently reviewed and selected studies for inclusion based on titles, abstracts and keywords (generated by the search strategy) to determine eligibility in terms of prehabilitation interventions, participants and protocols. Any disagreements were discussed until a consensus was found. If no consensus was found, a third reviewer (F.V.) determined the inclusion or exclusion of the articles in question. The two main authors retrieved the full texts of all relevant trials.
Next, inclusion or exclusion at the full-text review stage was performed independently for all articles by B.C. and A.C. and included information about participants, prehabilitation interventions, content of SOC and outcome measures that were not discussed in the abstracts. As with the first analysis, the included studies were compared, discussed, and, if no consensus was reached, assessed by the third reviewer (F.V.).
Data extraction and risk of methodological biases
Data from each article were extracted independently by B.C. and A.C. and reviewed blindly by F.V. These data included study characteristics, population characteristics, intervention details, methods and outcomes. The methodological quality of the studies was measured by the Joanna Briggs Institute RCT Critical Appraisal Tool35. This assessment list included 13 quality criteria that could be classified as high, moderate or low risk. The quality score for each study was determined according to the following thresholds: low risk of bias if at least 70% of responses were low risk; moderate risk if low risk responses were between 50% and 69%; and high risk of bias if less than 50% of responses were low risk36,37.
Missing data management
If the full-text article was not found or if data were missing from the studies, the corresponding authors were contacted by email. In particular, when the LOS was not reported in median and i.q.r., the corresponding authors were contacted to obtain these measures or the respective raw data for the authors' calculation. If the corresponding authors did not respond after three emails over a 3-month period, data were considered missing. Similarly, if authors could not provide full text, articles were excluded from the analysis.
Statistics
Postoperative complications were defined as the occurrence of a complication with a grade ≥1 according to the Clavien–Dindo classification33 (Table S1). LOS (total or ICU) was treated as a discrete ordinal variable ranging from 0 to X days and reported in one-day increments. Pain was measured by VAS and treated as a discrete ordinal variable ranging from 0 to 10 and reported with one-point increments. The distribution of numerical variables was reported with the median and i.q.r., when available.
OR and median from random effect models with 95% c.i.s were calculated and included all studies with available data represented by forest plots using R software version 3.6.1 (2019–07–05) with the meta (v 4.19-0) and metamedian (v 0.1.5) libraries38. Heterogeneity was assessed using the χ2 test and the I2 statistic. Tests were considered statistically significant if P<0.05. An additional subgroup analysis according to separate uni- or multimodal prehabilitation had been planned in the protocol to meet the objectives of the review. For every subgroup analysis, we performed similar random-effect models analysis.
To analyse the occurrence of complications, a random-effect OR comparison approach with 95% c.i. was performed. For LOS, a common approach was used to compare differences in medians with random effect. This meant restricting the analysis to items for which median and i.q.r. were available. Medians were not inferred from means as we decided to have a rigorous approach in data integration.
Results
Research strategy
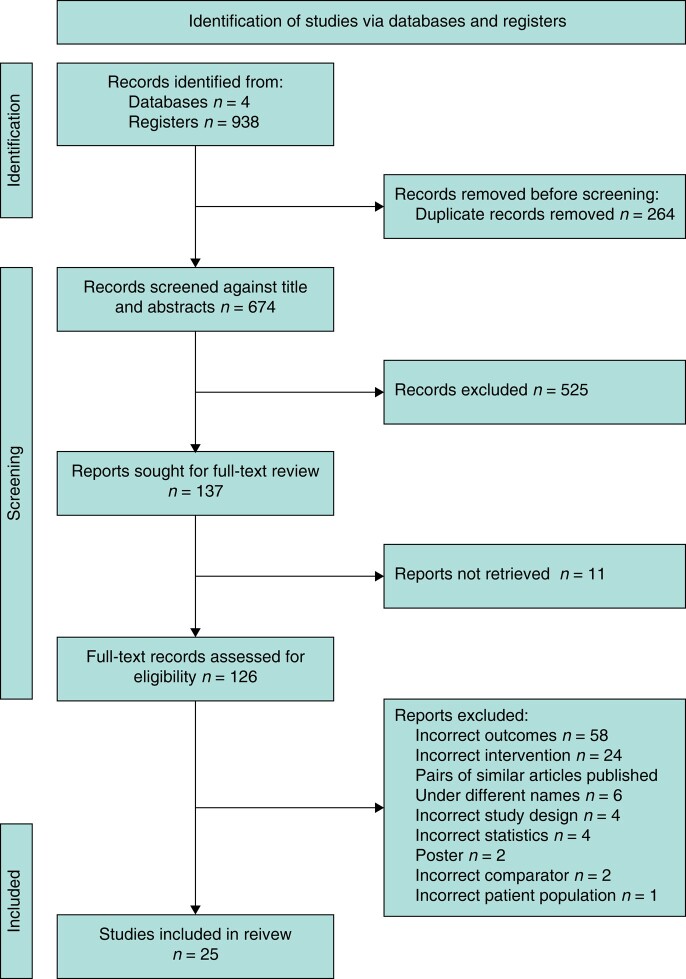
The selection process is summarized in the PRISMA 2020 flow diagram (Fig. 1)39. A total of 938 RCTs were identified and, after excluding duplicates and ineligibles, 25 RCTs were included in the meta-analysis and systematic review.
Fig. 1.
PRISMA flow chart
Missing data
Eighteen corresponding authors were contacted to retrieve missing data15,40–58, of which five supplied the relevant information40,43,52,55,56.
Study characteristics
Study characteristics are summarized in Table 1. Among the 25 studies included in the present review, 17 took place in Europe41,43,47–49,51,55,56,59–66 (1250 patients), four in Canada40,54,67,68 (263 patients), three in Australia44,50,69 (504 patients) and one in China70 (73 patients). The sample size ranged from 21 to 432 patients. Two studies were multicentre RCTs44,54 (532 patients), all others were monocentric. Most studies were coordinated in university hospitals, only four were led from regional hospitals50,52,59,60 (225 patients). None of the authors reported a conflict of interest. Manuscripts were published between 2010 and 2022.
Table 1.
Study characteristics
| Author and year | Sample size (total) | Age prehab. (years) | Age SOC (years) | Surgery | Surgical indication | Prehab. modality | Prehab. duration | Measured outcome | Adherence (%) |
|---|---|---|---|---|---|---|---|---|---|
| Au et al.40 | 42 | 61.4(7.8) | 58.4(6.1) | Prostatectomy | Cancer | PT | 5 | LOS | 81.5 |
| Barnejee et al.41 | 60 | 71.6(6.8) | 72.5(8.4) | Cystectomy | Cancer | PT | 3–6 | CD, LOS | NR |
| Barakat et al.51 | 124 | 73.8(6.5) | 72.9(7.9) | AAA repair | AAA | PT | 6 | LOS | NR |
| Berkel et al.43 | 57 | 74(7) | 73(6) | Colorectal | Cancer | PT | 2–4 | CD, LOS | 90 |
| Boden et al.44 | 432 | 65 (52–72) | 67.5 (56–75) | Major abdominal surgery | Multiple | PT, IMT | 6 | LOS | 94 |
| Chakravartty et al.47 | 28 | 44 (26–60) | 39 (24–66) | RYGB | Obesity | Nutrition | 4 | CD, LOS | NR |
| Dunne et al.48 | 37 | 61 (56–66) | 62 (53–72) | Hepatobiliary | Cancer | PT | 4 | CD, LOS | 94 |
| Fulop et al.56 | 184 | 70 (60–75) | 70 (64–75) | Colorectal | Cancer | PT, IMT, Nutrition, Cognition | 3–6 | CD, LOS | NR |
| Gloor et al.66 | 106 | 66 (24–90) | 65 (29–86) | Colorectal | Multiple | PT | 3–6 | CD | NR |
| Grąt et al.49 | 55 | 52 (47–58) | 50 (35–61) | Liver transplant | Cirrhosis | Nutrition | >2 | CD, LOS | 92.3 |
| Hollis et al.50 | 50 | 48.2(13.3) | 51.8(12.2) | General surgery | Multiple | Nutrition | 8 | CD, LOS | 93 |
| Hoogeboom et al.60 | 21 | 77(3) | 75(5) | Hip replacement | Arthrosis | PT | 3–6 | LOS | 91 |
| IJmker-Hemink et al.61 | 126 | 63.3(12) | 62.3(12.7) | Digestive, gynaecologic, orthopaedic, urologic | Multiple | Nutrition | 3 | LOS | 35 |
| Karenovics et al.62 | 164 | 64(13) | 64(10) | Thoracic | Cancer | PT | >4 | LOS | 87 |
| Karlsson et al.63 | 21 | 84 (76–85) | 74 (73–76) | Prostatectomy | Cancer | PT, IMT, Nutrition | 2 | CD, LOS | 97 |
| Liu et al.70 | 73 | 56.2(10.3) | 56.2(8.7) | AAA repair | AAA | PT, Nutrition, Cognition | 2 | CD, LOS | NR |
| Minnella et al.67 | 51 | 67.3(7.4) | 68.0(11.6) | Colorectal | Cancer | PT, Nutrition | 5 | CD, LOS | 63 |
| Minnella et al.68 | 70 | 69.7(10.2) | 66.0(10.2) | Major abdominal surgery, urologic | Multiple | PT, Nutrition, Cognition | 4 | CD, LOS | 83.3 |
| Moug et al.51 | 48 | 65.2(11.4) | 66.5(9.6) | Knee replacement | Arthrosis | PT | 14 | CD, LOS | 75 |
| Oosting et al.52 | 30 | 76.9(6.3) | 75.0(6.3) | RYGB | Obesity | PT | 5 | LOS | NR |
| Rolving et al.64 | 96 | 51.4(9.2) | 47.7(8.9) | Hepatobiliary | Cancer | Cognition | 6 | LOS | 24 |
| Santa Mina et al.54 | 100 | 61.2(8) | 62.2(6.9) | Colorectal | Cancer | PT | 6.5 | CD, LOS | 69.2 |
| Sebio García et al.55 | 40 | 70.9(6.1) | 69.4(9.4) | Liver transplant | Cirrhosis | PT, IMT | 7.5 | LOS | NR |
| Steffens et al.69 | 22 | 66 (46–70) | 62 (48–72) | General surgery | Multiple | PT, IMT | 2–6 | CD, LOS | 93 |
| Tew et al.65 | 53 | 74.6(5.5) | 74.9(6.4) | Hip replacement | Arthrosis | PT | 5 | LOS | 75.8 |
Age is expressed as mean(s.d.) or median (interquartile range (i.q.r.)), prehabilitation duration is expressed in weeks.
Prehab., prehabilitation group; SOC, ‘standard of care’; AAA, abdominal aortic aneurysm; CD, Clavien–Dindo classification; IMT, inspiratory muscle training; LOS, length of stay; NR, not reported; PT, physical therapy; RYGB, Roux-en-Y gastric bypass.
Population
Population characteristics are summarized in Table 1. Thirteen studies only included patients undergoing oncological operations (785 patients), four studies included patients both with and without cancer (849 patients) and eight studies included patients undergoing non-cancer-related surgery (457 patients). Patients’ co-morbidities are detailed in Table S2. The repartition of surgical procedures in the 25 studies was as follows: abdominal (11/24, 639 patients), thoracic (3/24, 277 patients), urologic (4/24, 272 patients), vascular (2/24, 177 patients) and orthopaedic (2/24, 51 patients). Three studies included different types of surgery (654 patients)44,61,64. The case mix was as follows: abdominal and urologic (432 patients), neurosurgery and abdominal (96 patients), urologic, abdominal, gynaecological and orthopaedic (126 patients).
Prehabilitation intervention and standard of care
The characteristics of prehabilitation interventions are summarized in Table 1. Programmes were mostly unimodal (n = 17 studies, 1198 patients), delivered in a healthcare facility and supervised by healthcare professionals (n = 14 studies, 1168 patients). The median duration of these prehabilitation programmes was 31.5 preoperative days (i.q.r. 27.5–41, 1984 patients). Each of the studies compared their prehabilitation programme to various SOCs according to local practice: patients were invited to either continue the routine they followed prior to surgery (11 studies, 861 patients)41,47,49,50,55,56,59,61,67,68,70 or they received instructions for unsupervised physical exercise and nutritional advice (13 studies, 1066 patients)40,43,44,48,51,52,54,60,63–66,69. Most studies evaluated the impact of PT alone40,41,43,48,51,52,54,59,60,62,65,66 (12 studies, 843 patients) or combined with nutrition or inspiratory muscle training44,63,67–69 (5 studies, 575 patients). Exercise modalities were either endurance training (9 studies, 386 patients) or high-intensity interval training (HIIT; 4 studies, 262 patients), or a mix of both (2 studies, 164 patients). Exercise modalities were not precisely described in four studies (663 patients).
The second most studied intervention was nutrition alone47,49,50,61 (4 studies, 259 patients) or combined with other modalities63,67,68 (3 studies, 647 patients). Details on prehabilitation interventions and SOC are presented in Table 2. When available, information on patients’ adherence to the protocol was collected (Table 1). The mean study recruitment rate was 58%, the mean adherence to the programmes was 78% and the mean attrition rate was 12%. No serious adverse event related to prehabilitation was reported.
Table 2.
Prehabilitation modalities
| Author and year | Prehabilitation intervention | Standard of care |
|---|---|---|
| Au et al.40 | Home-based moderate intensity aerobic and resistance exercises prescribed and demonstrated shortly after consenting to surgery. Manual detailing exercise prescription with supporting behaviour change strategies. |
Book on maintaining a healthy lifestyle after a prostate cancer diagnosis with no further exercise support. |
| Barnejee et al.41 | Two supervised training sessions per week in an exercise facility for 6 weeks. Fractionated high-intensity aerobic exercise (cycle ergometer). | Patients in the control group were advised to carry on with their lifestyles in the ‘usual way’. |
| Barakat et al.59 | Instructions and timetable to join hospital-based exercise classes, 3 times a week, 1-hour duration, during the 6 preoperative weeks. | Continued with their normal lifestyle, and avoid any additional, unsupervised exercises. |
| Berkel et al.43 | Three one-hour supervised sessions of physical therapy per week. High-intensity interval training on a cycle ergometer and resistance training. | Nutrition counselling and advice on smoking cessation. |
| Boden et al.44 | One 30-minute session with a physiotherapist for education and prevention of PPC, breathing exercises, delivery of a booklet. | Breathing exercise booklet. |
| Chakravartty et al.47 | 800 kcal VLCD regimen for 4 weeks. VLCD contained 3 pints of semi-skimmed milk, equivalent to 1704 ml (total intake of 800 kcal, 82 g carbohydrate, 61 g protein and 30 g fat), multivitamin and mineral supplementation, plus minimum of 2 l of energy-free liquid. | Continued with their usual diet. |
| Dunne et al.48 | 12 interval exercise sessions delivered by a cycle ergometer (moderate and vigorous intensity). | Patients were encouraged to follow clinical advice on exercise before surgery. |
| Fulop et al.56 | Information booklet, work diary, multimodal home-based exercise programme plus weekly in-hospital exercise sessions, deep breathing, incentive spirometry, nutritionist evaluation and oral nutritional supplementation, 60-minute session with a trained psychologist (techniques to reduce anxiety + lifestyle advice). | Not reported. |
| Gloor et al.66 | Training twice a week with a qualified physiotherapist, and once supervised at home. Each session was 90 min (10 min warm up, 35 min endurance training (HIT), 35 min strength training, 10 min cool down). | Continued with usual physical activities |
| Grat et al.49 | Probiotic capsules once/day until transplant (Lactococcus lactis PB411 (50.0%), Lactobacillus casei PB121 (25.0%), Lactobacillus acidophilus PB111 (12.5%) and Bifidobacterium bifidum PB211 (12.5%)). | Daily placebo. |
| Hollis et al.50 | VLCD programme (+ 2 cups vegetable salad, 2 l non-energy-free fluids, one teaspoon of vegetable oil). | Not reported. |
| Hoogeboom et al.60 | 60 min of supervised exercise sessions at the outpatient clinic twice a week for 3–6 weeks preoperatively. Patients were encouraged to exercise at home. | One group-based education session (early mobilization, surgery and anaesthesia, restricted movements, benefits of activity). |
| IJmker-Hemink et al.61 | Pre-cooked meals for 3 weeks: 6 protein-rich dishes per day (morning shake, 2 lunch dishes, snack, dinner and dessert for each day (avg energy 1553 kcal/day, avg protein 60.8 g/day)) + information leaflet describing personal protein requirements (1.2 g/kg body weight) and ‘protein-meter’ for protein intake. | Usual care with habitual diet followed. |
| Karenovics et al.62 | Up to 3 weekly supervised high-intensity training sessions using a cycle ergometer. Advice on active mobilization and risk factor management. | Not reported. |
| Karlsson et al.63 | 2–3 supervised sessions per week for 6 weeks. Inspiratory muscle training (30 breaths twice a day), high-intensity functional strength exercises. Difficulty was increased for progression. + Usual care | Ordinary preoperative information and recommendation of 150 min per week of moderate physical intensity. |
| Liu et al.70 | Aerobic and resistance exercises, respiratory training, nutritional counselling with whey protein supplementation, psychological adjustments (basic mental relaxation skills), conventional guidance. | Not reported. |
| Minnella et al.67 | Home-based programme (aerobic exercise and moderate continuous training 3 days per week after one demonstration session with a kinesiologist). Dietary advice + whey protein supplement (daily protein intake of 1.2 to 1.5 g/kg). | No specific intervention before surgery. |
| Minnella et al.68 | Same as Minnella 2018 + relaxation and imaging techniques. | No specific intervention before surgery. |
| Moug et al.51 | 8 weeks of graduated goals calculated from the baseline stepping count. Walking diary and use of a pedometer. Follow-up telephone calls. Support person (for example, spouse) to assist in their adherence to the programme. | Maintain their normal level of physical activity. |
| Oosting et al.52 | Home-based exercise programme with patient-tailored functional activities and walking. Pedometer (goal = minimum 30 min per day) + 30 min supervised sessions twice a week for 3–6 weeks. | Usual care: one session of instructions, a single group session supervised by a physical therapist 3 weeks before surgery. |
| Rolving et al.64 | Four 3-hour group sessions directed by a multidisciplinary team on interaction between cognition and pain perception, coping strategies, pacing principles, ergonomic directions, return to work, details about the surgical procedure. | Standard preoperative information. |
| Santa Mina et al.54 | Pelvic floor muscle exercise and total body exercises. 60 min of unsupervised, home-based, moderate intensity exercise 3–4 days per week. (Exercise manual with online exercises.) | Manual detailing a pelvic floor training regimen. |
| Sebio Garcia et al.55 | One hour supervised pulmonary prehabilitation programme 3–5 times per week. Moderate endurance (ergometer) and resistance training. | Usual care—no exercise training. |
| Steffens et al.69 | Exercise sessions: 1 h individualized, hospital-based training session once a week for 2–6 weeks (aerobic and endurance, respiratory, muscle strength) + personalized home exercises + activity tracker (goal = 30 min walk/day). | Nutritional counselling and advice on smoking cessation, reduction of alcohol intake. Instruction to maintain normal daily activities. |
| Tew et al.65 | Three hospital-based supervised exercise sessions on a cycle ergometer per week for the 4 consecutive weeks preceding their operation date. | Usual care, which comprised evidence-based medical optimization. |
PPC, postoperative pulmonary complication; VLCD, very low-calorie diet.
Quality of evidence
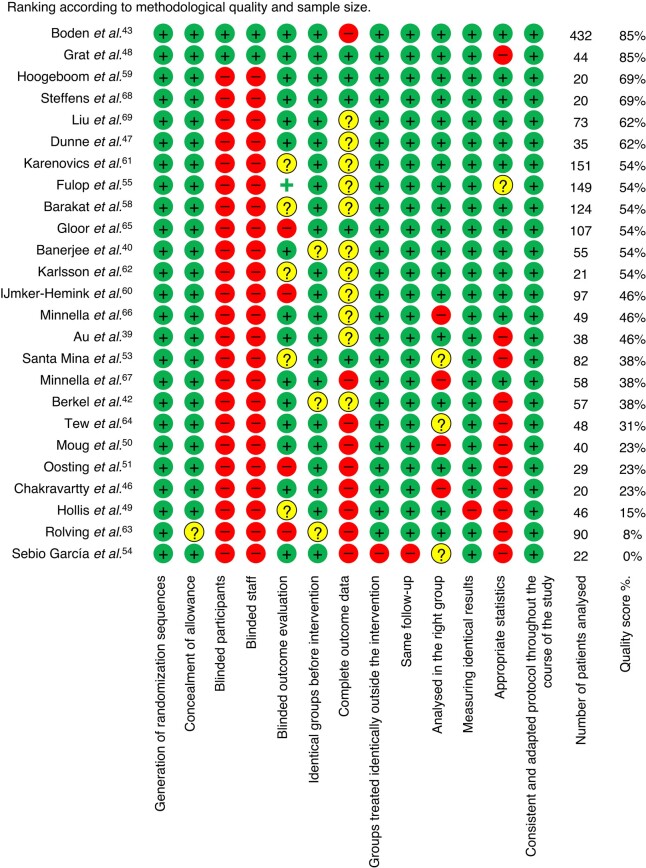
Data on quality of evidence are presented in Fig. 2. The mean score for overall quality of evidence was 45%, ranging from 0% to 89%. Two studies were at low risk of bias (score >70%, 476 patients)44,49 and 13 studies were at high risk of bias (score <50%, 627 patients)40,43,47,50–52,54,55,61,64,65,67,68, with 10 studies of moderate quality of evidence (score ≥50% and ≤70%, 755 patients)41,48,56,59,60,62,63,66,69,70. The funnel plot for the primary outcome is symmetrical, and more than 95% of the included studies are within the fixed-effect summary estimate, thereby not providing any evidence of publication bias, as shown in Fig. 3.
Fig. 2.
Quality assessment of included studies
Fig. 3.
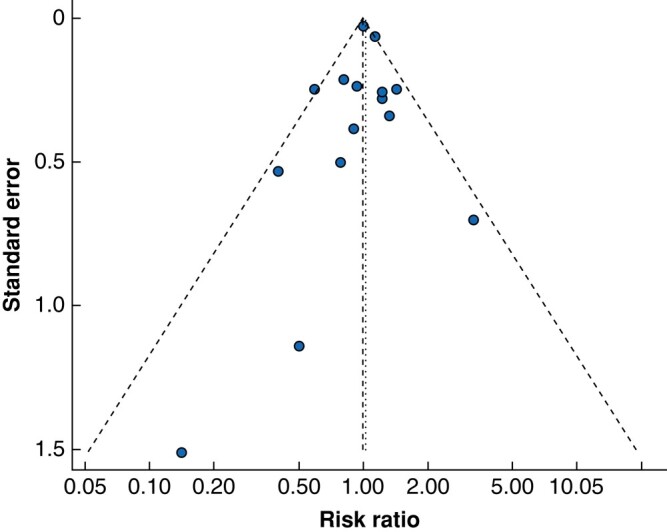
Funnel plot
Primary outcome of included studies
Only one study reported postoperative complications at postoperative day 30 as a primary outcome according to the Clavien–Dindo classification (57 patients)43. Six studies focused on postoperative complications without using the Clavien–Dindo classification as the primary outcome (937 patients)43,44,49,59,62,66. Eight studies used feasibility and acceptability of the prehabilitation programme as primary outcomes (352 patients)41,50–52,54,60,63,69. The focus of seven studies was mainly on prehabilitation’s influence on patients’ perioperative functional capacity (497 patients)40,48,55,56,67,68,70. One study measured postoperative pain without using the VAS64. None of the studies had LOS as a primary outcome.
Primary outcome: postoperative complications at postoperative day 30 according to the Clavien–Dindo classification
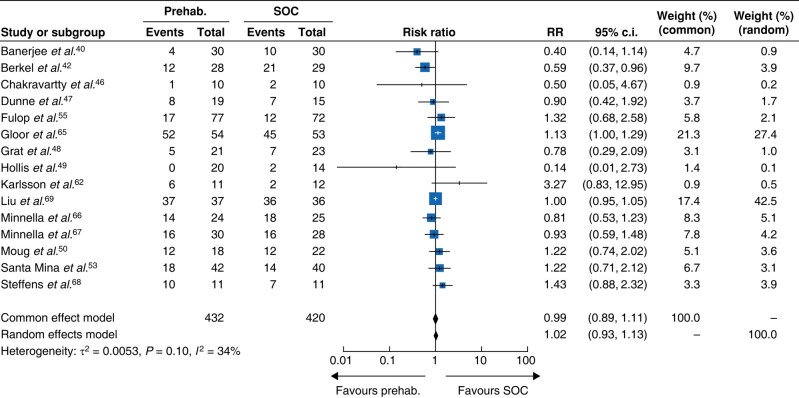
Fifteen studies collected data on postoperative complications according to the Clavien–Dindo classification at day 3041,43,47–51,54,56,63,66–70, allowing for analysis of 852 patients (Fig. 4). The procedures were mostly abdominal (11 studies, n = 588), followed by urologic (3 studies, n = 195) and thoracic (1 study, n = 73). Of these 14 studies, one found a significant reduction of postoperative complications in the prehabilitation group, all others showed no effect. Overall, no statistical difference was observed between the prehabilitation and SOC cohorts (OR = 1.02, 95% c.i. 0.93 to 1.13; P = 0.10; I2 = 34%, random effect, Fig. 4).
Fig. 4.
Impact of prehabilitation on postoperative complications according to the Clavien–Dindo classification
Events, number of complications; prehab., prehabilitation group; SOC, standard of care group; Total, total number of patients; Weight, study weight.
Secondary outcomes
Total length of hospital stay
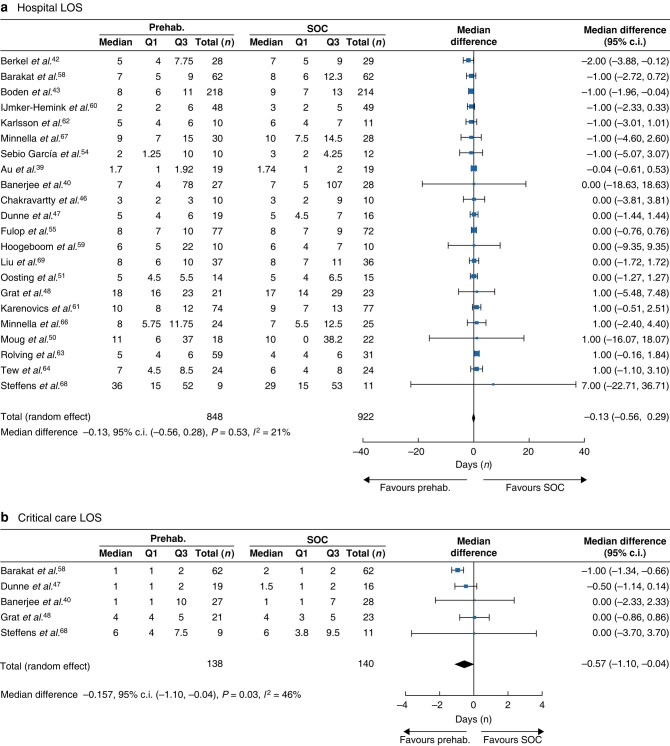
Twenty-two studies (1770 patients) measured the efficacy of prehabilitation on the total LOS40,41,43,44,47–49,51,52,55,56,59–65,67–70, with 1672 patients analysed. Most of these studies were in patients undergoing abdominal surgery (9 studies, 435 patients), followed by thoracic (3 studies, 246 patients), urologic (3 studies, 151 patients) and vascular (2 studies, 172 patients) surgery. Three studies included a combination of general, urologic, gynaecologic and orthopaedic surgeries representing 619 patients. Of those 22 studies, two found a reduction in the total LOS; however, overall, no statistical difference was observed between the prehabilitation and the SOC groups (median differences (MD) = –0.13 days; 95% c.i. −0.56 to 0.28; P = 0.53; I2 = 21%, Fig. 5a).
Fig. 5.
(a) Impact of prehabilitation on total hospital length of stay and (b) Impact of prehabilitation on critical care unit length of stay
LOS, length of stay; prehab., prehabilitation group; Q1, first quartile (25%); Q3, third quartile (75%); SOC, standard of care group; Total, total number of patients.
Length of stay in the ICU
Five studies measured the median LOS in the ICU41,48,49,59,69 with a total of 278 patients analysed. Of these five studies, one found a significant reduction in the ICU LOS. Procedures were mostly abdominal (three studies, 114 patients). A statistical difference was observed in favour of prehabilitation compared with the SOC groups (n = 278, MD = −0.57 days; 95% c.i. −1.10 to −0.04; P = 0.03; I2 = 46%, Fig. 5b). The ICU LOS was reduced by 28% in the prehabilitation compared to the SOC group.
Pain
None of the included studies measured pain with a VAS at postoperative D1. Among studies having a pain outcome, pain was not assessed using the specific method defined in the present study setup. Instead, pain was measured using other scales or was assessed at different time points, typically in the long term (postoperative months 3–6).
Subgroup analysis
No statistically significant difference in the effectiveness of prehabilitation according to the different prehabilitation modalities was found, either on postoperative complications for both unimodal prehabilitation (n = 478, RR 0.92, 95% c.i. 0.70 to 1.20; P = 0.10; I2 = 40%, random-effect model; Fig. S1) or multimodal prehabilitation (n = 374, RR 1.09, 95% c.i. 0.82 to 1.44; P = 0. 02; I2 = 63%, random-effect model; Fig. S2), or on total LOS for both unimodal (n = 371, MD = −0.14 days; 95% c.i. −0.69 to 0.42; P = 0.63; I2 = 43%; Fig. S3) and multimodal prehabilitation (n = 392, MD = −0.11 days; 95% c.i. −0.74 to 0.51; P = 0.63; I2 = 43%; Fig. S4). There was also no difference in the abdominal surgery group on postoperative complication rate (579 patients, RR = 1.03, 95% c.i. 0.83 to 1.26; P = 0.11; I2 = 36%; Fig. S5) or LOS (n = 435, MD = −0.25, 95% c.i. −0.86 to 0.36; P = 0.42; I2 = 1.55%; Fig. S6) or in the other surgery types group (n = 273, RR = 1.00, 95% c.i. 0.95 to 1.05; P = 0.31; I2 = 16%, random-effect model; n = 1237, MD = –0.06 days, 95% c.i. −0.60 to 0.48; P = 0.83; I2 = 40%; for postoperative complication and LOS, respectively; Fig. S7, S8, respectively).
Discussion
In this meta-analysis, which examined RCTs with prehabilitation programmes longer than 14 days and compared them to SOC for any type of elective surgery over the last 15 years, no significant difference was found between groups regarding the incidence of postoperative complications or total LOS. The authors identified a significant reduction of LOS in ICUs of approximately 28% in favour of prehabilitation as compared to SOC. The effect of prehabilitation on postoperative day 1 pain scores has not been studied in any included study. All analyses showed moderate statistical heterogeneity. Subgroup analyses did not identify increased efficacy of prehabilitation depending on modality, either uni- or multimodal, with moderate to significant heterogeneity, respectively. To the authors' knowledge, this meta-analysis is the first to include studies combining several types of surgeries and different prehabilitation modalities in the same analysis. The current methodology rigorously followed the international recommendations for conducting a systematic review and meta-analysis26,71.
These results on complication rate are contradictory to the last retrospective epidemiological data on more than 1500 patients72 as well as to a recent observational study73, and other meta-analyses focusing on abdominal and cardiovascular operations which showed that prehabilitation is effective on both clinical and economic outcomes43,49. Several explanations for this discrepancy are possible: heterogeneity in prehabilitation programmes limits statistical power, methodological bias may skew results, and target populations limit the generalizability of results.
First, programmes included in this meta-analysis were not standardized and did not always meet the guidelines issued since 2017 by the Macmillan74 or the Franco Carli groups75, the ERAS initiative9 or the nutritional recommendations of the European Society for Clinical Nutrition and Metabolism (ESPEN)76. Many studies to date have been conducted on small patient cohorts with varied programmes and outcomes, primarily focusing on safety, starting with physical exercise which could include either strength training, or aerobic exercise or high-intensity training or a combination of those, all aiming at improving patients’ functional status yet having different effects on the body. Also, despite a growing number of studies on the effectiveness of multimodal prehabilitation, a large majority of included RCTs tested only a unimodal intervention, mostly PT or nutrition77. Only 25% of the included studies focused on multimodal prehabilitation, inducing a probable lack of power to demonstrate the efficacy of this modality. Finally, only studies with prehabilitation programmes longer than 14 days were included. Although this has led to a selection bias and lack of power because it drastically limited the number of studies included, the criterion was chosen so we could focus on prehabilitation programmes designed for a duration compatible with a change in preoperative functional parameters13,78. This approach has been adopted by others25,44,61,79 and is based on the postulate that exercise, nutrition and brain function have an impact on immune system functions14,80,81, which are key players in surgical recovery82,83. In addition, exercise can increase physiological reserve and the body’s ability to cope with increased metabolic demand, while nutritional intervention can help reduce sarcopenia14. The fact that heterogeneity remains under 34% for all outcomes, despite different programmes and surgery types, validates the authors' approach of pooling all these data together to a certain extent. Including various prehabilitation modalities and surgery types allowed for a pragmatic analysis of prehabilitation in multiple surgical disciplines, enabling a broad generalizability of the results. Additionally, subgroup analysis based on prehabilitation type (uni- or multimodal) or type of surgery did not demonstrate a preference for prehabilitation over standard of care.
When evaluating the efficacy of prehabilitation, it raises questions about the type of outcome to be studied from both the patient’s and medical perspectives, aiming to provide the best patient-centred care. For instance, although postoperative delirium is a common complication among elderly patients that could potentially be mitigated by prehabilitation17, none of the included studies reported this outcome. To better meet patients’ needs, patient self-reported measures (PROM-10 or 29, for example)84,85 have been developed and will probably provide answers to those questions in the future.
Second, the dramatic variability in methodological quality limits the interpretation and generalizability of the results. This heterogeneity is partially explained by the inconsistency in prehabilitation programmes as discussed above, as well as the raw methodology. Indeed, an invariable feature associated with PT prehabilitation is the impossibility of conducting these studies in a patient-blinded setting. In addition, interventions in the SOC groups overwhelmingly comprised dietary advice booklets and PT, which could be a confounding factor. Moreover, the recruitment rate in these studies was relatively low (60% on average), creating a possible selection bias. The number of studies included was also drastically decreased by the choice of the complication reporting scale (Clavien–Dindo), which also produced a selection and evaluation bias. However, this classification is the most robust classification for complication evaluation and allows for reproducible and comparable results. This effectively translated in a heterogeneity of 34% for the impact of prehabilitation on postoperative complication when other recent meta-analysis had a heterogeneity ranging from 43% to 77%26,86. Of note, most studies took place in a European setting, limiting the generalizability of the results to other healthcare systems and world regions. Results on postoperative complication may also be driven by the high proportion of abdominal surgery (11 of 15 studies). However, even when conducting subgroup analysis specifically focusing on non-abdominal surgeries, no efficacy of prehabilitation on postoperative complications or total hospital length of stay was observed.
While the current analysis does not show an impact of prehabilitation on total hospital LOS, prehabilitation is associated with a decrease in ICU LOS. Along with existing literature on specific high-risk patient populations21,22,24,26,77,87,88, this provides compelling arguments for the potential benefits of prehabilitation in preoperative care for at-risk populations. It is important to note that a reduction in ICU LOS is a significant outcome in itself, contributing to improved resource utilization and potentially better patient outcomes, especially as high-dependency units are associated with a greater risk of delirium89, mortality90, post-traumatic stress disorder91 and higher financial burden92. Although this reduction in ICU LOS could be due to a decrease in severe complications, especially in frail patients, further studies are needed to provide direct evidence for this correlation. Indeed, while preoperative frailty (broadly defined by a multidimensional state of reduced physiological reserve93) has been identified as a major risk factor for delayed postoperative recovery10,11,94,95, most of the studies included in the review did not measure participants’ frailty before prehabilitation and did not adapt their programmes accordingly, which may explain this lack of effect of prehabilitation in the general population. A meta-analysis targeting frail patients undergoing abdominal surgery conducted by Daniels et al. in 202024 showed that a multimodal prehabilitation significantly reduced severe complications (Clavien–Dindo ≥III).
Patient adherence is another critical factor in the effectiveness of prehabilitation, which could potentially explain the current results. This is further highlighted by recent findings showing negative results of home-based prehabilitation for frail patients undergoing cancer surgery96. Adherence could be improved by integrating motivational interviews into prehabilitation programmes, by improving their accessibility (delivery of programmes via tele-coaching and real-time adaptation to patients’ difficulties in changing their lifestyle), or by using connected wearable devices both to monitor and prompt patients to comply better with their prehabilitation programme.
This also highlights the necessity for performance tools to stratify patients at risk for postoperative complications and furthermore patients who would benefit from prehabilitation. Current tools to evaluate patients’ frailty (such as the risk analysis index-administrative [RAI-A]97 that is to date the most performant frailty evaluation tool, or the American College of Surgeons National Surgical Quality Improvement Program [ACS NSQIP] surgical risk score, or Fried’s frailty score) based solely on clinical data lack performance98,99 and are barely used in clinical practice. Recently, new approaches integrating immune parameters, and in particular intracellular signalling, in addition to clinical data have made it possible to achieve unequalled predictive performance for postoperative infections (AUC 0.94 versus 0.63 when limited to clinical data using the ACS NSQIP surgical risk score100).
Considering the numerous challenges associated with prehabilitation, such as accurately identifying patients at risk for postoperative complications and determining who would benefit from prehabilitation, as well as ensuring sufficient adherence (which often requires effective coordination between healthcare workers, patients and caregivers) to ensure the programme is correctly followed and yields timely and effective results in an appropriate setting, it may be worth considering that a well-implemented unimodal programme could be sufficient. This is especially relevant because the current results did not demonstrate superior efficacy of multimodal compared to unimodal prehabilitation. Such a unimodal programme would still address patients’ needs by involving them as major participants in their care and recovery19.
In conclusion, this meta-analysis demonstrates positive and original results concerning the effect of prehabilitation programmes longer than 14 days on the reduction of ICU LOS. No effect on complications or hospital LOS criteria was found. In accordance with the Cochrane recommendations, an analysis of the effect on postoperative pain was not feasible according to the predefined criteria (that is, VAS at postoperative day one). These data suggest the importance of standardizing (both in prehabilitation modality and actual action within each modality) and evaluating prehabilitation programmes and improving the definition of patients most likely to benefit from them (that is, at-risk patients undergoing elective surgery). However, there was significant heterogeneity among the included studies, both in terms of methodology and populations targeted, which may limit the generalizability of the current findings. Additionally, this meta-analysis does not provide any insight into the long-term effects of prehabilitation on quality of life or functional outcomes.
Supplementary Material
Acknowledgements
A.C., B.C., B.G., and F.V. contributed equally to this study. The authors thank Amanda Woodward from Stanford medicine for her help in designing the detailed equation of the MeSH terms used in this meta-analysis.
Contributor Information
Amélie Cambriel, Department of Anesthesiology and Intensive Care, Hôpital Saint-Antoine, Assistance Publique-Hôpitaux de Paris, Paris, France; GRC 29, DMU DREAM, Sorbonne University, Assistance Publique-Hôpitaux de Paris, Paris, France; Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine, Stanford, California, USA.
Benjamin Choisy, Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine, Stanford, California, USA.
Julien Hedou, Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine, Stanford, California, USA.
Marie-Pierre Bonnet, GRC 29, DMU DREAM, Sorbonne University, Assistance Publique-Hôpitaux de Paris, Paris, France; Department of Anesthesia and Critical Care, Trousseau Hospital, Assistance Publique-Hôpitaux de Paris, Sorbonne University, Paris, France; Obstetrical Perinatal and Paediatric Epidemiology Research Team, Université Paris Cité, CRESS, EPOPé, INSERM, INRA, Paris, France.
Souad Fellous, Department of Anesthesiology and Intensive Care, Hôpital Saint-Antoine, Assistance Publique-Hôpitaux de Paris, Paris, France.
Jérémie H Lefevre, Sorbonne University and Department of Digestive Surgery, Hôpital Saint-Antoine, Assistance Publique-Hôpitaux de Paris, Paris, France.
Thibault Voron, Sorbonne University and Department of Digestive Surgery, Hôpital Saint-Antoine, Assistance Publique-Hôpitaux de Paris, Paris, France.
Dyani Gaudillière, Division of Plastic & Reconstructive Surgery, Department of Surgery, Stanford University, Stanford, California, USA.
Cindy Kin, Division of General Surgery, Department of Surgery, School of Medicine, Stanford University, Stanford, California, USA.
Brice Gaudillière, Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine, Stanford, California, USA.
Franck Verdonk, Department of Anesthesiology and Intensive Care, Hôpital Saint-Antoine, Assistance Publique-Hôpitaux de Paris, Paris, France; GRC 29, DMU DREAM, Sorbonne University, Assistance Publique-Hôpitaux de Paris, Paris, France; Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine, Stanford, California, USA.
Funding
The authors have no funding to declare.
Author contributions
Amélie Cambriel (Conceptualization, Methodology, Writing—original draft, Writing—review & editing), Benjamin Choisy (Conceptualization, Formal analysis, Methodology, Writing—original draft, Writing—review & editing), Julien Hedou (Formal analysis), Marie Pierre Bonnet (Conceptualization, Methodology, Writing—review & editing), Jeremie LeFevre (Writing—review & editing), Souad Fellous (Formal analysis), Thibault Voron (Writing—review & editing), Dyani Gaudilliere (Writing—original draft, Writing—review & editing), Cindy Kin (Writing—review & editing), Brice Gaudilliere (Conceptualization, Methodology, Supervision, Writing—review & editing), and Franck Verdonk (Conceptualization, Methodology, Supervision, Writing—original draft, Writing—review & editing)
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
Full data are available upon request to the corresponding author.
References
- 1. Tevis SE, Kennedy GD. Postoperative complications and implications on patient-centered outcomes. J Surg Res 2013;181:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GlobalSurg Collaborative and National Institute for Health Research Global Health Research Unit on Global Surgery . Global variation in postoperative mortality and complications after cancer surgery: a multicentre, prospective cohort study in 82 countries. Lancet Lond Engl 2021;397:387–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet Lond Engl 2012;380:1059–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg 2014;259:930–938 [DOI] [PubMed] [Google Scholar]
- 5. O’Brien WJ, Gupta K, Itani KMF. Association of postoperative infection with risk of long-term infection and mortality. JAMA Surg 2020;155:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel AS, Bergman A, Moore BW, Haglund U. The economic burden of complications occurring in major surgical procedures: a systematic review. Appl Health Econ Health Policy 2013;11:577–592 [DOI] [PubMed] [Google Scholar]
- 7. Statistiques annuelles des établissements de santé-enquête 2019 [Internet]. 2021. https://www.sae-diffusion.sante.gouv.fr/sae-diffusion/recherche.htm (accessed 21 September 2021)
- 8. Vonlanthen R, Slankamenac K, Breitenstein S, Puhan MA, Muller MK, Hahnloser D et al. The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 2011;254:907–913 [DOI] [PubMed] [Google Scholar]
- 9. Ljungqvist O, Young-Fadok T, Demartines N. The history of enhanced recovery after surgery and the ERAS society. J Laparoendosc Adv Surg Tech A 2017;27:860–862 [DOI] [PubMed] [Google Scholar]
- 10. Shinall MC Jr, Arya S, Youk A, Varley P, Shah R, Massarweh NN et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg 2020;155:e194620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McIsaac DI, Jen T, Mookerji N, Patel A, Lalu MM. Interventions to improve the outcomes of frail people having surgery: a systematic review. PLoS One 2017;12:e0190071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levett DZH, Grocott MPW. Cardiopulmonary exercise testing, prehabilitation, and Enhanced Recovery After Surgery (ERAS). Can J Anaesth 2015;62:131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Durrand J, Singh SJ, Danjoux G. Prehabilitation. Clin Med Lond Engl 2019;19:458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gillis C, Wischmeyer PE. Pre-operative nutrition and the elective surgical patient: why, how and what? Anaesthesia 2019;74:27–35 [DOI] [PubMed] [Google Scholar]
- 15. Valkenet K, van de Port IGL, Dronkers JJ, de Vries WR, Lindeman E, Backx FJG. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clin Rehabil 2011;25:99–111 [DOI] [PubMed] [Google Scholar]
- 16. Levett DZH, Grimmett C. Psychological factors, prehabilitation and surgical outcomes: evidence and future directions. Anaesthesia 2019;74:36–42 [DOI] [PubMed] [Google Scholar]
- 17. Humeidan ML, Reyes JPC, Mavarez-Martinez A, Roeth C, Nguyen CM, Sheridan E et al. Effect of cognitive prehabilitation on the incidence of postoperative delirium among older adults undergoing major noncardiac surgery: the Neurobics randomized clinical trial. JAMA Surg 2021;156:148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Topp R, Ditmyer M, King K, Doherty K, Hornyak J. The effect of bed rest and potential of prehabilitation on patients in the intensive care unit. AACN Clin Issues 2002;13:263–276 [DOI] [PubMed] [Google Scholar]
- 19. Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiol Clin 2015;33:17–33 [DOI] [PubMed] [Google Scholar]
- 20. Arora RC, Brown CH, Sanjanwala RM, McKelvie R. ‘NEW’ prehabilitation: a 3-way approach to improve postoperative survival and health-related quality of life in cardiac surgery patients. Can J Cardiol 2018;34:839–849 [DOI] [PubMed] [Google Scholar]
- 21. Hughes MJ, Hackney RJ, Lamb PJ, Wigmore SJ, Christopher Deans DA, Skipworth RJE. Prehabilitation before major abdominal surgery: a systematic review and meta-analysis. World J Surg 2019;43:1661–1668 [DOI] [PubMed] [Google Scholar]
- 22. Marmelo F, Rocha V, Moreira-Gonçalves D. The impact of prehabilitation on post-surgical complications in patients undergoing non-urgent cardiovascular surgical intervention: systematic review and meta-analysis. Eur J Prev Cardiol 2018;25:404–417 [DOI] [PubMed] [Google Scholar]
- 23. Moran J, Guinan E, McCormick P, Larkin J, Mockler D, Hussey J et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery 2016;160:1189–1201 [DOI] [PubMed] [Google Scholar]
- 24. Daniels SL, Lee MJ, George J, Kerr K, Moug S, Wilson TR et al. Prehabilitation in elective abdominal cancer surgery in older patients: systematic review and meta-analysis. BJS Open 2020;4:1022–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McIsaac DI, Gill M, Boland L, Hutton B, Branje K, Shaw J et al. Prehabilitation in adult patients undergoing surgery: an umbrella review of systematic reviews. Br J Anaesth 2022;128:244–257 [DOI] [PubMed] [Google Scholar]
- 26. Waterland JL, McCourt O, Edbrooke L, Granger CL, Ismail H, Riedel B et al. Efficacy of prehabilitation including exercise on postoperative outcomes following abdominal cancer surgery: a systematic review and meta-analysis. Front Surg 2021;8:628848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lau CSM, Chamberlain RS. Prehabilitation programs improve exercise capacity before and after surgery in gastrointestinal cancer surgery patients: a meta-analysis. J Gastrointest Surg 2020;24:2829–2837 [DOI] [PubMed] [Google Scholar]
- 28. Halliday LJ, Doganay E, Wynter-Blyth VA, Hanna GB, Moorthy K. The impact of prehabilitation on post-operative outcomes in oesophageal cancer surgery: a propensity score matched comparison. J Gastrointest Surg 2021;25:2733–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Minnella EM, Liberman AS, Charlebois P, Stein B, Scheede-Bergdahl C, Awasthi R et al. The impact of improved functional capacity before surgery on postoperative complications: a study in colorectal cancer. Acta Oncol 2019;58:573–578 [DOI] [PubMed] [Google Scholar]
- 30. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2019, p.726 [Google Scholar]
- 31. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 32. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196 [DOI] [PubMed] [Google Scholar]
- 34. Covidence [Internet]. 2021. Covidence—Better systematic review management. https://www.covidence.org/ (accessed 21 September 2021)
- 35. Farrah K, Young K, Tunis MC, Zhao L. Risk of bias tools in systematic reviews of health interventions: an analysis of PROSPERO-registered protocols. Syst Rev 2019;8:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goplen C, Verbeek W, Kang S, Jones A, Voaklander D, Churchill T et al. Preoperative opioid use is associated with worse patient outcomes after total joint arthroplasty: a systematic review and meta-analysis. BMC Musculoskelet Disord 2019;20:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis [Internet]. JBI; 2020. https://jbi-global-wiki.refined.site/space/MANUAL (accessed 21 September 2021)
- 38. McGrath S, Sohn H, Steele R, Benedetti A. Meta-analysis of the difference of medians. Biom J 2020;62:69–98 [DOI] [PubMed] [Google Scholar]
- 39. Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. doi: 10.1371/journal.pmed1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Au D, Matthew AG, Lopez P, Hilton WJ, Awasthi R, Bousquet-Dion G et al. Prehabilitation and acute postoperative physical activity in patients undergoing radical prostatectomy: a secondary analysis from an RCT. Sports Med Open 2019;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Banerjee S, Manley K, Shaw B, Lewis L, Cucato G, Mills R et al. Vigorous intensity aerobic interval exercise in bladder cancer patients prior to radical cystectomy: a feasibility randomised controlled trial. Support Care Cancer 2018;26:1515–1523 [DOI] [PubMed] [Google Scholar]
- 42. Barbalho-Moulim MC, Miguel GPS, Forti EMP, Campos Fdo A, Costa D. Effects of preoperative inspiratory muscle training in obese women undergoing open bariatric surgery: respiratory muscle strength, lung volumes, and diaphragmatic excursion. Clin Sao Paulo Braz 2011;66:1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Berkel AEM, Bongers BC, Kotte H, Weltevreden P, de Jongh FHC, Eijsvogel MMM et al. Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of a randomized clinical trial. Ann Surg 2021;275:e299–e306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boden I, Skinner EH, Browning L, Reeve J, Anderson L, Hill C et al. Preoperative physiotherapy for the prevention of respiratory complications after upper abdominal surgery: pragmatic, double blinded, multicentre randomised controlled trial. BMJ 2018;360:j5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Calatayud J, Casaña J, Ezzatvar Y, Jakobsen MD, Sundstrup E, Andersen LL. High-intensity preoperative training improves physical and functional recovery in the early post-operative periods after total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 2017;25:2864–2872 [DOI] [PubMed] [Google Scholar]
- 46. Cavill S, McKenzie K, Munro A, McKeever J, Whelan L, Biggs L et al. The effect of prehabilitation on the range of motion and functional outcomes in patients following the total knee or hip arthroplasty: a pilot randomized trial. Physiother Theory Pract 2016;32:262–270 [DOI] [PubMed] [Google Scholar]
- 47. Chakravartty S, Vivian G, Mullholland N, Shaikh H, McGrath J, Sidhu PS et al. Preoperative liver shrinking diet for bariatric surgery may impact wound healing: a randomized controlled trial. Surg Obes Relat Dis 2019;15:117–125 [DOI] [PubMed] [Google Scholar]
- 48. Dunne DFJ, Jack S, Jones RP, Jones L, Lythgoe DT, Malik HZ et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg 2016;103:504–512 [DOI] [PubMed] [Google Scholar]
- 49. Grąt M, Wronka KM, Lewandowski Z, Grąt K, Krasnodębski M, Stypułkowski J et al. Effects of continuous use of probiotics before liver transplantation: a randomized, double-blind, placebo-controlled trial. Clin Nutr Edinb Scotl 2017;36:1530–1539 [DOI] [PubMed] [Google Scholar]
- 50. Hollis G, Franz R, Bauer J, Bell J. Implementation of a very low calorie diet program into the pre-operative model of care for obese general elective surgery patients: outcomes of a feasibility randomised control trial. Nutr Diet 2020;77:490–498 [DOI] [PubMed] [Google Scholar]
- 51. Moug SJ, Mutrie N, Barry SJE, Mackay G, Steele RJC, Boachie C et al. Prehabilitation is feasible in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy and may minimize physical deterioration: results from the REx trial. Colorectal Dis 2019;21:548–562 [DOI] [PubMed] [Google Scholar]
- 52. Oosting E, Jans MP, Dronkers JJ, Naber RH, Dronkers-Landman CM, Appelman-de Vries SM et al. Preoperative home-based physical therapy versus usual care to improve functional health of frail older adults scheduled for elective total hip arthroplasty: a pilot randomized controlled trial. Arch Phys Med Rehabil 2012;93:610–616 [DOI] [PubMed] [Google Scholar]
- 53. Sahar W, Ajaz N, Haider Z, Jalal A. Effectiveness of pre-operative respiratory muscle training versus conventional treatment for improving post operative pulmonary health after coronary artery bypass grafting. Pak J Med Sci 2020;36:1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Santa Mina D, Hilton WJ, Matthew AG, Awasthi R, Bousquet-Dion G, Alibhai SMH et al. Prehabilitation for radical prostatectomy: a multicentre randomized controlled trial. Surg Oncol 2018;27:289–298 [DOI] [PubMed] [Google Scholar]
- 55. Sebio García R, Yáñez-Brage MI, Giménez Moolhuyzen E, Salorio Riobo M, Lista Paz A, Borro Mate JM. Preoperative exercise training prevents functional decline after lung resection surgery: a randomized, single-blind controlled trial. Clin Rehabil 2017;31:1057–1067 [DOI] [PubMed] [Google Scholar]
- 56. Fulop A, Lakatos L, Susztak N, Szijarto A, Banky B. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: a randomised clinical trial. Anaesthesia 2021;76:82–90 [DOI] [PubMed] [Google Scholar]
- 57. Woodfield JC, Clifford K, Wilson GA, Munro F, Baldi JC. Short-term high-intensity interval training improves fitness before surgery: a randomized clinical trial. Scand J Med Sci Sports 2022;32:856–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marchand AA, Houle M, O’Shaughnessy J, Châtillon CÉ, Cantin V, Descarreaux M. Effectiveness of an exercise-based prehabilitation program for patients awaiting surgery for lumbar spinal stenosis: a randomized clinical trial. Sci Rep 2021;11:11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barakat HM, Shahin Y, Khan JA, McCollum PT, Chetter IC. Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair: a randomized controlled trial. Ann Surg 2016;264:47–53 [DOI] [PubMed] [Google Scholar]
- 60. Hoogeboom TJ, Dronkers JJ, van den Ende CHM, Oosting E, van Meeteren NLU. Preoperative therapeutic exercise in frail elderly scheduled for total hip replacement: a randomized pilot trial. Clin Rehabil 2010;24:901–910 [DOI] [PubMed] [Google Scholar]
- 61. IJmker-Hemink VE, Wanten GJA, de Nes LCF, van den Berg MGA. Effect of a preoperative home-delivered, protein-rich meal service to improve protein intake in surgical patients: a randomized controlled trial. J Parenter Enteral Nutr 2021;45:479–489 [DOI] [PubMed] [Google Scholar]
- 62. Karenovics W, Licker M, Ellenberger C, Christodoulou M, Diaper J, Bhatia C et al. Short-term preoperative exercise therapy does not improve long-term outcome after lung cancer surgery: a randomized controlled study. Eur J Cardio-Thorac Surg 2017;52:47–54 [DOI] [PubMed] [Google Scholar]
- 63. Karlsson E, Farahnak P, Franzén E, Nygren-Bonnier M, Dronkers J, van Meeteren N et al. Feasibility of preoperative supervised home-based exercise in older adults undergoing colorectal cancer surgery—a randomized controlled design. PLoS One 2019;14:e0219158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rolving N, Nielsen CV, Christensen FB, Holm R, Bünger CE, Oestergaard LG. Preoperative cognitive-behavioural intervention improves in-hospital mobilisation and analgesic use for lumbar spinal fusion patients. BMC Musculoskelet Disord 2016;17:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tew GA, Batterham AM, Colling K, Gray J, Kerr K, Kothmann E et al. Randomized feasibility trial of high-intensity interval training before elective abdominal aortic aneurysm repair. Br J Surg 2017;104:1791–1801 [DOI] [PubMed] [Google Scholar]
- 66. Gloor S, Misirlic M, Frei-Lanter C, Herzog P, Müller P, Schäfli-Thurnherr J et al. Prehabilitation in patients undergoing colorectal surgery fails to confer reduction in overall morbidity: results of a single-center, blinded, randomized controlled trial. Langenbecks Arch Surg 2022;407:897–907 [DOI] [PubMed] [Google Scholar]
- 67. Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg 2018;153:1081–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Minnella EM, Awasthi R, Bousquet-Dion G, Ferreira V, Austin B, Audi C et al. Multimodal prehabilitation to enhance functional capacity following radical cystectomy: a randomized controlled trial. Eur Urol Focus 2021;7:132–138 [DOI] [PubMed] [Google Scholar]
- 69. Steffens D, Young J, Beckenkamp PR, Ratcliffe J, Rubie F, Ansari N et al. Feasibility and acceptability of a preoperative exercise program for patients undergoing major cancer surgery: results from a pilot randomized controlled trial. Pilot Feasibility Stud 2021;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu Z, Qiu T, Pei L, Zhang Y, Xu L, Cui Y et al. Two-week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: a randomized controlled trial. Anesth Analg 2020;131:840–849 [DOI] [PubMed] [Google Scholar]
- 71. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–784 [DOI] [PubMed] [Google Scholar]
- 72. Mouch CA, Kenney BC, Lorch S, Montgomery JR, Gonzalez-Walker M, Bishop K et al. Statewide prehabilitation program and episode payment in Medicare beneficiaries. J Am Coll Surg 2020;230:306–313.e6 [DOI] [PubMed] [Google Scholar]
- 73. Lee A, Shelton E, Bidwell S, Shankar K, Ando K, Gaudilliere B et al. Association of prehabilitation with postoperative opioid use in colorectal surgery: an observational cohort study. J Surg Res 2022;273:226–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Principles and guidance for prehabilitation within the management and support of people with cancer. https://www.macmillan.org.uk/dfsmedia/1a6f23537f7f4519bb0cf14c45b2a629/13225-source/prehabilitation-for-people-with-cancer.
- 75. Carli F, Bousquet-Dion G, Awasthi R, Elsherbini N, Liberman S, Boutros M et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg 2020;155:233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr Edinb Scotl 2017;36:1187–1196 [DOI] [PubMed] [Google Scholar]
- 77. Bolshinsky V, Li MHG, Ismail H, Burbury K, Riedel B, Heriot A. Multimodal prehabilitation programs as a bundle of care in gastrointestinal cancer surgery: a systematic review. Dis Colon Rectum 2018;61:124–138 [DOI] [PubMed] [Google Scholar]
- 78. Faithfull S, Turner L, Poole K, Joy M, Manders R, Weprin J et al. Prehabilitation for adults diagnosed with cancer: a systematic review of long-term physical function, nutrition and patient-reported outcomes. Eur J Cancer Care (Engl) 2019;28:e13023. [DOI] [PubMed] [Google Scholar]
- 79. Perry R, Herbert G, Atkinson C, England C, Northstone K, Baos S et al. Pre-admission interventions (prehabilitation) to improve outcome after major elective surgery: a systematic review and meta-analysis. BMJ Open 2021;11:e050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Childs CE, Calder PC, Miles EA. Diet and immune function. Nutrients 2019;11:1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev 2018;98:477–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gaudillière B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M et al. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med 2014;6:255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fragiadakis GK, Gaudillière B, Ganio EA, Aghaeepour N, Tingle M, Nolan GP et al. Patient-specific immune states before surgery are strong correlates of surgical recovery. Anesthesiology 2015;123:1241–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cong-Tri T. Qualité des soins perçue par le patient—Indicateurs PROMs et PREMs. 2021, p.134. https://www.has-sante.fr/upload/docs/application/pdf/2022-03/proms_prems_panorama_of_experiences_in_other_countries_vf.pdf
- 85. Graupner C, Kimman ML, Mul S, Slok AHM, Claessens D, Kleijnen J et al. Patient outcomes, patient experiences and process indicators associated with the routine use of patient-reported outcome measures (PROMs) in cancer care: a systematic review. Support Care Cancer 2021;29:573–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Duro-Ocana P, Zambolin F, Jones AW, Bryan A, Moore J, Quraishi-Akhtar T et al. Efficacy of supervised exercise prehabilitation programs to improve major abdominal surgery outcomes: a systematic review and meta-analysis. J Clin Anesth 2023;86:111053. [DOI] [PubMed] [Google Scholar]
- 87. Gillis C, Buhler K, Bresee L, Carli F, Gramlich L, Culos-Reed N et al. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta-analysis. Gastroenterology 2018;155:391–410.e4 [DOI] [PubMed] [Google Scholar]
- 88. Zheng YT, Zhang JX. Preoperative exercise and recovery after cardiac surgery: a meta-analysis. BMC Cardiovasc Disord 2020;20:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Arumugam S, El-Menyar A, Al-Hassani A, Strandvik G, Asim M, Mekkodithal A et al. Delirium in the intensive care unit. J Emerg Trauma Shock 2017;10:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Moitra VK, Guerra C, Linde-Zwirble WT, Wunsch H. Relationship between ICU length of stay and long-term mortality for elderly ICU survivors. Crit Care Med 2016;44:655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Righy C, Rosa RG, da Silva RTA, Kochhann R, Migliavaca CB, Robinson CC et al. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: a systematic review and meta-analysis. Crit Care 2019;23:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bagshaw SM, Tran DT, Opgenorth D, Wang X, Zuege DJ, Ingolfsson A et al. Assessment of costs of avoidable delays in intensive care unit discharge. JAMA Netw Open 2020;3:e2013913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Milder D, Pillinger N, Kam P. The role of prehabilitation in frail surgical patients: a systematic review. Acta Anaesthesiol Scand 2018;62:1356–1366 [DOI] [PubMed] [Google Scholar]
- 94. Barberan-Garcia A, Ubré M, Roca J, Lacy AM, Burgos F, Risco R. et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018;267:50–56 [DOI] [PubMed] [Google Scholar]
- 95. Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010;210:901–908 [DOI] [PubMed] [Google Scholar]
- 96. McIsaac DI, Hladkowicz E, Bryson GL, Forster AJ, Gagne S, Huang A et al. Home-based prehabilitation with exercise to improve postoperative recovery for older adults with frailty having cancer surgery: the PREHAB randomised clinical trial. Br J Anaesth 2022;129:41–48 [DOI] [PubMed] [Google Scholar]
- 97. McIsaac DI, Aucoin SD, van Walraven C. A Bayesian comparison of frailty instruments in noncardiac surgery: a cohort study. Anesth Analg 2021;133:366–373 [DOI] [PubMed] [Google Scholar]
- 98. National Guideline Centre (UK) . Evidence Review for Preoperative Risk Stratification Tools: Perioperative Care in Adults: Evidence Review C [Internet]. London: National Institute for Health and Care Excellence (NICE), 2020 [PubMed] [Google Scholar]
- 99. Verdonk F, Einhaus J, Tsai AS, Hedou J, Choisy B, Gaudilliere D et al. Measuring the human immune response to surgery: multiomics for the prediction of postoperative outcomes. Curr Opin Crit Care 2021;27:717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rumer KK, Hedou J, Tsai A, Einhaus J, Verdonk F, Stanley N et al. Integrated single-cell and plasma proteomic modeling to predict surgical site complications: a prospective cohort study. Ann Surg 2022;275:582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Full data are available upon request to the corresponding author.