Abstract
Transcriptome studies disentangle functional mechanisms of gene expression regulation and may elucidate the underlying biology of disease processes. However, the types of tissues currently collected typically assay a single post-mortem timepoint or are limited to investigating cell types found in blood. Noninvasive tissues may improve disease-relevant discovery by enabling more complex longitudinal study designs, by capturing different and potentially more applicable cell types, and by increasing sample sizes due to reduced collection costs and possible higher enrollment from vulnerable populations. Here, we develop methods for sampling noninvasive biospecimens, investigate their performance across commercial and in-house library preparations, characterize their biology, and assess the feasibility of using noninvasive tissues in a multitude of transcriptomic applications. We collected buccal swabs, hair follicles, saliva, and urine cell pellets from 19 individuals over three to four timepoints, for a total of 300 unique biological samples, which we then prepared with replicates across three library preparations, for a final tally of 472 transcriptomes. Of the four tissues we studied, we found hair follicles and urine cell pellets to be most promising due to the consistency of sample quality, the cell types and expression profiles we observed, and their performance in disease-relevant applications. This is the first study to thoroughly delineate biological and technical features of noninvasive samples and demonstrate their use in a wide array of transcriptomic and clinical analyses. We anticipate future use of these biospecimens will facilitate discovery and development of clinical applications.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12864-023-09875-4.
Keywords: Noninvasive RNA-sequencing, Transcriptomics, Methods, EQTLs, Hair follicles, Urine cell pellets
Introduction
Large scale transcriptomics has multiple applications in biomedical research. It provides insight into the functional consequences of genetic variation and aids our understanding of genetic determinants of disease mechanisms. Prospective applications include biomarker identification of disease risk, onset, prognosis, and treatment response, discovery of potential therapies, and assessing outcomes of in vitro perturbations of environmental or pharmacological exposures [1]. In the realm of cancer research, this approach has been fruitful in identifying early diagnostic markers, classifying cancer subtypes, identifying novel drug targets, and optimizing treatment choice [2–4]. In cases of rare, genetic disease, diagnosis is enhanced by inclusion of transcriptomic data due to improved detection and annotation of rare functional variants [5–7]. For common traits and diseases, discovery remains complicated by trait polygenicity, linkage disequilibrium, small effect sizes of variants, and widespread pleiotropy [8–11]. By integrating transcriptomic data with genetic information, loci with an effect on gene expression, known as molecular quantitative trait loci (molQTLs), lend potential interpretation to variants found by genome wide association studies (GWAS) [12–14].
Despite their promise, there are several barriers to transcriptome studies. Recent works suggest that elucidation of key pathways and molecular targets for a given trait requires transcriptomes from relevant tissues, i.e. cell types [15, 16], and contexts [17]. Dynamic changes in the transcriptome and molQTLs, which change over the course of development, disease, or environmental conditions, are suspected to lend even greater insight into the genetic architecture of the genome and are essential for using the transcriptome as a biomarker. At this time, whole blood and peripheral blood mononuclear cells (PBMCs) are the most readily collected tissue type and can be longitudinally sampled. However, the Genotype-Tissue Expression (GTEx) project, the largest and most comprehensive study of genetic regulation across post-mortem human tissues, showed that blood is an outlier in its gene expression regulatory mechanisms relative to other tissues of the body [18, 19], and the majority of expression quantitative trait loci (eQTLs) in linkage disequilibrium with GWAS variants are not found in whole blood [20]. Previous observations suggest this difference is driven by the unique functions and cell type composition of blood versus other tissues of the body [15, 16, 21, 22]. This biological difference poses major limitations to discovery in contexts where the cell types in blood are not centrally implicated in trait development or disease pathogenesis [18–20]. Sampling directly from meaningful tissue types, if the tissues of interest are known, provides more biologically relevant data. However, current approaches include surgical extractions, invasive biopsies, and post-mortem donations, which cannot be sampled over time and typically result in high cost and complicated logistics [23]. These factors also contribute to underrepresentation of minorities and vulnerable populations in most transcriptomic studies [24–27].
Thus, future transcriptome studies would benefit from sampling techniques that capture a broad array of cell types relevant to disease, enable use of longitudinal study designs for observation of context-specific effects, and facilitate rapid expansion to underserved populations to mitigate impending health disparities. Here, we investigate the use of noninvasive biospecimens to overcome these barriers. Early studies suggest buccal swabs, hair follicles, nasal swabs, saliva, and urine cell pellets may have potential use in clinical settings and for discovery [28–38], though no studies to date have comprehensively delineated sources of technical and biological variance in noninvasive tissue types, nor their performance across a wide array of transcriptomic and clinical analyses. Furthermore, no comparisons to invasive sample types have been made in terms of cell type composition or regulation of gene expression and splicing. In this study, we collected buccal swabs, hair follicles, saliva, and urine cell pellets from 19 individuals over 4 timepoints, and we prepared the samples for sequencing using both in-house and commercial library kits. Both the sample collection and low-cost library preparation methods used here are available open-access. We investigated the unique biology of noninvasive sample types and analyzed sources of technical variance, identified suitable invasive tissue type proxies, and demonstrated their use in transcriptomic and disease-relevant applications. We conclude that hair follicles and urine cell pellets are promising biospecimens for future study because of the sample quality, diversity of cell types captured, ease of inclusion in healthcare pipelines, and disease-relevant expression data.
Results
Noninvasive tissues are amenable to low-input library preparations
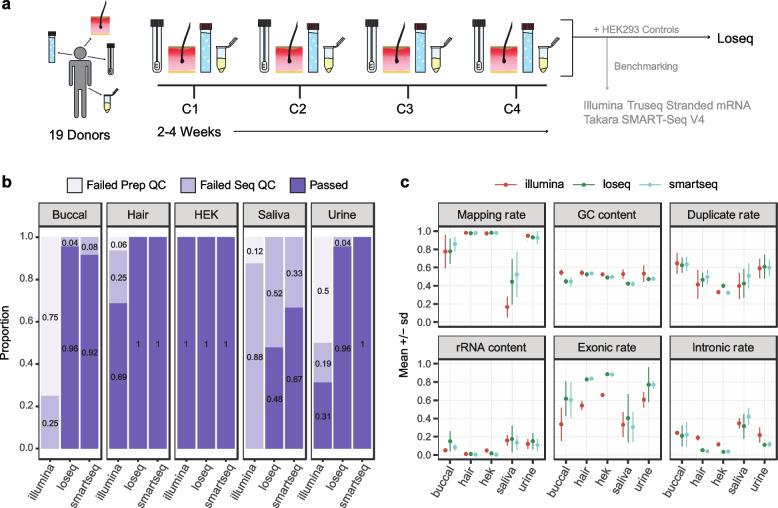
Buccal swabs, hair follicles, saliva, and urine cell pellets were collected from 19 healthy individuals at three to four separate time points, amounting to 300 unique biological samples (Fig. 1a). Briefly, participants deposited a saliva sample into an Oragene saliva collection kit, 10 hair follicles were plucked, and participants provided a buccal swab sample (see Methods and DOI: dx.doi.org/10.17504/protocols.io.kqdg3pjzzl25/v1). Saliva samples were stored according to kit instructions, and hair follicles and buccal swabs were flash frozen at -80C. Saliva, hair follicles, and buccal swabs were all able to be collected and stored within 9 min, on average. Urine samples were obtained at any time throughout the day, and underwent serial centrifugation before flash freezing the cell pellet at -80C. Individuals enrolled in the study provided genotyping data from 23andMe or Ancestry SNP arrays, or from low-pass whole genome sequencing provided by Gencove. Using the 1000 genomes database and k-nearest neighbors we find 15/19 individuals are of European descent, 3/19 are admixed Americans, and 1/19 is of East Asian ancestry.
Fig. 1.
Noninvasive sample study design and processing outcomes. a Four collections (C1-C4) of four noninvasive tissues were collected from 19 donors over the course of 2–4 weeks per donor. All samples were processed using our in-house method, Loseq, while a subset was prepared using commercially available kits. Two biological replicates of HEK293 cell controls were included in triplicate for all library preparations. b Proportion of samples passing per tissue type and preparation. Failed Prep QC = exceeded 600 bp average size or less than 2 nM yield. Failed Seq QC = protein-coding and lncRNA depth less than 1 million. c RNA-seq quality metrics for all sequenced samples for each tissue and library preparation
Total RNA yielded from noninvasive tissues was tissue-specific in yield and variability (buccal median = 1.05ug and IQR = 0.56ug, hair median = 1.44ug and IQR = 1.92ug, saliva median = 6.82ug and IQR = 8.60, urine median = 0.25ug and IQR = 1.54ug, Supp. Fig. 1c). Urine pellets (Levene P = 0.0002), hair follicles (P = 0.01) and buccal swabs (P = 0.05) exhibited significant donor-dependent variability in yield compared to other tissues (Supp. Fig. 1e). This observation agrees with clinically known interindividual differences in cell numbers found in urine [29], while hair follicle output appeared to be due to individual differences in hair texture (i.e. follicles more consistently remained attached to thicker, coarser hair). All samples were prepared for sequencing using a low-cost in-house library preparation we developed specifically for low-input bulk RNA applications, which uses template-switching oligo (TSO) and tagmentation chemistry and reduces cost by 83% and 68% per reaction compared to the Illumina TruSeq Stranded mRNA Library Prep and SMART-seq V4 kits, respectively (Supp. Tables 1 and 2). We herein refer to this method as Loseq.
To validate the consistency of our in-house method, we included 15 technical replicates per tissue in each Loseq library preparation batch. To compare performance across library preparation methods, 12 randomly selected samples of each tissue were prepared using the TakaraBio Smartseq V4 kit, a commercially available kit with similar chemistry to Loseq, and 16 samples were prepared using the Illumina TruSeq Stranded mRNA kit, one of the most frequently used kits in the transcriptomics field (Supp. Fig. 1a). For all preparation methods, 2 HEK293 cell samples were included in triplicate to serve as a quality control standard. In total, 472 noninvasive and 36 HEK293 transcriptomes were prepared across the three library preps. 485 of 508 samples passed pre-sequencing quality criteria of > 2 nM concentration and average library size < 600 bp and were sequenced on the Illumina NovaSeq S4 platform to a mean depth of 25.6 million total reads per sample (Supp. Fig. 1f, g).
Post-sequencing, we evaluated multiple quality control metrics returned from RNA-SeQC [39]. Ultimately, we found protein-coding and lncRNA read depth corresponded with high quality samples and consistent gene expression capture (Supp. Figure 1b and 2). Across all tissues, Illumina prepared samples yielded a lower number of reads, and we thus used a less stringent 1 million protein-coding and lncRNA depth threshold for cross-preparation comparisons. A 2.5 million threshold and a 5 million threshold were used for the Loseq-only and GTEx comparison analyses, respectively.
Looking across tissues and library preparations, we observe 109/118 buccal swabs, 113/118 hair follicles, 51/118 saliva, and 106/118 urine samples pass pre- and post-sequencing quality checks (Fig. 1b). Notably, hair performs well regardless of kit used and meets pre- and post-sequencing standards typical of traditionally used bulk RNA-sequencing samples (hair mean RIN = 8.9, Fig. 1c, Supp. Figs. 1 and 2). 8 of 16 urine and 12 of 16 buccal samples fail pre-sequencing checks for the Illumina kit. Given urine shows excellent performance via traditional RNA-sequencing quality metrics when low-input library preparation protocols are used (86/90 and 12/12 of samples pass QC for Loseq and Smartseq, respectively), we suspect this is driven by low and highly variable RNA yield not amenable to traditional bulk kits (median = 253.4 ng, Q1 = 98 ng, Q3 = 1642.2 ng, Supp. Fig. 1c, e). Buccal and saliva display higher rates of RNA degradation, as reflected by lower RIN (buccal mean RIN = 2.5, saliva mean RIN = 2.6) and computationally derived transcript integrity (buccal mean TIN = 39, saliva mean TIN = 30), as well as diminished performance apparent in other RNA-seq QC metrics (Fig. 1c, Supp. Figs. 1b and 2). The lower quality of these tissue types is likely resulting in the higher rate of failure across the different preparations. We found saliva to be a particularly poor biospecimen for transcriptomic study with few samples passing our thresholds (0/16 Illumina, 43/90 Loseq, and 8/12 of Smartseq pass QC). Looking across saliva collections we observe some donors more consistently pass or fail quality checkpoints (10 donors fail 3 or more collections, 8 donors fail 1 or fewer collections, Supp. Fig. 1a), suggesting donor-specific variables may play a larger role in determining the sample quality relative to other tissue types. Buccal, though not an ideal sample type (as shown by the metrics in Fig. 1c), performs well enough for use in targeted applications (86/90 and 11/12 pass QC for Loseq and Smartseq, respectively).
To compare gene expression patterns across libraries in an unbiased manner, we downsampled all QC-passed samples to a depth of 1 million by performing binomial sampling 5 times on the raw, unfiltered counts matrix and then taking the average. We found the majority of genes expressed in a tissue were captured regardless of preparation method (Supp. Fig. 3a) and there was high agreement in gene expression levels across library preparations (Supp. Fig. 3d). For most tissues, the number of genes detected was correlated with the RNA yield of the original extraction (Supp. Fig. 3e). One difference of note is Loseq tends to capture longer genes with lower GC content relative to the commercial kits (Supp. Fig. 3b, c). Principal component analysis (PCA) shows that the preparation method contributed minimally to variance observed across the samples (Supp. Fig. 4).
Unmapped reads capture tissue-specific microbial signatures
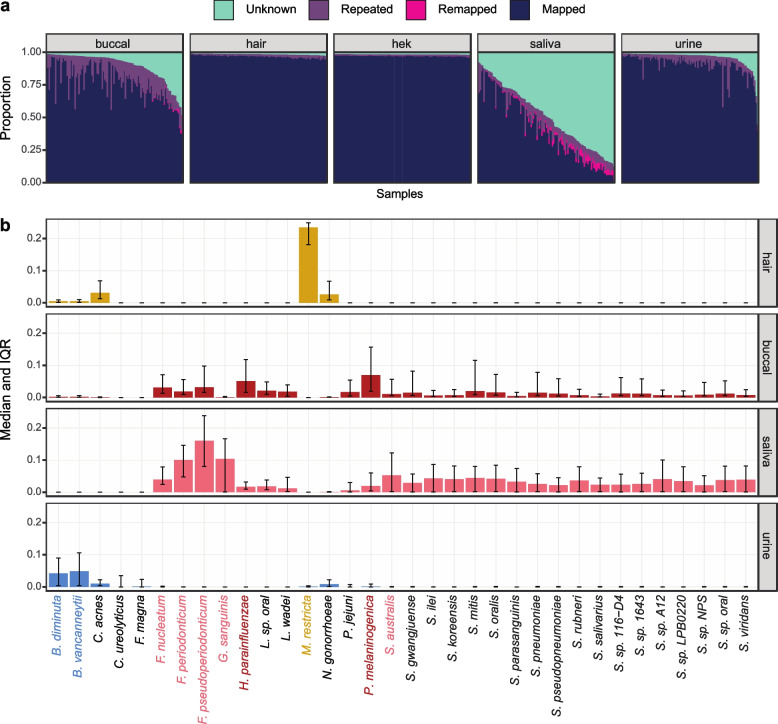
Since we observed a low mapping rate for buccal and saliva and because the noninvasive samples were collected from non-sterile human tissues, we decided to investigate biological and technical sources of unmapped reads in our samples (Supp. Fig. 5a). First, we remapped unmapped reads to microbial genomes using Decontaminer [40]. This process remapped only a small fraction of the unmapped reads (Fig. 2a, Supp. Fig. 5b), a somewhat unsurprising result given our library preparation is targeted to capture poly-A mRNA transcripts and most microbial transcripts are not polyadenylated. In saliva, the mapping rate to the human genome is negatively correlated with RNA yield, suggesting a presence of non-human RNA (Supp. Fig. 5e). Nonetheless, when we look at the top 0.5% most abundant remapped species we observe distinct microbial signatures across the noninvasive tissues that support previously known microbiota of the oral cavity, human skin, and genitourinary tract (Fig. 2b) [41–44]. In addition, we see high correlation in estimated species abundances across technical replicates suggesting that, despite the limitations in our library preparation approach, we are capturing real, replicable biological signal (Supp. Fig. 5c). Altogether, these findings support noninvasive samples may bear biological utility in follow-up microbiome studies using microbiome-specific or total RNA library preparations. Next, we used FastQC to identify overrepresented sequences in the remaining unmapped reads. These sequences were compiled across all tissues and samples into a list of 707 sequences, which were compared to primer and adapter sequences. From this analysis we observe 517 of the highly abundant reads were present in all tissues across all library preparations (“Repeated” in Fig. 2a, Supp. Fig. 5d). This suggests these reads are most likely technical byproducts from the sequencing process, but they may also be genomic regions that align poorly. Buccal and saliva display a greater proportion of reads of unknown origin, which may result from biological sources, like microbial species that our analysis did not identify, or from technical factors, like sample degradation leading to short sequences, overamplification, and poor alignment. Overall, especially for saliva and for many buccal samples, a large proportion of reads remained of unknown origin.
Fig. 2.
Classification of unmapped reads in noninvasive samples. a Proportion of reads per sample. Mapped = aligned to hg38. Remapped = aligned to microbial species using Decontaminer. Repeated = highly abundant reads identified by FastQC. Unknown = reads not mapped, remapped, or highly abundant. b Normalized proportion of reads remapping per species for each tissue. The top 0.5% most abundant microbes are shown. Highlighted species have a median abundance > 0.05 for that tissue
Sources of gene expression variance between individuals and noninvasive tissue types
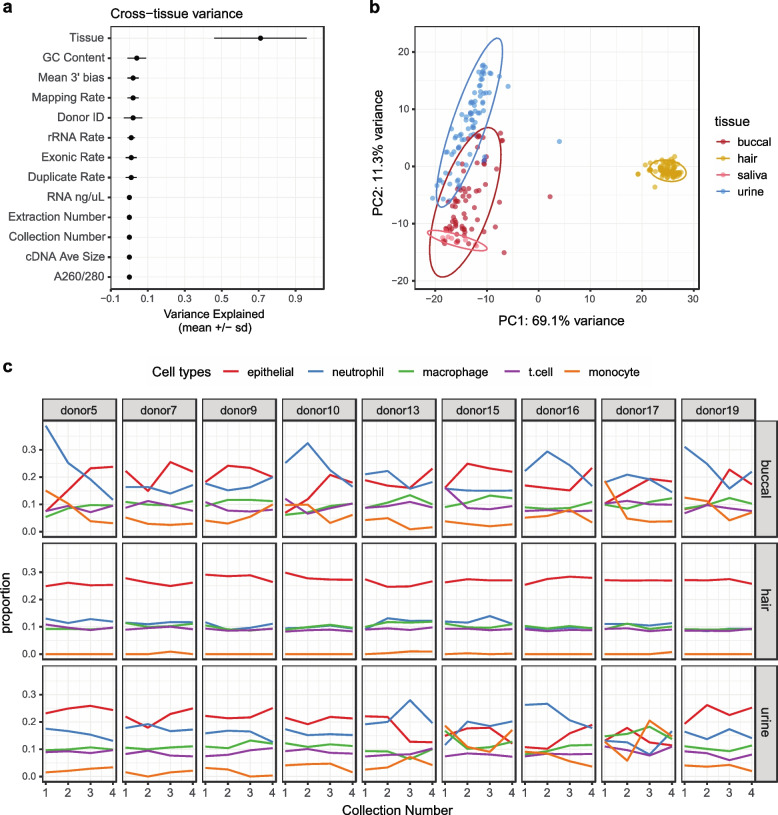
Next, we investigated gene expression variance due to noninvasive tissue type or individual. Saliva samples largely failed to pass post-sequencing quality standards and were excluded from several analyses for this reason. Using a mixed linear model to delineate biological and technical contributors to gene expression variability, we found tissue type is the main driver of variance across samples (Fig. 3a). These results were supported by PCA, which segregated samples primarily by tissue type, with hair forming a separate, distinct cluster from the other tissues (Fig. 3b).
Fig. 3.
Technical and biological sources of variance in noninvasive samples. a Factors contributing to variance in gene expression across tissues as determined by mixed linear modeling. b Principal component analysis using DESeq2 normalized counts and the top 1000 most variable genes. c GEDIT cell type proportion estimates across collections per donor. Only donors with samples passing QC for all collections are displayed here. Niche cell types were collapsed into larger categories (Supp. Fig. 7b), and the top 25% most abundant cell type categories across tissues are shown
Because underlying cell type composition frequently explains gene expression variance across samples and tissues [15, 16], we deconvolved our noninvasive samples using GEDIT [45] and the provided BlueCodeV2 single cell reference. From this we observed hair is primarily composed of epithelial cell types, buccal and urine capture both epithelial and immune cells, and saliva mostly contains neutrophils and monocytes. Looking across tissues, donors, and collections, hair was highly consistent in cell type abundance estimates, both within and across donors (Fig. 3c, Supp. Fig. 7a). On the other hand, urine and buccal samples were more variable. In these samples, cell type composition was occasionally consistent within and across donors, but there were also patterns where cell type composition changed across collections for the same donor (e.g. donor 5, buccal), showed a different pattern of abundance compared to other donors (e.g. donor 16, urine), or completely lacked any consistency (e.g. donor 17 urine). There was no consistent pattern of RNA yield correlating with cell type composition estimates (Supp. Fig. 7c), even though it is correlated to gene detection (Supp. Fig. 3e). When we used linear mixed modeling to identify biological and technical sources of gene expression variance within tissues, the individual donor was the primary contributor in buccal and urine (Supp. Fig. 6). Both of these results indicate that cell type compositions sampled from urine and buccal samples are potentially highly variable and donor specific. For hair, the relative contribution of technical factors and the donor of origin to gene expression variance is similar. As previously described, hair follicle data quality matches gold-standard RNA-sequencing data, which together with the low variance in cell type composition leads to highly consistent gene expression profiles.
Noninvasive tissue characteristics suggest potential invasive tissue type proxies
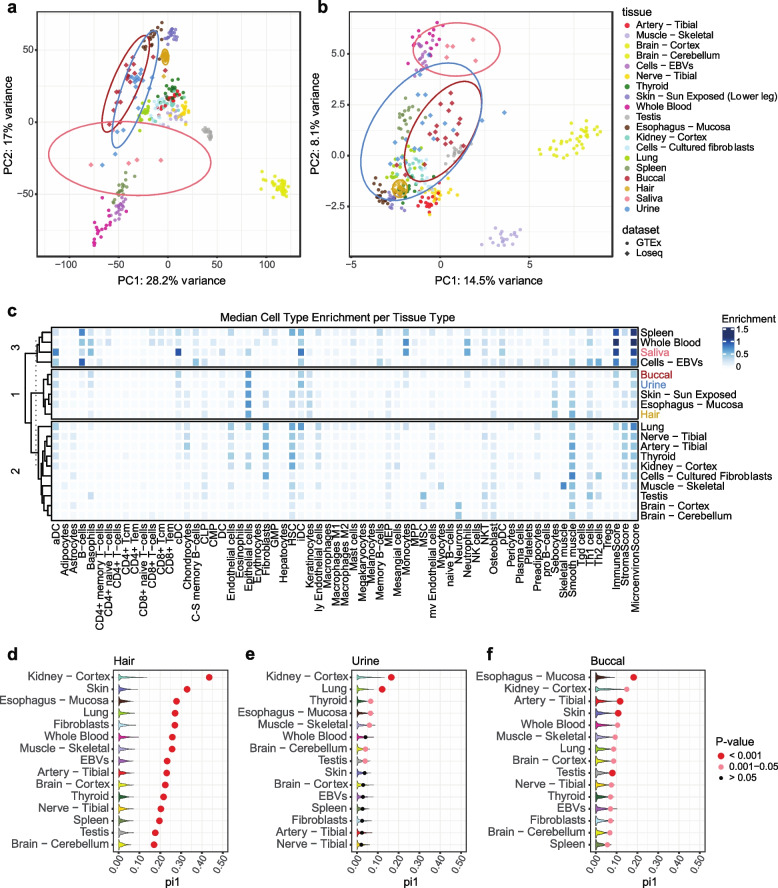
To investigate the biological similarity of noninvasive tissues to known, invasive sample types, we compared gene expression, splicing, and cell type enrichment patterns to GTEx. To do so, we first selected a single noninvasive sample per donor and per tissue with the highest protein-coding depth. Representative GTEx tissues were chosen based on k means clustering, and 19 samples of each tissue type were randomly selected. Both the noninvasive and GTEx samples were downsampled (see methods) to 5 million read counts to normalize for differences in total sequencing depth.
Using this data we projected the noninvasive samples onto the GTEx PCA space to observe global patterns of gene expression similarity (Fig. 4a). Hair clusters closely with esophageal mucosa and skin, saliva is proximal to spleen, blood, and EBVs, and buccal and urine are intermediaries between these groups. We repeated this analysis using splicing events generated from rMATS [46] (Fig. 4b). From this we recapitulate similar clustering patterns observed for expression.
Fig. 4.
Comparison of noninvasive samples to the GTEx dataset. a Noninvasive sample types projected onto the GTEx expression PCA space. Counts were normalized using DESeq2, centered and scaled, and the top 1000 most variable genes were used. Ellipses represent 95% confidence intervals. b Noninvasive sample types projected onto the top 1000 most variable rMATS splicing events in GTEx. c xCell cell type enrichment estimates per tissue. Tissues are clustered using k-means clustering. d, e, f GTEx eQTL replication estimates for hair, urine, and buccal samples. Dots show π1 calculated by selecting significant GTEx gene-variant pairs from the noninvasive data with sizing indicating permutation p-value significance. Violin plots show null π1 distributions generated from allele-frequency matched, randomly selected gene-variant pairs. 1000 permutations were performed
Since these clusters may reflect similarities in underlying cell types, we investigated this question by using xCell [47] to calculate cell type enrichment scores. Here we used xCell because it is the most comprehensive cell type database available, thus enabling analysis of diverse tissues and biospecimens, and we observed high concordance in cell type estimates between GEDIT and xCell for cell types present in both references (Supp. Fig. 8). From this analysis we replicated the same tissue clustering we observed by PCA except using cell type enrichment estimates (Fig. 4c). Because cell type sharing is highly predictive of shared gene regulatory mechanisms [26–29], this suggests gene expression regulatory mechanisms present in invasive tissues may be captured noninvasively.
To further explore how noninvasive samples may capture genetic regulatory variants discovered in postmortem tissues, we assessed replication of GTEx eQTLs in buccal, hair, and urine samples. With our sample size being insufficient for full eQTL discovery, we looked for enrichment of low p-values in our noninvasive dataset for the eVariant-eGene pairs previously discovered in GTEx. A null distribution was generated by randomly sampling allele-frequency matched eVariant-eGene pairs from our noninvasive data, and we calculated π1, an estimate of the true positive rate, in both the null datasets and when selecting the significant GTEx eVariant-eGene pairs. In hair, we find significant replication of GTEx pairs across all studied GTEx tissues, with kidney cortex and skin showing the highest degree of replication (π1 = 0.44 and π1 = 0.33, respectively, Fig. 4d). Buccal and urine are less homogenous tissue types, thus further decreasing our power especially as our sample size does not allow highly efficient approaches to correct for latent variation [48]. As such, they showed less clear signal across all tissues (Supp. Fig. 9). However, we did observe most significant enrichment for kidney cortex eVariant-eGene pairs in urine and esophageal mucosa signal enrichment in buccal (π1 = 0.17 and π1 = 0.18, respectively, Figs. 4e and 4f). Of note, kidney cortex has a low sample size relative to other tissues in GTEx and thus little power for discovery of more subtle eQTL effects. Thus, it is unclear whether the high replication of kidney eQTL signal across noninvasive tissues is due to similar biology or an abundance of common and/or high effect size eQTLs in this tissue. In all, our results suggest noninvasive tissues capture cell types and gene expression regulatory mechanisms present in invasive tissue types and may provide insight into disease processes affecting these tissues.
Sex-specific differences in gene expression in noninvasive samples
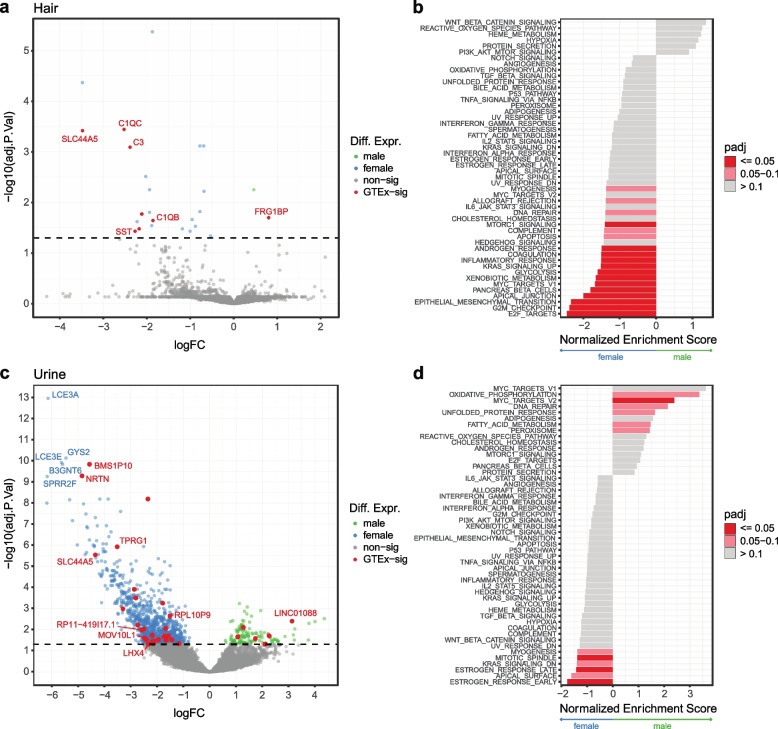
Because biological and environmental contexts play a major role in expression regulation, we aimed to explore whether RNA-sequencing from noninvasive tissues may be used for this purpose. To this end, we tested for sex-based differential expression and the replication and biological role of the discoveries. Using edgeR [49] and limma-voom [50], we were able to identify 25 and 1032 sex-based differentially expressed genes in hair and urine, respectively (Supp. Fig. 10a). In comparing hair to sun-exposed skin, 8 of the 25 significant hits were previously seen in GTEx and are highlighted in Fig. 5a. Looking across all GTEx tissues, 17 of the 25 genes were previously observed (Supp. Fig. 10b). Running FGSEA [51] on the hair results showed significant enrichment for E2F targets and G2M checkpoint pathways in females (Fig. 5b), and these are central to regulating the cell cycle and proliferation [52, 53]. Though nonsignificant, Wnt signaling, the top hit for males, has previously been reported to play a key role in hair loss prevention [54]. In urine, 33 of the 1032 significant findings were seen in kidney cortex, and, overall, 582 were differentially expressed in any GTEx tissue (Fig. 5c, Supp. Fig. 10b). Notably, estrogen response is greatly enriched in females (Fig. 5d). Estrogen signaling is central to many physiological processes in the kidney and is considered potentially protective against many renal diseases, though much remains unknown [55]. This analysis demonstrates the potential for noninvasive samples to elucidate underlying biology in a variety of potential contexts and assays.
Fig. 5.
Sex-based expression differences in noninvasive samples. a Volcano plot of sex-based differentially expressed genes in hair. Genes highlighted in red are replicated sun-exposed skin findings in GTEx. Dotted line indicates 0.05 significance threshold. b Hair FGSEA of all genes ranked by z-score and using the Hallmark Gene set from MSigDB. c Sex-based differentially expressed genes in urine cell pellets. Genes highlighted in red are replicated kidney cortex findings in GTEx. d Urine FGSEA of all genes ranked by z-score and using the Hallmark Gene set from MSigDB
Noninvasive samples may be leveraged for disease-relevant applications
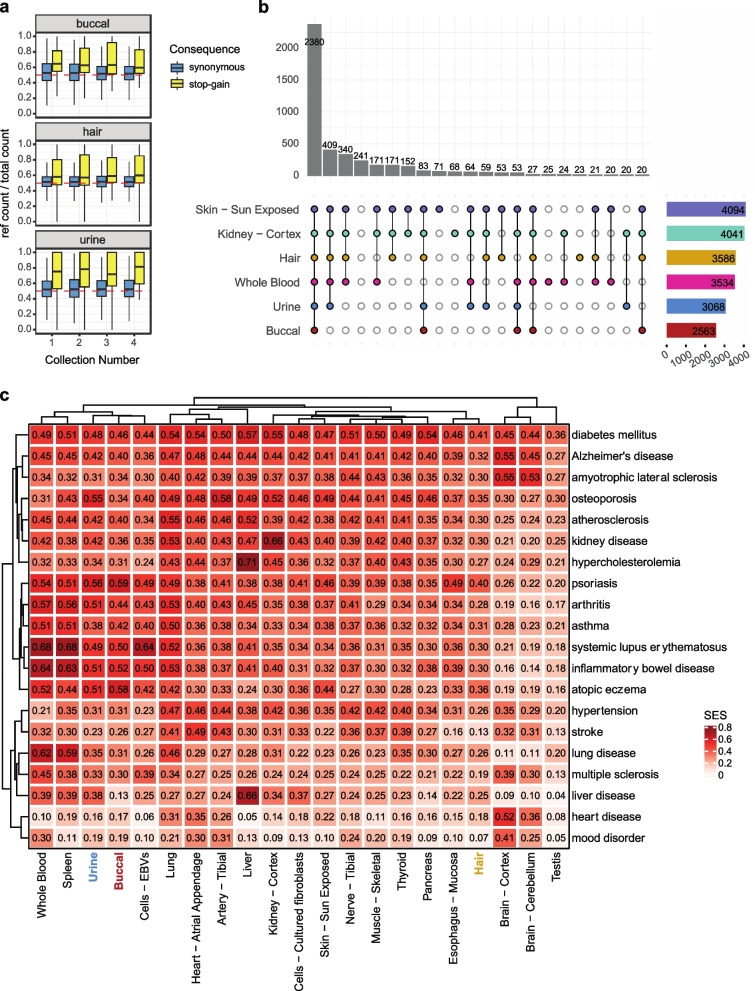
Allele specific expression (ASE) analysis compares allelic expression levels within the same individual, and it is an important tool for investigating rare and cis-regulatory variation, nonsense mediated decay, and genomic imprinting [56]. Here we quantified ASE using heterozygous sites across tissues. From this, we observe anticipated reference allele ratio patterns per individual (Supp. Fig. 11a) and depending on the SNP annotation (Supp. Fig. 11b), and we show robust nonsense mediated decay for stop-gain variants across all collections for buccal, hair, and urine samples (Fig. 6a). This suggests noninvasive sampling may be used to identify gene-disrupting variants that are a common focus of genetic diagnosis in rare disease.
Fig. 6.
Use of noninvasive samples in disease-relevant analyses. a ASE for annotated stop-gain variants vs synonymous. Only sites with > 16 total counts were included. b Genes with median expression > 0.1 TPM in a tissue were intersected with the OMIM gene set. Shown is the intersection of OMIM genes captured across tissues. c Capture of common disease signals in the OpenTargets database. SES = Σ(evidence scores of disease genes expressed in a tissue)/ Σ(evidence scores of disease genes expressed in any included tissue)
Application of noninvasive samples to rare and common disease was evaluated using the OMIM [57] and OpenTargets [58] repositories. For Mendelian disease, our samples captured a median of 80% (hair), 70% (urine), and 55% (buccal) of genes above a 0.1 TPM threshold (Supp. Fig. 13a). This capture is consistently high for hair samples across collections but shows donor-dependent consistency for urine (Supp. Fig. 13b). Clustering samples by median OMIM gene expression with GTEx recapitulated our prior proxy observations (Supp. Fig. 13c). In Fig. 6b, we show the overlapping gene sets for Mendelian genes with a median expression greater than 0.1 TPMs in a given tissue after employing GTEx expression thresholds within that tissue. The selected GTEx tissues shown are relatively minimally invasive or identified as most similar by clustering and eQTL replication. We see the vast majority of genes are captured in both noninvasive and invasive tissue types, indicating noninvasive samples may be a suitable biospecimen for studying gene expression and regulatory processes in many rare disease applications. Notably, we observe a subset of genes expressed in noninvasive samples that are not captured in whole blood (ex. 171 in hair, skin, and kidney only; 83 in all tissues except for blood). This suggests diseases where the primary tissue type affected is more akin to noninvasive samples may benefit more from the use of noninvasive sampling versus whole blood collection. Importantly, recent work from others investigating clinically accessible tissues further supports the conclusions we draw here [59]. In all, efforts to improve clinical genomics studies using transcriptomics may be further augmented by use of noninvasive samples – especially those that yield high-quality data and robust gene expression estimates—especially where invasive surgical sampling of tissues primarily affected is often not possible.
Looking at common disease, we first selected the most general OpenTargets ontology category for every disease included. We filtered for genes with greater than 5 sources of evidence and with tissue elevated specificity from the Human Protein Atlas database [60] (Supp. Fig. 12). Disease enrichment was calculated by summing together OpenTargets gene evidence scores (summed evidence score, SES) for genes with median expression greater than zero in a given tissue and dividing by the total possible summed evidence score. From this, we found buccal and urine captured diseases with a strong immunological component, much like whole blood and spleen (Fig. 6c). Urine additionally showed strong signal for kidney disease (SES = 0.42). Hair performed best for skin-related diseases (psoriasis SES = 0.40), but overall did not show strong enrichment for any particular disease. Because OpenTargets relies on current RNA-sequencing datasets and the majority use whole blood or related tissues, it is unsurprising given our cell type deconvolution analysis that buccal and urine more strongly capture the disease-relevant signals in the dataset compared to hair follicles. Our results here combined with our GTEx tissue comparisons suggest hair follicles may be well-suited for obtaining expression patterns not accessible in current study designs. Overall, these results suggest noninvasive samples bear promise for use in disease-relevant studies while providing the advantage of potentially longitudinal monitoring and greater enrollment.
Discussion
Discovery from transcriptomic data and its use in precision medicine is considerably limited by cost and access to biologically applicable biospecimens [18, 61]. As a result, most transcriptome studies have lagged behind GWAS in sample size, which now often include hundreds of thousands of individuals, and studies still need to increase enrollment from non-European populations. Further, disentangling causation and finding context-specific disease mechanisms is challenging using a single collection time point [17, 62]. To address these limitations, we sought to investigate low-cost, noninvasive RNA-sequencing as an alternative approach. From our study we observed hair follicles and urine cell pellets provide the highest quality data and perform best in functional genomics and clinical applications.
A primary advantage of noninvasive biospecimens over blood-related specimens to the transcriptomics field is the set of cell types captured. Shared cell type composition corresponds with shared regulation of gene expression and splicing [15, 16, 21, 22], and a major limitation of blood-related samples is that they represent a highly tissue-specific set of cell types. In noninvasive samples we observed greater similarity by expression, splicing, and genetic regulation to invasive GTEx tissues relative to GTEx whole blood. Cell type deconvolution analysis estimated noninvasive tissues to contain epithelial cells, myocytes, stromal cells, and others, all of which are unable to be captured using blood and play a key role in mechanisms of many diseases.
Several considerations should be taken into account when deciding to use noninvasive tissues. Generally, ease of sample collection and consistency of library preparation performance and quality is biospecimen-dependent. Additionally, the feasibility of clinical use and longitudinal study design varies depending on the tissue type. We observed high failure rates using buccal swabs and saliva, and though the data yielded from samples passing quality standards provides valuable insights and the samples themselves are simplest to collect, we believe use of these biospecimens should be reserved for specialized applications where health status of the oral cavity and/or upper gastrointestinal tract is primarily under study.
Though we found urine cell pellets to yield high quality RNA, the pellet itself may be minimal and difficult to visualize for some donors. This is especially true for healthy donors, who tend to shed fewer cells into their urine [29], and could introduce bias into future study designs if special care is not taken when using this tissue. Similarly, we found urine cell pellet RNA quantity to be variable and donor dependent. Others have aforementioned single cell approaches for urine specimens [30, 31], and we anticipate further development of these methods will better control for sampling inconsistency. Here, we showed urine cell pellets capture genetic regulatory mechanisms seen in the kidney as well as gene expression signatures relevant to kidney disease and diseases mediated via kidney functions. Given the enormous health burden kidney disease poses in the US and worldwide, and the central role the kidney plays in many diseases [63], methods for noninvasively monitoring kidney function and enabling early diagnosis could meaningfully improve morbidity and mortality. In addition to the analyses performed here, others have proposed laboratory protocols for propagating cells collected from urine for use in identifying disease mechanisms and novel treatments, or, in the case of stem cells, developing autologous cell therapies [32–34]. Overall, given further optimization, we expect urine holds the greatest potential for clinical use and discovery.
Hair follicles perform robustly using any library preparation and exhibit low technical variance across collections and donors. It should be noted that hair follicle collection does require additional training of personnel not necessarily needed for the other biospecimens. Also, fine versus coarse hair type played a role in determining the ease of collection, and we do observe slight differences in yield depending on the donor, though this did not impact sample performance. We do foresee the need to explore collection of hair follicles from other parts of the body when head hair is not available, and it is likely necessary in future studies to collect additional information regarding the use of cosmetics and medications applied to the head and skin. Here, we showed hair follicles result in consistent quality, cell types, and expression profiles across collections, and, despite our low sample size, we found significant replication of previously observed eQTLs across all GTEx tissues. Together, these findings suggest hair is a highly robust biospecimen with potentially broad applications, and, because of its consistency, biological perturbations due to disease, treatment, or other environmental exposures will likely be observable in clinical and longitudinal settings.
Notably, across all noninvasive tissues we observed a large majority of Mendelian disease genes were expressed. The ease and decreased invasiveness of noninvasive proxy biospecimens could facilitate greater use of transcriptome analyses in diagnosing rare, genetic disease [59], however, further work is needed to explore this possibility.
Our findings will need to be validated using larger, more diverse, and clinical cohorts. We expect noninvasive tissues may reduce Eurocentric sampling bias and enable sampling from more vulnerable populations, but this expectation will need to be measured against future study enrollment. Additionally, we used bulk RNA-sequencing, and the performance and features of noninvasive samples using single cell methods will need to be optimized and evaluated.
Conclusion
In this study we aimed to establish whether noninvasive sampling may be used to scale transcriptomic studies. From our work, we were able to characterize many of the technical and biological features of four possible noninvasive samples, and we showed their potential utility in both transcriptomic and disease-related applications. Overall, we find hair follicles and urine cell pellets to be the most promising biospecimens, and we propose advantages in terms of cost and study designs for pursuing noninvasive sampling. In all, noninvasive RNA-sequencing offers meaningful improvements to current transcriptomic approaches that could enable dramatic scaling in sample size and increased discovery potential. This scaling would bring closer parity with GWAS via transformational increases in power, thus better positioning transcriptomic studies for use in diagnostic and clinical applications.
Methods
Noninvasive sample collection
IRB approval was obtained for the study. 19 participants were recruited and consented, and four total collections of four tissue types were completed. The first collection occurred 6 months prior to confirm piloted procedures were ready to scale, and the remaining 3 collections were performed within a 2–4 week window per participant. 75 samples of each tissue type were obtained (1 participant provided only 3 collections). A detailed noninvasive sample collection protocol is provided on protocol.io (DOI: dx.doi.org/10.17504/protocols.io.kqdg3pjzzl25/v1).
Library preparation and sequencing
RNA extraction procedures are unique to each tissue type, and thus were performed separately for each tissue. Collections were randomized across extractions. All samples were prepared using our in-house method with 15 samples of each tissue type in duplicate. Takara Bio SMART-seq v4 and Illumina stranded mRNA prep kits were prepared according to the manual and using a random subset of 12 and 16 samples of each tissue type, respectively. In total, 6 library preparation plates were prepared, and 2 HEK cell samples were included in triplicate on each library prep plate. This resulted in 508 total samples. 485 samples passed yield and size pre-sequencing quality parameters (> 2 nM yield and < 600 bp average size). Samples were pooled by tissue type with HEK cell samples randomized across the library pools. Libraries were sequenced 2 × 150 bp on a Novaseq 6000 S4 flow cell. A detailed sample preparation protocol for RNA-extraction and our in-house method is provided on protocol.io (DOI: dx.doi.org/10.17504/protocols.io.kqdg3pjzzl25/v1).
Alignment, quantification of gene counts, and quality assessment
Adapter sequences were removed using Trimmomatic 0.36 [64]. Sequences were aligned to build 38 of the human genome with Gencode v35 annotation using STAR 2.7.3a [65] and Samtools 1.9 [66] set to GTEx mapping parameters [18]. Marking of duplicate reads was done using Picard 2.23.7 [67], and gene counts were quantified using featureCounts from Subread 1.6.5 [68]. QC results output from FastQC 0.11.3 [69], STAR, and RNA-SeQC 2.3.6 [39] were consolidated using MultiQC 1.8 [70]. QC filtering based on standard sequencing quality metrics or based on protein coding and lncRNA depth thresholds were found to be largely redundant (Supp. Fig. 2), and thus depth of mapped reads was used for its simplicity. Genes were determined to be expressed in a tissue and included in downstream analyses if raw counts > = 8 and TPMs > = 0.1 in > = 20% of samples within a given tissue type.
Unmapped reads analysis
Unmapped reads were output to fastq files during alignment. These reads were remapped using DecontaMiner 1.4 [40]. 23,488 bacterial, 21 fungal, and 11,120 viral genome references were downloaded as suggested in the Installation and User Guide. Default parameters were used to remove low quality, human ribosomal and mitochondrial reads. For BLASTn alignments to the reference databases, bacterial and fungi parameters included minimum length = 50 bp and gaps and mismatches = 2 bp. Gaps and mismatches were increased to 5 bp for viral genome remapping. Organisms were left unfiltered during initial remapping settings (Supp. Fig. 5a). For the analysis, we normalized the results by genomic length of the remapped species and by number of remapped reads per sample. We selected the top 0.5% of remapped species across all tissue types.
Technical and biological sources of unmapped reads were investigated by first removing all Decontaminer remapped reads from the unmapped fastqs, and then using FastQC to identify overrepresented sequences in the remaining reads. These sequences were compiled across all tissues and samples into a list of 707 sequences. Command line tools were used to filter and quantify the overrepresented read counts per sample. Computational and manual comparison to primer and adapter sequences as well as comparisons across preps and tissues were used to delineate the potential sources of the reads (Supp. Fig. 5d).
Downsampling
For analyses involving downsampling, binomial sampling was performed 5 times on the raw, unfiltered counts matrix and then taking the average. Binomial probability of success was set to the (desired depth)/(original depth), number of observations set to the total gene number, and trials set to the gene counts per a given gene. Samples were QCed for protein coding and lncRNA depth prior to downsampling. For comparisons across different library preparations, all samples passing a threshold depth of 1 million reads mapped to the human genome were included and then subsequently downsampled to 1 million (Supp. Fig. 2a,b,c). Loseq analyses were thresholded and downsampled to 2.5 million protein coding depth (Supp. Fig. 2d,e,f), with the exception of the cell type deconvolution analysis which was thresholded and downsampled to 5 million. All GTEx comparisons were made with both Loseq and GTEx thresholded and downsampled to 5 million.
Cross-preparation comparison
Median TPMs per gene were calculated within a given tissue, prep, and replicate group, and the Pearson correlation across replicate groups 1 and 2 for a given tissue and prep was quantified (Supp. Fig. 3). Overlap of gene expression capture was evaluated by taking the median TPMs within a tissue and prep, filtering genes with zero median expression, and determining the gene overlap using ComplexUpset 1.3.1 [71, 72] in R 3.6.0 (Supp. Fig. 3). Principal component analysis was performed using DESeq2 1.26.0 [73] VST normalized counts and by selecting for the top 500 most variable genes. Variance attributable to tissue and prep was done by performing linear regression per PC (PC ~ tissue + prep) followed by ANOVA with p-value correction based on the number of PCs tested (Supp. Fig. 4).
Loseq cross-sample variance assessment
Principle components analysis was done across tissues using DESeq2 1.26.0 VST normalized counts and by selecting for the top 1000 most variable genes. Correlation of technical variables with PCs was investigated via linear regression. VariancePartition 1.21.6 [74] was used to identify sources of gene expression variance within and across tissue types. The cross tissue VariancePartition model included collection, extraction, donor id, and tissue as random variables and rRNA rate, mapping rate, duplicate rate, exonic rate, 3’ bias, RNA concentration, a260/280, cDNA size, and GC content as fixed variables. Within tissue models were the same except tissue type was dropped as a variable (Supp. Fig. 6).
Cell type deconvolution of noninvasive samples
Deconvolution was done using GEDIT 1.7 [45] and the provided BlueCodeV1.0.tsv reference matrix. For the final analyses, cell types were collapsed into broader umbrella categories by adding the estimated proportions together (Supp. Fig. 7b). Only the top 25% most abundant cell types per tissue were considered when looking across collections, and only donors with all 4 collections passing QC in urine, hair, and buccal were included in the final plot. All donors are plotted in the supplement (Supp. Fig. 7a).
PCA projection of noninvasive samples onto GTEx
For Loseq, the sample with the highest protein coding and lncRNA depth passing the 2.5 million threshold was selected per donor and per tissue type (19 samples per hair and urine, 17 buccal, 5 saliva), and both Loseq and GTEx were downsampled to 5 million. 19 samples of each representative (see xCell section) GTEx tissue were randomly sampled. Counts were VST normalized using DESeq2 1.26.0. Principal components analysis was run on centered and scaled GTEx counts using the top 1000 most variable genes. The resulting PCA loadings were multiplied by GTEx and Loseq centered and scaled counts, and this data was plotted as shown in the main figure.
The splicing PCA was performed in the same manner except using an exon inclusion level matrix generated by rMATS 4.1.2 [46] as input. Splicing events with zero inclusion for any sample in a tissue were excluded. The rMATS results were filtered for events found to be significantly different across the select GTEx tissues and with inclusion levels greater than 2 standard deviations beyond the average inclusion (0.3). Again, PCA was run for the top 1000 most variable events in GTEx, and the loadings were multiplied by the GTEx and Loseq centered and scaled inclusion levels.
Cell type enrichment analysis of noninvasive and GTEx samples
75 samples were sampled from each GTEx tissue to approximately match the number of Loseq samples included (75 hair, 63 urine, 25 buccal, 5 saliva). GTEx and Loseq TPMs were deconvolved for enrichment using 64 cell type signatures in xCell 1.1.0 [47] in R 3.6.0. Select GTEx tissues were chosen using gene expression clustering of median TPMs per GTEx tissue. GTEx groups were established using k means, and the tissue with the highest sample size per group was selected as representative. Kidney cortex, esophageal mucosa and lung were added based on their proximity to noninvasive tissues we studied. Clustering of cell type enrichment was done by taking the median enrichment score per tissue and then using k means in ComplexHeatmap 2.2.0 [75].
GTEx eQTL replication analysis
Participants provided their genotyping data from 23andme (9 donors), Ancestry (2 donors), and Gencove (8 donors) platforms. Array data was imputed using the 1000 genomes phase 3 reference [76] and the Sanger Imputation Service [77]. Eagle 2.4.1 [78] and the 1000 genomes reference were used for VCF phasing. Monomorphic alleles, alleles with MAF < 0.05, multiallelic sites and indels were excluded from all analyses. This imputed, phased, and filtered VCF was used for eQTL and ASE analyses.
For the genotyping PCA, LD pruning was performed with PLINK 1.90-b3.29 [79] with a window size of 50, shift of 5, and r squared cutoff of 0.2. Only SNPs with a 100% genotyping rate and HWE 1e-5 were included. Ancestry was imputed by merging the donor VCF with the 1000 genomes VCF, excluding any individuals with relatedness > = 0.0625, running smartpca with eigensoft 6.1.3 [80], and using k-nearest neighbors to infer the ancestry population of our donors. Running smartpca on the donor samples alone revealed genotyping PC1 corresponded with ancestry, while PC2 corresponded with genotyping panel. Expression data from the collection with the highest protein coding and lncRNA depth was selected per donor and per tissue type (19 samples per hair and urine, 17 buccal). Genotyping PCs 1 and 2 were included as covariates in the eQTL analysis, as well as expression PCs with variance explained > 15% that accounted for global changes in gene expression (PC1 for hair and buccal and PCs 1 and 2 for urine). Counts were TMM and inverse normalized and filtered for genes with raw counts > = 6 and TPMs > = 0.1 (as is the GTEx standard). eQTL mapping was done on a per tissue basis using TensorQTL v.1.0.5 with the window set to 1 MB (following the GTEx parameters).
To calculate replication, each GTEx tissue was filtered for the top eVariant-eGene pair per gene with MAF > = 0.05, qvalue < = 0.05, and with effect size greater than the minimum observed in the lowest powered GTEx tissue (kidney cortex = 0.32). This was intersected with the pairs discovered in the noninvasive dataset, and pi1 was calculated using qvalue 2.18.0 [81] and R 3.6.0. Null datasets were of the same size as the overlapping significant GTEx pairs set and were generated by sampling allele-frequency matched eVariant-eGene pairs from the noninvasive data 1000 times. Pi1 was calculated per each dataset. Significance for enrichment was determined based on permutation p-value calculations (Supp. Fig. 9).
Differential expression analysis and FGSEA
Sex-based differential expression analysis was performed on a per tissue basis using edgeR 3.28.1 [49] and Limma-Voom 3.42.2 [50]. Counts were filtered based on GTEx parameters and TMM normalized prior to analysis. Multiple donor collections were adjusted for by treating the donor as a blocking variable and applying the duplicateCorrelation function in limma. GTEx sex-based differential expression results [82] were retrieved from the GTEx Portal (https://gtexportal.org/home/datasets) for overlap comparisons (Supp. Fig. 10b). For noninvasive tissues, a named list of sex-based differentially expressed genes and their t-scores was input into FGSEA 1.12.0 [51]. The Hallmark gene sets file was obtained from Molecular Signatures Database (http://www.gsea-msigdb.org/gsea/msigdb/collections.jsp#H) [83] and used for the analysis.
Loss of function detection using ASE
Allele-specific expression was calculated using ASEReadCounter 4.0.1.1 [56] and using the imputed, phased, and filtered VCF described in the GTEx replication analyses. Sites with fewer than 16 total counts were filtered from the analysis. The reference counts divided by total counts was assessed across tissues and donors, and one donor was removed due to extreme ratios and thus potential genotyping errors (Supp. Fig. 11a). Ensembl Variant Effect Predictor 5.28.1 [84] was used to annotate variant consequences, and the ratio of reference to total allele counts was compared given these annotations (Supp. Fig. 11b).
OMIM Mendelian disease gene overlap
Genes with Mendelian inheritance were downloaded from the OMIM database [57] (https://www.omim.org/). The donor sample with the highest protein coding and lncRNA depth was included per tissue. Tissues were filtered were genes meeting minimum GTEx expression thresholds, and then the remaining gene set was overlapped with OMIM genes (Supp. Fig. 13). ComplexUpset 1.3.1 was used to compare genes captured across noninvasive and select GTEx tissues.
OpenTargets evaluation of disease-relevancy
Data was retrieved from the OpenTargets database [58]. Disease ids were selected by choosing the broadest ontological category specific to a given disease (as provided on the OpenTargets platform). For the analysis, the association file incorporating all sources of evidence was used, and disease genes were included only if there were 5 or more sources of evidence (Supp. Fig. 12a). Loseq samples were thresholded and downsampled to a protein coding and lncRNA depth of 5 million, and samples with the highest depth per donor and tissue were selected. GTEx was similarly downsampled and 19 samples of each GTEx tissue were randomly selected. GTEx tissues were chosen based on their relevance to selected diseases. Genes were filtered based on GTEx parameters, and the median TPMs per tissue was calculated. 10,986 tissue-elevated genes were obtained from the Human Protein Atlas [60] (https://www.proteinatlas.org/humanproteome/tissue/tissue+specific), and each tissue was additionally filtered for tissue-elevated genes. In the end, the top 3,411 most expressed, tissue-elevated genes present in a tissue were analyzed, based on the tissue with the lowest number of genes passing post-expression and HPA filtering (Supp. Fig. 12b).
To calculate the summed evidence score, first, the original target overlap was calculated by intersecting the genes present across all tissues with the disease gene targets. Summing the evidence score for these genes resulted in the total possible score for a given disease. Then, the genes expressed in a particular tissue were overlapped with disease gene targets. The evidence scores for tissue-specific overlapping genes were summed together and then divided by the total possible score for a disease. This normalized score is the summed evidence score reported in the analysis (Supp. Fig. 12c).
Supplementary Information
Additional file 1: Supplementary Figure 1. a. Outcome of library preparation QC per donor, collection, and preparation. The crosses indicate a failed sample, and the numbers correspond to the collection. b. Measured RIN vs computationally-derived transcript integrity number (TIN) per sample. Passing is determined by 1 million protein-coding depth threshold. c. RNA yield distribution per tissue type. Buccal median = 1054.2ng, Q1 = 735.7ng, Q3 = 1292.55ng. Hair median = 1444.1ng, Q1 = 606.2ng, Q3 = 2530.5ng. Saliva median = 4705.4ng, Q1 = 2278.5ng, Q3 = 10920.35ng. Urine median = 253.4ng, Q1 = 98ng, Q3 = 1642.2ng. d. cDNA average size for Loseq and SmartSeq preparations across noninvasive tissues. e. RNA yield per donor and tissue. Each data point is a collection. Buccal Levene P = 0.05, Hair Levene P = 0.01, Saliva Levene P = 0.2, Urine Levene P = 0.0002. f. Total reads sequenced per sample, colored by prep. g. Categorization of gene types for uniquely mapped reads mapping to genes across tissues. Supplementary Figure 2. a-c quality statistics for all samples across all library preparations: a. b. Samples remaining and genes detected depending on depth threshold. Dotted line indicates preparation QC threshold of 1 million. c. Proportion of reads mapping to exonic, intergenic, and intronic genomic features. Pass indicator is based on preparation QC threshold of 1 million. d-f quality statistics for only Loseq samples: d. e. Samples remaining and genes detected depending on depth threshold. Dotted line indicates Loseq QC threshold of 2.5 million. f. Proportion of reads mapping to exonic, intergenic, and intronic genomic features. Pass indicator is based on Loseq QC threshold. Supplementary Figure 3. a. Intersection of genes with greater than zero median expression across library preparations. b. c. Comparison of gene length and GC content of genes uniquely captured in a given preparation. d. Comparison of median gene expression levels of library preparation replicates. The median of all replicate 1 samples (15) is compared against the median of all replicate 2 samples (15) per library preparation. The spearman correlation between these is shown. e. Comparison of the number of genes detected per tissue and library preparation depending on the RNA yield of the sample. Pearson correlation and p-value is shown. Supplementary Figure 4. a. b. Principal Component Analysis of all samples passing preparation QC thresholds. c. Percent variance explained per PC. d. ANOVA results for PC ~ tissue and PC ~ preparation. P-values are Bonferroni-corrected for the number of PCs tested (10). Supplementary Figure 5. a. Schematic of pipeline for assigning unmapped reads. Briefly, unmapped reads were remapped using decontaminer, normalized using a procedure akin to TPM normalization, then species of interest were filtered based on their abundance and replication across technical replicates. Remaining unmapped reads were investigated using FastQC. b. Total number of reads assigned to each category. Mapped = aligned to hg38. Remapped = aligned to microbial species using Decontaminer. Repeated = highly abundant reads identified by FastQC. Unknown = reads not mapped, remapped, or highly abundant. c. For each species included in the final analysis, spearman rank correlation between technical replicates is shown with the dots. Bar plot of species abundance with error bars is shown in the background for direct comparison (y-scale of abundance in Figure 2b). d. Breakdown of repeated sequence sharing across tissues and library preparations. e. Mapping rate per tissue and library preparation as a function of RNA yield. Pearson correlation and p-value is shown. Supplementary Figure 6. a. Variance in gene expression across tissues explained by technical and biological variables. Canonical correlation analysis shows correlation between variables used. b. c. Variance in gene expression within each tissue explained by technical and biological variables. Canonical correlation analysis shows correlation between variables used. Only Loseq samples were included in these analyses. Supplementary Figure 7. A. GEDIT cell type proportion estimates per collection and donor. Top 25% most abundant, condensed cell type categories are shown. b. Breakdown of all cell types included in the GEDIT reference. Binning into larger cell type categories is shown. c. Cell type proportion estimates looking across samples ordered from lowest to highest RNA yield. Supplementary Figure 8. a. xCell enrichment scores across the noninvasive and select GTEx tissues for cell types corresponding to the GEDIT collapsed cell type categories. Note that the enrichment score does not correspond to a proportion. b. Comparison of xCell enrichment scores and GEDIT proportions for cell types shared by both references. Only noninvasive tissues are shown. Supplementary Figure 9. a. b. c. Calculated pi1 versus the null pi1 distribution for every tissue in GTEx. Pi1 was calculated by selecting for significant variants (q-value <=0.05) with MAF > 0.05 and minimum effect size greater than the maximum minimum across GTEx tissues (kidney cortex 0.32) that were present in the noninvasive dataset. The null distribution was generated by performing 1000 samples of size equivalent to the number of overlapping gene-variant pairs used for the pi1 calculation for that tissue. d. e. f. Histograms of Loseq p-values for gene-variant pairs included in the pi1 calculation. Supplementary Figure 10. a. Number of sex-based upregulated genes per noninvasive tissue type. b. Per tissue overlap between significant DE genes in the noninvasive dataset with genes previously found to be significant in the GTEx dataset. c. Sex-based differential expression for buccal samples, with no significant genes. FGSEA shows some rank-based gene category enrichment for females. Supplementary Figure 11. a. Ratio of (reference allele count)/(total count) for all heterozygous sites per donor and tissue. b. Reference ratio breakdown per VEP annotation. Supplementary Figure 12. a. Total number of disease-relevant genes per OpenTargets ontology category. Genes with >= 5 separate sources of evidence were included in the final analysis. b. Number of genes per tissue following minimum expression level thresholding and overlap with the HPA tissue-elevated gene list. The top 3,411 most expressed genes per tissue were included in the analysis. c. Summed evidence scores (SESs) for all GTEx and noninvasive tissues. Supplementary Figure 13. a. Proportion and total OMIM gene capture per noninvasive tissue type, depending on minimum median TPM threshold. b. Proportion of OMIM gene capture per collection, donor, and tissue using a minimum expression threshold of 0.1 TPMs. c. Clustering of noninvasive and select GTEx tissues based on median OMIM gene expression. Supplementary Table 1. Breakdown of reaction cost (as of August 2022) per reagent using our in-house method (Loseq) versus TruSeq Stranded mRNA Library Prep and Takara SMART-seq V4 commercial kits. The cost of Ampure XP beads (cat# A63881) is excluded, but it is notably lower per reaction for Loseq and SMART-seq preparations due to smaller volume requirements. Supplementary Table 2. Comparison of total cost per reaction by library preparation. Loseq percent cost reduction calculated as: (1-(Loseq/Commercial kit))*100.
Acknowledgements
We would like to thank the members of the Lappalainen laboratory, most notably Kristina Buschur and Paul Hoffman, as well as William Mauck (Satija Laboratory, NYGC) and Stephanie Hao (Innovation Laboratory, NYGC). We thank the 19 donors for their participation and contribution to science.
Authors’ contributions
M.M., S.E.C, and T.L. designed the study. S.E.C. performed donor recruitment and enrollment. S.E.C. and M.M. optimized the in-house library preparation protocol. M.M. collected and prepared all donor samples for sequencing and performed or led all analyses herein. A.G. assisted in data generation. R.G. did the unmapped reads and splicing analyses. S.K. provided code, expertise, and supervision of eQTL analyses. M.M. wrote the manuscript. S.E.C., S.K., R.G., and T.L. edited the manuscript. All authors read and approved the final manuscript.
Funding
M.M. was supported by the NHGRI (F30HG011194). S.K., and T.L. were supported by the NHLBI (R01HL142028). R.G., A.G., and T.L. were supported by the NIA (R01AG057422). S.E.C. was supported by the NHGRI (1K99HG009916).
Availability of data and materials
The data set generated and analyzed in the study is available on dbGaP (accession number posted upon publication). Noninvasive sample collection and in-house library preparation methods are provided on protocols.io (dx.doi.org/10.17504/protocols.io.kqdg3pjzzl25/v1). Analysis code has been deposited in the GitHub repository: https://github.com/LappalainenLab/loseq.
Declarations
Ethics approval and consent to participate
IRB approval was granted by both Columbia University Human Research Protection Office and IRBs and by Biomedical Research Alliance of New York (BRANY) for the study, and informed consent was obtained from all 19 participants. All methods were carried out in accordance with the protocols laid out in the approved IRB and in accordance with current guidelines and regulations regarding human subject research. Consent for publication is not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stephane E. Castel and Tuuli Lappalainen contributed equally to this work.
Contributor Information
Molly Martorella, Email: mem2331@cumc.columbia.edu.
Stephane E. Castel, Email: stephanecastel@gmail.com
Tuuli Lappalainen, Email: tlappalainen@nygenome.org.
References
- 1.Zeggini E, Gloyn AL, Barton AC, Wain LV. Translational genomics and precision medicine: Moving from the lab to the clinic. Science. 2019;365:1409–1413. doi: 10.1126/science.aax4588. [DOI] [PubMed] [Google Scholar]
- 2.Supplitt S, Karpinski P, Sasiadek M, Laczmanska I. Current achievements and applications of transcriptomics in personalized cancer medicine. Int J Mol Sci. 2021;22:1422. doi: 10.3390/ijms22031422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27:1345–1356. doi: 10.1038/s41591-021-01450-2. [DOI] [PubMed] [Google Scholar]
- 4.Docking TR, et al. A clinical transcriptome approach to patient stratification and therapy selection in acute myeloid leukemia. Nat Commun. 2021;12:2474. doi: 10.1038/s41467-021-22625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings BB, et al. Improving genetic diagnosis in Mendelian disease with transcriptome sequencing. Sci Transl Med. 2017;9:09. doi: 10.1126/scitranslmed.aal5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremer LS, et al. Genetic diagnosis of Mendelian disorders via RNA sequencing. Nat Commun. 2017;8:15824. doi: 10.1038/ncomms15824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammadi P, et al. Genetic regulatory variation in populations informs transcriptome analysis in rare disease. Science. 2019;366:351–356. doi: 10.1126/science.aay0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manolio TA, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price AL, Spencer CCA, Donnelly P. Progress and promise in understanding the genetic basis of common diseases. Proc Biol Sci. 2015;282:20151684. doi: 10.1098/rspb.2015.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visscher PM, et al. 10 years of GWAS discovery: biology, function, and translation. Am J Hum Genet. 2017;101:5–22. doi: 10.1016/j.ajhg.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle EA, Li YI, Pritchard JK. An expanded view of complex traits: from polygenic to omnigenic. Cell. 2017;169:1177–1186. doi: 10.1016/j.cell.2017.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cano-Gamez E, Trynka G. From GWAS to function: using functional genomics to identify the mechanisms underlying complex diseases. Front Genet. 2020;11:424. doi: 10.3389/fgene.2020.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lappalainen T, MacArthur DG. From variant to function in human disease genetics. Science. 2021;373:1464–1468. doi: 10.1126/science.abi8207. [DOI] [PubMed] [Google Scholar]
- 14.Albert FW, Kruglyak L. The role of regulatory variation in complex traits and disease. Nat Publ Group. 2015;16:197–212. doi: 10.1038/nrg3891. [DOI] [PubMed] [Google Scholar]
- 15.Kim-Hellmuth S, et al. Cell type–specific genetic regulation of gene expression across human tissues. Science. 2020;369:8. doi: 10.1126/science.aaz8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donovan MKR, D’Antonio-Chronowska A, D’Antonio M, Frazer KA. Cellular deconvolution of GTEx tissues powers discovery of disease and cell-type associated regulatory variants. Nat Commun. 2020;11:955–1014. doi: 10.1038/s41467-020-14561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umans BD, Battle A, Gilad Y. Where are the disease-associated eQTLs? Trends Genet. 2021;37:109–124. doi: 10.1016/j.tig.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GTEx Consortium The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318–1330. doi: 10.1126/science.aaz1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mele M, et al. The human transcriptome across tissues and individuals. Science. 2015;348:660–665. doi: 10.1126/science.aaa0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamazon ER, et al. Using an atlas of gene regulation across 44 human tissues to inform complex disease- and trait-associated variation. Nat Genet. 2018;50:956–967. doi: 10.1038/s41588-018-0154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jagadeesh, KA. et al. Identifying disease-critical cell types and cellular processes across the human body by integration of single-cell profiles and human genetics. 2021;1–86. https://www.biorxiv.org/content/10.1101/2021.03.19.436212v1.full.pdf.
- 22.Hekselman I, Yeger-Lotem E. Mechanisms of tissue and cell-type specificity in heritable traits and diseases. Nat Rev Genet. 2020;21:137–150. doi: 10.1038/s41576-019-0200-9. [DOI] [PubMed] [Google Scholar]
- 23.GTEx Standard Operating Procedures Library | Programs | BBRB. https://biospecimens.cancer.gov/resources/sops/library.asp.
- 24.Shavers-Hornaday VL, Lynch CF, Burmeister LF, Torner JC. Why are African Americans under-represented in medical research studies? Impediments to participation. Ethn Health. 1997;2:31–45. doi: 10.1080/13557858.1997.9961813. [DOI] [PubMed] [Google Scholar]
- 25.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104:e16–e31. doi: 10.2105/AJPH.2013.301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luebbert R, Perez A. Barriers to clinical research participation among African Americans. J Transcult Nurs. 2016;27:456–463. doi: 10.1177/1043659615575578. [DOI] [PubMed] [Google Scholar]
- 27.Britton A, et al. Threats to applicability of randomised trials: exclusions and selective participation. J Health Serv Res Policy. 1999;4:112–121. doi: 10.1177/135581969900400210. [DOI] [PubMed] [Google Scholar]
- 28.Theda C, et al. Quantitation of the cellular content of saliva and buccal swab samples. Nat Publ Group. 2018;8:6944. doi: 10.1038/s41598-018-25311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bondue T, et al. Urine-derived epithelial cells as models for genetic kidney diseases. Cells. 2021;10:1413. doi: 10.3390/cells10061413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung MD, et al. Single-cell RNA sequencing of urinary cells reveals distinct cellular diversity in COVID-19-associated AKI. Kidney360. 2022;3:28–36. doi: 10.34067/KID.0005522021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latt KZ, et al. Urine single-Cell RNA sequencing in focal segmental glomerulosclerosis reveals inflammatory signatures. Kidney Int Rep. 2022;7:289–304. doi: 10.1016/j.ekir.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira Arcolino F, et al. Human urine as a noninvasive source of kidney cells. Stem Cells Int. 2015;2015:362562. doi: 10.1155/2015/362562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley MS, et al. Urine RNA processing in a clinical setting. Female Pelvic Med Reconstr Surg. 2017 doi: 10.1097/spv.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavathuparambil Abdul Manaph N, Al-Hawwas M, Bobrovskaya L, Coates PT, Zhou XF. Urine-derived cells for human cell therapy. Stem Cell Res Ther. 2018;1–12. 10.1186/s13287-018-0932-z. [DOI] [PMC free article] [PubMed]
- 35.Ng DL, et al. A diagnostic host response biosignature for COVID-19 from RNA profiling of nasal swabs and blood. Sci Adv. 2021;7:eabe5984. doi: 10.1126/sciadv.abe5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziegler CGK, et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell. 2021;184:4713–4733. doi: 10.1016/j.cell.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SJ, et al. Gene expression in head hair follicles plucked from men and women. Ann Clin Lab Sci. 2006;36:115–126. [PubMed] [Google Scholar]
- 38.Herrera-Rivero M, Hochfeld LM, Sivalingam S, Nöthen MM, Heilmann-Heimbach S. Mapping of cis-acting expression quantitative trait loci in human scalp hair follicles. BMC Dermatol. 2020;20:16. doi: 10.1186/s12895-020-00113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeLuca DS, et al. RNA-SeQC: RNA-seq metrics for quality control and process optimization. Bioinforma Oxf Engl. 2012;28:1530–1532. doi: 10.1093/bioinformatics/bts196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sangiovanni M, Granata I, Thind AS, Guarracino MR. From trash to treasure: detecting unexpected contamination in unmapped NGS data. BMC Bioinformatics. 2019;20:168. doi: 10.1186/s12859-019-2684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deo PN, Deshmukh R. Oral microbiome: unveiling the fundamentals. J Oral Maxillofac Pathol JOMFP. 2019;23:122–128. doi: 10.4103/jomfp.JOMFP_304_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. 2018;16:143–155. doi: 10.1038/nrmicro.2017.157. [DOI] [PubMed] [Google Scholar]
- 43.Qin J, et al. Characterization of the genitourinary microbiome of 1,165 middle-aged and elderly healthy individuals. Front Microbiol. 2021;12:673969. doi: 10.3389/fmicb.2021.673969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan MP, Pembroke JT. Brevundimonas spp: emerging global opportunistic pathogens. Virulence. 2018;9:480–493. doi: 10.1080/21505594.2017.1419116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadel BB, et al. The Gene Expression Deconvolution Interactive Tool (GEDIT): accurate cell type quantification from gene expression data. Gigascience. 2021;10:2. doi: 10.1093/gigascience/giab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen S, et al. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci. 2014;111:E5593–E5601. doi: 10.1073/pnas.1419161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017;1–14 10.1186/s13059-017-1349-1. [DOI] [PMC free article] [PubMed]
- 48.Stegle O, Parts L, Piipari M, Winn J, Durbin R. Using probabilistic estimation of expression residuals (PEER) to obtain increased power and interpretability of gene expression analyses. Nat Protoc. 2012;7:500–507. doi: 10.1038/nprot.2011.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinforma Oxf Engl. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Law CW, Chen Y, Shi W, Smyth GK. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15:R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korotkevich G. et al. Fast gene set enrichment analysis. 2021. 060012 Preprint at 10.1101/060012.
- 52.Ren B, et al. E2F integrates cell cycle progression with DNA repair, replication, and G2/M checkpoints. Genes Dev. 2002;16:245–256. doi: 10.1101/gad.949802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji S, Zhu Z, Sun X, Fu X. Functional hair follicle regeneration: an updated review. Signal Transduct Target Ther. 2021;6:1–11. doi: 10.1038/s41392-020-00441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi BY. Targeting Wnt/β-Catenin pathway for developing therapies for hair loss. Int J Mol Sci. 2020;21:4915. doi: 10.3390/ijms21144915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma H-Y, Chen S, Du Y. Estrogen and estrogen receptors in kidney diseases. Ren Fail. 2021;43:619–642. doi: 10.1080/0886022X.2021.1901739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castel SE, Levy-Moonshine A, Mohammadi P, Banks E, Lappalainen T. Tools and best practices for data processing in allelic expression analysis. Genome Biol. 2019;1–12. 10.1186/s13059-015-0762-6. [DOI] [PMC free article] [PubMed]
- 57.Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. OMIM org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43:D789–798. doi: 10.1093/nar/gku1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ochoa D, et al. Open targets platform: supporting systematic drug–target identification and prioritisation. Nucleic Acids Res. 2021;49:D1302–D1310. doi: 10.1093/nar/gkaa1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aicher JK, Jewell P, Vaquero-Garcia J, Barash Y, Bhoj EJ. Mapping RNA splicing variations in clinically accessible and nonaccessible tissues to facilitate Mendelian disease diagnosis using RNA-seq. Genet Med. 2020;22:1181–1190. doi: 10.1038/s41436-020-0780-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uhlén M, et al. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 61.DNA Sequencing Costs: Data. Genome.gov https://www.genome.gov/about-genomics/fact-sheets/DNA-Sequencing-Costs-Data.
- 62.Yao DW, O’Connor LJ, Price AL, Gusev A. Quantifying genetic effects on disease mediated by assayed gene expression levels. Nat Genet. 2020;52:626–633. doi: 10.1038/s41588-020-0625-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie Y, et al. Analysis of the global burden of disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2012;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li H, et al. The sequence alignment/Map format and SAMtools. Bioinforma Oxf Engl. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.“Picard Toolkit.” Broad Institute, GitHub Repository. 2019. http://broadinstitute.github.io/picard/.
- 68.Liao Y, Smyth GK, Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013;41:e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Babraham Bioinformatics - FastQC A Quality Control tool for High Throughput Sequence Data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- 70.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krassowski M, Arts M, Lagger C. krassowski/complex-upset: v1.3.3 (v1.3.3). Zenodo. 2021. 10.5281/zenodo.5762625.
- 72.Lex A, Gehlenborg N, Strobelt H, Vuillemot R, Pfister H. UpSet: Visualization of Intersecting Sets. IEEE Trans Vis Comput Graph. 2014;20:1983–1992. doi: 10.1109/TVCG.2014.2346248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:31–21. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoffman GE, Schadt EE. variancePartition: interpreting drivers of variation in complex gene expression studies. BMC Bioinformatics. 2016;17:483. doi: 10.1186/s12859-016-1323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32:2847–2849. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 76.1000 Genomes Project Consortium A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCarthy S, et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet. 2016;48:1279–1283. doi: 10.1038/ng.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loh P-R, et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet. 2016;48:1443–1448. doi: 10.1038/ng.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Price AL, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 81.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oliva M, et al. The impact of sex on gene expression across human tissues. Science. 2020;369:eaba3066. doi: 10.1126/science.aba3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Subramanian A, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McLaren W, et al. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17:122. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Figure 1. a. Outcome of library preparation QC per donor, collection, and preparation. The crosses indicate a failed sample, and the numbers correspond to the collection. b. Measured RIN vs computationally-derived transcript integrity number (TIN) per sample. Passing is determined by 1 million protein-coding depth threshold. c. RNA yield distribution per tissue type. Buccal median = 1054.2ng, Q1 = 735.7ng, Q3 = 1292.55ng. Hair median = 1444.1ng, Q1 = 606.2ng, Q3 = 2530.5ng. Saliva median = 4705.4ng, Q1 = 2278.5ng, Q3 = 10920.35ng. Urine median = 253.4ng, Q1 = 98ng, Q3 = 1642.2ng. d. cDNA average size for Loseq and SmartSeq preparations across noninvasive tissues. e. RNA yield per donor and tissue. Each data point is a collection. Buccal Levene P = 0.05, Hair Levene P = 0.01, Saliva Levene P = 0.2, Urine Levene P = 0.0002. f. Total reads sequenced per sample, colored by prep. g. Categorization of gene types for uniquely mapped reads mapping to genes across tissues. Supplementary Figure 2. a-c quality statistics for all samples across all library preparations: a. b. Samples remaining and genes detected depending on depth threshold. Dotted line indicates preparation QC threshold of 1 million. c. Proportion of reads mapping to exonic, intergenic, and intronic genomic features. Pass indicator is based on preparation QC threshold of 1 million. d-f quality statistics for only Loseq samples: d. e. Samples remaining and genes detected depending on depth threshold. Dotted line indicates Loseq QC threshold of 2.5 million. f. Proportion of reads mapping to exonic, intergenic, and intronic genomic features. Pass indicator is based on Loseq QC threshold. Supplementary Figure 3. a. Intersection of genes with greater than zero median expression across library preparations. b. c. Comparison of gene length and GC content of genes uniquely captured in a given preparation. d. Comparison of median gene expression levels of library preparation replicates. The median of all replicate 1 samples (15) is compared against the median of all replicate 2 samples (15) per library preparation. The spearman correlation between these is shown. e. Comparison of the number of genes detected per tissue and library preparation depending on the RNA yield of the sample. Pearson correlation and p-value is shown. Supplementary Figure 4. a. b. Principal Component Analysis of all samples passing preparation QC thresholds. c. Percent variance explained per PC. d. ANOVA results for PC ~ tissue and PC ~ preparation. P-values are Bonferroni-corrected for the number of PCs tested (10). Supplementary Figure 5. a. Schematic of pipeline for assigning unmapped reads. Briefly, unmapped reads were remapped using decontaminer, normalized using a procedure akin to TPM normalization, then species of interest were filtered based on their abundance and replication across technical replicates. Remaining unmapped reads were investigated using FastQC. b. Total number of reads assigned to each category. Mapped = aligned to hg38. Remapped = aligned to microbial species using Decontaminer. Repeated = highly abundant reads identified by FastQC. Unknown = reads not mapped, remapped, or highly abundant. c. For each species included in the final analysis, spearman rank correlation between technical replicates is shown with the dots. Bar plot of species abundance with error bars is shown in the background for direct comparison (y-scale of abundance in Figure 2b). d. Breakdown of repeated sequence sharing across tissues and library preparations. e. Mapping rate per tissue and library preparation as a function of RNA yield. Pearson correlation and p-value is shown. Supplementary Figure 6. a. Variance in gene expression across tissues explained by technical and biological variables. Canonical correlation analysis shows correlation between variables used. b. c. Variance in gene expression within each tissue explained by technical and biological variables. Canonical correlation analysis shows correlation between variables used. Only Loseq samples were included in these analyses. Supplementary Figure 7. A. GEDIT cell type proportion estimates per collection and donor. Top 25% most abundant, condensed cell type categories are shown. b. Breakdown of all cell types included in the GEDIT reference. Binning into larger cell type categories is shown. c. Cell type proportion estimates looking across samples ordered from lowest to highest RNA yield. Supplementary Figure 8. a. xCell enrichment scores across the noninvasive and select GTEx tissues for cell types corresponding to the GEDIT collapsed cell type categories. Note that the enrichment score does not correspond to a proportion. b. Comparison of xCell enrichment scores and GEDIT proportions for cell types shared by both references. Only noninvasive tissues are shown. Supplementary Figure 9. a. b. c. Calculated pi1 versus the null pi1 distribution for every tissue in GTEx. Pi1 was calculated by selecting for significant variants (q-value <=0.05) with MAF > 0.05 and minimum effect size greater than the maximum minimum across GTEx tissues (kidney cortex 0.32) that were present in the noninvasive dataset. The null distribution was generated by performing 1000 samples of size equivalent to the number of overlapping gene-variant pairs used for the pi1 calculation for that tissue. d. e. f. Histograms of Loseq p-values for gene-variant pairs included in the pi1 calculation. Supplementary Figure 10. a. Number of sex-based upregulated genes per noninvasive tissue type. b. Per tissue overlap between significant DE genes in the noninvasive dataset with genes previously found to be significant in the GTEx dataset. c. Sex-based differential expression for buccal samples, with no significant genes. FGSEA shows some rank-based gene category enrichment for females. Supplementary Figure 11. a. Ratio of (reference allele count)/(total count) for all heterozygous sites per donor and tissue. b. Reference ratio breakdown per VEP annotation. Supplementary Figure 12. a. Total number of disease-relevant genes per OpenTargets ontology category. Genes with >= 5 separate sources of evidence were included in the final analysis. b. Number of genes per tissue following minimum expression level thresholding and overlap with the HPA tissue-elevated gene list. The top 3,411 most expressed genes per tissue were included in the analysis. c. Summed evidence scores (SESs) for all GTEx and noninvasive tissues. Supplementary Figure 13. a. Proportion and total OMIM gene capture per noninvasive tissue type, depending on minimum median TPM threshold. b. Proportion of OMIM gene capture per collection, donor, and tissue using a minimum expression threshold of 0.1 TPMs. c. Clustering of noninvasive and select GTEx tissues based on median OMIM gene expression. Supplementary Table 1. Breakdown of reaction cost (as of August 2022) per reagent using our in-house method (Loseq) versus TruSeq Stranded mRNA Library Prep and Takara SMART-seq V4 commercial kits. The cost of Ampure XP beads (cat# A63881) is excluded, but it is notably lower per reaction for Loseq and SMART-seq preparations due to smaller volume requirements. Supplementary Table 2. Comparison of total cost per reaction by library preparation. Loseq percent cost reduction calculated as: (1-(Loseq/Commercial kit))*100.
Data Availability Statement
The data set generated and analyzed in the study is available on dbGaP (accession number posted upon publication). Noninvasive sample collection and in-house library preparation methods are provided on protocols.io (dx.doi.org/10.17504/protocols.io.kqdg3pjzzl25/v1). Analysis code has been deposited in the GitHub repository: https://github.com/LappalainenLab/loseq.