Abstract
Laryngopharyngeal reflux (LPR) is characterized by the reflux of gastric contents into the pharynx or larynx and often presents with symptoms including but not limited to cough, throat clearing, sore throat, globus, and dysphonia. Unlike gastroesophageal reflux disease (GERD), LPR is a relatively understudied syndrome, and knowledge regarding the diagnostic and treatment strategies, as well as the psychosocial impact continues to evolve. No singular test or procedure currently exists as a gold standard for LPR diagnosis. While laryngoscopy or pH monitoring may be positive, this does not exclude the contribution of non-gastroenterological processes. Prior research into psychosocial impact demonstrates a significant increase in symptom burden when comparing patients with laryngeal symptoms to controls and those with isolated GERD symptoms. However, these data are limited by the absence of physiologic data to correlate with the reported symptoms and survey responses. This knowledge gap highlights the need for further research to investigate the relationship between symptom burden and pathologic acid reflux on quality of life (QOL), anxiety, and depression. Ultimately, future studies to directly analyze these variables will help to guide treatment strategies and improve QOL in these patients.
Keywords: Extra esophageal reflux, Anxiety, Depression, Disease burden
Graphical Abstract
Introduction/Background
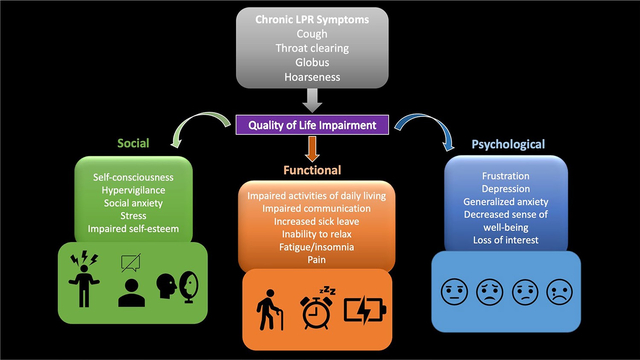
Laryngopharyngeal reflux (LPR) is a condition in which reflux of gastric contents into the esophagus leads to extra-esophageal symptoms such as cough, throat clearing, sore throat, globus, and dysphonia [1, 2]. Though originating through similar mechanisms, LPR and gastroesophageal reflux disease (GERD) differ in diagnostic and treatment guidelines, as well as their psychosocial impact. Additionally, LPR is not limited to a gastroenterological mechanism. Its symptoms can be caused by myriad conditions—such as voice disorders, medications, neurogenic, pulmonary, smoking, environment, or allergies. Further, even in the setting of LPR, laryngeal behavioral processes can augment symptoms. For instance, throat clearing and/or cough can persist due to learned hyper-responsive behaviors and similarly hoarseness can represent a biomechanical dysfunction.
For patients with typical GERD symptoms and no alarm features, guidelines suggest that an empiric proton pump inhibitor (PPI) trial can be considered. However, due to the inconsistent response to empiric PPI therapy in patients with LPR, upfront ambulatory reflux monitoring is recommended in patients with isolated laryngeal symptoms, whereas an empiric trial of PPIs is reasonable in patients with concomitant esophageal symptoms. Notably, current guidelines suggest that laryngoscopy is not sufficient to diagnose LPR [3]. Multichannel intraluminal impedance and pH monitoring also possesses limitations through both the snapshot in time at which the data are collected over a person’s variable symptom presentation as well as the multifactorial nature of the disease itself. Additionally, there are many limitations with oropharyngeal monitoring and this is not recommended as a diagnostic test by recent guidelines and best practices due to lack of evidence on its utility.
Obtaining an adequate diagnosis can have implications on treatment strategies for patients. In GERD, it is well understood that symptoms do not always follow pathology. Many patients with GERD symptoms experience significant quality of life impairment emotionally, physically, and socially [4]. This heightened levels of hypervigilance and anxiety in these patients occurs even in the absence of correlating physiological data, suggesting a multidimensional etiology of the observed burden in patients—specifically, the emotional distress about the presence of symptoms themselves [5, 6]. This understanding of the cognitive-affective contribution to esophageal symptom burden has evolved treatment guidelines to include psychological in addition to physiological treatment with proton pump inhibitors. Therapies targeting the gut-brain axis, such as hypnotherapy, cognitive behavioral therapy, diaphragmatic breathing, relaxation strategies, and anti-depressants can be very helpful in symptom improvement in these patients [6].
With regard to LPR, research into the symptom impact on QOL is much more limited, which can have significant implications on our understanding of how to adequately manage these patients. In the few studies over the last two decades that have examined this area, researchers have found that quality of life (QOL), depression, anxiety, and social functioning in patients presenting with laryngeal symptoms are significantly worse than control and GERD counterparts. Outcomes questionnaires that have been utilized in prior research studies include a number of surveys to assess the psychosocial burden such as Short-Form 36 (SF-36), and 12 (SF-12), Hospital Anxiety and Depression Scale (HADS), Sino-Nasal Outcome Test (SNOT-22), Reflux Symptom Index (RSI), Voice Handicap Index (VHI), and Health-Related Quality of Life (HRQOL). Survey domains of focus, scoring, and outcome measures are summarized in Table 1. Unfortunately, a significant limitation of these studies is the reliance on laryngoscopy and symptoms to diagnose LPR, as opposed to ambulatory reflux monitoring, making it challenging to fully understand that underlying pathophysiology driving symptoms and consequent impact on QOL.
Table 1.
Quality of life surveys
| Survey | Domains of focus | Scoring | Outcome measures |
|---|---|---|---|
|
| |||
| SF-36 [7, 8] SF-12 [9, 10] |
Physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, mental health | 0–100 | < 50 Abnormal |
| HADS [11, 12] | Depression and Anxiety | 0–21 | 8–10 Borderline > 11 Abnormal |
| SNOT-22 [13, 14] | Social/emotional impact, productivity, sleep | 0–110 | 8–17 Mild 23–48 Moderate 54–83 Severe |
| RSI [15, 16] | LPR Symptoms | 0–45 | > 13 Abnormal |
| VHI [17] | Emotional, physical, and functional aspects of voice disorders | 0–120 | 0–30 Mild 31–60 Moderate 60–120 Severe |
| HRQOL [18, 19] | Hoarseness, cough, throat clearing, swallowing, overall impact of acid reflux | 0–82 Voice 0–46 Cough 0–46 Throat 0–40 Swallow 0–100 Overall Impact |
Undefined, higher scores correlate with greater disease burden |
| EQ-5D [20] (Korean Modified) | Mobility, self-care, usual activities, pain/discomfort, anxiety, depression | 0–1 | 0–1 Abnormal (1 = full health) |
| EHAS [5, 21] | Hypervigilance, symptom-specific anxiety | 0–60 | > 21 Abnormal |
This goal of this review is to compile prior research on the psychosocial impact of laryngeal symptoms on patient QOL, anxiety, and depression, as well as identifying knowledge gaps for future research.
Review of Literature
There have been a handful of studies that examine QOL, including anxiety, depression, social interaction, and physical well-being, in patients with LPR symptoms and it has been repeatedly found that patients with LPR symptoms have worse quality of life emotionally, physically, and socially than patients with GERD symptoms only and controls. Prior literature can be separated by surveys used to evaluate QOL and how LPR was defined (listed in Table 2).
Table 2.
LPR inclusion criteria and survey selection
| Study | LPR definition | Surveys |
|---|---|---|
|
| ||
| Carrau et al. [22] | 1. Symptoms:* > 1 in the past month 2. Laryngoscopy: findings** of laryngitis in the past month 3. Other; New diagnosis (< 1mo) or relapsed not on treatment |
SF-36 |
| Cheung et al. [23] | 1. Symptoms: > 1 month in the past year 2. Laryngoscopy: VSL evidence of LPR, with a RFS of > 15 3. Other: Absence of upper respiratory tract infection in < 4 weeks, or allergic causes of laryngitis, or chronic respiratory disease |
HADS, VHI, SF-36 |
| Brown et al. [2] | 1. Symptoms: Presence of an accepted laryngeal symptom 2. Laryngoscopy: findings of laryngeal edema or erythema in posterior region 3. Other: RSI score > 13 |
SNOT-22, RSI |
| Lechien et al. [24] | 1. Symptoms: RSI score > 13 2. Laryngoscopy: RFS score > 7 |
RSI, SF-36, VHI |
| Siupsinskiene et al. [25] | 1. Symptoms: > 2 Laryngeal Symptoms both > 2 on Likert Scale for > 1 month 2. Laryngoscopy: findings of Laryngitis 3. Other: Absence of alternative diagnosis |
VHI, HADS, W-Bvas |
| Kim et al. [18] | 1. > 1 reflux event to larynx or pharynx on 24-h MII-pH monitoring 2. Laryngeal symptoms: globus, hoarseness, or chronic cough |
SF-12, RSI, HRQOL |
| Gong et al. [26] | 1. Symptoms: RSI score > 14 and Laryngeal symptoms at least once per week for > 1 month | EQ-5D (Korean modified) |
| Lenderking et al. [4] | Other: Patients receiving treatment at selected Otolaryngology clinics for LPR | Focus Group |
| Krause et al. [5] | 1. Symptoms: adults with laryngeal symptoms 2. pH Impedance: acid exposure time (AET) ≥ 6% on reflux monitoring |
EHAS, RSI, GerdQ |
Criteria are defined by a combination of symptoms, laryngoscopy findings, or pH-impedance monitoring parameters
Hoarseness, chronic cough (defined as cough > 1 month), globus, laryngospasm, chronic throat clearing, difficulty swallowing
Subglottic edema, ventricular obliteration, arytenoid erythema, vocal fold edema, diffuse laryngeal edema, posterior commissure hypertrophy, granuloma/granulation, and pachydermia larynges
Allergies, voice abuse, infection, sinus pathology, asthma, or active smoking
One of the first studies assessing QOL in patients with LPR symptoms was a systematic literature review by Lenderking et al. which found no prior studies on QOL in patients with LPR symptoms but did find several studies highlighting the impact of GERD on QOL. From these studies, the authors found evidence that QOL impairment in patients with GERD is significant and often these patients report higher rates of QOL impairment than those with other chronic diseases such as diabetes or hypertension [4]. Lenderking et al. also discussed the negative impact of laryngeal symptoms—specifically vocal dysfunction—on communication and social confidence. Due to absence of valid instruments to measure QOL in LPR at the time of the study, Lenderking et. al instead utilized studies regarding dysphonia and QOL to make assumptions on the burden of LPR on QOL; it was suspected that due to overlapping symptoms of dysphonia in vocal disorders and hoarseness with LPR, the negative social impact caused dysphonia (as determined by prior VHI studies) likely reflected the social consequences of the throat and voice changes precipitated by LPR. Focus groups later supported this suspicion as patients with laryngeal symptoms reported significant impact on self-esteem, frustration, stress, and relationships. Ultimately, the authors conclude that a disease specific instrument to assess impact of LPR on QOL is needed to qualify psychosocial impact more accurately and ultimately improve clinical care and therapeutic options [4].
The QOL studies conducted since this literature review can be differentiated by the definition of LPR and the outcomes measured (Table 2).
LPR Defined by Laryngeal Symptoms and/or Laryngoscopic/Endoscopic Findings
The following studies investigate QOL outcomes in patients with laryngeal symptoms and evidence of laryngeal abnormalities on laryngoscopic exam or endoscopic findings on upper GI endoscopy.
Brown et al. investigated 138 patients (60 LPR, 36 chronic rhino sinusitis, and 42 chronic rhinosinusitis + LPR) with chronic rhinosinusitis (CRS) and/or laryngeal symptoms with laryngoscopic evidence of laryngeal edema or erythema and found that those with CRS and laryngeal symptoms had significantly higher RSI and SNOT-22 scores when compared to patients with just CRS or laryngeal symptoms alone [2].
A prospective study by Carrau et al. utilized SF-36 to compare psychosocial impact in 117 patients with LPR (as defined by laryngeal symptoms and laryngoscopic findings as outlined in Table 2), patients with GERD, and healthy controls. Data for the GERD patients and healthy controls were derived from benchmark scores previously published for patients with GERD and the general US population. Analysis of this previous data against LPR patients using the same QOL measures found that seven of eight domains (Table 1) were significantly worse in patients with LPR when compared to healthy controls, with the most significant differences in social functioning and bodily pain. Additionally, social functioning and vitality domains in patients with LPR were significantly worse than patients with GERD; five of the six remaining domains had a clinically, but not statistically significant decrease for patients with LPR as compared to patients with GERD [22].
Cheung et al. compared 76 patients with LPR (as defined by laryngeal symptoms, reflux finding score > 15, and absence of alternative diagnosis) versus healthy controls in the Chinese population using the HADS, VHI, and SF-36. Patients with LPR had significantly higher rates of anxiety, clinically higher—but not statistically significant—rates of depression, significantly higher physical, emotional, and functional burden on the VHI survey, and worse outcomes across all SF-36 domains, most significantly in pain, general health perception, and social functioning with significantly increased sick leave and adverse social life impact, when compared to healthy controls [23].
Siupsinskiene et al. compared VHI, HADS, and well-being (W-Bvas) in 100 patients with laryngeal symptoms with findings of at least posterior laryngitis on laryngoscopy—21 of whom had esophagitis (Los Angeles grades A or B) on upper GI endoscopy and 79 without esophagitis—to healthy controls and assessed changes to these parameters after treatment with Omeprazole. Ultimately, researchers found that patients with laryngeal symptoms had worse scores on HADS, VHI and W-Bvas when compared to controls. There was no significant difference in QOL between patients with and without esophagitis. Treatment with omeprazole significantly improved QOL in both laryngeal subgroups patients [25].
Lechien et al. analyzed the impact of age on QOL in 80 patients. Patients were subdivided into three groups with suspected LPR before and after treatment with low-acid diet [27], lifestyle, and behavioral recommendations as well as twice daily Pantoprazole. Age groups were determined as follows: 18–39 (n = 21), 40–59 (n = 31), > 60 years (n = 28). All patients completed the RSI questionnaire and underwent reflux finding score (RFS) evaluation with video laryngostroboscopy. Inclusion criteria were RSI > 13 and RFS > 7. The only significant baseline difference between age groups was lower RSI score in patients > 60 years old compared to both younger age groups. However, all groups at baseline had abnormal RSI (23.94, 23.78, and 19.19, respectively) and RFS (10.00, 11.04, and 10.59, respectively) scores. Only one of the SF-36 domains was < 50 at baseline, which was vitality in the 18–39 age group. Otherwise, vitality, mental health, physical role limitations, and general health were the lowest scoring domains with scores between 50 and 65; 100 is regarded as perfect health, which suggests that though these values were not by definition meeting < 50 criteria for abnormality, the domain scoring was still markedly below what is considered perfect health at 50–65%. Significant improvements in RSI, VHI, and SF-36 (both physical and mental health) were found in all patient groups treated with Pantoprazole, lifestyle, and dietary interventions [24].
These studies highlight that patients with laryngeal symptoms and abnormal laryngoscopic findings seem to have greater disease burden than GERD patients, CRS patients, or healthy controls, but not more than laryngeal patients without laryngitis. In addition, one study suggests that patients with laryngeal symptoms had similar impact of symptom burden on quality of life, despite the presence/absence of Los Angeles grade A or B esophagitis.
LPR Defined by Symptoms Alone
One study defined LPR as individuals with an elevated RSI score, without concomitantly evaluating for physiologic evidence of disease.
Gong et al. examined QOL in 300 GERD patients (150 with and 150 without laryngeal symptoms) using a Korean version of EuroQol five dimensions (EQ-5D) questionnaire. A structured questionnaire on patient satisfaction and the Work Productivity and Activity Impairment questionnaire were also used. Results ultimately echoed prior studies in LPR QOL; the proportion of patients with laryngeal symptoms had more burden across domains than patients without laryngeal symptoms, with significant differences in pain/discomfort and anxiety/depression domains. Patients with laryngeal symptoms also had significantly lower satisfaction with treatment, greater sickness-related absent hours per week, and greater overall work impairment. This association with laryngeal symptoms and worsening QOL measures suggests that LPR imparts a greater burden on QOL than GERD [26].
This study similarly echoes the fact that patients with laryngeal symptoms seem to have increased QOL impairment when compared to patients with isolated GERD symptoms.
LPR Defined by Abnormal Ambulatory Reflux Testing
Two studies analyzed laryngeal symptoms in relation to ambulatory reflux testing to determine the association of objective data and QOL impairments.
Kim et al. analyzed the association between 24 h pH-impedance studies and QOL of 45 LPR patients using SF-12, RSI, and HRQOL and found that most parameters on 24 h pH-impedance testing had no or poor correlation with questionnaires. However, two parameters did have a strong correlation. Number of non-acid reflux events that reached the larynx and pharynx and total number of events that reached the larynx and pharynx were strongly associated with heartburn on RSI in regression analyses. The only significant association found in multiple regressions analyses was between the number of non-acid reflux events reaching the larynx/pharynx and the voice/hoarseness component of the HRQOL questionnaire [18].
Similarly, Krause et al. utilized the Esophageal Hypervigilance and Anxiety Scale (EHAS), a validated questionnaire utilized to assess hypervigilance and anxiety in patients with esophageal symptoms, in 77 patients with laryngeal symptoms with and without pathologic LPR (defined by acid exposure time (AET) on ambulatory reflux monitoring ≥ 6%). Twenty-two (29%) met criteria for an elevated acid exposure time. There were no significant differences between Gastroesophageal Reflux Disease Questionnaire (GerdQ) or RSI scores between groups. The authors found similarly elevated EHAS scores in both patient groups, suggesting that physiological burden does not fully account for psychological distress [5].
These studies suggest that perhaps cognitive-affective processes are interrelated with laryngeal symptom burden, which has implications in management of these patients.
Limitations of LPR Research
Review of prior research into the psychosocial impact of LPR emphasizes a significant burden across most life domains, especially quality of life, anxiety, depression, and social functioning. A significant limitation in many of the studies examining LPR and QOL lies in the absence of a consistent methodology to diagnose LPR, as well as the reliance on laryngoscopy to define patients with LPR, which is not recommended in current guidelines as laryngoscopy findings are ultimately subjective, with wide variability among examiners [3, 28]. Currently, ambulatory reflux monitoring with either prolonged wireless reflux monitoring or 24 h pH-impedance testing is the reference standard for the diagnosis of LPR, and data on QOL, depression, and anxiety in patients with LPR diagnosed based on these tests are lacking [3].
Opportunities for Future Investigation
As outlined above, laryngeal symptoms are associated with a significant burden on QOL, anxiety, depression, and social functioning. Studies discussing the mechanism of disease suggest that laryngeal symptoms are likely multifactorial [1, 22]. This is further supported by the fact that patients with LPR tend to respond poorly to traditional GERD therapies, suggesting there is likely another component, perhaps anxiety or hypervigilance, that is contributing to this observed impairment in psychosocial functioning [2, 5]. While cognitive-affective impact on symptomatic experience is an increasingly accepted notion in gastroesophageal reflux patients [4], prior literature suggests that there is likely a similar cognitive interplay in laryngeal symptoms on symptom outcomes, which warrants further investigation in order to better target treatments for these patients.
Conclusion
Research into LPR—diagnosis, treatment, and psychosocial burden—is a developing field that has shown LPR to be an increasingly complex disease that is somewhat different from its much more well-known and studied counterpart, GERD. One of these distinct differences is the impact on quality of life, anxiety, depression, and social functioning. Though research demonstrates that both GERD and laryngeal symptoms can cause impaired QOL, anxiety, and depression compared to controls, the literature suggests that patients with laryngeal symptoms suffer the greatest burden across most domains.
However, prior literature fails to use consistent LPR selection criteria and to incorporate guideline-driven diagnostic testing strategies into the interpretation of psychosocial burden, which ultimately limits our ability to optimize the patient treatment and outcomes. Further research into quality of life with specific physiological parameters is necessary to properly interpret and reduce burden, identify etiology, and develop streamlined treatment options for these complex patients [1].
Funding
This work was supported by NIH 5T32DK007202 (Ghosh, PI); NIH K23 DK125266 (Yadlapati, PI); NIH R03 DK135513 (Yadlapati, PI).
Footnotes
Conflict of interest RY has served as a consultant for Medtronic, Ironwood Pharmaceuticals, Phathom Pharmaceuticals, StatLink MD and Medscape. RY has received research support from Ironwood Pharmaceuticals. RY has served on advisory boards for RJS Mediagnostix with stocks.
References
- 1.Krause AJ, Walsh EH, Weissbrod PA, Taft TH, Yadlapati R. An update on current treatment strategies for laryngopharyngeal reflux symptoms. Ann N Y Acad Sci. 2022;1510:5–17. 10.1111/nyas.14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown HJ, Kuhar HN, Plitt MA, Husain I, Batra PS, Tajudeen BA. The Impact of Laryngopharyngeal Reflux on Patient-reported Measures of Chronic Rhinosinusitis. Ann Otol Rhinol Laryngol. 2020;129:886–893. 10.1177/0003489420921424. [DOI] [PubMed] [Google Scholar]
- 3.Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am J Gastroenterol. 2022;117:27–56. 10.14309/ajg.0000000000001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenderking WR, Hillson E, Crawley JA, Moore D, Berzon R, Pashos CL. The clinical characteristics and impact of laryngopharyngeal reflux disease on health-related quality of life. Value Health 2003;6:560–565. 10.1046/j.1524-4733.2003.65243.x. [DOI] [PubMed] [Google Scholar]
- 5.Krause AJ, Greytak M, Burger ZC, Taft T, Yadlapati R. Hypervigilance and Anxiety are Elevated Among Patients With Laryngeal Symptoms With and Without Laryngopharyngeal Reflux. Clin Gastroenterol Hepatol. Oct 26 2022;doi: 10.1016/j.cgh.2022.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yadlapati R, Gyawali CP, Pandolfino JE. AGA Clinical Practice Update on the Personalized Approach to the Evaluation and Management of GERD: Expert Review. Clin Gastroenterol Hepatol. 2022;20:984–994.e1. 10.1016/j.cgh.2022.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brazier JE, Harper R, Jones NM et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Bmj. 1992;305:160–164. 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016;4:2050312116671725. 10.1177/2050312116671725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah CH, Brown JD. Reliability and Validity of the Short-Form 12 Item Version 2 (SF-12v2) Health-Related Quality of Life Survey and Disutilities Associated with Relevant Conditions in the U.S. Older Adult Population. J Clin Med. Feb 29 2020;9. doi: 10.3390/jcm9030661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soh SE, Morello R, Ayton D, et al. (2021) Measurement properties of the 12-item Short Form Health Survey version 2 in Australians with lung cancer: a Rasch analysis. Health Qual Life Outcomes 19:157. doi: 10.1186/s12955-021-01794-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Djukanovic I, Carlsson J, Årestedt K. Is the Hospital Anxiety and Depression Scale (HADS) a valid measure in a general population 65–80 years old? A psychometric evaluation study. Health Qual Life Outcomes. Oct 4 2017;15:193. doi: 10.1186/s12955-017-0759-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. Feb 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 13.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34:447–454. 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 14.Toma S, Hopkins C. Stratification of SNOT-22 scores into mild, moderate or severe and relationship with other subjective instruments. Rhinology. 2016;54:129–133. 10.4193/Rhino15.072. [DOI] [PubMed] [Google Scholar]
- 15.Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16:274–277. 10.1016/s0892-1997(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 16.Abraham ZS, Kahinga AA. Utility of reflux finding score and reflux symptom index in diagnosis of laryngopharyngeal reflux disease. 10.1002/lio2.799. Laryngoscope Investigative Otolaryngology. 2022/06/01 2022;7:785–789. doi: 10.1002/lio2.799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murry T, Rosen CA. Outcome measurements and quality of life in voice disorders. Otolaryngol Clin North Am. 2000;33:905–916. 10.1016/s0030-6665(05)70251-6. [DOI] [PubMed] [Google Scholar]
- 18.Kim SI, Kwon OE, Na SY, Lee YC, Park JM, Eun YG. Association between 24-hour combined multichannel intraluminal impedance-pH monitoring and symptoms or quality of life in patients with laryngopharyngeal reflux. Clin Otolaryngol. 2017;42:584–591. 10.1111/coa.12817. [DOI] [PubMed] [Google Scholar]
- 19.Carrau RL, Khidr A, Gold KF et al. Validation of a quality-of-life instrument for laryngopharyngeal reflux. Arch Otolaryngol Head Neck Surg. 2005;131:315–320. 10.1001/archotol.131.4.315. [DOI] [PubMed] [Google Scholar]
- 20.McCaffrey N, Kaambwa B, Currow DC, Ratcliffe J. Health-related quality of life measured using the EQ-5D-5L: South Australian population norms. Health Qual Life Outcomes. Sep 20 2016;14:133. doi: 10.1186/s12955-016-0537-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taft TH, Triggs JR, Carlson DA et al. Validation of the oesophageal hypervigilance and anxiety scale for chronic oesophageal disease. Aliment Pharmacol Ther. 2018;47:1270–1277. 10.1111/apt.14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrau RL, Khidr A, Crawley JA, Hillson EM, Davis JK, Pashos CL. The impact of laryngopharyngeal reflux on patient-reported quality of life. Laryngoscope. 2004;114:670–674. 10.1097/00005537-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Cheung TK, Lam PK, Wei WI et al. Quality of life in patients with laryngopharyngeal reflux. Digestion. 2009;79:52–57. 10.1159/000205267. [DOI] [PubMed] [Google Scholar]
- 24.Lechien JR, Finck C, Huet K, et al. Impact of age on laryngopharyngeal reflux disease presentation: a multi-center prospective study. European Archives of Oto-Rhino-Laryngology. 2017/10/01 2017;274:3687–3696. doi: 10.1007/s00405-017-4671-z [DOI] [PubMed] [Google Scholar]
- 25.Siupsinskiene N, Adamonis K, Toohill RJ. Quality of life in laryngopharyngeal reflux patients. Laryngoscope. 2007;117:480–484. 10.1097/MLG.0b013e31802d83cf. [DOI] [PubMed] [Google Scholar]
- 26.Gong EJ, Choi KD, Jung HK et al. Quality of life, patient satisfaction, and disease burden in patients with gastroesophageal reflux disease with or without laryngopharyngeal reflux symptoms. J Gastroenterol Hepatol. 2017;32:1336–1340. 10.1111/jgh.13716. [DOI] [PubMed] [Google Scholar]
- 27.Koufman JA. Low-acid diet for recalcitrant laryngopharyngeal reflux: therapeutic benefits and their implications. Ann Otol Rhinol Laryngol. 2011;120:281–287. 10.1177/000348941112000501. [DOI] [PubMed] [Google Scholar]
- 28.Campagnolo AM, Priston J, Thoen RH, Medeiros T, Assunção AR. Laryngopharyngeal reflux: diagnosis, treatment, and latest research. Int Arch Otorhinolaryngol. 2014;18:184–191. 10.1055/s-0033-1352504. [DOI] [PMC free article] [PubMed] [Google Scholar]


