Abstract
Background
This Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) provides an overview of the mechanisms and treatment of obesity and hypertension.
Methods
The scientific support for this CPS is based upon published citations, clinical perspectives of OMA authors, and peer review by the Obesity Medicine Association leadership.
Results
Mechanisms contributing to obesity-related hypertension include unhealthful nutrition, physical inactivity, insulin resistance, increased sympathetic nervous system activity, renal dysfunction, vascular dysfunction, heart dysfunction, increased pancreatic insulin secretion, sleep apnea, and psychosocial stress. Adiposopathic factors that may contribute to hypertension include increased release of free fatty acids, increased leptin, decreased adiponectin, increased renin-angiotensin-aldosterone system activation, increased 11 beta-hydroxysteroid dehydrogenase type 1, reduced nitric oxide activity, and increased inflammation.
Conclusions
Increase in body fat is the most common cause of hypertension. Among patients with obesity and hypertension, weight reduction via healthful nutrition, physical activity, behavior modification, bariatric surgery, and anti-obesity medications mostly decrease blood pressure, with the greatest degree of weight reduction generally correlated with the greatest degree of blood pressure reduction.
Keywords: Adiposopathy, High blood pressure, Hypertension, Obesity
1. Introduction
Beginning in 2013, the Obesity Medicine Association (OMA) created and maintained an Adult “Obesity Algorithm” (i.e., educational slides and eBook) that underwent yearly updates by OMA authors and was reviewed and approved annually by the OMA Board of Trustees [1]. This current OMA Clinical Practice Statement (CPS) regarding obesity and hypertension was derived from extensive updates to the content of the 2021 OMA Adult Obesity Algorithm. This CPS is one of a series of OMA CPSs designed to assist clinicians in the care of their patients with the disease of obesity.
2. Obesity and high blood pressure/hypertension
Hypertension can be defined as arterial blood pressure readings that, when persistently elevated above ranges established by medical organizations, adversely affect patient health [2]. For this discussion, high blood pressure refers to the physiologic measurement of the blood flow force on arteries, while hypertension refers to the disease of persistent high blood pressure.
2.1. Blood pressure measurements
Blood pressure measurements can be affected by many factors. A key for potentially actionable longitudinal assessment and treatment of high blood pressure is consistency in how blood pressure is measured, with recognition that blood pressure can be affected by:
-
•
White-coat hypertension: Some patients may experience an increase in blood pressure due to anxiety and stress before and duringencounters with the clinician. Such patients may benefit from home blood pressure (BP) monitoring and/or ambulatory BP monitoring (ABPM) [2].
-
•
Masked hypertension: Some patients have normal blood pressure measurements in the clinician office, but elevated blood pressure upon ambulatory blood pressure monitoring or home blood pressure monitoring. Patients with masked hypertension are at risk for cardiovascular disease [3].
-
•
Overstimulation: Patients should avoid caffeine, energy drinks, decongestants, physical exercise, stressful situations, full bladder, and/or smoking for at least 30 minutes prior to BP measurement. For example, if a patient arrives to the medical office after experiencing frustration due to anxiety-producing traffic, then the patient should be given the opportunity to calm down in a quiet room for 30 minutes or longer. Patients with full bladder, [4] and/or who feel the need to urinate, may have increased blood pressure and should void prior to having blood pressure taken. Some patients with acute pain (e.g., immediately after a phlebotomy stick) or discomfort may also experience transient increased blood pressure; the high blood pressure due to acute pain should resolve once the pain and/or discomfort resolves.
-
•
On first measurement date, blood pressure should optimally be measured in both arms by repeated values separated by at least 1 min, with a record of the values and respective arms (left and right). Longitudinally, future BP measurement might best be measured on the same arm previously recorded as having the highest BP measurement.
-
•
Patients should have clothing removed from the arm, be seated with feet flat on the floor (i.e., not crossed), relaxed and quiet for 5–10 minutes prior to BP measurement. Crossed legs may increase blood pressure [5].
-
•
During the BP measurement, the patient should not talk, nor should the patient be asked questions (including medical questions) during the time blood pressure is being assessed.
-
•
Blood pressure should be obtained by trained medical personnel using a properly validated and calibrated blood pressure measurement device.
-
•
The cuff should be placed on around the skin of the upper arm (i.e., not over clothing).
-
•
The cuff type and size should be appropriate for the patient arm size [6].
-
•If blood pressure is taken by a manual cuff (i.e., sphygmomanometer):
-
oThe cuff should be placed one inch above the elbow bend, with the center of the cuff (often identified with a marker) aligned with the brachial artery, as found by palpation.
-
oThe cuff should be inflated until the radial pulse is no longer felt.
-
oThe cuff is then slowly deflated until the pulse is felt again. This number reading (mmHg) on the mercury column is the approximate systolic blood pressure.
-
oAfter deflating the cuff, and after waiting another 15 seconds, the bell of the stethoscope is placed over the brachial artery.
-
oThe cuff is then inflated 30 mmHg above the previous systolic blood pressure reading.
-
oThe cuff is then deflated (again) at 2 mmHg per second or beat.
-
oThe first of at least two regular beats is the systolic blood pressure.
-
oWhen the beat disappears entirely, this is the diastolic blood pressure.
-
oAfter obtaining BP readings, the cuff should be deflated, removed, and the systolic and diastolic BP should be recorded (in mmHg) immediately.
-
o
2.2. Differential diagnosis of hypertension
Among the more important considerations in evaluating high blood pressure in patients with obesity is ensuring an accurate diagnosis. While the adiposopathic effects of increased body fat are the most common cause of hypertension, simply because a patient has obesity does not negate the need to be mindful of other potential secondary causes that include [7]:
-
⁃
Pheochromocytoma
-
⁃
Primary hyperaldosteronism
-
⁃
Hypercortisolism
-
⁃
Hyperthyroidism
-
⁃
Renal artery stenosis
-
⁃
Kidney diseases
-
⁃
Side effects of concomitant therapies
-
⁃
Familial or genetic syndromes
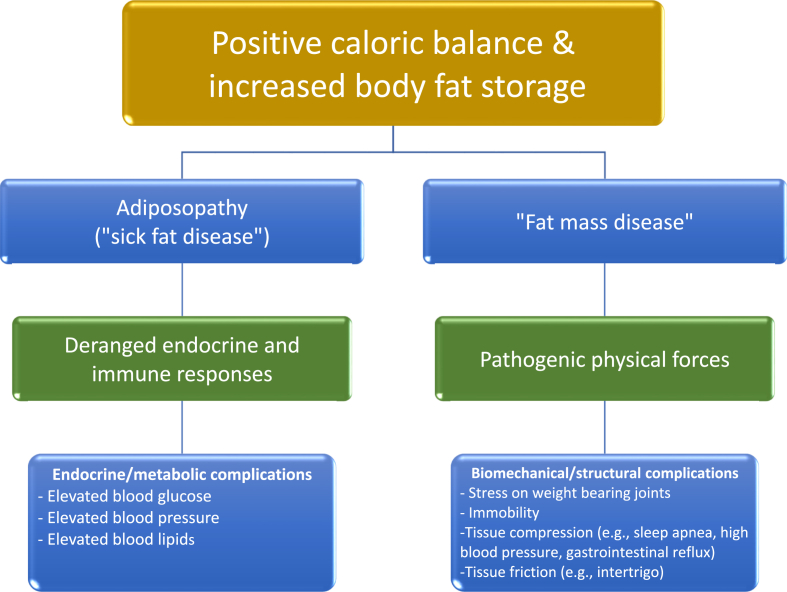
That said, overweight and obesity clearly increases the risk of hypertension [[8], [9], [10]]. As with obesity itself, hypertension is a chronic disease that represents a major risk factor for cardiovascular disease (CVD), which is the most common cause of mortality among patients with obesity and hypertension [11]. In the United States, hypertension is present in over 40% and 25% of individuals with obesity and overweight, respectively. Over 70% of individuals with hypertension have overweight or obesity [12,13]. When implementing comprehensive management of obesity, standards of care include healthful nutrition, physical activity, behavior modification, and medical treatment (e.g., anti-obesity medications and/or bariatric procedures) [14,15]. Regarding medications, it is best to avoid obesogenic medications, and instead select pharmacotherapy that not only improves the body composition of patients, but also improves the health of patients, with control of blood pressure being a key health metric [16]. Table 1 describes ten takeaways from this current OMA CPS regarding obesity and hypertension. Fig. 1 illustrates how positive caloric balance may lead to increase body fat storage. If during the process of body fat storage, adipocyte proliferation is impaired resulting in pathogenic adipocyte hypertrophy, then this may lead to “sick fat disease” (adiposopathy), as well as “fat mass disease.” The ensuing deranged endocrine and immune responses, as well as pathogenic physical forces, all may contribute to obesity complications such as an increase in blood pressure.
Table 1.
Ten takeaway messages: obesity and hypertension. The disease of obesity may promote the development of hypertension, which is a major risk factor for cardiovascular disease. See text for details.
| 1. | The disease of obesity may promote an increase in blood pressure, which if persistent, leads to the metabolic disease complication of hypertension. |
| 2. | Over 70% of individuals with hypertension have overweight or obesity. Hypertension is a major risk factor for cardiovascular disease (CVD); CVD is the most common cause of mortality among patients with obesity and hypertension. |
| 3. | Patients with obesity and hypertension should optimally undergo comprehensive obesity management (e.g., healthful nutrition, physical activity, behavior modification, and/or anti-obesity medications/bariatric procedures) [14] as well as global CVD risk reduction (e.g., optimal control of blood pressure, blood lipids, blood glucose, and smoking cessation) [2]. |
| 4. | In addition to unhealthful food intake contributing to positive caloric balance, increased dietary sodium and saturated fats can also increase blood pressure. Conversely, nutritional interventions that contribute to healthful weight reduction and reduced sodium intake can help prevent and treat hypertension, especially when accompanied by routine physical activity and behavior modification. |
| 5. | Obesity and “fat mass disease” can contribute to sleep apnea, kidney and renal vessel compression, perivascular adipose tissue (restricting blood vessel wall expansion) and increased cardiac output – all of which can increase blood pressure. |
| 6. | Mechanisms contributing to obesity-related high blood pressure include unhealthful nutrition, physical inactivity, insulin resistance, increased sympathetic nervous system activity, renal dysfunction, vascular dysfunction, heart dysfunction, increased pancreatic insulin secretion, sleep apnea, and psychosocial stress. |
| 7. | Adiposopathic factors that may contribute to high blood pressure include increased release of free fatty acids, increased leptin, decreased adiponectin, increased renin-angiotensin-aldosterone system activation, increased 11 beta-hydroxysteroid dehydrogenase type 1, reduced nitric oxide activity, and increased inflammation. |
| 8. | Bariatric surgery (e.g., gastric bypass and sleeve gastrectomy) can produce clinically meaningful reduction in both blood pressure and body weight. |
| 9. | Especially when accompanied by clinically meaningful weight reduction, many anti-obesity medications such as glucagon like peptide-1 (GLP-1) receptor agonists (e.g., semaglutide and liraglutide), GLP-1 and glucose-dependent insulinotropic peptide receptor agonist (e.g., tirzepatide), and some bariatric procedures, decrease blood pressure. Some adrenergic anti-obesity agents may initially increase blood pressure (i.e., sympathomimetics such as phentermine) with possible longer-term reduction in blood pressure (compared to baseline) after weight reduction. |
| 10. | Unless the medication has blood pressure raising effects (i.e., some adrenergic agents), the degree of reduction with anti-obesity medications generally correlates to the degree of weight reduction. |
Fig. 1.
Obesity, adiposopathy (sick fat disease), fat mass disease, and obesity complications. An increase in body fat can ultimately lead to endocrine/metabolic and biomechanical/structural abnormalities, potentially leading to complications such as increased blood pressure [17].
3. Conceptually, how does the “fat mass disease” of obesity contribute to hypertension?
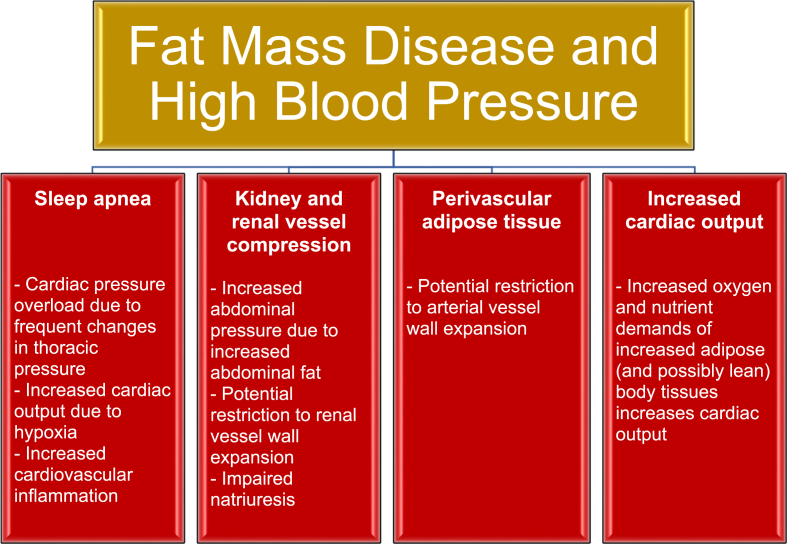
Fig. 2 illustrates how obesity may lead to fat mass disease. The increased body fat of obesity may result in pathogenic physical forces contributing to biomechanical/structural abnormalities, which in turn can lead to sleep apnea, kidney and renal vessel compression, restriction of arteriole wall expansion, and increased cardiac output – all that contribute to high blood pressure and the disease of hypertension [[8], [9], [10],18].
Fig. 2.
Fat mass disease and hypertension. Increased body fat leading to biomechanical and structural abnormalities (i.e., “fat mass disease”) may lead to hypertension through promotion of sleep apnea, kidney/renal vessel compression, perivascular adipose tissue, and increased cardiac output (heart rate x stroke volume) [[8], [9], [10],17,18].
4. Conceptually, how does the “sick fat disease” (adiposopathy) of obesity cause hypertension?
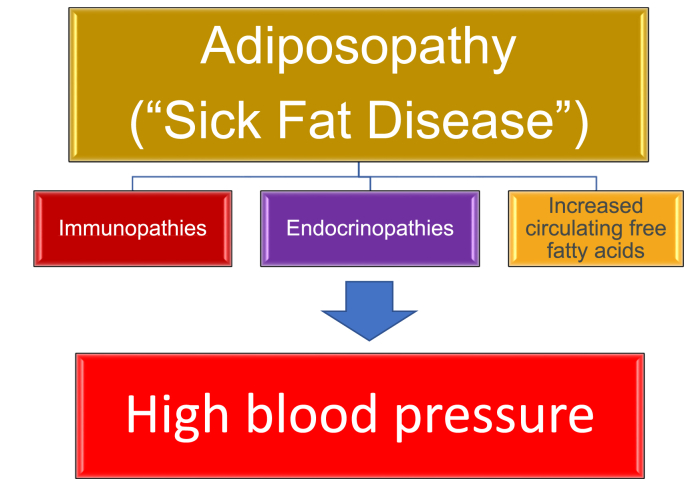
Independent of increased adiposity, high blood pressure can be caused by rare conditions such as pheochromocytoma, primary hyperaldosteronism, hypercortisolism, hyperthyroidism, renal artery stenosis, kidney diseases, side effects of concomitant therapies, and familial or genetic syndromes [7]. That said, increased body fat is by far the most common cause of hypertension. Estimates suggest the essential hypertension is attributable to increased adiposity in 78% in men and 65% in women [12,19] Other reports suggest up to 80% of essential hypertension is attributable to increased adiposity [20]. Anatomically, if during positive caloric balance proliferation and differentiation of adipose tissue is impaired in subcutaneous adipose tissue, then the energy overflow may result in fat deposition within and/or around other body tissues, such as the liver, muscle, and pancreas – potentially contributing to “fatty liver” and “fatty muscle” and lipotoxicity leading to insulin resistance [21]. This energy overflow may also increase fat deposition in the visceral region, as well as increase fat surrounding the blood vessels (i.e., perivascular fat), heart, and kidney [21]. An increase in the volume and activity of fat depots surrounding the arterial blood vessels, heart, and kidney may not only contribute to biomechanical dysfunction, but these fat depots may have pathogenic local and systemic immune and endocrine effects that contribute to high blood pressure [22]. In short, Fig. 3 illustrates how adiposopathic complications of obesity may lead to functional adiposopathy, or “sick fat disease,” resulting in immunopathies, endocrinopathies, and increased circulating free fatty acids, all that may contribute to high blood pressure [[8], [9], [10],18].
Fig. 3.
Adiposopathy and hypertension. Positive caloric balance, especially in an environment of limited adipocyte proliferation, often leads to dysfunctional adipocyte hypertrophy and adipose tissue accumulation (i.e., “sick fat disease”), which in turn leads to immunopathies, endocrinopathies, and increased circulating free fatty acids that may promote hypertension [[8], [9], [10],17,18].
5. Nutrition and blood pressure
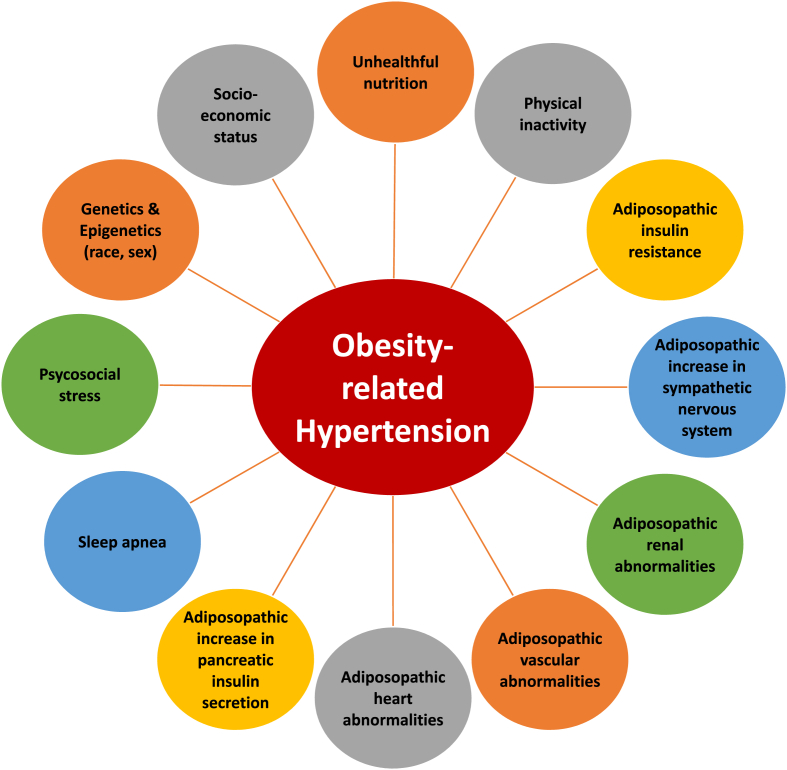
Fig. 4 describes various mechanisms accounting for obesity-related hypertension. Among these mechanisms includes unhealthful nutrition. Physiologically, eating stimulates both the anabolic parasympathetic nervous system [i.e., increases insulin secretion, increases peristalsis, increases gastrointestinal (GI) secretions] and catabolic sympathetic nervous system (promotes thermogenesis, decreases GI motility and secretions, helps maintain postprandial blood pressure through peripheral vasoconstriction to compensate for splanchnic vasodilation) [24,25]. Eating also increases blood flow to splanchnic vessels, increases release of vasoactive peptides, and causes gastric distention, which, along with effects upon autonomic nervous and baroreflex systems, can cause relative or absolute reduction in blood pressure (postprandial hypotension), increased heart rate (commonly detected by wearable heart rate technology after meals), and sometimes lightheadedness [24,26]. Postprandial hypotension may be misdiagnosed as “reactive hypoglycemia,” which has clinical relevance to patients who have undergone bariatric surgery [27]. Particularly in patients who experience significant reductions in blood pressure, when encountering a patient with lightheadedness after bariatric surgery, it is important to consider the potential of postprandial hypotension [28] in addition to the potential for postprandial hypoglycemia [29], as this might suggest the need to reduce or discontinue concomitant anti-hypertensive medications.
Fig. 4.
Obesity-related hypertension. Overweight and/or obesity may result in multiple mechanisms that help account for increased blood pressure.
Beyond the neurovascular effects of eating, unhealthful nutrition is an important driver of body fat accumulation and adipose tissue dysfunction [30]. In the absence of weight reduction among patients with pre-obesity or obesity, and compared to monounsaturated and polyunsaturated fats, increased saturated fat intake can increase blood pressure, possibly due to impaired arterial endothelial function [10]. Increased sodium intake can further increase blood pressure, especially among salt-sensitive patient populations [[31], [32], [33]]. Increased ultra-processed, refined carbohydrate intake may also increase blood pressure [34]. Specifically, the ultra-processing of food results in substantive changes to its matrices (i.e., highly degrades the food physical structure) that, especially when coupled with food additives and neo-formed contaminants, may not only affect absorption kinetics, satiety, glycemic response, and gut microbiota composition and function, but may also contribute to obesity, inflammation, oxidative stress, insulin resistance, and thus alterations in blood glucose, blood lipids, and blood pressure [35].
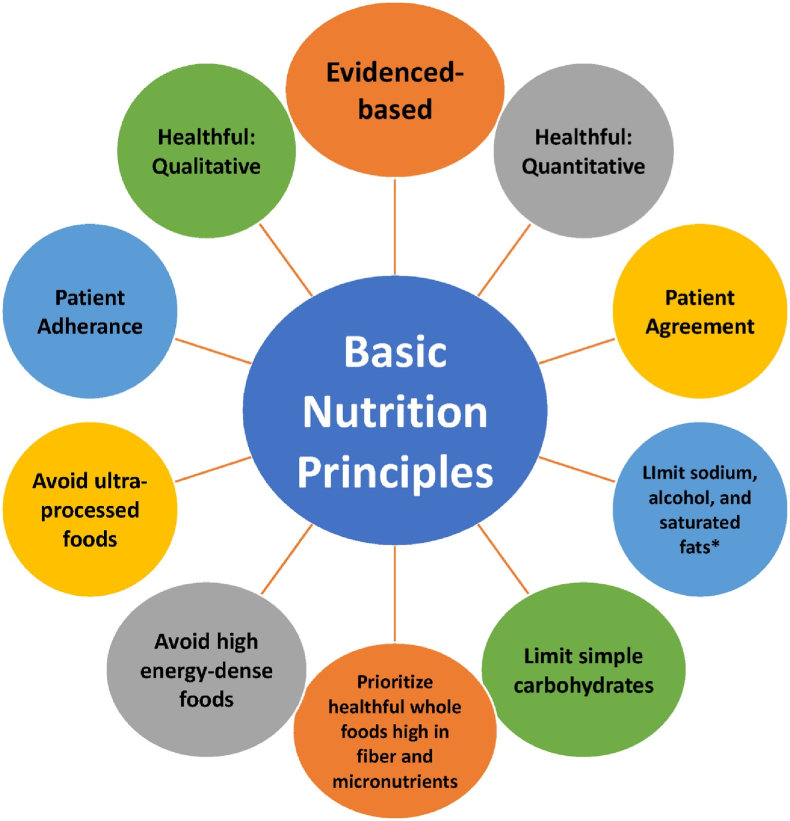
Fig. 5 illustrates healthful nutrition principles that may often improve hypertension, which are generally similar to nutritional recommendations regarding treatment of patients with increased body fat, increased blood sugars, and increased blood lipids [21]. In general, beyond the favorable blood pressure effects of a reduction in sodium intake and alcohol, and beyond the favorable effects of weight reduction alone, macronutrient consumption most associated with reduced blood pressure includes consumption of unprocessed, carbohydrate-rich diets that emphasize vegetables and fruits, low-fat dairy products, reduced saturated fat, total fat, and cholesterol, and replacement of refined/processed carbohydrate with either protein or with unsaturated fat [36].
Fig. 5.
Implementation of healthful nutrition may help treat metabolic abnormalities, such as high blood sugar, high blood lipids, and high blood pressure (Copied with permission from Ref. [21]).
6. Physical activity and blood pressure
Fig. 4 also illustrates physical inactivity as a contributor to both obesity and hypertension [37]. Routine physical activity has health benefits in improving body composition [38], facilitating weight reduction, and especially maintaining weight reduction [30]. In addition, regular physical activity may lower blood pressure, reduce cardiovascular risk, and improve cardiac remodeling. The cardiac remodeling from hypertension may result in pathogenic increases in myocyte hypertrophy, fibrosis, and risk of heart failure and mortality. Conversely, except for extreme physical exercise [39], the left ventricular hypertrophy in athletes is generally non-pathologic (i.e., lacks fibrosis). In patients with hypertension, physical activity may result in a favorable regression or prevention of left ventricular hypertrophy [40].
7. Adipose tissue, increased body fat, and hypertension
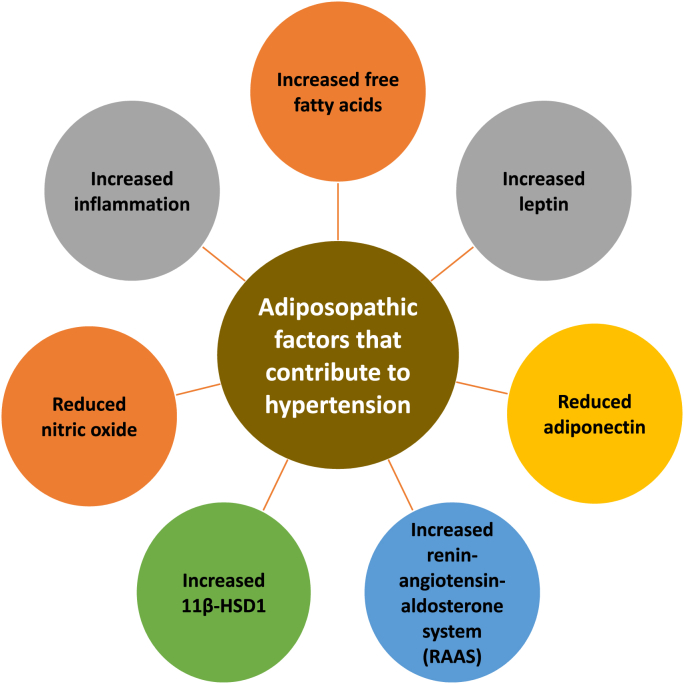
Fig. 6 describes adiposopathic factors that may contribute to high blood pressure. They include:
Fig. 6.
Illustrative adiposopathic factors that may contribute to hypertension. Beyond the adverse biomechanical effects of fat mass alone shown in Fig. 2, positive caloric balance may lead to adipocyte hypertrophy and adipose tissue accumulation with pathogenic adipose tissue (including perivascular adipose tissue) [23] endocrine and immune factors that may contribute to hypertension. 11β-HSD1: beta-hydroxysteroid dehydrogenase type 1.
7.1. Adiposopathy, insulin resistance, and pathogenic hormone & immune factors (Fig. 4, Fig. 6)
Positive caloric balance (especially when accompanied by physical inactivity and genetic predisposition) is often accompanied by pathogenic adipocyte hypertrophy and adipose tissue accumulation leading to adiposopathic endocrinopathies and immunopathies that may contribute to insulin resistance, type 2 diabetes mellitus, and hypertension [21] (Fig. 3). Hyperinsulinemia and insulin resistance may increase sodium retention [41,42], increase fluid volume [43], increase sympathetic nervous system activity (i.e., with increased vasoconstriction, increased heart rate and increased cardiac output) [44], reduce nitric oxide production (i.e., insulin is normally a vasodilator hormone; insulin resistance reduces vasodilation) [21], increase net inflammation (i.e., increased pro-inflammatory factors such as tumor necrosis factor and interleukin-6 and decreased anti-inflammatory factors such as adiponectin) [21]. These adverse consequences ofhyperinsulinemia and/or insulin resistance may contribute to vascular endothelial dysfunction and hypertension [45,46] (See Section 10). If adiposopathic insulin resistance leads to type 2 diabetes mellitus, then the development of diabetes nephropathy and renal dysfunction may exacerbate the inappropriate activation of the renin-angiotensin-aldosterone system and sympathetic nervous system, increase fluid overload, promote mitochondria dysfunction, excessive oxidative stress, and exacerbate systemic inflammation [47], leading to worsening high blood pressure [48].
7.2. Increased free fatty acids
Positive caloric balance in an environment of insufficient subcutaneous adipocyte proliferation often results in adipocyte hypertrophy and adipose tissue dysfunction. Subsequent adiposopathic endocrinopathies and immunopathies contribute to insulin resistance (Fig. 1, Fig. 3), with net increased release of free fatty acids into the circulation and potential fat deposition in the visceral region, liver, muscle, kidney, pancreas, and heart [21]. Increased circulating free fatty acids may contribute to endothelial dysfunction through impaired insulin signaling of nitric oxide production, oxidative stress [42], inflammation and the activation of the renin-angiotensin system and apoptosis in vascular endothelial cells [49]. Some saturated fatty acids may increase vascular inflammation (i.e., via cytokines such as tumor necrosis factor and interleukin-6) and oxidative stress [50,51], all contributing to increased blood pressure (See Fig. 6).
7.3. Increased leptin
Leptin is a peptide adipocytokine hormone primarily produced by adipocytes with anorexigenic signaling in the hypothalamus during times when the body has sufficient fat stores. Increased fat cell size increases leptin blood levels. It is hypothesized that through mechanisms such as activation of melanocortin receptors [42], leptin increases sympathetic nervous system activity to tissues such as the heart, blood vessels, and kidneys, resulting in increased cardiac output, increased vascular tone, and activation of the renin-angiotensin system, resulting in sodium retention and circulatory expansion – all leading to increased blood pressure [52,53] (See Fig. 6). Other ways in which increased leptin levels with obesity are proposed to contribute to high blood pressure include increased vascular stiffness due to vascular smooth muscle cell proliferation and migration via direct effects on the aorta as well as tunica media and adventitia of arteries and inside atherosclerotic plaques, activation of the immune system (both monocytes and T-cell), and generation of radical oxygen species [22].
7.4. Reduced adiponectin
Adiponectin is a peptide adipocytokine hormone produced primarily by adipose tissue. Adiponectin has anti-inflammatory effects, and enhances insulin sensitivity, reduces hepatic and muscle fat, and facilitates metabolism of lipids. Adiposopathic adipocyte hypertrophy and increased pro-inflammatory adipocytokine production reduces adiponectin production, such that obesity is often associated with reduced adiponectin levels [21]. Blood pressure may be increased when obesity-mediated reduced adiponectin levels limit adiponectin’s anti-inflammatory effects, increased vasodilatory nitric oxide production, suppression of sympathetic nervous system activity, and general improvement in endothelial function [54,55] (See Fig. 6).
7.5. Increased activation of the renin-angiotensin-aldosterone system (RAAS)
Angiotensinogen is mainly produced by the liver, with variably reported angiotensinogen production by larger adipose tissue depots such as subcutaneous adipose tissue [20,56,57], as well as smaller adipose tissue depots such as visceral adipose tissue [58] and perivascular adipose tissue [59]. Angiotensinogen levels are increased with obesity. Angiotensinogen is converted into angiotensin I by renal renin. Angiotensin I is converted to angiotensin II by angiotensin-converting enzyme (ACE). Angiotensin II has a role in oxidative stress, sympathetic activation [60], and is thus a vasoconstrictor that increases vascular peripheral resistance and increases blood pressure. Angiotensin II also stimulates the release of aldosterone, thus promoting sodium and water reabsorption by the kidneys. Just as angiotensin II can be produced by adipose tissue [20], aldosterone can also be produced by adipocytes in patients with obesity [61]. The increase in vascular vasoconstriction, coupled with increased water reabsorption increases blood pressure (See Fig. 6).
7.6. Increased 11 beta-hydroxysteroid dehydrogenase type 1 (11β-HSD1)
11β-HSD1 is an enzyme produced in adipose tissue (and liver) that converts inactive cortisone to active cortisol. Increased 11β-HSD1 activity with adipocyte hypertrophy and obesity may facilitate local glucocorticoid effects (“local Cushing’s syndrome”), even when circulating glucocorticoid levels are not elevated [21]. Among the adiposopathic consequences of increased local cortisol activity via increased 11β-HSD1 include increase lipolysis, increase lipotoxic release of free fatty acids, increase gluconeogenesis in the liver, and decrease glucose uptake in muscle. Specific to blood pressure, adiposopathic increases in 11β-HSD1 with obesity amplify glucocorticoid effects resulting in salt-sensitive hypertension [62] (See Fig. 6).
7.7. Reduced nitric oxide
Nitric oxide is synthesized by nitric oxide synthase in most all cell types, tissues, and organs in the human body [63]. This is especially so with the endothelial cells of blood vessels, where nitric oxide serves as a vaso-protective vasodilator. Endothelial nitric oxide synthase is also present in adipocytes and endothelial cells of perivascular adipose tissue [64]. Production of nitric oxide may be diminished by aging [65], oxidative stress caused by factors such as smoking [66], and excessive alcohol consumption [67], as well as factors that may concomitantly contribute to obesity, such as physical inactivity [68] and consumption of saturated fats (i.e., as opposed to unsaturated fats, vegetables, and fruits) [69,70]. Nitric oxide may also be decreased with obesity itself [71] via development of adiposopathic insulin resistance [21], immunopathies such as increased inflammatory factors [72] and reduced anti-inflammatory factors (i.e., adiponectin) [73]. Increased angiotensin II production is illustrative of an adiposopathic endocrinopathy that may promote oxidative stress and decrease endothelial nitric oxide function, leading to increased blood pressure [74,75] (See Fig. 6).
7.8. Increased inflammatory factors (e.g., tumor necrosis factor and interleukin 6)
Just as with their contribution to insulin resistance and type 2 diabetes mellitus [21], adipocytokines can contribute to high blood pressure. Leptin and adiponectin are examples of adipocytokines produced by adipose tissue, and their roles in contributing to high blood pressure were described in sections 7.2, 7.3. Two other adipocytokines that may be increased with obesity and often described to contribute to adiposopathic effects of obesity are tumor necrosis factor (TNF) and interleukin 6. TNF is an inflammatory adipocytokine produced by adipose tissue that may promote high blood pressure via increased production of angiotensinogen (See Section 7.4) [76], contribution to insulin resistance (See Section 7.1) [21], and worsening of endothelial dysfunction [45]. Interleukin-6 (IL-6) is another pro-inflammatory adipocytokine produced by adipose tissue that can also contribute to worsening endothelial function [45] and therefore may contribute to high blood pressure (See Fig. 6).
8. The brain, increased body fat, and hypertension
Physiologically, the central nervous system regulation of blood pressure (e.g., water and electrolyte balance, heart rate and baroreflex activity) occurs via sensory afferent signaling from organs such as the kidney, heart, and vasculature [77]. Similar afferent nervous system signaling can also occur from adipose tissue, representing an adipose tissue-brain-cardiovasculature pathway. As with afferent signaling from other body tissues, afferent signaling to the brain from adipose tissue can contribute to pathogenic efferent sympathetic responses [77]. From a hormone standpoint, increased body fat may lead to hyperleptinemia (and possibly hyperinsulinemia) which can lead to increase central nervous system (CNS) sympathetic activation among patients with obesity [77,78]. The effects of increased sympathetic nervous system signaling [8,9,18,24] include increased liver glycogenolysis and glucose production, increased skeletal muscle glucose uptake, and increased pancreatic insulin release. Regarding effects more directly related to blood pressure, increased sympathetic activation increases circulating free fatty acids (i.e., via increased adipocyte lipolysis), increases renin-angiotensin-aldosterone system activity, increases sodium and fluid retention, increases heart rate, increases cardiac output, increases arteriole vasoconstriction, increases peripheral arterial resistance, and decreased adiponectin, which are effects that may help facilitate high blood pressure [10,55,77,[79], [80], [81]] (See Fig. 4).
Another potential link between the brain and increased blood pressure is the melanocortin system [82]. Anorexigenic hormones or neurotransmitters such as leptin, insulin, and serotonin activate pro-opiomelanocortin neurons and inhibit Agouti-related peptide neurons. These effects activate melanocortin 4 receptors (MC4R) that signal reduced food intake and/or increased energy expenditure [83]. Melanocortin activation increases sympathetic nervous system activity and facilitates obesity-induced hypertension [84]. In fact, the potential to increase blood pressure represents challenges in developing MC4R agonists to promote weight reduction [84].
9. The kidneys, increased body fat, and hypertension
From a “fat mass disease” perspective, excessive intra‐abdominal and retroperitoneal fat may cause renal compression, which increases intrarenal pressure, decreases sodium excretion, and stimulates the renin-angiotensin-aldosterone system (RAAS) [85] (See Fig. 1, Fig. 2). From a “sick fat disease” perspective, “ectopic” adipose tissue surrounding the kidney may transmit lipotoxic immune and endocrine effects as the result of oxidative stress, mitochondrial dysfunction, and endoplasmic reticulum stress [22]. Systemically, adiposopathic release of adipocytokines may promote inflammation and enhance activity of the RAAS, increasing renal sodium and water reabsorption [86]. Increased adiposopathic sympathetic nervous system activity may further increase RAAS activity (See Section 8), which, in turn, further increases production of angiotensin II with circular increase in sympathetic nervous system activity [24,86], constriction of the efferent renal arterioles, increase in intraglomerular pressure, loss of nephrons, increase in renal tubular sodium reabsorption, and impairment of pressure natriuresis leading to fluid retention [42]. Another factor that may worsen obesity-related hypertension is adiposopathic chronic kidney disease due to causes beyond high blood pressure (i.e., kidney disease due to type 2 diabetes mellitus or obesity itself [87]). Thus, adiposopathic kidney disease is both a common cause of hypertension and a common complication of uncontrolled hypertension [88] (See Fig. 4).
10. The vasculature, increased body fat, and hypertension
Fig. 4 illustrates that adiposopathic vascular abnormalities represent another mechanism accounting for obesity-related hypertension. Endothelial dysfunction may occur due to pathogenic effects of obesity and increased perivascular adipose tissue on the inner lining of blood vessels, resulting in inflammation, resistance to vasodilation, impaired blood flow, and arterial stiffness [22,23,89], all potentially leading to high blood pressure. Other obesity-related factors that may contribute to endothelial dysfunction include insulin resistance, increased free fatty acids, increased leptin levels, decreased adiponectin levels, increased renin angiotensin aldosterone system activity, nitric oxide imbalance, and increase in pro-inflammatory factors and decrease in anti-inflammatory factors. Beyond obesity alone, endothelial dysfunction is also important in the pathogenesis of peripheral vascular disease, stroke, heart disease, diabetes, insulin resistance, chronic kidney failure, tumor growth, metastasis, venous thrombosis, and severe viral infectious diseases [90].
Increases in adiposopathic cytokine release increases mitochondrial stress (imbalance between reactive oxygen species [ROS] production and ROS clearance), increases ROS, and activates the RAAS [80,86]. Adiposopathy, inflammation (e.g., increased tumor necrosis factor & interleukin-6 with decreased adiponectin), lipotoxicity, food composition, lack of physical activity, and increases in RAAS can promote endothelial dysfunction and increased vasoconstriction and high blood pressure via [8,9,18,80,91,92]:
-
•
Increased angiotensinogen
-
•
Increased angiotensin II
-
•
Increased sympathetic nervous system activity
-
•
Increased endothelial vasoconstrictors
-
•
Decreased nitric oxide production
-
•
Decreased endothelia-dependent vasodilation
-
•
Increased arterial stiffness
11. The heart, increased body fat, and hypertension
Fig. 4 illustrates the heart as a contributor to obesity-related hypertension. Adverse consequences of obesity related to the heart that contribute to hypertension include increased blood volume, increased heart rate, increased cardiac output, and endothelial damage that may ultimately lead to heart failure [93], including heart failure with preserved ejection fraction [94].
Another way in which the heart may contribute to high blood pressure among patients with obesity is fluid retention. Natriuretic peptides are a group of hormones primarily produced by the heart, that regulate body fluid balance, blood pressure, and cardiovascular function. Atrial Natriuretic Peptide (ANP) is produced by the heart atria in response to increased blood volume and pressure, and functions to promote diuresis and natriuresis, thus promoting elimination of water and sodium, reducing blood volume, and lowering blood pressure. Brain Natriuretic Peptide (“B-type BNP”) is produced by the heart ventricles as the result of stretching of the heart muscle cells. BNP also promotes diuresis and natriuresis to reduce blood volume and blood pressure. Atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) are stimulated with increased ventricular filling [95]. Elevated active BNP and its inactive metabolite N-terminal pro B-type natriuretic peptide (NT-proBNP) help diagnose heart failure [95,96]. ANP and BMP have diuretic, natriuretic, and antihypertensive effects via inhibition of the RAAS [80]. While less so with ANP produced by atrial muscle cells, BNP produced by the left ventricle is decreased with obesity [95,97,98]. In fact, in patients with clinical heart failure, lower NT-proBNP levels in patients with overweight or obesity may not rule out diagnosis of HfpEF [96]. Loss of BNP natriuresis with obesity may contribute to sodium retention and increased blood pressure. Nutritional weight loss in patients with obesity may increase BNP [99].
12. The pancreas, increased body fat, insulin resistance, and hypertension
The adiposopathic endocrine and immune consequences of obesity often lead to insulin resistance and increased insulin secretion from the pancreas [21] (See Fig. 4). Insulin resistance represents a decrease in responsiveness of body tissues to the metabolic actions of insulin, such as a decrease in insulin-mediated glucose disposal [21,100]. A decreased in the responsiveness of the vasculature to insulin, and the decrease in nitric oxide production, may help promote vascular stiffness [100]. As with hyperleptinemia, the increased insulin blood levels with insulin resistance have variably been described to increase sympathetic nervous system activity and thus contribute to high blood pressure [78,101,102]. That said, hyperglycemia (and dyslipidemia) may act synergistically with hypertension to cause vascular and kidney injury, exacerbating hypertension [102] (See Fig. 4).
13. Sleep apnea and hypertension
Sleep apnea is a common complication of obesity. Sleep apnea is a potential contributor to hypertension [103] (See Fig. 4). Mechanisms that may help account for increased blood pressure with sleep apnea include pathogenic alterations in intrathoracic pressure, intermittent hypoxia, oxidative stress with increased reactive oxygen species, inflammation, neurohormonal dysregulation, increased sympathetic nervous system activation, vasoconstriction, endothelial dysfunction, and alterations in circadian rhythms [42,[104], [105], [106], [107]] (See Fig. 4).
14. Psychosocial stress and hypertension
As with depression, the relationship between obesity and psychosocial stress is bidirectional. Stress can contribute to obesity; obesity can contribute to stress [[108], [109], [110]]. Increase in acute mental stress may increase sympathetic nervous response, with the increased release of catecholamines leading to increased heart rate, increased cardiac output, vasoconstriction, and increase in blood pressure [111,112]. In some patients, chronic stress may also contribute to glucocorticoid excess (i.e., cortisol) [110] with potential increases in blood pressure [108] (See Fig. 4).
15. Genetics, epigenetics, sex, gender, race, ethnicity, obesity, and hypertension
With or without obesity, individuals have varying degrees of genetic susceptibility to hypertension [42]. Similarly, as with obesity itself, epigenetic factors may predispose to hypertension. Among the most applicable epigenetic alteration in gene expression [(i.e., without interfering with deoxynucleic acid (DNA) structure)] include: (a) DNA methylation, (b) histone modification, and (c) non-coding RNA [42,113].
It is suggested that hypertension has the highest impact on mortality of all pharmacologically modifiable cardiovascular risk factors [114]. Among those younger than 60 years of age, the prevalence of hypertension in males is higher than females, potentially related in part due to favorable effects of female estrogen on the vasculature and sympathetic nervous system [114]. Among those over 60 years of age, the prevalence of hypertension is higher in females than males [115]. As with males, an increase in body weight in females increases blood pressure [42], with 78% of new cases of “essential hypertension” in men and 65% in women reportedly attributable to excess body fat [116]. Regarding gender, gender affirming hormone therapy utilized by transgender individuals may have heterogenous responses, with unclear long-term effects on cardiovascular outcomes [117].
Race may also play a role in obesity and hypertension. Obesity increases cardiovascular disease risk factors in patients from South and East Asia [118,119]. Genetic predisposition to higher blood pressure may be an independent risk factor for hypertension and incident hypertension among those of Asian descent [120], with the caveat being that many of the identified genetic polymorphism markers of hypertension being derived from European and East Asian populations [121]. While some argue that race is a “social construct and not a defining biology [122],” African Americans and other people of African descent have a higher incidence of hypertension and related comorbidities compared to White individuals, with several identifiable genes helping to potentially account for racial differences regarding hypertension [123]. Regarding perceived racial discrimination and hypertension, the reported association is variable [[124], [125], [126], [127], [128]], with challenges regarding methodology of analyses, and likely most applicable when examined on an individual basis. Obesity also is also pathogenic among Latine individuals [129], who have high rates of cardiometabolic risk factors such as obesity, high blood glucose, high blood lipids, and high blood pressure [130]. Challenges exist in precisely characterizing unique genetic predispositions, due to the heterogeny in Latine individuals regarding race and ancestry, as well as variances in social composition and health-related behaviors [131].
16. Socio-economic status, obesity, and hypertension
Like obesity, lower socio-economic status is associated with higher rates of hypertension, especially among those with lower levels of education [132], and even within races [129,133]. Beyond lower education, factors that may contribute to higher blood pressure among socio-economically disadvantaged individuals include disparities resulting in less healthful nutrition (i.e., increased saturated fats and sodium) [134,135], less access to healthful nutrition (i.e., cost, food desserts) [136], lack of fruits and vegetables, less physical activity, more alcohol consumption, increased smoking, higher rates of dyslipidemia, increased psychosocial stress, discrimination, malnutrition in early childhood, poverty at older age, increased air pollution, reduced employment status, higher comorbid health conditions, and limitations to quality health care [133,137].
17. Treatment of obesity and hypertension
17.1. Medical nutrition therapy, physical activity, and behavior modification treatment of obesity
Nutritional intervention to reduce dietary sodium [138] and saturated fats may lower blood pressure (See Fig. 5), as typified by the Dietary Approaches to Stop Hypertension (DASH) [30]. DASH medical nutrition therapy effectively reduces blood pressure in patients with obesity, but may not necessarily promote clinically meaningful weight reduction [22]. However, when nutritional intervention, again such as the DASH diet, is accompanied by weight reduction management, and if clinically meaningful weight reduction is achieved, then the blood pressure lowering through nutritional intervention is substantially enhanced [22]. Dietary and moderate-intensity aerobic physical exercise may not only achieve clinically meaningful blood pressure reduction, but clinically meaningful weight reduction as well, with the caveat that the addition of moderate-intensity aerobic physical exercise to nutritional intervention may result in only marginal further reduction in body weight compared to nutritional intervention alone [22]. Overall, as noted with anti-obesity medications and bariatric surgery (See Table 2), the reported blood pressure reduction with healthful nutrition, physical activity, and/or behavior modification is widely variable, depending on the study, interventions, and duration of follow-up, with some reports demonstrating only modest blood pressure reduction, while other reporting blood pressure reductions as high as 16 mmHg systolic and 10 mmgHg diastolic [22].
Table 2.
Blood pressure and body weight changes compared to placebo after at least 1-year of treatment with anti-obesity medications approved for long-term treatment of obesity. [[11], [12], [13], [14],141,145,146] The listed blood pressures are not from head-to-head controlled clnical trials and only intended to provide a general overview of potentially expected blood pressure responses. Some of the reported values are pooled data, and may not be consistent with individual studies – especially those studies less than one year. The reported blood pressure reduction with bariatric surgery is heterogenous, depending on the study, procedure, and duration of follow-up with study suggesting an average of 9 mmHg reduction in systolic blood pressure, variable effects on diastolic blood pressure [147], and greater weight reduction associated with higher rates of hypertension remission [148].
| Anti-Obesity Medication | Systolic blood pressure (mmHg) | Diastolic blood pressure (mmHg) | % Weight reduction |
|---|---|---|---|
| Phentermine/topiramate | -3 | -1 | -9 |
| Naltrexone/bupropion | +1.5 | +1 | -4 |
| Orlistat | -1 | -1 | -3 |
| Liraglutide | -3 | -1 | -5 |
| Semaglutide | -5 | -2.4 | -12 |
| Tirzepatidea | -6 | -4 | -18% |
Tirzepatide is not approved for treatment of obesity at the time of this writing. Shown are the blood pressure effects of the tirzepatide 15 mg dose when studied in patients with obesity and without diabetes mellitus.
17.2. Bariatric surgery
Bariatric surgery (e.g., gastric bypass and sleeve gastrectomy) can produce clinically meaningful reduction in both blood pressure and body weight [22,139]. Fewer anti-hypertensive medications are typically required after bariatric surgery [140,141], with remission of hypertension after bariatric surgery reported as high as 50–75% [141,142]. Mechanistically, bariatric surgery may reduce RAAS activation, reduce systemic inflammation (i.e., with decreased vascular constriction, decreased arterial stiffness, and decreased sodium reabsorption), decrease insulin resistance (i.e., with decreased arterial stiffness), and decrease in sympathetic nervous system activation [143]. The reduced incidence of hypertension with bariatric surgery is consistent with the beneficial effects of bariatric surgery on other cardiometabolic health metrics, such as reduced incidence type 2 diabetes mellitus, reduced dyslipidemia, reduced ischemic heart disease, reduced cardiovascular mortality, and reduced rate of all-cause mortality [144]. The effects on blood pressure with bariatric surgery are widely variable, depending on the study, the procedure, and the duration of follow-up.
17.3. Anti-obesity medications
Anti-diabetes medications that reduce body weight generally reduce blood pressure [149]. Table 2 describes the blood pressure effects of anti-obesity medications. This table should be viewed with caution, in that the data does not represent blinded, randomized, controlled clinical trials directly comparing the listed anti-obesity medications. Phentermine is not listed because phentermine has not been evaluated in prospective, randomized clinical trials lasting one year or longer.
Phentermine is a sympathomimetic amine with adrenergic effects that is contraindicated in patients with cardiovascular disease (e.g., coronary artery disease, stroke, cardiac dysrhythmias, congestive heart failure, uncontrolled hypertension) [150]. In a 28 week study designed to evaluate phentermine/topiramate and its components, the phentermine 15 mg per day group experienced a placebo corrected 5% weight reduction, 1.7% systolic blood pressure reduction, and a 0.2% diastolic blood pressure reduction [151] This study is limited in that it was only 28 weeks (not a full year) and because the dose evaluated was phentermine 15 mg per day (with a more common prescribed dose being 37.5 mg per day) [141].
As can be seen from Table 2, many of the older anti-obesity medications have modest effects on both blood pressure and body weight. Phentermine/topiramate producesmore clinically meaningful weight reduction and blood pressure lowering than some of the older anti-obesity medications. Naltrexone (i.e., opioid antagonist [152]) has the potential to increase catecholamine concentrations [153]. Bupropion (i.e., inhibitor of neuronal reuptake of norepinephrine and dopamine used as an antidepressant and for smoking cessation [152]) can sometimes increase blood pressure [154]. The naltrexone/bupropion combination anti-obesity medication Prescribing Information lists increase in blood pressure and heart rate as a “Warning and Precaution” (https://contrave.com/storage/2022/02/Contrave_PI_CON-LC115.02_0222.pdf).
Among the more recent highly effective anti-obesity medications include semaglutide (i.e., a glucagon-like peptide 1 receptor agonist) and tirzepatide (a glucagon-like peptide 1 receptor agonist and glucose-dependent insulinotropic polypeptide receptor agonist, not FDA approved for obesity at the time of this writing) [155]. The Semaglutide Treatment Effects in People with Obesity (STEP) 1 trial was a 68-week trial in patients with overweight or obesity, but without diabetes mellitus. Body weight was reduced by ∼15% (with a 2.4% reduction in the placebo group) [145]. Semaglutide reduced placebo-corrected systolic blood pressure 5% and reduced diastolic blood pressure 2.4% [145]. Regarding tirzepatide, the SURMOUNT-1 study was a 72-week trial in patients with overweight or obesity, but without diabetes mellitus. In the tirzepatide 15 mg group, body weight was reduced by 21% (with a 3% reduction in the placebo group). Tirzepatide reduced placebo corrected reduction systolic blood pressure 6% and diastolic blood pressure 4.0% [146].
17.4. Obesity treatment effects on pulse/heart rate
Direct comparison obesity treatment effects on pulse/heart rate also presents challenges due to lack of comprehensive head-to-head clinical data, especially long-term clinical data. While weight reduction via healthful nutrition and physical activity seems to consistently improve heart rate variability [156], the reports of the effects of caloric restriction on heart rate are more variable, with results most often favoring a reduction in heart rate [157,158]. Both low energy diets and very low energy diets generally decrease heart rate (and decrease blood pressure) [159].
Regarding anti-obesity medications, in a chart review of 300 patients seen at a private weight management practice, phentermine treatment did not appear to increase heart rate (or blood pressure) after a mode of 52 weeks [160]. However, in a 28-week, randomized, head-to-head, controlled trial compared the combination of phentermine and topiramate extended-release with its components as monotherapies and with placebo adults with obesity, phentermine 15 mg increased heart rate by 1.1 beats per minute, phentermine HCL/topiramate extended release 15/92 mg decreased heart rate by 1.6 beats per minute, and placebo decreased heart rate 1.9 beats per minute. Thus, compared to placebo, phentermine 15 mg per day increased pulse rate 3.0 beats per minute while the heart rate effect of phentermine HCL/topiramate extended release 15/92 mg was generally similar to placebo; phentermine HCL/topiramate extended release 15/92 mg had greater weight loss than either placebo or phentermine alone [151]. In a randomized clinical trial, time-weighted average heart rate regarding the naltrexone/bupropion combination agent increase heart rate 0.7 beats/min in the naltrexone-bupropion group and 0.6 beats/min in the placebo group [161]. Orlistat may reduce resting heart rate 2 beats per minute [162], with a meta-analysis supporting that a weight loss of ≥10% associated with a significant reduction in heart rate, blood pressure, and systolic workload [163].
Glucagon-like peptide 1 receptor agonists may cause a small increase in heart rate. While some have suggested that glucagon-like peptide 1 receptor agonists may facilitate an imbalanced response of the autonomic nervous system (i.e., sympathetic relative to parasympathetic activity), the mechanisms are not definitively established [164]. According to their respective prescribing information, liraglutide for obesity may increase pulse rate 2–3 beats per minute (https://www.novo-pi.com/saxenda.pdf), semaglutide for obesity may increase pulse rate 1–4 beats per minute (https://www.novo-pi.com/wegovy.pdf), and tirzepatide for treatment of type 2 diabetes mellitus (not yet approved for obesity) may increase pulse rate 2–4 beats per minute (https://uspl.lilly.com/mounjaro/mounjaro.html#pi).
17.5. Medication treatment of hypertension in patients with obesity
Regarding the overall effect of anti-obesity therapies in patients with obesity-related, adiposopathic hypertension, healthful weight reduction often reduces blood pressure. Prior to introduction of some of the newer highly effective anti-obesity medications [155], the obesity treatment intervention most associated with longer term blood pressure control was bariatric surgery [148]. Longer-term clinical data will better define the longer-term blood pressure effects (and cardiovascular disease outcome effects) of more recent, novel anti-obesity medications [165].
Alternatively, regarding anti-hypertensive therapy body weight effects, some beta-blockers may increase body weight (e.g., propranolol, atenolol, and metoprolol). Peripheral alpha-1 antagonists [166], vasodilators such as hydralazine [167], and some older dihydropyridines calcium channel blockers such as nifedipine and amlodipine [16] may increase fluid retention, contribute to edema, and increase body weight. Non-dihydropyridines such as diltiazem and verapamil have less potential for edema (with edema also being common in patients with obesity) and thus these agents have less potential for weight gain [16]. Other anti-hypertensive medications less likely to result in weight gain include some beta blockers (i.e., carvedilol) [16,168], as well as diuretics (i.e., improvement in edema and congestive heart failure may result in weight loss) [169], angiotensin converting enzyme inhibitors [170], and angiotensin II receptor blockers [171].
18. Conclusions
This Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) on obesity and hypertension is designed to assist clinicians in better understanding the physiology and treatment of obesity. The information presented in this CPS may aid clinicians in improving the health and wellbeing of their patients with the disease of obesity and the adiposopathic complication of hypertension.
Transparency [172]
This manuscript was derived and edited from the 2021 Obesity Medicine Association (OMA) Obesity Algorithm. Beginning in 2013, OMA created and maintained an online Adult “Obesity Algorithm” (i.e., educational slides and eBook) that underwent yearly updates by OMA authors and was reviewed and approved annually by the OMA Board of Trustees.
Group composition
Over the years, the authors of the OMA Obesity Algorithm have represented a diverse range of clinicians, allied health professionals, clinical researchers, and academicians. The authors reflect a multidisciplinary and balanced group of experts in obesity science, patient evaluation, and clinical treatment.
Author contributions
TLC and AF reviewed and edited the manuscript. HEB served as the main medical writer, with initial assistance from another medical writer (see Acknowledgements).
Managing disclosures and dualities of interest
Potential dualities or conflicts of interest of the authors are listed in the Individual Disclosure section. Assistance of a medical writer paid by the Obesity Medicine Association is noted in the Acknowledgements section. Neither the prior OMA Obesity Algorithms, nor the publishing of this Clinical Practice Statement received outside funding. The authors of prior OMA Obesity Algorithms never received payment for their writing, editing, and publishing work. Authors of this Clinical Practice Statement likewise received no payment for their writing, editing, and publishing work. While listed journal Editors received payment for their roles as Editors, they did not receive payment for their participation as authors.
Individual Disclosures
TLC reports serving on speakers’ bureaus for Novo Nordisk and Eli Lilly.
AF reports serving on advisory boards for Novo Nordisk, Gelesis, Vivus, Jenny Craig, Found Health, Ms. Medicine, and Eli Lilly.
HEB’ research site institution has received research grants from 89Bio, Allergan, Alon Medtech/Epitomee, Altimmune, Amgen, Anji Pharma, AstraZeneca, Bionime, Boehringer Ingelheim, Eli Lilly, Esperion, Evidera, Fractyl, GlaxoSmithKline, HighTide, Home Access, Ionis, Kallyope, LG-Chem, Madrigal, Merck, New Amsterdam, Novartis, NovoNordisk, Pfizer, Satsuma, Selecta, Shionogi, TIMI, Viking, and Vivus. HEB has served as a consultant/advisor for 89Bio, Altimmune, Amgen, Boehringer Ingelheim, and Lilly.
Funding and acknowledgements
Initial medical writing support (funded by the Obesity Medicine Association) was provided by Savannah Logan, who adhered to Good Publication Practice (GPP3) guidelines and International Committee of Medical Journal Editors (ICMJE) recommendations. Otherwise, this manuscript received no funding.
Evidence
The content of the OMA Obesity Algorithm and this manuscript is supported by citations, which are listed in the References section.
Ethics review
This OMA Clinical Practice Statement manuscript was peer-reviewed and approved by the OMA Board of Trustee members prior to publication. Edits were made in response to reviewer comments and the final revised manuscript was approved by all the authors prior to publication. This submission did not involve human test subjects or volunteers.
Conclusions and recommendations
This Clinical Practice Statement is intended to be an educational tool that incorporates the current medical science and the clinical experiences of obesity specialists. The intent is to better facilitate and improve the clinical care and management of patients with pre-obesity and obesity. This Clinical Practice Statement should not be interpreted as “rules” and/or directives regarding the medical care of an individual patient. The decision regarding the optimal care of the patient with pre-obesity and obesity is best reliant upon a patient-centered approach, managed by the clinician tasked with directing an individual treatment plan that is in the best interest of the individual patient.
Updating
It is anticipated that sections of this Clinical Practice Statement may require future updates. The timing of such an update will depend on decisions made by Obesity Pillars Editorial team, with input from the OMA members and OMA Board of Trustees.
Disclaimer and limitations
Both the OMA Obesity Algorithms and this Clinical Practice Statement were developed to assist health care professionals in providing care for patients with pre-obesity and obesity based upon the best available evidence. In areas regarding inconclusive or insufficient scientific evidence, the authors used their professional judgment. This Clinical Practice Statement is intended to represent the state of obesity medicine at the time of publication. Thus, this Clinical Practice Statement is not a substitute for maintaining awareness of emerging new science. Finally, decisions by practitioners to apply the principles in this Clinical Practice Statement are best made by considering local resources, individual patient circumstances, patient agreement, and knowledge of federal, state, and local laws and guidance.
Declaration of AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) used Chat GPT to help list and categorize content. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Contributor Information
Tiffany Lowe Clayton, Email: lowepayne@campbell.edu.
Angela Fitch, Email: drfitch@knownwell.health.
Harold Edward Bays, Email: hbaysmd@outlook.com, http://www.lmarc.com.
References
- 1.Bays HE, McCarthy W, Burridge K, Tondt J, Karjoo S, Christensen S, Ng J, Golden A, Davisson L, Richardson L. Obesity Algorithm eBook, presented by the obesity medicine association. www.obesityalgorithm.org. 2021. https://obesitymedicine.org/obesity-algorithm/.
- 2.Bays H.E., Kulkarni A., German C., Satish P., Iluyomade A., Dudum R., et al. Ten things to know about ten cardiovascular disease risk factors - 2022. Am J Prev Cardiol. 2022;10 doi: 10.1016/j.ajpc.2022.100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzoni D. Masked hypertension: how to identify and when to treat? High Blood Pres Cardiovasc Prev. 2016;23:181–186. doi: 10.1007/s40292-016-0140-9. [DOI] [PubMed] [Google Scholar]
- 4.Choi E.J., Jeong D.W., Lee J.G., Lee S., Kim Y.J., Yi Y.H., et al. The impact of bladder distension on blood pressure in middle aged women. Korean J Fam Med. 2011;32:306–310. doi: 10.4082/kjfm.2011.32.5.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters G.L., Binder S.K., Campbell N.R. The effect of crossing legs on blood pressure: a randomized single-blind cross-over study. Blood Press Monit. 1999;vol. 4:97–101. [PubMed] [Google Scholar]
- 6.Siddiqui M., Calhoun D.A. Blood pressure measurement challenges in severely obese patients. Am J Hypertens. 2018;32:139–140. doi: 10.1093/ajh/hpy162. [DOI] [PubMed] [Google Scholar]
- 7.Bays H.E., Golden A., Tondt J. Thirty obesity myths, misunderstandings, and/or oversimplifications: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022;3 doi: 10.1016/j.obpill.2022.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bays H. Adiposopathy, "sick fat," Ockham's razor, and resolution of the obesity paradox. Curr Atherosclerosis Rep. 2014;16:409. doi: 10.1007/s11883-014-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bays H.E. Adiposopathy is "sick fat" a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 10.Landsberg L., Aronne L.J., Beilin L.J., Burke V., Igel L.I., Lloyd-Jones D., et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment--a position paper of the the Obesity Society and the American Society of Hypertension. Obesity. 2013;21:8–24. doi: 10.1002/oby.20181. [DOI] [PubMed] [Google Scholar]
- 11.Riaz H., Khan M.S., Siddiqi T.J., Usman M.S., Shah N., Goyal A., et al. Association between obesity and cardiovascular outcomes: a systematic review and meta-analysis of mendelian randomization studies. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leggio M., Lombardi M., Caldarone E., Severi P., D'Emidio S., Armeni M., et al. The relationship between obesity and hypertension: an updated comprehensive overview on vicious twins. Hypertens Res. 2017;40:947–963. doi: 10.1038/hr.2017.75. [DOI] [PubMed] [Google Scholar]
- 13.Bays H.E., Bazata D.D., Clark N.G., Gavin J.R., 3rd, Green A.J., Lewis S.J., et al. Prevalence of self-reported diagnosis of diabetes mellitus and associated risk factors in a national survey in the US population: SHIELD (Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes) BMC Publ Health. 2007;7:277. doi: 10.1186/1471-2458-7-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitch A., Alexander L., Brown C.F., Bays H.E. Comprehensive care for patients with obesity: an obesity medicine association position statement. Obesity Pillars. 2023;7 doi: 10.1016/j.obpill.2024.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis K.H., Fischer H., Ard J., Barton L., Bessesen D.H., Daley M.F., et al. Safety and effectiveness of longer-term phentermine use: clinical outcomes from an electronic health record cohort. Obesity. 2019;27:591–602. doi: 10.1002/oby.22430. [DOI] [PubMed] [Google Scholar]
- 16.Tondt J., Bays H.E. Concomitant medications, functional foods, and supplements: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitch A.K., Bays H.E. Obesity definition, diagnosis, bias, standard operating procedures (SOPs), and telehealth: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bays H., Ballantyne C. Adiposopathy: why do adiposity and obesity cause metabolic disease? Future Lipidol. 2006;1:389–420. [Google Scholar]
- 19.Aronow W.S. Association of obesity with hypertension. Ann Transl Med. 2017;5:350. doi: 10.21037/atm.2017.06.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schütten M.T.J., Houben A.J.H.M., Leeuw PWd, Stehouwer C.D.A. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology. 2017;32:197–209. doi: 10.1152/physiol.00037.2016. [DOI] [PubMed] [Google Scholar]
- 21.Bays H.E., Bindlish S., Clayton T.L. Obesity, diabetes mellitus, and cardiometabolic risk: an obesity medicine association (OMA) clinical practice statement (CPS) 2023. Obesity Pillars. 2023;5 doi: 10.1016/j.obpill.2023.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fantin F., Giani A., Zoico E., Rossi A.P., Mazzali G., Zamboni M. Weight loss and hypertension in obese subjects. Nutrients. 2019;11 doi: 10.3390/nu11071667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saxton S.N., Clark B.J., Withers S.B., Eringa E.C., Heagerty A.M. Mechanistic links between obesity, diabetes, and blood pressure: role of perivascular adipose tissue. Physiol Rev. 2019;99:1701–1763. doi: 10.1152/physrev.00034.2018. [DOI] [PubMed] [Google Scholar]
- 24.Thorp A.A., Schlaich M.P. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J Diabetes Res. 2015;2015 doi: 10.1155/2015/341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim K., Burke S.L., Head G.A. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension. 2013;61:628–634. doi: 10.1161/HYPERTENSIONAHA.111.00705. [DOI] [PubMed] [Google Scholar]
- 26.Trahair L.G., Horowitz M., Jones K.L. Postprandial hypotension: a systematic review. J Am Med Dir Assoc. 2014;15:394–409. doi: 10.1016/j.jamda.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen N.Q., Debreceni T.L., Burgstad C.M., Wishart J.M., Bellon M., Rayner C.K., et al. Effects of posture and meal volume on gastric emptying, intestinal transit, oral glucose tolerance, blood pressure and gastrointestinal symptoms after roux-en-Y gastric bypass. Obes Surg. 2015;25:1392–1400. doi: 10.1007/s11695-014-1531-4. [DOI] [PubMed] [Google Scholar]
- 28.Luciano G.L., Brennan M.J., Rothberg M.B. Postprandial hypotension. Am J Med. 2010;123:281.e1–281.e6. doi: 10.1016/j.amjmed.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Salehi M., Vella A., McLaughlin T., Patti M.E. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metabol. 2018;103:2815–2826. doi: 10.1210/jc.2018-00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander L., Christensen S.M., Richardson L., Ingersoll A.B., Burridge K., Golden A., et al. Nutrition and physical activity: an obesity medicine association (OMA) clinical practice statement 2022. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grillo A., Salvi L., Coruzzi P., Salvi P., Parati G. Sodium intake and hypertension. Nutrients. 2019;11 doi: 10.3390/nu11091970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rust P., Ekmekcioglu C. Impact of salt intake on the pathogenesis and treatment of hypertension. Adv Exp Med Biol. 2017;956:61–84. doi: 10.1007/5584_2016_147. [DOI] [PubMed] [Google Scholar]
- 33.Farquhar W.B., Edwards D.G., Jurkovitz C.T., Weintraub W.S. Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol. 2015;65:1042–1050. doi: 10.1016/j.jacc.2014.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiNicolantonio J.J., Lucan S.C. The wrong white crystals: not salt but sugar as aetiological in hypertension and cardiometabolic disease. Open heart. 2014;1 doi: 10.1136/openhrt-2014-000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juul F., Vaidean G., Lin Y., Deierlein A.L., Parekh N. Ultra-processed foods and incident cardiovascular disease in the framingham offspring study. J Am Coll Cardiol. 2021;77:1520–1531. doi: 10.1016/j.jacc.2021.01.047. [DOI] [PubMed] [Google Scholar]
- 36.Miller E.R., Erlinger T.P., Appel L.J. The effects of macronutrients on blood pressure and lipids: an overview of the DASH and OmniHeart trials. Current Cardiovascul Risk Reports. 2007;1:46–51. doi: 10.1007/s11883-006-0020-1. [DOI] [PubMed] [Google Scholar]
- 37.Purba E.N., Santosa H., Siregar F.A. The relationship of physical activity and obesity with the incidence of hypertension in adults aged 26-45 Years in medan. Open Access Maced J Med Sci. 2019;7:3464–3468. doi: 10.3889/oamjms.2019.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burridge K., Christensen S.M., Golden A., Ingersoll A.B., Tondt J., Bays H.E. Obesity history, physical exam, laboratory, body composition, and energy expenditure: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharalaya Z., Phelan D. Cardiac risk of extreme exercise. Sports Med Arthrosc Rev. 2019;27:e1–e7. doi: 10.1097/JSA.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 40.Hegde S.M., Solomon S.D. Influence of physical activity on hypertension and cardiac structure and function. Curr Hypertens Rep. 2015;17:77. doi: 10.1007/s11906-015-0588-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brosolo G., Da Porto A., Bulfone L., Vacca A., Bertin N., Scandolin L., et al. Insulin resistance and high blood pressure: mechanistic insight on the role of the kidney. Biomedicines. 2022;10 doi: 10.3390/biomedicines10102374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Meouchy P., Wahoud M., Allam S., Chedid R., Karam W., Karam S. Hypertension related to obesity: pathogenesis, characteristics and factors for control. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232012305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarafidis P.A., Bakris G.L. The antinatriuretic effect of insulin: an unappreciated mechanism for hypertension associated with insulin resistance? Am J Nephrol. 2007;27:44–54. doi: 10.1159/000098955. [DOI] [PubMed] [Google Scholar]
- 44.Valensi P. Autonomic nervous system activity changes in patients with hypertension and overweight: role and therapeutic implications. Cardiovasc Diabetol. 2021;20:170. doi: 10.1186/s12933-021-01356-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwaifa I.K., Bahari H., Yong Y.K., Noor S.M. Endothelial dysfunction in obesity-induced inflammation: molecular mechanisms and clinical implications. Biomolecules. 2020;10 doi: 10.3390/biom10020291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Usui I. Common metabolic features of hypertension and type 2 diabetes. Hypertens Res : Off J Japanese Societ Hypert. 2023;46:1227–1233. doi: 10.1038/s41440-023-01233-x. [DOI] [PubMed] [Google Scholar]
- 47.Jia G., Sowers J.R. Hypertension in diabetes: an update of basic mechanisms and clinical disease. Hypertension. 2021;78:1197–1205. doi: 10.1161/HYPERTENSIONAHA.121.17981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Buren P.N., Toto R. Hypertension in diabetic nephropathy: epidemiology, mechanisms, and management. Adv Chron Kidney Dis. 2011;18:28–41. doi: 10.1053/j.ackd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghosh A., Gao L., Thakur A., Siu P.M., Lai C.W.K. Role of free fatty acids in endothelial dysfunction. J Biomed Sci. 2017;24:50. doi: 10.1186/s12929-017-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joffe Y.T., Collins M., Goedecke J.H. The relationship between dietary fatty acids and inflammatory genes on the obese phenotype and serum lipids. Nutrients. 2013;5:1672–1705. doi: 10.3390/nu5051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall W.L. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr Res Rev. 2009;22:18–38. doi: 10.1017/S095442240925846X. [DOI] [PubMed] [Google Scholar]
- 52.Lu S.C., Akanji A.O. Leptin, obesity, and hypertension: a review of pathogenetic mechanisms. Metab Syndr Relat Disord. 2020;18:399–405. doi: 10.1089/met.2020.0065. [DOI] [PubMed] [Google Scholar]
- 53.Guyenet P.G., Stornetta R.L., Souza G., Abbott S.B.G., Brooks V.L. Neuronal networks in hypertension: recent advances. Hypertension. 2020;76:300–311. doi: 10.1161/HYPERTENSIONAHA.120.14521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabaratnam R., Svenningsen P. Adipocyte-Endothelium crosstalk in obesity. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.681290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim D.H., Kim C., Ding E.L., Townsend M.K., Lipsitz L.A. Adiponectin levels and the risk of hypertension: a systematic review and meta-analysis. Hypertension. 2013;62:27–32. doi: 10.1161/HYPERTENSIONAHA.113.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cassis L.A., Police S.B., Yiannikouris F., Thatcher S.E. Local adipose tissue renin-angiotensin system. Curr Hypertens Rep. 2008;10:93–98. doi: 10.1007/s11906-008-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koizumi M., Niimura F., Fukagawa M., Matsusaka T. Adipocytes do not significantly contribute to plasma angiotensinogen. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2016;17 doi: 10.1177/1470320316672348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y., Somers K.R., Becari C., Polonis K., Pfeifer M.A., Allen A.M., et al. Comparative expression of renin-angiotensin pathway proteins in visceral versus subcutaneous fat. Front Physiol. 2018;9:1370. doi: 10.3389/fphys.2018.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stanek A., Brożyna-Tkaczyk K., Myśliński W. The role of obesity-induced perivascular adipose tissue (PVAT) dysfunction in vascular homeostasis. Nutrients. 2021;13 doi: 10.3390/nu13113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masi S., Uliana M., Virdis A. Angiotensin II and vascular damage in hypertension: role of oxidative stress and sympathetic activation. Vasc Pharmacol. 2019;115:13–17. doi: 10.1016/j.vph.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Dinh Cat A.N., Friederich-Persson M., White A., Touyz R.M. Adipocytes, aldosterone and obesity-related hypertension. J Mol Endocrinol. 2016;57:F7–f21. doi: 10.1530/JME-16-0025. [DOI] [PubMed] [Google Scholar]
- 62.Bailey M.A. 11β-Hydroxysteroid dehydrogenases and hypertension in the metabolic syndrome. Curr Hypertens Rep. 2017;19:100. doi: 10.1007/s11906-017-0797-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tenopoulou M., Doulias P.T. Endothelial nitric oxide synthase-derived nitric oxide in the regulation of metabolism. F1000Research. 2020;9 doi: 10.12688/f1000research.19998.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Man A.W.C., Zhou Y., Xia N., Li H. Endothelial nitric oxide synthase in the perivascular adipose tissue. Biomedicines. 2022;10 doi: 10.3390/biomedicines10071754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shannon O.M., Clifford T., Seals D.R., Craighead D.H., Rossman M.J. Nitric oxide, aging and aerobic exercise: sedentary individuals to Master's athletes. Nitric Oxide. 2022;125–126:31–39. doi: 10.1016/j.niox.2022.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Messner B., Bernhard D. Smoking and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 67.Toda N., Ayajiki K. Vascular actions of nitric oxide as affected by exposure to alcohol. Alcohol Alcohol. 2010;45:347–355. doi: 10.1093/alcalc/agq028. [DOI] [PubMed] [Google Scholar]
- 68.van Niekerk E., Botha Le Roux S., Atzler D., Schwedhelm E., Böger R.H., van Rooyen J.M., et al. Blood pressure and nitric oxide synthesis capacity in physically active and inactive groups: the SABPA study. J Hum Hypertens. 2021;35:325–333. doi: 10.1038/s41371-020-0344-2. [DOI] [PubMed] [Google Scholar]
- 69.Martins M.A., Catta-Preta M., Mandarim-de-Lacerda C.A., Aguila M.B., Brunini T.C., Mendes-Ribeiro A.C. High fat diets modulate nitric oxide biosynthesis and antioxidant defence in red blood cells from C57BL/6 mice. Arch Biochem Biophys. 2010;499:56–61. doi: 10.1016/j.abb.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 70.Kobayashi J., Ohtake K., Uchida H. NO-rich diet for lifestyle-related diseases. Nutrients. 2015;7:4911–4937. doi: 10.3390/nu7064911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sansbury B.E., Hill B.G. Regulation of obesity and insulin resistance by nitric oxide. Free Radic Biol Med. 2014;73:383–399. doi: 10.1016/j.freeradbiomed.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aghamohammadzadeh R., Unwin R.D., Greenstein A.S., Heagerty A.M. Effects of obesity on perivascular adipose tissue vasorelaxant function: nitric oxide, inflammation and elevated systemic blood pressure. J Vasc Res. 2016;52:299–305. doi: 10.1159/000443885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Achari A.E., Jain S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ding J., Yu M., Jiang J., Luo Y., Zhang Q., Wang S., et al. Angiotensin II decreases endothelial nitric oxide synthase phosphorylation via AT(1)R nox/ROS/PP2A pathway. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.566410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou M.S., Schulman I.H., Raij L. Nitric oxide, angiotensin II, and hypertension. Semin Nephrol. 2004;24:366–378. doi: 10.1016/j.semnephrol.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 76.Pausova Z., Deslauriers B., Gaudet D., Tremblay J., Kotchen T.A., Larochelle P., et al. Role of tumor necrosis factor-α gene locus in obesity and obesity-associated hypertension in French Canadians. Hypertension. 2000;36:14–19. doi: 10.1161/01.hyp.36.1.14. [DOI] [PubMed] [Google Scholar]
- 77.Dalmasso C., Leachman J.R., Osborn J.L., Loria A.S. Sensory signals mediating high blood pressure via sympathetic activation: role of adipose afferent reflex. Am J Physiol Regul Integr Comp Physiol. 2020;318 doi: 10.1152/ajpregu.00079.2019. R379-r89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Russo B., Menduni M., Borboni P., Picconi F., Frontoni S. Autonomic nervous system in obesity and insulin-resistance—the complex interplay between leptin and central nervous system. Int J Mol Sci. 2021;22:5187. doi: 10.3390/ijms22105187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rossi R.C., Vanderlei L.C.M., Gonçalves A.C.C.R., Vanderlei F.M., Bernardo A.F.B., Yamada K.M.H., et al. Impact of obesity on autonomic modulation, heart rate and blood pressure in obese young people. Auton Neurosci. 2015;193:138–141. doi: 10.1016/j.autneu.2015.07.424. [DOI] [PubMed] [Google Scholar]
- 80.Kawarazaki W., Fujita T. The role of aldosterone in obesity-related hypertension. Am J Hypertens. 2016;29:415–423. doi: 10.1093/ajh/hpw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.von Schnurbein J., Manzoor J., Brandt S., Denzer F., Kohlsdorf K., Fischer-Posovszky P., et al. Leptin is not essential for obesity-associated hypertension. Obes Facts. 2019;12:460–475. doi: 10.1159/000501319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bays H. The melanocortin system as a therapeutic treatment target for adiposity and adiposopathy. Drugs R. 2006;7:289–302. doi: 10.2165/00126839-200607050-00002. [DOI] [PubMed] [Google Scholar]
- 83.Yang Y., Xu Y. The central melanocortin system and human obesity. J Mol Cell Biol. 2020;12:785–797. doi: 10.1093/jmcb/mjaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.da Silva A.A., do Carmo J.M., Wang Z., Hall J.E. Melanocortin-4 receptors and sympathetic nervous system activation in hypertension. Curr Hypertens Rep. 2019;21:46. doi: 10.1007/s11906-019-0951-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chrysant S.G. Pathophysiology and treatment of obesity-related hypertension. J Clin Hypertens. 2019;21:555–559. doi: 10.1111/jch.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cabandugama P.K., Gardner M.J., Sowers J.R. The renin angiotensin aldosterone system in obesity and hypertension: roles in the cardiorenal metabolic syndrome. Med Clin. 2017;101:129–137. doi: 10.1016/j.mcna.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kovesdy C.P., Furth S.L., Zoccali C., World Kidney Day Steering C. Obesity and kidney disease: hidden consequences of the epidemic. Can J Kidney Health Dis. 2017;4 doi: 10.1177/2054358117698669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamrahian S.M., Falkner B. Hypertension in chronic kidney disease. Adv Exp Med Biol. 2017;956:307–325. doi: 10.1007/5584_2016_84. [DOI] [PubMed] [Google Scholar]
- 89.Aroor A.R., Jia G., Sowers J.R. Cellular mechanisms underlying obesity-induced arterial stiffness. Am J Physiol Regul Integr Comp Physiol. 2018;314 doi: 10.1152/ajpregu.00235.2016. R387-r98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rajendran P., Rengarajan T., Thangavel J., Nishigaki Y., Sakthisekaran D., Sethi G., et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barton M., Baretella O., Meyer M.R. Obesity and risk of vascular disease: importance of endothelium-dependent vasoconstriction. Br J Pharmacol. 2012;165:591–602. doi: 10.1111/j.1476-5381.2011.01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Engin A. Endothelial dysfunction in obesity. Adv Exp Med Biol. 2017;960:345–379. doi: 10.1007/978-3-319-48382-5_15. [DOI] [PubMed] [Google Scholar]
- 93.Ashraf M.J., Baweja P. Obesity: the 'huge' problem in cardiovascular diseases. Mo Med. 2013;110:499–504. [PMC free article] [PubMed] [Google Scholar]
- 94.Redfield M.M., Borlaug B.A. Heart failure with preserved ejection fraction: a review. JAMA, J Am Med Assoc. 2023;329:827–838. doi: 10.1001/jama.2023.2020. [DOI] [PubMed] [Google Scholar]
- 95.Khalid U., Wruck L.M., Quibrera P.M., Bozkurt B., Nambi V., Virani S.S., et al. BNP and obesity in acute decompensated heart failure with preserved vs. reduced ejection fraction: the Atherosclerosis Risk in Communities Surveillance Study. Int J Cardiol. 2017;233:61–66. doi: 10.1016/j.ijcard.2017.01.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buckley L.F., Canada J.M., Del Buono M.G., Carbone S., Trankle C.R., Billingsley H., et al. Low NT-proBNP levels in overweight and obese patients do not rule out a diagnosis of heart failure with preserved ejection fraction. ESC Heart Fail. 2018;5:372–378. doi: 10.1002/ehf2.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bachmann K.N., Gupta D.K., Xu M., Brittain E., Farber-Eger E., Arora P., et al. Unexpectedly low natriuretic peptide levels in patients with heart failure. JACC Heart Fail. 2021;9:192–200. doi: 10.1016/j.jchf.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCord J., Mundy B.J., Hudson M.P., Maisel A.S., Hollander J.E., Abraham W.T., et al. Relationship between obesity and B-type natriuretic peptide levels. Arch Intern Med. 2004;164:2247–2252. doi: 10.1001/archinte.164.20.2247. [DOI] [PubMed] [Google Scholar]
- 99.Kistorp C., Bliddal H., Goetze J.P., Christensen R., Faber J. Cardiac natriuretic peptides in plasma increase after dietary induced weight loss in obesity. BMC Obes. 2014;1:24. doi: 10.1186/s40608-014-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hill M.A., Yang Y., Zhang L., Sun Z., Jia G., Parrish A.R., et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metab, Clin Exp. 2021;119 doi: 10.1016/j.metabol.2021.154766. [DOI] [PubMed] [Google Scholar]
- 101.Emdin M., Gastaldelli A., Muscelli E., Macerata A., Natali A., Camastra S., et al. Hyperinsulinemia and autonomic nervous system dysfunction in obesity. Circulation. 2001;103:513–519. doi: 10.1161/01.cir.103.4.513. [DOI] [PubMed] [Google Scholar]
- 102.da Silva A.A., do Carmo J.M., Li X., Wang Z., Mouton A.J., Hall J.E. Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Can J Cardiol. 2020;36:671–682. doi: 10.1016/j.cjca.2020.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pennings N., Golden L., Yashi K., Tondt J., Bays H.E. Sleep-disordered breathing, sleep apnea, and other obesity-related sleep disorders: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars. 2022;4 doi: 10.1016/j.obpill.2022.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brown J., Yazdi F., Jodari-Karimi M., Owen J.G., Reisin E. Obstructive sleep apnea and hypertension: updates to a critical relationship. Curr Hypertens Rep. 2022;24:173–184. doi: 10.1007/s11906-022-01181-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Prabhakar N.R., Peng Y.J., Nanduri J. Hypoxia-inducible factors and obstructive sleep apnea. J Clin Invest. 2020;130:5042–5051. doi: 10.1172/JCI137560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Y.E., Ren J. Association between obstructive sleep apnea and cardiovascular diseases. Acta Biochim Biophys Sin. 2022;54:882–892. doi: 10.3724/abbs.2022084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Makarem N., Alcántara C., Williams N., Bello N.A., Abdalla M. Effect of sleep disturbances on blood pressure. Hypertension. 2021;77:1036–1046. doi: 10.1161/HYPERTENSIONAHA.120.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van der Valk E.S., Savas M., van Rossum E.F.C. Stress and obesity: are there more susceptible individuals? Curr Obes Rep. 2018;7:193–203. doi: 10.1007/s13679-018-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Osborne M.T., Shin L.M., Mehta N.N., Pitman R.K., Fayad Z.A., Tawakol A. Disentangling the links between psychosocial stress and cardiovascular disease. Circ Cardiovasc Imaging. 2020;13 doi: 10.1161/CIRCIMAGING.120.010931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Christensen S.M., Varney C., Gupta V., Wenz L., Bays H.E. Stress, psychiatric disease, and obesity: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022;4 doi: 10.1016/j.obpill.2022.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spruill T.M. Chronic psychosocial stress and hypertension. Curr Hypertens Rep. 2010;12:10–16. doi: 10.1007/s11906-009-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ayada C., Toru Ü., Korkut Y. The relationship of stress and blood pressure effectors. Hippokratia. 2015;19:99–108. [PMC free article] [PubMed] [Google Scholar]
- 113.Bays H., Scinta W. Adiposopathy and epigenetics: an introduction to obesity as a transgenerational disease. Curr Med Res Opin. 2015;31:2059–2069. doi: 10.1185/03007995.2015.1087983. [DOI] [PubMed] [Google Scholar]
- 114.Regensteiner J.G., Reusch J.E.B. Sex differences in cardiovascular consequences of hypertension, obesity, and diabetes: JACC focus seminar 4/7. J Am Coll Cardiol. 2022;79:1492–1505. doi: 10.1016/j.jacc.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garcia M., Mulvagh S.L., Merz C.N., Buring J.E., Manson J.E. Cardiovascular disease in women: clinical perspectives. Circ Res. 2016;118:1273–1293. doi: 10.1161/CIRCRESAHA.116.307547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shariq O.A., McKenzie T.J. Obesity-related hypertension: a review of pathophysiology, management, and the role of metabolic surgery. Gland Surg. 2020;9:80–93. doi: 10.21037/gs.2019.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Connelly P.J., Clark A., Touyz R.M., Delles C. Transgender adults, gender-affirming hormone therapy and blood pressure: a systematic review. J Hypertens. 2021;39:223–230. doi: 10.1097/HJH.0000000000002632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bays H.E., Shrestha A., Niranjan V., Khanna M., Kambhamettu L. Obesity pillars roundtable: obesity and South asians. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bays H.E., Ng J., Sicat J., Look M. Obesity pillars roundtable: obesity and East asians. Obesity Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu X., Huang J., Wang L., Chen S., Yang X., Li J., et al. Genetic predisposition to higher blood pressure increases risk of incident hypertension and cardiovascular diseases in Chinese. Hypertension. 2015;66:786–792. doi: 10.1161/HYPERTENSIONAHA.115.05961. [DOI] [PubMed] [Google Scholar]
- 121.Gupta R. In: Hypertension and cardiovascular disease in Asia. Ram C.V.S., Teo B.W.J., Wander G.S., editors. Springer International Publishing; Cham: 2022. Relation of genetics and obesity with hypertension: an asian perspective; pp. 77–90. [Google Scholar]
- 122.Bays H.E., Gonsahn-Bollie S., Younglove C., Wharton S. Obesity pillars roundtable: body mass index and body composition in black and female individuals. Race-relevant or racist? Sex-relevant or sexist? Obesity Pillars. 2022 doi: 10.1016/j.obpill.2022.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zilbermint M., Hannah-Shmouni F., Stratakis C.A. Genetics of hypertension in african Americans and others of african descent. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gabriel A.C., Bell C.N., Bowie J.V., Hines A.L., LaVeist T.A., Thorpe R.J., Jr. The association between perceived racial discrimination and hypertension in a low-income, racially integrated urban community. Fam Community Health. 2020;43:93–99. doi: 10.1097/FCH.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 125.Couto P.F., Goto J.B., Bastos J.L. Blood pressure and interpersonal discrimination: systematic review of epidemiologic studies. Arq Bras Cardiol. 2012;99:956–963. doi: 10.1590/s0066-782x2012005000090. [DOI] [PubMed] [Google Scholar]
- 126.Brondolo E., Libby D.J., Denton E.G., Thompson S., Beatty D.L., Schwartz J., et al. Racism and ambulatory blood pressure in a community sample. Psychosom Med. 2008;70:49–56. doi: 10.1097/PSY.0b013e31815ff3bd. [DOI] [PubMed] [Google Scholar]
- 127.Steffen P.R., McNeilly M., Anderson N., Sherwood A. Effects of perceived racism and anger inhibition on ambulatory blood pressure in African Americans. Psychosom Med. 2003;65:746–750. doi: 10.1097/01.psy.0000079380.95903.78. [DOI] [PubMed] [Google Scholar]
- 128.Dolezsar C.M., McGrath J.J., Herzig A.J.M., Miller S.B. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol. 2014;33:20–34. doi: 10.1037/a0033718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bays H.E., Muñoz-Mantilla D.X., Morgan R., Nwizu C., Garcia T.T. Obesity pillars roundtable: obesity and diversity. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gomez S., Blumer V., Rodriguez F. Unique cardiovascular disease risk factors in hispanic individuals. Curr Cardiovasc Risk Rep. 2022;16:53–61. doi: 10.1007/s12170-022-00692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Elfassy T., Hazzouri A.Z.A., Cai J., Baldoni P.L., Llabre M.M., Rundek T., et al. Incidence of hypertension among US hispanics/latinos: the hispanic community health study/study of latinos, 2008 to 2017. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leng B., Jin Y., Li G., Chen L., Jin N. Socioeconomic status and hypertension: a meta-analysis. J Hypertens. 2015;33:221–229. doi: 10.1097/HJH.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 133.Glover L.M., Cain-Shields L.R., Wyatt S.B., Gebreab S.Y., Diez-Roux A.V., Sims M. Life course socioeconomic status and hypertension in african American adults: the jackson heart study. Am J Hypertens. 2020;33:84–91. doi: 10.1093/ajh/hpz133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Psaltopoulou T., Hatzis G., Papageorgiou N., Androulakis E., Briasoulis A., Tousoulis D. Socioeconomic status and risk factors for cardiovascular disease: impact of dietary mediators. Hellenic J Cardiol. 2017;58:32–42. doi: 10.1016/j.hjc.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 135.de Mestral C., Mayén A.L., Petrovic D., Marques-Vidal P., Bochud M., Stringhini S. Socioeconomic determinants of sodium intake in adult populations of high-income countries: a systematic review and meta-analysis. Am J Publ Health. 2017;107:e1–e12. doi: 10.2105/AJPH.2016.303629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bertoni A., Foy C., Hunter J., Quandt S., Vitolins M., Whitt-Glover M. A multilevel assessment of barriers to adoption of dietary Approaches to Stop hypertension (DASH) among african Americans of low socioeconomic status. J Health Care Poor Underserved. 2011;22:1205–1220. doi: 10.1353/hpu.2011.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abba M.S., Nduka C.U., Anjorin S., Mohamed S.F., Agogo E., Uthman O.A. Influence of contextual socioeconomic position on hypertension risk in low- and middle-income countries: disentangling context from composition. BMC Publ Health. 2021;21:2218. doi: 10.1186/s12889-021-12238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lai J.S., Aung Y.N., Khalid Y., Cheah S.C. Impact of different dietary sodium reduction strategies on blood pressure: a systematic review. Hypertens Res: Offic J Japanese Societ Hypert. 2022;45:1701–1712. doi: 10.1038/s41440-022-00990-5. [DOI] [PubMed] [Google Scholar]
- 139.Samson R., Ayinapudi K., Le Jemtel T.H., Oparil S. Obesity, hypertension, and bariatric surgery. Curr Hypertens Rep. 2020;22:46. doi: 10.1007/s11906-020-01049-x. [DOI] [PubMed] [Google Scholar]
- 140.Schiavon C.A., Bersch-Ferreira A.C., Santucci E.V., Oliveira J.D., Torreglosa C.R., Bueno P.T., et al. Effects of bariatric surgery in obese patients with hypertension: the GATEWAY randomized trial (gastric bypass to treat obese patients with steady hypertension) Circulation. 2018;137:1132–1142. doi: 10.1161/CIRCULATIONAHA.117.032130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cohen J.B., Gadde K.M. Weight loss medications in the treatment of obesity and hypertension. Curr Hypertens Rep. 2019;21:16. doi: 10.1007/s11906-019-0915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chang S.H., Stoll C.R., Song J., Varela J.E., Eagon C.J., Colditz G.A. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA surgery. 2014;149:275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Climent E., Oliveras A., Pedro-Botet J., Goday A., Benaiges D. Bariatric surgery and hypertension. J Clin Med. 2021:10. doi: 10.3390/jcm10184049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wiggins T., Guidozzi N., Welbourn R., Ahmed A.R., Markar S.R. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and meta-analysis. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I., et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 146.Jastreboff A.M., Aronne L.J., Ahmad N.N., Wharton S., Connery L., Alves B., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 147.Gloy V.L., Briel M., Bhatt D.L., Kashyap S.R., Schauer P.R., Mingrone G., et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cohen J.B. Hypertension in obesity and the impact of weight loss. Curr Cardiol Rep. 2017;19:98. doi: 10.1007/s11886-017-0912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liakos C.I., Papadopoulos D.P., Sanidas E.A., Markou M.I., Hatziagelaki E.E., Grassos C.A., et al. Blood pressure-lowering effect of newer antihyperglycemic agents (SGLT-2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors) Am J Cardiovasc Drugs: Drugs, Devices, Other Intervent. 2021;21:123–137. doi: 10.1007/s40256-020-00423-z. [DOI] [PubMed] [Google Scholar]
- 150.Bays H.E., Lazarus E., Primack C., Fitch A. Obesity pillars roundtable: phentermine – Past, present, and future. Obesity Pillars. 2022;3 doi: 10.1016/j.obpill.2022.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aronne L.J., Wadden T.A., Peterson C., Winslow D., Odeh S., Gadde K.M. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity. 2013;21:2163–2171. doi: 10.1002/oby.20584. [DOI] [PubMed] [Google Scholar]
- 152.Bays H.E., Fitch A., Christensen S., Burridge K., Tondt J. Anti-obesity medications and investigational agents: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022 doi: 10.1016/j.obpill.2022.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kotlyar M., al'Absi M., Brauer L.H., Grant J.E., Fong E., Kim S.W. Naltrexone effect on physiological and subjective response to a cold pressor task. Biol Psychol. 2008;77:233–236. doi: 10.1016/j.biopsycho.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Calvi A., Fischetti I., Verzicco I., Belvederi Murri M., Zanetidou S., Volpi R., et al. Antidepressant drugs effects on blood pressure. Frontiers Cardiovascul Medi. 2021;8 doi: 10.3389/fcvm.2021.704281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bays H.E., Burridge K., Richards J., Fitch A. Obesity Pillars roundtable: excessive weight reduction with highly effective anti-obesity medications (heAOMs) Obesity Pillars. 2022;4 doi: 10.1016/j.obpill.2022.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mattos S., Rabello da Cunha M., Barreto Silva M.I., Serfaty F., Tarvainen M.P., Klein M.R.S.T., et al. Effects of weight loss through lifestyle changes on heart rate variability in overweight and obese patients: a systematic review. Clin Nutr. 2022;41:2577–2586. doi: 10.1016/j.clnu.2022.09.009. [DOI] [PubMed] [Google Scholar]
- 157.Nicoll R., Henein M.Y. Caloric restriction and its effect on blood pressure, heart rate variability and arterial stiffness and dilatation: a review of the evidence. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19030751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Han X., Ren J. Caloric restriction and heart function: is there a sensible link? Acta Pharmacol Sin. 2010;31:1111–1117. doi: 10.1038/aps.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Christensen P., Bliddal H., Riecke B.F., Leeds A.R., Astrup A., Christensen R. Comparison of a low-energy diet and a very low-energy diet in sedentary obese individuals: a pragmatic randomized controlled trial. Clinical Obesity. 2011;1:31–40. doi: 10.1111/j.1758-8111.2011.00006.x. [DOI] [PubMed] [Google Scholar]
- 160.Hendricks E.J., Greenway F.L., Westman E.C., Gupta A.K. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity. 2011;19:2351–2360. doi: 10.1038/oby.2011.94. [DOI] [PubMed] [Google Scholar]
- 161.Nissen S.E., Wolski K.E., Prcela L., Wadden T., Buse J.B., Bakris G., et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA, J Am Med Assoc. 2016;315:990–1004. doi: 10.1001/jama.2016.1558. [DOI] [PubMed] [Google Scholar]
- 162.Martin J., Paquette C., Marceau S., Hould F.S., Lebel S., Simard S., et al. Impact of orlistat-induced weight loss on diastolic function and heart rate variability in severely obese subjects with diabetes. J Obesit. 2011;2011 doi: 10.1155/2011/394658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sharma A.M., Golay A. Effect of orlistat-induced weight loss on blood pressure and heart rate in obese patients with hypertension. J Hypertens. 2002;20:1873–1878. doi: 10.1097/00004872-200209000-00034. [DOI] [PubMed] [Google Scholar]
- 164.Greco C., Santi D., Brigante G., Pacchioni C., Simoni M. Effect of the glucagon-like peptide-1 receptor agonists on autonomic function in subjects with diabetes: a systematic review and meta-analysis. Diabetes Metabol J. 2022;46:901–911. doi: 10.4093/dmj.2021.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bays H.E., Fitch A., Christensen S., Burridge K., Tondt J. Anti-obesity medications and investigational agents: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obesity Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bryson C.M., Psaty BMMDPBM A review of the adverse effects of peripheral alpha-1 antagonists in hypertension therapy. Curr Contr Trials Cardiovasc Med. 2002;3:7. doi: 10.1186/1468-6708-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Messerli F.H. Vasodilatory edema: a common side effect of antihypertensive therapy. Curr Cardiol Rep. 2002;4:479–482. doi: 10.1007/s11886-002-0110-9. [DOI] [PubMed] [Google Scholar]
- 168.Messerli F.H., Bell D.S., Fonseca V., Katholi R.E., McGill J.B., Phillips R.A., et al. Body weight changes with beta-blocker use: results from GEMINI. Am J Med. 2007;120:610–615. doi: 10.1016/j.amjmed.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 169.Kumar A., Aronow W.S., Vadnerkar A., Sivan K., Mittal S. Effects of increased dose of diuretics on symptoms, weight, 6-minute walk distance, and echocardiographic measurements of left ventricular systolic and diastolic function in 51 patients with symptomatic heart failure caused by reduced left ventricular ejection fraction treated with beta blockers and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Am J Therapeut. 2009;16:5–7. doi: 10.1097/MJT.0b013e31813e6452. [DOI] [PubMed] [Google Scholar]
- 170.Santos E.L., de Picoli Souza K., Guimarães P.B., Reis F.C.G., Silva S.M.A., Costa-Neto C.M., et al. Effect of angiotensin converting enzyme inhibitor enalapril on body weight and composition in young rats. Int Immunopharm. 2008;8:247–253. doi: 10.1016/j.intimp.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 171.Zorad S., Dou J.T., Benicky J., Hutanu D., Tybitanclova K., Zhou J., et al. Long-term angiotensin II AT1 receptor inhibition produces adipose tissue hypotrophy accompanied by increased expression of adiponectin and PPARgamma. Eur J Pharmacol. 2006;552:112–122. doi: 10.1016/j.ejphar.2006.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Graham R., Mancher M., Miller Wolman D., Greenfield S., Steinberg E. National Academies Press; Washington (DC: 2011. Clinical practice guidelines we can trust. [PubMed] [Google Scholar]