Abstract
Background and Objectives
To examine the association of whole grain consumption and longitudinal change in global cognition, perceptual speed, and episodic memory by different race/ethnicity.
Methods
We included 3,326 participants from the Chicago Health and Aging Project who responded to a Food Frequency Questionnaire (FFQ), with 2 or more cognitive assessments. Global cognition was assessed using a composite score of episodic memory, perceptual speed, and the Mini Mental State Examination (MMSE). Diet was assessed by a 144-item FFQ. Linear mixed-effects models were used to estimate the association of intakes of whole grains and cognitive decline.
Results
This study involved 3,326 participants (60.1% African American [AA], 63.7% female) with a mean age of 75 years at baseline and a mean follow-up of 6.1 years. Higher consumption of whole grains was associated with a slower rate of global cognitive decline. Among AA participants, those in the highest quintile of whole grain consumption had a slower rate of decline in global cognition (β = 0.024, 95% CI [0.008–0.039], p = 0.004), perceptual speed (β = 0.023, 95% CI [0.007–0.040], p = 0.005), and episodic memory (β = 0.028, 95% CI [0.005–0.050], p = 0.01) compared with those on the lowest quintile. Regarding the amount consumed, in AA participants, those who consumed >3 servings/d vs those who consumed <1 serving/d had a slower rate of decline in global cognition (β = 0.021, 95% CI [0.005–0.036], p = 0.0093). In White participants, with >3 servings/d, we found a suggestive association of whole grains with global cognitive decline when compared with those who consumed <1 serving/d (β = 0.025, 95% CI [−0.003 to 0.053], p = 0.08).
Discussion
Among AA participants, individuals with higher consumption of whole grains and more frequent consumption of whole grain had slower decline in global cognition, perceptual speed, and episodic memory. We did not see a similar trend in White adults.
Introduction
Alzheimer disease and related dementia (ADRD) is a devastating neurodegenerative condition affecting more than 6 million Americans. The concurrent increase in the aging population and the prevalence of ADRD poses a significant burden on health care, social-economic, and public health systems. Primary prevention through modifiable lifestyle factors including diet has become a high public healthy priority. Previous evidence demonstrated that adherence to certain dietary patterns1 (i.e., MedDiet, Mediterranean-DASH Diet Intervention for Neurodegenerative Delay [MIND], diet, MIND diet or consumption of certain foods including green leafy vegetables2 and berries)2,3 was associated with slower cognitive decline in older adults across various race/ethnic groups.
The American Heart Association and Dietary Guidelines for Americans recommended whole grain as part of healthy dietary pattern.4 In the United States, total grain consumption (sum of refined and whole grains consumption) is exceeding the recommended daily intake levels5; however, whole grain consumption is falling short across the population.5 Whole grains preserve more fiber, polyphenols, minerals, and vitamins B and E6 compared with the refined-grain products. Previous epidemiologic studies demonstrated that whole grain consumption was associated with a lower risk of cardiovascular disease (CVD),7 type 2 diabetes,8 and other cardiometabolic diseases.9,10
Emerging evidence suggested that carotenoids (lutein, zeaxanthin, and β-cryptoxanthin) and polyphenol phytochemicals in whole grains11,12 such as phenols and flavonoids were associated with benefits in cognition. Recent evidence from a systematic review of 23 studies, including 4 randomized controlled trials and 19 observational studies suggests higher intakes of whole grain were associated with better score of mood and anxiety; however, inconsistent results were reported regarding cognition.13 In addition, there is limited evidence on how whole grain consumption relates to cognitive function, particularly, among different racial groups. The current evidence on relation of diet, nutrition, and risk of ADRD and cognitive function has been from cohorts and trials with predominantly White participants, which limited the generalization across different racial groups. Our previous research showed whole grain consumption as a subcomponent of a plant-based diet was significantly different between African American (AA) and White participants.14 Therefore, in this study, we aim to investigate the association of whole grain consumption and change of rate of global cognition, perceptual speed, and episodic memory among AA participants and White participants of the Chicago Healthy Aging Project.
Method
Study Population
The Chicago Health and Aging Project (CHAP), initiated in 1993, is a longitudinal, biracial, population-based study of Alzheimer disease (AD) dementia and other health conditions that enrolled individuals aged 65 years or older residing in neighborhoods on the south side of Chicago, Illinois.15 Of individuals who were identified from October 1993 to April 1997, 6,158 (79%) participated in an in-home interview, which collected information on demographic variables, health status, and current functioning. Participants' racial and ethnic identification were self-identification based on the 1990 census race classification.15 Physical and cognitive performance tests were also conducted as part of the in-home interview. We conducted in-home interview every 3 years repeatedly with up to 6 data collection cycles. The CHAP study is composed of 62% AA participants and 38% White participants and has a follow-up rate of 89% of surviving participants.
We included a total of 3,326 participants who responded to a dietary questionnaire, with 2 or more cognitive assessments and ApoE genotype assessment. We excluded participants with MMSE ≤10, with extreme body mass index (BMI) (<14 kg/m2 or >55 kg/m2, n = 202), and with implausible caloric intakes (<500 kcal or >3,800 kcal for women, <800 kcal or >4,200 kcal for men), or when an entire page or >50% of items were unanswered.16 Analysis was restricted to the first 10 years of follow-up after the initial FFQ.
Cognitive Function Assessment
At each cycle, during the in-home interview, 4 performance of cognitive functioning tests were conducted, consisting of 2 measures of episodic memory: immediate and delayed recall of 12 ideas contained in the East Boston Story,17,18 one measure of perceptual speed19,20: the oral form of the Symbol Digit Modalities Test,21 and the Mini-Mental State Examination,22 a widely used 30-item measure of global cognition. In a previous principal components analysis, all tests had loadings of 0.79 or more on a single factor accounting for 74% of the variance.23 Therefore, we used a summary measure including all 4 tests as an assessment of global cognition. Combining all 4 tests creates a global measure that reduces the effect of any skewness and floor or ceiling effects that can come from individual tests.
For each test, the raw scores were transformed to z scores using the mean and SDs from the total population at baseline. For global cognitive score, we created a composite z score by averaging the z scores of all 4 tests.23 With this approach, the cognitive function is therefore standardized with positive scores indicating higher performance.24 For the episodic memory score, we averaged the z scores of the East Boston test; the perceptual speed score was the z score from the Symbol Digit Modalities Test. The MMSE variable is highly skewed; if used independently, it would violate the normality assumptions of the linear mixed-effects models used in our analysis.
Dietary Assessment
Diet was assessed using a 144-item semiquantitative Food Frequency Questionnaire (FFQ) modified from the Harvard FFQ.25 Measurements of whole grain and refined-grain goods along with other aspects of diet were assessed by FFQ. FFQs were administered at each cycle. We used dietary data from the first available FFQ in this study. For each food, participants were asked on average the frequency of their consumption of specific foods or beverage with predetermined portion sizes over the preceding year.25 The modified FFQ was validated for use in older Chicago residents.25 The calculation of nutrient intake was based on the food composition databases of US Department of Agriculture and Harvard University, which has been updated over time to incorporate new food items and to reflect the changes in food composition. For each food, daily consumption was calculated by multiplying frequency per day by either the natural portion size or the serving sizes based on sex-specific mean portion sizes based on a national survey for older adults.25 Whole grain foods included dark bread, whole grain breakfast cereal, cornbread, and other grains i.e., kasha, couscous, bulgar, quinoa, tortilla, and popcorn. Refined grain included white bread, pita bread, or toast, English muffins or bagels, muffin, rice, pancakes or waffles, crackers, noodles, pasta, spaghetti, French toast, biscuit, pretzel, cold breakfast cereal, and PopTarts.
Assessment of Covariates
During the in-home interview, we used the 1990 US Census questionnaire collecting various social and demographic characteristics including age (years), sex (male/female), education (number of years in school), smoking status (never smoker vs current or former smoker), and ethnicity.23 Weight (in kg) and height (in meters) were measured and used to compute BMI (in kg/m2). History of diabetes, hypertension, heart disease, myocardial infarction or digitalis use, stroke, and medication use were self-reported.15 Participants' cognitive activities were assessed by the type and frequency of 7 cognitive activities. A composite score was calculated as the mean frequency across all items.23
Statistical Analysis
The primary outcome was rate of decline in global cognition. The secondary outcome variables were rate of decline in perceptual speed and episodic memory. Data were presented as mean and SDs for continuous variables and frequency (%) for categorical variables. We used linear mixed models to examine the association of whole grain consumption and the longitudinal change in global cognition, episodic memory, and perceptual speed. The mixed model allows for both within-individual and between-individual variations. All models allowed for both random slope and intercept. Whole grain consumption was modeled as both continuous (serving/d) and categorical variables in quintiles, with the lowest quintile as the reference category. Median values of each quintile were used to calculate the p value for the trend. In addition, we modeled the whole grain consumption as categorical variable based on the frequency of consumption. Model of longitudinal change in cognitive function, perceptual speed, and episodic memory included whole grain consumption, age, sex, APOE ε4 allele, education, calorie, cognitive activities, smoking status, time, and an interaction term of each covariate by time. Time was defined as years since baseline (time of first FFQ). We then further accounted for comorbidities (history of hypertension, diabetes, myocardial infarction, and stroke) and their respective interactions with time in the model. We conducted several sensitivity analyses to test the robustness of our findings by: (1) further adjusting for BMI, (2) excluding participants with MMSE ≤24, and (3) adjusting for physical activity. Our previous research showed that there are distinct dietary patterns between AA and White participants; therefore, we presented the results stratified by race. SAS version 9.4 was used for data analysis with a type 1 error rate for significance at 0.05, and all tests were 2-sided.
Standard Protocol Approvals, Registrations, and Patient Consents
The institutional review board of Rush University Medical Center approved this study. All CHAP participants provided written informed consent.
Data Availability
Investigators may request access to data of the CHAP cohort study through the Rush Institute for Healthy Aging data portal (riha.rush.edu/dataportal.html) pending proposal approval and completion of a Data Use Agreement.
Results
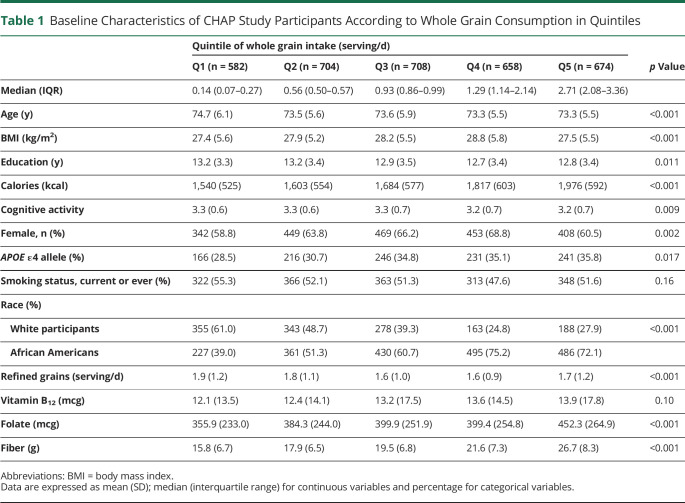
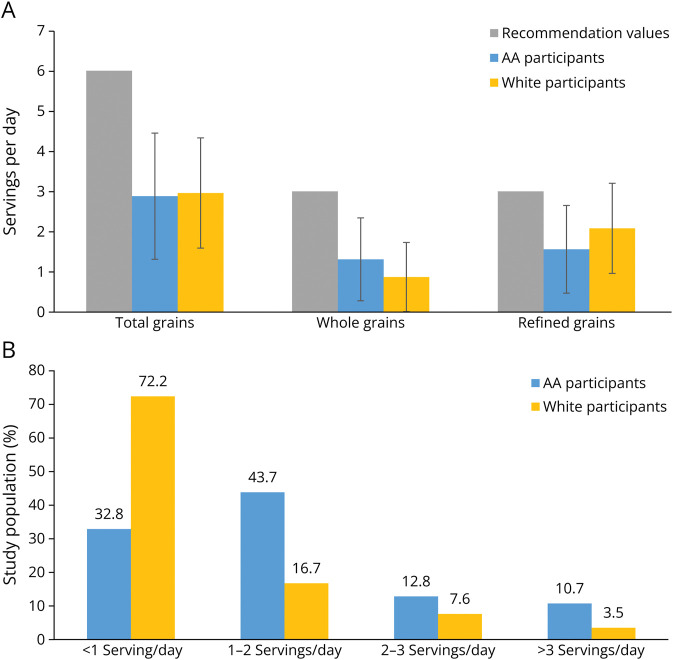
A total of 3,326 participants were included with an average of 3 observations per person and a total of 9,866 cognitive assessments (eFigure 1, links.lww.com/WNL/D178). Participant baseline characteristics are summarized in Table 1. Participants with higher whole grain consumption, on average, had higher intakes of calories, protein, fat, carbohydrate, folate, vitamin B12, and total fiber and a lower prevalence of comorbidities. There was a higher proportion of AA participants who consumed more servings of whole grains compared with that of White participants and a higher proportion of people with APOE ε4 in participants with high whole grain consumption. In the CHAP cohort, the average consumption of whole grain only met half of the recommendation values and AA adults having a slightly higher consumption of whole grains vs White participants (Figure 1A). Similarly, compared with White participants (28.8%), a higher proportion of AA participants (53.1%) had more than 1 serving/d of whole grains (Figure 1B).
Table 1.
Baseline Characteristics of CHAP Study Participants According to Whole Grain Consumption in Quintiles
| Quintile of whole grain intake (serving/d) | p Value | |||||
| Q1 (n = 582) | Q2 (n = 704) | Q3 (n = 708) | Q4 (n = 658) | Q5 (n = 674) | ||
| Median (IQR) | 0.14 (0.07–0.27) | 0.56 (0.50–0.57) | 0.93 (0.86–0.99) | 1.29 (1.14–2.14) | 2.71 (2.08–3.36) | |
| Age (y) | 74.7 (6.1) | 73.5 (5.6) | 73.6 (5.9) | 73.3 (5.5) | 73.3 (5.5) | <0.001 |
| BMI (kg/m2) | 27.4 (5.6) | 27.9 (5.2) | 28.2 (5.5) | 28.8 (5.8) | 27.5 (5.5) | <0.001 |
| Education (y) | 13.2 (3.3) | 13.2 (3.4) | 12.9 (3.5) | 12.7 (3.4) | 12.8 (3.4) | 0.011 |
| Calories (kcal) | 1,540 (525) | 1,603 (554) | 1,684 (577) | 1,817 (603) | 1,976 (592) | <0.001 |
| Cognitive activity | 3.3 (0.6) | 3.3 (0.6) | 3.3 (0.7) | 3.2 (0.7) | 3.2 (0.7) | 0.009 |
| Female, n (%) | 342 (58.8) | 449 (63.8) | 469 (66.2) | 453 (68.8) | 408 (60.5) | 0.002 |
| APOE ε4 allele (%) | 166 (28.5) | 216 (30.7) | 246 (34.8) | 231 (35.1) | 241 (35.8) | 0.017 |
| Smoking status, current or ever (%) | 322 (55.3) | 366 (52.1) | 363 (51.3) | 313 (47.6) | 348 (51.6) | 0.16 |
| Race (%) | ||||||
| White participants | 355 (61.0) | 343 (48.7) | 278 (39.3) | 163 (24.8) | 188 (27.9) | <0.001 |
| African Americans | 227 (39.0) | 361 (51.3) | 430 (60.7) | 495 (75.2) | 486 (72.1) | |
| Refined grains (serving/d) | 1.9 (1.2) | 1.8 (1.1) | 1.6 (1.0) | 1.6 (0.9) | 1.7 (1.2) | <0.001 |
| Vitamin B12 (mcg) | 12.1 (13.5) | 12.4 (14.1) | 13.2 (17.5) | 13.6 (14.5) | 13.9 (17.8) | 0.10 |
| Folate (mcg) | 355.9 (233.0) | 384.3 (244.0) | 399.9 (251.9) | 399.4 (254.8) | 452.3 (264.9) | <0.001 |
| Fiber (g) | 15.8 (6.7) | 17.9 (6.5) | 19.5 (6.8) | 21.6 (7.3) | 26.7 (8.3) | <0.001 |
Abbreviations: BMI = body mass index.
Data are expressed as mean (SD); median (interquartile range) for continuous variables and percentage for categorical variables.
Figure 1. Daily Intake (A) and Frequency of Intake (B) of Whole Grains Among CHAP Participants.
CHAP = Chicago Health and Aging Project.
Whole Grain Consumption, Refined-Grain Consumption, and Cognitive Decline
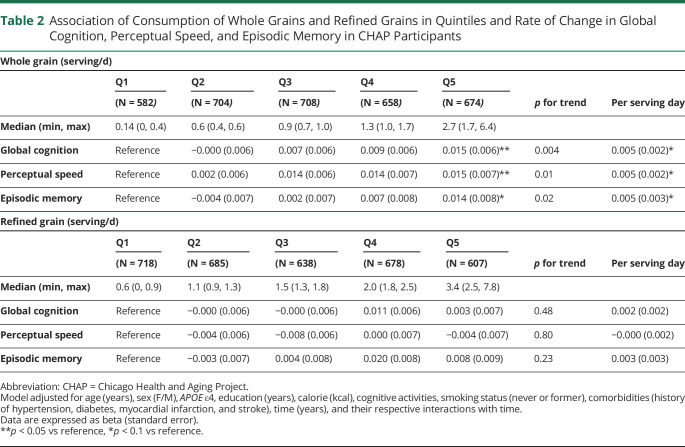
After adjusted for participant characteristics, higher whole grain consumption was associated with slower global cognitive decline (p for trend 0.004, Table 2). Participants in the highest quintile of consumption vs those in the lowest quintile had a slower rate of global cognitive decline (β = 0.015 ± 0.006, p = 0.013). Regarding individual cognitive tests, higher whole grain consumption was associated with slower change in rate of perceptual speed (p for trend 0.01) and episodic memory (p for trend 0.02). When compared with the lowest quintile, participants in the highest quintile of whole grain consumption had a slower rate of decline by β = 0.015 ± 0.007 (p = 0.026) for perceptual speed and by β = 0.014 ± 0.008 (p = 0.08) for episodic memory. There was no association between refined-grain consumption and change of rate of global cognition, perceptual speed, and episodic memory among study participants (Table 2).
Table 2.
Association of Consumption of Whole Grains and Refined Grains in Quintiles and Rate of Change in Global Cognition, Perceptual Speed, and Episodic Memory in CHAP Participants
| Whole grain (serving/d) | |||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | Per serving day | |
| (N = 582) | (N = 704) | (N = 708) | (N = 658) | (N = 674) | |||
| Median (min, max) | 0.14 (0, 0.4) | 0.6 (0.4, 0.6) | 0.9 (0.7, 1.0) | 1.3 (1.0, 1.7) | 2.7 (1.7, 6.4) | ||
| Global cognition | Reference | −0.000 (0.006) | 0.007 (0.006) | 0.009 (0.006) | 0.015 (0.006)** | 0.004 | 0.005 (0.002)* |
| Perceptual speed | Reference | 0.002 (0.006) | 0.014 (0.006) | 0.014 (0.007) | 0.015 (0.007)** | 0.01 | 0.005 (0.002)* |
| Episodic memory | Reference | −0.004 (0.007) | 0.002 (0.007) | 0.007 (0.008) | 0.014 (0.008)* | 0.02 | 0.005 (0.003)* |
| Refined grain (serving/d) | |||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | Per serving day | |
| (N = 718) | (N = 685) | (N = 638) | (N = 678) | (N = 607) | |||
| Median (min, max) | 0.6 (0, 0.9) | 1.1 (0.9, 1.3) | 1.5 (1.3, 1.8) | 2.0 (1.8, 2.5) | 3.4 (2.5, 7.8) | ||
| Global cognition | Reference | −0.000 (0.006) | −0.000 (0.006) | 0.011 (0.006) | 0.003 (0.007) | 0.48 | 0.002 (0.002) |
| Perceptual speed | Reference | −0.004 (0.006) | −0.008 (0.006) | 0.000 (0.007) | −0.004 (0.007) | 0.80 | −0.000 (0.002) |
| Episodic memory | Reference | −0.003 (0.007) | 0.004 (0.008) | 0.020 (0.008) | 0.008 (0.009) | 0.23 | 0.003 (0.003) |
Abbreviation: CHAP = Chicago Health and Aging Project.
Model adjusted for age (years), sex (F/M), APOE ε4, education (years), calorie (kcal), cognitive activities, smoking status (never or former), comorbidities (history of hypertension, diabetes, myocardial infarction, and stroke), time (years), and their respective interactions with time.
Data are expressed as beta (standard error).
**p < 0.05 vs reference, *p < 0.1 vs reference.
Association of Whole Grain Consumption and Cognitive Decline by Race
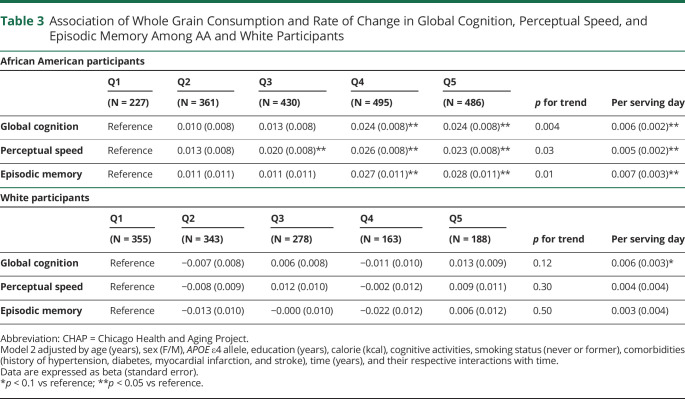
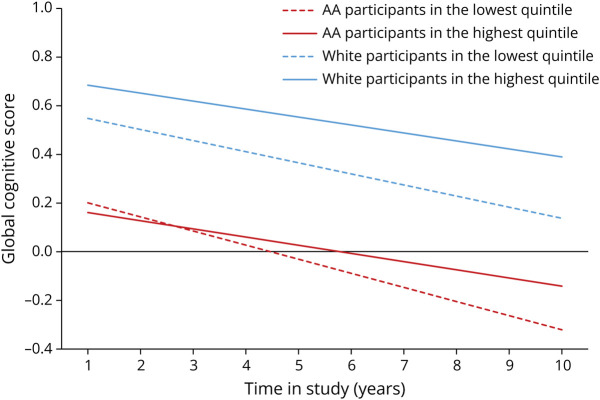
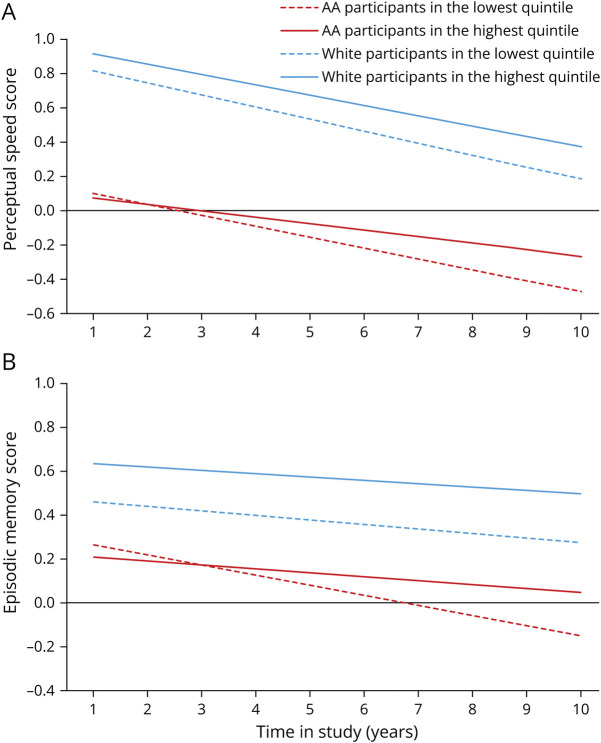
We observed the protective association between whole grain consumption and slower rate of decline in global cognition and individual tests of cognition in AA participants, but not in White participants (Table 3). Among AA adults, higher consumption of whole grains was associated with slower decline in global cognition (p for trend 0.004), perceptual speed (p for trend 0.03), and episodic memory (p for trend 0.01). Those in the highest quintile of consumption had a slower rate of decline by β = 0.024 ± 0.008 (p = 0.0043) in global cognition, β = 0.02 ± 0.008 (p = 0.025) in perceptual speed, and β = 0.027 ± 0.011 (p = 0.012) in episodic memory compared with the individuals in the lowest quintile of consumption. Among White participants, there was a suggestion of a protective association between whole grain consumption and rate of cognitive decline (p = 0.08). Whole grain consumption was not associated with a slower rate of cognitive decline among White participants (n = 1,249). As shown in Figure 2, rates of global decline among AAs in the highest quintile were attenuated by 38% when compared with individuals in the lowest quintile of whole grain consumption. We did not see a similar trend in White adults (Figure 2). Similarly, a slower rate of decline on perceptual speed (Figure 3A) and episodic memory (Figure 3B) was observed in AAs but not in White participants. When compared with years of cognitive aging, among AAs, those who were in the highest quintile of whole grain consumption had decline equivalent to being 8.5 years younger compared with those in the lowest quintile.
Table 3.
Association of Whole Grain Consumption and Rate of Change in Global Cognition, Perceptual Speed, and Episodic Memory Among AA and White Participants
| African American participants | |||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | Per serving day | |
| (N = 227) | (N = 361) | (N = 430) | (N = 495) | (N = 486) | |||
| Global cognition | Reference | 0.010 (0.008) | 0.013 (0.008) | 0.024 (0.008)** | 0.024 (0.008)** | 0.004 | 0.006 (0.002)** |
| Perceptual speed | Reference | 0.013 (0.008) | 0.020 (0.008)** | 0.026 (0.008)** | 0.023 (0.008)** | 0.03 | 0.005 (0.002)** |
| Episodic memory | Reference | 0.011 (0.011) | 0.011 (0.011) | 0.027 (0.011)** | 0.028 (0.011)** | 0.01 | 0.007 (0.003)** |
| White participants | |||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend | Per serving day | |
| (N = 355) | (N = 343) | (N = 278) | (N = 163) | (N = 188) | |||
| Global cognition | Reference | −0.007 (0.008) | 0.006 (0.008) | −0.011 (0.010) | 0.013 (0.009) | 0.12 | 0.006 (0.003)* |
| Perceptual speed | Reference | −0.008 (0.009) | 0.012 (0.010) | −0.002 (0.012) | 0.009 (0.011) | 0.30 | 0.004 (0.004) |
| Episodic memory | Reference | −0.013 (0.010) | −0.000 (0.010) | −0.022 (0.012) | 0.006 (0.012) | 0.50 | 0.003 (0.004) |
Abbreviation: CHAP = Chicago Health and Aging Project.
Model 2 adjusted by age (years), sex (F/M), APOE ε4 allele, education (years), calorie (kcal), cognitive activities, smoking status (never or former), comorbidities (history of hypertension, diabetes, myocardial infarction, and stroke), time (years), and their respective interactions with time.
Data are expressed as beta (standard error).
*p < 0.1 vs reference; **p < 0.05 vs reference.
Figure 2. Whole Grain Consumption and the Rate of Change in Global Cognitive Score Among African American and White Participants of the CHAP Study (N = 3,326).
The whole grain consumption was categorized into quintiles, with the lowest quintile as the referent group. Red dash line and the red solid line represent the lowest and highest quintiles of whole grain consumption among AA adults. Blue dash line and the blue solid line represent the lowest and highest quintiles of whole grain consumption among White adults. Model adjusted for age, sex, APOE ε4 allele, calorie intake, smoking status, cognitive activity, education, cardiovascular comorbidities, time, and time interaction with each condition. AAs = African Americans; CHAP = Chicago Health and Aging Project.
Figure 3. Whole Grain Consumption and the Rate of Change in Perceptual Speed Score (A) and Memory Score (B) Among African American and White Participants of the CHAP Study (N = 3,326).
The whole grain consumption was categorized into quintiles, with the lowest quintile as the referent group. Red dash line and the red solid line represent the lowest and highest quintiles of whole grain consumption among AA adults. Blue dash line and the blue solid line represent the lowest and highest quintiles of whole grain consumption among White adults. Model adjusted for age, sex, APOE ε4 allele, calorie intake, smoking status, cognitive activity, education, cardiovascular comorbidities, time, and time interaction with each condition. AAs = African Americans; CHAP = Chicago Health and Aging Project.
In sensitivity analyses, we further adjusted the model for BMI. The results remained similar. Among AA participants, those in the highest quintile had a slower decline in global cognition by β = 0.024 ± 0.008 (p = 0.0032) compared with those in the lowest quintile; per 1 serving/d increase in whole grain consumption was associated with a slower rate of decline in global cognition by β = 0.006 ± 0.002 (p = 0.0078). After further excluded participants with MMSE scores less than or equal to 24 (n = 441), among AA participants (n = 1,636), for global cognition, per 1 serving increase in whole grain consumption was associated with a slower rate of cognitive decline by β = 0.007 ± 0.002 (p = 0.0077); those in the highest quintile had a slower cognitive decline by β = 0.023 ± 0.009 (p = 0.0094) compared with those in the lowest quintile. Last, we further adjusted physical activity. The results remained unchanged. Among AA participants, those in the highest quintile had a slower cognitive decline by β = 0.024 ± 0.008 (p = 0.0038) compared with those in the lowest quintile. Among White participants, for global cognition, whole grain consumption was not associated with a slower rate of cognitive decline.
Association of Whole Grain Consumption and Cognitive Decline by the Frequency of Consumption
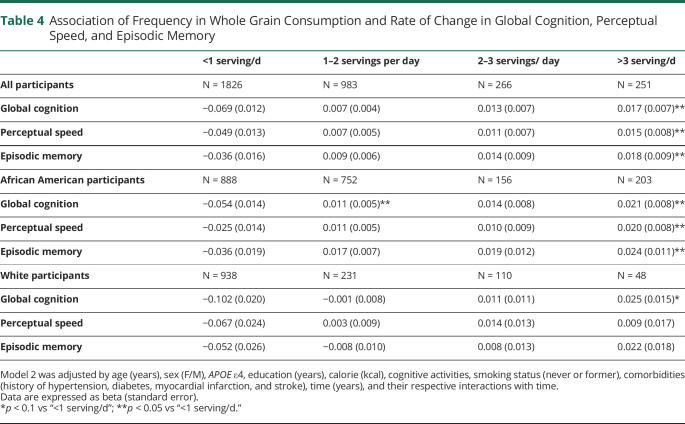
We further examined the association between the frequency of consuming whole grain products and cognitive decline (Table 4). Among all participants, those who consumed >3 serving/d (more than half of the recommendation values) had a slower rate of decline in global cognition (p = 0.012), perceptual speed (p = 0.049), and episodic memory (p = 0.048) vs those who consumed <1 serving/d. Among AA adults, compared with the individuals consuming whole grain <1 serving/d, those who consumed >3 servings/d had a slower rate of decline in global cognition β = 0.021 ± 0.008 (p = 0.0093), perceptual speed by β = 0.020 ± 0.008 (p = 0.015), and episodic memory by β = 0.024 ± 0.011 (p = 0.032) (Table 4). Among AA adults, those who had 1–2 serving per day also had a slower rate of decline in global cognition (β = 0.011 ± 0.005, p = 0.025) and episodic memory (β = 0.017 ± 0.007, p = 0.011) compared with the lowest consumption group. Among White adults, there is a marginal statistical significance when comparing those who consumed whole grain >3 servings/d with those who consumed <1 serving/d (β = 0.025 ± 0.015, p = 0.08).
Table 4.
Association of Frequency in Whole Grain Consumption and Rate of Change in Global Cognition, Perceptual Speed, and Episodic Memory
| <1 serving/d | 1–2 servings per day | 2–3 servings/ day | >3 serving/d | |
| All participants | N = 1826 | N = 983 | N = 266 | N = 251 |
| Global cognition | −0.069 (0.012) | 0.007 (0.004) | 0.013 (0.007) | 0.017 (0.007)** |
| Perceptual speed | −0.049 (0.013) | 0.007 (0.005) | 0.011 (0.007) | 0.015 (0.008)** |
| Episodic memory | −0.036 (0.016) | 0.009 (0.006) | 0.014 (0.009) | 0.018 (0.009)** |
| African American participants | N = 888 | N = 752 | N = 156 | N = 203 |
| Global cognition | −0.054 (0.014) | 0.011 (0.005)** | 0.014 (0.008) | 0.021 (0.008)** |
| Perceptual speed | −0.025 (0.014) | 0.011 (0.005) | 0.010 (0.009) | 0.020 (0.008)** |
| Episodic memory | −0.036 (0.019) | 0.017 (0.007) | 0.019 (0.012) | 0.024 (0.011)** |
| White participants | N = 938 | N = 231 | N = 110 | N = 48 |
| Global cognition | −0.102 (0.020) | −0.001 (0.008) | 0.011 (0.011) | 0.025 (0.015)* |
| Perceptual speed | −0.067 (0.024) | 0.003 (0.009) | 0.014 (0.013) | 0.009 (0.017) |
| Episodic memory | −0.052 (0.026) | −0.008 (0.010) | 0.008 (0.013) | 0.022 (0.018) |
Model 2 was adjusted by age (years), sex (F/M), APOE ε4, education (years), calorie (kcal), cognitive activities, smoking status (never or former), comorbidities (history of hypertension, diabetes, myocardial infarction, and stroke), time (years), and their respective interactions with time.
Data are expressed as beta (standard error).
*p < 0.1 vs “<1 serving/d”; **p < 0.05 vs “<1 serving/d.”
Discussion
In this study, we observed that whole grain consumption was associated with slower rate of decline in global cognition, perceptual speed, and episodic memory. The observed association of whole grain consumption and slower cognitive decline was predominant in AA adults. By contrast, there was no association between consumption of refined grains and cognitive function.
Whole grain consumption remains low in most countries.26 The Dietary Guidelines for Americans recommends adherence to a healthy dietary pattern, which includes grains, at least half of which are whole grain.27 However, whole grain consumption is suboptimal in US adults, with more than 90% not meeting the recommendation intakes.27 Among 3,326 CHAP participants, the average consumption of grain products is 3.2 serving/d with half from whole grains. In addition, we observed that more AA adults (67.2%) who had intakes >1 servings per day than White adults (37.8%).
Whole grains are nutrient dense, containing greater amount of minerals, trace elements, vitamins, and polyphenols compared with refined flours and foods.28 For instance, the refined whole grain wheat may have lost up to 58% fiber content, 90% of various minerals, 61% folate, and 79% of vitamin E.29 Whole grain has been included as part of recommended dietary patterns i.e., the Mediterranean dietary pattern,30 the Dietary Approaches to Stop Hypertension diet,31 and the MIND diet.1 Higher adherence to these dietary patterns has been associated with slower cognitive decline in various populations.1,32 As part of the dietary pattern, intakes of whole grains, nuts, and legumes were positively associated with better cognitive functions over 11 years of follow-up among adults aged 65 years or older without cognitive impairment.32 Higher intakes of whole grains in young adulthood were associated with better cognitive function later in life according to evidence from the Bogalusa heart study.33 Evidence from the Whitehall II study demonstrated that lower consumption of whole grains was part of a dietary pattern associated with higher inflammatory markers contributing to an accelerated cognitive decline at older ages.34
Whole grain is a great source of vitamins B and E, phytochemicals, carotenoids, and flavonoids.28 The antioxidant and anti-inflammatory properties of these bioactive compounds have been suggested as potential mechanisms of neuroprotection. High intakes of vitamin E from dietary source has been associated with lower risk of incident AD.35 Dietary carotenoids intakes36 and circulating alpha-carotene37 were positively associated with a higher score for cognition and lower risk of incident AD.38 A recent study that included more than 7,000 middle-aged and older adults demonstrated higher serum carotenoids was associated with lower risk of all-cause dementia.39 Vitamin B12 has been associated with slower cognitive decline among very old participants,40 and serum vitamin B12 was inversely associated with risk of dementia.41 Folic acid fortification of cereal grain products has been mandated since 1998. However, the association of folic acid with cognition is still inconclusive,40,42 and further investigations are warranted.
Emerging research on the microbiota-gut-brain axis suggested that fiber is a key contributor to the effects of nutrition on the brain.43 Evidence from observational studies demonstrated higher dietary fiber intake was associated with better cognitive outcomes.44-47 Although not fully understood, the potential mechanism underlying the effects of dietary fiber on cognitive function include (1) dietary fiber influences gut microbiota composition and function, (2) improvements in the immune system, and (3) blood lipid levels.43
In our previous study, we presented that AA adults had different dietary patterns than White adults in the CHAP study.14 This race-specific association may be partially explained by the differences in background diet among minorities groups. AA participants had significantly lower calorie intake and lower intake from animal foods, refined grain products, and significantly higher consumption of sugar-sweetened beverages and whole grains. Our previous research demonstrated a race-specific association of a healthful plant-based dietary pattern and cognitive function among AA participants.14 Higher intakes of whole grain may represent a relatively healthier dietary pattern. The results remain unchanged after we adjusted for other dietary factors in the models. Previous evidence suggests whole grain consumption was inversely associated with risk of coronary heart disease.48 AAs have higher prevalence of risk of cardiometabolic diseases i.e., diabetes, hypertension, and stroke. The protective association of whole grain on cognition may be through lowering the risk of cardiometabolic diseases.
Among White participants, those in the highest consumption quintile of whole grains had a baseline level of cognition (β = 0.14) higher than those in the lowest consumption quintile. By contrast, a difference of β = 0.04 was observed in AA participants when comparing the extreme quintiles at baseline. If whole grain consumption is indicative of a healthy dietary pattern, the discrepancy we observed at baseline year in White adults might reflect the effect of diet on cognition from prior years i.e., midlife.
Social and demographic factors may affect the relation of diet and cognition among older adults with robust association between diet and cognition observed among individuals with low social-economic status.49,50 The association of whole grain consumption and slower cognitive decline remained significant among AA participants but not White participants after we adjusted for potential confounding of social-economic status (i.e., education, cognitive activates) in the model. It is worth noting that diets are collectively determined and shaped by multiple factors including cultural norms, economic status, and available resources. And these factors are often subject to external structural forces, for instance, poverty and racism, which play a crucial role in shaping individuals' food choices and overall diet. We previously reported that the racial differences in the level of late-life cognition were significantly attenuated when considering demographic, health-related, and other social structural factors.51 The observed association in AAs could be partially due to the overall higher consumption and more participants who met the recommendations consuming >3 serving/d. The higher consumption may partly explain the protective association among AAs. Nevertheless, evidence from this study suggests that meeting the daily recommendation of whole grain consumption might potentially have cognitive benefits.
There are some limitations to be considered. First, the dietary data were self-reported using FFQ, which could be prone to recall error and misclassification of dietary intake although the validity of FFQ has been demonstrated using objective biochemical markers in older adults.25,52 To address the potential recall error related to cognitive decline, we excluded participants with MMSE less than 10 and for sensitivity, less than 24. Second, we examined the association of whole grain consumption and cognitive decline using the first available FFQ, which limited us to assess the causal association of dietary changes over time and the changes in cognitive function. The study demographic represents older adults from the south side of Chicago, which may potentially limit the generalization of our findings to populations with different socioeconomic status. Last, we included participants with measurement of APOE ε4 allele in the final model, which potentially reduced the sample size. Because AA adults have a higher prevalence of developing CVD, the race-specific association between diet and cognitive function could be mediated by the effects of diet on CVD. Although we have adjusted for multiple potential confounders for CVD comorbidities during follow-up in this analysis, we cannot completely rule out residual confounding. We must caution against a causal interpretation of findings given the observational study design. Future studies of different racial groups with larger sample sizes are warranted to validate our findings and to further investigate the causal role of whole grain on cognition. Strengths of this study include the prospective design in a biracial community cohort and the in-person evaluation of cognitive function.
In this study, AA adults had higher whole grain consumption compared with White participants. Among AAs, higher intake and more frequent intakes of whole grain were associated with slower rate of decline in global cognition, perceptual speed, and episodic memory. In White participants, higher consumption was not significantly associated with cognitive decline. The results presented in this study could be informative in facilitating tailored dietary recommendations for diverse populations.
Acknowledgment
The authors thank the participants of the CHAP study for their dedication to research and all research assistants, psychologists, and physicians of the CHAP study.
Glossary
- AAs
African Americans
- AD
Alzheimer disease
- ADRD
Alzheimer disease and related dementia
- BMI
body mass index
- CHAP
Chicago Health and Aging Project
- CVD
cardiovascular disease
- FFQ
Food Frequency Questionnaire
- MMSE
Mini Mental State Examination
Appendix. Authors
| Name | Location | Contribution |
| Xiaoran Liu | Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL; Department of Internal Medicine, Rush University Medical Center, Chicago, IL | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Todd Beck, MSc | Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL; Department of Internal Medicine, Rush University Medical Center, Chicago, IL | Analysis or interpretation of data |
| Klodian Dhana, MD, PhD | Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL; Department of Internal Medicine, Rush University Medical Center, Chicago, IL | Drafting/revision of the article for content, including medical writing for content |
| Pankaja Desai | Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL; Department of Internal Medicine, Rush University Medical Center, Chicago, IL | Drafting/revision of the article for content, including medical writing for content |
| Kristin R. Krueger, PhD | Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL; Department of Internal Medicine, Rush University Medical Center, Chicago, IL | Drafting/revision of the article for content, including medical writing for content |
| Christy C. Tangney, PhD, MS, BA | Department of Clinical Nutrition & Preventive Medicine, Rush University Medical Center, Chicago, IL | Drafting/revision of the article for content, including medical writing for content |
| Thomas M. Holland | Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL; Department of Internal Medicine, Rush University Medical Center, Chicago, IL | Drafting/revision of the article for content, including medical writing for content |
| Puja Agarwal, PhD | Department of Internal Medicine, Rush University Medical Center, Chicago, IL | Drafting/revision of the article for content, including medical writing for content |
| Denis A. Evans, PhD | Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL; Department of Internal Medicine, Rush University Medical Center, Chicago, IL | Study concept or design |
| Kumar B. Rajan, PhD | Rush Institute for Healthy Aging, Rush University Medical Center, Chicago, IL; Department of Internal Medicine, Rush University Medical Center, Chicago, IL | Major role in the acquisition of data; study concept or design |
Study Funding
This article has been funded by the Alzheimer's Association (AA) (AARG-22-928311), NIH (R01AG03154), NIH (R01AG051635), NIH (RF1AG057532), and NIH (R01AG058679).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015-1022. doi: 10.1016/j.jalz.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67(8):1370-1376. doi: 10.1212/01.wnl.0000240224.38978.d8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal P, Holland TM, Wang Y, Bennett DA, Morris MC. Association of strawberries and anthocyanidin intake with Alzheimer's dementia risk. Nutrients. 2019;11(12):3060. doi: 10.3390/nu11123060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenstein AH, Appel LJ, Vadiveloo M, et al. 2021 dietary guidance to improve cardiovascular health: a scientific statement from the American heart association. Circulation. 2021;144(23):e472-e487. doi: 10.1161/CIR.0000000000001031 [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025, 9th ed; 2020. DietaryGuidelines.gov [Google Scholar]
- 6.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010;23(1):65-134. doi: 10.1017/S0954422410000041 [DOI] [PubMed] [Google Scholar]
- 7.Bechthold A, Boeing H, Schwedhelm C, et al. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2019;59(7):1071-1090. doi: 10.1080/10408398.2017.1392288 [DOI] [PubMed] [Google Scholar]
- 8.Schwingshackl L, Hoffmann G, Lampousi AM, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363-375. doi: 10.1007/s10654-017-0246-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwingshackl L, Schwedhelm C, Hoffmann G, et al. Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2017;8(6):793-803. doi: 10.3945/an.117.017178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlesinger S, Neuenschwander M, Schwedhelm C, et al. Food groups and risk of overweight, obesity, and weight gain: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2019;10(2):205-218. doi: 10.1093/advances/nmy092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ndolo VU, Beta T. Distribution of carotenoids in endosperm, germ, and aleurone fractions of cereal grain kernels. Food Chem. 2013;139(1-4):663-671. doi: 10.1016/j.foodchem.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 12.Călinoiu LF, Vodnar DC. Whole grains and phenolic acids: a review on bioactivity, functionality, health benefits and bioavailability. Nutrients. 2018;10(11):1615. doi: 10.3390/nu10111615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross AB, Shertukde SP, Livingston Staffier K, Chung M, Jacques PF, McKeown NM. The relationship between whole-grain intake and measures of cognitive decline, mood, and anxiety-a systematic review. Adv Nutr. 2023;14(4):652-670. doi: 10.1016/j.advnut.2023.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Dhana K, Barnes LL, et al. A healthy plant-based diet was associated with slower cognitive decline in African American older adults: a biracial community-based cohort. Am J Clin Nutr. 2022;116(4):875-886. doi: 10.1093/ajcn/nqac204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago health and aging Project (CHAP). J Alzheimers Dis. 2003;5:349-355. doi: 10.3233/jad-2003-5501 [DOI] [PubMed] [Google Scholar]
- 16.Morris MC, Evans DA, Tangney CC, et al. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr. 2005;81(2):508-514. doi: 10.1093/ajcn.81.2.508 [DOI] [PubMed] [Google Scholar]
- 17.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci. 1991;57(3-4):167-178. doi: 10.3109/00207459109150691 [DOI] [PubMed] [Google Scholar]
- 18.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179-193. doi: 10.1037/0882-7974.17.2.179 [DOI] [PubMed] [Google Scholar]
- 19.Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61(6):812-816. doi: 10.1212/01.wnl.0000083989.44027.05 [DOI] [PubMed] [Google Scholar]
- 20.Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12):2322-2326. doi: 10.1212/01.wnl.0000147473.04043.b3 [DOI] [PubMed] [Google Scholar]
- 21.Smith A. Symbol Digit Modalities Test (SDMT): Manual. Western Psychological; 1982. [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 23.Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999;54(3):P155-P160. doi: 10.1093/geronb/54b.3.p155 [DOI] [PubMed] [Google Scholar]
- 24.Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA. Change in cognitive function in older persons from a community population: relation to age and Alzheimer disease. Arch Neurol. 1999;56(10):1274-1279. doi: 10.1001/archneur.56.10.1274 [DOI] [PubMed] [Google Scholar]
- 25.Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol. 2003;158(12):1213-1217. doi: 10.1093/aje/kwg290 [DOI] [PubMed] [Google Scholar]
- 26.Drewnowski A, McKeown N, Kissock K, et al. Perspective: why whole grains should be incorporated into nutrient-profile models to better capture nutrient density. Adv Nutr. 2021;12(3):600-608. doi: 10.1093/advances/nmaa172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025, 9th ed; 2020. [Google Scholar]
- 28.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010;23(1):65-134. doi: 10.1017/S0954422410000041 [DOI] [PubMed] [Google Scholar]
- 29.Truswell AS. Cereal grains and coronary heart disease. Eur J Clin Nutr. 2002;56:1-14. doi: 10.1038/sj.ejcn.1601283 [DOI] [PubMed] [Google Scholar]
- 30.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. doi: 10.1056/NEJMoa1800389 [DOI] [PubMed] [Google Scholar]
- 31.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-sodium collaborative research group. New Engl J Med. 2001;344(1):3-10. doi: 10.1056/NEJM200101043440101 [DOI] [PubMed] [Google Scholar]
- 32.Wengreen H, Munger RG, Cutler A, et al. Prospective study of dietary approaches to stop hypertension- and mediterranean-style dietary patterns and age-related cognitive change: the cache county study on memory, health and aging. Am J Clin Nutr. 2013;98(5):1263-1271. doi: 10.3945/ajcn.112.051276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fortune NC, Harville EW, Guralnik JM, et al. Dietary intake and cognitive function: evidence from the Bogalusa Heart Study. Am J Clin Nutr. 2019;109(6):1656-1663. doi: 10.1093/ajcn/nqz026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozawa M, Shipley M, Kivimaki M, Singh-Manoux A, Brunner EJ. Dietary pattern, inflammation and cognitive decline: the Whitehall II prospective cohort study. Clin Nutr. 2017;36(2):506-512. doi: 10.1016/j.clnu.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris MC, Evans DA, Bienias JL, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287(24):3230-3237. doi: 10.1001/jama.287.24.3230 [DOI] [PubMed] [Google Scholar]
- 36.Yuan C, Fondell E, Ascherio A, et al. Long-term intake of dietary carotenoids is positively associated with late-life subjective cognitive function in a prospective study in US women. J Nutr. 2020;150(7):1871-1879. doi: 10.1093/jn/nxaa087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Dhana K, Furtado JD, et al. Higher circulating α-carotene was associated with better cognitive function: an evaluation among the MIND trial participants. J Nutr Sci. 2021;10:e64. doi: 10.1017/jns.2021.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan C, Chen H, Wang Y, Schneider JA, Willett WC, Morris MC. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: a community-based cohort of older adults. Am J Clin Nutr. 2021;113(1):200-208. doi: 10.1093/ajcn/nqaa303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beydoun MA, Beydoun HA, Fanelli-Kuczmarski MT, et al. Association of serum antioxidant vitamins and carotenoids with incident Alzheimer disease and all-cause dementia among US adults. Neurology. 2022;98(21):e2150-e2162. doi: 10.1212/WNL.0000000000200289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris MC, Evans DA, Bienias JL, et al. Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch Neurol. 2005;62(4):641-645. doi: 10.1001/archneur.62.4.641 [DOI] [PubMed] [Google Scholar]
- 41.Smith AD, Warren MJ, Refsum H. Chapter six–vitamin B12. In: Eskin NAM, ed. Advances in Food and Nutrition Research. Academic Press; 2018:215-279. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Bao G, Liu D, et al. The association between folate and Alzheimer's disease: a systematic review and meta-analysis. Front Neurosci. 2021;15:661198. doi: 10.3389/fnins.2021.661198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muth AK, Park SQ. The impact of dietary macronutrient intake on cognitive function and the brain. Clin Nutr. 2021;40(6):3999-4010. doi: 10.1016/j.clnu.2021.04.043 [DOI] [PubMed] [Google Scholar]
- 44.Berti V, Murray J, Davies M, et al. Nutrient patterns and brain biomarkers of Alzheimer's disease in cognitively normal individuals. J Nutr Health Aging. 2015;19(4):413-423. doi: 10.1007/s12603-014-0534-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding B, Xiao R, Ma W, Zhao L, Bi Y, Zhang Y. The association between macronutrient intake and cognition in individuals aged under 65 in China: a cross-sectional study. BMJ Open. 2018;8(1):e018573. doi: 10.1136/bmjopen-2017-018573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gopinath B, Flood VM, Kifley A, Louie JCY, Mitchell P. Association between carbohydrate nutrition and successful aging over 10 years. J Gerontol A Biol Sci Med Sci. 2016;71:1335-1340. doi: 10.1093/gerona/glw091 [DOI] [PubMed] [Google Scholar]
- 47.Vercambre MN, Boutron-Ruault MC, Ritchie K, Clavel-Chapelon F, Berr C. Long-term association of food and nutrient intakes with cognitive and functional decline: a 13-year follow-up study of elderly French women. Br J Nutr. 2009;102(3):419-427. doi: 10.1017/S0007114508201959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu S, Manson JE, Stampfer MJ, et al. A prospective study of whole-grain intake and risk of type 2 diabetes mellitus in US women. Am J Public Health. 2000;90(9):1409-1415. doi: 10.2105/ajph.90.9.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weng PH, Chen JH, Chiou JM, et al. The effect of lifestyle on late-life cognitive change under different socioeconomic status. PLoS One. 2018;13(6):e0197676. doi: 10.1371/journal.pone.0197676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parrott MD, Shatenstein B, Ferland G, et al. Relationship between diet quality and cognition depends on socioeconomic position in healthy older adults. J Nutr. 2013;143(11):1767-1773. doi: 10.3945/jn.113.181115 [DOI] [PubMed] [Google Scholar]
- 51.Wilson RS, Rajan KB, Barnes LL, Weuve J, Evans DA. Factors related to racial differences in late-life level of cognitive function. Neuropsychology. 2016;30(5):517-524. doi: 10.1037/neu0000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tangney CC, Bienias JL, Evans DA, Morris MC. Reasonable estimates of serum vitamin E, vitamin C, and beta-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. J Nutr. 2004;134(4):927-934. doi: 10.1093/jn/134.4.927 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Investigators may request access to data of the CHAP cohort study through the Rush Institute for Healthy Aging data portal (riha.rush.edu/dataportal.html) pending proposal approval and completion of a Data Use Agreement.