Abstract
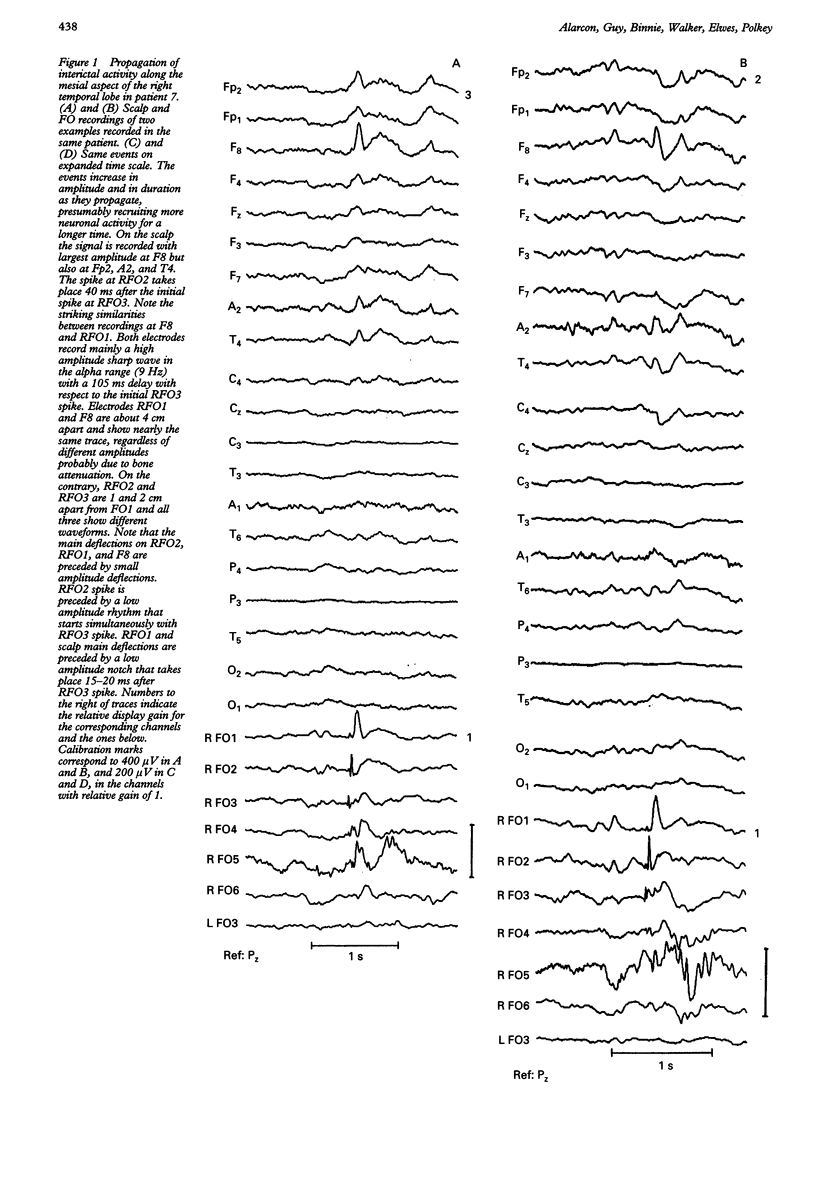
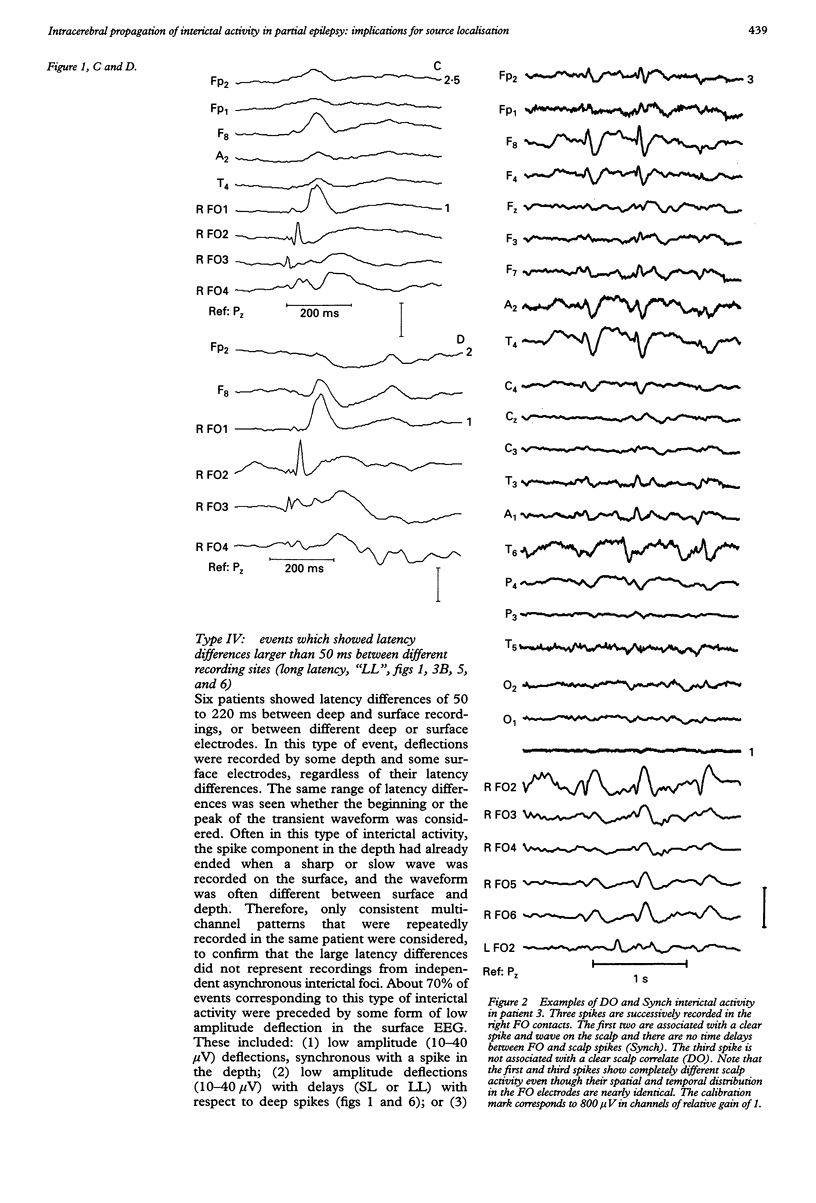
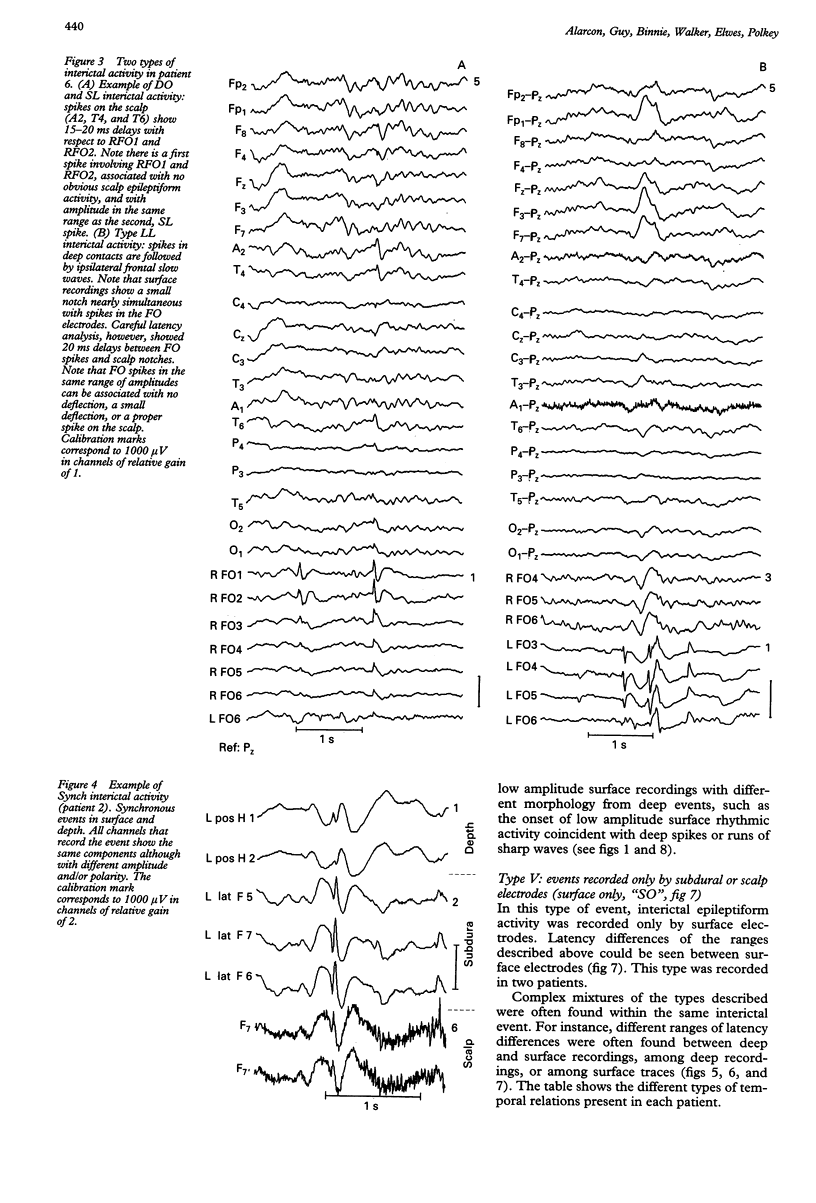
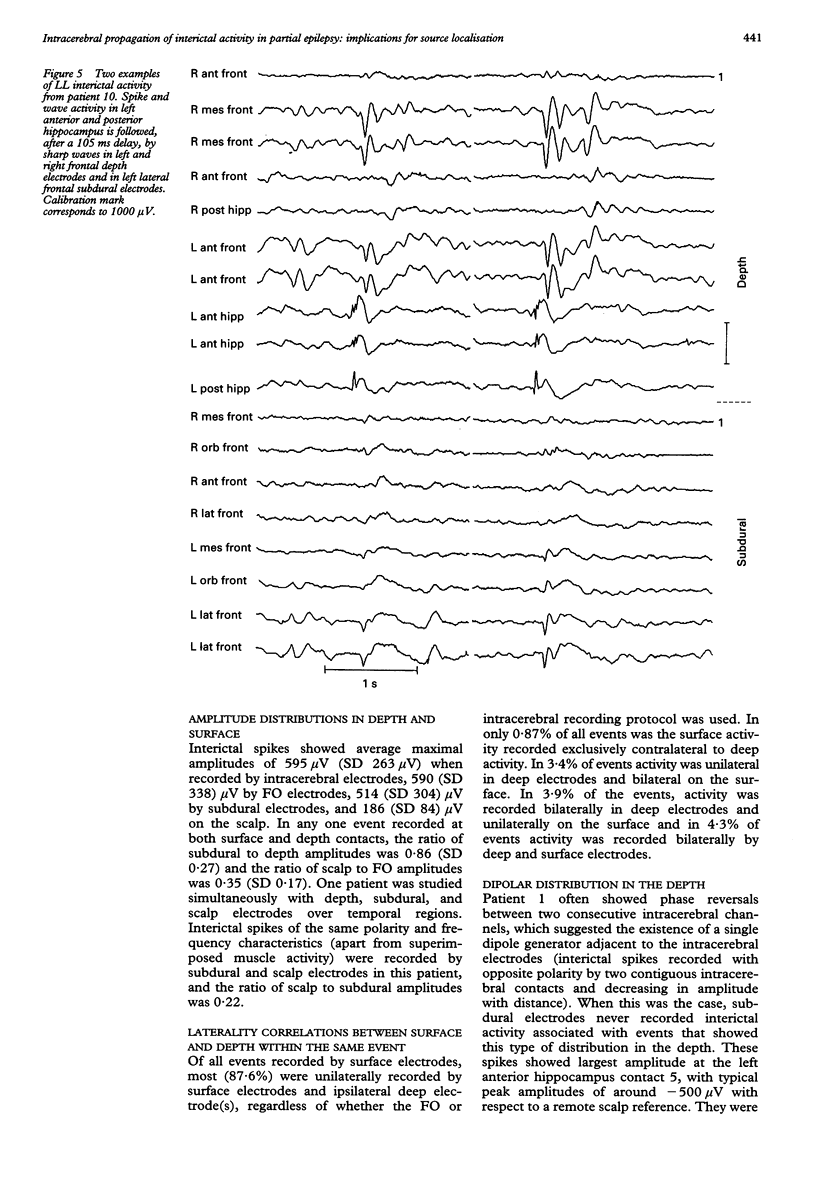
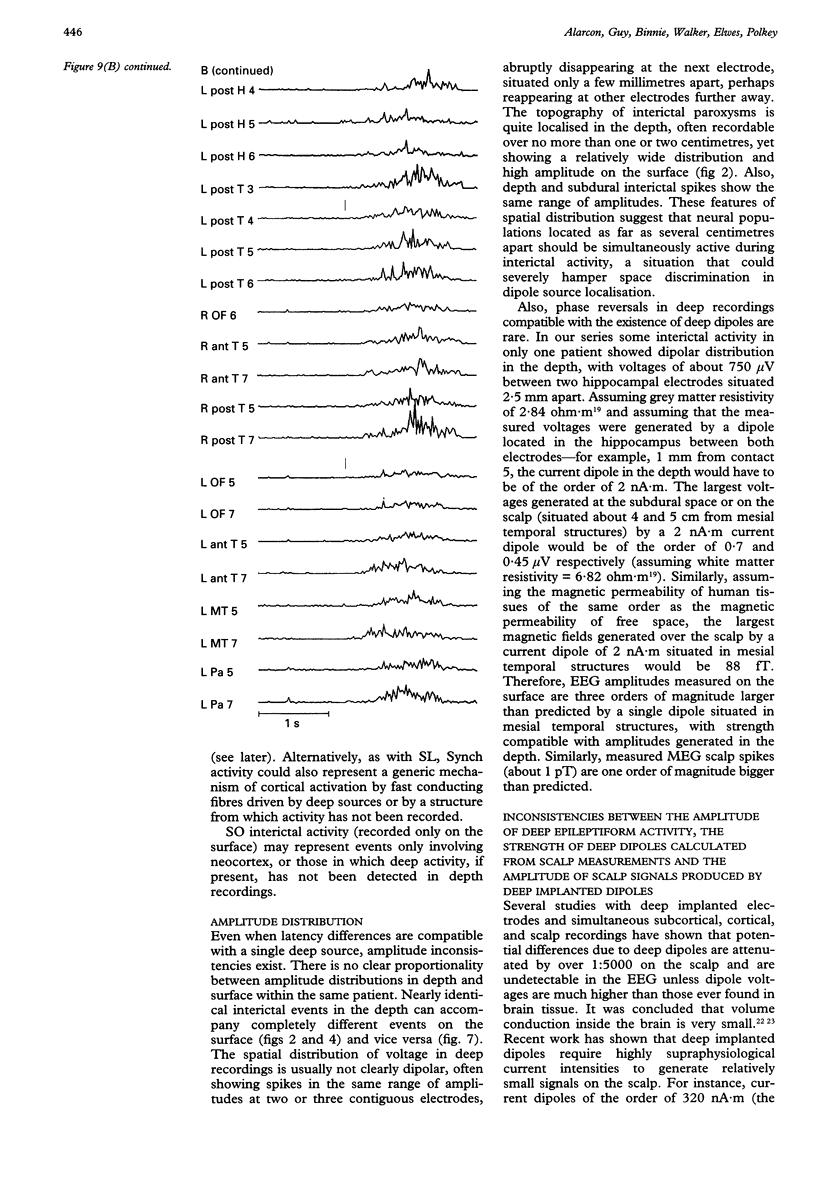
The hypothesis that focal scalp EEG and MEG interictal epileptiform activity can be modelled by single dipoles or by a limited number of dipoles was examined. The time course and spatial distribution of interictal activity recorded simultaneously by surface electrodes and by electrodes next to mesial temporal structures in 12 patients being assessed for epilepsy surgery have been studied to estimate the degree of confinement of neural activity present during interictal paroxysms, and the degree to which volume conduction and neural propagation take part in the diffusion of interictal activity. Also, intrapatient topographical correlations of ictal onset zone and deep interictal activity have been studied. Correlations between the amplitudes of deep and surface recordings, together with previous reports on the amplitude of scalp signals produced by artificially implanted dipoles suggest that the ratio of deep to surface activity recorded during interictal epileptiform activity on the scalp is around 1:2000. This implies that most such activity recorded on the scalp does not arise from volume conduction from deep structures but is generated in the underlying neocortex. Also, time delays of up to 220 ms recorded between interictal paroxysms at different recording sites show that interictal epileptiform activity can propagate neuronally within several milliseconds to relatively remote cortex. Large areas of archicortex and neocortex can then be simultaneously or sequentially active via three possible mechanisms: (1) by fast association fibres directly, (2) by fast association fibres that trigger local phenomena which in turn give rise to sharp/slow waves or spikes, and (3) propagation along the neocortex. The low ratio of deep-to-surface signal on the scalp and the simultaneous activation of large neocortical areas can yield spurious equivalent dipoles localised in deeper structures. Frequent interictal spike activities can also take place independently in areas other than the ictal onset zone and their interictal propagation to the surface is independent of their capacity to trigger seizures. It is concluded that: (1) the deep-to-surface ratios of electromagnetic fields from deep sources are extremely low on the scalp; (2) single dipoles or a limited number of dipoles are not adequate for surgical assessment; (3) the correct localisation of the onset of interictal activity does not necessarily imply the onset of seizures in the region or in the same hemisphere. It is suggested that, until volume conduction and neurophysiological propagation can be distinguished, semiempirical correlations between symptomatology, surgical outcome, and detailed presurgical modeling of the neocortical projection patterns by combined MEG, EEG, and MRI could be more fruitful than source localization with unrealistic source models.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison T., McCarthy G., Wood C. C., Darcey T. M., Spencer D. D., Williamson P. D. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. J Neurophysiol. 1989 Sep;62(3):694–710. doi: 10.1152/jn.1989.62.3.694. [DOI] [PubMed] [Google Scholar]
- Amaral D. G., Price J. L. Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol. 1984 Dec 20;230(4):465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Barth D. S., Baumgartner C., Sutherling W. W. Neuromagnetic field modeling of multiple brain regions producing interictal spikes in human epilepsy. Electroencephalogr Clin Neurophysiol. 1989 Nov;73(5):389–402. doi: 10.1016/0013-4694(89)90088-6. [DOI] [PubMed] [Google Scholar]
- Binnie C. D., Dekker E., Smit A., Van der Linden G. Practical considerations in the positioning of EEG electrodes. Electroencephalogr Clin Neurophysiol. 1982 Apr;53(4):453–458. doi: 10.1016/0013-4694(82)90010-4. [DOI] [PubMed] [Google Scholar]
- Binnie C. D., Elwes R. D., Polkey C. E., Volans A. Utility of stereoelectroencephalography in preoperative assessment of temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 1994 Jan;57(1):58–65. doi: 10.1136/jnnp.57.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COBB W., SEARS T. A. A study of the transmission of potentials after hemispherectomy. Electroencephalogr Clin Neurophysiol. 1960 May;12:371–383. doi: 10.1016/0013-4694(60)90012-2. [DOI] [PubMed] [Google Scholar]
- COOPER R., WINTER A. L., CROW H. J., WALTER W. G. COMPARISON OF SUBCORTICAL, CORTICAL AND SCALP ACTIVITY USING CHRONICALLY INDWELLING ELECTRODES IN MAN. Electroencephalogr Clin Neurophysiol. 1965 Feb;18:217–228. doi: 10.1016/0013-4694(65)90088-x. [DOI] [PubMed] [Google Scholar]
- Cohen D., Cuffin B. N., Yunokuchi K., Maniewski R., Purcell C., Cosgrove G. R., Ives J., Kennedy J. G., Schomer D. L. MEG versus EEG localization test using implanted sources in the human brain. Ann Neurol. 1990 Dec;28(6):811–817. doi: 10.1002/ana.410280613. [DOI] [PubMed] [Google Scholar]
- Cuffin B. N., Cohen D., Yunokuchi K., Maniewski R., Purcell C., Cosgrove G. R., Ives J., Kennedy J., Schomer D. Tests of EEG localization accuracy using implanted sources in the human brain. Ann Neurol. 1991 Feb;29(2):132–138. doi: 10.1002/ana.410290204. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr, Driver M. V., Falconer M. A. Electrophysiological correlates of pathology and surgical results in temporal lobe epilepsy. Brain. 1975 Mar;98(1):129–156. doi: 10.1093/brain/98.1.129. [DOI] [PubMed] [Google Scholar]
- HUGHES J. R. A statistical analysis on the location of EEG abnormalities. Electroencephalogr Clin Neurophysiol. 1960 Nov;12:905–909. doi: 10.1016/0013-4694(60)90139-5. [DOI] [PubMed] [Google Scholar]
- Hosek R. S., Sances A., Jr, Jodat R. W., Larson S. J. The contributions of intracerebral currents to the EEG and evoked potentials. IEEE Trans Biomed Eng. 1978 Sep;25(5):405–413. doi: 10.1109/TBME.1978.326337. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva F. H., Witter M. P., Boeijinga P. H., Lohman A. H. Anatomic organization and physiology of the limbic cortex. Physiol Rev. 1990 Apr;70(2):453–511. doi: 10.1152/physrev.1990.70.2.453. [DOI] [PubMed] [Google Scholar]
- Lütkenhöner B. Frequency-domain localization of intracerebral dipolar sources. Electroencephalogr Clin Neurophysiol. 1992 Feb;82(2):112–118. doi: 10.1016/0013-4694(92)90153-9. [DOI] [PubMed] [Google Scholar]
- Mauguière F. A consensus statement on relative merits of EEG and MEG. European Concerted Action on Biomagnetism, Lyon meeting, November 26 and 27, 1991. Electroencephalogr Clin Neurophysiol. 1992 May;82(5):317–319. doi: 10.1016/0013-4694(92)90001-x. [DOI] [PubMed] [Google Scholar]
- McBride M. C., Binnie C. D., Janota I., Polkey C. E. Predictive value of intraoperative electrocorticograms in resective epilepsy surgery. Ann Neurol. 1991 Oct;30(4):526–532. doi: 10.1002/ana.410300404. [DOI] [PubMed] [Google Scholar]
- Nicholson P. W. Specific impedance of cerebral white matter. Exp Neurol. 1965 Dec;13(4):386–401. doi: 10.1016/0014-4886(65)90126-3. [DOI] [PubMed] [Google Scholar]
- Ricci G. B., Romani G. L., Salustri C., Pizzella V., Torrioli G., Buonomo S., Peresson M., Modena I. Study of focal epilepsy by multichannel neuromagnetic measurements. Electroencephalogr Clin Neurophysiol. 1987 Apr;66(4):358–368. doi: 10.1016/0013-4694(87)90204-5. [DOI] [PubMed] [Google Scholar]
- Rose D. F., Sato S., Smith P. D., Porter R. J., Theodore W. H., Friauf W., Bonner R., Jabbari B. Localization of magnetic interictal discharges in temporal lobe epilepsy. Ann Neurol. 1987 Sep;22(3):348–354. doi: 10.1002/ana.410220311. [DOI] [PubMed] [Google Scholar]
- Salustri C., Chapman R. M. A simple method for 3-dimensional localization of epileptic activity recorded by simultaneous EEG and MEG. Electroencephalogr Clin Neurophysiol. 1989 Dec;73(6):473–478. doi: 10.1016/0013-4694(89)90257-5. [DOI] [PubMed] [Google Scholar]
- Sarvas J. Basic mathematical and electromagnetic concepts of the biomagnetic inverse problem. Phys Med Biol. 1987 Jan;32(1):11–22. doi: 10.1088/0031-9155/32/1/004. [DOI] [PubMed] [Google Scholar]
- Stefan H., Schneider S., Abraham-Fuchs K., Pawlik G., Feistel H., Bauer J., Neubauer U., Huk W. J., Holthoff V. The neocortico to mesio-basal limbic propagation of focal epileptic activity during the spike-wave complex. Electroencephalogr Clin Neurophysiol. 1991 Jul;79(1):1–10. doi: 10.1016/0013-4694(91)90150-3. [DOI] [PubMed] [Google Scholar]
- Sutherling W. W., Crandall P. H., Cahan L. D., Barth D. S. The magnetic field of epileptic spikes agrees with intracranial localizations in complex partial epilepsy. Neurology. 1988 May;38(5):778–786. doi: 10.1212/wnl.38.5.778. [DOI] [PubMed] [Google Scholar]
- Wieser H. G., Elger C. E., Stodieck S. R. The 'foramen ovale electrode': a new recording method for the preoperative evaluation of patients suffering from mesio-basal temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1985 Oct;61(4):314–322. doi: 10.1016/0013-4694(85)91098-3. [DOI] [PubMed] [Google Scholar]
- van Veelen C. W., Debets R. M., van Huffelen A. C., van Emde Boas W., Binnie C. D., Storm van Leeuwen W., Velis D. N., van Dieren A. Combined use of subdural and intracerebral electrodes in preoperative evaluation of epilepsy. Neurosurgery. 1990 Jan;26(1):93–101. doi: 10.1097/00006123-199001000-00013. [DOI] [PubMed] [Google Scholar]


