Abstract
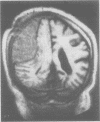
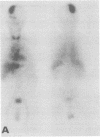
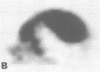
A 68 year old man with a 10 year history of apparently benign IgM kappa paraproteinaemia presented with dysarthria, left hemiparesis, and a sensory peripheral neuropathy. A calcified right temporoparietal extradural mass was shown by scintigraphy with 123I-serum amyloid P component to contain amyloid. There were no extracranial amyloid deposits. Clinical improvement followed craniotomy and partial resection of tissue which consisted of amyloid and a mixed mononuclear cell infiltrate. The amyloid fibrils consisted of the framework 1 region of the variable domain of monoclonal kappa IV immunoglobulin light chains. There was a prominent B-cell clonal immunoglobulin gene rearrangement in the tumour tissue, supporting a diagnosis of lymphoplasmacytic lymphoma, but no sign of systemic lymphoma. Neurological state, tumour volume, and quantity of amyloid have remained static for two years after treatment with chlorambucil.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr R., Lampert P. Intrasellar amyloid tumor. Acta Neuropathol. 1972;21(1):83–86. doi: 10.1007/BF00688003. [DOI] [PubMed] [Google Scholar]
- Cohen M., Lanska D., Roessmann U., Karaman B., Ganz E., Whitehouse P., Gambetti P. Amyloidoma of the CNS. I. Clinical and pathologic study. Neurology. 1992 Oct;42(10):2019–2023. doi: 10.1212/wnl.42.10.2019. [DOI] [PubMed] [Google Scholar]
- DeCastro S., Sparks J. R., Lapey J. D., Freidberg S. R. Amyloidoma of the gasserian ganglion. Surg Neurol. 1976 Dec;6(6):357–359. [PubMed] [Google Scholar]
- Ellie E., Vergier B., Duche B., Rivel J., Vital C., Loiseau P. Local amyloid deposits in a primary central nervous system lymphoma. Study of a stereotactic brain biopsy. Clin Neuropathol. 1990 Sep-Oct;9(5):231–233. [PubMed] [Google Scholar]
- Eriksson L., Sletten K., Benson L., Westermark P. Tumour-like localized amyloid of the brain is derived from immunoglobulin light chain. Scand J Immunol. 1993 Jun;37(6):623–626. doi: 10.1111/j.1365-3083.1993.tb01673.x. [DOI] [PubMed] [Google Scholar]
- Ferreiro J. A., Bhuta S., Nieberg R. K., Verity M. A. Amyloidoma of the skull base. Arch Pathol Lab Med. 1990 Sep;114(9):974–976. [PubMed] [Google Scholar]
- Giordano A., Horne D. G., Gudbrandsson F., Meyerhoff W. Temporal bone amyloidoma. Otolaryngol Head Neck Surg. 1983 Feb;91(1):104–108. doi: 10.1177/019459988309100122. [DOI] [PubMed] [Google Scholar]
- Hawkins P. N., Lavender J. P., Pepys M. B. Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990 Aug 23;323(8):508–513. doi: 10.1056/NEJM199008233230803. [DOI] [PubMed] [Google Scholar]
- Hu E., Trela M., Thompson J., Lowder J., Horning S., Levy R., Sklar J. Detection of B-cell lymphoma in peripheral blood by DNA hybridisation. Lancet. 1985 Nov 16;2(8464):1092–1095. doi: 10.1016/s0140-6736(85)90686-5. [DOI] [PubMed] [Google Scholar]
- Klobeck H. G., Bornkamm G. W., Combriato G., Mocikat R., Pohlenz H. D., Zachau H. G. Subgroup IV of human immunoglobulin K light chains is encoded by a single germline gene. Nucleic Acids Res. 1985 Sep 25;13(18):6515–6529. doi: 10.1093/nar/13.18.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle R. A., Garton J. P. The spectrum of IgM monoclonal gammopathy in 430 cases. Mayo Clin Proc. 1987 Aug;62(8):719–731. doi: 10.1016/s0025-6196(12)65225-2. [DOI] [PubMed] [Google Scholar]
- Kyle R. A., Lust J. A. Monoclonal gammopathies of undetermined significance. Semin Hematol. 1989 Jul;26(3):176–200. [PubMed] [Google Scholar]
- Lipper S., Kahn L. B. Amyloid tumor. A clinicopathologic study of four cases. Am J Surg Pathol. 1978 Jun;2(2):141–145. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Matsumoto T., Tani E., Maeda Y., Natsume S. Amyloidomas in the cerebellopontine angle and jugular foramen. Case report. J Neurosurg. 1985 Apr;62(4):592–596. doi: 10.3171/jns.1985.62.4.0592. [DOI] [PubMed] [Google Scholar]
- Moreno A. J., Brown J. M., Brown T. J., Graham G. D., Yedinak M. A. Scintigraphic findings in a primary cerebral amyloidoma. Clin Nucl Med. 1983 Nov;8(11):528–530. doi: 10.1097/00003072-198311000-00003. [DOI] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J. J., Tomiyasu U., MacKay A., Wilson C. B. Central nervous system amyloid presenting as a mass lesion. Report of two cases. J Neurosurg. 1982 Mar;56(3):439–442. doi: 10.3171/jns.1982.56.3.0439. [DOI] [PubMed] [Google Scholar]
- Vidal R. G., Ghiso J., Gallo G., Cohen M., Gambetti P. L., Frangione B. Amyloidoma of the CNS. II. Immunohistochemical and biochemical study. Neurology. 1992 Oct;42(10):2024–2028. doi: 10.1212/wnl.42.10.2024. [DOI] [PubMed] [Google Scholar]
- Wiltshaw E. The natural history of extramedullary plasmacytoma and its relation to solitary myeloma of bone and myelomatosis. Medicine (Baltimore) 1976 May;55(3):217–238. doi: 10.1097/00005792-197605000-00002. [DOI] [PubMed] [Google Scholar]