Abstract
Background
Cardiovascular calcification, characterized by deposition of calcium phosphate in the arterial wall and heart valves, is associated with cardiovascular morbidity and mortality and is commonly seen in aging, diabetes, and chronic kidney disease. Whether evidence‐based interventions could significantly attenuate cardiovascular calcification progression remains uncertain.
Methods and Results
We conducted a systematic review of randomized controlled trials involving interventions, compared with placebo, another comparator, or standard of care, to attenuate cardiovascular calcification. Included clinical trials involved participants without chronic kidney disease, and the outcome was cardiovascular calcification measured using radiological methods. Quality of evidence was determined by the Cochrane risk of bias and Grading of Recommendations, Assessment, Development, and Evaluations assessment. Forty‐nine randomized controlled trials involving 9901 participants (median participants 104, median duration 12 months) were eligible for inclusion. Trials involving aged garlic extract (n=6 studies) consistently showed attenuation of cardiovascular calcification. Trials involving 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors (n=14), other lipid‐lowering agents (n=2), hormone replacement therapies (n=3), vitamin K (n=5), lifestyle measures (n=4), and omega‐3 fatty acids (n=2) consistently showed no attenuation of cardiovascular calcification with these therapies. Trials involving antiresorptive (n=2), antihypertensive (n=2), antithrombotic (n=4), and hypoglycemic agents (n=3) showed mixed results. Singleton studies involving salsalate, folate with vitamin B6 and 12, and dalcetrapib showed no attenuation of cardiovascular calcification. Overall, Cochrane risk of bias was moderate, and the Grading of Recommendations, Assessment, Development, and Evaluations assessment for a majority of analyses was moderate certainty of evidence.
Conclusions
Currently, there are insufficient or conflicting data for interventions evaluated in clinical trials for mitigation of cardiovascular calcification. Therapy involving aged garlic extract appears most promising, but evaluable studies were small and of short duration.
Keywords: cardiovascular risk, coronary artery calcification, randomized controlled trials, systematic review, valvular calcification, vascular calcification
Subject Categories: Vascular Disease
Nonstandard Abbreviations and Acronyms
- AGE
aged garlic extract
Clinical Perspective.
What Is New?
Forty‐nine randomized controlled trials (n=9901) evaluating interventions that may attenuate cardiovascular calcification progression in the general population without kidney disease were assessed in this systematic review.
The systematic review showed there are insufficient or conflicting data for mitigation of cardiovascular calcification; although therapy involving aged garlic extract appears most promising, evaluable studies were small and of short duration.
What Are the Clinical Implications?
Although vascular calcification is associated with increased cardiovascular outcomes, it is not clear that adverse outcomes are causally mediated by calcification or that interventions to attenuate vascular calcification would reduce cardiovascular outcomes (eg, statin therapy, known to reduce cardiovascular events, does not reduce and can increase vascular calcification, as outlined in this review, but should not be avoided as a result).
Cardiovascular calcification is a heterogeneous process characterized by deposition and accumulation of calcium phosphate in the arterial wall and cardiac valves, and is considered a hallmark of aging. It is a pervasive complication of chronic kidney disease (CKD), diabetes, dyslipidemia, and hypertension, as well as chronic inflammatory conditions, 1 , 2 and is associated with increased cardiovascular morbidity and mortality. Consequently, cardiovascular calcification has been considered a risk factor that may prompt strategies to reduce the burden of cardiovascular disease.
The pathogenesis of cardiovascular calcification is not fully understood. It is recognized as a complex cell‐regulated process, determined by an interplay of genetic and epigenetic factors and the inherent tendency of vascular smooth muscle cells to acquire an osteogenic phenotype in response to certain environmental cues (eg, metabolic, endocrine, and inflammatory stimuli). 3 Arterial calcification is often categorized as intimal, typically associated with atherosclerotic plaque, or medial, a more diffuse arteriosclerotic process that can lead to vascular stiffening, abnormal coronary perfusion, and cardiac hypertrophy. 4 Although histologically distinct, intimal and medial calcification can coexist in the same arterial segment, and synergistically predict adverse cardiovascular outcomes. 3 Coronary artery calcification (CAC) is one of the most studied calcification processes and widely considered pathognomonic for atherosclerosis. 5 Although severity of CAC correlates with plaque burden and future risk of cardiovascular events, there is a complex relationship between the extent/type of calcification and plaque stability. Calcifications at other sites, including aortic, peripheral arteries, and valvular, also often associated with atherosclerosis, predict cardiovascular risk, although sometimes independent of atherosclerosis. 6 , 7
As well as anatomical distribution and location within the vessel wall, the size of calcifications also has important functional consequences. Smaller calcifications (microcalcifications) in the tunica intima may increase plaque instability, promote local stress in the aortic valve leaflets, and trigger inflammation pathways. Microcalcifications can aggregate to form larger calcifications (macrocalcification), which can have divergent effects on tissue function. In the tunica intima, calcification may decrease plaque vulnerability, whereas it causes vessel stiffening when present in the tunica media and impaired aortic valve function when present in the valve leaflet. Importantly, however, with contemporaneous imaging modalities, we are only able to visualize macrocalcifications.
Despite associative data between cardiovascular calcification and adverse outcomes, there is a lack of clinical evidence that supports improved clinical outcomes with attenuation of calcification progression. Thus, cardiovascular calcification is considered a surrogate marker of cardiovascular disease but may not represent a therapeutic target in clinical practice. Despite this, pharmaceutical agents and lifestyle measures have been proposed to attenuate calcification burden, and numerous clinical trials have been reported to evaluate efficacy of these interventions in mitigating its progression. One such systematic review of 77 prospective clinical trials was published highlighting interventions to attenuate cardiovascular calcification in people with CKD, 8 with magnesium and sodium thiosulfate reported as potentially beneficial to attenuate cardiovascular calcification progression in that population. No systematic review to date has investigated clinical trials of all interventions reported to target cardiovascular calcification in the general population. This systematic review addresses evidence‐based strategies to attenuate the progression of cardiovascular calcification in people without CKD, as reported in randomized controlled trials (RCTs).
METHODS
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement. The protocol is registered in the International Prospective Register of Systematic Reviews (2020 CRD42021251625) (available at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=251625).
Inclusion Criteria
This systematic review included RCTs evaluating all reported interventions to attenuate cardiovascular calcification progression in the general, non‐CKD population. The search included studies looking at vascular, arterial, aortic, valvular, and coronary calcification, as well as terms including dense calcium and calcified plaque (Data S1). Included studies (1) had participants who were not prespecified to have CKD and were not kidney transplant recipients; (2) compared an intervention with either placebo or another comparative treatment; (3) radiologically measured arterial or valvular calcification as a primary or secondary outcome with computed tomography, positron emission tomography, or intravascular ultrasound techniques; and (4) evaluated interventions that were pharmacological agents and nonpharmacological measures.
Trials specifically targeting CKD populations were excluded. Other excluded trials were nonhuman studies, in vitro studies, non‐English language studies, and studies that were not RCTs (including review articles, retrospective studies, prospective nonrandomized trials, observational studies, protocol papers, and conference abstracts). Furthermore, studies addressing surrogate measures of cardiovascular calcification, including carotid intima media thickness, pulse wave velocity, serum calcification propensity, and calciprotein particles, were excluded.
Search Strategy
Embase and MEDLINE were searched without restrictions on date of publication or number of trial participants. The complete search strategy is shown in Data S1. Two authors (S.M. and N.D.T.) independently screened titles and abstracts of all articles, and studies that did not meet inclusion criteria were discarded. Full texts for all remaining studies were subsequently retrieved and evaluated.
Data Extraction, Quality Assessment and Statistical Analysis
Two authors (S.M. and N.D.T.) assessed each trial, and extracted data on participant number and characteristics, study duration, interventions, comparators, and measurement of cardiovascular calcification. Where trials yielded >1 publication, findings were collated, and the publication with the most complete data was used for analysis.
Quality of methodology for each RCT was assessed using the risk of bias assessment tool developed by the Cochrane Bias Methods Group. 9 The following factors were assessed: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and outcome assessors, (4) attrition bias, (5) selective outcome reporting, and (6) any other bias (eg, study design). Furthermore, quality of evidence for each intervention was assessed by the Grading of Recommendations, Assessment, Development, and Evaluation approach. Each study was estimated to be of high, moderate, low, or very low evidence. 10 Two authors (S.M. and N.D.T.) independently conducted risk of bias and Grading of Recommendations, Assessment, Development, and Evaluation assessments. This systematic review had institutional ethics approval, and no informed consent was required for the study. The authors declare that all supporting data are available within the article and its online supplementary files.
RESULTS
Search Results
A Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram is shown in the Figure representing search results. A total of 5573 potentially relevant records were identified through database searches. After duplicates were removed, 4539 records were screened by title and abstract, and a remaining 177 records were identified for full‐text review. A total of 49 studies met inclusion criteria and were included in this review. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59
Figure . Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram.
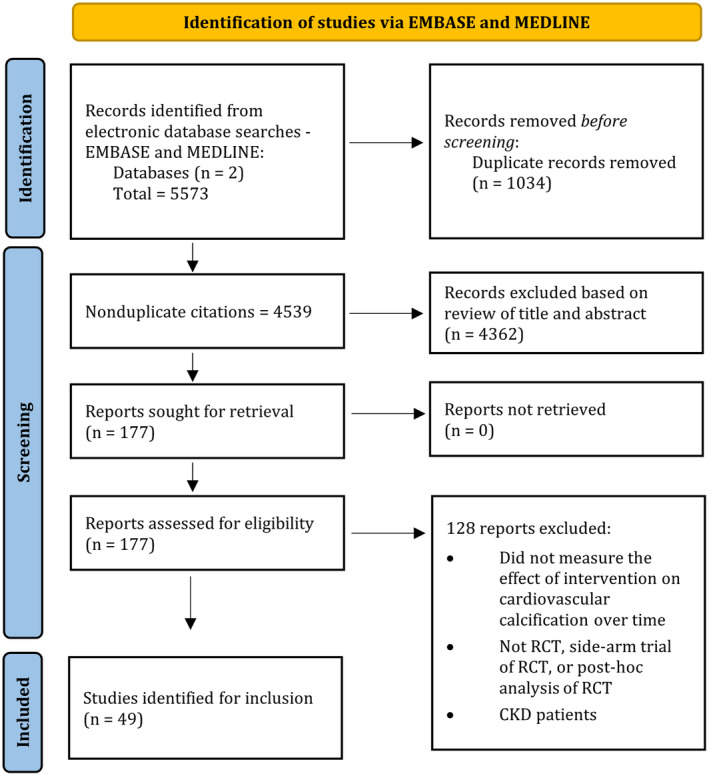
CKD indicates chronic kidney disease; and RCT, randomized controlled trial.
Study Characteristics
Tables 1, 2, 3, 4, 5, 6, 7 through 1, 2, 3, 4, 5, 6, 7 display study characteristics, target population, and outcome of 49 included RCTs. Included studies had a total of 9901 participants (median participants 104, average participants 202, range 19–1005; median study duration 12 months, range 3–72 months). Arterial or valvular calcification was the primary outcome in 27 studies. Interventions studied in at least 2 RCTs included HMG‐CoA (3‐hydroxy‐3‐methylglutaryl coenzyme A) reductase inhibitors, other lipid‐lowering agents, aged garlic extract (AGE), antiresorptive therapies, antihypertensive agents, hormone replacement therapies (HRTs), lifestyle measures, vitamin K, omega‐3 fatty acids, oral hypoglycemic agents, and antithrombotic therapies. There were single trials for each of salsalate (a platelet‐sparing nonsteroidal anti‐inflammatory drug), dalcetrapib (a cholesteryl ester transfer protein inhibitor with antilipid actions), and multivitamins containing folate and vitamins B12 and B6.
Table 1.
Summary of HMG‐CoA Reductase Inhibitor (n=14) and Other Lipid‐Lowering Agent (n=2) Study Characteristics and Cardiovascular Calcification Outcome
| Author and year | Mean study duration, mo | Target population | Study sample size | Intervention | Comparator | Primary or secondary end point | Imaging modality | Site | Reported outcomes | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| Arad 2005 11 | 48 | Age 50–70 y with known CAC | 1005 | 20 mg atorvastatin daily, 1 g vitamin C daily, 1000 IU vitamin E daily | Placebo | Secondary | CT | Coronary artery | CAC (AU); 331±421 treatment vs 323±385 control (P=0.80) | No significant attenuation |
| Cowell 2005 12 | 25 | Calcific aortic stenosis | 155 | 80 mg atorvastatin daily | Placebo | Primary | CT | Aortic valve | Valvular calcification (mean %±SD); 22.3±21 treatment vs 21.7±21 control (P=0.93) | No significant change in aortic valve calcium score |
| Dichtl 2008 13 | 28 | Calcific aortic stenosis | 47 | 20 mg atorvastatin daily | Placebo | Primary, aortic valve calcification; | CT | Aortic valve and coronary artery | Not reported; P=n.s. | No significant attenuation |
| secondary, CAC | ||||||||||
| Egede 2013 50 | 12 | ST‐segment–elevation myocardial infarction | 87 | 40 mg rosuvastatin daily | 5 mg rosuvastatin daily | Primary | VH‐IVUS | Coronary artery | Dense calcium (mean %±SD); 0.8±3.7 treatment vs −0.3±3.2 control (P=0.19) | No significant attenuation of dense calcium |
| Hougaard 2020 53 | 12 | ST‐segment–elevation myocardial infarction | 87 | 80 mg atorvastatin daily and ezetimibe 10 mg daily | 80 mg atorvastatin daily | Secondary | OCT | Nonculprit study plaque in noninfarct‐related coronary artery | Calcium arc, median m (IQR); 43.7 (−32.9 to 172.8) treatment vs 20.7 (−9.9 to 205.2) control (P=0.77) | No significant attenuation in arcs of calcific plaque |
| Houslay 2006 14 | 24 | Calcific aortic stenosis | 102 | 80 mg atorvastatin daily | Placebo | Primary | CT | Coronary artery | CAC (log AU); 0.234±0.037 treatment vs 0.167±0.034 control (P=0.18) | No significant attenuation |
| Lee 2016 52 | 3 | Acute coronary syndrome requiring percutaneous coronary intervention | 70 | Ezetimibe/simvastatin 10/40 mg daily | Pravastatin 20 mg daily | Secondary | VH‐IVUS | Coronary artery | Dense calcium mm3 (mean±SD); −0.2±5.4 treatment vs 0.1±1.8 control (P=0.746) | No significant attenuation of dense calcium |
| Longenecker 2016 15 | 22 | HIV infected | 147 | 10 mg rosuvastatin daily | Placebo | Secondary | CT | Coronary artery | CAC (%); 15% treatment vs 6% control (P=0.19) | No significant attenuation |
| Lo 2015 51 | 12 | HIV infected with subclinical coronary atherosclerosis | 40 | Stepwise escalation from 20 mg then 40 mg atorvastatin daily | Placebo | Secondary | CT | Coronary artery | CAC mean Agatston score, median (IQR); 1.7 (0.0 to 28.0) treatment vs 0.9 (0.0 to 18.5) control (P=0.74) | No significant attenuation |
| Miyoshi 2018 16 | 12 | Hypercholesterolemia and CAC | 156 | Treatment A: 2 mg pitavastatin daily plus 1800 mg eicosapentenoic acid daily | 2 mg pitavastatin daily | Primary | CT | Coronary artery | CAC (%); 42% treatment A vs 34% control (P=0.88); 44% treatment B vs 34% control (P=0.80) | No significant attenuation |
| Treatment B: 4 mg pitavastatin daily | ||||||||||
| Park 2016 49 | 12 | Coronary artery disease | 312 | 40 mg rosuvastatin daily | 10 mg rosuvastatin daily | Primary | VH‐IVUS | Coronary artery | Difference in dense calcium % (95% CI) between treatment vs control; 0.98 (3.25 to 1.27) (P=0.391) | No significant attenuation |
| Petri 2011 17 | 24 | SLE | 200 | 40 mg atorvastatin daily | Placebo | Primary | CT | Coronary artery | CAC loge (95% CI) treatment minus control; −0.08 (−0.39 to 0.23) (P=0.62) | No significant attenuation |
| Plazak 2011 18 | 12 | SLE (CKD with CrCl <30 excluded) | 60 | 40 mg atorvastatin daily | Placebo | Primary | CT | Coronary artery | Calcium score (Agatston); 32.1±39.1 to 59.5±64.4 (P<0.05) with control; 44.8±50.6 to 54.9±62.5 with treatment (P=n.s.) | Attenuation by statin compared with placebo |
| Raggi 2005 19 | 12 | Postmenopausal women aged 55–75 y, hypercholesterolemia, and presence of or at increased risk of coronary heart disease | 615 | 80 mg atorvastatin daily | 40 mg pravastatin daily | Primary | EBCT | Coronary artery | Calcium volume score (% mean±SD); 20.1±30.8 treatment vs 19.8±34.8 control (P=0.64) | No significant attenuation |
| Schmermund 2006 20 | 12 | Age 32–80 y, dyslipidemia and no history of ischemic heart disease | 471 | 80 mg atorvastatin daily | 10 mg atorvastatin daily | Primary | EBCT | Coronary artery | CAC, % mean (95% CI); 27 (20.8 to 33.1) treatment, 25 (19.1 to 30.8) control (P=0.65) | No significant attenuation with higher dose statin |
| Terry 2007 21 | 12 | Age 21–75 y, dyslipidemia and presence of CAC. Diabetes excluded. | 80 | 80 mg simvastatin daily | Placebo | Primary | CT | Coronary artery and abdominal aorta | CAC; 9% treatment vs 5% control (P=0.12) | No significant attenuation |
AU indicates Agatston units; CAC, coronary artery calcification; CKD, chronic kidney disease; CrCl, creatinine clearance; CT, computed tomography; EBCT, electron‐beam computed tomography; HMG‐CoA, 3‐hydroxy‐3‐methylglutaryl coenzyme A; IQR, interquartile range; n.s., not significant; OCT, optical coherence tomography; SLE, systemic lupus erythematosus; and VH‐IVUS, virtual histology intravascular ultrasound.
Table 2.
Summary of AGE Study Characteristics and Cardiovascular Calcification Outcome (6 Studies)
| Author and year | Mean study duration, mo | Target population | Study sample size | Intervention | Comparator | Primary or secondary end point | Imaging modality | Site | Reported outcomes | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| Budoff 2009 22 | 12 | CAC score >30 | 65 | 250 mg AGE daily plus 100 μg vitamin B12, 300 μg folic acid, 12.5 mg vitamin B6, and 100 mg L‐arginine daily | Placebo | Primary | EBCT | Coronary artery | CAC, % (mean); 6.8% treatment vs 26.5% control (P=0.005) | Significant attenuation by AGE+supplements compared with placebo |
| Serum creatinine >1.4 mg/dL were excluded | ||||||||||
| Budoff 2004 23 | 12 | Coronary artery disease | 19 | 4 mL AGE (at 305 g/L of extracted solid) | Placebo | Primary | EBCT | Coronary artery |
Calcium score, % (mean±SD); 7.5±9.4 treatment vs 22.2±18.5 control (P=0.046) |
Significant attenuation with AGE compared with placebo |
| Serum creatinine >1.4 mg/dL excluded | ||||||||||
| Matsumoto 2014 24 | 12 | Aged 40–75 y with metabolic syndrome | 55 | 2400 mg AGE daily | Placebo | Primary | CCTA | Coronary artery | Dense calcium, % (mean±SD); 0.2±1.4 treatment vs 0.2±1.7 control (P=0.99) | No significant attenuation of dense calcium coronary plaque volume |
| Serum creatinine >1.4 mg/dL excluded | ||||||||||
| Shaikh 2020 25 | 12 | Aged 30–75 y with type 2 diabetes | 80 | 2400 mg AGE daily | Placebo | Secondary | CCTA | Coronary | Dense calcium, % (mean); 69% treatment vs 33% control (P=0.82) | No significant attenuation of dense calcium coronary plaque volume |
| Serum creatinine >1.4 mg/dL excluded | ||||||||||
| Wlosinska 2020 27 | 12 | Aged 40–75 y with confirmed CAC on CT | 104 | 1200 mg AGE twice daily | Placebo | Primary | CT | Coronary artery | CAC progression, OR 2.95 (95% CI, 1.05–8.27) in favor of treatment arm (P<0.05) | Significant attenuation with AGE compared with placebo |
| Serum creatinine >140 μmol/L excluded | ||||||||||
| Zeb 2012 26 | 12 | Firefighters with CAC score >10 | 65 | 1200 mg AGE and 120 mg coenzyme Q10 daily | Placebo | Primary | CT | Coronary artery | CAC, Agatston units (mean±SD); 32±6 treatment vs 58±8 control (P=0.01) | Significant attenuation with AGE compared with placebo |
| Serum creatinine >1.4 mg/dL excluded |
AGE indicates aged garlic extract; CAC, coronary artery calcification; CCTA, cardiac computed tomography angiography; CT, computed tomography; EBCT, electron‐beam computed tomography; and OR, odds ratio.
Table 3.
Summary of Hormone Replacement Therapy Study Characteristics and Cardiovascular Calcification Outcome (3 Studies)
| Author and year | Mean study duration, mo | Target population | Study sample size | Intervention | Comparator | Primary or secondary end point | Imaging modality | Site | Reported outcomes | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| Basaria 2015 28 | 36 | ≥60 y with low to low‐normal testosterone levels | 308 | 7.5 g of 1% testosterone gel | Placebo | Primary | CT | Common carotid artery | CAC rate of change, Agatston units/year; mean difference (95% CI); −10.8 (−45.7 to 24.2) (P=0.54) | No significant attenuation |
| Serum creatinine >2.5 mg/dL excluded | ||||||||||
| Budoff 2017 29 | 12 | Men aged ≥65 y with symptomatic low testosterone levels (<275 ng/dL) | 170 | 1% testosterone gel | Placebo | Secondary | CT | Coronary artery | CAC rate of change Agatston units/year; mean difference (95% CI); −27 (−80 to 26) (P=0.31) | No significant attenuation |
| eGFR <60 mL/min per 1.73 m2 excluded | ||||||||||
| Harman 2014 30 | 48 | Postmenopausal women aged 42–58 y | 727 | 0.45 mg oral conjugated equine estrogen daily or 50 μg transdermal 17ß‐estradiol daily, each with 200 mg cyclical progesterone | Placebo | Secondary | CT | Coronary artery | CAC (hormone group minus placebo), % (95% CI);−3.6 (−11.4 to 4.1) for oral estrogen,(P=0.36); −2.1 (−10 to 5.7) for transdermal estrogen, (P=0.59) | No significant attenuation |
CAC indicates coronary artery calcification; CT, computed tomography; and eGFR, estimated glomerular filtration rate.
Table 4.
Summary of Vitamin K Study Characteristics and Cardiovascular Calcification Outcome (5 Studies)
| Author and year | Mean study duration, mo | Target population | Study sample size | Intervention | Comparator | Primary or secondary end point | Imaging modality | Site | Reported outcomes | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| Bellinge 2022 31 | 3 | Aged ≥40 y with diabetes and known CAC | 154 | Vitamin K1 (10 mg/d) and colchicine (0.5 mg/d) | Placebo/placebo vitamin K (10 mg/d)/placebo colchicine (0.5 mg/d)/placebo | Primary | 18 F‐NaF PET | Coronary artery and aorta | Coronary TBRmax, mean difference for treatment compared with placebo±SD; 0.00±0.16 and 0.01±0.17 for vitamin K and colchicine, respectively (P>0.05) | No significant attenuation of TBRmax with vitamin K1 or colchicine compared with placebo |
| Brandenburg 2017 32 | 12 | Minimally symptomatic aortic valve calcification | 99 | 2 mg vitamin K1 (phytomenadione) daily | Placebo | Primary | CT | Aortic valve | Aortic valve calcification volume, mL (mean±SD); 78±165 treatment vs 181±234 control (P=0.04) | Significant attenuation of progression by vitamin K compared with placebo |
| Diederichsen 2022 54 | 24 | Aortic valve calcification score >300 arbitrary units | 365 | 720 μg menaquinone‐7 (vitamin K2) daily +25 μg vitamin D | Placebo | Primary | CT | Aortic valve | Aortic valve calcification (Agatston units) of intervention compared with placebo, mean (95% CI); 17 (−86 to 53) (P=0.64) | No significant attenuation in valvular calcification by vitamin K2 and D |
| Shea 2009 33 | 36 | Men and postmenopausal women aged 60–80 y | 388 | 500 μg vitamin K1 (phyloquinone) daily | Multivitamin | Primary | CT | Coronary artery | CAC progression, mean Agatston score (95% CI); 27 (15 to 38) treatment vs 37 (24 to 50) control (P=0.26) | No significant attenuation. Significant attenuation among adherent participants and those with preexisting mild CAC |
| Zwakenberg 2019 34 | 6 | Age >40 y with diabetes, cardiovascular disease, and eGFR >30 | 68 | 360 μg menaquinone‐7 daily | Placebo | Secondary | PET‐CT for TBR | Femoral artery, other vascular beds examined post hoc | Femoral arterial TBRmax with treatment compared with control; mean (95% CI); 0.25 (−0.02 to 0.51) (P=0.06) | No significant attenuation on any vascular bed with menaquinone‐7 supplementation |
18F‐NaF indicates sodium fluoride F18; CAC, coronary artery calcification; CT, computed tomography; eGFR, estimated glomerular filtration rate; PET, positron emission tomography; and TBR, target‐to‐background ratio.
Table 5.
Summary of Lifestyle (n=4) and Antihypertensive (n=2) Study Characteristics and Cardiovascular Calcification Outcome
| Author and year | Mean study duration, mo | Target population | Study sample size | Intervention | Comparator | Primary or secondary end point | Imaging modality | Site | Reported outcomes | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| Henzel 2021 58 | 15 | Nonobstructive coronary atherosclerosis | 92 | Adherence to the Dietary Approaches to Stop Hypertension model and increase physical activity+optimal medical therapy | Optimal medical therapy | Secondary | CT, angiogram | Coronary artery | Dense calcium mm 3 , mean±SD; 33.8±68.9 treatment vs 30.2±52.5 control (P=0.78) | No difference in dense calcium changes were observed between groups |
| Fitch 2012 59 | 12 | HIV‐infected and metabolic syndrome | 50 | 2×2 factorial study, lifestyle+metformin, lifestyle+placebo | No lifestyle+metformin, no lifestyle+placebo | Primary | CT | Coronary artery | CAC score, mean±SD; −1±2 metformin vs 33±17 control, (P=0.004); 8±6 LSM vs 21±14 no LSM (P=0.82) | No significant attenuation of CAC with lifestyle modification compared with placebo. Significant attenuation of CAC by metformin compared with placebo |
| Kuller 2011 35 | 48 | Overweight women aged 52–62 y with no history of cardiovascular disease | 508 | Strict dietary goals, caloric targets, and physical activity | Health education | Secondary | EBCT | Coronary artery | Coronary calcium, mean±SD; 4±13 treatment vs 4±10 control among participants with no baseline coronary calcium; 75±102 treatment vs 70±142 control among participants with baseline coronary calcium 10; no P values provided | No significant attenuation with lifestyle intervention |
| Lehmann 2011 36 | 36 | Coronary artery disease | 96 | Mediterranean diet and stress reduction | Written advice only | Primary | EBCT | Coronary artery | CAC progression, median factor changes (IQR); 1.46 (1.16–2.19) treatment vs 1.41 (1.20–1.79) control (P=0.68) | No significant attenuation with lifestyle intervention |
| Motro 2000 43 | 36 | Aged 55–80 y with hypertension and cardiovascular risk factors | 201 | 30 mg nifedipine daily | 25 mg hydrochlorothiazide daily and 2.5 mg amiloride daily (co‐amilozide) | Secondary | CT | Coronary | Coronary calcium, mean %; 40% treatment vs 78% control (P=0.02) | Significant attenuation by nifedipine compared with co‐amilozide |
| Motro 2007 44 | 72 (4.5–6 y) | Stable angina pectoris with coronary artery disease | 518 | 60 mg nifedipine daily | Placebo | Secondary | CT | Coronary | Coronary calcification, median (IQR); 73 (5–244) treatment vs 55 (7–305) control (P=0.85) | No significant attenuation |
CAC indicates coronary artery calcium; CT, computed tomography; EBCT, electron‐beam computed tomography; HIV, human immunodeficiency virus; IQR, interquartile range; and LSM, lifestyle modification.
Table 6.
Summary of Antithrombotic (n=4) and Antiresorptive Therapy (n=2) Study Characteristics and Cardiovascular Calcification Outcome
| Author and year | Mean study duration, mo | Target population | Study sample size | Intervention | Comparator | Primary or secondary end point | Imaging modality | Site | Reported outcomes | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| Lee 2017 37 | 6 | Aged 40–70 y with type 2 diabetes and coronary artery narrowing | 40 | 300 mg sarpogrelate daily+100 mg aspirin daily | 100 mg aspirin daily | Primary | CT | Coronary artery | CAC, mean±SD; 192±53 to 189±45 treatment vs 96±36 to 99±035 control (P=0.29 between groups) | No significant attenuation |
| Lee 2019 38 | 12 | Aged 30–80 y with diabetes and coronary artery narrowing | 100 | 200 mg cilostazol daily | 100 mg aspirin daily | Secondary | CT | Coronary artery | CAC, mean; 54.6 with treatment vs 63.1 with control (P=0.235) | No significant attenuation |
| Lee 2018 39 | 12 | Nonvalvular AF | 120 | 20 mg rivaroxaban daily | Warfarin titrated to INR 2–3 | Primary | CT | Coronary artery | Coronary calcified plaque volume mm3, median (IQR); 0.8 (0 to 9.8) treatment vs 3.9 (0 to 29.2) control (P=0.22) | Significant attenuation with rivaroxaban compared with warfarin only on multivariate analysis (P=0.026) |
| Win 2019 40 | 12 | Nonvalvular AF | 66 | 5 mg apixaban twice daily | Warfarin titrated to INR 2–3 | Primary | CT | Coronary artery | Calcified coronary plaque volume 15.1±30.3 treatment vs 18.1±41.2 control (P=0.45) | Significant attenuation with apixaban compared with warfarin only on multivariate analysis (P=0.005) |
| Kranenburg 2018 41 | 12 | Pseudoxanthoma elasticum with calcification of lower limb vessels | 74 | Cyclical 20 mg/kg etidronate for 2 weeks every 12 weeks | Placebo | Secondary | CT | Femoral artery, with other vascular beds evaluated post hoc | Femoral arterial calcification %, median (IQR); −4 (−10 to 7) treatment vs 8 (−1 to 20) control (P=0.001) | Attenuation of femoral arterial calcification by etidronate |
| Bartstra 2020 60 post hoc trial* | Attenuation of VC in all other vascular beds except coronary arteries and carotid siphon | |||||||||
| Pawade 2021 42 | 24 | Aged >50 y with aortic valve calcification | 150 | Treatment A: 60 mg denosumab subcutaneous every 6 months | Placebo | Primary | CT | Aortic valve | Aortic valve calcium score, Agatston units, mean (95% CI); 343 (198 to 804) treatment A vs 354 (76 to 675) control (P=0.41); 326 (138 to 813) treatment B vs 354 (76 to 675) (P=0.49) | No significant attenuation with denosumab or alendronate |
| Treatment B: 70 mg alendronic acid oral daily |
AF indicates atrial fibrillation; CAC, coronary artery calcium; CT, computed tomography; INR, international normalized ratio; IQR, interquartile range; and VC, vascular calcification.
*Post hoc analysis of study by Kranenburg (2018). 41
Table 7.
Summary of Omega‐3 Fatty Acids (n=2), Oral Hypoglycemic Agents (n=2), and Other Singleton Therapy (n=3) Study Characteristics and Cardiovascular Calcification Outcome (n=3 Studies)
| Author and year | Mean study duration, mo | Target population | Study sample size | Intervention | Comparator | Primary or secondary end point | Imaging modality | Site | Reported outcomes | Interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| Alfaddagh 2017 56 | 30 | Stable coronary artery disease on HMG‐CoA reductase inhibitors | 285 | Omega‐3 fatty acid (1.86 g eicosapentaenoic acid and 1.5 g docosahexaenoic acid) daily | No omega‐3 treatment | Secondary | CCTA | Coronary artery | Calcified plaque volume, % median (IQR); 39.1 (−5.2 to 118.1) treatment vs 57.4 (4.3 to 146.6) (P=0.18) | No significant attenuation in calcified plaque |
| Budoff 2020 55 | 18 | Coronary atherosclerosis | 80 | Omega‐3 fatty acid (4 g icosapent ethyl) daily | Placebo | Secondary | CCTA | Coronary artery | Dense calcium, % mean±SD; 0.0±0.5 treatment vs 0.4±1.2 control (P=0.0531) | No significant attenuation in dense calcium |
| Davidson 2010 45 | 17 (72 wk) | Aged 45–85 y with type 2 diabetes | 299 | 15–45 mg pioglitazone daily aiming for fasting blood glucose of 7.8 mmol/L or lower | 1–4 mg glimepiride daily aiming for fasting blood glucose of 7.8 mmol/L or lower | Secondary | EBCT | Coronary artery | CAC between treatment and control groups not quantified | No significant attenuation |
| Nozue 2016 57 | 11 | Type 2 diabetes who underwent successful elective PCI | 28 | 50 mg sitagliptin daily | Continue antidiabetic medication at time of randomization | Secondary | IVUS | Non‐PCI lesion in coronary artery | Calcified plaque volume, mm3, mean±SD; 2.1±0.9 to 3.2±1.8 treatment (P=0.06); 2.3±1.7 to 4.8±3.5 (P=0.04); between‐group P value difference not reported | No significant attenuation of calcified plaque |
| Hauser 2016 46 | 30 | Overweight men and women on stable HMG‐CoA reductase inhibitor therapy with coronary heart disease and creatinine clearance >60 mL/min per 1.73 m 2 | 257 | 3.5 g salsalate daily | Placebo | Secondary | CT | Coronary artery | Calcified plaque volume, mm3, mean (95% CI) (placebo minus treatment); −5 (−13 to 2) (P=0.17) | No significant attenuation |
| Hodis 2009 47 | 37 | Aged ≥40 y, with fasting homocysteine level ≥8.5 μmol/L | 506 | 5 mg folic acid daily, 0.4 mg vitamin B12 daily, and 50 mg vitamin B6 daily | Placebo | Secondary | CT | Coronary artery | CAC, median (IQR); 0 (0 to 43) treatment vs 0 (0 to 52) control (P=0.82) | No significant attenuation |
| Joshi 2016, post‐hoc analysis of the dal‐PLAQUE trial 48 | 6 (in post‐hoc; 24 in original dal‐PLAQUE trial) | Coronary heart disease or cardiovascular risk factors | 130 | 600 mg dalcetrapib daily | Placebo | Secondary | CT | Aortic arch, ascending aorta, carotid artery, and coronary artery | Coronary artery calcium with treatment minus control Agatston units, mean (95% CI); −61 (−171 to 48) (P=0.263) | No significant attenuation in any arterial territory |
CAC indicates coronary artery calcium; CT, computed tomography; CCTA, coronary computed tomography angiography; dal‐PLAQUE, Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non‐invasive multimodality imaging; EBCT, electron‐beam computed tomography; HMG‐CoA, 3‐hydroxy‐3‐methylglutaryl coenzyme A; IQR, interquartile range; IVUS, intravascular ultrasound; and PCI, percutaneous coronary intervention.
Study Interventions
Lipid‐Lowering Agents
Fourteen trials evaluated the effect of HMG‐CoA reductase inhibitors in mitigating progression of cardiovascular calcification in 3477 participants (median participants 151, range 47–1005; median duration 12 months, range 12–48 months) (Table 1). 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 49 , 50 , 51 Attenuation of vascular calcification was the primary outcome in 9 trials, with coronary arteries being the most common site evaluated (n=13). Aortic valvular calcification was also evaluated in 2 trials. 12 , 13 There was participant heterogeneity in the HMG‐CoA reductase trials, which included target populations with, but not limited to, dyslipidemia, HIV infection, systemic lupus erythematosus, and calcific aortic stenosis. Confirmed presence of cardiovascular calcification at baseline was required in some but not all trials. Twelve trials used computed tomography or electron beam, 3 trials used intravascular ultrasound combined with virtual histology software techniques to measure dense calcium or calcified plaque, and 1 trial used optical coherence tomography harnessing catheter‐guided infrared light to measure calcification burden.
Thirteen of the 14 trials did not demonstrate significant cardiovascular calcification attenuation with HMG‐CoA reductase inhibitors, including 9 placebo‐controlled trials. The largest trial with the longest duration of follow‐up by Arad et al evaluated the impact of 20 mg atorvastatin, 1 g vitamin C, and 1000 IU vitamin E daily compared with placebo at 48 months in 1005 participants aged 50 to 70 years with coronary artery calcium score at or above the 80th percentile. 11 In this study, there was no significant difference in the secondary outcome of CAC at 4 years between treatment and control groups. Addition of ezetimibe to HMG‐CoA reductase inhibitors, evaluated by Hougaard et al and Lee et al, did not significantly attenuate cardiovascular calcification. 52 , 53
Aged Garlic Extract
Six trials evaluated the effect of AGE in attenuating the progression of cardiovascular calcification in 388 participants (median participants 65, range 19–104) (Table 2). 22 , 23 , 24 , 25 , 26 , 27 All trials were placebo controlled, 12 months in duration, and evaluated coronary arteries as the site. Participants had cardiovascular risk factors or confirmed presence of CAC, and those with CKD (serum creatinine >124 μmol/L for women or >140 μmol/L for men) were excluded in all trials.
Four of 6 trials demonstrated significant attenuation of cardiovascular calcification with AGE supplementation compared with placebo. The largest of these trials was a double‐blinded placebo‐controlled RCT with 104 participants by Wlosinska et al, which found that AGE significantly reduced progression of annular CAC compared with placebo over 12 months. 27 However, 2 trials that evaluated percentage change in dense calcium in coronary arteries did not demonstrate attenuation despite higher doses of AGE (2400 mg daily) compared with placebo at 12 months. 24 , 25
Hormone Replacement Therapies
Three trials examined the clinical impact of HRTs in mitigating progression of cardiovascular calcification in 1205 participants (Table 3). 28 , 29 , 30 , 31 None of the included trials that investigated HRTs (estrogen in postmenopausal populations and testosterone replacement in men with testosterone deficiency) reached their primary or secondary outcome in attenuating calcification.
Vitamin K
Five trials evaluated the effect of vitamin K in attenuating progression of cardiovascular calcification in 1074 participants (median participants 154, range 68–388; median duration 12 months, range 3–36 months) (Table 4). 32 , 33 , 34 , 35 , 54 All included RCTs evaluated calcification attenuation as their primary outcome, but there was heterogeneity in the site evaluated, including various vascular beds and valvular calcification. Two trials used computed tomography to identify cardiovascular calcification, and 2 used positron emission tomography scans to assess the target‐to‐background ratio as a measure of calcification burden.
Three hundred eighty‐eight men and postmenopausal women aged 60 to 80 years were included in the largest trial by Shea et al, with a mean study duration of 36 months and randomization to vitamin K 500 μg daily or multivitamin. 34 In the primary intention‐to‐treat analysis, there was no significant attenuation at the coronary arteries between groups; however, when results were restricted to participants >85% adherent to the intervention, or who had mild CAC at baseline, attenuation was observed. Only 1 RCT evaluating vitamin K achieved the primary outcome. 33
Lifestyle Measures
Lifestyle measures were investigated as strategies to attenuate cardiovascular calcification in 4 RCTs, with a total of 746 participants (average study duration 28 months) (Table 5). 36 , 37 , 58 , 59 CAC was evaluated in all trials, and no trial demonstrated efficacy with nonpharmacological measures, specifically diet and exercise.
Antithrombotic and Anticoagulant Therapies
Four RCTs evaluated the impact of antithrombotic or anticoagulant therapy in attenuating progression of cardiovascular calcification (Table 6). 38 , 39 , 40 , 41 Agents evaluated included those with antiplatelet mechanisms, including sarpogrelate and cilostazol, and direct oral anticoagulant drugs, rivaroxaban and apixaban. Both sarpogrelate and cilostazol were compared with aspirin in 140 participants, and there was no significant benefit in mitigating calcification progression. 37 , 38 Direct oral anticoagulant drugs were compared with the vitamin K antagonist warfarin (international normalized ratio of 2–3) in 104 patients with nonvalvular atrial fibrillation. Apixaban and rivaroxaban were associated with reduced cardiovascular calcification progression compared with warfarin on multivariate analysis. 39 , 40
Antiresorptive Agents
Two trials presented the impact of antiresorptive therapy in 224 participants and involved a heterogeneous population (Table 6) 42 , 43 ; 1 study involved 74 participants with confirmed pseudoxanthoma elasticum, and 1 study involved 150 participants with calcific aortic stenosis. The former trial compared etidronate to placebo, and as a secondary outcome, attenuation of femoral artery calcification was demonstrated. 41 , 60 However, Pawade et al reported no significant differences in aortic valve calcification in individuals aged >50 years with either denosumab or alendronate compared with placebo. 42
Antihypertensive Therapy
Two trials examined the effect of antihypertensive agents on cardiovascular calcification as a secondary outcome (Table 5) 44 , 45 involving 719 participants with cardiovascular risk factors. In the trial by Motro et al, there was significant attenuation by nifedipine when compared with combination treatment with hydrochlorothiazide and amiloride. 43 In contrast, a placebo‐controlled RCT by Motro et al, which included 518 participants with angiographic, radiographic, or clinical evidence of ischemic heart disease, demonstrated no significant attenuation with nifedipine. 44
Oral Hypoglycemic Agents
Fitch et al investigated the effect of both lifestyle modification and metformin compared with placebo on CAC in a 2×2 factorial RCT in 50 participants with HIV and metabolic syndrome (Table 5). 59 Metformin‐treated participants had significantly less progression of CAC over 1 year of follow‐up even after stratifying for antiretroviral therapy. Non‐placebo‐controlled trials by Davison et al and Nozue et al investigated the effect of oral hypoglycemics on CAC among 327 participants with type 2 diabetes (Table 7). Pioglitazone was compared with glimepiride, and sitagliptin to control (usual diabetic medication), and in both studies, intervention did not demonstrate attenuation of cardiovascular calcification.
Omega‐3 Fatty Acids
Two trials evaluated the effect of omega‐3 fatty acids compared with placebo on cardiovascular calcification (Table 7). 55 , 56 No significant attenuation of calcification was observed with treatment compared with placebo in 365 participants.
Singleton Therapies
Three other singleton therapies were examined and are listed in Table 7. 46 , 47 , 48 They included salsalate, folate with vitamin B6 and 12, and dalcetrapib against placebo. No significant differences in cardiovascular calcification attenuation were observed with treatment compared with placebo or standard of care.
Risk of Bias and Grading of Recommendations, Assessment, Development, and Evaluations Assessment
Table S1 summarizes the risk of bias assessment for included RCTs. Random sequence generation and allocation concealment were reported with low risks of bias in 100% and 37% of trials, respectively. Blinding of participants and investigators to the allocated intervention and blinding to outcomes were reported with low risk of bias in 69% and 90% of trials, respectively.
Table S2 summarizes the Grading of Recommendations, Assessment, Development, and Evaluations assessment for quality of evidence of studies for each intervention. A majority of analyses were based on moderate certainty of evidence, primarily due to risk of bias, imprecision of reporting, and publication bias. Imprecision grading was based on small event numbers. All trials included were RCTs.
DISCUSSION
This is the first systematic review of RCTs evaluating the impact of all interventions reported to attenuate progression of cardiovascular calcification in clinical trials involving the general population and not specifically targeting patients with CKD. Forty‐nine trials were included, with a variable range of study participants (median 104 participants) and study duration (median 12 months). Six trials had >500 participants each, and 17 trials were 24 months or more in study duration. Treatment benefit was consistently seen with the use of AGE, whereas vitamin K supplementation, direct anticoagulant therapy, antiresorptive, antihypertensive, and hypoglycemic agents had insufficient evidence to affirm treatment benefit. HMG‐CoA reductase inhibitors, which had the largest number of included trials, as well as other lipid‐lowering agents, antithrombotic therapies, lifestyle measures (diet and exercise), HRTs, omega‐3 fatty acids, salsalate, folic acid, and dalcetrapib were unlikely to mitigate cardiovascular calcification progression (Table 8).
Table 8.
Summary of Evidence‐Based Interventions Reported to Attenuate Cardiovascular Calcification
| Probably reduces progression | Possibly reduces progression | Unlikely to reduce progression |
|---|---|---|
| Aged garlic extract | Vitamin K | Lipid‐lowering agents |
| Hypoglycemic agents | Antithrombotic therapy | |
| Antiresorptive therapy | Hormone replacement therapy | |
| Antihypertensive therapy | Salsalate | |
| Direct anticoagulant therapy* | Folic acid | |
| Dalcetrapib | ||
| Lifestyle (diet and exercise) | ||
| Omega‐3 fatty acids |
Compared with warfarin.
Significant heterogeneity was identified across included clinical trials with respect to target populations. Participant characteristics in RCTs included preexisting cardiovascular risk factors (eg, coronary artery disease, dyslipidemia, type 2 diabetes, and valvular disease), proinflammatory conditions (eg, systemic lupus erythematosus, pseudoxanthoma elasticum, and HIV infection), and varying ages, body mass index, and menopausal status for women. Heterogeneity was also seen in trials with regard to baseline cardiovascular calcification in participants, target of cardiovascular calcification site (eg, coronary arteries, aortic valve, femoral arteries), and the comparator (alternative intervention, standard of care, or placebo).
HMG‐CoA reductase inhibitor therapy is important in managing dyslipidemia and mitigating progression of atherosclerotic disease, by means of plaque stabilization, and is therefore prescribed as primary and secondary prevention to reduce cardiovascular risk. 61 In our systematic review of RCTs, HMG‐CoA reductase inhibitors (statins) were the most frequently evaluated intervention. Thirteen of 14 trials did not demonstrate calcification attenuation, 9 of which were placebo‐controlled trials, including trials of several dosing regimens from 10 mg rosuvastatin daily, and up to 80 mg atorvastatin in 5 RCTs. Higher doses did not demonstrate significant calcification attenuation. One trial (Plazak et al) reported that 40 mg atorvastatin was superior to placebo in attenuating progression of CAC among participants with SLE 18 ; however, these results were not reproduced in a larger trial involving similar participants by Petri et al. 17
Discordance in cardiovascular outcomes with statin therapy, in its application as secondary prevention, yet failure to mitigate cardiovascular calcification, may be due to inherent properties of HMG‐CoA reductase inhibitors in accelerating plaque calcification. A retrospective cohort analysis of the CAC Consortium, evaluating 28 025 individuals, highlighted that statin users had higher CAC compared with nonstatin users (281 versus 107 Agatston score). 62 It has been postulated that accelerated calcification of atheromatous lesions, which may be seen in people on statin therapy, might be protective through plaque stabilization, prevention of plaque rupture, and reduction in incident acute coronary syndromes. 63 , 64 High‐intensity statin therapy may increase calcification density while at the same time reducing the lipid‐rich necrotic core, making plaque less likely to rupture and decreasing cardiovascular event risk, but paradoxically increasing the Agatston score.
The basis of estrogen supplementation in postmenopausal women is underpinned by the well‐established acceleration of cardiovascular risk with endogenous estrogen withdrawal following menopause. Disappointingly, the Women's Health Initiative trial of conjugated estrogens compared with placebo in postmenopausal women who had undergone hysterectomies demonstrated no significant cardiovascular benefit with HRT in 10 739 women, but revealed a higher risk of stroke, venous thromboembolism, and breast cancer. 65 Results from a follow‐up study of 1079 women highlighted that the mean CAC score was lower among women in the treatment arm, 66 but CAC was not measured before randomization, and therefore attenuation of calcification progression over time cannot be argued. Three RCTs evaluating HRTs against placebo (estrogen supplementation for 727 postmenopausal women and testosterone supplementation for 478 men with testosterone deficiency) were included in this systematic review. Unfortunately, none of these studies demonstrated significant attenuation of calcification progression, and consistent with this finding, HRT is not recommended as first line for prevention of cardiovascular disease.
Two meta‐analyses have demonstrated cholesterol‐lowering effects of garlic supplementation. 67 , 68 Mechanisms by which AGE confers beneficial effects on atherosclerotic disease include prevention of vascular smooth muscle cell proliferation, mitigating entry of lipids into the arterial wall, and mediating low‐density lipoprotein oxidation. 26 , 69 , 70 The largest RCT evaluating AGE also reported a beneficial effect on inflammation, with measurable reductions in IL‐6 (interleukin 6), which is postulated to exert a positive effect on CAC scores. 27 In our systematic review, we presented 6 placebo‐controlled RCTs examining the impact of AGE on cardiovascular calcification attenuation. Four trials demonstrated significant attenuation of progression of cardiovascular calcification as its primary outcome. These RCTs, however, were limited by moderate risk of bias (by attrition, industry involvement, and lack of reporting across Cochrane risk of bias domains), small sample sizes (median 65 participants), and short study duration (median 12 months), reflecting challenges in translating its effects in clinical practice.
Increasing studies demonstrate the impact of vitamin K antagonists, for example warfarin, in accelerating cardiovascular calcification, including retrospective non‐CKD cohorts 71 , 72 and 2 recent prospective RCTs, which compared warfarin with direct oral anticoagulant drugs. 39 , 40 Warfarin may impair the vitamin K‐dependant γ‐carboxylation of MGP (matrix Gla protein), a potent inhibitor of mineralization. The mechanism of action of MGP is still not fully understood but thought to involve inhibition of Bone morphogenetic protein‐driven osteogenic differentiation of vascular smooth muscle cells and direct binding to nascent calcium phosphate crystals and blocking their deposition in the arterial wall. 73 In 2 RCTs in our systematic review, reporting on 186 participants with nonvalvular atrial fibrillation, warfarin demonstrated increased calcified plaque evolution when compared with direct oral anticoagulant drugs (rivaroxaban and apixaban, which work independent of vitamin K). However, although vitamin K antagonism is thought to contribute to cardiovascular calcification, it is not clear whether vitamin K supplementation represents a therapeutic approach to ameliorate calcification in non‐CKD populations. Only 1 of 5 trials evaluating the efficacy of vitamin K (against placebo or multivitamins) in mitigating vascular and valvular calcification progression reached its primary outcome. Subgroup analysis by Shea et al, however, demonstrated less progression among participants with excellent treatment adherence (>85%) or who had mild baseline CAC. 34 Similarly, post hoc analysis of a 2×2 factorial trial by Bellinge et al showed beneficial effects of vitamin K supplementation in mitigating burden of positron emission tomography‐positive lesions (as a measure of calcification). 74
Nonpharmacological approaches, specifically exercise and diet, are important strategies to modify cardiovascular risk; however, RCTs evaluating the impact of lifestyle measures on cardiovascular calcification attenuation in our systematic review did not show benefit. The WOMAN (Woman on the Move Through Activity and Nutrition) trial by Kuller et al was a large, randomized trial of 508 participants investigating the impact of lifestyle intervention, which included a low‐fat, low‐calorie diet and 150 minutes of weekly moderate‐intensity exercise. 36 At 48 months, there were no differences in calcification burden between treatment arms. Lehmann et al similarly demonstrated that prescription of an intensive 12‐month lifestyle program compared with written advice only did not attenuate CAC at 3 years. 37 In alignment with these findings, nonrandomized studies have suggested that physical activity may enhance progression of CAC. A prospective cohort study recruited >25 000 participants, and demonstrated that physically active participants had accelerated CAC regardless of baseline CAC. 75 Similarly, a large prospective observational study by DeFina et al highlighted that participants with high physical activity had presence of CAC. 66 Interestingly, this was in the absence of increased all‐cause or cardiovascular mortality at a 10‐year follow‐up even with clinically significant CAC levels. Mechanisms by which exercise may be associated with cardiovascular calcification remain unknown but are postulated to be secondary to hyperdynamic coronary circulation and systemic inflammatory responses in exercise increasing shear forces. 76 Our systematic review included 4 RCTs that do not support routine exercise prescription as a means to mitigate calcification, although recognizing that increased physical activity remains important in optimizing cardiovascular risk in primary practice in general.
Observational studies have reported associations between CAC and dietary factors including lower carbohydrate intake and higher lipid intake (especially saturated fatty acids), 77 , 78 , 79 although not all studies consistently report this association. 80 PREDIMED (Prevención con Dieta Mediterránea [Prevention With Mediterranean Diet]) was the first RCT designed to evaluate beneficial effects of a Mediterranean diet on primary prevention of cardiovascular disease, with a substudy reporting a 1% increase in total saturated fatty acid intake associated with an 8% increase in the prevalence of moderate‐to‐severe CAC. 77 Few clinical trials, however, have used dietary measures as interventions to assess an effect on vascular calcification. One RCT, by Henzel et al, reported on 92 participants with nonobstructive coronary atherosclerosis randomized to a dietary and lifestyle intervention (involving dietitian follow‐up and prescription of the Dietary Approaches to Stop Hypertension nutrition model) compared with optimal medical therapy alone. 68 After a mean of 67 weeks of follow‐up, there was a significant reduction in progression of atherosclerosis and noncalcified plaque with the dietary intervention but no difference in the dense calcium changes between groups.
Antihypertensive therapy and optimizing glycemic control in patients with diabetes are established measures to reduce complications including cardiovascular events, stroke, and CKD. However, there is paucity of data, specifically RCT evidence, evaluating the impact of these strategies on attenuation of cardiovascular calcification. The Coronary Calcification study, a side‐arm study of a larger trial by Poole‐Wilson, 81 assessed the impact of nifedipine on progression of CAC score (using dual‐energy computed tomography) every 2 years over a 6‐year period. Change in calcium score was not significantly different between treatment and placebo groups. 45 The landmark EDIC (Epidemiology of Diabetes Interventions and Complications) trial was conducted as an observational follow‐up of the 1993 DCCT (Diabetes Control and Complications Trial) and investigated the legacy effect of intensive glycemic control (Hemoglobin A1c 7.2% in intensive arm, 9.1% in conventional arm) on diabetic complications including CAC. 82 There were 1205 participants included in this follow‐up, and there was lasting benefit of intensive glycemic control, up to 9 years following completion of the DCCT, on cardiovascular calcification attenuation despite loss of glycemic separation between treatment arms. However, the impact of treatment over time can only be inferred because baseline CAC scores were not obtained in either the DCCT or EDIC studies. Both trials were excluded from our systematic review, underpinning the paucity of randomized data on addressing metabolic risk to attenuate calcification progression. One placebo‐controlled RCT also assessed the impact of hypoglycemic agents in reducing calcification progression in non‐CKD populations. Fitch et al demonstrated that metformin significantly attenuated CAC progression compared with placebo after 1 year of follow‐up, postulating that metformin may have vasoprotective effects and mitigate plaque progression by AMP‐kinase activation and secondary regulation of cholesterol and fatty acid oxidation. 59
There are no RCTs evaluating the impact of aspirin on cardiovascular calcification. Sarpogrelate is a selective serotonin 2A (5‐HT2A) receptor antagonist that inhibits platelet aggregation. Lee et al examined the combined effect of sarpogrelate and aspirin compared with aspirin alone on CAC, and demonstrated no significant additive benefit of sarpogrelate in mediating attenuation. 38 Similarly, Lee et al demonstrated no significant difference between cilosatzol, a phosphodiesterase‐3 inhibitor, and aspirin on coronary calcified plaque volume progression. 39 There is insufficient RCT evidence to affirm quantifiable cardiovascular calcification attenuation with aspirin; however, epidemiological evidence from longitudinal data suggests a net favorable cardiovascular outcome with aspirin prescription in participants with elevated CAC scores. 83 Therefore, the Cardiac Society of Australia and New Zealand recommends asymptomatic CAC testing among patients aged 45 to 75 years with intermediate cardiovascular risk, and aspirin therapy is suggested for patients with CAC scores >100. 84
One of the mechanisms mediating valvular calcification is preferential osteoblastic differentiation in the valve, stimulated by the RANK (receptor activator of nuclear factor kappa‐β) ligand. Therefore, therapies targeting the RANK ligand and other antiresorptive agents have been well studied, but its use in clinical practice to mitigate cardiovascular calcification remains contentious. A majority of RCTs are studied in participants with CKD, and a systematic review by Xu et al reported possible attenuation of cardiovascular calcification progression with antiresorptive therapy in this population, although most trials were small and of short duration. 8 Two small RCTs were included in our systematic review that evaluated antiresorptive therapy in the non‐CKD population. Pawade et al demonstrated no amelioration of valvular calcification in 150 participants with either antiresorptive therapy compared with placebo, 43 in stark contrast with the trial by Kranenburg et al, which demonstrated favorable response with etidronate compared with placebo in patients with pseudoxanthoma elasticum. 42 However, both trials were small, with heterogeneous vascular targets and populations, and therefore, there is insufficient evidence to support the use of antiresorptive therapy for cardiovascular calcification attenuation in the general population.
Based on our systematic review, there is insufficient and conflicting data for interventions evaluated in RCTs for the mitigation of cardiovascular calcification. AGE therapies appear most promising, but the evaluated studies were small and of short duration, with moderate Cochrane risk of bias. These findings require confirmation with randomized trials with larger cohorts. Interestingly, HMG‐CoA reductase inhibitors and lifestyle interventions, including low‐caloric diet and exercise, were associated with higher cardiovascular calcification in participants, without impact on mortality or incident adverse coronary outcomes. This may be secondary to the inherent properties of calcification in plaque stabilization and prevention of plaque rupture. Although vitamin K antagonism has a well‐established role in cardiovascular calcification propagation, our systematic review demonstrates that the vitamin K pathway may not represent a therapeutic target to mitigate this in a non‐CKD cohort. Results presented in our systematic review should be interpreted with caution due to the heterogeneity in study populations and exclusion criteria across trials. Also, given the nature of many of the clinical trials evaluating vascular calcification, and the size and heterogeneity of these studies (eg, different populations and different measurements of vascular or valvular calcification), unfortunately meta‐analyses were not able to be performed, nor would they be any more meaningful than the descriptive data presented in our study.
Critically, we await definitive evidence that targeting vascular or valvular calcification translates to improved patient outcomes. Therefore, future clinical trials of interventions to slow cardiovascular calcification progression should prioritize recruitment of larger cohorts with longer durations of follow‐up, and report on cardiovascular outcomes so its application in clinical practice can be cautiously considered. This is especially true in the general population, because calcification in arterial and valvular tissues develops over the course of decades. It is important to also note that although vascular calcification is associated with worse cardiovascular outcomes, it is not clear that adverse outcomes are causally mediated by calcification or that interventions to attenuate vascular calcification would be expected to reduce cardiovascular outcomes. This is exemplified by the fact that statin therapy and lifestyle interventions, known to reduce cardiovascular events, do not reduce, and can increase vascular calcification, as highlighted in RCTs in this systematic review, likely through stabilizing plaque via calcification. Therefore, with a general goal of management to reduce cardiovascular events, these beneficial interventions should not be avoided because of concerns for increased vascular calcification.
Sources of Funding
None.
Disclosures
E.R.S. is a stockholder of Calciscon AG (Nidau, Switzerland), which commercializes the T50 serum calcification propensity test. He also reports research funding from Sanofi and Baxter. N.D.T. reports research funding and honoraria from Amgen, Sanofi, and Takeda. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S2
Acknowledgments
Study design: S.M., E.R.S., M.K.T., S.‐J.T., N.D.T. Literature search, study selection, and data extraction: S.M., N.D.T. Supervision: E.R.S., M.K.T., N.D.T. Each author contributed important intellectual content during article drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All data and supporting materials have been provided with the published article.
This article was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031676
For Sources of Funding and Disclosures, see page 17.
References
- 1. Quaglino D, Boraldi F, Fofaro FD. Cell and molecular biology of septins. Int Rev Cell Mol Biol. 2020;310:289–339. [DOI] [PubMed] [Google Scholar]
- 2. Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–1605. doi: 10.2215/CJN.02120508 [DOI] [PubMed] [Google Scholar]
- 3. Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lanzer P, Hannan FM, Lanzer JD, Janzen J, Raggi P, Furniss D, Schuchardt M, Thakker R, Fok PW, Saez‐Rodriguez J, et al. Medial arterial calcification: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;78:1145–1165. doi: 10.1016/j.jacc.2021.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging. 2018;11:127–142. doi: 10.1016/j.jcmg.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 6. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta‐analysis. Vasc Health Risk Manag. 2009;5:185–197. doi: 10.2147/VHRM.S4822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Topaz G, Yehezkel E, Benchetrit S, Korzets Z, Arnson Y, Kitay‐Cohen Y, Pereg D, Cohen‐Hagai K. Non‐coronary cardiac calcifications and outcomes in patients with heart failure. J Cardiol. 2021;77:83–87. doi: 10.1016/j.jjcc.2020.07.019 [DOI] [PubMed] [Google Scholar]
- 8. Xu C, Smith ER, Tiong MK, Ruderman I, Toussaint ND. Interventions to attenuate vascular calcification progression in chronic kidney disease: a systematic review of clinical trials. J Am Soc Nephrol. 2022;33:1011–1032. doi: 10.1681/ASN.2021101327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman A, Savovic J, Schulz K, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, Schünemann HJ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. 2005;46:166–172. doi: 10.1016/j.jacc.2005.02.089 [DOI] [PubMed] [Google Scholar]
- 12. Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid‐lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876 [DOI] [PubMed] [Google Scholar]
- 13. Dichtl W, Alber HF, Feuchtner GM, Hintringer F, Reinthaler M, Bartel T, Süssenbacher A, Grander W, Ulmer H, Pachinger O, et al. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg). Am J Cardiol. 2008;102:743–748. doi: 10.1016/j.amjcard.2008.04.060 [DOI] [PubMed] [Google Scholar]
- 14. Houslay ES, Cowell SJ, Prescott RJ, Reid J, Burton J, Northridge DB, Boon NA, Newby DE. Progressive coronary calcification despite intensive lipid‐lowering treatment: a randomised controlled trial. Heart. 2006;92:1207–1212. doi: 10.1136/hrt.2005.080929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Longenecker CT, Sattar A, Gilkeson R, McComsey GA. Rosuvastatin slows progression of subclinical atherosclerosis in patients with treated HIV infection. AIDS. 2016;30:2195–2203. doi: 10.1097/QAD.0000000000001167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miyoshi T, Kohno K, Asonuma H, Sakuragi S, Nakahama M, Kawai Y, Uesugi T, Oka T, Munemasa M, Takahashi N. Effect of intensive and standard pitavastatin treatment with or without eicosapentaenoic acid on progression of coronary artery calcification over 12 months: prospective multicenter study. Circ J. 2018;82:532–540. doi: 10.1253/circj.CJ-17-0419 [DOI] [PubMed] [Google Scholar]
- 17. Petri MA, Kiani AN, Post W, Christopher‐Stine L, Magder LS. Lupus Atherosclerosis Prevention Study (LAPS). Ann Rheum Dis. 2011;70:760–765. doi: 10.1136/ard.2010.136762 [DOI] [PubMed] [Google Scholar]
- 18. Plazak W, Gryga K, Dziedzic H, Tomkiewicz‐Pajak L, Konieczynska M, Podolec P, Musial J. Influence of atorvastatin on coronary calcifications and myocardial perfusion defects in systemic lupus erythematosus patients: a prospective, randomized, double‐masked, placebo‐controlled study. Arthritis Res Ther. 2011;13:R117. doi: 10.1186/ar3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raggi P, Davidson M, Callister TQ, Welty FK, Bachmann G, Hecht H, Rumberger J. Aggressive versus moderate lipid‐lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation. 2005;112:563–571. doi: 10.1161/CIRCULATIONAHA.104.512681 [DOI] [PubMed] [Google Scholar]
- 20. Schmermund A, Achenbach S, Budde T, Buziashvilli Y, Förster A, Friedrich G, Henein M, Kerkhoff G, Knollmann F, Kukharchuk V. Effect of intensive versus standard lipid‐lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double‐blind trial. Circulation. 2006;113:427–437. doi: 10.1161/CIRCULATIONAHA.105.568147 [DOI] [PubMed] [Google Scholar]
- 21. Terry JG, Carr JJ, Kouba EO, Davis DH, Menon L, Bender K, Chandler ET, Morgan T, Crouse JR III. Effect of simvastatin (80 mg) on coronary and abdominal aortic arterial calcium (from the coronary artery calcification treatment with Zocor [CATZ] study). Am J Cardiol. 2007;99:1714–1717. doi: 10.1016/j.amjcard.2007.01.060 [DOI] [PubMed] [Google Scholar]
- 22. Budoff MJ, Ahmadi N, Gul KM, Liu ST, Flores FR, Tiano J, Takasu J, Miller E, Tsimikas S. Aged garlic extract supplemented with B vitamins, folic acid and l‐arginine retards the progression of subclinical atherosclerosis: a randomized clinical trial. Prev Med. 2009;49:101–107. doi: 10.1016/j.ypmed.2009.06.018 [DOI] [PubMed] [Google Scholar]
- 23. Budoff MJ, Takasu J, Flores FR, Niihara Y, Lu B, Lau BH, Rosen RT, Amagase H. Inhibiting progression of coronary calcification using aged garlic extract in patients receiving statin therapy: a preliminary study. Prev Med. 2004;39:985–991. doi: 10.1016/j.ypmed.2004.04.012 [DOI] [PubMed] [Google Scholar]
- 24. Matsumoto S, Nakanishi R, Li D, Alani A, Rezaeian P, Prabhu S, Abraham J, Fahmi MA, Dailing C, Flores F, et al. Aged garlic extract reduces low attenuation plaque in coronary arteries of patients with metabolic syndrome in a prospective randomized double‐blind study. J Nutr. 2016;146:427S–432S. doi: 10.3945/jn.114.202424 [DOI] [PubMed] [Google Scholar]
- 25. Shaikh K, Kinninger A, Cherukuri L, Birudaraju D, Nakanishi R, Almeida S, Jayawardane E, Shekar C, Flores F, Hamal S, et al. Aged garlic extract reduces low attenuation plaque in coronary arteries of patients with diabetes: a randomized, double‐blind, placebo‐controlled study. Exp Ther Med. 2020;19:1457–1461. doi: 10.3892/etm.2019.8371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zeb I, Ahmadi N, Nasir K, Kadakia J, Larijani VN, Flores F, Li D, Budoff MJ. Aged garlic extract and coenzyme Q10 have favorable effect on inflammatory markers and coronary atherosclerosis progression: a randomized clinical trial. J Cardiovasc Dis Res. 2012;3:185–190. doi: 10.4103/0975-3583.98883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wlosinska M, Nilsson AC, Hlebowicz J, Hauggaard A, Kjellin M, Fakhro M, Lindstedt S. The effect of aged garlic extract on the atherosclerotic process‐a randomized double‐blind placebo‐controlled trial. BMC Complement Med Ther. 2020;20:132. doi: 10.1186/s12906-020-02932-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Basaria S, Harman SM, Travison TG, Hodis H, Tsitouras P, Budoff M, Pencina KM, Vita J, Dzekov C, Mazer NA, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with lowor low‐normal testosterone levels: a randomized clinical trial. JAMA. 2015;314:570–581. doi: 10.1001/jama.2015.8881 [DOI] [PubMed] [Google Scholar]
- 29. Budoff MJ, Ellenberg SS, Lewis CE, Mohler ER, Wenger NK, Bhasin S, Barrett‐Connor E, Swerdloff RS, Stephens‐Shields A, Cauley JA, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317:708–716. doi: 10.1001/jama.2016.21043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, Hopkins PN, Lobo RA, Manson JE, Merriam GR, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161:249–260. doi: 10.7326/M14-0353 [DOI] [PubMed] [Google Scholar]
- 31. Bellinge JW, Francis RJ, Lee SC, Vickery A, Macdonald W, Gan SK, Chew GT, Phillips M, Lewis JR, Watts GF, et al. The effect of Vitamin‐K1 and Colchicine on Vascular Calcification Activity in subjects with Diabetes Mellitus (ViKCoVaC): a double‐blind 2×2 factorial randomized controlled trial. J Nucl Cardiol. 2022;29:1855–1866. doi: 10.1007/s12350-021-02589-8 [DOI] [PubMed] [Google Scholar]
- 32. Brandenburg VM, Reinartz S, Kaesler N, Krüger T, Dirrichs T, Kramann R, Peeters F, Floege J, Keszei A, Marx N, et al. Slower progress of aortic valve calcification with vitamin K supplementation: results from a prospective interventional proof‐of‐concept study. Circulation. 2017;135:2081–2083. doi: 10.1161/CIRCULATIONAHA.116.027011 [DOI] [PubMed] [Google Scholar]
- 33. Shea MK, O'Donnell CJ, Hoffmann U, Dallal GE, Dawson‐Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89:1799–1807. doi: 10.3945/ajcn.2008.27338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zwakenberg SR, De Jong PA, Bartstra JW, Van Asperen R, Westerink J, De Valk H, Slart RHJA, Luurtsema G, Wolterink JM, De Borst GJ, et al. The effect of menaquinone‐7 supplementation on vascular calcification in patients with diabetes: a randomized, double‐blind, placebo‐controlled trial. Am J Clin Nutr. 2019;110:883–890. doi: 10.1093/ajcn/nqz147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuller LH, Pettee Gabriel KK, Kinzel LS, Underwood DA, Conroy MB, Chang Y, Mackey RH, Edmundowicz D, Tyrell KS, Buhari AM, et al. The Women on the Move Through Activity and Nutrition (WOMAN) study: final 48‐month results. Obesity (Silver Spring). 2012;20:636–643. doi: 10.1038/oby.2011.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lehmann N, Paul A, Moebus S, Budde T, Dobos GJ, Michalsen A. Effects of lifestyle modification on coronary artery calcium progression and prognostic factors in coronary patients—3‐year results of the randomized SAFE‐LIFE trial. Atherosclerosis. 2011;219:630–636. doi: 10.1016/j.atherosclerosis.2011.08.050 [DOI] [PubMed] [Google Scholar]
- 37. Lee DH, Chun EJ, Hur JH, Min SH, Lee JE, Oh TJ, Kim KM, Jang HC, Han SJ, Kang DK, et al. Effect of sarpogrelate, a selective 5‐HT2A receptor antagonist, on characteristics of coronary artery disease in patients with type 2 diabetes. Atherosclerosis. 2017;257:47–54. doi: 10.1016/j.atherosclerosis.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 38. Lee DH, Chun EJ, Oh TJ, Kim KM, Moon JH, Choi SH, Park KS, Jang HC, Lim S. Effect of cilostazol, a phosphodiesterase‐3 inhibitor, on coronary artery stenosis and plaque characteristics in patients with type 2 diabetes: ESCAPE study. Diabetes Obes Metab. 2019;21:1409–1418. doi: 10.1111/dom.13667 [DOI] [PubMed] [Google Scholar]
- 39. Lee J, Nakanishi R, Li D, Shaikh K, Shekar C, Osawa K, Nezarat N, Jayawardena E, Blanco M, Chen M, et al. Randomized trial of rivaroxaban versus warfarin in the evaluation of progression of coronary atherosclerosis. Am Heart J. 2018;206:127–130. doi: 10.1016/j.ahj.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 40. Win TT, Nakanishi R, Osawa K, Li D, Susaria SS, Jayawardena E, Hamal S, Kim M, Broersen A, Kitslaar PH, et al. Apixaban versus warfarin in evaluation of progression of atherosclerotic and calcified plaques (prospective randomized trial). Am Heart J. 2019;212:129–133. doi: 10.1016/j.ahj.2019.02.014 [DOI] [PubMed] [Google Scholar]
- 41. Kranenburg G, de Jong PA, Bartstra JW, Lagerweij SJ, Lam MG, Norel JO, Risseeuw S, Van Leeuwen R, Imhof SM, Verhaar HJ, et al. Etidronate for prevention of ectopic mineralization in patients with pseudoxanthoma elasticum. J Am Coll Cardiol. 2018;71:1117–1126. doi: 10.1016/j.jacc.2017.12.062 [DOI] [PubMed] [Google Scholar]
- 42. Pawade TA, Doris MK, Bing R, White AC, Forsyth L, Evans E, Graham C, Williams MC, van Beek EJR, Fletcher A, et al. Effect of denosumab or alendronic acid on the progression of aortic stenosis: a double‐blind randomized controlled trial. Circulation. 2021;143:2418–2427. doi: 10.1161/CIRCULATIONAHA.121.053708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Motro M, Shemesh J. Calcium channel blocker nifedipine slows down progression of coronary calcification in hypertensive patients compared with diuretics. Hypertension. 2001;37:1410–1413. doi: 10.1161/01.HYP.37.6.1410 [DOI] [PubMed] [Google Scholar]
- 44. Motro M, Kirwan BA, De Brouwer S, Poole‐Wilson PA, Shemesh J. Tracking coronary calcification and atherosclerotic lesions in patients with stable angina pectoris undergoing nifedipine therapy. Cardiology. 2007;107:165–171. doi: 10.1159/000095308 [DOI] [PubMed] [Google Scholar]
- 45. Davidson MH, Beam CA, Haffner S, Perez A, Dagostino R, Mazzone T. Pioglitazone versus glimepiride on coronary artery calcium progression in patients with type 2 diabetes mellitus: a secondary end point of the CHICAGO study. Arterioscler Thromb Vasc Biol. 2010;30:1873–1876. doi: 10.1161/ATVBAHA.110.207696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hauser TH, Salastekar N, Schaefer EJ, Desai T, Goldfine HL, Fowler KM, Weber GM, Welty F, Clouse M, Shoelson SE, et al. Effect of targeting inflammation with salsalate: the TINSAL‐CVD randomized clinical trial on progression of coronary plaque in overweight and obese patients using statins. JAMA Cardiol. 2016;1:413–423. doi: 10.1001/jamacardio.2016.0605 [DOI] [PubMed] [Google Scholar]
- 47. Hodis HN, Mack WJ, Dustin L, Mahrer PR, Azen SP, Detrano R, Selhub J, Alaupovic P, Liu CR, Liu CH, et al. High‐dose B vitamin supplementation and progression of subclinical atherosclerosis: a randomized controlled trial. Stroke. 2009;40:730–736. doi: 10.1161/STROKEAHA.108.526798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Joshi FR, Rajani NK, Abt M, Woodward M, Bucerius J, Mani V, Tawakol A, Kallend D, Fayad ZA, Rudd JFH. Does vascular calcification accelerate inflammation? A substudy of the dal‐PLAQUE trial. J Am Coll Cardiol. 2016;67:69–78. doi: 10.1016/j.jacc.2015.10.050 [DOI] [PubMed] [Google Scholar]
- 49. Park SJ, Kang SJ, Ahn JM, Chang M, Yun SC, Roh JH, Lee PH, Park HW, Yoon SH, Park DW, et al. Effect of statin treatment on modifying plaque composition: a double‐blind, randomized study. J Am Coll Cardiol. 2016;67:1772–1783. doi: 10.1016/j.jacc.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 50. Egede R, Jensen LO, Hansen HS, Hansen KN, Junker A, Thayssen P. Influence of high‐dose lipid lowering treatment compared to low‐dose lipid lowering treatment on plaque composition assessed by intravascular ultrasound virtual histology in patients with ST‐segment elevation acute myocardial infarction: the VIRHISTAMI trial. EuroIntervention. 2013;8:1182–1189. doi: 10.4244/EIJV8I10A182 [DOI] [PubMed] [Google Scholar]
- 51. Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV. Effects of statin therapy on coronary artery plaque volume and high‐risk plaque morphology in HIV‐infected patients with subclinical atherosclerosis: a randomised, double‐blind, placebo‐controlled trial. Lancet HIV. 2015;2:e52–e63. doi: 10.1016/S2352-3018(14)00032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee JH, Shin DH, Kim BK, Ko YG, Choi D, Jang Y, Hong MK. Early effects of intensive lipid‐lowering treatment on plaque characteristics assessed by virtual histology intravascular ultrasound. Yonsei Med J. 2016;57:1087–1094. doi: 10.3349/ymj.2016.57.5.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hougaard M, Hansen HS, Thayssen P, Maehara A, Antonsen L, Junker A, Mintz GS, Jensen LO. Influence of ezetimibe on plaque morphology in patients with ST elevation myocardial infarction assessed by optical coherence tomography: an OCTIVUS sub‐study. Cardiovasc Revasc Med. 2020;21:1417–1424. doi: 10.1016/j.carrev.2019.04.021 [DOI] [PubMed] [Google Scholar]
- 54. Diederichsen ACP, Lindholt JS, Möller S, Ovrehus KA, Auscher S, Lambrechtsen J, Hosbond SE, Alan DH, Urbonaviciene G, Becker SW, et al. Vitamin K2 and D in patients with aortic valve calcification: a randomized double‐blinded clinical trial. Circulation. 2022;145:1387–1397. doi: 10.1161/CIRCULATIONAHA.121.057008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Budoff MJ, Bhatt DL, Kinninger A, Lakshmanan S, Muhlestein JB, Te VT, May HT, Shaikh K, Shekar C, Roy SK, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020;41:3925–3932. doi: 10.1093/eurheartj/ehaa652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alfaddagh A, Elajami TK, Ashfaque H, Saleh M, Bistrian BR, Welty FK. Effect of eicosapentaenoic and docosahexaenoic acids added to statin therapy on coronary artery plaque in patients with coronary artery disease: a randomized clinical trial. J Am Heart Assoc. 2017;6:e006981. doi: 10.1161/JAHA.117.006981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nozue T, Fukui K, Koyama Y, Fujii H, Kunishima T, Hikita H, Hibi K, Miyazawa A, Michishita I. Effects of sitagliptin on coronary atherosclerosis evaluated using integrated backscatter intravascular ultrasound in patients with type 2 diabetes: rationale and design of the TRUST study. Heart Vessels. 2016;31:649–654. doi: 10.1007/s00380-015-0662-4 [DOI] [PubMed] [Google Scholar]
- 58. Henzel J, Kępka C, Kruk M, Makarewicz‐Wujec M, Wardziak L, Trochimiuk P, Dzielinska Z, Demkow M. High‐risk coronary plaque regression after intensive lifestyle intervention in nonobstructive coronary disease: a randomized study. JACC Cardiovasc Imaging. 2021;14:1192–1202. doi: 10.1016/j.jcmg.2020.10.019 [DOI] [PubMed] [Google Scholar]
- 59. Fitch K, Abbara S, Lee H, Stravrou E, Sacks R, Michel T, Hemphill L, Torriani M, Grinspoon S. Effects of lifestyle modification and metformin on atherosclerotic indices among HIV‐infected patients with the metabolic syndrome. AIDS. 2012;26:587–597. doi: 10.1097/QAD.0b013e32834f33cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bartstra JW, de Jong PA, Kranenburg G, Wolterink JM, Isgum I, Wijsman A, Wolf B, den Harder AM, Mali WPTM, Spiering W. Etidronate halts systemic arterial calcification in pseudoxanthoma elasticum. Atherosclerosis. 2020;292:37–41. doi: 10.1016/j.atherosclerosis.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 61. Chou R, Cantor A, Dana T, Wagner J, Ahmed AY, Fu R, Ferencik M. Statin use for the primary prevention of cardiovascular disease in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;328:754–771. doi: 10.1001/jama.2022.12138 [DOI] [PubMed] [Google Scholar]
- 62. Trivedi R, Blaha M, Blumenthal R, Cainzos‐Archirica M. The Agatston coronary artery calcium score in statin users: recent insights from the CAC Consortium and pathways forward. Am Coll Cardiol. 2021. Accessed November 16, 2023. https://www.acc.org/Latest‐in‐Cardiology/Articles/2021/01/19/14/27/The‐Agatston‐Coronary‐Artery‐Calcium‐Score‐in‐Statin‐Users [Google Scholar]
- 63. Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzco EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. doi: 10.1016/j.jacc.2015.01.036 [DOI] [PubMed] [Google Scholar]
- 64. Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A, Lin FY, Kumar A, Hadamitzky M, Kim YJ, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475–1484. doi: 10.1016/j.jcmg.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 65. Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE. Estrogen therapy and coronary‐artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513 [DOI] [PubMed] [Google Scholar]
- 66. Allison MA, Manson JE, Langer RD, Carr JJ, Rossouw JE, Pettinger MB, Phillips L, Cochrane BB, Eaton CB, Greenland P, et al. Oophorectomy, hormone therapy, and subclinical coronary artery disease in women with hysterectomy: the Women's Health Initiative coronary artery calcium study. Menopause. 2008;15:639–647. doi: 10.1097/gme.0b013e31816d5b1c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Neil HA, Silagy CA, Lancaster T, Hodgeman J, Vos K, Moore JW, Jones L, Cahill J, Fowler GH. Garlic powder in the treatment of moderate hyperlipidaemia: a controlled trial and meta‐analysis. J R Coll Physicians Lond. 1996;30:329–334. [PMC free article] [PubMed] [Google Scholar]
- 68. Warshafsky S, Kamer RS, Sivak SL. Effect of garlic on total serum cholesterol. A meta‐analysis. Ann Intern Med. 1993;119:599–605. doi: 10.7326/0003-4819-119-7_Part_1-199310010-00009 [DOI] [PubMed] [Google Scholar]
- 69. Rahman K, Billington D. Dietary supplementation with aged garlic extract inhibits ADP‐induced platelet aggregation in humans. J Nutr. 2000;130:2662–2665. doi: 10.1093/jn/130.11.2662 [DOI] [PubMed] [Google Scholar]
- 70. Lau BH. Suppression of LDL oxidation by garlic. J Nutr. 2001;131(3s):985S–988S. doi: 10.1093/jn/131.3.985S [DOI] [PubMed] [Google Scholar]
- 71. Plank F, Beyer C, Friedrich G, Stühlinger M, Hintringer F, Dichtl W, Wildauer M, Feuchtner G. Influence of vitamin K antagonists and direct oral anticoagulation on coronary artery disease: a CTA analysis. Int J Cardiol. 2018;260:11–15. doi: 10.1016/j.ijcard.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 72. Tastet L, Pibarot P, Shen M, Clisson M, Côté N, Salaun E, Arsenault M, Bédard É, Capoulade R, Puri R, et al. Oral anticoagulation therapy and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2019;73:1869–1871. doi: 10.1016/j.jacc.2019.01.043 [DOI] [PubMed] [Google Scholar]
- 73. Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20 [DOI] [PubMed] [Google Scholar]
- 74. Bellinge JW, Francis RJ, Lee SC, Bondonno NP, Sim M, Lewis JR, Watts GF, Schultz CJ. The effect of vitamin K1 on arterial calcification activity in subjects with diabetes mellitus: a post hoc analysis of a double‐blind, randomized, placebo‐controlled trial. Am J Clin Nutr. 2022;115:45–52. doi: 10.1093/ajcn/nqab306 [DOI] [PubMed] [Google Scholar]
- 75. Sung KC, Hong YS, Lee JY, Lee SJ, Chang Y, Ryu S, Zhao D, Cho J, Guallar E, Liam JAC. Physical activity and the progression of coronary artery calcification. Heart. 2021;107:1710–1716. doi: 10.1136/heartjnl-2021-319346 [DOI] [PubMed] [Google Scholar]
- 76. Merghani A, Maestrini V, Rosmini S, Cox AT, Dhutia H, Bastiaenan R, David S, Yeo TJ, Narain R, Malhotra A, et al. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation. 2017;136:126–137. doi: 10.1161/CIRCULATIONAHA.116.026964 [DOI] [PubMed] [Google Scholar]
- 77. Guasch‐Ferré M, Babio N, Martínez‐González MA, Corella D, Ros E, Martín‐Peláez S, Estruch R, Arós F, Gómez‐Gracia E, Fiol M, et al. Dietary fat intake and risk of cardiovascular disease and all‐cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr. 2015;102:1563–1573. doi: 10.3945/ajcn.115.116046 [DOI] [PubMed] [Google Scholar]
- 78. Park HA, Lee JS, Kuller LH. Relationship between premenopausal dietary intake and postmenopausal subclinical atherosclerosis. Atherosclerosis. 2006;186:420–427. doi: 10.1016/j.atherosclerosis.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 79. Bruscato NM, da Luz PL, Werle BM, Schvartzman PR, Kesties J, Vivian L, De Carli W, Moriguchi EH. Coronary artery calcification and dietary intake in asymptomatic men. Braz J Med Biol Res. 2021;54:e11371. doi: 10.1590/1414-431x2021e11371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Richardson LA, Basu A, Chien LC, Alman AC, Snell‐Bergeon JK. Associations of the Mediterranean‐style dietary pattern score with coronary artery calcification and pericardial adiposity in a sample of US adults. Nutrients. 2022;14:3385. doi: 10.3390/nu14163385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Poole‐Wilson PA, Lubsen J, Kirwan BA, Van Dalen FJ, Wagener G, Danchin N, Just H, Fox KAA, Pocock SJ, Clayton TC, et al. Effect of long‐acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet. 2004;364:849–857. doi: 10.1016/S0140-6736(04)16980-8 [DOI] [PubMed] [Google Scholar]
- 82. Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JYC, Zinman B, Jacobson A, Sun W, Lachin JM, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes. 2006;55:3556–3565. doi: 10.2337/db06-0653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Miedema MD, Duprez DA, Misialek JR, Blaha MJ, Nasir K, Silverman MG, Blankstein R, Budoff MJ, Greenland P, Folsom AR. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the MultiEthnic Study of Atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7:453–460. doi: 10.1161/CIRCOUTCOMES.113.000690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liew G, Chow C, van Pelt N, Younger J, Jelinek M, Chan J, Hamilton‐Craig C. Cardiac Society of Australia and New Zealand position statement: coronary artery calcium scoring. Heart Lung Circ. 2017;26:1239–1251. doi: 10.1016/j.hlc.2017.05.130 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2


