Abstract
Background
COVID‐19 stressed hospitals and may have disproportionately affected the stroke outcomes and treatment of Black and Hispanic individuals.
Methods and Results
This retrospective study used 100% Medicare Provider Analysis and Review file data from between 2016 and 2020. We used interrupted time series analyses to examine whether the COVID‐19 pandemic exacerbated disparities in stroke outcomes and reperfusion therapy. Among 1 142 560 hospitalizations for acute ischemic strokes, 90 912 (8.0%) were Hispanic individuals; 162 752 (14.2%) were non‐Hispanic Black individuals; and 888 896 (77.8%) were non‐Hispanic White individuals. The adjusted odds of mortality increased by 51% (adjusted odds ratio [aOR], 1.51 [95% CI, 1.34–1.69]; P<0.001), whereas the rates of nonhome discharges decreased by 11% (aOR, 0.89 [95% CI, 0.82–0.96]; P=0.003) for patients hospitalized during weeks when the hospital's proportion of patients with COVID‐19 was >30%. The overall rates of motor deficits (P=0.25) did not increase, and the rates of reperfusion therapy did not decrease as the weekly COVID‐19 burden increased. Black patients had lower 30‐day mortality (aOR, 0.70 [95% CI, 0.69–0.72]; P<0.001) but higher rates of motor deficits (aOR, 1.14 [95% CI, 1.12–1.16]; P<0.001) than White individuals. Hispanic patients had lower 30‐day mortality and similar rates of motor deficits compared with White individuals. There was no differential increase in adverse outcomes or reduction in reperfusion therapy among Black and Hispanic individuals compared with White individuals as the weekly COVID‐19 burden increased.
Conclusions
This national study of Medicare patients found no evidence that the hospital COVID‐19 burden exacerbated disparities in treatment and outcomes for Black and Hispanic individuals admitted with an acute ischemic stroke.
Keywords: COVID‐19, disparities, equity, stroke
Subject Categories: Cerebrovascular Disease/Stroke
Nonstandard Abbreviations and Acronyms
- CMS
Centers for Medicare and Medicaid Services
- GWTG
Get With The Guidelines
Clinical Perspective.
What Is New?
Using data on 1 265 084 hospitalizations for acute ischemic stokes in adults aged ≥65 years, we did not find evidence that the COVID‐19 pandemic exacerbated racial and ethnic disparities in 30‐day mortality, severe strokes, nonhome discharges, or the use of reperfusion therapies.
Patients with stroke hospitalized during weeks when the proportion of hospitalized patients with COVID‐19 was ≥30% experienced 51% higher odds of mortality but no reduction in the use of reperfusion therapies.
In these contemporary data, there continue to be persistent disparities in the use of thrombolytic therapy and endovascular thrombectomy.
What Are the Clinical Implications?
This study helps physicians and policymakers to understand the impact of COVID‐19 on disparities in stroke outcomes.
Further efforts are needed to improve access to reperfusion therapy for underserved populations.
Stroke is a leading cause of death and disability in the United States, with Black individuals bearing a disproportionate burden. In the United States, approximately 7.6 million individuals report having suffered a stroke, with approximately 795 000 experiencing a new or recurrent stroke annually. 1 Strokes are the primary source of serious long‐term disability, the fifth leading cause of death, and are responsible for 1 out of every 19 deaths in the United States. 1 Black individuals face a 50% higher likelihood of stroke, 2 a 70% higher population stroke mortality rate, 2 , 3 and suffer more severe strokes than White individuals. 4 , 5 , 6 Additionally, Black individuals are significantly more likely to experience physical disability and dementia than White individuals after a stroke. 7 , 8 , 9
Health care inequity is a public health crisis. It has been estimated that achieving racial equity would have resulted in 1.63 million fewer deaths of Black individuals between 1990 and 2020. 10 Health care disparities have many causes, including an inequitable distribution of resources, fewer economic and educational opportunities, unequal access to health care, differences in where marginalized patients receive their health care, and differential treatment within hospitals of minoritized patients compared with their White counterparts. 11 Provider‐level disparities can stem from conscious and unconscious bias, physician–patient racial discordance, and a scarcity of minority providers. 12 Although health care disparities have many causes, the COVID‐19 pandemic may serve as a stress test to identify hospital pathology (ie, hospital practices or systems that lead to adverse health outcomes) that might not be evident at rest (ie, before the pandemic).
Thus, the COVID‐19 pandemic presents a unique opportunity to explore whether the extreme pandemic‐induced stress on hospitals and their workforce exacerbated racial disparities in stroke outcomes. 13 Although it is well established that historically marginalized populations have been disproportionately affected by COVID‐19, 14 the extent to which hospitals under stress may have contributed to worse outcomes among minoritized individuals remains unclear. Using national Medicare data, this study addresses 2 questions: (1) Did patients hospitalized for stroke have higher rates of mortality, severe strokes, and nonhome discharges, and lower rates of reperfusion therapy (thrombolytic therapy and endovascular thrombectomy) in hospitals during weeks with a high COVID‐19 burden compared with weeks with a low COVID‐19 burden? (2) Were these changes greater among Black and Hispanic patients than White patients? Examining the results of this stress test could provide important insights into the role that hospitals play in perpetuating disparities and lay the groundwork for policies to mitigate these effects.
METHODS
This study was approved by the Columbia University Institutional Review Board, which determined that informed consent was unnecessary. This study was covered by a data use agreement with the Centers for Medicare and Medicaid Services (CMS). The CMS data used in this study can be obtained by submitting a request for research identifiable data to the Research Data Assistance Center, which is then submitted to the CMS for review. The Strengthening the Reporting of Observational Studies in Epidemiology statement was used to guide the reporting of this study. 15 Data were analyzed between March 2023 and May 2023.
Data Source
This retrospective cohort study used patient‐level data from the 100% Medicare Provider Analysis and Review file and the Master Beneficiary Summary file from between 2016 and 2020. These databases included beneficiary demographic information (age, sex, and self‐reported race and ethnicity [Asian, Black, Hispanic, North American Native, White, other {no additional details available on the other category}], which is captured at the time of Social Security enrollment); International Classification of Diseases and Related Health Problems, Tenth Revision (ICD‐10) diagnosis and procedure codes; source of admission; date of admission; admission status (elective, urgent, emergent); payer status; discharge destination; date of death; and hospital identifiers. These data were merged with the CMS Impact Files, which included information on hospital characteristics (geographic region, rurality, number of beds, average daily census, disproportionate share hospital percentage, and resident‐to‐bed ratio).
Study Population
We identified 1 265 084 patients aged ≥65 years who were hospitalized with a primary diagnosis of acute ischemic stroke between January 1, 2016, and December 31, 2020 (Table S1; Figure S1). Patients were categorized as Black, Hispanic, or non‐Hispanic White using the Research Triangle Institute race code. 16 We excluded admissions in December 2020 to observe 30‐day outcomes for patients admitted in November 2020 (21 843); admissions with missing race (9953); patients who were neither non‐Hispanic White (hereafter referred to as White), Black, or Hispanic (48 015); unknown admission urgency (10 121); elective admissions (28 335); admissions for stroke transferred out (2225); transfer in from hospice (82); and hospitalizations in hospitals not in the CMS Impact File (1950) (Figure S1). In cases where patients were transferred to another hospital, we linked the transfers into 1 episode of care and attributed outcomes and therapies to the final admission (the transfer‐in admission). The final analytic cohort consisted of 1 142 560 hospitalizations.
Statistical Analysis
The outcomes of interest were (1) 30‐day mortality, (2) motor deficits (monoplegia, hemiplegia or hemiparesis, paraplegia or paraparesis, quadriplegia or quadriparesis, other paralytic syndromes), (3) nonhome discharges (death or discharge to a skilled nursing facility or nursing home, inpatient rehabilitation facility, long‐term care hospital, or hospital transfer), and (4) reperfusion therapy (thrombolytic therapy or endovascular thrombectomy). The first aim of this study was to determine whether patients hospitalized with a stroke had different rates of these outcomes during weeks with a high hospital COVID‐19 burden compared with weeks with a low hospital COVID‐19 burden. The unit of analysis was the acute hospitalization for acute ischemic stroke. Consider the exemplar outcome of 30‐day mortality:
where f is the logit function, Y ikt is the outcome (30‐day mortality) for patient i in hospital k at time t, RaceEthnic i is race and ethnicity (Black, Hispanic, White), Week t is the underlying linear weekly time trend, and Month t is a set of monthly indicators to control for seasonal variation around the weekly time trend. COVIDmonth t is a set of monthly indicators that control for deviations from the seasonally adjusted weekly time trend during the COVID‐19 pandemic. The exposure of interest, COVIDburden kt , is a weekly measure of the hospital proportion of all Medicare inpatients who tested positive for COVID‐19, classified as 0% to 2.0%, 2.1% to 10%, 10.1% to 20%, 20.1% to 30%, and >30%. We controlled for the patient‐level covariates X i , demographics (age, sex, race, and ethnicity), admission status (urgent, emergent), admission source (community, hospital, skilled nursing facility/nursing home, other), dual eligibility (enrollment in both Medicare and Medicaid), COVID‐19, body mass index, National Institutes of Health Stroke Scale, time elapsed from prior stroke, stroke location (eg, anterior cerebral, middle cerebral), prior cardiac procedure (percutaneous coronary intervention, coronary artery bypass graft surgery, heart valve surgery), functional status (wheelchair, care provider, supplemental oxygen), and individual Elixhauser comorbidities (Table S1). 17 , 18 We then expanded the baseline model to control for hospital characteristics: rurality, resident‐to‐bed ratio, disproportionate share percentage, the proportion of non‐Hispanic Black and Black patients admitted with stroke, hospital volume of patients with stroke, and geographic region. We repeated these analyses for the other outcomes of interest. We did not control for the National Institutes of Health Stroke Scale in the analyses for motor deficits because the National Institutes of Health Stroke Scale includes motor function as 1 of its components. Patients previously hospitalized with an acute ischemic stroke with motor deficits were not considered to have a new motor deficit.
The second aim of this study was to determine whether Black and Hispanic patients hospitalized during weeks with a high hospital COVID‐19 burden experienced worse outcomes than White patients under those conditions compared with patients hospitalized during weeks with a low hospital COVID‐19 burden. To characterize these differences in outcomes, we modified the baseline model to include an interaction between a linear specification of the weekly COVID‐19 burden and the indicator for race and ethnicity. Because the primary goal of the study was to describe the association between hospital stress and changes in disparities, we considered this second aim to be the primary analysis.
All statistical analyses were performed using STATA/MP version 17.0 (StataCorp). We used cluster robust variance estimators to account for the clustering of observations within hospitals. We estimated adjusted rates and outcomes using average marginal effects. The threshold for statistical significance was 2‐sided P<0.05. We did not adjust for multiple comparisons to avoid obscuring potential associations between hospital stress and widening disparities. 19 , 20
RESULTS
Patient Population
This study was based on data from 1 142 560 hospitalizations for acute ischemic stroke. Among these, 623 993 (54.6%) were women, 90 912 (8.0%) were Hispanic individuals, 162 752 (14.2%) were non‐Hispanic Black individuals, 888 896 (77.8%) were non‐Hispanic White individuals, and the mean (SD) age was 79.0 (8.7) years (Table 1). Black (69 033 [42.4%]) and Hispanic individuals (42 878 [47.2%]) were more likely to be dually enrolled compared with White individuals (143 170 [16.1%]). Black (5261 [3.2%]) and Hispanic individuals (2300 [2.5%]) were more likely to be on dialysis compared with White individuals (7018 [0.8%]). Hospital characteristics are described in Table 2. Twenty‐four percent of the hospitals were classified as rural (741), 11.5% (351) had a disproportionate share hospital percentage of ≥50%, and 10.1% (306) had ≥50% Black or Hispanic patients.
Table 1.
Patient Characteristics
| Characteristics | No. of patients | |||
|---|---|---|---|---|
| Total | White | Black | Hispanic | |
| (N=1 142 560) | (N=888 896) | (N=162 752) | (N=90 912) | |
| Race and ethnicity | ||||
| White | 888 896 (77.8) | |||
| Black | 162 752 (14.2) | |||
| Hispanic | 90 912 (7.96) | |||
| Hospital COVID‐19 burden | ||||
| Before COVID‐19 | 936 007 (81.9) | 729 679 (82.1) | 132 494 (81.4) | 73 834 (81.2) |
| 0.0%–2.0% | 70 069 (6.13) | 56 835 (6.39) | 7869 (4.83) | 5365 (5.9) |
| 2.1%–10.0% | 94 851 (8.3) | 72 111 (8.11) | 15 090 (9.27) | 7650 (8.41) |
| 10.1%–20.0% | 28 941 (2.53) | 21 236 (2.39) | 5056 (3.11) | 2649 (2.91) |
| 20.1%–30.0% | 8321 (0.73) | 6153 (0.69) | 1444 (0.89) | 8321 (0.73) |
| >30.0% | 4371 (0.38) | 2882 (0.32) | 799 (0.49) | 690 (0.76) |
| Age, y (SD) | 79 (8.7) | 79.6 (8.6) | 76.1 (8.4) | 77.7 (8.5) |
| Sex | ||||
| Men | 518 567 (45.4) | 405 859 (45.7) | 68 991 (42.4) | 43 717 (48.1) |
| Women | 623 993 (54.6) | 483 037 (54.3) | 93 761 (57.6) | 47 195 (51.9) |
| Urgency | ||||
| Emergent | 984 753 (86.2) | 759 217 (85.4) | 146 977 (90.3) | 78 559 (86.4) |
| Urgent | 157 807 (13.8) | 129 679 (14.6) | 15 775 (9.7) | 12 353 (13.6) |
| Admission source | ||||
| Community | 982 720 (86) | 754 077 (84.8) | 146 300 (89.9) | 82 343 (90.6) |
| Hospital | 118 961 (10.4) | 101 374 (11.4) | 11 398 (7) | 6189 (6.8) |
| SNF/nursing home | 26 369 (2.3) | 21 878 (2.5) | 3332 (2.1) | 1159 (1.3) |
| Other | 14 510 (1.3) | 11 567 (1.3) | 1722 (1.1) | 1221 (1.3) |
| Dual eligible | 255 090 (22.3) | 143 179 (16.1) | 69 033 (42.4) | 42 878 (47.2) |
| Medicare advantage | 418 398 (36.6) | 294 214 (33.1) | 74 275 (45.6) | 49 909 (54.9) |
| Body mass index | ||||
| Underweight | 25 252 (2.2) | 19 162 (2.2) | 4552 (2.8) | 1538 (1.7) |
| Morbid obesity | 32 762 (2.9) | 24 380 (2.7) | 6469 (4) | 1913 (2.1) |
| NIH Stroke Scale Score | ||||
| 0–9 | 400 850 (35.1) | 321 425 (36.2) | 53 296 (32.8) | 26 129 (28.7) |
| 10–19 | 88 084 (7.7) | 67 739 (7.6) | 13 571 (8.3) | 6774 (7.5) |
| 20–29 | 43 101 (3.8) | 33 157 (3.7) | 6479 (4) | 3465 (3.8) |
| ≥30 | 6711 (0.6) | 5111 (0.6) | 1049 (0.6) | 551 (0.6) |
| Missing | 603 814 (52.9) | 461 464 (51.9) | 88 357 (54.3) | 53 993 (59.4) |
| Time elapsed from prior stroke | ||||
| No prior stroke | 1 053 056 (92.2) | 823 199 (92.6) | 146 458 (90) | 83 399 (91.7) |
| ≤30 d | 35 997 (3.2) | 27 302 (3.1) | 5617 (3.5) | 3078 (3.4) |
| 31–60 d | 11 259 (1) | 8137 (0.9) | 2185 (1.3) | 937 (1) |
| 61–90 d | 7516 (0.7) | 5375 (0.6) | 1515 (0.9) | 626 (0.7) |
| 91–180 d | 15 151 (1.3) | 10 858 (1.2) | 3025 (1.9) | 1268 (1.4) |
| 181–360 d | 19 581 (1.7) | 14 025 (1.6) | 3952 (2.4) | 1604 (1.8) |
| Stroke location | ||||
| Precerebral, unspecified | 6121 (0.5) | 4939 (0.6) | 800 (0.5) | 382 (0.4) |
| Cerebral, unspecified | 64 758 (5.7) | 52 478 (5.9) | 8047 (4.9) | 4233 (4.7) |
| Carotid | 52 739 (4.6) | 43 529 (4.9) | 5405 (3.3) | 3805 (4.2) |
| Anterior cerebral | 14 836 (1.3) | 10 902 (1.2) | 2652 (1.6) | 1282 (1.4) |
| Middle cerebral | 274 997 (24.1) | 216 599 (24.4) | 37 380 (23) | 21 018 (23.1) |
| Posterior | 53 735 (4.7) | 42 927 (4.8) | 6354 (3.9) | 4454 (4.9) |
| Vertebral | 13 077 (1.1) | 10 290 (1.2) | 1832 (1.1) | 955 (1.1) |
| Cerebellar | 27 152 (2.4) | 21 206 (2.4) | 3764 (2.3) | 2182 (2.4) |
| Basilar | 12 291 (1.1) | 9134 (1) | 2104 (1.3) | 1053 (1.2) |
| Other | 622 854 (54.5) | 476 892 (53.7) | 94 414 (58) | 51 548 (56.7) |
| Prior procedures | ||||
| PCI | 95 508 (8.4) | 79 272 (8.9) | 10 317 (6.3) | 5919 (6.5) |
| CABG | 6282 (6.9) | 76 581 (8.6) | 7540 (4.6) | 6282 (6.9) |
| Heart valve surgery | 28 146 (2.5) | 24 796 (2.8) | 1805 (1.1) | 1545 (1.7) |
| Functional status | ||||
| Wheelchair | 11 547 (1) | 8475 (1) | 2157 (1.3) | 915 (1) |
| Supplemental oxygen | 16 874 (1.5) | 14 479 (1.6) | 1640 (1) | 755 (0.8) |
| Dependent on provider | 12 230 (1.1) | 7318 (0.8) | 2856 (1.8) | 2056 (2.3) |
| COVID‐19 | 2521 (0.2) | 1611 (0.2) | 549 (0.3) | 361 (0.4) |
| Dialysis | 14 579 (1.3) | 7018 (0.8) | 5261 (3.2) | 2300 (2.5) |
| Elixhauser comorbidities | ||||
| Congestive heart failure | 241 452 (21.1) | 182 931 (20.6) | 40 848 (25.1) | 17 673 (19.4) |
| Cardiac arrhythmias | 429 517 (37.6) | 354 561 (39.9) | 46 367 (28.5) | 28 589 (31.5) |
| Valvular heart disease | 118 171 (10.3) | 97 415 (11) | 13 089 (8) | 7667 (8.4) |
| Pulmonary circulation | 52 086 (4.6) | 40 283 (4.5) | 8600 (5.3) | 3203 (3.5) |
| Peripheral vascular disorder | 105 112 (9.2) | 84 245 (9.5) | 13 103 (8.1) | 7764 (8.5) |
| Hypertension, uncomplicated | 634 268 (55.5) | 498 792 (56.1) | 83 272 (51.2) | 52 204 (57.4) |
| Hypertension, complicated | 384 819 (33.7) | 284 494 (32) | 70 019 (43) | 30 306 (33.3) |
| Chronic pulmonary disease | 197 024 (17.2) | 160 785 (18.1) | 25 127 (15.4) | 11 112 (12.2) |
| Diabetes, uncomplicated | 185 960 (16.3) | 132 455 (14.9) | 32 550 (20) | 20 955 (23.1) |
| Diabetes, complicated | 257 976 (22.6) | 176 325 (19.8) | 52 946 (32.5) | 28 705 (31.6) |
| Hypothyroidism | 216 292 (18.9) | 186 609 (21) | 15 003 (9.2) | 14 680 (16.2) |
| Renal failure | 253 444 (22.2) | 185 114 (20.8) | 49 127 (30.2) | 19 203 (21.1) |
| Liver disease | 17 252 (1.5) | 12 150 (1.4) | 3208 (2) | 1894 (2.1) |
| Peptic ulcer disease | 6480 (0.6) | 4958 (0.6) | 969 (0.6) | 553 (0.6) |
| AIDS/HIV | 842 (0.1) | 317 (0.04) | 418 (0.3) | 107 (0.1) |
| Lymphoma | 7630 (0.7) | 6062 (0.7) | 1101 (0.7) | 467 (0.5) |
| Metastatic cancer | 21 959 (1.9) | 17 662 (2) | 2980 (1.8) | 1317 (1.5) |
| Solid tumor | 43 019 (3.8) | 34 169 (3.8) | 6081 (3.7) | 2769 (3.1) |
| Rheumatoid arthritis | 36 760 (3.2) | 30 132 (3.4) | 4440 (2.7) | 2188 (2.4) |
| Coagulopathy | 52 979 (4.6) | 41 452 (4.7) | 7520 (4.6) | 4007 (4.4) |
| Fluid and electrolyte disorder | 237 960 (20.8) | 179 391 (20.2) | 40 004 (24.6) | 18 565 (20.4) |
| Anemia, blood loss | 4165 (0.4) | 3242 (0.4) | 654 (0.4) | 269 (0.3) |
| Anemia, deficiency | 34 705 (3) | 25 488 (2.9) | 6728 (4.1) | 2489 (2.7) |
| Alcohol abuse | 31 901 (2.8) | 23 960 (2.7) | 5528 (3.4) | 2413 (2.7) |
| Drug abuse | 14 731 (1.3) | 9112 (1) | 4610 (2.8) | 1009 (1.1) |
| Psychoses | 7495 (0.7) | 4721 (0.5) | 2092 (1.3) | 682 (0.8) |
| Depression | 135 776 (11.9) | 115 388 (13) | 12 061 (7.4) | 8327 (9.2) |
| Outcomes | ||||
| Death within 30 d | 147 485 (12.9) | 121 003 (13.6) | 15 758 (9.7) | 10 724 (11.8) |
| Motor deficit | 553 785 (48.5) | 426 510 (48) | 83 132 (51.1) | 44 143 (48.6) |
| Nonhome discharge | 616 917 (54) | 482 613 (54.3) | 91 858 (56.4) | 42 446 (46.7) |
| Endovascular thrombectomy | 33 989 (3) | 27 487 (3.1) | 3966 (2.4) | 2536 (2.8) |
| Thrombolytic therapy | 113 397 (9.9) | 89 228 (10) | 14 750 (9.1) | 9419 (10.4) |
Values are number (percent) unless indicated otherwise. CABG indicates coronary artery bypass grafting; NIH, National Institutes of Health; PCI, percutaneous coronary intervention; and SNF, skilled nursing facility.
Table 2.
Hospital Characteristics
| Characteristics | Hospitals, n (%) |
|---|---|
| Total no. of beds | |
| <50 | 433 (14.2) |
| 51–149 | 1082 (35.6) |
| 150–249 | 667 (21.9) |
| 250–499 | 643 (21.1) |
| ≥500 | 219 (7.2) |
| Resident‐to‐bed ratio | |
| 0 | 1951 (64.1) |
| >0–0.10 | 516 (17) |
| 0.11–0.20 | 209 (6.9) |
| 0.21–0.40 | 184 (6) |
| ≥0.41 | 184 (6) |
| Rurality | |
| Rural hospital | 741 (24.3) |
| Large urban hospital | 1233 (40.5) |
| Other urban hospital | 1070 (35.2) |
| Region | |
| New England | 128 (4.2) |
| Middle Atlantic | 342 (11.2) |
| South Atlantic | 555 (18.2) |
| East North Central | 466 (15.3) |
| East South Central | 280 (9.2) |
| West North Central | 228 (7.5) |
| West South Central | 418 (13.7) |
| Mountain | 199 (6.5) |
| Pacific | 381 (12.5) |
| Puerto Rico | 47 (1.5) |
| Disproportionate share percentage | |
| 0%–9.9% | 183 (6) |
| 10.0%–24.9% | 1008 (33.1) |
| 25.0%–49.9% | 1502 (49.3) |
| ≥50.0% | 351 (11.5) |
| Proportion of minority patients (strokes) | |
| <5% | 847 (27.8) |
| 5.0%–9.9% | 709 (23.3) |
| 10.0%–24.9% | 726 (23.9) |
| 25.0%–49.9% | 456 (15) |
| ≥50.0% | 306 (10.1) |
| Volume of stroke hospitalizations | |
| <50 | 456 (15) |
| 50–249 | 835 (27.4) |
| 250–749 | 956 (31.4) |
| ≥750 | 797 (26.2) |
Association of Hospital COVID‐19 Burden With Changes in Clinical Outcomes Overall and by Race and Ethnicity
Among all patients, 30‐day mortality rates increased more during weeks with a high weekly hospital COVID‐19 burden compared with the prepandemic period (P<0.001). Although mortality increased during the pandemic compared with the prepandemic period (P<0.001), increases in the hospital COVID‐19 burden were not associated with increases in the rate of severe strokes (P=0.25) (Figure 1; Table S2). The adjusted odds of mortality increased by 34% (adjusted OR [aOR], 1.34 [95% CI, 1.25–1.45]; P<0.001), 32% (aOR, 1.32 [95% CI, 1.19–1.46]; P<0.001), and 51% (aOR, 1.51 [95% CI, 1.34–1.69]; P<0.001) for patients hospitalized during weeks with a COVID‐19 burden of 10.1% to 20%, 20.1% to 30%, and >30%, respectively. Nonhome discharges decreased slightly during the pandemic (P<0.001). These findings did not change substantially after also controlling for hospital characteristics (Figure 1).
Figure 1. Changes in mortality, motor deficits, nonhome discharges, and reperfusion therapy in patients with stroke as a function of the weekly hospital COVID‐19 burden.
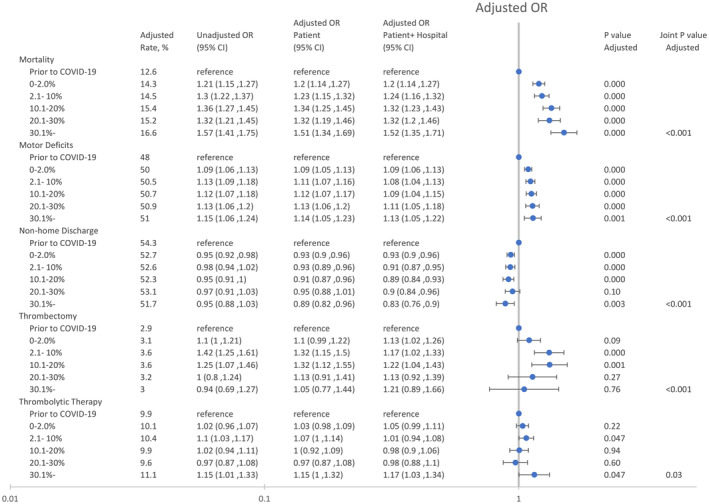
The patient model was adjusted for patient age, race, ethnicity, and patient risk (see text), and the patient+hospital model was adjusted for patient age, race, ethnicity, patient risk, and hospital characteristics (see text). The unadjusted model was adjusted for patient age, race, and ethnicity. P values and adjusted rates are based on the patient model. OR indicates odds ratio.
Black and Hispanic individuals were less likely to die, whereas Black individuals were more likely to be admitted with severe strokes compared with White individuals (Figure 2). Compared with White individuals, Black and Hispanic individuals had 30% (aOR, 0.70 [95% CI, 0.69–0.72]; P<0.001) and 9% lower odds (aOR, 0.91 [95% CI, 0.88–0.95]; P<0.001) of 30‐day mortality compared with White individuals, respectively. However, Black individuals had 14% (aOR, 1.14 [95% CI, 1.12–1.16]; P<0.001) higher odds of motor deficits than White individuals. Black patients (aOR, 0.95 [95% CI, 0.92–0.97]; P<0.001) were slightly less likely to be discharged to a nonhome setting than White patients.
Figure 2. Association of race and ethnicity with mortality, motor deficits, nonhome discharges, and reperfusion therapy in patients with stroke.
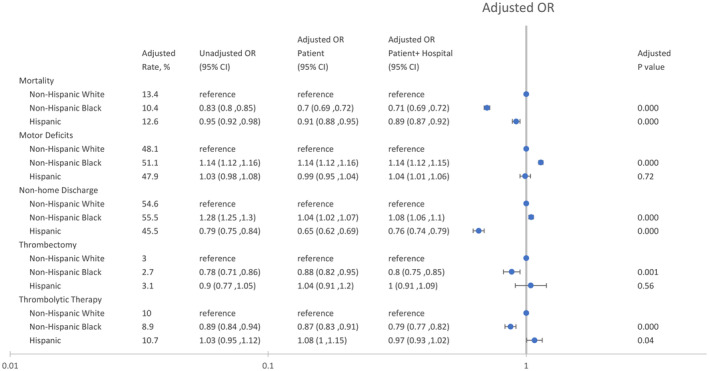
The patient model was adjusted for patient age, race, ethnicity, and patient risk (see text), and the patient+hospital model was adjusted for patient age, race, ethnicity, patient risk, and hospital characteristics (see text). The unadjusted model was adjusted for patient age, race, and ethnicity. P values and adjusted rates are based on the patient model. OR indicates odds ratio.
Black and Hispanic patients did not experience a greater change in mortality or motor deficits than White patients as the weekly hospital COVID‐19 burden increased (Figure 3). These findings were not changed after adjusting for hospital characteristics.
Figure 3. Changes in mortality, motor deficits, nonhome discharges, and reperfusion therapy in patients with stroke as a function of the weekly hospital COVID‐19 burden interacted with race and ethnicity.
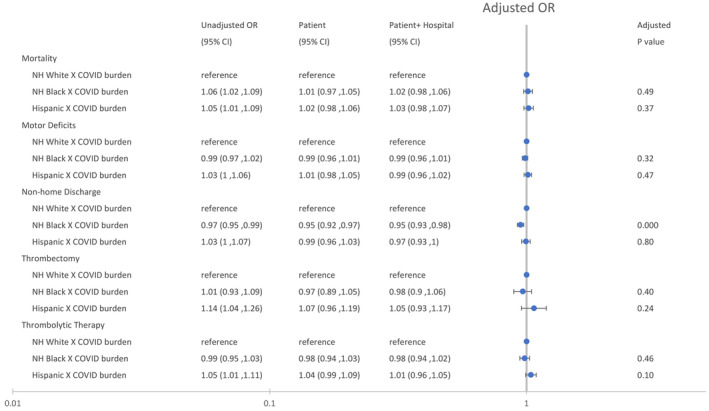
The patient model was adjusted for patient age, race, ethnicity, and patient risk (see text), and the patient+hospital model was adjusted for patient age, race, ethnicity, patient risk, and hospital characteristics (see text). The unadjusted model was adjusted for patient age, race, and ethnicity. P values are based on the patient model. NH indicates non‐Hispanic; and OR, odds ratio.
Association of Hospital COVID‐19 Burden With Changes in Reperfusion Therapy Overall and by Race and Ethnicity
Among all patients, reperfusion rates increased during weeks with a high weekly COVID‐19 burden (Figure 1). The adjusted odds of endovascular thrombectomy increased by 32% (aOR, 1.32 [95% CI, 1.15–1.50]; P<0.001) and 32% (aOR, 1.32 [95% CI, 1.12–1.55]; P<0.001) for patients hospitalized during weeks with a COVID‐19 burden of 2.1% to 10% and 10.1% to 20%, respectively. The adjusted odds of thrombolytic therapy increased slightly during weeks with a COVID‐19 burden >30% (aOR, 1.15 [95% CI, 1.00–1.32]; P=0.047).
Black individuals were less likely to receive thrombectomy (aOR, 0.88 [95% CI, 0.82–0.95]; P=0.001) or thrombolytic therapy (aOR, 0.87 [95% CI, 0.83–0.91]; P<0.001) than White individuals (Figure 2). Hispanic individuals had similar rates of thrombectomy (aOR, 1.04 [95% CI, 0.91–1.20]; P=0.56) and slightly higher rates of thrombolytic therapy (aOR, 1.08 [95% CI, 1.00–1.15]; P=0.04) compared with White individuals (Figure 2). These findings remained largely unchanged after adjusting for hospital characteristics.
There was no evidence that disparities in thrombectomy (P=0.40) and thrombolytic therapy (P=0.46) for Black patients were increased during weeks with a high COVID‐19 burden (Figure 3). These findings were not changed after adjusting for hospital characteristics.
DISCUSSION
Using national Medicare data on 1 142 560 hospitalizations from between 2016 and 2020, we found that patients hospitalized with an acute ischemic stroke during the first year of the pandemic had a higher risk of mortality during weeks when the proportion of inpatients with COVID‐19 was elevated compared with before the pandemic. After controlling for patient and hospital characteristics, including infection with COVID‐19, patients admitted with a stroke during weeks with a high or very high COVID‐19 burden faced up to 30% to 50% higher odds of 30‐day mortality. However, we did not find that patients were more likely to present with more severe strokes or to be discharged to a nonhome setting in hospitals stressed by COVID‐19. Moreover, patients with stroke were not less likely to receive reperfusion therapy during these weeks.
In terms of racial disparities, Black and Hispanic individuals were less likely to die, but Black individuals were more likely to present with more severe strokes than White individuals. Moreover, Black individuals were less likely to receive reperfusion therapy than White individuals. Although COVID‐19 has disproportionately impacted historically marginalized populations, we found no evidence that the pandemic exacerbated disparities for patients hospitalized with a stroke. We have also previously reported that COVID‐19 did not exacerbate surgical disparities. 21 , 22 In particular, although the pandemic was associated with a higher risk of postoperative death, patients without insurance or Medicaid insurance did not experience greater increases in mortality than patients with private insurance during the pandemic. 21 We also separately reported that although the CMS moratorium on elective surgery led to a 51% reduction in elective surgeries, minoritized individuals did not experience a greater reduction in surgical use than White individuals. 22 The findings that, under conditions of extreme stress, hospitals treat vulnerable individuals similarly to nonvulnerable individuals do not alleviate foundational concerns about health equity. However, these findings do suggest that, at least in some cases, these disparities are not exacerbated by hospitals and their physician and nonphysician workforce.
Stroke care during the pandemic could have been impacted by disruptions to usual processes of care, or by delay and deferral of care among patients experiencing stroke symptoms. Based on data from the GWTG (Get With The Guidelines) Stroke national registry, previous studies reported increases in mortality rates and stroke severity during the pandemic and no differences in the use of reperfusion therapy. 23 , 24 In contrast, although we also found increases in mortality, we found increases in the use of reperfusion therapy and no increase in stroke severity. The discrepancies in the findings may be due in part to differences in patient samples. Although the GWTG Stroke registry is a convenience sample that includes patients of all ages at a subset of hospitals participating in the GWTG quality improvement initiatives, our study is based on a larger population‐based sample of elderly Medicare patients and includes patients at a much broader range of hospitals. Additionally, our study examined the effect of the pandemic as a function of the weekly hospital COVID‐19 burden, whereas previous studies compared the prepandemic to the pandemic period by combining all of the patients in the pandemic period into 1 group, regardless of the inpatient COVID‐19 burden. Although these earlier studies examined the impact of COVID‐19 on the overall treatment and outcomes of patients with ischemic strokes, to our knowledge, no other study has examined the effects of COVID‐19 on disparities in stroke outcomes and treatment.
Although we found no evidence that the COVID‐19 pandemic, acting as a hospital stress test, exacerbated racial disparities in stroke care, the underlying disparities in stroke severity were nonetheless concerning. Black individuals had 14% higher odds of presenting with a motor deficit than White individuals, even after controlling for baseline differences in risk and hospital characteristics. The magnitude of these differences is especially troubling, because racial disparities in stroke severity were reported at least 20 years ago. 4 , 5 , 6 The observation that Black individuals have lower mortality rates and yet worse poststroke outcomes may, in part, reflect racial differences in preferences for more aggressive end‐of‐life care. 25 We also found disparities in the use of reperfusion therapy, as reported by others. 26 , 27 , 28 , 29 , 30 These disparities may be due to delayed hospital presentation because of delays in seeking medical treatment, less frequent use of emergency medical services, longer waiting times in the emergency department, as well as reduced access to stroke‐certified hospitals. 31 , 32 , 33 , 34 Notably, we found no evidence of greater reductions in the use of reperfusion therapy in Black and Hispanic patients compared with White patients during the pandemic. In 2020, the American Heart Association issued a Call to Action to address the differential access to health care services that is contributing to persistent racial disparities in cardiovascular health. 35 Although our study suggests that COVID‐19 did not exacerbate racial disparities in stroke care, our results expose persistent disparities in stroke outcomes that need to be addressed. These racial disparities are most likely caused by upstream social determinants of health and structural racism that preferentially allocates health care resources to White individuals over historically marginalized groups who have less access to high‐quality care because of where they live and because of differences in wealth and income. 36 , 37 Targeted efforts to improve stroke outcomes in historically underserved populations could include prehospital triage programs that refer patients with suspected large‐vessel occlusions to endovascular‐capable stroke centers and the use of mobile stroke units to reduce door‐to‐needle time. 6
Limitations
Our study has several limitations. First, our study is a nonrandomized study using retrospective data, which makes it difficult to make causal inferences. Second, our findings are based on administrative data that, unlike clinical data, may not capture the full complexity of patient disease and outcomes. Despite this, our models were able to control for key clinical characteristics such as National Institutes of Health Stroke Scale, the time elapsed from a prior stroke, and stroke location. Notably, administrative data are widely used by researchers to answer policy‐relevant questions because they are population‐based and comprehensive in scope. 38 , 39 , 40 Third, our study only examined the first year of the pandemic and may not reflect changes that occurred later in the pandemic. However, we chose to limit our study to the early phase of the pandemic because we assumed that hospitals were least prepared to deal with COVID‐19 during this early stage, making this period most relevant to use for a hospital stress test. Fourth, because our study is limited to patients aged ≥65 years, and Black individuals are more likely than White individuals to have a stroke at an early age (the age‐ and sex‐adjusted incidence rate ratio of strokes for Black compared with White individuals is 4.02 for patients aged 45 to 54 years versus 0.86 for those aged ≥85 years), and patients aged <65 years comprise 38% of individuals hospitalized for stroke, our findings may underestimate the impact of COVID‐19 on stroke disparities. 1 , 41
CONCLUSIONS
In this study of 1.1 million hospitalizations for stroke, the COVID‐19 pandemic did not exacerbate disparities in stroke treatment and outcomes for Black and Hispanic individuals. Our finding that COVID‐19 acting as a hospital stress test failed to worsen disparities is reassuring. Nonetheless, there are persistent disparities in the use of reperfusion therapy and in stroke severity that must be addressed. Reducing these disparities may require efforts to promote education focusing on early recognition of brain attacks and the value of emergency medical services for obtaining timely care, improving access to reperfusion therapy, increasing provider cultural competency, and expanding the numbers of underrepresented minority physicians, in addition to the traditional focus on risk factor modification. 12 , 42 , 43
Sources of Funding
This work was supported by a grant from the National Institute on Aging (R01AG074492) and the Department of Anesthesiology and Perioperative Medicine at the University of Rochester School of Medicine and Dentistry. The funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; review or approval of the article; or decision to submit the article.
Disclosures
Dr Joynt Maddox reports consulting for Centene Corporation and Humana Inc. and grants/contracts from the Journal of the American Medical Association, the National Heart, Lung, and Blood Institute, and the National Institute on Aging. Dr Bender reports consulting for the Stryker Corporation. The remaining authors have no disclosures to report.
Supporting information
Data S1
Acknowledgments
The principal investigator (L.G.G.) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031221
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2. Stroke and African Americans . U.S. Department of Health and Human Services, Office of Minority Health. 2022. Accessed April 13, 2022. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=28#:~:text=African%20Americans%20are%2050%20percent,compared%20to%20non%2DHispanic%20whites.
- 3. Murphy SL, Xu J, Kochanek KD, Arias E, Tejada‐Vera B. Deaths: final data for 2018. Natl Vital Stat Rep. 2021;69:1–83. [PubMed] [Google Scholar]
- 4. Kuhlemeier KV, Stiens SA. Racial disparities in severity of cerebrovascular events. Stroke. 1994;25:2126–2131. doi: 10.1161/01.str.25.11.2126 [DOI] [PubMed] [Google Scholar]
- 5. Jones MR, Horner RD, Edwards LJ, Hoff J, Armstrong SB, Smith‐Hammond CA, Matchar DB, Oddone EZ. Racial variation in initial stroke severity. Stroke. 2000;31:563–567. doi: 10.1161/01.str.31.3.563 [DOI] [PubMed] [Google Scholar]
- 6. Bosch PR, Karmarkar AM, Roy I, Fehnel CR, Burke RE, Kumar A. Association of Medicare‐Medicaid dual eligibility and race and ethnicity with ischemic stroke severity. JAMA Netw Open. 2022;5:e224596. doi: 10.1001/jamanetworkopen.2022.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones EM, Okpala M, Zhang X, Parsha K, Keser Z, Kim CY, Wang A, Okpala N, Jagolino A, Savitz SI, et al. Racial disparities in post‐stroke functional outcomes in young patients with ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29:104987. doi: 10.1016/j.jstrokecerebrovasdis.2020.104987 [DOI] [PubMed] [Google Scholar]
- 8. Burke JF, Freedman VA, Lisabeth LD, Brown DL, Haggins A, Skolarus LE. Racial differences in disability after stroke: results from a nationwide study. Neurology. 2014;83:390–397. doi: 10.1212/WNL.0000000000000640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clark DG, Boan AD, Sims‐Robinson C, Adams RJ, Amella EJ, Benitez A, Lackland DT, Ovbiagele B. Differential impact of index stroke on dementia risk in African‐Americans compared to whites. J Stroke Cerebrovasc Dis. 2018;27:2725–2730. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caraballo C, Massey DS, Ndumele CD, Haywood T, Kaleem S, King T, Liu Y, Lu Y, Nunez‐Smith M, Taylor HA, et al. Excess mortality and years of potential life lost among the black population in the US, 1999‐2020. JAMA. 2023;329:1662–1670. doi: 10.1001/jama.2023.7022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kapral MK. Kenton award lecture‐stroke disparities research: learning from the past, planning for the future. Stroke. 2023;54:379–385. doi: 10.1161/STROKEAHA.122.039562 [DOI] [PubMed] [Google Scholar]
- 12. Levine DA, Duncan PW, Nguyen‐Huynh MN, Ogedegbe OG. Interventions targeting racial/ethnic disparities in stroke prevention and treatment. Stroke. 2020;51:3425–3432. doi: 10.1161/STROKEAHA.120.030427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. French G, Hulse M, Nguyen D, Sobotka K, Webster K, Corman J, Aboagye‐Nyame B, Dion M, Johnson M, Zalinger B, et al. Impact of hospital strain on excess deaths during the COVID‐19 pandemic ‐ United States, July 2020–July 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1613–1616. doi: 10.15585/mmwr.mm7046a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mackey K, Ayers CK, Kondo KK, Saha S, Advani SM, Young S, Spencer H, Rusek M, Anderson J, Veazie S, et al. Racial and ethnic disparities in COVID‐19‐related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med. 2021;174:362–373. doi: 10.7326/M20-6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 16. Research Triangle Institute (RTI) Race Code . Accessed April 10, 2023. https://resdac.org/cms‐data/variables/research‐triangle‐institute‐rti‐race‐code
- 17. ELIXHAUSER COMORBIDITY SOFTWARE REFINED FOR ICD‐10‐CM . Accessed April 13, 2023. https://hcup‐us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp#:~:text=The%20Elixhauser%20Comorbidity%20Indices%20Refined%20for%20ICD%2D10%2DCM%20is,%2Dday%2C%20all%2Dcause%20readmission
- 18. Elixhauser: stata module to calculate Elixhauser index of comorbidity. 2015. Accessed April 1, 2021. https://ideas.repec.org/c/boc/bocode/s458077.html.
- 19. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. doi: 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 20. Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Glance LG, Dick AW, Shippey E, McCormick PJ, Dutton R, Stone PW, Shang J, Lustik SJ, Lander HL, Gosev I, et al. Association between the COVID‐19 pandemic and insurance‐based disparities in mortality after major surgery among US adults. JAMA Netw Open. 2022;5:e2222360. doi: 10.1001/jamanetworkopen.2022.22360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glance LG, Chandrasekar EK, Shippey E, Stone PW, Dutton R, McCormick PJ, Shang J, Lustik SJ, Wu IY, Eaton MP, et al. Association between the COVID‐19 pandemic and disparities in access to major surgery in the US. JAMA Netw Open. 2022;5:e2213527. doi: 10.1001/jamanetworkopen.2022.13527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Srivastava PK, Zhang S, Xian Y, Xu H, Rutan C, Alger HM, Walchok JG, Williams JH, de Lemos JA, Decker‐Palmer MR, et al. Treatment and outcomes of patients with ischemic stroke during COVID‐19: an analysis from get with the guidelines‐stroke. Stroke. 2021;52:3225–3232. doi: 10.1161/STROKEAHA.120.034414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tong X, King SMC, Asaithambi G, Odom E, Yang Q, Yin X, Merritt RK. COVID‐19 pandemic and quality of care and outcomes of acute stroke hospitalizations: the Paul Coverdell national acute stroke program. Prev Chronic Dis. 2021;18:E82. doi: 10.5888/pcd18.210130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burke JF, Feng C, Skolarus LE. Divergent poststroke outcomes for black patients: lower mortality, but greater disability. Neurology. 2019;93:e1664–e1674. doi: 10.1212/WNL.0000000000008391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsia AW, Edwards DF, Morgenstern LB, Wing JJ, Brown NC, Coles R, Loftin S, Wein A, Koslosky SS, Fatima S, et al. Racial disparities in tissue plasminogen activator treatment rate for stroke: a population‐based study. Stroke. 2011;42:2217–2221. doi: 10.1161/STROKEAHA.111.613828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwamm LH, Reeves MJ, Pan W, Smith EE, Frankel MR, Olson D, Zhao X, Peterson E, Fonarow GC. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121:1492–1501. doi: 10.1161/CIRCULATIONAHA.109.881490 [DOI] [PubMed] [Google Scholar]
- 28. Aparicio HJ, Carr BG, Kasner SE, Kallan MJ, Albright KC, Kleindorfer DO, Mullen MT. Racial disparities in intravenous recombinant tissue plasminogen activator use persist at primary stroke centers. J Am Heart Assoc. 2015;4:e001877. doi: 10.1161/JAHA.115.001877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Otite FO, Saini V, Sur NB, Patel S, Sharma R, Akano EO, Anikpezie N, Albright K, Schmidt E, Hoffman H, et al. Ten‐year trend in age, sex, and racial disparity in tPA (alteplase) and thrombectomy use following stroke in the United States. Stroke. 2021;52:2562–2570. doi: 10.1161/STROKEAHA.120.032132 [DOI] [PubMed] [Google Scholar]
- 30. Kim Y, Sharrief A, Kwak MJ, Khose S, Abdelkhaleq R, Salazar‐Marioni S, Zhang GQ, Sheth SA. Underutilization of endovascular therapy in black patients with ischemic stroke: an analysis of state and nationwide cohorts. Stroke. 2022;53:855–863. doi: 10.1161/STROKEAHA.121.035714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhattacharya P, Mada F, Salowich‐Palm L, Hinton S, Millis S, Watson SR, Chaturvedi S, Rajamani K. Are racial disparities in stroke care still prevalent in certified stroke centers? J Stroke Cerebrovasc Dis. 2013;22:383–388. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.018 [DOI] [PubMed] [Google Scholar]
- 32. Karve SJ, Balkrishnan R, Mohammad YM, Levine DA. Racial/ethnic disparities in emergency department waiting time for stroke patients in the United States. J Stroke Cerebrovasc Dis. 2011;20:30–40. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 33. King DF, Trouth AJ, Adams AO. Factors preventing African Americans from seeking early intervention in the treatment of ischemic strokes. J Natl Med Assoc. 2001;93:43–46. [PMC free article] [PubMed] [Google Scholar]
- 34. Shen YC, Sarkar N, Hsia RY. Structural inequities for historically underserved communities in the adoption of stroke certification in the United States. JAMA Neurol. 2022;79:777–786. doi: 10.1001/jamaneurol.2022.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 36. Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–398. doi: 10.1146/annurev-publhealth-031210-101218 [DOI] [PubMed] [Google Scholar]
- 37. Yearby R, Clark B, Figueroa JF. Structural racism In historical and modern US health care policy. Health Aff (Millwood). 2022;41:187–194. doi: 10.1377/hlthaff.2021.01466 [DOI] [PubMed] [Google Scholar]
- 38. Desai NR, Ross JS, Kwon JY, Herrin J, Dharmarajan K, Bernheim SM, Krumholz HM, Horwitz LI. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316:2647–2656. doi: 10.1001/jama.2016.18533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Finkelstein A, Ji Y, Mahoney N, Skinner J. Mandatory Medicare bundled payment program for lower extremity joint replacement and discharge to institutional Postacute care: interim analysis of the first year of a 5‐year randomized trial. JAMA. 2018;320:892–900. doi: 10.1001/jama.2018.12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Regenbogen SE, Cain‐Nielsen AH, Norton EC, Chen LM, Birkmeyer JD, Skinner JS. Costs and consequences of early hospital discharge after major inpatient surgery in older adults. JAMA Surg. 2017;152:e170123. doi: 10.1001/jamasurg.2017.0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stoke Facts (CDC) . CDC. Accessed August 1, 2023. https://www.cdc.gov/stroke/facts.htm#print.
- 42. Ikeme S, Kottenmeier E, Uzochukwu G, Brinjikji W. Evidence‐based disparities in stroke care metrics and outcomes in the United States: a systematic review. Stroke. 2022;53:670–679. doi: 10.1161/STROKEAHA.121.036263 [DOI] [PubMed] [Google Scholar]
- 43. Skolarus LE, Sharrief A, Gardener H, Jenkins C, Boden‐Albala B. Considerations in addressing social determinants of health to reduce racial/ethnic disparities in stroke outcomes in the United States. Stroke. 2020;51:3433–3439. doi: 10.1161/STROKEAHA.120.030426 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


