Abstract
Background
Data are rare about the incidence of severe Fontan‐associated liver disease (FALD) and its association with mortality. We sought to: (1) estimate the probability of developing severe FALD in patients who undergo the Fontan procedure (Fontan patients), compared with severe liver complications in patients with a ventricular septal defect; (2) assess the severe FALD‐mortality association; and (3) identify risk factors for developing severe FALD.
Methods and Results
Using the Quebec Congenital Heart Disease database, a total of 512 Fontan patients and 10 232 patients with a ventricular septal defect were identified. Kaplan‐Meier curves demonstrated significantly higher cumulative risk of severe FALD in Fontan patients (11.95% and 52.24% at 10 and 35 years, respectively), than the risk of severe liver complications in patients with a ventricular septal defect (0.50% and 2.75%, respectively). At 5 years, the cumulative risk of death was 12.60% in patients with severe FALD versus 3.70% in Fontan patients without FALD (log‐rank P=0.0171). Cox proportional hazard models identified significant associations between the development of severe FALD and congestive heart failure and supraventricular tachycardia, with hazard ratios (HRs) of 2.36 (95% CI, 1.38–4.02) and 2.45 (95% CI, 1.37–4.39), respectively. More recent Fontan completion was related to reduced risks of severe FALD, with an HR of 0.95 (95% CI, 0.93–0.97) for each more recent year.
Conclusions
This large‐scale population‐based study documents that severe FALD in Fontan patients was associated with a >3‐fold increase in mortality. The risk of FALD is time‐dependent and can reach >50% by 35 years after the Fontan operation. Conditions promoting poor Fontan hemodynamics were associated with severe FALD development.
Keywords: congenital heart defects, Fontan procedure, liver diseases, mortality, risk factor
Subject Categories: Etiology, Clinical Studies, Mechanisms
Nonstandard Abbreviations and Acronyms
- FALD
Fontan‐associated liver disease
Clinical Perspective.
What Is New?
Hospitalization attributable to Fontan‐associated liver disease (FALD) is frequent after Fontan operation; at 10 and 35 years after Fontan completion, the cumulative incidence of FALD hospitalization reaches 12% and 50%, respectively.
Severe FALD is associated with a 3‐fold increase in mortality risk among patients who undergo the Fontan procedure.
Factors associated with the development of severe FALD include heart failure and supraventricular arrhythmias.
What Are the Clinical Implications?
Protocols for FALD surveillance are suggested to enable early detection and treatment of severe FALD and are expected to be helpful in decreasing the medical burden of FALD.
Better understanding of Fontan circulation is needed to inform the mechanism that may lead to reduction in the frequency of severe FALD development and suggest effective prevention and treatment.
Since its introduction in 1968, Fontan palliation surgery has improved the survival of patients with single‐ventricle physiology worldwide, with 20‐ and 30‐year survival rates of 61%, and 43%, respectively. 1 , 2 , 3 However, this unique physiological and anatomic configuration leads to an increased risk of extracardiac complications related mainly to passive flow and low cardiac output. 3
Fontan‐associated liver disease (FALD) is one of the most prevalent extracardiac complications derived from the Fontan operation. 4 The cause of FALD remains hitherto a clinical dilemma. Nonetheless, most revisions point to a multifactorial cause. 5
Liver fibrosis appears to be universal in patients who undergo the Fontan procedure (Fontan patients), but the clinical significance of this fibrosis is yet to be established. 6 Definition, diagnosis, and classification of this complication are subjects of continuous debate. 4 , 5
FALD by itself is thought to be associated with increased morbidity. A cohort of 60 Fontan patients reported a 22% incidence of adverse hepatic‐related outcomes, with a mean interval of 18±5 years since Fontan completion. However, this trial does not have sufficient statistical power to link FALD with mortality, nor was it able to evaluate which risk factors are associated with clinically overt FALD. 7 Notwithstanding increased awareness and detection, the burden of FALD and its relationship to mortality has not been determined to date.
We hypothesized that severe FALD is related to increased mortality in patients after Fontan operation. Our objectives were 3‐fold: to estimate the incidence of severe FALD, to assess its association with mortality, and to identify post‐Fontan risk factors for developing severe FALD.
Methods
The authors declare that all supporting data are available within the article (and its online supplementary files). The data will not be made available to other researchers for purposes of reproducing the results or replicating the procedure because of confidentiality agreements governed by Regie de l'Assurance Maladie du Quebec. Our administrative database has scrambled identifications and prevents the necessity for informed consent.
Data Source
In Quebec, Canada, all residents are assigned a unique health care number from birth. The Quebec congenital heart disease (CHD) database was created by merging 3 administrative databases: the administrative medical claims database, the provincial hospital discharge summary database, and the death registry. 8 The Quebec CHD database contains longitudinal, demographic, diagnostic, and therapeutic records of all Quebec residents with CHD. 8 , 9 The database is composed of 132 071 patients with CHD with up to 35 years of follow‐up (from January 1, 1983, to December 31, 2017). This study was approved by the McGill University Health Centre ethics board. Given our data source is an administrative database that covers the entire province of Quebec, loss of follow‐up happens in our study only if patients moved out of the province. Public data show that the emigration rate of Quebec is as low as 0.15% per year. It is thus reasonable to assume that loss of follow‐up is not an issue in our study that could detriment the internal and external validity of our study findings. 9
Study Design and Population
Retrospective cohort studies were adopted for all 3 study objectives. Using the Quebec CHD database, a cohort of Fontan patients was assembled, including patients who had a history of Fontan operation defined by procedural codes that were available for all patients in the database. Patients were excluded if they died within 30 days after Fontan completion as death in these cases was most likely attributed to surgical mortality. To evaluate the association between Fontan and the incidence of severe FALD, we used patients with ventricular septal defects (VSDs) as the control group, as previously described, 10 because there is no known association between VSD and liver disease. For each Fontan patient, a total of 20 patients with VSD were randomly selected and matched on age, sex, and surgery calendar time, using the date of Fontan completion as the index date. We thus established a retrospective longitudinal cohort of Fontan patients and patients with VSD, referred to as the Fontan‐VSD cohort. This cohort was used to estimate and compare the incidence of severe FALD in Fontan patients and severe liver complications in patients with VSD, for the purpose of assessing the extra burden of severe liver complication in Fontan patients compared with general patient population with CHD. Severe FALD and severe liver complications were defined as having a hospitalization attributable to liver disease using International Classification of Diseases, Ninth Revision (ICD‐9), and International Classification of Diseases, Tenth Revision (ICD‐10), codes (Tables S1 and S2). Thus, our definition of FALD is a genuinely significant form of liver disease. To assess the association between severe FALD and mortality, each Fontan patient with severe FALD was matched with 2 Fontan patients who did not have severe FALD on a propensity score that was calculated using sex, age at Fontan, calendar year of Fontan, and diagnosis of atrial fibrillation, congestive heart failure (CHF), heart block, pulmonary hypertension, arterial hypertension, acute renal failure, supraventricular tachycardia, and protein‐losing enteropathy (see Table S3 for the ICD‐9/ICD‐10 codes for identification). By adjusting these factors using propensity score matching, the 2 groups with and without FALD were made alike in the probability of developing FALD, except the true occurrence of FALD, thus to reduce confounding for assessing the association between FALD and mortality. In addition, using propensity score matching enables the calculation of absolute risk of mortality associated to FALD by means of Kaplan‐Meier curve analysis, with adjustment for selected sociodemographics and comorbidities. For each patient, the propensity score of developing FALD was calculated using a binary logistic regression model including the aforementioned 11 sociodemographics and comorbidities (Figure S1). Nearest neighbor matching was used to select the patients with the closest propensity scores to comprise the matching sets. However, if the closest propensity score for a patient with FALD was >0.2 SDs away, the patient was dropped from the analysis. The index date (time 0) for matching was defined as the first hospitalization date of the patient with severe FALD. For each matching set, the follow‐up time was also matched between the patients with and without severe FALD by taking the shortest duration of the 3 patients in the matching set.
The Fontan cohort was used for identifying risk factors of FALD development. To fully account for the temporal relationship between risk factors and outcomes, we defined risk factors in the time intervals preceding the diagnosis of the outcomes. Thus, comorbidities, such as CHF, that were diagnosed during the same hospitalization with FALD were not considered.
The time‐dependent comorbidities were defined by ICD‐9 and ICD‐10 codes from hospitalization records between the time of the Fontan operation and the end of follow‐up. For each patient, the end of follow‐up was the time of first FALD diagnosis, death, or the end of the database observation, whichever comes early. The Fontan patients were coded as comorbidity free until they received the first diagnosis of the comorbidities. For example, if a patient has a diagnosis of atrial fibrillation in the first year after Fontan operation, his/her atrial fibrillation coding will be 0 (absent) before the time of the atrial fibrillation diagnosis and 1 (presence) since this time point. In addition, we only counted incident comorbidities, excluding those comorbidities that happened before Fontan completion. This is based on the presumption that the completion of the Fontan pathway will correct some, if not all, derived complications of native single‐ventricle physiology.
Statistical Analysis
Estimation of Severe Liver Complications in the Fontan‐VSD Cohort
Descriptive statistics include frequencies, percentage, and interquartile ranges. Kaplan‐Meier curve analysis was used to illustrate the cumulative probability of severe FALD with log‐rank test for assessing group differences.
Association Between Severe FALD and Mortality
The survival probabilities for patients with severe FALD and propensity score–matched non‐FALD patients were estimated using Kaplan‐Meier curves and compared with the log‐rank test. The hazard ratio (HR) for the association between an incident of severe FALD and mortality was estimated using a Cox proportional hazard model stratified on the matched sets.
Predictors of Severe FALD
In addition to the year of Fontan operation, a group of 8 known comorbidities associated with poor Fontan hemodynamics and outcomes (Table) were considered as potential predictors of severe FALD. These comorbidities were identified from an in‐depth literature review for potential predictors of FALD. 2 , 3 , 4 , 5 , 6 , 7 , 10 , 11 , 12 , 13 , 14 To consider the fact that these comorbidities started at different times after Fontan operation, we described their incidence using time intervals. Specifically, the following time intervals after Fontan operation were used for calculating their incidence: <1, 1 to 3, 3 to 5, 5 to 10, and >10 years. In the Cox proportional hazard model for assessing the significant predictors of severe FALD, the comorbidities were coded as time‐varying variables. HRs and 95% CIs were reported for the effects of the potential predictors.
Table 1.
Incidence of Selected Comorbidities Among Patients <60 Years Who Underwent the Fontan Procedure and Who Survived 30 Days After Fontan
| Comorbidity | Post‐Fontan, y | ||||
|---|---|---|---|---|---|
| <1 (N=98) | 1–3 (N=92) | 3–5 (N=76) | 5–10 (N=65) | >10 (N=50) | |
| FALD+ | |||||
| CHF | 5 (5.1) | 6 (6.5) | 4 (5.3) | 4 (6.2) | 7 (14.0) |
| AF | 2 (2.0) | 0 | 4 (5.3) | 9 (13.8) | 16 (32.0) |
| Pulmonary hypertension | 1 (1.0) | 0 | 2 (2.6) | 1 (1.5) | 3 (6.0) |
| Heart block | 1 (1.0) | 1 (1.1) | 1 (1.3) | 3 (4.6) | 6 (12.0) |
| Atrial hypertension | 3 (3.1) | 2 (2.2) | 2 (2.6) | 1 (1.5) | 4 (8.0) |
| Acute renal failure | 1 (1.0) | 1 (1.1) | 4 (5.3) | 1 (1.5) | 5 (10.0) |
| Supraventricular tachycardia | 0 | 3 (3.3) | 2 (2.6) | 2 (3.1) | 13 (26.0) |
| Protein‐losing enteropathy | 3 (3.1) | 2 (2.2) | 2 (2.6) | 1 (1.5) | 3 (6.0) |
| Comorbidity | <1 (N=414) | 1–3 (N=372) | 3–5 (N=324) | 5–10 (N=297) | >10 (N=238) |
|---|---|---|---|---|---|
| FALD− | |||||
| CHF | 19 (4.6) | 14 (3.8) | 11 (3.4) | 13 (4.3) | 16 (6.7) |
| AF | 4 (1.0) | 9 (2.4) | 8 (2.5) | 13 (4.3) | 31 (13.0) |
| Pulmonary hypertension | 8 (1.9) | 7 (1.9) | 5 (1.5) | 2 (0.7) | 2 (0.8) |
| Heart block | 5 (1.2) | 6 (1.6) | 4 (1.2) | 4 (1.4) | 7 (2.9) |
| Atrial hypertension | 5 (1.2) | 4 (1.1) | 6 (1.8) | 5 (1.7) | 9 (3.8) |
| Acute renal failure | 4 (1.0) | 4 (1.1) | 4 (1.2) | 8 (2.8) | 6 (2.5) |
| Supraventricular tachycardia | 5 (1.2) | 3 (0.8) | 6 (1.8) | 8 (2.8) | 24 (10.1) |
| Protein‐losing enteropathy | 3 (0.7) | 2 (0.5) | 3 (0.9) | 5 (1.7) | 0 |
Data are given as number (percentage) of each group. AF indicates atrial fibrillation; CHF, congestive heart failure; FALD, Fontan‐associated liver disease; FALD−, patients without FALD; and FALD+, patients with FALD.
Analyses were conducted using SAS statistical software version 9.4 (SAS Institute, Cary, NC).
Results
The Fontan cohort included 512 patients matched to a total of 10 232 patients with VSDs (Figure 1). The median follow‐up time was 12.40 years (interquartile range, 3.80–19.70 years).
Figure 1. Population derivation flowchart.
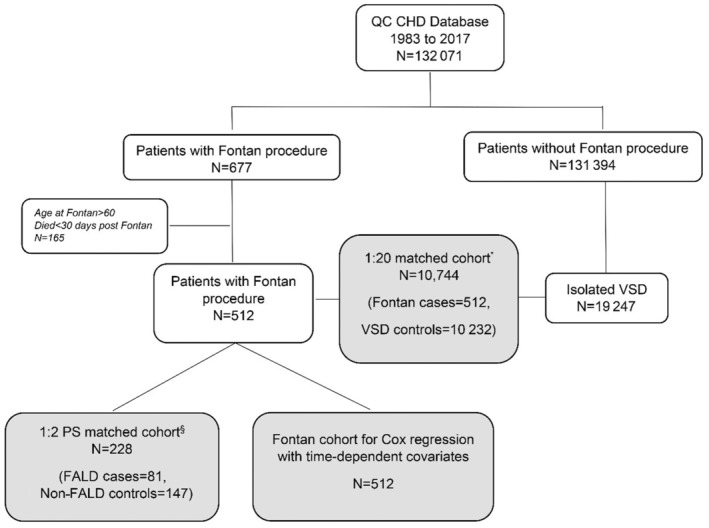
§Propensity score matching (PS) takes sex, age at Fontan, year of Fontan, atrial fibrillation, stroke, congestive heart failure, heart block, ventricular arrhythmia, pulmonary hypertension, and arterial hypertension into account. Fontan‐associated liver disease (FALD) cases and non‐FALD controls were matched at the time of the first FALD diagnosis. CHD indicates congenital heart disease; QC, Quebec; and VSD, ventricular septal defect.
A total of 98 (19.14%) Fontan patients developed severe FALD versus 78 (0.76%) patients with severe liver complications in the VSD group (P<0.0001). In the Fontan cohort, the cumulative risk of developing severe FALD started soon after Fontan completion and continued to increase throughout the entire follow‐up period up to 35 years after Fontan. It reached 11.95% and 52.24% at 10 and 35 years, respectively, compared with 0.50% and 2.75%, respectively, for the VSD group (log‐rank P<0.0001) (Figure 2).
Figure 2. Risk of developing severe Fontan‐associated liver disease (FALD)/severe liver complications (SLCs) in matched Fontan vs ventricular septal defect (VSD) cohorts.
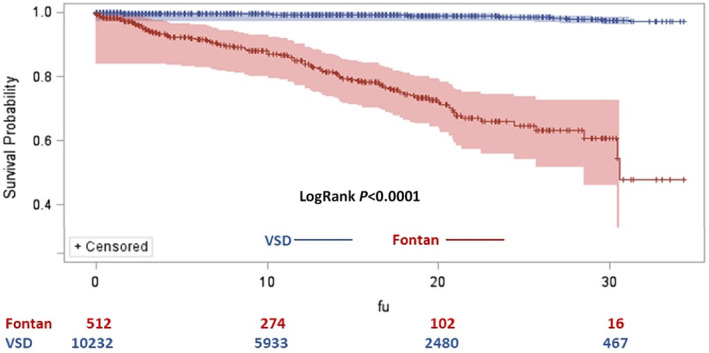
Ten‐year cumulative risk of developing severe FALD/SLCs in patients who undergo the Fontan procedure (Fontan patients) is 11.95%; and in patients with VSD, it is 0.50%. Long‐term (35‐year) cumulative risk of developing severe FALD/SLCs in Fontan patients is 52.24%; and in patients with VSD, it is 2.75%. Log‐rank P<0.0001. FU indicates follow‐up.
Association Between Severe FALD and Mortality Among Fontan Patients
Among the 512 Fontan patients, 52 died during the study observation period. Of the 52 deaths, 27% happened within 6 months with another 10% that happened between 6 months and 1 year after Fontan operation. Of the deaths, 31%, 15%, and 17% happened 1 to 5, 5 to 10, and >10 years after Fontan operation, respectively. The cumulative mortality risks were ≈8%, 10%, and 13% by 5, 10, and 20 years of follow‐up, respectively (Figure S2).
The median age at the first hospitalization for severe FALD was 15.31 (interquartile range, 4.83–23.29) years in the Fontan group. Among the 98 patients who developed severe FALD, 17 were removed because of no available propensity score–matching controls. The rest of 81 Fontan patients with severe FALD were matched to 147 Fontan patients without severe FALD. Kaplan‐Meier curve analyses showed a 5‐year mortality rate of 12.60% in patients with severe FALD versus 3.70% in patients without FALD (log‐rank P=0.0171) (Figure 3). In the Cox proportional hazard model, mortality risk in the severe FALD group was 3.4 times higher than that in Fontan patients without severe FALD (HR, 3.38 [95% CI, 1.14–9.99]; P=0.0275).
Figure 3. Comparison of survival probability in patients with severe Fontan‐associated liver disease (FALD) vs patients without severe FALD.
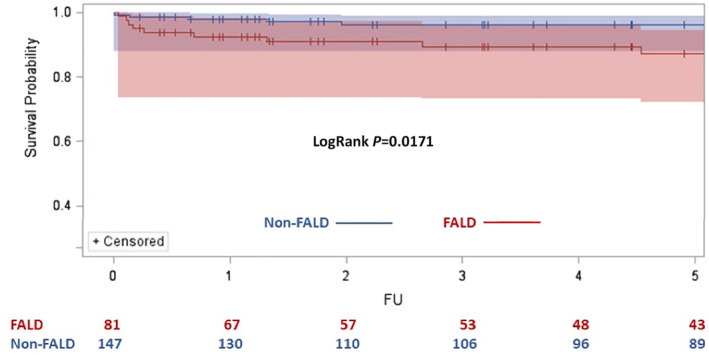
Cumulative risk of death is 12.62% in patients with severe FALD and 3.75% in patients without severe FALD (non‐FALD). Log‐rank P=0.0171. Non‐FALD indicates without severe FALD; and FU, follow‐up.
Risk Factors for Developing Severe FALD
The Table shows the incidence of the comorbidities a priori selected as potential predictors for developing severe FALD at varying time intervals after Fontan procedures. At 10 years or beyond after the Fontan operation, comorbidities with the highest incidence in the severe FALD group were CHF (14.0%), atrial fibrillation (32.0%), supraventricular tachycardia (26.0%), and heart block (12.0%). Fontan patients without severe FALD had a lower incidence of cardiovascular comorbidities, with CHF present in 6.7%, atrial fibrillation present in 13.0%, supraventricular tachycardia present in 10.1%, and heart block present in 2.90% of patients (Table).
The Cox proportional hazard model showed that both CHF and supraventricular tachycardia were independently associated with the development of severe FALD, with HRs of 2.36 (95% CI, 1.38–4.02) and 2.45 (95% CI, 1.37–4.39), respectively (Figure 4). Given the same length of follow‐up and same incidence of cardiovascular comorbidities including heart failure and supraventricular tachycardia, patients who had the Fontan operation at an earlier era had higher risk of developing FALD than those who had the operation in more recent years, with an HR of 0.95 (95% CI, 0.93–0.97) for each 1‐year increment.
Figure 4. Forest plot illustrating the identified risk factors of severe Fontan‐associated liver disease (FALD) in patients who undergo the Fontan procedure.
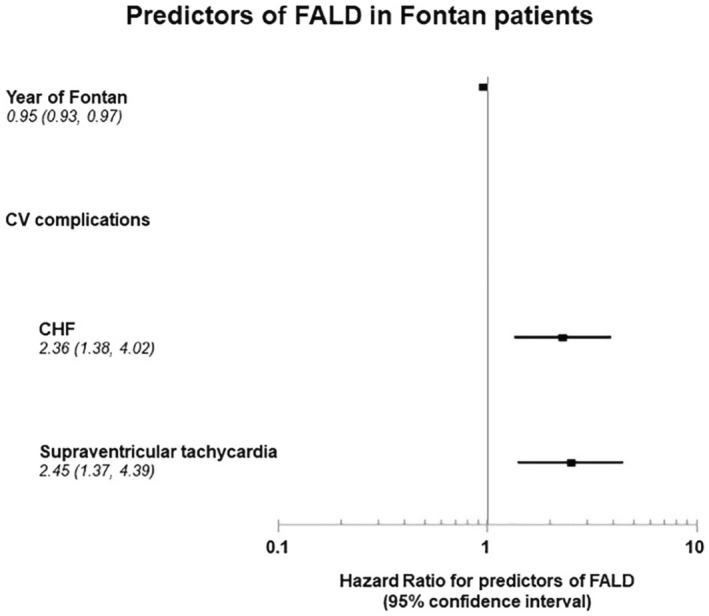
Results of Cox regression. (Comorbidities act as time‐dependent variables.) CHF indicates congestive heart failure; and CV, cardiovascular.
Discussion
This is a large‐scale population‐based study that assessed the incidence of FALD, its association with mortality, and the risk factors of developing severe FALD. The study reports that the cumulative risk of developing severe FALD was 11.95% and 52.24% at 10 and 35 years after Fontan operation, respectively. Compared with Fontan patients without severe FALD, those with severe FALD had a 3.4‐fold increase in mortality risk. Among Fontan patients, heart failure and supraventricular tachycardia were the 2 comorbidities that were significantly associated with an increased risk of severe FALD, supporting the notion that Fontan physiology is associated with the complex interplay between extracardiac Fontan complications and Fontan function. Moreover, the calendar year of Fontan operation completion was associated with severe FALD risk. The later the Fontan operation in calendar years, the smaller was the risk for severe FALD, with a 10% risk reduction for each 1‐year increment.
Incidence of Severe FALD
We used hospital discharge diagnoses attributable to severe liver complications as the end point for FALD, as they are more accurate than outpatient diagnoses. Few studies have tried to associate FALD with clinically significant advanced liver disease. 7 , 15 In a retrospective cohort of 60 patients, Lindsay et al reported a 22% incidence of adverse hepatic outcomes, with FALD occurring at a mean of 18.5 years of follow‐up. Similar to our definition of severe FALD, adverse hepatic outcomes in the cohort of Lindsay et al were liver failure, hepatorenal syndrome, and hepatic encephalopathy, pointing to a clinically symptomatic synthetic hepatic dysfunction. 7 Our findings exhibit a similar incidence of severe FALD occurrence (19.14% at a mean of 12.40 [interquartile range, 3.80–19.70] years), with a constant increase in the cumulative probability of severe FALD over time, reflecting the ongoing process of liver dysfunction in the Fontan population.
Literature has not reached an agreement on the definition of FALD. The diagnosis of FALD is based on the interpretation of imaging and laboratory findings of each Fontan patient, where the clinicians and treating group reach an agreement on what to call FALD. In this study, we focus on severe FALD, defined as a liver complication that leads to hospitalization. We thus link the diagnosis of FALD to what is clinically meaningful in terms of health care use. Using the severe FALD diagnosis, our study observed an ≈10% cumulative incidence of severe FALD within 10 years after Fontan operation. This finding is consistent with previous work on invasive hepatic screening in Fontan patients, which showed that liver fibrosis was almost universal, with 1 of 4 Fontan patients having high‐grade liver fibrosis early after Fontan completion. 6
Our definition of severe FALD yielded similar results in the incidence of FALD to what has been reported in literature. In a recent study of 1260 Fontan patients using imaging (mainly) and biopsy for FALD diagnosis, 54% were expected to be alive, free of liver cirrhosis or hepatocellular carcinoma, at 30 years after Fontan completion. 11 This result is consistent with our findings of a cumulative risk of 52.2% of severe FALD at 35 years after Fontan operation. However, variations in the incidence of FALD were seen in many studies 5 , 7 , 15 , 16 , 17 that were at least partially attributable to the lack of a standardized definition and staging of FALD. Our definition used ICD‐9/ICD‐10 codes for liver disease that are well known and universal and vary less in time, as imaging surveillance for FALD is a tool introduced later in the course of FALD surveillance (>2000s). 16 We hope that our carefully designed attempt in defining severe FALD with an administrative database would promote efforts in diagnosing and defining FALD, leading to a clear and standard way to measure FALD.
There has been an increase in the frequency of FALD over time. Figure S3 shows the frequency of severe FALD hospitalizations in Quebec CHD database increased from 2% in 1990 to 8% in 2017. The increase might be partly attributable to the increased awareness of FALD in recent years. It might also be related to the aging of the Fontan patient population, enabled by their prolonged survival. 3 , 12
Our results support previous findings that FALD may not be a silent bystander, but a progressive condition that leads to significant hospitalizations and complications in the long‐term follow‐up of Fontan patients. 5 , 7 , 16 , 18
Viral diseases affecting the liver accounted for only 3% of the events in our study. The possible role of infectious causes in the genesis of FALD remains unclear. Indeed, viral hepatitis is not considered a driver of the condition, but rather an associated hepatic insult that may precipitate FALD. 5 , 17 On the basis of our findings, viral hepatitis does not appear to be a preponderant factor in the genesis of severe FALD. Further analyses are needed to understand the role of infectious diseases affecting the liver and their association with FALD genesis.
Severe FALD and Mortality
This study shows that the mortality risk in Fontan patients with severe FALD is significantly higher than in Fontan patients without FALD (HR, 3.38 [95% CI, 1.14–9.99]; P=0.0275). In our cohort, mortality was seen early after severe FALD, with a 12.6% mortality rate at 5 years and a median time between FALD and death of 0.48 years (interquartile range, 0.12–2.67 years). Our findings are consistent with what other studies have reported involving liver disease in Fontan patients. In a Japanese nationwide survey, liver disease was the cause of death in 17.8% of Fontan patients during the study period. 17
The foremost cause of death is yet to be defined, as the nature of our database prevents us from identifying the precise end process leading to death. Further analysis will be needed to clarify if gastrointestinal tract bleeding, neoplastic disorder, CHF, or liver failure is the leading cause for decreased survival in Fontan patients with FALD.
Although some similar pathophysiological mechanisms exist between FALD and congestive cirrhosis from other forms of heart failure, the differences are important. Fontan patients have a unique physiological model and disease trajectory. Moreover, little is known about the impact that congestive cirrhosis has on patients with chronic heart failure. 10 Notwithstanding, in 22 579 patients surviving orthotopic cardiac transplant, the predicted mortality was related to the Model for End‐Stage Liver Disease (MELD‐XI) score. 11 In patients with a high MELD‐XI score (>16.4) in the early and late postoperatory period, the 30‐day, 1‐year, and 5‐year mortality hazard ratios were 1.85, 1.76, and 1.42, respectively, when compared with patients with lower MELD‐XI scores (<10.5). There was an absolute decrease of 8.20% in 5‐year survival in the high MELD‐XI score group compared with the lower MELD‐XI score group (78.2% versus 70.0%; P<0.001). When compared with our results, we found striking similarities, as appreciated in the Kaplan‐Meier curve analysis (Figure 3), which shows that mortality risk started early and continued for 5 years, with 3.4 times increase in patients with severe FALD and an absolute increase in mortality of 8.9% when compared with Fontan patients without severe FALD. 11
Risk Factors for Severe FALD
Congestive heart failure was a risk factor for developing severe FALD. FALD severity categorized using an imaging approach has been associated with impaired Fontan hemodynamics, predominantly with increased hepatic wedge pressure and single‐ventricle end‐diastolic pressure. 18
In a cohort of 261 Fontan patients, the hazard for CHF‐related death increased progressively after 10 years post‐Fontan. 12 Increased Fontan pressure was an independent predictor for CHF‐related death and a described risk factor for FALD development. 4 , 12 , 18 The present analysis brings into a context that the development of FALD is strongly associated with cardiovascular comorbidities, such as CHF/Fontan failure. Further analyses are needed to evaluate the possible causality.
The relationship between severe FALD and supraventricular tachycardia can be elucidated by the association between adequate Fontan function and regular heart rhythm. 13 , 14 It is well recognized that tachyarrhythmias trigger Fontan failure, impair flow dynamics, decrease cardiac output, and increase central venous pressure, leading to further liver damage. 13 , 14 , 19
The findings reported by a large‐scale meta‐analysis of 5859 Fontan patients indicated an earliest surgical era of Fontan completion was strongly related to increased mortality. 20 The present study identified a recent calendar year of Fontan completion compared with earlier years (<1990) as a protective factor for severe FALD development. This protective effect probably reflected the better understanding of single‐ventricle physiology and the evolution of surgical methods in recent era. For example, the instauration of the lateral tunnel and extracardiac conduits has replaced classic atriopulmonary connections as the main surgical approach, leading to better Fontan hemodynamics. 3 , 14 , 19 , 20 Future studies are needed to determine how the native anatomy, cardiac function, and detailed surgical interventions may impact FALD development and subsequent mortality.
It is crucial to identify the natural history of FALD as multiple reports have suggested that liver fibrosis is almost universal after Fontan completion. 6 Further detailed evaluations are needed to better understand the nature of this condition with the purpose of reducing the disease progression leading to severe FALD.
Limitations
This study used administrative databases in which diagnoses could be misclassified because of coding errors. We minimized the misclassification of CHD diagnoses by using all available data from the 3 provincial databases. We further manually audited the CHD diagnoses on random samples of 28% of subjects. 8 , 21 Our analysis may result in a misclassification bias as some severe FALD cases might occur undiagnosed or be subclinical. We chose a hard outcome, such as hospitalization, relying on discharge diagnoses known also to be prone to misclassification, to define predictor variables and FALD. Our database with 35 years of follow‐up, tracking health services, is well suited to study severe FALD resulting in FALD hospitalizations. We based our definition on established liver complications that we were able to identify in our data sources. We defined severe FALD as having at least 1 hospitalization with any diagnosis of severe liver disease. The constructed codes for our definition of FALD were based on our clinical expertise and previous data on congestive liver injury. 10 , 22 , 23 Moreover, our study design was not intended to address the important pre‐Fontan clinical determinants of FALD. It was also not designed to examine the importance of the hemodynamics of the Fontan circulation, native patient anatomy, different single‐ventricle pathway palliation, systemic ventricle dysfunction, or medication use. We used ICD‐9/ICD‐10 codes to define the comorbidities, as previously validated by our group in CHD 9 , 21 and by others. 24 Future studies with more granular preoperative hemodynamics and anatomic data are needed to confirm the study findings. Preoperative liver and cardiac function tests are important baseline factors for assessing FALD development and outcomes. As these data were not available in our database, we could not include them in our analyses. Although it would have been interesting to assess specifically if death in Fontan patients is relative to redo surgical interventions, heart transplant, or heart‐liver transplant, our study was not designed to provide data on the specific cause of death in Fontan patients. Death relative to redo interventions is a time‐dependent end point that requires weighted estimates of predictors at various time windows. Limited by the number of deaths at different time windows, despite our large population‐based sample, our study is not sufficiently powered for this analysis. Moreover, our study does not enable us to establish causal inference about the time of onset and the cause and the duration of liver dysfunction, nor do our findings explore the impact of interventions that are important in guiding clinical inquiry. Even so, we initiated the development of the prediction model for FALD with available data sources. Our use of the VSD group as a control group allows us to establish a risk profile of one group compared with another within the same population. Our application of propensity score matching mitigates confounding as much as possible with our data sources. Despite these limitations, the internal validity of our findings relative to our objectives has enabled us to provide first‐time estimates of the association between FALD and mortality, its predictors, and cumulative incidence from a matched population‐based cohort analysis.
Conclusions
We observed that severe FALD may affect >50% of Fontan patients at 30 years of follow up. Our findings suggest that severe FALD in Fontan patients was associated with a >3‐fold increase in mortality compared with Fontan patients without severe FALD. Factors associated with the development of severe FALD included CHF and supraventricular arrhythmias. The time course between cardiac deterioration and advanced hepatic disease needs to be better elucidated. Clinical studies are needed to characterize the impact of FALD on long‐term Fontan trajectories.
Sources of Funding
None.
Disclosures
Dr Guerrero‐Chalela is supported by the Beth Raby Adult Congenital Heart Disease Fellowship, Jewish General Hospital, Montreal, Canada. Dr Marelli is supported by the Heart and Stroke Foundation of Canada and the Canadian Institutes of Health Research. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figures S1–S3
Acknowledgments
This work was performed in the McGill Adult Unit for Congenital Heart Disease Excellence (MAUDE Unit), McGill University Health Centre, Montreal, Quebec, Canada.
This article was sent to John L. Jefferies, MD, MPH, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.024034
See Editorial by Haeffele and McElhinney.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. doi: 10.1136/thx.26.3.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart. 2016;102:1081–1086. doi: 10.1136/heartjnl-2015-307467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, Dahl SH, Cannon BC, O'Leary PW, Driscoll DJ, et al. 40‐year follow‐up after the Fontan operation long‐term outcomes of 1,052 patients. J Am Coll Cardiol. 2015;66:1700–1710. doi: 10.1016/j.jacc.2015.07.065 [DOI] [PubMed] [Google Scholar]
- 4. Daniels CJ, Bradley EA, Landzberg MJ, Aboulhosn J, Beekman RH, Book W, Gurvitz M, John A, John B, Marelli A, et al. Fontan‐associated liver disease: proceedings from the American College of Cardiology Stakeholders Meeting, October 1 to 2, 2015, Washington DC. J Am Coll Cardiol. 2017;70:3173–3194. doi: 10.1016/j.jacc.2017.10.045 [DOI] [PubMed] [Google Scholar]
- 5. Gordon‐Walker TT, Bove K, Veldtman G. Fontan‐associated liver disease: a review. J Cardiol. 2019;74:223–232. doi: 10.1016/j.jjcc.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 6. Goldberg DJ, Surrey LF, Glatz AC, Dodds K, O'Byrne ML, Lin HC, Fogel M, Rome JJ, Rand EB, Russo P, et al. Hepatic fibrosis is universal following Fontan operation, and severity is associated with time from surgery: a liver biopsy and hemodynamic study. J Am Heart Assoc. 2017;6:e004809. doi: 10.1161/JAHA.116.004809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindsay I, Johnson J, Everitt MD, Hoffman J, Yetman AT. Impact of liver disease after the Fontan operation. Am J Cardiol. 2015;115:249–252. doi: 10.1016/j.amjcard.2014.10.032 [DOI] [PubMed] [Google Scholar]
- 8. Marelli AJ, Ionescu‐Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749–756. doi: 10.1161/CIRCULATIONAHA.113.008396 [DOI] [PubMed] [Google Scholar]
- 9. Bouchardy J, Therrien J, Pilote L, Ionescu‐Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319 [DOI] [PubMed] [Google Scholar]
- 10. Xanthopoulos A, Starling RC, Kitai T, Triposkiadis F. Heart failure and liver disease: cardiohepatic interactions. JACC Hear Fail. 2019;7:87–97. doi: 10.1016/j.jchf.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 11. Grimm JC, Shah AS, Magruder JT, Kilic A, Valero V, Dungan SP, Tedford RJ, Russell SD, Whitman GJR, Sciortino CM. MELD‐XI score predicts early mortality in patients after heart transplantation. Ann Thorac Surg. 2015;100:1737–1743. doi: 10.1016/j.athoracsur.2015.07.026 [DOI] [PubMed] [Google Scholar]
- 12. Khairy P, Fernandes SM, Mayer JE, Triedman JK, Walsh EP, Lock JE, Landzberg MJ. Long‐term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559 [DOI] [PubMed] [Google Scholar]
- 13. Ghai A, Harris L, Harrison DA, Webb GD, Siu SC. Outcomes of late atrial tachyarrhythmias in adults after the Fontan operation. J Am Coll Cardiol. 2001;37:585–592. doi: 10.1016/s0735-1097(00)01141-4 [DOI] [PubMed] [Google Scholar]
- 14. Ishizaki U, Nagao M, Shiina Y, Fukushima K, Takahashi T, Shimomiya Y, Matsuo Y, Inai K, Sakai S. Prediction of Fontan‐associated liver disease using a novel cine magnetic resonance imaging “vortex flow map” in the right atrium. Circ J. 2018;82:2143–2151. doi: 10.1253/circj.CJ-17-1260 [DOI] [PubMed] [Google Scholar]
- 15. Pundi K, Pundi KN, Kamath PS, Cetta F, Li Z, Poterucha JT, Driscoll DJ, Johnson JN. Liver disease in patients after the Fontan operation. Am J Cardiol. 2016;117:456–460. doi: 10.1016/j.amjcard.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 16. Nii M, Inuzuka R, Inai K, Shimada E, Shinohara T, Kogiso T, Ono H, Ootsuki S, Kurita Y, Takeda A, et al. Incidence and expected probability of liver cirrhosis and hepatocellular carcinoma after Fontan operation. Circulation. 2021;144:2043–2045. doi: 10.1161/circulationaha.121.056870 [DOI] [PubMed] [Google Scholar]
- 17. Kuwabara M, Niwa K, Toyoda T, Shirai T, Tateno S, Ohuchi H, Tanaka Y, Ichida F, Fujisawa T, Akagi T, et al. Liver cirrhosis and/or hepatocellular carcinoma occurring late after the Fontan procedure: a nationwide survey in Japan. Circ J. 2018;82:1155–1160. doi: 10.1253/circj.CJ-17-1053 [DOI] [PubMed] [Google Scholar]
- 18. Schleiger A, Salzmann M, Kramer P, Danne F, Schubert S, Bassir C, Müller T, Müller HP, Berger F, Ovroutski S. Severity of Fontan‐associated liver disease correlates with Fontan hemodynamics. Pediatr Cardiol. 2020;41:736–746. doi: 10.1007/s00246-020-02291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dennis M, Zannino D, du Plessis K, Bullock A, Disney PJS, Radford DJ, Hornung T, Grigg L, Cordina R, d'Udekem Y, et al. Clinical outcomes in adolescents and adults after the Fontan procedure. J Am Coll Cardiol. 2018;71:1009–1017. doi: 10.1016/j.jacc.2017.12.054 [DOI] [PubMed] [Google Scholar]
- 20. Schwartz I, McCracken CE, Petit CJ, Sachdeva R. Late outcomes after the Fontan procedure in patients with single ventricle: a meta‐analysis. Heart. 2018;104:1508–1514. doi: 10.1136/heartjnl-2017-312807 [DOI] [PubMed] [Google Scholar]
- 21. Marelli AJ, Mackie AS, Ionescu‐Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115:163–172. doi: 10.1161/CIRCULATIONAHA.106.627224 [DOI] [PubMed] [Google Scholar]
- 22. Waseem N, Limketkai BN, Kim B, Woreta T, Gurakar A, Chen PH. Risk and prognosis of acute liver injury among hospitalized patients with hemodynamic instability: a nationwide analysis. Ann Hepatol. 2018;17:119–124. doi: 10.5604/01.3001.0010.7543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Udo R, Maitland‐van der Zee AH, Egberts TCG, den Breeijen JH, Leufkens HGM, van Solinge WW, De Bruin ML. Validity of diagnostic codes and laboratory measurements to identify patients with idiopathic acute liver injury in a hospital database. Pharmacoepidemiol Drug Saf. 2016;25:21–28. doi: 10.1002/pds.3824 [DOI] [PubMed] [Google Scholar]
- 24. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S3


