Abstract
Salmonella typhimurium uses of a type III protein secretion system encoded at centisome 63 of its chromosome to deliver effector molecules into the host cell. These proteins stimulate host cell responses such as reorganization of the actin cytoskeleton and activation of transcription factors. One of these effector proteins is SptP, a tyrosine phosphatase that causes disruption of the host cell actin cytoskeleton. A characteristic feature of many substrates of type III secretion systems is their association with specific cytoplasmic chaperones which appears to be required for secretion and/or translocation of these proteins into the host cell. We report here the identification of SicP, a 13-kDa acidic polypeptide that is encoded immediately upstream of sptP. A loss-of-function mutation in sicP resulted in drastically reduced levels of SptP but did not affect sptP expression, indicating that SicP exerts its effect posttranscriptionally. Pulse-chase experiments demonstrated that the loss of SicP leads to increased degradation of SptP. In addition, we show that SicP binds to SptP directly and that the binding site is located between residues 15 and 100 of the tyrosine phosphatase. Taken together, these results indicate that SicP acts as a specific chaperone for SptP.
A number of bacterial pathogens have evolved a specialized protein secretion system (type III) to deliver a battery of effector proteins into the host cell to interfere with or stimulate cellular responses for their own benefit (13). Salmonella typhimurium encodes one such system in a pathogenicity island located at centisome 63 of its chromosome (12). This type III secretion apparatus is required for the stimulation of signal transduction pathways leading to a variety of cellular responses, such as reorganization of the actin cytoskeleton, activation of transcription factors, and in some cells, initiation of programmed cell death (13). These responses are essential for pathogenicity, as they allow Salmonella to gain access to an intracellular environment and help to establish the inflammatory diarrhea that often follows infection with these microorganisms.
Several substrates of the centisome 63 type III secretion system of Salmonella have been identified (8, 18, 19, 21–23, 29, 36). Some of these proteins are involved in the secretion process itself (6) or in the translocation of effector molecules into the host cell (7, 11, 36). Other secreted proteins, however, are bona fide effector molecules (18, 19, 21, 23, 36). One of these proteins is SptP, a tyrosine phosphatase that is translocated into host cells and is required for the full display of virulence (11, 23). The amino-terminal half of SptP is homologous to the Yersinia YopE and Pseudomonas aeruginosa ExoS secreted toxins, while the carboxy-terminal half shows sequence similarity with the catalytic domain of the Yersinia tyrosine phosphatase YopH (23). As for these toxins, microinjection of SptP into host cells results in disruption of the actin cytoskeleton (11).
A characteristic feature of type III secretion systems is that substrate proteins are often associated with specific chaperones (32–34). These chaperones, which are most often encoded in the vicinity of the cognate substrate protein, are thought to aid the secretion process and/or prevent the premature association of secreted proteins, thereby protecting them from degradation (5, 10, 26, 31–33, 35). Although there is little primary sequence similarity among the different chaperones, they do share a number of structural features such as small size, low isoelectric point, and predicted α-helical secondary structure (34). We describe here the identification and characterization of SicP, a specific chaperone for the S. typhimurium secreted tyrosine phosphatase SptP.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. typhimurium wild-type strain SL1344 and its derivative SB237, which carries a loss-of-function mutation in sptP, have been described previously (23). S. typhimurium strains were grown in L broth containing 0.3 M sodium chloride to allow optimal expression of the components and targets of the invasion-associated type III secretion system.
Strain and plasmid constructions.
A strain carrying a nonpolar mutation in sicP was constructed as follows. Plasmid pSB417 (23) was partially digested with EcoRV and ligated to a cassette containing a modified aminoglycoside 3′-phosphotransferase (aphT) gene which lacks a transcription terminator (14). This ligation yielded plasmid pSB673, which carries an insertion of the aphT cassette in the sicP gene oriented in the same direction as the transcription of this gene. A KpnI-SacI fragment of pSB673, carrying the mutated sicP gene and flanking sequences, was cloned into the KpnI and SacI sites of the suicide vector pGP704 (27). The resulting plasmid, pSB674, was then mobilized into wild-type S. typhimurium SL1334 by conjugation and wild-type sicP was replaced with the insertion mutant allele by homologous recombination, yielding the sicP mutant strain SB747. The correct insertion of the sicP::aphT allele was verified by Southern hybridization. Plasmid pSB674 was also mobilized into the S. typhimurium SL1344 derivative strain SB550, which carries an insertion of the reporter gene xylE into the PvuII site of sptP (9), yielding strain SB748.
The sicP-complementing plasmid pSB679 was constructed by cloning the BstYI-PvuII fragment of pSB417, which carries the sicP gene, into the BamHI and HincII sites of pACYC184 (4). The expression of sicP in the resulting plasmid was driven by the tetracycline promoter. Plasmid pSB680 expressing epitope-tagged SicP was constructed by cloning a PCR-amplified fragment (5′ primer, 5′-GCGCGAATTCTGTTCCGATGCGTAGTGAATGGC-3′; 3′ primer, 5′-AACTCCATGGCTACTTTAGCATATTCCTGCAGTAT-3′) that contains sicP plus 200 bp of upstream sequence into the NcoI and EcoRI sites of pSB616, a derivative of pBAD24 (17) encoding an 18-amino-acid epitope tag from the adenovirus E4-6/7 protein that can be recognized by the monoclonal antibody M45 (28). Construction of plasmid pSB667, a derivative of pBAD18 (17) which encodes hilA under the control of the araBAD promoter, will be described elsewhere (9). Plasmids expressing glutathione S-transferase (GST) fused to different truncated forms of SptP were constructed by PCR amplification of different fragments of SptP and subsequent cloning of the products into the BamHI and EcoRI sites of pGEX-KG (16).
Western immunoblotting of bacterial culture supernatants and whole-cell lysates.
S. typhimurium strains were grown in 2 ml of L broth containing 0.3 M NaCl to an optical density at 600 nm (OD600) of 1.0. Bacterial cultures were centrifuged at 12,000 × g for 10 min; supernatants were transferred to a clean microcentrifuge tube and spun again at 12,000 × g for 10 min. Proteins from bacterium-free culture supernatants and bacterial pellets were separated by discontinuous sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, N.H.), and SptP was detected by immunoblotting with an anti-SptP monoclonal antibody followed by enhanced chemiluminescence (Pierce).
C2,3O assay.
Overnight cultures of the different bacterial strains were diluted in 50 ml of L broth to an OD600 of 0.050 and grown at 37°C with mild agitation. At different times, 3-ml aliquots of the cultures were removed, the OD600 was measured, and bacteria were recovered by centrifugation. Bacterial cells were washed once with 20 mM potassium phosphate buffer (pH 7.2), resuspended in APB buffer (100 mM potassium phosphate buffer [pH 7.5], 10% [vol/vol] acetone), and disrupted by sonication for 1 min. Sonicated cells were centrifuged at 12,000 × g for 5 min, and supernatants were transferred to new tubes. The catechol 2,3-dioxygenase (C2,3O) activity in cell lysates was measured in 4-ml cuvettes containing 100 μl of bacterial lysate, 400 μl of APB buffer, and 2.5 ml of 100 μM potassium phosphate buffer (pH 8.0). The reaction was started by adding 10 μl of 100 mM catechol, and the absorbance at 375 nm was measured over time in a spectrophotometer. Protein concentration was measured by using a commercial kit as instructed by the manufacturer (Pierce). Dioxygenase activity was expressed as micromoles of catechol per minute per milligram of protein.
Pulse-chase labeling.
To facilitate expression of SicP and SptP under the labeling growth conditions, plasmid pSB667, which expresses HilA under the control of the araBAD promoter, was introduced into both wild-type S. typhimurium and its isogenic sicP derivative. Cultures of the different strains of S. typhimurium were grown overnight at 37°C in M9-Casamino Acids medium (M9 salts supplemented with 1% Casamino Acids, 0.2% glucose, 0.1% nicotinic acid, and 0.001% histidine) and subsequently diluted 1:20 into 3 ml of M9 medium supplemented with 0.1% each amino acid except methionine and cysteine. After 3 h of incubation at 37°C, the cells were washed with M9 inducing medium (M9 salts supplemented with 0.1% each amino acid except methionine and cysteine, 0.2% glycerol, 0.1% nicotinic acid, and 0.02% arabinose). Cells were resuspended in 5 ml of M9 inducing medium and grown for 2 h at 37°C. Bacteria were labeled with 500 μCi of Pro-Mix (mixture of [35S]methionine and [35S]cysteine; Amersham) for 2 min and chased with 0.3% unlabeled methionine and 0.5% Casamino Acids. At different times after labeling, 750 μl of culture was removed and proteins were precipitated by adding 8% trichloroacetic acid. Protein pellets were washed in cold acetone and resuspended in radioimmunoprecipitation assay buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1% Triton, 0.5% deoxycholate, 0.1% SDS), and samples were adjusted so as to contain the same number of radioactive counts. Equal amounts of radioactively labeled GST-SptP1-146 were added to each sample (to serve as an internal control) before immunoprecipitation with an anti-SptP monoclonal antibody for 2 h at room temperature. Protein A-Sepharose 4B beads (70 μl of a 50% slurry) were then added to each sample, incubated for 1 h, and washed four times in radioimmunoprecipitation assay buffer. The bound proteins were then eluted in 50 mM Tris (pH 8.0) containing 0.4% SDS. Samples were separated in an SDS–10% polyacrylamide gel, and labeled proteins were visualized by fluorography with Amplify (Amersham). Quantitation of labeled proteins was carried out by phosphoimager (Storm 860; Molecular Dynamics) analysis. Radioactive labeling of GST-SptP1-146 was carried out by incubating a culture of a strain of E. coli carrying plasmid pSB689 (which encodes GST-SptP1-146) (11) in the presence of 60 μCi of Pro-Mix for 2 min and subsequently precipitating the labeled proteins with 8% trichloroacetic acid.
Far-Western blotting.
Whole-cell lysates of S. typhimurium or E. coli strains (100 μg) or purified GST fusion proteins (2 to 10 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 2.5% skim milk in buffer A (50 mM Tris [pH 7.5], 100 mM sodium acetate, 150 mM sodium chloride, 1 mM dithiothreitol, 1 mM EDTA, 5 mM MgCl2, 0.3% Tween 20) for 1 h. E. coli strains expressing epitope-tagged SicP or carrying the vector only were grown in 20 ml of L broth to an OD600 of 1.0 in the presence of 0.002% arabinose. Cells were pelleted and sonicated in buffer A. Supernatants of bacterial lysates in buffer A were incubated with the membranes at 4°C for 4 h. SicP-interacting proteins were visualized by sequential incubation of the membranes with monoclonal antibody M45 and horseradish peroxidase-conjugated anti-mouse antibody, followed by enhanced chemiluminescence.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper has been deposited in GenBank under accession no. AF060857.
RESULTS
Identification of S. typhimurium SicP.
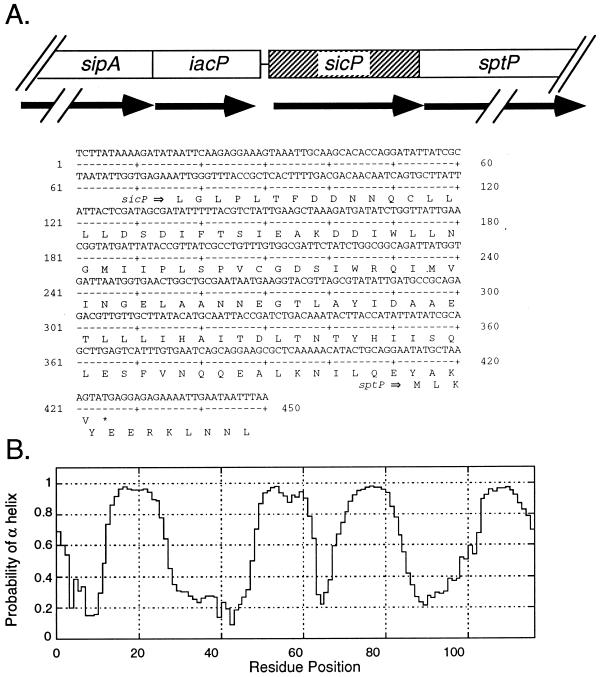
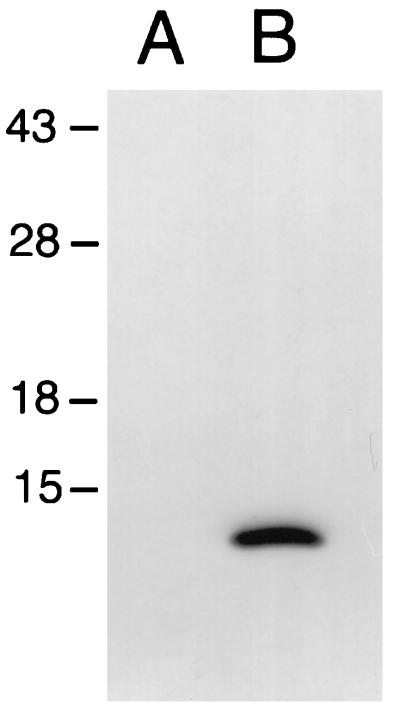
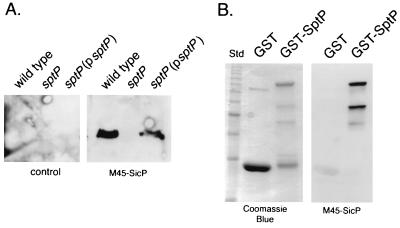
Most substrates of type III secretion systems are associated with specific intracellular chaperones (34). Such association appears to be required for the translocation of these proteins into the host cell. These chaperones are most often encoded in the vicinity of their cognate target proteins. In an effort to identify a putative chaperone for SptP, we determined the nucleotide sequence of the region located immediately upstream of its coding gene and identified an open reading frame (ORF) which is predicted to be in the same transcriptional unit as sptP (Fig. 1A). This ORF does not possess an AUG initiation codon, although it has a UUG codon located at an appropriate distance from a consensus ribosome binding site which would result in the production of a 116-amino-acid polypeptide with a predicted molecular mass of 13 kDa. To investigate whether this ORF was capable of encoding a polypeptide, an M45 epitope tag from the adenovirus E4-6/7 protein was fused to the carboxy terminus of the putative protein. Whole-cell lysates and culture supernatants of an S. typhimurium strain carrying either pSB680, the plasmid encoding the tagged protein, or the vector alone were examined by immunoblot analysis with a monoclonal antibody that recognizes the M45 epitope. A polypeptide with a calculated molecular mass of 14 kDa was detected in whole-cell lysates of the strain carrying pSB680 but was absent in whole-cell lysates of the strain carrying the plasmid vector alone (Fig. 2) or in culture supernatants of either of the two strains (data not shown). The size of the expressed polypeptide is in good agreement with the size of the predicted polypeptide plus the epitope tag, suggesting that initiation of translation occurs at the UUG codon (Fig. 1). The product of this ORF will be referred to as SicP. Further analysis of the predicted SicP protein sequence revealed that it has a calculated isoelectric point of 4.0 and a predicted α-helical secondary structure that extends throughout the entire length of the protein (Fig. 1B). Relative small size, α-helical secondary structure, and low isoelectric point are characteristic features of the chaperones associated with substrates of type III secretion systems, suggesting that SicP may perform an equivalent function in S. typhimurium. Comparison of the predicted amino acid sequence of SicP with sequences in the available databases revealed significant sequence similarity (23% identity and 43% similarity throughout the sequence) to IpgA, a protein encoded in the virulence plasmid of Shigella spp. (Fig. 3) (1). Although the function of this protein has not been investigated, it is intriguing that it is also encoded in the immediate vicinity of the invasion-associated type III secretion system of Shigella spp., suggesting that SicP and IpgA may perform similar functions.
FIG. 1.
Identification of the S. typhimurium sicP gene. (A) Nucleotide sequence and localization of the sicP gene in the centisome 63 pathogenicity island. (B) Probability of SicP residues being in an α helix. Secondary structure analysis was carried out at the web site of the BioMolecular Engineering Research Center of Boston University (Boston, Mass.) (http://bmerc-www.bu.edu/protein-seq/).
FIG. 2.
The sicP ORF encodes a polypeptide. The sicP ORF was fused at its 3′ end to a nucleotide sequence encoding the M45 epitope tag derived from the adenovirus E4-6/7 protein and expressed in S. typhimurium. Whole-cell lysates of S. typhimurium carrying a plasmid expressing the epitope-tagged SicP protein (lane B) or the plasmid vector alone (lane A) were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with a monoclonal antibody that recognizes the M45 epitope. Numbers to the left indicate the positions (in kilodaltons) of molecular weight markers.
FIG. 3.
Sequence alignment of S. typhimurium SicP and Shigella flexneri IpgA. The alignment was constructed by using the program BESTFIT from the Genetics Computer Group package from the University of Wisconsin. Identical residues are indicated with vertical lines, and conserved substitutions are indicated by periods or colons.
A loss-of-function mutation in sicP causes decreased intracellular and extracellular levels of SptP.
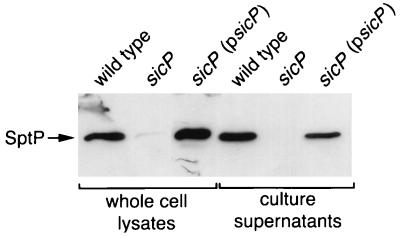
The predicted structure of SicP in conjunction with the location of its coding gene suggests that it may function as a chaperone for the S. typhimurium secreted tyrosine phosphatase SptP. We therefore examined the effect of a loss-of-function mutation in sicP on the levels of SptP. An S. typhimurium strain carrying a nonpolar mutation in sicP was constructed by allele replacement as described in Materials and Methods, and the levels of SptP in the whole-cell lysate and culture supernatant of the resulting mutant strain were compared to those of the wild type. As shown in Fig. 4, the levels of SptP were dramatically reduced in both the culture supernatant and the whole-cell lysate of the sicP mutant. Introduction into this mutant of the complementing plasmid pSB679, which encodes sicP, restored the wild-type levels of SptP in both culture supernatant and the whole-cell lysate. These results indicate that the observed effect of the sicP mutation on SptP levels was due to the absence of SicP and not to a polar effect on the downstream sptP gene.
FIG. 4.
Effect of a loss-of-function mutation in sicP on the intracellular and extracellular levels of SptP. Whole-cell lysate and culture supernatant proteins from wild-type S. typhimurium, its isogenic sicP mutant strain SB747, or the same mutant carrying the sicP-complementing plasmid pSB679 (psicP) were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with an anti-SptP monoclonal antibody. The arrow indicates the position of SptP.
We then examined the possibility that SicP affects the level of SptP by acting at the transcriptional level. The sicP nonpolar loss-of-function mutation was introduced into the chromosome of S. typhimurium SB550, which carries a transcriptional fusion of sptP to xylE, a Pseudomonas putida Tol plasmid gene that encodes C2,3O (38). The C2,3O levels in the resulting strain SB748 were compared to those of the parent strain SB550. As shown in Fig. 5, there was no significant difference in the expression levels of sptP in these two strains, indicating that SicP must exert its effect on the levels of SptP posttranscriptionally.
FIG. 5.
Effect of a loss-of-function mutation in sicP on the expression of sptP. The levels of C2,3O in S. typhimurium SB550, which carries an sptP::xylE gene fusion, and in its derivative strain SB748, which carries a loss-of-function mutation in sicP, were measured as indicated in Materials and Methods. Units are expressed as micromoles of C2,3O per minute per microgram of protein.
We also investigated the effect of the sicP mutation on the levels of secreted proteins other than SptP by examining the protein profiles of culture supernatant preparations of the S. typhimurium sicP mutant strain. Other than the absence of SptP, no difference was detected between the profiles of Coomassie blue-stained proteins of the wild-type strain and the sicP mutant strain, supporting the notion that SicP exerts its function exclusively on SptP (data not shown).
SicP affects the stability of SptP.
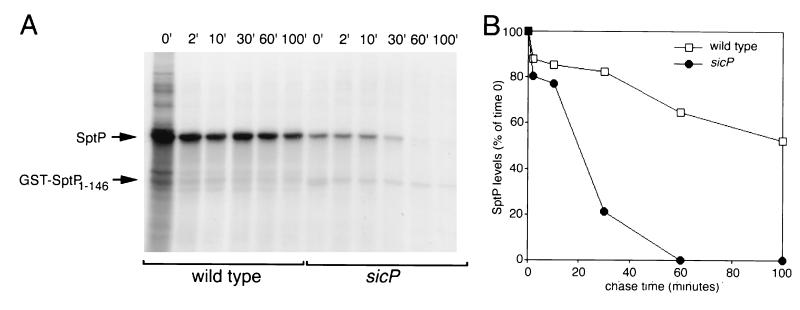
To address the possibility that SicP affects the levels of SptP by influencing its stability, we performed pulse-chase experiments. Cultures of wild-type and sicP S. typhimurium strains were pulse-labeled with [35S]methionine and [35S]cysteine for 2 min and then chased with cold amino acids. The levels of labeled SptP over time were then determined by immunoprecipitation and phosphorimaging analysis as indicated in Materials and Methods. After a 100-min chase with cold amino acids, almost 60% of pulse-labeled [35S]SptP was still detectable in wild-type S. typhimurium (Fig. 6). In contrast, in the sicP mutant, only 20% of the [35S]SptP remained detectable 20 min after pulse-labeling, and by 60 min essentially all [35S]SptP had been degraded (Fig. 6). These results indicate that SicP affects the level of SptP by influencing its stability.
FIG. 6.
Absence of SicP results in decreased SptP stability. (A) Wild-type S. typhimurium SL1344 and its isogenic sicP mutant strain SB747 were pulse-labeled with [35S]methionine and [35S]cysteine for 2 min and chased with cold amino acids for 100 min. At the time points indicated (in minutes), samples were removed, immunoprecipitated with an anti-SptP monoclonal antibody, and subjected to SDS-PAGE. Labeled proteins were visualized by fluorography. The position of SptP and that of the internal control GST-SptP1-146 (see Materials and Methods) are indicated by arrows. (B) Phosphorimager scanning of the gel shown in panel A. The intensity of the SptP band at each time point was standardized to the intensity of the internal GST-SptP1-146 control.
SicP binds to SptP.
If SicP acts as a chaperone, it must be able to bind its cognate target protein. The ability of SicP to bind SptP directly was therefore investigated by far-Western blot analysis. A carboxy-terminal M45 epitope-tagged SicP was used as a probe in this assay since this protein was shown to complement the sicP mutant phenotype, indicating that the presence of the tag does not interfere with SicP function. Whole-cell lysates of wild-type S. typhimurium SL1344, its isogenic sptP mutant strain SB237, and SB237 carrying the sptP-complementing plasmid pSB450 were separated by SDS-PAGE and immobilized onto nitrocellulose membranes. The membranes were incubated with bacterial lysates of either an E. coli strain expressing the M45 epitope-tagged SicP or the same strain carrying the vector plasmid only. Blots were subsequently treated with a monoclonal antibody that recognizes the M45 epitope tag. A 60-kDa polypeptide corresponding to the molecular weight of SptP was detected in cell lysates of wild-type S. typhimurium SL1344 and the sptP mutant strain SB237 carrying pSB450 but not in lysates of SB237 without the complementing plasmid (Fig. 7A). The detection of the 60-kDa polypeptide was dependent on the presence of epitope-tagged SicP, as this band was not detected in membranes that were treated with whole-cell lysates of E. coli carrying the vector only. When membranes were stripped and reprobed with an anti-SptP monoclonal antibody, a 60-kDa band that exactly overlapped with the SicP-interacting protein was detected (data not shown), indicating that this protein is most likely SptP. To further confirm that SicP can bind SptP, purified GST-SptP and purified GST were immobilized onto nitrocellulose membranes and probed with M45-SicP as described above. As shown in Fig. 7B, M45-SicP bound to purified GST-SptP but not to purified GST. Taken together, these results indicate that SicP can bind directly to SptP.
FIG. 7.
Binding of SicP to SptP. (A) Whole-cell lysates from wild-type S. typhimurium, its isogenic sptP mutant strain SB237, or the same mutant carrying the sptP-complementing plasmid pSB450 (psptP) were separated by SDS-PAGE, transferred to a nitrocellulose membrane, probed with whole-cell lysates of E. coli expressing M45-SicP or carrying the empty cloning vector (control), and subsequently incubated with monoclonal antibody M45. (B) Purified GST and GST-SptP were separated by SDS-PAGE and either stained with Coomassie blue or immobilized on a nitrocellulose membrane, which was subsequently probed with a whole-cell lysate of E. coli expressing M45-SicP as indicated above.
Mapping the SicP binding site within SptP.
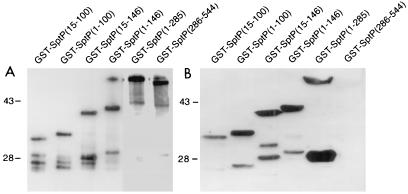
To determine the binding site of SicP within SptP, fusion proteins of GST to the amino (GST-SptP1-285)- and carboxy (GST-SptP286-544)-terminal halves of SptP were constructed and probed for SicP binding. Whole-cell lysates of E. coli strains expressing the different GST-SptP fusions were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and probed with either the M45 epitope-tagged SicP to detect binding or anti-GST antibodies to analyze the expression levels of the fusion protein. As shown in Fig. 8, M45-SicP bound to the GST-SptP1-285 but not the GST-SptP286-544 protein fusion, indicating that the SicP binding site must be located in the amino-terminal half of SptP. To further delineate the SicP binding site, a series of amino-terminal deletions of SptP (SptP1-146, SptP15-146, SptP1-100, and SptP15-100) were constructed, expressed as GST fusion proteins in E. coli, and probed for SicP binding. As shown in Fig. 8B, M45-SicP effectively bound to all truncated forms of SptP, delineating the SicP binding site between residues 15 and 100 of SptP. This finding is in good agreement with the chaperone binding sites mapped on other substrates of type III secretion systems such as the Yersinia outer proteins YopE (between residues 15 and 50) and YopH (between residues 20 and 70) (5, 31, 33, 35).
FIG. 8.
Mapping of the SicP binding domain in SptP. Whole-cell lysates of E. coli expressing GST fused to different fragments of SptP were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with either an anti-GST antibody (to examine protein expression) (A) or a whole-cell lysate of E. coli expressing M45-SicP followed by a monoclonal antibody directed to the M45 epitope (B). Numbers to the left of each panel indicate the positions (in kilodaltons) of molecular weight standards. Ten times more protein was loaded in each lane of the gel probed with M45-SicP.
DISCUSSION
Most substrate proteins of type III secretion systems are believed to be associated to specific cytoplasmic chaperones or partitioning factors (34). Unlike other well-characterized chaperones such as GroEL or Hsp70 (3, 20), these proteins have a rather narrow binding specificity and appear to lack nucleotide-binding or nucleotide-hydrolyzing activities. Although the type III secretion-associated chaperones identified so far exhibit little amino acid sequence similarity, they share a number of properties consistent with a common function: a relatively small size (15 to 18 kDa), a low isoelectric point, and a predominantly α-helical secondary structure. In addition, they are usually encoded in the vicinity of their cognate target proteins. We have described here the identification and characterization of SicP, an S. typhimurium protein that meets all criteria to be a specific chaperone for SptP, a substrate of the centisome 63 type III secretion system that is translocated into the host cell and disrupts the actin cytoskeleton (11).
The function of the type III-associated chaperones has been studied most extensively in bacterial pathogens of the genus Yersinia (5, 10, 31–33, 35). The role of these chaperones in the function of this secretion system is not clearly understood and in some instances is the subject of some controversy. Deletion analyses of the Yersinia Yop proteins have established the existence of well-defined independent domains that are involved in their secretion and translocation into the host cell (5, 31, 35). In general, the first 10 to 20 amino acids of the Yop proteins are thought to be required for their secretion, whereas an immediately adjacent domain of 60 to 70 residues is thought to be involved in their translocation into the host cell (5, 31, 35). The binding site on the translocated Yops for their specific chaperones has been mapped to a region that overlaps their translocation domains (31, 35). Consistent with the similarity in the structural organization of this family of proteins, we have mapped the binding site of SicP to a domain located between residues 15 and 100 of SptP, further strengthening the implication that SicP performs a function similar to that of the Yop chaperones.
At issue is the role of this family of chaperones in the secretion process. It is clear that in Yersinia, absence of a given chaperone (e.g., SycE or SycH) results in reduced secretion of the cognate substrate protein (e.g., YopE or YopH) (5, 10, 31, 33, 35). In some instances, it is not known how much of this reduction is due to degradation of the target protein in the absence of the chaperone or is a consequence of the direct involvement of the chaperones in the secretion process itself. It is known, however, that secretion of the Yop proteins can take place in the absence of the chaperones, albeit requiring certain mutant backgrounds (e.g., yopD and yopB) (35) or the removal of the chaperone binding site from the cognate proteins (5, 31, 35). Thus, it has remained controversial whether the function of these chaperones is to deliver the secreted target proteins to the type III secretion machinery, to maintain them in a secretion competent state, to prevent premature association with other cognate secreted products leading to degradation, or a combination of some of these possible functions. More recently, Cheng et al. have proposed the existence of two independent type III secretion mechanisms for the YopE protein, which may explain some of the previous conflicting results (5). One pathway is encoded within the first 15 amino acids and is independent of the function of its specific chaperone. The other pathway is mediated by a domain located between residues 15 and 100 and requires the function of the SycE chaperone protein. Our results cannot address the possibility that SicP plays a direct role in secretion since a loss-of-function mutation in sicP resulted in a drastic reduction of the levels of SptP in both culture supernatants and whole-cell lysates of S. typhimurium. However, our results clearly showed that such a reduction is not due to a potential influence of SicP on the transcription of sptP, as we found equivalent expression levels of sptP::xylE in both wild-type and sicP mutant strains. In fact, our pulse-chase experiments indicate that the absence of SicP results in the premature degradation of SptP. Our results suggest that SicP may function similarly to the Shigella invasion-associated protein IpgC, which is thought to prevent the premature association and subsequent degradation of the Ipa invasins within the bacterial cytoplasm (26). This hypothesis is supported by the observation that SicP is capable of specifically binding to SptP in vitro. S. typhimurium secretes at least 10 proteins through its invasion-associated type III secretion system (12). Which if any of these proteins forms a cytoplasmic complex with SptP in the absence of SicP is not known. Candidate proteins include SipB, SipC, or SipD which are required for the translocation of SptP into host cells although they are not necessary for its secretion (11). The S. typhimurium sicP mutant strain exhibits trace amounts of SptP secretion. This may imply that SicP does not play a direct role in SptP secretion. However, additional studies will be necessary to test this hypothesis.
Although the precise location of the secretion signal in SptP has not been determined, such a signal must be located at the amino terminus since the first 100 amino acids of SptP are efficiently secreted by S. typhimurium (11a). Recently, Anderson and Schneewind have proposed the existence of a translationally coupled secretion signal encoded in the RNA sequence of the first ∼60 nucleotides of the Yersinia YopE and YopN mRNAs (2). Similar to the cases for Yersinia spp. (30) and Shigella spp. (25), secretion of type III proteins in Salmonella is stimulated upon contact with eukaryotic cells (15, 37). Unlike Yersinia spp., however, in both Shigella and Salmonella spp. such stimulation does not require de novo protein synthesis (15, 25, 37). Rather, it appears that these bacteria accumulate an intracellular pool of effector proteins destined to be secreted which are rapidly exported upon reception of the host cell-derived signal. Consistent with this hypothesis, S. typhimurium is capable of inducing type III-dependent host cell responses in the presence of chloramphenicol (15, 24). Therefore, a secretion mechanism that is coupled to translation is unlikely to be involved in the type III delivery of S. typhimurium effectors to the host cell. In this context, a putative secretion pathway that involves chaperones such as SicP may be more likely to be the physiologically relevant pathway in Salmonella infections.
SicP shows 43% protein sequence similarity with a Shigella protein, IpgA (1). IpgA is encoded in immediate proximity to IcsB, a protein involved in intercellular spread. Although it is unknown whether IcsB is a target of the invasion-associated type III secretion system of Shigella and the role of IpgA in intercellular spread has not been investigated, it is tempting to speculate that IpgA may function as a chaperone of IcsB.
In summary, we have identified a protein, SicP, that acts as a chaperone for the tyrosine phosphatase SptP, a substrate of the centisome 63 type III secretion system of S. typhimurium. Since the absence of SicP results in almost complete degradation of SptP, it remains to be established whether in addition to stabilizing its cognate target protein, this chaperone plays a role in secretion.
ACKNOWLEDGMENTS
We thank members of the Galán laboratory for critical review of the manuscript.
This work was supported by Public Health Service grant AI30492 from the National Institutes of Health. J.E.G. is an investigator of the American Heart Association.
REFERENCES
- 1.Allaoui A, Mounier J, Prevost M C, Sansonetti P J, Parsot C. icsB: a Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol Microbiol. 1992;6:1605–1616. doi: 10.1111/j.1365-2958.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 3.Burston S G, Clarke A R. Molecular chaperones: physical and mechanistic properties. Essays Biochem. 1995;29:125–136. [PubMed] [Google Scholar]
- 4.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1155. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng L W, Anderson D M, Schneewind O. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol Microbiol. 1997;24:757–765. doi: 10.1046/j.1365-2958.1997.3831750.x. [DOI] [PubMed] [Google Scholar]
- 6.Collazo C, Galán J E. Requirement of exported proteins for secretion through the invasion-associated type III system in Salmonella typhimurium. Infect Immun. 1996;64:3524–3531. doi: 10.1128/iai.64.9.3524-3531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collazo C, Galán J E. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol. 1997;24:747–756. doi: 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- 8.Collazo C M, Zierler M K, Galán J E. Functional analysis of the Salmonella typhimurium invasion genes invI and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol. 1995;15:25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 9.Eichelberg, K., and J. E. Galán. Unpublished data.
- 10.Frithz-Lindsten E, Rosqvist R, Johansson L. The chaperone-like protein YerA of Yersinia pseudotuberculosis stabilizes YopE in the cytoplasm but is dispensible for targeting to the secretion loci. Mol Microbiol. 1995;16:635–647. doi: 10.1111/j.1365-2958.1995.tb02426.x. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y, Galán J E. The Salmonella spp. protein tyrosine phosphatase SptP is translocated into host cells and disrupts the host-cell cytoskeleton. Mol Microbiol. 1998;27:359–368. doi: 10.1046/j.1365-2958.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 11a.Fu, Y., and J. E. Galán. Unpublished data.
- 12.Galán J E. Molecular bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 13.Galán J E, Bliska J B. Cross-talk between bacterial pathogens and their host cells. Annu Rev Cell Dev Biol. 1996;12:219–253. doi: 10.1146/annurev.cellbio.12.1.221. [DOI] [PubMed] [Google Scholar]
- 14.Galán J E, Ginocchio C, Costeas P. Molecular and functional characterization of the Salmonella typhimurium invasion gene invA: homology of InvA to members of a new protein family. J Bacteriol. 1992;17:4338–4349. doi: 10.1128/jb.174.13.4338-4349.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginocchio C, Olmsted S B, Wells C L, Galán J E. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–724. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 16.Guan K-L, Dixon J E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Ann Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 17.Guzman L M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardt W-D, Galán J E. A secreted Salmonella protein with homology to an avirulence determinant of plant pathogenic bacteria. Proc Natl Acad Sci USA. 1997;94:9887–9892. doi: 10.1073/pnas.94.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardt W-D, Urlaub H, Galán J E. A target of the centisome 63 type III protein secretion system of Salmonella typhimurium is encoded by a cryptic bacteriophage. Proc Natl Acad Sci USA. 1998;95:2574–2579. doi: 10.1073/pnas.95.5.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 21.Kaniga K, Trollinger D, Galán J E. Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J Bacteriol. 1995;177:7078–7085. doi: 10.1128/jb.177.24.7078-7085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaniga K, Tucker S C, Trollinger D, Galán J E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaniga K, Uralil J, Bliska J B, Galán J E. A secreted tyrosine phosphatase with modular effector domains encoded by the bacterial pathogen Salmonella typhimurium. Mol Microbiol. 1996;21:633–641. doi: 10.1111/j.1365-2958.1996.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 24.Macbeth K J, Lee C A. Prolonged inhibition of bacterial protein synthesis abolishes Salmonella invasion. Infect Immun. 1993;61:1544–1546. doi: 10.1128/iai.61.4.1544-1546.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ménard R, Sansonetti P J, Parsot C. The secretion of the Shigella flexneri Ipa invasins is induced by the epithelial cell and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ménard R, Sansonetti P J, Parsot C, Vasselon T. The IpaB and IpaC invasins of Shigella flexneri associate in the extracellular medium and are partitioned in the cytoplasm by a specific chaperon. Cell. 1994;79:515–529. doi: 10.1016/0092-8674(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 27.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obert S, O’Connor R J, Schmid S, Hearing P. The adenovirus E4-6/7 protein transactivates the E2 promoter by inducing dimerization of a heteromeric E2F complex. Mol Cell Biol. 1994;14:1333–1346. doi: 10.1128/mcb.14.2.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pegues D A, Hantman M J, Behlau I, Miller S I. PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol. 1995;17:169–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- 30.Rosqvist R, Magnusson K E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schesser K, Frithz-Lindsten E, Wolf-Watz H. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J Bacteriol. 1996;178:7227–7233. doi: 10.1128/jb.178.24.7227-7233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wattiau P, Bernier B, Deslée P, Michiels T, Cornelis G R. Individual chaperones required for Yop secretion by Yersinia. Proc Natl Acad Sci USA. 1994;91:10493–10497. doi: 10.1073/pnas.91.22.10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wattiau P, Cornelis G R. SycE, a chaperone-like protein of Yersinia enterocolitica involved in the secretion of YopE. Mol Microbiol. 1993;8:123–131. doi: 10.1111/j.1365-2958.1993.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 34.Wattiau P, Woestyn S, Cornelis G R. Customized secretion chaperones in pathogenic bacteria. Mol Microbiol. 1996;20:255–262. doi: 10.1111/j.1365-2958.1996.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 35.Woestyn S, Sory M P, Boland A, Lequenne O, Cornelis G R. The cytosolic SycE and SycH chaperones of Yersinia protect the region of YopE and YopH involved in translocation across eukaryotic cell membranes. Mol Microbiol. 1996;20:1261–1271. doi: 10.1111/j.1365-2958.1996.tb02645.x. [DOI] [PubMed] [Google Scholar]
- 36.Wood M W, Rosqvist R, Mullan P B, Edwards M H, Galyov E E. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol Microbiol. 1996;22:327–338. doi: 10.1046/j.1365-2958.1996.00116.x. [DOI] [PubMed] [Google Scholar]
- 37.Zierler M, Galán J E. Contact with cultured epithelial cells induces the secretion of the Salmonella typhimurium invasion protein InvJ. Infect Immun. 1995;63:4024–4028. doi: 10.1128/iai.63.10.4024-4028.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zukowski M M, Gaffney D F, Speck D, Kauffman M, Findell A, Wisecup A, Lecocq J-P. Chromogenic identification of genetic regulatory signals in Bacillus subtilis based on expression of a cloned Pseudomonas gene. Proc Natl Acad Sci USA. 1983;80:1101–1105. doi: 10.1073/pnas.80.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]