Abstract
PURPOSE
Trastuzumab deruxtecan (T-DXd) is a human epidermal growth factor 2 (HER2)–directed antibody-drug conjugate approved in HER2-expressing breast and gastric cancers and HER2-mutant non–small-cell lung cancer. Treatments are limited for other HER2-expressing solid tumors.
METHODS
This open-label phase II study evaluated T-DXd (5.4 mg/kg once every 3 weeks) for HER2-expressing (immunohistochemistry [IHC] 3+/2+ by local or central testing) locally advanced or metastatic disease after ≥1 systemic treatment or without alternative treatments. The primary end point was investigator-assessed confirmed objective response rate (ORR). Secondary end points included safety, duration of response, progression-free survival (PFS), and overall survival (OS).
RESULTS
At primary analysis, 267 patients received treatment across seven tumor cohorts: endometrial, cervical, ovarian, bladder, biliary tract, pancreatic, and other. The median follow-up was 12.75 months. In all patients, the ORR was 37.1% (n = 99; [95% CI, 31.3 to 43.2]), with responses in all cohorts; the median DOR was 11.3 months (95% CI, 9.6 to 17.8); the median PFS was 6.9 months (95% CI, 5.6 to 8.0); and the median OS was 13.4 months (95% CI, 11.9 to 15.5). In patients with central HER2 IHC 3+ expression (n = 75), the ORR was 61.3% (95% CI, 49.4 to 72.4), the median DOR was 22.1 months (95% CI, 9.6 to not reached), the median PFS was 11.9 months (95% CI, 8.2 to 13.0), and the median OS was 21.1 months (95% CI, 15.3 to 29.6). Grade ≥3 drug-related adverse events were observed in 40.8% of patients; 10.5% experienced adjudicated drug-related interstitial lung disease (ILD), with three deaths.
CONCLUSION
Our study demonstrates durable clinical benefit, meaningful survival outcomes, and safety consistent with the known profile (including ILD) in pretreated patients with HER2-expressing tumors receiving T-DXd. Greatest benefit was observed for the IHC 3+ population. These data support the potential role of T-DXd as a tumor-agnostic therapy for patients with HER2-expressing solid tumors.
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2) is a transmembrane tyrosine kinase receptor involved in the stimulation of cell proliferation, differentiation, and survival.1 HER2 overexpression can occur in a range of solid tumors, including breast, gastric, biliary tract, bladder, pancreatic, and gynecological tumors.2 HER2 overexpression is associated with a biologically aggressive tumor phenotype, poor prognosis, increased risk of disease recurrence, and limited benefit from chemotherapy.1,3-5 HER2-directed therapy is standard of care for HER2-expressing unresectable or metastatic breast cancer, HER2-positive locally advanced or metastatic gastric cancers, colorectal and gastroesophageal junction adenocarcinomas, and HER2-mutant non–small-cell lung cancer.6-9 However, many patients with other HER2-expressing solid tumors will progress on standard therapy, with poor prognosis and limited alternatives.5,10-13 This represents an opportunity to improve outcomes for such patients with novel HER2-targeted therapeutics.
CONTEXT
Key Objective
What is the efficacy and safety of trastuzumab deruxtecan (T-DXd; 5.4 mg/kg once every 3 weeks) in previously treated patients with locally advanced or metastatic human epidermal growth factor 2 (HER2)–expressing (immunohistochemistry [IHC] 3+/2+) solid tumors?
Knowledge Generated
DESTINY-PanTumor02 demonstrated that treatment with T-DXd resulted in durable responses across multiple tumor types, alongside clinically meaningful rates of progression-free survival and overall survival, with the greatest benefit observed in the HER2 IHC 3+ population. The safety profile was consistent with the known profile for T-DXd, including the incidence of interstitial lung disease (ILD).
Relevance (G.F. Fleming)
-
T-DXd provides meaningful benefit for patients with multiple types of solid tumors that express HER2, particularly for those whose tumors express HER2 at the 3+ level on central review.*
*Relevance section written by JCO Associate Editor Gini F. Fleming, MD.
Trastuzumab deruxtecan (T-DXd) is a HER2-directed antibody-drug conjugate composed of a humanized immunoglobulin G1 anti-HER2 monoclonal antibody, a tetrapeptide-based cleavable linker, and a potent topoisomerase I inhibitor payload.14 T-DXd is currently approved in the United States and European Union for treatment of HER2-expressing breast cancer and HER2-positive gastric or gastroesophageal junction adenocarcinoma and in the United States and Japan for HER2-mutant non–small cell lung cancer.15-17 In early-phase studies, T-DXd demonstrated antitumor activity in a range of HER2-expressing malignancies, including colorectal, salivary gland, biliary tract, and endometrial cancer.18 In August 2023, T-DXd was granted breakthrough therapy designations in the United States for adult patients with unresectable or metastatic HER2-positive (immunohistochemistry [IHC] 3+) solid tumors that have progressed after prior treatment and have no satisfactory alternatives and for patients with HER2-positive (IHC 3+) metastatic colorectal cancer who have received ≥2 prior treatment regimens.19 The aim of this study (ClinicalTrials.gov identifier: NCT04482309) was to assess the efficacy and safety of T-DXd in patients with selected, locally advanced, metastatic, or unresectable HER2-expressing solid tumors.
METHODS
Study Design and Participants
This open-label, multicenter, phase II study (ClinicalTrials.gov identifier: NCT04482309) evaluated the efficacy and safety of T-DXd 5.4 mg/kg once every 3 weeks in patients with previously treated HER2-expressing solid tumors in seven cohorts.
Eligible patients were age 18 years or older; had histologically confirmed locally advanced, unresectable, or metastatic biliary tract, bladder, cervical, endometrial, ovarian, pancreatic, or other solid cancers (excluding breast, colorectal, gastric, and non–small-cell lung cancers); who progressed after ≥1 systemic treatment or had no satisfactory alternative treatment options; Eastern Cooperative Oncology Group performance status of 0-120; HER2-overexpressing tumors with IHC 3+/2+ (local or central testing) scored using current ASCO/College of American Pathology guidelines for scoring HER2 in gastric cancer21; and had ≥1 investigator-assessed measurable lesion on the basis of RECIST 1.1.22 Patients with noninfectious interstitial lung disease (ILD)/pneumonitis requiring steroids, or if suspected ILD/pneumonitis could not be ruled out by imaging at screening, were excluded. HER2 expression for eligibility was based on local assessment, where available. Otherwise, eligibility was determined by central testing. HER2 IHC status was assessed centrally using HER2 HercepTest (DAKO) and scored according to gastric-specific criteria. Prior HER2-targeted therapy was permitted. Eligibility criteria are provided in Appendix 2, online only.
The study Protocol (online only) was approved by the institutional review board at each site and was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice, the Declaration of Helsinki, and local regulations on the conduct of clinical research. All patients provided written informed consent before study participation.
Procedures
T-DXd was administered intravenously once every 3 weeks at 5.4 mg/kg of body weight. RECIST scans were performed at screening and every 6 weeks until documented disease progression (RECIST 1.1) or withdrawal of consent. Treatment continued until documented disease progression (RECIST 1.1), withdrawal of consent, or when discontinuation criteria were met. Dose interruptions and/or reduction and supportive therapy were permitted for clinically significant and/or unacceptable toxicity. For suspected ILD/pneumonitis, treatment was interrupted pending evaluation, and all events were followed until resolution (including after discontinuation) regardless of severity (Appendix 2).
End Points
The primary end point was investigator-assessed confirmed objective response rate (ORR), defined as the proportion of patients with a confirmed complete or partial response by RECIST 1.1 (Appendix 2). Secondary efficacy end points included duration of response (DOR; time from date of first documented response [complete or partial] until the date of documented progression or death in the absence of disease progression); disease control rate (percentage of patients with a best objective response of confirmed complete response or partial response, or with stable disease for at least 5 weeks after first dose); progression-free survival (PFS; time from first dose until date of objective disease progression or death regardless of withdrawal or receipt of another cancer therapy); and overall survival (OS; time from date of first dose until death due to any cause). An independent central review per RECIST 1.1 was performed and reported alongside the investigator-assessed results for secondary outcomes. Exploratory endpoints included subgroup analysis by HER2 status.
Secondary safety end points included the occurrence of adverse events (including drug-related adverse events, serious adverse events, and adverse events of special interest [ILD/pneumonitis and left ventricular dysfunction]) and changes in vital sign measurements and standard clinical laboratory parameters. Adverse events were coded and graded according to the Medical Dictionary for Regulatory Activities (version 26.0) and National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0). Potential cases of ILD/pneumonitis were evaluated by an independent adjudication committee.
Statistical Analysis
A sample size of 40 patients per cohort was determined to provide sufficient precision for the estimation of objective response in each cohort (eg, for ORR 35%, exact CI would be 20.6 to 51.7). Efficacy and safety results are presented by cohort and overall on the basis of the full analysis set (patients who received at least one dose of study medication). Outcomes are reported in all patients enrolled by local and central testing; subgroup analyses by HER2 status are reported as confirmed by central testing alone. Descriptive statistics were used to summarize each end point. Kaplan-Meier estimations were used to describe DOR, PFS, and OS. Exact 95% CIs for binomial proportions were calculated using the Clopper-Pearson method.
RESULTS
Between October 7, 2020, and July 7, 2022, a total of 268 patients with HER2-expressing solid tumors were enrolled from >120 sites across 15 countries. Of them, 267 (99.6%) patients received at least one dose of study treatment and were included in the full analysis set; one patient withdrew before receiving treatment (Appendix Fig A1).
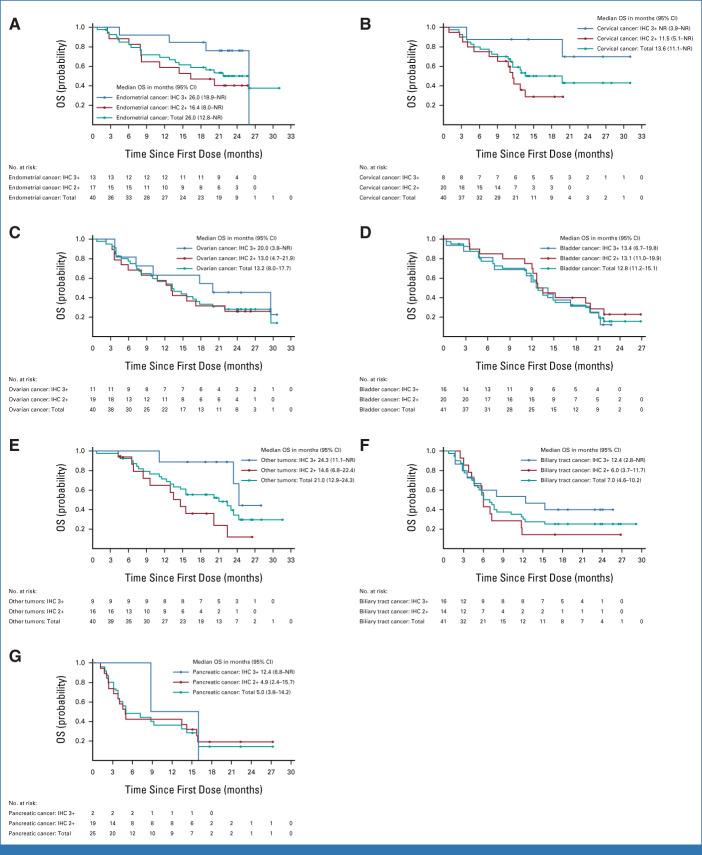
The median age was 62 (range, 23-85) years. Patients had received a median of two lines of prior therapy (range, 0-12; Table 1). Across all cohorts, 40.8% had received ≥three prior lines, and 14.2% had received prior HER2 therapy (trastuzumab [12.4%], pertuzumab [1.9%], zanidatamab [1.5%], trastuzumab emtansine [1.1%], trastuzumab duocarmazine [0.4%], and/or tucatinib [0.4%]). The other tumors cohort included patients with salivary gland cancer (n = 19), malignant neoplasm of unknown primary site (n = 5), extramammary Paget disease (n = 3), cutaneous melanoma (n = 2), oropharyngeal neoplasm (n = 2), adenoid cystic carcinoma, head and neck cancer, lip and/or oral cavity cancer, esophageal adenocarcinoma, intestinal adenocarcinoma, appendiceal adenocarcinoma, esophageal squamous cell carcinoma, testicular cancer, and vulvar carcinoma (all n = 1).
TABLE 1.
Demographics and Baseline Clinical Characteristics
In total, 202 patients were enrolled on the basis of local HER2 testing, and 65 patients were enrolled on the basis of central HER2 testing. According to HER2 testing for eligibility, 111 patients were enrolled with IHC 3+ expression, 151 with IHC 2+ expression, and five with IHC 1+ expression (Table 1). On the basis of central testing, there were 75 patients with IHC 3+ expression, 125 with IHC 2+ expression, 25 with IHC 1+ expression, 30 with IHC 0 expression, and 12 patients were unknown, owing to unavailable/unevaluable samples for central testing (Appendix Table A1).
At data cutoff (June 8, 2023), the median follow-up duration across all cohorts was 12.75 months (range, 0.4-31.6); 235 patients had discontinued treatment (progressive disease [n = 167, 62.5%], any adverse event [n = 32, 12.0%], death during study [n = 18, 6.7%], patient decision [n = 11, 4.1%], investigator decision [n = 4, 1.5%], unknown [n = 2, 0.7%], lost to follow-up [n = 1, 0.4%]), and 32 (12.0%) patients remained on treatment. The median number of 21-day treatment cycles for all patients was eight.
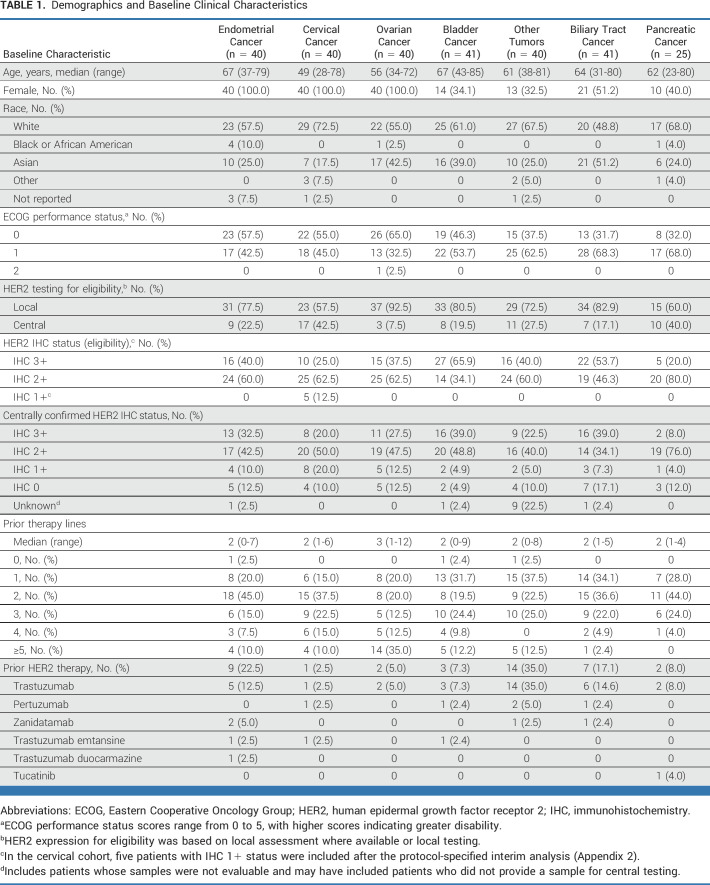
Among the 267 patients, 99 patients (37.1%; [95% CI, 31.3 to 43.2]) had a confirmed objective response by investigator assessment. Investigator-assessed ORRs in all patients by cohort (Fig 1 and Appendix Table A2) were 57.5% for endometrial (95% CI, 40.9 to 73.0), 50.0% for cervical (95% CI, 33.8 to 66.2), 45.0% for ovarian (95% CI, 29.3 to 61.5), 39.0% for bladder (95% CI, 24.2 to 55.5), 30.0% for other tumors (95% CI, 16.6 to 46.5), 22.0% for biliary tract (95% CI, 10.6 to 37.6), and 4.0% for pancreatic (95% CI, 0.1 to 20.4). In patients with centrally confirmed HER2 IHC 3+ expression (n = 75), investigator-assessed ORRs by cohort (Fig 1) were 84.6% for endometrial (n = 13 [95% CI, 54.6 to 98.1]), 75.0% for cervical (n = 8 [95% CI, 34.9 to 96.8]), 63.6% for ovarian (n = 11 [95% CI, 30.8 to 89.1]), 56.3% for bladder (n = 16 [95% CI, 29.9 to 80.2]), 44.4% for other tumors (n = 9 [95% CI, 13.7 to 78.8]), 56.3% for biliary tract (n = 16 [95% CI, 29.9 to 80.2]), and 0% for pancreatic cancer (n = 2). In the pancreatic cohort, no objective response was observed in the first 15 patients, and the cohort was closed for further recruitment according to prespecified futility criterion, by which time 25 patients had been enrolled. Investigator-assessed ORRs by central IHC 3+/2+ status are provided in Figure 1A.
FIG 1.
Investigator-assessed responses as per RECIST 1.1. (A) ORR across tumor cohorts, according to HER2 status by central testing. aResponses in the other tumors cohort include responses in extramammary Paget disease, oropharyngeal neoplasm, head and neck cancer, and salivary gland cancer. (B) The maximum change in tumor size, according to tumor type. Patients with IHC 3+ status (central testing) are marked with a dot. The other tumors cohort includes responses in extramammary Paget disease, head and neck cancer, oropharyngeal neoplasm, and salivary gland cancer. (C) DOR in patients with an objective response, according to tumor type. DOR was defined as the time from the date of first documented response (complete response or partial response) until the date of documented progression, or death in the absence of disease progression. Response was determined by investigator assessment according to RECIST 1.1 and required confirmation after the first observed response at least 4 weeks later. Censored patients are marked with a rounded dot, patients who stopped responding are marked with a triangular dot, and patients with a complete response are marked with a square dot. BTC, biliary tract cancer; DOR, duration of response; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; ORR, objective response rate.
Responses were observed in patients who received (n = 38; 36.8% [95% CI, 21.8 to 54.0]) or did not receive (n = 227; 37.4% [95% CI, 31.1 to 44.1]) prior HER2 therapy. Across all tumor types, 100 patients (37.5% [95% CI, 31.6 to 43.6]) had a confirmed ORR by independent central review. By cohort, ORRs by independent central review in all patients were 57.5% for endometrial (95% CI, 40.9 to 73.0), 37.5% for cervical (95% CI, 22.7 to 54.2), 42.5% for ovarian (95% CI, 27.0 to 59.1), 41.5% for bladder (95% CI, 26.3 to 57.9), 35.0% for other tumors (95% CI, 20.6 to 51.7), 26.8% for biliary tract (95% CI, 14.2 to 42.9), and 12.0% for pancreatic (95% CI, 2.5 to 31.2).
The investigator-assessed median DOR (Fig 1C and Appendix Table A2) across all cohorts was 11.3 months (95% CI, 9.6 to 17.8), ranging from 5.7 months in the pancreatic cohort to 22.1 months in the other tumors cohort; median DOR was not reached in the endometrial cohort. In all HER2 subgroups, the longest median DOR was in patients with IHC 3+ (22.1 months [95% CI, 9.6 to not reached]).
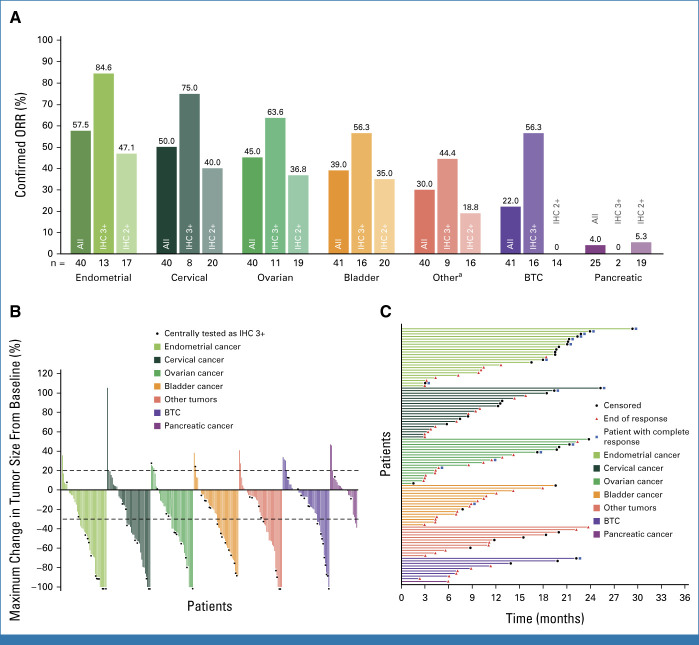
The investigator-assessed median PFS (Fig 2 and Appendix Table A2) was 6.9 months (95% CI, 5.6 to 8.0), ranging from 3.2 months in the pancreatic cohort to 11.1 months in the endometrial cohort. In all HER2 subgroups, the longest median PFS was in patients with IHC 3+ (11.9 months [95% CI, 8.2 to 13.0]). PFS by tumor cohort and HER2 status is provided in Figure 2 and Appendix Table A2.
FIG 2.
Kaplan-Meier estimates of PFS, according to tumor type. (A) Endometrial cancer, (B) cervical cancer, (C) ovarian cancer, (D) bladder cancer, (E) other tumors, (F) biliary tract cancer, and (G) pancreatic cancer. IHC, immunohistochemistry; NR, not reached; PFS, progression-free survival.
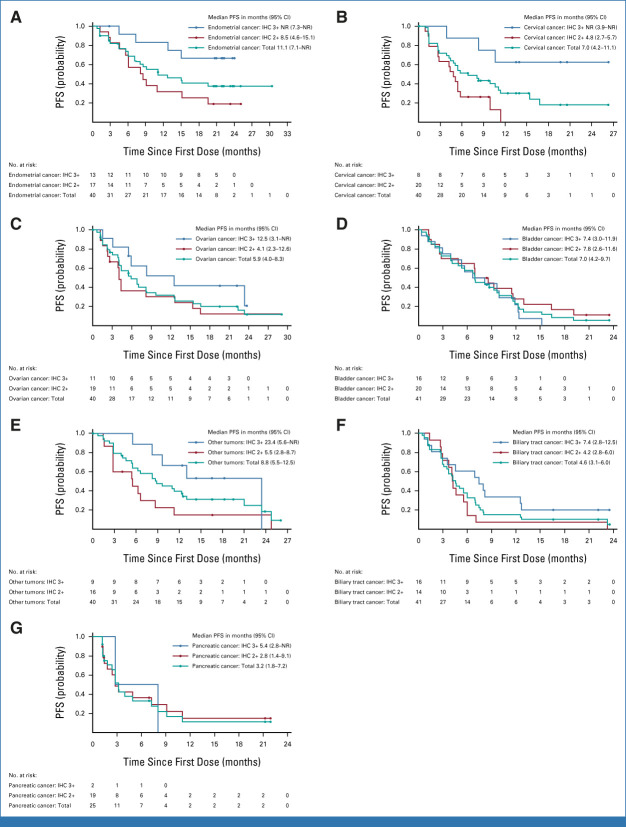
Across all cohorts, the median OS (Fig 3 and Appendix Table A2) was 13.4 months (95% CI, 11.9 to 15.5; 66% maturity), ranging from 5.0 months in the pancreatic cohort to 26.0 months in the endometrial cohort. In all HER2 subgroups, the longest median OS was in patients with IHC 3+ (21.1 months [95% CI, 15.3 to 29.6]). OS by tumor cohort and HER2 status is provided in Figure 3 and Appendix Table A2.
FIG 3.
Kaplan-Meier estimates of OS, according to tumor type. (A) Endometrial cancer, (B) cervical cancer, (C) ovarian cancer, (D) bladder cancer, (E) other tumors, (F) biliary tract cancer, and (G) pancreatic cancer. IHC, immunohistochemistry; NR, not reached; OS, overall survival.
Percentage change of target lesion size from baseline and a full breakdown of efficacy in the other tumors cohort are shown in Appendix Fig A2 and Appendix Table A3, respectively.
Among 267 treated patients (median follow-up of 12.75 months), ≥1 investigator-assessed drug-related adverse event was experienced by 226 (84.6%) patients (Table 2), with the most common being nausea (55.1%), anemia (27.7%), diarrhea (25.8%), vomiting (24.7%), and fatigue (24.7%). Grade 3 or higher drug-related adverse events occurred in 109 (40.8%) patients, with the most common being neutropenia (10.9%) and anemia (10.9%). Serious drug-related adverse events occurred in 36 (13.5%) patients. Drug-related adverse events led to discontinuation in 23 (8.6%) patients and dose reduction in 54 (20.2%) patients. Drug-related adverse events and non–drug-related adverse events resulting in death occurred in four (1.5%) and 19 (7.1%) patients, respectively. Adjudicated drug-related events of ILD/pneumonitis occurred in 28 (10.5%) patients, with the majority as low grade (grade 1, n = 7 [2.6%]; grade 2, n = 17 [6.4%]). There was one (0.4%) grade 3 event and three (1.1%) fatal adjudicated drug-related cases of ILD/pneumonitis, one each in the biliary tract, endometrial, and other tumors cohorts. Non-drug–related adverse events are provided in Appendix Table A4.
TABLE 2.
Incidence of Drug-Related Adverse Events
DISCUSSION
In this phase II study, T-DXd demonstrated durable responses across multiple tumor types, alongside clinically meaningful PFS and OS in pretreated patients. The highest response rates and longest DOR, PFS, and OS were observed in tumors with IHC 3+ expression. Responses were also observed irrespective of prior HER2 therapy.
HER2 protein expression, gene amplification, and gene mutation have been identified as therapeutic targets in multiple tumor types.23 However, HER2-targeted therapy is not currently approved beyond breast, gastric, colorectal, and lung cancer.5,15,24 The tumor types investigated here were predefined on the basis of epidemiological frequency, prevalence of HER2 expression, and unmet medical need.2,5 Investigations are supported by phase I clinical data of T-DXd and encouraging results from the HERALD phase II basket trial which assessed T-DXd in advanced solid tumors with HER2 amplification.18,25
Of note are the magnitudes of benefit observed in the endometrial, cervical, and ovarian cohorts; the highest ORRs were observed in these cohorts across all studied tumor types (57.5% for endometrial, 50.0% for cervical, 45.0% for ovarian). To the best of our knowledge, this is the first report of a HER2-directed antibody-drug conjugate in these gynecological tumors. In the endometrial cohort, 77.5% of patients had ≥two prior lines of therapy. The ORR in patients with HER2 IHC 3+ expression was 84.6%. In all patients with endometrial cancer, median PFS and OS were 11.1 months and 26.0 months, respectively. The clinically significant response and survival rates observed in this study are encouraging for HER2-expressing endometrial cancers, which are typically associated with high risk for progression and poor survival rates.10 In the cervical cohort, 85.0% of patients had ≥two prior lines of therapy, and the ORR in patients with HER2 IHC 3+ expression was 75.0%. The median OS in this cohort was 13.6 months in all patients, not reached in IHC 3+ patients, and 11.5 months in IHC 2+ patients. These data are promising in a cohort with few treatment options and a typically low response rate to treatment.11 The median number of prior treatments in the ovarian cohort was three, and 35.0% of patients had five or more prior lines of therapy; the median OS was 13.2 months in all patients and 20.0 months in patients with HER2 IHC 3+ expression. The results from this study further support use of a HER2 antibody-drug conjugate for treating ovarian cancer, and the outcomes are promising for a disease subgroup with a high mortality rate.12,26
Although there was only one investigator-assessed responder in the pancreatic cohort (4.0%; closed to recruitment with 25 patients enrolled), when assessed by independent central review, three responses were observed (12.0%). PFS and OS results showed potential in the late-line pancreatic cancer setting; however, it is challenging to draw conclusions from this cohort owing to the low patient numbers, particularly in the IHC 3+ group.
Biliary tract cancer (BTC) is uncommon12 but has a high mortality rate13 and limited clinical benefit from second-line chemotherapy.27 The phase II trial of T-DXd in patients with unresectable or recurrent HER2-expressing BTCs showed promising activity in patients with HER2-positive (IHC 3+ and IHC 2+/in-situ hybridization+) BTC.28 The data in the DESTINY-PanTumor02 trial further support HER2 as a therapeutic target in BTC where an ORR of 56.3% and OS of 12.4 months were observed in patients with IHC 3+ tumors.
Safety findings for T-DXd in this trial were consistent with the established safety profile.15 A risk of pulmonary adverse events, primarily ILD/pneumonitis, has been observed in patients receiving T-DXd and is an important consideration for these patients.29,30 Although most cases of adjudicated drug-related ILD in this trial were low-grade and manageable and overall incidence was consistent with that in previous studies,31 three adjudicated drug-related ILD/pneumonitis-related deaths occurred. Multidisciplinary guidelines for diagnosing and managing T-DXd–related ILD/pneumonitis have been published.29 T-DXd–related ILD/pneumonitis can be safely managed with a multidisciplinary team, who should manage the ILD/pneumonitis jointly with the medical oncologist and may include a primary care physician, nurse practitioner, pulmonologist, pathologist, pharmacist, infectious disease specialist, and radiologist. Patients should be proactively monitored for ILD/pneumonitis, and suspected cases should be actively managed by a multidisciplinary team; T-DXd treatment should be interrupted in the event of grade 1 ILD/pneumonitis, and the event must resolve before treatment may resume.29
This tumor-agnostic biomarker-driven approach represents an innovative application of the principles of precision medicine.5 Despite the prospects of the tumor-agnostic strategy, only six drugs have received US Food and Drug Administration approval on the following basis: pembrolizumab for microsatellite instability high, mismatch repair deficient, or tumor mutational burden high tumors; dostarlimab for mismatch repair deficient tumors; larotrectinib or entrectinib for tumors with NTRK gene fusions; dabrafenib plus trametinib for tumors with BRAF V600E mutations; and selpercatinib for tumors with RET gene fusions.32 As with those studies, this trial has a clear rationale on the basis of preclinical/clinical data and demonstrates meaningful antitumor activity across endometrial, cervical, ovarian, bladder, biliary tract, and other tumor cohorts.
A tumor-agnostic investigative approach has some limitations, most notably the single-arm nature of the studies. It was not possible to include a single comparator, given the range of tumor types that were included. Another potential limitation is the few patients included with HER2 IHC 1+ tumors. The protocol allowed for recruitment of patients with HER2 IHC 1+ tumors once 3 of 15 responders within a cohort had been observed in centrally confirmed HER2 IHC 3+ or IHC 2+ tumors. However, only the cervical cohort prospectively opened enrollment to patients with IHC 1+ tumors as recruitment in other cohorts was complete by the time response rate data were available on the first 15 patients. There is limited evidence available from this study in HER2-low patients, a population of growing clinical interest after the approval of T-DXd in HER2-low breast cancer.15 The few responses in patients who were determined to be IHC 1+/0 on retrospective central testing suggest that further exploration in patients with IHC 1+ tumors is warranted beyond breast cancer.
In this global, multicenter phase II study, treatment with T-DXd demonstrated robust clinical activity providing durable clinical benefit for pretreated patients with selected HER2-expressing solid tumors. The observed safety profile, including ILD, was consistent with that in previously reported studies of T-DXd. These data provide clinical evidence for antitumor activity of T-DXd across multiple tumor types, suggesting potential tumor-agnostic activity in patients with HER2-expressing solid tumors.
ACKNOWLEDGMENT
We thank the patients who participated in the study, as well as their families and caregivers. We also thank the staff and investigators at all the study sites and the following AstraZeneca employees: Pippa Hadland for publication leadership, interpretation of the data, and scientific review of the manuscript; Nassim Morsli for clinical leadership of the study, interpretation of the data, and scientific review of the manuscript; Chiedozie Anoka, Fabiola Cecchi, Flavia Michelini, Jose David Hernandez Chagui, Mark Gustavson, and Rob McEwen for interpretation and/or scientific review of the manuscript; Aleksandra Lorenc, Anna Dobrowolska, Jin Sakong, Magdalena Zakrzewska, Marta Czekaj, and Nicholas Holoweckyj for conduct of the study/data; Ahmed Mohamed, Anubhavini Chaudhry, Chetan Shatapathy, Joao Simoes, and Nataliya Kuptsova-Clarkson for safety analyses and data interpretation; and Ann Smith and Kai Chen for statistical analyses. Under guidance of the authors and in accordance with GPP, medical writing support was provided by Neil Patel, MSc, Helios Medical Communications, Cheshire, UK, which was funded by AstraZeneca.
Investigators are listed at Appendix 1.
APPENDIX 1. LIST OF INVESTIGATORS
Australia
Olivia Newton-John Cancer Research Institute: Hui Gan
Olivia Newton-John Cancer Research Institute: Jermaine Coward
Chris O'Brien Lifehouse: Michelle Harrison
Linear Clinical Research: Tarek Meniawy
ICON Cancer Care: Jermaine Coward
Belgium
Reinier de Graaf Gasthuis: Lemonitsa Mammatas
Reinier de Graaf Gasthuis: Annelie Vulink
CHU de Liège—Domaine Sart Tilman: Guy Jerusalem
CHU de Liège—Domaine Sart Tilman: Joëlle Collignon
Universitair Ziekenhuis Brussels: Sofie Joris
UZ Leuven: Toon van Gorp
Canada
Center intégré de cancérologie du CHU de Québec—Université Laval, Hôpital de l’Enfant-Jésus: Olivier Dumas
McGill University—Jewish General Hospital: Cristiano Ferrario
Czech Republic
Fakultni nemocnice, klinika onkologie a radioterapie: Stanislav John
Masarykuv onkologicky ustav: Maria Zvarikova
Fakultni nemocnice Olomouc: Bohuslav Melichar
Nemocnice Na Bulovce: Michal Zikan
Fakultni nemocnice v Motole: Katerina Kopeckova
India
Tata Memorial Hospital: Vikas Ostwal
Artemis Hospitals: Hari Goyal
Rajiv Gandhi Cancer Institute & Research Centre: Vineet Talwar
Tata Medical Center: Bivas Biswas
Italy
Policlinico Universitario A Gemelli Domenica Lorusso IEO—European Institute of Oncology: Nicoletta Colombo
A.O. Ospedale Niguarda Ca’ Granda: Salvatore Siena
Poland
Narodowy Instytut Onkologii im. Marii Skłodowskiej-Curie: Iwona Ługowska
Uniwersyteckie Centrum Kliniczne: Jacek Jassem
Szpital Uniwersytecki w Krakowie: Piotr Wysocki
Centrum Onkologii im. Łukaszczyka: Bogdan Żurawski
Uniwersytecki Szpital Kliniczny w Poznaniu: Jacek Mackiewicz
The Netherlands
Antoni van Leeuwenhoek Hospital—Netherlands Cancer Institute: Neeltje Steeghs
Universitair Medisch Centrum Groningen: Mathilde Jalving
Russia
GBUZ Saint Petersburg clinical scientific and practical center: Vladimir Moiseyenko
Moscow City Oncology Hospital #62: Daniil Stroyakovskiy
AO Medsi (Moscow region): Anastasiya Mochalova
Russian Scientific Center of Roentgeno-Radiology: Yulia Kreynina
N.N. Blokhin Medical Research Center of Oncology: Elena Artamonova
Kaluga Regional Clinical Oncology Dispensary GBUZ KO “KOKOD”: Igor Kudryavtsev
Hadassah Medical Moscow—Oncology Department: Dmitry Gornastolev
Clinical Hospital “RZHD-Medicine”: Konstantin Penkov
Clinical Hospital “RZHD-Medicine”: Aleksandr Vasiliev
LLC Evromedservis: Konstantin Penkov
Spain
Madrid, H.U. La Paz, Oncología: Andrés Redondo Sánchez
Hospital Universitario 12 de Octubre: Luis Manuel Manso Sanchez
Clínica Universidad de Navarra: Antonio Gonzalez Martin
Hospital Universitario Reina Sofía: Alberto Moreno Vega
Hospital Universitario Vall d’Hebrón: Ana Oaknin Benzaken
Hospital General Universitario de Valencia, Oncologia: Cristina Caballero Díaz
Madrid, H.C.S. Carlos, Oncología: Aranzazu Manzano Fernández
Madrid, H.C.S. Carlos, Oncología: Gonzalo Fernandez Hinojal
South Korea
Seoul National University Hospital: Do-Youn Oh
Severance Hospital, Yonsei University Health System: Jung-Yun Lee
Samsung Medical Center: Seung Tae Kim
Asan Medical Center: Kyung Hae Jung
Taiwan
Taipei Veterans General Hospital: Yee Chao
Taipei Veterans General Hospital: Yi-Ping Hung
Department of Oncology, Chi-Mei Hospital—Liouying: Sheng-Yen Hsiao
National Taiwan University Hospital—Oncology: Chia-Chi Lin
Veterans General Hospital Taichung: Chien-Hsing Lu
Linkou Chang Gung Memorial Hospital: Jen-Shi Chen
Thailand
Maharaj Nakorn Chiang Mai Hospital: Busyamas Chewaskulyong
Division of Medical Oncology, Srinagarind Hospital: Jarin Chindaprasirt
King Chulalongkorn Memorial Hospital: Napa Parinyanitikul
Chulabhorn Hospital: Teerapat Ungtrakul
Songklanagarind Hospital, Prince of Songkla University: Arunee Dechaphunkul
Bangkok, Oncology Unit, Pramongkutklao H.: Naiyarat Prasongsook
Medical Oncology Unit, Department of Internal Medicine, HRH Princess Mahachakri Sirindhorn Medical Center, Faculty of Medicine: Chanchai Charonpongsuntorn
The United Kingdom
The Royal Marsden NHS Foundation Trust: Susana Banerjee
Christie Hospital: Mairead McNamara
United States of America
MD Anderson Cancer Center: Funda Meric-Bernstam
Memorial Sloan Kettering Cancer Center: Vicky Makker
Dana-Farber Cancer Institute: Jennifer Veneris
Dana-Farber Cancer Institute: Panagiotis Konstantinopoulos
Icahn School of Medicine at Mount Sinai: Deborah Doroshow
The University of Chicago Medical Center: Gini Fleming
University of Washington—Seattle Cancer Care Alliance: John Liao
IU Health Ball Memorial Hospital Physicians, Inc: Jonathan Berkowitz
St Joseph Heritage Healthcare: Ian Anderson
City of Hope Comprehensive Cancer Center: Daneng Li
Duke University: Jennifer Choe
Duke University: James Abbruzzese
APPENDIX 2. METHODS
Patients
Male and female patients were at least 18 years of age at the time of giving signed informed consent. Patients with locally advanced, unresectable, or metastatic solid tumors with histology specific to respective cohorts, who have progressed after at least one prior systemic treatment for metastatic or advanced disease, or who have no satisfactory alternative treatment option, were recruited; patients with prior human epidermal growth factor receptor 2 (HER2)–targeted therapy were permitted. The following are the respective cohorts for patient inclusion:
Cohort 1 (biliary tract cancer): metastatic or advanced biliary tract cancers, including intrahepatic or extrahepatic cholangiocarcinoma and tumors arising in the ampulla of Vater or gallbladder
Cohort 2 (bladder cancer): metastatic or advanced urothelial carcinoma, including transitional cell or predominantly transitional cell carcinoma of the renal pelvis, ureter, urinary bladder, or urethra
Cohort 3 (cervical cancer): metastatic or advanced cervical carcinoma
Cohort 4 (endometrial cancer): metastatic or advanced endometrial carcinoma
Cohort 5 (ovarian cancer): metastatic or advanced epithelial ovarian carcinoma
Cohort 6 (pancreatic cancer): metastatic or advanced pancreatic cancer
Cohort 7 (other tumors): metastatic or advanced rare tumors with HER2 overexpression (immunohistochemistry [IHC] 3+ and 2+), excluding the tumors mentioned above, and breast, non–small-cell lung, gastric, and colorectal cancers
Patients must have had HER2 overexpression (IHC 3+ or IHC 2+) as determined by local or central assessment scored using current ASCO/College of American Pathologists guidelines for scoring HER2 in gastric cancer. Central assessment may have been offered on the basis of site need. For each cohort, 1-6, up to 10 IHC 1+ patients may have been included if ≥3 objective responses were observed in the first 15 patients with confirmed HER2 overexpression (IHC 3+ or IHC 2+) by central testing. For the other tumors cohort (cohort 7), only patients with HER2 overexpression (IHC 3+ or IHC 2+) were enrolled. Patients must have provided an existing formalin-fixed paraffin-embedded (FFPE) tumor sample for tissue-based IHC staining to centrally determine HER2 expression and other correlatives. The mandatory FFPE tumor sample needed to have been obtained at the time of diagnosis of metastatic or locally advanced, unresectable, solid tumors (most recent pre-enrollment tumor sample must have been provided). Specimens with limited tumor content and fine needle aspirates were inadequate for defining tumor HER2 status. Patients were also required to have measurable target disease assessed by the investigator on the basis of RECIST 1.1, an Eastern Cooperative Oncology Group performance status of 0-1, left ventricular ejection fraction ≥50% by either echocardiography or multiple-gated acquisition scan within 28 days before treatment assignment, adequate organ function within 14 days before trastuzumab deruxtecan (T-DXd) administration, and adequate treatment washout period before study drug treatment.
Patients were excluded from the study if they had a known somatic DNA mutation of HER2 (ERBB2) without tumoral HER2 expression, primary diagnosis of adenocarcinoma of the breast, adenocarcinoma of the colon or rectum, adenocarcinoma of the gastric body or gastroesophageal junction, or non–small-cell lung cancer. Substance abuse or any other medical conditions (eg, clinically significant cardiac or psychological conditions) that may, in the opinion of the investigator, have interfered with the patient's participation in the clinical study or evaluation of the clinical study results also warranted exclusion from the study.
Central HER2 Testing
Tumor tissue samples collected from patients will be analyzed for HER2 status by a central laboratory designated by the sponsor using a validated assay. Tumor lesions used to acquire samples for HER2 testing were not target lesions, unless there were no other lesions suitable for biopsy. Samples with limited tumor content and fine needle aspirate specimens were considered not acceptable.
Treatment and Responses
Patients received a dose of 5.4 mg/kg once every 3 weeks, and the number of treatment cycles with T-DXd until RECIST 1.1 disease progression and withdrawal of consent parameters were not fixed. On commencing study treatment, patients continued receiving T-DXd until RECIST 1.1 disease progression, withdrawal of consent, or any of the discontinuation criteria were met.
T-DXd was administered using an intravenous bag containing 5% (w/v) dextrose injection infusion solution and delivered through an intravenous administration set with a 0.2 or 0.22 μm filter. The standard infusion time for T-DXd was approximately 90 minutes for the first infusion. If the first infusion was well tolerated and the participant did not experience an infusion-related reaction, the minimum infusion time for subsequent cycles was at least 30 minutes. If there were interruptions during the infusion, the total infusion time was not allowed to exceed 3 hours at room temperature. The participant's weight at screening (baseline) was used to calculate the initial dose. If, during treatment, the participant's weight changed by ≥10%, the participant's dose was recalculated on the basis of the participant's updated weight.
All dose modifications (interruption, reduction, and/or discontinuation) should be based on the worst preceding toxicity. Dosing was interrupted (or discontinued in the case of a dose-limiting toxicity), and supporting therapy was administered as required. On improvement of an adverse event leading to dose interruption, T-DXd therapy could be resumed at the same dose. If a further episode of the same adverse event, or a different adverse event, required dose interruption, therapy could be restarted at a reduced dose on improvement (dose level 1: 4.4 mg/kg of body weight once every 3 weeks; dose level 2: 3.2 mg/kg of body weight once every 3 weeks). Treatment-emergent adverse events were assessed by the study investigator as related to use of T-DXd.
Interstitial Lung Disease/Pneumonitis
Interstitial lung disease (ILD) is considered an important identified risk based on a comprehensive cumulative review of potential ILD/pneumonitis cases reviewed by the independent ILD Adjudication Committee, the available safety data from the clinical development program, available data from recent epidemiology/literature, biological plausibility, and safety information from drugs of similar class. High-resolution computed tomography and pulmonary function were measured at baseline and at the time of suspected ILD/pneumonitis events. Pulmonologist consultation, pulse oximetry (SpO2), arterial blood gases if clinically indicated, and one blood sample were collected for pharmacokinetics as soon as ILD/pneumonitis was suspected, if feasible.
Multidisciplinary guidelines for diagnosing and managing T-DXd-related ILD/pneumonitis have been published and are available at Swain et al.29
Visit Responses
For all patients, the RECIST tumor response data were used to determine each patient's visit response according to RECIST 1.1. They were also used to determine if a patient had progressed in accordance with RECIST and their best objective response to study treatment.
Baseline radiological tumor assessments were performed no more than 28 days before the start of study treatment and were performed as close as possible to the start of study treatment. Postbaseline tumor assessments by the investigator were performed at the following time points:
Every 6 weeks (±1 week) relative to the date of first dose of T-DXd, until RECIST 1.1-defined radiological disease progression
Tumor assessment scans continued if patients discontinued T-DXd owing to toxicity without progression until progressive disease was detected
If an unscheduled assessment was performed and the patient had not progressed, every attempt should have been made to complete the subsequent assessments at their scheduled visits. This schedule was followed to minimize any unintentional bias caused by some patients being assessed at a different frequency from other patients.
At each visit, patients were assigned a RECIST 1.1 visit response of complete response, partial response, stable disease, or progressive disease, using the information from target lesions, nontarget lesions, and new lesions and depending on the status of their disease compared with baseline and previous assessments. If a patient had a tumor assessment that could not be evaluated, the patient was assigned a visit response of not evaluable unless there was evidence of progression, in which case the response was assigned as progressive disease.
Interim Analyses
Interim efficacy analyses were performed using the centrally determined analysis set after 15 centrally determined HER2-eligible patients within a cohort had the opportunity to complete two scheduled postbaseline scans according to RECIST 1.1. Safety data were reviewed alongside efficacy to support any decision to expand the inclusion criteria or size of a cohort. No adjustment for multiple testing was planned for this study.
Once 15 patients within a cohort were centrally determined as having HER2 IHC 3+ or IHC 2+ and had the opportunity to complete at least two scheduled postbaseline scans according to RECIST 1.1, the following applied:
For each tumor-specific cohort (cohorts 1-6), the inclusion criteria were expanded to include up to 10 IHC 1+ patients, if three or more responses were observed in the first 15 patients. If one or two responses were observed in the first 15 patients, the cohort continued recruiting without change. If zero responses were observed in the first 15 patients, the cohort was closed to further recruitment
For the other tumors cohort (cohort 7), if one or more responses were observed in the first 15 patients, the cohort continued recruiting without change. If zero responses were observed in the first 15 patients, the cohort was closed to further recruitment
During the study, both the bladder and cervical cohorts met the protocol-specified criteria to open recruitment of IHC 1+ patients, and only the cervical cohort prospectively recruited patients who were 1+ after this point and so available data for 1+ patients are very limited. The cohorts for biliary tract cancer, endometrial cancer, and ovarian cancer had almost fully enrolled to 40 patients at the time the first 15 centrally confirmed patients were evaluable for response. Recruitment to the pancreatic cohort was closed (March 24, 2022) as zero responses in the first 15 patients had been observed (Appendix Table A5)
Statistical Analyses
All RECIST 1.1 assessments, whether scheduled or unscheduled, were included in the calculation of efficacy variables, regardless of whether a patient discontinued study treatment or received another anticancer therapy. At the time of final analysis, all efficacy end points were summarized by cohort for the full analysis set. Selected efficacy end points were also summarized by cohort for the centrally determined efficacy analysis set (Appendix Table A6).
TABLE A1.
HER2 Status at Baseline, Local versus Central Test Results
TABLE A2.
Efficacy by Tumor Cohort
TABLE A3.
Efficacy by Tumor Type in the Other Tumors Cohort
TABLE A4.
Safety (non–drug-related AEs)
Table A5.
Visit Response Summary
Table A6.
Efficacy Analyses Summary
FIG A1.
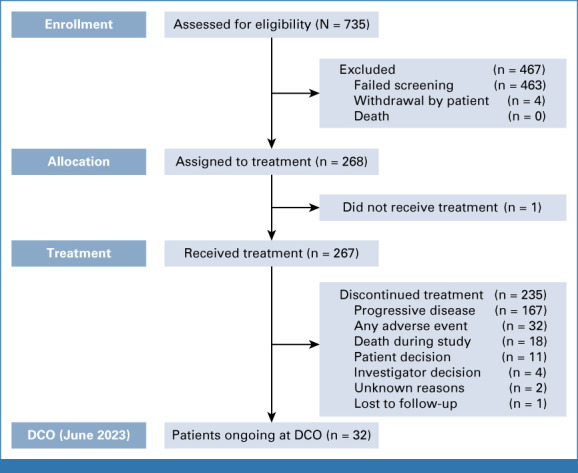
Patient disposition. DCO, data cutoff.
FIG A2.
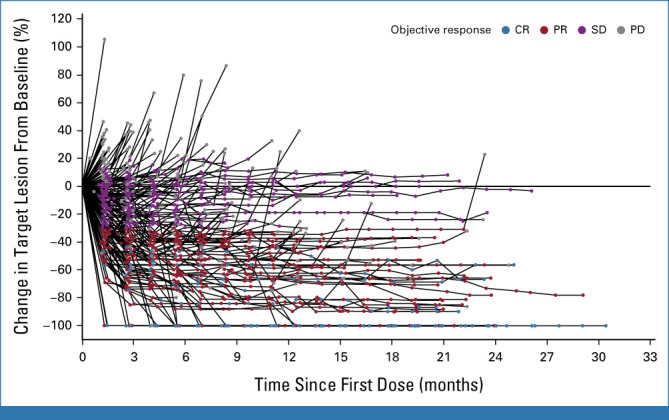
Target lesions size, percentage change from baseline over time (full analysis set). CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Funda Meric-Bernstam
Employment: MD Anderson Cancer Center
Consulting or Advisory Role: F. Hoffmann-La Roche, Zymeworks, Infinity Pharmaceuticals, Black Diamond Therapeutics, Eisai, OnCusp Therapeutics, Lengo Therapeutics, Tallac Therapeutics, Karyopharm Therapeutics, Biovica, AstraZeneca, Seattle Genetics, LOXO-Oncology, PACT Pharmaceuticals, Apeiron Biologics, EcoR1 Capital, Menarini Group, Theratechnologies, LegoChem Biosciences, Calibr
Research Funding: Novartis (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst), Genentech (Inst), Calithera Biosciences (Inst), Debiopharm Group (Inst), Bayer (Inst), Aileron Therapeutics (Inst), PUMA Biotechnology (Inst), CytomX Therapeutics (Inst), Jounce Therapeutics (Inst), Zymeworks (Inst), Curis (Inst), Pfizer (Inst), eFFECTOR Therapeutics (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), GlaxoSmithKline (Inst), Seattle Genetics (Inst), Taiho Pharmaceutical Co (Inst), Klus Pharma (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: European Organisation for Research and Treatment of Cancer (EORTC), European Society for Medical Oncology (ESMO), Cholangiocarcinoma Foundation
Vicky Makker
Consulting or Advisory Role: Eisai, Merck, Karyopharm, ArQule, Takeda, IBM Watson, GlaxoSmithKline, Clovis, Faeth, Novartis, Duality, ITeos Therapeutics, Kartos Therapeutics, Lilly
Research Funding: Lilly, AstraZeneca, Eisai, Merck, Bristol-Myers Squibb, Karyopharm Therapeutics, Takeda, Clovis, Bayer, Zymeworks, Duality, Faeth Therapeutics
Travel, Accommodations, Expenses: Eisai, Merck, Karyopharm Therapeutics
Other Relationship: IBM
Ana Oaknin
Consulting or Advisory Role: AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Immunogen, Genmab, Mersana Therapeutics, GSK, Deciphera Pharmaceutial, AGENUS, Corcept Therapeutics, Eisai, F. Hoffmann-La Roche, Merck Sharp & Dohme, Novocure, Sattucklabs, Sutro Biopharma, iTheos, Seagen, OneXerna Therapeutics, Inc, Regeneron Pharmaceuticals, Inc, Exelixis
Research Funding: AbbVie Deutschland (Inst), Advaxis Inc (Inst), Aeterna Zentaris (Inst), Aprea Therapeutics (Inst), Clovis Oncology Inc (Inst), EISAI limited LTD (Inst), F. Hoffmann-La Roche LTD (Inst), Regeneron Pharmaceuticals (Inst), Bristol Myers Squibb International Corporation (BMS) (Inst), Immunogen (Inst), MedImmune (Inst), Merck Sharp & Dohme (Inst), Tesaro (Inst), Amgen (Inst), Millennium Pharmaceuticals Inc (Inst), PharmaMar (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche
Do-Youn Oh
Consulting or Advisory Role: AstraZeneca (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year), Merck Serono (less than $10,000 USD in a single calendar year), Genentech/Roche (less than $10,000 USD in a single calendar year), Bayer (less than $10,000 USD in a single calendar year), Taiho Pharmaceutical (less than $10,000 USD in a single calendar year), ASLAN Pharmaceuticals (less than $10,000 USD in a single calendar year), Halozyme (less than $10,000 USD in a single calendar year), Zymeworks (less than $10,000 USD in a single calendar year), Celgene (less than $10,000 USD in a single calendar year), Basilea (less than $10,000 USD in a single calendar year), BeiGene (less than $10,000 USD in a single calendar year), Turning Point Therapeutics (less than $10,000 USD in a single calendar year), Yuhan (less than $10,000 USD in a single calendar year), Arcus Biosciences (less than $10,000 USD in a single calendar year), IQVIA (less than $10,000 USD in a single calendar year), MSD Oncology (less than $10,000 USD in a single calendar year)
Research Funding: AstraZeneca, Novartis, Array BioPharma, Eli Lilly, SERVIER, BeiGene, MSD, Handok
Susana Banerjee
Stock and Other Ownership Interests: Percihealth (shares of a publicly traded company equal or less than $50,000 in value, or an equity interest in a privately held company equal or less than 5%)
Honoraria: AstraZeneca, GSK, Clovis (less than $10,000 USD in a single calendar year), Pfizer (less than $10,000 USD in a single calendar year), Immunogen, MSD Oncology, Mersana, Roche, Takeda, Amgen (less than $10,000 USD in a single calendar year), Novacure
Consulting or Advisory Role: GlaxoSmithKline ($10,000 USD or above in a single calendar year), MSD Oncology, Mersana, AstraZeneca, Seagen, OncXerna Therapeutics, Shattuck Labs, Immunogen, Regeneron ($10,000 USD or above in a single calendar year), Novartis ($10,000 USD or above in a single calendar year), Epsilogen ($10,000 USD or above in a single calendar year)
Research Funding: GSK ($10,000 USD or above in a single calendar year), AstraZeneca ($10,000 USD or above in a single calendar year)
Travel, Accommodations, Expenses: GSK (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year), Verastem (less than $10,000 USD in a single calendar year)
Antonio González-Martín
Consulting or Advisory Role: Roche (less than $10,000 USD in a single calendar year), Tesaro/GSK (less than $10,000 USD in a single calendar year), Clovis (less than $10,000 USD in a single calendar year), AstraZenenca (less than $10,000 USD in a single calendar year), MSD (less than $10,000 USD in a single calendar year), Genmab (less than $10,000 USD in a single calendar year), Immunogen (less than $10,000 USD in a single calendar year), Oncoinvent (less than $10,000 USD in a single calendar year), Pfizer/EMD Serono (less than $10,000 USD in a single calendar year), Amgen (less than $10,000 USD in a single calendar year), Mersana (less than $10,000 USD in a single calendar year), SOTIO (less than $10,000 USD in a single calendar year), SUTRO (less than $10,000 USD in a single calendar year), Macrogenics (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year), Alkermes (less than $10,000 USD in a single calendar year), HederaDx (less than $10,000 USD in a single calendar year), Novocure (less than $10,000 USD in a single calendar year), Seagen (less than $10,000 USD in a single calendar year), Takeda (less than $10,000 USD in a single calendar year)
Speakers' Bureau: Roche (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year), Tesaro/GSK (less than $10,000 USD in a single calendar year), PharmaMar (less than $10,000 USD in a single calendar year), Clovis Oncology (less than $10,000 USD in a single calendar year), MSD Oncology (less than $10,000 USD in a single calendar year)
Research Funding: Roche ($10,000 USD or above in a single calendar year), Tesaro/GSK ($10,000 USD or above in a single calendar year)
Travel, Accommodations, Expenses: Roche ($10,000 USD or above in a single calendar year), AstraZeneca ($10,000 USD or above in a single calendar year), PharmaMar ($10,000 USD or above in a single calendar year), Tesaro/GSK (less than $10,000 USD in a single calendar year), MSD Oncology (less than $10,000 USD in a single calendar year)
Kyung Hae Jung
Consulting or Advisory Role: Roche Korea (less than $10,000 USD in a single calendar year), AstraZeneca Korea (less than $10,000 USD in a single calendar year), Celgene Korea (less than $10,000 USD in a single calendar year), Eisai Korea (less than $10,000 USD in a single calendar year), Takeda Pharmaceuticals Korea (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year), Everest Medicine (less than $10,000 USD in a single calendar year), Merck (less than $10,000 USD in a single calendar year), Bixink (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year), Eisai (less than $10,000 USD in a single calendar year), Pfizer (less than $10,000 USD in a single calendar year), Daiichi Sankyo/AstraZeneca (less than $10,000 USD in a single calendar year), MSD (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year)
Iwona Ługowska
Stock and Other Ownership Interests: Amgen, Roche, BMS, Janssen, AstraZeneca, Agenus, Macrogenics, Celon, MSD, Menarini, Pfizer, Sanofi, BeiGene, Jacobio, Loxo, Rhizen, Takeda, Cullinan Oncology
Research Funding: Roche, AGENUS
Travel, Accommodations, Expenses: BMS
Luis Manso
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Novartis, Pfizer, Tesaro, Eisai, Lilly, Clovis Oncology, Pierre Fabre, GSK
Speakers' Bureau: Roche/Genentech, Novartis, Pfizer, AstraZeneca, Lilly, Tesaro
Travel, Accommodations, Expenses: Tesaro, MSD Oncology, GSK
Aránzazu Manzano
Consulting or Advisory Role: GSK (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year)
Speakers' Bureau: GSK (less than $10,000 USD in a single calendar year), AstraZeneca Spain (less than $10,000 USD in a single calendar year), PharmaMar (less than $10,000 USD in a single calendar year), Sanofi (less than $10,000 USD in a single calendar year), MSD Oncology (less than $10,000 USD in a single calendar year), LEO Pharma (less than $10,000 USD in a single calendar year), Rovi (less than $10,000 USD in a single calendar year)
Research Funding: AstraZeneca ($10,000 USD or above in a single calendar year)
Travel, Accommodations, Expenses: GlaxoSmithKline (less than $10,000 USD in a single calendar year), AstraZeneca Spain (less than $10,000 USD in a single calendar year)
Bohuslav Melichar
Honoraria: Roche (less than $10,000 USD in a single calendar year), Pfizer (less than $10,000 USD in a single calendar year), Bristol Myers Squibb (less than $10,000 USD in a single calendar year), Astellas Pharma (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year), MSD (less than $10,000 USD in a single calendar year), Merck Serono (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year), Eisai (less than $10,000 USD in a single calendar year), Lilly (less than $10,000 USD in a single calendar year)
Consulting or Advisory Role: Roche (less than $10,000 USD in a single calendar year), Pfizer (less than $10,000 USD in a single calendar year), Bristol Myers Squibb (less than $10,000 USD in a single calendar year), Astellas Pharma (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year), MSD (less than $10,000 USD in a single calendar year), Merck Serono (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year), Eisai (less than $10,000 USD in a single calendar year), Lilly (less than $10,000 USD in a single calendar year)
Travel, Accommodations, Expenses: Bristol Myers Squibb (less than $10,000 USD in a single calendar year), Merck Serono, AstraZeneca, MSD
Salvatore Siena
Stock and Other Ownership Interests: Guardant Health (shares of a publicly traded Company equal or less than $50,000 in value, or an equity interest in a privately held Company equal or less than 5%), Myriad Genetics (shares of a publicly traded Company equal or less than $50,000 in value, or an equity interest in a privately held Company equal or less than 5%)
Consulting or Advisory Role: Bayer (less than $10,000 USD in a single calendar year), Bristol Myers Squibb (less than $10,000 USD in a single calendar year), Daiichi Sankyo (less than $10,000 USD in a single calendar year), Merck (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year), CheckmAb (less than $10,000 USD in a single calendar year), Agenus (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year), GSK (less than $10,000 USD in a single calendar year), MSD Oncology (less than $10,000 USD in a single calendar year), Pierre Fabre (less than $10,000 USD in a single calendar year), Seagen (less than $10,000 USD in a single calendar year), T-One Therapeutics (less than $10,000 USD in a single calendar year)
Research Funding: MSD Oncology ($10,000 USD or above in a single calendar year)
Patents, Royalties, Other Intellectual Property: Amgen
Travel, Accommodations, Expenses: Amgen, Bayer, Roche
Daniil Stroyakovskiy
Speakers' Bureau: Roche/Genentech, Bristol Myers Squibb, BioCad, AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca, Novartis, Roche
Anitra Fielding
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Yan Ma
Employment: AstraZeneca, BeiGene
Stock and Other Ownership Interests: BeiGene
Travel, Accommodations, Expenses: AstraZeneca
Soham Puvvada
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Research Funding: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Norah Shire
Employment: AstraZeneca
Leadership: Anzu Partners
Jung-Yun Lee
Consulting or Advisory Role: AstraZeneca, MSD, Roche, Takeda
Research Funding: Clovis Oncology ($10,000 USD or above in a single calendar year), Immunogen ($10,000 USD or above in a single calendar year), Janssen Oncology ($10,000 USD or above in a single calendar year), Merck ($10,000 USD or above in a single calendar year), MSD ($10,000 USD or above in a single calendar year), Synthon ($10,000 USD or above in a single calendar year), Eisai ($10,000 USD or above in a single calendar year), Mersana ($10,000 USD or above in a single calendar year), Ascendis Pharma ($10,000 USD or above in a single calendar year), AstraZeneca ($10,000 USD or above in a single calendar year), Novartis ($10,000 USD or above in a single calendar year), OncoQuest Pharmaceutical ($10,000 USD or above in a single calendar year), Roche ($10,000 USD or above in a single calendar year), Seagen ($10,000 USD or above in a single calendar year), Takeda ($10,000 USD or above in a single calendar year)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the ASCO 2023 annual meeting, Chicago, IL, June 2-6, 2023.
SUPPORT
Supported by and sponsored by AstraZeneca in collaboration with Daiichi Sankyo. In March 2019, AstraZeneca entered into a global development and commercialization collaboration agreement with Daiichi Sankyo for trastuzumab deruxtecan (T-DXd; DS-8201). In collaboration with the authors, both AstraZeneca and Daiichi Sankyo assisted with data interpretation, writing of the report, reviewing the manuscript, and the decision to submit for publication.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at: https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/.
AUTHOR CONTRIBUTIONS
Conception and design: Funda Meric-Bernstam, Salvatore Siena, Soham Puvvada, Norah Shire, Jung-Yun Lee
Provision of study materials or patients: Ana Oaknin, Antonio González-Martín, Kyung Hae Jung, Luis Manso, Bohuslav Melichar, Salvatore Siena, Daniil Stroyakovskiy
Collection and assembly of data: Funda Meric-Bernstam, Do-Youn Oh, Antonio González-Martín, Aránzazu Manzano, Bohuslav Melichar, Salvatore Siena, Soham Puvvada, Norah Shire, Jung-Yun Lee
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Funda Meric-Bernstam
Employment: MD Anderson Cancer Center
Consulting or Advisory Role: F. Hoffmann-La Roche, Zymeworks, Infinity Pharmaceuticals, Black Diamond Therapeutics, Eisai, OnCusp Therapeutics, Lengo Therapeutics, Tallac Therapeutics, Karyopharm Therapeutics, Biovica, AstraZeneca, Seattle Genetics, LOXO-Oncology, PACT Pharmaceuticals, Apeiron Biologics, EcoR1 Capital, Menarini Group, Theratechnologies, LegoChem Biosciences, Calibr
Research Funding: Novartis (Inst), AstraZeneca (Inst), Taiho Pharmaceutical (Inst), Genentech (Inst), Calithera Biosciences (Inst), Debiopharm Group (Inst), Bayer (Inst), Aileron Therapeutics (Inst), PUMA Biotechnology (Inst), CytomX Therapeutics (Inst), Jounce Therapeutics (Inst), Zymeworks (Inst), Curis (Inst), Pfizer (Inst), eFFECTOR Therapeutics (Inst), AbbVie (Inst), Boehringer Ingelheim (Inst), Guardant Health (Inst), Daiichi Sankyo (Inst), GlaxoSmithKline (Inst), Seattle Genetics (Inst), Taiho Pharmaceutical Co (Inst), Klus Pharma (Inst), Takeda (Inst)
Travel, Accommodations, Expenses: European Organisation for Research and Treatment of Cancer (EORTC), European Society for Medical Oncology (ESMO), Cholangiocarcinoma Foundation
Vicky Makker
Consulting or Advisory Role: Eisai, Merck, Karyopharm, ArQule, Takeda, IBM Watson, GlaxoSmithKline, Clovis, Faeth, Novartis, Duality, ITeos Therapeutics, Kartos Therapeutics, Lilly
Research Funding: Lilly, AstraZeneca, Eisai, Merck, Bristol-Myers Squibb, Karyopharm Therapeutics, Takeda, Clovis, Bayer, Zymeworks, Duality, Faeth Therapeutics
Travel, Accommodations, Expenses: Eisai, Merck, Karyopharm Therapeutics
Other Relationship: IBM
Ana Oaknin
Consulting or Advisory Role: AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Immunogen, Genmab, Mersana Therapeutics, GSK, Deciphera Pharmaceutial, AGENUS, Corcept Therapeutics, Eisai, F. Hoffmann-La Roche, Merck Sharp & Dohme, Novocure, Sattucklabs, Sutro Biopharma, iTheos, Seagen, OneXerna Therapeutics, Inc, Regeneron Pharmaceuticals, Inc, Exelixis
Research Funding: AbbVie Deutschland (Inst), Advaxis Inc (Inst), Aeterna Zentaris (Inst), Aprea Therapeutics (Inst), Clovis Oncology Inc (Inst), EISAI limited LTD (Inst), F. Hoffmann-La Roche LTD (Inst), Regeneron Pharmaceuticals (Inst), Bristol Myers Squibb International Corporation (BMS) (Inst), Immunogen (Inst), MedImmune (Inst), Merck Sharp & Dohme (Inst), Tesaro (Inst), Amgen (Inst), Millennium Pharmaceuticals Inc (Inst), PharmaMar (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche
Do-Youn Oh
Consulting or Advisory Role: AstraZeneca (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year), Merck Serono (less than $10,000 USD in a single calendar year), Genentech/Roche (less than $10,000 USD in a single calendar year), Bayer (less than $10,000 USD in a single calendar year), Taiho Pharmaceutical (less than $10,000 USD in a single calendar year), ASLAN Pharmaceuticals (less than $10,000 USD in a single calendar year), Halozyme (less than $10,000 USD in a single calendar year), Zymeworks (less than $10,000 USD in a single calendar year), Celgene (less than $10,000 USD in a single calendar year), Basilea (less than $10,000 USD in a single calendar year), BeiGene (less than $10,000 USD in a single calendar year), Turning Point Therapeutics (less than $10,000 USD in a single calendar year), Yuhan (less than $10,000 USD in a single calendar year), Arcus Biosciences (less than $10,000 USD in a single calendar year), IQVIA (less than $10,000 USD in a single calendar year), MSD Oncology (less than $10,000 USD in a single calendar year)
Research Funding: AstraZeneca, Novartis, Array BioPharma, Eli Lilly, SERVIER, BeiGene, MSD, Handok
Susana Banerjee
Stock and Other Ownership Interests: Percihealth (shares of a publicly traded company equal or less than $50,000 in value, or an equity interest in a privately held company equal or less than 5%)
Honoraria: AstraZeneca, GSK, Clovis (less than $10,000 USD in a single calendar year), Pfizer (less than $10,000 USD in a single calendar year), Immunogen, MSD Oncology, Mersana, Roche, Takeda, Amgen (less than $10,000 USD in a single calendar year), Novacure
Consulting or Advisory Role: GlaxoSmithKline ($10,000 USD or above in a single calendar year), MSD Oncology, Mersana, AstraZeneca, Seagen, OncXerna Therapeutics, Shattuck Labs, Immunogen, Regeneron ($10,000 USD or above in a single calendar year), Novartis ($10,000 USD or above in a single calendar year), Epsilogen ($10,000 USD or above in a single calendar year)
Research Funding: GSK ($10,000 USD or above in a single calendar year), AstraZeneca ($10,000 USD or above in a single calendar year)
Travel, Accommodations, Expenses: GSK (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year), Verastem (less than $10,000 USD in a single calendar year)
Antonio González-Martín
Consulting or Advisory Role: Roche (less than $10,000 USD in a single calendar year), Tesaro/GSK (less than $10,000 USD in a single calendar year), Clovis (less than $10,000 USD in a single calendar year), AstraZenenca (less than $10,000 USD in a single calendar year), MSD (less than $10,000 USD in a single calendar year), Genmab (less than $10,000 USD in a single calendar year), Immunogen (less than $10,000 USD in a single calendar year), Oncoinvent (less than $10,000 USD in a single calendar year), Pfizer/EMD Serono (less than $10,000 USD in a single calendar year), Amgen (less than $10,000 USD in a single calendar year), Mersana (less than $10,000 USD in a single calendar year), SOTIO (less than $10,000 USD in a single calendar year), SUTRO (less than $10,000 USD in a single calendar year), Macrogenics (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year), Alkermes (less than $10,000 USD in a single calendar year), HederaDx (less than $10,000 USD in a single calendar year), Novocure (less than $10,000 USD in a single calendar year), Seagen (less than $10,000 USD in a single calendar year), Takeda (less than $10,000 USD in a single calendar year)
Speakers' Bureau: Roche (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year), Tesaro/GSK (less than $10,000 USD in a single calendar year), PharmaMar (less than $10,000 USD in a single calendar year), Clovis Oncology (less than $10,000 USD in a single calendar year), MSD Oncology (less than $10,000 USD in a single calendar year)
Research Funding: Roche ($10,000 USD or above in a single calendar year), Tesaro/GSK ($10,000 USD or above in a single calendar year)
Travel, Accommodations, Expenses: Roche ($10,000 USD or above in a single calendar year), AstraZeneca ($10,000 USD or above in a single calendar year), PharmaMar ($10,000 USD or above in a single calendar year), Tesaro/GSK (less than $10,000 USD in a single calendar year), MSD Oncology (less than $10,000 USD in a single calendar year)
Kyung Hae Jung
Consulting or Advisory Role: Roche Korea (less than $10,000 USD in a single calendar year), AstraZeneca Korea (less than $10,000 USD in a single calendar year), Celgene Korea (less than $10,000 USD in a single calendar year), Eisai Korea (less than $10,000 USD in a single calendar year), Takeda Pharmaceuticals Korea (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year), Everest Medicine (less than $10,000 USD in a single calendar year), Merck (less than $10,000 USD in a single calendar year), Bixink (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year), Eisai (less than $10,000 USD in a single calendar year), Pfizer (less than $10,000 USD in a single calendar year), Daiichi Sankyo/AstraZeneca (less than $10,000 USD in a single calendar year), MSD (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year)
Iwona Ługowska
Stock and Other Ownership Interests: Amgen, Roche, BMS, Janssen, AstraZeneca, Agenus, Macrogenics, Celon, MSD, Menarini, Pfizer, Sanofi, BeiGene, Jacobio, Loxo, Rhizen, Takeda, Cullinan Oncology
Research Funding: Roche, AGENUS
Travel, Accommodations, Expenses: BMS
Luis Manso
Consulting or Advisory Role: Roche/Genentech, AstraZeneca, Novartis, Pfizer, Tesaro, Eisai, Lilly, Clovis Oncology, Pierre Fabre, GSK
Speakers' Bureau: Roche/Genentech, Novartis, Pfizer, AstraZeneca, Lilly, Tesaro
Travel, Accommodations, Expenses: Tesaro, MSD Oncology, GSK
Aránzazu Manzano
Consulting or Advisory Role: GSK (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year)
Speakers' Bureau: GSK (less than $10,000 USD in a single calendar year), AstraZeneca Spain (less than $10,000 USD in a single calendar year), PharmaMar (less than $10,000 USD in a single calendar year), Sanofi (less than $10,000 USD in a single calendar year), MSD Oncology (less than $10,000 USD in a single calendar year), LEO Pharma (less than $10,000 USD in a single calendar year), Rovi (less than $10,000 USD in a single calendar year)
Research Funding: AstraZeneca ($10,000 USD or above in a single calendar year)
Travel, Accommodations, Expenses: GlaxoSmithKline (less than $10,000 USD in a single calendar year), AstraZeneca Spain (less than $10,000 USD in a single calendar year)
Bohuslav Melichar
Honoraria: Roche (less than $10,000 USD in a single calendar year), Pfizer (less than $10,000 USD in a single calendar year), Bristol Myers Squibb (less than $10,000 USD in a single calendar year), Astellas Pharma (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year), MSD (less than $10,000 USD in a single calendar year), Merck Serono (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year), Eisai (less than $10,000 USD in a single calendar year), Lilly (less than $10,000 USD in a single calendar year)
Consulting or Advisory Role: Roche (less than $10,000 USD in a single calendar year), Pfizer (less than $10,000 USD in a single calendar year), Bristol Myers Squibb (less than $10,000 USD in a single calendar year), Astellas Pharma (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year), MSD (less than $10,000 USD in a single calendar year), Merck Serono (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year), Eisai (less than $10,000 USD in a single calendar year), Lilly (less than $10,000 USD in a single calendar year)
Travel, Accommodations, Expenses: Bristol Myers Squibb (less than $10,000 USD in a single calendar year), Merck Serono, AstraZeneca, MSD
Salvatore Siena
Stock and Other Ownership Interests: Guardant Health (shares of a publicly traded Company equal or less than $50,000 in value, or an equity interest in a privately held Company equal or less than 5%), Myriad Genetics (shares of a publicly traded Company equal or less than $50,000 in value, or an equity interest in a privately held Company equal or less than 5%)
Consulting or Advisory Role: Bayer (less than $10,000 USD in a single calendar year), Bristol Myers Squibb (less than $10,000 USD in a single calendar year), Daiichi Sankyo (less than $10,000 USD in a single calendar year), Merck (less than $10,000 USD in a single calendar year), Novartis (less than $10,000 USD in a single calendar year), CheckmAb (less than $10,000 USD in a single calendar year), Agenus (less than $10,000 USD in a single calendar year), AstraZeneca (less than $10,000 USD in a single calendar year), GSK (less than $10,000 USD in a single calendar year), MSD Oncology (less than $10,000 USD in a single calendar year), Pierre Fabre (less than $10,000 USD in a single calendar year), Seagen (less than $10,000 USD in a single calendar year), T-One Therapeutics (less than $10,000 USD in a single calendar year)
Research Funding: MSD Oncology ($10,000 USD or above in a single calendar year)
Patents, Royalties, Other Intellectual Property: Amgen
Travel, Accommodations, Expenses: Amgen, Bayer, Roche
Daniil Stroyakovskiy
Speakers' Bureau: Roche/Genentech, Bristol Myers Squibb, BioCad, AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca, Novartis, Roche
Anitra Fielding
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Yan Ma
Employment: AstraZeneca, BeiGene
Stock and Other Ownership Interests: BeiGene
Travel, Accommodations, Expenses: AstraZeneca
Soham Puvvada
Employment: AstraZeneca
Stock and Other Ownership Interests: AstraZeneca
Research Funding: AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Norah Shire
Employment: AstraZeneca
Leadership: Anzu Partners
Jung-Yun Lee
Consulting or Advisory Role: AstraZeneca, MSD, Roche, Takeda
Research Funding: Clovis Oncology ($10,000 USD or above in a single calendar year), Immunogen ($10,000 USD or above in a single calendar year), Janssen Oncology ($10,000 USD or above in a single calendar year), Merck ($10,000 USD or above in a single calendar year), MSD ($10,000 USD or above in a single calendar year), Synthon ($10,000 USD or above in a single calendar year), Eisai ($10,000 USD or above in a single calendar year), Mersana ($10,000 USD or above in a single calendar year), Ascendis Pharma ($10,000 USD or above in a single calendar year), AstraZeneca ($10,000 USD or above in a single calendar year), Novartis ($10,000 USD or above in a single calendar year), OncoQuest Pharmaceutical ($10,000 USD or above in a single calendar year), Roche ($10,000 USD or above in a single calendar year), Seagen ($10,000 USD or above in a single calendar year), Takeda ($10,000 USD or above in a single calendar year)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Najjar MK, Manore SG, Regua AT, et al. : Antibody-drug conjugates for the treatment of HER2-positive breast cancer. Genes (Basel) 13:2065, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan M, Schwaederle M, Arguello D, et al. : HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev 34:157-164, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, et al. : Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177-182, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Xing F, Gao H, Chen G, et al. : CMTM6 overexpression confers trastuzumab resistance in HER2-positive breast cancer. Mol Cancer 22:6, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh D-Y, Bang Y-J: HER2-targeted therapies – a role beyond breast cancer. Nat Rev Clin Oncol 17:33-48, 2020 [DOI] [PubMed] [Google Scholar]
- 6.Owen DH, Singh N, Ismaila N, et al. : Therapy for stage IV non–small-cell lung cancer with driver alterations: ASCO living guideline, version 2022.2. J Clin Oncol 41:e10-e20, 2023 [DOI] [PubMed] [Google Scholar]
- 7.Shah MA, Kennedy EB, Alarcon-Rozas AE, et al. : Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO guideline. J Clin Oncol 41:1470-1491, 2023 [DOI] [PubMed] [Google Scholar]
- 8.Giordano SH, Franzoi MA, Temin S, et al. : Systemic therapy for advanced human epidermal growth factor receptor 2–positive breast cancer: ASCO guideline update. J Clin Oncol 40:2612-2635, 2022 [DOI] [PubMed] [Google Scholar]
- 9.Cervantes A, Adam R, Roselló S, et al. : Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 34:10-32, 2023 [DOI] [PubMed] [Google Scholar]
- 10.Diver E J, Foster R, Rueda BR, et al. : The therapeutic challenge of targeting HER2 in endometrial cancer. Oncologist 20:1058-1068, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gadducci A, Cosio S: Pharmacological treatment of patients with metastatic, recurrent or persistent cervical cancer not amenable by surgery or radiotherapy: State of art and perspectives of clinical research. Cancers (Basel) 12:2678, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Fuchs HE, et al. : Cancer statistics, 2022. CA Cancer J Clin 72:7-33, 2022 [DOI] [PubMed] [Google Scholar]
- 13.Tariq NU, McNamara MG, Valle JW: Biliary tract cancers: Current knowledge, clinical candidates and future challenges. Cancer Manag Res 11:2623-2642, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogitani Y, Aida T, Hagihara K, et al. : DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 22:5097-5108, 2016 [DOI] [PubMed] [Google Scholar]
- 15.US Food and Drug Administration (FDA) : ENHERTU (fam-trastuzumab deruxtecan-nxki): Highlights of prescribing information (PI). 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761139s024lbl.pdf
- 16.European Medicines Agency (EMA) : ENHERTU (trastuzumab deruxtecan): Summary of product characteristics (SmPC) [Internet]. 2023. https://www.ema.europa.eu/en/documents/product-information/enhertu-epar-product-information_en.pdf
- 17.Daiichi Sankyo : Press release. ENHERTU® approved in Japan as first HER2 directed therapy for patients with HER2 mutant metastatic non-small cell lung cancer. 2023. https://www.daiichisankyo.com/files/news/pressrelease/pdf/202308/20230823_E.pdf
- 18.Tsurutani J, Iwata H, Krop I, et al. : Targeting HER2 with trastuzumab deruxtecan: A dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov 10:688-701, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daiichi Sankyo : Press release. ENHERTU® granted two breakthrough therapy designations in U.S. for patients across multiple HER2 expressing cancers. 2023. https://www.daiichisankyo.com/files/news/pressrelease/pdf/202308/20230831_E2.pdf
- 20.Oken MM, Creech RH, Tormey DC, et al. : Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649-656, 1982 [PubMed] [Google Scholar]
- 21.Bartley AN, Washington MK, Ventura CB, et al. : HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: Guideline from the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Arch Pathol Lab Med 140:1345-1363, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Schwartz LH, Litière S, de Vries E, et al. : RECIST 1.1—Update and clarification: From the RECIST committee. Eur J Cancer 62:132-137, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omar N, Yan B, Salto-Tellez M: HER2: An emerging biomarker in non-breast and non-gastric cancers. Pathogenesis 2:1-9, 2015 [Google Scholar]
- 24.Stinchcombe T, Monk BJ, Okines AF, et al. : SGNTUC-019: Phase 2 basket study of tucatinib and trastuzumab in previously treated solid tumors with HER2 alterations (trial in progress). J Clin Oncol 39, 2021. (suppl 15; abstr TPS3151) [Google Scholar]
- 25.Taniguchi H, Yagisawa M, Satoh T, et al. : Tissue-agnostic efficacy of trastuzumab deruxtecan (T-DXd) in advanced solid tumors with HER2 amplification identified by plasma cell-free DNA (cfDNA) testing: Results from a phase 2 basket trial (HERALD/EPOC1806). J Clin Oncol 41, 2023. (suppl 16; abstr 3014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erickson BK, Zeybek B, Santin AD, et al. : Targeting human epidermal growth factor receptor 2 (HER2) in gynecologic malignancies. Curr Opin Obstet Gynecol 32:57-64, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamarca A, Palmer DH, Wasan HS, et al. : Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol 22:690-701, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohba A, Morizane C, Kawamoto Y, et al. : Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial). J Clin Oncol 40, 2022. (suppl 16; abstr 4006) [DOI] [PubMed] [Google Scholar]
- 29.Swain SM, Nishino M, Lancaster LH, et al. : Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis—Focus on proactive monitoring, diagnosis, and management. Cancer Treat Rev 106:102378, 2022 [DOI] [PubMed] [Google Scholar]
- 30.Rugo HS, Bianchini G, Cortes J, et al. : Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open 7:100553, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell CA, Modi S, Iwata H, et al. : Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open 7:100554, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tateo V, Marchese PV, Mollica V, et al. : Agnostic approvals in oncology: Getting the right drug to the right patient with the right genomics. Pharmaceuticals (Basel) 16:614, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at: https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/.