Abstract
Background and Objectives
Prior observational studies for autoimmune encephalitis (AE) have mostly focused on outcomes after acute immunotherapies with better outcomes associated with earlier immunotherapy use. However, the impact of long-term immunotherapy and its association with clinical relapse is not well known.
Methods
We conducted a retrospective study of consecutive patients meeting published clinical criteria for AE evaluated at UC San Diego and Rady Children's Hospital from January 2007 to November 2021. Survival analysis and Cox multivariable regression models were used to evaluate relapse risk using rituximab exposure as a time-dependent variable. Pooled and age-stratified analyses were performed.
Results
A total of 204 pediatric and 380 adult participants were screened of which 30 pediatric and 75 adult participants were included. The most common antibody subtype in both cohorts was anti-NMDA receptor (76% in pediatric, 34% in adult). Relapses occurred in 31% of pediatric antibody-positive, 40% of adult antibody-positive, and 20% of adult antibody-negative cases. Times to first relapse (TTFR) were 10.6 ± 7.4 months (pediatric antibody-positive), 13.1 ± 24.5 months (adult antibody-positive), and 6.9 ± 3.8 months (adult antibody-negative). Rituximab was the most common second-line immunotherapy used. Combining pediatric and adult data, rituximab use was associated with a 71% lower hazard for time to first relapse (hazard ratio [HR] 0.29, 95% CI 0.09–0.85) and 51% lower hazard for recurring relapses (HR 0.49, 95% CI 0.9–1.26). The HR for TTFR with rituximab use in children was 0.30 (95% CI 0.05–1.69), 0.29 (95% CI 0.07–1.29) in adults, 0.32 in non-NMDA antibody-positive encephalitis (95% CI 0.07–1.39), and 0.42 (95% CI 0.07–2.67) for anti-NMDAR.
Discussion
Relapses are common in pediatric and adult patients with AE, although less frequently in anti-NMDARE. Using a rigorous survival model, we demonstrate a substantial benefit of rituximab use for reducing relapse rates in AE, especially for the adult population.
Classification of Evidence
This study provides Class IV evidence that rituximab is associated with a lower hazard to relapse in patients with AE.
Introduction
Autoimmune encephalitis (AE) is a category of inflammatory brain disease that is increasingly recognized as a treatable cause of encephalitis, the most common being anti-NMDA receptor (NMDAR) encephalitis.1,2 Data from Olmstead County published in 2015 estimated a prevalence of 6.5 per 100,000 persons (incidence 0.4 per 100,000 person-years) for antibody-positive AE and 1.3 per 100.000 persons (incidence 0.1 per 100,000 person-years) for antibody-negative AE.3 The pathophysiology of AE is hypothesized to be autoantibodies directed at neuronal surface, synaptic, or intracellular targets causing specific clinical syndromes.4 The generation of autoantibodies is believed to be triggered by either viral infections or immunologic responses to benign or malignant tumors, although the causes of many forms of AE are unknown.3 Patients with AE present with a subacute encephalopathy with cognitive dysfunction, and neuropsychiatric symptoms often accompanied by new onset seizures, movement disorders, sleep disorders, catatonia, or acute psychosis.1,5,6
Existing data on relapses are mostly limited to relapse frequencies from retrospective studies and lack specific data on time to first relapse (TTFR) and the number of recurring relapses in relation to immunotherapy. The wide range of relapse frequencies reported are attributable to many factors, including sample size, diversity of patient population, criteria defined for relapse, length of follow-up time, and differences in acute and chronic treatment. For anti-NMDAR encephalitis cohorts, 8.3%–32.6% of patients relapse, generally within the first 2–3 years, but relapse up to 7 years after the initial attack has been reported.7-10 Relapse frequencies for anti–leucine-rich glioma-inactivated 1 (LGI1) encephalitis, anti–contactin-associated protein-like 2 (CASPR2) encephalitis, and anti–gamma-aminobutyric acid encephalitis have been reported to be 15.4%–40%, 25%, and 9%, respectively.11,12 The data on antibody-negative AE are even more limited. Relapse frequencies for antibody-negative AE have been recorded as 35.1% in a pediatric-specific study and 11.8% in a single, multicenter retrospective study for both pediatric and adult combined cases.13,14
Regarding treatment, early initiation of immunotherapy is an independent predictor of good outcome for a modified Rankin Scale (mRS) 0–2, especially for anti-NMDAR AE.7,15 First-line immunotherapies include high-dose steroids, IV immunoglobulins (IVIG), or plasma exchange (PLEX). Second-line immunotherapies include rituximab, cyclophosphamide, and tocilizumab, which are reserved for more severe cases in which patients do not quickly respond to first-line immunotherapy and/or require intensive care unit (ICU) admission.16 One of the main challenges in the clinical management of AE is the decision to administer second-line immunotherapy at initial presentation and the continuation of immunotherapy after the first episode. The chosen immunotherapy often varies among different institutions, and there is a lack of consensus for the duration of immunotherapy use to prevent relapses.17 Although rituximab use has been reported to reduce relapses, the studies so far report only the relapse frequencies and not risk ratios.4,15,16 To our knowledge, there are no studies evaluating relapse rate for AE accounting for different exposure times to immunotherapy. In this study, we aimed to evaluate whether second-line immunotherapy, specifically rituximab, decreased the risk of relapse in AE and whether there were differences in relapse rates and relapse risks with rituximab use between pediatric and adult cases and between antibody-positive and antibody-negative AE cases.
Methods
Design and Participants
We conducted a retrospective cohort study of consecutive patients treated at Rady Children's Hospital San Diego (RCHSD) and the University of California San Diego Health (UCSD) between January 2007 and November 2021 who were diagnosed with antibody-positive AE or antibody-negative AE. We screened all eligible participants using the diagnostic criteria set forth by Graus et al.1 by reviewing available data in the medical record: subacute onset (<3 months) of memory changes, altered mental status or psychiatric symptoms, and at least one of the following: (1) new focal neurologic findings, (2) seizures not explained by prior seizure disorder, (3) CSF pleocytosis (white blood cell >5 cells/mm3), or (4) MRI features suggestive of encephalitis. Participants were included in the study if they met the consensus criteria for seropositive AE, definitive limbic encephalitis, anti-NMDAR encephalitis, Hashimoto/steroid-responsive encephalopathy, or seronegative AE.1 Patients were excluded if they (1) did not meet clinical criteria, (2) had alternative diagnoses that explained the cause of encephalitis, (3) had insufficient data to reasonably apply the inclusion criteria, and/or (4) did not receive any portion of their acute care or initial hospital care at RCHSD or UCSD. Additional cases were excluded if the participants did not meet published diagnostic criteria1 on retrospective chart review.
For participants with preexisting developmental delay, which included any history of gross motor, fine motor and/or speech delay requiring therapy, or special education assistance, we confirmed through the chart that any changes in their behavior or decline in motor skills at the time of their encephalitis presentation were acute changes from baseline. Similarly, for those with a history of epilepsy, we confirmed that they were either controlled with antiseizure medication or stable off medication, and any seizure at the time of encephalitis presentation was a change from baseline. Psychiatric diseases, including any history of depression, anxiety, or other primary mood disorder, were confirmed to be stable or controlled on medication before the onset of encephalitis symptoms.
Study participants were identified using the Slicer Dicer function in the Epic electronic medical record. Search terms included “AE,” “AE due to NMDA receptor,” “limbic encephalitis,” “paraneoplastic cerebellar degeneration,” “limbic encephalitis with LGI1 antibody, “limbic encephalitis with anti-NMDA,” “paraneoplastic limbic encephalitis,” “paraneoplastic encephalitis,” and/or “other encephalitis and encephalomyelitis.” The ICD-10 code for other antibody-specific AE overlaps with the code for AE and limbic encephalitis.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the UCSD Institutional Review Board. As a minimal risk records-based study, informed consent was waived.
Definitions
Antibody-positive cases were defined as antibody positivity in serum, CSF, or both. Cases were considered antibody-negative if no antibodies in the serum or CSF were identified at the time of initial or follow-up evaluation. CSF pleocytosis was defined as white blood cell count of >5 cells/μL, and elevated CSF protein was defined as any value >45 mg/dL. Abnormal MRI included any T2 changes, abnormal contrast enhancement, diffusion restriction, or presence of hemorrhage. Abnormal EEG included any routine, video, or continuous monitoring demonstrating focal/generalized slowing, focal/generalized epileptiform discharges, and/or seizures.
A relapse was defined as any acute worsening of neuropsychiatric, seizures, or other neurologic symptoms after a period of at least 1 month of clinical stability after acute immunotherapy treatment as determined by a chart review. TTFR was defined as the time from the date of clinical stability (date of hospital discharge or approximate date of outpatient stability) to the onset of the first relapse.
Acute immunotherapy was defined as any immunotherapies given at the time of the patient's initial presentation and/or hospitalization. Long-term, or chronic, immunotherapy after the initial episode was defined as any immunotherapies that were continued or started after the initial episode and/or immunotherapies with effects lasting ≥3 months. This included oral prednisone taper for ≥3 months, IV methylprednisolone, IVIG or PLEX with repeated treatment cycles for ≥3 months, rituximab given at initial onset with or without continued infusions, cyclophosphamide scheduled after hospital discharge, or oral immunotherapies started after hospitalization. We considered a complete induction treatment course of rituximab to be 375 mg/m2 for 4 doses, 750 mg/m2 for 2 doses, or 1,000 mg for 2 doses. We considered maintenance rituximab dosing as 500–750 mg/m2 or 1,000 mg. We defined total exposure time to rituximab as 12 months from the last loading dose or 12 months from the last maintenance dose, accounting for any pauses in therapy between acute and chronic immunotherapy. The exposure time was chosen based on the data from multiple sclerosis studies with similar rituximab dosing regimen that demonstrated chronic B-cell suppression up to 12 months18,19
Statistical Analyses
Descriptive data analyses included assessments of frequencies, means, and medians as appropriate. Survival analysis and Cox regression models were used to evaluate hazard ratios (HRs) for any relapse associated with acute immunotherapy use, ICU admission, presence of tumor, and tumor removal, adjusting for age of disease onset, sex, and presence of tumor when appropriate. The same methodology was applied to evaluate rituximab's effect on relapse rates, with rituximab modeled as a time-varying covariate. The analysis was focused on rituximab because it was the most common second-line immunotherapy. HRs for TTFR and recurring relapses were compared between participants who received rituximab and participants who did not receive rituximab using a Cox proportional hazard model. The adjusted analysis accounted for variables with face validity based on prior knowledge. We used a backward model selection method retaining variables that alter the point estimate by a minimum of 10%. Analyses were performed using R statistical software, and p values <0.05 were considered statistically significant.
Data Availability
Anonymized data and data sets not published within this article will be made available by request from any qualified investigator.
Results
Study Participants and Demographics
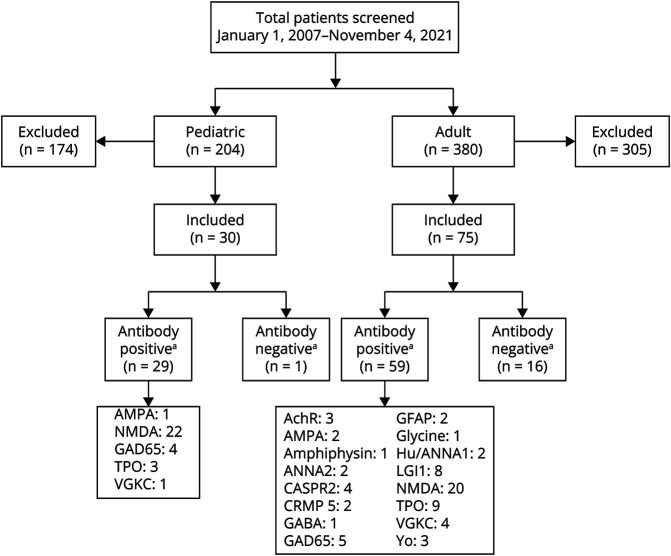
A total of 204 pediatric patients were identified through a search of Rady Children's Hospital records, 30 of whom fully met study inclusion criteria (Figure 1). The most common diagnoses initially misattributed as AE in the pediatric cohort were acute disseminated encephalomyelitis (35%, n = 61) and systemic lupus erythematous (11% n = 19). For adults, 380 potential participants were identified through electronic medical record review, 75 of whom met study criteria (Figure 1). The most common diagnoses initially misattributed as AE in the adult cohort were acute disseminated encephalomyelitis (11%, n = 33) and unspecified encephalitis not meeting published diagnostic criteria for seronegative AE (11%, n = 33). The mean age of presentation for pediatric and adult participants was 12.2 and 45.8 years, respectively, with 63% female participants in the pediatric cohort and 52% female participants in the adult cohort (Table 1). Follow-up data, defined as any time after initial hospital discharge, were available for all pediatric patients, 92% of adult antibody-positive and 94% of antibody-negative adult patients (Table 2).
Figure 1. Study Population.
AchR = acetylcholine receptor; AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; ANNA2 (Ri) = antineuronal nuclear autoantibody type 2; CASPR2 = contactin-associated protein-like 2; CRMP5 = CV2/collapsin response mediator protein; GABA = gamma-aminobutyric acid; GAD65 = glutamic acid decarboxylase 65; GFAP = glial fibrillary acidic protein; Hu/ANNA1 = antineuronal nuclear autoantibody type 1; LGI1 = leucine-rich glioma-inactivated 1; TPO = thyroid peroxidase; VGKC = voltage-gated potassium channel; Yo = Purkinje cell cytoplasmic antibody type 1. aTwo patients had more than one autoantibody.
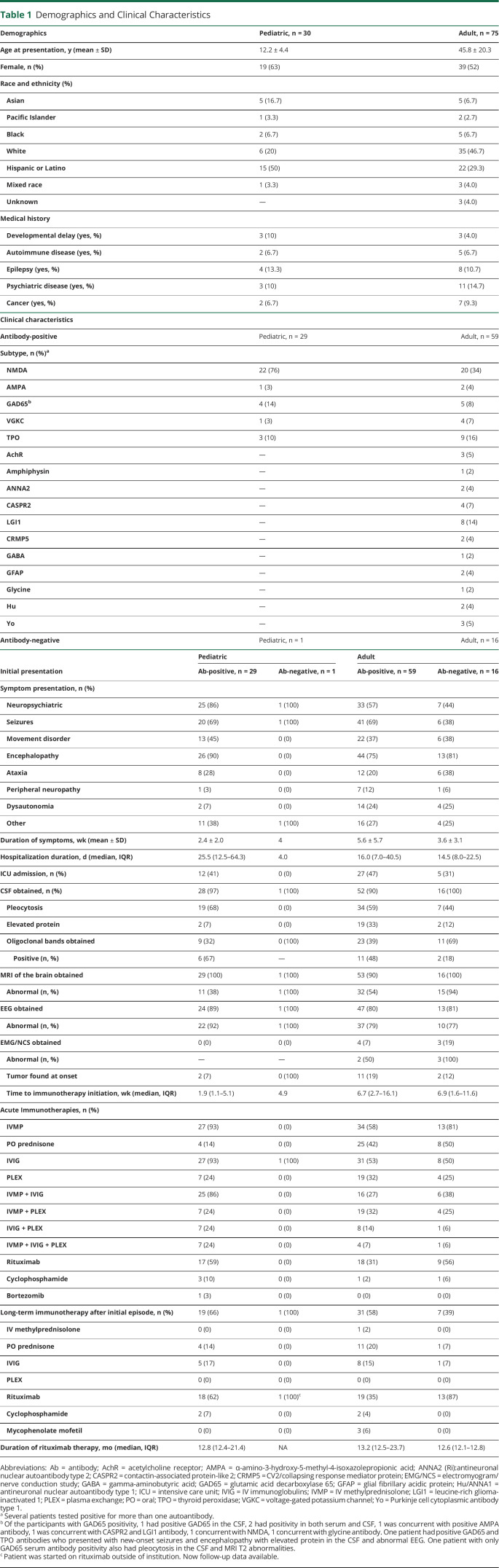
Table 1.
Demographics and Clinical Characteristics
| Demographics | Pediatric, n = 30 | Adult, n = 75 |
| Age at presentation, y (mean ± SD) | 12.2 ± 4.4 | 45.8 ± 20.3 |
| Female, n (%) | 19 (63) | 39 (52) |
| Race and ethnicity (%) | ||
| Asian | 5 (16.7) | 5 (6.7) |
| Pacific Islander | 1 (3.3) | 2 (2.7) |
| Black | 2 (6.7) | 5 (6.7) |
| White | 6 (20) | 35 (46.7) |
| Hispanic or Latino | 15 (50) | 22 (29.3) |
| Mixed race | 1 (3.3) | 3 (4.0) |
| Unknown | — | 3 (4.0) |
| Medical history | ||
| Developmental delay (yes, %) | 3 (10) | 3 (4.0) |
| Autoimmune disease (yes, %) | 2 (6.7) | 5 (6.7) |
| Epilepsy (yes, %) | 4 (13.3) | 8 (10.7) |
| Psychiatric disease (yes, %) | 3 (10) | 11 (14.7) |
| Cancer (yes, %) | 2 (6.7) | 7 (9.3) |
| Clinical characteristics | ||
| Antibody-positive | Pediatric, n = 29 | Adult, n = 59 |
| Subtype, n (%)a | ||
| NMDA | 22 (76) | 20 (34) |
| AMPA | 1 (3) | 2 (4) |
| GAD65b | 4 (14) | 5 (8) |
| VGKC | 1 (3) | 4 (7) |
| TPO | 3 (10) | 9 (16) |
| AchR | — | 3 (5) |
| Amphiphysin | — | 1 (2) |
| ANNA2 | — | 2 (4) |
| CASPR2 | — | 4 (7) |
| LGI1 | — | 8 (14) |
| CRMP5 | — | 2 (4) |
| GABA | — | 1 (2) |
| GFAP | — | 2 (4) |
| Glycine | — | 1 (2) |
| Hu | — | 2 (4) |
| Yo | — | 3 (5) |
| Antibody-negative | Pediatric, n = 1 | Adult, n = 16 |
| Initial presentation | Pediatric | Adult | ||
| Ab-positive, n = 29 | Ab-negative, n = 1 | Ab-positive, n = 59 | Ab-negative, n = 16 | |
| Symptom presentation, n (%) | ||||
| Neuropsychiatric | 25 (86) | 1 (100) | 33 (57) | 7 (44) |
| Seizures | 20 (69) | 1 (100) | 41 (69) | 6 (38) |
| Movement disorder | 13 (45) | 0 (0) | 22 (37) | 6 (38) |
| Encephalopathy | 26 (90) | 0 (0) | 44 (75) | 13 (81) |
| Ataxia | 8 (28) | 0 (0) | 12 (20) | 6 (38) |
| Peripheral neuropathy | 1 (3) | 0 (0) | 7 (12) | 1 (6) |
| Dysautonomia | 2 (7) | 0 (0) | 14 (24) | 4 (25) |
| Other | 11 (38) | 1 (100) | 16 (27) | 4 (25) |
| Duration of symptoms, wk (mean ± SD) | 2.4 ± 2.0 | 4 | 5.6 ± 5.7 | 3.6 ± 3.1 |
| Hospitalization duration, d (median, IQR) | 25.5 (12.5–64.3) | 4.0 | 16.0 (7.0–40.5) | 14.5 (8.0–22.5) |
| ICU admission, n (%) | 12 (41) | 0 (0) | 27 (47) | 5 (31) |
| CSF obtained, n (%) | 28 (97) | 1 (100) | 52 (90) | 16 (100) |
| Pleocytosis | 19 (68) | 0 (0) | 34 (59) | 7 (44) |
| Elevated protein | 2 (7) | 0 (0) | 19 (33) | 2 (12) |
| Oligoclonal bands obtained | 9 (32) | 0 (100) | 23 (39) | 11 (69) |
| Positive (n, %) | 6 (67) | — | 11 (48) | 2 (18) |
| MRI of the brain obtained | 29 (100) | 1 (100) | 53 (90) | 16 (100) |
| Abnormal (n, %) | 11 (38) | 1 (100) | 32 (54) | 15 (94) |
| EEG obtained | 24 (89) | 1 (100) | 47 (80) | 13 (81) |
| Abnormal (n, %) | 22 (92) | 1 (100) | 37 (79) | 10 (77) |
| EMG/NCS obtained | 0 (0) | 0 (0) | 4 (7) | 3 (19) |
| Abnormal (n, %) | — | — | 2 (50) | 3 (100) |
| Tumor found at onset | 2 (7) | 0 (100) | 11 (19) | 2 (12) |
| Time to immunotherapy initiation, wk (median, IQR) | 1.9 (1.1–5.1) | 4.9 | 6.7 (2.7–16.1) | 6.9 (1.6–11.6) |
| Acute Immunotherapies, n (%) | ||||
| IVMP | 27 (93) | 0 (0) | 34 (58) | 13 (81) |
| PO prednisone | 4 (14) | 0 (0) | 25 (42) | 8 (50) |
| IVIG | 27 (93) | 1 (100) | 31 (53) | 8 (50) |
| PLEX | 7 (24) | 0 (0) | 19 (32) | 4 (25) |
| IVMP + IVIG | 25 (86) | 0 (0) | 16 (27) | 6 (38) |
| IVMP + PLEX | 7 (24) | 0 (0) | 19 (32) | 4 (25) |
| IVIG + PLEX | 7 (24) | 0 (0) | 8 (14) | 1 (6) |
| IVMP + IVIG + PLEX | 7 (24) | 0 (0) | 4 (7) | 1 (6) |
| Rituximab | 17 (59) | 0 (0) | 18 (31) | 9 (56) |
| Cyclophosphamide | 3 (10) | 0 (0) | 1 (2) | 1 (6) |
| Bortezomib | 1 (3) | 0 (0) | 0 (0) | 0 (0) |
| Long-term immunotherapy after initial episode, n (%) | 19 (66) | 1 (100) | 31 (58) | 7 (39) |
| IV methylprednisolone | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| PO prednisone | 4 (14) | 0 (0) | 11 (20) | 1 (7) |
| IVIG | 5 (17) | 0 (0) | 8 (15) | 1 (7) |
| PLEX | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Rituximab | 18 (62) | 1 (100)c | 19 (35) | 13 (87) |
| Cyclophosphamide | 2 (7) | 0 (0) | 2 (4) | 0 (0) |
| Mycophenolate mofetil | 0 (0) | 0 (0) | 3 (6) | 0 (0) |
| Duration of rituximab therapy, mo (median, IQR) | 12.8 (12.4–21.4) | NA | 13.2 (12.5–23.7) | 12.6 (12.1–12.8) |
Abbreviations: Ab = antibody; AchR = acetylcholine receptor; AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; ANNA2 (Ri):antineuronal nuclear autoantibody type 2; CASPR2 = contactin-associated protein-like 2; CRMP5 = CV2/collapsing response mediator protein; EMG/NCS = electromyogram/nerve conduction study; GABA = gamma-aminobutyric acid; GAD65 = glutamic acid decarboxylase 65; GFAP = glial fibrillary acidic protein; Hu/ANNA1 = antineuronal nuclear autoantibody type 1; ICU = intensive care unit; IVIG = IV immunoglobulins; IVMP = IV methylprednisolone; LGI1 = leucine-rich glioma-inactivated 1; PLEX = plasma exchange; PO = oral; TPO = thyroid peroxidase; VGKC = voltage-gated potassium channel; Yo = Purkinje cell cytoplasmic antibody type 1.
Several patients tested positive for more than one autoantibody.
Of the participants with GAD65 positivity, 1 had positive GAD65 in the CSF, 2 had positivity in both serum and CSF, 1 was concurrent with positive AMPA antibody, 1 was concurrent with CASPR2 and LGI1 antibody, 1 concurrent with NMDA, 1 concurrent with glycine antibody. One patient had positive GAD65 and TPO antibodies who presented with new-onset seizures and encephalopathy with elevated protein in the CSF and abnormal EEG. One patient with only GAD65 serum antibody positivity also had pleocytosis in the CSF and MRI T2 abnormalities.
Patient was started on rituximab outside of institution. Now follow-up data available.
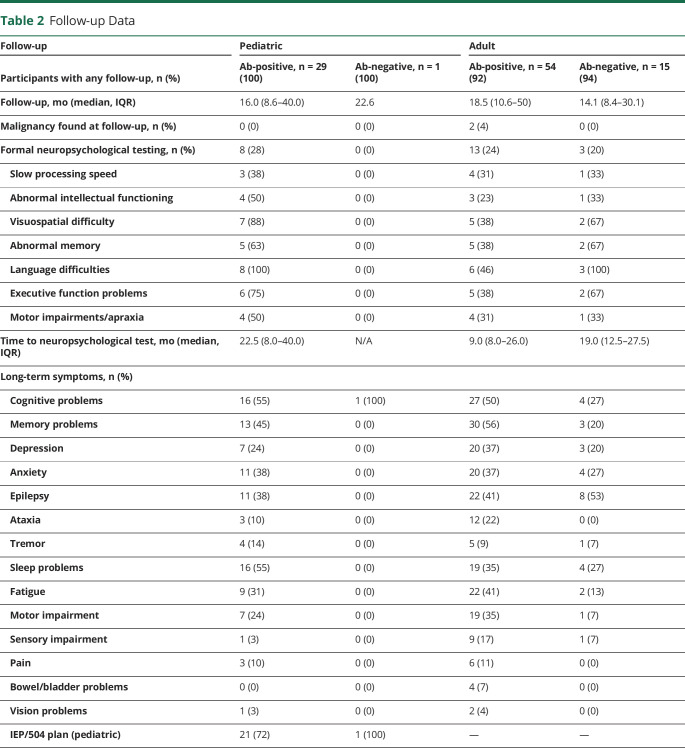
Table 2.
Follow-up Data
| Follow-up | Pediatric | Adult | ||
| Participants with any follow-up, n (%) | Ab-positive, n = 29 (100) | Ab-negative, n = 1 (100) | Ab-positive, n = 54 (92) | Ab-negative, n = 15 (94) |
| Follow-up, mo (median, IQR) | 16.0 (8.6–40.0) | 22.6 | 18.5 (10.6–50) | 14.1 (8.4–30.1) |
| Malignancy found at follow-up, n (%) | 0 (0) | 0 (0) | 2 (4) | 0 (0) |
| Formal neuropsychological testing, n (%) | 8 (28) | 0 (0) | 13 (24) | 3 (20) |
| Slow processing speed | 3 (38) | 0 (0) | 4 (31) | 1 (33) |
| Abnormal intellectual functioning | 4 (50) | 0 (0) | 3 (23) | 1 (33) |
| Visuospatial difficulty | 7 (88) | 0 (0) | 5 (38) | 2 (67) |
| Abnormal memory | 5 (63) | 0 (0) | 5 (38) | 2 (67) |
| Language difficulties | 8 (100) | 0 (0) | 6 (46) | 3 (100) |
| Executive function problems | 6 (75) | 0 (0) | 5 (38) | 2 (67) |
| Motor impairments/apraxia | 4 (50) | 0 (0) | 4 (31) | 1 (33) |
| Time to neuropsychological test, mo (median, IQR) | 22.5 (8.0–40.0) | N/A | 9.0 (8.0–26.0) | 19.0 (12.5–27.5) |
| Long-term symptoms, n (%) | ||||
| Cognitive problems | 16 (55) | 1 (100) | 27 (50) | 4 (27) |
| Memory problems | 13 (45) | 0 (0) | 30 (56) | 3 (20) |
| Depression | 7 (24) | 0 (0) | 20 (37) | 3 (20) |
| Anxiety | 11 (38) | 0 (0) | 20 (37) | 4 (27) |
| Epilepsy | 11 (38) | 0 (0) | 22 (41) | 8 (53) |
| Ataxia | 3 (10) | 0 (0) | 12 (22) | 0 (0) |
| Tremor | 4 (14) | 0 (0) | 5 (9) | 1 (7) |
| Sleep problems | 16 (55) | 0 (0) | 19 (35) | 4 (27) |
| Fatigue | 9 (31) | 0 (0) | 22 (41) | 2 (13) |
| Motor impairment | 7 (24) | 0 (0) | 19 (35) | 1 (7) |
| Sensory impairment | 1 (3) | 0 (0) | 9 (17) | 1 (7) |
| Pain | 3 (10) | 0 (0) | 6 (11) | 0 (0) |
| Bowel/bladder problems | 0 (0) | 0 (0) | 4 (7) | 0 (0) |
| Vision problems | 1 (3) | 0 (0) | 2 (4) | 0 (0) |
| IEP/504 plan (pediatric) | 21 (72) | 1 (100) | — | — |
Clinical Characteristics of Acute Presentation
In the pediatric cohort, 29 of the 30 participants were antibody-positive, with the most common antibody being against NMDAR (n = 22, 76%). In the adult cohort, 59 of the 75 participants were antibody-positive, with the most common being NMDAR (n = 20, 34%). The remaining 11 pediatric and 16 adult participants were antibody-negative but met clinical criteria for seronegative AE. Tumors were found in 7% of pediatric antibody-positive, 19% of adult antibody-positive, and 12% of adult antibody-negative cases. Sixty-eight percent of pediatric antibody-positive, 59% of adult antibody-positive, and 44% of adult antibody-negative cases had a CSF pleocytosis. Oligoclonal bands, when checked, were present in 67% of pediatric antibody-positive, 48% of adult antibody-positive, and 18% of adult antibody-negative cases. MRI was abnormal more frequently in the adult cohort (54% antibody-positive, 94% antibody-negative) compared with the pediatric cohort (38% antibody-positive, 100% antibody-negative). EEGs in both cohorts demonstrated some abnormality in most cases (92% pediatric antibody-positive, 100% pediatric antibody-negative, 79% adult antibody-positive, 77% adult antibody-negative). Table 1 summarizes the demographic and clinical presentations for pediatric and adult cohorts.
Follow-up
The median follow-up durations were 16.0 months (pediatric antibody-positive), 22.6 months (pediatric antibody-negative), 18.5 months (adult antibody-positive), and 14.1 months (adult antibody-negative). Three patients in the adult antibody-positive group did not have identifiable tumors at their initial presentation but were found to have tumors at some point during their follow-up (anti-NMDAR associated with a desmoid type fibromatosis tumor, anti-Hu associated with renal and breast cancers, and anti-LGI1 associated with lung cancer). Long-term symptoms were commonly reported in all cohorts. In the pediatric cohort, 72% of antibody-positive and the one antibody-negative case required some form of individualized education plan (IEP) or 504 plans for school accommodations. In the adult cohort, cognitive (50%) and memory (56) were the most common long-term symptoms in the antibody-positive cohort, and epilepsy (53%) was the most common long-term symptom in the antibody-negative cohort. Table 2 summarizes follow-up data for both cohorts.
Acute and Chronic Immunotherapy Treatment
The median time to immunotherapy (time from symptom onset to the first day of any immunotherapy) was 1.9 weeks for pediatric antibody-positive, 4.9 weeks for the single pediatric antibody-negative case, 6.7 weeks for adult antibody-positive, and 6.9 weeks for adult antibody-negative (Table 1). Most participants received IV methylprednisolone (93% antibody-positive pediatric, 58% adult antibody-positive, 81% adult antibody-negative) as first-line immunotherapy. Treatment with IVIG was more common in the pediatric cohorts (93%) than adults (52%). Therapeutic apheresis (PLEX) was used in 24% antibody-positive pediatric, 19% antibody-positive adult, 25% antibody-negative adult, and none in the antibody-negative pediatric participants. The most common second-line acute immunotherapy was rituximab (59% antibody-positive pediatric, 31% antibody-positive adult, 56% antibody-negative adult). Cyclophosphamide was used in 3 pediatric and 2 adult patients for acute treatment. One pediatric patient received 2 cycles of bortezomib, a proteosome inhibitor against plasma cells, for refractory anti-NMDAR encephalitis. Of the chronic immunotherapy options, rituximab was the most common exposure (62% antibody-positive pediatric, 36% antibody-positive adult, 33% antibody-negative adult). The median duration on rituximab therapy was 12.8 months (pediatric antibody-positive), 13.2 months (adult antibody-positive), and 12.6 months (adult antibody-negative), which we calculated to include total exposure time to rituximab (Table 1). A total of 12 patients in both cohorts combined had more than one rituximab treatment cycle. A description of other chronic immunotherapies used is summarized in Table 1.
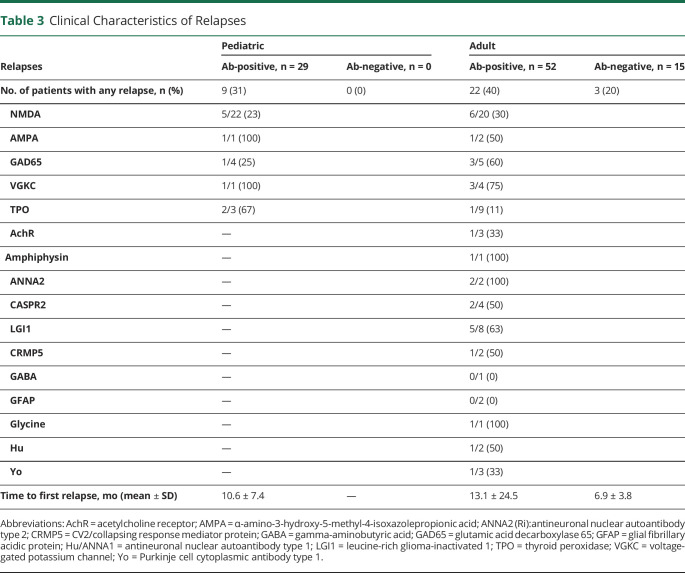
Relapses
In the pediatric cohort, 31% of antibody-positive participants had at least one clinical relapse. The one seronegative pediatric patient did not have a documented clinical relapse. In the adult cohort, 40% of antibody-positive and 20% of antibody-negative cases had at least one relapse. Within the antibody-positive cases, there were fewer total relapses observed in the NMDAR cohorts (23% pediatric and 30% adult) compared with pooled non-NMDAR autoantibodies. The mean TTFR was 10.6 ± 7.4 months (pediatric antibody-positive), 13.1 ± 24.5 months (adult antibody-positive), and 6.9 ± 3.8 months (adult antibody-negative). Table 3 summarizes the relapse frequencies and TTFR.
Table 3.
Clinical Characteristics of Relapses
| Relapses | Pediatric | Adult | ||
| Ab-positive, n = 29 | Ab-negative, n = 0 | Ab-positive, n = 52 | Ab-negative, n = 15 | |
| No. of patients with any relapse, n (%) | 9 (31) | 0 (0) | 22 (40) | 3 (20) |
| NMDA | 5/22 (23) | 6/20 (30) | ||
| AMPA | 1/1 (100) | 1/2 (50) | ||
| GAD65 | 1/4 (25) | 3/5 (60) | ||
| VGKC | 1/1 (100) | 3/4 (75) | ||
| TPO | 2/3 (67) | 1/9 (11) | ||
| AchR | — | 1/3 (33) | ||
| Amphiphysin | — | 1/1 (100) | ||
| ANNA2 | — | 2/2 (100) | ||
| CASPR2 | — | 2/4 (50) | ||
| LGI1 | — | 5/8 (63) | ||
| CRMP5 | — | 1/2 (50) | ||
| GABA | — | 0/1 (0) | ||
| GFAP | — | 0/2 (0) | ||
| Glycine | — | 1/1 (100) | ||
| Hu | — | 1/2 (50) | ||
| Yo | — | 1/3 (33) | ||
| Time to first relapse, mo (mean ± SD) | 10.6 ± 7.4 | — | 13.1 ± 24.5 | 6.9 ± 3.8 |
Abbreviations: AchR = acetylcholine receptor; AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; ANNA2 (Ri):antineuronal nuclear autoantibody type 2; CRMP5 = CV2/collapsing response mediator protein; GABA = gamma-aminobutyric acid; GAD65 = glutamic acid decarboxylase 65; GFAP = glial fibrillary acidic protein; Hu/ANNA1 = antineuronal nuclear autoantibody type 1; LGI1 = leucine-rich glioma-inactivated 1; TPO = thyroid peroxidase; VGKC = voltage-gated potassium channel; Yo = Purkinje cell cytoplasmic antibody type 1.
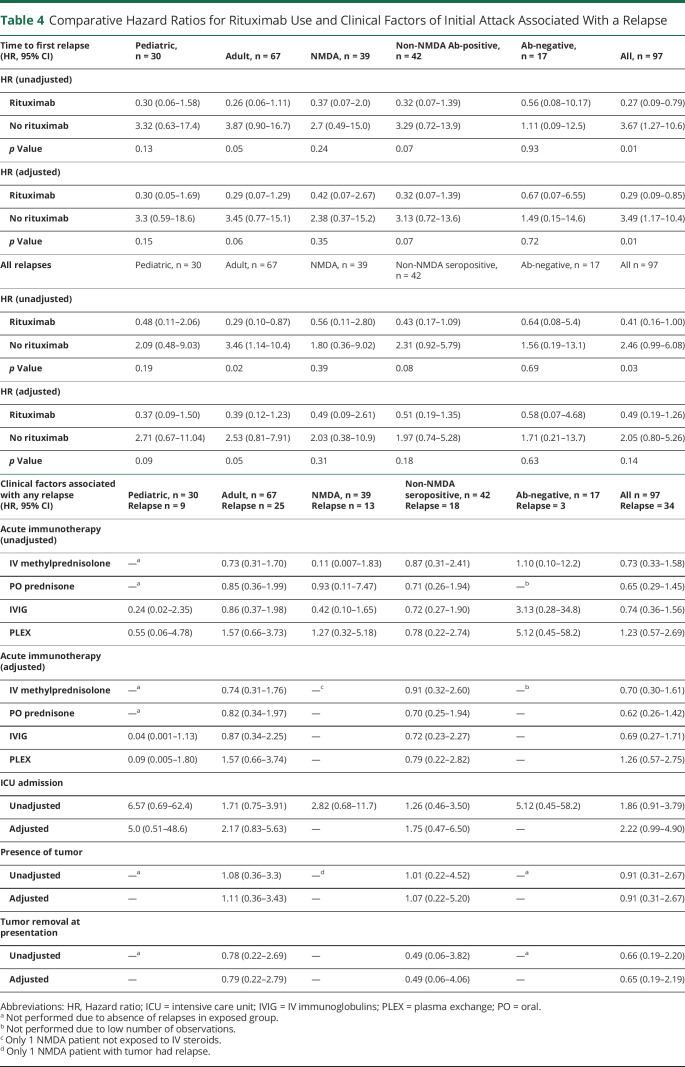
We applied a Cox proportional hazard model to calculate the HRs for acute immunotherapies, ICU admission, presence of tumor, and tumor removal at acute presentation associated with the presence of any clinical relapse (Table 4). Our models for acute immunotherapy and ICU admission adjusted for sex and age at onset at initial presentation except for NMDAR and antibody-negative groups because of the low number of relapses. None of the acute immunotherapies had a significant reduction in relapse risk. ICU admission at presentation was associated with higher relapse risk with an adjusted HR of 2.22 (95% CI 0.99–4.90). There were no significant associations between relapse and other immunotherapies, the presence of tumor, or tumor removal at the time of initial presentation.
Table 4.
Comparative Hazard Ratios for Rituximab Use and Clinical Factors of Initial Attack Associated With a Relapse
| Time to first relapse (HR, 95% CI) | Pediatric, n = 30 | Adult, n = 67 | NMDA, n = 39 | Non-NMDA Ab-positive, n = 42 | Ab-negative, n = 17 | All, n = 97 |
| HR (unadjusted) | ||||||
| Rituximab | 0.30 (0.06–1.58) | 0.26 (0.06–1.11) | 0.37 (0.07–2.0) | 0.32 (0.07–1.39) | 0.56 (0.08–10.17) | 0.27 (0.09–0.79) |
| No rituximab | 3.32 (0.63–17.4) | 3.87 (0.90–16.7) | 2.7 (0.49–15.0) | 3.29 (0.72–13.9) | 1.11 (0.09–12.5) | 3.67 (1.27–10.6) |
| p Value | 0.13 | 0.05 | 0.24 | 0.07 | 0.93 | 0.01 |
| HR (adjusted) | ||||||
| Rituximab | 0.30 (0.05–1.69) | 0.29 (0.07–1.29) | 0.42 (0.07–2.67) | 0.32 (0.07–1.39) | 0.67 (0.07–6.55) | 0.29 (0.09–0.85) |
| No rituximab | 3.3 (0.59–18.6) | 3.45 (0.77–15.1) | 2.38 (0.37–15.2) | 3.13 (0.72–13.6) | 1.49 (0.15–14.6) | 3.49 (1.17–10.4) |
| p Value | 0.15 | 0.06 | 0.35 | 0.07 | 0.72 | 0.01 |
| All relapses | Pediatric, n = 30 | Adult, n = 67 | NMDA, n = 39 | Non-NMDA seropositive, n = 42 | Ab-negative, n = 17 | All n = 97 |
| HR (unadjusted) | ||||||
| Rituximab | 0.48 (0.11–2.06) | 0.29 (0.10–0.87) | 0.56 (0.11–2.80) | 0.43 (0.17–1.09) | 0.64 (0.08–5.4) | 0.41 (0.16–1.00) |
| No rituximab | 2.09 (0.48–9.03) | 3.46 (1.14–10.4) | 1.80 (0.36–9.02) | 2.31 (0.92–5.79) | 1.56 (0.19–13.1) | 2.46 (0.99–6.08) |
| p Value | 0.19 | 0.02 | 0.39 | 0.08 | 0.69 | 0.03 |
| HR (adjusted) | ||||||
| Rituximab | 0.37 (0.09–1.50) | 0.39 (0.12–1.23) | 0.49 (0.09–2.61) | 0.51 (0.19–1.35) | 0.58 (0.07–4.68) | 0.49 (0.19–1.26) |
| No rituximab | 2.71 (0.67–11.04) | 2.53 (0.81–7.91) | 2.03 (0.38–10.9) | 1.97 (0.74–5.28) | 1.71 (0.21–13.7) | 2.05 (0.80–5.26) |
| p Value | 0.09 | 0.05 | 0.31 | 0.18 | 0.63 | 0.14 |
| Clinical factors associated with any relapse (HR, 95% CI) | Pediatric, n = 30 Relapse n = 9 |
Adult, n = 67 Relapse n = 25 |
NMDA, n = 39 Relapse n = 13 |
Non-NMDA seropositive, n = 42 Relapse = 18 |
Ab-negative, n = 17 Relapse = 3 |
All n = 97 Relapse = 34 |
| Acute immunotherapy (unadjusted) | ||||||
| IV methylprednisolone | —a | 0.73 (0.31–1.70) | 0.11 (0.007–1.83) | 0.87 (0.31–2.41) | 1.10 (0.10–12.2) | 0.73 (0.33–1.58) |
| PO prednisone | —a | 0.85 (0.36–1.99) | 0.93 (0.11–7.47) | 0.71 (0.26–1.94) | —b | 0.65 (0.29–1.45) |
| IVIG | 0.24 (0.02–2.35) | 0.86 (0.37–1.98) | 0.42 (0.10–1.65) | 0.72 (0.27–1.90) | 3.13 (0.28–34.8) | 0.74 (0.36–1.56) |
| PLEX | 0.55 (0.06–4.78) | 1.57 (0.66–3.73) | 1.27 (0.32–5.18) | 0.78 (0.22–2.74) | 5.12 (0.45–58.2) | 1.23 (0.57–2.69) |
| Acute immunotherapy (adjusted) | ||||||
| IV methylprednisolone | —a | 0.74 (0.31–1.76) | —c | 0.91 (0.32–2.60) | —b | 0.70 (0.30–1.61) |
| PO prednisone | —a | 0.82 (0.34–1.97) | — | 0.70 (0.25–1.94) | — | 0.62 (0.26–1.42) |
| IVIG | 0.04 (0.001–1.13) | 0.87 (0.34–2.25) | — | 0.72 (0.23–2.27) | — | 0.69 (0.27–1.71) |
| PLEX | 0.09 (0.005–1.80) | 1.57 (0.66–3.74) | — | 0.79 (0.22–2.82) | — | 1.26 (0.57–2.75) |
| ICU admission | ||||||
| Unadjusted | 6.57 (0.69–62.4) | 1.71 (0.75–3.91) | 2.82 (0.68–11.7) | 1.26 (0.46–3.50) | 5.12 (0.45–58.2) | 1.86 (0.91–3.79) |
| Adjusted | 5.0 (0.51–48.6) | 2.17 (0.83–5.63) | — | 1.75 (0.47–6.50) | — | 2.22 (0.99–4.90) |
| Presence of tumor | ||||||
| Unadjusted | —a | 1.08 (0.36–3.3) | —d | 1.01 (0.22–4.52) | —a | 0.91 (0.31–2.67) |
| Adjusted | — | 1.11 (0.36–3.43) | — | 1.07 (0.22–5.20) | — | 0.91 (0.31–2.67) |
| Tumor removal at presentation | ||||||
| Unadjusted | —a | 0.78 (0.22–2.69) | — | 0.49 (0.06–3.82) | —a | 0.66 (0.19–2.20) |
| Adjusted | — | 0.79 (0.22–2.79) | — | 0.49 (0.06–4.06) | — | 0.65 (0.19–2.19) |
Abbreviations: HR, Hazard ratio; ICU = intensive care unit; IVIG = IV immunoglobulins; PLEX = plasma exchange; PO = oral.
Not performed due to absence of relapses in exposed group.
Not performed due to low number of observations.
Only 1 NMDA patient not exposed to IV steroids.
Only 1 NMDA patient with tumor had relapse.
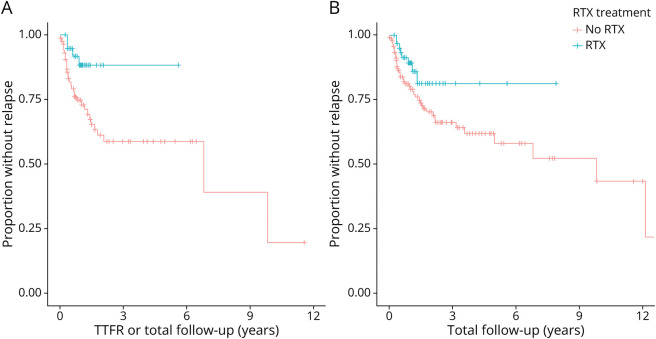
We next evaluated the HRs for TTFR and recurring relapses using a Cox proportional hazard model with rituximab exposure as a time-dependent variable (Table 4). Other second-line immunotherapies were not included in the analysis because of the low number of observations. The adjusted analysis for TTFR accounted for age at onset, sex, and presence of tumor; the adjusted analysis for recurring relapses accounted for IV steroid use, time to immunotherapy, and presence of tumor. In a pooled analysis of adults and children for TTFR, rituximab use was associated with an unadjusted HR of 0.27 (95% CI 0.09–0.79) and an adjusted HR of 0.29 (95% CI 0.09–0.85), suggesting no substantial confounding by the face validity covariates. Rituximab use was also associated with reduced hazard to relapse in a repeated measures Cox analysis accounting for all relapses over time, with an unadjusted HR of 0.41 (95% CI 0.16–1.00). After adjustment, the HR was 0.49 (95% CI 0.19–1.26). Figure 2 shows the Kaplan-Meier survival curves for TTFR and recurring relapses. We conducted stratified analyses for pediatric and adult cohorts, combined adult and pediatric NMDAR-positive cases, combined adult and pediatric non-NMDA antibody-positive cases, and combined adult and pediatric antibody-negative cases (Table 4). In our subanalyses for recurring relapses, rituximab treatment in adults was associated with an unadjusted HR of 0.29 (95% CI 0.10–0.87) and an adjusted HR of 0.39 (95% CI 0.12–1.23, p = 0.05). There was a trend toward lower HR for TTFR in the adult cohort (adjusted HR 0.29, 95% CI 0.07–1.29, p = 0.06) with rituximab use and non-NMDA antibody-positive cohort (adjusted HR 0.32, 95% CI 0.07–1.39, p = 0.07) with rituximab use.
Figure 2. Survival Analysis of Relapse Rates for Combined Pediatric and Adult Cohorts.
Kaplan-Meier curves for time to first relapse (A) and overall relapse rate over the total follow-up period (B) in both pediatric and adult patients.
The results of our study provide Class IV evidence that rituximab is associated with a lower hazard to relapse in patients with AE.
Discussion
In our study, we observed a substantial reduction in TTFR and recurring relapses with rituximab treatment using a rigorous survival model accounting for immunotherapy exposure time. The effect of rituximab on TTFR risk was significant even after adjusting for covariates of interest. The effect of rituximab on reducing recurring relapses is less clear, and exposure to IV steroids may confound this observed effect. However, IV steroid use was not a confounder in the analysis for TTFR, and exposure to first-line immunotherapies individually did not have a significant impact on relapse risk. In addition, we found that 31% of pediatric and 40% of adult AE patients experience relapses. The observed relapses are less frequent in anti-NMDAR encephalitis. There is an overall increased risk of relapse associated with ICU admission.
The analyses of relapse risk for AE thus far have been limited to descriptive reports of total relapses over time or logistic regression models. A single-center prospective cohort study from China of 44 anti-LGI1 and 35 anti-CASPR2 patients reported a relapse frequency of 13.6% for LGI1 and 20% for CASPR2.20 Among the patients with relapses, none received rituximab during their acute immunotherapy course, although 2 patients were treated with cyclophosphamide, 3 with mycophenolate mofetil, and 1 with azathioprine.20 In an analysis of long-term outcomes from rituximab treatment in 358 patients with anti-NMDAR, LGI1, CASPR2, or GAD65 AE from a German registry cohort, relapses occurred in 19% for anti-NMDAR, 20% for anti-LGI1, and 11% for anti-CASPR2.21 Fewer relapses occurred after rituximab treatment for LGI1 (20% vs 31%) and CASPR2 (11% vs 14%), although the differences were not statistically significant. Duration and dosage of rituximab did not significantly affect the mRS at the final follow-up time (median 41 months) using multivariate logistic regression models.21 This study did not factor in time-dependent treatment effects from rituximab, and the analysis was not performed in the context of relapses. In a pediatric-specific study focusing on antibody-negative AE, relapsing disease was higher in antibody-negative cases compared with anti-NMDAR (35.1% and 22.2%, respectively) using a mixed linear model, although this difference did not achieve statistical significance, and direct effects of second-line immunotherapy were not studied.14 In our study, the analysis of relapses in the pediatric seronegative cohort was limited due to only one participant who met diagnostic criteria. In our analysis of rituximab treatment, there were no observed significant effects on relapse rate with rituximab use in the antibody-negative cases. Although sample size was limited in this subset, this could indicate that when these antibodies cannot be demonstrated, B-cell–depleting therapy may not be effective.
A meta-analysis of anti-NMDAR encephalitis using multivariable logistic regression models sought to evaluate factors at initial presentation associated with mRS and relapsing disease.22 In their cohort of 410 patients in the relapsing model, adolescent age at onset was associated with increased odds of relapse (OR 2.18, 95% CI 1.18–4.15), whereas rituximab (OR 0.17, 95% CI 0.05–0.42) and IVIG use for 6 months or longer (OR 0.16, 95% CI 0.07–0.33) were associated with a nonrelapsing course.22 There was no significant association with ICU admission or tumor association. The results of our survival model, which included 76% pediatric and 34% adult anti-NMDAR encephalitis cases, align with their findings. Owing to the low number of relapses (n = 1) in the paraneoplastic cases, we were not powered to apply the survival analysis in this study. However, this limitation suggests that relapses may be lower in paraneoplastic anti-NMDA cases. An international consensus statement on the treatment of pediatric anti-NMDAR encephalitis recommended IV corticosteroids for all patients with possible addition of IVIG and PLEX.23 Chronic immunosuppression, including rituximab, is recommended for severe cases only.23 Our study did not find a significant reduction in relapses associated with rituximab use in the anti-NMDAR cohort. However, the fewer relapses overall support the notion that anti-NMDAR cases are more likely to be monophasic and provide supportive evidence for the treatment approach outlined by the consensus statement.
As noted earlier, previous studies using multivariate logistic regression models did not account for the timing of rituximab treatment and various interval dosing. Therefore, we established a survival model for TTFR and recurring relapse risk that factored in the chronic effects of rituximab given during the acute treatment period and subsequent treatment doses, adjusting for potential confounders. The distribution of patients on long-term immunotherapy in our study differs slightly from other studies that separate rituximab therapy into acute therapy and rituximab redosing groups. Because we considered rituximab to be a chronic immunotherapy due to its lasting effects on B-cell suppression, those who received rituximab during the acute treatment period were included in the long-term immunotherapy group. The median durations of rituximab therapy were 12.8 months in the pediatric group, 13.2 months in adult antibody-positive group, and 12.6 months in the adult antibody-negative group, which included total exposure time to rituximab. This meant that most of our cohort received a short course of rituximab either during the acute treatment phase or after a clinical relapse. Interestingly, most relapses occurred within 2–3 years with or without rituximab, with fewer relapses observed in the patients treated with rituximab. After this initial period, relapses leveled off, although most patients did not remain on rituximab after the first 1–2 years. This suggests that most patients may not need long-term immunotherapy beyond the first few years after the initial attack. However, the median follow-up times for all cohorts in the study fell within this window. Thus, this interpretation should be used with caution as more work is needed to determine factors contributing to relapses within the first 2–3 years with or without continued immunotherapy with longer follow-up duration to capture all relapses over time.
Currently, there are no standardized treatment guidelines for the long-term management of AE.16,17 Even among providers with extensive experience in treating AE, there are varying practice preferences. In a survey of AE Alliance Clinicians Network providers, 50% will prescribe maintenance immunotherapy for a patient with a positive neuronal surface antibody, 70% will treat with maintenance immunotherapy after a second relapse for neuronal surface antibody-positive cases or antibody-negative cases, and 20%–40% will treat patients with a positive intracellular antibody.17 One study suggests the potential benefit of using maintenance rituximab beyond the initial treatment period in AE including antibody-negative cases to improve functional outcomes, but the specific relationship with rituximab treatment exposure was not explored.24 Our study provides additional data on specific AE subtypes that may be more likely to be monophasic and those more prone to relapses. In our analysis, there was a reduced relapse hazard for the anti-NMDAR cohort, but this did not achieve nominal statistical significance likely due to the sample size. In our subanalyses, there was an overall higher frequency of relapses over time and favorable response to rituximab in all groups based on the point estimate, although the results did not achieve nominal statistical significance. In the pediatric cohort, this is likely due to the fact that most cases were anti-NMDAR. Based on our results, adult patients and non-NMDA antibody-positive cases should be considered earlier for second-line immunotherapy, especially for the reduction in TTFR risk.
Although not the main focus of this study, several salient points in our follow-up data are worth mentioning. In our follow-up data, patients who underwent formal neuropsychological testing revealed that many patients have chronic neurocognitive impairments with abnormal finding in all cohorts despite reported good motor outcomes. In addition, in our pediatric cohort, most patients require IEPs. Previous observational studies, mostly around anti-NMDAR encephalitis, demonstrated good functional outcomes defined by mRS at 24 months after acute treatment.7 However, more recent studies suggest persistent cognitive and neuropsychological symptoms despite initial therapy.25 Future studies on the effects of immunotherapy should include specific long-term neuropsychological outcomes as these symptoms may be exacerbated by recurrence of clinical relapses.
The limitations of this study include the retrospective nature of the study and the lack of the commercially available expanded panel antibody testing for patients who presented more than 5 years ago. It is possible that some of the antibody-negative cases that were diagnosed during a time of limited commercial testing would be antibody-positive if evaluated at a later time. The relative rarity of non-NMDA antibody-positive cases did not allow for further analyses into factors contributing to relapses for specific antibody subtypes. In addition, the definition of a relapse varied as there is no formal definition based on objective biomarkers or ancillary testing criteria. Thus, we were only able to rely on documentation in the medical chart as determined by the treating clinician. While there was follow-up up to 13 years in our cohorts, the median follow-up duration for both pediatric and adult cohorts was less than 2 years, which may not be adequate to capture all potential relapses. Our predetermined definition of total treatment time on rituximab of 12 months also poses a potential issue as we recognize that B-cell repopulation rates differ among individual patients and vary by age groups. Finally, we were unable to analyze the relapse risks using other second-line and chronic immunotherapies because of the limited number of cases to adequately power the analysis.
Overall, the strengths of this study include the robust survival analysis methodology and the capture of both pediatric and adult relapse data. In the evaluation of disease-modifying therapy, we accounted for chronic effects of B-cell suppression that affect relapses over time. We evaluated antibody-positive cases and antibody-negative cases fulfilling consensus clinical criteria for AE and compared potential factors contributing to relapses. Because antibody-negative cases are more difficult to diagnose, we applied a strict criterion based on previous consensus recommendations and manually inspected each clinical chart to determine study eligibility. Owing to the heterogeneity of clinical presentations in pediatric and adult patients, NMDA vs non-NMDA AE, we were able to stratify and compare the results among these different cohorts.
Future prospective studies in AE should apply standardized inclusion criteria for antibody-positive and antibody-negative AE with a follow-up period of at least 10 years to capture all potential relapses over time with more data collection on the acute and chronic effects from disease-modifying therapies. Immunotherapy exposure times for individual patients should be based on their B-cell repopulation rates for B-cell–deleting therapy and pharmacokinetic measurements for other immunotherapies. Further characterization of relapses and immunotherapy effects on relapses are critical in future clinical trial designs in AE to provide evidence-based guidance on the approach to both acute and long-term immunotherapy management.
Acknowledgment
The authors thank Hyeri You and Lin Liu for their biostatistical guidance from the University of California San Diego Altman Clinical and Translational Research Institute.
Glossary
- AE
autoimmune encephalitis
- CASPR2
contactin-associated protein-like 2
- HR
hazard ratio
- ICD-10
International Classification of Diseases, Tenth Revision
- ICU
intensive care unit
- IEP
individualized education plan
- IVIG
IV immunoglobulins
- LGI1
Leucine-rich glioma-inactivated 1
- mRS
modified Rankin Scale
- NMDAR
NMDA receptor
- PLEX
plasma exchange
- RCHSD
Rady Children's Hospital San Diego
- TTFR
time to first relapse
- UCSD
University of California San Diego Health
Appendix. Authors
| Name | Location | Contribution |
| Jennifer H. Yang, MD | University of California, San Diego; Rady Children's Hospital San Diego | Study concept and design; major role in acquisition of data; analysis and interpretation of data; drafting/revising the manuscript for content |
| Emilie Liu, BA, BS | University of California, San Diego | Major role in acquisition of data; drafting/revising the manuscript for content |
| Linda Nguyen, MD, PhD | University of California, San Diego; Rady Children's Hospital San Diego | Major role in acquisition of data; revising the manuscript for content |
| Anastasie Dunn-Pirio, MD, MSc | University of California, San Diego | Revising the manuscript for content |
| Jennifer S. Graves, MD, PhD, MAS | University of California, San Diego; Rady Children's Hospital San Diego | Study concept and design; analysis and interpretation of data; revising the manuscript for content |
Footnotes
Editorial, page 985
Class of Evidence: NPub.org/coe
Study Funding
The authors report no targeted funding.
Disclosure
J.H. Yang, E. Liu, and L. Nguyen report no disclosures relevant to the manuscript; A. Dunn-Pirio reports no disclosures relevant to the manuscript; J.S. Graves has received grant or clinical trial funding from the NMSS, UCSD, Octave, Biogen, EMD-Serono, and ABM. She serves on a steering committee for a clinical trial with Novartis and served on advisory boards for TG therapeutics, Genentech, and Bayer unrelated to the current work. Go to Neurology.org/N for full disclosures.
References
- 1.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404. doi: 10.1016/S1474-4422(15)00401-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54(7):899-904. doi: 10.1093/cid/cir1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubey D, Pittock SJ, Kelly CR, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166-177. doi: 10.1002/ana.25131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gastaldi M, Thouin A, Vincent A. Antibody-mediated autoimmune encephalopathies and immunotherapies. Neurotherapeutics. 2016;13(1):147-162. doi: 10.1007/s13311-015-0410-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cellucci T, Van Mater H, Graus F, et al. Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e663. doi: 10.1212/NXI.0000000000000663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbagallo M, Vitaliti G, Pavone P, Romano C, Lubrano R, Falsaperla R. Pediatric autoimmune encephalitis. J Pediatr Neurosci. 2017;12(2):130-134. doi: 10.4103/jpn.JPN_185_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157-165. doi: 10.1016/S1474-4422(12)70310-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66(1):11-18. doi: 10.1002/ana.21756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabilondo I, Saiz A, Galán L, et al. Analysis of relapses in anti-NMDAR encephalitis. Neurology. 2011;77(10):996-999. doi: 10.1212/WNL.0b013e31822cfc6b [DOI] [PubMed] [Google Scholar]
- 10.Chiang S, Garg T, Hu A, et al. Pearls & Oy-sters: relapse of anti-NMDA receptor encephalitis after prior first- and second-line immunotherapy. Neurology. 2018;90(20):936-939. doi: 10.1212/WNL.0000000000005517 [DOI] [PubMed] [Google Scholar]
- 11.van Sonderen A, Ariño H, Petit-Pedrol M, et al. The clinical spectrum of Caspr2 antibody–associated disease. Neurology. 2016;87(5):521-528. doi: 10.1212/WNL.0000000000002917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shan W, Yang H, Wang Q. Neuronal surface antibody-medicated autoimmune encephalitis (limbic encephalitis) in China: a multiple-center, retrospective study. Front Immunol. 2021;12:621599. doi: 10.3389/fimmu.2021.621599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gastaldi M, Mariotto S, Giannoccaro MP, et al. Subgroup comparison according to clinical phenotype and serostatus in autoimmune encephalitis: a multicenter retrospective study. Eur J Neurol. 2020;27(4):633-643. doi: 10.1111/ene.14139 [DOI] [PubMed] [Google Scholar]
- 14.Lee S, Kim HD, Lee JS, Kang HC, Kim SH. Clinical features and treatment outcomes of seronegative pediatric autoimmune encephalitis. J Clin Neurol. 2021;17(2):300-306. doi: 10.3988/jcn.2021.17.2.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nosadini M, Mohammad SS, Ramanathan S, Brilot F, Dale RC. Immune therapy in autoimmune encephalitis: a systematic review. Expert Rev Neurother. 2015;15(12):1391-1419. doi: 10.1586/14737175.2015.1115720 [DOI] [PubMed] [Google Scholar]
- 16.Shin Y-W, Lee S-T, Park K-I, et al. Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord. 2018;11:1756285617722347. doi: 10.1177/1756285617722347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abboud H, Probasco J, Irani SR, et al. Autoimmune encephalitis: proposed recommendations for symptomatic and long-term management. J Neurol Neurosurg Psychiatry. 2021;92(8):897-907. doi: 10.1136/jnnp-2020-325302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676-688. doi: 10.1056/NEJMoa0706383 [DOI] [PubMed] [Google Scholar]
- 19.Bar-Or A, Calabresi PAJ, Arnold D, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63(3):395-400. doi: 10.1002/ana.21363 [DOI] [PubMed] [Google Scholar]
- 20.Guo K, Liu X, Lin J, et al. Clinical characteristics, long-term functional outcomes and relapse of anti-LGI1/Caspr2 encephalitis: a prospective cohort study in Western China. Ther Adv Neurol Disord. 2022;15:17562864211073203. doi: 10.1177/17562864211073203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thaler FS, Zimmermann L, Kammermeier S, et al. Rituximab treatment and long-term outcome of patients with autoimmune encephalitis: real-world evidence from the GENERATE registry. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1088. doi: 10.1212/NXI.0000000000001088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nosadini M, Eyre M, Molteni E, et al. Use and safety of immunotherapeutic management of N-Methyl-d-Aspartate receptor antibody encephalitis: a meta-analysis. JAMA Neurol. 2021;78(11):1333-1344. doi: 10.1001/jamaneurol.2021.3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nosadini M, Thomas T, Eyre M, et al. International consensus recommendations for the treatment of pediatric NMDAR antibody encephalitis. Neurol Neuroimmunol Neuroinflamm. 2021;8(5):e1052. doi: 10.1212/NXI.0000000000001052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee W-J, Lee S-T, Byun J-I, et al. Rituximab treatment for autoimmune limbic encephalitis in an institutional cohort. Neurology. 2016;86(18):1683-1691. doi: 10.1212/WNL.0000000000002635 [DOI] [PubMed] [Google Scholar]
- 25.Yeshokumar AK, Gordon-Lipkin E, Arenivas A, et al. Neurobehavioral outcomes in autoimmune encephalitis. J Neuroimmunol. 2017;312:8-14. doi: 10.1016/j.jneuroim.2017.08.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data and data sets not published within this article will be made available by request from any qualified investigator.