Abstract
Introduction
Nutritional ultrasound (US) is an emerging technique in clinical nutrition for the morphological and structural study of muscle mass. Currently, all definitions of malnutrition include the measurement of muscle mass; however, there is no single way to assess it. It is necessary to develop new techniques to identify muscle involvement in malnutrition that are valid, standardised, reliable, accurate and profitable.
Objective
To value the new muscle US techniques aimed to measure muscle and functional status, to make a more accurate diagnosis and a better prediction of complications and morbidity and mortality in patients at nutritional risk. Primary outcome: to assess the feasibility of US or muscle US techniques in both nutritional diagnosis and follow-up in a nutritional intervention programme.
Methods and analysis
Disease-Related caloric-protein malnutrition EChOgraphy (DRECO) is a prospective, multicentre (25 Spanish hospitals), uncontrolled clinical study in standard clinical practice to value the usefulness of nutritional US (muscle US) in the nutritional diagnosis and follow-up, over 3–6 months, after standard nutritional clinical practice intervention and physical activity, to control their disease-related malnutrition. 1000 patients are expected to be included in.
Discussion
This study will standardise nutritional US measures. It will validate and define specific cut-off values for nutritional US and correlate it with already well-known nutritional tools such as Subjective Global Assessment or Global Leadership Initiative on Malnutrition criteria. Thus, muscle US will become not only a tool to diagnose malnutrition, but it will also be integrated in the daily practice to evaluate nutritional interventions.
Ethics and dissemination
All DRECO study materials have been approved by each of the IRB/IEC of all the sites enrolled (either approval of the own IRB/IEC or validating the approval of the IRB/IEC of another hospital). The study has been registered with ClinicalTrials.gov, on 27 June 2022. The results from this study will be presented at scientific conferences and in peer-reviewed scientific journals.
Trial registration number
Keywords: nutrition & dietetics, ultrasound, nutritional support
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Multicentre, prospective, medium-term study in which a large sample (1000 patients) is expected to be recruited.
First study designed as a real-world study to evaluate the feasibility of nutritional ultrasound (US), led by senior researchers wide experienced in clinical nutrition.
Validation of classical tools and new morphofunctional assessment techniques (US and bioelectrical impedance) is proposed.
Non-randomised clinical practice study, so it will not be possible to adequately analyse the effect of nutritional intervention.
It is restricted to patients on hospital discharge, so it cannot be generalised to the entire population of people at risk of malnutrition.
Introduction
Disease-related malnutrition (DRM) can occur when there is a deficient supply of energy, protein and/or other nutrients, depending on the nutritional needs of everyone at different times of their life cycle or health or disease circumstances. This deficiency induces effects on body composition and tissue and organ function and results in clinical consequences: increased morbidity and mortality associated with different disease processes.1
In 2019, the Global Leadership Initiative on Malnutrition (GLIM) criteria were published,2 providing a different vision of how to assess the malnourished patient. These criteria are divided into both phenotypic and aetiological criterion:
Phenotypic criterion:
Weight loss (%): >5% within past 6 months, or >10% beyond 6 months.
Low body mass index (BMI; kg/m2): <20 if <70 years, or <22 if >70 years. Asia: <18.5 if <70 years, or <20 if >70 years.
Reduced muscle mass: reduced by validated body composition measuring techniques.
Aetiological criterion
Overall, 50% of energy requirements> 1 week, or any reduction for >2 weeks, or any chronic gastrointestinal condition that adversely impacts food assimilation or absorption.
Inflammation: acute disease/injury or chronic disease related.
There are techniques for nutritional assessment using assessment tools aimed at morphofunctional diagnosis of malnutrition,3 in addition to the classical nutritional parameters, such as weight loss, BMI, folds, circumferences, albumin, lymphocytes, cholesterol and intake. New advanced parameters are being incorporated into clinical nutrition and their incorporation into clinical practice is of increasing interest, such as measures derived from bioelectrical impedance (BIA) and phase angle (PhA), dynamometry, functional tests, C reactive protein (CRP)/prealbumin ratio and muscle ultrasound (US) (see figure 1).
Figure 1.
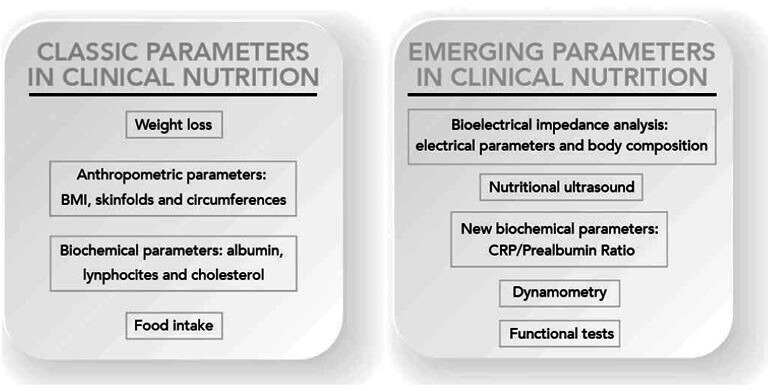
Update of nutritional evolution parameters. BMI, body mass index; CRP, C reactive protein. Reproduced with permission from the authors.3
From a scientific point of view, the following nutritional assessment techniques are being incorporated.
Muscle US
The application of US for the morphological and structural study of muscle mass is an emerging technique. Currently, there are different validation studies on the measurement technique. The US technique determines the surface area of the muscle in transverse and longitudinal position. With US analysis, it is possible to measure key parameters of muscle architecture, such as muscle volume and muscle fascicle length. Although there are different muscle structures that can be evaluated, many of the studies focus on the quadriceps rectus femoris (QRF) or on combinations of various muscle groups involving large muscle bundles with functional importance to the patient in terms of gait. Measurement of the rectus femoris of the quadriceps is one of the most referenced measurements due to its correlation with strength and tests of execution or functional performance. It is necessary to develop new techniques to identify muscle involvement in malnutrition that are valid, standardised, reliable, accurate and profitable. Currently, all definitions of malnutrition include the measurement of muscle mass involvement; however, there is no single way to assess it. The classic imaging techniques such as dual-energy x-ray absorptiometry, CT and MRI are considered the gold standard, but they have difficulties in their clinical application under normal practice conditions. US has the advantage of being inexpensive, portable and does not involve ionising radiation. Several studies have confirmed the reliability of this technique to measure the size of the quadriceps muscle in a healthy population.4 Studies on the reliability of rectus femoris US have been published with an intraclass coefficient of variation of 0.97 (95% CI: 0.92 to 0.99) for the test–retest reliability of US.
The American Society for Parenteral and Enteral Nutrition (ASPEN), among the criteria for the diagnosis of malnutrition in adults, recommends including an evaluation of fat and muscle deposits. Specialists must incorporate techniques that properly help to identify the loss of muscle and fat mass for a correct diagnosis of malnutrition. Implementing these evaluation techniques and instruments is challenging and remains a work in progress.5 Muscle ultrasonography correlates with body composition measurement techniques such as BIA and anthropometry in patients with cancer.6 In adults with cystic fibrosis muscle US measurements, particularly the mean muscular area rectus anterior, are related to the nutritional status and respiratory function of these patients.7
The GLIM has recently appointed a working group to provide consensus-based guidance on assessment of skeletal muscle mass and its role in the malnutrition diagnostic and assessment process. They support the use of US, particularly in settings where its practical applicability provides potential for patient follow-up through repeated measurements, but it requires standardisation through experienced operators, and repeated measurements performed by the same individual. They also encourage further validation studies for the US.8
Bioelectrical impedance (BIA)
BIA is used as a tool to obtain data that helps to better understand the patient’s nutritional status, being a non-invasive, inexpensive and easily transportable technique. Vector analysis and PhA provide direct data, not being necessary to be later adjusted using formulas or mathematical models, as it is needed with simple or multifrequency BIA or multifrequency.9 This method is based on the analysis of the two bioimpedance vectors: resistance (R) and capacitive reactance (Xc). Resistance is defined as the opposition to a flow of electric current through a circuit component, medium or substance, providing information about biological fluids, and therefore, related to tissue hydration. A decrease in the resistance/height ratio will indicate swelling or third space; conversely, an increased ratio will indicate dehydration. Reactance is the effect on an electrical current caused by a material’s ability to store energy in cell membranes, so it is related to the cell mass and the integrity of its membranes. A decrease in Xc indicates loss of cell mass. This cell mass is the sum of all metabolically active cells, being the central parameter in the evaluation of nutritional status, since the reduction of cell mass is typically related to malnutrition.10
A recent study conducted by Fernandez-Jimenez et al found that a low standardised phase angle (SPhA) malnutrition value (SPhA < −0.3) was significantly associated with a higher mortality HR (HR 7.87, 95% CI 2.56 to 24.24, p<0.001). This biological marker could therefore be incorporated among the screening tools and mortality risk assessment in this population.11
Dynamometry
Dynamometry is one of the six criteria to define malnutrition according to ASPEN.12 It is extremely sensitive to nutritional status changes, so it is particularly useful to track nutritional therapy or interventions results, even in the short-term and medium term. It has mostly been used to predict postsurgical complications including elderly patients.13 The results obtained are compared with the population averages by age and sex. Sanchez et al14 presented reference values for hand dynamometry using a Jamar hand dynamometer for a Spanish population, providing cut-off points to define malnutrition. They concluded that hand dynamometry is associated with lean mass, which supports its usefulness in nutritional assessment.
Although the new GLIM consensus-based guidance on assessment of skeletal muscle mass does not include dynamometry as a marker of muscle mass,8 the authors hereby signing this article have previously studied dynamometry as a marker of muscle mass, suggesting that GLIM criterion and dynamometry are associated with a higher mortality rate in both hospitalised and outpatient oncology patients.15 16
Functional tests
These tests are a series of physical activities related to mobility, walking or balance. Their results are related to those of scales that assess instrumental activities of daily living (ADL). The most common are the ‘Timed Up and Go test’ (TUG), the ‘Gait Speed Test’ and the ‘Short Physical Performance Battery (SPPB)’ test that includes three tests (balance, gait speed and get up and walk).17
Besides, the decrease in physical performance, evaluated by the SPPB test or hand grip strength, has been shown to be elevated in patients with colorectal cancer prior to surgery, and it was related to an increase in postoperative complications and mortality.15
Study objectives
The objective of this study is to value the new muscle US techniques aimed to measure muscle and functional status, to make a more accurate diagnosis and a better prediction of complications and morbidity and mortality in patients at nutritional risk. This main objective is developed in primary and secondary objectives as it follows.
Primary objective
To assess the feasibility of US or muscle US techniques in both nutritional diagnosis and follow-up, over 3–6 months, in a nutritional intervention programme.
Secondary objectives
To determine the association between muscle morphological parameters (nutritional US of the leg (area, circumference, axis and adipose tissue), total abdominal and pre-peritoneal parameters measured by nutritional US and the nutritional and functional status of the patient, as well as their prognostic value in hospitalised patients.
To establish an association between US as a diagnostic value of malnutrition as compared with the diagnostic gold standard (Subjective Global Assessment (SGA) and GLIM criteria).
-
To determine the US cut-off points associated with the diagnosis of malnutrition and sarcopenia using the following tools:
Measurement of body composition using impedance techniques (report: PhA, body cell mass (BCM), hydration, fat free mass and lean mass index.
Muscle strength and capacity to perform physical activity after the intervention: dynamometry and TUG.
Criteria for sarcopenia.
To assess association with inflammatory activity markers: high-sensitivity CRP/prealbumin.
To assess US changes in patient follow-up.
To establish an association of US results as predictors of morbidity and mortality (stay, mortality at 3 and 6 months, readmissions and in-hospital complications).
Patient–participant involvement and feasibility of study design
Disease-Related caloric-protein malnutrition EChOgraphy (DRECO) is a prospective, multicentre, uncontrolled clinical study in standard clinical practice to value the usefulness of nutritional US (muscle US) in the nutritional diagnosis and follow-up of patients over a period of 3–6 consecutive months, after standard nutritional clinical practice intervention and physical activity to control their DRM.
The study may be considered non-interventional, since patients will undergo nutritional interventions and the standard treatment planned by their physician for treatment according to his/her standard clinical practice, and the only addition to the standard measurement and follow-up techniques of the patient will be the performance of a muscle US measurement using equipment provided to the centre for this purpose.
Patients over 18 years of age who, in the first week of hospital admission in medical-surgical areas, excluding critical patients, have an assessment of risk of malnutrition according to the Malnutrition Universal Screening Tool (MUST) and SARC-F (SARC-F is an acronym of five domains included in the questionnaire: (1) Strength, (2) Assistance with walking, (3) Rising from a chair, (4) Climbing stairs and (5) Falls) screening test using Remote consultation on MAlnutrition in the Primary Practice (R-MAPP).
If the results show a moderate or high risk of malnutrition, these patients will be invited to participate in the study and will undergo the morphofunctional assessment, an US study and the SGA. This study is registered under ClinicalTrials.gov (NCT05433831).
Figure 2.
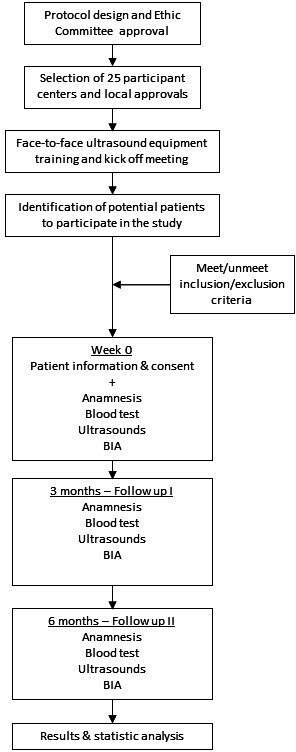
The schedule of enrolment, interventions and assessments. BIA, bioelectrical impedance.
Inclusion criteria
Patients admitted to hospital who in the first week of admission have moderate or high risk of malnutrition according to the MUST and SARC-F screening test using R-MAPP.
Patients aged 18–85 years.
Patient who agrees to participate in the study and signs the informed consent.
Exclusion criteria
Hepatic impairment—aspartate aminotransferase/alanine aminotransferase three times upper limit of normal.
Chronic kidney failure—glomerular filtration rate<45 mL/min.
Patients with previous intensive care unit stay during the study admission.
Cancer patients on palliative treatment or Eastern Cooperative Oncology Group≥ 3.
Orthopaedic disease that does not allow adequate walking.
Patients with known dementia or others not related to a significant neurological or psychiatric disorder, or any other psychological condition that may interfere with the conduct of the study.
Patients with eating disorders.
Life expectancy of less than 6 months.
Patients unable to adequately complete the clinical laboratory assessments required for the study protocol.
Sample size calculation
There are no previous clinical trials focusing on this objective published in the literature. We report a study in patients with chronic kidney disease on haemodialysis (HD)18 where measurement of the rectus femoris cross-sectional muscle area (RFCSA) was validated for the diagnosis of malnutrition related to this condition. RFCSA compared with bioimpedance spectroscopy had higher area under the curve (AUC, 0.686 vs 0.581), sensitivity (72.8% vs 65.8%) and specificity (55.6% vs 53.9%). The AUC of RFCSA was higher for the risk of protein-energy wasting (PEW) in male (0.74, 95% CI: 0.66 to 0.82) and female patients (0.80, 95% CI: 0.70 to 0.90) (both p<0.001). Gender-specific RFCSA values (males<6.00 cm2; females<4.47 cm2) indicated that HD patients with lower RFCSA were eight times more likely to have PEW (AOR=8.63, 95% CI: 4.80 to 15.50, p<0.001).
Our study aims to establish the feasibility of nutritional US measurements at different ages in both sexes to apply to patients with nutritional risk worldwide. For this purpose, the electronic case report form (CRF) will be programmed with the sample distributed by quotas to cover 50% men and 50% women, as well as 10-year age ranges. Age-stratified sampling is designed to obtain representative results of different ages and could be associated with the results of Subjective Global Assessment (SGA), BIA and dynamometry. The variability of measurements should be adjusted for sex, age and anthropometric parameters such as height.
It is estimated that 1000 patients with nutritional risk will be discharged from 20 to 25 healthcare centres throughout Spain and that at least 60% of the population will complete the 3-to-6-month follow-up of the study. Due to the special pandemic situation, a higher-than-expected dropout rate is expected at 6 months than under normal conditions (40% are estimated not to complete the 6-month follow-up for any reason).
Study conduct
The physicians participating in the study will be responsible for assessing the suitability of inclusion for each patient.
Patients will be consecutively recruited by the physician as they are assessed daily in their clinical practice at the hospital and found to have a risk of malnutrition according to the MUST/SARC-F (R-MAPP) screening test.
Before inclusion, the investigator must check the inclusion and exclusion criteria and obtain their informed consent.
The physician will be responsible for applying nutritional intervention and physical activity treatment according to standard clinical practice, as well as for clinical monitoring of patients. The treatment prescribed to each patient is not the objective of this study and is how the patient will experience changes that must be recorded with the different techniques described and with the muscle US involved in this study.
All physicians participating in the study must have been previously trained in the use of the US equipment and materials provided for the study, as well as in the use of the electronic CRF for data entry designed for this study.
Throughout the entire study, monthly meetings are held with all participants on Thursdays at 8:30 and on Fridays at 8:30 with the study’s central committee. The objective of these meetings is to monitor the status of the study at each participating centre, to resolve doubts and to make sure that all techniques and measurements are properly made according to previous training.
Nutritional US techniques and measurements
US accuracy highly depends on the skills of the technician. Point training using rectus femoris phantom has shown to improve the accuracy of measurements.19 Before starting the study, a training session was held. All study participants were required to attend, and they had the opportunity to practice with the same US machine that was going to be used in the study in phantom patients. Besides, several videos explaining detailed measurements technique were recorded. These videos were proactively shared with all researchers and available anytime at the study on-line electronic data capture platform.
Beyond, once the study finishes, all Digital Imaging and Communications in Medicine (DICOM) images gathered will be analysed to develop a semiautomated algorithm that helps diagnose the patient’s nutritional status. Subsequently, once the algorithm is available, the individual and manual US measurements will be contrasted with the data showed by the automatic algorithm, thus minimising the interobserver and intraobserver correlation. This work will have its own analysis and publication plan.
Abdominal and anterior thigh muscle measurements are performed using a commercially available portable US system with a 4–10 cm linear tube (UProbe L6C Ultrasound Scanner, Guangzhou Sonostar Technologies, Guangzhou, Guangdong, China). The funder of the study provided an US machine to each of the participants hospitals.
Quadriceps rectus femoris (QRF) US
With the patient lying supine with knees extended and relaxed, US measurements of unilateral (right side) QRF is performed at each participating centre by an experienced medical sonographer blinded to the clinical data and other results of nutritional assessment. The acquisition site is located two-thirds of the way along the femur length, measured between the anterior superior iliac spine and the upper edge of the patella. The transducer is placed perpendicular to the long axis of the thigh with excessive use of contact gel and minimal pressure to avoid compression of the muscle. All parameters are taken as an average of three consecutive measurements in the dominant leg. We measure the transversal axis of the cross-sectional area in cm2, the X-axis and Y-axis in mm, which corresponded to the linear measurement of the distance between the muscular limits of the rectus femoris (lateral and anteroposterior), the X-axis/Y-axis ratio and the total fat tissue in mm. All US parameters were also standardised divided by height squared (in cm2 for rectus femoris). The DICOM images of the QRF USs will be kept for later analysis (figure 3).
Figure 3.
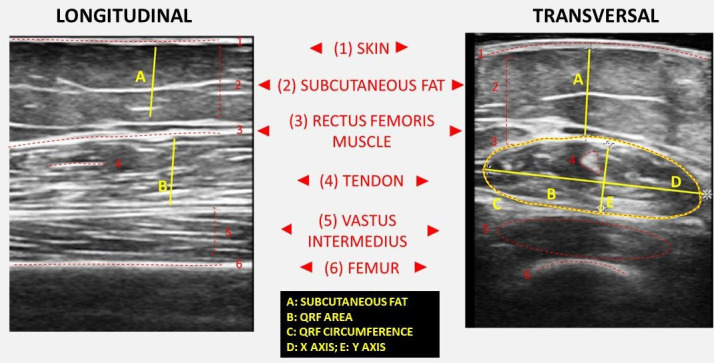
Comparison of longitudinal and transversal sections of the quadriceps rectus femoris muscle area ultrasound. Functional measures and main anatomical structures are represented.
Abdominal US
The second component of nutritional US is the evaluation of fat at the level of the abdominal wall.20 The location of the measurement point is set at the midpoint between the xiphoid appendix and the navel on the midline. The patient remains in a supine position in a situation of relaxation and the image is taken during the unforced expiration, in a transverse plane using the same linear probe perpendicular to the skin. In the cross-section, the anatomical structures that are visualised are ordered from the most superficial layer corresponding to the epidermis, followed by the layer of subcutaneous, superficial and deep adipose tissue. Then the two muscles of the anterior rectum of the abdomen that join in the central part in the linea alba are identified.20 We measure both total and superficial subcutaneous adipose tissue and the pre-peritoneal visceral adipose tissue. The DICOM images of the abdominal USs will be kept for later analysis (figure 4).
Figure 4.
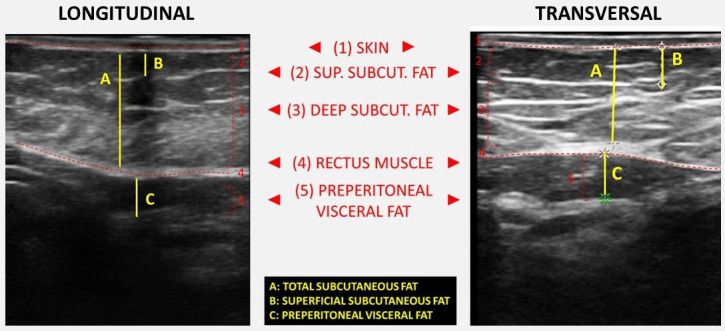
Comparison of longitudinal and transversal sections of the abdominal area ultrasound. Functional measures and main anatomical structures are represented.
Bioelectrical impedance (BIA)
Total body BIA (50 kHz frequency) (Tanita BC-420MA BIA analyzer, Tanita Corporation, Arlington Heights, Illinois, USA) was used to determine PhA (degrees), total body water (%), fat mass (kg), lean mass (kg), BCM (kg) and appendicular skeletal muscle mass (kg).
Since interval fluid balance is more sensible to the change of oedema, BIA analysis can be affected in oedematous patients.21 Therefore, extreme PhA values and/or non-coherent reactance/resistance ratios will be discarded, as a control measure, to detect patients with oedema and fluid balance change.
Timed Up and Go test (TUG)
The TUG test was used to assess functionality. A coloured tape was marked 3 m away from an armless chair in which participants were sitting. Participants were asked to walk 3 m, turn around the marked tape and return to the chair as fast as they could. A timer was set as soon as the patient stood up from the chair and was stopped when the patient was seated again. At least one practice trial was performed before the test. Being that a TUG Score of ≥20 s is identified as a cut-off point for severe sarcopenia, TUG was considered in this study.22
Handgrip strength test
Handgrip strength was determined using the Jamar dynamometer (J A Preston Corporation, New York, New York, USA). The dominant hand was tested. Three measurements of both media and maximum value were taken. The ASPEN has included the assessment of grip strength by dynamometer as one of the six criteria to define malnutrition.23 In this study, the cut-off values defined for the Spanish population will be considered.14
Although some quality-of-life tests, such as SF-36 or ADL test, were initially considered in the study protocol, they were finally rejected because, in real clinical practice, these tests are not used with the patient profile included in this study.
Follow-up period
The planned follow-up period for each patient will be 3–6 months from the inclusion visit.
The investigating physician will perform at least one first inclusion visit, and a follow-up visit at 3 and 6 months for each patient. A follow-up period of 6 months was established since it is common clinical practice in these patients, and with the aim of making the results more generalisable.
It is planned that the same physician attends the three visits to the patient (baseline, 3 and 6 months), to minimise the interpersonal variability in the measurements.
Study duration
The study is planned to last 18 months to detect patients at risk of malnutrition, recruitment, field work, monitoring and data analysis.
An estimated 2–3 months will be needed to plan the coordination and distribution of the work in the hospitalisation and outpatient clinic areas for the selection of candidate patients. It will take 6–9 months to recruit patients. From the start of the study, the database will be completed, and preliminary analyses will be performed. The final analysis will be performed when the follow-up is completed together with writing of the related work that will require 4–6 months to complete.
Outcome measures
A list of the outcomes of interest is provided in box 1.
Box 1. Study outcomes.
Primary outcomes
Nutritional ultrasound measurements: ultrasound with 4–10 cm linear probe*
Abdominal ultrasound: total, superficial and pre-peritoneal adipose tissue (measured in cm)
Muscle ultrasound: area, circumference, axes and adipose tissue (measured in cm)
Secondary outcomes
Sociodemographic data:
Age
Sex
Educational level
Toxic habits
Medical history
Risk of sarcopenia and moderate-to-high malnutrition based on Malnutrition Universal Screening Tool (MUST) and SARC-F (SARC-F is an acronym of five domains included in the questionnaire: (1) Strength, (2) Assistance with walking, (3) Rising from a chair, (4) Climbing stairs and (5) Falls) screening test using Remote consultation on MAlnutrition in the Primary Practice (R-MAPP)
Subjective Global Assessment Questionnaire
Anthropometric data:
Current body weight (measured or estimated)
Usual weight
Adjusted weight (adjusted weight in obese subjects, dry weight without oedema in malnourished subjects)
Height (measured or estimated)
Body mass index
Arm circumference
Bioelectrical impedance data (model (50 kHz)):**
Total body water, L
Extracellular water, L
Intracellular water, L
Fat free mass (lean mass, kg)
Fat mass, kg
Body cell mass, kg
Appendicular skeletal muscle mass, kg
Skeletal muscle mass index, kg
Per cent hydration
Body fat (%)
Blood biochemistry data (at baseline visit, at 3 and 6 months):
Albumin
Prealbumin
C reactive protein
Total cholesterol
Lymphocytes
Bioelectrical impedance data (model (50 kHz)):
Age
Sex
Educational level
Toxic habits
Medical history
Risk of sarcopenia and moderate-to-high malnutrition based on MUST and SARC-F screening test using R-MAPP
Functional parameters
Timed Up and Go test: patient sits in a chair and is told to get up (timing starts), walks 3 m, comes back and sits in the initial chair (timing ends). Interpretation: <20 s: normal, > 20 s: increased risk of falling.
Dynamometry. Three measurements of the dominant hand will be made recording the mean and maximum, measured in kilograms. Jamar dynamometers are most used in international studies and have several grip positions.
Current patient status
Hospital stays, mortality at 3 and 6 months, hospital readmissions and complications, if occurring, and their consequences (resolved/unresolved) must be recorded in the form.
Adherence
Attendance to study follow-up visits.
*The equipment provided for the study is the UProbe L6C Ultrasound Scanner (linear transducer 7.5–10 kHz) that allows depths up to 100 mm. Manufactured by Guangzhou Sonostar Technologies, China.
**Each healthcare center could use the BIA device they already owned. The most used device among all participants was AKERN branded.
Data analysis plan
Data analysis will be performed using SPSS V.22.0 software.
Quantitative variables will be expressed as mean±SD. The comparison between qualitative variables will be performed using the χ2 test with Fisher’s correction when necessary. Quantitative variables will be analysed using a Kolmogorov-Smirnov test. Differences between quantitative variables will be analysed using Student’s t-test or ANOVA test (for two or more samples, respectively) and non-parametric tests (Mann-Whitney or Kruskal-Wallis) will be used when the variables to be analysed do not follow a normal distribution.
The kappa coefficient will be used to assess agreement between techniques in diagnosis of malnutrition.
The association between variables will be studied using Spearman or Pearson correlations according to normality.
The thresholds for translation into clinical practice will be presented as cut-off points that will be estimated by AUC ROC curves. Centiles will also be considered.
The significant associations between muscle US parameters and the objective clinical variables in the univariate analysis will then be analysed in multivariate logistic regression models which also control other confounding variables. To assess which nutritional tool best predicts the risk of mortality during admission (and readmission), we will perform multivariate logistic regression models, in which the dependent variable will be in-hospital mortality (or readmission) based on the different tools applied (eg, US, PhA, SGA criteria, GLIM, Lean Mass Index (LMI)), also controlling for sex, the presence of previous comorbidities and other variables showing association in the univariate study.
For all calculations, a probability p less than 0.05 for two tails will be considered significant.
Recording of adverse reactions
Adverse reactions reporting is not the objective of the study. The investigator should proceed as usual and through the channels established in the healthcare system if any adverse effect occurs during follow-up. It will only be recorded in the follow-up if the patient must leave the study for this reason for statistical purposes.
Handling of missing data
No formal imputation will be made for the different analyses; therefore, all estimates will be obtained using all available data (available data only).
Since the study will be recorded using an electronic CRF, the necessary consistency filters and alerts for missing data will be programmed to validate and store the information, to minimise missing data and prevent the entry of incorrect or out of range data.
Ethics
General aspects
This study will be conducted in accordance with current regulations, accepted international ethical standards of Good Clinical Practice (CPMP/ICH/135/95), the principles laid down in the latest version of the Declaration of Helsinki, RD 1591/2009 and Circular No. 07/2004 regulating clinical research with medical devices.
Informed consent
Before inclusion in the study and after considering the suitability of patient inclusion, all participating physicians must offer the patient information about the study using a patient information sheet, invite the patient to participate in it, answer their questions and request completion of the informed consent form that will be kept in their own file.
Evaluation by an ethics committee
All DRECO study materials have been approved by each of the IRB/IEC of all the sites enrolled (either approval of the own IRB/IEC or validating the approval of the IRB/IEC of another hospital).
Confidentiality
The study data will be entered into an automated file owned by the sponsor. The analysis of study results will be made from an anonymised database, that is, dissociated, with no personal data, so that no subject can be identified or identifiable. This study database will be extracted from the electronic CRF and will include data from physician records, impedance recordings, and muscle US images. Data from different sources will be linked from the patient code and will not include personal data. All data in the file owned by the sponsor will be treated confidentially. The sponsor undertakes not to transfer data to third parties.
Dissemination
The results from this study will be presented at international and national scientific conferences, and in peer-reviewed scientific journals.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Discussion
There is a growing interest in the literature on the evaluation of muscle mass by US.20 Its current clinical utility focuses on measuring the involvement of muscle mass to assess the nutritional status of a patient.24 The further step that it is being investigated in this clinical study, is that muscle US becomes not only a tool to assess the diagnosis of malnutrition but to integrate it in the routine clinical practices to evaluate nutritional interventions.
The evaluation of the nutritional US should enable clinical decisions based on its results to permit the adjustment and individualisation of the nutritional therapeutic and physical exercise plan, along with functional recovery.20
To the best of our knowledge, this is going to be the largest study (sample size=1000) using nutritional US in patients with nutritional risk. Current scientific evidence is limited, and it is expected that such a large population will allow us to validate and define specific cut-off values for nutritional US and get its correlation with already well-known nutritional tools such as SGA or GLIM criteria.25
This study stands out for the use of several morphofunctional assessment techniques in patients with DRM in real clinical practice. Beyond its large sample, it is the first study with this design, as a real-world study, to evaluate the feasibility of nutritional US.
The emerging field of US assessment of muscle mass only highlights the need for a standardisation of measurement technique as Perkisas et al26 outline in their recently published 2022 SARCUS update. This update provides the approach of muscle assessment according to the most recent literature and anatomical landmarks for 39 different muscles. Besides, the discussion about four new muscle parameters that are added to the five that were previously considered is also presented26 and some of these parameters have been correlated with PhA27 and they will be analysed in our present protocol. Our ongoing study is intended to standardise these outstanding technique measures, to apply this technique widely soon. Recruited patients were at risk of malnutrition, so the results will be very interesting for routine clinical practice and nutritional care, in this patient profile, easily generalisable and free to use with publication.
Supplementary Material
Footnotes
Twitter: @golveirafuster
Contributors: All authors have identified the research question and were responsible for the conception and design of the protocol and the study. JMGA, DB, DADL and GO conducted study investigation. GGR has managed funding acquisition. All authors have been involved in drafting the manuscript and revising it critically for intellectual content. All authors read and approved the final manuscript.
Funding: The study was supported by Abbott Laboratories.
Competing interests: JMGA, DB, DADL and GO declare no conflict of interest. GGR is an employee of Abbott Laboratories.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Soeters PB, Reijven PLM, van Bokhorst-de van der Schueren MAE, et al. A rational approach to nutritional assessment. Clin Nutr 2008;27:706–16. 10.1016/j.clnu.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 2.Jensen GL, Cederholm T, Correia MITD, et al. GLIM criteria for the diagnosis of malnutrition: A consensus report from the global clinical nutrition community. JPEN J Parenter Enteral Nutr 2019;43:32–40. 10.1002/jpen.1440 [DOI] [PubMed] [Google Scholar]
- 3.García Almeida JM, García García C, Bellido Castañeda V, et al. New approach to nutrition. assessment of the patient’s nutritional status: function and body composition. Nutr Hosp 2018;35. Available: http://revista.nutricionhospitalaria.net/index.php/nh/article/view/2027 [Google Scholar]
- 4.Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr 2004;23:1226–43. 10.1016/j.clnu.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 5.Norman K, Stobäus N, Pirlich M, et al. Bioelectrical phase angle and impedance vector analysis--clinical relevance and applicability of impedance parameters. Clin Nutr 2012;31:854–61. 10.1016/j.clnu.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 6.López-Gómez JJ, Benito-Sendín Plaar K, Izaola-Jauregui O, et al. Muscular Ultrasonography in Morphofunctional assessment of patients with Oncological pathology at risk of malnutrition. Nutrients 2022;14:1573. 10.3390/nu14081573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sánchez-Torralvo FJ, Porras N, Ruiz-García I, et al. Usefulness of muscle Ultrasonography in the nutritional assessment of adult patients with cystic fibrosis. Nutrients 2022;14:3377. 10.3390/nu14163377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compher C, Cederholm T, Correia MITD, et al. Guidance for assessment of the muscle mass Phenotypic criterion for the global leadership initiative on malnutrition diagnosis of malnutrition. JPEN J Parenter Enteral Nutr 2022;46:1232–42. 10.1002/jpen.2366 [DOI] [PubMed] [Google Scholar]
- 9.Ní Bhuachalla ÉB, Daly LE, Power DG, et al. Computed tomography diagnosed Cachexia and Sarcopenia in 725 oncology patients: is nutritional screening capturing hidden malnutrition? is nutritional screening capturing hidden malnutrition in oncology patients? J Cachexia Sarcopenia Muscle 2018;9:295–305. 10.1002/jcsm.12258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Werf A, Langius JAE, de van der Schueren MAE, et al. Percentiles for Skeletal muscle index, area and radiation Attenuation based on computed tomography imaging in a healthy Caucasian population. Eur J Clin Nutr 2018;72:288–96. 10.1038/s41430-017-0034-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-Jiménez R, Dalla-Rovere L, García-Olivares M, et al. Phase angle and Handgrip strength as a Predictor of disease-related malnutrition in admitted patients: 12-month mortality. Nutrients 2022;14:1851. 10.3390/nu14091851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White JV, Guenter P, Jensen G, et al. Consensus statement: Academy of nutrition and Dietetics and American society for parenteral and Enteral nutrition: characteristics recommended for the identification and documentation of adult malnutrition (Undernutrition). JPEN J Parenter Enteral Nutr 2012;36:275–83. 10.1177/0148607112440285 [DOI] [PubMed] [Google Scholar]
- 13.Barata AT, Santos C, Cravo M, et al. Handgrip Dynamometry and patient-generated subjective global assessment in patients with Nonresectable lung cancer. Nutr Cancer 2017;69:154–8. 10.1080/01635581.2017.1250923 [DOI] [PubMed] [Google Scholar]
- 14.Sánchez Torralvo FJ, Porras N, Abuín Fernández J, et al. Normative reference values for hand grip Dynamometry in Spain. Nutr Hosp 2018;35:98–103. 10.20960/nh.1052 [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Torralvo FJ, González-Poveda I, García-Olivares M, et al. Poor physical performance is associated with postoperative complications and mortality in preoperative patients with colorectal cancer. Nutrients 2022;14:1484. 10.3390/nu14071484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Contreras-Bolívar V, Sánchez-Torralvo FJ, Ruiz-Vico M, et al. GLIM criteria using hand grip strength adequately predict six-month mortality in cancer Inpatients. Nutrients 2019;11:2043. 10.3390/nu11092043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Podsiadlo D, Richardson S. The timed «up & go»: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 18.Sahathevan S, Khor B-H, Singh BKS, et al. Association of ultrasound-derived Metrics of the quadriceps muscle with protein energy wasting in Hemodialysis patients: A multicenter cross-sectional study. Nutrients 2020;12:3597. 10.3390/nu12113597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakanishi N, Inoue S, Tsutsumi R, et al. Rectus Femoris mimicking ultrasound phantom for muscle mass assessment: design. J Clin Med 2021;10:2721. 10.3390/jcm10122721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Almeida JM, García-García C, Vegas-Aguilar IM, et al. Nutritional ultrasound: Conceptualisation, technical considerations and Standardisation, Endocrinología, diabetes Y Nutrición. Endocrinología, Diabetes y Nutrición 2023;70:74–84. 10.1016/j.endinu.2022.03.008 Available: 10.1016/j.endinu.2022.03.008 [DOI] [PubMed] [Google Scholar]
- 21.Nakanishi N, Tsutsumi R, Okayama Y, et al. Monitoring of muscle mass in critically ill patients: comparison of ultrasound and two bioelectrical impedance analysis devices. J Intensive Care 2019;7:61. 10.1186/s40560-019-0416-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:601. 10.1093/ageing/afz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of nutrition and Dietetics/American society for parenteral and Enteral nutrition: characteristics recommended for the identification and documentation of adult malnutrition (Undernutrition). J Acad Nutr Diet 2012;112:730–8. 10.1016/j.jand.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 24.Connolly B, MacBean V, Crowley C, et al. Ultrasound for the assessment of peripheral Skeletal muscle architecture in critical illness: a systematic review. Crit Care Med 2015;43:897–905. 10.1097/CCM.0000000000000821 [DOI] [PubMed] [Google Scholar]
- 25.Huo Z, Chong F, Yin L, et al. Accuracy of the GLIM criteria for diagnosing malnutrition: A systematic review and meta-analysis. Clin Nutr 2022;41:1208–17. 10.1016/j.clnu.2022.04.005 [DOI] [PubMed] [Google Scholar]
- 26.Perkisas S, Bastijns S, Baudry S, et al. Application of ultrasound for muscle assessment in Sarcopenia: 2020 SARCUS update. Eur Geriatr Med 2021;12:45–59. 10.1007/s41999-020-00433-9 [DOI] [PubMed] [Google Scholar]
- 27.Primo D, Izaola O, Gómez JJL, et al. Correlation of the phase angle with muscle ultrasound and quality of life in obese females. Dis Markers 2022;2022:7165126. 10.1155/2022/7165126 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


